Abstract
Background
Stroke recognition systems have been developed to reduce time delays, however, a comprehensive triaging score identifying stroke subtypes is needed to guide appropriate management. We aimed to develop a prehospital scoring system for rapid stroke recognition and identify stroke subtype simultaneously.
Methods and findings
In prospective database of regional emergency and stroke center, Clinical Information, Vital signs, and Initial Labs (CIVIL) of 1,599 patients suspected of acute stroke was analyzed from an automatically-stored electronic health record. Final confirmation was performed with neuroimaging. Using multiple regression analyses, we determined independent predictors of tier 1 (true-stroke or not), tier 2 (hemorrhagic stroke or not), and tier 3 (emergent large vessel occlusion [ELVO] or not). The diagnostic performance of the stepwise CIVIL scoring system was investigated using internal validation. A new scoring system characterized by a stepwise clinical assessment has been developed in three tiers. Tier 1: Seven CIVIL-AS3A2P items (total score from –7 to +6) were deduced for true stroke as Age (≥ 60 years); Stroke risks without Seizure or psychiatric disease, extreme Sugar; “any Asymmetry”, “not Ambulating”; abnormal blood Pressure at a cut-off point ≥ 1 with diagnostic sensitivity of 82.1%, specificity of 56.4%. Tier 2: Four items for hemorrhagic stroke were identified as the CIVIL-MAPS indicating Mental change, Age below 60 years, high blood Pressure, no Stroke risks with cut-point ≥ 2 (sensitivity 47.5%, specificity 85.4%). Tier 3: For ELVO diagnosis: we applied with CIVIL-GFAST items (Gaze, Face, Arm, Speech) with cut-point ≥ 3 (sensitivity 66.5%, specificity 79.8%). The main limitation of this study is its retrospective nature and require a prospective validation of the CIVIL scoring system.
Conclusions
The CIVIL score is a comprehensive and versatile system that recognizes strokes and identifies the stroke subtype simultaneously.
Introduction
Stroke remains with a high burden in societies, and improving the recognition of a stroke can help reduce this burden [1]. “Time-is-brain” is a crucial concept in ischemic stroke management [2,3], the importance of early recanalization has been further addressed after the success of endovascular recanalization therapies [4]. In the case of hemorrhagic stroke, early surgical interventions can be beneficial in selected patients [5,6]. Candidates for urgent interventions should be transported to an appropriately-equipped hospital, however, treatment can be delayed due to various reasons [7].
Stroke recognition systems have been developed to reduce time delay in community and hospital settings [8,9]. Various scales and scoring systems have been developed, but there is still no consensus on which scale perform better. [10,11]. Previous systems have issues related to false positives and false negatives, making it difficult for stroke specialists to handle a considerable number of patients [12,13]. In addition, most research has covered only one aspect of stroke, ‘true stroke or not’ or ‘selection of emergent large vessel occlusion (ELVO),’ and clinical parameters can be subjective even after thorough training. Finally, previously published systems have a greater focus on reducing pre-hospital delay [14,15]. Therefore, a comprehensive triaging system for considering next-step treatments should address to reduce the workload at stroke centers with acceptable sensitivity and specificity.
In this context, we have developed a new scoring system using Clinical Information, baseline Vital sign, and Initial Labs (CIVIL). Here, we aimed to evaluate the feasibility of the CIVIL system and compare with previous screening systems in suspicious acute stroke patients.
Methods
Study population
This electronic health record-based observational cohort study was performed in a tertiary referral hospital from January 2012 to December 2015. Care in the tertiary stroke center fulfilled the Brain Attack Coalition’s standardized criteria, and the stroke unit had also obtained certification from the Korean Stroke Association [16]. The regional emergency medical center serves the southern part of Gyeonggi Province of South Korea with a population of approximately four million, and it’s emergency room (ER) has approximately 89,000 patients annually [17]. Previously, we developed the stroke recognition system ‘Cubic S model’ (S1 Fig), which is based on the Electronic Medical Record (EMR) system for suspected stroke patients in the ER [18]. It is based on common signs and symptoms and contains three domains: time, body-part involvement, and symptomatic presentations. When suspicious stroke patients visit the ER, ER physicians check the presence of three domains: sudden onset, one-sided involvement of face/arm/leg, and 6 representative symptoms of stroke.
Each dataset was automatically stored in the database from a prospectively registered critical pathway system for rapid thrombolysis in suspicious stroke patient. The data has been used to improve the quality of registered data through monthly reports. Inclusion criteria for this study were (a) acute neurologic manifestations within 6 hours, (b) acute thrombolysis code activating cases who meets all three domains of EMR-based Cubic S system, and (c) final confirmation with clinical and imaging findings by stroke neurologists. Exclusion criteria were: (a) incorrect activation of an acute thrombolysis code, (b) onset-to-door time > 6 hours, and (c) uncertain final diagnosis due to incomplete study. The study protocol was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-MED-MDB-16-407). Informed consent was waived because of the study’s retrospective nature.
