Abstract
Introduction
Contrast-induced acute kidney injury (CI-AKI) is associated with high risks of morbidity and mortality. Hyperbilirubinemia might have some renal protection but with no clear cutoff value for protection. Related studies are typically on limited numbers of patients and only in conditions of vascular intervention.
Methods
We performed this study to elucidate CI-AKI in patients after contrast-enhanced computed tomography (CCT). The outcomes were CI-AKI, dialysis and mortality. Patients were divided to three groups based on their serum levels of total bilirubin: ≤1.2 mg/dl, 1.3–2.0 mg/dl, and >2.0 mg/dl.
Results
We enrolled a total of 9,496 patients who had received CCT. Patients with serum total bilirubin >2.0 mg/dl were associated with CI-AKI. Those undergoing dialysis had the highest incidence of PC-AKI (p<0.001). No difference was found between the two groups of total bilirubin ≤1.2 and 1.3–2.0 mg/dl. Patients with total bilirubin >2mg/dl were associated with CI-AKI (OR = 1.89, 1.53–2.33 of 95% CI), dialysis (OR = 1.40, 1.01–1.95 of 95% CI) and mortality (OR = 1.63, 1.38–1.93 of 95% CI) after adjusting for laboratory data and all comorbidities (i.e., cerebrovascular disease, coronary artery disease, peripheral arterial disease, and acute myocardial infarction, diabetes mellitus, hypertension, gastrointestinal bleeding, cirrhosis, peritonitis, ascites, hepatoma, shock lung and colon cancer). We concluded that total bilirubin level >2 mg/dl is an independent risk factor for CI-AKI, dialysis and mortality after CCT. These patients also had high risks for cirrhosis or hepatoma.
Conclusion
This is the first study providing evidence that hyperbilirubinemia (total bilirubin >2.0 mg/dl) being an independent risk factor for CI-AKI, dialysis and mortality after receiving CCT. Most patients with total bilirubin >2.0mg/dl had cirrhosis or hepatoma.
Introduction
The nephrotoxicity of iodinated contrast media is well-known, and that is also the major cause of acute kidney injury (AKI). Formerly called contrast-induced nephropathy, it has now been called contrast-induced AKI (CI-AKI). The incidence of CI-AKI is high, with 12 to 50% morbidity [1–4]. Despite of its nephrotoxicity, the iodine-containing contrast medium is required to obtain good quality images. Therefore, its nephrotoxicity seems inevitable in clinical practice. Identified risk factors for CI-AKI are the following: impaired baseline renal function [5, 6], type and dose of contrast material [5, 7], conditions associated with reduced renal perfusion (such was heart failure [8], medications (angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blockers (ARB), non-steroidal anti-inflammatory drug (NSAID), diuretics, and metformin [9]), volume depletion like diarrhea or vomiting and sepsis. Despite of such knowledge on risk factors and the preventive measure with volume expansion, the incidence of CI-AKI remains high. This indicates other undiscovered risk factors for CI-AKI.
Bile acid or bilirubin with low water solubility can cause cast formation within the low pH microenvironment of distal nephrons. Bile cast cholemic nephrosis or bile nephrosis has been reported since 1953 [10]. Later related studies were done in rabbits 1957 [11], on tubular injury in 1958 [12], and on autopsy [13]. Since 2000, only a few studies have been reported on renal toxicity related to bile acid or bilirubin [14–16]. Bilirubin is an endogenous circulating antioxidant with protective role on kidney damages [17]. Also, bilirubin has ability anti-inflammatory, complete inhibitory and lipid-lowering properties[18]. Patients with Gilbert’s syndrome may experience mild jaundice and the condition was recently shown to reduce all-cause mortality by half [19]. Hypobilirubinemia was reported in 2012 to be a possible risk factor for end-stage kidney disease (ESRD), independent of the estimated glomerular filtration rate (eGFR) [20]. A recent study also supported the protective role of hyperbilirubinemia on renal functions (0.5±0.2 vs. 0.7±0.3 mg/dl, p<0.001) [21]. On the other hand, contradicting results were reported in another study as detailed below [22]. In that observational large hospital-based study of 2,678 adult outpatients, the total bilirubin was found to be inversely associated with eGFR in both non-diabetic (r = -0.17; p < 0.0001) and diabetic patients (r = -0.14; p < 0.05). Some issues remain controversial between serum bilirubin and renal function. First, there is no consensus regarding serum bilirubin in terms of its nephrotoxicity or renal protective role. Second, even if it is true for renal protection or nephrotoxicity, their effective cutoff values of hyperbilirubinemia remain unclear. Third, the effect of hyperbilirubinemia on CI-AKI was reported only in patients undergoing diagnostic angiography [23] or in coronary intervention [21] [24]. In the above clinical scenarios, the conditions are relatively simple. No studies have been done specifically on the relationship between serum bilirubin levels and CI-AKI after contrast-enhanced computed tomography (CCT). Fourth, the contrast volume, a possible risk factor for CI-AKI was not constant across studies. Finally, case numbers are not sufficiently large enough for adjusting confounding factors. Therefore, in the study, we determined the association between serum bilirubin and renal function after CCT (at a fixed volume of contrast volume, 100ml).
Methods and materials
Study design and patient population
We used in our hospital (Taichung Veterans General Hospital) a historical cohort that consisted data of 20,018 non-dialytic adult patients who had received the non-ionic iso-osmolar contrast medium, iodixanol (Visipaque, Chicago, IL, USA), for enhanced CT imaging during an approximately 15-year period (June 1, 2008 to March 31, 2015). The data recorded for each patient included a baseline serum level of creatinine and total bilirubin within two days before CCT. They were used to evaluate the association between serum bilirubin and renal outcome after CCT. Exclusion criteria were those with pre-existing AKI (defined according to KDIGO practice guideline[25]), with recent exposures to contrast media over the previous 30 days, volume of contrast medium not being to 100 ml (regular contrast volume for CCT), baseline serum levels of total bilirubin and creatinine within the two days before CCT not available, and post-contrast serum creatinine within one week after CCT not available.
Our study was approved by the institute review board of Taichung Veterans General Hospital approved this study (IRB TCVGH No:F15059). Patient informed consent was waived due to the pure data analysis nature of the study. There was no formal protocol for the prevention of contrast-induced nephropathy at this hospital over the study period.
Baseline data retrieval and definition
The baseline stages of chronic kidney diseases (CKD) was calculated using the equation of modification of diet in renal disease (MDRD) equation [26]: eGFR (ml/min per 1.73 m2) = 186*SCr-1.154 *Age-0.203*0.742 (if female). Medical records of patients were screened for all comorbid conditions: such as, cardiovascular and cerebrovascular disease (cerebrovascular attack, coronary artery disease, peripheral arterial disease, and acute myocardial infarction), metabolic disease (diabetes mellitus, and hypertension), gastrointestinal bleeding, liver disease (cirrhosis, peritonitis, ascites and hepatoma), shock and malignancy (lung and colon cancer). Medications screened were ACEi, ARB, NSAID, aspirin, aminoglycoside, loop diuretics, steroid, statin, and H2-blocker (ranitidine and famotidine).
