Summary
Southeast Asia is a hotspot for emerging infectious diseases, including those with pandemic potential. Emerging infectious diseases have exacted heavy public health and economic tolls. Severe acute respiratory syndrome rapidly decimated the region's tourist industry. Influenza A H5N1 has had a profound effect on the poultry industry. The reasons why southeast Asia is at risk from emerging infectious diseases are complex. The region is home to dynamic systems in which biological, social, ecological, and technological processes interconnect in ways that enable microbes to exploit new ecological niches. These processes include population growth and movement, urbanisation, changes in food production, agriculture and land use, water and sanitation, and the effect of health systems through generation of drug resistance. Southeast Asia is home to about 600 million people residing in countries as diverse as Singapore, a city state with a gross domestic product (GDP) of US$37 500 per head, and Laos, until recently an overwhelmingly rural economy, with a GDP of US$890 per head. The regional challenges in control of emerging infectious diseases are formidable and range from influencing the factors that drive disease emergence, to making surveillance systems fit for purpose, and ensuring that regional governance mechanisms work effectively to improve control interventions.
This is the third in a Series of six papers on health in southeast Asia
Introduction
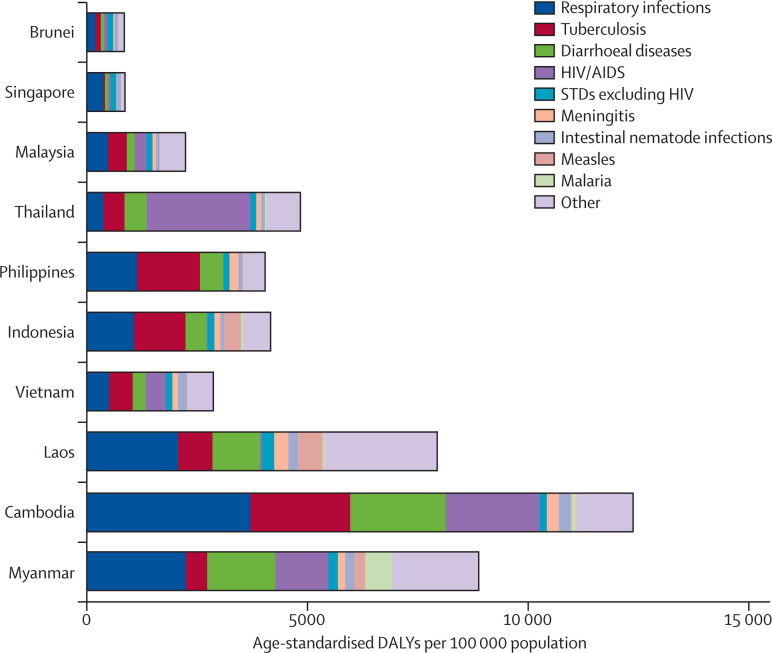
Emerging infectious diseases result from complex, dynamic systems in which biological, social, ecological, and technological processes interconnect. Southeast Asia is a loosely defined geopolitical region that is characterised and shaped by differing environmental, ecological, and economic factors. These factors are discussed in more detail in other reports in this Series. As a consequence, the region shoulders a great diversity of communicable disease and, closely associated with development, a heavy burden in countries with the lowest incomes (see figure ).1 The region has been at the centre of global attention regarding emerging infectious diseases, with the threat of diseases with pandemic potential receiving particular attention. Although the focus of this paper is emerging infectious diseases, as can be seen in the figure, the burden of infectious diseases is substantial. In low-income countries in particular, respiratory infections and diarrhoeal diseases are especially important. For the purposes of this paper, we define southeast Asia as the ten member countries of the Association of Southeast Asian Nations (ASEAN), a region with growing geopolitical influence in view of Asia's global economic ascendancy. The ASEAN countries are Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand, and Vietnam.
Figure.
The burden of communicable disease in southeast Asian countries, 2004
Data are from WHO Global Burden of Disease, 2004 update.1 DALYs=disability-adjusted life-years. STDs=sexually transmitted diseases.
Definitions of emerging infectious diseases vary—for example, to reflect whether concepts such as drug resistance are included.2, 3 For the purposes of this report we use the WHO definition of diseases that are “newly recognised, newly evolved or occurred previously but have shown an increase in incidence or expansion of geographical, vector or host range”4 and include pathogens showing drug resistance within this definition. The table summarises several infections that have attracted attention in recent years.
Table.
Summary of selected emerging infectious diseases in southeast Asia
| Primary transmission | Comments | |
|---|---|---|
| Emerging diseases | ||
| Avian influenza A H5N1 | Zoonotic (close contact with poultry) | 325 reported cases, 224 deaths in Indonesia, Vietnam, Thailand, Cambodia, Laos, and Myanmar5 |
| Pandemic influenza A H1N1 (2009) | Respiratory | 5290 reported cases, eight deaths in all ten countries6 |
| SARS | Respiratory | 331 reported probable cases, 44 deaths in Singapore, Vietnam, Thailand, Malaysia, and Indonesia7 |
| Nipah virus | Zoonotic (close contact with pigs) | First known human cases in Malaysia; 276 cases, 106 deaths in Malaysia and Singapore8 |
| Re-emerging diseases | ||
| Chikungunya fever | Vector-borne | Endemic in many southeast Asian countries; re-emerged in Singapore (2008), Malaysia (2007),9 Thailand (2009), and Indonesia (2010) |
| Dengue fever | Vector-borne | Originated in southeast Asia; 398 340 cases and 1596 deaths in 2008 with high burden in Indonesia, Vietnam, Thailand, Malaysia, the Philippines, Myanmar, and Cambodia; estimated 253 000 DALYs lost in 20041 |
| Japanese encephalitis | Vector-borne and zoonotic | Only 68 reported cases in Thailand in 2009;10 estimated 243 000 DALYs lost in 20041 |
| Rabies | Zoonotic (bite or scratch from rabid animal) | 587 cases and deaths in 2009 in Indonesia, the Philippines, Vietnam, Myanmar, and Thailand10 |
| HIV/AIDS | Sexual, injecting drug use, vertical | High adult HIV prevalence (more than 0·5%) in Thailand, Cambodia, and Myanmar, with more than 200 000 HIV-positive people in Thailand, Vietnam, Indonesia, and Myanmar;11 estimated 2 952 000 DALYs lost in 20041 |
| Streptococcus suis | Zoonotic (close contact with pigs) | Case reports from Thailand and Vietnam12 |
| Leptospirosis | Zoonotic (skin contact with urine of rodents | 5697 cases and 83 deaths in 2009 with high burden in Thailand and reported cases in Indonesia and Myanmar10 |
| Drug-resistant diseases | ||
| MDR tuberculosis | Respiratory | 2332 cases in 2008;13 high-burden countries are the Philippines, Myanmar, Indonesia, and Thailand |
| XDR tuberculosis | Respiratory | Detected in Myanmar, the Philippines, Singapore, Thailand, and Vietnam13 |
| MDR Plasmodium falciparum malaria | Vector-borne | Documented on Cambodia's border with Thailand14 |
SARS=severe acute respiratory syndrome. DALYs=disability-adjusted life-years. MDR=multidrug resistant. XDR=extensively drug resistant.
