Abstract
Background
Although amoxicillin–clavulanate is the recommended first-line empirical oral antibiotic treatment for non-severe exacerbations in children with bronchiectasis, azithromycin is also often prescribed for its convenient once-daily dosing. No randomised controlled trials involving acute exacerbations in children with bronchiectasis have been published to our knowledge. We hypothesised that azithromycin is non-inferior to amoxicillin-clavulanate for resolving exacerbations in children with bronchiectasis.
Methods
We did this parallel-group, double-dummy, double-blind, non-inferiority randomised controlled trial in three Australian and one New Zealand hospital between April, 2012, and August, 2016. We enrolled children aged 1–19 years with radiographically proven bronchiectasis unrelated to cystic fibrosis. At the start of an exacerbation, children were randomly assigned to oral suspensions of either amoxicillin–clavulanate (22·5 mg/kg, twice daily) and placebo or azithromycin (5 mg/kg per day) and placebo for 21 days. We used permuted block randomisation (stratified by age, site, and cause) with concealed allocation. The primary outcome was resolution of exacerbation (defined as a return to baseline) by 21 days in the per-protocol population, with a non-inferiority margin of −20%. We assessed several secondary outcomes including duration of exacerbation, time to next exacerbation, laboratory, respiratory, and quality-of-life measurements, and microbiology. This trial was registered with the Australian/New Zealand Registry (ACTRN12612000010897).
Findings
We screened 604 children and enrolled 236. 179 children had an exacerbation and were assigned to treatment: 97 to amoxicillin–clavulanate, 82 to azithromycin). By day 21, 61 (84%) of 73 exacerbations had resolved in the azithromycin group versus 73 (84%) of 87 in the amoxicillin–clavulanate group. The risk difference showed non-inferiority (−0·3%, 95% CI −11·8 to 11·1). Exacerbations were significantly shorter in the amoxicillin–clavulanate group than in the azithromycin group (median 10 days [IQR 6–15] vs 14 days [8–16]; p=0·014). Adverse events were attributed to the trial medication in 17 (21%) of 82 children in the azithromycin group versus 23 (24%) of 97 in the amoxicillin–clavulanate group (relative risk 0·9, 95% CI 0·5 to 1·5).
Interpretation
By 21 days of treatment, azithromycin is non-inferior to amoxicillin–clavulanate for resolving exacerbations in children with non-severe bronchiectasis. In some patients, such as those with penicillin hypersensitivity or those likely to have poor adherence, azithromycin provides another option for treating exacerbations, but must be balanced with risk of treatment failure (within a 20% margin), longer exacerbation duration, and the risk of inducing macrolide resistance.
Funding
Australian National Health and Medical Research Council.
Introduction
Bronchiectasis is a chronic pulmonary disorder defined as dilatation of bronchi that presents clinically with a chronic productive cough.1 Despite being recognised increasingly in non-Indigenous children1, 2 and adults,3 and no longer considered an orphan disease,1 it remains a neglected condition.
Although there is no strict definition of a bronchiectasis exacerbation, it is generally characterised by increasing cough, and in adults by increased sputum volume and purulence.1 In children, exacerbations cause increased parental anxiety and stress, adversely affect quality of life,4 and when severe (requiring hospital admission) they can negatively affect future lung function.5, 6 Few randomised controlled trials have been done to provide evidence for how to best treat exacerbations.7 Those that have been published involved small numbers of participants, many were open label, and none included children.
Although exacerbations are triggered often by viral infections,4 antibiotics are commonly used.8, 9, 10 Treatment is guided ideally by lower airway microbiology but such data are often unavailable in children unable to provide sputum for culture. Thus, empirical antibiotics are frequently necessary. The Australian and New Zealand guidelines for bronchiectasis recommend amoxicillin–clavulanate as the first-line empirical oral antibiotic therapy for non-severe exacerbations in children.11 European guidelines do not name the antibiotics but refer to a paper that recommends amoxicillin-clavulanate.10, 12 Amoxicillin–clavulanate is active against Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis, the three bacterial pathogens detected most often in high densities in the lower airways of children with bronchiectasis.13 However, amoxicillin–clavulanate requires multiple dosing per day and can cause gastrointestinal symptoms. Oral azithromycin is attractive as an alternative first-line therapy because of its long half-life, which means it can be taken less frequently, and is well-tolerated by children. In disadvantaged communities, twice-daily dosing of antibiotics such as amoxicillin–clavulanate is not always feasible, and might result in children with exacerbations receiving suboptimal treatment, risking continuing symptoms and antibiotic resistance. Three randomised controlled trials14 did not show that azithromycin was superior to amoxicillin–clavulanate for treating paediatric community-acquired pneumonia. However, azithromycin is still used in some settings as first-line treatment of acute exacerbations in children with underlying bronchiectasis.15
Research in context.
Evidence before this study
Before the study began, we searched PubMed and Cochrane databases for randomised controlled trials involving bronchiectasis. We repeated these searches on Jan 8, 2018. We used the terms “bronchiectasis” and “controlled trials”. We then searched using keywords “antibiotics”, “bronchiectasis”, and “exacerbation” in the same databases. Our searches were restricted to reports in English. We found six randomised controlled trials of antibiotic treatment of exacerbations in adults, of which only one was placebo-controlled. The remaining five compared two different antibiotics, none of which were macrolides, and three were open-label. All involved small numbers of patients (range 18–43) and none was in children. During the study, studies on the long-term use of macrolides in adults and children with bronchiectasis were published, but there are still no strong data on antibiotics in acute exacerbations of bronchiectasis in children.
Added value of this study
To our knowledge, this is the first randomised controlled trial comparing amoxicillin–clavulanate with azithromycin for treating exacerbations in children with bronchiectasis. It shows that, within a 20% margin, azithromycin is non-inferior to amoxicillin–clavulanate for resolving symptoms at 21 days when treating acute exacerbations of bronchiectasis, but takes significantly longer to resolve.
Implications of all the available evidence
Although azithromycin is non-inferior to amoxicillin-clavulanate for treating non-severe acute exacerbations of bronchiectasis in children, the exacerbation might take significantly longer to resolve, and the risk of inducing macrolide resistance should be considered. Although azithromycin might be used cautiously for some patients, such as those with penicillin hypersensitivity or for whom less frequent dosing might improve adherence, amoxicillin–clavulanate remains the first choice empirical antibiotic.
