Summary
Background
Previous studies on the pneumonia outbreak caused by the 2019 novel coronavirus disease (COVID-19) were based on information from the general population. Limited data are available for pregnant women with COVID-19 pneumonia. This study aimed to evaluate the clinical characteristics of COVID-19 in pregnancy and the intrauterine vertical transmission potential of COVID-19 infection.
Methods
Clinical records, laboratory results, and chest CT scans were retrospectively reviewed for nine pregnant women with laboratory-confirmed COVID-19 pneumonia (ie, with maternal throat swab samples that were positive for severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) who were admitted to Zhongnan Hospital of Wuhan University, Wuhan, China, from Jan 20 to Jan 31, 2020. Evidence of intrauterine vertical transmission was assessed by testing for the presence of SARS-CoV-2 in amniotic fluid, cord blood, and neonatal throat swab samples. Breastmilk samples were also collected and tested from patients after the first lactation.
Findings
All nine patients had a caesarean section in their third trimester. Seven patients presented with a fever. Other symptoms, including cough (in four of nine patients), myalgia (in three), sore throat (in two), and malaise (in two), were also observed. Fetal distress was monitored in two cases. Five of nine patients had lymphopenia (<1·0 × 10⁹ cells per L). Three patients had increased aminotransferase concentrations. None of the patients developed severe COVID-19 pneumonia or died, as of Feb 4, 2020. Nine livebirths were recorded. No neonatal asphyxia was observed in newborn babies. All nine livebirths had a 1-min Apgar score of 8–9 and a 5-min Apgar score of 9–10. Amniotic fluid, cord blood, neonatal throat swab, and breastmilk samples from six patients were tested for SARS-CoV-2, and all samples tested negative for the virus.
Interpretation
The clinical characteristics of COVID-19 pneumonia in pregnant women were similar to those reported for non-pregnant adult patients who developed COVID-19 pneumonia. Findings from this small group of cases suggest that there is currently no evidence for intrauterine infection caused by vertical transmission in women who develop COVID-19 pneumonia in late pregnancy.
Funding
Hubei Science and Technology Plan, Wuhan University Medical Development Plan.
Introduction
The type of pneumonia caused by the 2019 novel coronavirus disease (COVID-19) is a highly infectious disease, and the ongoing outbreak has been declared by WHO as a global public health emergency.1, 2 COVID-19 pneumonia was first reported in Wuhan, Hubei Province, China, in December, 2019, followed by an outbreak across Hubei Province and other parts of the country.1, 2 A study in The Lancet by Huang and colleagues2 reported the epidemiological, clinical, laboratory, and radiological characteristics, as well as treatment and clinical outcomes, of patients with laboratory-confirmed COVID-19 pneumonia. However, Huang and colleagues’ report mainly focused on non-pregnant adults. The clinical characteristics and vertical transmission potential of COVID-19 pneumonia in pregnant women is unknown. Urgent questions that need to be addressed promptly include whether pregnant women with COVID-19 pneumonia will develop distinct symptoms from non-pregnant adults, whether pregnant women who have confirmed COVID-19 pneumonia are more likely to die of the infection or to undergo preterm labour, and whether COVID-19 could spread vertically and pose risks to the fetus and neonate. Answers to these questions are essential for formulating the principles of obstetric treatment for pregnant women with COVID-19 infection. Therefore, to facilitate efforts, both in China and globally, to prevent and control COVID-19 pneumonia in children and pregnant women,3 we retrospectively collected and analysed detailed clinical data from pregnant women with laboratory-confirmed COVID-19 infection at Zhongnan Hospital of Wuhan University, Wuhan, China. In this study we present clinical features of pregnant women with confirmed COVID-19 pneumonia and examine the vertical transmission potential of COVID-19.
Research in context.