Key messages.
-
•
Southeast Asia is a diverse region that is undergoing rapid social, environmental, and demographic change.
-
•
The emergence of new ecological niches means that the region is likely to remain a hotspot for emerging infectious diseases.
-
•
Governance of infectious disease control is challenging, with overlapping institutional roles and responsibilities. The region also is politically complex, with some intranational and international tensions that have the potential to further hinder control.
-
•
There has been substantial investment in surveillance capacity in recent years, but it remains weak in many areas.
-
•
Research in the region that practically informs policy and practice is scarce. Research areas demanding attention include the development of predictive surveillance (including the potential risks associated with social and environmental changes) and priority setting within health systems to allow response to surges in demand and to improve equity, effectiveness, and efficiency.
We review the past decade's experience of emerging infectious diseases in southeast Asia and reflect on the epidemiological driving forces behind these diseases, the regional diversity regarding human and animal public health capacity, progress and shortfalls in regional disease surveillance, and the challenges to governance faced at national and international levels. We draw attention to what we believe are crucially important challenges, briefly provide case studies to illustrate some of these challenges, and offer insights into what steps might be taken to improve control of emerging infectious diseases.
The burden and diversity of emerging infectious diseases
During the past decade, novel viruses, particularly those causing severe acute respiratory syndrome (SARS) and avian influenza A H5N1, have attracted international concern, attention, and investment in southeast Asia. These two diseases, although undoubtedly exerting major public health and economic burdens, represent only part of a rich tapestry of many pathogens that have emerged to pose a public health threat within the region in recent years (table and panel 1 ).5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Recent outbreaks of Nipah virus and artemisinin-resistant Plasmodium falciparum, for example, both of which have emerged within the region itself, have also focused national, regional, and international attention on the threat posed by emerging infectious diseases, and in particular on southeast Asia as the epicentre of these diseases.
Panel 1. A brief overview of selected newly emerging infectious diseases in southeast Asia.
Nipah virus
In recent decades, the only major infectious disease in man to have probably emerged in southeast Asia was severe febrile encephalitis during infection with Nipah virus. Occurring in peninsular Malaysia and Singapore in late 1998 to early 1999, the outbreak resulted in the deaths of more than 100 people in these two countries (at a case fatality rate of around 40%),8 which is twice as many as were killed by severe acute respiratory syndrome (SARS) across all of southeast Asia and just under half as many as have died of avian influenza A H5N1. Most of those infected worked in the pig farming and meat production industries, reflecting the major form of transmission, which was close contact between pigs and human beings. Despite eventual containment of the outbreak through a mass cull of more than a million pigs, a related virus has since emerged outside southeast Asia, causing outbreaks in Bangladesh and India.
SARS
In 2003, southeast Asia's unique combination of strong links with other Asian countries alongside a multitude of intercontinental connections (three of the world's 30 busiest airports are now found in southeast Asia—Bangkok, Jakarta, and Singapore) facilitated the regional and global spread of the SARS coronavirus from its origins in southern China, a close neighbour. Guests infected in a hotel in Hong Kong (where an infected doctor from China was staying) unknowingly carried the virus to several countries including Vietnam and Singapore.15 Outbreaks occurred in both of these countries and cases were reported throughout the region, although Singapore was the most severely affected with 33 deaths compared with 11 across the rest of southeast Asia.7 Singapore was also implicated in international transmission to outside the region. Although the global outbreak ended in July, 2003, a further laboratory-acquired infection was reported in Singapore in September, 2003.
H5N1 influenza
In the same year as SARS spread through southeast Asia, the region began to experience outbreaks of another emerging infectious disease, H5N1 influenza, which had again spread from southern China.16 Although the very high mortality in domestic poultry (approaching 100%) was alarming, the number of human infections that were occurring and the deaths of many of those infected (human case fatality in southeast Asia was just under 70%)5 caused greater concern. The previous rapid worldwide dissemination of the SARS coronavirus fuelled fears that the H5N1 virus, were it to become readily transmissible between humans and retain some of its pathogenic potential, could spread rapidly and result in an influenza pandemic that might kill millions, result in untold economic disruption, and threaten global security.17 Despite these grave concerns, the H5N1 virus has yet to cause an influenza pandemic owing to an inability to achieve sustained human-to-human spread (although there is evidence to support some human-to-human transmission events).18, 19 However, the threat still remains. The virus continues to circulate in wild birds worldwide, causing outbreaks in poultry in several southeast Asian countries, and, in 2010, cases in human beings have been reported in southeast Asia in Cambodia, Indonesia, and Vietnam.
Artemisinin-resistant falciparum malaria
Reports of reduced Plasmodium falciparum clearance rates during treatment with artemisinin (in combination therapy as well as monotherapy) have surfaced in southeast Asia, namely on the Thailand–Cambodia border, since 2004.14 These reports have attracted much regional and international concern, especially in view of southeast Asia's historical role in the emergence and spread of parasite resistance to chloroquine and sulfadoxine–pyrimethamine, and the reliance of the global Roll Back Malaria campaign on artemisinin combination therapy. Although a containment programme, currently funded by the Bill & Melinda Gates Foundation, is in place, there is an acceptance that “the actual geographic extent of resistance is unknown”.20
The role of southeast Asia as a hotspot for emerging diseases is further illustrated by the less recent but certainly no less important emergence of new cholera and dengue variants that continue to greatly affect both regional and global health. The variant of Vibrio cholerae 01 El Tor causing the present (seventh) pandemic first emerged in Indonesia in 1961. Moreover, the first major outbreaks of the haemorrhagic form of dengue were reported in Manila, Philippines, and Bangkok, Thailand, in the 1950s, and the southeast Asian strains have contributed greatly to global spread of dengue—causing outbreaks of haemorrhagic disease throughout the Americas, for example.21 Japanese encephalitis, another arbovirus that is highly endemic to southeast Asia, is thought to have evolved in the region and has subsequently spread across Asia and to parts of Australia.22 Other threats of notable concern to the region, but receiving little attention, include increasing rates of antibiotic resistance among enteric pathogens such as Campylobacter 23 and increasing incidence of food-borne trematodiases in parts of southeast Asia.
Despite southeast Asia's importance with respect to emerging infectious diseases, frailties and differences in surveillance systems within the region make estimation of the burden and diversity of disease and any cross-country comparisons difficult. As we note in this report, the likelihood of widespread under-reporting of emerging infectious diseases means that knowledge is scarce and prevention and response hampered. But perhaps a greater challenge is determination of the risk of emerging infectious diseases that arises from influences within the region, and development of strategies that address both public health prevention, containment, and mitigation imperatives and socioeconomic realities.
