Summary
The objective of this review was to identify and critique over forty years of peer‐reviewed literature concerned with the transmission of canine zoonoses to Aboriginal people and determine the zoonotic organisms documented in dogs in Australian Aboriginal communities. A systematic literature search of public health, medical and veterinary databases identified 19 articles suitable for critical appraisal. Thirteen articles documented the occurrence of recognized zoonotic organisms in dogs in Aboriginal communities, including Toxocara canis, Dirofilaria immitis, Streptococcus dysgalactiae, Rickettsia felis, Sarcoptes scabiei and Giardia. Currently, there is definitive evidence indicating that dogs act as a reservoir for human scabies in Aboriginal communities. However, there is a need for large‐scale, high‐quality, comparative studies of dogs and humans from the same household to assess the occurrence and importance of transmission of S. scabiei and other diseases between dogs and humans. These studies should use current genetic and molecular techniques along with traditional techniques to identify and type organisms in order to better understand their epidemiology. This review has revealed that there is a lack of high‐quality comparative studies to determine whether dogs are contributing to human disease by transmitting zoonoses. Our recommendations differ significantly from current public health policy and may have substantial implications for human and dog health.
Keywords: Aboriginal, dogs, parasites, scabies, zoonoses
Impacts.
This critical review of over 40 years of published research reveals a lack of high‐quality comparative studies to determine whether dogs are contributing to human disease in Aboriginal communities.
The aim of this study was to provide the public health audience with a summary of zoonotic organisms that have been found in dogs and humans within Aboriginal Australian communities.
A better understanding of the epidemiology of zoonotic diseases is essential to direct health care funding where it is most needed
1. Introduction
For decades, the shared environment of Australian Aboriginal people and unhealthy dogs has raised public health concerns because of the assumed risk of zoonoses. In many Aboriginal communities, dogs are often diseased and malnourished, reflecting the condition of stray or unwanted dogs from mainstream communities (Raw, 2001).
Historically, Aboriginal Australians adopted the dingo (Canis dingo Meyer, 1793) into their community (Meggitt, 1965). Following the colonization of Australia, Aboriginal people embraced the European dog (Canis lupus familiaris) which has resulted in numerous problems that were not previously seen. Dingoes breed once per year (Catling, Corbett, & Newsome, 1992; Corbett, 1995) compared with domestic dogs which will breed twice per year and have a greater number of pups in their litters (Catling et al., 1992). This has resulted in many communities being burdened with canine overpopulation and a poor state of dog health which not only affects animal welfare but human social welfare (Constable, Brown, Dixon, & Dixon, 2008). This is a cause for concern and has resulted in the implementation of numerous dog health programmes since the mid‐1980s (English, 2000).
Previous researchers have documented various zoonotic organisms being carried by community dogs resulting in the suggestion that dogs may play a role in the human disease burden (Currie, 1995; Shield, 1992; Wilks, 2000). The effect of resultant dog health programmes on Aboriginal health has been the focus of considerable debate. The main concern is that Aboriginal health funding is redirected to dog health under the assumption that improving dog health will improve community health (Currie, 1995). The debate was largely extinguished in the Northern Territory following the research conducted by Walton et al. (1999, 2004), that used microsatellite typing to show that scabies mites from dogs and humans group separately in a phylogenetic dichotomous tree which they suggested demonstrated separate transmission cycles. The ramifications of this have been that many communities are now under the impression that dogs pose no significant public health risks. Several reviews have been published trying to assess this risk, but none have explained their methods, nor critiqued the research using a specified system (Currie, 1995; Gaskin, Bentham, Cromar, & Fallowfield, 2007; Raw, 2001). Zoonotic and public health literature reviews have been scrutinized for their lack of methodological soundness in review techniques, because they are more likely to contain bias or errors (Waddell et al., 2009).
Therefore, despite over 40 years of research, we present here the first critical review of canine zoonoses in Australian Aboriginal communities, using a systematic methodology.
The aim of this study was to provide the public health audience with a summary of zoonotic organisms that have been found in dogs and humans within Aboriginal Australian communities, their zoonotic potential and their importance to the human disease burden based on evidence and methodological soundness. Through this review, we better assess the public health risks that dogs pose in Aboriginal Australian communities.
We identify directions for future high‐quality, evidence‐based research to address current gaps in knowledge. Our recommendations differ significantly from current public health policy and have substantial implications for human and dog health.
2. Methods
2.1. Literature search strategy
A database search of several public health, medical and veterinary databases including Medline, Web of Science, Embase, Scopus, Biosis reviews, APAIS Health, CINAHL, Zoological record, CABI Abstracts, EBM Reviews was undertaken using combinations of the words, “zoonoses” OR “zoonotic” OR “disease” OR “parasites” AND “dogs” AND “Australia” AND “Aboriginal”. Frequent authors were also searched, and searches through the reference lists of eligible papers were also studied and included if eligible.
2.2. Selection of articles for review
Database searches returned 43 articles, and a further nine articles were retrieved by studying the reference lists of all papers and searching for common authors. Of these, 19 articles were eligible for inclusion. These articles have been summarized in Appendix S1. Only articles that were consistent with the aim and were published in peer‐reviewed journals were eligible for inclusion (Figure 1).
Figure 1.
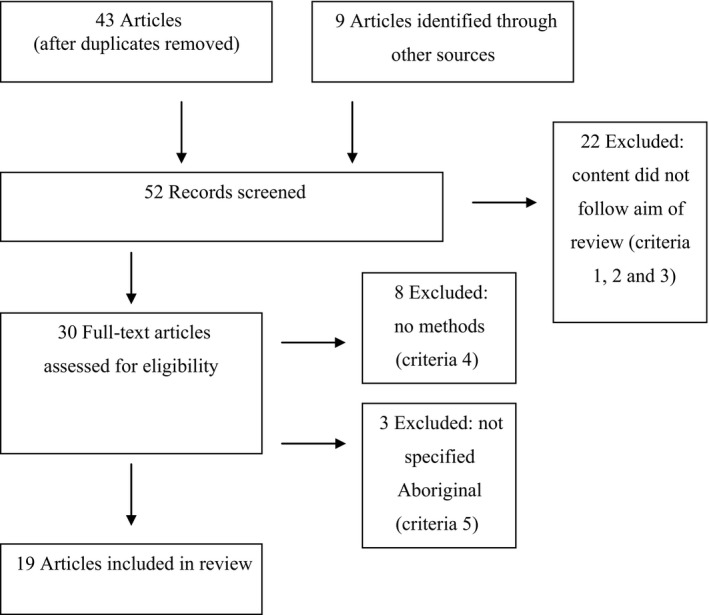
Flow diagram of papers through the exclusion and critiquing process
2.3. Exclusion criteria
A checklist was developed and papers excluded if:
They did not contain any zoonotic information pertaining to dogs (or did not distinguish between companion animals).
