Abstract
To investigate the current genotypes of circulating human adenovirus (HAdV) strains, we molecularly genotyped HAdV in the nasopharyngeal aspirates (NPAs) of patients with acute lower respiratory tract infections (ALRTIs) and attempted to determine their associations with clinical symptoms. A total of 4751 NPA samples were collected from 4751 patients admitted to Hunan Provincial People's Hospital from September 2007 to March 2014, of which 447 (9.4%) samples were HAdV positive. Fourteen different HAdV types were identified; HAdV types 1 to 7 (HAdV 1‐7) were identified in 95.7% of the 447 NPA samples with HAdV‐7 and HAdV‐3 being the most prevalent. In addition, 93.3% (417 of 447) of patients were younger than 5 years. The incidence of HAdV infection peaked in summer. Different HAdV types showed a predilection for different age groups and different seasonal distribution patterns. Coinfection of HAdVs and other respiratory viruses was detected in 63.3% (283 of 447) of the HAdV‐positive samples. The most common clinical diagnosis was pneumonia and the most common symptoms were fever and cough. In comparison with children infected with HAdV‐3 alone, those infected with HAdV‐7 alone had an increased frequency of severe pneumonia involvement (11.6% vs 32.4%; P = 0.031), higher intensive care unit admission rates (7.0% vs 26.5%; P = 0.019), and a longer length of hospital stay (P = 0.03). Mixed infections in younger children were associated with a longer hospital stay (P = 0.023). Our results demonstrate the recent changes in the trends of circulating HAdV genotypes associated with ALRTIs in Hunan China.
Keywords: acute lower respiratory tract infection, adenovirus, children, hospitalization, polymerase chain reaction
1. INTRODUCTION
Human adenoviruses (HAdVs) are a common cause of respiratory tract infections in children, accounting for 5% to 10% of all lower respiratory tract infections (LRTIs) in children.1, 2 The major clinical manifestations of HAdV respiratory infections include fever, cough, sore throat, and tonsillitis and are accompanied by gastrointestinal symptoms, such as vomiting and diarrhea.3, 4 Asymptomatic latent infection may occur in moderate self‐limiting HAdV infection cases, whereas multiple organ failure or even death has been recorded in severe cases.1, 2 Pulmonary sequelae occur in 14% to 60% of patients with pneumonia caused by HAdV infection.5, 6, 7
To date, 79 HAdV types have been identified and are classified into seven species (A‐G).8, 9, 10, 11, 12, 13, 14 HAdV types 1 to 7 account for approximately 80% of all HAdV‐infected cases in children.5, 6, 15, 16 HAdV‐3 and HAdV‐7 are closely associated with LRTIs.5, 6, 15, 16 HAdV‐7 may lead to severe respiratory tract infections and HAdV‐7 infection is associated with a high incidence of death and severe sequelae.16, 17, 18
The dominant type and detection rate of HAdV vary in different countries and eras. Epidemic outbreaks of HAdV‐3 and HAdV‐7 infections occurred in children during the 1950s and 1960s in China.16, 17 HAdV‐3 and HAdV‐7 infections are currently the most severe infections in Northern China with mortality rates of 16.6% to 33.3%. The incidence and severity of HAdV infections have decreased gradually since the 1980s,16, 17, 19, 20 although an epidemic outbreak of HAdV‐7 infection occurred in a northern city of Shanxi Province (China) in January 2009.19 Subsequently, an epidemic outbreak of HAdV‐55 infection, which was confirmed to be an intertypic recombinant virus according to complete genomic sequencing and was designated as a new genotype after bioinformatics analysis, was detected in January 2013 in China.21, 22 Currently, the molecular and clinical characterization of HAdV infection in Chinese children is limited. Therefore, the current study aimed to explore the molecular epidemiology and clinical features of HAdV infection in hospitalized children with acute lower respiratory tract infections (ALRTIs) in Hunan, China.
2. MATERIALS AND METHODS
2.1. Ethics statement
The study protocol was approved by the Ethical Review Committee of Hunan People's Hospital. The parents or guardians of all the participants in this cooperative study provided written informed consent.
2.2. Patients and sample collection
Respiratory virus surveillance was performed in 4751 patients who were enrolled from September 2007 to March 2014 at Hunan People's Hospital. The patients in this study were children diagnosed with ALRTIs (including pneumonia, acute bronchitis, and bronchiolitis). ALRTIs were diagnosed on the basis of clinical and radiologic findings. Enrollment of children in the study followed the diagnosis of dominant symptoms of an acute or worsening cough or a clinical presentation that suggested an LRTI with a duration of less than or equal to 28 days. The age of the children in our study ranged from 1 day to 14 years.
All nasopharyngeal aspirate (NPA) samples were collected 1 to 3 days after admission to the hospital. Approximately 1 to 2 mL of deep NPA samples was obtained using a disposable sterile suction tube that was inserted 7 to 8 cm into the throat via the nose. NPA samples were then transferred to a sterile collection tube after normal saline was added. A virus protection solution (2 mL; containing 200 U/mL of penicillin, 200 U/mL of streptomycin, 200 U/mL of amphotericin B, and 0.125% bovine serum albumin [BSA]) was added to each sample. The virus protection solution and NPA were mixed and then the mixture was immediately placed in a −80°C freezer. The samples were sent to the China National Center for Disease Control and Prevention (Supporting Information Figure 1).
2.3. Data collection
The general demographics, including age, sex, and NPA sample collection time, were recorded. The details of the clinical findings and disease severity were obtained from the medical records after the patients’ discharge from the hospital. Patients coinfected with bacteria, mycoplasma, and chlamydia were excluded from the statistical data of the clinical profiles of HAdV infection cases.
