Abstract
Introduction
Frequent HIV testing of at‐risk individuals is crucial to detect and treat infections early and prevent transmissions. We assessed the effect of reminders on HIV retesting uptake.
Methods
The study was conducted within a programme involving four facilities providing free‐of‐charge HIV, syphilis and hepatitis B and C testing and counselling in northern Thailand. Individuals found HIV negative and identified at risk by counsellors were invited to participate in a three‐arm, open‐label, randomized, controlled trial comparing: (a) “No Appointment & No Reminder” (control arm); (b) “No Appointment but Reminder”: short message service (SMS) sent 24 weeks after the enrolment visit to remind booking an appointment, and sent again one week later if no appointment was booked; and (c) “Appointment & Reminder”: appointment scheduled during the enrolment visit and SMS sent one week before appointment to ask for confirmation; if no response: single call made within one business day. The primary endpoint was a HIV retest within seven months after the enrolment visit. The cost of each reminder strategy was calculated as the sum of the following costs in United States dollars (USD): time spent by participants, counsellors and hotline staff; phone calls made; and SMS sent. The target sample size was 217 participants per arm (651 overall).
Results
Between April and November 2017, 651 participants were randomized. The proportion presenting for HIV retesting within seven months was 11.2% (24/215) in the control arm, versus 19.3% (42/218) in “No Appointment but Reminder” (p = 0.023) and 36.7% (80/218) in “Appointment & Reminder” (p < 0.001). Differences in proportions compared to the control arm were respectively +8.1% (95% CI: +1.4% to +14.8%) and +25.5% (+17.9% to +33.2%). The incremental cost‐effectiveness ratios of “No Appointment but Reminder” and “Appointment & Reminder” compared to the control arm were respectively USD 0.05 and USD 0.14 per participant for each 5% increase in HIV retesting uptake within seven months.
Conclusions
Scheduling an appointment and sending a reminder one week before was a simple, easy‐to‐implement and affordable intervention that significantly increased HIV retesting uptake in these at‐risk individuals. The personal phone call to clients probably contributed, and also improved service efficiency.
Keywords: reminder, appointment, text messaging, cell phone, testing, retesting
1. Introduction
Ensuring that individuals at risk of HIV infection are frequently tested is essential to treat new infections as early as possible after virus acquisition and, thus, prevent new transmissions. However, in 2018, only 79% of people living with HIV worldwide knew their status [1] and 1.7 million new HIV infections occurred [2].
After a generalized HIV epidemic which started in the late 1980s, Thailand is now facing an epidemic mainly concentrated in key populations, in particular in men who have sex with men [3]. In 2018, Thailand reported that it achieved the first UNAIDS 90% target [1, 4]. However, it also reported that 53% of newly diagnosed individuals had a CD4 cell count <200 cells/mm3 at diagnosis and that 18,000 people died of AIDS‐related causes [3], suggesting that HIV was often diagnosed too late. In this context, the Thai government is supporting all initiatives to increase access to HIV testing and linkage to care.
The 2019 World Health Organization (WHO) guidelines on HIV testing services suggested a wide range of approaches to increase demand for HIV testing services [5] but no specific strategies to retain at‐risk individuals into HIV prevention/sexual health programmes – with or without pre‐exposure prophylaxis – notably those involving reminders. The wide availability of mobile phones (8.2 billion subscriptions worldwide in 2018, including 6.5 billion in developing countries [6]) may provide opportunities to improve health outcomes due to their low cost [7], convenience and variety of communication means (calls, voice messages, short message service (SMS), mobile applications). For example, SMS‐based interventions have been found effective in improving adherence to antiretroviral therapy in HIV‐infected people [8] and WHO recommend their use in this context [7]. SMS reminders also have the potential to increase the uptake of frequent HIV testing in uninfected at‐risk individuals [9]. However, previous studies had limitations: they either were observational [10, 11, 12] or assessed the effect of sending SMS reminders to retest participants presenting with a suspected acute HIV infection one month after a first negative test, a very specific situation [13].
We conducted a randomized controlled trial in Thailand to evaluate whether reminders could increase the uptake of HIV retesting by at‐risk individuals.
2. Methods
2.1. Study setting
“Napneung” was designed as a research project aimed at evaluating new methods to increase the frequency of HIV testing by at‐risk individuals [14]. Trained nurses and medical technologists provided free‐of‐charge anonymous and confidential testing and counselling in four settings in Chiang Mai and Chiang Rai, two medium‐sized cities in northern Thailand. All individuals aged 15 years or above were welcome for testing and counselling, regardless of gender, sexual orientation, risk behaviour or history of HIV testing. Outreach of at‐risk individuals relied on distribution of vouchers in public places, posters, social media and digital advertising campaigns. After an appointment made through a 24/7 telephone line or online through the project website, clients were provided with pre‐ and post‐test counselling and rapid testing for HIV, syphilis, hepatitis B surface antigen and hepatitis C antibodies in less‐than‐one‐hour sessions. While waiting for the test results, clients were invited to complete self‐administered questionnaires about their sociodemographic and behavioural characteristics on a tablet computer. All clients were provided with a unique study identification card and a secret code so that those presenting for retesting could be linked to previously recorded data. No incentives were provided for presenting for retesting.
