Abstract
Objective
A small thigh circumference is associated with an increased risk of diabetes, cardiovascular diseases, and total mortality. The purpose of this study was to evaluate the association between thigh circumference and hypertension in the middle-aged and elderly population.
Methods
A total of 9520 individuals aged 40 years and older with measurement of thigh circumference were available for analysis. The measurement of thigh circumference was performed directly below the gluteal fold of the thigh. The association of thigh circumference with hypertension was tested in logistic regression analyses and reported as odds ratio (OR) with 95% CI.
Results
Thigh circumference was negatively correlated with systolic blood pressure, diastolic blood pressure, fasting glucose, and total cholesterol. Compared with the lowest thigh circumference tertile group, the risk of hypertension was significantly lower in the highest tertile group, both in overweight individuals (OR 0.68; 95% CI 0.59–0.79, P < 0.001) and obese individuals (OR 0.51; 95% CI 0.38–0.70, P < 0.001).
Conclusion
In the present study, large thigh circumference is associated with lower risk of hypertension in overweight and obese Chinese individuals.
Keywords: thigh circumference, hypertension, overweight, obesity
Introduction
Hypertension, characterized by chronically elevated blood pressure (BP) above 140/90 mmHg, is a major public health problem affecting more than 1 billion people worldwide (1, 2). Despite multiple methods to prevent and manage high blood pressure, the global incidence and prevalence of hypertension continues to increase significantly (3). Convincing evidence demonstrated that hypertension, untreated or uncontrolled, is a crucial contributor to cardiovascular disease, stroke, and chronic kidney diseases (2). Consequently, hypertension is the leading cause of mortality and disability globally, which results in a huge health burden (4). Startlingly, more than half of individuals with elevated BP were unaware of their hypertensive status in numerous surveys, partly owing to hypertension rarely showing symptoms in the early stages (3, 4, 5). Hence, hypertension is a silent killer.
Previous research has demonstrated that intra-abdominal visceral deposition of adipose tissue, which characterizes upper-body obesity, plays a significant role in the development of hypertension (6, 7). Conversely, subjects with lower-body obesity exhibited a favorable metabolic profile (8). Due to the advantages of simplicity, convenience, harmlessness, and low cost, anthropometric measurements, especially circumferences, are useful options for estimating fat distribution and body composition, particularly in large-scale population-based studies. Certain circumferences have shown good performance in evaluating nutritional status, assessing obesity, screening for ectopic lipid deposits, and identifying decline of muscle mass (9, 10, 11). Moreover, some abnormal circumferences are marks of increased risk of certain diseases (12). For instance, a small thigh circumference was associated with greater risk of diabetes (13), cardiovascular diseases, and total mortality (14) in both men and women. Additionally, it is well established that waist circumference is positively correlated with blood pressure (15) and that a high waist–thigh circumference ratio is a risk factor for hypertension (16). Data indicate that thigh circumference may be an indicator of hypertension.
To date, however, evidence from large-scale populations about the association between thigh circumference and hypertension is scarce. The aim of the present study was to examine the relationship between thigh circumference and blood pressure.
Methods
Study population
The study is a part of the Risk Evaluation of cAncers in Chinese diabeTic Individuals: A lONgitudinal (REACTION) study, which was a community-based cross-sectional study conducted among 259,657 Chinese individuals aged 40 years and older, from 2011 to 2012 (17). The study design and methods have been described previously in detail (17, 18, 19). The data presented in this study are based on the baseline survey of subsamples from the Chongming District in Shanghai, China. A total of 9930 eligible subjects participated in the research. The individuals with data missing for thigh circumference, blood pressure (BP), and metabolic variables were excluded. The final model included 9520 subjects (3095 men and 6425 women).
Data collection
A standardized questionnaire was used by certified medical workers to gather essential information, including sex, age, smoking and alcohol consumption habits, physical activity, education status, and previous medical history. Anthropometric measurements were performed by trained physicians using standard protocols. Thigh circumference was measured directly below the gluteal fold of the thigh. The final thigh circumference is the average value of left thigh circumference and right thigh circumference. BP was measured with an automated electronic device (OMRON Model1 Plus; Omron Company, Kyoto, Japan) after the subjects sat down and rested quietly for >5 min. BP measurements were taken three times and the mean was recorded as the BP result. Overweight was defined as 24 kg/m2 ≤ BMI < 28 kg/m2 and obesity was defined as BMI ≥ 28 kg/m2.
Biochemical measurements
Peripheral venous blood samples were collected after 10 h of overnight fasting. Plasma glucose level was assessed by the glucose oxidase method (ADVIA-1650 Chemistry System, Bayer). Triglycerides, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and total cholesterol were measured by an automatic analyzer (Hitachi 7080). Fasting insulin was assessed by RIA (Linco Research). Insulin resistance was evaluated by the homeostasis model of assessment for insulin resistance (HOMA-IR).