2.4. DNA/RNA extraction
Viral DNA and RNA were extracted from 140 μL of each NPA sample using a QIAamp Viral DNA and RNA Mini Kit (Qiagen, Shanghai, China) according to the manufacturer's protocol. Random hexamer primers and the Superscript II RH‐reverse transcriptase (Invitrogen, Carlsbad, CA) were used to synthesize complementary DNA (cDNA).
2.5. HAdV detection
Nested polymerase chain reaction (PCR) and DNA sequencing of the HAdV hexon gene HVR1‐6 were used in the current study as they are more rapid and efficient compared with classical serologic testing.23, 24 The AdhexF1/R1 and AdhexF2/R2 primer pairs24 were used to amplify a 688‐ to 821‐bp fragment of the hexon gene. The PCR program used was 94°C for 3 minutes, followed by 35 cycles of 94°C for 30 seconds, 52°C for 30 seconds, 72°C for 60 seconds, and a final 8‐minute extension at 72°C.
2.6. Screening for other respiratory viruses
From September 2007 to March 2012, 3495 NPA samples were randomly collected from 3495 children with ALRTI. In addition to HAdVs, the samples were also screened for human respiratory syncytial virus (HRSV), human metapneumovirus (HMPV), influenza viruses A and B (IFVA and IFVB), parainfluenza virus types 1 to 3 (PIV1‐3), human rhinoviruses (HRVs), human coronaviruses (HCoV‐NL63 and HCoV‐HKU1), and human bocavirus (HBoV) using PCR as described in previous studies.25, 26 From April 2012 to March 2014, 1256 NPA samples were collected, and 11 respiratory viruses were detected by real‐time PCR as described previously27, 28, 29 (Figure 1).
Figure 1.
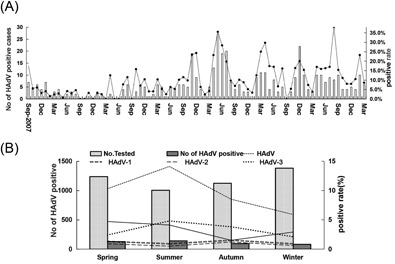
A, Monthly distribution of cases of HAdV infection during the study period. B, Seasonal distribution of cases of HAdV infection between September 2007 and March 2014. HAdV, human adenovirus
2.7. Nucleotide sequence analysis
All of the amplification products of HAdV were sent to Beijing Tianyi Huiyuan Bioscience & Technology, Inc, for sequencing. BLAST was performed using the US National Center for Biotechnology (NCBI) GenBank database. The phylogenetic tree of the sequencing results for each HAdV type was constructed using MEGA 4.0 software (http://www.megasoftware.net). The partial gene sequences were compared with MEGA 4.0 software using ClustalW, and the phylogenetic tree was plotted via the Neighbor‐Joining method. The Bootstrap value was set to 1000 and the reference sequences were selected from GenBank. The reference strains of the different HAdV types that were selected from GenBank were as follows: HAdV1‐AB330082, HAdV2‐AF542120, HAdV3‐AY854173, HAdV5‐FJ943614, HAdV6‐AB434209, HAdV7‐GU230898, HAdV55‐KP896483, HAdV21‐AY008279, HAdV37‐AB500123, HAdV40‐X51782, and HAdV41‐AB330122.
2.8. Statistical analysis
Normally distributed continuous data are presented as the mean ± SD and were analyzed using the Student t test or ANOVA followed by Tukey's post hoc test, as appropriate. Non‐normally distributed continuous data are presented as the medians (interquartile range) and were analyzed using the Mann‐Whitney U test. Categorical data are expressed as frequencies and were analyzed using the χ 2 test or Fisher exact test, as appropriate. Multivariate logistic regression analysis was performed to identify independent risk factors. All analyses were performed using SPSS 20.0 (IBM, Armonk, NY). P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Clinical demographics
NPA samples were collected from 4751 patients hospitalized for ALRTIs from September 2007 to March 2014, including 3059 males and 1692 females (male/female, 1.81/1). Patient age ranged from 1 day to 168 months old (Supporting Information Figure 2). There were 197 patients with acute bronchitis, 1003 with bronchiolitis, and 3551 with pneumonia.
3.2. HAdV types
Of the 4751 patients, 447 patients were HAdV positive and the detection rate was 9.4%. The proportions of patients carrying HAdV in each year from September 2007 to March 2014 are listed in Supporting Information Table 1.
Fourteen different HAdV types were detected in the 447 HAdV‐positive samples. HAdV‐7 showed the highest detection rate (156 of 447) compared with that of other HAdV types, followed by HAdV‐3 (150 of 447), HAdV‐1 (54 of 447), HAdV‐2 (38 of 447), HAdV‐4 (13 of 447), HAdV‐6 (9 of 447), HAdV‐5 (8 of 447), HAdV‐14 (6 of 447), HAdV‐55 (3 of 447), HAdV‐57 (3 of 447), HAdV‐41 (3 of 447), HAdV‐21 (2 of 447), HAdV‐40 (1 of 447), and HAdV‐37 (1 of 447).
The main epidemic strain of HAdV changed over the years. There was a switch in the most prevalent type from HAdV‐3 to HAdV‐7. HAdV‐3 infection was most prevalent from September 2007 to August 2011, and HAdV‐7 infection was most prevalent from September 2010 to August 2013, whereas HAdV‐1 infection was the predominant strain in September 2007 and August 2008. HAdV‐2 infection was the predominant HAdV infection from September 2013 to March 2014. Infections of other types were only sporadic (Supporting Information Table 1).
