Abstract
Background
An ongoing outbreak of pneumonia associated with a novel coronavirus was reported in Wuhan city, Hubei province, China. Affected patients were geographically linked with a local wet market as a potential source. No data on person-to-person or nosocomial transmission have been published to date.
Methods
In this study, we report the epidemiological, clinical, laboratory, radiological, and microbiological findings of five patients in a family cluster who presented with unexplained pneumonia after returning to Shenzhen, Guangdong province, China, after a visit to Wuhan, and an additional family member who did not travel to Wuhan. Phylogenetic analysis of genetic sequences from these patients were done.
Findings
From Jan 10, 2020, we enrolled a family of six patients who travelled to Wuhan from Shenzhen between Dec 29, 2019 and Jan 4, 2020. Of six family members who travelled to Wuhan, five were identified as infected with the novel coronavirus. Additionally, one family member, who did not travel to Wuhan, became infected with the virus after several days of contact with four of the family members. None of the family members had contacts with Wuhan markets or animals, although two had visited a Wuhan hospital. Five family members (aged 36–66 years) presented with fever, upper or lower respiratory tract symptoms, or diarrhoea, or a combination of these 3–6 days after exposure. They presented to our hospital (The University of Hong Kong-Shenzhen Hospital, Shenzhen) 6–10 days after symptom onset. They and one asymptomatic child (aged 10 years) had radiological ground-glass lung opacities. Older patients (aged >60 years) had more systemic symptoms, extensive radiological ground-glass lung changes, lymphopenia, thrombocytopenia, and increased C-reactive protein and lactate dehydrogenase levels. The nasopharyngeal or throat swabs of these six patients were negative for known respiratory microbes by point-of-care multiplex RT-PCR, but five patients (four adults and the child) were RT-PCR positive for genes encoding the internal RNA-dependent RNA polymerase and surface Spike protein of this novel coronavirus, which were confirmed by Sanger sequencing. Phylogenetic analysis of these five patients' RT-PCR amplicons and two full genomes by next-generation sequencing showed that this is a novel coronavirus, which is closest to the bat severe acute respiatory syndrome (SARS)-related coronaviruses found in Chinese horseshoe bats.
Interpretation
Our findings are consistent with person-to-person transmission of this novel coronavirus in hospital and family settings, and the reports of infected travellers in other geographical regions.
Funding
The Shaw Foundation Hong Kong, Michael Seak-Kan Tong, Respiratory Viral Research Foundation Limited, Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited, Marina Man-Wai Lee, the Hong Kong Hainan Commercial Association South China Microbiology Research Fund, Sanming Project of Medicine (Shenzhen), and High Level-Hospital Program (Guangdong Health Commission).
Introduction
The Health Commission of Hubei province, China, first announced a cluster of unexplained cases of pneumonia on Dec 31, 2019.1 27 patients were initially reported, which was subsequently revised to 41 on Jan 11, 2020, with seven severe cases and one death.2 Some patients were reported to have radiographic ground-glass lung changes; normal or lower than average white blood cell lymphocyte, and platelet counts; hypoxaemia; and deranged liver and renal function. Most were said to be geographically linked to the Huanan seafood wholesale market, which was subsequently reported by journalists to be selling freshly slaughtered game animals.3 To date, no evidence of person-to-person transmission or affected health-care workers has been published in the scientific literature. The Chinese health authority said that the patients initially tested negatively for common respiratory viruses and bacteria, but later tested positive for a novel coronavirus.2 The virus was soon isolated and its genome sequenced by a number of Chinese scientists.4 The virus was tentatively named by WHO as the 2019 novel coronavirus (2019-nCoV). Here, we report the epidemiological, clinical, radiological, laboratory, and genomic findings of a family cluster of five patients in Shenzhen who had a history of travel to Wuhan, and one other family member who has not travelled to Wuhan.
Research in context.
Evidence before this study
We searched PubMed on Jan 13, 2020, with no starting date limitations, using the terms “family”, “pneumonia”, “Wuhan”, “coronavirus”, and “novel” for articles in English. Our search did not reveal any reports of novel coronavirus pneumonia in Wuhan before 2020. We only noted family clusters of pneumonia due to the novel severe acute respiratory syndrome (SARS) coronavirus in 2003, and Middle East respiratory syndrome coronavirus in 2012.
Added value of this study
The epidemiological, clinical, laboratory, radiological, and microbiological findings of unexplained pneumonia in a Shenzhen family cluster connected to a Wuhan hospital were presented. The diagnostic tests from relevant clinical samples confirmed the presence of a novel coronavirus in five of six patients with radiological changes of viral pneumonia. The phylogenetic analysis of this novel coronavirus suggested its linkage to a possible animal source.
Implications of all the available evidence
Although this novel coronavirus might have first originated from animals and now jumped into humans, the possibility of person-to-person transmission could not be excluded, as seen in this family cluster with no known history of exposure to markets or animals, and rapid intercity spread might be possible by air travel. Vigilant epidemiological control in the community and health-care facilities is important to prevent another SARS-like epidemic.
Methods
Cases
On Jan 10, 2020, we initially enrolled two patients who initially presented to The University of Hong Kong-Shenzhen Hospital (Shenzhen, Guangdong province, China) with fever, respiratory symptoms, and pulmonary infiltrates on chest radiographs. Subsequently, between Jan 11, and Jan 15, 2020, five other members of this family also presented to our hospital for the assessment of their health conditions.
We recorded and analysed the history, physical findings, and haematological, biochemical, radiological, and microbiological investigation results. All laboratory procedures for clinical samples have been previously reported.5 Briefly, nasopharyngeal and throat swabs and stool and urine samples were taken and put into viral transport media. Plasma was separated from EDTA bottles and serum were separated from clotted blood bottles.
This study was approved by the Institutional Review Board of The University of Hong Kong-Shenzhen Hospital (number [2015]90). We obtained written consent from the patients.
Respiratory and diarrhoeal pathogen detection
Respiratory samples of the patients were tested for influenza A and B viruses and respiratory syncytial virus using the Xpert Xpress Flu/RSV assay (GeneXpert System, Cepheid, Sunnyvale, CA, USA) according to the manufacturer's instructions.6 To detect the presence of 18 respiratory virus targets and four bacteria (including adenovirus, coronaviruses [HCoV-229E, HCoV-Nl63, HCoV-Oc43, HCoV-HKU1, and MERS-CoV], human metapneumovirus, respiratory syncytial virus, human rhinovirus or enterovirus, influenza A viruses [H1, H1-2009 and H3], influenza B virus, parainfluenza viruses [types 1–4], Bordetella pertussis, Bordetella parapertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae), samples were tested using BioFire FilmArray Respiratory Panel 2 plus (bioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions.7 The two faecal samples were taken from the patients who had diarrhoea as part of their symptoms, and the samples were tested by BioFire FilmArray Gastrointestinal panel (bioMérieux) for 22 diarrhoeal pathogens.
