Abstract
Background
Body dysmorphic disorder (BDD) is a prevalent and disabling preoccupation with a slight or imagined defect in appearance. Trials have investigated the use of serotonin reuptake inhibitors (SRIs) and cognitive behaviour therapy (CBT) for BDD.
Objectives
To assess the efficacy of pharmacotherapy, psychotherapy or a combination of both treatment modalities for body dysmorphic disorder.
Search methods
We searched the Cochrane Depression, Anxiety and Neurosis Trial Register (December 2007), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 4, 2007), MEDLINE (January 1966 to December 2007), and PsycINFO (1967 to December 2007). Ongoing and unpublished trials were located through searching the metaRegister of Controlled Trials, the CRISP and WHO ICTRP search portals (databases searched in December 2007), and through contacting key researchers and pharmaceutical companies. Additional studies were located through study reference lists.
Selection criteria
Randomised controlled trials (RCTs) of patients meeting DSM or ICD diagnostic criteria for BDD, in which the trials compare pharmacotherapy, psychotherapy or multi‐modal treatment groups with active or non‐active control groups. Short or long‐term trials were eligible.
Data collection and analysis
Two review authors independently assessed RCTs for inclusion in the review, collated trial data, and assessed trial quality. Investigators were contacted to obtain missing data. Summary effect sizes for dichotomous and continuous outcomes were calculated using a random effects model and heterogeneity was assessed.
Main results
Two pharmacotherapy and three psychotherapy trials were eligible for inclusion in the review, with data from four short‐term RCTs (169 participants) available for analysis. Response data from a single placebo‐controlled trial of fluoxetine suggested overall superiority of medication relative to placebo (relative risk (RR) 3.07, 95% CI 1.4 to 6.72, n = 67). Symptom severity was also significantly reduced in the RCTs of fluoxetine and clomipramine (relative to desipramine), as well as in the two CBT trials (WMD ‐44.96, 95% CI ‐54.43 to ‐35.49, n = 73). A low relapse rate (4/22) was demonstrated in one trial of CBT.
Authors' conclusions
Results from the small number of available RCTs suggest that SRIs and CBT may be useful in treating patients with BDD. The findings of these studies need to be replicated. In addition, future controlled studies in other samples, such as adolescents, and using other selective SRIs, as well as a range of psychological therapy approaches and modalities (alone and in combination), are essential in supplementing the sparse data currently available.
Plain language summary
Medication, psychotherapy, or a combination of both, in treating body dysmorphic disorder
Body dysmorphic disorder (BDD) is a condition characterised by a distressing and disabling preoccupation with an imagined or slight defect in appearance. This causes people with this disorder either significant distress or disrupts their daily functioning (or both). There has been a growing recognition that BDD is common, and is associated with significant illness and disability. There is also some evidence that it may respond to pharmacotherapy and psychotherapy. Our systematic review of randomised controlled trials assesses the effects of drug treatment or psychotherapy when used on their own or in combination. We found five eligible trials, including three of psychotherapy (cognitive behavioural therapy (CBT) and exposure and response prevention (ERP)) and two of medication (the serotonin reuptake inhibitors (SRIs) fluoxetine and clomipramine). In the only placebo‐controlled medication trial included in our review, people with BDD treated with fluoxetine were more likely to respond (56%, 19 out of 34) than those allocated placebo (18%, 6 out of 33). Symptoms became less severe after treatment with both medication and psychotherapy. Adverse events were mild to moderate in severity and none of the people in the active treatment groups were reported to have dropped out of the studies because of treatment‐emergent adverse events. There is preliminary evidence from one trial of CBT that the effects of CBT may persist once treatment has ended. Treatment response in the medication trials was not effected by the degree to which people had insight into their condition. Although few controlled trials have been done, and those that have been conducted were small, indicating that our findings should be used with caution unless confirmed by larger studies (some of which are ongoing), the results suggest that treatment with both medication or psychotherapy can be effective in treating the symptoms of body dysmorphic disorder.
Background
Description of the condition
Body dysmorphic disorder (BDD) is a condition characterised by a distressing and disabling preoccupation with an imagined or slight defect in appearance. Patients with BDD are typically fixated on more than one body part over the course of the illness (Phillips 1993; Phillips 1997a; Veale 1996), with common foci of distress including, but not limited to the skin, hair, and nose. Although currently classified as a somatoform disorder, some have argued that BDD should be conceptualised as a mood disorder, or as an obsessive compulsive spectrum disorder (OCSD) (Hollander 1993). Cases of "dysmorphophobia" have been described for more than a century, but the disorder received increased clinical and research attention after its incorporation into the official psychiatric nomenclature in the DSM‐IIIR (Diagnostic and Statistical Manual, version III revised) in 1987 (APA 1987; Phillips 1991).
There has been a growing recognition that BDD is prevalent, and is associated with significant comorbidity, disability and social or occupational impairment. Patients with BDD have been reported as having low levels of self‐esteem (Rosen 1998) and impaired mental health ‐ related quality of life (Phillips 2003; Phillips 2004) relative to normal controls. Preliminary evidence from surveys indicates that approximately a quarter of patients with BDD may have attempted suicide in their lifetimes (Phillips 1997a; Veale 1996b). Patients may undergo multiple cosmetic procedures, usually with little positive effect on their symptoms (Veale 1996; Veale 2000, Tignol 2007).
Estimates of the prevalence of BDD have ranged between 1% (Faravelli 1997) and 4‐5% (Bohne 2002a; Bohne 2002b) of the nonclinical population, with even higher prevalence rates in specialised medical settings (Phillips 2000). Evidence concerning the gender distribution of BDD is inconsistent. (Rosen 1996; Veale 1996; Frare 2004; Phillips 1997a; Phillips 2000). Males and females may have somewhat different kinds of concerns, with males generally more preoccupied with their height, genitals, body hair and build, and females with their breasts, hips, legs and body weight (Phillips 1997a; Phillips 2006b). Individuals with BDD are frequently diagnosed with comorbid psychiatric disorders (Phillips 1993; Veale 1996), the most common being major depression, social phobia and obsessive compulsive disorder (Phillips 1997a).
The validity of the DSM distinction between BDD and delusional disorder, somatic type has been questioned. In a study assessing the prevalence of delusionality amongst 100 participants diagnosed with BDD, approximately half (52%) had at some point been absolutely convinced that their physical imperfections were real (Phillips 1994). Furthermore, open‐label (Phillips 2001b), retrospective (Phillips 1994; Phillips 2001a) and prospective (Phillips 2006d) studies have found little evidence that BDD patients with and without insight have a different pharmacotherapy response. Nevertheless, it has been suggested that delusional BDD may represent a more severe form of the disorder (Eisen 2004). This receives some support from the finding of a positive association between delusionality and response to the SRI clomipramine in a RCT cross‐over trial (Hollander 1999). Evidence of the lack of consensus about the classification of BDD is apparent in the ability to diagnose patients according to DSM‐IV criteria as being afflicted with both BDD and delusional disorder, and the definition in the ICD‐10, the standard diagnostic system employed outside of the United States, of BDD as a subgroup of hypochondrial disorder (WHO 1992).
Description of the intervention
There appears to be evidence that BDD responds to pharmacotherapy, and to SRIs in particular. Case studies (Hollander 1989), retrospective case series (Phillips 1994), and open‐label trials (Phillips 2003, Phillips 2006e) all contribute to the impression that SRIs are effective, and perhaps preferentially effective. In a chart review study of 90 patients, an improvement in BDD symptoms was observed for 63.2% of patents in response to a range of SRI's (Phillips 2001a). This study also detected high relapse rates (84% (31) of 37 participants) following discontinuation of SSRIs (Phillips 2003). Conversely, despite the apparent widespread use of antipsychotics to treat BDD (Saxena 2001), the only placebo‐controlled study of the efficacy of this medication class in treating 28 patients with BDD was not able to detect any differences following the eight week open‐label augmentation of the SSRI fluoxetine with pimozide (Phillips 2005b).
Theoretical considerations suggest that psychotherapy may also be useful in treating BDD (Veale 1996). While evidence of the effectiveness of psycho‐analysis is contested (Phillips 2001b), findings of an increasing number of open studies (Wilhelm 1999) and RCTs (Veale 1996b) support the effectiveness of cognitive behaviour therapy (CBT) in treating BDD. CBT treatment modalities typically include exposure with response prevention (ERP) and changing beliefs underlying patients' dissatisfaction with their bodies by focusing on cognitive processes such as self‐focused attention and rumination. These methods are frequently augmented with information provided to the patient concerning the nature of BDD, and with reverse role playing strategies. Although there is some evidence that both the behavioural (Marks 1988; McKay 1997) and cognitive (Geremia 2001) aspects of CBT are effective in isolation, the relative contribution of these respective elements in combined treatment requires further investigation. Nevertheless, there are preliminary indications that the behavioural component of CBT may result in lower relapse rates that those found in SRI trials (McKay 1997; McKay 1999).
How the intervention might work
Although the pathogenesis of BDD is not well understood, preliminary evidence of a response of BDD symptoms to SRIs is consistent with impaired modulation of neural activity by the serotonergic system. Abnormalities in brain structure (Gabbay 2003, Rauch 2003) and function (Feusner 2007), as well as cognitive impairments (Hanes 1998; Deckersbach 2000) have also been detected in subjects with the disorder, though evidence of the causal status of these factors vis‐a‐vie BDD is lacking. Both psychotherapy and pharmacotherapy have demonstrated normalising effects on brain morphology and activation in OCD (Baxter 1992), a disorder frequently conceptualised as lying on the same spectrum of disorders as BDD.
Why it is important to do this review
To date only one systematic review and meta‐analyses of the pharmacotherapy or psychotherapy of BDD has been conducted (Williams 2006). The authors concluded that both modalities are effective in treating BDD, but that CBT is more effective than medication therapies in treating this disorder. The latter conclusion was based on indirect comparisons of trials and should therefore be interpreted cautiously. Differences in the control groups employed in the different modes of treatment also raises doubts regarding their comparability (placebo controls are typically not feasible in psychotherapy trials). Moreover, the authors of this review did not control for the possibility of multiple sources of bias undermining the validity of their conclusions. These included (a) the synthesis of outcomes from both controlled and non‐controlled trials, and (b) the inclusion of multiple effect sizes from individual studies in the same comparison. Our review was conducted in accordance with guidelines proposed by the Cochrane collaboration to minimise bias in determining the relative efficacy and acceptability of pharmacotherapy and psychotherapy in treating BDD.
