Executive summary
Essential medicines satisfy the priority health-care needs of the population. Essential medicines policies are crucial to promoting health and achieving sustainable development. Sustainable Development Goal 3.8 specifically mentions the importance of “access to safe, effective, quality and affordable essential medicines and vaccines for all” as a central component of Universal Health Coverage (UHC), and Sustainable Development Goal 3.b emphasises the need to develop medicines to address persistent treatment gaps.
The recognition of the importance of essential medicines is not new. At the 1985 Nairobi Conference on the Rational Use of Drugs, government representatives and other stakeholders proposed a comprehensive set of essential medicines policies. 30 years later, The Lancet's Commission on Essential Medicines Policies convened to explore these questions: what progress has been achieved? What challenges remain to be addressed? Which lessons have been learned to inform future approaches? And how can essential medicines policies be harnessed to promote UHC and contribute to the global sustainable development agenda? This report addresses these questions, with the intent to reposition essential medicines policies on the global development agenda.
The Commission identified five areas that are crucial to essential medicines policies: paying for a basket of essential medicines, making essential medicines affordable, assuring the quality and safety of medicines, promoting quality use of medicines, and developing missing essential medicines. The Commission located essential medicines policies within the context of current global debates about balancing trade and intellectual property policies with human rights, assuring health security, strengthening people-centred health systems, and advancing access to essential technologies. In all policy areas, particular attention was paid to furthering equity in access, strengthening relevant institutions, and creating accountability. For each policy area, the Commission made actionable recommendations, thereby reaffirming essential medicines policies as a central pillar of the global health and development agenda.
Paying for a basket of essential medicines to promote sustainable access for all
Globally, a quarter of all health expenditure is on medicines. In many countries, the main source of financing for medicines is direct payment by the individual and households—this source is both highly inequitable and inefficient, and its reduction is a key target for UHC. Furthermore, the Commission found that the available data on pharmaceutical expenditure in many countries lack sufficient detail on the types of medicines procured or sold, public and private sector spending, and the degree of access by key population subgroups.
For this report, the Commission developed a new model-based global estimate of the total financing that would be needed to achieve universal access to a basic package of essential medicines in low-income and middle-income countries (LMICs). A costing model was developed on the basis of disease prevalence, current or projected consumption of medicines, and international reference prices. Using two consumption scenarios, the Commission estimated that between US$77·4 and $151·9 billion (or $13 to $25 per capita) is required to finance a basic package of 201 essential medicines (378 dosage forms) in all LMICs. Yet in 2010, the majority of low-income countries (LICs) and 13 out of 47 middle-income countries, spent less than $13 per capita on pharmaceuticals. Thus, the Commission confirmed that many people worldwide do not have access to even a limited basket of essential medicines. Countries should adapt the Commission's model to their national contexts to create a locally relevant estimate as a benchmark for measuring performance on essential medicines. The Commission's recommendations on financing of essential medicines are:
-
•
Governments and national health systems must provide adequate financing to ensure inclusion of essential medicines in the benefit packages provided by the public sector and all health insurance schemes.
-
•
Governments and national health systems must implement policies that reduce the amount of out-of-pocket spending on medicines.
-
•
The international community must fulfil its human rights obligations to support governments of LICs in financing a basic package of essential medicines for all, if they are unable to do so domestically.
-
•
Governments and national health systems must invest in the capacity to accurately track expenditure on medicines, especially essential medicines, in both the public and private sectors, disaggregated between prepaid and out-of-pocket expenditure, and among important key populations.
Making essential medicines affordable is necessary to achieve equity in access
The affordability of essential medicines is a core challenge for any health system working to achieve UHC, and therefore features prominently on the global agenda. The complexity of the problem of affordability illustrates the urgent need for comprehensive policy solutions; no single policy alone can solve this problem.
The lack of medicines pricing information makes it difficult for consumers—both individuals and health systems—to make informed decisions about purchasing medicines. Scarcity of data also impedes assessments of whether individuals and households face financial barriers when making out-of-pocket payments for medicines, and creates a barrier to cross-national comparisons that could inform the setting of benchmarks and the establishment of appropriate and effective pricing policies.