The consequences of emerging infectious diseases in southeast Asia stretch far beyond a narrow purview of public health. The estimated cost of SARS to east and southeast Asia was US$18 billion, which is roughly US$2 million per person infected.24 In southeast Asia, the sudden collapse in demand for the service industry was a dominant feature in this cost, particularly in view of a tourist industry reliant on the 35 million tourists arriving every year from outside the region.25 Indeed, the relation between public health and socioeconomic effects is by no means linear. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in the UK illustrated this effect. Fear, anxiety, and changes in behaviour and the effect on the tourist industry have unpredictable consequences. Before 2009, the World Bank estimated a possible global cost of an influenza pandemic at around US$1·25 to 2 trillion.26 The Asian Development Bank estimated a shock to demand might cost southeast Asia up to US$283 billion.27 Moreover, the costs of emerging infectious diseases, including pandemics, do not fall evenly. Although emerging infectious diseases have until now disproportionately affected low-income countries,1 and the poorest within society were affected the most,28 pandemics have the potential to disrupt highly interconnected and high-income areas such as Singapore.27, 29
The economic consequences of H5N1 influenza have been different from those of SARS in southeast Asia. The region's tourist industry collapsed overnight as a result of SARS. Although affected, the industry has been affected less by H5N1. The poultry industry, by contrast, was profoundly affected. Control policies during the 2003–04 H5N1 outbreak in Vietnam led to the culling of 45 million birds at an estimated cost of almost US$118 million.30 Furthermore, insufficient reimbursement after a ban on the sale of so-called backyard poultry in Vietnam lowered the household income for poor families disproportionately by comparison with wealthier families.26 In Thailand, where the highly industrialised poultry export market is an important contributor to the national economy, exports were banned.31 In 2003, poultry meat exports were worth US$597·6 million. A year later, they had had fallen by 93% to US$43·5 million.32 Avian influenza continues to exact an economic strain in the region, with new cases in poultry or wild birds reported this year from Cambodia, Vietnam, Laos, and Myanmar (Indonesia is endemic for H5N1 in birds, but does not report current outbreaks). Surprisingly, the potential economic effect of other emerging infectious diseases in southeast Asia, and analyses of the operational capacity of health systems to respond, have received little robust research attention.
Important factors leading to the emergence of infectious diseases
Driving forces in southeast Asia
Southeast Asia is a hotspot for emerging infectious diseases—in particular, zoonotic and vector-borne diseases—as a result of many factors including population growth, mobility, and urbanisation, and environmental changes such as agriculture and livestock intensification, deforestation, and climate change. Many, if not all, of these interlinked driving forces, although occurring in other parts of the world, have particularly important effects on emerging infectious diseases in southeast Asia (webappendix p 1).2, 3, 33 Indeed, the factors that coalesce in east and southeast Asia to increase the risk of emerging infectious diseases can be considered at three levels: (1) as a region containing diverse zoonotic and vector-borne pathogens, and thus a primary source of emerging infectious disease; (2) as a region in which the high density, proximity, and mobility of human beings and animal reservoirs provide fertile conditions for transmission between species, within human populations, and across geographic areas; and (3) as a region with ecological factors that allow rapid pathogen mutation and host adaptation—for example, Dengue, reassortments of influenza virus, and emergence of drug resistance.34, 35, 36
Population growth and urbanisation
Human population growth and increasing density are important independent predictors of emerging infectious diseases.2 The population in southeast Asia, which is currently estimated at around 580 million, has increased by more than 30% since 1990.37 Increasing population density not only affects the spread of infectious diseases directly (eg, through increased human-to-human contact), but also underpins many other ecological driving forces such as changing land use, agriculture, and livestock intensification.38
In addition to rapid population growth, southeast Asia is rapidly becoming more urbanised, with low-income countries seeing the most striking changes. Around 48% of people in the region live in urban areas, a figure which is expected to grow to more than 70% by 2050 (webappendix p 1).37 Urbanisation is associated with changes in social structures, increased personal mobility, and extended and changing social networks.39 It is also a driving force behind some vector-borne diseases—for example, dengue, which has seen a resurgence in southeast Asia during the past 50 years. This resurgence has been linked to the establishment of (often impoverished) periurban areas in which the collection and storage of water, because of a lack of reliable water and sanitation systems and the accumulation of social detritus such as used tyres, provide breeding sites for Aedes aegypti mosquitoes.21
Birth rates, which are highest in the poorest countries in the region, also have consequences for infectious disease transmission because of the effect of immunologically naive individuals who perpetuate epidemics. Fortunately, the demographic shift towards decreased birth and mortality rates across southeast Asia might help to lower the transmission of some diseases, and there is evidence of such an effect on dengue in Thailand.40
Population movements and animal trade
Increasing regional population mobility, including both documented and undocumented travel as well as increases in international population movements across national boundaries, is an important feature of southeast Asia. The Mekong Basin subregion, which includes Thailand, Cambodia, Laos, Vietnam, Myanmar, and China, has seen a sharp increase in cross-border migration in recent years. Much of this migration is driven by poverty, with migrant workers moving from the low-income countries of Laos, Cambodia, and Myanmar to Thailand, a middle-income country. Thailand is estimated to have 1·5–2 million immigrants from neighbouring countries, and about 150 000 refugees. Large-scale migration of economic and political refugees, including the frequent movements of hill tribe populations along with their livestock, present substantial challenges to cross-border disease control in the Mekong Basin subregion. Furthermore, undocumented migrants often live in unhygienic and overcrowded conditions (particularly in camps such as those along the Thailand–Myanmar border) with poor access to health services, with infectious diseases such as malaria being an important cause of morbidity and death.14, 41, 42, 43
In addition to human movements, increased cross-border trade of livestock and wildlife is also a concern. Trading centres, for example, can act as mixing bowls for “humans and dozens of other species before they are shipped to other markets, sold locally, or even freed and sent back into the wild”.44 Data for wildlife trade is scarce, although some have estimated that in east and southeast Asia, tens of millions of wild animals cross borders each year regionally and to more distant countries around the world for use as food, pets, or in traditional medicine.44 Figures suggest that the export of many different species of wild animals increased between 1998 and 2007, although the licit export of birds fell substantially after major importers such as the European Union imposed restrictions in response to H5N1 influenza. Along with trade, the natural movements of migratory birds and bats within, to, and from the region are also a key influence for several emerging infectious diseases such as H5N1 influenza, Japanese encephalitis, and Nipah virus.
Water and sanitation
In terms of population coverage, water and sanitation systems are improving in southeast Asia, with the region generally on track to meet the Millennium Development Goal targets. This progress is encouraging in view of the association between water and sanitation systems and the burden of diarrhoeal diseases across southeast Asia in low-income countries (webappendix p 2), along with links to vector-borne diseases, as we have mentioned. However, population growth and urbanisation mean that the number of people in southeast Asia using unimproved sanitation and drinking water systems in urban areas is actually increasing, having risen by 20 million between 1990 and 2006.