Objectives
To determine the effectiveness of medication, psychotherapy or a combination of both treatment modalities in combating body dysmorphic disorder, relative to placebo and other comparison groups.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of pharmacotherapy, psychotherapy, or a combination of these modalities for the treatment of body dysmorphic disorder were considered for inclusion. Studies employing cross‐over as well as parallel designs were potentially eligible. Published and unpublished trials were considered, with no language restrictions applied.
Types of participants
All participants diagnosed with body dysmorphic disorder according to the criteria of the Diagnostic and Statistical Manual (DSM‐III‐R (APA 1987) or DSM‐IV (APA 1994)), or the International Classification of Diseases (ICD‐9 (WHO 1975) or ICD‐10 (WHO 1992)), irrespective of age, inpatient or outpatient status, presence of comorbidity, or poor insight.
Types of interventions
Pharmacotherapy RCTs of all medication agents (excluding experimental agents, such as neuropeptides) were assessed for inclusion. This included trials of benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), anticonvulsants and antipsychotics.
With the addition of further trials in future updates of the review, pharmacotherapy interventions will be classified as either benzodiazepines, SSRIs, TCAs, anticonvulsants or antipsychotics. A separate category labelled 'Other medication' will be reserved for agents with miscellaneous mechanisms of action.
Psychotherapy RCTs of all forms of psychotherapy were potentially eligible for inclusion. This included behavioural modification and cognitive restructuring programmes (and their combination in the form of cognitive‐behavioural therapy), as well as psychodynamic, relaxation, gestalt, interpersonal and supportive therapies. The third generation of psychotherapies (mindfulness, acceptance and commitment therapy, compassion‐focused therapy) were also eligible for inclusion.
With the addition of further trials in future updates of the review, psychotherapy treatments will be classified according to whether they consist primarily of behaviour modification, cognitive restructuring, their combination (cognitive‐behavioural therapy) or third generation CBT interventions. Separate categories will also be created for interpersonal and psychodynamic interventions. Other psychotherapy modalities, such as relaxation, gestalt and supportive therapies will be classified as 'Other psychotherapies'.
Trials employing both group‐based and individual sessions of psychotherapy were considered. Group‐based treatments were only included on the condition that they employed a cluster randomisation design. Both long‐term interventions (defined for the purposes of this review as > 16 weeks for medication and psychotherapy) and short‐term interventions were considered for inclusion in this review.
Planned treatment comparisons
Pharmacotherapy versus placebo
Pharmacotherapy versus alternative medication
Pharmacotherapy versus psychotherapy
Psychotherapy versus waiting list/usual care
Psychotherapy versus alternative psychotherapy model
Combined pharmacotherapy/psychotherapy versus placebo
Combined pharmacotherapy/psychotherapy versus pharmacotherapy
Combined pharmacotherapy/psychotherapy versus psychotherapy
Types of outcome measures
Primary outcomes
1. Treatment response (number of responders) were determined from the improvement item of the Clinical Global Impressions scale (CGI‐I), a widely used categorical measure of treatment response in which responders are defined as having a change item score of 1 = "very much" or 2 = "much" improved (Guy 1976).
2. The effect of interventions on symptom severity were determined using summary statistics from standardised instruments such as the Yale Brown Obsessive Compulsive Scale, modified for body dysmorphic disorder (BDD‐YBOCS) (Phillips 1997b) and the Body Dysmorphic Disorder Examination (BDDE) (Rosen 1996).
3. The effectiveness of treatment in long‐term maintenance/discontinuation trials was assessed by means of the total number of people who relapsed by trial end‐point (according to criteria used by the trial investigators).
Secondary outcomes
Scores on rating scales for disorders other than BDD, including:
1. The impact of treatment on delusionality, as measured by the Brown Assessment of Beliefs Scale (BABS), the only scale of delusionality that can easily be applied to BDD patients (Eisen 1998)
2. Reduction of comorbid symptoms of depression and obsessive‐compulsive disorder (OCD). This was assessed for depression using scales such as the Beck Depression Inventory (BDI) (Beck 1987), the Hamilton Depression scale (HAM‐D) (Hamilton 1959), and the Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979). Comorbid OCD symptoms were assessed using data obtained from either the Yale Brown Obsessive Compulsive Scale (YBOCS) (Goodman 1989) or the National Institute of Mental Health scale, modified for OCD (NIMH‐OCD).
3. The effectiveness of treatment was assessed with measures of: Quality of life and functional disability, such as the Sheehan Disability Scale (SDS), which includes subscales to assess work, social and family‐related impairment (Sheehan 1996)
4. The acceptability of treatment was determined by: a) The total proportion of participants who withdrew from the RCTs due to treatment emergent adverse events. This was assessed as a surrogate measure of medication acceptability, in the absence of other more direct indicators of acceptability. b) The most common drug‐related adverse events (defined as those occurring in at least 20% of the participants given medication), as well as significant differences in the rate of occurrence of drug‐related adverse events between medication and control groups. This was described as part of the narrative review.
Search methods for identification of studies
Electronic searches
1. The Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR‐Studies) was searched in December 2007 using the following search strategy;
Diagnosis = "Body Dysmorphic Disorder" or Dysmorphophobia
2. The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2007) was searched using the terms '"body dysmorphic disorder" or dysmorphophobia
3. Additional searches were carried out on MEDLINE (via PubMed, 1966 ‐ December 2007) and PsycINFO (1972‐ December 2007). The MEDLINE search query was derived from the highly sensitive search strategy developed by Robinson and Dickersin (Robinson 2002) for identifying controlled trials in PubMed. The exact search queries used to identify trials in these databases is provided as an appendix to this review.
4. Ongoing trials were located using the metaRegister of Controlled Trials database (mRCT) (http://www.controlled‐trials.com), the National Institute of Health's Computer Retrieval of Information on Scientific Projects (CRISP) service (http://crisp.cit.nih.gov) and the WHO international clinical trials registry platform (ICTRP) search portal (http://www.who.int/trialsearch). The search terms 'body dysmorphic disorder' and 'dysmorphophobia' were entered separately into the search interface for the CRISP database, and in combination (using the OR boolean operator) when searching the mRCT and ICTRP databases.
Searching other resources
Reference Lists The bibliographies of all identified trials were checked for additional studies.
Personal Communication Published and unpublished trials were obtained from key researchers, as identified by the frequency with which they are cited in the bibliographies of RCTs and open‐label studies.
Data collection and analysis
Selection of studies
RCTs that were potentially eligible for inclusion after an initial screening of their abstracts by one of the review authors (JI) were independently assessed by two authors (DS & JI), based on information included in the main body of the trial report, or its abstract, in cases in which the article was not accessible.
Data extraction and management
Spreadsheet forms were designed for the purpose of recording descriptive information, summary statistics of the outcome measures, the quality scale ratings, and associated commentary. This data was subsequently exported to the Review Manager (RevMan) software. Where information was missing, the review authors contacted investigators by email in an attempt to obtain it.
The following information was collated independently by two review authors (CS and JI) from each trial that satisfied the inclusion criteria:
1. Description of the trials, including primary researcher and year of publication
2. Characteristics of the interventions, including the number of participants randomised to the treatment and control groups, and in psychotherapeutic trials, the form of psychotherapy practiced. In RCTs employing medication, the name of the medication, the class to which it belongs (e.g. SSRIs, benzodiazepines), and the doses and duration of medication used were also recorded.
3. Characteristics of trial methodology, including the diagnostic (e.g. DSM‐IV (APA 1994)) and exclusionary criteria employed, the screening instrument used (e.g. the Structured Clinical Interview for DSM‐IV (SCID) (Spitzer 1996)), the inclusion of comorbidity ‐‐ including major depression and OCD, the use of a placebo run‐in and a minimal severity criterion, the number of centres involved, and the trial's methodological quality.
4. Characteristics of participants, including their gender distribution, the mean and range of their ages, and the mean length of time that they have been diagnosed with BDD.
5. Outcome measures employed, and summary continuous (means and standard deviations) and dichotomous (number of responders) data. Additional information was included, such as whether data reflected the intent‐to‐treat (ITT) with last observation carried forward (LOCF) or completer/observed cases (OC) sample, the drop‐out rates of participants randomised to the active intervention and control groups, as well as the proportion of drop‐outs who stopped participating due to treatment‐emergent adverse effects.
6. Quality assessment, including the number of randomised participants who were not included in the analysis (lost to follow‐up (LTF)), whether blinding occurred for the assessor/s, participants, or those who administered the active intervention, as well as whether the allocation of medication was randomised and the allocation sequence was concealed (the methods used in implementing these respective bias reduction measures were also documented).
Assessment of risk of bias in included studies
Data for trial characteristics which have been recognised as potential sources of bias, such as the method used in generating the allocation sequence, how allocation was concealed, whether outcome assessment was blinded, and the number of participants who where lost to follow up, were independently determined by two raters (CS and JI), as part of the data collation process. This is regarded as necessary given doubts concerning the usefulness of an overall quality score from a scale composed of multiple items (Higgins 2008).
The quality of the trials was also assessed by one of the review authors (JI) using the CCDAN Quality Rating Scale (CCDAN‐QRS) (Moncrieff 2001). This 23‐item scale assesses a range of features such as sample size, the duration of the intervention, inclusion and exclusion criteria, and whether or not the power of the trial to detect a treatment effect was calculated.
Measures of treatment effect
Dichotomous data Relative risk of response to treatment and number needed to benefit (NNTB) was calculated for the dichotomous outcome of interest (CGI‐I or related measure). Relative risk was used instead of odds ratios, as odd ratios are less easily interpreted. Odds ratios also tend to overestimate the size of the treatment effect when interpreted as relative risks. This is especially the case when the occurrence of the outcome of interest is common (as anticipated in this review, with an expected response greater than 20%) (Deeks 2008). NNTB is defined as the inverse of the absolute risk difference due to the active intervention. In this review it is used to indicate the number of patients who require treatment with either pharmacotherapy or psychotherapy, relative to a control, before a single additional patient responds to active treatment.
Continuous data Weighted means were calculated for continuous summary data obtained from studies employing identical scales. Alternatively, in cases in which different versions of the same scale are employed, such as both the 10 and 12 item YBOCS‐BDD, the standardised weighted mean was determined. This method of analysis standardises the differences between the means of the treatment and control group in terms of the variability observed in the trial.
Unit of analysis issues
Trials with more than one treatment group Unit‐of‐analysis bias may be introduced from trials testing the efficacy of fixed doses of medication through comparing the summary statistics for multiple groups against the same placebo control (Deeks 2008). Although no dose‐comparison studies were included in this review, this bias will be controlled for in future editions of this review by pooling the means and standard deviations across all of the treatment arms as a function of the number of participants in each arm. The same form of bias resulting from the inclusion of trials comparing the efficacy of more than two interventions will be circumvented in future updates of this review by means of a multiple‐treatments meta‐analysis (Higgins 2008b).