Evidence before this study
We searched PubMed and the China National Knowledge Infrastructure database for articles published up to Feb 6, 2020, using the keywords “novel coronavirus”, “2019 novel coronavirus”, “2019-nCoV”, “pneumonia”, “coronavirus”, “Wuhan”, AND “novel” ,“pregnancy”, “maternal infection”, AND “fetal infection” for articles published in both Chinese and English. We found two articles: one titled Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, published in The Lancet, and another titled Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, published in the New England Journal of Medicine. We identified no published studies on pregnant women with the 2019 novel coronavirus disease (COVID-19) infection.
Added value of this study
We retrospectively reviewed clinical records, laboratory findings, and chest CT scans for nine pregnant women with laboratory-confirmed COVID-19 pneumonia. Evidence of vertical transmission was assessed by testing for the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in amniotic fluid, cord blood, breastmilk, and neonatal throat swab samples from six of nine patients. All nine women were in the third trimester. Seven presented with fever without chill. Other symptoms included cough (in four of nine patients), myalgia (in three), sore throat (in two), and malaise (in two). Fetal distress occurred in two cases. Five of the nine patients had lymphopenia (<1·0 × 10⁹ cells per L). Three patients had increased aminotransferase concentrations. None of the patients developed severe COVID-19 pneumonia or died. Nine livebirths were recorded. No severe neonatal asphyxia was observed. All nine livebirths had a 1-min Apgar score of 8–10 and 5-min Apgar score of 9–10. Amniotic fluid, cord blood, neonatal throat swab, and breastmilk samples from six of the nine patients were tested for SARS-CoV-2, and all results were negative.
Implications of all the available evidence
The clinical characteristics of COVID-19 pneumonia in pregnant women were similar to those of non-pregnant adult patients with COVID-19 pneumonia. Based on data from this small group of patients, there is currently no evidence of vertical transmission in pregnant women who develop COVID-19 pneumonia in the third trimester.
Methods
Study design and patients
We did a retrospective review of medical records from nine pregnant women with COVID-19 pneumonia admitted to Zhongnan Hospital of Wuhan University from Jan 20 to Jan 31, 2020. Diagnosis of COVID-19 pneumonia was based on the New Coronavirus Pneumonia Prevention and Control Program (4th edition) published by the National Health Commission of China.4 All nine pregnant women with COVID-19 pneumonia tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by use of quantitative RT-PCR (qRT-PCR) on samples from the respiratory tract.
This study was reviewed and approved by the Medical Ethical Committee of Zhongnan Hospital of Wuhan University (approval number 2020004). Written informed consent was obtained from each enrolled patient.
Data collection
We reviewed clinical records, laboratory findings, and chest CT scans for all nine pregnant women. All information was obtained and curated with a customised data collection form. Two study investigators (JG and XY) independently reviewed the data collection forms to verify data accuracy.
Maternal throat swab samples were collected and tested for SARS-CoV-2 with the Chinese Center for Disease Control and Prevention (CDC) recommended Kit (BioGerm, Shanghai, China), following WHO guidelines for qRT-PCR.5, 6, 7 All samples were processed simultaneously at the Department of Clinical Laboratory of Zhongnan Hospital and State Key Laboratory of Virology/Institute of Medical Virology, School of Basic Medical Sciences, Wuhan University. Positive confirmatory cases of COVID-19 infection were defined as those with a positive test result from either laboratory.
Amniotic fluid samples from patients with COVID-19 pneumonia were obtained via direct syringe aspiration at the time of delivery. Cord blood and neonatal throat swab samples were collected immediately after delivery in the operating room. Additionally, breastmilk samples from patients with COVID-19 pneumonia were collected after their first lactation. Evidence of vertical transmission was evaluated by testing for the presence of SARS-CoV-2 in these clinical samples. Sample collection was successful in six cases (patients 2, 4–6, 8, and 9). Among the three patients from whom sample collection was not successful, patient 1 was diagnosed after caesarean section, thus no sample was obtained. Patients 3 and 7 underwent caesarean section at night, thus immediate sample collection was not possible. All samples were processed at the State Key Laboratory of Virology/Institute of Medical Virology, School of Basic Medical Sciences, Wuhan University, for further testing. Sample collection, processing, and laboratory testing complied with WHO guidance.6 All samples, as described above, were tested for SARS-CoV-2 by use of qRT-PCR with the CDC recommended Kit. The test results were confirmed by nested RT-PCR with designed primers. For the nested RT-PCR assay, total RNA was extracted from samples by use of the TRIzol LS reagent (Invitrogen, Carlsbad, CA, USA), followed by reverse transcription by use of the one-step RT-PCR Kit (TaKaRa, Dalian, China). Primers were designed on the basis of the sequence of Wuhan-Hu-1 (MN908947). Partial S segment sequences (nt 21730-22458) were amplified with primers: 5′-CTCAGGACTTGTTCTTACCTT-3′ and 5′-CAAGTGCACAGTCTAC-AGC-3′.