Definition of hypertension
Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure (DBP) ≥ 90 mmHg, or current use of antihypertensive medications (20).
Statistical analysis
Normally distributed continuous variables were presented as means ± s.d., and variables with skewed distribution were reported as medians (interquartile range) and log transformed to approximate normality before analysis. Categorical variables were described by percentage (%). P values were calculated by Student’s t-test (for continuous parametric variables) or nonparametric Mann–Whitney U test (for continuous nonparametric variables) and by Chi-squared tests for categorical variables. Multiple linear regression analyses were used to assess the association between thigh circumference and metabolic features. Multivariate logistic regression models were applied to estimate the odds ratios (ORs) for hypertension. Potential confounding variables including age, gender, smoking, alcohol drinking, physical activity, educational status, C-reactive protein (CRP), adiponectin, BMI, and HOMA-IR were controlled in the regression models. Data management and statistical analysis were performed with SPSS software (version 23.0). The significance level was set at P < 0.05, and P values were provided for two-sided tests.
Ethics statement
The study protocol was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from all participants.
Results
Characteristics of participants with or without incident hypertension
The mean thigh circumference was 53.3 ± 5.0 cm for men and 52.6 ± 4.7 cm for women (P < 0.001), respectively. Subjects with hypertension were more likely to be older, a smoker, and with a higher BMI, waist circumference, fasting blood glucose, HOMA-IR, total cholesterol and triglyceride level (Table 1).
Table 1.
Baseline characteristics of the study participants.
| Characteristics | Men | P value | Women | P value | ||
|---|---|---|---|---|---|---|
| Normotension | Hypertension | Normotension | Hypertension | |||
| n | 1363 | 1732 | 3586 | 2839 | ||
| Age, year(s) | 56.23 ± 7.71 | 58.83 ± 7.24 | <0.001 | 53.49 ± 7.68 | 57.90 ± 7.23 | <0.001 |
| Smoking (yes), % | 39.5 | 45.7 | 0.005 | 3.8 | 5.9 | <0.001 |
| Alcohol (yes), % | 48.3 | 48.9 | 0.392 | 10.2 | 10.6 | 0.08 |
| Education, % | ||||||
| 0–6 | 16.1 | 21.3 | 0.001 | 19 | 30.1 | <0.001 |
| 7–9 | 51.5 | 48.5 | 48.8 | 49.2 | ||
| ≥10 | 32.4 | 30.2 | 32.3 | 20.7 | ||
| Physical activity | ||||||
| Low | 70.2 | 71.6 | 0.541 | 72.3 | 72.9 | 0.536 |
| Moderate | 21.7 | 20.1 | 20.2 | 19.9 | ||
| High | 8.1 | 8.3 | 7.5 | 7.2 | ||
| SBP, mmHg | 121 ± 10 | 144 ± 17 | <0.001 | 118 ± 11 | 144 ± 16 | <0.001 |
| DBP, mmHg | 77 ± 7 | 87 ± 9 | <0.001 | 75 ± 7 | 85 ± 10 | <0.001 |
| BMI, kg/m2 | 24.04 ± 3.20 | 25.62 ± 3.23 | <0.001 | 23.80 ± 2.93 | 25.46 ± 3.28 | <0.001 |
| Waist circumference, cm | 85.2 ± 9.5 | 89.8 ± 9.5 | <0.001 | 80.9 ± 9.5 | 86.4 ± 11.4 | <0.001 |
| WHR | 0.89 ± 0.07 | 0.91 ± 0.06 | <0.001 | 0.85 ± 0.08 | 0.09 ± 0.16 | <0.001 |
| FPG, mmol/L | 6.35 ± 1.79 | 6.73 ± 1.91 | <0.001 | 5.91 ± 1.34 | 6.49 ± 1.82 | <0.001 |
| Insulin, µU/L | 5.40 (3.80–7.70) | 6.50 (4.50–9.00) | <0.001 | 6.40 (4.80–8.60) | 7.70 (5.60–10.70) | <0.001 |
| HOMA‐IR | 1.53 (0.99–2.30) | 1.88 (1.29–2.75) | <0.001 | 1.65 (1.24–2.38) | 2.24 (1.54–3.19) | <0.001 |
| CRP, μg/mL | 4.89 ± 6.12 | 5.24 ± 6.18 | 0.462 | 4.63 ± 5.39 | 5.27 ± 6.11 | 0.427 |
| Adiponectin, µg/mL | 12.6 (7.9–15.7) | 10.9 (6.7–13.2) | 0.023 | 11.9 (7.2–14.6) | 9.5 (6.2–12.6) | 0.014 |
| Triglycerides, mmol/L | 1.32 (0.94–2.04) | 1.53 (1.06–2.33) | <0.001 | 1.19 (0.87–1.73) | 1.52 (1.09–2.20) | <0.001 |
| TC, mmol/L | 4.49 ± 0.99 | 4.61 ± 1.01 | 0.002 | 4.58 ± 1.04 | 4.85 ± 1.03 | <0.001 |
| LDL-c, mmol/L | 2.53 ± 0.73 | 2.58 ± 0.75 | 0.077 | 2.58 ± 0.77 | 2.72 ± 0.79 | 0.094 |
| HDL-c, mmol/L | 1.17 ± 0.32 | 1.15 ± 0.32 | 0.066 | 1.26 ± 0.31 | 1.24 ± 0.31 | 0.097 |
| Thigh circumference, cm | 53.2 ± 5.0 | 53.4 ± 5.1 | 0.143 | 52.6 ± 4.5 | 52.6 ± 4.7 | 0.825 |
Continuous data are shown as means ± s.d. or medians with interquartile range and categorical data as percentage (%). P values were calculated by Student’s t-test (for continuous parametric variables) or nonparametric Mann–Whitney U test (for continuous nonparametric variables) and by Chi-squared tests for categorical variables.