They did not contain any information about an organism recognized as a zoonosis. (However, research was included if the organism's ability to cause disease in humans was still unknown).
The main aim was to test the efficiency of a microbiological technique.
They did not describe their methods of research.
They did not specify whether the research pertained to Aboriginal people or dogs from their communities (or a location commonly known as an Aboriginal community).
2.4. Critiquing tools
Appendix S2 illustrates how included articles were critiqued using the Crowe Critical Appraisal Tool (CCAT) (Crowe & Sheppard, 2011). Briefly, the CCAT is an appraisal tool that allows reviewers to evaluate a paper by dividing it into categories and scoring each category out of five based on descriptors. There are a total of eight categories;
Preamble (Title, Abstract and text overall).
Introduction (Background and Objective).
Design (Research design, measure, bias).
Sampling (method, size, protocol).
Data collection (method, protocol).
Ethical matters (participant ethics and researcher ethics).
Results (analysis, integration, interpretation, outcome).
Discussion (interpretation, generalization, conclusion).
Two reviewers used the Crowe critical appraisal tool to systematically summarize the strengths and weaknesses of each study by following the above criteria. Reference was made to Dohoo, Martin, and Stryhn (2003) and Lewis‐Beck, Bryman, and Liao (2004) for information regarding sampling and research design.
2.5. Ethical matters
Ethics approval was not required for this review as there was no human or animal intervention. The authors state there are no conflict of interests or funding sources to be declared.
3. Results
The majority of research in this area is not recent (i.e. many papers with large sample sizes were published before 1994). Most studies were opportunistic, had small sample sizes and did not compare pathogens in humans with those in dogs. The few comparative studies almost never compared dogs and people from the same household. Furthermore, the pathogenicity of many of the organisms found has not been determined.
Of the 19 papers included in the review, six were short contributions (Hii et al., 2011; Jenkins & Andrew, 1993; Lee & Hampson, 1996; Meloni, Lymbery, Thompson, & Gracey, 1988; Schnagl & Holmes, 1978; Thompson, Meloni, Hopkins, Deplazes, & Reynoldson, 1993). We did not find any eligible papers prior to 1974 with the bulk of studies undertaken between 1990 and 2000. Papers were published in medical, veterinary, public health and parasitology journals. The majority were in medical journals; however, after 2000, this shifted to parasitology. Four papers determined the prevalence of a wide range of parasites (Jenkins & Andrew, 1993; Meloni, Thompson, Hopkins, Reynoldson, & Gracey, 1993; Shield et al., 2015; Thompson, Meloni, et al., 1993). The remaining 15 papers targeted 12 zoonotic organisms (Table 1).
Table 1.
Number of published papers according to zoonotic organism and symptoms recorded in people
| Organism | Number of papers/Reference | Symptoms in people |
|---|---|---|
| Dirofilaria immitis | 1/Welch and Dobson (1974a) | Pulmonary lesions, retinal granuloma. |
| D. immitis and Toxocara canis | 1/Welch et al. (1979) | Visceral larval migrans, Ocular larval migrans |
| Microsporum canis | 1/Kaminski and Green (1977) | Rare cause of Tinea capitis |
| Coronavirus‐like particles |
2/Schnagl et al. (1978) Schnagl and Holmes (1978) |
Unknown, possible gastroenteritis |
| Giardia spp. |
2/Meloni et al. (1988) Hopkins et al. (1997) |
Acute diarrhoea, weight loss and abdominal pain. |
| Spirochaetes |
2/Lee and Hampson (1994) Lee and Hampson (1996) |
Unknown, possible cause of diarrhoea and dehydration |
| Parasites (Wide range) |
4/Meloni et al. (1993) Jenkins and Andrew (1993) Thompson et al. (1993) Shield et al. (2015) |
Range of acute gastroenteritis |
| Sarcoptes scabiei |
2/Walton et al. (1999) Walton et al. (2004) |
Skin lesions, secondary bacterial infection |
| Ancylostoma spp. | 1/Palmer et al. (2007) | Eosinophilic enteritis, cutaneous larval migrans |
| Blastocystis | 1/Parkar et al. (2007) | Unknown |
| Rickettsia felis | 1/Hii et al. (2011) | Fever, rash, headache, abdominal pain, nausea, vomiting, and diarrhoea, as well as central nervous system involvement (photo‐phobia, hearing loss, and/or meningism) |
| Streptococcus dysgalactiae (SDSE) | 1/Schrieber et al. (2014) | Unknown |
Two reviewers using CCAT appraisal methods were often in agreement on scores and differed by only 1 or 2 points occasionally (See Appendix S2 for averaged results). Most papers received a low score for design, sampling, data collection and ethical matters.
3.1. Design and sampling
Only five of 19 articles described the research design of the study albeit briefly (Hopkins et al., 1997; Meloni et al., 1993; Schrieber, Towers, Muscatello, & Speare, 2014; Walton et al., 1999, 2004). Five were purely observational and documented the presence of zoonotic organisms in dogs only (Hii et al., 2011; Jenkins & Andrew, 1993; Lee & Hampson, 1996; Palmer et al., 2007; Thompson, Meloni, et al., 1993). Thirteen also examined samples from humans to see whether disease transmission had occurred.
The most renowned comparative studies conducted on possible transfer of zoonotic organisms from dogs to people in Aboriginal communities are those of Walton et al. (1999, 2004). The studies included a large number of scabies mites but a limited number of hosts, 16 people and 17 dogs. Within‐household comparisons were only applicable to mites from a four‐week‐old baby and three puppies. The results of these studies are discussed further on in this paper.
Welch and Dobson (1974a) conducted research to assess the prevalence of antibodies to the dog heartworm, Dirofilaria immitis in Caucasian and Aboriginal Australians. This was achieved by screening blood samples for anti‐D. immitis antibodies in both Aboriginal and Caucasian sera and compared with a miniature mass radiography for lesions that appeared to coincide with D. immitis. The Aboriginal sample was quite large (n = 323); however, the random Caucasian sample consisted of only 38 individuals. The miniature mass radiography of Aboriginal participants, completed by the Queensland Department of Health, found no lesions. In comparison, five Caucasians from Queensland were found to have lesions in the same year, but it is unknown whether they were the same individuals who participated in the study. Regardless, the prevalence of canine dirofilariasis significantly correlated with the mean titre of individuals testing positive to anti‐D. immitis antibodies, indicating that D. immitis prevalence in dogs will increase a person's exposure.