2.2. Study design and population
Within the Napneung project, we implemented a three‐arm randomized controlled study to evaluate and compare the effect of scheduling appointments and sending reminders on the uptake of HIV retesting among at‐risk individuals (ClinicalTrials.gov: NCT02752152). This study was nested within another randomized study designed to compare the efficacy of three counselling methods in terms of propensity to present for retesting [14].
Clients were eligible to participate in the study if they were aged 18 years or above, tested HIV negative and reported risks of HIV infection. Guidelines used to consider that individuals at risk of HIV acquisition were those from key populations [15]. However, the definition of an individual's risk and its assessment are difficult. In this study, we decided to rely on counsellors, who were trained to base their assessment on clients' self‐reported behaviours during the last six months and to be cautious about intention‐behaviour gaps [16]. This operational definition is closer to the 2019 WHO guidelines, which recommend HIV retesting at least annually in people who have ongoing HIV‐related risks [5].
Consenting participants were randomly assigned 1:1:1 to one of the following three arms:
“No Appointment & No Reminder” (control arm): at the enrolment visit, the counsellor encouraged the client to present for HIV retesting within three to six months or even within less than three months if the perceived risk was high; then, no further contact was made;
“No Appointment but Reminder”: at the enrolment visit, the counsellor encouraged the client to present for HIV retesting within three to six months or even within less than three months if the perceived risk was high; 24 weeks later, the following SMS was sent: “Time to visit Napneung again! Please make an appointment at [hotline phone number]”; if no appointment was booked within one week, the same SMS was sent again once; or
“Appointment & Reminder”: at the enrolment visit, the counsellor and the client agreed on a date and time for the next HIV test, within three to six months or even within less than three months if the perceived risk was high; one week before the scheduled appointment, the following SMS was sent: “Napneung: appointment on [date and time] at [testing facility]. Please reply 'Yes' to confirm”; one business day later, if the client did not reply to the SMS, one phone call was made by our hotline staff to clarify the appointment status.
Randomization was performed with a block size of six and stratified per counsellor and by counselling method received as part of the three‐arm counselling study.
2.3. Reminders
All participants randomized to arms “No Appointment but Reminder” or “Appointment & Reminder” were invited to provide a mobile phone number. Those who did not provide a phone number or did not agree for the use of their phone number to receive reminders were eligible to participate in the study but were not sent any reminders. Mobile phone numbers and all participant data were collected anonymously on tablet computers, encrypted, transmitted and stored in real‐time within a central database hosted on a secured server with restricted access and extended traceability features. Verification of mobile phone numbers was performed and documented immediately after entry by sending a test SMS to participants. Reminders for retesting were only sent to participants who agreed for the use of their phone number to receive reminders. SMS were sent at 5.45pm every day by an automated system according to criteria and characteristics recorded in the study database, including participant’s preferred language (Thai, Burmese, Shan or English). Reminders were not sent to participants who presented for retesting earlier than scheduled. Phone calls were made by the project hotline staff between 4pm and 8pm. After the first retest visit (if any), participants were offered the same reminder procedures for further retest visits until the end of the study follow‐up period (31 January 2019), unless opting out.
2.4. Endpoints
The primary endpoint was a HIV retest at any of the study facilities within seven months after the enrolment visit, and the secondary endpoint was a HIV retest at any of the study facilities within 12 months.
2.5. Sample size calculation
We assumed that 25% of participants in the control arm and 40% in each experimental arm would present for HIV retesting within seven months after the enrolment visit. One interim analysis for efficacy was planned when half of participants have reached the primary endpoint assessment timepoint, but this analysis was not performed because the target accrual for the final analysis had already been reached by that time. The overall two‐sided type I error for pairwise comparisons between the control arm and each experimental arm was set to 0.001 for the interim analysis and 0.049 for the final analysis, with adjustment for multiple comparisons using Sidak correction, and the power was set to 90%. Based on these parameters and Fisher's exact tests, the final analysis was planned when 217 participants per arm, that is, 651 overall, reached the primary endpoint assessment timepoint.
2.6. Statistical analyses
For each arm, we calculated the proportions of participants presenting for retesting within seven months and within twelve months after enrolment and their 95% confidence intervals (CI) using the Clopper‐Pearson method. Comparisons of these proportions between each experimental arm and the control arm were performed using two‐sided Fisher’s exact tests. The effect of each experimental arm relative to the control arm was expressed as a difference in proportions, with 95% CI based on the normal approximation to the binomial distribution. We assessed whether the effect of the reminder methods on HIV retesting uptake within seven months was different across counselling methods by testing the interaction between the effects of reminder and of counselling methods on HIV retesting uptake in a logistic regression model (more details are provided in the footnotes of Table S1). A comparison between the two experimental arms was performed in a post hoc analysis.