We therefore tested whether daily oral azithromycin was non-inferior to oral amoxicillin–clavulanate for resolving exacerbations by 21 days of treatment. We also assessed the effect of the interventions on exacerbation duration, time-to-next respiratory exacerbation, lung function, quality of life, systemic inflammation, antibiotic resistance, and treatment-related adverse effects, and describe the point prevalence and diversity of respiratory viruses, Mycoplasma pneumoniae and Chlamydiales spp during exacerbations.
Methods
Study design and participants
We did this parallel-group, double-dummy, double-blind, placebo-controlled randomised trial in four hospitals in Australia and New Zealand between April, 2012, and August, 2016. The study protocol has been published.16 An independent data safety and monitoring committee monitored the study.
Children (aged 1–19 years) attending respiratory clinics in three tertiary paediatric hospitals (Brisbane, Sydney, and Auckland) and general paediatric clinics (Darwin) were eligible for enrolment if they had received a diagnosis of bronchiectasis from a respiratory physician (based on their clinical features and results of a chest high-resolution CT scan) within the past 5 years, were followed up regularly by a respiratory physician, and had at least two respiratory exacerbations in the previous 18 months.
We excluded children if they had a current or recent severe exacerbation of bronchiectasis (dyspnoea, hypoxia [SpO2 <90% in air], or were admitted to hospital) in the previous 8 weeks; had cystic fibrosis or liver dysfunction; had hypersensitivity to β lactam or macrolide antibiotics; had detection of Pseudomonas aeruginosa within the past 4 months or past or current infection with non-tuberculous mycobacteria; had received short-term β lactam or macrolide antibiotics within the past 3 weeks; or were receiving treatment for cancer. Children who were taking long-term antibiotics (>4 weeks) for managing their bronchiectasis were not excluded, but the maintenance antibiotic was ceased while taking the study medication. Participating hospitals' human research ethics committees approved the study. Parents or primary caregivers gave written informed consent before their child participated in the study. Children aged older than 12 years also provided their written assent.
Randomisation and masking
Randomisation was stratified by site (Brisbane, Darwin, Sydney, or Auckland), age (≤5 years, >5 years), and underlying cause (either [post-infectious or idiopathic], or [immunodeficiency, aspiration, primary ciliary dyskinesia, or other]). The computer-generated permuted block (block size 2–8) randomisation allocation sequence (1:1 ratio) was prepared by a statistician not in the study team. At the time of an exacerbation, the child was allocated to the next number on the randomisation list maintained by the local hospital pharmacist (except in Darwin, where the list was maintained by the Brisbane pharmacist). The statistician and the trial pharmacist had no involvement in the study after sequence preparation and allocation respectively.
The participants, caregivers, study coordinators at various sites, and the investigators were all masked to treatment assignment until the data analysis was completed. Participants received one active trial medication and equivalent volumes of placebo for the other trial medications. The placebo was manufactured by the Institute of Drug Technology Australia (Melbourne, VIC, Australia) and had a similar taste and colour to their respective antibiotics.17 Both active medications (amoxicillin–clavulanate and azithromycin) were repackaged and relabelled so that both antibiotics and their respective placebos were provided in identical opaque bottles. All trial medications were supplied as dry powder to be reconstituted at home by adding equal volumes of water for placebo and active medication.
Procedures
When beginning treatment of an exacerbation (day 1), children were randomly assigned to receive oral suspensions of either amoxicillin–clavulanate (22·5 mg/kg twice daily) and placebo or azithromycin (5 mg/kg per day) and placebo. All treatments continued for 21 days, unless the child exited the study. Children whose exacerbation did not resolve by 21 days received open-label oral amoxicillin–clavulanate (usual treatment). Study drugs were administered by the family or caregivers. Adherence was assessed by return of study medication bottles. Children were followed up for 6 months after the exacerbation or until the next exacerbation after finishing the study treatment, whichever was earlier. Only a single exacerbation per enrolled child was included in the study.
Deep nasal swabs were collected at recruitment (baseline), immediately before starting treatment for the exacerbation (day 1), and at cessation on day 21, and placed into skimmed milk tryptone glucose glycerol broth and transported to the laboratory for storage at −80°C. Swabs were then cultured for common respiratory bacteria and phenotypic antibiotic resistance testing was done with established methods.16, 17 Nucleic acids were also extracted from the broth of nasal swabs collected on day 1 and tested for 16 respiratory viruses, M pneumoniae, and Chlamydiales spp using real-time PCR assays as described previously.16, 17
Spirometry was done at baseline, and at the beginning and end of an exacerbation in children able to do this test (aged ≥6 years) and the FEV1% predicted was recorded.
Research nurses contacted the parents or caregivers each month by telephone, email, or through an online survey to collect data about any exacerbations. Parents and caregivers were also asked to contact the study coordinator during business hours, or the on-call physician for the study after-hours, when they thought their child was having an exacerbation. The child was then assessed for their suitability to start the study medication and encouraged to attend the clinic to be seen by one of the study doctors and started on the study medication upon fulfilling the definition of an exacerbation. Study participants who were in remote and rural areas or who were unable to attend the clinic had the study medication and quality-of-life questionnaire sent to them. While the child was taking trial medication, their parent or caregiver kept a daily cough diary and an adverse events diary and could contact the research staff if they suspected that any adverse effects were related to treatment.
A non-severe exacerbation was defined as an increase in cough frequency, a change in character of the cough from dry to wet, or an increase in sputum volume or purulence for at least 3 days and unaccompanied by dyspnoea, hypoxia (SpO2 <90% in air), or need for hospital admission according to the treating clinician. Resolution of an exacerbation was defined a priori by cough scores from the daily symptom diary returning to baseline for at least 2 days,18 accompanied by resolution of any new additional symptoms and signs associated with the episode. Research nurses telephoned parents and caregivers on day 3 and day 10 to record adverse events, and clinical reviews, including examination of the symptom diaries, were done on day 14 and day 21.16 The day of resolution was then determined and recorded. Baseline state of each child was determined at enrolment with a validated cough diary card.18
Outcomes
The primary outcome was the proportion of children whose exacerbations resolved at any time within 21 days of treatment.16 For children who withdrew from the study, or received additional antibiotic treatment, their exacerbation was categorised as non-resolved. Secondary clinical outcomes were hospital admission, exacerbation duration (persistence of symptoms until return to baseline state), time to next exacerbation, change in the forced expiratory volume in 1 s percent (FEV1%) predicted, parent cough-specific quality-of-life score,19 and treatment-related adverse events.