Lee and Hampson (1992) found that Aboriginal people from various communities in the Kimberley area had intestinal spirochaetal bacteria in their faeces. In addition, another study of dogs from Fitzroy Crossing and Jarvis Bay were also found to have morphologically similar spirochaetes in their faeces to humans (Lee & Hampson, 1996). Multilocus enzyme electrophoresis of spirochaetes isolated from a dog with diarrhoea was closely related genetically to spirochaetes recovered from Aboriginal children with whom the dog lived (Lee & Hampson, 1994).
Kaminski and Green (1977) conducted a large‐scale study on the prevalence of Tinea capitis in Aboriginal communities. In the community of Maningrida, they found that 25.3% of children with tinea capitis were due to the “Maningrida” type variant of Microsporum canis. This variant was also found in four cats and two dogs in the community.
Schrieber et al. (2014) reported the finding of an identical strain of Streptococcus dysgalactiae subsp. equisimilis (SDSE), also known as group G and C streptococci, in the throat of a child and their dog. This study specifically used samples from a human and a dog from within the same household.
The only other study we could find that conducted comparative studies on potentially zoonotic organisms isolated from dogs and people in Aboriginal communities was that of Schnagl and Holmes (1978; Schnagl, Holmes, & Mackay‐Scollay, 1978) who found coronavirus‐like particles in faeces from both dogs and humans in Aboriginal communities. However, it is largely unknown what these particles are, what they do, and whether their presence in both dogs and people indicates disease.
The sample sizes for five papers (Meloni et al., 1993; Shield et al., 2015; Thompson, Meloni, et al., 1993; Welch & Dobson, 1974b; Welch, Dobson, & Freeman, 1979) were considerable, providing precise estimates of prevalence in dogs in the Kimberley region (Meloni et al., 1993; Thompson, Meloni, et al., 1993), various locations around Queensland (Welch & Dobson, 1974b), Central Australia (Welch et al., 1979) and Arnhem Land (Shield et al., 2015). Two papers were from the same study, although considered different organisms (Meloni et al., 1993; Thompson, Meloni, et al., 1993). The prevalence of Toxocara canis and Dirofilaria immitis differed markedly among locations, highlighting the need for dog control and education programmes to be targeted towards the risks faced by each community.
The study by Jenkins and Andrew (1993) had a much smaller sample size of 15, presumably because it was an opportunistic study made possible by a culling programme by the local council. Therefore, the results indicate “presence of an organism” rather than prevalence or disease freedom.
Parkar et al. (2007) compared PCR detection directly from faeces to in vitro propagation for the detection of Blastocystis. The study included dogs sourced from Aboriginal communities but did not state the communities from which the dogs were sourced nor include samples from people in the communities for comparison.
Hii et al. (2011) also used PCR assays to assess the prevalence of Rickettsia felis in dogs at Maningrida Aboriginal community in the Northern Territory. This was an opportunistic study of 130 dogs included in a desexing programme operated by the Animal Management in Rural and Remote Indigenous Communities (AMRRIC) organization.
3.2. Ethical matters
Fourteen papers published from 1974 to 2007 did not discuss ethical approval of their study; nine of these included the use of human data. Only seven of 19 papers scored any points in the ethical matters category. Only two papers discussed the use of informed consent when taking samples from Aboriginal people and their dogs (Schrieber et al., 2014; Walton et al., 1999). Many papers thanked nurses and participants, and two papers recognized the support of the community council (Jenkins & Andrew, 1993; Welch et al., 1979).
While informed consent is not mentioned in many of the papers, it may still have been received. Regardless, papers should clearly state the ethical procedures undertaken within Aboriginal communities whether samples are coming from humans or their dogs.
3.3. Sarcoptes results
Walton et al. (1999) stated that the human‐derived scabies mites and the dog‐derived scabies mites in the same Aboriginal community had different transmission cycles based on them grouping in separate clusters within a phylogenetic dichotomous tree. However, a re‐analysis of the data using reticulated networks rather than dichotomous trees to represent the evolutionary history of S. scabiei in the Aboriginal community showed that both human‐to‐human and dog‐to‐human transmission cycles occur and that both are important for control programmes (Morrison, 2005). Therefore, the method of analysis can significantly affect interpretation of results. In this case, failure to consider reticulate evolution led to an important zoonotic transmission pathway being overlooked.
4. Discussion
The continuing health disparities seen between Indigenous and non‐Indigenous Australians are often related to socioeconomic factors and the harsh living conditions experienced within rural and remote Indigenous communities. Some of these health issues could be attributed to canine zoonotic diseases due to the presence of large free‐ranging dog populations and the general lack of veterinary services in these areas. Often zoonotic diseases are not reported and instead labelled under symptom‐based headings such as gastroenteric disease, which not only down plays their importance but reduces the likelihood of identifying zoonotic organisms and risk practices associated with their transmission.
4.1. Skin infections
4.1.1. Microsporum canis
Fungal skin infections are common among Aboriginal people living in the humid regions of northern Australia. A granular variant of Trichophyton rubrum was reported to be responsible for the most common endemic ringworm cases (Green & Kaminski, 1973; Koh et al., 2003). Kaminski and Green (1977) isolated a variant of Microsporum canis from 21 Aboriginal children suffering from tinea capitis in the Maningrida community and found two dogs to be possible reservoirs of this variant.
4.2. Scabies
Scabies is a debilitating skin condition in Aboriginal communities caused by the mite Sarcoptes scabiei. It is important because the resultant trauma to the skin can lead to subsequent bacterial infection. In some Aboriginal communities, scabies has been shown to underlie up to 70% of streptococcal pyoderma (Currie & Carapetis, 2000). Dog‐derived scabies mites have been experimentally shown to burrow, lay eggs and defecate in human skin initiating papular lesions (Estes, Kummel, & Arlian, 1983).
Smith and Claypoole (1967) documented 22 cases of human infestation with Sarcoptes scabiei var canis in which members of a household were living with a dog diagnosed with sarcoptic mange. The characteristic features included the sudden onset of intensely pruritic papules and vesicles in areas of contact with pets, extreme difficulty in demonstrating mites in humans and excellent responses to treatment with scabicides.