Outcome data retrieval and outcome definitions
Primary outcome, PC-AKI, was defined according to the KDIGO practice guideline[25]: absolute increase of serum creatinine levels ≥0.3 mg/dl from baseline within 48 h, or ≥ 50% within 7 days after CCT [4]. Considering that urine volume had not been regularly collected, we therefore did not include the criterion of urine volume for AKI, as that was in the guideline of KDIGO. The secondary endpoint was the need of emergent hemodialysis within 30 days after CCT (as identified by the first recorded procedure of hemodialysis within 30 days after CCT). All the indication and timing for urgent hemodialysis for patients were the same as described below: refractory fluid overload even with diuretics, severe hyperkalemia (>6.5 meq/L) even after medication, >100 mg/dl of blood urea nitrogen, >6 mg/dl of serum creatinine, metabolic acidosis (<7.2), uremic encephalopathy, uremic bleeding and uremic pericarditis.
Patients were divided to three groups according to serum levels of total bilirubin: ≤1.2 mg/dl, 1.3~2.0 mg/dl, and >2.0 mg/dl. The first cutoff value was 1.2 mg/dl which just exceeded the normal range. Also, the Youden index was used to determine the cutoff value to predict AKI was set to >1.2 mg/dl of total bilirubin within 30 days after CCT (S1 Fig). The other cutoff value of total bilirubin> 2.0 mg/dl followed the Child–Pugh classification[27]. The incidence of AKI in patients with total bilirubin > 2.0 mg/dl was also classified into different three liver conditions (cirrhosis, hepatoma and no cirrhosis or hepatoma) in S2 Table. Furthermore, the incidence of AKI in patients without any liver conditions was classified according to serum levels of total bilirubin in S3 Table.
Statistical analyses
Quantitative data were expressed as mean ± standard deviation. Nominal and categorical variables were compared using the Chi-square likelihood ratio or Fisher exact test with bonferroni post-hoc analyses to detect differences between data pairs. Continuous variables were compared using the nonparametric Wilcoxon test. The stepwise multivariate logistic regression analysis was used to examine the independent association of PC-AKI with patient-related characteristics and comorbidities. In model 1, we adjusted for all comorbidities. They included cerebrovascular disease, coronary artery disease, peripheral arterial disease, and acute myocardial infarction, diabetes mellitus, hypertension, gastrointestinal bleeding, cirrhosis, peritonitis, ascites, hepatoma, shock lung and colon cancer. In model 2, in addition to adjusting for the above comorbidities, we further adjusted for the following: stage of CKD, hemoglobin, serum sodium, serum potassium, prothrombin time, international normalized ratio, the usage of aspirin, aminoglycoside, loop diuretics, ACEi, ARB, NSAID, and the use of fluid replacement >1 liter on the day of CCT. The associations between serum bilirubin level (>2.0 mg/dl) and the characteristics and risks of PC-AKI, and dialysis within 30 days after CCT were calculated by odds ratio (OR) and 95% confidence interval (CI). A two-sided p value of <0.05 represented statistical significance. The SPSS software (Statistical Package for the Social Science, version 20.0, Armonk, NY, USA) was used for statistical analyses.
Results
Initially, we recruited a total 20,018 who received CCT patients for this study. After exclusion, a total of 9,496 patients of these patients without missing data were enrolled in the final study cohort (Fig 1). Their baseline characteristics are list in Table 1, according to three categories based on serum levels of total bilirubin (ie. ≤1.2, 1.3~2.0, and >2.0 mg/dl). Patients with higher levels of total bilirubin had the following characteristics: older (p = 0.012), more males (p<0.001), lower serum albumin levels (p<0.001), more with hyponatremia (p<0.001), longer prothrombin time (p<0.001), more metabolic acidosis (p<0.001), fewer cerebrovascular attacks (p<0.001), more cirrhosis (p<0.001), more hepatoma (p<0.001), fewer lung cancer (p<0.001), more shocks (p<0.001), more peritonitis (p<0.001), more ascites (p<0.001), and more gastrointestinal bleeding (p<0.001). Patients with higher total bilirubin also received the medications as follow: less NSAIDs (p<0.001), more aspirin (p<0.001), more aminoglycosides (p<0.001), more loop diuretics (p<0.001), less ARB (p = 0.003), less steroid (p<0.001), less statin (p<0.001). They also received more fluid replacement (>1 liter) (p = 0.009). Higher total bilirubin was associated with more PC-AKI (p<0.001) and more incidence of urgent dialysis (p<0.001).
Fig 1. Algorithm of patient selection.
Table 1. Baseline characteristics of patients.
| Total bilirubin | ≤1.2 (mg/dl) | 1.3–2.0 (mg/dl) | >2.0 (mg/dl) | All | P value |
|---|---|---|---|---|---|
| Numbers | 7173 | 995 | 1368 | 9496 | |
| Age (years) | 64.63±16.31 | 62.62±16.66 | 65.88±15.89 | 64.91±16.29 | 0.012 |
| ≧65 years | 3783 (52.7%) | 527 (55.2%) | 765 (55.9%) | 5075 (53.4%) | 0.051 |
| Female | 2818 (39.3%) | 301(31.5%) | 415(30.3%) | 3534 (37.2%) | <0.001 |
| Stages of CKD | <0.001 | ||||
| 1 | 2730 (38.1%) | 350 (36.6%) | 541 (39.5%) | 3621 (38.1%) | |
| 2 | 2292 (32.0%) | 306 (32.0%) | 371 (27.1%) | 2969 (31.3%) | |
| 3a | 866 (12.1%) | 140 (14.7%) | 176 (12.9) | 1182 (12.4%) | |
| 3b | 651 (9.1%) | 91 (9.5%) | 129 (9.4%) | 871 (9.2%) | |
| 4 | 381 (5.3%) | 49(5.1%) | 106(7.7%) | 536(5.6%) | |
| 5 | 253(3.5%) | 19(2.0%) | 45(3.3%) | 317(3.3%) | |