Processing after critical pathway activation
Initial assessment and activation of acute thrombolysis code was performed by ER physicians. Education of ER physicians and nurses was routinely performed every 6 to 12 months by stroke neurologists. Immediately after the activation of acute thrombolysis code, stroke neurologists assessed the patients. All patients except those with contraindications to contrast use underwent computed tomography (CT) scan with angiography. Simultaneously, neurologists meticulously investigated clinical information, baseline vital signs, initial laboratory findings, and stroke images. In cases where recanalization treatments were needed, intravenous recombinant tissue plasminogen activator and/or endovascular therapies were implemented according to critical pathway in our institute. Clinical information was recorded including age, sex, prior medical histories (hypertension, diabetes mellitus, previous stroke occurrence, seizure or syncope, and psychiatric history) from the patient, care-givers, or paramedics. Initial neurological manifestations were assessed with the Cubic S model. Baseline vital signs were comprised of blood pressure, pulse rate, and body temperature, and initial laboratory findings (glucose level and oxygen saturation) were also included.
Confirmation of final diagnosis
The adjudication meeting comprised of stroke specialists was held weekly for final diagnoses of all patients according to the three tiers; as stroke mimic vs. true stroke, ischemic vs. hemorrhagic stroke, non-ELVO vs. ELVO. Final diagnosis was determined after review of ER chart, imaging and laboratory studies for differential diagnoses. True stroke was diagnosed when the neurologic exam was compatible with supportive imaging evidence of CT and/or magnetic resonance imaging including diffusion weighted image. Transient cerebral ischemic attack was classified into the true stroke and ischemic stroke group. Stroke mimics were designated when the clinical details were compatible with non-vascular etiologies. Initial CT angiography confirmed hemorrhagic stroke and large artery occlusion. ELVO was designated as occlusion of the internal carotid artery, M1 or M2 segment of the middle cerebral artery, or basilar artery [19,20].
Development of new scoring system
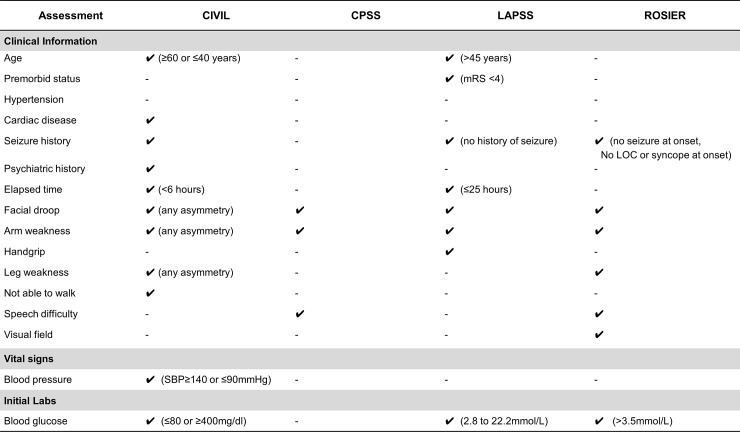
We intended to develop a new scoring system that is characterized by a “stepwise clinical assessment system which enables rapid discrimination of patients suspected of acute stroke,” potential variables were assessed including Clinical Information, Vital signs, and Initial Labs (CIVIL) used in ER and prehospital settings. There were 23 clinical findings, four vital signs, and two laboratory findings. We analyzed these variables by the three tiers; stroke mimic vs. true stroke, ischemic vs. hemorrhagic stroke, non-ELVO vs. ELVO. At the first tier (stroke mimic vs. true stroke), we assigned +1 to positive variables for true stroke (odds ratio [OR] > 1.0) and -1 to negative variables (OR <1.0). In the second tier, items suggestive of hemorrhagic stroke were derived (only positive scores). Finally, to discriminate ELVO from non-ELVO, we applied the gaze to face-arm-speech-time (GFAST) scoring system [21]. For evaluation of performance, the CIVIL system was compared with three previous recognition systems for acute stroke: Cincinnati Prehospital Stroke Scale (CPSS) [14], Los Angeles Prehospital Stroke Screen (LAPSS) [15], and Recognition Of Stroke In the Emergency Room (ROSIER) system [8].
Statistical analysis
Differences between the two groups at each steps were analyzed using χ2 or Student t-test for categorical and continuous variables, respectively. Significant variables from univariate analyses (p<0.05) were assessed with multivariate logistic regression models for deduction of scoring items (enter method). Associations were presented as odds ratios (OR) with corresponding 95% confidence interval (CI). Internal validation using receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut off point for each steps. Diagnostic performance including sensitivity, specificity, positive predictive value, negative predictive value, and Youden index were assessed for each cut-off point. We performed all analyses using SPSS 25.0 for Windows (SPSS Inc., Chicago, Ill).
Results
Patient assessment
The flow chart of study population is shown in S2 Fig. A total of 1,621 patients were screened by acute thrombolysis code activation. Sixty-two patients were excluded, and the remaining 1,559 suspected stroke patients were enrolled, of these, true stroke was confirmed in 1,153 (74.0%). Causes of stroke mimicking symptoms were metabolic disease (18.0%), drug intoxication (15.0%), peripheral neuropathy (14.3%), psychogenic disorder (14.3%), seizure (13.8%), infectious disease (7.4%), syncope (6.9%), and tumorous condition (3.4%). True stroke patients comprised of ischemic stroke (n = 894, 77.5%) and hemorrhagic stroke (n = 259, 22.5%), and the number of ischemic stroke patient requiring recanalization therapy was 291 (32.6%).