3.3. Characteristics of patients infected with HAdV
The age of the 447 patients with HAdV infection (male:female, 1.55:1) ranged from 1 day to 144 months (mean, 24.2 ± 21.3 months). Of the 447 patients, 93.3% (417 of 447) were younger than 5 years of age and 71.6% of patients (320 of 447) were younger than 3 years of age, including 13 newborns. The HAdV infection rates varied significantly among the different age groups. The HAdV detection rate was the highest in 3 to 4 years old children and was lowest in children less than 6 months old (χ 2 = 76.87; P = 0.000; Supporting Information Figure 2). HAdV‐7 showed the highest detection rate among 4 to 5 years old children (χ 2 = 14.28; P = 0.022). HAdV‐3 was the predominant HAdV infection type in children aged 3 to 4 years old (χ 2 = 51.18; P = 0.000). HAdV‐1 was the most common type in children aged 2 to 3 years old (χ 2 = 22.01; P = 0.010; Supporting Information Figure 3). The detection rates of the other types showed no clear age distribution (data not shown).
3.4. Epidemiology of HAdV infection
The seasonal and monthly distribution of HAdV infection is shown in Figure 1. Over the 6.5 years of surveillance, HAdV infections were detected during all months except September, October, and December of 2008 and March, May, June, and September of 2009. The number of HAdV infection cases significantly increased in 2011, especially in May and June, and HAdV‐7 infection was dominant during this period. The seasonal distribution exhibited statistically significant differences in the positive rates of HAdV among the four different seasons. The highest detection rate was observed in summer, which had statistically significant differences (χ 2 = 48.93; P = 0.000). HAdV‐3 was the predominant HAdV infection type during summer (χ 2 = 17.5; P = 0.001). HAdV‐7 had the highest detection rate during spring (χ 2 = 21.41; P = 0.000). By contrast, HAdV‐1 and HAdV‐2 showed no obvious seasonal distribution.
3.5. Clinical profile of HAdV infection
For the 447 patients with HAdV infection, 279 patients infected with a single HAdV or infected with HAdV and another respiratory virus were enrolled for further analysis. The other 168 cases tested positive for chlamydophila pneumoniae, mycoplasma pneumoniae, or a typical bacterial infection in addition to HAdV and were excluded to avoid the influence of nonviral factors, such as bacteria, upon comparison of clinical symptoms. The demographic data and clinical manifestations of the 279 patients with HAdV infection are shown in Tables 1 and 2. The mean age of these 279 patients was 20 months old, and most patients were aged 1‐ to 2‐year old (P = 0.013), with a sex ratio of 1.68:1 (male:female). The 279 patients included 237 (85.0%) cases diagnosed with pneumonia, 18 (6.5%) cases diagnosed with bronchiolitis, and 24 (8.6%) cases diagnosed with bronchitis. Fever was one of the most common symptoms after HAdV infection (77.1%, 215 of 279), and 48.4% (135 of 279) of patients had a temperature greater than 39°C. The mean duration of fever was 6 days, and 22.6% of patients had a fever lasting more than 1 week. High fever was observed in 62.2% (56 of 90) of patients infected with HAdV‐7, and 35.6% (32 of 90) of patients manifested a fever lasting more than 1 week. Other clinical manifestations of HAdV‐positive children included cough (93.2%), rales (87.8%), wheezing (38.0%), cyanosis or dyspnea (21.8%), and decreased breath sounds (5.0%). Extrapulmonary manifestations included diarrhea (12.2%), vomiting (9.7%), neurological symptoms (7.2%), and rash (9.0%).
Table 1.
Demographic data and clinical characteristics of hospitalized children with lower respiratory tract infection associated with adenovirus, according to the type
| Characteristics | HAdV‐1, n = 33, % | HAdV‐2, n = 24, % | HAdV‐3, n= 97, % | HAdV‐7, n = 90, % | HAdV‐4, n = 10, % | Other type, n = 25, % | Total, n = 279, % |
|---|---|---|---|---|---|---|---|
| Age, IQR, mo | 24 (8‐35) | 23 (9.2‐37.5) | 20 (8.5‐38.5) | 18.5 (9‐36) | 10 (8‐17) | 11 (8‐20) | 20 (9‐37) |
| Male | 21 (63.6) | 15 (62.5) | 58 (59.7) | 52 (57.8) | 9 (90.0) | 20 (80.0) | 175 (62.7) |
| Most common diagnostic terms | |||||||
| Pneumonia | 28 (84.9) | 15 (62.5) | 83 (85.6) | 80 (88.9) | 9 (90.0) | 22 (88.0) | 237 (85.0) |
| Bronchiolitis | 4 (12.1) | 5 (20.8) | 5 (5.2) | 4 (4.4) | 0 | 0 | 18 (6.5) |
| Bronchitis | 1 (3.0) | 4 (16.7) | 9 (9.3) | 6 (6.7) | 1 (10.0) | 3 (8.0) | 24 (8.6) |
| Underlying disease | 0 | 0 | 5 (5.2) | 5 (5.6) | 0 | 3 (12.0) | 13 (4.7) |
| Outcome LOS, median (IQR), d | 5 (6‐8) | 6 (5‐7.75) | 7 (6‐9.5) | 9 (7‐11) | 6 (6‐7.75) | 8 (6.75‐10) | 7 (6‐10) |
| Coinfection | 22 (66.7) | 19 (79.2) | 54 (55.7) | 56 (62.2) | 2 (20.0) | 23 (92.0) | 175 (62.7) |
