Abstract
Respiratory viral infections are a major cause of morbidity and mortality in solid organ transplant recipients. Early detection of a viral etiology of a LRTI in a febrile transplant recipient can theoretically reduce the use of antibiotics, trigger modification of immunosuppression and prompt appropriate isolation procedures to reduce nosocomial infections. We retrospectively evaluated pediatric abdominal organ transplant recipients hospitalized with respiratory illnesses to determine the viral pathogens identified by various methods including multiplex RT‐PCR performed on nasopharyngeal or endotracheal aspirates. Among 30 symptomatic subjects (median age, 2.5 yr) evaluated using this methodology, 25 (83%) were positive for at least one virus. Rhinovirus was the most frequently identified virus (14 subjects). RSV was identified in five subjects with associated mortality of 40%. Parainfluenza, influenza, metapneumovirus, and adenovirus were also identified. This study indicates that rhinovirus is a significant cause of morbidity in this single center cohort of pediatric abdominal organ transplant recipients.
Keywords: viral pneumonia, bronchiolitis, rhinovirus, immunocompromise, child, infant, transplantation
Abbreviations
- BAL
bronchoalveolar lavage
- CMV
cytomegalovirus
- EBV
Epstein‐Barr virus
- ETT
endotracheal tube
- LRTI
lower respiratory tract infection
- LRTVI
lower respiratory tract viral infection
- NP
nasopharyngeal washing
- PCP
Pneumocystis pneumonia
- RSV
respiratory syncytial virus
- RT‐PCR
reverse‐transcriptase polymerase chain reaction.
Infection is the leading cause of mortality in solid organ transplant recipients 1. Respiratory viral infections are a major cause of morbidity and mortality in these immunocompromised hosts 1, 2. Respiratory viruses such as RSV, influenza, parainfluenza, adenovirus, rhinovirus and other picornaviruses, are increasingly recognized as significant pathogens in these populations 2, 3. Early detection of a viral etiology of LRTIs in a febrile transplant recipient can prevent overuse of antibiotics, reduce the need for BAL, and trigger implementation of appropriate infection control precautions 4, 5. Proper diagnosis can also guide appropriate reduction in immunosuppression, antiviral treatment when available and ultimately improve outcomes 4, 5.
More established diagnostic tests such as viral culture, antigen detection by monoclonal antibodies, and serology are either insufficiently sensitive or too slow to be clinically useful 4, 5. This has led to increased utilization of qualitative and quantitative RT‐PCR to detect DNA due to its ability to rapidly and specifically identify viral pathogens 6, 7. Multiplex RT‐PCR offers impressive overall sensitivity and specificity of 98.4 and 96.4%, respectively, for detection of viral pathogens 8.
Several recent large case series and a pooled analysis report the epidemiology of community acquired respiratory viral infections in immunocompromised hosts 4, 5, 6, 7, 9, 10, 11, 12, 13, 14, 15. Pediatric data primarily were obtained from hematopoietic and lung transplant recipients who have undergone BAL 5, 7, 15. However, there is still a paucity of data for liver, intestinal, and multivisceral organ transplant recipients 13, 14. We retrospectively evaluated the medical records of pediatric abdominal organ transplant recipients hospitalized with respiratory illness to determine the viral pathogens implicated in LRTIs particularly when multiplex RT‐PCR is performed on nasopharyngeal or endotracheal aspirates collected from symptomatic patients.
Methods
The use of a commercial laboratory (ViraCor Laboratories, Lee's Summit, MO, USA) for RT‐PCR detection of respiratory viruses was first introduced in our institution in mid 2008. Subjects between six months and 18 yr of age admitted to Holtz Children's Hospital, Jackson Health System, Miami, Florida, between October 1, 2008, and April 30, 2011, were identified by a review of the physician billing database of the University of Miami Miller School of Medicine for the assigned diagnostic related group numbers for respiratory compromise or failure and liver, kidney, intestinal, or multivisceral transplant. To ensure that all subjects were identified, the Jackson Health System database was also searched for children who had undergone solid organ transplantation and were coded at discharge with diagnoses of bronchiolitis, viral pneumonia, respiratory tract viral infections, and pneumonitis. The retrospective study was approved by the University of Miami Investigational Review Board, and informed consent was waived.
