Abstract
Introduction:
There is considerable controversy on the accuracy of Kidney Injury Molecule-1 (KIM-1) in prediction of acute kidney injury (AKI) in children. Therefore, the present study intends to provide a systematic review and meta-analysis of the value of this biomarker in predicting AKI in children.
Methods:
An extensive search was performed on the Medline, Embase, Scopus and Web of Science databases by the end of 2019. Cohort and case-control studies on children were included. Urinary KIM-1 levels were compared between AKI and non-AKI groups. Findings were reported as an overall standardized mean difference (SMD) with a 95% confidence interval (CI). Also, the overall area under the receiver operating characteristic (ROC) curve (AUC) of KIM-1 in predicting AKI in children was calculated.
Results:
Data from 13 articles were included. Urinary KIM-1 levels in children with stage 1 AKI were higher than the non-AKI group only when assessed within the first 12 hours after admission (SMD = 0.95; 95% CI: 0.07 to 1.84; p = 0.034). However, urinary KIM-1 levels in children with stage 2-3 AKI were significantly higher than non-AKI children (p <0.01) at all times. The AUC of urinary KIM-1 in predicting AKI in children was 0.69 (95% CI: 0.62 to 0.77).
Conclusion:
Based on the available evidence, KIM-1 seems to have moderate value in predicting AKI in children. Since previous meta-analyses have provided other urinary and serum biomarkers that have better discriminatory accuracy than KIM-1, so it had better not to use KIM-1 in predicting AKI in children.
Key Words: Acute Kidney Injury, Renal Insufficiency, HAVCR1 protein, human, Hepatitis A Virus Cellular Receptor 1
Introduction:
Acute kidney injury (AKI) is one of the major public health problems worldwide, with a high incidence and many new cases annually (1). There are many complications that can result from this condition, including metabolic acidosis, elevated blood potassium levels, uremia, and changes in fluid balance. Long-term complications of AKI also include cardiovascular disease, stroke, and heart failure.
Children with AKI mainly die from cardiovascular diseases and infections (2). Current research suggests that the use of preventive strategies and rapid diagnosis of AKI can lead to a significant reduction in the burden of AKI (3). Prompt diagnosis and treatment of the disease will enable slowing down the progression of the disease and prevent it from causing lasting complications, such as chronic kidney failure. Despite significant advances in medical knowledge, delayed identification of AKI can occur in some cases, and this may lead to persistent damage (4-6). Therefore, researchers are looking for diagnostic methods for early AKI identification.
In recent years, serum and urine biomarkers have been suggested as reliable methods for rapid diagnosis of renal diseases, they have been shown to have better prognostic value compared to other techniques (7-9). These factors include serum creatinine, cystatin C, neutrophil gelatinase-associated lipocalin (NGAL) protein, and Kidney Injury Molecule-1 (KIM-1) (10-12).
KIM-1 is a membrane protein, which is not detectable in serum/urine of healthy individuals. However, KIM-1 is widely expressed in proximal tubule cells after ischemia and toxic conditions, and has been reported to be an appropriate marker in the diagnosis of AKI (13, 14). Systematic reviews and meta-analyses of adult studies suggest that urinary KIM-1 levels can be an appropriate marker for early detection of AKI (14, 15).
As can be seen, these meta-analyses were mainly performed on adults, while the number of studies on children has increased in recent years. In addition, there is considerable controversy on the accuracy of KIM-1 in prediction of AKI in children. Therefore, the present study intends to provide a systematic review and meta-analysis of the value of this biomarker in predicting AKI in children.
Methods:
- Study design
This meta-analysis was designed based on the guidelines for Meta-analysis of Epidemiology Statement, to evaluate the value of urinary KIM-1 level in predicting AKI in children (16).
- Search strategy
To achieve the objectives of the present study, extensive searches were conducted on Medline (via PubMed), Embase, Scopus, and Web of Science by the end of 2019. The search strategy was based on words related to KIM-1 and AKI. Then, by combining these words with appropriate tags in the databases, searches were performed and relevant articles were screened. To find additional articles or unpublished data, manual search was performed in the bibliography of relevant studies as well as search in Google and Google Scholar search engines. The search query used in Medline database is reported in appendix 1.
- Selection criteria
PICO was defined as follows: P: paediatric patients with AKI, I: urinary KIM-1, C: compare with non-AKI children, and outcome: discriminatory accuracy of KIM-1. In the present study, cohort and case-control studies on the accuracy of KIM-1 in predicting AKI in children were included. Studies were included if AKI was confirmed by a standard procedure and urine samples were obtained from all participants. Duplicate studies, review studies, studies without a non-AKI group, and adult studies were excluded from the present study.