| Chest radiopraphic finding: consolidation | 1 (3.0) | 0 | 12 (12.4) | 28 (31.1) | 0 | 3 (12.0) | 44 (15.8) |
| Severe pneumonia | 3 (9.1) | 1 (4.2) | 11 (11.3) | 30 (33.3) | 0 | 4 (16.0) | 49 (17.6) |
| Admission to ICU | 1 (3.0) | 1 (4.2) | 5 (5.2) | 17 (33.3) | 0 | 1 (4.0) | 25 (9.0) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of hospital stay.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Clinical manifestations from hospitalized children infected with lower respiratory tract infection associated with adenovirus, according to the type
| Characteristics | HAdV‐1, n = 33, % | HAdV‐2, n = 24,% | HAdV‐3, n = 97, % | HAdV‐7, n = 90,% | HAdV‐4, n = 10,% | Other types, n = 25, % | Total, n = 279 |
|---|---|---|---|---|---|---|---|
| Fever | 18 (54.6) | 16 (66.7) | 77 (79.4) | 77 (85.6) | 8 (80.0) | 19 (76.0) | 215 (77.1) |
| High fever, temperature >39°C | 7 (21.2) | 9 (37.5) | 44 (45.4) | 56 (62.2) | 6 (60.00) | 13 (52.00) | 135 (48.4) |
| Duration of fever, IQR, d | 3 (1.5‐5) | 4 (3‐6.75) | 5 (4‐7) | 7 (5‐11.5) | 4.5 (25‐5.5) | 6 (3.75‐8.75) | 6 (4‐9) |
| Duration of fever >7 d | 3 (9.1) | 3 (12.5) | 18 (18.6) | 32 (35.6) | 1 (10.0) | 6 (24.0) | 63 (22.9) |
| Cough | 31 (93.9) | 24 (100.0) | 87 (89.7) | 85 (94.4) | 10 (100.0) | 23 (92.0) | 260 (93.2) |
| Wheezing | 14 (42.4) | 7 (29.2) | 37 (38.1) | 36 (40.0) | 1 (10.0) | 11 (44.0) | 106 (38.0) |
| Cyanosis | 0 | 1 (4.2) | 6 (6.2) | 10 (11.1) | 0 | 2 (8.0) | 19 (6.8) |
| Tachypnea | 3 (9.1) | 1 (4.2) | 16 (16.5) | 17 (18.9) | 0 | 5 (20.0) | 42 (15.1) |
| Rales | 29 (87.9) | 20 (83.3) | 80 (82.5) | 84 (93.3) | 10 (100.0) | 22 (88.0) | 245 (87.8) |
| Decreased breath sounds | 0 | 0 | 6 (6.2) | 8 (9.0) | 0 | 0 | 14 (5.0) |
| Extrapulmonary manifestations | 0 | ||||||
| Diarrhea | 1 (3.0) | 4 (16.7) | 11 (11.3) | 17 (18.9) | 0 | 1 (4.0) | 34 (12.2) |
| Vomiting | 0 | 1 (4.2) | 11 (11.3) | 14 (15.6) | 1 (10.00) | 0 | 27 (9.7) |
| Rash | 1 (3.0) | 3 (12.5) | 9 (9.3) | 12 (13.3) | 0 | 0 | 25 (9.0) |
| Seizure | 1 (3.0) | 2 (8.3) | 2 (2.1) | 1 (1.1) | 0 | 0 | 6 (2.2) |
| Mental alteration | 0 | 0 | 3 (3.1) | 11 (12.2) | 0 | 0 | 14 (5.0) |
Abbreviations: HAdV, human adenovirus; IQR, interquartile range.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Of the 279 HAdV‐infected patients, 106 (38.0%) had a single HAdV infection and 173 (62.0%) were coinfected with more than one virus. The dominant respiratory viruses found to be coinfected with HAdV were RSV, HRV, HBoV, and PIV3. We compared the clinical characteristics between the single HAdV infected group and HAdV coinfected group. Patients infected with HAdV alone (n = 106) were significantly older than patients coinfected with HAdV and another virus (n = 173 and P = 0.01). The coinfection rate (44.5%) was higher in children aged 6 to 24 months old compared with children of other age groups. Indeed, in our study, a longer hospital stay occurred more often in children in the HAdV virus coinfected group (P = 0.023). No significant difference was found in clinical manifestations except wheezing when comparing patients with a single HAdV‐virus infection and patients with a HAdV coinfection. Variables including age, wheezing, intensive care unit (ICU) admission, supplemental oxygen requirement, severe pneumonia, and the length of hospital stay (LOS; required ≥ 7 days) were entered into the multivariate analyses, and wheezing and duration of hospitalization greater than or equal to 7 days were associated with HAdV coinfection (odds ratio [OR], 1.82; 95% confidence interval [CI], 1.063‐3.116; P = 0.029 and OR, 1.98; 95% CI, 1.179‐3.337; P = 0.01, respectively).
The demographic and clinical profiles of patients with a single HAdV infection are shown in Tables 3 and 4, which indicate that the predominant types of HAdV in single viral infections were HAdV‐7 (34 cases) and HAdV‐3 (43 cases). In our study, children with infections of HAdV‐7 alone and HAdV‐3 alone did not significantly differ with regard to age, sex, fever, presence or absence of underlying diseases, average duration of fever, cough, wheezing, rales, tachypnea, rash, vomiting, and diarrhea (Table 3). However, infection with HAdV‐7 alone was associated with more severe clinical outcomes than infection with HAdV3 alone. Of the 34 patients infected with the single HAdV‐7, 32.4% (11 of 34 patients) were diagnosed with severe pneumonia, which was significantly higher than the 11.6% (5 of 43 patients) of patients infected with the single HAdV‐3 (P = 0.031; Table 4). Pneumonia consolidation and admission to the ICU were more common in patients with a single HAdV‐7 infection (P = 0.013 and 0.019, respectively; Table 4). HAdV‐7 infection resulted in patients having a longer hospital stay compared with that of patients infected with HAdV‐3 (P = 0.03; Table 4).
Table 3.