Cross‐over trials Primary outcome summary statistics for cross‐over trials with an adequate wash‐out period were calculated separately from RCTs which employ a parallel design (adequate wash‐out will be defined as a minimum of ine month for trials employing psychotherapy, and a minimum of two weeks in the case of medication trials ‐‐ data from trials assessing the efficacy of agents with extended half‐lives, such as the SSRI, fluoxetine (Gury 1999), would require longer wash‐out periods before being included). In cases in which the wash‐out period is of an insufficient duration, or in which the small number of cross‐over trials does not justify the separate analysis of the summary statistics, only treatment and placebo/comparator data from the first treatment period would be combined with the data from parallel RCTs.
Dealing with missing data
All analyses of dichotomous data were intention‐to‐treat (ITT). The total number of participants randomised to the different comparison groups were used as the denominator in comparisons of treatment response. Only data from trials that provide information on the original group size (prior to drop‐outs) were included in these analyses. Preference was given to the inclusion of summary statistics for continuous outcome measures derived from mixed effects models, followed by last observation carried forward (LOCF) and observed cases (OC) summary statistics (in that order). This is in line with evidence that ME methods are more robust to bias than LOCF analyses (Verbeke 2000).
Assessment of heterogeneity
Heterogeneity of treatment response, that is whether the differences among the results of trials were greater than would be expected by chance alone, was assessed visually from a forest plot of relative risk, as well as by means of the chi‐square test of heterogeneity. A significance level of less than 0.10 was interpreted as evidence of heterogeneity, as the chi‐square statistic is reported to possess less power when the number of trials is small (Deeks 2008).
In addition, the I‐square heterogeneity statistic reported by RevMan was used to quantify the consistency of the trial results within each comparison (Higgins 2003). Differences on continuous measures in medication efficacy between the group being compared was assessed by means of Deeks' stratified test of heterogeneity (Deeks 2001). This method subtracts the sum of the chi‐square statistics for each of the groups from the total chi‐square for the subgroup analysis, to provide a measure (Qb) of heterogeneity between groups. Differences in treatment response on the CGI‐I was determined by whether the confidence intervals of the subgroups overlap. This method was chosen in preference to the stratified test, due to inaccuracies in the calculation in RevMan of the chi‐square statistic for dichotomous measures (Deeks 2008).
Assessment of reporting biases
The authors planned to visually inspect a funnel plot of treatment response in order to detect small trial effects, including those resulting from publication bias. However, this was not feasible in the current version of the review, given the small number of included trials.
Data synthesis
All comparisons were stratified by treatment mode (pharmacotherapy, psychotherapy, or multimodal interventions). A random effects model was employed for the analysis of both dichotomous and continuous outcome measures. As this model includes both within‐study sampling error and between‐studies variation, there is less risk of committing a Type I error (falsely concluding that there is a treatment effect when there is none) through overestimating the precision of effect size estimates, than would be the case were the fixed effect model employed (Hunter 2000).
Subgroup analysis and investigation of heterogeneity
A series of comparisons between subgroups, as defined by a number of prespecified clinical and methodological criteria, were planned in order to identify the source of systematic differences across studies on both primary outcome measures according to:
1. trial duration, with interventions of eight weeks or less compared to longer short‐term interventions (>8 to 16 weeks), to determine whether treatment efficacy depends on trial duration.
2. whether or not trials included BDD participants with delusions or major depressive episodes. These analyses were deemed necessary given speculation that delusional and non‐delusional BDD represents the same disorder (Phillips 2006) and the finding that changes in the status of BDD and major depression are correlated over time (Phillips 2006c)
3. the use of waiting list compared to usual care in psychotherapy trials
However, these subgroup analyses could not be conducted in the current version of the review, due to the small number of trials and inadequate reporting of participant demographics.
In recognition of the possibility of differential effects for interventions belonging to these different categories, it was planned to stratify primary outcomes by medication class or type of psychotherapy, however, the limited number of eligible trials did not warrant their stratification (see Types of intervention section for information on categories).
Sensitivity analysis
Sensitivity analyses were planned to assess the degree to which the findings obtained on the primary outcome measures were influenced by the following criteria
1. Treatment response versus non‐response as the unit of comparison in determining medication efficacy.
2. Whether or not trials possess a cross‐over or parallel group design. This analysis would only be performed in comparing the summary statistics from parallel group trials with those from cross‐over trials which possess adequate wash‐out periods (defined in terms of the half‐life of the respective medication/s, or in trials employing psychotherapy, as a minimum of one month duration).
3. The influence of missing data on treatment response.
For the current version of the review, the analysis to determine the robustness of this review's findings to the use of treatment response versus non‐response as the unit of comparison was not conducted, as only a single trial provided summary statistics on the CGI‐I (Phillips 2002). Comparison of the effect of trial design was also not possible, as the only included RCT to employ a cross‐over design (Hollander 1999) was not comparable to the other medication trial in terms of the control group employed. Finally, the planned analysis of the effect of including outcome data for the loss‐to‐follow up (LTF) sample could also not be performed, as none of the RCTs reported any LTF amongst participants.
Results
Description of studies
Results of the search
The search of the CCDAN Controlled Trials Register (CCDANCTR) yielded eight results. The PubMed and psycINFO searches retrieved 138 and 25 articles, respectively. All of the abstracts retrieved from CCDANCTR were selected for independent assessment by two raters, as were 69 from the PubMed and eight from the psycINFO databases. All five of the included trials were retrieved through PubMed, with psycINFO yielding four and the CCDANCTR‐Studies database yielding two. The search for unpublished trials resulted in the retrieval of 13 documents from the mRCT database, and 10 each from the CRISP and ICTRP search portals. Five of these studies are regarded as potentially eligible for inclusion in future updates of this review (described in the section on ongoing studies, below).
Included studies
A total of two pharmacotherapy (96 participants) and three psychotherapy (83 participants) trials were eligible for inclusion in the review. A16 week cross‐over comparison of the serotonin noradrenergic reuptake inhibitor (SNRI) clomipramine (mean dose: 138 mg/d) with the TCA desipramine (mean dose: 147 mg/d) (Hollander 1999) in 23 patients with BDD reported clomipramine as significantly more effective on all of the primary outcomes (CGI, modified for BDD, BDD‐YBOCS, NIMH‐OCD). Similar results were obtained when comparing the efficacy of 12 weeks of placebo‐controlled fluoxetine treatment (mean dose: 78 mg/d) in a sample of 67 participants (Phillips 2002).
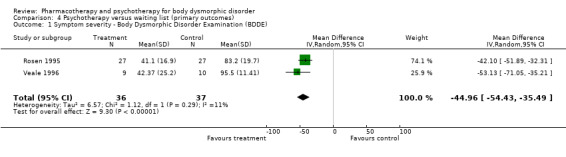
Two of the three psychotherapy trials (Rosen 1996 and Veale 1996) compared the efficacy of 12 weeks of CBT to waiting list comparison groups. Rosen 1996 observed significant improvement in 27 patients on all symptom measures after treatment with 8 2‐hour sessions of CBT consisting of both ERP and psycho‐education components. Twenty‐two of the 27 subjects (81.5%) receiving CBT were reported as no longer meeting diagnostic criteria on the BDDE at posttreatment, with over three‐quarters (20/26) of the patients having recovered at follow‐up 4.5 months after treatment ended. Veale 1996 randomised 19 patients to a waiting list or 12 sessions of CBT involving attention training and ERP, and detected a significant reduction on BDD symptom severity on the BDDE and the BDD‐YBOCS.
The remaining psychotherapy trial (McKay 1997) investigated the effectiveness of six months of maintenance treatment following a six week course of behavioural therapy (ERP). Although the investigators did not detect differences in BDD symptom severity on the BDD‐YBOCS at the end of the maintenance phase between the 5 participants receiving maintenance treatment versus the five who were randomised not to receive it, significant reductions on measures of anxiety and depression were observed.
There were substantially fewer male participants in the psychotherapy (7.2%) than the medication trials (38.9%), with one of the CBT trials (Rosen 1995) composed exclusively of female participants.
Trials excluded from quantitative analyses The reviewers were unable to extract summary statistics from McKay 1997 for inclusion in the meta‐analyses.
Excluded studies
Trials of citalopram (Phillips 2003), escitalopram (Phillips 2006e) and fluvoxamine (Perugi 1996, Phillips 1998) and venlafaxine (Allen 2008a) were excluded due to a lack of a control group. Three medication augmentation trials were excluded. Treatment with fluoxetine was augmented with pimozide (Phillips 2005b) and olanzapine (Phillips 2005c) in two of these trials, while buspirone was added to a range of SSRIs in the third (Phillips 1996). The augmentation trials will be assessed for inclusion in the update of a separate Cochrane Review of pharmacotherapy augmentation strategies in treatment‐resistant anxiety disorders (Ipser 2006).
Ongoing studies The CRISP, mRCT and WHO ICTRP databases provided information for five ongoing trials: a 13 week fluoxetine RCT for children and adolescents aged 10‐16 years with BDD (NCT00245635), a 6 month relapse prevention trial for 58 responders to 14 weeks of open‐label treatment with escitalopram (NCT00149799), a 24 week trials comparing 22 sessions of individual, manual‐based CBT with a waiting list (NCT00106223), and an augmentation RCT providing CBT to 20 patients who have received at least 12 weeks of treatment with an SRI (NCT00211809). A comparison of interpersonal psychotherapy with treatment as usual is also planned (1K23MH076934‐01A1). In addition, a 12 week trial of fluoxetine for adolescents with BDD has been suspended (NCT00029471).
Risk of bias in included studies
Allocation
Generation of Allocation Sequence Only one of the trials (Phillips 2002) described the manner in which the randomisation sequence was allocated. A computer‐generated urn procedure was employed in this study.
Allocation Concealment The randomisation list was kept by a technician with no clinical contact in the only trial reporting the method used to conceal treatment allocation (Phillips 2002).
Blinding
Outcome assessment was explicitly described as blinded in three of the short term trials (Hollander 1999, Phillips 2002; Rosen 1995). These included both medication RCTs, the only trials in which the assessors were blinded to adverse events as well.
Incomplete outcome data
Patient withdrawals were restricted to the medication trials. A total dropout rate of 14.6% (14/96) was observed following treatment with medication. The dropout rates for the primary intervention and comparison groups (including desipramine in the crossover trial) were 16.1% and 6.3%, respectively.