Each identified medical record was retrospectively reviewed. Subjects were included only if they were successfully tracheally extubated for more than 24 h immediately after transplant surgery; thereby excluding respiratory failure in the immediate postoperative period. Subjects were excluded if they had no evidence of increased work of breathing (tachypnea, retractions, wheezing, rales) or hypoxemia requiring oxygen therapy even if they had a positive antigen, culture, or viral PCR result. Collected data included demographic data, dates of admission and testing; the results of any respiratory sample tested to confirm or exclude a respiratory infection including bacterial and/or viral culture results, the presence of symptoms of fever, tachypnea, the description of the respiratory system examination, the presence of hypoxemia requiring oxygen therapy and any abnormal chest X‐ray finding, immunosuppression administered in the first post‐transplant month, and outcome.
Immunosuppression regimens differ for kidney, liver, and intestinal transplants but with some overlap. All recipients receive high‐dose steroids on induction and are maintained on steroids and tacrolimus. Prophylaxis for CMV with either ganciclovir or valganciclovir is administered for three months. All recipients received PCP prophylaxis. Recipients <24 months of age received monthly palivizumab while hospitalized. All inpatients also underwent weekly screening for CMV and EBV by RT‐PCR performed on blood samples.
The treating physician determined what type of sample and test would be used to evaluate symptomatic patients. LRTVI is diagnosed by positivity for viruses by any method on any respiratory sample (NP, ETT sample, or BAL) and clinical symptoms as described earlier. Viruses were detected by variable methods as ordered by the clinicians including antigen detection via immunoflourescence, viral culture or viral nucleic acid detection via ViraCor™ viral respiratory panel (Viracor‐IBT Laboratories, Lee's Summit, MO, USA) performed on clinical specimens. The following 12 viruses are included in the molecular assay: adenovirus, influenza A, influenza A subtype H1, influenza A subtype H3, influenza B, metapneumovirus, parainfluenza 1, 2, 3, rhinovirus, RSV A and RSV B. Bacterial superinfections were identified by a heavy growth of at least one organism at the time of identification of a viral pathogen. Death following a positive test without resolution of symptoms was recorded as mortality associated with that virus. Incomplete recovery was defined as a patient who was discharged with a new oxygen requirement.
Results
Seventy‐four medical records were evaluated representing 77 hospitalizations. Forty‐four of these admissions were associated with PCR testing of respiratory samples. Thirty‐eight admissions were excluded because the subjects had respiratory insufficiency in the immediate postoperative period. Nine additional subjects were excluded because they had no lower respiratory tract symptomatology at the time of testing. Of the remaining 30 symptomatic subjects, 25 (81%) were positive for at least one virus. In the remaining five symptomatic subjects, no virus was identified and no bacterial infection was confirmed.
Demographic characteristics and clinical conditions and symptoms for the 25 subjects with viral respiratory infection are summarized in Table 1. The median age of the subjects at the time of transplant was 14 months (range, 4–104) and at the time of presentation was 24 months (range 12–108). The transplants were performed between December 2005 and February 2011. Seventy‐two percent of study subjects received an intestinal graft and 28% received a liver graft only. One patient received a multivisceral transplant combined with a kidney transplant. No isolated kidney transplant recipient was admitted for pneumonia during this time period. Three subjects developed a LRTVI within the first month after transplantation; 11 were diagnosed between one and six months post‐transplant, and 11 were diagnosed >6 months after transplant.
Table 1.
Demographic and clinical data for abdominal organ transplants with viral LRTIs
| Characteristics | All viruses N = 30 | Rhinovirus (N = 11) |
|---|---|---|
| Age (months) Median (range) | 24 (11–108) | 19 (11–108) |
| Months elapsed since transplant | 29 (1–270) | 23 (2–105) |
| Fever | 20 (65) | 8 (67) |
| Abnormal clinical examination | 25 (80) | 7 (58) |
| O2 requirement | 23 (74) | 8 (67) |
| Abnormal CXR | 22 (71) | 8 (67) |
| Bronchoscopy | 5 | 0 |
| Lung biopsy | 0 | 0 |
| Mortality | 4 (13) | 0 |
All subjects received high‐dose steroids on induction and were maintained on steroids and tacrolimus. One liver recipient and ten intestinal recipients also received additional immunosuppression on induction (nine thymoglobulin, two daclizumab and one rituximab). Two liver recipients and nine intestinal recipients received additional immunosuppression in the first month post‐transplant for management of acute rejection (OKT3 5, thymoglobulin 4, rituximab 4, daclizumab 1). In the 25 subjects with an identified viral pathogen, RSV was identified in five (20%) by antigen detection and subsequently confirmed by viral culture in two. These five subjects were not evaluated for viruses by multiplex RT‐PCR. Rhinovirus was the most frequently detected viral pathogen by RT‐PCR, identified in 11 subjects; two were co‐infections with influenza and pneumocystis, respectively. Influenza and parainfluenza accounted for four cases and three cases, respectively. Adenovirus was identified in two cases; one of these cases was also persistently positive for parainfluenza type 3. Finally, there was one case of metapneumovirus. The seasonal distribution of the respiratory pathogens is shown in the Fig. 1. Human rhinovirus and RSV occurred year round. Parainfluenza type 3 occurred in the late spring and early summer.