- Data extraction and risk of bias assessment
The method of collecting and evaluating the data is described in detail in our previous meta-analyses (17-20). In summary, after searching and removing duplicates, two independent researchers reviewed the titles and abstracts of records and then full-texts of potentially eligible articles were assessed. Disagreements were resolved in consultation with a third reviewer. The data collection checklist was designed based on the PRISMA statement guidelines (21). Extracted data included first author's name, year of publication, sample size, age and sex distribution of patients, patients’ setting, AKI definition criteria, KIM-1 level assay method, time interval between patient’s admission and KIM-1 level assessment, the mean and standard deviation of urinary KIM-1 level, area under the receiver operating characteristic curve (AUC), and sensitivity and specificity of KIM-1 in predicting AKI in children. The risk of bias was assessed using the proposed guidelines in QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies (22).
- Statistical analysis
Data were recorded as mean and standard deviation, AUC, sensitivity, and specificity of KIM-1 in predicting AKI in children. Most studies reported median and interquartile range instead of mean and standard deviation. Therefore, Cochrane's proposed method was used to estimate the mean and standard deviation (23). All analyses were performed in STATA 14.0 statistical program and “metan” command was used. Findings were presented as standardized mean difference (SMD) with a 95% confidence interval (95% CI) to compare the mean urinary KIM-1 level in AKI group with non-AKI group.
Since the time interval between admission and KIM-1 assessment varied between 0 and 96 hours in the included studies, analyses were performed in three time subgroups including 0-12 hours, 12-24 hours, and 24-96 hours. Also, in the eligible studies, patients were divided into three groups based on AKI severity, including stage 1 (or high-risk) AKI, stages 2-3 (or injury and failure) and all severities (stages 1-3). For this reason, the analysis was also performed based on these subgroups. For this purpose, being high risk was considered as stage 1 AKI, injury was deemed equivalent to stage 2 AKI, and failure was deemed equivalent to stage 3 AKI.
An additional analysis was performed to pool the AUCs reported for urinary KIM-1 level in predicting AKI in children. In this section, the AUCs of urinary KIM-1 with their 95% CI were recorded in the statistical program and an overall AUC was reported.
Heterogeneity between studies was assessed using I2 test and p value less than 0.1 were considered significant (indicating heterogeneity). In addition, publication bias was assessed using the funnel Plot (Egger's tests) (24).
Results:
- Characteristics of included studies
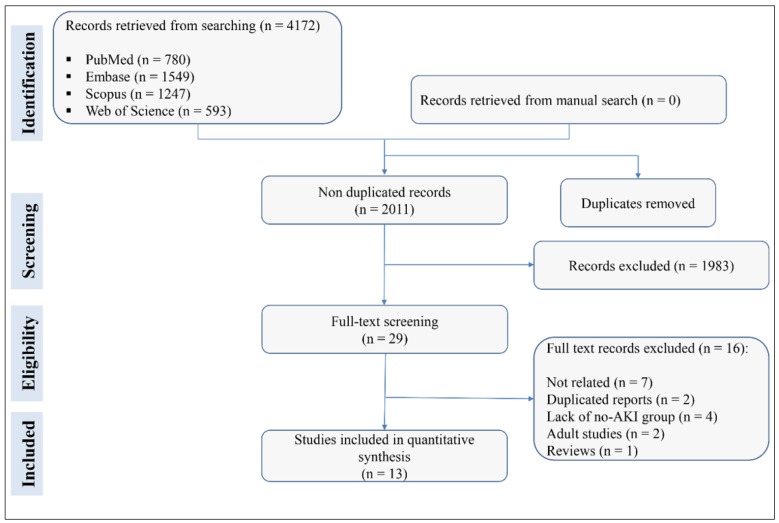
A search of databases yielded 2011 non-duplicated studies. During screening, 29 articles were reviewed in detail, and finally the data of 13 articles were included in the present meta-analysis (25-37) (Figure 1). There were 8 cohort and 5 case-control studies. The sample sizes ranged from 33 to 252 children. The total sample size was 1620 children (825 of whom were boys). Identification of AKI in 8 studies were based on Kidney Disease Improvement Global Outcomes (KDIGO) criteria, based on Pediatric Risk, Injury, Failure, and End-stage kidney disease (pRIFLE) criteria in 4 studies and based on AKI network definition in 1 study. The interval between admission of patients and assessment of urinary KIM-1 levels ranged from 0 to 96 hours. All studies used the ELISA method to check the urine level of KIM-1 and all of them had frozen the specimens at -80 °C prior to examination. Table 1 shows the characteristics of the included studies.
Figure 1.
Flow diagram of screening and selection of eligible studies. AKI: Acute kidney injury.
Table 1.