2.7. Intervention costs and cost‐effectiveness
We calculated the total cost of each reminder strategy as the sum of the following costs (where applicable): time spent by participants, counsellors and hotline staff; phone calls made; and SMS sent. Cost of time spent by participants was estimated based on actual time spent with study staff and on the monthly income that they reported in the self‐administered questionnaire or, if null or missing, on an imputed monthly income corresponding to the minimum wage in Chiang Mai. Cost of time spent by counsellors and hotline staff was estimated based on actual time spent with participants and on their monthly income. We estimated the main fixed costs, but excluded them from the cost calculations because the information technology (IT) infrastructure to send SMS reminders could have been used for many more clients than those who participated in this study [17]. All costs were adjusted for the consumer price index as of 2015 and converted from Thai Baht (THB) to United States dollars (USD) at the yearly average exchange rate for 2015 (THB 1 = USD 0.02919). The incremental cost‐effectiveness ratio of each experimental arm compared to the control arm was calculated as the difference in mean costs per participant between these two strategies divided by the difference in proportions of participants presenting for retesting within seven months between these two strategies.
2.8. Ethical considerations
All procedures performed in this study were in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. The study protocol was reviewed and approved by the ethics committees of the Faculty of Associated Medical Sciences, Chiang Mai University, and of Chiangrai Prachanukroh Hospital. An Advisory Committee reviewed the study progress annually. In addition, the study was reviewed and discussed quarterly by a Community Advisory Board.
3. Results
3.1. Study population
Between 25 April 2017 and 1 December 2017, a total of 651 participants were randomized: 215 to “No Appointment & No Reminder,” 218 to “No Appointment but Reminder” and 218 to “Appointment & Reminder” (Figure 1). The distribution of participants’ characteristics and counselling arm was similar between arms (Table 1). Of the 651 participants, 359 (55%) were men, median age was 24 (interquartile range (IQR), 22 to 31) years, 401 (63%) had never been tested for HIV, 236 (37%) had at least two sexual partners in the last three months and 261 (41%) used condoms inconsistently in the last three months. No protocol deviations occurred during the study.
Figure 1.
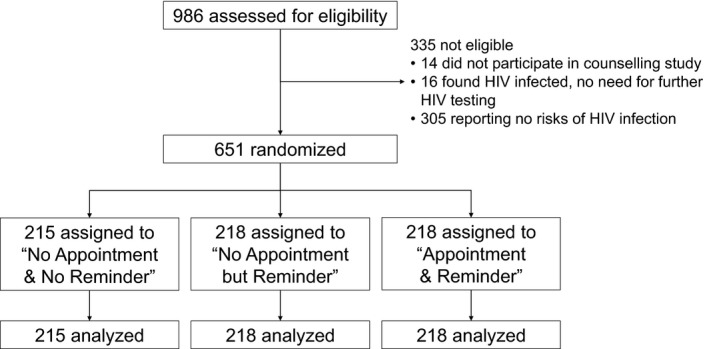
CONSORT diagram of the disposition of participants.
Table 1.
Participants' characteristics by arm
| Participants' characteristics | No appointment & no reminder (N = 215) | No appointment but reminder (N = 218) | Appointment & reminder (N = 218) | Overall |
|---|---|---|---|---|
| Gender | ||||
| Male | 123 (57%) | 120 (55%) | 116 (53%) | 359 (55%) |
| Female | 91 (42%) | 98 (45%) | 99 (45%) | 288 (44%) |
| Male‐to‐female transgender | 1 (<1%) | 0 (0%) | 3 (1%) | 4 (1%) |
| Age <25 years | 124 (58%) | 131 (60%) | 122 (56%) | 377 (58%) |
| Country | ||||
| Thailand | 211 (98%) | 213 (98%) | 214 (98%) | 638 (98%) |
| Myanmar | 4 (2%) | 2 (1%) | 3 (1%) | 9 (1%) |
| Other | 0 (0%) | 3 (1%) | 1 (<1%) | 4 (1%) |
| Pursued higher education | 131/212 (62%) | 129/214 (60%) | 130/217 (60%) | 390/643 (61%) |
| Man who has ever had sex with men | 57 (27%) | 54 (25%) | 42 (19%) | 153 (24%) |
| Previously tested for HIV | 84/209 (40%) | 79/213 (37%) | 73/215 (34%) | 236/637 (37%) |
| Counselling method received | ||||
| Standard counselling | 89 (41%) | 86 (39%) | 90 (41%) | 265 (41%) |
| Computer‐assisted counselling | 88 (41%) | 93 (43%) | 88 (40%) | 269 (41%) |
| On‐demand counselling | 38 (18%) | 39 (18%) | 40 (18%) | 117 (18%) |
| Agreed timeline for retesting | ||||
| Within less than three months after enrolment visit | 22 (10%) | 25 (11%) | 25 (11%) | 72 (11%) |