Reverse transcription, in-house conventional RT-PCR and sequencing
Reverse transcription was done using the SuperScript IV reverse transcriptase (Invitrogen, Carlsbad, USA) as previously described.8 The reaction mixture (10 μL) contained 5·5 μL of RNA, 2 μL of 5 ×SuperScript IV buffer, 0·5 μL of 100 mM dithiothreitol, 0·5 μL of 10 mM deoxynucleotide triphosphate (dNTP) mixture, 0·5 μL of 50 μM random hexamers, 0·5 μL of SuperScript IV reverse transcriptase (200 U/μL), and 0·5 μl of RNase-free water. The mixtures were incubated at 23°C for 10 min, followed by 50°C for 10 min and 80°C for 10 min. The PCR mixture (25 μL) contained 1 μL of cDNA, 2·5 μL of 10X PCR buffer II, 2 μL of 25 mM MgCl2, 0·5 μL of 10 mM dNTP mix, 2·5 μL of each 10 μM forward and reverse primer, 0·125 μL of AmpliTaq Gold Polymerase (Applied Biosystems, Foster City, USA; 5 U/μL), and nuclease-free water.
The first set of primers was the forward primer (5′-CAAGTGGGGTAAGGCTAGACTTT-3′) and the reverse primer (5′-ACTTAGGATAATCCCAACCCAT-3′) targeting 344 bp of RNA-dependent RNA polymerase (RdRp) gene of all severe acute respiratory syndrome (SARS)-related coronaviruses. The second set of primers was designed after our first 2019-nCoV genome sequence by Nanopore sequencing from the positive clinical samples: the forward primer (5′-CCTACTAAATTAAATGATCTCTGCTTTACT-3′) and the reverse primer (5′-CAAGCTATAACGCAGCCTGTA-3′) targeting the 158 bp of Spike (S) gene of this novel coronavirus. These sets were used for PCR using an automated thermocycler (Applied Biosystems) with a hot start at 95°C for 10 min, followed by 50 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min. The PCR products were detected by agarose gel electrophoresis. The PCR products with correct target size were purified using QIAquick Gel Extraction Kit (Qiagen). Both strands of PCR products were sequenced with an ABI 3500xl Dx Genetic Analyzer (Applied Biosystems) using the PCR primers. During the set up of the assays, we initialy used SARS-CoV cDNA as a positive control for RdRp assay and gene-synthesised fragment for Spike assay. Thereafter, diluted samples from positive patients were used as the positive control for both assays. All positive results were confirmed by Sanger sequencing.
In-house one-step real-time RT-PCR assay
A total of 140 μL of respiratory, urine, stool, serum, or plasma samples from each patient was subjected to RNA extraction into 50 μL elutes using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). Forward (5′-CCTACTAAATTAAATGATCTCTGCTTTACT-3′) and reverse (5′-CAAGCTATAACGCAGCCTGTA-3′) primers targeting the S gene of this novel coronavirus were used for the assay. Real-time RT-PCR assay was done using QuantiNova SYBR Green RT-PCR Kit (Qiagen) in a LightCycler 480 Real-Time PCR System (Roche, Basel, Switzerland), as previously described.9 Each 20 μL reaction mixture contained 10 μL of 2 ×QuantiNova SYBR Green RT-PCR Master Mix, 0·2 μL of QN SYBR Green RT-Mix, 1 μM of each 10 μM forward and reverse primers, and 5 μL of RNA and nuclease-free water. Reactions were incubated at 50°C for 10 min and 95°C for 2 min, followed by 45 cycles at 95°C for 5 s and 60°C for 30 s, and then subjected to melting curve analysis (95°C for 5 s, 65°C for 1 min, followed by a gradual increase in temperature to 97°C with continuous recording of fluorescence).
Whole-genome sequencing and genome analysis by bioinformatics
Whole-genome sequencing was done using Oxford Nanopore MinION device (Oxford Nanopore Technologies, Oxford, UK) supplemented by Sanger sequencing. RNA was extracted from host cell-depleted nasopharyngeal and sputum samples using a QIAamp Viral RNA Mini Kit, as described previously.10, 11, 12 Whole-genome amplification of the coronavirus was done using a sequence-independent single-primer amplification approach, as described previously.13 Bioinformatics analyses were done using an in-house pipeline. Details on the library preparation and bioinformatics analysis are described in the appendix (pp 1–2). The consensus sequence of HKU-SZ-002a (accession number MN938384) and HKU-SZ-005b (accession number MN975262) have been deposited into GenBank. Raw reads, after excluding human reads, have been deposited into BioProject (accession number PRJNA601630).
Phylogenetic tree construction
Phylogenetic trees were constructed using MEGA X software using the RT-PCR amplicons of partial RdRp and S gene regions of the strains detected in this study and other related coronaviruses.14 The trees of the amplicons were constructed using maximum likelihood methods with bootstrap values calculated from 1000 trees, with human coronavirus 229E as outgroup. The phylogenetic tree of the full-length genome was constructed by use of the neighbour-joining method using the Tamura-Nei model with a gamma distribution. The bootstrap values were calculated from 1000 trees and values only greater than 70 were displayed.
