Abstract
Context
The steroidogenic enzyme aromatase (CYP19A1) is required for estrogen biosynthesis from androgen precursors in the ovary and extragonadal tissues. The role of aromatase, and thus estrogens, is best illustrated by genetic variations of the CYP19A1 gene leading to aromatase deficiency or excess.
Objective
The objective of this work is to characterize novel CYP19A1 variants.
Design, setting, and patients
Variants causing aromatase deficiency were suspected in four 46,XX children of African and Indian origin by careful clinical phenotyping. Sequencing of the CYP19A1 gene identified novel variants. Minigene experiments, aromatase activity assay, and computational, and histological analysis were used to characterize the variants.
Main outcome measure and results
CYP19A1 variants were found in all patients: a deletion in intron 9 leading to p.P423_H503del, a delins variant at p.P154, and point variants p.V161D, p.R264C, p.R375C. Except for R264C, all variants showed a loss of function. Protein structure and dynamics studies were in line with functional assays. The 2 female patients with delins variants manifested with ambiguous genitalia at birth. Histologic investigation revealed normal ovarian tissue on one side and a streak gonad on the other. Two female patients presented with abnormal pubertal development and polycystic ovaries.
Conclusion
In girls, aromatase deficiency usually manifests at birth, but diagnosis may also be made because of abnormal pubertal development or ovarian torsion due to (poly)cystic ovaries. The ovary harboring CYP19A1 variants may present as streak gonad or appears normal at birth, but is then at very high risk to produce cysts with aging and is therefore prone to ovarian torsion.
Keywords: aromatase deficiency, CYP19A1, disorder of sexual development, virilization, estrogen synthesis, hyperandrogenism
The CYP19A1 gene encodes the steroidogenic enzyme aromatase on chromosome 15q21.2 (OMIM 107910; RefSeq NM_000103.3). It spans over 123 kb and consists of 9 coding exons (spanning over 30 kb, exons 2-10). For the CYP19A1 gene, a large number of alternative first exons and 9 different transcriptional start sites with individual promoters have been described [1, 2]. Several tissues use their specific promoters leading to a very complex tissue-specific regulation of CYP19A1 expression. Metabolic activities of aromatase include the biosynthesis of estrone from androstenedione, estriol from 16-hydroxytestosterone, and 17β-estradiol from testosterone [3]. Of these, androstenedione and testosterone are the common physiological steroid substrates of aromatase [3-5]. Recently 16β-OH-androstenedione was also reported to be a substrate for aromatase [6]. Although the human ovary is the principal site of estrogen production, even the testis produces estrogens, and extragonadal synthesis occurs from circulating C19 steroids in bone, breast, brain, adipose, and other tissues through aromatase expressed in these tissues. The role of aromatase and thus estrogens for human biology is best illustrated in disease states, both deficiency and excess.
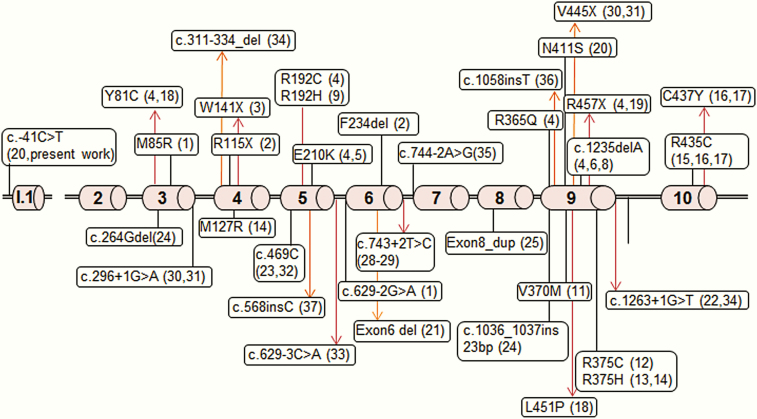
Aromatase deficiency (OMIM 613546) is a rare, autosomal recessive disorder that was first described in 1991 by Shozu et al in a mother who experienced virilisation during pregnancy due to a 46,XX fetus with aromatase deficiency that presented with severe virilization of the external genitalia at birth [7]. Meanwhile, about 50 cases of aromatase deficiency due to variants in CYP19A1 have been reported, both in female and male patients (Table 1 and Fig.1) [7-42]. During pregnancy, aromatase deficiency of the fetus typically manifests in the pregnant mother by progressive virilization due to the inability of the placenta to aromatize androgens derived from the fetal adrenals. These androgens also virilize the 46,XX fetus. During infancy and childhood, aromatase deficiency may go unnoticed, although, in some girls, abdominal problems may be seen due to ovarian cysts caused by a lack of feedback regulation of estrogens to the hypothalamic-pituitary-gonadal axis [34]. At puberty, girls without aromatase activity are not able to produce estrogens from androgen precursors for pubertal development, for example, breast and uterine development. Young men with aromatase deficiency usually present in the second decade of life with low bone mass and unfused epiphyses leading to persistent growth into adulthood with extremely tall stature [43]. In addition, aromatase deficiency is reported to cause disturbances of the lipid profile and insulin sensitivity (eg, metabolic syndrome), and it may also cause male infertility and affect bone quality.
Table 1.
Summary of all reported human CYP19A1/aromatase mutations and their characteristics
| No. of patients | Familial | Consanguinity | Mutation Allele 1 | Mutation Allele 2 | Karyotype | Sex of rearing | Age at Presentation | Phenotype | Virilization of Baby at Birth | Virilization of Mother | Spontaneous Puberty | Ovary/Testis Phenotype | Functional Testing | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Yes | NK | M85R | c.629-2G>A | 46,XX | F | Birth | Ambiguous genitalia at birth, puberty absent and bone age delayed, virilizing signs at pubertal age | Yes | NK | NK | NK | M85R: no activity | (8) |
| 2 | Yes | Likely | R115X | R115X | 46,XX | M + F | 14/8 | P1 (M): At 14 y, undescended testes and hypospadias, presence of ovaries with cysts and small uterus. P2 (F): At 8 y, 1 cm phallus, 2 urogenital openings, pubic pilosity | Yes (Prader III in P1 and II in P2) | No | Yes | Histo P1: ovarian follicle cysts | ND | (9) |
| 2 | Yes | Yes | W141X | P1: W141X; P2: missing allele/deletion | 46,XX | F | 2/5 | Hypertrophic clitoris and prepuce with single urogenital opening; low estradiol, elevated LH and FSH | Yes (Prader IV) | Yes | No | Normal at presentation | ND | (10) |
| 2 | No | No | E210K | E210K | 46,XX | F | Birth | P1: Ambiguous genitalia at birth, spontaneous pubertal development Tanner 4 with virilization signs; menarche at 12 y. P3: Clitorial enlargement with complete labial fusion and urogenital sinus with single phalloscrotal opening; gonads nonpalpable. Spontaneous puberty starting at 9 y, Tanner 3 at 12 y with signs of virilization and elevated gonadotropins | Yes (Prader II in P1; IV in P3) | No | Yes | Multiple cysts in both ovaries at age 18 y (P1) and at 9 y (P3) | ND | (11) |
| 1 | No | No | E210K | E210K | 46,XY | M | 25 | 29 y, tall, eunuchoid habitus, cryptorchidism, bone pain, metabolic syndrome | NA | NK | Yes | Histo: hypotrophic seminiferous tubules with mature Sertoli cells. Germ cell depletion without spermatogenic development. Scarce, mature Leydig cells in small groups. | ND | (12) |
| 1 | No | No | E210K | c.1235delA | 46,XX | F | Birth | Ambiguous genitalia and virilization (Prader V), PCOS in childhood; spontaneous breast development at age 8 y | Yes (Prader V) | Yes | Yes (partial) | Large ovarian follicles at 5 mo; Histo: normal stroma, numerous follicles. At 7.7 y: increased size of uterus with multiple ovarian cysts | c.1235delA: no activity | (11) (13- 15) |
| 1 | No | No | E210K | Y81C | 46,XX | M to F | Birth | Phallic enlargement, hypospadias, nonpalpable gonads. Delayed bone age at 6 y | Yes (Prader IV) | Yes | NA | Bilateral ovaries with cysts | ND | (11) |
| 1 | No | No | R192C | R192C | 46,XX | F | Birth | Ambiguous genitalia, 2 cm phallus | Yes (Prader IV) | Yes | NA | Normal by ultrasound at years 3 | ND | (11) |
| 1 | No | No | R192C | R457X | 46,XX | F | Birth | Ambiguous genitalia, 1 cm phallus and complete labial fusion. Single phalloscrotal opening. No gonads palpable. | Yes | No | Yes | At 10 y, enlarged ovaries by ultrasound | ND | (11) |
| 2 | Yes | Yes | R192H | R192H | 46,XX and 46,XY | F + M | F at birth; M at 15 mo | F: Clitoral hypertrophy and labial fusion; M: hypospadia and cryptorchidism | F: yes (Prader IV) | No | NK | Histo of ovary at 1 year: normal. | 19% of WT | (1) |
| 1 | No | Yes | R365Q | R365Q | 46,XY | M | 28 | 28 y, continuous growth and unfused epiphysis, infertility, skeletal pain, metabolic syndrome | NA | NK | Yes | Histo: hypospermatogenesis and germ cell arrest | 0.4% of WT | (16) |
| 1 | No | Likely | V370M | V370M | 46,XX | F | Birth | Ambiguous genitalia (small phallic structure without hypospadias); not palpable gonads. Urogenital sinus | Yes (Prader V) | Yes | NA | Ovaries not visualized at birth | ND | (17) |