CRP, C-reactive protein; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, the homeostatic model assessment of insulin resistance; LDL-c, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; WHR, waist–hip ratio.
The thigh circumference of hypertensive patients is lower than normotensive subjects
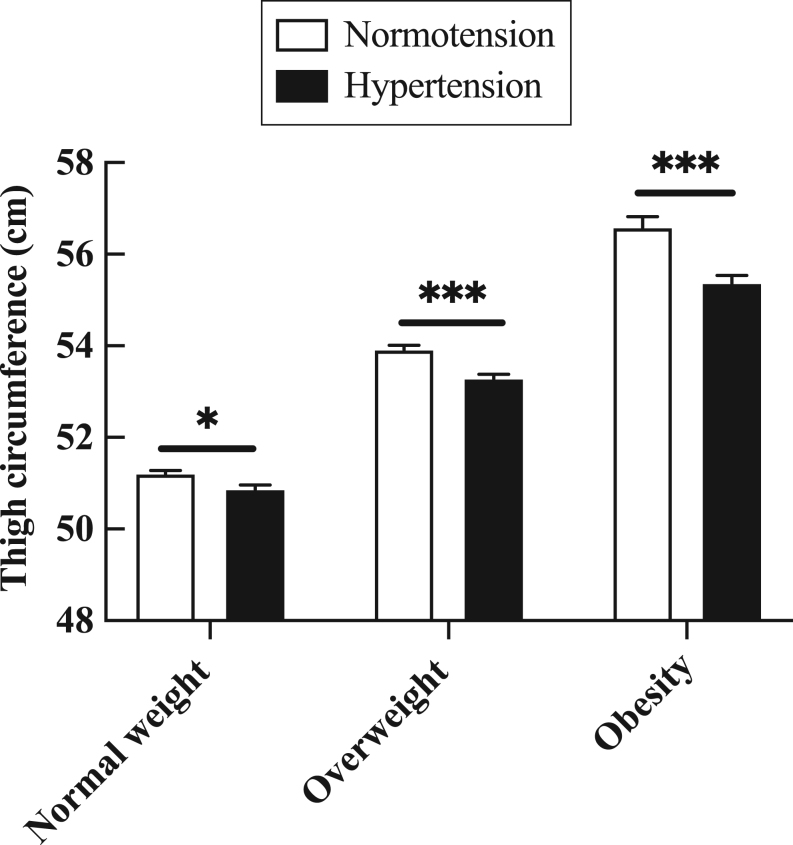
Compared with normotensive individuals, the thigh circumference of subjects with hypertension was lower (normal weight: 51.2 ± 4.2 cm vs 50.9 ± 4.2 cm, P = 0.011; overweight: 53.9 ± 4.4 cm vs 53.3 ± 4.4 cm, P < 0.001; obese: 56.6 ± 5.1 cm vs 55.4 ± 5.5 cm, P < 0.001) (Fig. 1).
Figure 1.
The thigh circumference was smaller in subjects with hypertension than individuals with normotension. Data are presented as mean ± s.e.m. P values were calculated by Student’s t-test. *P < 0.05; **P < 0.01, ***P < 0.001.