Role of the funding sources
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
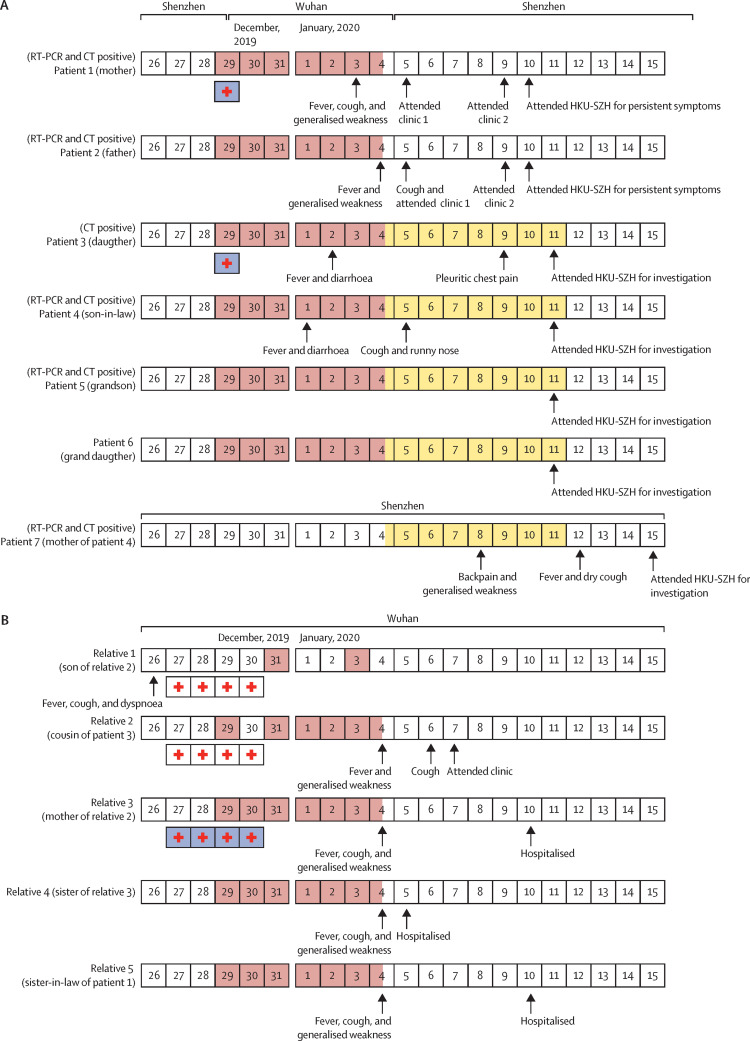
The family cluster of six patients (patients 1–6) flew from Shenzhen to Wuhan on Dec 29, 2019, and flew back to Shenzhen on Jan 4, 2020 (figure 1 ). This travel period overlapped with the time period after the announcement of the first case of Wuhan pneumonia (symptom onset on Dec 12, 2019) according to the Chinese health authority.2 They had no history of contact with animals, visits to markets including the Huanan seafood wholesale market in Wuhan, or eating game meat in restaurants. The family stayed in the same hotel throughout their travel. Patients 1 and 2 stayed in one room and patients 3–6 stayed in another room. After patient 4 developed fever and diarrhoea on Jan 1, 2020, patients 5 and 6 stayed in the same room as patients 1 and 2, and patient 3 stayed with patient 4. Patients 1–6 had met with their relatives (relatives 2–5: one female cousin and three aunts of patient 3) every day during their stay in Wuhan for meals. Relative 4 made frequent visits to the wet market but not the Huanan seafood wholesale market, which had been implicated by the health authority to be the epidemic centre. Relatives 2–5 have developed fever, cough, and weakness since Jan 4, 2020. Patients 1 and 3 had visited relative 1, aged 1 year, and the son of relative 2, on Dec 29, 2019, in a Wuhan hospital, who had been treated in hospital for febrile pneumonia (relative 2 accompanied relative 1 in the hospital overnight; relative 1 later recovered and was discharged home on Dec 31, 2019). Patient 3, but not patient 1, had worn a surgical mask during the hospital visit. The incubation period was estimated to be between 3 and 6 days. Patients 1–4 were symptomatic, and they only presented to our hospital (The University of Hong Kong-Shenzhen Hospital, Shenzhen) 6–10 days after symptom onset. For the two asymptomatic children (patients 5 and 6), patient 5 had ground-glass lung opacities identified by CT scan. Unlike patient 5, who was aged 10 years and non-compliant to parental guidance, patient 6, who was aged 7 years and reported by her mother to wear a surgical mask for most of the time during the period in Wuhan, was not found to be infected by virological or radiological investigations. The blood tests and CT scan of patient 6 were normal. After they returned to Shenzhen on Jan 4, 2020, patients 3–6 stayed in the same household of patient 7 (mother of patient 4) until Jan 11, 2020. Patient 7, who did not go to Wuhan or visit Shenzhen markets in the preceding 14 days, developed back pain and generalised weakness and attended the outpatient clinic at another local hospital on Jan 8, 2020. She was given cefaclor for 3 days with no improvement. She developed fever and dry cough and attended the same outpatient clinic and was treated with intravenous cefazolin (two doses) on Jan 12, 2020. She was admitted to our hospital on Jan 15, 2020, due to persistent symptoms.
Figure 1.
Chronology of symptom onset of the Shenzhen family cluster and their contacts in Wuhan
Dates filled in red are the dates on which patients 1–6 had close contacts with their relatives (relatives 1–5). Dates filled in yellow are the dates on which patients 3–6 stayed with patient 7. The boxes with an internal red cross are the dates on which patients 1 and 3 or relatives 1, 2, and 3 had stayed overnight (white boxes) at or had visited (blue boxes) the hospital in which relative 1 was admitted for febrile pneumonia. The information of relatives 1–5 was provided by patient 3. No virological data were available.
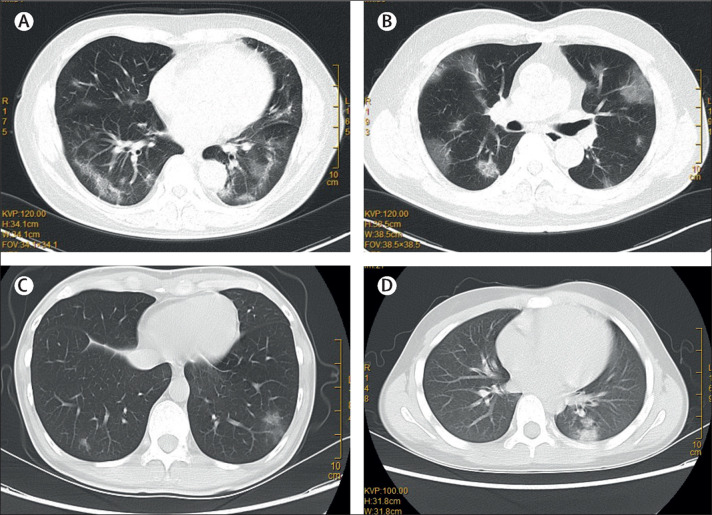
Of the six patients with pulmonary infiltrates (patients 1–5 and patient 7) on CT scans, three were male and three were female, with ages ranging 10–66 years (table 1 ). Four had chronic comorbidities and five had history of fever. The three older patients (aged >60 years: patients 1, 2, and 7) had dry cough and generalised weakness. Patient 4 had productive cough. Patients 3 and 4 were younger adults and had diarrhoea and upper respiratory tract symptoms including sore throat, nasal congestion, and rhinorrhoea. Patient 3 also had pleuritic chest pain. Except for patient 4, all six had normal or lower than average total white blood cell counts. The three older patients (patients 1, 2, and 7) all had substantially increased C-reactive protein, fibrinogen, and lactate dehydrogenase levels. Patients 1 and 2 also had lymphopenia, mild thrombocytopenia, and extended activated thromboplastin time. All six patients showed multifocal patchy ground-glass opacities, especially around the peripheral parts of the lungs on CT scans, which were compatible with changes seen in viral pneumonia (figure 2 ). No other clinical or radiological changes of lung congestion, fibrosis, or cancer to explain these ground-glass lung changes, or any concomitant radiological changes of dense consolidation, pleural effusion, lymphadenopathy, or pneumomediastinum were seen.