Statistical analysis
Statistical analysis was done with SPSS, version 20.0. Continuous variables were directly expressed as a range. Categorical variables were expressed as number (%).
Role of the funding source
The funding agencies did not participate in study design, data collection, data analysis, or writing of the report. The corresponding authors were responsible for all aspects of the study to ensure that issues related to the accuracy or integrity of any part of the work were properly investigated and resolved. The final version was approved by all authors.
Results
The nine pregnant women were all in their third trimester, and all underwent caesarean section. All patients had a history of epidemiological exposure to COVID-19. The age range of the patients was 26–40 years, and the range of gestational weeks at admission was 36 weeks to 39 weeks plus 4 days. None of the patients had underlying diseases such as diabetes, chronic hypertension, or cardiovascular disease. One patient, however, had gestational hypertension since 27 gestational weeks, while another developed pre-eclampsia at 31 gestational weeks. Both of these patients were in a stable condition during pregnancy. Additionally, one patient was found to have influenza virus infection upon admission to hospital (table 1 ; appendix).
Table 1.
Maternal clinical and laboratory characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||||||
| Date of admission | Jan 20 | Jan 25 | Jan 27 | Jan 26 | Jan 27 | Jan 27 | Jan 28 | Jan 29 | Jan 30 | ·· | |
| Age (years) | 33 | 27 | 40 | 26 | 26 | 26 | 29 | 28 | 34 | ·· | |
| Gestational age on admission | 37 weeks, 2 days | 38 weeks, 2 day | 36 weeks | 36 weeks, 2 days | 38 weeks,1 day | 36 weeks, 3 days | 36 weeks, 2 days | 38 weeks | 39 weeks, 4 days | ·· | |
| Epidemiological history | Yes (exposure to relevant environment)* | Yes (contact with infected person) | Yes (contact with infected person) | Yes (exposure to relevant environment)* | Yes (exposure to relevant environment)* | Yes (contact with infected person) | Yes (contact with infected person) | Yes (contact with infected person) | Yes (exposure to relevant environment)† | 9 (100%) | |
| Other family members affected | No | Yes | Yes | No | No | Yes | No | Yes | No | 4 (44%) | |
| Onset to delivery (days) | 1 | 6 | 4 | 3 | 1 | 4 | 2 | 2 | 7 | ·· | |
| Complications | Influenza | None | Gestational hypertension | Pre-eclampsia | Fetal distress | None | PROM | Fetal distress | PROM | ·· | |
| Signs and symptoms | |||||||||||
| Fever on admission | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 (78%) | |
| Post-partum fever | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | 6 (67%) | |
| Myalgia | No | Yes | No | No | Yes | Yes | No | No | No | 3 (33%) | |
| Malaise | No | No | No | No | Yes | Yes | No | No | No | 2 (22%) | |
| Rigor | No | No | No | No | No | No | No | No | No | 0 | |
| Cough | Yes | Yes | Yes | No | No | Yes | No | No | No | 4 (44%) | |
| Dyspnoea | No | No | No | Yes | No | No | No | No | No | 1 (11%) | |
| Sore throat | No | No | No | No | No | Yes | Yes | No | No | 2 (22%) | |
| Diarrhoea | No | No | No | Yes | No | No | No | No | No | 1 (11%) | |
| Chest pain | No | No | No | No | No | No | No | No | No | 0 | |
| Laboratory characteristics | |||||||||||
| White blood cell count (×109 cells per L) | 6·15 | 5·07 | 8·78 | 7·63 | 9·34 | 5·57 | 10·61 | 9·96 | 7·08 | ·· | |