| Within three to six months after enrolment visit | 193 (90%) | 193 (89%) | 193 (89%) | 579 (89%) |
| Risks of HIV infection | ||||
| Inconsistent condom use in last three months | 80/209 (38%) | 88/213 (41%) | 93/213 (44%) | 261/635 (41%) |
| ≥2 sexual partners in last three months | 83/208 (40%) | 78/211 (37%) | 75/212 (35%) | 236/631 (37%) |
| Positive syphilis test | 12 (6%) | 5 (2%) | 6 (3%) | 23 (4%) |
| STI symptoms | 8/207 (4%) | 7/205 (3%) | 9/207 (4%) | 24/619 (4%) |
| Ever had an STI | 30/208 (14%) | 17/214 (8%) | 22/212 (10%) | 69/634 (11%) |
| Ever received benefits in exchange of sex | 9/208 (4%) | 6/210 (3%) | 15/207 (7%) | 30/625 (5%) |
| Ever provided benefits in exchange of sex | 14/209 (7%) | 18/210 (9%) | 15/211 (7%) | 47/630 (7%) |
| Ever had sex outdoors | 30/211 (14%) | 29/212 (14%) | 23/211 (11%) | 82/634 (13%) |
| Ever injected drugs | 6/214 (3%) | 5/215 (2%) | 8/216 (4%) | 19/645 (3%) |
3.2. HIV retesting
In the primary analysis at seven months, the proportion of participants who had presented for HIV retesting was 11.2% (24/215; 95% CI: 7.3% to 16.2%) in “No Appointment & No Reminder,” as compared to 19.3% (42/218; 95% CI: 14.3% to 25.1%) in “No Appointment but Reminder” (p = 0.023) and to 36.7% (80/218; 95% CI: 30.3% to 43.5%) in “Appointment & Reminder” (p < 0.001) (Figure 2a). Differences in proportions compared to the control arm were respectively +8.1% (95% CI: +1.4% to +14.8%) and +25.5% (95% CI: +17.9% to +33.2%). In a post hoc analysis comparing the two experimental arms, the p‐value was <0.001 and the difference in proportions was +17.4% (95% CI: +9.2% to +25.7%). No interaction was observed between reminder and counselling methods (Table S1).
Figure 2.
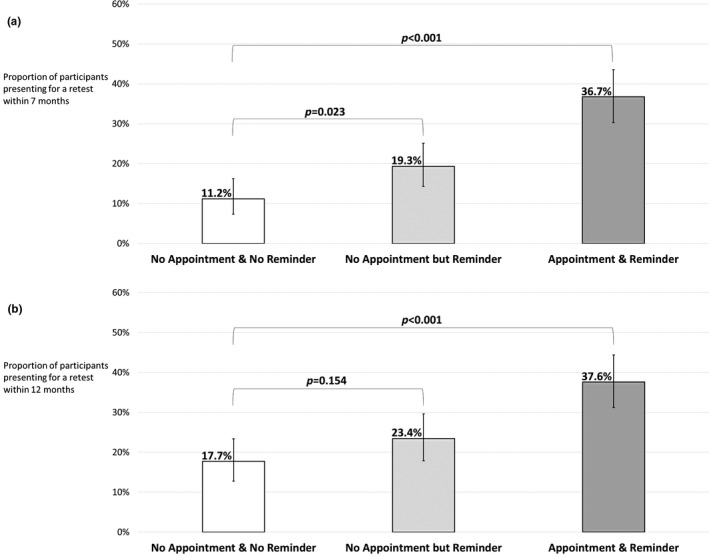
Participants retested for HIV within (a) seven months (b) twelve months by arm. “No Appointment & No Reminder”: clients encouraged to present for HIV retesting within three to six months or even within less than three months if the perceived risk was high, then no further contact made; “No Appointment but Reminder”: short message service (SMS) sent 24 weeks after the enrolment visit to remind booking an appointment, and sent again one week later if no appointment was booked; “Appointment & Reminder”: appointment scheduled during the enrolment visit and SMS sent one week before appointment to ask for confirmation; if no response: single call made within one business day. Bars represent 95% confidence intervals calculated using the Clopper–Pearson method. The overall two‐sided type I error for the final analysis was set to 0.049 for pairwise comparisons between the control arm and each experimental arm, that is, 0.0248 per comparison using Sidak correction. p‐values were derived from two‐sided Fisher’s exact tests. In a post hoc analysis, the p‐value for the comparison between the two experimental arms was <0.001 (a) 0.002 (b).
In the secondary analysis at 12 months, the proportion of participants who had presented for HIV retesting was 17.7% (38/215; 95% CI: 12.8% to 23.4%) in “No Appointment & No Reminder,” as compared to 23.4% (51/218; 95% CI: 17.9% to 29.6%) in “No Appointment but Reminder” (p = 0.15) and to 37.6% (82/218; 95% CI: 31.2% to 44.4%) in “Appointment & Reminder” (p < 0.001) (Figure 2b). In a post hoc analysis comparing the two experimental arms, the p‐value was 0.002. Of note, while no participant tested HIV positive within the first seven months, one in “No Appointment & No Reminder” tested positive between seven and twelve months.
By the end of the study follow‐up period, that is, within a median of 17.8 (IQR, 16.2 to 19.8) months after the enrolment visit, the proportions of participants who had presented for HIV retesting were 21.9% (47/215) in “No Appointment & No Reminder,” 26.2% (57/218) in “No Appointment but Reminder” and 37.6% (82/218) in “Appointment & Reminder.”
3.3. Delivery and outcome of retest reminders in experimental arms
A detailed description of delivery and outcome of retest reminders within the seven months after the enrolment visit is provided in Figures S1 and S2.