The secondary laboratory outcomes were changes in peripheral white blood cell count and C-reactive protein (CRP), and nasal respiratory bacterial pathogens, including their antibiotic susceptibilities, between day 1 and day 21, as well as respiratory viruses and atypical pathogens on day 1. The laboratory outcomes were available only in a subset of children because not all children underwent venepuncture or had a second nasal swab taken.
Serious adverse events were defined a priori as any unexpected medical occurrence that resulted in death or was life-threatening, caused significant disability or incapacity, or hospital admission. All serious adverse events were reported to an independent data safety monitoring board. If serious adverse events occurred during the 21-day study period and judged to be associated with the trial medication, the trial drug was ceased.
Statistical analysis
We based the sample size on a non-inferiority hypothesis comparing oral azithromycin with amoxicillin–clavulanate for resolution by 21 days. The true between-group difference was assumed to be zero. The non-inferiority margin was defined as an absolute difference of 20%, and we expected 80% of exacerbations to resolve in the amoxicillin–clavulanate group. The size of the non-inferiority margin was chosen by the respiratory physicians in the study and determined by what, in their view, was a clinically acceptable loss of efficacy compensated for by azithromycin's dosing profile and potential for increased treatment adherence from daily supervised use in remote and rural settings. The resolution percentage was informed by a pilot study of 15 children treated with oral amoxicillin–clavulanate for a non-severe exacerbation in which 12 (80%) children had their symptoms resolved by day 21. To achieve 90% power with a one-sided α of 0·025, we would require a sample size of 170 children (85 per arm). Because the primary outcome was to be captured in all randomly assigned participants, we did not account for attrition in the sample size calculations. We defined azithromycin to be non-inferior to amoxicillin–clavulanate if the lower bound of the 95% CI for the risk difference in proportions of children whose exacerbation resolved was greater than −20·0%.
We present summary statistics as medians (IQR) for continuous data and as number (%) for categorical data. We also present normally distributed data as means (SD) in the appendix. We analysed the primary endpoint in the per-protocol population with intention-to-treat as sensitivity analyses, each with a two-sided 95% CI.20, 21 The per-protocol analysis included only children who received the study drugs for 21 days and had no protocol deviation that could affect efficacy. It also included any child who switched to intravenous antibiotics because of worsening symptoms and treatment failure. We compared the primary outcome in each group using a generalised linear regression model with binomial family and identity link to calculate the absolute difference in risk of resolution.
We analysed secondary endpoints in children who had data available, per protocol, with sensitivity analyses done for the intention-to-treat population We limited analysis of bacteriological data from nasal swabs to participants who provided paired swabs when at day 1 and day 21.
Median regression compared the median duration of exacerbation in days and time-to-next exacerbation in days between the two groups. For children whose symptoms did not resolve within 21 days and the days to achieve cure was unknown, the maximum time-to-resolution value obtained in the cohort was assigned. We used Cox proportional hazard modelling and Kaplan–Meier survival analysis to compare the two groups for the time-to-next exacerbation. We confirmed the proportional hazards assumption was not violated using Schoenfeld residuals and visually using log–log plots. We also compared the parent cough-specific quality-of-life scores at day 21 (or the higher value within 21 days) in the two treatment groups by linear regression with the main effect being the treatment group, and score at baseline included as a covariable, based on the minimum important score difference of 0·9.19
We did an additional a-priori sensitivity analysis of the primary and secondary endpoints in participants not receiving long-term macrolides at the time of their exacerbation. We used the Statistical Package for Social Science (version 25) and Stata (version 15.0) to analyse the data. All tests were two-tailed, 95% CIs were reported where appropriate, and statistical significance was set at p less than 0·05.
The statistical analysis plan was approved by the study investigators and the data and safety management committee before the data were analysed. Data were analysed before revealing the arm to which each child was allocated.
This trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12612000010897).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, or data interpretation, or the writing of the report. VG, ABC, MJB, and RSW had access to the raw data. The corresponding author had full access to all the data in the study and the final responsibility for the decision to submit for publication.
Results
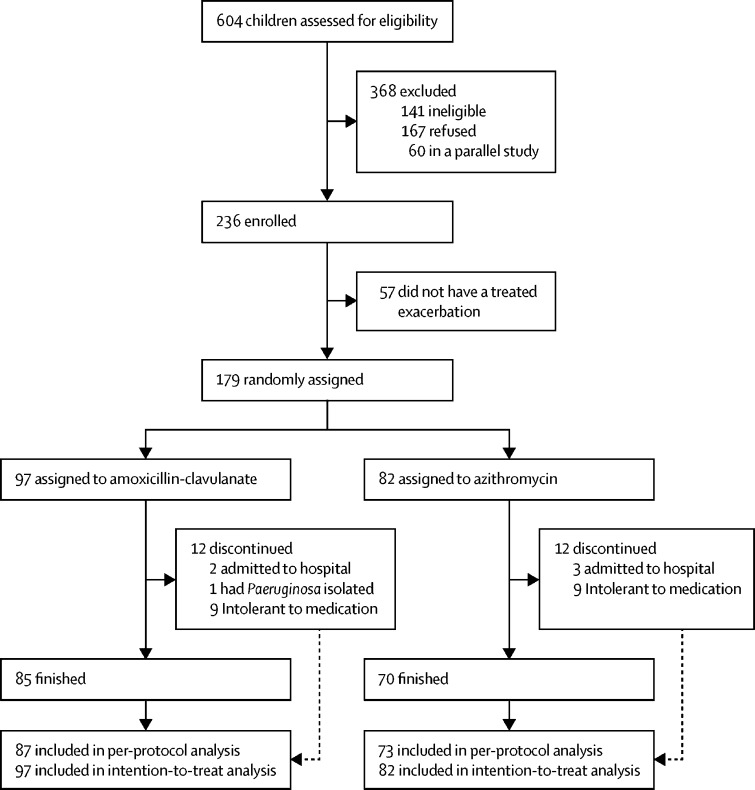
463 children met the inclusion criteria from 604 screened for the study (figure 1 ). Parents of 167 children declined participation, another 60 children were already enrolled in a parallel trial and also declined, while 57 were recruited, but did not have a treated exacerbation during the study period. Overall, 179 children had an exacerbation and were randomly assigned to either amoxicillin–clavulanate (n=97) or azithromycin (n=82) between April 17, 2012, and Aug 30, 2016. Recruitment continued beyond the planned sample size of 170 because each centre had their own randomisation list with block randomisation, and some of the children recruited from remote or rural areas were given the study medication during their baseline visit to start at the time of their next exacerbation. The last follow-up visit was on Feb 27, 2017.