Characteristics of the included studies
| Author; year; country | Study type | Setting | Age | Sample size | No. of boys | AKI definition | Timing (hrs) |
|---|---|---|---|---|---|---|---|
| Askenazi; 2012; USA | Case-Control | AKI suspected | Neonates | 33 | 17 | AKI Network | 0 to 96 |
| Carvalho Pedrosa; 2015; Brazil | Cohort | Chemotherapy induced AKI | <18 | 64 | 26 | KDIGO | 24, 48, 72, 96 |
| Dong; 2017; USA | Case-Control | Post-cardiopulmonary surgery AKI | <18 | 150 | 77 | KDIGO | 2, 6, 12 |
| Du; 2010; USA | Cohort | AKI suspected | 11.4 | 252 | 126 | KDIGO | 0 |
| Gist; 2017; USA | Cohort | Post-cardiopulmonary surgery AKI | <1 | 94 | 63 | KDIGO | 6 |
| Kandur; 2016; Turkey | Case-Control | ICU admitted AKI | 1 to 17 | 60 | 33 | KDIGO | 24 |
| Krawczeski; 2011; USA | Case-Control | Post-cardiopulmonary surgery AKI | <18 | 220 | 110 | KDIGO | 0, 6, 12, 24 |
| Lagos-Arevalo; 2014; Canada | Cohort | AKI suspected | < 18 | 160 | 58 | KDIGO | 0 to 24 |
| McCaffrey; 2015; UK | Cohort | AKI suspected | <18 | 49 | 26 | pRIFLE criteria | 0 |
| Parikh; 2013; USA | Cohort | Post-cardiopulmonary surgery AKI | <18 | 311 | 171 | pRIFLE criteria | 6, 12, 24, 48, 72, 96 |
| Peco-Antić; 2013; Serbia | Cohort | Post-cardiopulmonary surgery AKI | 1.6 | 112 | 65 | pRIFLE criteria | 2, 6, 24, 48 |
| Sarafidis; 2012; Greece | Case-Control | Asphyxia-associated AKI | Neonates | 35 | 21 | KDIGO | 24, 72 |
| Westhoff; 2016; Germany | Cohort | AKI suspected | <10 | 80 | 32 | pRIFLE criteria | 0 |
AKI: Acute kidney injury; KDIGO: Kidney Disease Improving Global Outcomes; pRIFLE: Pediatric Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; Timing: Time interval between admission and kidney injury molecule-1 assessment.
- Risk of bias and publication bias assessment
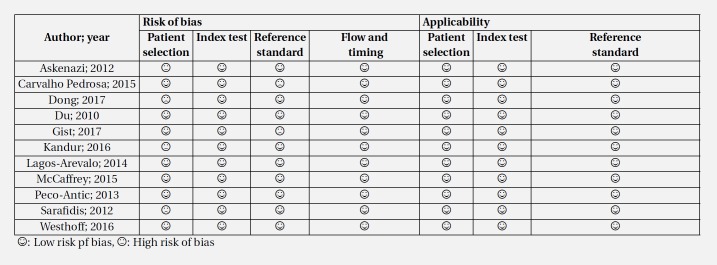
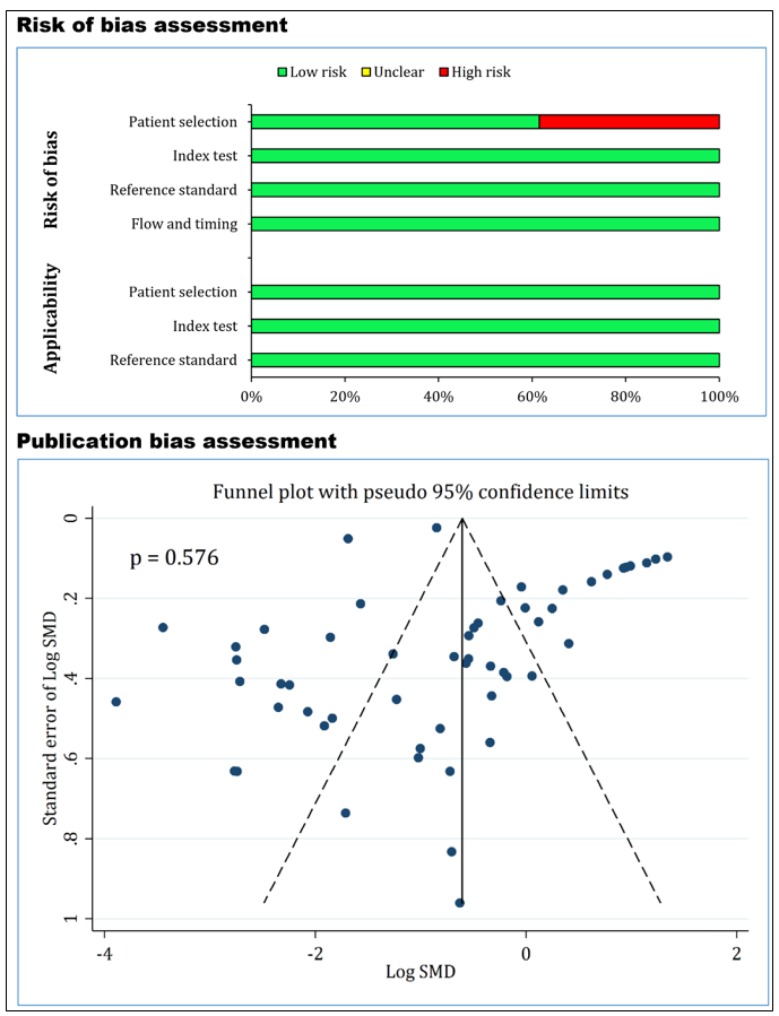
The quality control of studies was performed based on QUADAS-2 criteria. Since the design of 5 studies was case-control, patient selection was associated with bias in these 5 studies and therefore, they were marked as having high-risk of bias. In other cases, the risk of bias and applicability were low risk (Table 2 and Figure 2). The analysis also revealed no evidence of publication bias in the present study (p = 0.576) (Figure 2).