Of the 218 participants in arm “No Appointment but Reminder,” 28 (13%) called for a new appointment before receiving the SMS reminders (including four who did not receive them due to technical issues), 6 (3%) after receiving the first SMS and 8 (4%) after receiving the second SMS, and 176 (81%) did not call.
Of the 218 participants in arm “Appointment & Reminder,” 4 (2%) confirmed the retest appointment despite not receiving reminders due to technical issues, 17 (8%) confirmed it by SMS reply (13 on the day of SMS receipt (median of 47 (IQR, 13 to 96) minutes after), 3 on the following day and 1 two days later), 59 (27%) confirmed it after receiving the subsequent phone call, and 138 (63%) did not confirm it. Of the 80 participants who presented for HIV retesting within seven months, 37 presented on the same day as originally scheduled at the enrolment visit, 16 earlier than scheduled (median of 2 (IQR, 1 to 5) days before) and 27 later than scheduled (median of 6 (IQR, 2 to 9) days after).
Of the 401 participants who were sent SMS reminders, 125 (31%) did not receive them, for the following reasons (several possible): SMS blocked by the participant's mobile operator (n = 82), participant's mobile phone number no longer existing (n = 30) or gateway error (n = 14).
3.4. Intervention costs and cost‐effectiveness
We estimated that the total fixed cost was about USD 15,000, including developing the IT infrastructure, developing an in‐house software to send SMS messages and the protocol for SMS delivery, and others. The mean cost per participant of the reminder strategies was USD 0.15 for “No Appointment & No Reminder,” USD 0.22 for “No Appointment but Reminder” and USD 0.84 for “Appointment & Reminder,” mostly reflecting the costs associated with time spent by participants and study staff (Table 2). The incremental cost‐effectiveness ratios of “No Appointment but Reminder” and “Appointment & Reminder” compared to “No Appointment & No Reminder” were respectively USD 0.05 and USD 0.14 per participant for each 5% increase in uptake of HIV retesting within seven months, and that of “Appointment & Reminder” compared to “No Appointment but Reminder” was USD 0.18.
Table 2.
Details of mean costs per participant for each reminder strategy from a societal perspective, in USD
| Mean costs per participant | No appointment & no reminder (N = 215) | No appointment but reminder (N = 218) | Appointment & reminder (N = 218) |
|---|---|---|---|
| Costs borne by participants | USD 0.09 | USD 0.10 | USD 0.19 |
| Time spent a | USD 0.03 | USD 0.03 | USD 0.17 |
| Phone calls made | USD 0.06 | USD 0.07 | USD 0.01 |
| SMS sent | None | None | USD 0.01 |
| Counsellor‐related costs | None | None | USD 0.35 |
| Time spent b | None | None | USD 0.35 |
| Hotline staff related costs | USD 0.06 | USD 0.12 | USD 0.30 |
| Time spent b | USD 0.06 | USD 0.08 | USD 0.17 |
| Phone calls made | None | None | USD 0.08 |
| SMS sent | None | USD 0.04 | USD 0.05 |
| Total mean cost per participant | USD 0.15 | USD 0.22 | USD 0.84 |
All costs were adjusted for the consumer price index as of 2015 and converted from THB to USD at the yearly average exchange rate for 2015 (THB 1 = USD 0.02919). SMS, short message service; USD, United States dollars.
Cost of time spent by participants was estimated based on actual time spent with study staff and on the monthly income that they reported in the self‐administered questionnaire or, if null or missing, on an imputed monthly income corresponding to the minimum wage in Chiang Mai (USD 280.22);
Cost of time spent by counsellors and hotline staff was estimated based on actual time spent with participants and on their monthly income.
4. Discussion
In this randomized trial, we found that individuals at risk of HIV infection were significantly more likely to present for HIV retesting within seven months after a first visit if they had an appointment scheduled during their first visit and were reminded of it (37%) or received no appointment but a reminder (19%) than if they received no appointment and no reminder (11%).
Uptake of HIV retesting in the arm where no appointment was scheduled in advance but an SMS reminder was sent (followed by a second one a week later if no appointment was made) was significantly higher than in the control arm, but remained low. Sending SMS reminders had a significant but limited positive effect, increasing from 11% to 19% the proportion of clients presenting for HIV retesting. Interestingly, the proportion of those who called for an appointment before receiving any SMS reminder was 13% (28/218), consistent with the 11% observed in the arm with no intervention. Sending one SMS reminder had little additional effect (3%; 6/218), and a second SMS reminder one week later as well (4%; 8/218). Using phone call reminders may have been more effective than sending SMS reminders to increase HIV retesting uptake.
The most active strategy, that is, when an appointment was scheduled in advance and a reminder sent, was the most successful. The higher success of this strategy compared to the other experimental strategy may be partly because reminders were sent earlier, the content of the SMS reminder was more personalised or calling participants was more effective than SMS [12] although, by design, the specific contribution of these components of the intervention could not be assessed. Interestingly, only 8% (17/218) confirmed the appointment by SMS reply, while 27% (59/218) confirmed it after receiving the subsequent phone call. This finding suggests that the provider's proactive and personalised attitude was a key component explaining the success of this intervention. One additional advantage of the most successful strategy is that after calling the participant, the hotline officer recorded immediately in the system whether the retest appointment was confirmed or cancelled. Therefore, the counsellors knew the status of the appointment several days prior to the scheduled appointment and managed their time more efficiently than in the other two reminder strategies.