Figure 1.
Trial profile
The intention-to-treat population included all children who were randomly assigned and took at least one dose of the study medication, the per-protocol population includes children who completed treatment, including those who were admitted to hospital, but excluding one child who discontinued treatment because of Pseudomonas aeruginosa isolation and 18 who were intolerant to the treatment.
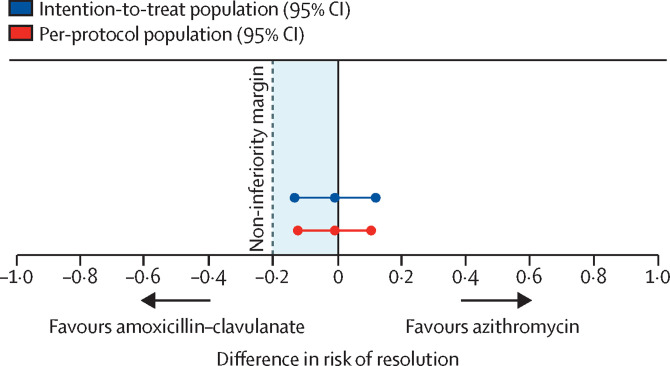
Characteristics of the two groups were similar at baseline (table 1 ) and at the beginning of an exacerbation (appendix p 10). Of the 94 children who completed 3 weeks of treatment and returned the empty bottles, medication adherence was 48 (96%) of 50 in the amoxicillin–clavulanate group and 38 (86%) of 44 in the azithromycin group (p=0·14). In the per-protocol population, the number of children whose exacerbation symptoms had resolved by day 21 was 73 (83·9%) of 87 in the amoxicillin–clavulanate group versus 61 (83·6%) of 73 in the azithromycin group. The risk difference was −0·3% (95% CI −11·8 to 11·1), falling within the a-priori 20% non-inferiority margin (figure 2 ). In a sensitivity analysis of the intention-to-treat population, exacerbations had resolved by day 21 in 75 (77·3%) of 97 children in the amoxicillin-clavulanate group and 63 (76·8%) of 82 in the azithromycin group, with a risk difference of −0·5% (95% CI −12·9 to 11·9).
Table 1.
Baseline characteristics
| Amoxicillin–clavulanate group (n=97) | Azithromycin group (n=82) | |
|---|---|---|
| Sociodemographic | ||
| Age (years) | 6·8 (4·3–10·1) | 6·4 (4·0–9·0) |
| Male | 55 (57%) | 40 (49%) |
| Indigenous ethnicity | 37 (38%) | 33 (40%) |
| Medical history | ||
| History of preterm birth (<37 weeks) | 22/90 (24%) | 21/79 (27%) |
| Breastfeeding (ever in infancy) | 69 (71%) | 61 (74%) |
| Tobacco smoke exposure | 29 (30%) | 26 (32%) |
| Age at diagnosis of bronchiectasis (years) | 4·3 (2·1–7·5) | 3·6 (2·2–6·1) |
| Number of lobes affected | 3 (2–4) | 3 (2–4) |
| Non-hospital admission exacerbations in past 12 months (n=173) | 3 (1–5) | 3 (1–5) |
| Hospital admissions in past 2 years for bronchiectasis exacerbations (n=178) | 1 (0–2) | 1 (0–2) |
| Long-term antibiotics (>4 weeks)* | 54 (56%) | 43 (52%) |
| Long-term macrolides | 35 (36%) | 32 (39%) |
| Underlying cause | ||
| Post-infectious | 57 (59%) | 47 (57%) |
| Idiopathic | 19 (20%) | 12 (15%) |
| Immunodeficiency | 5 (5%) | 3 (4%) |
| Aspiration | 8 (8%) | 8 (10%) |
| Primary ciliary dyskinesia | 3 (3%) | 3 (4%) |
| Other | 3 (3%) | 6 (7%) |
| Comorbidities | ||
| Tracheomalacia | 13 (13%) | 6 (7%) |
| Syndromic (eg, trisomy 21) | 4 (4%) | 8 (10%) |
| Asthma | 19 (20%) | 10 (12%) |
| Examination findings | ||
| Weight (kg) | 22·9 (15·8–39·2) | 22·3 (16·6–36·7) |
| Oxygen saturation (n=163) | 98·5 (98–100) | 99 (98–99) |
| Cough score17 (n=177) | 1 (0–2) | 1 (0–2) |
| Digital clubbing (n=178) | 22/96 (23%) | 11/82 (13%) |
| Chest wall deformity | 24 (25%) | 19 (23%) |
| Wheeze | 2 (2%) | 2 (2%) |
| Crackles | 8 (8%) | 2 (2%) |
| FEV1% predicted (n=99) | 88 (77–101) | 85 (79–95) |
| PC-QoL score18 (n=175) | 6·3 (4·6–6·8) | 6·2 (4·5–7·0) |
| Serum biomarkers | ||
| White blood cell count (×109 per L; n=92) | 8·2 (6·8–9·3) | 8·5 (7·1–10·1) |
| CRP (mg/L; n=94) | 2 (2–2) | 2 (2–2) |
Data are median (IQR) or n (%) unless stated otherwise. CRP=C-reactive protein. FEV1%=forced expiratory volume in 1 s percent. PC-QoL=parent cough-specific quality of life.
The only other long-term antibiotic used in these children was co-trimoxazole, by 19 in the amoxicillin-clavulanate group and 11 in the azithromycin group.
Figure 2.