Table 2.
Quality assessment of included studies based on QUADAS-2 recommendations
Figure 2.
Risk of bias and publication bias assessment of the included studies. There is no evidence of publication bias (p = 0.576).
- Comparison of mean urinary KIM-1 levels in children with and without AKI
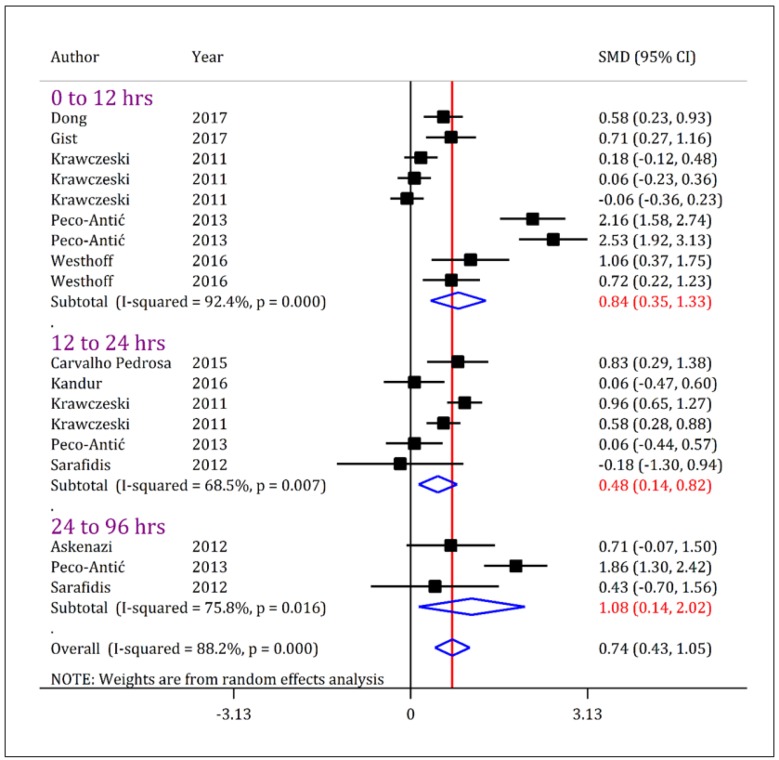
Urinary KIM-1 levels were significantly higher in children with AKI compared to non-AKI children, regardless of severity of AKI. As figure 3 shows, the urinary level of KIM-1 in children with all intensities of AKI (stage 1-3) was higher than non-AKI children during first 12 hours after admission (SMD = 0.84; 95% CI: 0.35 to 1.33; p = 0.001), 24-12 hours (SMD = 0.48; 95% CI: 0.14 to 0.82; p = 0.006) and between 96-24 hours (SMD = 1.08; 95% CI: 0.14 to 2.02; p = 0.024).
Figure 3.
Forest plot for standardized mean difference (SMD) of urine kidney injury molecule-1 (KIM-1) between acute kidney injury (AKI) patients with all severities (stage 1/risk, stage 2/injury, and stage 3/failure) and non-AKI patients at different time cut offs. The urinary level of KIM-1 in AKI-patients is higher than non-AKI patients. CI: Confidence interval.