| Low or normal leukocyte count (<9·5×109 cells per L) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 7 (78%) | |
| Lymphocyte count (×109 cells per L) | 1·59 | 0·56 | 0·46 | 2·83 | 0·69 | 0·66 | 0·87 | 1·53 | 1·47 | ·· | |
| Lymphopenia (<109 cells per L) | No | Yes | Yes | No | Yes | Yes | Yes | No | No | 5 (56%) | |
| C-reactive protein concentration (mg/L) | 20·3 | 14·4 | 33·4 | 3·3 | 28·2 | 18·2 | NA | 6·2 | 24·9 | ·· | |
| Elevated C-reactive protein (>10 mg/L) | Yes | Yes | Yes | No | Yes | Yes | NA | No | Yes | 6 (75%)‡ | |
| Elevated ALT (>45 U/L) or AST (>35 U/L) | Yes | No | Yes | Yes | No | No | No | No | No | 3 (33%) | |
| ALT (U/L) | 2093 | 9 | 62 | 54 | 18 | 14 | 6 | 16 | 12 | ·· | |
| AST (U/L) | 1263 | 24 | 71 | 76 | 24 | 23 | 15 | 22 | 21 | ·· | |
| Confirmatory test done (SARS-CoV-2 quantitative RT-PCR) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 (100%) | |
| CT evidence of pneumonia | |||||||||||
| Typical signs of viral infection | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 8 (89%) | |
| Delivery | |||||||||||
| Method of delivery | C-section | C-section | C-section | C-section | C-section | C-section | C-section | C-section | C-section | ·· | |
| Indication for C-section | Severely elevated ALT or AST; COVID-19 pneumonia | Mature; COVID-19 pneumonia | History of C-section (×2); COVID-19 pneumonia | Pre-eclampsia; COVID-19 pneumonia | Fetal distress; COVID-19 pneumonia | History of stillbirth (×2); COVID-19 pneumonia | PROM; COVID-19 pneumonia | Fetal distress; COVID-19 pneumonia | PROM; COVID-19 pneumonia | ·· | |
| Treatment after delivery | |||||||||||
| Oxygen support (nasal cannula) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 (100%) | |
| Antiviral therapy | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | 6 (67%) | |
| Antibiotic therapy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 (100%) | |
| Use of corticosteroid | No | No | No | No | No | No | No | No | No | 0 | |
PROM=premature rupture of membrane. NA=not applicable. ALT=alanine transaminase. AST=aspartate transaminase. COVID-19=2019 novel coronavirus disease. C-section=caesarean section. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Exposure to Hankou, the area in Wuhan where the epidemic was first detected.
A university where the patient works, and a gathering of people.
Data missing for one patient.
Seven of the nine patients presented with a fever without chills, but none had a high fever (body temperature >39°C). Patients’ body temperatures fluctuated within a range of 36·5–38·8°C. The two patients with a normal body temperature before caesarean section both had post-partum fever (range 37·8–39·3°C). Other symptoms of an upper respiratory tract infection were also observed: four patients had a cough, three had myalgia, two reported a sore throat, and two indicated malaise. Additionally, one patient showed obvious gastrointestinal symptoms. Another patient had shortness of breath and pre-eclampsia. However, none of the nine patients developed severe pneumonia, requiring mechanical ventilation, or died of COVID-19 pneumonia, as of Feb 4, 2020. Pregnancy complications that appeared after the onset of COVID-19 infection included fetal distress (in two of nine patients) and premature rupture of the membrane (in two of nine; table 1).
All patients were given oxygen support (nasal cannula) and empirical antibiotic treatment. Six patients were administered antiviral therapy (table 1).