Clinical manifestations from hospitalized children infected with single HAdV‐3 and HAdV‐7, single and coinfection
| Clinical symptoms and signs | Single infection, n = 106, % | Coinfection, n = 173, % | P | Single HAdV‐3, n = 43, % | Single HAdV‐7, n = 34, % | P |
|---|---|---|---|---|---|---|
| Fever | 85 (80.2) | 130 (75.1) | 0.331 | 36 (83.7) | 30 (88.2) | 0.815 |
| High fever | 51 (48.1) | 84 (48.6) | 0.943 | 22 (12.7) | 21 (61.8) | 0.352 |
| Duration of fever >7 d | 21 (19.8) | 41 (23.7) | 0.448 | 8 (18.6) | 10 (29.4) | 0.266 |
| Duration of fever, IQR, d | 6 (4‐7.5) | 6 (3‐10) | 0.8 | 6 (4.25‐7.75) | 7 (5‐8.25) | 0.18 |
| Cough | 98 (92.5) | 162 (93.6) | 0.702 | 39 (90.7) | 31 (91.2) | 1 |
| Wheezing | 30 (28.3) | 76 (43.9) | 0.009 | 10 (23.3) | 11 (32.4) | 0.373 |
| Rales | 94 (88.7) | 151 (87.3) | 0.729 | 39 (90.7) | 32 (94.1) | 0.689 |
| Tachypnea | 16 (15.1) | 30 (17.3) | 0.624 | 6 (14.0) | 7 (20.6) | 0.44 |
| Cyanosis | 6 (5.7) | 13 (7.5) | 0.551 | 3 (7.0) | 3 (8.8) | 1 |
| Decreased breath sounds | 6 (5.7) | 8 (4.6) | 0.7 | 1 (2.3) | 2 (5.9) | 0.835 |
| Rash | 12 (11.3) | 13 (7.5) | 0.28 | 5 (11.6) | 4 (11.8) | 1 |
| Diarrhea | 12 (11.3) | 22 (12.7) | 0.729 | 5 (11.6) | 8 (23.5) | 0.166 |
| Vomiting | 12 (11.3) | 15 (8.7) | 0.467 | 3 (7.00) | 6 (17.7) | 0.276 |
| Seizure | 3 (2.8) | 3 (1.7) | 0.677 | 1 (2.3) | 0 | N |
| Mental alteration | 4 (3.8) | 10 (5.8) | 0.456 | 0 | 5 (14.7) | N |
Abbreviations: IQR, interquartile range; N, not applicable.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 4.
Demographic data of and outcome from hospitalized children infected with single HAdV‐3 infection and HAdV‐7 infection, single infection and coinfection
| Single infection, n = 106, % | Coinfection, n = 173, % | P | Single HAdV‐3,n = 43, % | Single HAdV‐7,n = 34, % | P | |
|---|---|---|---|---|---|---|
| Age, median (IQR), mo | 27.5 (12‐41) | 17 (9‐32.5) | 0.01 | 36 (13‐41) | 23.5 (8.75‐36) | 0.29 |
| <6 | 14 (13.2) | 26 (15.0) | 7 (16.3) | 3 (8.8) | ||
| 6‐12 | 11 (10.4) | 36 (20.8) | 3 (6.98) | 7 (20.6) | ||
| 13‐24 | 21 (19.8) | 41 (23.7) | 8 (18.6) | 7 (20.6) | ||
| 25‐36 | 13 (12.3) | 30 (17.3) | 3 (7.0) | 6 (17.7) | ||
| 37‐48 | 30 (28.3) | 15 (8.7) | 17 (39.5) | 8 (23.5) | ||
| 49‐60 | 8 (7.6) | 15 (8.7) | 1 (2.3) | 1 (2.9) | ||
| >60 | 9 (8.5) | 10 (5.8) | 0.007 | 4 (9.3) | 2 (5.9) | 0.47 |
| Male | 64 (60.4) | 112 (64.7) | 0.464 | 24 (55.8) | 20 (58.8) | 0.791 |
| Underlying disease | 6 (5.7) | 7 (4.1) | 0.361 | 3 (7.0) | 3 (8.8) | 1 |
| Lobe consolidation | 18 (17.0) | 26 (15.0) | 0.664 | 5 (11.6) | 12 (35.3) | 0.013 |
| Supplemental oxygen | 13 (12.3) | 31 (17.9) | 0.208 | 6 (14.0) | 9 (26.5) | 0.168 |
| Severe pneumonia | 12 (11.3) | 35 (20.2) | 0.054 | 5 (11.6) | 11 (32.4) | 0.031 |
| Admission to ICU | 6 (5.7) | 19 (11.0) | 0.131 | 3 (7.0) | 9 (26.5) | 0.019 |
| LOS, median (IQR), d | 7 (6‐9) | 8 (6‐10) | 0.023 | 7 (6‐8) | 9 (6.75‐11) | 0.03 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of hospital stay.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.6. Other respiratory viruses
The other respiratory viruses detected in patients infected with HAdV are shown in Figure 2. There were 283 (63.3%) patients coinfected with other types of viruses among the 447 HAdV‐positive patients. RSV and PIV3 were the most common concomitant infections with HAdV infection, occurring in 115 and 90 patients, respectively. Infections with HBoV (n = 85), HRV (n = 76), HMPV (n = 28), PIV1 (n = 13), IFVB (n = 14), HCoV‐HKU1 (n = 6), IFVA (n = 4), PIV2 (n = 4), and NL63 (n = 6) were also found in patients.
Figure 2.