| 2 | Yes | Yes | R375C | R375C | 46,XX and 46,XY | F + M | Birth | P1, 46,XX, F, 28 y: DSD at birth, sexual infantilism and virilization, PCOS, tall stature. P2, 46,XY, M, 24 y: tall, osteopenia, delayed bone maturation, eunuchoid, macroorchidism | Yes (P1) | Yes | No in P1 (F), yes in P2 (M) | Histo: ovaries with PCOS phenotype | 0.2% of WT | (18) |
| 2 | Yes | Yes | R375H | R375H | 46,XY | M | 27 | Bone pain and recurrent forearm fractures, progressive overgrowth | NA | NK | Yes | Borderline sperm count and motility | ND | (19) |
| 1 | No | No | R375H | M127R | 46,XY | M | 25 | Continuing linear growth, eunuchoid body habitus, adiposity, and diffuse bone pain | NA | NK | Yes | Histo: hypospermatogenesis | R375H: 7% of WT; M127R: 0% | (20) |
| 1 | No | No | R435C | R435C | 46,XX | F | Birth | Ambiguous genitalia; sexual infantilism, PCO | Yes (Prader IV) | Yes | Yes, partial | Bilateral cystic ovaries at age 13.5 y | 1.5% of WT | (21) |
| 2 | Yes | Yes | F234del | F234del | 46,XX | M + F | Birth | P1, m: Ambiguous genitalia (phallic enlargement and hypospadia). Gynecomastia at puberty caused gonadectomy. P2, F: Ambiguous genitalia. 2 cm phallus, partial labial fusion. | Yes (Prader IV) | Yes | Yes | Histo: cystic ovaries | 19% of WT | (21) |
| 1 | Yes | Yes | c.452-621-c.628 + 803del | c.452-621-c.628 + 803del | 46,XX | F | Birth | Labial fusion, excess of clitoral skin | Yes | Yes | No | Small ovaries observed with MRI | 0% | (21) |
| 1 | No | NK | C437Y | R435C | 46,XX | F | Birth | Ambiguous genitalia at birth; 14 y: primary amenorrhea, no breast development, enlarged clitoris, pubic hair Tanner stage III, sexual infantilism, PCO | Yes | Only acne | No | Multiple bilateral cysts | C437Y: 0% of WT R435C: 1.1% of WT | (22, 23) |
| 1 | No | No | L451P | Y81C | 46,XY | M | 24 | Eunuchoid proportions, bilateral genua valga, incomplete fused epiphyses, osteopenia | NA | No | Yes | Testes of normal size and consistency | L451P: 3.1% of WT Y81C:14.3% of WT | (24) |
| 1 | NK | NK | R457X | R457X | 46,XX | F | NK | F, virilization | Yes | NK | NK | NK | ND | (25) |
| 1 | No | NK | N411S | c.–41C>T | 46,XX | F | Birth | At birth ambiguous genitalia, clitoral hypertrophy, thin dividing wall between urethral opening and a normal vaginal introitus with a hymen altus | Yes | Yes | NA | Normal by ultrasound | N411S: 0% of WT c.-41C>T: 50% of WT | (26) |
| 1 | No | Yes | c.629-1453_744- 486_del | c.629- 1453_744- 486_del | 46,XX | M + F | Birth | At 1 mo: ambiguous genitalia, gonads not palpable, phallus 1 cm, uterus by ultrasound | Yes (Prader III) | No | NA | Small ovaries by ultrasound | ND | (27) |
| 1 | No | Yes | c.1263 + 1G>T | c.1263 + 1G>T | 46,XX | M | Birth | Ambiguous genitalia of unknown cause at birth. At 21 y: undescended testes, hypoplastic scrotum, hypoplastic uterus | Yes (Prader V) | No | Abnormal | Histo: streak gonads | ND | (28) |
| 1 | No | Yes | c.469C_del | c.469C_del | 46,XY | M | Birth | Investigated after birth because of severe maternal virilization. Found low estrogen levels, high androstenedione, normal free testosterone. | NA | Yes | NA | Normal | ND | (29) |
| 1 | No | Yes | c.264G_del | c.1036_1037ins 23bp | 46,XX | F | Birth | Ambiguous genitalia, 3 cm clitoromegaly; PCOS later | Yes (Prader IV) | Yes | NA | Large ovaries with cysts | c.264G_del < 0.3% of WT; 23bp_del < 0.3% of WT | (30) |
| 1 | No | Yes | Ala306_Ser314_ dup | Ala306_ Ser314_dup (likely) | 46,XX | F | Birth | At birth ambiguous genitalia. Lack of pubertal development. Streak ovaries. At 32 y, osteopenia and fracture, tall stature, central obesity, borderline hypertension, impaired fasting glucose | Yes (Prader III) | NK | No | Streak ovaries | ND | (31) |
| 1 | No | No | c.1263 + 5G>A | c.1263 + 5G>A | 46,XX | F | 4 | At 4, labioscrotal fusion, 1.5 cm clitoromegaly, perineal urethra. At 13.5 y, delayed bone age, small uterus, follicular cysts in ovaries. | Yes | No | Abnormal (B1, P4) | PCOS | ND | (32, 42) |
| 1 | No | Yes | c.743 + 2T>C | c.743 + 2T>C | 46,XX | F | Birth | At birth: ambiguous genitalia, enlarged phallus, gonads not palpable | Yes (Prader IV-V) | Yes | NA | ND | 0.3% of WT | (7, 33) |
| 1 | No | No | V445X | c.296 + 1G>A | 46,XX | F | Birth | At birth: masculine- appearing external genitalia, enlarged phallus, complete fusion of posterior labioscrotal folds | Yes (Prader V) | Yes | Yes (Tanner 4) | At birth: slightly enlarged ovaries. At 2 y: enlarged ovaries with several cysts, fallopian tubes and uterus normal in appearance, histopathology showed many normal-appearing large tertiary follicles with an oocyte within a cumulus oophorus projecting into antrum. | ND | (34, 35) |
| 2 | Yes | Yes | c.469C_del | c.469C_del | 46,XY and 46,XX | M + F | 16 (M | P1 (M): 16 y, no gynecomastia or other abnormalities, open epiphyses, congenital hearing loss of 85%, delayed bone age | NA and NK (F) | No | M: yes and F: no | Normal by clinical exam | ND | (36) |
| 1 | No | Yes | c.629-3C>A– frameshift and stop codon 8 nt after exon 5 | c.629-3C>A– frameshift and stop codon 8 nt after exon 5 | 46,XY | M | 27 | Normal sex characteristics, spontaneous erections sufficient for intercourse, genua valga, kyphoscoliosis, pectus carinatus. Retarded bone maturation. Abnormal glucose and lipid metabolism. | NA | Yes | Yes | Below threshold sperm count (1 mL/ mL), 100% immotile spermatozoa. Oligoazoospermia | ND | (37) |
| 1 | No | NK | c.311-334_del | c.1263 + 1G>T → Y361X | 46,XY | M | 27 | At 27 y, height 193 cm, eunuchoid, unfused epiphyses, osteopenia, increase fasting insulin, mild astenozoospermia and history of right cryptorchidism | NA | NK | Yes | History of cryptorchidism. Borderline FSH and inhibin B at age 27 y | ND | (38) |
| 1 | No | Yes | c.744-2A>G | c.744-2A>G | 46,XX | F | Birth | Ambiguous genitalia at birth; 19 mo: gonads not palpable, penis-like phallus 1.5 cm, single penoscrotal urethral opening, labioscrotal fusion defect | Yes (Prader IV) | Yes | NA | Very small ovaries by ultrasound | ND | (39) |
| 1 | NK | NK | c.1058insT | c.1058insT | 46,XY | M | 26 | 190 cm tall, hypertension, hyperuricemia, hypercholesterolemia | NA | NK | Yes | NK | ND | (40) |
| 3 | Yes | Yes | c.568insC → L190P leading to S199X | c.568insC → L190P leading to S199X | 46,XX | F | Birth | Clitoromegaly, partial to complete labial fusion | Yes (Prader II-III) | Yes | NA | Hypoplastic ovaries by imaging studies | ND | (41) |
Abbreviations: DSD, disorder of sex development; F, female; FSH, follicle-stimulating hormone; LH, luteinizing hormone; M, male; MRI, magnetic resonance imaging; NA, not applicable; ND, not determined; NK, not known; P1, patient 1; P2, patient 2 etc; PCOS, polycystic ovary syndrome; WT, wild-type.
Figure 1.
Localization of reported CYP19A1 variants. The coding exons 2 to 10 and the alternative exon I.1 of the aromatase CYP19A1 gene are shown.
Aromatase excess (OMIM 139300) has been found in a few, mostly familial cases of gynecomastia associated with accelerated growth and bone maturation due to excessive peripheral estrogen production. The main causes of aromatase excess include chromosomal rearrangements altering regulatory elements of the CYP19A1 gene expression or a higher expression of an alternative first exon enhancing CYP19A1 transcription [44, 45]. By contrast, aromatase deficiency is almost exclusively caused by variants altering the coding sequence of the CYP19A1 gene, such as point mutations, deletions, and insertions, or splicing mutations (Table 1 and Fig. 1).
In this study, we describe several novel variants in CYP19A1. A homozygote splice-site deletion in the CYP19A1 gene was found in an African 46,XX neonate presenting with typical clinical manifestations during pregnancy and at birth. An indel variant in exon 5, together with the c.–41C>T variant in the CYP19A1 promoter that had been described earlier, was found in an Indian 46,XX child presenting with an unsolved disorder of sex development at age 3 years. Novel point variants were identified in 2 more unrelated adolescent girls in India who presented with poor breast development and polycystic ovaries. To address the impact of the novel CYP19A1 variants manifesting at different time points of sex development and originating from diverse populations, we performed genetic, functional, and structural studies and compared the findings of novel variants in the CYP19A1 gene with the published literature.