Data from laboratory tests showed that five of the nine pregnant women with COVID-19 pneumonia had lymphopenia (<1·0 × 10⁹ cells per L). Six patients had elevated concentrations of C-reactive protein (>10 mg/L). Three had increased concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), one of whom had ALT reaching 2093 U/L and AST reaching 1263 U/L. Additionally, seven patients had a normal white cell count, with none of the patients having a white cell count below the normal range (table 1).
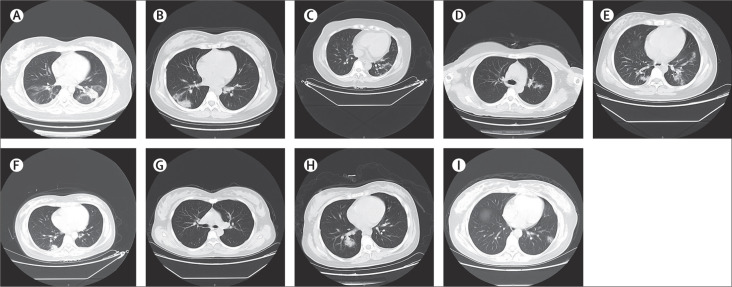
All nine patients had a chest CT scan. Eight patients showed typical findings of chest CT images—multiple patchy ground-glass shadows in lungs2 (figure ).
Figure.
Chest CT scans (transverse plane) of nine patients
(A) Patient 1: left-sided patchy consolidation and multiple bilateral ground-glass opacities. (B) Patient 2: subpleural patchy consolidation in the right lung and slightly infiltrated shadows around left bronchus. (C) Patient 3: bilateral multiple ground-glass opacities, prominent on the left. (D) Patient 4: left-sided patchy ground-glass opacity. (E) Patient 5: multiple ground-glass opacities bilaterally. (F) Patient 6: right-sided subpleural patchy consolidation. (G) Patient 7: bilateral clear lung fields with no obvious ground-glass opacities. (H) Patient 8: multiple bilateral ground-glass opacities, prominent on the right. (I) Patient 9: multiple bilateral ground-glass opacities.
Nine livebirths were recorded. No fetal death, neonatal death, or neonatal asphyxia was observed. Four patients had preterm labour, but all beyond 36 gestational weeks. Two of the four premature neonates at 36 gestational weeks plus 2 days had a birthweight lower than 2500 g (table 2 ). Neonate 4 had a birthweight of 1880 g and the pregnancy was complicated by pre-eclampsia. Neonate 7 had a birthweight of 2460 g. All nine livebirths had a 1-min Apgar score of 8–9 and a 5-min Apgar score of 9–10 (table 2). Neonate 1 had a mild increase in myocardial enzymes on the day of birth (myoglobin 170·8 ng/mL and creatine kinase-myocardial band 8·5 ng/mL), but without any clinical symptoms.
Table 2.
Neonatal outcomes
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | n (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gestational age at delivery | 37 weeks, 2 days | 38 weeks, 3 days | 36 weeks | 36 weeks, 2 days | 38 weeks, 1 day | 36 weeks, 3 days | 36 weeks, 2 days | 38 weeks | 39 weeks, 4 days | ·· |
| Birthweight (g) | 2870 | 3730 | 3820 | 1880 | 2970 | 3040 | 2460 | 2800 | 3530 | ·· |
| Low birthweight (<2500 g) | No | No | No | Yes | No | No | Yes | No | No | 2 (22%) |
| Premature delivery | No | No | Yes | Yes | No | Yes | Yes | No | No | 4 (44%) |
| Apgar score (1 min, 5 min) | 8, 9 | 9, 10 | 9, 10 | 8, 9 | 9, 10 | 9, 10 | 9, 10 | 9, 10 | 8, 10 | ·· |
| Severe neonatal asphyxia | No | No | No | No | No | No | No | No | No | 0 |
| Neonatal death | No | No | No | No | No | No | No | No | No | 0 |
| Fetal death or stillbirth | No | No | No | No | No | No | No | No | No | 0 |
The presence of SARS-CoV-2 was tested in amniotic fluid, cord blood, neonatal throat swab, and breastmilk samples collected from six patients. Neither the Kit recommended by CDC nor our in-house nested RT-PCR assays detected SARS-CoV-2 in these samples.