The treatment of household dogs with sarcoptic mange is important in preventing and treating human cases of scabies. The initial reaction is sufficient for secondary bacterial infection (Smith & Claypoole, 1967) with bacteria such as Streptococcus pyogenes or Staphylococcus aureus, which can lead to post‐infective complications including acute post‐streptococcal glomerulonephritis (Bandi & SaiKuMar, 2013; Hoy et al., 2012).
The studies conducted by Walton et al. (1999, 2004) have been used as evidence of a lack of zoonotic transmission of the scabies mite. However, re‐analysis of the data using more appropriate methods showed that dog‐to‐human transmission occurred multiple times and was an important component of the epidemiology of human scabies (Morrison, 2005). Given this and the above evidence from other studies, the conclusions of Walton et al. (1999, 2004) that “control programmes for human scabies in endemic areas do not require resources directed against zoonotic infection from dogs,” are incorrect. Successful mitigation of the effects of scabies in Aboriginal communities must include the control of sarcoptic mange in dogs.
4.3. Respiratory infections
Dirofilaria immitis is a filarial nematode responsible for heartworm in dogs and can be transmitted to humans by mosquitoes. Because mosquitoes are the vector, D. immitis transmission is more common in areas where mosquitoes are endemic. This may explain the high frequency of D. immitis in blood mounts of dogs sourced from Queensland communities compared with those from Central Australia (Welch et al., 1979). A recent study in the Wet Tropics of Far North Queensland found D. immitis at high prevalence (72.7%) in wild dingoes in low‐density housing areas (Smout, Skerratt, Butler, Johnson, & Congdon, 2016). The heartworm life cycle has five larval stages (L1–L5). Adult heartworms are generally present in the pulmonary arteries but may be found in the right ventricle, right atrium and caudal vena cava in heavy infections. Mature females release microfilariae (L1) into the host's bloodstream. The vector mosquito that feeds on an infected dog, the primary host, picks up the microfilariae where they undergo two moults to L3 stage. The mosquito transmits infective larvae (L3) when it subsequently feeds on a human, the secondary host. In the dog, the larvae mature in the muscle sheath, subcutaneous and adipose tissue to L5 stage and the immature adults migrate to the heart and pulmonary artery where they mature to adults. A similar transmission pattern has been proposed for humans where adult nematodes are eventually washed into the pulmonary artery and become lodged in the lungs causing pulmonary nodules (Narine, Brennan, Gilfillan, & Hodge, 1999). These pulmonary nodules have been mistaken for tuberculosis and metastatic tumours (Narine et al., 1999; Ro et al., 1989). Aboriginal people have extremely high rates of tuberculosis with 6.2 cases per 100,000 population in 2008 versus 0.9 cases per 100,000 population of non‐Indigenous people born in Australia (Barry, Waring, Stapledon, & Konstantinos, 2012). In 1974, Welch and Dobson (1974b) reported that fluorescent antibody tests (FAT) found 65 (20.1%) of 323 sera collected from Aboriginal participants had positive titres of human anti‐D. immitis antibodies. This correlated significantly with the prevalence of canine dirofilariasis. All of the Aboriginal participants underwent miniature mass radiography for tuberculosis by the Queensland Department of Health; however, no D. immitis‐like lesions were found in the lungs. These results could indicate that a high exposure to D. immitis may provide protective immunity against infection in people in Aboriginal communities (Welch & Dobson, 1974b).
4.3.1. Toxocara canis
Although classically associated with ocular and visceral larva migrans, Toxocara infection is now known to manifest more commonly as non‐classic or covert toxocariasis where clinical signs include wheezing and asthma, pulmonary infiltrates and eosinophilia (Feldman & Parker, 1992; Sharghi, Schantz, & Hotez, 2000). Welch et al. (1979) reported T. canis in about 75% of dogs from most areas in Queensland. Although a recent national study found a low prevalence of T. canis in domestic dogs in veterinary clinics and refuges (1.2%) (Palmer, Thompson, Traub, Rees, & Robertson, 2008), a recent wild dog survey, which may better reflect the zoonotic risk from free‐ranging community dogs, reported prevalences of 46% (Smout, Thompson, & Skerratt, 2013). Mizgajska (2001) concluded that the prevalence of human toxocariasis is proportional to soil contamination with infective eggs of Toxocara spp. Shield et al. (2015) found 21% of people seropositive for T. canis in their studies in Arnhem Land in the mid 1990s. Toxocariasis is now being heralded as the most common human parasitic worm infection in the United States, and with its high prevalence in developing countries, it is considered that its global importance may be greatly underestimated (Hotez & Wilkins, 2009).
4.4. Gastroenteric infections
4.4.1. Giardia
Giardia duodenalis (syn Giardia intestinalis; G. lamblia) is the most common intestinal parasite of humans in developed countries (Thompson, 2000). The highly variable symptoms of giardiasis include persistent diarrhoea, abdominal pain and rapid weight loss (Thompson, Reynoldson et al., 1993). It is now commonly thought that although dogs can carry strains of Giardia which are potentially infective to humans, transmission mainly occurs among humans (Hopkins et al., 1997; Robertson & Thompson, 2002). In support of this Hopkins et al. (1997) found differences in genotypes of Giardia isolated from 13 Aboriginal people from Fitzroy Crossing and nine dogs that had been culled from the same area. They found that the samples separated into four different genetic groups. All of the human and three dog isolates were contained in groups 1 and 2, while groups 3 and 4 consisted entirely of Giardia samples from dogs. In contrast, Traub et al. (2004) studied zoonotic Giardia transmission in a remote community in India and found that Giardia isolates derived from dogs were placed within the human genetic groupings. Furthermore, humans residing in a house that owned dogs and where one dog was infected with Giardia were significantly more likely to be infected, than humans that did not have a dog or a dog infected with Giardia. In addition, genetically identical isolates were found in dogs and humans from the same household in two cases. Together, these are taken as strong evidence in support of the potential for zoonotic transmission.