Risk difference for resolution of exacerbations with azithromycin versus amoxicillin–clavulanate
The median time to resolution of exacerbation was 4 days shorter in the amoxicillin–clavulanate group than in the azithromycin group in both the per-protocol and intention-to-treat analyses (table 2 ). By contrast, the median time to next exacerbations was similar in both groups (table 2, appendix p 6). The likelihood of having an exacerbation during follow-up was similar between groups: in the per-protocol, the hazard ratio was 0·8 (95% CI 0·5–1·2; p=0·3) and in the intention-to-treat group it was 0·8 (95% CI 0·6–1·2; p=0·5). Similarly, we detected no significant differences between groups for changes in inflammatory biomarkers, FEV1% predicted, or parent cough-specific quality-of-life scores (table 3 ).
Table 2.
Time to resolution and to next exacerbation
| Amoxicillin–clavulanate group (n=97) | Azithromycin group (n=82) | p value | |
|---|---|---|---|
| Per-protocol analyses | |||
| Time to resolution (days) | 10 (6–15) | 14 (8–16) | 0·014 |
| Time to next exacerbation (days)* | 85 (30–180) | 91 (38–180) | 0·81 |
| Intention-to-treat analyses | |||
| Time to resolution (days) | 10 (6–15) | 14 (7–16) | 0·013 |
| Time to next exacerbation (days)* | 75 (26–180) | 90·5 (37–180) | 0·52 |
Data are median (IQR).
Data were censored at 180 days.
Table 3.
Laboratory, respiratory, and quality of life results
|
Amoxicillin–clavulanate group |
Azithromycin group |
Difference between change in groups (95% CI)* | |||
|---|---|---|---|---|---|
| Day 1 | Day 21 | Day 1 | Day 21 | ||
| Per-protocol analyses | |||||
| White blood cell count (n=43) | 9·1 (7·3 to 10·5) | 8·2 (6·7 to 9·9) | 8·8 (7·2 to 10·6) | 7·7 (6·5 to 9·1) | 0·0 (−1·7 to 1·7) |
| CRP (n=46) | 2·0 (2·0 to 5·1) | 2·0 (2·0 to 2·0) | 2·0 (2·0 to 9·9) | 2·0 (2·0 to 2·0) | −0·3 (−2·8 to 2·2) |
| FEV1% predicted, (n=36) | 81·0 (72·0 to 90·0) | 84·0 (71·5 to 94·5) | 74·0 (66·0 to 89·0) | 82·5 (72·0 to 96·6) | 3·0 (−3·2 to 9·2) |
| PC-QoL (n=143) | 4·2 (3·3 to 5·2) | 6·3 (5·3 to 6·9) | 4·5 (3·3 to 5·2) | 6·0 (4·8 to 6·8) | −0·2 (−0·8 to 0·5) |
| Intention-to-treat analyses | |||||
| White blood cell count (n=45) | 8·8 (7·0 to 10·5) | 8·2 (6·7 to 9·9) | 8·8 (7·2 to 10·6) | 7·7 (6·5 to 9·1) | −0·6 (−2·1 to 0·9) |
| CRP (n=48) | 2·0 (2·0 to 5·1) | 2·0 (2·0 to 2·0) | 2·0 (2·0 to 9·9) | 2·0 (2·0 to 2·0) | 0·0 (−2·4 to 2·4) |
| FEV1% predicted (n=37) | 81·0 (68·5 to 90·0) | 83·0 (71·0 to 93·0) | 73·5 (66·0 to 89·0) | 82·5 (72 to 96·6) | 4·0 (−2·5 to 10·5) |
| PC-QoL (n=153) | 4·3 (3·4 to 5·3) | 6·3 (5·3 to 6·9) | 4·5 (3·4 to 5·7) | 6·0 (4·8 to 6·8) | −0·2 (−0·9 to 0·4) |
Data are median (IQR). CRP=C-reactive protein. FEV1%=forced expiratory volume in 1 s percent. PC-QoL=parent cough-specific quality of life.
To compare difference between groups, median regression with 95% CI is reported.
Of the 113 children who provided paired nasal swab specimens on day 1 and day 21, 74 bacterial pathogens were cultured (H influenzae, S pneumoniae, M catarrhalis, or Staphylococcus aureus) from 55 (48·7%) children on day 1 (appendix pp 11–12). The bacteriological profile in nasal swabs, including carriage of azithromycin-resistant organisms, was similar in both treatment groups at the start of an exacerbation (appendix pp 11–12). One child whose sputum contained Pseudomonas aeruginosa withdrew from the trial to begin anti-pseudomonal therapy. By day 21, the number of children carrying these pathogens had more than halved and all patients carrying S pneumoniae on day 1 had cleared it, except for one child in the amoxicillin–clavulanate group. Of the children whose swabs contained pathogens at day 21, four (29%) of 14 in the amoxicillin–clavulanate group and eight (80%) of ten who received azithromycin carried azithromycin-resistant organisms. The azithromycin-resistant S aureus isolates in both treatment groups did not change during the study (appendix pp 11–12).
Of the 147 children with a nasal swab taken at the beginning of an exacerbation, 70 (47·6%) had a virus identified: 37 (48·1%) of 77 in the amoxicillin–clavulanate group and 33 (47·1%) of 70 in the azithromycin group. Among the 70 children with a nasal swab positive for a respiratory virus, two viruses were detected in 11 children, while one child had three viruses present. Rhinovirus was the most common virus detected (n=50, 71·4%) followed by parainfluenza 1–3 (n=8, 11·4%), respiratory syncytial virus (n=4, 5·7%), human metapneumovirus, adenovirus, influenza A or B, human coronavirus-HKU1 and OC43, and enterovirus (n=3 each, 4·3%), human bocavirus (n=2, 1·3%), and human polyomavirus WU (n=1, 0·6%). M pneumoniae and C pneumoniae were identified in one child each.
Overall, 17 (20·7%) of 82 children in the azithromycin group had one or more symptom attributed to the trial medication (nausea, vomiting, diarrhoea, or rash) compared with 23 (23·7%) of 97 in the amoxicillin–clavulanate group (relative risk 0·9, 95% CI 0·5–1·5). Of these 40 children, nine children in each group withdrew from the study early and started open-label antibiotics because of vomiting repeatedly the trial medication (relative risk 1·2, 95% CI 0·5–2·8). A further three children in the azithromycin group and two in the amoxicillin–clavulanate group were admitted to hospital for worsening respiratory symptoms during the trial (relative risk 1·8, 95% CI 0·3–10·3; table 4 ).
Table 4.