- Comparison of mean urinary KIM1 levels between stage 1 AKI and non-AKI patients
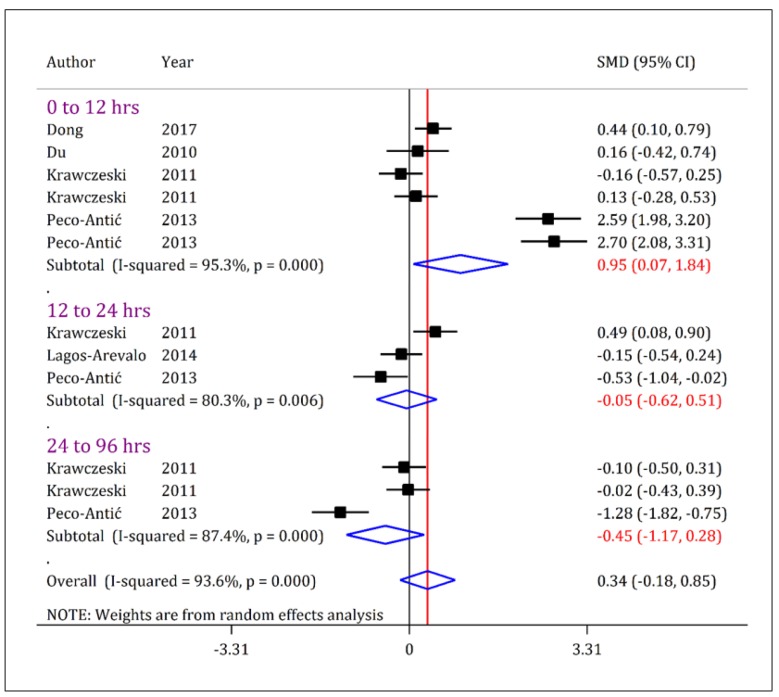
The urinary level of KIM-1 in children with stage 1 AKI was higher than the non-AKI group only when examined within the first 12 hours of admission (SMD = 0.95; 95% CI: 0.07 to 1.84; p = 0.034). However, 12-24 hours (SMD = -0.05; 95% CI: -0.62 to 0.51; p = 0.859) and 24-96 hours (SMD = -0.45; 95% CI: -1.17 to 0.28; p = 0.226) after admission, there was no difference between stage 1 AKI and non-AKI groups (Figure 4).
Figure 4.
Forest plot for standardized mean difference (SMD) of urine kidney injury molecule-1 (KIM-1) between acute kidney injury (AKI) patients in stage 1/risk and non-AKI patients at different time cut offs. The urinary level of KIM-1 in AKI-patients with a severity of stage 1/risk is slightly higher than non-AKI patients only when assessed during the first 12-hours after admission. CI: Confidence interval.
- Evaluation of mean urinary KIM-1 levels in stage 2-3 of AKI and non-AKI patients
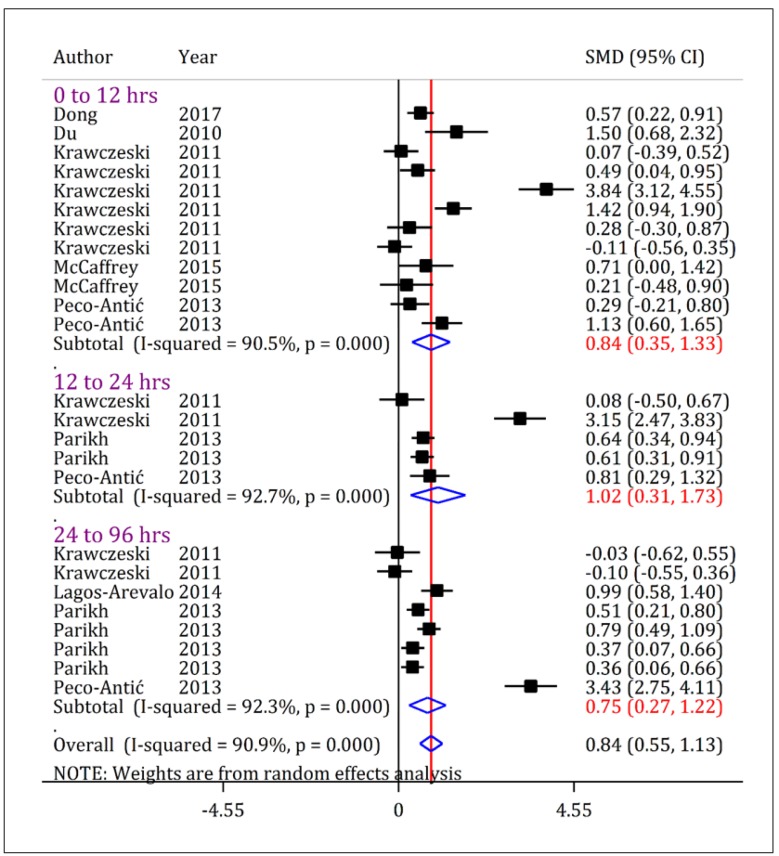
Urinary KIM-1 level in children with stage 2-3 AKI was significantly higher than non-AKI children. As figure 5 shows, the urinary KIM-1 level in children with stage 2-3 AKI were higher than non-AKI children within the first 12 hours (SMD = 0.84; 95% CI: 0.35 to 1.33; p = 0.001), 24-12 hours (SMD = 1.02; 95% CI: 0.31 to 1.73; p = 0.005) and 96-24 hours (SMD = 0.75; 95% CI: 0.27 to 1.22; p = 0.002) after admission.
Figure 5.
Forest plot for standardized mean difference (SMD) of urine kidney injury molecule-1 (KIM-1) between acute kidney injury (AKI) patients with stage 2-3/injury-failure severity and non-AKI patients at different time cut offs. The urinary level of KIM-1 in AKI-patients with a severity of stage 2-3/risk is higher than non-AKI patients in all assessed time points. CI: Confidence interval.