Large thigh circumference is associated with lower prevalence of hypertension in overweight and obese individuals
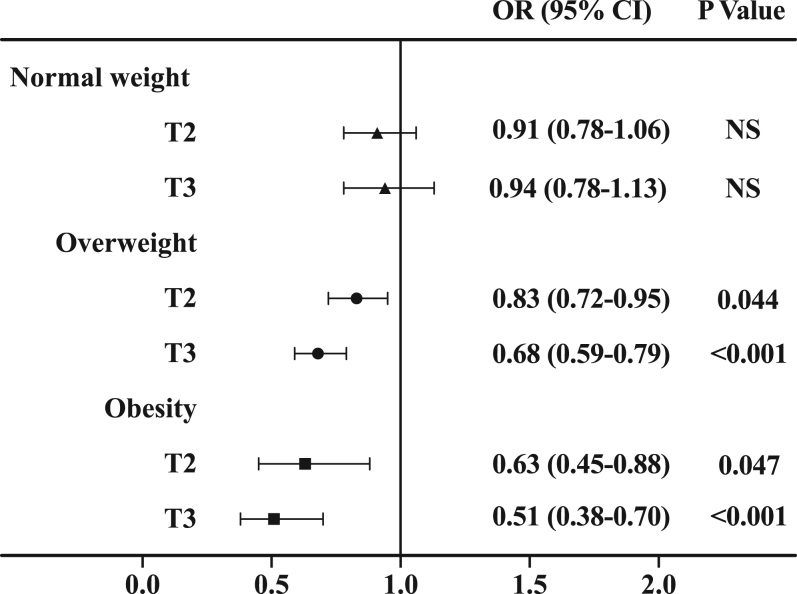
The subjects were divided into three groups (T1, T2, and T3) according to the tertiles of thigh circumference. The tertile ranges of thigh circumference were ≤51.2, 51.2–55.3, and ≥55.3 cm in men and ≤50.7, 50.7–54.4, and ≥54.4 cm in women, respectively. Along with the increased tertile of thigh circumference, the prevalence of hypertension gradually decreased in both overweight (men: 64.6% vs 61.3% vs 55.1%, P = 0.046; women: 53.8% vs 48.1% vs 43.5%, P = 0.004) and obese individuals (men: 79.5% vs 75.2% vs 71.5%, P = 0.047; women: 74.5% vs 69.3% vs 59.0%, P < 0.001).
We used multivariate logistic regression models, with the lowest tertile group (T1) as a reference, to evaluate the association between thigh circumference and risk of incident hypertension. As is shown in Table 2, the ORs for hypertension decreased when thigh circumference tertiles increased (P < 0.001). In the highest tertile group (T3), the OR (95% CI) for hypertension was 0.83 (0.71–0.96) in overweight individuals and 0.56 (0.41–0.75) in obese individuals compared with the T1 group, after adjusting for age, gender, smoking, alcohol consumption, education status, physical activity, CRP, and adiponectin (Model 2). Moreover, after further adjustment for BMI and waist circumference, the association between thigh circumference and hypertension remained statistically significant in overweight individuals (OR 0.68; 95% CI, 0.59–0.79; P < 0.001) and obese individuals (OR 0.51; 95% CI, 0.38–0.70; P < 0.001) (Model 4). Therefore, large thigh circumference was associated with a lower risk of hypertension among overweight and obese individuals, independent of BMI and waist circumference. Additionally, the correlation between thigh circumference and hypertension was stronger in obese individuals than in overweight individuals (Fig. 2). However, we did not observe a significant association between thigh circumference and hypertension in normal weight individuals (P > 0.05).
Table 2.
Adjusted ORs (95% CI) of hypertension according to tertiles of thigh circumference.
| Characteristics | T1 | T2 | T3 | P value for trend |
|---|---|---|---|---|
| (n = 3177) | (n = 3187) | (n = 3156) | ||
| Normal weight (n = 4172) | ||||
| Model 1 | 1 | 0.97 (0.84–1.12) | 1.05 (0.87–1.26) | 0.72 |
| Model 2 | 1 | 1.02 (0.87–1.20) | 1.09 (0.98–1.35) | 0.21 |
| Model 3 | 1 | 0.91 (0.79–1.06) | 0.95 (0.78–1.14) | 0.49 |
| Model 4 | 1 | 0.91 (0.78–1.06) | 0.94 (0.78–1.13) | 0.45 |
| Overweight (n = 3864) | ||||
| Model 1 | 1 | 0.87 (0.76–0.99) | 0.86 (0.75–0.98) | 0.024 |
| Model 2 | 1 | 0.86 (0.75–0.99) | 0.83 (0.71–0.96) | 0.020 |
| Model 3 | 1 | 0.85 (0.73–0.99) | 0.69 (0.59–0.81) | <0.001 |
| Model 4 | 1 | 0.83 (0.72–0.95) | 0.68 (0.59–0.79) | <0.001 |
| Obese (n = 1484) | ||||
| Model 1 | 1 | 0.67 (0.48–0.93) | 0.57 (0.42–0.77) | 0.001 |
| Model 2 | 1 | 0.65 (0.46–0.90) | 0.56 (0.41–0.75) | 0.001 |
| Model 3 | 1 | 0.64 (0.46–0.89) | 0.52 (0.38–0.70) | <0.001 |
| Model 4 | 1 | 0.63 (0.45–0.88) | 0.51 (0.38–0.70) | <0.001 |
Model 1: adjusted for age and sex; Model 2: further adjusted for smoking, drinking, physical activity, education status, CRP, and adiponectin; Model 3: further adjusted for BMI; Model 4: further adjusted for waist circumference. T1 is the reference group. Multivariate logistic regression models were applied.