Table 1.
Summary of clinical features and laboratory results of the family cluster infected with 2019 novel coronavirus, at presentation
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 7 | ||
|---|---|---|---|---|---|---|---|
| Relationship | Mother of patient 3 | Father of patient 3 | Daughter of patients 1 and 2 | Son-in-law of patients 1 and 2 | Grandson of patients 1 and 2 | Mother of patient 4 in Shenzhen | |
| Age (years) | 65 | 66 | 37 | 36 | 10 | 63 | |
| Sex | Female | Male | Female | Male | Male | Female | |
| Occupation | Retired | Retired | Office worker | Architect | Student | Retired | |
| Chronic medical illness | Hypertension; benign intracranial tumour treated by gamma knife | Hypertension | None | Chronic sinusitis | None | Diabetes | |
| Interval between symptom onset and arrival at Wuhan (days) | 5 (hospital exposure) | 6 | 4 (hospital exposure) | 3 | NA | NA | |
| Interval between admission to hospital and symptom onset (days) | 7 | 6 | 9 | 10 | NA | 7 | |
| Presenting symptoms and signs | .. | .. | .. | .. | .. | .. | |
| Fever | + | + | + | + | − | + | |
| Cough | + (dry) | + (dry) | − | + (productive) | − | + (dry) | |
| Generalised weakness | + | + | − | − | − | + | |
| Nasal congestion | − | − | + | − | − | − | |
| Rhinorrhoea | − | − | − | + | − | − | |
| Sneezing | − | − | − | + | − | − | |
| Sore throat | − | − | + | − | − | − | |
| Pleuritic chest pain | − | − | + | − | − | − | |
| Diarrhoea | − | − | + (3 days, 5–6 times per day) | + (4 days, 7–8 times per day) | − | − | |
| Body temperature (°C) | 39·0 | 39·0 | 36·2 | 36·5 | 36·5 | 39·0 | |
| Oximetry saturation (%) | 94% | 96% | NA | NA | NA | NA | |
| Haemoglobin (g/dL); (male normal range 13·3–17·1; female normal range 11·5–14·8) | 13·1 | 15·6 | 15·0 | 15·2 | 14·6 | 13·0 | |
| White blood cell count (× 109 cells per L); (normal range 3·9–9·9) | 4·8 | 4·2 | 5·6 | 11·4 (↑) | 6·5 | 4·3 | |
| Neutrophil count (× 109 cells per L); (normal range 2·0–7·4) | 4·0 | 3·2 | 3·1 | 8·1 (↑) | 3·2 | 2·7 | |
| Lymphocyte count (× 109 cells per L); (normal range 1·1–3·6) | 0·6 (↓) | 0·7 (↓) | 2·2 | 2·7 | 2·8 | 1·2 | |
| Platelet count (× 109 cells per L); (normal range 162–341) | 157 (↓) | 118 (↓) | 224 | 196 | 197 | 205 | |
| Prothrombin time (s); (normal range 11·0–14·5) | 12·6 | 12·5 | 13·0 | 13·0 | 13·1 | 12·9 | |
| International normalised ratio | 1·0 | 1·0 | 1·0 | 1·0 | 1·0 | 1·0 | |
| Activated partial thromboplastin time (s); (normal range 26·0–40·0) | 45·4 (↑) | 45·3 (↑) | 36·0 | 31·4 | 34·0 | 35·8 | |
| D-dimer (μg/mL); (normal range 0·0–0·5) | 0·6 (↑) | 0·3 | NA | NA | NA | 0·6 (↑) | |
| Fibrinogen (g/dL); (normal range 2·0–4·0) | 6·2 (↑) | 5·1 (↑) | 3·8 | 3·8 | 2·9 | 4·5 (↑) | |
| C-reactive protein (mg/L); (normal range 0·0–5·0) | 55·6 (↑) | 34·2 (↑) | 0·5 | 4·9 | 0·2 | 44·9 (↑) | |
| Albumin (g/L); (normal range 35·0–52·0) | 39·4 | 38·5 | 50·4 | 48·1 | 49·1 | 41·2 | |
| Bilirubin (μmol/L); (normal range 0·0–21·0) | 6·9 | 5·9 | 9·3 | 8·9 | 3·6 | 10·4 | |
| Alkaline phosphatase (U/L); (normal range 35–105) | 68 | 56 | 56 | 48 | 211 (↑) | 66 | |
| Alanine aminotransferase (U/L); (normal range 0·0–33·0) | 14·2 | 13·9 | 25·9 | 20·2 | 23·9 | 17·3 | |
| Aspartate aminotransferase (U/L); (normal range 0·0–32·0) | 20·5 | 23·3 | 27·4 | 18·1 | 28·2 | 27·6 | |
| Urea (mmol/L); (normal range 2·8–8·1) | 3·5 | 5·7 | 3·1 | 5·2 | 5·6 | 4·9 | |
| Creatinine (μmol/L); (normal range 44–80) | 53 | 93 (↑) | 67 | 87 (↑) | 51 | 55 | |
| Sodium (mmol/L); (normal range 136–145) | 136 | 133 (↓) | 142 | 141 | 141 | 139 | |
| Potassium (mmol/L); (normal range 3·5–5·1) | 3·2 (↓) | 3·7 | 3·7 | 3·7 | 3·9 | 3·8 | |
| Creatine kinase (U/L); (normal range 0–170) | 42 | 109 | 50 | 137 | 78 | 143 | |
| Lactate dehydrogenase (U/L); (normal range 135–214) | 286 (↑) | 232 (↑) | 192 | 176 | 194 | 252 (↑) | |
| Amylase (U/L); (normal range 28–100) | NA | NA | 70 | 61 | 61 | NA | |
NA=not available. +=positive. –=negative. ↑=above normal range. ↓=below normal range.
Figure 2.