Discrimination
- The AUC of urinary KIM-1 level in diagnosis of pediatric AKI
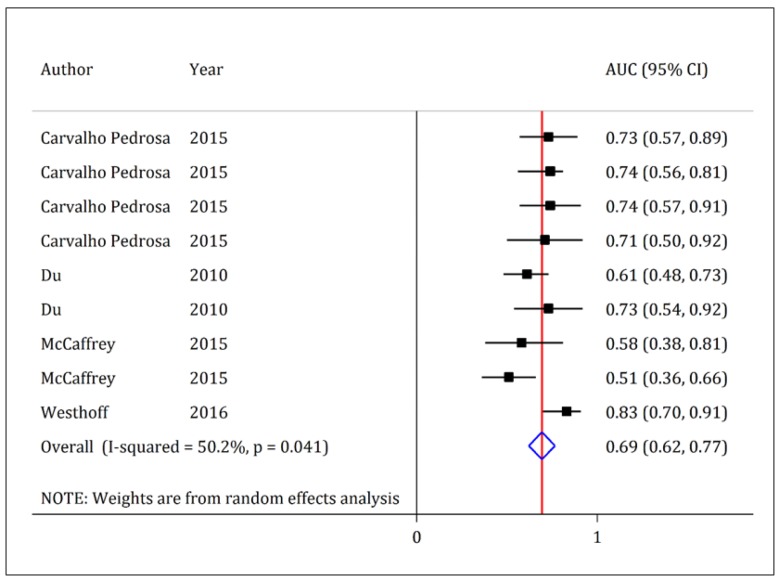
In four studies, AUC of urinary KIM-1 was reported with a 95% CI (26, 28, 33, 37). Pooled analysis showed that AUC of urinary KIM-1 in prediction of AKI was 0.69 (95% CI: 0.62 to 0.77).
- Sensitivity and specificity of urinary KIM-1 level in diagnosis of pediatric acute kidney injury
In the beginning of the present study, it was decided to evaluate the discriminatory power of urinary KIM-1 based on sensitivity, specificity and diagnostic odds ratio. To achieve this goal, we needed data of true positive, false positive, true negative and false negative. But such information was not reported in the studies.
Only three studies reported the sensitivity and specificity of urinary KIM-1 level in prediction of AKI. In the first study, Carvalho et al. showed that the best cut-off point for KIM-1 in predicting chemotherapy-induced AKI was 6.2 ng / mg of creatinine. In this cut-off, urinary KIM-1 had a sensitivity and specificity of 73.1% and 92.1%, respectively (26). Another study by Sarafidis et al. showed that based on the best cut-off point (cut-off = 569.8 pg / ml), KIM-1 had a sensitivity and specificity of 40% and 86%, respectively (36). Finally, Westhoff et al. reported sensitivity and specificity of 54.5% and 96.5%, respectively, for KIM-1; indicating that the best cut-off point for this urine biomarker was 2235 pg / ml (37).
Discussion:
The findings of the present study showed that mean urinary KIM-1 level in children with stage 2-3 AKI was significantly higher than the non-AKI group. It was also found that the level of this biomarker in stage 1 AKI patients was only higher than the non-AKI group when assessed within the first 12 hours of admission. However, the AUC of urinary KIM-1 in prediction of AKI was 0.69, which is in the poor to fair range.
Figure 6.
Area under the curve (AUC) of kidney injury molecule-1 (KIM-1) in diagnosis of acute kidney injury in children. The discriminatory power of KIM-1 in detection of acute kidney injury is poor to fair (AUC = 0.69; 95% confidence interval: 0.62 to 0.77).
Pooled analysis in the present study showed that the urinary level of KIM-1 was significantly higher in children with AKI compared to non-AKI cases. However, with a closer look at the findings, we will find that the obtained SMD is often below 1, which is in the poor to moderate effect size range. Therefore, KIM-1 may not be a good biomarker for the prediction of AKI in children. In addition, the AUC of this biomarker being poor to fair generally indicates that the discriminatory accuracy of KIM-1 is moderate at best.
Several methods have been suggested for assessing the discriminatory power of a biomarker. Although AUC calculation is the most common method in this field, it should be kept in mind that this test is a primary test and we require additional assessment such as sensitivity and specificity. However, sensitivity and specificity of KIM-1 were only reported in three studies. The sensitivity of KIM-1 to predict AKI in children was between 40% and 73.1% and its specificity was between 86% and 96.5%. This sensitivity and specificity were reported based on a wide range cut-off points. Therefore, we could not pool the data in this section. However, it seems that urinary KIM-1's sensitivity to predict pediatric AKI is poor to fair.
Other findings of the present study indicate the weakness of KIM-1 in differentiating patients at risk of AKI (stage 1 AKI) from non-AKI patients. It seems that the level of KIM-1 in the urine would increase significantly only when the patient is in the advanced stages of the AKI (injury or failure phase). This is a major limitation for KIM-1 in predicting AKI in children.