The contrasting results seen between the Aboriginal and the Indian communities may be because dogs from Aboriginal communities experience a higher level of interaction with other dogs and have less opportunity to eat human faeces (Traub et al., 2004). Giardia isolates are prone to competitive exclusion, enabling selection of host‐specific Giardia assemblages (Thompson, 2000). It is also possible that the source of dog samples within the Aboriginal study is biased and may therefore be masking zoonotic transmission. According to Hopkins et al. (1997), the dog isolates used in the study were retrieved from culled dogs from the same area as the human participants (n = 9). However, there is nothing to suggest that the dogs used in the study were from the same household as human participants or that the dogs were owned by anyone. Generally, dogs that are euthanased by councils are unhealthy, stray dogs. Sampling from the stray/wild dog population might bias the result because the major form of Giardia transmission, dog to dog, would predominately select for dog‐specific Giardia assemblages. Further research is required to ascertain the potential and frequency of zoonotic transmission of Giardia from dogs to their owners in Aboriginal communities.
4.4.2. Ancylostoma spp.
Palmer et al. (2007) investigated the public health significance of hookworms in Australia and found that Ancylostoma caninum, the dog hookworm, had the highest prevalence (14%) in Aboriginal communities. It is interesting to note that with such a high incidence of A. caninum in communities, we did not recover any reports of eosinophilic enteritis. An Australian study implicated A. caninum as the leading cause of human eosinophilic enteritis (Prociv & Croese, 1990). It is possible that this disease is masked by other health issues in Aboriginal communities.
The detection of Ancylostoma ceylanicum for the first time in Australia in 10.9% of domestic dogs found positive for hookworm (Palmer et al., 2007) and in Australian wild dogs (Smout et al., 2013) warrants further investigation given this parasite's potential to infect dogs and cats and cause a patent infection in humans (Inpankaew et al., 2014; Ngui, Lim, Traub, Mahmud, & Mistam, 2012). Koehler et al. (2013) recorded the first two cases of A. ceylanicum in humans in Western Australia and suggest, as there was no record of travel outside of Australia, the infection was autochthonous and derived from dogs or cats.
4.4.3. Rickettsia felis
R. felis, the agent of fleaborne spotted fever in humans, is typically transmitted through the bite of an infected cat flea (Ctenocephalides felis). Clinical infection ranges from fever, headaches, chills, muscle aches, joint pain and possible eschar at the bite site to a more severe, multisystemic disease as a result of a widespread vasculitis (Maina et al., 2012; Teoh et al., 2016). Molecular techniques have been used to identify R. felis infection in cat fleas from multiple sites in Western Australia (Schloderer, Owen, Clark, Stenos, & Fenwick, 2006). Williams, Izzard, Graves, Stenos, and Kelly (2011) reported R. felis infection in two adults and three children from Victoria. Previously, many infections in Australia may have been misdiagnosed as murine typhus as serological diagnosis was not specific and was typically confounded by cross‐reactivity with typhus group rickettsiae (Teoh et al., 2016). Dogs are often infested with cat fleas, and infected dogs can appear physically healthy, which may be characteristic of reservoir hosts of R. felis (Hii et al., 2011). Further study is needed to determine the pathogenicity of this infection in dogs.
4.5. Zoonoses that are evident in Aboriginal community dogs but have not yet been researched
4.5.1. Coronavirus‐like particles
Schnagl et al. (1978) reported that coronavirus‐like particles were equally prevalent in humans with or without symptoms of diarrhoea. The proportion of children who excreted the viral particles increased with age. Viruses found in humans and dogs were indistinguishable morphologically (Schnagl et al., 1978). The authors concluded that given there are few reports of canine enteric coronaviruses it is difficult to gauge how widespread and important they are, not only as a health risk to dogs, but also to humans (Schnagl & Holmes, 1978).
4.5.2. Intestinal spirochaetes
Lee and Hampson (1992, 1994, 1996) investigated intestinal spirochaetes in faecal samples of humans, pigs and dogs. Although they are found more commonly when diarrhoea is present, their pathogenicity is still largely unknown. Spirochaetes are possibly commensal organisms of the intestine that are flushed out during bouts of diarrhoea (Leach, Lee, & Stubbs, 1973). Therefore, it is unknown what impact spirochaetes may have on Aboriginal health.
4.5.3. Streptococcus dysgalactiae subsp. equisimilis
Schrieber et al. (2014) identified an identical strain of SDSE from pharyngeal swabs of a child and dog from the same household. Once considered a commensal organism, recent studies have shown that through horizontal gene transfer SDSE may be gaining virulence genes from Streptococcus pyogenes, thus elevating its potential importance as a human pathogen (Brandt & Spellerberg, 2009).
4.6. Recommendations
Identifying all factors including those of the shared environment that may or may not contribute to disease is extremely important to the improvement of health outcomes for Aboriginal Australians. This review has revealed that there have not been enough high‐quality comparative studies to determine whether dogs are contributing significantly to the disease burden of Aboriginal communities by transmitting zoonoses.
Aboriginal health researchers have expressed concern that further research into canine zoonoses may result in government funding being shifted from child health to fund dog health programmes, irrespective of the evidence of whether it will substantially benefit the health of Aboriginal children (Currie, 1995). It is outside the scope of this paper to predict future funding decisions and distribution of resources. However, we believe that we have shown that there is insufficient scientific evidence to prove that zoonotic transmission is not important. There is evidence that zoonotic transmission is occurring with S. scabiei being transmitted from dogs to people. A coordinated approach is necessary and dog health should be added to the list of potentially hazardous environmental issues to be addressed within Aboriginal communities. Dog health programmes are still extremely important for animal welfare and providing relevant information to pet owners in Aboriginal Australian communities regardless of zoonotic potential. Furthermore, as there can be no disputing the significant problem of dog bites as a serious canine zoonotic issue (Abreu & D'adonna, 2009); the safety of community members from pack dogs should be motivation enough for the employment of animal control officers in Aboriginal communities.
Despite 30 years of dog health programmes, and over 40 years of research, we could only find 19 studies that had published their findings in peer‐reviewed journals. We are aware of many other research projects which have been conducted in Aboriginal communities, such as that of Wilks and Williamson (1998), but the results of these studies could not be found in peer‐reviewed journals and may result in research being repeated in an already over‐researched Aboriginal population.
In addition, the small amount of comparative studies conducted almost never included people and dogs from the same household. Studies which only used culled dogs for isolates are not always reliable as most are stray dogs with minimal human contact. While it can be assumed that funding constraints make it difficult to invest time in building relationships and employing local Aboriginal research assistants and research candidates, the benefits of local knowledge would far outweigh these issues and ensure an accurate sample of the community as a whole, improving the quality, extent and impact of research results.