1. Case Reports, Materials, and Methods
A. Case Reports
Family 1. An 8-day-old newborn was referred to the clinic of A.L. for assessment of ambiguous genitalia. The infant was the seventh child of second-degree consanguineous African parents. The first child was stillborn (Fig. 2). There was maternal virilization during the second pregnancy and the newborn who was declared “male,” died on the first day of life (with no further details available). After delivery, signs of virilization slowly decreased in the mother, and she subsequently gave birth to 4 healthy sons without suffering from virilization again during these pregnancies. However, during the seventh pregnancy, the mother, age 36 years, again developed progressive signs of virilization from 12th week of gestation, with facial and abdominal hirsutism, facial acne, clitoral hypertrophy, and deepening of the voice. No drugs or environmental toxins were suspected of influencing the pregnancy. Biochemical workup of the mother revealed marked hyperandrogenism with high testosterone levels (eg, 45.1 nmol/L at 32/40 weeks of pregnancy, normal range is 2.2-10.7 nmol/L). Ultrasound and magnetic resonance imaging studies after delivery revealed enlarged ovaries containing multiple follicles consistent with polycystic ovaries. However, the signs of virilization decreased slowly after birth, and serum testosterone levels normalized within 1 month.
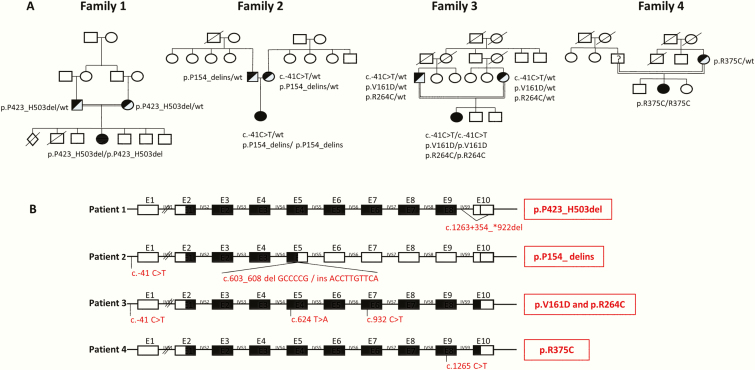
Figure 2.
Genetic characterization of 4 patients harboring novel CYP19A1 variants. A, Family pedigrees show consanguinity in 3 families. B, Diagram showing localization and specific variations in the CYP19A1 gene with respect to exon-intron boundaries. Black-filled exon rectangles show exons that are translated into RNA/protein sequences despite the genetic variations.
The baby was born at term by spontaneous vaginal delivery. Birth weight, length, and head circumference were normal (3500 g/50 cm/35 cm). Neonatal adaptation was also normal, but the baby had ambiguous genitalia without presenting other dysmorphic features. Genital examination showed a 1.5 cm cliterophallus with a single meatus opening at the base of the genital tubercle and a complete posterior labial fusion (Prader IV). The genital folds were scrotalized, and no gonads were palpable (Fig. 3). Pelvic ultrasound showed a uterus, whereas the gonads were not visible. Karyotype was 46,XX. Biochemical workup revealed normal electrolytes, 17α-hydroxyprogesterone, cortisol, and renin levels, ruling out classic 21-hydroxylase deficiency with congenital adrenal hyperplasia (CAH) (Table 2). Testosterone levels were slightly elevated at birth but decreased to normal levels by 1 month, whereas other androgen levels were within reference ranges. Estradiol levels were very low (Table 2). Follow-up revealed that the patient did not have any distress as seen in cortisol deficiency. Thus virilization of the 46,XX baby with concurrent virilization of the mother during pregnancy suggested aromatase deficiency in the child.
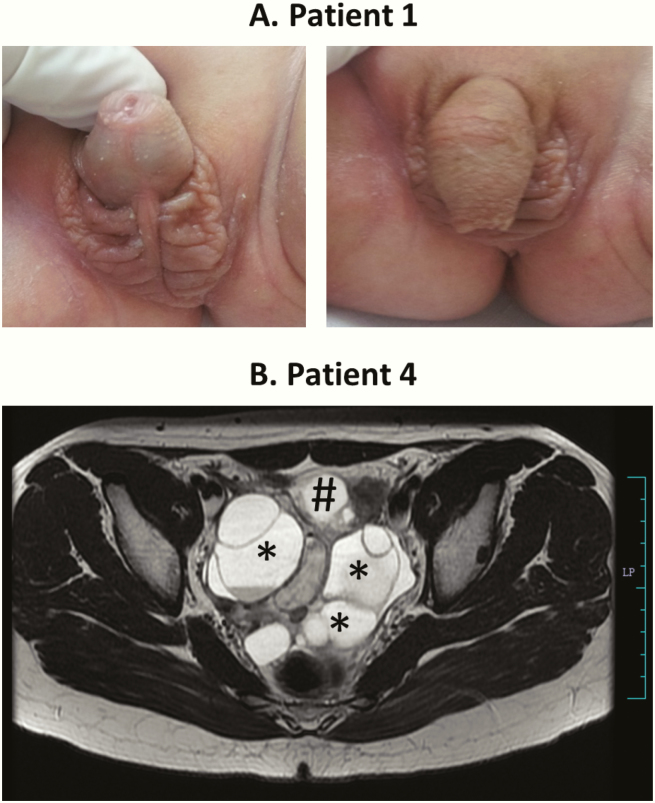
Figure 3.
Phenotype of girls with CYP19A1 mutations. A, Picture of the ambiguous genitalia at birth found in the 46,XX disorder of sex development index patient 1. Note the phallus-like tubercle with a single meatus opening at the base and the complete labioscrotal fusion (Prader IV). The photograph is shown with the permission of the parents. B, Magnetic resonance imaging of the pelvis in patient 4 at age 14 years showing a large, multiloculated, cystic mass (6.8 × 9.6 × 6.5 cm) involving bilateral adnexa (*), displacing the uterus (#) anteriorly and inferiorly. Few of the cysts are showing hemorrhage. Both ovaries cannot be separately visualized.
Table 2.
Clinical and biochemical characteristics of four 46,XX patients with aromatase deficiency
| Laboratory Findings | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt ID | Origin | Maternal virilization | Age at Manifestation/Follow-up | Genital Phenotype | Pubertal Development (Tanner stage) | Ovarian Phenotype | T, nmol/L | DHEAS, µmol/L | 4A, nmol/L | 17OHP, nmol/L | Cortisol, nmol/L | E2, pmol/L | LH, IU/L | FSH, IU/L |
| 1 | African | Yes | Birth | Prader IV | NA | NK | 3.41 | 55.9 | ||||||
| 10 d | 2.03 | 1.34 | 4.85 | 11.92 | 504 | |||||||||
| 1 mo | 0.19 | 0.35 | 0.8 | 3.85 | 305 | < 18 | ||||||||
| 4 mo (minipuberty) | 0.38 | 1.04 | ||||||||||||
| 2 | Indian | No | Birth | Atypical genitalia | NA | |||||||||
| 3 y | Prader IV | NA | See Figure 3. | < 0.45 | 5 | 745a | NM | < 1 | 12 | |||||
| 3 | Indian | Maybe | 14 y | Clitoromegaly | B3/P4/A3 | Polycystic ovaries, ovarian torsion | 2.06 | 1.93 | 2.03 | 648a | 58.7 | 39.9 | 10.98 | |
| 4 | Indian | Yes | 14 y | Clitoromegaly | B3/P4/A3 | Polycystic ovaries | 2.6 | 3.58 | 1.38 | 634a | < 18 | 32 | 37 | |
Laboratory values outside the normative range are shown in bold.
Abbreviations: 4A, androstenedione; 17OHP, 17α-hydroxyprogesterone; DHEAS, dehydroepiandrosterone-sulfate; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NA, not applicable; NK, not known; NM, not measurable; Pt, patient; T, testosterone.
aAdrenocorticotropin-stimulated 60-minute values.
Patient 2. This 46,XX infant was referred to the clinic of V.P.P. for workup of ambiguous genitalia at age 3 years. She was the only child of nonconsanguineous Indian parents and was noted to have a phallic structure with absent gonads at birth. There was no history of maternal virilization or hirsutism during pregnancy. Evaluation elsewhere had ruled out CAH. Karyotype was 46,XX, and laparoscopy had been performed at age 8 months and revealed a uterus and fallopian tubes. Unilateral gonad biopsy was suggestive of ovarian tissue. At age 3 years, she presented with a well-formed phallus with a single opening at the tip with good prepucial skin and fused labioscrotal folds with rugosity (Prader IV). Gonads were not palpable. Biochemical workup revealed normal cortisol response to an adrenocorticotropin stimulation test (60 minutes, 745 nmol/L), normal 17-hydroxyprogesterone, undetectable testosterone, elevated follicle-stimulating hormone (FSH), and prepubertal luteinizing hormone (LH) (Table 2). A 100 cell count karyotype was repeated, which again revealed only 46,XX cell lines. Laparoscopy was also repeated and confirmed earlier findings; gonadal biopsy from the other side revealed primordial follicles with ovarian stroma. Because of normal stimulated serum cortisol and 17-hydroxyprogesterone, P450 oxidoreductase deficiency (PORD) was considered less likely and aromatase deficiency was suspected.