In a systematic review with the aim of examining the value of KIM-1 in diagnosis of AKI in children and adults, Wang et al. showed that the AUC, sensitivity and specificity of KIM-1 in prediction of AKI after cardiac surgery were 0.71, 76% and 0.84%, respectively (15). Although the findings of the study by Wang et al. are in line with the findings of the present study, there are major differences between the two studies. First, Wang et al.'s study pooled the findings of studies on adults and children; and second, out of the 15 included studies, only 3 were studies on children. Therefore, the findings of the study by Wang et al. could not be generalized to the pediatric community.
Along with KIM-1, there are other biomarkers such as cystatin C and NGAL that studies have cited as reliable indicators of AKI. Three previous meta-analyses showed that urinary and serum levels of cystatin C and NGAL had good to excellent discriminatory accuracy in predicting AKI. In the first study, Nakhjavan-Shahraki et al. showed that sensitivity and specificity of serum cystatin C in predicting AKI in children were 85% and 61%, respectively. The AUC of serum and urine cystatin C in predicting AKI were 0.83 and 0.85, respectively (4). In the other two meta-analyses, Izadi et al. showed that the serum level of NGAL in predicting AKI was 87% sensitive and 88% specific, and its urinary level had a sensitivity and specificity of 92% in predicting AKI. The AUC of serum and urinary NGAL in AKI prediction was 0.94 and 0.97, respectively (5, 6). Therefore, it seems that the use of NGAL and cystatin C biomarkers in predicting AKI is superior to KIM-1 in children.
Limitations
In the beginning of the present study, it was decided to calculate the overall sensitivity, specificity, and diagnostic odds ratio of KIM-1 in predicting AKI in children, but after searching and entering studies it became clear that such analysis was not possible. On the other hand, out of the 13 included studies, 5 were case-controls. In this type of design, the research team is aware of the existence of AKI in patients from the beginning, and this may lead to some degree of bias. Also, since a wide range of cut-off points were reported for KIM-1 in the studies, we were unable to reach a unique cut-off point for urinary KIM-1 in predicting AKI in children.
Conclusion:
Based on available evidence, KIM-1 appears to have a moderate value in predicting childhood AKI. Since previous meta-analyses have shown urinary and serum biomarkers that have better discriminatory accuracy than KIM-1, it is better not to use urinary KIM-1 in predicting AKI in children.
Acknowledgment
We are grateful to Dr. Behnaz Bazargani and Prof. Nematollah Ataei for their valuable consultations.
Funding
This study was funded and supported by Tehran University of Medical Sciences (TUMS); Grant no. 95-04-184-33088.
Conflict of interest
There is no conflict of interest.
Author contributions
Study design: Mahmoud Yousefifard, Mostafa Hosseini
Data gathering: Mojtaba Fazel, Arash Sarveazad, Mahmoud Yousefifard
Analysis and interpreting the results: Mahmoud Yousefifard, Mostafa Hosseini, Kosar Mohammad Ali
Drafting the manuscript: Mahmoud Yousefifard
Critically revising the paper: All authors
All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Authors ORCID
Mojtaba Fazel: 0000-0003-0463-4257
Arash Sarveazad: 0000-0001-9273-1940
Kosar Mohammad Ali: 0000-0001-5533-2924
Mahmoud Yousefifard: 0000-0001-5181-4985
Mostafa Hosseini: 0000-0002-1334-246X
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau J-L, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23(2):194. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakhjavan-Shahraki B, Yousefifard M, Ataei N, Baikpour M, Ataei F, Bazargani B, et al. Accuracy of cystatin C in prediction of acute kidney injury in children; serum or urine levels: which one works better? A systematic review and meta-analysis. BMC Nephrol. 2017;18(1) doi: 10.1186/s12882-017-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izadi A, Yousefifard M, Nakhjavan-Shahraki B, Baikpour M, Mirzay Razaz J, Ataei N, et al. Value of plasma/serum neutrophil gelatinase-associated lipocalin in detection of pediatric acute kidney injury; a systematic review and meta-analysis. Int J Pediatr. 2016;4(11):3815–36. [Google Scholar]
- 6.Izadi A, Yousefifard M, Nakhjavan-Shahraki B, Baikpour M, Mirzay Razaz J, Hosseini M. Diagnostic value of Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) in detection of pediatric acute kidney injury; a systematic review and meta-analysis. Int J Pediatr. 2016;4(11):3875–95. [Google Scholar]
- 7.Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22(1):101–8. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 8.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. The Lancet. 2005;365(9466):1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 9.Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17(2):127–32. doi: 10.1097/MNH.0b013e3282f4e525. [DOI] [PubMed] [Google Scholar]
- 10.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H. Kidney injury molecule-1 in renal disease. J Pathol. 2010;220(1):7–16. doi: 10.1002/path.2642. [DOI] [PubMed] [Google Scholar]