Canine zoonotic organisms that still need to be studied in Aboriginal Australian communities include the following: Ancylostoma ceylanicum, Strongyloides stercoralis, Campylobacter, Zoonotic Salmonella, Streptococcal spp., Staphlococcal spp., Leptospira interrogans, Echinococcus granulosus, Dipylidium caninum, Spirometra erinacei, Cryptosporidium and Cystoisospora canis.
Current public health approaches to helminth infections are directed at investigating anthroponotic routes of infection (Crompton, Montresor, Nesheim, & Savioli, 2003). Whereas addressing zoonotic origins may be more appropriate. Identification of human parasitic infections such as Trichuris trichiura using egg morphology alone may be inadequate and may have previously led to cases of misidentification due to morphological similarity to T. vulpis (Dunn, Columbus, Aldeen, Davis, & Carroll, 2002). The recent findings of A. ceylanicum in dogs (Palmer et al., 2007), dingoes (Smout et al., 2013) and humans (Koehler et al., 2013) in Australia should ensure caution is used when diagnosing infections such as A. duodenale in humans. The development of advanced, PCR‐based techniques allows for differentiation between hookworm species using DNA isolated from eggs in faeces and soil (Traub, Inpankaew, Sutthikornchai, Sukthana, & Thompson, 2008) and enables a better understanding of the epidemiology of A. ceylanicum infection (Smout et al., 2013).
To achieve the most from further research, it would be wise to invest time in the Aboriginal community, communicate with relevant community groups and workers and employ Aboriginal locals (such as the animal management worker) on the project team. The establishment of early contact with community based health services can result in collaboration with Aboriginal health workers and other research projects that may already be underway in the community. It is extremely important to conduct comparative studies using samples from dogs and humans from the same household, along with samples from the stray/wild dog population. Samples from both necropsied and live animals would be ideal. And finally, it is imperative that results are published in peer‐reviewed journals for the benefit of everyone involved, present and future.
Supporting information
Smout F, Schrieber L, Speare R, Skerratt LF. More bark than bite: Comparative studies are needed to determine the importance of canine zoonoses in Aboriginal communities. A critical review of published research. Zoonoses Public Health. 2017;64:495–504. 10.1111/zph.12354
References
- Abreu, C. , & D'adonna, A . (2009). Supporting animal management in Aboriginal and Torres Strait Islander governments In: Working Group for Aboriginal and Torres Strait Islander Environmental Health (ed.) 7th National Aboriginal and Torres Strait Islander Environmental Health Conference. Kalgoorlie, WA: Australian Government, Department of Health and Ageing website. [Google Scholar]
- Bandi, K. M. , & SaiKuMar, C. (2013). Sarcoptic mange: A zoonotic ectoparasitic skin disease. Journal of Clinical and Diagnostic Research, 7, 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, C. , Waring, J. , Stapledon, R. , & Konstantinos, A. (2012). Tuberculosis notifications in Australia, 2008 and 2009. Communicable Diseases Intelligence Quarterly Report, 36, 82. [DOI] [PubMed] [Google Scholar]
- Brandt, C. M. , & Spellerberg, B. (2009). Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clinical Infectious Diseases, 49, 766–772. [DOI] [PubMed] [Google Scholar]
- Catling, P. C. , Corbett, L. K. , & Newsome, A. E. (1992). Reproduction in captive and wild dingoes (Canis familiaris dingo) in temperate and arid environments of Australia. Wildlife Research, 19, 195–209. [Google Scholar]
- Constable, S. , Brown, G. , Dixon, R. M. , & Dixon, R. J . (2008). Healing the hand that feeds you: Exploring solutions for dog and community health and welfare in Australian Indigenous Cultures. International Journal of Interdisciplinary Sciences, 3, 220–229. [Google Scholar]
- Corbett, L. (1995). The dingo in Australia and Asia. Sydney: University of New South Wales Press Ltd. [Google Scholar]
- Crompton, D. W. T. , Montresor, A. , Nesheim, M. C. , & Savioli, L. (2003). Controlling disease due to helminth infections. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Crowe, M. , & Sheppard, L. (2011). A review of critical appraisal tools show they lack rigor: Alternative tool structure is proposed. Journal of clinical epidemiology, 64, 79–89. [DOI] [PubMed] [Google Scholar]
- Currie, B. (1995). Dogs and human health in Aboriginal communities: How important are zoonoses? Aboriginal and Torres Strait Islander Health Information Bulletin, 20, 19–29. [Google Scholar]
- Currie, B. J. , & Carapetis, J. R. (2000). Skin infections and infestations in Aboriginal communities in northern Australia. Australasian Journal of Dermatology, 41, 139–143. [DOI] [PubMed] [Google Scholar]
- Dohoo, I. , Martin, W. , & Stryhn, H . (2003). Veterinary epidemiologic research. Charlottetown, Canada: AVC Inc. [Google Scholar]
- Dunn, J. J. , Columbus, S. T. , Aldeen, W. E. , Davis, M. , & Carroll, K. C. (2002). Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. Journal of Clinical Microbiology, 40, 2703–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English, A . (2000). A review of the history and aims of dog health programs in Australia. In Proceedings of the Big Lick Conference (Darwin, Australia). [Google Scholar]
- Estes, S. A. , Kummel, B. , & Arlian, L. (1983). Experimental canine scabies in humans. Journal of the American Academy of Dermatology, 9, 397–401. [DOI] [PubMed] [Google Scholar]
- Feldman, G. J. , & Parker, H. W. (1992). Visceral larva migrans associated with the hypereosinophilic syndrome and the onset of severe asthma. Annals of Internal Medicine, 116, 838–840. [DOI] [PubMed] [Google Scholar]
- Gaskin, S. , Bentham, R. , Cromar, N. , & Fallowfield, H. (2007). The Zoonotic potential of dogs in Aboriginal communities in Central Australia. Environmental Health, 7, 36–45. [Google Scholar]
- Green, A. C. , & Kaminski, G. W. (1973). Trichophyton rubrum infections in Northern Territory Aborigines. Australasian Journal of Dermatology, 14, 101–120. [DOI] [PubMed] [Google Scholar]