Figure 2.
Adjusted ORs of hypertension according to tertiles of thigh circumference. The ORs with corresponding 95% CIs were adjusted for age, gender, life factors, CRP, adiponectin, BMI, and waist circumference. T1 is the reference group.
Subgroup analyses
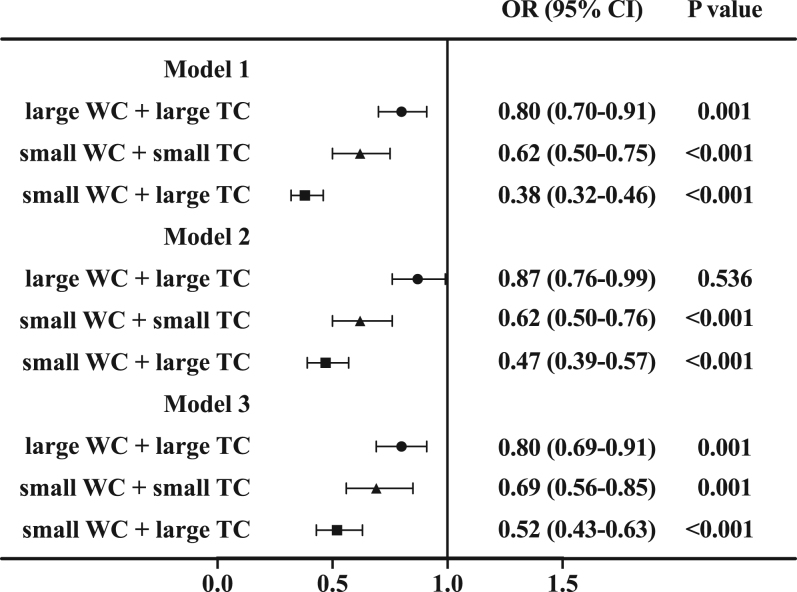
To evaluate the effect of waist circumference on the association of thigh circumference and blood pressure, we further combined thigh circumference and waist circumference in statistical analysis. We divided participants into four subgroups based on the medians of thigh circumference and waist circumference. The OR for hypertension was lower in the large thigh circumference with small waist circumference subgroup than in other subgroups (OR 0.52; 95% CI, 0.43–0.63; P < 0.001) after adjustment for age, sex, life factor, CRP, adiponectin, and BMI in overweight and obese individuals (Fig. 3). Individuals with small thigh circumference and large waist circumference form the reference group.
Figure 3.
The association of thigh circumference with hypertension in different subgroups of waist circumference. WC, waist circumference; TC, thigh circumference. Model 1 was unadjusted; Model 2 was adjusted for age, sex, life factor, CRP, and adiponectin; Model 3 was further adjusted for BMI. Large waist circumference with small thigh circumference is the reference group.
Thigh circumference is negatively correlated with SBP and DBP
Multiple linear regression analyses indicated that thigh circumference was positively correlated with HDL-c, triglycerides, and insulin and was negatively correlated with SBP, DBP, fasting glucose, and total cholesterol (Table 3).
Table 3.