| Laboratory data of blood | |||||
| Hemoglobin (g/dl) | 12.14±2.53 | 12.60±2.69 | 12.02±2.76 | 12.17±2.59 | <0.001 |
| Albumin (g/dl) | 3.47±0.72 | 3.29±0.75 | 3.12±0.75 | 3.40±0.74 | <0.001 |
| Calcium (mg/dl) | 8.04±1.63 | 7.84±1.66 | 7.88±1.54 | 8.00±1.62 | <0.001 |
| Sodium (meq/L) | 137.96±5.60 | 136.98±6.35 | 136.00±6.07 | 137.58±5.79 | <0.001 |
| Potassium (mg/dl) | 4.09±0.70 | 4.03±0.78 | 4.06±0.81 | 4.08±0.72 | 0.043 |
| Uric acid (mg/dl) | 6.59±2.56 | 7.72±3.83 | 6.70±3.14 | 6.68±2.74 | 0.043 |
| Prothrombin time (s) | 11.48±4.78 | 12.63±4.61 | 14.18±7.20 | 12.02±5.30 | <0.001 |
| pH | 6.87±0.83 | 6.99±0.77 | 6.95±0.78 | 6.90±0.81 | <0.001 |
| HCO3- (mmo/L) | 23.96±5.28 | 23.40±5.13 | 22.81±5.03 | 23.69±5.23 | <0.001 |
| Comorbidity | |||||
| Diabetes mellitus | 2116 (38.1%) | 276 (28.9%) | 422 (30.8%) | 2814 (29.6%) | 0.529 |
| Hypertension | 3529 (49.2%) | 469(49.1%) | 606 (44.3%) | 4604 (48.5%) | 0.004 |
| Cerebrovascular attack | 1114 (15.5%) | 139 (14.6%) | 132(9.6%) | 1385 (14.6%) | <0.001 |
| Peripheral arterial disease | 178 (2.5%) | 16 (1.7%) | 28 (2.0%) | 222 (2.3%) | 0.224 |
| Cirrhosis | 560 (7.8%) | 208 (21.8%) | 459 (33.6%) | 1227 (12.9%) | <0.001 |
| Hepatoma | 501 (7.0%) | 152 (15.9%) | 358 (26.2%) | 1011 (10.6%) | <0.001 |
| Colon cancer | 796 (11.1%) | 74 (7.7%) | 101 (7.4%) | 971 (10.2%) | <0.001 |
| Lung cancer | 1466 (20.4%) | 110 (11.5%) | 139 (10.2%) | 1715 (18.1%) | <0.001 |
| Atrial fibrillation | 630 (8.8%) | 124 (13.0%) | 130 (10.2%) | 884 (9.3%) | <0.001 |
| Coronary arterial disease | 1179 (16.4%) | 184 (19.3%) | 204 (14.9%) | 1567 (4.6%) | 0.020 |
| Myocardial infarction | 346 (4.8%) | 34 (3.6%) | 58 (4.2%) | 438 (4.6%) | 0.168 |
| Shock | 123 (1.7%) | 24 (2.5%) | 55 (4.0%) | 202 (2.1%) | <0.001 |
| Peritonitis | 128 (1.8%) | 32 (3.4%) | 72 (5.3%) | 232 (2.4%) | <0.001 |
| Ascites | 70 (1.0%) | 20 (2.1%) | 66 (4.8%) | 156 (1.6%) | <0.001 |
| Gastrointestinal bleeding | 355 (4.9%) | 65 (6.8%) | 113(8.3%) | 533(5.6) | <0.001 |
| Medication | |||||
| Non-steroidal anti-inflammatory drugs | 3666 (51.1%) | 391 (40.9%) | 503 (36.8%) | 4560 (48.0%) | <0.001 |
| Aspirin | 1393 (19.4%) | 180 (18.8%) | 192 (14.0%) | 1765 (18.6%) | <0.001 |
| Aminoglycoside | 3092 (43.1%) | 456 (47.7%) | 686 (50.1%) | 4234 (44.6%) | <0.001 |
| Loop diuretics | 3866 (53.9%) | 585 (61.3%) | 888 (64.9%) | 5339 (56.2%) | <0.001 |
| Angiotensin-converting-enzyme inhibitor | 643 (9.0%) | 97 (10.2%) | 120 (8.8%) | 860 (9.1%) | 0.446 |
| Angiotensin receptor blockers | 1466 (20.4%) | 178 (18.6%) | 227 (16.6%) | 1871 (19.7%) | 0.003 |
| Steroid | 1561 (21.8%) | 155 (16.2%) | 179 (13.1%) | 1895 (20.2%) | <0.001 |
| Statin | 711 (9.9%) | 77 (8.1%) | 76 (5.6%) | 864 (9.1%) | <0.001 |
| Ranitidine | 654 (9.1%) | 71 (7.4%) | 116 (8.5%) | 841 (8.9%) | 0.198 |
| Famotidine | 1147 (16.0%) | 149 (15.6%) | 223 (16.3%) | 1519 (16.0%) | 0.902 |
| Fluid replacement > 1000c.c. | 1426 (19.9%) | 199 (20.8%) | 322 (23.5%) | 1947 (20.5%) | 0.009 |
| Acute kidney injury | 569 (7.9%) | 89 (9.3%) | 225 (16.4%) | 883 (9.3%) | <0.001 |
| Dialysis within 30 days | 304 (4.2%) | 47 (4.9%) | 98 (7.2%) | 449 (4.7%) | <0.001 |
Chi-square test and one-way ANOVA.
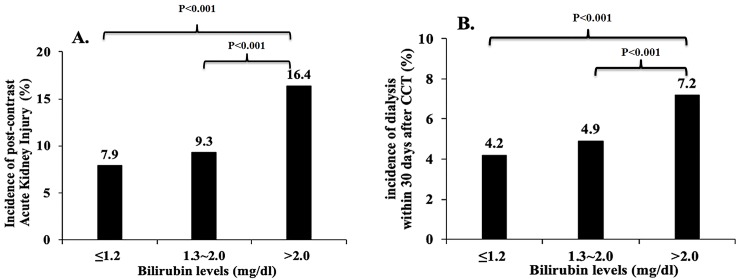
Patients in the group with total bilirubin>2mg/dl had more AKI (16.4% vs. 9.3% vs. 7.9%) compared to the other two groups with lower levels (≤1.2 mg/dl (p<0.001) and 1.3~2.0 mg/dl (p<0.001))(Fig 2A). Similarly, patients with total bilirubin>2mg/dl were prone to receive urgent hemodialysis compared to the other two groups of patients (≤1.2 mg/dl (p<0.001) or 1.3~2.0 mg/dl (p<0.001)) (Fig 2B). No difference was found between the two groups with lower total bilirubin levels regarding AKI or the incidence of urgent dialysis.
Fig 2. Renal outcomes after contrast-enhanced computed tomography in all patients divided by different serum levels of bilirubin.
2A. Incidence of acute kidney after contrast-enhanced computed tomography (CCT) divided by different serum levels of total bilirubin. No statistical significance was noticed between the groups of ≤1.2 mg/dl and 1.3–2.0 mg/dl of serum bilirubin (7.9 vs. 6.3%). Differences are statistically significant between groups of ≤1.2 mg/dl and >2.0 mg/dl of serum bilirubin (7.9 vs. 16.4%, p<0.001); and between 1.3–2.0 mg/dl and >2.0 mg/dl of serum bilirubin (9.3 vs. 16.4%, p<0.001). 2B. Incidence of urgent dialysis within 30 days after contrast-enhanced computed tomography (CCT) divided by different serum levels of total bilirubin. No statistical significance was found between the groups of ≤1.2 mg/dl and 1.3–2.0 mg/dl of serum bilirubin (4.2 vs. 4.9%). Differences are statistically significant between groups of ≤1.2 mg/dl and >2.0 mg/dl of serum bilirubin (4.2 vs. 7.2%, p<0.001); and between 1.3–2.0 mg/dl and >2.0 mg/dl of serum bilirubin (4.9 vs. 7.2%, p<0.001).
Total bilirubin>2mg/dl was associated with AKI (OR = 2.05, 1.72–2.45 of 95% CI), urgent dialysis (OR = 1.52, 1.14–2.02 of 95% CI) and mortality (OR = 1.82, 1.59–2.08 of 95% CI) (Table 2) after adjusting all comorbidities in model 1 (Table 1). After further adjusting for comorbidities, medications and laboratory data in model 2 (Table 1), total bilirubin >2 mg/dl was still associated with more AKI (OR = 1.89, 1.53–2.33 of 95% CI), dialysis (OR = 1.40, 1.01–1.95 of 95% CI) and mortality (OR = 1.63, 1.38–1.93 of 95% CI) in all patients (Table 2). Therefore, total bilirubin >2 mg/dl is an independent risk factor for AKI, dialysis and mortality after CCT in this cohort.