Discussion
We report clinical data from nine pregnant women with laboratory-confirmed COVID-19 pneumonia. The clinical characteristics of these patients with COVID-19 infection during pregnancy were similar to those of non-pregnant adults with COVID-19 infection, as previously reported.2 None of the nine patients developed severe pneumonia or died, as of Feb 4, 2020. Notably, based on our findings in these nine patients, there is currently no evidence to suggest that development of COVID-19 pneumonia in the third trimester of pregnancy could lead to the occurrence of severe adverse outcomes in neonates and fetal infection that might be caused by intrauterine vertical transmission.
Pregnant women are particularly susceptible to respiratory pathogens and severe pneumonia, because they are at an immunosuppressive state, and physiological adaptive changes during pregnancy (eg, diaphragm elevation, increased oxygen consumption, and oedema of respiratory tract mucosa) can render them intolerant to hypoxia. For example, the 1918 influenza pandemic caused a mortality rate of 2·6% in the overall population, but 37% among pregnant women.8 Pregnant women were reported to be at an increased risk of complications from the pandemic H1N1 2009 influenza virus infection, and were more than four times more likely to be admitted to hospital than the general population (relative risk 4·3 [95% CI 2·3–7·8]).9 Wong and colleagues10 also reported that around 50% of pregnant women who developed SARS were admitted to the intensive care unit, around 33% of pregnant women with SARS required mechanical ventilation, and the mortality rate was as high as 25% for these women. In the current study, we treated nine pregnant women with COVID-19 pneumonia in 11 days from Jan 20 to Jan 31, 2020. Considering that SARS-CoV-2 has up to 85% sequence similarity with SARS,11, 12, 13, 14 although none of our patients developed severe pneumonia or died of COVID-19 infection, we should be alert to the possibility that the disease course and prognosis of COVID-19 pneumonia could follow the same trend as SARS in pregnant women. However, our observations are based on a small number of cases and the time between illness onset and delivery was short.
According to our study, pregnant women with COVID-19 pneumonia showed a similar pattern of clinical characteristics to non-pregnant adult patients, as recently reported.2, 15 Common symptoms at the onset of COVID-19 pneumonia for these women included a fever and cough, whereas less common symptoms were myalgia, malaise, sore throat, diarrhoea, and shortness of breath. Laboratory tests indicated that lymphopenia is also likely to occur. Additionally, increased concentrations of ALT or AST might be one of the clinical manifestations. However, none of these symptoms was present in every patient, nor were the symptoms specific to pregnant women with COVID-19 pneumonia. By contrast, chest CT might have a high diagnostic value because of its typical images of virus infection, high accuracy with a low false negative rate, and time efficiency. Therefore, we recommend that, in addition to using nucleic acid tests as the gold standard for the diagnosis of COVID-19 pneumonia, relevant clinical examinations are done, including blood counts and chest CT and a comprehensive evaluation of a patient's medical history, epidemiological exposure, and symptoms.
All nine pregnant women in this study underwent a caesarean section. Indications for a caesarean section included severe pre-eclampsia, a history of caesarean sections, and fetal distress. Importantly, uncertainty about the risk of intrapartum mother-to-child transmission by vaginal delivery was another reason for carrying out caesarean sections. Four of the nine patients had preterm labour. However, the causes of premature birth were not related to COVID-19 pneumonia: one patient had severe pre-eclampsia, one had a history of two stillbirths, one had a history of two caesarean sections and irregular contractions; and one had premature rupture of the membrane for about 12 h and suspected intrauterine infection. Moreover, one neonate in our study had a birthweight of 1880 g at 36 gestational weeks plus 2 days, and this case was complicated by pre-eclampsia. Furthermore, the time period from onset of COVID-19 pneumonia to admission was only 3 days for this patient; therefore we reasoned that the fetal intrauterine growth restriction was more likely to be a symptom associated with pre-eclampsia. Importantly, all nine livebirths had a 1-min Apgar score of 8–9 and 5-min Apgar score of 9–10. One infant had a mild increase in myocardial enzymes on the day of birth, but without any clinical symptoms. None of the neonates needed special paediatric treatment.