Adjusted regression coefficients of thigh circumference with metabolic parameters.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| β (s.e.m.) | P | β (s.e.m.) | P | β (s.e.m.) | P | β (s.e.m.) | P | |
| SBP (mmHg) | −0.143 (0.084) | <0.001 | −0.146 (0.089) | <0.001 | −0.145 (0.090) | <0.001 | −0.160 (0.089) | <0.001 |
| DBP (mmHg) | −0.102 (0.046) | <0.001 | −0.111 (0.049) | <0.001 | −0.110 (0.049) | <0.001 | −0.123 (0.049) | <0.001 |
| FPG (mmol/L) | −0.032 (0.005) | 0.020 | −0.033 (0.005) | 0.018 | −0.034 (0.005) | 0.013 | −0.040 (0.005) | 0.004 |
| 2-h glucose (mmol/L) | 0.029 (0.020) | 0.268 | 0.049 (0.021) | 0.086 | 0.050 (0.021) | 0.079 | 0.047 (0.021) | 0.099 |
| Insulin (μU/mL) | 0.145 (0.016) | <0.001 | 0.153 (0.016) | <0.001 | 0.122 (0.017) | <0.001 | 0.098 (0.017) | <0.001 |
| HOMA-IR | 0.122 (0.006) | <0.001 | 0.136 (0.005) | <0.001 | 0.107 (0.006) | <0.001 | 0.084 (0.006) | <0.001 |
| TC (mmol/L) | −0.115 (0.005) | <0.001 | −0.116 (0.005) | <0.001 | −0.119 (0.005) | <0.001 | −0.119 (0.005) | <0.001 |
| LDL-c (mmol/L) | −0.021 (0.004) | 0.422 | −0.018 (0.004) | 0.529 | −0.018 (0.004) | 0.539 | −0.018 (0.004) | 0.536 |
| HDL-c (mmol/L) | 0.276 (0.001) | <0.001 | 0.281 (0.001) | <0.001 | 0.280 (0.001) | <0.001 | 0.282 (0.001) | <0.001 |
| Triglycerides (mmol/L) | 0.068 (0.003) | <0.001 | 0.070 (0.003) | 0.013 | 0.068 (0.001) | 0.016 | 0.063 (0.001) | 0.025 |
Fasting insulin, HOMA-IR, and triglycerides were log-transformed before analysis. Model 1: adjusted for age, sex. Model 2: further adjusted for smoking, drinking, physical activity, education status, CRP, and adiponectin. Model 3: further adjusted for BMI. Model 4: further adjusted for waist circumference. Multiple linear regression analyses were used.
Discussion
In this study, we found that large thigh circumference is significantly associated with lower prevalence of hypertension in overweight and obese individuals, independent of age, BMI, and waist circumference. Moreover, thigh circumference is negatively correlated with both SBP and DBP.
Differences in body fat distribution patterns have long been linked to certain metabolic disease risks (21, 22). Previous studies have reported that upper-body and lower-body fat have opposite (harmful vs beneficial) correlations with long-term blood pressure and the risk of developing diabetes (23). Advanced and accurate measuring techniques such as dual-energy X-ray absorptiometry and CT are often used to assess body fat and muscle distribution. However, these sophisticated, time-consuming, and costly techniques are not always feasible, particularly in large-scale population studies. Due to convenience, harmlessness, and low cost, simple anthropometric measurements, such as waist circumferences, are often preferable. Large waist circumference is a proxy of abnormal accumulation of visceral fat along with adverse metabolism (15, 24). Conversely, large thigh circumference is considered to be protective for diabetes and cardiovascular disease, and a small thigh circumference seems to be associated with an increased risk of developing heart disease (14, 25).
There are two likely causes for the hypertensive risk difference between different thigh circumference groups. On the one hand, a small thigh circumference may involve low subcutaneous thigh fat. Several studies reported that, independently of high abdominal fat, low subcutaneous thigh fat contributed to adverse lipid and glucose metabolism, while high subcutaneous thigh fat was associated with favourable lipid and glucose metabolism (26). It is established that thigh subcutaneous adipose is negatively associated with fasting glucose, post load glucose, and HOMA-IR (27, 28). Moreover, greater thigh-fat deposition exhibited a more favorable metabolic profile including lower triglycerides and LDL-c (26), and thigh adipocytes were resistant to epinephrine-stimulated lipolysis, presumably due to an increase in alpha-adrenergic receptors (29). Therefore, the adverse glucose and lipid metabolism that mediates vascular dysfunction may link low thigh circumference to increased risk of elevated blood pressure.
On the other hand, small thigh circumference may indicate a low thigh skeletal muscle mass, which is closely associated with increased risk factors for hypertension and diabetes (30, 31). Clear evidence demonstrated that obese individuals with sarcopenia have a higher risk of hypertension than patients with obesity or sarcopenia alone (31, 32). Obesity increases the risk of hypertension, a decline in thigh skeletal muscle mass increases this risk further. This may partially explain why we only found a stronger correlation of small thigh circumference and increased risk of developing hypertension in overweight and obese subjects. Additionally, skeletal muscle is the predominant site for insulin-stimulated glucose uptake, a lower thigh skeletal muscle mass may induce or promote dysfunctional glucose metabolism.