Adverse events
| Amoxicillin–clavulanate (n=97) | Azithromycin (n=82) | |
|---|---|---|
| Nausea | 13 (13·4%) | 9 (11·0%) |
| Vomiting | 9 (9·3%) | 9 (11·0%) |
| Diarrhoea | 15 (15·5%) | 6 (7·3%) |
| Rash | 0 (0%) | 2 (2·4%) |
| Hospital admission while receiving medication | 2 (2·1%) | 3 (3·7%) |
| Any | 23 (23·7%) | 17 (20·7%) |
Data are number of children (%); a child could have more than one adverse event.
Of the 18 children who withdrew from the study but were not admitted to hospital, 15 received open-label amoxicillin–clavulanate as per the study protocol. The remaining three children, all in the azithromycin group, received either co-trimoxazole, azithromycin, or both amoxicillin–clavulanate and prednisolone. Continued vomiting was not reported by children receiving open-label antibiotics without the accompanying placebo. A sensitivity analysis excluding the 45 children taking long-term macrolides did not alter our findings (appendix pp 4, 5, 7, 8). Post-hoc subgroup analyses based on age (<5 vs >5 years) and identification of a virus (present or absent) at the beginning of exacerbation are shown in the appendix (p 8).
Discussion
We found that 3 weeks of oral azithromycin was non-inferior (within a 20% margin) to 3 weeks of oral amoxicillin–clavulanate for resolving symptoms in 179 children experiencing a non-severe exacerbation of their bronchiectasis. However, the duration of the exacerbation was shorter by a median of 4 days in children receiving amoxicillin–clavulanate than in those who had azithromycin. There were no significant differences between groups for the outcomes of the time to next exacerbation, inflammatory markers, FEV1% predicted or quality-of-life scores, but azithromycin resistance in bacterial pathogens was more common in the azithromycin group. Gastrointestinal problems, including nausea and diarrhoea, were the most common drug-related adverse events with no significant difference between the two antibiotics. Although adherence was 10 percentage points better in the amoxicillin–clavulanate group, this difference was not statistically significant. Although both antibiotics were generally well tolerated, nine children in each treatment group withdrew from the trial because of vomiting. Almost half the children had respiratory viruses detected at the beginning of an exacerbation, while atypical pathogens were rare. The 20% non-inferiority margin is relatively wide, but without placebo-controlled trials of amoxicillin–clavulanate or azithromycin to guide the treatment of bronchiectasis exacerbations in children, the non-inferiority margin was based on respiratory physicians' expert opinion for a clinically acceptable loss of efficacy compensated for by azithromycin's more favourable dosing profile.
This trial is the first to compare antibiotic efficacy for treating exacerbations in children with bronchiectasis. In the past two decades, bronchiectasis has been recognised increasingly as a relatively common, but often underdiagnosed and under-researched chronic disease.1 Because the lower airway microbial profiles in children with bronchiectasis differ from adults with this disorder and from children with cystic fibrosis,22 having data from children with bronchiectasis is important. This trial is also the first to compare amoxicillin–clavulanate with azithromycin using a non-inferior hypothesis that was determined a priori. Amoxicillin–clavulanate was used as the reference treatment because in Australia and New Zealand it is the recommended first-line antibiotic for outpatient management of children with bronchiectasis when lower airway specimens are unavailable.11 Similarly, the latest European adult guidelines12 refer to an earlier publication listing amoxicillin–clavulanate as the first option for acute exacerbations in adults with bronchiectasis, but without P aeruginosa infection; however, the guidelines do not explicitly recommend amoxicillin–clavulanate in this setting.10
Although antibiotics underpin the treatment of bronchiectasis exacerbations,12 the optimum duration of treatment and dosing regimen is unknown. European guidelines recommend 14 days of therapy, but stated that the level of evidence was very low and based on intravenous rather than oral antibiotics. Although 2 weeks is the standard in routine clinical practice, we chose 3 weeks of antibiotics in this trial on the basis of our prior data. In our prospective cohort study23 of 69 children followed up for 900 child-months, 36 (23%) of 158 exacerbations were treated with intravenous antibiotics following persistence of symptoms—ie, the exacerbation had not resolved. Generally, patients were admitted to hospital 3–5 weeks following the initiation of oral antibiotics and resolution occurred within the next 2 weeks. In addition, from our pilot work involving 15 children, based on validated diary cards,18 we found that six (40%) were cough free by day 7, nine (60%) by day 14, and 12 (80%) by day 21 and day 28.
The long half-life of azithromycin allows a more convenient dosing schedule than does that of amoxicillin–clavulanate, making it an attractive alternative when adherence is particularly difficult and directly supervised therapy is feasible. Azithromycin has good activity against S pneumoniae, M catarrhalis, and atypical pathogens, although the latter may not be so important in this patient population.22 At least for some cases, azithromycin might not be as effective as amoxicillin–clavulanate at eradicating H influenzae,24 which is the predominant pathogen in the lower airways of children with bronchiectasis,22 which is why we used a non-inferiority design in this trial.
Several studies have compared the efficacies of azithromycin and amoxicillin–clavulanate for treating lower respiratory tract infections in children and adults. A Cochrane review14 included 12 studies in adults and three in children comparing 3–5 days of treatment with azithromycin to 5–10 days of either amoxicillin or amoxicillin–clavulanate for treating lower respiratory infections. The review showed that clinical failure, microbial eradication, and adverse events measured after 10–14 days were not significantly different between treatment groups. However, most studies were of uncertain methodological quality. A subgroup analysis of adults with acute bronchitis showed that clinical failure was significantly lower in the azithromycin group.14 Nevertheless, none of these studies included patients with bronchiectasis or used a pre-defined non-inferiority hypothesis, which limits the validity of their overall conclusion.20 By contrast, an investigator-blind randomised controlled trial24 of 730 children with acute otitis media (with tympanocentesis to identify bacterial pathogens), reported that amoxicillin–clavulanate was significantly more efficacious than azithromycin at producing clinical cures (90·5% vs 80·9%, p<0·001). It also showed that azithromycin failed to eradicate H influenzae for 47% of children, despite the in-vitro susceptibility of most H influenzae strains to azithromycin.