- 12.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25(10):2177–86. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ucakturk A, Avci B, Genc G, Ozkaya O, Aydin M. Kidney injury molecule-1 and neutrophil gelatinase associated lipocalin in normoalbuminuric diabetic children. J Pediatr Endocrinol Metab. 2016;29(2):145–51. doi: 10.1515/jpem-2015-0138. [DOI] [PubMed] [Google Scholar]
- 14.Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, et al. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PLoS One. 2014;9(1):e84131. doi: 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Wang WJ, Zhou CC, Cen D. Accuracy of urinary kidney injury molecule-1 in predicting acute kidney injuries associated with cardiac surgery: a systematic review and meta-analysis. Int J Clin Exp Med. 2019;12(6):6570–8. [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology. JAMA: the journal of the American Medical Association. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Yousefifard M, Baikpour M, Ghelichkhani P, Asady H, Darafarin A, Esfahani MRA, et al. Comparison of Ultrasonography and Radiography in Detection of Thoracic Bone Fractures; a Systematic Review and Meta-Analysis. Emergency. 2016;4(2):55. [PMC free article] [PubMed] [Google Scholar]
- 18.Yousefifard M, Baikpour M, Ghelichkhani P, Asady H, Nia KS, Jafari AM, et al. Screening Performance Characteristic of Ultrasonography and Radiography in Detection of Pleural Effusion; a Meta-Analysis. Emergency. 2016;4(1) [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahimi A, Yousefifard M, Kazemi HM, Rasouli HR, Asady H, Jafari AM, et al. Diagnostic accuracy of chest ultrasonography versus chest radiography for identification of pneumothorax: a systematic review and meta-analysis. Tanaffos. 2014;13(4):29–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini M, Yousefifard M, Aziznejad H, Nasirinezhad F. The Effect of Bone Marrow–Derived Mesenchymal Stem Cell Transplantation on Allodynia and Hyperalgesia in Neuropathic Animals: A Systematic Review with Meta-Analysis. Biol Blood Marrow Transplant. 2015;21(9):1537–44. doi: 10.1016/j.bbmt.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med. 2011;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2011. [Google Scholar]
- 24.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, et al. Urine biomarkers predict acute kidney injury in newborns. J Pediatr. 2012;161(2):270–5. doi: 10.1016/j.jpeds.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho Pedrosa D, Macedo de Oliveira Neves F, Cavalcante Meneses G, Pinheiro Gomes Wirtzbiki G, da Costa Moraes CA, Costa Martins AM, et al. Urinary KIM-1 in children undergoing nephrotoxic antineoplastic treatment: a prospective cohort study. Pediatr Nephrol. 2015;30(12):2207–13. doi: 10.1007/s00467-015-3178-3. [DOI] [PubMed] [Google Scholar]
- 27.Dong L, Ma Q, Bennett M, Devarajan P. Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr Nephrol. 2017;32(12):2351–60. doi: 10.1007/s00467-017-3748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y, Zappitelli M, Mian A, Bennett M, Ma Q, Devarajan P, et al. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol. 2011;26(2):267–74. doi: 10.1007/s00467-010-1673-0. [DOI] [PubMed] [Google Scholar]
- 29.Gist KM, Goldstein SL, Wrona J, Alten JA, Basu RK, Cooper DS, et al. Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol. 2017;32(9):1611–9. doi: 10.1007/s00467-017-3655-y. [DOI] [PubMed] [Google Scholar]
- 30.Kandur Y, Gonen S, Fidan K, Soylemezoglu O. Evaluation of urinary KIM-1, NGAL, and IL-18 levels in determining early renal injury in pediatric cases with hypercalciuria and/or renal calculi. Clin Nephrol. 2016;86(2):62–9. doi: 10.5414/CN108843. [DOI] [PubMed] [Google Scholar]
- 31.Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58(22):2301–9. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennett MR, Sabbisetti V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol. 2015;30(4):665–76. doi: 10.1007/s00467-014-2987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaffrey J, Coupes B, Chaloner C, Webb NJ, Barber R, Lennon R. Towards a biomarker panel for the assessment of AKI in children receiving intensive care. Pediatr Nephrol. 2015;30(10):1861–71. doi: 10.1007/s00467-015-3089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–88. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peco-Antic A, Ivanisevic I, Vulicevic I, Kotur-Stevuljevic J, Ilic S, Ivanisevic J, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem. 2013;46(13-14):1244–51. doi: 10.1016/j.clinbiochem.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Sarafidis K, Tsepkentzi E, Agakidou E, Diamanti E, Taparkou A, Soubasi V, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol. 2012;27(9):1575–82. doi: 10.1007/s00467-012-2162-4. [DOI] [PubMed] [Google Scholar]
- 37.Westhoff JH, Fichtner A, Waldherr S, Pagonas N, Seibert FS, Babel N, et al. Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr Nephrol. 2016;31(12):2353–63. doi: 10.1007/s00467-016-3418-1. [DOI] [PubMed] [Google Scholar]