Quality Score The average quality score on the CCDAN‐QRS for the RCTs was 24 (range: 12‐39) out of a maximum of 46 points. On this scale, one of the five trials provided adequate details of the side effects experienced by each group and recorded the number and reasons for withdrawal by group. Only two RCTs explicitly declared the source of funding for the trials.
The pharmacotherapy trials obtained a higher quality rating score on average than the psychotherapy trials (31.5 versus 19.3, respectively), largely due to inadequate reporting of the outcomes and aspects of the trial methodology in the latter.
Effects of interventions
Outcome summary statistics from two pharmacotherapy and two CBT randomised controlled trials were included in the analyses. Information regarding the design, interventions, participants and outcomes of these trials is provided in the "Description of studies" section of this review.
1. Pharmacotherapy versus placebo
Primary outcome measures
Treatment response (Graph 1.1) Treatment response on the CGI‐I was significantly higher following treatment with medication (fluoxetine) than placebo (55.9% and 18.2% respectively, Relative Risk (RR) 3.07, 95%CI 1.40 to 6.72, n = 67) in the only trial to include this outcome measure (Phillips 2002). This is equivalent to a number needed to benefit (NNTB) of 2.7.
Symptom severity (Graph 1.2) Administration of fluoxetine also resulted in a statistically significant reduction of approximately 6 points on the BDD‐YBOCS symptom severity scale, relative to placebo (number of trials (N) = 1, Weighted Mean Difference (WMD) ‐5.90, 95%CI ‐10.52, ‐1.28, number of participants (n) = 67)
Secondary outcome measures
1. Delusionality Medication was reported in the trial report for the RCT of fluoxetine as demonstrating equivalent efficacy in reducing BDD symptom severity in both delusional and non‐delusional participants (Phillips 2002). Delusionality was significantly reduced in treatment responders versus non‐responders from both treatment groups combined (F = 9.5, p < 0.001).
2. Comorbid depression and OCD symptoms (Graphs 2.1 to 2.3) Depression scores were also significantly reduced in participants administered fluoxetine (N = 1, WMD = ‐7.00, 95%CI ‐11.94 to ‐2.06, n = 67) (Graph 2.1). Fluoxetine failed to improve quality of life on the mental health subscale of the 36‐Item Short‐Form Health Survey (Ware 1993) (N = 1, WMD = 9.1, 95%CI ‐1.05 to 19.25, n = 67) (Graph 2.2). However, this agent was effective in reducing functional disability on the Range of Impaired Functioning Tool (Leon 1999) (WMD = ‐3.00, 95%CI ‐4.65 to ‐1.35, n = 67) (Graph 2.3).
3. Quality of life/functional disability No trials contributed data to this outcome
4. Acceptability Treatment with fluoxetine led to more than 20% of 34 participants experiencing insomnia, drowsiness and stomach/abdominal discomfort (in order of decreasing incidence) (Phillips 2002). Stomach or abdominal discomfort (chi‐squared = 7.5, p < 0.01) and drowsiness (chi‐squared = 4.4, p < 0.05) were the only side effects to occur significantly more frequently in the medication than placebo group, with approximately a third of the participants experiencing these symptoms following treatment with fluoxetine. No drug‐related adverse events were regarded as severe by the authors. 2. Pharmacotherapy versus alternative medication
Primary outcome measures 1. Treatment response No trials contributed data to this outcome.
2. Symptom severity (Graph 3.1) There was evidence from the single study comparing clomipramine with desipramine that clomipramine reduced BDD symptom severity on the BDD‐YBOCS (WMS ‐5.72, 95%CI ‐11.17, ‐0.27, n = 23).
Secondary outcome measures 1. Delusionality Clomipramine was reported to be more effective than desipramine in reducing symptom severity amongst both delusional and non‐delusional patients (assessed using the Fixity of Beliefs scale (Foa 1995)) (Hollander 1999). Higher levels of delusionality were associated with greater treatment response on the CGI‐BDD after treatment with clomipramine (r = ‐0.56, p = 0.007). A reduction in symptoms of delusionality was observed in the clomipramine, but not the desipramine group.
2. Comorbid depression and OCD symptoms Depression and OCD symptom severity scores were reported as decreasing to a greater extent following treatment with clomipramine than desipramine (t = 2.44, p = 0.02 and t = 3.01, p = 0.007, respectively) (Hollander 1999). The diagnosis of both social anxiety and OCD was found to be unrelated to outcome response in Hollander 1999.
3. Quality of life/functional disability Clomipramine was also reported as reducing functional disability on the Schneier Disability Profile (Schneier 1994) to a greater extent than desipramine after a minimum of 4 weeks of treatment with both agents (Hollander 1999).
4. Acceptability Clomipramine and desipramine resulted in high rates of dry mouth, sedation or tiredness, constipation, sweating and tremor, and insomnia. Although the frequency of these side effects did not differ significantly between the two medication groups (averaging 64.8% versus 41.6% for clomipramine and desipramine, respectively), the only subjects in this review to withdraw due to adverse events were 4 patients receiving desipramine (Hollander 1999). The particular side effects resulting in drop out in this trial were not specified. 3. Psychotherapy versus waiting list
Primary outcome measures 1. Treatment response No trials contributed data to this outcome.
2. Symptom reduction (Graph 4.1) Psychotherapy (CBT) reduced symptom severity scores on the BDDE by 45 points, relative to subjects receiving no treatment (N = 2, WMD = ‐44.96, 95%CI ‐54.43 to ‐35.49, n = 73).
3. Relapse The treatment gains following eight 2‐hourly sessions of CBT, as reported by Rosen 1995, were maintained at 4.5 months post‐treatment, with only 4 of the 22 participants who no longer met diagnostic criteria on the BDDE at the end of treatment having relapsed by the follow‐up assessment. Significantly, 3 of the 5 participants who still satisfied the criteria for BDD immediately after treatment no longer did so at follow‐up.
Secondary outcome measures 1. Delusionality No trials contributed data to this outcome.
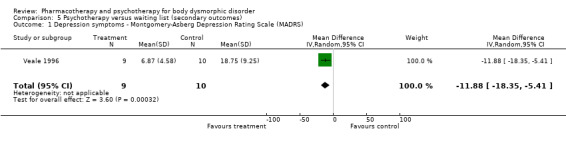
2. Comorbid depression and OCD symptoms (Graph 5.1) Depression scores were significantly reduced after 12 weeks of treatment with CBT in the one trial that reported this outcome (N = 1, WMD = ‐11.88, 95%CI ‐18.35 to ‐5.41, n = 19).
3. Quality of life/functional disability No trials contributed data to this outcome.
4. Acceptability No trials contributed data to this outcome.
Discussion
The findings of this review indicate that both pharmacotherapy (clomipramine, fluoxetine) and psychotherapy (CBT) may be effective in the treatment of BDD.
Fluoxetine demonstrated a significantly higher global response to treatment with medication than placebo on the CGI‐I (Phillips 2002). More than three times as many people responded on this scale in the medication group, which is equivalent to approximately three patients having to be treated with this agent before an additional person responds, relative to those who would have responded to placebo alone (ie. NNTB). The observation that clomipramine was more effective at decreasing symptom severity scores than desipramine is consistent with the findings of open‐label trials of medications which more selectively target the serotonergic system, such as citalopram (Phillips 2003) and escitalopram (Phillips 2006e).
Cognitive behavioural therapy was implemented in both short‐term psychotherapy trials, thus demonstrating that this modality may be effective in the treatment of BDD. There is also preliminary evidence that the effects of treatment with CBT may persist once treatment has ended (Rosen 1995). The only data on the efficacy of long‐term treatment with psychotherapy are inconclusive, as six months of ERP maintenance treatment (and emergency support where required) did not result in significant reductions in BDD symptom severity (McKay 1997).
Despite some promising findings, there are a number of qualifications that need to be made with respect to the strength of the evidence upon which they are based. Firstly, the small sample size and number of RCTs in the comparisons conducted, as well as inadequacies of reporting (and possibly trial methodology) limit the strength of the conclusions that can be drawn. It was also not possible to compare the efficacy of different forms of psychotherapy, or to comment on the relative efficacy of the cognitive or behavioural modification components of CBT, as data from RCTs was only available for studies combining both aspects of treatment. The power to detect the efficacy of pharmacotherapy was compromised by the necessity of analysing data separately from the two included trials, as differences in study design prohibited synthesis of data from these trials. Finally, the review authors were not able to exclude the possibility of bias being introduced through dependence of publication in this field on study outcomes.
There is some evidence that fluoxetine and CBT may be effective in treating co‐morbid symptoms of depression in BDD. Functional disability was significantly reduced in a trial of clomipramine (Hollander 1999), a noteworthy finding given the relatively high levels of functional disability suffered by those with BDD compared to other related disorders (Frare 2004). However, treatment with another SRI, fluoxetine, failed to improve quality of life as measured by the Medical Outcomes Study 36‐Item short‐form Health Survey (SF‐36) (Phillips 2002). This could be due in part to the large proportion of participants who experienced treatment‐emergent adverse event in this trial (82%). Nevertheless, no drop‐outs due to adverse events were observed in this review following treatment with the SRIs, suggesting that this medication class is well tolerated in treating BDD.
There were insufficient data for a systematic evaluation of differences in treatment response between delusional and non‐delusional participants. The conceptualisation of the delusional variant as representing a more severe form of the disorder receives some support from the discovery in one of these trials of a positive relationship between delusionality and response to the SSRI, clomipramine (Hollander 1999). Patients with poor insight were also significantly less likely to respond to placebo than less delusional participants in the fluoxetine study (Phillips 2002). Indirect evidence that delusional and non‐delusional BDD represent differences of severity rather than kind also comes from failure to observe differences in the response of these groups in placebo‐controlled and open‐label augmentation studies of the SSRI fluoxetine with the antipsychotics pimozide (Phillips 2005b) and olanzapine (Phillips 2005c), respectively.
The evidence database does not currently support conclusions regarding the relative efficacy of pharmacotherapy versus psychotherapy in treating BDD. Difficulties in obtaining equivalent control groups when conducting head‐to‐head comparisons may be compounded in indirect comparisons of these treatment modalities. The frequent finding of larger effects in RCTs of CBT than antidepressants (eg NICE 2005; Williams 2006) needs to be considered in light of the possibility that wait‐list controls in the former might inflate effect size estimates when compared to placebo‐controlled pharmacotherapy trials. Assessing the comparability of trials of psychotherapy and pharmacotherapy is further complicated by differences between modalities in the quality of reporting of trial methodology and outcomes. It is unclear, for instance, to what extent the results of psychotherapy RCTs in this review generalise to the highly comorbid populations that typically present in clinical practice, as the included studies did not provide data on prevalence of psychiatric comorbidity in their samples. The use of different measures of symptom severity in medication and psychotherapy trials also hinders cross‐modality comparisons. Finally, the absence of a follow‐up phase in three of the trials makes it difficult to assess whether or not the effects of CBT are more persistent than pharmacotherapy.