Table 2. Association between serum bilirubin > 2.0 mg/dL and the risk of acute kidney injury, dialysis within 30 days, and death after contrast-enhanced computerized tomography (CCT).
| Odds Ratio | 95%CI | P value | |
|---|---|---|---|
| Risk of acute kidney injury after CCT | |||
| Unadjusted | 2.23 | (1.90–2.63) | <0.001** |
| Adjusted, model 1 | 2.05 | (1.72–2.45) | <0.001** |
| Adjusted, model 2 | 1.89 | (1.53–2.33) | <0.001** |
| Risk of dialysis within 30 days after CCT | |||
| Unadjusted | 1.71 | (1.36–2.16) | <0.001** |
| Adjusted, model 1 | 1.52 | (1.14–2.02) | 0.005** |
| Adjusted, model 2 | 1.40 | (1.01–1.95) | 0.044* |
| Risk of death after CCT | |||
| Unadjusted | 1.98 | (1.76–2.24) | <0.001** |
| Adjusted, model 1 | 1.82 | (1.59–2.08) | <0.001** |
| Adjusted, model 2 | 1.63 | (1.38–1.93) | <0.001** |
Definition of acute kidney injury is an absolute increment of serum creatinine ≥0.3 mg/dl from baseline within 48 hours or ≥50% within 7 days after contrast-enhanced computerized tomography (CCT). Model 1, adjusted for the comorbidities listed in Table 1. Model 2, adjusted for the stage of CKD, hemoglobin, serum sodium, serum potassium, prothrombin time, international normalized ratio, the usage of aspirin, aminoglycoside, loop diuretics, ACE inhibitors/ARB, non-steroidal anti-inflammatory drugs, the use of fluid replacement > 1 liter on the day of CCT, plus covariates listed in Model 1.
*: p<0.05
**: p<0.01.
Compared to no cirrhosis or hepatoma, either with cirrhosis or hepatoma, patients are prone to have total bilirubin>2.0mg/dl (34% vs. 10%, p<0.001; 27.8% vs. 10%, p<0.001; respectively) (Fig 3). Because total bilirubin> 2 mg/dl is more common in patients with liver conditions, we further analyzed the association between total bilirubin >2 mg/dl and renal outcome in patients with baseline liver diseases (Table 3). The total bilirubin> 2 mg/dl was still associated with more AKI (OR = 3.50, 2.14–5.72 of 95% CI), and mortality (OR = 2.21, 1.58–3.11 of 95% CI) in patients with cirrhosis. Similarly, total bilirubin >2 mg/dl was also associated with more AKI (OR = 3.24, 1.89–5.56 of 95% CI), and mortality (OR = 2.41, 1.65–3.50 of 95% CI) in patients with hepatoma (Table 3). The ORs for AKI, dialysis and mortality are more significant in the cirrhosis group (3.50 vs. 1.89, 1.55 vs. 1.40; 2.21 vs. 1.63) (Table 3) or the hepatoma group (3.24 vs. 1.89; 1.35 vs. 1.40; 2.41 vs. 1.63) (Table 3) than in whole patients group (Table 2). In summary, patients with worse liver conditions (total bilirubin>2 mg/dl) in baseline cirrhosis or hepatoma (Table 3) had higher and additional risk of worse renal outcomes than whole population (Table 2).
Fig 3. Incidence of serum bilirubin > 2.0 mg/dl in patients with different liver conditions.
Differences are statistically significant between groups of serum bilirubin > 2.0mg/dl in patients with cirrhosis and without liver conditions (34 vs. 10%, p<0.001), and between groups of patients with hepatoma and without liver conditions (27.8 vs. 10%, p<0.001).
Table 3. Association between serum bilirubin > 2.0 mg/dl and the risk of acute kidney injury, dialysis within 30 days and death after contrast-enhanced computerized tomography (CCT) in patients with liver cirrhosis and liver cancer.
| Odds Ratio | 95%CI | P value | ||
|---|---|---|---|---|
| Patients with liver cirrhosis and serum bilirubin > 2.0 mg/dl | ||||
| Risk of acute kidney injury after CCT | ||||
| Unadjusted | 3.55 | (2.47- | 5.09) | <0.001** |
| Adjusted, model 1 | 3.69 | (2.50- | 5.44) | <0.001** |
| Adjusted, model 2 | 3.50 | (2.14- | 5.72) | <0.001** |
| Risk of dialysis within 30 days after CCT | ||||
| Unadjusted | 1.48 | (0.94- | 2.33) | 0.093 |
| Adjusted, model 1 | 1.58 | (0.91- | 2.75) | 0.103 |
| Adjusted, model 2 | 1.55 | (0.77- | 3.11) | 0.220 |
| Risk of death within 30 days after CCT | ||||
| Unadjusted | 2.29 | (1.80- | 2.91) | <0.001** |
| Adjusted, model 1 | 2.36 | (1.81- | 3.08) | <0.001** |
| Adjusted, model 2 | 2.21 | (1.58- | 3.11) | <0.001** |
| Patients with liver cancer and serum bilirubin > 2.0 mg/dl | ||||
| Risk of acute kidney injury after CCT | ||||
| Unadjusted | 3.50 | (2.36- | 5.18) | <0.001** |
| Adjusted, model 1 | 3.47 | (2.26- | 5.33) | <0.001** |
| Adjusted, model 2 | 3.24 | (1.89- | 5.56) | <0.001** |
| Risk of dialysis within 30 days after CCT | ||||
| Unadjusted | 1.37 | (0.76- | 2.48) | 0.296 |
| Adjusted, model 1 | 1.33 | (0.67- | 2.66) | 0.413 |
| Adjusted, model 2 | 1.35 | (0.52- | 3.52) | 0.536 |
| Risk of death within 30 days after CCT | ||||
| Unadjusted | 2.33 | (1.79- | 3.03) | <0.001** |
| Adjusted, model 1 | 2.26 | (1.70- | 3.00) | <0.001** |
| Adjusted, model 2 | 2.41 | (1.65- | 3.50) | <0.001** |
Definition of acute kidney injury is an absolute increment of serum creatinine ≥0.3 mg/dl from baseline within 48 hours or ≥50% within 7 days after contrast-enhanced computerized tomography (CCT). Model 1, adjusted for the comorbidities listed in Table 1. Model 2, adjusted for the stage of CKD, hemoglobin, serum sodium, serum potassium, prothrombin time, international normalized ratio, the usage of aspirin, aminoglycoside, loop diuretics, ACE inhibitors/ARB, non-steroidal anti-inflammatory drugs, the use of fluid replacement > 1 liter on the day of CCT, plus covariates listed in Model 1.