Nearly one third of the clients who were sent SMS reminders did not receive them: many SMS were automatically blocked by mobile operators, a common issue with the use of automated SMS sending systems. Sending manually SMS reminders after notification that the automated SMS was blocked may add significant cost. Some mobile phone numbers were no longer existing, suggesting that some clients changed their phone number. Implementing phone call reminders in settings where changing mobile phone numbers is frequent may therefore be challenging. Sending reminders through instant messaging applications may be an alternative but a significant part of young people cannot afford mobile Internet access.
Results from the cost analysis indicate that the two experimental reminder strategies were affordable, each with a total mean cost per client of less than USD 1. Both strategies were highly cost‐effective in terms of HIV retesting uptake. Compared to the control arm, the additional mean cost per participant to increase by 5% the uptake of HIV retesting within seven months was less than USD 0.15, showing that the costs associated with these strategies are not a limitation for implementation. Indeed, we did not include fixed costs in the cost calculations, but one can assume that even a costly software developed at a national level for all HIV testing facilities would remain highly cost‐effective.
Strengths of our study were the rigorous methodology in a setting with experience in clinical research implementation, the appropriateness of the study population and the absence of bias that we could suspect. A limitation was that we were not able to know whether clients presented for HIV retesting in other facilities. However, there is no reason to expect a difference between arms in the proportion of such clients. This proportion was probably small given the high satisfaction rate reported at the first visit [14]. Another limitation was that the selection of study participants was based on a risk assessment performed by counsellors, which could vary across counsellors. However, randomization was stratified per counsellor.
5. Conclusions
Scheduling an appointment and sending a reminder one week before was a simple, easy‐to‐implement and affordable intervention that significantly increased the uptake of HIV retesting in this population of at‐risk individuals. The personal phone call to clients probably contributed, and also improved service efficiency. This intervention can be implemented at country level – including in resource‐limited settings – and has the potential to increase the retention of at‐risk individuals into HIV prevention services.
Competing interest
The authors declare no competing interest.
Authors' Contributions
NS, PA, JYM, LD, SC and GJ conceived and designed the study. LD created the IT tools. NS and LS analysed the data. NS, PA, JYM, LD, LS, SC, SA, WK, PL, VS, JA, CR, WS, NNGH and GJ interpreted the data. NS drafted the manuscript and all other authors revised the manuscript. All authors have read and approved the final manuscript.
Supporting information
Figure S1. Delivery and outcome of retest reminders for the 218 participants in “No Appointment but Reminder.”
Figure S2. Delivery and outcome of retest reminders for the 218 participants in “Appointment & Reminder.”
Table S1. Participants retested for HIV within seven months by combination of counselling and reminder methods
Acknowledgements
The authors thank all study participants. The authors also thank the members of the Napneung Advisory Committee: Sumet Ongwandee, Sombat Thanprasertsuk and Nakorn Premsri (Department of Diseases Control, Ministry of Public Health, Nonthaburi, Thailand), Suchada Chaivoot (National Health Security Office, Nonthaburi, Thailand), Mukta Sharma (World Health Organization, Thailand Country Office, Nonthaburi, Thailand), Chutima Jaruwat and Withun Wongthip (Provincial Public Health Office, Chiang Mai, Thailand), Eric Fleutelot (French Embassy to Thailand, Bangkok, Thailand), Pongthorn Chanlearn (M‐Plus, Chiang Mai, Thailand), Ratchadet Reaukhamfu (Caremat, Chiang Mai, Thailand), Bram Press and Namisi Jate (MAP Foundation, Chiang Mai, Thailand), Boonnium Wongjaikham (Thai AIDS Treatment Action Group, Chiang Mai, Thailand), Thitipong Sangyoung (Thai Drug Users' Network, Chiang Mai, Thailand), Monthira Metha (Fah Mai