Evidence is currently lacking for the effectiveness of administering multiple forms of intervention concurrently in treating BDD (ie. combining medications or psychotherapy with pharmacotherapy), despite the widespread use of multiple interventions in clinical practice. Ongoing research on adding CBT to a minimum of 12 weeks of treatment with a SSRI (NCT00211809) will help determine whether there is any basis for speculation that combining pharmacotherapy and psychotherapy might further increase treatment response relative to either intervention on its own. Preliminary evidence from small placebo‐controlled studies and case series studies suggest that antipsychotics may not be useful when used in combination with an SSRI (Phillips 2005b; Phillips 2005c).
The conclusion that pharmacotherapy and psychotherapy may be efficacious in treating BBD is consistent with the findings of the only other meta‐analysis of treatment of this disorder (Williams 2006).This is encouraging, given the stricter inclusion criteria employed in this review. The finding in Williams 2006 of a significantly larger overall treatment effect for five CBT trials (three of which have been excluded from this review ‐ Khemlani‐Patel 2001; Neziroglu 1996; Wilhelm 1999) versus five trials of medication (including those in this review, as well as one case series study of citalopram (Phillips 2003) and two of fluvoxamine (Perugi 1996; Phillips 1998)), should be approached with caution, however, not only because of the indirect nature of this comparison, but also as it combines data from case series studies and RCTs, some which contribute multiple effect sizes.
The small number of trials employing rigorous designs in assessing the treatment of BDD is noteworthy, given the high prevalence and morbidity associated with this condition. The lack of controlled trials and the paucity of research on the pathogenesis of this disorder indicate that it is a field which deserves additional attention and funding.
Authors' conclusions
Implications for practice.
The results from the small number of RCTs reviewed suggest that certain SRIs and CBT may be effective in treating patients with BDD. Tolerability was acceptable for both modalities, as indicated by high compliance rates, and no drop‐outs due to treatment‐emergent adverse events with SRIs. There is also preliminary evidence that the effects of treatment with CBT may persist once treatment has ended. Although it was not an aim of this review to assess augmentation studies, there is little evidence in the literature that adding antipsychotics to treatment for non‐responders to SRIs is an effective treatment strategy.
Implications for research.
The small number of controlled trials included in this review reflects the paucity of research in the treatment of a prevalent and disabling disorder. Additional research into the relative efficacy of cognitive and behavioural therapy is warranted, as is the assessment of whether adding cognitive restructuring to behaviour therapy improves compliance rates, as has been speculated by some (Geremia 2001). Controlled trials of other established psychotherapy modalities and third generation psychotherapies (eg. mindfulness therapy) may also be warranted. Open‐label studies of promising selective SRIs, such as escitalopram and citalopram, should be followed up with randomised placebo‐controlled trials. The contribution of these trials to knowledge regarding the effectiveness of particular interventions in treating BDD would be enhanced if they were reported in a manner consistent with the guidelines of the CONSORT statement (Moher 2001).
Additional data are needed to address several areas, including the efficacy of treatment over the longer‐term, the value of medication augmentation strategies for non‐responders, and the efficacy of combination medication and multimodal treatment strategies (pharmacotherapy and psychotherapy). Future research should also focus on gender differences in treatment response and whether findings of this review translate to other age groups (ie adolescents). Finally, comparability between treatment of BDD with pharmacotherapy and psychotherapy would be enhanced through agreement on the use of a common standardised outcome scale.
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2008 | Amended | Converted to new review format. |
Acknowledgements
This work is supported by the MRC Unit on Anxiety Disorders. The reviewers would like to thank Dr Andrea Allen for providing outcome data for Hollander 1999 .
Appendices
Appendix 1. Search strategies for electronic databases
MEDLINE:
(randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ("latin square" [tw]) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh]) AND ( "body dysmorphic disorder" [tw] OR dysmorphophobia [tw])
PsycINFO:
search strategy included the following search query: ("randomisation" OR "randomization") OR "controlled" AND ("body dysmorphic disorder" OR dysmorphia).
Data and analyses
Comparison 1. Pharmacotherapy versus placebo (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment response ‐ Clinical Global Impressions Scale ‐ Improvement item (CGI‐I) | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [1.40, 6.72] |
| 2 Symptom severity ‐ BDD Yale Brown Obsessive Compulsive scale | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
1.1. Analysis.

Comparison 1 Pharmacotherapy versus placebo (primary outcomes), Outcome 1 Treatment response ‐ Clinical Global Impressions Scale ‐ Improvement item (CGI‐I).
1.2. Analysis.

Comparison 1 Pharmacotherapy versus placebo (primary outcomes), Outcome 2 Symptom severity ‐ BDD Yale Brown Obsessive Compulsive scale.
Comparison 2. Pharmacotherapy versus placebo (secondary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression symptoms ‐ Hamilton Depression Scale (HAM‐D) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐11.94, ‐2.06] |
| 2 Quality of life ‐ 36‐Item Short‐Form Health Survey (SF‐36) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 9.10 [‐1.05, 19.25] |
| 3 Functional disability ‐ Range of Impaired Functioning Tool (RIFT) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐4.65, ‐1.35] |
2.1. Analysis.

Comparison 2 Pharmacotherapy versus placebo (secondary outcomes), Outcome 1 Depression symptoms ‐ Hamilton Depression Scale (HAM‐D).
2.2. Analysis.

Comparison 2 Pharmacotherapy versus placebo (secondary outcomes), Outcome 2 Quality of life ‐ 36‐Item Short‐Form Health Survey (SF‐36) .
2.3. Analysis.

Comparison 2 Pharmacotherapy versus placebo (secondary outcomes), Outcome 3 Functional disability ‐ Range of Impaired Functioning Tool (RIFT).
Comparison 3. Pharmacotherapy versus alternative medication (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom severity ‐ BDD Yale Brown Obsessive Compulsive scale | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
3.1. Analysis.

Comparison 3 Pharmacotherapy versus alternative medication (primary outcomes), Outcome 1 Symptom severity ‐ BDD Yale Brown Obsessive Compulsive scale.
Comparison 4. Psychotherapy versus waiting list (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom severity ‐ Body Dysmorphic Disorder Examination (BDDE) | 2 | 73 | Mean Difference (IV, Random, 95% CI) | ‐44.96 [‐54.43, ‐35.49] |
4.1. Analysis.
Comparison 4 Psychotherapy versus waiting list (primary outcomes), Outcome 1 Symptom severity ‐ Body Dysmorphic Disorder Examination (BDDE).
Comparison 5. Psychotherapy versus waiting list (secondary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression symptoms ‐ Montgomery‐Asberg Depression Rating Scale (MADRS) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐11.88 [‐18.35, ‐5.41] |
5.1. Analysis.
Comparison 5 Psychotherapy versus waiting list (secondary outcomes), Outcome 1 Depression symptoms ‐ Montgomery‐Asberg Depression Rating Scale (MADRS).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hollander 1999.
| Methods | DESIGN
Description: Randomised, flexible dose, double blind, cross‐over trial, 2 week single blind placebo run‐in BLINDING Participants: Unclear Assessors: Yes Administrators: Yes ALLOCATION CONCEALMENT Method: Unclear RANDOMISATION Method: Unclear |
|
| Participants | SAMPLE
Description: 40 DSM‐III‐R patients (35 randomised); average age: 34.5 years; duration of illness: 18.1 years; 57.5% male; baseline severity on BDD‐YBOCS: SCREENING Primary diagnosis: clinical interview Comorbidity: SCID‐I |
|
| Interventions | Description:clomipramine (25 ‐250 mg/d; mean dose: 138 mg/d) versus desipramine (25 ‐250 mg/d; mean dose: 147 mg/d) x 16 weeks | |
| Outcomes | Primary outcomes: BDD‐YBOCS (10 item), BDD‐CGI, BDD‐NIMH (mod)
Secondary outcomes: YBOCS, SADS, FNES, SDP, FBQ Data estimation: OC (?) |
|
| Notes | INDUSTRY SUPPORT
Industry funded: No
Medication provided by industry: No
Any of the authors work for industry: No ADDITIONAL INFORMATION Drop‐out rates: 1 on chlomipramine, 5 on desipramine (for 12 weeks) Quality rating score: 23 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McKay 1997.
| Methods | DESIGN
Description: Randomised, parallel, maintenance trial, unblinded (assumed) BLINDING Participants: No Assessors: No Administrators: No ALLOCATION CONCEALMENT Method: No RANDOMISATION Method: Unclear |
|
| Participants | SAMPLE
Description: 10 DSM‐III‐R BDD, 60% female, average age: 31.2 years SCREENING Primary diagnosis: SCID Comorbidity: Unclear |
|
| Interventions | Description: 6 week uncontrolled behavioural therapy (exposure with response prevention) followed by 6 month controlled maintenance period (included in review) | |
| Outcomes | BAT; BDD‐YBOCS; BDI; BAI (no distinction made between primary and secondary outcomes) Data estimation: ITT |
|
| Notes | INDUSTRY SUPPORT
Industry funded: Unclear
Medication provided by industry: N/A
Any of the authors work for industry: No ADDITIONAL INFORMATION Drop‐out rates: 0 Quality rating score: 16 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Phillips 2002.
| Methods | DESIGN
Description: Randomized, placebo‐controlled, parallel, flexible dose, 1 week single blind placebo run‐in BLINDING Participants: Unclear Assessors: Yes Administrators: Yes ALLOCATION CONCEALMENT Method: clinician kept code RANDOMISATION Method: computer generated urn procedure |
|
| Participants | SAMPLE
Description: 67 DSM‐IV BDD, 68.7% female, average age: 32.1 years, average duration of illness: 14.5 years, 64.2% MDD, baseline severity on BDD‐YBOCS: 31.5 (fluoxetine) 30.8 (placebo) SCREENING Primary diagnosis: BDD‐DM Comorbidity: SCID‐P; SCID‐PD |
|
| Interventions | Description: fluoxetine (20‐80mg/d; mean dose: 77.7 mg/d) vs placebo (20‐80mg/d; mean dose: 76 mg/d) x 12 weeks | |
| Outcomes | Primary outcomes: BDD‐YBOCS,
Secondary outcomes: CGI‐I, BDD‐CGI, BDD‐NIMH,BABS, HAM‐D, BPRS, SOFAS, GAF Data estimation: LOCF |
|
| Notes | INDUSTRY SUPPORT
Industry funded: No
Medication provided by industry: Yes
Any of the authors work for industry: Yes ADDITIONAL INFORMATION Drop‐out rates: 0 on fluoxetine and placebo Quality rating score: 39 3 patients in the fluoxetine and placebo groups received ongoing psychotherapy (not CBT) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rosen 1995.