Figure 1.
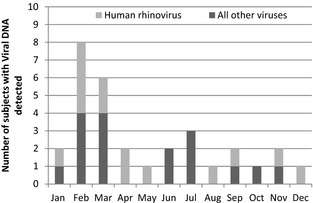
Distribution of respiratory viral infections by month for all infections. Gray boxes represent rhinovirus infections. Black boxes represent all other infections.
Six patients became ill during their initial transplant hospitalization. Two of these had positive diagnostic tests within 48 h of ICU admission, suggesting that they were harboring a virus at the time of transplantation. In one case, the virus appeared to be contracted in the intensive care unit; three became symptomatic while hospitalized on the ward. Five patients became symptomatic on the ward during a subsequent hospitalization. The remaining 14 cases appeared to be contracted in the community. Three patients were identified as having a bacterial superinfection – one mycobacterium and two pseudomonas. The overall mortality was 24%. There was no mortality in the subjects in which no pathogen was identified. Three subjects succumbed due to respiratory failure related to a LRTI; two with RSV and one with adenovirus. RSV had 40% mortality despite attempts to decrease immunosuppression and administration of palivizumab. Two patients with rhinovirus and one with RSV had prolonged complex hospitalizations and died of other causes. One patient with adenovirus died of respiratory failure but had no evidence of adenovirus at the time of death following treatment with cidofovir. Nine patients required oxygen supplementation at discharge.
Discussion
This retrospective study was designed to describe the distribution of clinically significant LRTVIs in a pediatric solid organ transplant population other than lung transplant recipients. Rhinovirus was detected in 44% of subjects; it was the sole identified pathogen in 38% of subjects. A similar distribution of viruses was reported in 154 immunocompetent children hospitalized over a 15‐yr period with viral respiratory infections when several diagnostic tests were used to identify infectious agents including blood culture, viral culture, direct fluorescence antibody, serology, and PCR for pneumococcal 16. A virus was detected in 45% of cases; influenza A, RSV, and parainfluenza viruses were most common. Rhinovirus, which in the above‐mentioned study was only detected by culture, an inferior detection method, was associated with only 5% of infections. Using molecular techniques in symptomatic children in a childcare setting, Fairchok et al. recently demonstrated that rhinovirus was the most commonly identified organism 17. Furthermore, this virus accounted for the greatest number of febrile and wheezing episodes and the highest total visits to a healthcare provider.
Difficulty growing rhinovirus in culture coupled with the fact that rhinovirus prefers a temperature of <35 °C led to a previous assumption that rhinovirus was confined to infections of the upper airway 10, 18. However, when either nasopharyngeal aspirates or BAL samples from patients hospitalized with lower respiratory tract disease were assayed by monoclonal antibodies and direct fluorescent antibody staining, rhinovirus was detected in 25% of subjects 19. Human rhinovirus has been demonstrated in endobronchial biopsies taken from infants with persistent respiratory symptoms by in situ RNA hybridization 18, 20. Furthermore, in subjects with a single viral infection, the decline in the human rhinovirus viral load was associated with clinical improvement 19.
Several studies performed in adult immunocompromised patients have employed molecular testing 4, 5, 7, 10, 13. Among 60 adult lung transplant recipients, 112 respiratory tract infections were evaluated by viral PCR. Viral nucleic acid was detected in 51 cases, but rhinovirus in only three 10. When RT‐PCR was used to detect 11 viruses including human rhinovirus in 117 symptomatic patients, human rhinovirus (24%) was the virus most frequently detected in BAL specimens 5. Vu et al. recently summarized over 34 studies reporting on respiratory infections in lung transplant recipients accounting for over 4000 samples 12. The distribution of viral infection varied widely depending on such factors as whether upper or only lower respiratory infections are studied and the number of viruses detected by the more sensitive molecular methodology. When a greater number of viruses can be detected by molecular methods, coronavirus and rhinovirus are most frequently detected.