Positive rate of 12 respiratory tract viruses in 4751 pediatric patients diagnosed with ALRTI in Hunan between September 2007 and March 2014. ALRTI, acute lower respiratory tract infections; HAdV, human adenovirus; HBoV, human bocavirus; HMPV, human metapneumovirus; HRV, human rhinovirus; IFVA, influenza virus; PIV1, parainfluenza virus type 1; RSV, respiratory syncytial virus
4. DISCUSSION
HAdV causes severe LRTIs in children and may lead to epidemic outbreaks. In this 6.5‐year epidemiological study of HAdV in NPA obtained from children hospitalized for ALRTIs, the detection rate of HAdV was 9.4%, which was similar to that of a previous report (4%‐10% for HAdV infections of LRTI cases).1, 2 During the study period, the HAdV infection rate was highest from September 2010 to August 2011. Interestingly, HAdV outbreaks were reported in 2011 in Hangzhou (China)30 and in 2011 in Taiwan.31
Age is a known risk factor for LRTIs caused by HAdV. Most studies have indicated that HAdV is the major pathogen that causes LRTIs in children aged 6 months to 5 years old.32 Severe LRTIs often occur in infants aged 6 to 24 months old. Children less than 6 months old often show immunity to HAdV because of maternal antibodies.3, 5, 6, 16, 20 This study also indicated that the majority of HAdV infections occurred in patients from 6 months to 5 years old, whereas the HAdV detection rate was higher in children aged 3 to 4 years. The reason for this difference may be the close contact of kindergarteners between the ages of 3 to 4 years. However, HAdV infection was most common in children aged 0 to 12 months old in Southern Palestine33 and 6 to 23 months old in Korea.6
HAdV infection was sporadically found throughout the year, and its incidence was relatively higher in winter and spring.6, 16, 20 However, in the current study, the highest HAdV detection rate was in summer, which suggested that HAdV infection in children manifested seasonal and geographic variation. This study did not show any statistically significant difference in the sex distribution of HAdV infection, in contrast to previous reports that suggested that HAdV infection was more frequent in males.23
In this study, the 14 HAdV types were not evenly distributed during the study period. The distribution of HAdV types attained epidemic proportions, lasting 2 or 3 years in each cycle.6, 34, 35, 36 HAdV‐3 and HAdV‐7 infections were the major prevalent HAdV infections among hospitalized children in Hunan (Southern China), similar to reports from Korea,6 Argentina,34 and Chongqing (China),15, 16 but in contrast to reports from Malaysia,37 Israel,38 and Hong Kong.5 In the current study, HAdV‐3 infection epidemics were sustained for three years (2008‐2010). The significant increase in HAdV‐7 infection between 2010 and 2011 represents an undetected outbreak in local children, which is consistent with the reported outbreaks in Chongqing (China).15, 16 However, in provinces of mainland China, such as Hangzhou30 and Jiangsu,35 most respiratory disease outbreaks were associated with HAdV‐3 infection. From 2012 to 2014, HAdV‐7 was the dominant HAdV infection type in Hunan. HAdV‐3 and HAdV‐7 infections result in severe pneumonia, and HAdV‐7 infection is found in children at a young age and results in epidemic outbreaks with high mortality and severe sequelae.6, 17, 18 Studies from the United States (2004‐2006) suggest that HAdV‐7 infection has not been implicated to have a strong association with severe morbidity possibly because of a stabilization of circulating HAdV‐7 genotypes in the United States, as was recently apparent in Iowa for the adenovirus type 7d2 strain, with subsequent increases in herd immunity and reductions in HAdV‐7 infections.23 A Korean (1997‐2007) report suggested that the intensity of outbreaks caused by HAdV‐7 decreased markedly and did not circulate endemically. Because the HAdV‐7 genome types were relatively limited in Korea, stabilization of the circulating HAdV‐7 genome types and the subsequent increase in herd immunity might have resulted in the decline of HAdV‐7 activity in Korea.39 By contrast, HAdV‐7 infection is a major cause of LRTIs in children in China.32 There was a high frequency of infection of HAdV‐7 and HAdV‐3 in pediatric pneumonia in Chongqing and Beijing, and HAdV‐7 infection was associated with a higher incidence of severe pneumonia,15, 40 which reinforces the findings of the current study.
HAdV‐4 and HAdV‐14 were the most common HAdV types associated with fever and respiratory infection among US military recruits, whereas they were uncommon in children with ALRTIs.23 In Chongqing (China), 3089 samples were collected from hospitalized children with pneumonia from June 2009 to May 2014, of which 208 samples were positive for HAdV and only one sample was positive for HAdV‐4 and HAdV‐14.15, 16 In the current study, 13 patients were infected with HAdV‐4 and 6 patients were infected HAdV‐14, which accounted for 2.9% and 1.3% of all patients infected with HAdVs, respectively. Cases of infections with HAdV‐21, HAdV‐37, HAdV‐57, and HAdV‐41 were rarely observed in children,39 which was consistent with the current study.
Gene recombination plays an important role in the molecular evolution of HAdV and can lead to a novel type with epidemic outbreaks or dominant substitutions, such as HAdV‐55. HAdV‐55 is an intertypic recombinant virus of HAdV‐11 and HAdV‐14 and was recently described by complete genomic sequencing and designated as a new genotype after bioinformatics analysis.22 We also found that HAdV‐55 exists and circulates in children. With only six confirmed HAdV‐55 cases, it is difficult to analyze its clinical effect. The long‐term surveillance of specific types of HAdV that can cause human infections is important, especially for newly identified genotypes, as an exploration of their clinical effect is much needed.
The most common clinical ALRTI diagnosis was pneumonia (85.0%), followed by bronchitis and bronchiolitis. Most patients showed mild symptoms with good prognosis. Fever and cough were the most common manifestations of HAdV infection, which was consistent with previous studies.6 HAdV‐7 infection was associated with a higher fatality rate than HAdV‐3 infection, which was consistent with previous reports.17, 18, 19, 20 HAdV infection, particularly HAdV‐7 infection, was strongly associated with severe morbidity, including required supplemental oxygen, an increased ICU stay, and a relatively longer hospitalization.6, 15, 16, 17, 18 In this surveillance study, patients with HAdV‐7 infection alone presented more severe pneumonia, relatively longer hospital stay, and more frequent transfer to the ICU compared with those in patients with HAdV‐3 infection alone (all P < 0.05).