Representative images of the thoracic CT scans showing multifocal ground-glass changes in the lungs of patient 1 (A), patient 2 (B), patient 3 (C), and patient 5 (D)
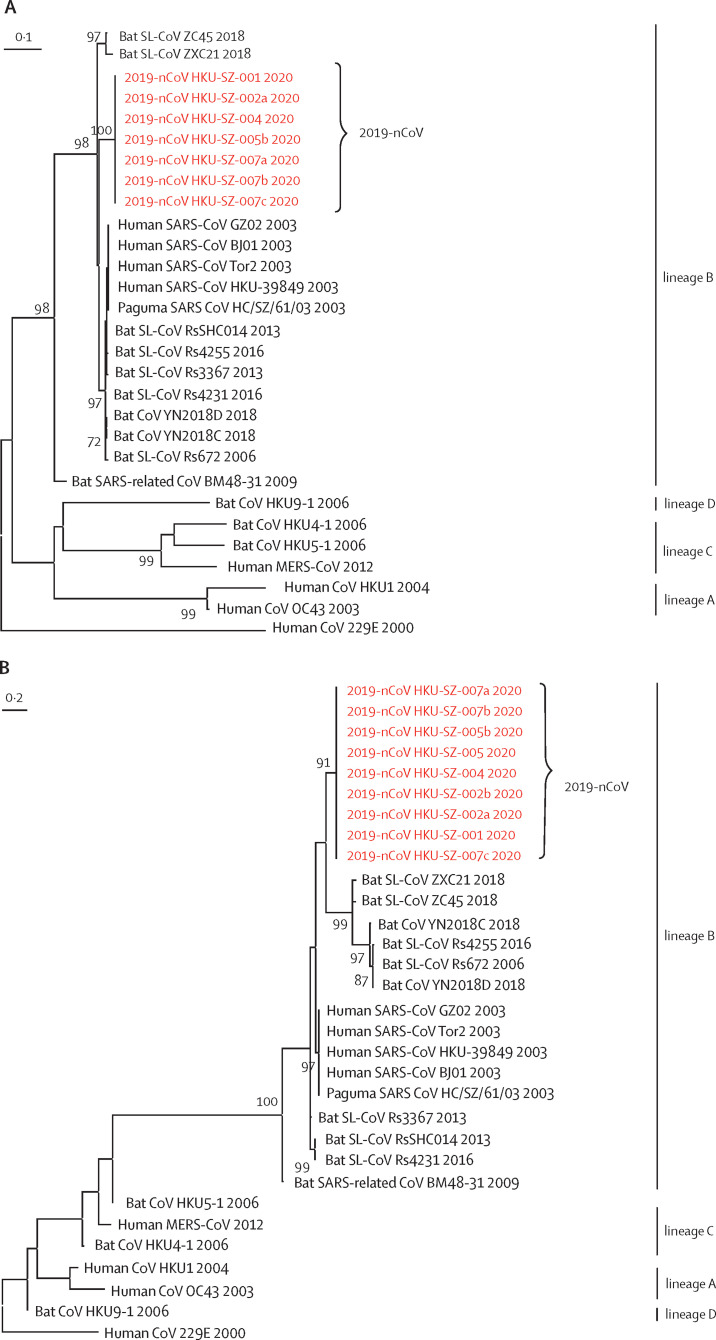
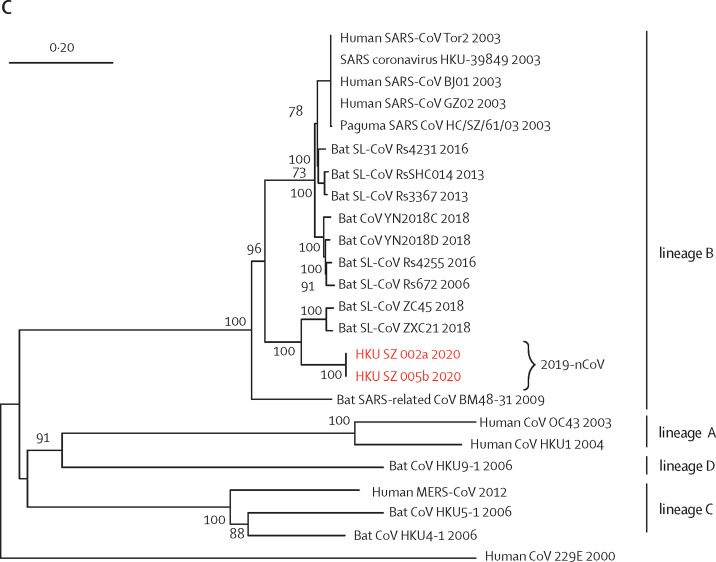
All respiratory samples were negative on two point-of-care multiplex PCR systems for 18 respiratory viral and four bacterial targets. The two faecal samples from patients 3 and 4 who had preceding diarrhoea were negative on a multiplex PCR assay for common diarrhoeal viruses, bacteria, and parasites (table 2 ). The respiratory samples of patients 1, 2, 4, 5, and 7 were positive for both RdRp and S genes by conventional RT-PCR, and for the S gene by real-time RT-PCR, which were confirmed by Sanger sequencing of all amplicons (appendix pp 3–5). Although the respiratory samples of patient 3 were negative for both RdRp and S gene (collected 9 days after symptom onset), she was still regarded as an infected case because she was strongly epidemiologically linked to the Wuhan hospital exposure and radiologically showing multifocal ground-glass lung opacities. Only the serum sample of patient 2 was positive and all other patients' serum, urine, and faecal samples were negative for this novel coronavirus. Phylogenetic analysis of the PCR products showed that the amplicon sequences of both RdRp and S genes from these five patients were novel (figure 3 ) and different from other known human or animal coronaviruses, including the SARS and bat SARS-related coronaviruses.
Table 2.
Microbiological findings from clinical specimens collected from the family cluter infected with 2019 novel coronavirus, at presentation
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 7 | |||
|---|---|---|---|---|---|---|---|---|
| Interval between sample collection and symptom onset (days) | 7 | 6 | 9 | 10 | NA | 7 | ||
| Conventional RT-PCR | .. | .. | .. | .. | .. | .. | ||
| Nasopharyngeal swab | .. | .. | .. | .. | .. | .. | ||
| RdRp | + | + | ND | + | ND | + | ||
| Spike | + | + | ND | + | + | + | ||
| Throat swab | .. | .. | .. | .. | .. | .. | ||
| RdRp | NA | NA | ND | ND | ND | + | ||
| Spike | NA | NA | ND | + | + | + | ||
| Serum | .. | .. | .. | .. | .. | .. | ||
| RdRp | ND | ND | NA | NA | NA | NA | ||
| Spike | ND | + | NA | NA | NA | NA | ||
| Plasma | .. | .. | .. | .. | .. | .. | ||
| RdRp | NA | NA | ND | ND | ND | NA | ||
| Spike | NA | NA | ND | ND | ND | NA | ||
| Urine | .. | .. | .. | .. | .. | .. | ||
| RdRp | ND | ND | ND | ND | ND | NA | ||
| Spike | ND | ND | ND | ND | ND | NA | ||
| Stool | .. | .. | .. | .. | .. | .. | ||
| RdRp | NA | NA | ND | ND | ND | NA | ||
| Spike | NA | NA | ND | ND | ND | NA | ||
| Real-time RT-PCR (spike gene) | .. | .. | .. | .. | .. | .. | ||
| Nasopharyngeal swab | + (Ct 31) | + (Ct 27) | ND | + (Ct 31) | ND | + (Ct 27) | ||
| Throat swab | NA | NA | ND | ND | + (Ct 40) | + (Ct 33) | ||
| Sputum | NA | NA | NA | NA | + (Ct 27) | + (Ct 25) | ||
| Serum | ND | + (Ct 40) | NA | NA | ND | NA | ||
| Plasma | NA | NA | ND | ND | ND | ND | ||
| Urine | ND | ND | ND | ND | ND | NA | ||
| Stool | NA | NA | ND | ND | ND | ND | ||
| FilmArray RP2 plus (nasopharyngeal swab only) | ND | ND | ND | ND | ND | ND | ||
| Xpert Xpress Flu/RSV (nasopharyngeal swab only) | ND | ND | ND | ND | ND | ND | ||
| FilmArray GI panel (faecal sample only) | NA | NA | ND | ND | NA | NA | ||
Ct values for real-time RT-PCR presented in parentheses. Ct=cycle threshold. NA=not available. +=positive. ND=not detected. RdRp=RNA-dependent RNA polymerase. RP2=respiratory panel 2. Flu=influenza. RSV=respiratory syncytial virus. GI=gastrointestinal.