There are limited data about the epidemiology of viral infections in pediatric solid organ transplant recipients. A multi‐institutional retrospective review identified 79 of 576 pediatric lung transplant recipients who experienced 101 respiratory viral infections involving either the upper and/or lower respiratory tract in the first year post‐transplant 15. The most frequently detected virus was adenovirus (25 cases), followed by rhinovirus (22 cases), RSV (21 cases), parainfluenza virus (19 cases), influenza virus, and enterovirus. These authors attributed the 20% incidence of rhinovirus infection to the use of molecular testing.
Liu et al. identified younger age at the time of transplant and a cyclosporine‐based immunosuppressive regimen as independent risk factors for the development of a respiratory viral infection in lung transplant recipients. The same appears to hold true for abdominal organ transplant recipients. Between Jan 1, 2006, and December 31, 2011, 254 children <10 yrs of age underwent abdominal organ transplants at the Holtz Children's Hospital, Jackson Health System, Miami, Florida, with liver transplant and intestinal transplant recipients accounting for 57 and 24% of organ transplants, respectively. Kidney recipients accounted for 18% of transplants. Among the abdominal organ transplants with LRTIs, intestinal recipients who receive greater immunosuppression, in part via higher target calcineurin inhibitor concentrations, accounted for over two‐thirds of LRTVI. Furthermore, children less than a year of age at the time of transplant accounted for one‐third of abdominal organ transplants but two‐thirds of LRTVI.
Significant mortality from RSV infection was observed in our cohort. Pohl et al. previously described 17 episodes of RSV infection among 493 pediatric liver transplant recipients 21. Four subjects, all with a history of previous lung disease experienced respiratory failure, and two died. All four developed infection within the first postoperative month. In contrast, among 173 kidney transplant recipients, five developed RSV infection. The youngest was 11 months at transplant and there were no deaths 22. In the present study, one death occurred in a 12‐month‐old subject receiving rituximab and high‐dose steroids for hemolytic anemia and the second occurred in a patient who was likely infected just prior to surgery. Like LRTVI in general, younger age and greater immunosuppression appear to be risk factors for serious RSV disease.
There are several limitations to the present study. Prospective studies of non‐symptomatic patients suggest that PCR may be overly sensitive and that not all viruses detected will produce significant disease 4. In a previous study, the viral detection yield from prospectively collected specimens from asymptomatic patients using PCR was two times greater than culture and was four times greater than fluorescent antibody detection 4. Similarly, when either nasopharyngeal aspirates or BAL samples from symptomatic hospitalized patients were assayed by monoclonal antibodies and direct fluorescent antibody staining, rhinovirus was detected in 25% of subjects 19. In the current study, only symptomatic patients were included and a bacterial co‐infection was only detected in one of 12 symptomatic cases where rhinovirus was detected.
Our results may not be generalizable to other transplant populations and/or other transplant centers. Our population is relatively young and therefore immunologically naïve, which may impact susceptibility to infection on exposure. Our results may also simply reflect the subjects' exposure to virus in our community. Unexpectedly, we did not detect any significant CMV disease which is frequently cited as a common cause of pneumonitis in the transplant population 1, 2, 14. Although the viral respiratory panel did not include CMV, CMV should have been detected by shell vial assay. The low incidence of CMV pneumonitis is more likely due to the use of viral prophylaxis.
Findings from the current study contribute to the limited available data about the epidemiology of viral LRTIs in immunocompromised hospitalized pediatric patients. In the present study of 30 pediatric abdominal transplant recipients with symptoms of a lower respiratory tract illness evaluated by multiplex RT‐PCR in addition to other common diagnostic tests, 83% were positive for at least one virus. This implies that the use of RT‐PCR significantly improves the yield of clinical samples in immunocompromised hosts with respiratory symptoms. Rhinovirus was detected in 36% of symptomatic subjects but was associated with no deaths, indicating that rhinovirus may be a significant cause of morbidity but not mortality in pediatric abdominal organ transplant recipients.
Authors' Contributions
The study was designed by TTT and GEM who acquired and analyzed the data and drafted the manuscript. AK provided additional data. AK and IAG critically reviewed the final manuscript.