The rate of coinfection by HAdV and other respiratory viruses is high in patients with HAdV infection.41, 42 We found that the rate of coinfection of HAdV and other respiratory viruses was 62.0% (173 of 279), which was similar to a report from Israel.41 Comparative studies revealed similar epidemiological and clinical features among patients infected with HAdV and those coinfected with HAdV and another respiratory virus. Similar to our findings, Foulongne et al reported that the hospital stay increased in hMPV/RSV coinfected children compared with those infected by one type of virus.43 However, some studies have reported that there are no clinical differences between patients with respiratory infections caused by one type of agent and those caused by multiple viruses detected in NPAs from hospitalized children.44, 45 From an empirical point of view, it might be logical to expect that infection with two or more types of viruses could aggravate the severity of illness; we believe that studies should focus on dual versus multiple infections as the different characteristics of the virus can cause a bias when analyzing the results. One report has indicated that the detection of viral load in respiratory specimens may be helpful for possible progress in identifying the true respiratory pathogen and may serve as an important clue to determine the virus that may have a greater influence on the clinical severity of the illness in a mixed infection.46 As a result, further studies are needed.
This study has several limitations. First, it was a single‐center study and the results may not be generalized to other settings. Second, our experiments did not determine the severity of other confounding factors, such as virus combinations, that could affect outcomes. Further, this study may also suffer from a selection bias as it did not include patients who consulted outpatient departments. Additional multicenter studies are needed to establish the epidemiology of HAdV.
In conclusion, this study revealed the HAdV types and epidemiology of HAdV among hospitalized children in Hunan (China) from September 2007 to March 2014. Further HAdV type analysis is essential as different HAdV types result in different diseases with possible epidemic outbreak potential. Analysis of the clinical data of children infected with different HAdV types may contribute to early diagnosis and treatment of adenovirus infection.
5. SUMMARY
The incidence of adenovirus infection presented a yearly increase during the study period. The predominant type, HAdV‐7, was associated with serious clinical implications compared with the other types.
CONFLICTS OF INTEREST
The authors declared that there are no conflicts of interest.
Supporting information
Supporting information
Supporting information
Supporting information
Supplemental Table 1. Distribution of different HAdV types among 447 children hospitalized for adenovirus infection between September 2007 and March 2014
Supporting information
ACKNOWLEDGMENTS
This study was funded by the China National Center for Disease Control and Prevention and Hunan Provincial Natural Science Foundation of China (07JJ5055) and National Special Fund for the Ministry of Health (201202010).
Xie L, Zhang B, Xiao N, et al. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J Med Virol. 2019;91:392–400. 10.1002/jmv.25333
References
REFERENCES
- 1. Chen HL, Chiou SS, Hsiao HP, et al. Respiratory adenoviral infections in children: a study of hospitalized cases in southern Taiwan in 2001–2002. J Trop Pediatr. 2004;50(5):279‐284. [DOI] [PubMed] [Google Scholar]
- 2. Yun BY, Kim MR, Park JY, Choi EH, Lee HJ, Yun CK. Viral etiology and epidemiology of acute lower respiratory tract infections in Korean children. Pediatr Infect Dis J. 1995;14(12):1054‐1059. [DOI] [PubMed] [Google Scholar]
- 3. Kwon HJ, Rhie YJ, Seo WH, et al. Clinical manifestations of respiratory adenoviral infection among hospitalized children in Korea. Pediatr Int. 2013;55(4):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun Q, Jiang W, Chen Z, et al. Epidemiology and clinical features of respiratory adenoviral infections in children. Eur J Pediatr. 2014;173(4):441‐444. [DOI] [PubMed] [Google Scholar]
- 5. Chau S‐K, Lee S, Peiris MJS, et al. Adenovirus respiratory infection in hospitalized children in Hong Kong: serotype–clinical syndrome association and risk factors for lower respiratory tract infection. Eur J Pediatr. 2014;173(3):291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong JY, Lee HJ, Piedra PA, et al. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32(10):1423‐1429. [DOI] [PubMed] [Google Scholar]
- 7. Murtagh P, Giubergia V, Viale D, Bauer G, Pena HG. Lower respiratory infections by adenovirus in children: Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr Pulmonol. 2009;44(5):450‐456. [DOI] [PubMed] [Google Scholar]
- 8. Gerd liebert U, Bergs S, Ganzenmueller T, Hage E, Heim A. Human mastadenovirus type 70: a novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J Gen Virol. 2015;96(9):2734‐2742. [DOI] [PubMed] [Google Scholar]
- 9. Iaconelli M, Valdazogonzález B, Equestre M, et al. Molecular characterization of human adenoviruses in urban wastewaters using next generation and Sanger sequencing. Water Res. 2017;121:240‐247. [DOI] [PubMed] [Google Scholar]
- 10. Liu EB, Ferreyra L, Fischer SL, et al. Genetic analysis of a novel human adenovirus with a serologically unique hexon and a recombinant fiber gene. PLOS One. 2011;6(9):e24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsushima Y, Shimizu H, Kano A, et al. Genome sequence of a novel virus of the species human adenovirus d associated with acute gastroenteritis. Genome Announc. 2013;1(1):e00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsushima Y, Shimizu H, Kano A, et al. Novel human adenovirus strain, Bangladesh. Emerging Infect Dis. 2012;18(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh MP, Seto J, Liu EB, et al. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J Clin Microbiol. 2011;49(10):3482‐3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshitomi H, Sera N, Gonzalez G, Hanaoka N, Fujimoto T. First isolation of a new type of human adenovirus (genotype 79), species Human mastadenovirus B (B2) from sewage water in Japan. J Med Virol. 2017;89(7):1192‐1200. [DOI] [PubMed] [Google Scholar]