The main focus of this study was to investigate the possibility of intrauterine transmission of COVID-19 infection. We chose to test amniotic fluid, cord blood, and neonatal throat swab samples at birth to ascertain the possibility of intrauterine fetal infection. Notably, all the samples tested in our study were collected in the operating room at the time of the caesarean section, thus guaranteeing that the samples were not contaminated and best represented fetal intrauterine conditions. Our results show that SARS-CoV-2 was negative in all of the above samples, suggesting that no intrauterine fetal infections occurred as a result of COVID-19 infection during a late stage of pregnancy. Our findings are in accordance with what was observed in SARS, which has a similar sequence to SARS-CoV-2.14 Previous studies have already shown no evidence of perinatal SARS infection among infants born to mothers who developed SARS infection during pregnancy.10, 16
However, our observation of no fetal infection caused by intrauterine vertical transmission could be affected by our small sample size and the stage of pregnancy at the onset of COVID-19 infection. All patients in the study were recruited in their third trimester, so we were unable to ascertain the possibility of intrauterine vertical transmission during the first or second trimester. For example, rubella infection in the first trimester can affect more than 50% of fetuses via intrauterine infection, whereas by the end of the second trimester the incidence rate is reduced by half.17
We did not collect samples of vaginal mucosa or shedding in birth canals, which prevented us from analysing whether COVID-19 could be transmitted during vaginal delivery. Notably, however, our results showed that breastmilk samples from mothers with COVID-19 infection appeared to be free from SARS-CoV-2.
On Feb 6, 2020, a neonate born to a pregnant woman with COVID-19 pneumonia tested positive for SARS-CoV-2 infection 36 h after birth.18, 19 Although many important clinical details of this single case were not available at the time of writing this report, there are reasonable concerns that COVID-19 could be contracted in the womb. Reportedly, the pregnant woman had developed fever for 8 h and was suspected to have COVID-19 pneumonia on the basis of her typical chest CT image before admission; an emergency caesarean section was subsequently done, which was followed by confirmation of COVID-19 pneumonia. Moreover, the neonate's throat swab sample was collected approximately 30 h after birth, thus providing no direct evidence for intrauterine infection. Additionally, no direct testing of intrauterine tissue samples such as amniotic fluid, cord blood, or placenta was done to confirm that the COVID-19 infection in the neonate was due to intrauterine transmission. Therefore, we cannot conclude whether or not intrauterine COVID-19 infection occurred in this particular case. Nonetheless, this single case of an infected neonate suggests that we should pay special attention to prevent infections in newborn babies born to mothers with COVID-19 pneumonia.
This study is limited by the small sample size and retrospective method. Several considerations should be taken into account when interpreting the findings. First, all enrolled patients were in the third trimester. The effect of COVID-19 infection on the fetus in the first or second trimester of pregnancy remains to be clarified. Second, whether vaginal delivery increases the risk of mother-to-child intrapartum transmission, and whether uterine contraction could increase the possibility of the virus ascending, needs to be further investigated. Third, the risk of infection in pregnant women and the effects of the time or mode of delivery on pregnancy outcomes were not evaluated. Fourth, whether COVID-19 could damage the placenta, which represents an important link in vertical transmission, also needs to be further investigated. Future investigations of these issues and follow-up studies of pregnant women with COVID-19 infection, as well as neonates, will be necessary to ascertain the safety and health of mothers and babies exposed to SARS-CoV-2.
In summary, the symptoms of pregnant women with COVID-19 pneumonia were diverse, with the main symptoms being fever and cough. We found no evidence for vertical transmission in late pregnancy. Considering the significance of this ongoing global public health emergency, although our conclusions are limited by the small sample size, we believe that the findings reported here are important for understanding the clinical characteristics and vertical transmission potential of COVID-19 infection in pregnant women.