Clinical information, baseline vital signs, and initial labs (CIVIL)
Tables 1 and 2 summarizes detailed findings according to the final diagnosis. In the first tier (stroke mimic vs. true stroke), true stroke patients were older and male-dominant. History of stroke risk factor were more frequent in the true stroke group, whereas history of seizure or psychiatric disease were less common. From clinical manifestations, “after awakening”, lateralizing symptoms, “not ambulating”, and “not able to grasp” were more prevalent in the true stroke group, while “mental change” was more frequent in the stroke mimic group. From vital-sign and initial laboratory findings, systolic and diastolic BP were higher in the true stroke group. In contrast, the stroke mimic group included more patients with low systolic BP (≤90mmHg) and extreme glucose level (initial glucose <80 or ≥400 mg/dl).
Table 1. Clinical information, vital signs, and initial labs (CIVIL).
| Stroke- mimic (n = 406) | True stroke (n = 1,153) | pa | Ischemic stroke (n = 894) | Hemorrhagic stroke (n = 259) | pb | |
| Clinical information | ||||||
| Age, years | 62.2 ± 15.8 | 64.5 ± 14.2 | 0.012 | 65.6 ± 14.0 | 60.5 ± 14.5 | <0.001 |
| Age ≥ 60 years, n (%) | 230 (56.7) | 743 (64.4) | 0.005 | 613 (68.6) | 130 (50.2) | <0.001 |
| Age ≤ 40 years, n (%) | 38 (9.4) | 57 (4.9) | 0.001 | 40 (4.5) | 17 (6.6) | 0.172 |
| Male, n (%) | 200 (49.3) | 699 (60.6) | <0.001 | 561 (62.8) | 138 (53.3) | 0.004 |
| Onset-to-door time (minute) | 172.6 ± 209.9 | 182.9 ± 205.2 | 0.118 | 197.9 ± 210.0 | 131.2 ± 178.7 | <0.001 |
| Onset-to-door ≥ 90 min, n (%) | 173 (42.6) | 466 (40.4) | 0.439 | 314 (35.1) | 152 (58.7) | <0.001 |
| Prior history, n (%) | ||||||
| Hypertension | 170 (41.9) | 606 (52.6) | <0.001 | 473 (52.9) | 133 (51.4) | 0.355 |
| Diabetes | 100 (24.6) | 250 (21.7) | 0.419 | 214 (23.9) | 36 (13.9) | <0.001 |
| Cardiac diseases | 69 (17.0) | 263 (22.8) | <0.001 | 244 (27.3) | 19 (7.3) | <0.001 |
| Previous stroke | 78 (19.2) | 242 (21.0) | 0.120 | 199 (22.3) | 43 (16.6) | 0.028 |
| Seizure or psychiatric history | 89 (21.9) | 21 (1.8) | <0.001 | 19 (2.1) | 2 (0.8) | 0.192 |
| Clinical manifestations | ||||||
| Time | ||||||
| “Sudden”, n (%) | 365 (89.9) | 1038 (90.0) | 0.172 | 786 (87.9) | 252 (97.3) | <0.001 |
| “After awakening”, n (%) | 32 (7.9) | 114 (9.9) | 0.021 | 105 (11.7) | 9 (3.5) | <0.001 |
| “As unusual”, n (%) | 13 (3.2) | 34 (2.9) | 0.476 | 31 (3.5) | 3 (1.2) | 0.034 |
| Body-spatial | ||||||
| “one-side arm”, n (%) | 144 (35.5) | 821 (71.2) | <0.001 | 642 (71.8) | 179 (69.1) | 0.221 |
| “one-side leg”, n (%) | 120 (29.6) | 720 (62.4) | <0.001 | 557 (62.3) | 163 (62.9) | 0.457 |
| “one-side face”, n (%) | 72 (17.7) | 401 (34.8) | <0.001 | 306 (34.2) | 95 (36.7) | 0.255 |
| “any asymmetry’, n (%) | 205 (50.5) | 958 (83.1) | <0.001 | 763 (85.3) | 195 (75.3) | <0.001 |
| Symptoms | ||||||
| “not ambulating”, n (%) | 79 (19.5) | 508 (44.1) | <0.001 | 388 (43.4) | 120 (46.3) | 0.222 |
| “not able to speak”, n (%) | 185 (45.6) | 576 (50.0) | 0.077 | 447 (50.0) | 129 (49.8) | 0.506 |
| “not able to grasp”, n (%) | 37 (9.1) | 206 (17.9) | <0.001 | 164 (18.3) | 42 (16.2) | 0.245 |
| “mental change” *, n (%) | 156 (38.4) | 193 (16.7) | <0.001 | 92 (10.3) | 101 (39.0) | <0.001 |
| “abnormal sensation”, n (%) | 56 (13.8) | 162 (14.1) | 0.247 | 138 (15.4) | 24 (9.3) | 0.006 |
| “visual disturbance”, n (%) | 4 (1.0) | 18 (1.6) | 0.165 | 17 (1.9) | 1 (0.4) | 0.062 |
| Baseline vital signs | ||||||
| SBP, mmHg | 137.7 ± 28.9 | 151.1 ± 28.6 | <0.001 | 147.7 ± 25.4 | 163.0 ± 34.9 | <0.001 |
| SBP ≥ 160mmHg, n (%) | 100 (24.6) | 473 (41.0) | <0.001 | 327 (36.6) | 146 (56.4) | <0.001 |
| SBP ≥ 140mmHg, n (%) | 202 (49.8) | 801 (69.5) | <0.001 | 598 (66.9) | 203 (78.4) | <0.001 |
| SBP ≤ 90mmHg, n (%) | 12 (3.0) | 3 (0.3) | <0.001 | 1 (0.1) | 2 (0.8) | 0.128 |
| DBP, mmHg | 81.9 ± 34.7 | 86.8 ± 16.4 | 0.018 | 85.6 ± 15.5 | 90.6 ± 18.7 | 0.005 |
| Pulse rate, bpm | 84.2 ± 19.1 | 82.9 ± 15.5 | 0.454 | 82.8 ± 15.9 | 83.2 ± 13.9 | 1.000 |
| Body temperature, °C | 36.5 ± 0.8 | 36.5 ± 0.5 | 1.000 | 36.5 ± 0.4 | 36.4 ± 0.6 | 0.173 |
| Initial laboratory findings | ||||||