- 15. Callaway Z, Kim SH, Kim JY, Kim DW, Kim CK. Adenovirus infection with serious pulmonary sequelae in Korean children. Clin Respir J. 2011;5(2):92‐98. [DOI] [PubMed] [Google Scholar]
- 16. Wo Y, Lu QB, Huang DD, et al. Epidemical features of HAdV‐3 and HAdV‐7 in pediatric pneumonia in Chongqing, China. Arch Virol. 2015;160(3):633‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Q, Zheng Q, Liu Y, Wadell G. Molecular epidemiology of adenovirus types 3 and 7 isolated from children with pneumonia in Beijing. J Med Virol. 1996;49(3):170‐177. [DOI] [PubMed] [Google Scholar]
- 18. Zhang ZJ, Wang ZL, Cao YP, et al. Acute respiratory infections in childhood in Beijing: An etiological study of pneumonia and bronchiolitis. Chin Med J. 1986;99(9):695‐702. [PubMed] [Google Scholar]
- 19. Tang L, Wang L, Tan X, Xu W. Adenovirus serotype 7 associated with a severe lower respiratory tract disease outbreak in infants in Shaanxi Province, China. Virol J. 2011;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao JMWZ. Adenovirus analysis in pediatric pneumonia of Beijing area during the year of 1964‐1967 and 1974‐1977. Chin J Pediatr. 1980;18:149. [Google Scholar]
- 21. Li X, Kong M, Su X, et al. An outbreak of acute respiratory disease in China caused by human adenovirus type B55 in a physical training facility. Int J Infect Dis. 2014;28:117‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu QB, Tong YG, Wo Y, et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009‐2012. Influenza Other Respir Viruses. 2014;8(3):302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gray GC, Mccarthy T, Lebeck MG, et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004‐2006. Clin Infect Dis. 2007;45(9):1120‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu X, Erdman DD. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch Virol. 2006;151(8):1587‐1602. [DOI] [PubMed] [Google Scholar]
- 25. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljunglindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891‐12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bellau‐Pujol S, Vabret A, Legrand L, et al. Development of three multiplex RT‐PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126(1‐2):53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real‐time reverse‐transcription polymerase chain reaction assays. J Infect Dis. 2007;196(9):1321‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kantola K, Sadeghi M, Antikainen J, et al. Real‐time quantitative PCR detection of four human bocaviruses. J Clin Microbiol. 2010;48(11):4044‐4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinberg GA, Schnabel KC, Erdman DD, et al. Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol. 2013;57(3):254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie L, Yu XF, Sun Z, et al. Two adenovirus serotype 3 outbreaks associated with febrile respiratory disease and pharyngoconjunctival fever in children under 15 years of age in Hangzhou, China, during 2011. J Clin Microbiol. 2012;50(6):1879‐1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsou TP, Tan BF, Chang HY, et al. Community outbreak of Adenovirus, Taiwan, 2011. Emerg Infect Dis. 2012;18(11):1825‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu C, Xiao Y, Zhang J, et al. Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007 to 2012. BMC Infect Dis. 2015;15(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qurei L, Seto D, Salah Z, Azzeh M. A molecular epidemiology survey of respiratory adenoviruses circulating in children residing in Southern Palestine. PLOS One. 2012;7(8):e42732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barrero PR, Valinotto LE, Tittarelli E, Mistchenko AS. Molecular typing of adenoviruses in pediatric respiratory infections in Buenos Aires, Argentina (1999‐2010). J Clin Virol. 2012;53(2):145‐150. [DOI] [PubMed] [Google Scholar]
- 35. Lu MP, Ma LY, Zheng Q, Dong LL, Chen ZM. Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J Pediatr. 2013;9(4):346‐349. [DOI] [PubMed] [Google Scholar]
- 36. Sutton RNP, Pullen HM, Blackledge P, Brown EH, Sinclair L, Swift PN. Adenovirus type 7; 1971‐74. Lancet. 1976;2(7993):987‐991. [DOI] [PubMed] [Google Scholar]
- 37. Abd‐Jamil J, Teoh BT, Hassan EH, Roslan N, Abubakar S. Molecular identification of adenovirus causing respiratory tract infection in pediatric patients at the University of Malaya Medical Center. BMC Pediatr. 2010;10(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mandelboim M, Dror P, Azar R, Bromberg M, Mendelson E. Adenovirus infections in hospitalized patients in Israel: epidemiology and molecular characterization. J Clin Microbiol. 2011;49(2):597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee J, Choi EH, Lee HJ. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991‐2007). J Med Virol. 2010;82(4):624‐631. [DOI] [PubMed] [Google Scholar]
- 40. Deng J, Qian Y, Zhao LQ, et al. [Identification and typing of adenoviruses from pediatric patients with acute respiratory infections in Beijing from 2003 to 2008]. Zhonghua Er Ke Za Zhi. 2010;48(10):739‐743. [PubMed] [Google Scholar]
- 41. Hindiyeh MY, Keller N, Mandelboim M, et al. High rate of human bocavirus and adenovirus coinfection in hospitalized Israeli children. J Clin Microbiol. 2008;46(1):334‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin Y, Zhang R, Xie Z, et al. Prevalence of adenovirus in children with acute respiratory tract infection in Lanzhou, China. Virol J. 2013;10(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foulongne V, Guyon G, Rodière M, et al. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25(4):354‐359. [DOI] [PubMed] [Google Scholar]
- 44. Scotta MC, Chakr VCBG, De moura A, et al. Respiratory viral coinfection and disease severity in children: a systematic review and meta‐analysis. J Clin Virol. 2016;80:45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brand HK, De Groot R, Galama JMD, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47:393‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emily TM, Jane K, Anna W, Englund JA. Multiple versus single virus respiratory infectiongs:viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012;6(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supplemental Table 1. Distribution of different HAdV types among 447 children hospitalized for adenovirus infection between September 2007 and March 2014
Supporting information