- Hii, S.‐F. , Kopp, S. R. , Thompson, M. F. , O'Leary, C. A. , Rees, R. L. , & Traub, R. J. (2011). Molecular evidence of Rickettsia felis infection in dogs from northern territory, Australia. Parasites & Vectors, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, R. M. , Meloni, B. P. , Groth, D. M. , Wetherall, J. D. , Reynoldson, J. A. , & Thompson, R. C. A. (1997). Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. The Journal of Parasitology, 44–51. [PubMed] [Google Scholar]
- Hotez, P. J. , & Wilkins, P. P. (2009). Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLoS Neglected Tropical Diseases, 3, e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy, W. E. , White, A. V. , Dowling, A. , Sharma, S. K. , Bloomfield, H. , Tipiloura, B. T. , … McCredie, D. A. (2012). Post‐streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney International, 81, 1026–1032. [DOI] [PubMed] [Google Scholar]
- Inpankaew, T. , Schär, F. , Dalsgaard, A. , Khieu, V. , Chimnoi, W. , Chhoun, C. , … Odermatt, P. (2014). High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerging Infectious Diseases, 20, 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, D. , & Andrew, P. (1993). Intestinal parasites in dogs from an aboriginal community in New South Wales. Australian Veterinary Journal, 70, 115–116. [DOI] [PubMed] [Google Scholar]
- Kaminski, G. W. , & Green, A. C. (1977). Tinea capitis in Aboriginal Children at Maningrida, Northern Territory, Australia. Australasian Journal of Dermatology, 18, 88–97. [DOI] [PubMed] [Google Scholar]
- Koehler, A. V. , Bradbury, R. S. , Stevens, M. A. , Haydon, S. R. , Jex, A. R. , & Gasser, R. B. (2013). Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning‐coupled approach. Electrophoresis, 34, 1720–1728. [DOI] [PubMed] [Google Scholar]
- Koh, K. J. , Parker, C. J. , Ellis, D. H. , Pruim, B. , Leysley, L. , & Currie, B. J. (2003). Use of terbinafine for tinea in Australian Aboriginal communities in the Top End. Australasian Journal of Dermatology, 44, 243–249. [DOI] [PubMed] [Google Scholar]
- Leach, W. , Lee, A. , & Stubbs, R. (1973). Localization of bacteria in the gastrointestinal tract: A possible explanation of intestinal spirochaetosis. Infection and Immunity, 7, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , & Hampson, D. (1992). Intestinal spirochaetes colonizing aborigines from communities in the remote north of Western Australia. Epidemiology and Infection, 109, 133–141. [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , & Hampson, D. (1994). Genetic characterisation of intestinal spirochaetes and their association with disease. Journal of Medical Microbiology, 40, 365. [DOI] [PubMed] [Google Scholar]
- Lee, J. , & Hampson, D. (1996). The prevalence of intestinal spirochaetes in dogs. Australian Veterinary Journal, 74, 466–467. [DOI] [PubMed] [Google Scholar]
- Lewis‐Beck, M. S. , Bryman, A. , & Liao, T. F. (2004). The Sage encyclopedia of social science research methods, Vol 1. Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Maina, A. N. , Knobel, D. L. , Jiang, J. , Halliday, J. E. , Feikin, D. R. , Cleaveland, S. , … Richards, A. L. (2012). Rickettsia fells infection in febrile patients, Western Kenya, 2007–2010. [DOI] [PMC free article] [PubMed]
- Meggitt, M . (1965). The association between Australian Aborigines and dingoes In: Leeds A. A. V. & Vayda A. P. (Eds.) Man, culture, and animals. American Association for the Advancement of Science (pp. 7–26). Washington, DC: American Association for the Advancement of Science. [Google Scholar]
- Meloni, B. , Lymbery, A. , Thompson, R. , & Gracey, M. (1988). High prevalence of Giardia lamblia in children from a WA aboriginal community. The Medical Journal of Australia, 149, 715. [DOI] [PubMed] [Google Scholar]
- Meloni, B. , Thompson, R. , Hopkins, R. , Reynoldson, J. , & Gracey, M. (1993). The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. The Medical Journal of Australia, 158, 157–159. [DOI] [PubMed] [Google Scholar]
- Mizgajska, H. (2001). Eggs of Toxocara spp. in the environment and their public health implications. Journal of Helminthology, 75, 147–151. [PubMed] [Google Scholar]
- Morrison, D. A. (2005). Networks in phylogenetic analysis: New tools for population biology. International Journal for Parasitology, 35, 567–582. [DOI] [PubMed] [Google Scholar]
- Narine, K. , Brennan, B. , Gilfillan, I. , & Hodge, A. (1999). Pulmonary presentation of Dirofilaria immitis (canine heartworm) in man. European Journal of Cardio‐Thoracic Surgery, 16, 475–477. [DOI] [PubMed] [Google Scholar]
- Ngui, R. , Lim, Y. A. L. , Traub, R. , Mahmud, R. , & Mistam, M. S. (2012). Epidemiological and genetic data supporting the transmission of ancylostoma ceylanicum among human and domestic animals. PLoS neglected tropical diseases, 6(2), e1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, C. , Thompson, R. , Traub, R. , Rees, R. , & Robertson, I. (2008). National study of the gastrointestinal parasites of dogs and cats in Australia. Veterinary Parasitology, 151, 181–190. [DOI] [PubMed] [Google Scholar]
- Palmer, C. S. , Traub, R. J. , Robertson, I. D. , Hobbs, R. P. , Elliot, A. , While, L. , … Thompson, R. (2007). The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum . Veterinary Parasitology, 145(3), 304–313. [DOI] [PubMed] [Google Scholar]
- Parkar, U. , Traub, R. , Kumar, S. , Mungthin, M. , Vitali, S. , Leelayoova, S. … Thompson, R. (2007). Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology, 134, 359–367. [DOI] [PubMed] [Google Scholar]
- Prociv, P. , & Croese, J. (1990). Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum . The Lancet, 335, 1299–1302. [DOI] [PubMed] [Google Scholar]
- Raw, L. (2001). Human health in relation to pets in urban and Indigenous communities In Canyon R., & Speare R. (Eds.), Rural and remote environmental health 1. Brisbane: The Australasian College of Tropical Medicine. [Google Scholar]
- Ro, J. Y. , Tsakalakis, P. J. , White, V. A. , Luna, M. A. , Chang‐Tung, E. G. , Green, L. … Ayala, A. G. (1989). Pulmonary dirofilariasis: The great imitator of primary or metastatic lung tumor. Human Pathology, 20, 69–76. [DOI] [PubMed] [Google Scholar]