Patient 3. Patient 3, from India, presented at age 14 years with an acute abdomen due to ovarian torsion. She was the second child of consanguineous parents (Fig. 2). Past medical history was suggestive for some maternal virilization during pregnancy, but at birth, the girl was recorded to be healthy, although retrospectively the prominence of the clitoris was present since birth. Pubertal development started at around age 11 years with pubic and axillary hair, and voice change was noted; breast development started after age 12.5 years, and growth of facial hair in the past 6 months was noted. Physical exam revealed a weight of 40.6 kg (P10), height 160 cm (P50), blood pressure 90/50 mm Hg, Ferriman Gallway Score 4, and Tanner stage B3/P4/A3. Exam of the external genitalia revealed a slightly enlarged clitoris (> 1 cm). Biochemical workup showed elevated LH, FSH, and testosterone with rather low estradiol (Table 2). Ultrasound and laparoscopy revealed polycystic ovaries on both sides with torsion of the left ovary.
Patient 4. Patient 4, from India, born to consanguineous parents, presented at age 14 years for abdominal pain and oligomenorrhea. No maternal virilization was reported during pregnancy. The girl likely had atypical genitalia at birth, but this was not recorded. Pubertal development started at around age 10 to 11 years with menarche at around 12 years. Breast development was poor, and clitoromegaly was claimed. At presentation, her weight was 50 kg (P50), height 155 cm (P25), and blood pressure was 90/60 mm Hg. No skeletal anomalies were found, and Tanner stage was B3P4A3. The genital exam showed a clitoromegaly of 20 mm, a common urogenital sinus, and posterior labial fusion. Ultrasound and magnetic resonance imaging revealed enlarged polycystic ovaries (Fig. 3). Laboratory findings were abnormal for elevated LH, FSH, and testosterone, whereas estradiol was not detectable (Table 2).
B. Genetic Studies
Genetic studies of rare steroid disorders were approved by the cantonal ethics’ commission of Bern, Switzerland. Written informed consent for the genetic studies was obtained from parents and teenage minors. DNA was extracted from blood samples of the patient and both parents as available. The CYP19A1 gene (NM_000103.3 or NG_007982.1) was sequenced from genomic DNA using a candidate exon-by-exon approach, as previously described [1]. Additional primers were used to characterize the deletion found at the boundary of exon/intron 9 and the deletion/insertion in exon 5; these primer sequences are available on request.
For the minigene experiment for genetic workup of patient 1, the wild-type (wt) and variant genomic CYP19A1 sequence comprising exon 9 to exon 10 – 3’UTR was cloned in a pcDNA3.1 vector. These vectors were then transiently transfected in HEK293 cells using Lipofectamine 2000 (Invitrogen AG, Life Technologies). Forty-eight hours after transfection, total RNA was extracted and reverse transcribed. Finally, transcripts of wt and variant vectors were polymerase chain reaction amplified. Polymerase chain reaction products were run on an agarose gel and visualized, as well as subjected to direct sequencing (Microsynth AG).
C. Analysis of Conservation of Amino Acids of CYP19A1
We used ConSurf analysis to study the evolutionary conservation of amino acids (aa) in CYP19A1 based on structural and phylogenetic relationships between the sequences from different species [46, 47]. All CYP19A1 sequences in the Uniprot database were searched, and the best quality sequences based on 3 rounds of iterated Phi-BLAST search were kept for further analysis. Multiple-sequence alignment of selected sequences was made to compare the position of variants described in this report with aa located at those positions in different species (Figs. 4 and 5).
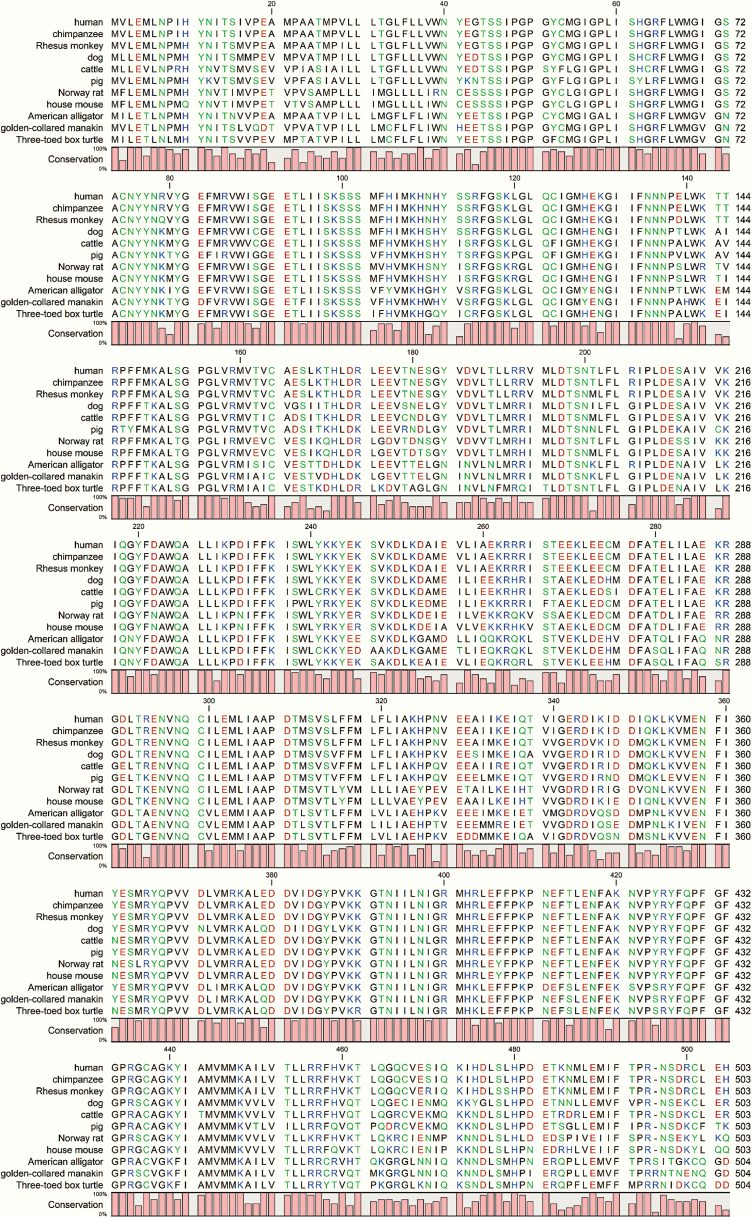
Figure 4.
Multiple sequence alignment of affected CYP19A1 amino acid sequences from patients showing that all variants described in this report are conserved in higher primates. The valine 161 residue is well conserved across species and in some cases, a similar amino acid (isoleucine) was found (alligator, manakin, and turtle). The CYP19A1 proteins from human, chimpanzee, rhesus monkey and pig have an arginine at the position 264, whereas dog, cow, and mouse have a histidine, and rat, American alligator, golden-collared manakin, and turtle have glutamine at amino acid position 264. Considering the critical role of arginine 375 in heme binding, it was found to be conserved across species and no substitutions were identified in all the CYP19A1 sequences analyzed.
Figure 5.
Multiple sequence alignment of CYP19A1 amino acid sequences from patients is shown. All the variants described in this report are conserved in higher primates as shown in Fig. 4.
D. CYP19A1 Protein Structure Modeling
In silico mutagenesis of the 3-dimensional protein models of CYP19A1, according to the variations identified in the patients, were performed as described earlier [1] using the 3- dimensional x-ray crystal structure of human aromatase (3S79) obtained from the Protein Data Bank database [48]. Model building was performed with programs YASARA [49] and WHATIF [50]. A screening of rotamer libraries was used to minimize stearic clashes with the neighboring residues that resulted from the substitution of aa. Molecular dynamics (MD) simulations were used to optimize the aa side chains. The final model was refined by a 1000 ps MD simulation using the AMBER ff15ipq forcefield [51]. Structure models were drawn using the software Pymol (www.pymol.org), and rendering of images was performed with POVRAY (www.povray.org).
E. Predicting the Effect of Protein Stability Using Site-Directed Mutator
The site-directed mutator (SDM) tool [52] described by Topham and colleagues [53] was used for predicting the effect of CYP19A1 variants. SDM software uses environment-specific substitution frequencies within homologous protein families to calculate a stability score. The structures of variants used for analysis were generated using the built-in program ANDANTE [54]. SDM provides a pseudo-δ G score for stability prediction and also a potential impact of genetic variants for causing disease.
F. In vitro Functional Testing of CYP19A1 Variants
The human wt CYP19A1 complementary DNA (cDNA) expression vector was described previously [1]. The new deletion constructs were created according to the cDNA sequences identified by the genetic analysis and minigene experiments. For point mutations, site-directed mutagenesis was performed to obtain the specific cDNA variants. HEK293 cells were transiently transfected with wt and variant CYP19A1 vectors for 48 hours (Lipofectamine 2000). Aromatase activity was then measured by the tritiated water release assay using cold/hot androstenedione as substrate (10 nM/[1β-3H(N)]-androstene-3,17-dione; 20 000 cpm/well) as previously reported [1, 55]. Data represent the mean ± SD of 3 independent experiments performed in duplicate. The student t test was used to test the significance (P ≤ .05).
G. Tissue Histology Studies
Formalin-embedded tissue biopsy material obtained by laparoscopy of patient 2 (see “Case Reports”) was available for histology studies. Tissue was investigated microscopically after hematoxylin and eosin staining. Tissue was compared to ovarian tissues originating from one representative, age-matched normal girl, and one girl with Turner syndrome (TS).