| Methods | DESIGN
Description: Randomised, parallel, no treatment‐controlled, parallel trial, uncontrolled follow‐up BLINDING Participants: No Assessors: Yes Administrators: No ALLOCATION CONCEALMENT Method: No RANDOMISATION Method: Unclear |
|
| Participants | SAMPLE
Description: 54 DSM‐III‐R & DSM‐IV, 100% female, average age: 36.5 years, baseline severity on BDDE: 83.9 (CBT) 89.9 (waiting list) SCREENING Primary diagnosis: BDDE Comorbidity: None |
|
| Interventions | Description: Cognitive‐behavior therapy (information about model, exposure therapy, cognitive restructuring, thought stopping, response prevention) versus no‐treatment control x 8‐12 weeks | |
| Outcomes | BDDE, BSQ, MBSRQ‐AE, BSI, RSS (no distinction made between primary and secondary outcomes) Data estimation: ITT |
|
| Notes | INDUSTRY SUPPORT
Industry funded: Unclear
Medication provided by industry: N/A
Any of the authors work for industry: No ADDITIONAL INFORMATION Drop‐out rates: 0 for both groups Quality rating score: 30 Excluded patients who suffered from clear physical abnormalities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Veale 1996.
| Methods | DESIGN
Description: Randomised, waiting‐list control, parallel, non‐blinded (presumably) BLINDING Participants: No Assessors: No Administrators: No ALLOCATION CONCEALMENT Method: None RANDOMISATION Method: Unclear |
|
| Participants | SAMPLE
Description: 19 DSM‐IV BDD, 89.5% female, average age: 35.4 years, average duration of illness: 14.8 years, baseline severity on YBOCS: 22 (CBT) 21.18 (waiting list) SCREENING Primary diagnosis: Unclear Comorbidity: Unclear |
|
| Interventions | Description: Cognitive‐behaviour therapy (information, exposure & response prevention, cognitive restructuring) versus waiting list x 12 weeks | |
| Outcomes | BDD‐YBOCS, BDDE, MADRS, SPAI, HADI, DS (no distinction made between primary and secondary outcomes) Data estimation: Unclear |
|
| Notes | INDUSTRY SUPPORT
Industry funded: Unclear
Medication provided by industry: N/A
Any of the authors work for industry: No ADDITIONAL INFORMATION Drop‐out rates: Not provided Quality rating score: 12 Significant differences between groups on social phobia score |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Acronyms for scales: BAT: Behavioral Avoidance Test; BDD‐DM: Body Dysmorphic Disorder Diagnostic Module; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BSI: Brief Symptom Inventory; BSQ: Body Shape Questionnaire; DS: Derriford Scales; FBQ: Fixity of Beliefs Questionnaire; FNES: Fear of Negative Evaluation Scale; HADS: Hospital Anxiety and Depression Scale; HAM‐D: Hamilton Depression Scale; LIFE‐RIFT: Longitudinal Interval Follow‐up Evaluation ‐ Range of Impaired Functioning Tool; MADRS: Montgomery‐Asberg Depression rating scale; MBSRQ‐AES: Multidimensional Body Self‐Relations Questionnaire ‐ Appearance Evaluation Scale; RSES: Rosenberg Self‐Esteem Scale; SADS: Social Avoidance and Distress Scale; SCID‐PD: Structured Clinical Interview of DSM, Personality Disorders; SDP: Schneier Disability Profile; SDS: Sheehan Disability Scale; SF‐36: Medical Outcomes Study 36‐Item Short‐Form Health Survey; SPAI: Social Phobia and Anxiety Inventory; YBOCS: Yale Brown Obsessive Compulsive Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2008a | No control group |
| Campisi 1995 | No control group |
| Geremia 2001 | No control group |
| Gomez‐Perez 1994 | No control group |
| Khemlani‐Patel 2001 | No control group |
| Neziroglu 1993 | No control group |
| Neziroglu 1996 | No control group |
| Perugi 1996 | No control group |
| Phillips 1996 | No control group and augmentation study |
| Phillips 1998 | No control group |
| Phillips 2003 | No control group |
| Phillips 2005b | Augmentation trial. Included in an upcoming cochrane review of pharmacotherapy augmentation strategies in treatment‐resistant anxiety disorders |
| Phillips 2005c | Augmentation trial. |
| Phillips 2006e | No control group |
| Wilhelm 1999 | No control group |
Characteristics of ongoing studies [ordered by study ID]
1K23MH076934‐01A1.
| Trial name or title | Interpersonal Psychotherapy for Body Dysmorphic Disorder |
| Methods | No information |
| Participants | No information |
| Interventions | IPT versus treatment as usual |
| Outcomes | BDD severity, interpersonal distress, and social adjustment |
| Starting date | 11 January 2007 |
| Contact information | elizabeth_didie@brown.edu |
| Notes |
NCT00029471.
| Trial name or title | Pharmacotherapy of Body Dysmorphic Disorder in Adolescents |
| Methods | Interventional, Treatment, Randomized, Double‐Blind, Placebo Control, Parallel Assignment, Safety/Efficacy Study |
| Participants | Adolescents (12 to 18 years) with DSM‐IV BDD |
| Interventions | Fluoxetine (Prozac) versus placebo (sugar pill) |
| Outcomes | No information |
| Starting date | April 1999 |
| Contact information | No information |
| Notes | Study ID numbers: R01 MH58750; DSIR CT‐M |
NCT00106223.
| Trial name or title | Treatment Study Investigating New Cognitive Behavioral Therapy Treatment Manual for Body Dysmorphic Disorder |
| Methods | 22 sessions of individual, manual‐based CBT |
| Participants | DSM‐IV diagnosis of BDD, score higher than 23 on BDD‐YBOCS, free of psychiatric comorbidity, alcohol abuse or dependence within 3 months prior, not suicidal or homicidal |
| Interventions | CBT versus waiting list |
| Outcomes | Body dysmorphic disorder symptom severity, Functioning and life satisfaction, Depressive symptoms, Anxiety symptoms |
| Starting date | April 2004 |
| Contact information | Kara Watts; tel: 617‐643‐3079; KLWatts@partners.org |
| Notes | Study ID numbers; R34 MH70490 ; 2004‐P‐000478/7 ; DATR A2‐AIR |
NCT00149799.
| Trial name or title | Effectiveness of Escitalopram in the Treatment of Body Dysmorphic Disorder |
| Methods | Open‐label phase, during which all participants will receive escitalopram for 14 weeks, followed by randomized, double‐blind, placebo control, parallel assignment, efficacy study |
| Participants | DSM‐IV diagnosis of BDD within 6 months of study start, score higher than 24 on BDD‐YBOCS, alcohol abuse or dependence within 3 months prior, not suicidal or homicidal, expected number: 128 |
| Interventions | Escitalopram (Lexapro) versus placebo |
| Outcomes | Relapse of Body Dysmorphic Disorder Symptoms, Month 6 Functioning and life satisfaction, Depressive symptoms, Anxiety symptoms |
| Starting date | May 2005 |
| Contact information | Kara L. Watts; tel: 617‐643‐3079; KLWatts@partners.org |
| Notes | Study ID numbers: R01 MH72854 ; 2004‐P‐002305 ; DSIR 83‐ATSO |
NCT00211809.
| Trial name or title | CBT as an Adjunct to SRIs in the Treatment of BDD |
| Methods | Randomized, Double‐Blind, Active Control, Parallel Assignment, Efficacy Study |
| Participants | 20 DSM‐IV BDD patients aged 16 through 65. Score of 20 or greater on the BDD‐YBOCS, on a stable, therapeutic does of an SRI (at least 12 weeks on the SRI with 8 weeks at a therapeutic dose: acceptable medications (therapeutic daily doses) are citalopram (40mg), clomipramine (150mg), fluoxetine (40mg), fluvoxamine (150mg), paroxetine (40mg), sertraline (50mg), and venlafaxine (150mg). |
| Interventions | CBT versus relaxation and stress management training (RSMT); augmentation of SRIs |
| Outcomes | BDD‐YBOCS, BDD‐CGI, BABS, BDI‐II, BAI |
| Starting date | No information |
| Contact information | Bryann Baker, tel: (212) 492‐9449, bryann.baker@mssm.edu |
| Notes | Study ID numbers: GCO 00‐0211PS* |
NCT00245635.
| Trial name or title | Fluoxetine in Pediatric Body Dysmorphic Disorder |
| Methods | Interventional, Treatment, Randomized, Double‐Blind, Placebo Control, Parallel Assignment, Safety/Efficacy Study |
| Participants | 10‐16 years of age with a diagnosis of BDD, expected enrollment of 37 |
| Interventions | Fluoxetine versus placebo x 14 weeks |
| Outcomes | No information |
| Starting date | November 2004 |
| Contact information | Suah Kim, tel: (212) 369‐5123, suah.kim@mssm.edu |
| Notes | Study ID numbers ‐ GCO#02‐1020 ; FD‐R‐002337 |
Differences between protocol and review
Differences between the protocol and first version of the review (31 October, 2008)
The original protocol specified that studies meeting DSM criteria would be included. We have decided, in the interests of inclusivity, to include studies meeting ICD criteria as well.
Adverse responses to treatment as part of the narrative review was included under secondary outcomes in the section on "Types of outcome measures". Side‐effects classified by the trial authors as severe, as well as significant differences between the medication and control groups in the prevalence of the most common drug‐related adverse events (defined as occurring in at least 20% of the participants given medication) for the included trials were described as part of the narrative review.
Weighted mean differences were used instead of standardised mean differences (as specified in the protocol for this review) for the primary outcome of symptom severity reduction for trials of psychotherapy. The initial rationale for specifying the use of SMD instead of WMD for the symptom severity reduction outcome was to facilitate comparison of the effect sizes for the pharmacotherapy and psychotherapy trials. It was subsequently decided not to conduct this comparison, given doubts regarding the validity of conclusions regarding the relative efficacy of pharmacotherapy and psychotherapy based on indirect comparison and the small number of trials involved.