Agriculture and changing land use
Human-induced changes in land use are key driving forces of emerging infectious diseases and also modify the transmission of endemic infections.33 Agriculture occupies around 25% of the land in southeast Asia, with the total agricultural area having increased by more than 8% between 1990 and 2008 (webappendix p 1). Moreover, there has been a particularly large increase, of more than 30% across the region, in the land area used for rice cultivation.32 Development of rice paddies can promote transmission of vector-borne diseases such as Japanese encephalitis through their role as vector-breeding sites and by attracting water birds, which are the natural reservoir of Japanese encephalitis. Transmission between birds and mosquitoes is further amplified by transmission in pigs.21 Countries such as Cambodia, Indonesia, Laos, and Myanmar are at risk of increases in Japanese encephalitis because of the combination of intensified rice and pig farming and the absence of vaccination programmes and surveillance.45 In addition to an increased potential for transmission of Japanese encephalitis, the attraction of various birds to rice paddies has also been associated with increased risk of H5N1 outbreaks in Thailand and Vietnam.46
Deforestation is continuing across most countries in the region (webappendix p 1). Human encroachment and fragmentation of wildlife habitats through processes such as deforestation increase interactions between wildlife, human beings, and livestock, and thus the potential for pathogens to cross species barriers. In Malaysia, changes in movements and densities of fruit bats due to deforestation, wildfires, and plantation of fruit orchards, along with the intensification of pig farming close to fruit-bat habitats, have all been postulated as influences for the emergence of Nipah virus as a zoonosis in Malaysia.8, 33, 47
Livestock production
Intensive livestock production is increasingly prevalent across southeast Asia. The density of poultry has at least doubled in most countries between 1990 and 2008, and increased more than three-fold in countries such as Myanmar, Laos, and Brunei (webappendix p 1). Increased poultry density is associated with the cumulative number of H5N1 cases in human beings at country level across the region (webappendix p 3). Although backyard and village farms remain the predominant environment for poultry producers in most low-income countries in southeast Asia, industrial production systems dominate in others, such as Thailand.30, 48 Where poultry production is in a backyard setting or is on a small scale, investment in biosecurity is likely to be low and ill-coordinated. Many species often coexist and the potential for cross-species transmission can be increased. However, as intensive production of single species in large-scale industrial and commercial sectors is becoming more dominant, although this setting might reduce the risk of cross-species infection, these sites might also act as amplifiers of disease during the emergence of large-scale outbreaks. Moreover, cross-infection can still occur within the marketplace, where economic imperatives can over-ride public health concerns.39
In concert with poultry production, pig farming is also intensifying across the region, with densities having at least doubled since 1990 in Myanmar, Laos, Vietnam, Indonesia, and the Philippines (webappendix p 1). This trend is arguably a cause for concern in view of the role of pigs in the transmission of zoonoses such as Nipah virus, Japanese encephalitis, and influenza.
Climate
Vector-borne and waterborne diseases are both strongly affected by climate. For example, the strength of El Niño was a predictor for dengue outbreaks in Thailand49 and Vietnam.50 Since arthropod vectors tend to be most active at high temperatures, and because water scarcity during droughts often leads to poor sanitation, climate change can be expected to drive the spread of vector-borne diseases and diarrhoeal illnesses in southeast Asia.
Drug resistance
In addition to the aforementioned demographic and environmental factors, which can drive the emergence of novel diseases and increase the incidence, prevalence, or geographic scope of existing ones, the importance of public health system factors as influences, in particular for the emergence of newly resistant strains, should not be underestimated. Irrational drug use, frail public health systems,34 and the wide availability of counterfeit and substandard drugs are factors with particular relevance in southeast Asia.51
During the past five decades, southeast Asia has been the epicentre of the evolution and spread of resistance to all important classes of antimalarial drugs. In the 1950s and 1960s, the Thailand–Cambodia border was the site of emergence of chloroquine and sulfadoxine–pyrimethamine resistance in P falciparum. This resistance subsequently spread across Asia and then Africa. Within the past 10 years, reduced susceptibility to the artemisinins has been documented in Cambodia,52 and concerns that it could spread have raised much concern within the international community. Surveillance information about the scale of artemisinin-resistant malaria in the region remains poor, however. Likewise, surveillance data for drug-resistant tuberculosis in the region are scarce, especially for low-income countries (Laos, for example, reports no multidrug-resistant cases).
Surveillance systems
Surveillance systems are the foundation on which disease control systems sit. They can serve several functions including anticipation of the emergence of diseases, support of outbreak responses, and facilitation of the monitoring and evaluation of responses. Although the observation and analyses of factors can be used to predict the emergence of infectious diseases, currently such predictive surveillance lacks specificity and sensitivity. As with any surveillance system, the completeness of data is problematic. For example, no cases of human Japanese encephalitis or leptospirosis were reported from either Laos or Cambodia during 2009, and no cases of rabies in human beings were reported from Laos in 2009, even though all diseases were reported in other neighbouring countries and the likelihood of disease seems high.
With most human pathogens originating as zoonoses, surveillance systems have until now relied on surveillance of animals and human beings. However, as in other resource-poor regions, capacity for animal health surveillance in southeast Asia is underdeveloped.53 Major constraints include the absence of specific government policies and legal frameworks for surveillance and control of zoonoses, as well as inadequate resources, insufficient animal–human public health cooperation, coordination, and collaboration, frail laboratory facilities, and weak and disconnected reporting systems.54 The emergence of SARS and the H5N1 and H1N1 (2009) viruses focused minds and brought investment. In Laos, for example, until 2004 there was almost no national infrastructure for communicable disease control.55 Initially, the Law on Hygiene, Disease Prevention and Health Promotion (2001) was the only law that addressed communicable diseases, and it principally applied to prevention rather than control and response.56 In the wake of H5N1 influenza outbreaks, however, the government established several new institutions to strengthen national capacities, including a National Coordination Committee on Communicable Diseases, a National Emerging Infectious Disease Control Office, and a Centre for Laboratory and Epidemiology that has recently been designated the national focal point for the implementation of the International Health Regulations.56 Similarly, the governments of Vietnam, Thailand, Indonesia, Cambodia, Malaysia, and the Philippines have all set up new institutional bodies, strengthened diagnostic laboratory capacity, and improved coordination mechanisms. Although gaps in national planning and surveillance systems persist, countries in southeast Asia have made substantial progress towards effective prevention and control of infectious diseases.57
Crucially, surveillance systems for influenza have started to integrate elements of animal health, particularly poultry-related incidents.57 For example, rapid response teams have been mobilised and trained to improve community-based surveillance in 331 districts in Indonesia. In Laos and Cambodia, a substantial amount of donor funding has been used to support pandemic preparedness and responses, including development of surveillance systems. Laboratory capacity to handle influenza viruses has improved—for example, through the building of biosafety level 3 laboratories in Indonesia and Cambodia for virus sequencing.57
Regional public health institutions have become sensitive to the threat posed by zoonoses. ASEAN member states have recently endorsed a Regional Mechanism on Animal Health and Zoonoses, to develop a unified framework against threats from animal diseases.58 Moreover, the integration of animal and human health has been at the centre of WHO's Asia-Pacific Strategy on Emerging Diseases—an ambitious strategic framework that aims to develop mechanisms for information sharing between the animal and human health sectors both at regional and country levels, in partnership with the Food and Agriculture Organization and the World Organization for Animal Health.59 The overlapping functions of some regional institutions and their substantially different geographic coverages are described on webappendix p 4
These initiatives are emerging in a complex regional environment, however. At a national level, thriving private health-care sectors in many countries increasingly pose challenges to reporting systems, with some being either unwilling or unable to provide information.60 Similar challenges arise from decentralised health systems—for example, in Indonesia and the Philippines, where local health authorities have become less active in case reporting compared with other countries.61 Where vertical disease-specific surveillance programmes have been developed, such as in Cambodia, there is a risk that parallel surveillance and laboratory testing systems, especially those funded through investments related to pandemic influenza preparedness or other global health initiatives, draw on limited existing capacity and contribute to a duplication of efforts and inefficient use of resources.62 In addition to the regional initiatives that we have described, several collaborative surveillance programmes and support structures exist, with input from the Western Pacific Regional Office and the South East Asia Regional Office of WHO (which themselves split southeast Asia along political lines that are different from those of ASEAN). These programmes include the Mekong Basin Disease Surveillance network (an innovative cross-border initiative; panel 2 ), the Southeast Asian Medical Information Centre, ASEAN, and the Asia-Pacific Economic Cooperation forum.