2. Results
A. Phenotype-Genotype Characterization
We found novel CYP19A1 variants in 4 girls (Fig. 2) manifesting either at birth with atypical genitalia or puberty with poor breast development, clitoromegaly, abnormal menstrual bleeding, polycystic ovaries, and ovarian torsion (Fig. 3). Three mothers had signs of virilization during pregnancy (Table 2). Biochemical analysis revealed elevated androgens during pregnancy and in the neonate (in family 1), and in the adolescent girls with incomplete pubertal development (patients 3 and 4). Gonadotropins LH and FSH were elevated in the pubertal girls, whereas FSH was also abnormally high in the 3-year-old (Table 2). CAH due to 21-hydroxylase (and PORD) was ruled out by normal serum cortisol and 17-hydroxyprogesterone levels (after adrenocorticotropin stimulation) in all patients, and with urinary steroid profiling on spot urine by gas chromatography–mass spectrometry in patient 2 (data not shown) [56].
Genetic analysis of patient 1 revealed a homozygote splice site deletion at the exon-intron 9-3’ boundary (Fig. 2). Both consanguineous parents were heterozygote carriers of this variant. The 4 healthy brothers were not available for analysis. Characterization of this novel variant by minigene experiments revealed that the coded transcript contained the wt CYP19A1 sequence from exon 2 to exon 9, but then found a stop codon within intron 9 after addition of 6 aa due to the intronic splice site deletion that prevented correct splicing. The corresponding protein p.423_503del therefore lacked exon 10 completely and was only 426 aa long compared to 503 aa of the wt protein.
In patient 2, compound heterozygote variants of the CYP19A1 gene were found. The previously described promoter c.–41C>T variant and a novel deletion/insertion in exon 5 at c.603_608 led to a truncated aromatase protein that was predicted to have no enzyme activity (p.P154_delins) (25). Both parents were carriers of the p.P154_delins variant, and the mother carried, in addition, the c-41C>T variant (Fig. 2). Other family members were phenotypically normal and not available for genetic studies.
Patient 3 had 2 point mutations in exons 5 and 7, corresponding to c.624T>A (p.V161D) and c.932C>T (p.R264C) together with the promoter variant c.–41C>T. The consanguineous parents carried all these variants on 1 allele. Siblings were not affected and were not available for our study.
Finally, a homozygote point mutation in exon 9 was found in patient 4, c.1265C>T, p.R375C. Of the consanguineous parents, only the mother’s DNA was available for genetic analysis. She was found to carry the variant on 1 allele (Fig. 2).
B. Bioinformatic Characterization of the CYP19A1 Variants
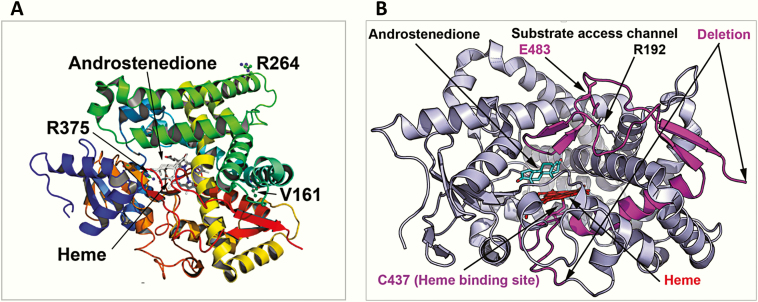
Multiple-sequence alignment of affected CYP19A1 aa sequences from the patients is shown in Fig. 5. All mutations described in this report are conserved in higher primates (Fig. 4). An analysis of different aromatase sequences across species revealed valine 161 is well conserved across species, and in some cases, a similar aa (isoleucine) was found (alligator, manakin, and turtle). The V161D replacement was predicted to be less stable compared to wt CYP19A1, with a δ G value of –2.5 compared to wt protein. The substitution of valine 161 for an aspartate introduces a buried charge and a hydrophilic residue in the core of the protein, which would cause reduced stability. Further, the helix 144 to 165 is also involved in protein stabilization, and the introduction of a charged residue is likely to cause reduced flexibility (Fig. 6A).
Figure 6.
Structural location of CYP19A1 variants. A, Point mutations. Here a ribbons model of CYP19A1 protein colored in the rainbow from amino terminus (violet) to carboxy terminus (red) is shown and the bound substrate, androstenedione, and central heme molecule are depicted as stick models. Heme is ligated to the protein via cysteine 437 residue and forms the catalytic center of the CYP19A1 protein. The substitution of valine 161 for aspartate introduces a buried charge and a hydrophilic residue in the core of the protein, which would cause reduced stability of the structure. The R264 residue on aromatase is surface exposed and located on the G helix of the CYP19A1 structure, which is part of the substrate access channel, and it has been widely reported to be flexible in cytochromes P450. The R375 residue is involved in binding of heme propionate at the catalytic center of the CYP19A1, and its change to cysteine will lead to loss of heme binding and a nonfunctional protein. B, Identified deletion/insertion variations. The P423_H503delins mutation results in loss of critical amino acids in aromatase, including the heme ligating cysteine 437, and would, therefore, result in an inactive enzyme that would also be unstable and subject to degradation.
R264C (rs700519) is a common polymorphic variant of CYP19A1 (CYP19A1*4). The R264C variant of CYP19A1 is present at a lower frequency in the European population, with a minor allele frequency (MAF) of 0.02 in the 1000 Genomes database but is more prevalent in the East Asian (MAF 0.17), South East Asian (MAF 0.24) and African (MAF 0.18) populations. The R264 residue on the aromatase is on the surface and located on the G helix of the CYP19A1 structure (Fig. 6A), which is part of the substrate access channel, and it has been widely reported to be flexible in cytochrome P450 proteins. The R264 residue is also the central residue of a 3-arginine cluster that is part of a consensus sequence for several kinases. Therefore, R264 may have a role both in the regulation of substrate access and posttranslational modifications of aromatase. Compared to the R263 position in aromatase, the R264 position is variable among species. The human, chimpanzee, rhesus monkey and pig have an arginine at the position 264; dog, cow, and mouse have a histidine, whereas the rat, American alligator, golden-collared manakin, and turtle have glutamine at position 264. As part of the KRRR sequence (AA 262-265), lysine 262 and arginine 263 are conserved across species and no variations were found at these positions, and at position 265, either arginine or lysine is present across species (Fig. 4).
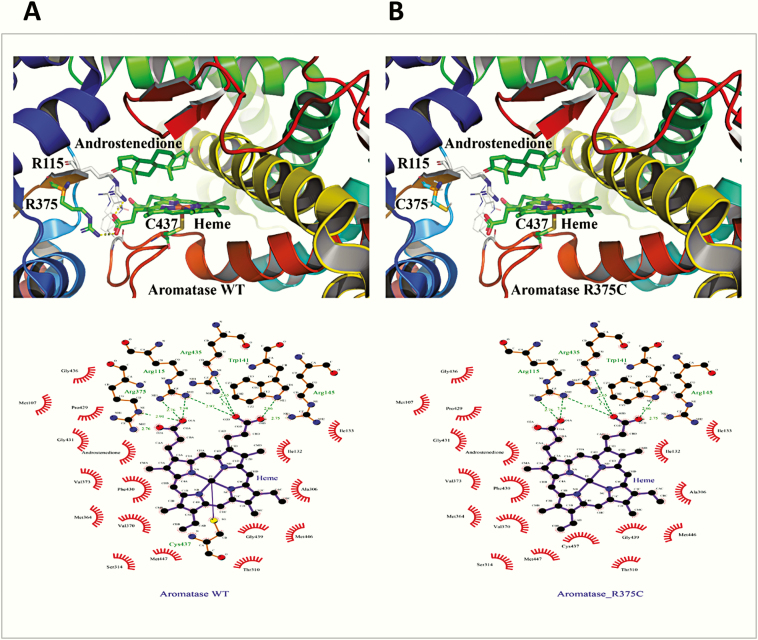
The R375 residue is involved in binding of heme propionate at the catalytic center of the CYP19A1, and its change to cysteine will lead to loss of heme binding, resulting in a nonfunctional protein, which is consistent with a complete loss of activity observed for this mutation (Figs. 6A and 7). Considering the critical role of arginine 375 in heme binding, it was found to be conserved across species, and no substitutions were identified in all CYP19A1 sequences analyzed (Fig. 4).
Figure 7.
Critical role of arginine 375 in heme binding. A and B, Structures of A, wild-type (WT), and B, R375C aromatase, shown as a ribbons model. The R375 residue is essential for the binding of heme at the catalytic center of the A, CYP19A1, and its change to B, cysteine, will lead to loss of heme binding, resulting in a nonfunctional protein. A complete loss of activity was observed for the R375C variant. Therefore, the arginine 375 residue has a critical role in heme binding and activity of aromatase. These observations are further substantiated by the conservation of R375 residue in aromatase across species, and no substitutions were identified in all CYP19A1 sequences analyzed (Fig. 4). C and D, A close-up of heme ligation in aromatase C, WT and D, R375C. D, After the change of arginine 375 to cysteine, one of the bonds holding the heme in place, formed between the propionate group in heme and arginine 375, is disrupted. The heme in aromatase, similar to other cytochrome P450 proteins, is held together by several bonds between the propionates of heme and arginines or histidine/tryptophan groups located at the catalytic center. Disruption of these linkages will lead to an unstable heme-binding site in aromatase and loss of heme, which would cause a nonfunctional protein. All the critical arginines (115, 145, and 375), as well as tryptophan 141 and cysteine 427, are conserved across species (Fig. 4), and no variation is observed at any of these places, confirming the importance of these amino acids in the function of aromatase.