This online publication has been corrected. The corrected version first appeared at thelancet.com on March 23, 2020
Data sharing
With the permission of the corresponding authors, we can provide participant data without names and identifiers, but not the study protocol, statistical analysis plan, or informed consent form. Data can be provided after the Article is published. Once the data can be made public, the research team will provide an email address for communication. The corresponding authors have the right to decide whether to share the data or not based on the research objectives and plan provided.
Acknowledgments
Acknowledgments
This study was supported by Hubei Science and Technology Plan (grant number 2017ACB640) and Wuhan University Medical Development Plan (grant number TFJC2018001).
Contributors
YZ, WH, and HY made substantial contributions to the study concept and design. HC was in charge of the manuscript draft. JG took responsibility for obtaining written consent from patients, obtaining ethical approval, collecting samples, and confirming data accuracy. CW participated in drafting the manuscript, and revising it on the basis of reviewers’ comments. XY made substantial contributions to data acquisition, analysis, and interpretation. DZ was the paediatrician in charge of treatment of the newborn babies. DX, QG, JL, and JL were the obstetricians of the pregnant women, and were responsible for data collection and confirmation. WH and FL were in charge of the laboratory tasks, including sample processing and detection. WZ made substantial revisions to the manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Huixia Yang, Email: yanghuixia@bjmu.edu.cn.
Wei Hou, Email: houwei@whu.edu.cn.
Yuanzhen Zhang, Email: zhangyuanzhen@whu.edu.cn.
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The State Council's Joint Prevention and Control Mechanism for Pneumonia Epidemic in Response to New Coronavirus Infection Notice on prevention and control of pneumonia in children and pregnant women with new coronavirus infection. Feb 3, 2020. http://www.ljxw.gov.cn/news-93789.shtml (in Chinese).
- 4.National Health Commission of China New coronavirus pneumonia prevention and control program (4th edn) Jan 22, 2020. http://www.gov.cn/zhengce/zhengceku/2020-01/28/5472673/files/0f96c10cc09d4d36a6f9a9f0b42d972b.pdf (in Chinese).
- 5.WHO Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Interim guidance. Jan 12, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf
- 6.WHO Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance. Jan 17, 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117
- 7.Corman VM, Landt O, Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history. Laeknabladid. 2008;94:737–745. (in Icelandic). [PubMed] [Google Scholar]
- 9.Jamieson DJ, Honein MA, Rasmussen SA. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 10.Wong SF, Chow KM, Leung TN. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YZ. Novel 2019 coronavirus genome. Jan 21, 2020. http://virological.org/t/novel-2019-coronavirus-genome/319
- 13.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P, Yang XL, Wang XG. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shek CC, Ng PC, Fung GP. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 17.Bouthry E, Picone O, Hamdi G, Grangeot-Keros L, Ayoubi JM, Vauloup-Fellous C. Rubella and pregnancy: diagnosis, management and outcomes. Prenat Diagn. 2014;34:1246–1253. doi: 10.1002/pd.4467. [DOI] [PubMed] [Google Scholar]
- 18.Niu Y, Yue H. Wuhan Tongji Hospital diagnoses first case of neonatal infection with new coronavirus. Feb 5, 2020. http://society.people.com.cn/n1/2020/0205/c1008-31572959.html (in Chinese).
- 19.Zhang Z, Wang C, Gao CC. Neonatal coronavirus expert confirmed at 30 hours of birth: vertical transmission from mother to infant. Feb 5, 2020. http://www.cnr.cn/hubei/yuanchuang/20200205/t20200205_524961963.shtml (in Chinese).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
With the permission of the corresponding authors, we can provide participant data without names and identifiers, but not the study protocol, statistical analysis plan, or informed consent form. Data can be provided after the Article is published. Once the data can be made public, the research team will provide an email address for communication. The corresponding authors have the right to decide whether to share the data or not based on the research objectives and plan provided.