*: p <0.05
**: p <0.01
The incidence of AKI in total bilirubin> 2 mg/dl according to different baseline liver conditions were shown in Fig 4. In patients with cirrhosis (Fig 4A), hepatoma (Fig 4B), or without cirrhosis or hepatoma (Fig 4C), patients with total bilirubin >2.0mg/dl also had more CI-AKI than patients lower levels of total bilirubin (p<0.001). This result also indicated that total bilirubin > 2 mg/dl was independent risk factors for CI-AKI in different baseline liver conditions. In patients with total bilirubin> 2 mg/dl (n = 1368), baseline characteristics were shown in S2 Table according to baseline liver conditions. Moreover, patients with total bilirubin > 2 mg/dl but without cirrhosis or hepatoma (n = 7826) were also shown S3 Table. Non-cirrhosis or hepatoma related hyperbilirubinemia (>2 mg/dl) was associated with older age (p<0.001), hypoalbuminemia (p<0.001), longer prothrombin time (p<0.001), more metabolic acidosis (p<0.001), less lung cancer (p<0.001), less cerebrovascular attack (p<0.001), less NSAID (p<0.001) and steroid usage (p<0.001), more aminoglycoside usage (p<0.001), and more fluid replacement (p = 0.017).
Fig 4. Acute kidney injury after contrast-enhanced computed tomography (CCT) in patients with different liver conditions divided by different serum levels of total bilirubin.
4A. Incidence of acute kidney injury after contrast-enhanced computed tomography (CCT) divided by different serum levels of total bilirubin in patients with cirrhosis. No statistical significance was noticed between the groups of ≤ 1.2 mg/dl and 1.3–2.0 mg/dl of serum bilirubin (6.6 vs. 7.2%). Differences are statistically significant between groups of ≤ 1.2 mg/dl and > 2.0 mg/dl of serum bilirubin (6.6 vs. 20.5%, p<0.001), and between 1.3–2.0 mg/dl and >2.0 mg/dl of serum bilirubin (7.2 vs. 20.5%, p<0.001). 4B. Incidence of acute kidney injury after contrast-enhanced computed tomography (CCT) divided by different serum levels of total bilirubin in patients with hepatoma. No statistical significance was noticed between the groups of ≤1.2 mg/dl and 1.3–2.0 mg/dl of serum bilirubin (6.4% vs. 9.2%). Differences are statistically significant between groups of ≤ 1.2 mg/dl and > 2.0 mg/dl of serum bilirubin (6.4 vs. 20.9%, p<0.001), and between 1.3–2.0 mg/dl and >2.0 mg/dl of serum bilirubin (9.2 vs. 20.9%, p<0.001). 4C. Incidence of acute kidney injury after contrast-enhanced computed tomography (CCT) divided by different serum levels of total bilirubin in patients without hepatoma or cirrhosis. No statistical significance was noticed between the groups of ≤1.2 mg/dl and 1.3–2.0 mg/dl of serum bilirubin (8.1% vs. 9.6%). Differences are statistically significant between groups of ≤ 1.2 mg/dl and > 2.0 mg/dl of serum bilirubin (8.1 vs. 13.4%, p<0.001), and between 1.3–2.0 mg/dl and >2.0 mg/dl of serum bilirubin (9.6 vs. 13.4%, p<0.001).
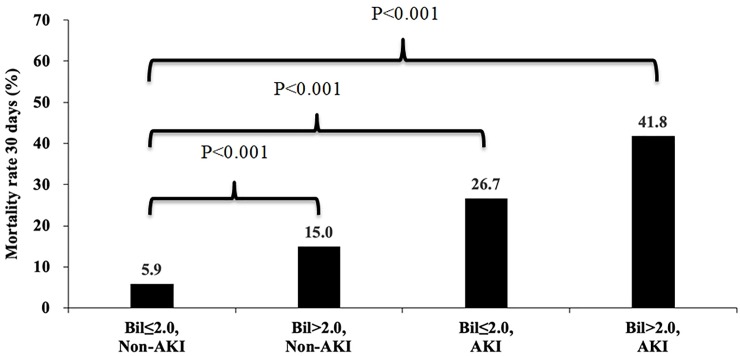
Post-CCT mortality is shown in Fig 5. Patients with total bilirubin levels ≤2.0 mg/dl and without AKI had the lowest mortality. For patients with either total bilirubin >2.0mg/dl or with AKI, mortality was higher (15% vs. 5.9%, p<0.001; 26.7% vs. 5.9%, p<0.001). Patients with simultaneously AKI and total bilirubin >2.0mg/dl had the highest mortality (41.8% vs. 5.9%, p<0.001).
Fig 5. Compared to no AKI and ≤ 2.0 mg/dl of serum bilirubin, patient mortality within 30 days after contrast-enhanced computed tomography (CCT) increased if AKI had occurred (26.7%, p<0.001) or > 2.0 mg/dl of serum bilirubin (15.0%, p<0.001).
Patients with both AKI and > 2.0 mg/dl of serum bilirubin had the highest risk of mortality (41.8%, p<0001).
Discussion
We conduct this study to elucidate the association between serum total bilirubin and CI-AKI in patients undergoing CCT. Previous studies on this issue only involved in patients undergoing angiography for coronary [21] [24] or with peripheral arterial intervention [23]. Our study is the first on patients undergoing CCT and it has largest of cases focusing on the association between total bilirubin and renal function. Previous studies were focused on relatively simple condition likely due to small numbers of cases and hard to adjust for confounding factors. Patients underwent CCT have more complicated comorbidities (e.g. shock, bleeding, cirrhosis, and hepatoma) and therefore more cases are required to analyze common clinical scenarios of CI-AKI after CCT than those after vascular intervention. Negative or inconclusive results on the relationship between bilirubin and renal functions were observed mostly in patients with multiple comorbidities such as hemorrheological disorders, infectious diseases, and decompensated heart failure. These conditions confound the effect of bilirubin on prognosis and the results obtained should be interpreted with caution. The importance of this study is the large number of cases (>9,000) so that all comorbidities and any other confounding factors could be adjusted for.
Mild elevation of serum bilirubin has renal protective funtion related to antioxidative effects of bilirubin [17–19, 21, 28–34]. Bilirubin can bind to albumin [17] and exhibits protein anitoxidative, anti-inflammatory, complete inhibitory and lipid-lowering properties [18]. It may also protect against all-cause mortality and CV diseases. For patients of Gilbert’s syndrome with mild jaundice the incidence of all-cause mortality is lowerd by half [19]. In a Korean study on a cohort (n = 1,458) with primary IgA nephropathy [28], quartile levels of bilirubin of patients were divided into: <0.4 mg/dL, 0.4–0.5 mg/dL, 0.6–0.7 mg/dL, and >0.8 mg/d. They found that the level of bilirubin was negatively associated with the incidence of ESRD. In another Korean study (1,363 patients) [29], total serum bilirubin was positively associated with eGFR and negatively associated with proteinuria. But their highest level of total bilirubin was only 0.77±0.22 mg/dl. In 2012, 544 patients receiving coronary intervention experienced fewer CI-AKI with higher serum levels of bilirubin [21]. The dovodomg values of bilirubin were ≤0.5 mg/dl, 0.5–0.7 mg/dl, and >0.7 mg/dl. Another study druing the same year showed that hypobilirubinemia is a possible risk factor of ESRD [20]. Their dividing values of bilirubin were <0.55, 0.59, 0.56, 0.47, and 0.36 mg/dl [20]. Bilirubin could attenuate cyclosporine-induced nephropathy of tubular injury by inhibiting oxidative stress and apoptosis in HK-2 cells [33]. With higher levels of bilirubin, the greater suppression on oxidative stress might attenuate the progression of CKD [17, 34]. Importantly, all the above clinical studies are in support of renal protection by bilirubin were based on mild elevation of bilirubin levels oftern within normal range (<1.2 mg/dl). That is why one of cutoff levels of bilirbuin in our study is 1.2 mg/dl.