| Glucose, mg/dl | 150.0 ± 101.6 | 146.8 ± 60.1 | 0.510 | 144.0 ± 60.5 | 156.4 ± 57.8 | 0.051 |
| Extreme glucose level†, n (%) | 24 (5.9) | 14 (1.2) | <0.001 | 11 (1.2) | 3 (1.2) | 1.000 |
| Oxygen saturation, % | 99.3 ± 3.2 | 99.5 ± 2.5 | 0.062 | 99.6 ± 2.2 | 99.2 ± 3.2 | 0.082 |
SBP means systolic blood pressure, and DBP indicates diastolic blood pressure. *”mental change” was defined when a decrease in the level of consciousness below drowsiness was observed on initial neurological examination. †initial blood glucose level ≤ 80 or ≥ 400 mg/dl, pa = Stroke mimic vs. true stroke, pb = Ischemic stroke vs. hemorrhagic stroke
Table 2. Clinical information, vital signs, and initial labs (CIVIL) between ELVO and non-ELVO patients.
| ELVO stroke (n = 291) | Non-ELVO stroke (n = 603) | p | |
|---|---|---|---|
| Clinical information | |||
| Age, years | 68.1 ± 13.6 | 64.4 ± 14.0 | <0.001 |
| Male, n (%) | 171 (58.8) | 390 (64.7) | 0.087 |
| Onset-to-door time (minute) | 191.8 ± 208.8 | 200.9 ± 210.7 | 0.045 |
| Prior history, n (%) | |||
| Hypertension | 160 (55.0) | 313 (51.9) | 0.388 |
| Diabetes | 59 (20.3) | 155 (25.7) | 0.075 |
| Cardiac problems | 115 (39.5) | 129 (21.4) | <0.001 |
| Previous stroke | 57 (19.6) | 142 (23.5) | 0.182 |
| Manifestation | |||
| Time domain | |||
| “Sudden”, n (%) | 260 (89.3) | 526 (87.2) | 0.363 |
| “After awakening”, n (%) | 30 (10.3) | 75 (12.4) | 0.354 |
| “As unusual”, n (%) | 10 (3.4) | 21 (3.5) | 0.972 |
| Body-spatial domain | |||
| “one-side arm”, n (%) | 241 (82.8) | 401 (66.5) | <0.001 |
| “one-side leg”, n (%) | 217 (74.6) | 340 (56.4) | <0.001 |
| “one-side face”, n (%) | 112 (38.5) | 194 (32.2) | 0.062 |
| “any asymmetry’, n (%) | 257 (88.3) | 506 (83.9) | 0.081 |
| Symptom domain | |||
| “not ambulating”, n (%) | 159 (54.6) | 229 (38.0) | <0.001 |
| “not able to speak”, n (%) | 188 (64.6) | 259 (43.0) | <0.001 |
| “not able to grasp”, n (%) | 53 (18.2) | 111 (18.4) | 0.944 |
| “mental change” *, n (%) | 59 (20.3) | 33 (5.5) | <0.001 |
| “abnormal sensation”, n (%) | 14 (4.8) | 124 (20.6) | <0.001 |
| “visual disturbance”, n (%) | 9 (3.1) | 8 (1.3) | 0.070 |
| “gaze deviation”, n (%) | 197 (67.7) | 48 (8.0) | <0.001 |
| Baseline vital signs | |||
| SBP, mmHg | 143.1 ± 26.4 | 149.9 ± 24.7 | <0.001 |
| DBP, mmHg | 83.5 ± 16.0 | 86.7 ± 15.1 | 0.003 |
| Pulse rate, bpm | 84.2 ± 18.1 | 82.1 ± 14.7 | 0.064 |
| Body temperature, °C | 36.4 ± 0.4 | 36.5 ± 0.4 | 0.004 |
| Initial laboratory findings | |||
| Glucose, mg/dl | 142.4 ± 49.3 | 144.8 ± 65.2 | 0.571 |
| Oxygen saturation, % | 99.5 ± 1.7 | 99.7 ± 2.4 | 0.202 |
| Stroke characteristics | |||
| NIHSS, median (IQR) | 16 (12–20) | 3 (1–6) | <0.001 |
| TOAST classification, n (%) | <0.001 | ||
| Large artery disease | 69 (23.7) | 105 (17.4) | |
| Cardioembolism | 157 (54.0) | 106 (17.6) | |
| Small artery disease | 0 (0.0) | 134 (22.2) | |
| Others | 65 (22.3) | 258 (42.8) | |
| Vessel occlusion, n (%) | |||
| ICA | 90 (30.9) | - | |
| M1 | 102 (35.1) | - | |
| M2 | 7 (16.2) | - | |
| BA | 33 (11.3) | - | |
| Others | 19 (6.5) | - | |
| tPA use, n (%) | 155 (53.3) | 83 (13.8) | <0.001 |
| Endovascular treatment, n (%) | 150 (51.5) | 0 (0.0) | <0.001 |
*”mental change” was defined when a decrease in the level of consciousness below drowsiness was observed on initial neurological examination. SBP = systolic blood pressure, DBP = diastolic blood pressure, NIHSS = National Institute of Health Stroke Scale, ICA = internal carotid artery, BA = basilar artery, tPA = tissue plasminogen activator
In the second tier (ischemic vs. hemorrhagic stroke), the ischemic stroke group was older and had a higher proportion of males than the hemorrhagic stroke group. The patients with hemorrhagic stroke had shorter onset-to-door time. History of stroke risk factor was more prevalent in the ischemic stroke group. “Sudden onset” was more frequent in the hemorrhagic stroke, while “after awakening” and “as unusual” were more common in the ischemic stroke. The ischemic stroke group showed more common “any asymmetry. “Mental change” was more prevalent in the hemorrhagic stroke group, while “abnormal sensation” was more frequent in the ischemic stroke group. Systolic and diastolic BP were higher in the hemorrhagic stroke group.