Panel 2. The Mekong Basin Disease Surveillance initiative—cross-border surveillance and response.
The Mekong Basin Disease Surveillance (MBDS) initiative was established in 1999 with the core values of “mutual trust, transparency [and] cooperative spirit”.63 Encompassing Cambodia, Laos, Myanmar, Thailand, and Vietnam as well as China's Yunnan province and Guangxi Zhuang autonomous region, the MBDS network straddles both the WHO South East Asia Regional Office and Western Pacific Regional Office regions, aiming to facilitate cross-border cooperation in surveillance and control of infectious disease.
A network of cross-border surveillance collaborations underpins the project, each consisting of two community-based surveillance sites, one on each side of the border, which report cases from a defined list of infectious diseases (webappendix p 6).
These sites have been responsible for notable successes of the MBDS—for example, the discovery of a Laotian infected with influenza A H5N1 in Thailand and the subsequent joint Lao–Thai investigation. Another example was the joint Lao–Thai investigation of a cholera outbreak that spread from Thailand to Laos with identification of the source, enabling coordinated control measures to be implemented. The scope of the project extends beyond joint monitoring and investigations. For example, cross-border medical care from Thailand was dispatched to Myanmar after cyclone Nargis in 2008. The completion of a regional tabletop pandemic preparedness exercise in Siem Reap, Cambodia, in 2007, further serves as an example of international collaboration through the initiative.
The signing of a new Memorandum of Understanding in 200764 reflected these successes, and the latest MBDS Action Plan63 seeks to further cross-border cooperation with activities that include:
-
•
Establishment of two new cross-border sites per country per year.
-
•
Regular meetings between participants and leaders at cross-border sites to discuss progress and share experiences.
-
•
Annual documentation of outbreak investigations or exercises at each site.
-
•
Ensuring sufficient clinical capacity, health-care workers, and personal protective equipment as well as adequate capacity for patient isolation and quarantine.
Successes of the MBDS initiative have shown the potential for collaborative efforts between resource-poor nations to meet WHO's 2005 International Health Regulations. MBDS might be a potential model to establish similar networks in other regions worldwide and to strengthen existing informal collaborations in regions with national tensions, such as the Middle East Consortium on Infectious Disease Surveillance (Israel, Jordan, and the Palestinian Authority).65
In an effort to address some of the weaknesses in surveillance in southeast Asia, several collaborative programmes for infectious disease research have been undertaken in association with Western countries. Some of these collaborations are very well integrated into the existing health system structure—for example, the Institut Pasteur, whose facilities have become national institutes in several major provinces of Vietnam. The Institut Pasteur network also includes a facility in Phnom Penh in close collaboration with the Ministry of Health in Cambodia, and another is under construction in Laos in formal association with the Laotian Ministry of Health. In Thailand, the Ministry of Public Health is also actively collaborating with the US Centers for Disease Control and Prevention to work on emerging infectious and tropical diseases, and the Thai and US armies have a collaborative infectious disease research laboratory, the Armed Forces Research Institute of Medical Sciences, which developed from a cholera research laboratory in 1958.66 The Wellcome Trust centres in Thailand, Vietnam, and Laos have had a longstanding presence, and the London School of Hygiene and Tropical Medicine has a research collaborating centre in the region, in Thailand. Indonesia's recent experience of collaborating with external infectious disease laboratories has been more problematic. Before closing down in 2008, the US Naval Medical Research Unit 2 in Indonesia was charged with political accusations of offering questionable benefit to Indonesians during its 30 years of operation and of alleged improper use and export of viral specimens67—themes that were to resonate globally with the ongoing debate about the sharing of biological materials and benefits that might accrue from the development of vaccines (panel 3 ).
Panel 3. Indonesia, virus sharing, and equitable access to vaccines.
In February, 2007, amid growing international concern over the threat of pandemic influenza, Indonesia's health minister announced that her country would no longer share avian influenza A H5N1 virus samples with WHO. This controversial decision was triggered by a dispute over property rights between the Indonesian Government and an Australian company that had used viral strains from Indonesia to produce and market an H5N1 influenza vaccine. Indonesia argued that the incident exposed wider issues of exploitation and global inequalities—pharmaceutical companies obtain, free of charge, viral samples that are shared by developing countries with WHO, then patent the resulting products and sell them at prohibitively expensive prices, thus providing benefits disproportionately to high-income countries.68
The controversy forced WHO and its member states to reconsider the current approach to global influenza surveillance and the sharing of biological materials, and to create new mechanisms for benefit sharing. To this aim, in May, 2007, the World Health Assembly adopted a resolution that promoted the “transparent, fair and equitable sharing of the benefits arising from the generation of information, diagnostics, medicines, vaccines and other technologies”,68 while reasserting the need for timely sharing of both information and biological samples with the Global Influenza Surveillance Network. Additionally, it established an intergovernmental meeting to consider further actions aimed at ensuring fair and equitable distribution of pandemic influenza vaccines.69
In 2008, after many debates and negotiations, the Indonesian Government agreed to share H5N1 influenza sequences (but not the viral samples) through the new Global Initiative on Sharing Avian Influenza Data.70 The dispute and its underlying issues, however, have not been settled. In 2009, Indonesia did not share any samples with the Global Influenza Surveillance Network, including those from pandemic influenza A H1N1. Moreover, the initial efforts to create a more equitable framework for the purchase and distribution of vaccines have not produced any substantial results thus far. Disagreement has arisen because high-income countries are reluctant to accept the suggestion of legally binding obligations to share the benefits of vaccines that accrue from sharing of biological samples, whereas many low-income and middle-income countries, notably Indonesia, Thailand, and Brazil, want to see binding obligations.71
Capacity for health-service response
As in many developing areas of the world, veterinary services in several southeast Asian countries are weak, and biosecurity in animal farms is poor. Although Thailand remains a regional example of success in the control of H5N1 virus outbreaks in birds, and has invested heavily in biosecurity, the animal health systems of low-income countries in the region are weak.72, 73
In terms of health-care resources for treatment of emerging infectious diseases in human patients, low-income countries face major constraints. For example, three countries in the region (Laos, Cambodia, and Indonesia) spend less per head than has been estimated to be necessary for health system functions to meet the Millennium Development Goals.57 There are substantial shortages of human resources in some countries in the region.74 The density of health-care professionals in five of the ten countries (Cambodia, Indonesia, Laos, Myanmar, and Vietnam) is lower than the level defined by WHO as adequate. The availability of health-care facilities as proxied by number of hospital beds per head is also very low in Laos, Cambodia, and Indonesia.75 With existing weakness in health system capacity, many countries in southeast Asia are at risk of being unable to adequately respond to emerging threats from new and re-emerging diseases or to surges in demand that might accompany these diseases. A study that included five southeast Asian countries showed that wide disparities exist in resource capacity not only in aggregate between countries, but also within countries. The northeast of Thailand has, for example, gaps in some health service resources that are more similar to the distribution in Laos and Cambodia than that of central Thailand. Ongoing research within our group suggests that these disparities probably result in inequitable rates of preventable mortality from emerging infectious diseases.60, 76
Regional coordination and support
As we have noted, there have been important initiatives aimed at strengthening the control of emerging infectious diseases regionally. To differing degrees, ASEAN, the Ayeyawady-Chao Phraya-Mekong Economic Cooperation Strategy, and the Asia-Pacific Economic Cooperation forum have all endorsed transnational cooperation in joint action with the WHO South East Asia and Western Pacific Regional Offices. Beyond surveillance, for example, the ASEAN Secretariat has managed a Singapore-based regional stockpile of 500 000 courses of antiviral drugs for the benefit of ASEAN member states (a regional collaborative stockpile that European institutions were unable to achieve). This initiative complements another supply of an additional 500 000 treatment courses that have already been distributed to ASEAN member states on the basis of population size. Another example of regional solidarity is the support given on the international stage of the UN by states such as Thailand that are sympathetic to Indonesia's stance on virus sharing.