Figure 3.
Phylogenetic trees of genetic sequences
(A) Amplicon fragments of RNA-dependent RNA polymerase of patients 1, 2, 4, 5, and 7. (B) Amplicon fragments of Spike gene of patients 1, 2, 4, 5, and 7. (C) The full genome sequences of strains from patients 2 and 5. Red text indicates the coronavirus (CoV) strains detected in the patients in the present study. 2019-nCoV is 2019 novel coronavirus. HKU-SZ-001 refers to the strain detected in the nasopharyngeal swab of patient 1; HKU-SZ-002a refers to strain detected in the nasopharyngeal swab of patient 2; HKU-SZ-002b refers to strain detected in the serum sample of patient 2; HKU-SZ-004 refers to the strain detected in the nasopharyngeal swab of patient 4; HKU-SZ-005 refers to the strain detected in the throat swab of patient 5; HKU-SZ-005b refers to the strain detected in the sputum sample of patient 5; HKU-SZ-007a refers to the strain detected in the nasopharyngeal swab of patient 7; HKU-SZ-007b refers to the strain detected in the throat swab of patient 7; and HKU-SZ-007c refers to the strain detected in the sputum sample of patient 7 (appendix p 6). The NCBI GenBank accession numbers of the genome sequences are MN938384 (HKU-SZ-002a), MN975262 (HKU-SZ-005b), MG772934 (Bat SL-CoV ZXC21), MG772933 (Bat SL-CoV ZC45), AY274119 (hSARS-CoV Tor2), AY278491 (SARS coronavirus HKU-39849), AY278488 (hSARS-CoV BJ01), AY390556 (hSARS-CoV GZ02), AY515512 (Paguma SARS CoV HC/SZ/61/03), KY417146 (Bat SL-CoV Rs4231), KC881005 (Bat SL-CoV RsSHC014), KC881006 (Bat SL-CoV Rs3367), MK211377 (Bat CoV YN2018C), MK211378 (Bat CoV YN2018D), KY417149 (Bat SL-CoV Rs4255), FJ588686 (Bat SL-CoV Rs672), NC014470 (Bat SARS-related CoV BM48-31), EF065513 (Bat CoV HKU9-1), AY391777 (hCoV OC43), NC006577 (hCoV HKU1), NC019843 (hMERS CoV), NC009020 (Bat CoV HKU5-1), NC009019 (Bat CoV HKU4-1), and NC002645 (hCoV 229E).
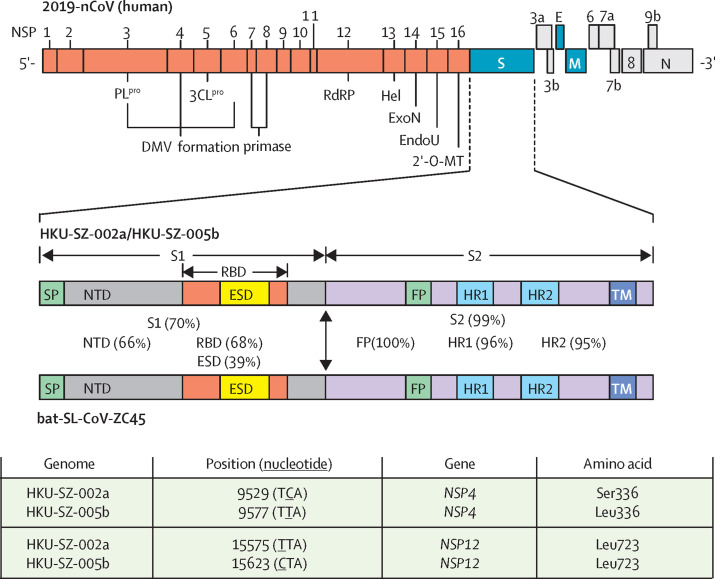
Two complete virus genomes (HKU-SZ-002a and HKU-SZ-005b) were sequenced using Nanopore technology and showed a novel coronavirus that is most closely related to those of the bat SARS-like coronavirus bat-SL-CoVZXC21 (NCBI accession number MG772934) and bat-SL-CoVZC45 (NCBI accession number MG772933). Their genome organisation is typical of a lineage B betacoronavirus. The size of the virus genomes from patient 2 (HKU-SZ-002a) and patient 5 (HKU-SZ-005b) are around 29·8 kilobases with GC content of 38% (appendix p 6). HKU-SZ-002a and HKU-SZ-005b differ from each other by only two bases. One of them is a non-synonymous mutation at amino acid position 336 of non-structural protein 4 (Ser336 for HKU-SZ-002a; Leu336 for HKU-SZ-005b; figure 4 ). Although amino acid sequence of the N-terminal domain of Spike subunit 1 of this novel coronavirus is approximately 66% identical to those of the SARS-related coronaviruses, and the core domain of the receptor binding domain of this novel coronavirus has about 68% amino acid identity with those of the SARS-related coronavirus, the protein sequence of the external subdomain region of receptor binding domain of Spike subunit 1 has only 39% identity, which might affect the choice of human receptor and therefore the biological behaviour of this virus (figure 4).
Figure 4.
Genome organisation of 2019-nCoV and the amino acid identities of different subunits and domains of the Spike between human 2019-nCoV strains (HKU-SZ-002a and HKU-SZ-005b) and bat-SL-CoV-ZC45
2′-O-MT=2′-O-ribose methyltransferase. 3CLpro=3C-like protease. DMV=double-membrane vesicles. E=envelope. EndoU=endoribonuclease. ESD=external subdomain. ExoN=exonuclease. FP=fusion peptide. Hel=helicase. HR1=heptad repeat 1. HR2=heptad repeat 2. M=membrane. N=nucleocapsid. NSP=non-structural protein. NTD=N-terminal domain. ORF=open reading frame. PLpro=papain-like protease. RBD=receptor binding domain. S=spike. S1=subunit 1. S2=subunit 2. SP=signal peptide. TM=transmembrane domain.