Tier 1: Stroke mimic vs. true stroke: CIVIL-AS3A2P
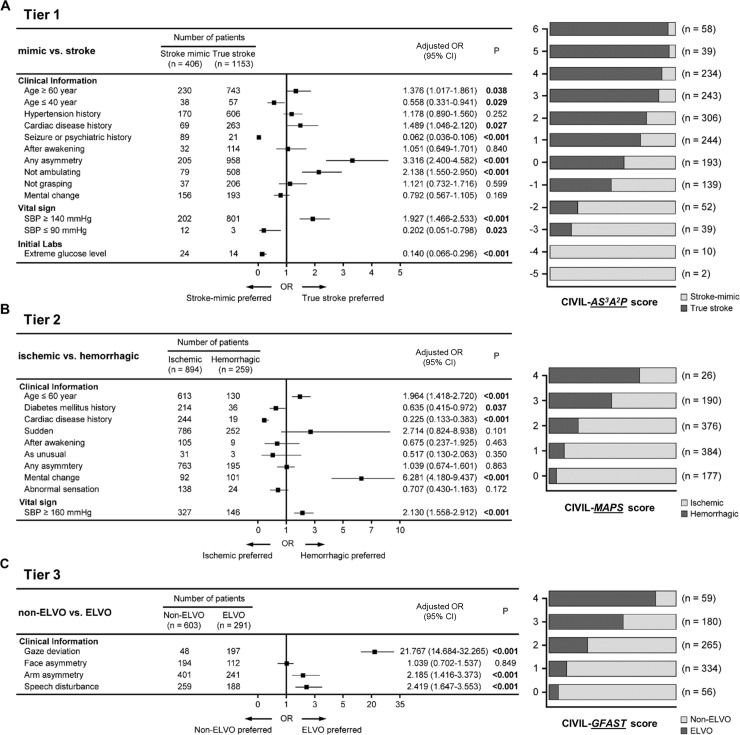
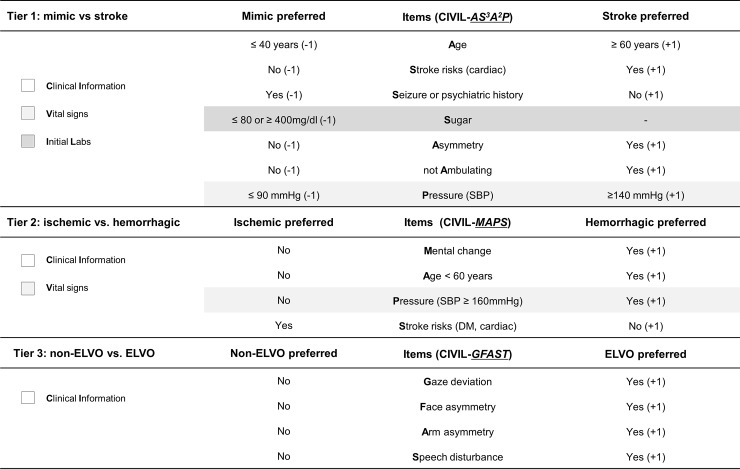
We determined independently significant factors in the CIVIL system using multiple regression analysis (Fig 1A). Age (≥60 years), Stroke risks (history of cardiac disease), “any Asymmetry”, and “not Ambulating” were positive discriminatory items for diagnosis of true stroke, while younger Age (≤40 years), history of Seizure or psychiatric disease were negative discriminatory items. In vital signs and laboratory data, high BP (systolic BP ≥140mmHg) was a positive discriminatory item, and low BP (systolic BP ≤90mmHg) and extreme Sugar level (≤80 or ≥400 mg/dL) were included as negative discriminatory items. Asymmetric leg weakness, non-lateralizing symptoms, mental change, and initial oxygen saturation were indiscriminate. The CIVIL-AS3A2P score was finally determined as overall 7 items (Fig 2): 5 clinical items, 1 vital sign, and 1 initial laboratory finding. The total score ranged from -5 to +6. Retrospective validation on 1,559 suspected stroke patients determined an optimal cut-off point for stroke diagnosis as ≥ +1. At this cut-off point, the diagnostic performance of CIVIL-AS3A2P score was as follows: sensitivity 82.1%, specificity 56.4%, positive predictive value (PPV) 84.3%, and negative predictive value (NPV) 52.6% (Youden’s index 0.385). We compared the performance of the CIVIL-ASAP score to the CPSS, LAPSS, and ROSIER scales in our data set. The sensitivity and specificity of these established recognition systems were 90.4% and 29.1% in CPSS, 69.7% and 67.7% in LAPSS, 93.8% and 34.0% in ROSIER. In ROC curve analysis, CIVIL-AS3A2P score had a superior diagnostic performance than the other three systems per area under the curve (S3 Fig, 0.767 in CIVIL-ASAP vs. 0.751 in ROSIER vs. 0.687 in LAPSS vs. 0.597 in CPSS). Comparisons among early stroke recognition scales were described in Fig 3.