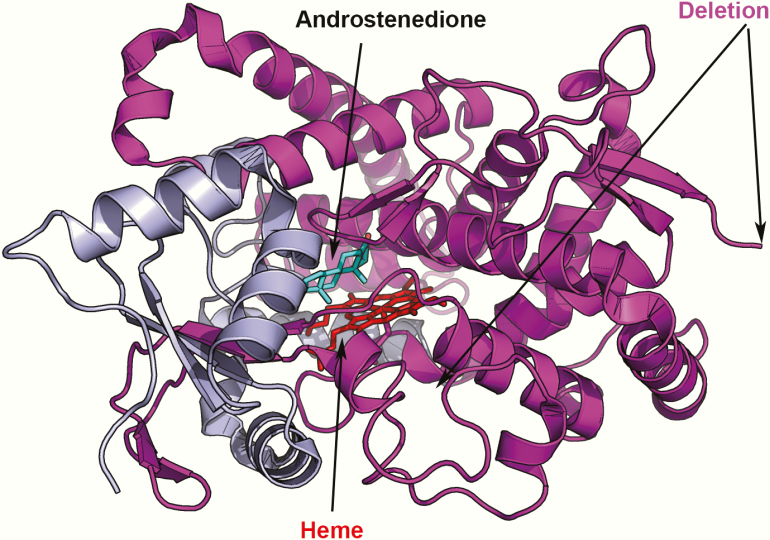
The P154delins variant results in a truncated protein, which would be degraded (Fig. 8). The P423_H503delins mutation results in the loss of critical aa in aromatase, including the heme-ligating cysteine 437 (Fig. 6B), and would, therefore, result in an inactive enzyme that would also be unstable and subject to degradation.
Figure 8.
The P154delIns protein variant lacks most of the critical residues of aromatase required for catalytic function because of truncation (shown in magenta). Such a small protein, without functionally important structural features, will be misfolded and degraded, leading to a complete loss of aromatase activity.
C. In vitro Functional Characterization of the Identified CYP19A1 Variants
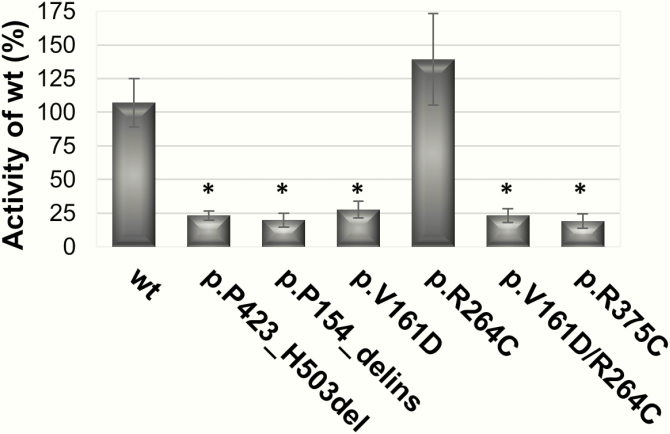
All CYP19A1 variants were tested in HEK293 cells for their ability to convert androstenedione to estrone using the tritiated water-release assay, as previously described [1, 57]. Both deletion/insertion variants and the 2 point variants p.V161D and p.R375C showed complete loss of aromatase activity (Fig. 9). By contrast, the p.R264C variation had similar aromatase activity as the wt CYP19A1. The double variant p.V161D and p.R264C, however, showed complete loss of function, like the p.V161D single variant. The functional effect of the promoter variant c.–41C>T on CYP19A1 expression has been previously described [25].
Figure 9.
Aromatase activity of identified CYP19A1 variants. Variants were built into the mammalian expression vector containing wt CYP19A1 cDNA. HEK293 cells were transiently transfected with wt and variant CYP19A1 plasmids, and aromatase activity was assessed by the tritiated water-release assay testing conversion of androstenedione to estrone. Compared to wt, all variants except p.R264C showed complete loss of activity. *P value is less than or equal to .05.
D. Histological Description of an Aromatase-Deficient Ovary in Infancy
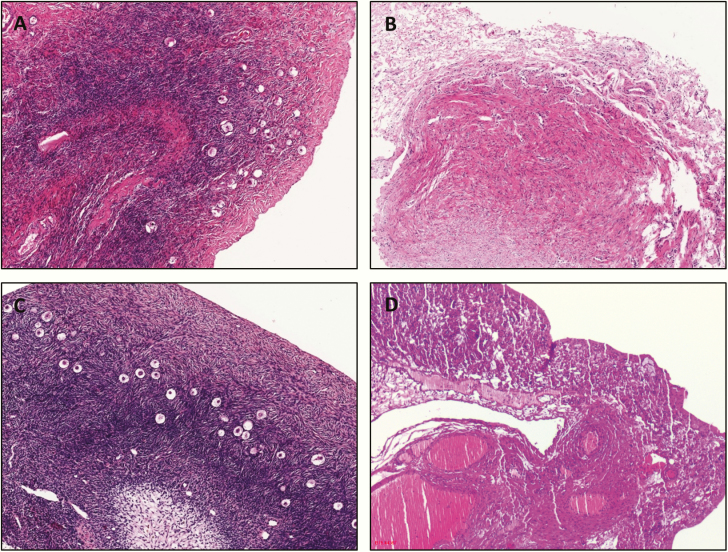
Ovarian tissue for histological evaluation was available from patient 2. At age 3 years, histology showed a normal-appearing ovary with normal-looking stroma and follicles on one side (Fig. 10A) compared to a healthy control ovary of the same age (Fig. 10C). By contrast, on the contralateral side, a streak gonad was identified macroscopically, with histological ovarian-like stroma without follicles (Fig. 10B) as found in typical TS (see age-matched picture of TS in Fig. 10D).
Figure 10.
Histology of an ovary originating from a toddler with aromatase deficiency. A, Hematoxylin and eosin (HE) histology picture of one ovary of patient 2 showing a normal ovary with normal stroma and follicles. B, HE histology of the contralateral ovary showing a streak gonad without follicles. C, HE picture of a normal, age-matched ovary. D, HE picture of a typical Turner syndrome streak ovary, age matched.
3. Discussion and Conclusion
There are only a few inborn errors of steroidogenesis that cause virilization of 46,XX individuals. The first group of disorders causes not only sex hormone disturbances but also glucocorticoid deficiency, and is therefore called CAH. This group comprises 3β-hydroxysteroid dehydrogenase (HSD3B2), 21-hydroxylase (CYP21A2), and 11-hydroxylase (CYP11B1) deficiencies. The second group with normal glucocorticoid production comprises aromatase deficiency (CYP19A1) and PORD, although in PORD stimulated cortisol production is often also compromised [56, 58]. Furthermore, mothers virilize during pregnancy only with disorders of the second group, which affect the aromatization of fetal androgens by the fetal-placental unit.
All girls with CYP19A1 deficiency reported in this study had normal cortisol production but suffered from androgen excess that manifested in utero (in 3 out of 4), at birth (in 2 to 3 out of 4), or puberty (in 1 or 2 out of 4) (see Table 2). Whether a detailed examination of the external genitalia would have revealed some atypical findings in patient 3 at birth remains speculative. So far, most reported 46,XX individuals with aromatase deficiency manifested at birth with moderate to severe virilization of the external genitalia (Prader III-V) (n = 25/34; Table 1). This has led to male sex assignment at birth in three 46,XX individuals, 2 of whom were reassigned to the female sex after diagnosis in childhood. Signs of virilization during pregnancy have been described in about half of mothers carrying an affected child. During infancy and childhood, ovarian cysts were noted in several and were either symptomatic or seen by imaging studies. At puberty, incomplete development with poor breast development, menstrual cycle anomalies, polycystic ovaries, and signs of androgen excess (eg, hirsutism, acne, enlarged clitoris) are typically due to estrogen deficiency and elevated androgens. Late diagnosis at pubertal age is described in less than one-third of reported 46,XX aromatase-deficient female patients. Of the 15 reported female individuals older than pubertal age, 4 showed no signs of spontaneous puberty, 6 had incomplete development, and only 5 spontaneously reached Tanner 5 (Table 1).
By contrast, affected 46,XY individuals typically present at around age 20 to 30 years with extremely tall stature due to failure of growth arrest. Only if the diagnosis is known in the family or maternal virilization during pregnancy raised suspicion, may diagnosis be made earlier in boys. We also suspect that the diagnosis is less often made in boys than girls because the clinical picture is less remarkable and manifests only in early adulthood when genetic disorders are less suspected; this may be evidenced by fewer reported cases of male patients in the literature (n = 13/47; Table 1), which does not reflect the autosomal recessive inheritance and the true incidence.
Owing to enlarged ovaries with cysts or polycystic ovaries, torsion of an ovary is a frequent complication that may occur early in life. This complication can be prevented by very low-dose estrogen replacement starting early in life [34, 59]. At puberty, sex hormone replacement therapy is required to allow for complete pubertal development and normal menstrual bleeding. So far no successful pregnancy has been described in a woman with aromatase deficiency. However, it is not excluded that this could be possible with the help of assisted reproduction, as recently demonstrated in a woman with 17-hydroxylase (CYP17A1) deficiency, a steroid enzyme essential for the production of all sex hormones [60]. Histologic investigations of ovarian tissue of female patients carrying CYP19A1 variants are scarce. We had a chance to investigate ovarian biopsy material in patient 2 and found a normal-looking ovary on one side and a streak gonad on the other. In the literature, ovaries are often reported to look normal or only slightly abnormal in size by imaging studies in very young girls, although smaller cysts may be found very early when specifically searched for. Imaging studies later during infancy and childhood mostly reveal abnormal ovarian cysts, which may resemble the picture of a polycystic ovary by the age of puberty (Table 1). This resulted in ovarian torsion in several cases, even at a very young age.