However, not all studies supported the same conclusion [14, 22, 35–42]. For example, Targher et al[22], did an observational study with 2,678 patients and they found that total bilirubin was inversely associated with eGFR in both non-diabetic (r = -0.17; p<0.0001) and diabetic patients (r = -0.14; p<0.05). In another study, the increased total bilirubin levels are independently associated with decreasing eGFR [35]. The authors hypothesized that the discrepancy in conclusion with the mainstream literature maybe due to nonalcoholic fatty liver disease [35]. Similarly, the benefits of hyperbilirubinemia might be confounded by other factors like: cholemic nephrosis [14, 38], cholestasis [39], infection with malaria [40, 41] and spontaneous bacterial peritonitis [36], and heart failure [42]. On the contrary, in patients with nonfulminant hepatitis A, lower levels of bilirubin are associated with fewer [37]. In the vent of hepatopathy, total bilirubin level is no longer associated with the protective effects from cardiovascular disease. Such findings highlighted the problematic use of total bilirubin measures in any investigation of the protective effects of bilirubin [43]. These studies with negative findings also indicated that patients with severe liver disease are more likely to develop hepatic encephalopathy and hepatorenal syndrome. That result is consistent with ours in that total bilirubin level >2 mg/dl was mostly in patients with cirrhosis or hepatoma and total bilirubin >2.0mg/dl is associated with CI-AKI after CCT.
Some new information has emerged from this study. First, with abnormally severe hyperbilirubinemia (>1.2 mg/dl), we found that the higher the total bilirubin, the more CI-AKI after CCT, especially > 2.0 mg/dl of total bilirubin. The renal protection by mild elevations of total bilirubin was all < 1.2 mg/dl, or within the normal range. Second, the mild elevation of total bilirubin maybe contributed by unconjugated bilirubin rather than by conjugated bilirubin. In patients with total bilirubin > 2.0mg/dl, mostly due to “patological” hyperbilibubinemia related to cirrhosis or hepatoma. Third, 10% patients without cirrhosis or hepatoma still had their total bilirubin > 2.0mg/dl, and they still had more CI-AKI after CCT. Total bilirubin > 2.0mg/dl is therefore an independent risk factor for CI-AKI, which indicated an association between CI-AKI and total bilirubin level > 2.0mg/dl.
The inciden PC-AKI in patients with total bilirubin> 2.0 mg/dl was 16.4%. This is the frist sutdy to point out the incidence of PC-AKI regarding hyperbilirubinemia. The incidence of PC-AKI varied from 12 to 50% according to different baseline conditions[2–4, 44–46]. The incidence of PC-AKI in patients with total bilirubin> 2.0 mg/dl maybe relatively low because of the following reasons. First, we recruited both inpatients and outpatients. Overally, this population was all patients receiveing CCT with relatively mild disease. Most patients had good baseline renal function (66.6% with baseline eGFR>60 ml/min.1.732m2) and without anemia (12.02 g/dl of mean Hb). Only 30.8% patients had DM and shock was only noticed in 4.0% patients. Second, 33.6% patients had cirrhosis and 26.2% patients had hepatoma. Patients with liver disease may have less skeletal muscle mass, which made less creatine storage, followed by less conversion of creatine to creatinine[47]. Creatinine-based formula may underestiate renal dysfunction[47]. Finally, in this study, we point out the importance of the incidence of PC-AKI in patients with total bilirubin>2.0 mg/dl. Therefore, without previous studies for comparison, the incidence (16.4%) cannot be considered as too high or too low.
CI-AKI is primarily a condition of glomerular hypotension related to vasoconstriction [48]. Biliriun had the ability to neuralize reactive oxidative species(ROS) [49]. Increased renal ROS leads renin releases, followed by more secretino of angiotenin II [50]. ROS’s effect on efferent vasocontriction is reduced[48], causing AKI by the reduced intra-glomeurlar pressure. In an animal study, moderate hyperbilirubinemia prevents angiotenin II-dependent hypertension by decreasing vascular oxidative stress [51]. The blocked angiotensin II may also reduce vasoconstrction over efferent arterioles [48]. Moreover, hyperbilirubinemia may also lower both artery pulse pressure [52] and intraglomerula pressure[17]. Therefore, severe hyperbilirubinemia may lower pre-renal perfusion by decreassed intraglomerrular perssure, predisposing CI-AKI. Another cause could be direct tubular damages by biliruin. Followed by the condition of pre-renal AKI, CI-AKI is a result of due to tubular injuries. The association between hyperbilirubinemia and AKI in the condition of liver cirrhosis might be partially explained by a direct cytotoxicity and tubular obstruction mediated via bile casts [38, 53, 54]. Bile cast nephropathy or cholemic nephrosis has been largely forgotten in the modern medical literature, a phenomenon which maybe due to the lack of renal biopsy in most pateints with hyperbilirubinemia.Bile casts are analogous to ‘myeloma’ or myoglobin casts, as they have direct toxic effects on tubular epithelium with an obstructive capacity, which further predispose contrast related tubular injury in CI-AKI.
There are some limitations in this study. First, because this is a retrospective study, we did not regularly collect direct/indirect bilirubin and iron/ferritin (associated with hemoglobin degradation) before CCT. However, total bilirubin is the most easily obtained data in clinical practice to remind clinicians to avoid CI-AKI. Moreover, it is a novel finding to inform clinicians that hyperbilirubinemia is an independent risk factor for CI-AKI in addition to patients with liver dysfunction. In particular, patients with total bilirubin> 2 mg/dl without baseline liver conditions are easily missed. We highlight this strong association based on current data. If no data of bilirubin, we should also check icteric sclera in our daily practice. More detailed role of direct or indirect bilirubin on renal function after contrast exposure needs further studies in the future. Second, the mechanism of direct injury by severe hyperbilirubinemia in CI-AKI remains to be explored.
Conclusions
We reported here for the first time, evidence that severe hyperbilirubinemia (total bilirubin > 2.0 mg/dl) is an independent risk factor for CI-AKI, dialysis and mortality after CCT. Most patients with total bilirubin > 2.0mg/dl also had cirrhosis or hepatoma. Clinicians should identify total bilirubin>2.0mg/dl even without cirrhosis or hepatoma.
Supporting information
(AUC = 0.579, with 34.09% of sensitivity and 78.75% of specificity).