All six patients were admitted to hospital under isolation, supportive care, and remained stable as of Jan 20, 2020.
Discussion
We report here a familial cluster of unexplained pneumonia due to 2019-nCoV. Six of seven family members had radiological changes of viral pneumonia, among whom five (patients 1, 2, 4, 5, and 7) tested positive for 2019-nCoV by RT-PCR. Five patients (patients 1, 2, 3, 4, and 7) had associated symptoms at the time of presentation. Complete genome sequences of the two strains from patients 2 and 5 showed almost complete nucleotide identity with each other, and were closest to the bat SARS-related coronaviruses reported in 2018. Several possible scenarios of transmission exist. The first and most likely scenario is that one virologically documented patient with pneumonia (patient 1) acquired the infection from a Wuhan hospital while visiting their relative (relative 1) and then patients 1–5 transmitted the virus to patient 7 on returning to Shenzhen. The second scenario is that patients 1–5 have directly acquired the infection from relatives 2–5 and transmitted it to patient 7 on returning to Shenzhen. But this scenario is less likely because patients 1–5 developed symptoms before relatives 2–5. The third scenario is that patients 1–5 acquired the infection from an unknown common source in Wuhan and transmitted it to patient 7 when back in Shenzhen. For the patients' relatives (relatives 2–5), they could have acquired the infection from the hospital or the community, although no virological confirmation was possible and they had no animal contacts, game food, or visits to the Huanan seafood wholesale market. Notably, patient 1 or patient 3 who had visited Wuhan hospital might have been infectious before symptom onset because patient 5 was shedding virus without symptoms. These findings suggested that person-to-person transmission and intercity spread of 2019-nCoV by air travel are possible, supporting reports of infected Chinese travellers from Wuhan being detected in other geographical regions.
Many of the epidemiological, clinical, laboratory, and radiological features of this novel coronavirus pneumonia were similar to those of SARS patients in 2003.8, 15, 16 The incubation period of the Wuhan pneumonia appeared similar to that of SARS. The attack rate is rather high, up to 83% if we included the five patients (patients 1, 2, 3, 4, and 5) with unexplained ground-glass radiological changes of the lungs on CT scan as the case definition in this family outbreak after visiting Wuhan. A rather unexpected finding from the lung CT scan of patient 5, which was done on the insistence by the nervous parents, also showed ground-glass pneumonic changes. Patient 5 was later confirmed virologically to have an asymptomatic infection. Although asymptomatic patients with SARS were uncommon, they were documented in our retrospective study in the minor 2004 SARS outbreak after reopening of the wildlife market in Guangzhou.17 Notably, patients 3 and 4 were afebrile at presentation to our hospital. These cryptic cases of walking pneumonia might serve as a possible source to propagate the outbreak. Further studies on the epidemiological significance of these asymptomatic cases are warranted.
The symptoms of this novel pneumonia were also non-specific. The three oldest patients in this family with comorbidities had more severe systemic symptoms of generalised weakness and dry cough. As expected, they might have decreased total white blood cell, lymphocyte, or platelet counts, with also extended activated thromboplastin time and increased C-reactive protein level. The multifocal ground-glass changes on lung CT scan were typical of viral pneumonia. Their lung involvement was also more diffuse and extensive than those of the younger patients, whose blood test results were largely normal. Patient 4, who had a history of chronic sinusitis, might have a bacterial superinfection because he had a productive cough instead of a dry cough. He also had a high white blood cell count, although the bacterial test was negative.
Interestingly, the two younger adults (patients 3 and 4) initially had diarrhoea, which was also reported in 10·6% (15 of 142) of our SARS patients at presentation;18 however, the subsequent faecal samples of patients 3 and 4 that were collected 9–10 days after symptom onset were negative for the virus after the diarrhoea had long subsided. Up to 30% of patients with Middle East respiratory syndrome coronavirus (MERS-CoV) also have diarrhoea.19 Subgenomic RNA indicating viral replication was seen in faecal samples of patients with MERS.20 Moreover, MERS-CoV was shown to survive in simulated fed gastrointestinal juice and the ability to infect intestinal organoid models.20 Diarrhoea and gastrointestinal involvement are well known in coronavirus infections of animals and humans.21
On microbiological testing, we did not find any evidence of other known respiratory viral or bacterial infections, but specific RT-PCR assays for two widely separated genome targets—the highly conserved RdRp and the highly variable S genes—were positive for this novel 2019-nCoV. Two complete genome sequences of this novel coronavirus were recovered from the nasopharyngeal swab of patient 2 and the sputum sample of patient 5 with an earlier cycle threshold value indicating a higher viral load. Patient 2 had more underlying comorbidities and clinical features and radiological findings of more severe disease than the other patients included here. Moreover, the serum sample of patient 2 was also positive for 2019-nCoV, which might indicate some virus spillover from the more severely infected lung into the systemic circulation, as previously reported in patients with SARS.22 Sputum samples were available for testing from patients 5 and 7. The cycle threshold values of the sputum samples were 8–13 cycles earlier than those of throat swabs, indicating higher viral loads detected in the lower respiratory tract. This finding is consistent with the observations in patients with MERS who had higher viral loads in lower respiratory tract samples than in upper respiratory tract samples.23 Thus, repeat testing of upper respiratory tract samples or testing of lower respiratory tract samples are warranted in clinically suspected cases with an initially negative result in nasopharyngeal or throat swab. Unlike our patients in the 2003 SARS outbreak,22 we found no evidence of viral shedding in urine and faeces in these six patients. However, improved systematic serial collection and testing of an increased number of such samples is warranted.
Coronaviruses are enveloped, positive-sense, single-stranded RNA viruses, capable of rapid mutation and recombination. They are classified into alphacoronaviruses and betacoronaviruses, which both have their gene source from bats and are mainly found in mammals such as bats, rodents, civets, and humans; and gammacoronaviruses and deltacoronaviruses, which both have their gene source from birds and are mainly found in birds.24, 25, 26 Phylogenetic analysis of the PCR amplicon fragments from five of our six patients and the complete virus genome of 29·8 kilobases from patients 2 and 5 showed that the virus is a novel betacoronavirus belonging to the lineage B or subgenus sarbecovirus, which also includes the human SARS coronavirus. The genome of our virus strains are phylogenetically closest to the bat SARS-related coronaviruses first found in the Chinese horseshoe bats, Rhinolophus sinicus, captured in Zhoushan, Zhejiang province, China, between 2015 and 2017.27 Notably, the first SARS-related coronavirus was also discovered in the R sinicus found in Hong Kong, and central and south China in 2005.28, 29 The full virus genome had about an 89% nucleotide identity with bat-SL-CoVZC45, which makes it a new species. Moreover, the Spike protein of our virus has an 84% nucleotide identity with the bat-SL-CoVZC45 coronavirus and an 78% nucleotide identity with the human SARS coronavirus. Although substantial genetic differences exist between this and other betacoronaviruses, cross reactions in RT-PCR or antibody assays for SARS or other betacoronaviruses are possible if the primers and antigenic epitopes are not carefully chosen, as previously reported.30 Further studies on the optimal diagnostic tests are warranted.