Fig 1. Results of multiple regression analysis and distribution of patients according to the CIVIL scores.
(A) Tier 1: CIVIL-AS3A2P score consisted of Age (≤40 years or ≥60 years), Stroke risk (cardiac disease history) without Seizure or psychiatric history, extreme Sugar level (≤80 or ≥400mg/dl), any Asymmetry, not Ambulating, and Pressure (SBP≤90 mmHg or ≥140mmHg). (B) Tier 2: CIVIL-MAPS included Mental change, Age (≤60 years), Pressure (SBP≥160mmHg), and no Stroke risks (history of diabetes mellitus or cardiac disease). (C) Tier 3: To identify emergent large vessel occlusion patients, GFAST score was incorporated into the CIVIL scoring system (Gaze, Face asymmetry, Arm asymmetry, Speech disturbance).
Fig 2. Summary of items and scoring in the CIVIL system.
In tier 1 (CIVIL-AS3A2P), 7 items which included clinical information (white), vital signs (grey), and initial labs (dark grey) were used. Stroke-preferred items were assigned positive points and stroke mimic preferred items were negative points (ranged from ―5 to +6). Tier 2 (CIVIL-MAPS) allocated 4 items with clinical information and vital signs, and the GFAST system was applied in tier 3 (CIVIL-GFAST) for the selection of ELVO patients.
Fig 3. Descriptive comparison of various early stroke recognition scales.
CIVIL = Clinical Information, Vital signs, and Initial Labs, CPSS = Cincinnati Prehospital Stroke Scale, LAPSS = Los Angeles Prehospital Stroke Screen, ROSIER = Recognition Of Stroke In the Emergency Room.
Tier 2: Ischemic vs. hemorrhagic stroke: CIVIL-MAPS
To differentiate between ischemic and hemorrhagic stroke, second tier analysis using multiple regression was conducted (Fig 1B). MAPS: Mental change, Age below 60 years, high blood Pressure (systolic BP ≥160mmHg), no Stroke risk (without history of diabetes or cardiac disease) were positive discriminatory items for diagnosis of hemorrhagic stroke (Fig 2). The CIVIL-MAPS score consisted of 3 clinical items and 1 vital sign, and the total score ranged from 0 to +4. Retrospective validation on the 1,153 true stroke patients determined an optimal cut-off point for hemorrhagic stroke diagnosis as ≥ 2. At this cut-off point, the diagnostic performance of CIVIL-MAPS score was as follows: sensitivity of 47.5%, specificity of 85.4%, PPV of 50.6%, NPV of 83.8% (Youden’s index 0.329).
Tier 3: Non-ELVO vs. ELVO: CIVIL-GFAST
In the final tier, we applied the GFAST score to select ELVO patients (Fig 1C). The score was calculated as the sum of positive symptoms: Gaze deviation, Face asymmetry, Arm asymmetry, and Speech disturbance (Fig 2). Retrospective validation on 894 ischemic stroke patients determined the optimal cut-off point for ELVO diagnosis as ≥ 3. At this cut-off point, the diagnostic performance of CIVIL-GFAST score was as follows: sensitivity of 66.5%, specificity of 79.8%, PPV of 54.6%, NPV of 86.7% (Youden’s index 0.463). The CIVIL scoring system is summarized in Fig 2.
Discussion
Our data support that the CIVIL scoring system is feasible for identifying suspicious acute stroke patients in a stepwise fashion: true stroke or not, hemorrhagic stroke or not, and ELVO or not. In addition, step-by-step acronyms (AS3A2P, MAPS, and GFAST) can be used in a wide range of fields of prehospital and ER-based situations to serve as triaging tools for patients to be easily remembered.
The CIVIL scoring system can help us to differentiate different types of stroke at the same time. Acute stroke is an urgent condition that requires rapid evaluation and proper management because the longer a stroke goes untreated, the greater the brain damage (time is brain) [22]. Efficient triaging is important for acute stroke patients to guide proper disposition and early interventions, which may be entirely decisive in some cases [23,24]. Due to limited time window for thrombolytic therapy, numerous prehospital scoring systems for early recognition of ischemic stroke have been developed [8,14,15]. Recently, endovascular recanalization therapy in ELVO patients has been proven as the standard treatment, consequently, several scoring systems to recognize ELVO have been addressed [19–21]. However, current scoring systems have focused only on one aspect of stroke identification: stroke versus stroke mimic, or ELVO discrimination [25]. In addition, little has been elucidated to distinguish hemorrhagic stroke from ischemic stroke especially in situations with limited imaging facilities [26]. To the best of our knowledge, there has been no definitive scoring system that integrates various aspects of stroke diagnosis.