The implementation of programmes for emerging infectious diseases in southeast Asia owes much to the financial and technical assistance of donor countries, private philanthropists, or development agencies such as the Asian Development Bank, the World Bank, the Global Fund, and the Rockefeller Foundation. The ASEAN stockpiles of antiviral drugs, for instance, were funded by the Japanese Government with a US$30 million grant within the wider scheme of the ASEAN-Japan Integration Fund. Additionally, the ASEAN Secretariat has long received support from the Australian Government through AusAid, the US Government, and the European Union, and the Mekong Basin Disease Surveillance network has been funded by the Rockefeller Foundation, among others. At the national level, many countries have benefited from substantial financial support associated with H5N1 influenza, as well as HIV/AIDS, tuberculosis, and malaria.
The large flow of foreign funding has undoubtedly contributed to strengthening of public health capacity in southeast Asia. But concerns have been raised. Some observers argue that foreign investment reflects the interests of donor countries or mainstream trends in public health—interests that are not necessarily aligned with public health priorities of recipient countries.77, 78 For example, many programmes focus on high-profile diseases such as H5N1 influenza, HIV/AIDS, tuberculosis, and malaria, but other diseases that carry a heavy burden of morbidity and mortality in the region are neglected, including traditional childhood diseases, emerging vector-borne diseases, and respiratory infections. A further concern is that disease-focused programmes that receive substantial funding are often poorly integrated within the wider health systems of recipient countries. Many initiatives in southeast Asia signify potentially important reforms—for example, the Linked Response in Cambodia, which aims to improve integration of vertical programmes for HIV, tuberculosis, and maternal and child health, and the incorporation of programmes initiated in response to H5N1 influenza into the broader control programme for emerging infectious diseases in Laos.55
Overarching many of the challenges to governance that we have outlined is a diversity of domestic political institutions, and tensions between and within countries that have the potential to hamper regional efforts to prevent and control emerging infectious diseases. Southeast Asia has witnessed major political upheavals during the past decade, with military coups (in Thailand), democratic reform (Indonesia), and a shift, albeit at differing paces, from Marxism to free market economies (Vietnam, Cambodia, Laos). Tensions also exist between and within countries—for example, a lingering border dispute centred on Preah Vihear temple between Thailand and Cambodia, an ongoing ethnic separatist insurgency in the south of Thailand, recent violence associated with elections in the Philippines, and terrorist attacks on tourist areas in Indonesia. The ongoing military dictatorship in Myanmar is a continuing regional concern. Emerging infectious diseases too have the potential to fan the flames of ethnic tensions. Recently, pigs—raised predominantly by non-Muslims in Egypt and Malaysia, both countries with predominantly Muslim populations—were a focus of concern regarding the H1N1 and Nipah viruses, respectively. These political, ethnic, and religious tensions all have the potential to create instability that affects the emergence and response to emerging infectious diseases.
Conclusion
Southeast Asia, a region that is home to some 600 million people, is also the home to many driving forces of emerging infectious diseases. The region is an acknowledged hotspot for risk, with new, emergent, and resurgent infectious diseases exploiting ecological niches that result in large part from man's influence on his environment. The pace of environmental transitions that are being witnessed in parts of southeast Asia makes the emerging infectious disease a reality. Moreover, many of the factors that influence emerging infectious diseases, from climate change to increased global demand for cheap protein from industrialised poultry production are the result of powerful forces, many of which are difficult to change. Southeast Asia is likely to remain a hotspot for emerging infectious diseases, including those diseases with pandemic potential.
The challenges that face the region therefore include reforming or modifying of upstream driving forces of emerging infectious diseases, prediction with improved accuracy of where and what diseases are likely to emerge, improvement of the governance, financing, and operational capacity of surveillance systems such that animal and human systems are coherently and strategically aligned, and use of timely generation of data and information to identify feasible and appropriate responses (panel 4 ). Animal and public health systems need to be made fit for purpose, not only to provide for domestic needs, but also to prevent, contain, or mitigate the emergence and spread of infectious diseases. And the most crucial weapon in our public health armamentarium is surveillance—a system that needs to be improved.
Panel 4. Recommendations.
-
•
There is an increasing trend towards regional coordination, cooperation, and information sharing in southeast Asia. This trend should be complemented by a commitment to address imbalances in health system capacity. The European Union model for structural funds could provide a way forward.
-
•
Emphasis on avian influenza A H5N1 with concomitant funding has meant the relative neglect of lower profile diseases such as Japanese encephalitis and rabies. Although generic capacity building across emerging infectious diseases is to be welcomed (for example, through the International Ministerial Conference on Animal and Pandemic Influenza79) this process needs to be built on with sustained and strategically focused funding.
-
•
Investment in the region needs to be sustained to ensure robust, resilient, and flexible institutional capacity.
-
•
Research needs to be done to improve understanding of the factors that are associated with risk of emerging infectious diseases.
-
•
Surveillance capacity needs to be strengthened, especially in low-income countries, and needs to be timely, coordinated regionally, and inform national and regional control priorities.
-
•
Predictive analyses need to be strengthened, including through the development of more robust datasets on factors associated with emerging infectious diseases such as changes in land use.