Clinic, Chiang Mai, Thailand) and Maleerat Vandriesten (Empower, Chiang Mai, Thailand); All Napneung partners, in particular: ACCESS, CAREMAT, EMPOWER, MAP Foundation, M‐Plus, Namklong Colorful, PIMAN Center, PLAN International, Thai AIDS Treatment Action Group and Thai Drug Users' Network; Chiangrai Prachanukroh Hospital, Nakornping Hospital, Sanpatong Hospital, Sarapee Hospital and STIs Clinic of the Office of Disease Prevention and Control Region 1; Chiang Rai Rajabhat University and Mae Fah Luang University; Provincial Public Health Offices; and Department of Disease Control, Thai Ministry of Public Health; The members of the Napneung project team: Giuliana Zegarra, Paporn Mongkolwat, Prueksalada Khiaokham, Suphakrit Jitrarat and Wanlee Kongnim (project coordination); Ampika Kaewbundit, Areerat Kongphonoi, Chutharat Kasemrat, Duanpen Panyasak, Jiraporn Khamkon, Kanyanee Preechapongmit, Krisana Suphayos, Laddawan Laomanit, Nantawan Wangsaeng, Nilawan Chetacha, Ninutcha Paengsai, Panaddar Phutthakham, Paporn Mongkolwat, Phornphimon Moolnoi, Prapan Luangsook, Subenya Natthanaboon, Tanawan Samleerat, Thipsuda Krueyot, Tiwacha Thimakam, Wanlee Kongnim, Warunee Khamjakkaew, Warunee Srisuk and Woottichai Khamduang (testing and counselling); Boonnium Wongjaikham, Chalathon Jantarapanya, Chittiwa Phumsawai, Jamreon Teeravittayakul, Kanjana Saengkham, Malee Kaewprom, Monthira Metha, Nipa Chomphupa, Prapatsorn Chaisopa, Ratchadet Reaukhamfu, Rodjana Yeepua, Saidao Sanko, Sirintorn Juntong, Sompol Punyasriwichai, Supaluck Nammuang, Thitipong Sangyoung and Wipawan Wongsawasdi (PHPT Community Advisory Board in Chiang Mai); Akrkapong Preechapongmit, Anupab Jampatong, Anyamanee Khattiya, Apirak Jittarat, Areerat Kongponoi, Bancha Kumsiri, Boonrod Suwanpa, Chalita Sakrew, Chopet Nobpharat, Daoruang Sangchan, Daranrat Nuntasonbut, Dawaree Tongcheepcha, Dumrongdech Reunsung, Ekapan Chaiwangras, Jedsada Srijanta, Jinjutha Darasilp, Jirayut Sitasoy, Kajorndej Panichadul, Kajorndej Surapanichadul, Kamolpop Moungtanee, Kamonporn Preechapongmit, Kanok Fuslin, Kanyanee Kaewmamueng, Khwanchanok Saenkriang, Kitipong Daenpen, Kittikun Jantaracha, Kritsana Suphayos, Kulnaree Klomkaew, Kulthida Mueangchanta, Lukkana Sitiritkawin, Maliwan Budsuwan, Manthana Nunta, Mathurada Kanthalee, Metinee Khamwiang, Nattachai Kumket, Nattarika Prommatiam, Natthaporn Chinda, Nawakhun Prungrathanasen, Nonthacha Yodying, Nutnicha Parjarnthit, Paradee Anantakul, Parit Maimun, Pawita Tuithemwong, Phanusporn Moonnoo, Phetay Sakbunyuen, Pilaiwan Yodprasert, Pimwaree Jaiai, Pongpak Sananta, Pornpavee Saleesongsom, Pranee Srikampan, Prapatsorn Alongkornpradub, Ramida Burapa, Ramida Burapa, Rattanai Soparat, Ruekdee Kaewmuangma, Rutchawat Fakkaew, Sangdao Somsri, Saowalak Panyawong, Sarawin Phunyim, Sedtawut Kunnakulsoontorn, Sunirat Manemaung, Sunun Manowan, Supakit Anantakul, Supakorn Tulapong, Supanut Krittitasema, Supawadee Pongprapass, Suphakrit Jitrarat, Suporn Watanaporn, Sureepon Pratumma, Sutikarn Jarenjai, Suttipong Kawilapat, Tanakorn Khamwiang, Tanongsak Suriyakaew, Thanapol Wongkhamnan, Thanyalark Seeti, Theerapong Pinta, Thitipong Luecha, Tidarat Peungtonang, Torraporn Maneerat, Tunya Boonruean, Unyamanee Chantra, Visaka Ruankaew, Wachirapong Teerasawatt, Warakon Aranpitak, Warintorn Fakkaew, Warunee Srisuk, Wasana Chaiwong, Wasinee Poonpon, Watchara Kunthanang, Woottichai Sriwichai, Woranet Wangkhummuen, Woraphan Doungtip, Yatika Theptanee, Yokfah Meena, Yudtana Panyawong, Yupin Buain, Yuthana and Yuthaphom Hongsorn (outreach); Niphatta Mungkhala, Pongsak Pirom and Sakawrut Jitharidkul (hotline); Pra‐ornsuda Sukrakanchana, Rukchanok Peongjakta and Suwalai Chalermpantmetagul (monitoring); Ampika Kaewbundit, Chutharat Kasemrat, Jiraporn Khamkon, Jutatip Kaewmalee, Laddawan Laomanit, Nantawan Wangsaeng, Patcharida Insee, Phornphimon Moolnoi, Pimpinun Punyathi, Rumpaiphorn Saisom and Thamonphat Phitak (laboratory); Aye Min Latt, Hark Murng, Naruporn Rawanchaikul and Sineenart Nupradit (translation); Kanchana Than‐in‐at, Marin Inta, Natthanidnan Sricharoen, Nirattiya Jaisieng, Ratchapat Jitharidkul, Sanuphong Chailoet and Wasan Wongwai (Data Centre); Pongsak Pirom and Sakawrut Jitharidkul (administration); Caroline Huron, Claire Le Moigne, Ludovic Barra, Nitima Chaiboonruang, Thanchanok Sriwised, Thibaut Vallet and Tidarat Tritungtrakul (finance).