The WHO international clinical trials registry platform (ICTRP) search portal (http://www.who.int/trialsearch) has been added to the section describing the method employed in identifying unpublished and ongoing studies (Search methods for identification of studies ‐ electronic searches).
Contributions of authors
Jonathan Ipser was responsible for compiling the original protocol, responding to editorial comments, and writing the methodology and results section of the review; Candice Sander and Jonathan screened studies for inclusion, extracted data from the RCTs and wrote the interpretive components of the review (discussion and conclusion). Dan Stein provided assistance as a content expert and coordinator, resolved disagreements in identifying studies for inclusion and provided feedback on draft versions of the protocol and review.
Jonathan Ipser stands as guarantor of this review.
Sources of support
Internal sources
MRC Research Unit on Anxiety and Stress Disorders, South Africa.
External sources
University of Cape Town, Cape Town, South Africa.
Declarations of interest
Potential conflicts of interest for individual reviewers
Jonathan Ipser has no known conflicts of interest outside of his employment by the MRC Unit on Anxiety Disorders.
Candice Sander has no known conflicts of interest. Dan Stein has received research grants and/or consultancy honoraria from Astrazeneca, Eli‐Lilly, GlaxoSmithKline, Lundbeck, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, and Wyeth. He has participated in a number of ongoing trials, and has presented data from some of these trials on behalf of the sponsoring companies.
New
References
References to studies included in this review
Hollander 1999 {published data only}
- Hollander E, Allen A, Kwon J, Aronowitz B, Schmeidler J, Wong C, Simeon D. Clomipramine vs des ipramine crossover trial in body dysmorphic disorder. Archives of General Psychiatry 1999;56:1033‐1039. [DOI] [PubMed] [Google Scholar]
McKay 1997 {published data only}
- McKay D. Two‐year follow‐up of behavioral treatment and maintenance for body dysmorphic disorder. Behavior Modification 1999;23(4):620‐9. [DOI] [PubMed] [Google Scholar]
- McKay D, Todaro J, Neziroglu F, Campisi T, Moritz EK, Yaryura‐Tobias JA. Body dysmorphic disorder: A preliminary evaluation of treatment and maintenance using exposure with response prevention. Behaviour Research and Therapy 1997;35(1):67‐70. [DOI] [PubMed] [Google Scholar]
Phillips 2002 {published data only}
- Phillips. Change in psychosocial functioning and quality of life of patients with body dysmorphic disorder treated with fluoxetine: A placebo‐controlled study. Psychosomatics 2004;45(5):438‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo‐controlled trial of fluoxetine in body dysmorphic disorder. Archives of General Psychiatry 2002;59:381‐388. [DOI] [PubMed] [Google Scholar]
Rosen 1995 {published data only}
- Rosen JC, Reiter J, Orosan P. Cognitive‐behavioral body image therapy for body dysmorphic disorder. Journal of Consulting and Clinical Psychology 1995;63(2):263‐269. [DOI] [PubMed] [Google Scholar]
Veale 1996 {published data only}
- Veale D, Gournay K, Dryden W, Boocock A, Shah F, Willson R, Walburn J. Body dysmorphic disorder: A cognitive behavioural model and pilot randomised controlled trial. Behavioural Research and Therapy 1996;34:717‐729. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 2008a {published data only}
- Allen A, Hadley SJ, Kaplan A, Simeon D, Friedberg J, Priday L, Baker BR, Greenberg JL, Hollander E. An open‐label trial of venlafaxine in body dysmorphic disorder . CNS Spectr ums 2008 ; 13 ( 2 ):138‐44. [DOI] [PubMed] [Google Scholar]
Campisi 1995 {published data only}
- Campisi TA. Exposure and response prevention in the treatment of body dysmorphic disorder. Dissertations Abstracts International: Section B: The Sciences and Engineering 1995;56:7036. [Google Scholar]
Geremia 2001 {published data only}
- Geremia GM, Neziroglu F. Cognitive therapy in the treatment of body dysmorphic disorder. Clinical Psychology and Psychotherapy 2001;8:243‐251. [Google Scholar]
Gomez‐Perez 1994 {published data only}
- Gomez‐Perez JC, Marks IM, Gutierrez‐Fisac JL. Dysmorphobia: Clinical features and outcome with behaviour therapy. European Psychiatry 1994;9:229‐235. [Google Scholar]
Khemlani‐Patel 2001 {published data only}
- Khemlani‐Patel S. Cognitive and behaviour therapy for body dysmorphic disorder: A comparative investigation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2001;62:1087. [Google Scholar]
Neziroglu 1993 {published data only}
- Neziroglu FA, Yaryura‐Tobias JA. Body dysmorphic disorder: phenomenology and case descriptions. Behavioural Psychotherapy 1993;21:27‐36. [Google Scholar]
Neziroglu 1996 {published data only}
- Neziroglu F, McKay D, Todaro J, Yaryura‐Tobias JA. Effects of cognitive behavior therapy on persons with body dysmorphic disorder and comorbid axis II diagnoses. Behavior Therapy 1996;27:67‐77. [Google Scholar]
Perugi 1996 {published data only}
- Perugi G, Giannotti D, Vaio S, Frare F, Saettoni M, Cassano GB. Fluvoxamine in the treatment of body dysmorphic disorder. International Clinical Psychopharmacology 1996;11(4):274‐54. [DOI] [PubMed] [Google Scholar]
Phillips 1996 {published data only}
- Phillips KA. An open study of buspirone augmentation of serotonin‐reuptake inhibitors in body dysmorphic disorder. Psychopharmacology Bulletin 1996;32(1):175‐80. [PubMed] [Google Scholar]
Phillips 1998 {published data only}
- Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. Journal of Clinical Psychiatry 1998;59(4):165‐71. [DOI] [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Dwight MM, Eisen JL, Rasmussen SA. Delusionality and response in open‐label fluvoxamine in body dysmorphic disorder. Journal of Clinical Psychiatry 2001;62(2):87‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2003 {published data only}
- Phillips KA, Najjar F. An open‐label study of citalopram in body dysmorphic disorder. Journal of Clinical Psychiatry 2003;64(6):715‐20. [DOI] [PubMed] [Google Scholar]
Phillips 2005b {published data only}
- Phillips KA. Placebo‐controlled study of pimozide augmentation of fluoxetine in body dysmorphic disorder. American Journal of Psychiatry 2005;162(2):377‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2005c {published data only}
- Phillips KA. Olanzapine augmentation of fluoxetine in body dysmorphic disorder. American Journal of Psychiatry 2005;162(5):1022‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2006e {published data only}
- Phillips KA. An open‐label study of escitalopram in body dysmorphic disorder. International Clinical Psychopharmacology 2006;21(3):177‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelm 1999 {published data only}
- Wilhelm S, Otto MW, Lohr B, Deckersbach T. Cognitive behavior group therapy for body dysmorphic disorder: a case series. Behaviour Research and Therapy 1999;37:71‐75. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
1K23MH076934‐01A1 {unpublished data only}
- Didie ER. Interpersonal Psychotherapy for Body Dysmorphic Disorder. CRISP ( http://crisp.cit.nih.gov/ ) 2008 [accessed on 30 October, 2008]. [Grant Number: 1K23MH076934‐01A1]
NCT00029471 {published data only}
- NIMH. Pharmacotherapy of Body Dysmorphic Disorder in Adolescents. controlled‐trials.com 2008 [accessed on 30 October, 2008]. [ClinicalTrials.gov identifier: NCT00029471]
NCT00106223 {published data only}
- Wilhelm S. Treatment study investigating new cognitive behavioral therapy treatment manual for body dysmorphic disorder. controlled‐trials.com 2008 [accessed on 30 October, 2008]. [ClinicalTrials.gov identifier :NCT00106223]
NCT00149799 {published data only}
- Wilhelm S, Phillips KA. Effectiveness of Escitalopram in the Treatment of Body Dysmorphic Disorder. controlled‐trials.com 2008 [accessed on 30 October, 2008]. [ClinicalTrials.gov identifier: NCT00149799]
NCT00211809 {published data only}
- Allen A. CBT as an Adjunct to SRIs in the Treatment of BDD. controlled‐trials.com 2008 [accessed on 30 October, 2008]. [ClinicalTrials.gov identifier: NCT00211809]
NCT00245635 {published data only}
- Hollander E. Fluoxetine in Pediatric Body Dysmorphic Disorder. controlled‐trials.com 2008 [accessed on 30 October, 2008]. [ClinicalTrials.gov identifier: NCT00245635]
Additional references
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Baxter 1992
- Baxter Jr lr, Schwartz JM, Berman KS, Szuba MP, Guze BH, Mazziotta JC, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive‐compulsive disorder. Archives of General Psychiatry 1992;49:681‐689. [DOI] [PubMed] [Google Scholar]
Beck 1987
- Beck AT, Steer R. Beck Depression Inventory: Manual. San Antonio, Texas: Psychological Corporation, 1987. [Google Scholar]
Bohne 2002a
- Bohne A, Wilhelm S, Keuthen NJ, Florin I, Baer L, Jenike MA. Prevalence of body dysmorphic disorder in a German college student sample . Psychiatry Res earch 2002 ; 109 ( 1 ):101‐4. [DOI] [PubMed] [Google Scholar]
Bohne 2002b
- Bohne A, Keuthen NJ, Wilhelm S, Deckersbach T, Jenike MA. Prevalence of symptoms of body dysmorphic disorder and its correlates: a cross‐cultural comparison . Psychosomatics 2002 ; 43 ( 6 ):486‐90. [DOI] [PubMed] [Google Scholar]
Deckersbach 2000
- Deckersbach T, Savage CR, Phillips KA, Wilhelm S, Buhlmann U, Rauch SL, Baer L, Jenike MA. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society 2000;6(6):673‐81. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis.. In: Egger M, Davey SG, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. BMJ Publication Group, 2001. [Google Scholar]
Deeks 2008
- Deeks J, Higgins JPT, Altman D. editors. Analysing and presenting results. In: Higgins JPT, Green S editor(s). Cochrane Reviewers’ Handbook 5.0.0 [updated February] Section 9;. Chichester, UK: John Wiley & Sons, Ltd., 2008. [Google Scholar]
Eisen 1998
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: Reliability and Validity. American Journal of Psychiatry 1998;155(1):102‐108. [DOI] [PubMed] [Google Scholar]
Eisen 2004
- Eisen JL, Phillips KA, Coles ME, Rasmussen SA. Insight in obsessive compulsive disorder and body dysmorphic disorder. Comprehensive Psychiatry 2004;45(1):10‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faravelli 1997