The major strength of this study is that the analysis is performed in different BMI classifications. Many potential covariates were considered in the analysis to minimize the impact of confusing risk factors. However, there are several potential limitations to consider in this study. First, we could not quantify the thigh muscle mass, intermuscular adipose tissue, and subcutaneous fat accumulation by more accurate radiographic measures owing to limitations of epidemiological screening conditions. Therefore, the amount and size of thigh subcutaneous adipocyte and intermuscular adipocyte and thigh muscle mass are not clear. Second, since the current study is a cross-sectional analysis, we cannot draw conclusions on causality from our findings. Third, it is unclear whether our findings in overweight and obese middle‐aged and older Chinese subjects can be generalized to normal-weight or younger people of other ethnicities.
Conclusions
In summary, large thigh circumference is independently associated with lower prevalence of hypertension, while small thigh circumference is linked to higher prevalence of hypertension in overweight and obese Chinese individuals. Based on the observations, it is necessary to include thigh circumference in routine anthropometric measurements, when evaluating populations at high risk of hypertension, cardiovascular diseases, or diabetes.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the National Natural Science Foundation of China (81670743, 81370953); Shanghai Health System Outstanding Young Talents Training Program (XYQ2013098); and Shanghai Health and Family Planning Commission (21740173).
Availability of data
This study is a part of the Risk Evaluation of cAncers in Chinese diabeTic Individuals: A lONgitudinal (REACTION) study. All data analyzed in this study are based on REACTION study. The data are held in a secure and confidential database that can only be assessed by members of the REACTION group. The REACTION study website is http://www.rjh.com.cn/pages/neifenmike/REACTION/index.shtml.
Ethics approval and consent to participants
This study protocol was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from all participants.
Author contribution statement
Z Y, L Q, and Q S designed the study. Y N, W Z, X L, H Z, N L, H G, J W, and G N recruited the subjects, processed samples, and contributed to acquisition of data. Z Y and J S analyzed the data. J S wrote the manuscript. Z Y revised the manuscript.
Acknowledgements
The authors thank all the subjects who participated in the study and the hospital staffs for their contribution in sample and data collection.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Hypertension 2018. 71 1269–1324. ( 10.1161/HYP.0000000000000066) [DOI] [PubMed] [Google Scholar]
- 2.Fisher NDL, Curfman G. Hypertension-A public health challenge of global proportions. JAMA 2018. 320 1757–1759. ( 10.1001/jama.2018.16760) [DOI] [PubMed] [Google Scholar]
- 3.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, et al A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the lancet commission on hypertension. Lancet 2016. 388 2665–2712. ( 10.1016/S0140-6736(16)31134-5) [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016. 388 1659–1724. ( 10.1016/S0140-6736(16)31679-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, et al Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013. 310 959–968. ( 10.1001/jama.2013.184182) [DOI] [PubMed] [Google Scholar]
- 6.Sullivan CA, Kahn SE, Fujimoto WY, Hayashi T, Leonetti DL, Boyko EJ. Change in intra-abdominal fat predicts the risk of hypertension in Japanese Americans. Hypertension 2015. 66 134–140. ( 10.1161/HYPERTENSIONAHA.114.04990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eastwood SV, Tillin T, Wright A, Mayet J, Godsland I, Forouhi NG, Whincup P, Hughes AD, Chaturvedi N. Thigh fat and muscle each contribute to excess cardiometabolic risk in South Asians, independent of visceral adipose tissue. Obesity 2014. 22 2071–2079. ( 10.1002/oby.20796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism: Clinical and Experimental 1991. 40 733–740. ( 10.1016/0026-0495(91)90093-c) [DOI] [PubMed] [Google Scholar]
- 9.Berkley J, Mwangi I, Griffiths K, Ahmed I, Mithwani S, English M, Newton C, Maitland K. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. JAMA 2005. 294 591–597. ( 10.1001/jama.294.5.591) [DOI] [PubMed] [Google Scholar]
- 10.Manco M, Bedogni G, Marcellini M, Devito R, Ciampalini P, Sartorelli MR, Comparcola D, Piemonte F, Nobili V. Waist circumference correlates with liver fibrosis in children with non-alcoholic steatohepatitis. Gut 2008. 57 1283–1287. ( 10.1136/gut.2007.142919) [DOI] [PubMed] [Google Scholar]
- 11.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. European Heart Journal 2007. 28 850–856. ( 10.1093/eurheartj/ehm026) [DOI] [PubMed] [Google Scholar]