In summary, an outbreak of novel coronavirus is ongoing at Wuhan in the winter of 2019–20. Similar to the 2003 SARS outbreak in Guangzhou, Wuhan is also a rapidly flourishing capital city of the Hubei province and the traffic hub of central China. Moreover, both outbreaks were initially connected to wet markets where game animals and meat were sold. In the case of SARS, person-to-person transmission was efficient and super-spreading events had led to major outbreaks in hotels and hospitals. Learning from the SARS outbreak, which started as animal-to-human transmission during the first phase of the epidemic, all game meat trades should be optimally regulated to terminate this portal of transmission. But as shown in this study, it is still crucial to isolate patients and trace and quarantine contacts as early as possible because asymptomatic infection appears possible (as shown in one of our patients), educate the public on both food and personal hygiene, and alert health-care workers on compliance to infection control to prevent super-spreading events. Unlike the 2003 SARS outbreak, the improved surveillance network and laboratory capability of China was able to recognise this outbreak within a few weeks and announced the virus genome sequences that would allow the development of rapid diagnostic tests and efficient epidemiological control. Our study showed that person-to-person transmission in family homes or hospital, and intercity spread of this novel coronavirus are possible, and therefore vigilant control measures are warranted at this early stage of the epidemic.
Acknowledgments
Acknowledgments
This study was partly supported by the Shaw Foundation Hong Kong; Michael Seak-Kan Tong; Respiratory Viral Research Foundation; Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited; Marina Man-Wai Lee; the Hong Kong Hainan Commercial Association South China Microbiology Research Fund; Sanming Project of Medicine in Shenzhen, China (SZSM201911014 and SZSM201612096); and the High Level-Hospital Program, Health Commission of Guangdong Province, China.
Contributors
JF-WC and K-YY had roles in the study design, clinical management, patient recruitment, data collection, data analysis, data interpretation, literature search, and writing of the manuscript. SY, K-HK, KK-WT, HChu, CC-YY, RW-SP, H-WT, SK-FL, K-HC, VK-MP, W-MC, JDI, J-PC, VC-CC, and HChe had roles in the experiments, data collection, data analysis, and data interpretation. JY, CK-MH, FX, and JL had roles in recruitment, data collection, and clinical management. All authors reviewed and approved the final version of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Centre for Health Protection of the Hong Kong Special Administrative Region Government CHP closely monitors cluster of pneumonia cases on Mainland. Dec 31, 2019. https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htm
- 2.Centre for Health Protection of the Hong Kong Special Administrative Region Government CHP provides further information on cluster of pneumonia cases in Wuhan. Jan 12, 2020. https://www.info.gov.hk/gia/general/202001/12/P2020011200710.htm
- 3.Juan D. Wuhan wet market closes amid pneumonia outbreak. ChinaDaily. Jan 1, 2020 https://www.chinadaily.com.cn/a/202001/01/WS5e0c6a49a310cf3e35581e30.html [Google Scholar]
- 4.Cohen J. American Association for the Advancement of Science; Washington, DC: Jan 11, 2020. Chinese researchers reveal draft genome of virus implicated in Wuhan pneumonia outbreak.https://www.sciencemag.org/news/2020/01/chinese-researchers-reveal-draft-genome-virus-implicated-wuhan-pneumonia-outbreak [Google Scholar]
- 5.To KK, Chan KH, Li IW, et al. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol. 2010;82:1–7. doi: 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To KKW, Yip CCY, Lai CYW, et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Chan KH, To KKW, Li PTW, et al. Evaluation of NxTAG Respiratory pathogen panel and comparison with xTAG respiratory viral panel fast v2 and film array respiratory panel for detecting respiratory pathogens in nasopharyngeal aspirates and swine/avian-origin influenza A subtypes in culture isolates. Adv Virol. 2017;2017 doi: 10.1155/2017/1324276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JF, Zhang AJ, Chan CC, et al. Zika virus infection in dexamethasone-immunosuppressed mice demonstrating disseminated infection with multi-organ involvement including orchitis effectively treated by recombinant type I interferons. EBioMedicine. 2016;14:112–122. doi: 10.1016/j.ebiom.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KKW, Chan WM, Li KSM, et al. High prevalence of four novel astrovirus genotype species identified from rodents in China. J Gen Virol. 2017;98:1004–1015. doi: 10.1099/jgv.0.000766. [DOI] [PubMed] [Google Scholar]
- 11.Woo PC, Lau SK, Teng JL, et al. Metagenomic analysis of viromes of dromedary camel fecal samples reveals large number and high diversity of circoviruses and picobirnaviruses. Virology. 2014;471–73:117–125. doi: 10.1016/j.virol.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tse H, Tsang AK, Tsoi HW, et al. Identification of a novel bat papillomavirus by metagenomics. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewandowski K, Xu Y, Pullan ST, et al. Metagenomic nanopore sequencing of influenza virus direct from clinical respiratory samples. J Clin Microbiol. 2019;58:e00963–e01019. doi: 10.1128/JCM.00963-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau SK, Feng Y, Chen H, et al. Severe acute respiratory syndrome (SARS) coronavirus ORF8 protein is acquired from SARS-related coronavirus from greater horseshoe bats through recombination. J Virol. 2015;89:10532–10547. doi: 10.1128/JVI.01048-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che XY, Di B, Zhao GP, et al. A patient with asymptomatic severe acute respiratory syndrome (SARS) and antigenemia from the 2003–2004 community outbreak of SARS in Guangzhou, China. Clin Infect Dis. 2006;43:e1–e5. doi: 10.1086/504943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng VC, Hung IF, Tang BS, et al. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;38:467–475. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Li C, Zhao G, et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3 doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung WK, To KF, Chan PK, et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung IF, Cheng VC, Wu AK, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10:1550–1557. doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo PC, Lau SK, Lam CS, et al. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau SK, Woo PC, Li KS, et al. Discovery of a novel coronavirus, China Rattus coronavirus HKU24, from Norway rats supports the murine origin of Betacoronavirus 1 and has implications for the ancestor of Betacoronavirus lineage A. J Virol. 2015;89:3076–3092. doi: 10.1128/JVI.02420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu D, Zhu C, Ai L, et al. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau SK, Woo PC, Li KS, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Shi Z, Yu M, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 30.Che XY, Qiu LW, Liao ZY, et al. Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J Infect Dis. 2005;191:2033–2037. doi: 10.1086/430355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.