-
•
International and domestic governance of surveillance of animal and human infectious diseases need to be strategically aligned across geographic, institutional, disease, and host boundaries, and avoid duplication of effort
-
•
Analyses of operational prevention, containment, and mitigation capacity are needed to inform investment linked to global, regional, and domestic public health and economic priorities
During the past decade, a multitude of national and regional initiatives have developed across animal and human health sectors in response to the threat of emerging infectious diseases. Very substantial sums have been invested in emerging infectious diseases in the region, in large part in response to HIV/AIDS, tuberculosis, malaria, and, more recently, SARS and H5N1 influenza. Yet the coordination, governance, and sustainability of regional control efforts in the face of global economic pressures remain a significant challenge.
Search strategy and selection criteria
We searched peer-reviewed English language literature through PubMed and grey literature published since 2000. We focused on factors leading to the emergence of infectious diseases in Association of Southeast Asian Nations countries, surveillance capacity, and governance of control systems. We searched institutional websites (for example, WHO, the Food and Agriculture Organization, the World Organisation for Animal Health, and donor agencies), and analysed primary data derived from these sources to provide an overview of crucially important issues related to emerging infectious diseases in southeast Asia during the past decade. Data were analysed to identify trends for upstream driving forces for emerging infectious diseases in the region. The ongoing portfolio of research of the London School of Hygiene and Tropical Medicine's Communicable Diseases Policy Research Group based in the region was also reviewed.
Acknowledgments
Acknowledgments
The paper is part of a Series funded by the China Medical Board, the Rockefeller Foundation, and Atlantic Philanthropies.
Contributors
All authors contributed equally to the conceptualisation, literature search, analysis, and drafting of the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.WHO . Global burden of disease: 2004 update. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Emerging zoonoses. http://www.who.int/zoonoses/emerging_zoonoses/en/ (accessed Nov 16, 2010).
- 5.WHO Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2010_08_31/en/index.html (accessed Sept 9, 2010).
- 6.WHO Pandemic (H1N1) 2009—update 58. http://www.who.int/csr/don/2009_07_06/en/index.html (accessed Sept 9, 2010).
- 7.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/index.html (accessed Sept 21, 2010).
- 8.Lo MK, Rota PA. The emergence of Nipah virus, a highly pathogenic paramyxovirus. J Clin Virol. 2008;43:396–400. doi: 10.1016/j.jcv.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Leo YS, Chow AL, Tan LK, Lye DC, Lin L, Ng LC. Chikungunya outbreak, Singapore, 2008. Emerg Infect Dis. 2009;15:836–837. doi: 10.3201/eid1505.081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Organisation for Animal Health World Animal Health Information Database Interface. http://www.oie.int/wahis/public.php?page=home (accessed Nov 16, 2010).
- 11.UNAIDS . Report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2008. [Google Scholar]
- 12.Wertheim HF, Nguyen HN, Taylor W. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One. 2009;4:e5973. doi: 10.1371/journal.pone.0005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 14.Dondorp AM, Yeung S, White L. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 15.Peiris JSM, Yuen KY, Osterhaus ADME, Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 16.Li KS, Guan Y, Wang J. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 17.Dry S, Leach M, editors. Epidemics: science, governance and social justice. Earthscan; London, UK: 2010. [Google Scholar]
- 18.Wang H, Feng Z, Shu Y. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371:1427–1434. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- 19.Ungchusak K, Auewarakul P, Dowell SF. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 20.Global Fund to Fight AIDS Tuberculosis and Malaria Cambodia Round 9 Proposal Form for Malaria. 2009. http://www.theglobalfund.org/grantdocuments/9CAMM_1809_0_full.pdf (accessed Nov 16, 2010).
- 21.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12 suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 22.Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003;77:3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodhidatta L, Vithayasai N, Eimpokalarp B, Pitarangsi C, Serichantalergs O, Isenbarger DW. Bacterial enteric pathogens in children with acute dysentery in Thailand: increasing importance of quinolone-resistant Campylobacter. Southeast Asian J Trop Med Public Health. 2002;33:752–757. [PubMed] [Google Scholar]
- 24.Asian Development Bank . Asian development outlook 2003 update. Asian Development Bank; Manila, Philippines: 2003. Assessing the impact and cost of SARS in Developing Asia.http://www.adb.org/documents/books/ado/2003/update/sars.pdf (accessed Nov 16, 2010). [Google Scholar]
- 25.ASEANWEB Tourist arrivals in ASEAN. http://www.aseansec.org/stat/Table28.pdf (accessed Sept 9, 2010).
- 26.Brahmbhatt M. Economic impacts of avian influenza propagation. First International Conference on Avian Influenza in Humans; Institut Pasteur, Paris, France; June 29, 2006.
- 27.Bloom E, de Wit V, Jose MJ Carangal-San. Potential economic impact of an avian flu pandemic on Asia. Asian Development Bank; Manila, Philippines: 2005. [Google Scholar]
- 28.Murray CJL, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368:2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 29.McKibben W, Sidorenko A. Global macroeconomic consequences of pandemic influenza. Lowy Institute for International Policy; Sydney, Australia: 2006. [Google Scholar]
- 30.Rushton J, Viscarra R, Guernebleich E, Mcleod A. Impact of avian influenza outbreaks in the poultry sectors of five South East Asian countries (Cambodia, Indonesia, Lao PDR, Thailand, Viet Nam) outbreak costs, responses and potential long term control. Proc Nutr Soc. 2005;61:491–541. [Google Scholar]
- 31.Taha FA. How highly pathogenic avian influenza (H5N1) has affected world poultry-meat trade. United States Department of Agriculture; Washington DC, USA: 2007. [Google Scholar]
- 32.UN Food and Agriculture Organization FAOSTAT. http://faostat.fao.org/default.aspx (accessed Sept 9, 2010).
- 33.Patz JA, Daszak P, Tabor GM. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coker R, Atun R, McKee M, editors. Health systems and the challenge of communicable diseases. Open University Press; Buckingham, UK: 2008. [Google Scholar]
- 35.Smith GJ, Vijaykrishna D, Bahl J. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 36.Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol. 2003;3:19–28. doi: 10.1016/s1567-1348(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 37.UN Department of Economic and Social Affairs . World population prospects: the 2008 revision. United Nations; New York, NY, USA: 2009. [Google Scholar]
- 38.Pimentel D, Cooperstein S, Randell H. Ecology of increasing diseases: population growth and environmental degradation. Hum Ecol. 2007;35:653–668. doi: 10.1007/s10745-007-9128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nat Med. 2004;10(12 suppl):S70–S76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings DA, Iamsirithaworn S, Lessler JT. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 2009;6:e1000139. doi: 10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivirojana N, Punpuing S. Health and mortality differentials among Myanmar, Laos and Cambodian migrants in Thailand. Public Health. 2008. http://paa2009.princeton.edu/download.aspx?submissionId=91913 (accessed Nov 16, 2010).