Although finding that azithromycin was non-inferior to amoxicillin–clavulanate for resolving symptoms of non-severe bronchiectasis exacerbations in children by day 21, the median time to resolution was 4 days longer in the azithromycin group. This finding should be taken into account when choosing antibiotics because bronchiectasis exacerbations can cause substantial morbidity and disease burden, including impaired quality of life and probably child care or school absenteeism for children, and work absence for parents.4
The major concern with the indiscriminate use of azithromycin is the induction of macrolide resistance and the risk of treatment failure.25 Indeed, in this trial almost one quarter of children who had respiratory bacterial pathogens in their nasal swabs at the beginning of an exacerbation had azithromycin-resistant strains. This finding is consistent with earlier studies and presumably reflects common usage of macrolides in these patients and in the community.15 Unlike other respiratory pathogens, azithromycin-resistant S aureus strains might persist following cessation of the antibiotic,26 and is of concern, especially in settings where meticillin-resistant S aureus is already prevalent.15, 26 Indigenous children who, as in this study, are over-represented among patients with bronchiectasis,27 also have high rates of local and invasive infections with S aureus, including meticillin-resistant S aureus.28 Increasing macrolide-resistance could therefore complicate the treatment of extrapulmonary infections in these high-risk populations.
Notwithstanding the novelty of our study, it has several limitations. There were unequal numbers of patients in the two treatment groups, probably as a result of block randomisation and site-specific stratification based on age and cause, leading to 16 concurrent randomisation lists. Nevertheless, the groups were evenly matched. Second, the optimum antibiotic doses for treating bronchiectasis exacerbations are unknown. The commonly used dose for the 7:1 formulation of amoxicillin–clavulanate available in Australia and New Zealand of 45 mg/kg per day is efficacious for treating children with protracted bacterial bronchitis;29 hence, the pragmatic dosing chosen for the study. However, 80 mg/kg per day of amoxicillin is recommended for treating childhood pneumonia in settings of antibiotic resistance.30 Similarly, without studies comparing different doses of azithromycin to treat lower respiratory tract infections or bronchiectasis exacerbations, we used the commonly recommended 5 mg/kg per day regimen, which corresponded with total weekly dosing of 30–35 mg/kg used successfully in a trial17 of long-term azithromycin to reduce exacerbation frequency in children with bronchiectasis. Third, the time to next exacerbation was based on parental recall of symptoms. Although some exacerbations might not have been recorded, there is no reason to believe that this would have been unevenly distributed between treatment groups. Fourth, only children not requiring hospital admission for an exacerbation were randomly assigned, which could explain such favourable resolution rates (77%). However, enrolling children deemed unwell enough to need intravenous antibiotics to an oral antibiotic trial is ethically unacceptable. Last, because most children are unable to expectorate, deep nasal swabs were collected as described previously.17 Although such specimens reflect poorly the lower airway microbiology, knowing nasopharyngeal carriage is more important from a community perspective because this is the principal reservoir for spreading antibiotic-resistant bacteria.25
In conclusion, when treating children with a non-severe exacerbation of their bronchiectasis, we found that oral azithromycin was non-inferior within a 20% margin to oral amoxicillin–clavulanate. The compromise when using azithromycin instead of amoxicillin–clavulanate is that the exacerbation may last as much as 4 days longer, and the potential risks of inducing macrolide resistance should also be considered. Azithromycin should therefore be regarded only as an alternative treatment for bronchiectasis exacerbations in children with penicillin hypersensitivity or if there is a high-risk of poor adherence with multiple daily dosing and directly observed therapy is possible. Further randomised controlled trials in other populations of patients with bronchiectasis are needed to validate the results of the present study. Until these data are available, amoxicillin–clavulanate remains the empirical first-line therapy of choice for treating non-severe exacerbations of bronchiectasis in children.
Acknowledgments
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council (NHMRC; project grant number 1019834,) the NHMRC Centre for Research Excellence in Lung Health of Aboriginal and Torres Strait Islander Children (1040830), and Cure Kids, Auckland, New Zealand (3702764). VG was supported by an NHMRC post-graduate scholarship (1075119). ABC is supported by an NHMRC practitioner fellowship (1058213). K-AFO was supported by a NHMRC CRE fellowship (10450830). We thank the children and their families for participating in the study. We also thank the research personnel, including Greta Busch (Centre for Children's Health Research) for help with data management and data cleaning, research nurses and staff Joanne Tuppin, Michelle Lewis, Sandra Goodwin and Samantha Gardiner (Centre for Children's Health Research), Clare Mckay (Menzies School of Health Research), Karen Mckay (Sydney Children's Hospital Network), and Charmaine Mobberley for their help with data collection, and Jane Gaydon (Queensland Paediatric Infectious Diseases Laboratory, Children's Health Queensland) and Jemima Beissbarth (Menzies School of Health Research) for processing the specimens. We also thank Alan Isles, Nitin Kapur, Claire Wainwright, Leanne Gauld, and Scott Burgess helping to recruit patients from their clinics. We are grateful to the members of the data safety and monitoring committee (Alan Isles, Mark Chatfield, Craig Mellis, Chris Blyth) who generously provided their time and expertise throughout the study including overseeing the statistical analyses plan.