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank the Clinical Informatics Research and Development Center of Taichung Veterans General Hospital for assistance in data collection, Miss Lin, Fen-Yi for assistance in data preparation. The authors thank the Biostatistics Task Force of Taichung Veterans General Hospital and Mr. Chen, Jun-Peng for help in statistics.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by grant TCVGH-1063601B and TCVGH-1073604C from Taichung Veterans General Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The Controversy of Contrast-Induced Nephropathy With Intravenous Contrast: What Is the Risk? American journal of kidney diseases: the official journal of the National Kidney Foundation. 2019. Epub 2019/09/02. 10.1053/j.ajkd.2019.05.022 . [DOI] [PubMed] [Google Scholar]
- 2.Davenport MS, Cohan RH, Ellis JH. Contrast media controversies in 2015: imaging patients with renal impairment or risk of contrast reaction. AJR American journal of roentgenology. 2015;204(6):1174–81. Epub 2015/03/03. 10.2214/AJR.14.14259 . [DOI] [PubMed] [Google Scholar]
- 3.Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719–28. Epub 2013/04/13. 10.1148/radiol.13122276 . [DOI] [PubMed] [Google Scholar]
- 4.McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267(1):106–18. Epub 2013/01/31. 10.1148/radiol.12121823 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern EF, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney international. 1995;47(1):254–61. Epub 1995/01/01. 10.1038/ki.1995.32 . [DOI] [PubMed] [Google Scholar]
- 6.Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. The New England journal of medicine. 1989;320(3):143–9. Epub 1989/01/19. 10.1056/NEJM198901193200303 . [DOI] [PubMed] [Google Scholar]
- 7.Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. The American journal of medicine. 1989;86(6 Pt 1):649–52. Epub 1989/06/01. 10.1016/0002-9343(89)90437-3 . [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Li HL, Bei WJ, Guo XS, Chen SQ, Islam SMS, et al. Association of left ventricular ejection fraction with contrast-induced nephropathy and mortality following coronary angiography or intervention in patients with heart failure. Therapeutics and clinical risk management. 2017;13:887–95. Epub 2017/08/05. 10.2147/TCRM.S137654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. European radiology. 2011;21(12):2527–41. Epub 2011/08/26. 10.1007/s00330-011-2225-0 . [DOI] [PubMed] [Google Scholar]
- 10.Holmes TW Jr. The histologic lesion of cholemic nephrosis. The Journal of urology. 1953;70(5):677–85. Epub 1953/11/01. 10.1016/s0022-5347(17)67968-0 . [DOI] [PubMed] [Google Scholar]
- 11.Fajers CM. Experimental studies in cholemic nephrosis. 3. The effect of cholemia by itself and combined with ten minutes' unilateral renal ischemia on the kidneys of hydrated rabbits as judged by some renal function tests. Acta pathologica et microbiologica Scandinavica. 1957;41(1):44–55. Epub 1957/01/01. . [PubMed] [Google Scholar]
- 12.Pasero G, Tamagnini G. [Cholemic tubulo-nephrosis; functional symptomatology of the kidney in icteric syndromes]. Omnia therapeutica Supplemento. 1958;36(1–2):1–35. Epub 1958/01/01. . [PubMed] [Google Scholar]
- 13.Sant SM, Purandare NM. CHOLEMIC NEPHROSIS—AN AUTOPSY AND EXPERIMENTAL STUDY. Journal of postgraduate medicine. 1965;11:79–89. Epub 1965/04/01. . [PubMed] [Google Scholar]
- 14.Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. Journal of nephrology. 2006;19(2):229–33. Epub 2006/06/01. . [PubMed] [Google Scholar]
- 15.Bredewold OW, de Fijter JW, Rabelink T. A case of mononucleosis infectiosa presenting with cholemic nephrosis. NDT plus. 2011;4(3):170–2. Epub 2011/06/01. 10.1093/ndtplus/sfr038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, Chang A. Jaundice-associated acute kidney injury. NDT plus. 2009;2(1):82–3. Epub 2009/02/01. 10.1093/ndtplus/sfn149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boon AC, Bulmer AC, Coombes JS, Fassett RG. Circulating bilirubin and defense against kidney disease and cardiovascular mortality: mechanisms contributing to protection in clinical investigations. American journal of physiology Renal physiology. 2014;307(2):F123–36. Epub 2014/04/25. 10.1152/ajprenal.00039.2014 . [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Pant P, Basu S, Rao GR, Khanna HD. Oxidative stress in neonatal hyperbilirubinemia. Journal of tropical pediatrics. 2007;53(1):69–71. Epub 2006/12/13. 10.1093/tropej/fml060 . [DOI] [PubMed] [Google Scholar]
- 19.Horsfall LJ, Nazareth I, Pereira SP, Petersen I. Gilbert's syndrome and the risk of death: a population-based cohort study. Journal of gastroenterology and hepatology. 2013;28(10):1643–7. Epub 2013/05/25. 10.1111/jgh.12279 . [DOI] [PubMed] [Google Scholar]
- 20.Oda E, Aoyagi R, Aizawa Y. Hypobilirubinemia might be a possible risk factor of end-stage kidney disease independently of estimated glomerular filtration rate. Kidney & blood pressure research. 2012;36(1):47–54. Epub 2012/07/27. 10.1159/000339027 . [DOI] [PubMed] [Google Scholar]
- 21.Huang SS, Huang PH, Wu TC, Chen JW, Lin SJ. Association of serum bilirubin with contrast-induced nephropathy and future cardiovascular events in patients undergoing coronary intervention. PloS one. 2012;7(8):e42594 Epub 2012/08/11. 10.1371/journal.pone.0042594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Targher G, Zoppini G, Cesare Guidi G, Lippi G. Relationship between serum bilirubin and kidney function in non-diabetic and diabetic individuals. Kidney international. 2009;75(8):863 Epub 2009/04/02. 10.1038/ki.2008.677 . [DOI] [PubMed] [Google Scholar]
- 23.Vuruskan E, Saracoglu E. Bilirubin Levels are Associated With Contrast-Induced Nephropathy in Peripheral Artery Disease. Angiology. 2017;68(8):728–33. Epub 2016/11/18. 10.1177/0003319716679340 . [DOI] [PubMed] [Google Scholar]
- 24.Luo E, Wang D, Qiao Y, Zhu B, Liu B, Hou J, et al. Association of total bilirubin with contrast-induced nephropathy in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Coronary artery disease. 2019. Epub 2019/09/17. 10.1097/mca.0000000000000783 . [DOI] [PubMed] [Google Scholar]
- 25.Okusa MD, Davenport A. Reading between the (guide)lines—the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney international. 2014;85(1):39–48. Epub 2013/09/27. 10.1038/ki.2013.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145(4):247–54. 10.7326/0003-4819-145-4-200608150-00004 . [DOI] [PubMed] [Google Scholar]
- 27.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. The British journal of surgery. 1973;60(8):646–9. Epub 1973/08/01. 10.1002/bjs.1800600817 . [DOI] [PubMed] [Google Scholar]
- 28.Chin HJ, Cho HJ, Lee TW, Na KY, Oh KH, Joo KW, et al. The mildly elevated serum bilirubin level is negatively associated with the incidence of end stage renal disease in patients with IgA nephropathy. Journal of Korean medical science. 2009;24 Suppl:S22–9. Epub 2009/02/12. 10.3346/jkms.2009.24.S1.S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin HS, Jung YS, Rim H. Relationship of serum bilirubin concentration to kidney function and 24-hour urine protein in Korean adults. BMC nephrology. 2011;12:29 Epub 2011/06/29. 10.1186/1471-2369-12-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–66. Epub 1998/05/30. 10.2337/diabetes.47.6.859 . [DOI] [PubMed] [Google Scholar]