Tran TT, Gonzalez IA, Tekin A, McLaughlin GE. Lower respiratory tract viral infections in pediatric abdominal organ transplant recipients: A single hospital inpatient cohort study.
References
- 1. Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther 2007: 12(4 Pt B): 627–638. [PubMed] [Google Scholar]
- 2. Vigil KJ, Adachi JA, Chemaly RF. Viral pneumonias in immunocompromised adult hosts. J Intensive Care Med 2010: 25: 307–326. [DOI] [PubMed] [Google Scholar]
- 3. Kumar D, Humar A. Respiratory viral infections in transplant and oncology patients. Infect Dis Clin North Am 2010: 24: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis 2009: 11: 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garbino J, Gerbase MW, Wunderli W, et al. Lower respiratory viral illnesses. Improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med 2004: 170: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 6. Templeton KE, Scheltinga SA, Van Den Eeden WC, et al. Improved diagnosis of the etiology of community‐acquired pneumonia with real‐time polymerase chain reaction. Clin Infect Dis 2005: 41: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rovida F, Percivalle E, Zavattoni M, et al. Monoclonal antibodies versus reverse transcription‐PCR for detection of respiratory viruses in the patient population with respiratory tract infections admitted to hospital. J Med Virol 2005: 75: 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahony J, Chong S, Merante F, et al. Development of a respiratory virus panel (RVP) test for detection of twenty human respiratory viruses using multiplex PCR and a fluid microbead‐based assay. J Clin Microbiol 2007: 45: 2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garbino J, Soccal PM, Aubert JD, et al. Respiratory viruses in bronchoalveolar lavage: A hospital‐based cohort study in adults. Thorax 2009: 64: 399–404. [DOI] [PubMed] [Google Scholar]
- 10. Weinberg A, Lyu DM, Li S, Marquesen J, Zamora MR. Incidence and morbidity of human metapneumovirus and other community acquired respiratory virus is lung transplant recipients. Transpl Infect Dis 2010: 12: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costa C, Bergallo M, Astegiano S, et al. Detection of human rhinoviruses and a lower respiratory tract of lung transplant recipients. Arch Virol 2011: 156: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vu DL, Bridevaux P‐O, Aubert J‐D, Soccal PM, Kaiser L. Respiratory viruses and lung transplant recipients: A critical review and pooled analysis of clinical studies. Am J Transplant 2011: 11: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez‐Medrano F, Aguado JM, Lizasoain M, et al. Clinical implications of respiratory virus infections in solid organ transplant recipients: A prospective study. Transplantation 2007: 84: 851–856. [DOI] [PubMed] [Google Scholar]
- 14. Bonatti H, Pruett TL, Brandacher G, et al. Pneumonia in solid organ recipients: Spectrum of pathogens in 217 episodes. Transplant Proc 2009: 41: 371. [DOI] [PubMed] [Google Scholar]
- 15. Liu M, Worley S, Arrigain P, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis 2009: 11: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community‐acquired pneumonia in hospitalized children. Pediatrics 2004: 113: 701–707. [DOI] [PubMed] [Google Scholar]
- 17. Fairchok MP, Martin ET, Chambers S, et al. Epidemiology of viral respiratory tract infections in a process that could cohort of infants and toddler's attending daycare. J Clin Virol 2010: 49: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malmstrom K, Pitkaranta A, Carpen O, et al. Human rhinovirus endobronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol 2006: 118: 591–596. [DOI] [PubMed] [Google Scholar]
- 19. Gerna G, Piralla A, Rovida F, et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms at hospitalized immunocompetent and immunocompromised patients. J Med Virol 2009: 81: 1498–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costa C, Bergallo M, Sidoti F, et al. What role for human rhinoviruses in the lower respiratory tract? New Microbiol 2009: 32: 115–117. [PubMed] [Google Scholar]
- 21. Pohl C, Green M, Wald ER, Ledesma‐Medina J. Respiratory syncytial virus infections in pediatric liver transplant recipients. J Infect Dis 1992: 165: 166–169. [DOI] [PubMed] [Google Scholar]
- 22. Miller RB, Chavers BM. Respiratory syncytial virus infections and pediatric renal transplant recipients. Pediatr Nephrol 1996: 10: 213–215. [DOI] [PubMed] [Google Scholar]