- 12.Yang GR, Yuan SY, Fu HJ, Wan G, Zhu LX, Bu XL, Zhang JD, Du XP, Li YL, Ji Y, et al Neck circumference positively related with central obesity, overweight, and metabolic syndrome in Chinese subjects with type 2 diabetes: Beijing Community Diabetes Study 4. Diabetes Care 2010. 33 2465–2467. ( 10.2337/dc10-0798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung KJ, Kimm H, Yun JE, Jee SH. Thigh circumference and diabetes: obesity as a potential effect modifier. Journal of Epidemiology 2013. 23 329–336. ( 10.2188/jea.je20120174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitmann BL, Frederiksen P. Thigh circumference and risk of heart disease and premature death: prospective cohort study. BMJ 2009. 339 b3292 ( 10.1136/bmj.b3292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okosun IS, Cooper RS, Rotimi CN, Osotimehin B, Forrester T. Association of waist circumference with risk of hypertension and type 2 diabetes in Nigerians, Jamaicans, and African-Americans. Diabetes Care 1998. 21 1836–1842. ( 10.2337/diacare.21.11.1836) [DOI] [PubMed] [Google Scholar]
- 16.Seidell JC, Bakx JC, De Boer E, Deurenberg P, Hautvast JG. Fat distribution of overweight persons in relation to morbidity and subjective health. International Journal of Obesity 1985. 9 363–374. [PubMed] [Google Scholar]
- 17.Ning G. & Reaction Study Group. Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. Journal of Diabetes 2012. 4 172–173. ( 10.1111/j.1753-0407.2012.00182.x) [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Yan C, Liu G, Niu Y, Zhang W, Lu S, Li X, Zhang H, Ning G, Fan J, et al Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: a cross-sectional analysis. Scientific Reports 2016. 6 37288 ( 10.1038/srep37288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin L, Yang Z, Gu H, Lu S, Shi Q, Xing Y, Li X, Li R, Ning G, Su Q. Association between serum uric acid levels and cardiovascular disease in middle-aged and elderly Chinese individuals. BMC Cardiovascular Disorders 2014. 14 26 ( 10.1186/1471-2261-14-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018. 39 3021–3104. ( 10.1093/eurheartj/ehy339) [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of Internal Medicine 2005. 165 777–783. ( 10.1001/archinte.165.7.777) [DOI] [PubMed] [Google Scholar]
- 22.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arteriosclerosis, Thrombosis, and Vascular Biology 2014. 34 1820–1826. ( 10.1161/ATVBAHA.114.303035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano Y, Vongpatanasin W, Ayers C, Turer A, Chandra A, Carnethon MR, Greenland P, de Lemos JA, Neeland IJ. Regional fat distribution and blood pressure level and variability: the Dallas heart study. Hypertension 2016. 68 576–583. ( 10.1161/HYPERTENSIONAHA.116.07876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentile CL, Weir TL, Cox-York KA, Wei Y, Wang D, Reese L, Moran G, Estrada A, Mulligan C, Pagliassotti MJ, et al The role of visceral and subcutaneous adipose tissue fatty acid composition in liver pathophysiology associated with NAFLD. Adipocyte 2015. 4 101–112. ( 10.4161/21623945.2014.978662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil-Smoller S, Allison MA, Shadyab AH, Wild RA, Sun Y, et al Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. European Heart Journal 2019. 40 2849–2855. ( 10.1093/eurheartj/ehz391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, et al Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005. 48 301–308. ( 10.1007/s00125-004-1637-7) [DOI] [PubMed] [Google Scholar]
- 27.Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obesity Research 2003. 11 104–111. ( 10.1038/oby.2003.18) [DOI] [PubMed] [Google Scholar]
- 28.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. & Hoorn Study. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 2004. 27 372–377. ( 10.2337/diacare.27.2.372) [DOI] [PubMed] [Google Scholar]
- 29.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW. Relation of body fat distribution to metabolic complications of obesity. Journal of Clinical Endocrinology and Metabolism 1982. 54 254–260. ( 10.1210/jcem-54-2-254) [DOI] [PubMed] [Google Scholar]
- 30.Han P, Yu H, Ma Y, Kang L, Fu L, Jia L, Chen X, Yu X, Hou L, Wang L, et al The increased risk of sarcopenia in patients with cardiovascular risk factors in Suburb-Dwelling older Chinese using the AWGS definition. Scientific Reports 2017. 7 9592 ( 10.1038/s41598-017-08488-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, Lee WC, Park YG, Kim MK, Park YM. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PLoS ONE 2014. 9 e86902 ( 10.1371/journal.pone.0086902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, Kim HK, Jang HJ, Lee JG, Park HY, et al Sarcopenic obesity as an independent risk factor of hypertension. Journal of the American Society of Hypertension 2013. 7 420–425. ( 10.1016/j.jash.2013.06.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is a part of the Risk Evaluation of cAncers in Chinese diabeTic Individuals: A lONgitudinal (REACTION) study. All data analyzed in this study are based on REACTION study. The data are held in a secure and confidential database that can only be assessed by members of the REACTION group. The REACTION study website is http://www.rjh.com.cn/pages/neifenmike/REACTION/index.shtml.



 This work is licensed under a
This work is licensed under a