- 31.Roh DD, Kamanna VS, Kirschenbaum MA. Oxidative modification of low-density lipoprotein enhances mesangial cell protein synthesis and gene expression of extracellular matrix proteins. American journal of nephrology. 1998;18(4):344–50. Epub 1998/07/08. 10.1159/000013363 . [DOI] [PubMed] [Google Scholar]
- 32.Mietus-Snyder M, Friera A, Glass CK, Pitas RE. Regulation of scavenger receptor expression in smooth muscle cells by protein kinase C: a role for oxidative stress. Arteriosclerosis, thrombosis, and vascular biology. 1997;17(5):969–78. Epub 1997/05/01. 10.1161/01.atv.17.5.969 . [DOI] [PubMed] [Google Scholar]
- 33.Oh SW, Lee ES, Kim S, Na KY, Chae DW, Kim S, et al. Bilirubin attenuates the renal tubular injury by inhibition of oxidative stress and apoptosis. BMC nephrology. 2013;14:105 Epub 2013/05/21. 10.1186/1471-2369-14-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markan S, Kohli HS, Sud K, Ahuja M, Ahluwalia TS, Sakhuja V, et al. Oxidative stress in primary glomerular diseases: a comparative study. Molecular and cellular biochemistry. 2008;311(1–2):105–10. Epub 2008/01/26. 10.1007/s11010-008-9701-0 . [DOI] [PubMed] [Google Scholar]
- 35.Targher G, Bosworth C, Kendrick J, Smits G, Lippi G, Chonchol M. Relationship of serum bilirubin concentrations to kidney function and albuminuria in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001–2006. Clinical chemistry and laboratory medicine. 2009;47(9):1055–62. Epub 2009/07/29. 10.1515/CCLM.2009.244 . [DOI] [PubMed] [Google Scholar]
- 36.Terg R, Gadano A, Cartier M, Casciato P, Lucero R, Munoz A, et al. Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver international: official journal of the International Association for the Study of the Liver. 2009;29(3):415–9. Epub 2008/09/23. 10.1111/j.1478-3231.2008.01877.x . [DOI] [PubMed] [Google Scholar]
- 37.Choi HK, Song YG, Han SH, Ku NS, Jeong SJ, Baek JH, et al. Clinical features and outcomes of acute kidney injury among patients with acute hepatitis A. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2011;52(3):192–7. Epub 2011/08/10. 10.1016/j.jcv.2011.07.013 . [DOI] [PubMed] [Google Scholar]
- 38.van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney international. 2013;84(1):192–7. Epub 2013/03/15. 10.1038/ki.2013.78 . [DOI] [PubMed] [Google Scholar]
- 39.Rafat C, Burbach M, Brocheriou I, Zafrani L, Callard P, Rondeau E, et al. Bilirubin-associated acute tubular necrosis in a kidney transplant recipient. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013;61(5):782–5. Epub 2013/03/08. 10.1053/j.ajkd.2012.11.046 . [DOI] [PubMed] [Google Scholar]
- 40.Misra DP, Das S, Pattnaik M, Singh SC, Jena RK. Relationship of hepatic and renal dysfunction with haemorrheological parameters in Plasmodium falciparum malaria. The Journal of the Association of Physicians of India. 2011;59:552–6. Epub 2012/02/18. . [PubMed] [Google Scholar]
- 41.Tangpukdee N, Elshiekh SB, Phumratanaprapin W, Krudsood S, Wilairatana P. Factors associated with acute renal failure in falciparum malaria infected patients. The Southeast Asian journal of tropical medicine and public health. 2011;42(6):1305–12. Epub 2012/02/04. . [PubMed] [Google Scholar]
- 42.Koyama S, Sato Y, Tanada Y, Fujiwara H, Takatsu Y. Early evolution and correlates of urine albumin excretion in patients presenting with acutely decompensated heart failure. Circulation Heart failure. 2013;6(2):227–32. Epub 2013/02/12. 10.1161/CIRCHEARTFAILURE.112.000152 . [DOI] [PubMed] [Google Scholar]
- 43.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Experimental biology and medicine (Maywood, NJ). 2003;228(5):568–71. Epub 2003/04/24. 10.1177/15353702-0322805-29 . [DOI] [PubMed] [Google Scholar]
- 44.Rudnick MR, Leonberg-Yoo AK, Litt HI, Cohen RM, Hilton S, Reese PP. The Controversy of Contrast-Induced Nephropathy With Intravenous Contrast: What Is the Risk? American journal of kidney diseases: the official journal of the National Kidney Foundation. 2020;75(1):105–13. Epub 2019/09/02. 10.1053/j.ajkd.2019.05.022 . [DOI] [PubMed] [Google Scholar]
- 45.Dekkers IA, van der Molen AJ. Propensity Score Matching as a Substitute for Randomized Controlled Trials on Acute Kidney Injury After Contrast Media Administration: A Systematic Review. AJR American journal of roentgenology. 2018;211(4):822–6. Epub 2018/08/08. 10.2214/AJR.17.19499 . [DOI] [PubMed] [Google Scholar]
- 46.Ellis JH, Khalatbari S, Yosef M, Cohan RH, Davenport MS. Influence of Clinical Factors on Risk of Contrast-Induced Nephrotoxicity From IV Iodinated Low-Osmolality Contrast Material in Patients With a Low Estimated Glomerular Filtration Rate. AJR American journal of roentgenology. 2019;213(5):W188–w93. Epub 2019/07/04. 10.2214/AJR.19.21424 . [DOI] [PubMed] [Google Scholar]
- 47.Cocchetto DM, Tschanz C, Bjornsson TD. Decreased rate of creatinine production in patients with hepatic disease: implications for estimation of creatinine clearance. Therapeutic drug monitoring. 1983;5(2):161–8. Epub 1983/06/01. 10.1097/00007691-198306000-00002 . [DOI] [PubMed] [Google Scholar]
- 48.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. Journal of the American Society of Nephrology: JASN. 2017;28(4):1023–39. Epub 2017/02/02. 10.1681/ASN.2016060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neuzil J, Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS letters. 1993;331(3):281–4. Epub 1993/10/04. 10.1016/0014-5793(93)80353-v . [DOI] [PubMed] [Google Scholar]
- 50.Welch WJ, Ott CE, Guthrie GP Jr., Kotchen TA. Mechanism of increased renin release in the adrenalectomized rat. Adrenal insufficiency and renin. Hypertension (Dallas, Tex: 1979). 1983;5(2 Pt 2):I47–52. Epub 1983/03/01. 10.1161/01.hyp.5.2_pt_2.i47 . [DOI] [PubMed] [Google Scholar]
- 51.Vera T, Granger JP, Stec DE. Inhibition of bilirubin metabolism induces moderate hyperbilirubinemia and attenuates ANG II-dependent hypertension in mice. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297(3):R738–43. Epub 2009/07/03. 10.1152/ajpregu.90889.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhuiyan AR, Srinivasan SR, Chen W, Sultana A, Berenson GS. Association of serum bilirubin with pulsatile arterial function in asymptomatic young adults: the Bogalusa Heart Study. Metabolism: clinical and experimental. 2008;57(5):612–6. Epub 2008/04/30. 10.1016/j.metabol.2007.12.003 . [DOI] [PubMed] [Google Scholar]
- 53.Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–36. Epub 1993/07/01. 10.1016/0016-5085(93)90031-7 . [DOI] [PubMed] [Google Scholar]
- 54.Haessler H, Rous P, Broun GO. THE RENAL ELIMINATION OF BILIRUBIN. The Journal of experimental medicine. 1922;35(4):533–52. Epub 1922/03/31. 10.1084/jem.35.4.533 [DOI] [PMC free article] [PubMed] [Google Scholar]