This scoring system features a stepwise approach to triage stroke suspicious patients. When paramedics in emergency medical service (EMS) or ER physicians proceed step by step, they will be able to properly classify stroke patients who require rapid treatment. CIVIL-AS3A2P initially differentiates patients with true stroke from stroke mimics. In this first tier, it is important not to exclude potential patients who need. In this context, as we expected, CIVIL-AS3A2P showed relatively high sensitivity and low specificity for including all possible candidates. Second (CIVIL-MAPS) and third tiers (CIVIL-GFAST) showed low sensitivity and high specificity so that patients in need of urgent treatments (thrombolysis and/or endovascular therapy) could be selected effectively. The CIVIL scoring system enables rapid identification of patients delivered to the ER with high sensitivity to identify the actual stroke, and also enables the recognition of hemorrhagic stroke and ELVO with high specificity.
The CIVIL scoring system included objective vital signs and laboratory findings as well as clinical information. Previous scoring systems consisting of clinical manifestations may be affected by the examiner’s experience and special training is needed to reduce inter-observer variability [27]. Some validation studies on early recognition scoring systems reported high variability in inter-observer reliability ranging from 69% to 90% [14,28]. To compensate for these variations, ROSIER [8] and LAPSS [15] included laboratory finding such as blood glucose levels. For this reason, the CIVIL scoring system contained vital signs in addition to laboratory findings designed to apply more objective parameters. Moreover, the extreme values of vital signs and laboratory findings-blood glucose level ≤ 80 or ≥ 400mg/dl and systolic BP ≥140 mmHg or ≤ 90 mmHg help to discriminate stroke mimic conditions such as sepsis, shock, or syncope. Therefore, our new scoring system could overcome some potential limitations of other previous scoring systems by including objective and quantitative items.
In this study, the CIVIL scoring system was developed for use in both ER and prehospital settings. The selection of acute stroke patients in the prehospital and emergent setting continue to be the subject of research due to the time-dependent nature of stroke [27]. Various early recognition systems have been used in the EMS to properly transport stroke patients to more appropriate centers. However, there have been several limitations including inconvenience, imperfect accuracy, and time-consuming training [19,20]. Moreover, an increase in items adds complexity to the system for rapid evaluation [29]. The CIVIL scoring system applies an intuitive and easy-to-remember acronym for EMS and other medical professionals to be easily used. We applied simple and familiar GFAST to improve accessibility in the third tier for the identification of ELVO patients.
Our study has some limitations. First, it is an observational study with retrospective nature; however, all information has been automatically stored in a prospectively-collecting database at a large regional emergency and stroke center. Second, data with time windows over 6 hours were not covered in the current study. From recent trials, mechanical thrombectomy is indicated up to 24 hours after stroke onset. Nevertheless, most patients with onset to treatment time less than 6 hours need more urgent treatment regardless of core-penumbra mismatch, so that our recognition system can more properly apply to those patients. Third, there are limits in the conclusions that can be drawn regarding the performance of the CIVIL system in patients with posterior circulation acute ischemic stroke. This scoring system was designed to focus on patients with anterior circulation which is supported by the current guideline for endovascular treatment. Finally, the sensitivity of tier 2 and 3 are less than 80%, the results should be interpreted with caution. In the future, prospective validation of the CIVIL scoring system should include a systematic education program for paramedics to improve performance.
In conclusion, the CIVIL scoring system can be used as a comprehensive and versatile tool to recognize true stroke and identify stroke subtypes simultaneously.
Supporting information
(DOC)
(XLSX)
(Cubic S model)
(PDF)
(PDF)
(PDF)
List of abbreviations
- CIVIL
Clinical Information, baseline Vital sign, and Initial Labs
- CPSS
Cincinnati Prehospital Stroke Scale
- CT
computed tomography
- ELVO
emergent large vessel occlusion
- EMR
Electronic Medical Record
- EMS
emergency medical service
- ER
emergency room
- GFAST
gaze to face-arm-speech-time
- LAPSS
Los Angeles Prehospital Stroke Screen
- OR
odds ratio
- ROC
receiver operating characteristic
- ROSIER
Recognition Of Stroke In the Emergency Room
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Korea Centers for Disease Control and Prevention (KCDC, M2020-A0258-00012) and the Korean Stroke Society (KSS).