- 42.Delacollette C, D'Souza C, Christophel E. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health. 2009;40:674–691. [PubMed] [Google Scholar]
- 43.WHO SEARO. WHO WPRO . Malaria in the Greater Mekong subregion: regional and country profiles. World Health Organization; New Delhi, India: 2010. [Google Scholar]
- 44.Karesh WB, Cook RA, Bennett EL, Newcomb J. Wildlife trade and global disease emergence. Emerg Infect Dis. 2005;11:1000–1002. doi: 10.3201/eid1107.050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert M, Xiao X, Pfeiffer DU. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc Natl Acad Sci USA. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chua KB, Goh KJ, Wong KT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 48.Scoones I. Avian influenza: science, policy and politics. Earthscan; London, UK: 2010. [Google Scholar]
- 49.Tipayamongkholgul M, Fang CT, Klinchan S, Liu CM, King CC. Effects of the El Nino-southern oscillation on dengue epidemics in Thailand, 1996–2005. BMC Public Health. 2009;9:422. doi: 10.1186/1471-2458-9-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thai KT, Cazelles B, Nguyen NV. Dengue dynamics in Binh Thuan province, southern Vietnam: periodicity, synchronicity and climate variability. PLoS Negl Trop Dis. 2010;4:e747. doi: 10.1371/journal.pntd.0000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newton PN, Dondorp A, Green M, Mayxay M, White NJ. Counterfeit artesunate antimalarials in southeast Asia. Lancet. 2003;362:169. doi: 10.1016/S0140-6736(03)13872-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 53.Butler D. Disease surveillance needs a revolution. Nature. 2006;440:6–7. doi: 10.1038/440006a. [DOI] [PubMed] [Google Scholar]
- 54.Narain J, Bhatia R. The challenge of communicable diseases in the WHO south-east Asia region. Bull World Health Organ. 2010;88:162. doi: 10.2471/BLT.09.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Sa J, Mounier-Jack S, Darapheak C. Responding to pandemic influenza in Cambodia and Loa PDR: challenges in moving from strategy to operation. Southeast Asian J Trop Med Public Health. 2010;41:1104–1115. [PubMed] [Google Scholar]
- 56.International Federation of Red Cross and Red Crescent Societies International Disaster Response Laws Rules and Principles Programme . Legal Preparedness for Responding to Disasters and Communicable Disease Preparedness: study report. International Federation of Red Cross and Red Crescent Societies/Asian Development Bank; Kuala Lumpur, Malaysia: 2009. [Google Scholar]
- 57.Hanvoravongchai P, Adisasmito W, Chau PN, Conseil A, de Sa J, Krumkamp R. Pandemic influenza preparedness and health systems challenges in Asia: results from rapid analyses in 6 Asian countries. BMC Public Health. 2010;10:322. doi: 10.1186/1471-2458-10-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ASEAN Regional mechanism on animal health and zoonoses endorsed. ASEAN Secretariat, press release. May 12, 2010. http://www.aseansec.org/24668.htm#Article-2 (accessed Nov 16, 2010).
- 59.WHO . Asia Pacific strategy for emerging diseases technical papers. WHO Regional Office for South-East Asia; New Delhi, India: 2010. [Google Scholar]
- 60.Putthasri W, Lertiendumrong J, Chompook P, Tangcharoensathien V, Coker R. Capacity of Thailand to contain an emerging influenza pandemic. Emerg Infect Dis. 2009;15:423–432. doi: 10.3201/eid1503.080872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adisasmito W. Health system and pandemic influenza preparedness: results from a rapid situational analysis (RSA) in Jakarta and Bali. Outbreak, Surveillance and Investigation Reports. 2010;1:1–9. [Google Scholar]
- 62.Touch S, Grundy J, Hills S. The rationale for integrated childhood meningoencephalitis surveillance: a case study from Cambodia. Bull World Health Organ. 2009;87:320–324. doi: 10.2471/BLT.08.052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mekong Basin Disease Surveillance Action plan 2008–2013. http://www.ghsi.org/downloads/MBDS_Action_Plan.pdf (accessed Nov 16, 2010).
- 64.Mekong Basin Disease Surveillance Regional pandemic influenza table-top exercise. http://un-influenza.org/files/asia_pacific/simex/26_-_mbds.pdf (accessed Nov 16, 2010).
- 65.Kimball AM, Moore M, French HM. Regional infectious disease surveillance networks and their potential to facilitate the implementation of the international health regulations. Med Clin North Am. 2008;92:1459–1471. doi: 10.1016/j.mcna.2008.06.001. xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.AFRIMS . Armed Forces Research Institute of Medical Sciences (AFRIMS) Brochure. Armed Forces Research Institute of Medical Sciences; Bangkok, Thailand: 2008. [Google Scholar]
- 67.Azly E. Call for closure of NAMRU-2 in Indonesia increasing. Antara News (Jakarta) June 27, 2008. http://www.accessmylibrary.com/article-1G1-180680585/news-focus-call-closure.html (accessed Nov 16, 2010).
- 68.Fidler DP. Influenza virus samples, international law, and global health diplomacy. Emerg Infect Dis. 2008;14:88–94. doi: 10.3201/eid1401.070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO . Pandemic influenza preparedness: sharing of influenza viruses and access to vaccines and other benefits. Sixtieth World Health Assembly Resolution. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- 70.Fedson DS. Meeting the challenge of influenza pandemic preparedness in developing countries. Emerg Infect Dis. 2009;15:365–371. doi: 10.3201/eid1503.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fidler DP. Negotiating equitable access to influenza vaccines: global health diplomacy and the controversies surrounding avian influenza H5N1 and pandemic influenza H1N1. PLoS Med. 2010;7:e1000247. doi: 10.1371/journal.pmed.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scoones I, Forster P. The international response to highly pathogenic avian influenza: science, policy and politics. STEPS Centre; Brighton, UK: 2008. [Google Scholar]
- 73.FAO . Livestock policies and poverty reduction in Africa, Asia and Latin America. Pro-poor livestock polict initiative policy brief. United Nations Food and Agriculture Organization; Rome, Italy: 2005. [Google Scholar]
- 74.Kanchanachitra C, Lindelow M, Johnston T. Human resources for health in southeast Asia: shortages, distributional challenges, and international trade in health services. Lancet. 2011 doi: 10.1016/S0140-6736(10)62035-1. published online Jan 25. [DOI] [PubMed] [Google Scholar]
- 75.Chongsuvivatwong V, Phua KH, Yap MT. Health and health-care systems in southeast Asia: diversity and transitions. Lancet. 2011 doi: 10.1016/S0140-6736(10)61507-3. published online Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krumkamp R, Kretzschmar M, Rudge JW. Health service resource needs for pandemic influenza in developing countries: a linked transmission dynamics, interventions and resource demand model. Epidemiol Infect. 2010 doi: 10.1017/S0950268810002220. published online Oct 5. [DOI] [PubMed] [Google Scholar]
- 77.Calain P. From the field side of the binoculars: a different view on global public health surveillance. Health Policy Plan. 2007;22:13–20. doi: 10.1093/heapol/czl035. [DOI] [PubMed] [Google Scholar]
- 78.Shiffman J. Donor funding priorities for communicable disease control in the developing world. Health Policy Plan. 2006;21:411–420. doi: 10.1093/heapol/czl028. [DOI] [PubMed] [Google Scholar]
- 79.UN, World Bank. International financial and technical assistance report. International Ministerial Conference on Animal and Pandemic Influenza; Hanoi, Vietnam; April 20–21, 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.