Furthermore, streak ovaries or absent/nonvisualized ovaries have been reported with CYP19A1 deficiency [17, 27, 30]. Thus it appears that aromatase deficiency might lead to an earlier destruction of the ovary than with other steroid biosynthetic disorders such as lipoid CAH due to steroidogenic acute regulatory protein deficiency. In STAR deficiency, lipid overload destroys the gonad over time and a PCOS-like phenotype is not characteristic [61]. Studies of ovary development have shown that concerted activity of aromatase (as well as other steroidogenic enzymes such as HSD17s and HSD3B2), steroid hormones (eg, estrogens and progesterone), and gonadotropins (eg, FSH) is essential for normal primordial follicle pool development during fetal life as well as for normal follicle maturation and ovulation in the adult ovary [62]. In the human fetal ovaries, expression of aromatase and estrogen receptors is upregulated in the second trimester when primordial folliculogenesis is fully active [63]. By contrast, mice lacking aromatase activity develop ovaries with diminished oocyte density [64, 65]. In addition, coordinated action of estrogens and FSH are also crucial for the proper timing of folliculogenesis [66]. In aromatase deficiency lack of estrogens results in abnormal gonadotropin feedback, which affects follicle growth in the ovaries. It has been shown that for the formation of (poly-)cystic ovaries elevated FSH plays a critical role [67, 68]. Overall, these facts from the literature may explain the ovarian phenotypes (both streak gonads and polycystic ovaries) seen with aromatase deficiency. Based on this knowledge and clinical experience (eg, [34]) (preventive) treatment of polycystic ovaries with estrogen replacement normalizing FSH should be advised in all patients with aromatase deficiency.
Human CYP19A1 variants described so far are summarized in Table 1. They span over all exons and comprise all types of genetic variations, as shown in Fig. 1. The genotype-phenotype correlation seems doubtful, but structure-function prediction has revealed consistent results with few exceptions. We found novel CYP19A1 variants in 4 affected girls. These variants were all predicted to severely affect enzyme activity, and functional tests confirmed this prediction. Novel deletion-insertion variants of CYP19A1 found in patients 1 and 2 received the label “pathogenic” when characterized according to American College of Medical Genetics and Genomics (ACMG) standards and guidelines [69]. In silico structure-function studies of the identified CYP19A1 variations showed loss of the heme-binding site due to the R375C variation and was predicted to cause a loss of function. In the case of the V161D variant, a loss of protein stability leading to degradation was predicted. This novel point mutation found in patient and family 3 is likely pathogenic according to ACMG characterization and was found in combination with known variants –41C>T and R264C. Variants R264C and R375C have been described previously [18, 70]. In line with our results, R375C was found to cause a complete loss of function. Although in silico structural prediction could not provide a clear analysis for the R264C variant, we found that the R264C variant showed activity similar to wt CYP19A1. The R264C variation is known in the literature as a polymorphism of CYP19A1, which seems common in Southeast Asia [70]. However, this CYP19A1 variant may affect enzyme function when it is present in combination with specific variants of cytochrome P450 oxidoreductase (POR), as reported in a recent study [70]. Partial loss of function of aromatase activity has been found with CYP19A1 variant R192H [1] and with several POR variants [56, 71-73]. Patients carrying these mutations manifest with variable degrees of virilization either at birth or puberty.
In summary, we describe clinical, genetic, and functional implications of novel CYP19A1 variants identified in 4 girls originating from Africa and India. CYP19A1 deficiency seems to cause maternal virilization in more than 50% of pregnancies and manifest with some degree of ambiguous genitalia in more than 70% of 46,XX newborns. Additionally, about 80% of affected girls are unable to enter or complete puberty spontaneously and need hormonal replacement therapies. The ovary with aromatase deficiency develops a polycystic ovary phenotype in most cases, but streak gonads are also seen. Normal aromatase activity in concerted action with other steroid enzymes and hormones as well as gonadotropins seem essential for proper ovarian follicle pool development during fetal life and for follicle maturation and ovulation during the reproductive years.
Acknowledgments
Financial Support: This work was supported in part by a grant from the Swiss National Science Foundation (SNF 320030-146127 to C.E.F.).
Glossary
Abbreviations
- CAH
congenital adrenal hyperplasia
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- MAF
minor allele frequency
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The data sets generated and analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Bouchoucha N, Samara-Boustani D, Pandey AV, et al. Characterization of a novel CYP19A1 (aromatase) R192H mutation causing virilization of a 46,XX newborn, undervirilization of the 46,XY brother, but no virilization of the mother during pregnancies. Mol Cell Endocrinol. 2014;390(1-2):8–17. [DOI] [PubMed] [Google Scholar]
- 2. Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84(12):4677–4694. [DOI] [PubMed] [Google Scholar]
- 3. Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–355. [DOI] [PubMed] [Google Scholar]
- 4. Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77(1-2):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Nardo G, Gilardi G. Human aromatase: perspectives in biochemistry and biotechnology. Biotechnol Appl Biochem. 2013;60(1):92–101. [DOI] [PubMed] [Google Scholar]
- 6. Neunzig J, Milhim M, Schiffer L, et al. The steroid metabolite 16(β)-OH-androstenedione generated by CYP21A2 serves as a substrate for CYP19A1. J Steroid Biochem Mol Biol. 2017;167:182–191. [DOI] [PubMed] [Google Scholar]
- 7. Shozu M, Akasofu K, Harada T, Kubota Y. A new cause of female pseudohermaphroditism: placental aromatase deficiency. J Clin Endocrinol Metab. 1991;72(3):560–566. [DOI] [PubMed] [Google Scholar]
- 8.Richter-Unruh. Aromatase deficiency: delayed puberty in a German girl is due to 2 new mutations of the CYP19 gene. Paper presented at: 90th Annual Meeting of the Endocrine Society; June 2008, San Francisco, CA. Abstr P2-590. [Google Scholar]
- 9. Ozen S, Atik T, Korkmaz O, et al. Aromatase deficiency in two siblings with 46, XX karyotype raised as different genders: a novel mutation (p.R115X) in CYP19A1 gene [Published online ahead of print April 10, 2019]. J Clin Res Pediatr Endocrinol. 10.4274/jcrpe.galenos.2019.2018.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludwikowski B, Heger S, Datz N, Richter-Unruh A, González R. Aromatase deficiency: rare cause of virilization. Eur J Pediatr Surg. 2013;23(5):418–422. [DOI] [PubMed] [Google Scholar]
- 11. Marino R, Perez Garrido N, Costanzo M, et al. Five new cases of 46,XX aromatase deficiency: clinical follow-up from birth to puberty, a novel mutation, and a founder effect. J Clin Endocrinol Metab. 2015;100(2):E301–E307. [DOI] [PubMed] [Google Scholar]
- 12. Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61–70. [DOI] [PubMed] [Google Scholar]
- 13. Belgorosky A, Pepe C, Marino R, et al. Hypothalamic-pituitary-ovarian axis during infancy, early and late prepuberty in an aromatase-deficient girl who is a compound heterocygote for two new point mutations of the CYP19 gene. J Clin Endocrinol Metab. 2003;88(11):5127–5131. [DOI] [PubMed] [Google Scholar]
- 14. Pepe CM, Saraco NI, Baquedano MS, et al. The cytochrome P450 aromatase lacking exon 5 is associated with a phenotype of nonclassic aromatase deficiency and is also present in normal human steroidogenic tissues. Clin Endocrinol (Oxf). 2007;67(5):698–705. [DOI] [PubMed] [Google Scholar]
- 15. Guercio G, Di Palma MI, Pepe C, et al. Metformin, estrogen replacement therapy and gonadotropin inhibition fail to improve insulin sensitivity in a girl with aromatase deficiency. Horm Res. 2009;72(6):370–376. [DOI] [PubMed] [Google Scholar]
- 16. Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337(2):91–95. [DOI] [PubMed] [Google Scholar]
- 17. Ludwig M, Beck A, Wickert L, et al. Female pseudohermaphroditism associated with a novel homozygous G-to-A (V370-to-M) substitution in the P-450 aromatase gene. J Pediatr Endocrinol Metab. 1998;11(5):657–664. [DOI] [PubMed] [Google Scholar]
- 18. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. [DOI] [PubMed] [Google Scholar]
- 19. Baykan EK, Erdoğan M, Özen S, Darcan Ş, Saygılı LF. Aromatase deficiency, a rare syndrome: case report. J Clin Res Pediatr Endocrinol. 2013;5(2):129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maffei L, Rochira V, Zirilli L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf). 2007;67(2):218–224. [DOI] [PubMed] [Google Scholar]
- 21. Lin L, Ercan O, Raza J, et al. Variable phenotypes associated with aromatase (CYP19) insufficiency in humans. J Clin Endocrinol Metab. 2007;92(3):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci U S A. 1993;90(24):11673–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom). J Clin Endocrinol Metab. 1994;78(6):1287–1292. [DOI] [PubMed] [Google Scholar]