References
- 1.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016; 15(9):913–24. 10.1016/S1474-4422(16)30073-4 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. Time is brain—quantified. Stroke. 2006; 37(1):263–6. 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 3.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010; 375(9727):1695–703. 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387(10029):1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira Manoel AL, Goffi A, Zampieri FG, Turkel-Parrella D, Duggal A, Marotta TR, et al. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit Care. 2016; 20:272 10.1186/s13054-016-1432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014; 9(7):840–55. 10.1111/ijs.12309 [DOI] [PubMed] [Google Scholar]
- 7.Jiang B, Ru X, Sun H, Liu H, Sun D, Liu Y, et al. Pre-hospital delay and its associated factors in first-ever stroke registered in communities from three cities in China. Sci Rep. 2016; 6:29795 10.1038/srep29795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nor AM, Davis J, Sen B, Shipsey D, Louw SJ, Dyker AG, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 2005; 4(11):727–34. 10.1016/S1474-4422(05)70201-5 [DOI] [PubMed] [Google Scholar]
- 9.Purrucker JC, Hametner C, Engelbrecht A, Bruckner T, Popp E, Poli S. Comparison of stroke recognition and stroke severity scores for stroke detection in a single cohort. J Neurol Neurosurg Psychiatry. 2015; 86(9):1021–8. 10.1136/jnnp-2014-309260 [DOI] [PubMed] [Google Scholar]
- 10.Walker GB, Zhelev Z, Henschke N, Fridhandler J, Yip S. Prehospital Stroke Scales as Screening Tools for Early Identification of Stroke and Transient Ischemic Attack. Stroke. 2019; 50(10):e285–e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhelev Z, Walker G, Henschke N, Fridhandler J, Yip S. Prehospital stroke scales as screening tools for early identification of stroke and transient ischemic attack. Cochrane Database Syst Rev. 2019; 4:CD011427 10.1002/14651858.CD011427.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nor AM, McAllister C, Louw SJ, Dyker AG, Davis M, Jenkinson D, et al. Agreement between ambulance paramedic- and physician-recorded neurological signs with Face Arm Speech Test (FAST) in acute stroke patients. Stroke. 2004; 35(6):1355–9. 10.1161/01.STR.0000128529.63156.c5 [DOI] [PubMed] [Google Scholar]
- 13.Oostema JA, Konen J, Chassee T, Nasiri M, Reeves MJ. Clinical predictors of accurate prehospital stroke recognition. Stroke. 2015; 46(6):1513–7. 10.1161/STROKEAHA.115.008650 [DOI] [PubMed] [Google Scholar]
- 14.Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999; 33(4):373–8. 10.1016/s0196-0644(99)70299-4 [DOI] [PubMed] [Google Scholar]
- 15.Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000; 31(1):71–6. 10.1161/01.str.31.1.71 [DOI] [PubMed] [Google Scholar]
- 16.Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, et al. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke. 2005; 36(7):1597–616. 10.1161/01.STR.0000170622.07210.b4 [DOI] [PubMed] [Google Scholar]
- 17.Yang HJ, Jeon W, Yang HJ, Kwak JR, Seo HY, Lee JS. The Clinical Differences between Urgent Visits and Non-Urgent Visits in Emergency Department During the Neonatal Period. J Korean Med Sci. 2017; 32(11):1870–5. 10.3346/jkms.2017.32.11.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Hong JM, Choi SC, Jung YS. "Cubic S Model": A for Early Recognition of Acute Ischemic Stroke Patients. J Korean Soc Emerg Med. 2010;21(3):313–320. [Google Scholar]
- 19.Perez de la Ossa N, Carrera D, Gorchs M, Querol M, Millan M, Gomis M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014; 45(1):87–91. 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 20.Teleb MS, Ver Hage A, Carter J, Jayaraman MV, McTaggart RA. Stroke vision, aphasia, neglect (VAN) assessment-a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices. J Neurointerv Surg. 2017; 9(2):122–6. 10.1136/neurintsurg-2015-012131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheitz JF, Abdul-Rahim AH, MacIsaac RL, Cooray C, Sucharew H, Kleindorfer D, et al. Clinical Selection Strategies to Identify Ischemic Stroke Patients With Large Anterior Vessel Occlusion: Results From SITS-ISTR (Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Registry). Stroke. 2017; 48(2):290–7. 10.1161/STROKEAHA.116.014431 [DOI] [PubMed] [Google Scholar]
- 22.Fransen PS, Berkhemer OA, Lingsma HF, Beumer D, van den Berg LA, Yoo AJ, et al. Time to Reperfusion and Treatment Effect for Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2016; 73(2):190–6. 10.1001/jamaneurol.2015.3886 [DOI] [PubMed] [Google Scholar]
- 23.Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000; 55(11):1649–55. 10.1212/wnl.55.11.1649 [DOI] [PubMed] [Google Scholar]
- 24.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004; 363(9411):768–74. 10.1016/S0140-6736(04)15692-4 [DOI] [PubMed] [Google Scholar]
- 25.Brandler ES, Sharma M, Sinert RH, Levine SR. Prehospital stroke scales in urban environments: a systematic review. Neurology. 2014; 82(24):2241–9. 10.1212/WNL.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainin M, Teuschl Y, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol. 2007; 6(6):553–61. 10.1016/S1474-4422(07)70005-4 [DOI] [PubMed] [Google Scholar]
- 27.Vidale S, Agostoni E. Prehospital stroke scales and large vessel occlusion: A systematic review. Acta Neurol Scand. 2018; 138(1):24–31. 10.1111/ane.12908 [DOI] [PubMed] [Google Scholar]
- 28.Demeestere J, Garcia-Esperon C, Lin L, Bivard A, Ang T, Smoll NR, et al. Validation of the National Institutes of Health Stroke Scale-8 to Detect Large Vessel Occlusion in Ischemic Stroke. J Stroke Cerebrovasc Dis. 2017; 26(7):1419–26. 10.1016/j.jstrokecerebrovasdis.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 29.Turc G, Maier B, Naggara O, Seners P, Isabel C, Tisserand M, et al. Clinical Scales Do Not Reliably Identify Acute Ischemic Stroke Patients With Large-Artery Occlusion. Stroke. 2016; 47(6):1466–72. 10.1161/STROKEAHA.116.013144 [DOI] [PubMed] [Google Scholar]