- Faravelli C, Salvatori S, Galassi F, Aiazza L, Drei C, Cabras P. Epidemiology of somatoform disorders: A community survey in Florence. Social Psychiatry and Psychiatric Epidemiology 1997;32(1):24‐29. [DOI] [PubMed] [Google Scholar]
Feusner 2007
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry 2007;64(12):1417‐25. [DOI] [PubMed] [Google Scholar]
Foa 1995
- Foa EB, Kozak MJ, Goodman WK, Hollander E, Jenike MA, Rasmussen SA. DSM‐IV field trial: obsessive‐compulsive disorder. American Journal of Psychiatry 1995;152(1):90‐6. [DOI] [PubMed] [Google Scholar]
Frare 2004
- Frare F, Perugi G, Ruffolo G, Toni C. Obsessive‐compulsive disorder and body dysmorphic disorder: a comparison of clinical features. European Psychiatry 2004;19:292‐298. [DOI] [PubMed] [Google Scholar]
Gabbay 2003
- Gabbay V, Asnis GM, Bello JA, Alonso CM, Serras SJ, O’Dowd MA. New onset of body dysmorphic disorder following frontotemporal lesion. Neurology 2003;61:123‐5. [DOI] [PubMed] [Google Scholar]
Goodman 1989
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale‐Brown Obsessive Compulsive Scale. I. Development, use, and reliability . Arch ives of Gen eral Psychiatry. 1989 ; 46 ( 11 ):1006‐11. [DOI] [PubMed] [Google Scholar]
Gury 1999
- Gury C, Cousin F. Pharmacokinetics of SSRI antidepressants: half‐life and clinical applicability. Encephale 1999;25(5):470‐476. [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU assessment manual for psychopharmacology. Washington, DC: U.S. National Institute of Health, 1976. [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50‐5. [DOI] [PubMed] [Google Scholar]
Hanes 1998
- Hanes KR. Neuropsychological performance in body dysmorphic disorder. Journal of the International Neuropsycholog ical Society 1998;4(2):167‐71. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JTP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(6):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Reviewers’ Handbook 5.0.0 [updated February] Section 8;. Chichester, UK: John Wiley & Sons, Ltd., 2008. [Google Scholar]
Higgins 2008b
- Higgins JPT, Deeks J, Altman D, Editors. Special topics in statistics. Higgins JPT, Green S, Editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February] Section 16. Chichester, UK: John Wiley & Sons Ltd, 2008. [Google Scholar]
Hollander 1989
- Hollander E, Liebowitz MR, Winchel R, Klumker A, Klein DF. Treatment of body‐dysmorphic disorder with serotonin reuptake blockers. American Journal of Psychiatry 1989;146(6):768‐770. [DOI] [PubMed] [Google Scholar]
Hollander 1993
- Hollander E, Phillips KA. Body image and experience disorders: Body dysmorphic and depersonalization disorders. In: Hollander E editor(s). Obssessive Compulsive‐Related Disorders. Washington DC: American Psychiatric Press, 1993:17‐48. [Google Scholar]
Hunter 2000
- Hunter JE, Schmidt FL. Fixed effects vs. random effects meta‐analysis models: Implications for cumulative research knowledge. International Journal of Selection and Assessment 2000;8(4):275‐292. [Google Scholar]
Ipser 2006
- JC Ipser, P Carey, Y Dhansay, N Fakier, S Seedat, DJ Stein. Pharmacotherapy augmentation strategies in treatment‐resistant anxiety disorders . Cochrane Database of Systematic Reviews 2006 , Issue 4 . [DOI] [PubMed] [Google Scholar]
Leon 1999
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE‐RIFT): a brief measure of functional impairment. Psychological Medicine 1999;29:869‐878. [DOI] [PubMed] [Google Scholar]
Marks 1988
- Marks I, Mishan J. Dysmorphophobic avoidance with disturbed bodily perception: A pilot study of exposure therapy. British Journal of Psychiatry 1988;152:674‐678. [DOI] [PubMed] [Google Scholar]
McKay 1999
- McKay D. Two‐year follow‐up of behavioral treatment and maintenance for body dysmorphic disorder. Behavior Modification 1999;23(4):620‐629. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials . Lancet 2001 ; 357 ( 9263 ):1191‐4. [PubMed] [Google Scholar]
Moncrieff 2001
- Moncrieff J, Churchill R, Drummond DC, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Methods in Psychiatric Research 2001;10(3):126‐33. [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
NICE 2005
- National Institute for Clinical Excellence. The management of post traumatic stress disorder in primary and secondary care . London : NICE , 2005 . [Google Scholar]
Phillips 1991
- Phillips KA. Body dysmorphic disorder: The distress of imagined ugliness. American Journal of Psychiatry 1991;148(9):1138‐1149. [DOI] [PubMed] [Google Scholar]
Phillips 1993
- Phillips KA, McElroy SL, Keck PE, Pope HG, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. American Journal of Psychiatry 1993;150(2):302‐308. [DOI] [PubMed] [Google Scholar]
Phillips 1994
- Phillips KA, McElroy SL, Keck PE, Hudson JI, Pope HG. A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacology Bulletin 1994;30(2):179‐186. [PubMed] [Google Scholar]
Phillips 1997a
- Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. Journal of Nervous and Mental Disease 1997;185(9):570‐577. [DOI] [PubMed] [Google Scholar]
Phillips 1997b
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: Development, reliability and validity of a modified version of the Yale‐Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin 1997;33(1):17‐22. [PubMed] [Google Scholar]
Phillips 2000
- Phillips KA, Dufresne RG, Wilkel CS, Vittorio CC. Rate of body dysmorphic disorder in dermatology patients. Journal of the American Academy of Dermatology 2000;42:436‐441. [DOI] [PubMed] [Google Scholar]
Phillips 2001a
- Phillips KA, Albertini RS, Siniscalchi JM, Khan A, Robinson M. Effectiveness of pharmacotherapy for body dysmorphic disorder: A chart‐review study. Journal of Clinical Psychiatry 2001;62:721‐727. [DOI] [PubMed] [Google Scholar]
Phillips 2001b
- Phillips KA, Grant J, Siniscalchi J, Albertini RS. Surgical and nonpsychiatric medical treatment of patients with body dysmorphic disorder. Psychosomatics 2001;42(6):504‐510. [DOI] [PubMed] [Google Scholar]
Phillips 2004
- Phillips KA, Rasmussen SA. Change in psychosocial functioning and quality of life of patients with body dysmorphic disorder treated with fluoxetine: A place‐controlled study. Psychosomatics 2004;45(5):438‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2006
- Phillips KA, Menard, W, Pagano ME, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: Clinical features and course of illness. Journal of Psychiatric Research 2006;40(2):95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2006b
- Phillips KA, Menard W, Fay C. Gender similarities and differences in 200 individuals with body dysmorphic disorder. Comprehensive Psychiatry 2006;47(2):77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2006c
- Phillips KA, Stout RL. Associations in the longitudinal course of body dysmorphic disorder with major depression, obsessive‐compulsive disorder, and social phobia. Journal of Psychiatric Research 2006;40(4):360‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2006d
- Phillips KA, Menard W, Pagano ME, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. Journal of Psychiatric Resear ch 2006;40(2):95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rauch 2003
- Rauch SL, Phillips KA, Segal E, Makris N, Shin LM, Whalen PJ, Jenike MA, Caviness VS Jr, Kennedy DN. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research 2003;122(1):13‐9. [DOI] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐153. [DOI] [PubMed] [Google Scholar]
Rosen 1996
- Rosen JC, Reiter J. Development of the Body Dysmorphic Disorder Examination. Behaviour Research and Therapy 1996;34(9):755‐766. [DOI] [PubMed] [Google Scholar]
Rosen 1998
- Rosen JC, Ramirez E. A comparison of eating disorders and body dysmorphic disorders on body image and psychological adjustment. Journal of Psychosomatic Research 1998;44(3/4):441‐449. [DOI] [PubMed] [Google Scholar]
Saxena 2001
- Saxena S, Winograd A, Dunkin JJ. A retrospective review of clinical characteristics and treatment response in body dysmorphic disorder versus obsessive‐compulsive disorder . Journal of Clinical Psychiatry 2001 ; 62 :67‐72. [DOI] [PubMed] [Google Scholar]
Schneier 1994
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, Street L, Bene D, Liebowitz MR. Functional impairment in social phobia . J ournal of Clin ical Psychiatry 1994 ; 55 ( 8 ):322‐31. [PubMed] [Google Scholar]
Sheehan 1996
- Sheehan DV, Harnett‐Sheehan K, Raj BA. The measurement of disability. International Clinical Psychopharmacology 1996;11 Suppl 3:89‐95. [DOI] [PubMed] [Google Scholar]
Spitzer 1996
- Spitzer R, Williams J, Gibbons M. Instructional manual for the structured clinical interview for DSM‐IV.. New York: New York State Psychiatric Institute., 1996. [Google Scholar]
Tignol 2007
- Tignol J, Biraben‐Gotzamanis L, Martin‐Guehl C, Grabot D, Aouizerate B. Body dysmorphic disorder and cosmetic surgery: Evolution of 24 subjects with a minimal defect in appearance 5 years after their request for cosmetic surgery. Euro pean Psychiatry 2007; 22 ( 8 ):520‐4. [DOI] [PubMed] [Google Scholar]
Veale 1996b
- Veale D, Boocock A, Gournay K, Dryden W, Shah F, Willson R, Walburn J. Body dysmorphic disorder: A survey of fifty cases. British Journal of Psychiatry 1996;169:196‐201. [DOI] [PubMed] [Google Scholar]
Veale 2000
- Veale, D. Outcome of cosmetic surgery and 'DIY' surgery in patients with body dysmorphic disorder. Psychiatric Bulletin 2000;24:218‐221. [Google Scholar]
Verbeke 2000
- Verbeke M, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer, 2000. [Google Scholar]
Ware 1993
- Ware JE Jr. SF‐36 Health Survey Manual and Interpretation Guide. Boston: Health Institute New England Medical Center, 1993. [Google Scholar]
WHO 1975
- World Health Organisation. The ICD‐9 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organisation, 1975. [Google Scholar]
WHO 1992
- World Health Organisation. ICD‐10 International statistical classification of diseases and related health problems : based on the International Statistical Classification of Diseases and Related Health Problems, ICD. Geneva: World Health Organisation, 1992. [Google Scholar]
Williams 2006
- Williams J, Hadjistavropoulos T, Sharpe D. A meta‐analysis of psychological and pharmacological treatments for Body Dysmorphic Disorder. Beha viour Research and Therapy 2006;44:99‐111. [DOI] [PubMed] [Google Scholar]