Funding
The Napneung project was supported by a grant from Expertise France through the 5% Initiative program (14SANIN204).
Salvadori, N. , Adam, P. , Mary, J.‐Y. , Decker, L. , Sabin, L. , Chevret, S. , Arunothong, S. , Khamduang, W. , Luangsook, P. , Suksa‐ardphasu, V. , Achalapong, J. , Rouzioux, C. , Sirirungsi, W. , Ngo‐Giang‐Huong, N. and Jourdain, G. Appointment reminders to increase uptake of HIV retesting by at‐risk individuals: a randomized controlled study in Thailand. J Int AIDS Soc. 2020; 23(4):e25478
References
- 1. Joint United Nations Programme on HIV/AIDS . Global AIDS update 2019 ‐ Communities at the centre [Internet]. Switzerland: UNAIDS; [cited 2019 Jul 31]. Available from: https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS . UNAIDS Data 2019 [Internet]. Switzerland: UNAIDS; [cited 2019 Jul 31]. Available from: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf [Google Scholar]
- 3. Joint United Nations Programme on HIV/AIDS . Country factsheets, Thailand, 2018 [Internet]. [cited 2020 Jan 7]. Available from: https://www.unaids.org/en/regionscountries/countries/thailand
- 4. Joint United Nations Programme on HIV/AIDS . Fast‐Track ‐ Ending the AIDS epidemic by 2030 [Internet]. Switzerland: UNAIDS; [cited 2019 Jul 31]. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf [Google Scholar]
- 5. World Health Organization . Consolidated guidelines on HIV testing services for a changing epidemic [Internet]. 2019. Nov [cited 2020 Jan 8]. Available from: https://www.who.int/publications-detail/consolidated-guidelines-on-hiv-testing-services-for-a-changing-epidemic
- 6. International Telecommunication Union . Key ICT indicators for developed and developing countries and the world (totals and penetration rates) [Internet]. [cited 2019 Jul 15]. Available from: https://www.itu.int/en/ITU-D/Statistics/Documents/statistics/2018/ITU_Key_2005-2018_ICT_data_with%2520LDCs_rev27Nov2018.xls
- 7. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach [Internet]. 2nd ed Geneva: World Health Organization; 2016. [cited 2019 Jul 15]. (WHO Guidelines Approved by the Guidelines Review Committee). Available from: http://www.ncbi.nlm.nih.gov/books/NBK374294/ [Google Scholar]
- 8. Amankwaa I, Boateng D, Quansah DY, Akuoko CP, Evans C. Effectiveness of short message services and voice call interventions for antiretroviral therapy adherence and other outcomes: A systematic review and meta‐analysis. PLoS One [Internet]. 2018. Sep 21 [cited 2019 Jul 15];13(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6150661/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paschen‐Wolff MM, Restar A, Gandhi AD, Serafino S, Sandfort T. A systematic review of interventions that promote frequent HIV testing. AIDS Behav. 2019;23(4):860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bourne C, Knight V, Guy R, Wand H, Lu H, McNulty A. Short message service reminder intervention doubles sexually transmitted infection/HIV re‐testing rates among men who have sex with men. Sex Transm Infect. 2011;87(3):229–31. [DOI] [PubMed] [Google Scholar]
- 11. Burton J, Brook G, McSorley J, Murphy S. The utility of short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing: a controlled before and after study. Sex Transm Infect. 2014;90(1):11–3. [DOI] [PubMed] [Google Scholar]
- 12. Nyatsanza F, McSorley J, Murphy S, Brook G. ‘It’s all in the message’: the utility of personalised short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing‐a repeat before and after study. Sex Transm Infect. 2016;92(5):393–5. [DOI] [PubMed] [Google Scholar]
- 13. Mugo PM, Wahome EW, Gichuru EN, Mwashigadi GM, Thiong’o AN, et al. Effect of text message, phone call, and in‐person appointment reminders on uptake of repeat HIV testing among outpatients screened for acute HIV infection in Kenya: A Randomized Controlled Trial. PLoS ONE. 2016;11:e0153612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salvadori N, Decker L, Ngo‐Giang‐Huong N, Mary J‐Y, Chevret S, Arunothong S, et al. Impact of counseling methods on HIV retesting uptake in at‐risk individuals: a randomized controlled study. AIDS Behav. 2019. 10.1007/s10461-019-02695-2 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Consolidated guidelines on HIV testing services. Geneva: WHO, 2015. [PubMed] [Google Scholar]
- 16. Sniehotta FF, Scholz U, Schwarzer R. Bridging the intention–behaviour gap: Planning, self‐efficacy, and action control in the adoption and maintenance of physical exercise. Psychol Health. 2005;20(2):143–60. [Google Scholar]
- 17. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA. 1996;276(15):1253–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Delivery and outcome of retest reminders for the 218 participants in “No Appointment but Reminder.”
Figure S2. Delivery and outcome of retest reminders for the 218 participants in “Appointment & Reminder.”
Table S1. Participants retested for HIV within seven months by combination of counselling and reminder methods