- Robertson, I. D. , & Thompson, R. C. (2002). Enteric parasitic zoonoses of domesticated dogs and cats. Microbes and Infection, 4, 867–873. [DOI] [PubMed] [Google Scholar]
- Schloderer, D. , Owen, H. , Clark, P. , Stenos, J. , & Fenwick, S. G. (2006). Rickettsia felis in fleas, Western Australia. Emerging Infectious Diseases, 12, 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnagl, R. , & Holmes, I. (1978). Coronavirus‐like particles in stools from dogs, from some country areas of Australia. The Veterinary Record, 102, 528–529. [DOI] [PubMed] [Google Scholar]
- Schnagl, R. , Holmes, I. , & Mackay‐Scollay, E. (1978). Coronavirus‐like particles in Aboriginals and non‐Aboriginals in Western Australia. The Medical Journal of Australia, 1, 307. [PubMed] [Google Scholar]
- Schrieber, L. , Towers, R. , Muscatello, G. , & Speare, R. (2014). Transmission of Streptococcus dysgalactiae subsp. equisimilis between child and dog in an Aboriginal Australian community. Zoonoses and Public Health, 61, 145–148. [DOI] [PubMed] [Google Scholar]
- Sharghi, N. , Schantz, P. , & Hotez, P.J. (2000). Toxocariasis: An occult cause of childhood neuropsychological deficits and asthma? In: Seminars in pediatric infectious diseases (pp. 257–260). [Google Scholar]
- Shield, J . (1992). Some problems of dog health and control in Aboriginal and Islander Communities in North Queensland In Proceedings of the 1st Annual Conference of Urban Animal Management (Mackay, Australia). [Google Scholar]
- Shield, J. , Aland, K. , Kearns, T. , Gongdjalk, G. , Holt, D. , Currie, B. , & Provic, P . (2015). Intestinal parasites of children and adults in a remote Aboriginal community of the Northern Territory, Australia, 1994–1996. Western Pacific Surveillance and Response 6. [DOI] [PMC free article] [PubMed]
- Smith, E. B. , & Claypoole, T. F. (1967). Canine scabies in dogs and in humans. JAMA: The Journal of the American Medical Association, 199, 59–64. [PubMed] [Google Scholar]
- Smout, F. A. , Skerratt, L. F. , Butler, J. R. , Johnson, C. N. , & Congdon, B. C. (2016). Dingoes (Canis dingo Meyer, 1793) continue to be an important reservoir host of Dirofilaria immitis in low density housing areas in Australia. Veterinary Parasitology, 215, 6–10. [DOI] [PubMed] [Google Scholar]
- Smout, F. A. , Thompson, R. , & Skerratt, L. F. (2013). First report of Ancylostoma ceylanicum in wild canids. International Journal for Parasitology: Parasites and Wildlife, 2, 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh, Y.T. , Hii, S.F. , Graves, S. , Rees, R. , Stenos, J. , & Traub, R.J . (2016) Evidence of exposure to Rickettsia felis in Australian patients. One Health 2, 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, R. (2000). Giardiasis as a re‐emerging infectious disease and its zoonotic potential. International Journal for Parasitology, 30, 1259–1267. [DOI] [PubMed] [Google Scholar]
- Thompson, R. , Meloni, B. , Hopkins, R. , Deplazes, P. , & Reynoldson, J. (1993). Observations on the endo and ectoparasites affecting dogs and cats in Aboriginal communities in the north west of Western Australia. Australian Veterinary Journal, 70, 268–270. [DOI] [PubMed] [Google Scholar]
- Thompson, R. , Reynoldson, J. , & Mendis, A. (1993). Giardia and giardiasis. Advances in Parasitology, 32, 71–160. [DOI] [PubMed] [Google Scholar]
- Traub, R. J. , Inpankaew, T. , Sutthikornchai, C. , Sukthana, Y. , & Thompson, R. (2008). PCR‐based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Veterinary Parasitology, 155, 67–73. [DOI] [PubMed] [Google Scholar]
- Traub, R. , Monis, P. , Robertson, I. , Irwin, P. , Mencke, N. , & Thompson, R. (2004). Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology, 128, 253–262. [DOI] [PubMed] [Google Scholar]
- Waddell, L. , Raji, A. , Sargeant, J. , Parker, S. , Deckert, A. , & McEwen, S. (2009). The methodological soundness of literature reviews addressing three potential zoonotic public health issues. Zoonoses and Public Health, 56, 477–489. [DOI] [PubMed] [Google Scholar]
- Walton, S. F. , Choy, J. L. , Bonson, A. , Valle, A. , McBroom, J. , Taplin, D. … Kemp, D. J. (1999). Genetically distinct dog‐derived and human‐derived Sarcoptes scabiei in scabies‐endemic communities in northern Australia. The American Journal of Tropical Medicine and Hygiene, 61, 542–547. [DOI] [PubMed] [Google Scholar]
- Walton, S. , Dougall, A. , Pizzutto, S. , Holt, D. , Taplin, D. , Arlian, L. , … Kemp, D. (2004). Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia. International Journal for Parasitology, 34, 839–849. [DOI] [PubMed] [Google Scholar]
- Welch, J. S. , & Dobson, C. (1974a). Antibodies to Dirofilaria Immitis in Causasian and Aboriginal Australians diagnosed by immunofluorescence and passive arthus hypersensitivity. The American Journal of Tropical Medicine and Hygiene, 23, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Welch, J. S. , & Dobson, C. (1974b). The prevalence of antibodies to Dirofilaria immitis in aboriginal and caucasian Australians. Transactions of the Royal Society of Tropical Medicine and Hygiene, 68, 466–472. [DOI] [PubMed] [Google Scholar]
- Welch, J. , Dobson, C. , & Freeman, C. (1979). Distribution and diagnosis of dirofilariasis and toxocariasis in Australia. Australian Veterinary Journal, 55, 265–274. [DOI] [PubMed] [Google Scholar]
- Wilks, K . (2000). Sustainable Dog Health Programs Are Possible: West Australian Experiences in Remote Management and Service Delivery In Proceedings of a conference on dog health programs in Indigenous communities. (Darwin, Australia). [Google Scholar]
- Wilks, K. , & Williamson, P . (1998). The dog health program in Aboriginal communities‐a method for dog management in remote Aboriginal communities In: Proceedings of the 7th Annual Conference of Urban Animal Management, Mackay, Australia. [Google Scholar]
- Williams, M. , Izzard, L. , Graves, S. R. , Stenos, J. , & Kelly, J. J. (2011). First probable Australian cases of human infection with Rickettsia felis (cat‐flea typhus). Medical Journal of Australia, 194, 41–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