- 24. Chen Z, Wang O, Nie M, et al. Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Mol Cell Endocrinol. 2015;399:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hauri-Hohl A, Meyer-Böni M, Lang-Muritano M, Hauri-Hohl M, Schoenle EJ, Biason-Lauber A. Aromatase deficiency owing to a functional variant in the placenta promoter and a novel missense mutation in the CYP19A1 gene. Clin Endocrinol (Oxf). 2011;75(1):39–43. [DOI] [PubMed] [Google Scholar]
- 26. Dursun F, Ceylaner S. A novel homozygous CYP19A1 gene mutation: aromatase deficiency mimicking congenital adrenal hyperplasia in an infant without obvious maternal virilisation. J Clin Res Pediatr Endocrinol. 2019;11(2):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazen I, McElreavey K, Elaidy A, Kamel AK, Abdel-Hamid MS. Aromatase deficiency due to a homozygous CYP19A1 mutation in a 46,XX Egyptian patient with ambiguous genitalia. Sex Dev. 2017;11(5-6):275–279. [DOI] [PubMed] [Google Scholar]
- 28. Deladoëy J, Flück C, Bex M, Yoshimura N, Harada N, Mullis PE. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. J Clin Endocrinol Metab. 1999;84(11):4050–4054. [DOI] [PubMed] [Google Scholar]
- 29. Zhu WJ, Cheng T, Zhu H, et al. Aromatase deficiency: a novel compound heterozygous mutation identified in a Chinese girl with severe phenotype and obvious maternal virilization. Mol Cell Endocrinol. 2016;433:66–74. [DOI] [PubMed] [Google Scholar]
- 30. Gagliardi L, Scott HS, Feng J, Torpy DJ. A case of aromatase deficiency due to a novel CYP19A1 mutation. BMC Endocr Disord. 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saraco N, Nesi-Franca S, Sainz R, et al. An intron 9 CYP19 gene variant (IVS9 + 5G>A), present in an aromatase-deficient girl, affects normal splicing and is also present in normal human steroidogenic tissues. Horm Res Paediatr. 2015;84(4):275–282. [DOI] [PubMed] [Google Scholar]
- 32. Harada N, Ogawa H, Shozu M, et al. Biochemical and molecular genetic analyses on placental aromatase (P-450AROM) deficiency. J Biol Chem. 1992;267(7):4781–4785. [PubMed] [Google Scholar]
- 33. Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab. 1997;82(6):1739–1745. [DOI] [PubMed] [Google Scholar]
- 34. Janner M, Flück CE, Mullis PE. Impact of estrogen replacement throughout childhood on growth, pituitary-gonadal axis and bone in a 46,XX patient with CYP19A1 deficiency. Horm Res Paediatr. 2012;78(4):261–268. [DOI] [PubMed] [Google Scholar]
- 35. Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab. 2004;89(12):6025–6029. [DOI] [PubMed] [Google Scholar]
- 36. Herrmann BL, Saller B, Janssen OE, et al. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab. 2002;87(12):5476–5484. [DOI] [PubMed] [Google Scholar]
- 37. Lanfranco F, Zirilli L, Baldi M, et al. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–635. [DOI] [PubMed] [Google Scholar]
- 38. Unal E, Yıldırım R, Taş FF, Demir V, Onay H, Haspolat YK. Aromatase deficiency due to a novel mutation in CYP19A1 gene. J Clin Res Pediatr Endocrinol. 2018;10(4):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mittre Hervé MH, Kottler ML, Pura M. Human gene mutations. Gene symbol: CYP19. Disease: aromatase deficiency. Hum Genet. 2004;114(2):224. [PubMed] [Google Scholar]
- 40. Akçurin S, Türkkahraman D, Kim WY, Durmaz E, Shin JG, Lee SJ. A novel null mutation in P450 aromatase gene (CYP19A1) associated with development of hypoplastic ovaries in humans. J Clin Res Pediatr Endocrinol. 2016;8(2):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abstracts of the 51st Annual Meeting of the European Society for Paediatric Endocrinology (ESPE). Leipzig, Germany. September 20–23, 2012. Horm Res Paediatr. 2012;78(Suppl.1):1–349. [DOI] [PubMed] [Google Scholar]
- 42. Portrat-Doyen SFM, Forest MG, Nicolino M, Morel Y, Chatelain PC. Female pseudohermaphroditism (FHP) resulting from aromatase(P40arom) deficiency associated with a novel mutation R457X in the CYP19 gene. Horm Res. 1996;46(Suppl 2):14. [Google Scholar]
- 43. Bulun SE. Clinical review 78: aromatase deficiency in women and men: would you have predicted the phenotypes? J Clin Endocrinol Metab. 1996;81(3):867–871. [DOI] [PubMed] [Google Scholar]
- 44. Shozu M, Sebastian S, Takayama K, et al. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med. 2003;348(19):1855–1865. [DOI] [PubMed] [Google Scholar]
- 45. Stratakis CA, Vottero A, Brodie A, et al. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998;83(4):1348–1357. [DOI] [PubMed] [Google Scholar]
- 46. Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38(Web Server issue):W529–W533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glaser F, Pupko T, Paz I, et al. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19(1):163–164. [DOI] [PubMed] [Google Scholar]
- 48. Ghosh D, Lo J, Morton D, et al. Novel aromatase inhibitors by structure-guided design. J Med Chem. 2012;55(19):8464–8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins. 2004;57(4):678–683. [DOI] [PubMed] [Google Scholar]
- 50. Vriend G. WHAT IF: a molecular modeling and drug design program. J Mol Graph. 1990;8(1):52–6, 29. [DOI] [PubMed] [Google Scholar]
- 51. Debiec KT, Cerutti DS, Baker LR, Gronenborn AM, Case DA, Chong LT. Further along the road less traveled: AMBER ff15ipq, an original protein force field built on a self-consistent physical model. J Chem Theory Comput. 2016;12(8):3926–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Worth CL, Bickerton GR, Schreyer A, et al. A structural bioinformatics approach to the analysis of nonsynonymous single nucleotide polymorphisms (nsSNPs) and their relation to disease. J Bioinform Comput Biol. 2007;5(6):1297–1318. [DOI] [PubMed] [Google Scholar]
- 53. Topham CM, Srinivasan N, Blundell TL. Prediction of the stability of protein mutants based on structural environment-dependent amino acid substitution and propensity tables. Protein Eng. 1997;10(1):7–21. [DOI] [PubMed] [Google Scholar]
- 54. Smith RE, Lovell SC, Burke DF, Montalvao RW, Blundell TL. Andante: reducing side-chain rotamer search space during comparative modeling using environment-specific substitution probabilities. Bioinformatics. 2007;23(9):1099–1105. [DOI] [PubMed] [Google Scholar]
- 55. Pandey AV, Kempná P, Hofer G, Mullis PE, Flück CE. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol Endocrinol. 2007;21(10):2579–2595. [DOI] [PubMed] [Google Scholar]
- 56. Pandey AV, Flück CE. NADPH P450 oxidoreductase: structure, function, and pathology of diseases. Pharmacol Ther. 2013;138(2):229–254. [DOI] [PubMed] [Google Scholar]
- 57. Lephart ED, Simpson ER. Assay of aromatase activity. Methods Enzymol. 1991;206:477–483. [DOI] [PubMed] [Google Scholar]
- 58. Parween S, Fernández-Cancio M, Benito-Sanz S, et al. Molecular basis of CYP19A1 deficiency in a 46, XX patient with R550W mutation in POR: expanding the PORD phenotype [Published online ahead of print February 15, 2020]. J Clin Endocrinol Metab. 10.1210/clinem/dgaa076 [DOI] [PubMed] [Google Scholar]
- 59. Burckhardt MA, Obmann V, Wolf R, Janner M, Flück CE, Mullis PE. Ovarian and uterine development and hormonal feedback mechanism in a 46 XX patient with CYP19A1 deficiency under low dose estrogen replacement. Gynecol Endocrinol. 2015;31(5):349–354. [DOI] [PubMed] [Google Scholar]
- 60. Bianchi PH, Gouveia GR, Costa EM, et al. Successful live birth in a woman with 17α-hydroxylase deficiency through IVF frozen-thawed embryo transfer. J Clin Endocrinol Metab. 2016;101(2):345–348. [DOI] [PubMed] [Google Scholar]
- 61. Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab. 1997;82(5):1511–1515. [DOI] [PubMed] [Google Scholar]
- 62. Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fowler PA, Anderson RA, Saunders PT, et al. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab. 2011;96(6):1754–1762. [DOI] [PubMed] [Google Scholar]
- 64. Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71(5):1712–1723. [DOI] [PubMed] [Google Scholar]
- 65. Dutta S, Mark-Kappeler CJ, Hoyer PB, Pepling ME. The steroid hormone environment during primordial follicle formation in perinatal mouse ovaries. Biol Reprod. 2014;91(3):68. [DOI] [PubMed] [Google Scholar]
- 66. Wang C, Zhou B, Xia G. Mechanisms controlling germline cyst breakdown and primordial follicle formation. Cell Mol Life Sci. 2017;74(14):2547–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. [DOI] [PubMed] [Google Scholar]
- 68. Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21(5):551–583. [DOI] [PubMed] [Google Scholar]
- 69. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parween S, DiNardo G, Baj F, Zhang C, Gilardi G, Pandey AV. Differential effects of variations in human P450 oxidoreductase on the aromatase activity of CYP19A1 polymorphisms R264C and R264H. J Steroid Biochem Mol Biol. 2020;196:105507. [DOI] [PubMed] [Google Scholar]
- 71. Flück CE, Pandey AV. Impact on CYP19A1 activity by mutations in NADPH cytochrome P450 oxidoreductase. J Steroid Biochem Mol Biol. 2017;165(Pt A):64–70. [DOI] [PubMed] [Google Scholar]
- 72. Flück CE, Mallet D, Hofer G, et al. Deletion of P399_E401 in NADPH cytochrome P450 oxidoreductase results in partial mixed oxidase deficiency. Biochem Biophys Res Commun. 2011;412(4):572–577. [DOI] [PubMed] [Google Scholar]
- 73. Udhane SS, Parween S, Kagawa N, Pandey AV. Altered CYP19A1 and CYP3A4 activities due to mutations A115V, T142A, Q153R and P284L in the human P450 oxidoreductase. Front Pharmacol. 2017;8:580. [DOI] [PMC free article] [PubMed] [Google Scholar]