Contributors
ABC conceived the study. ABC, PSM, KG, PvA, K-AFO, PJ, and IBM designed the study. VG, ABC, PSM, CAB, GBM, HMB, PVA, and HP recruited participants. HP and GBM collected data. AC randomly assigned patients and dispensed medications. VG coordinated the study and follow-up, and did the statistical analysis. VG, ABC, MJB, and RSW had access to the raw data and RSW supervised the statistical analyses. VG wrote the first draft of the article. ABC and KG revised the article. All authors edited the article and approved the final version.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Goyal V, Grimwood K, Marchant J, Masters IB, Chang AB. Pediatric bronchiectasis: no longer an orphan disease. Pediatr Pulmonol. 2016;51:450–469. doi: 10.1002/ppul.23380. [DOI] [PubMed] [Google Scholar]
- 2.McCallum GB, Binks MJ. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front Pediatr. 2017;5:27. doi: 10.3389/fped.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redondo M, Keyt H, Dhar R, Chalmers JD. Global impact of bronchiectasis and cystic fibrosis. Breathe (Sheff) 2016;12:222–235. doi: 10.1183/20734735.007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non-cystic fibrosis bronchiectasis. Chest. 2012;141:1018–1024. doi: 10.1378/chest.11-0679. [DOI] [PubMed] [Google Scholar]
- 5.Munro KA, Reed PW, Joyce H. Do New Zealand children with non-cystic fibrosis bronchiectasis show disease progression? Pediatr Pulmonol. 2011;46:131–138. doi: 10.1002/ppul.21331. [DOI] [PubMed] [Google Scholar]
- 6.Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis what influences lung function stability? Chest. 2010;138:158–164. doi: 10.1378/chest.09-2932. [DOI] [PubMed] [Google Scholar]
- 7.King PT, Holmes PW. Use of antibiotics in bronchiectasis. Rev Recent Clin Trials. 2012;7:24–30. doi: 10.2174/157488712799363280. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers JD, Smith MP, Mchugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186:657–665. doi: 10.1164/rccm.201203-0487OC. [DOI] [PubMed] [Google Scholar]
- 9.Ip M, Shum D, Lauder I, Lam WK, So SY. Effect of antibiotics on sputum inflammatory contents in acute exacerbations of bronchiectasis. Respir Med. 1993;87:449–454. doi: 10.1016/0954-6111(93)90072-8. [DOI] [PubMed] [Google Scholar]
- 10.Woodhead M, Blasi F, Ewig S. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect. 2011;17(suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang AB, Bell SC, Torzillo PJ. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med J Aust. 2015;202:21–23. doi: 10.5694/mja14.00287. [DOI] [PubMed] [Google Scholar]
- 12.Polverino E, Goeminne PC, McDonnell MJ. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50:1700629. doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 13.Grimwood K, Bell SC, Chang AB. Antimicrobial treatment of non-cystic fibrosis bronchiectasis. Expert Rev Anti Infect Ther. 2014;12:1277–1296. doi: 10.1586/14787210.2014.952282. [DOI] [PubMed] [Google Scholar]
- 14.Laopaiboon M, Panpanich R, Swa Mya K. Azithromycin for acute lower respiratory tract infections. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD001954.pub4. CD001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hare KM, Leach AJ, Morris PS. Impact of recent antibiotics on nasopharyngeal carriage and lower airway infection in indigenous Australian children with non-cystic fibrosis bronchiectasis. Int J Antimicrob Agents. 2012;40:365–369. doi: 10.1016/j.ijantimicag.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Chang AB, Grimwood K, Wilson AC. Bronchiectasis exacerbation study on azithromycin and amoxycillin-clavulanate for respiratory exacerbations in children (best-2): study protocol for a randomized controlled trial. Trials. 2013;14:53. doi: 10.1186/1745-6215-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valery PC, Morris PS, Byrnes CA. Long-term azithromycin for indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (bronchiectasis intervention study): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2013;1:610–620. doi: 10.1016/S2213-2600(13)70185-1. [DOI] [PubMed] [Google Scholar]
- 18.Chang AB, Newman RG, Carlin JB, Phelan PD, Robertson CF. Subjective scoring of cough in children: parent-completed vs child-completed diary cards vs an objective method. Eur Respir J. 1998;11:462–466. doi: 10.1183/09031936.98.11020462. [DOI] [PubMed] [Google Scholar]
- 19.Newcombe PA, Sheffield JK, Chang AB. Minimally important change in a parent-proxy quality-of-life questionnaire for pediatric chronic cough. Chest. 2011;139:576–580. doi: 10.1378/chest.10-1476. [DOI] [PubMed] [Google Scholar]
- 20.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C. Reporting of noninferiority and equivalence randomized trials: extension of the consort 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 21.Gotzsche PC. Lessons from and cautions about noninferiority and equivalence randomized trials. JAMA. 2006;295:1172–1174. doi: 10.1001/jama.295.10.1172. [DOI] [PubMed] [Google Scholar]
- 22.De Vries JJV, Chang AB, Marchant JM. Comparison of bronchoscopy and bronchoalveolar lavage findings in three types of suppurative lung disease. Pediatr Pulmonol. 2018;53:467–474. doi: 10.1002/ppul.23952. [DOI] [PubMed] [Google Scholar]
- 23.Kapur N, Masters IB, Morris PS, Galligan J, Ware R, Chang AB. Defining pulmonary exacerbation in children with non-cystic fibrosis bronchiectasis. Pediatr Pulmonol. 2012;47:68–75. doi: 10.1002/ppul.21518. [DOI] [PubMed] [Google Scholar]
- 24.Hoberman A, Dagan R, Leibovitz E. Large dosage amoxicillin/clavulanate, compared with azithromycin, for the treatment of bacterial acute otitis media in children. Pediatr Infect Dis J. 2005;24:525–532. doi: 10.1097/01.inf.0000164794.50281.1a. [DOI] [PubMed] [Google Scholar]
- 25.Serisier DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med. 2013;1:262–274. doi: 10.1016/S2213-2600(13)70038-9. [DOI] [PubMed] [Google Scholar]
- 26.Hare KM, Grimwood K, Chang AB. Nasopharyngeal carriage and macrolide resistance in indigenous children with bronchiectasis randomized to long-term azithromycin or placebo. Eur J Clin Microbiol Infect Dis. 2015;34:2275–2285. doi: 10.1007/s10096-015-2480-0. [DOI] [PubMed] [Google Scholar]
- 27.Goyal V, Grimwood K, Marchant J, Masters IB, Chang AB. Does failed chronic wet cough response to antibiotics predict bronchiectasis? Arch Dis Child. 2014;99:522–525. doi: 10.1136/archdischild-2013-304793. [DOI] [PubMed] [Google Scholar]
- 28.Mcmullan BJ, Bowen A, Blyth CC. Epidemiology and mortality of staphylococcus aureus bacteremia in Australian and New Zealand children. JAMA Pediatr. 2016;170:979–986. doi: 10.1001/jamapediatrics.2016.1477. [DOI] [PubMed] [Google Scholar]
- 29.Marchant J, Masters IB, Champion A, Petsky H, Chang AB. Randomised controlled trial of amoxycillin clavulanate in children with chronic wet cough. Thorax. 2012;67:689–693. doi: 10.1136/thoraxjnl-2011-201506. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca W, Hoppu K, Rey LC, Amaral J, Qazi S. Comparing pharmacokinetics of amoxicillin given twice or three times per day to children older than 3 months with pneumonia. Antimicrob Agents Chemother. 2003;47:997–1001. doi: 10.1128/AAC.47.3.997-1001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.