Medicines benefit packages guide procurement and reimbursement for affordable essential medicines. Compiling these packages necessitates building capacity at national level to translate findings from evidence (including health technology assessments) to local contexts, and to use the findings as inputs in decision making (including when to intervene to influence pricing). Governments and other purchasers of medicines can expand their transparent sharing of information to increase efficiency and avoid duplication of efforts.
The Commission's recommendations on making essential medicines affordable are:
-
•
Governments and health systems must create and maintain information systems for routine monitoring of data on the affordability of essential medicines, as well as price and availability, in the public and private sectors.
-
•
Governments must implement a comprehensive set of policies to achieve affordable prices for essential medicines.
-
•
Governments and health systems must develop national capacity to create medicines benefit packages that guide procurement and reimbursement for affordable essential medicines.
-
•
Governments, national health systems, and the pharmaceutical industry must promote transparency by sharing health and medicines information.
Assuring the quality and safety of medicines is needed to prevent harm to patients
Despite impressive progress, serious problems with medicine quality and safety remain, particularly in LMICs. These problems threaten the health of people and waste resources. Quality and safety of medicines are compromised when manufacturers, whether by accident or intent, produce substandard products, and when the supply chain allows unsafe, and sometimes dishonest, practices during transport and delivery. Current regulatory capacity and enforcement are insufficient in most LMICs.
Global and national regulatory structures therefore require considerable and urgent reform to assure the quality and safety of medicines. The large donor programmes for AIDS, tuberculosis, and malaria treatments have helped to advance strategies on quality procurement, such as the WHO/UN Prequalification Programme. A clear trend towards international regulatory collaboration and electronic communications has emerged. These trends can now be leveraged to ensure continued progress for the full array of essential medicines for all countries.
The Commission's recommendations on assuring the quality and safety of essential medicines are:
-
•
Global efforts must be made to promote the harmonisation of quality assurance efforts through the use of an international standard regulatory dossier that covers both format and content.
-
•
WHO should evolve the WHO/UN Prequalification Programme to maintain a moving focus on new essential medicines.
-
•
Payers and procurement agencies must adopt good procurement practices that incorporate effective and transparent quality assurance mechanisms.
-
•
Governments must redirect the activities of national regulatory agencies towards those that add value and reduce duplication of effort, and engage with a system for independent and public assessment of the performance of NMRAs.
-
•
Regulatory agencies must encourage the involvement of other stakeholders and the general public in promoting the quality and safety of essential medicines.
-
•
WHO and national governments must establish concrete targets and a public accountability mechanism for the performance of national regulatory authorities.
Promoting quality use of essential medicines leads to better health outcomes and can achieve considerable efficiencies
Medicines can treat diseases and alleviate suffering, but only when a patient receives and takes the right medicine to treat the symptom or disease, in the right formulation and dose, at the right time, and for the right duration. When any of these conditions are not met, problems with medicines use ensue. These problems include overuse (as with opioids in some settings), underuse (as in many countries with poor access to opioids for the management of severe pain), misuse (as when antibiotics are taken for a viral disease), and unnecessarily expensive use (as when brand-name medicines are used despite the existence of a lower-priced, quality-assured generic alternative). As UHC enables more people to have access to medicines, problems with the use of medicines threaten to undermine the potential benefits by harming individuals, reducing the efficacy of medicines (if antimicrobial resistance develops), and jeopardising the financial stability of health systems.
Problems of inappropriate use do not arise from a single root cause—thus, addressing them requires complex and coordinated interventions. The Commission's recommendations focus on strategies that enable collaboration among patients, health-care providers, insurers, supply chain managers, and others (including the pharmaceutical industry), to incentivise and support quality medicines use. Strong institutions with the capacity to generate evidence and implement evidence-informed policies are crucial. The benefits of these efforts will include improving clinical, public health, economic, and ethical outcomes.
The Commission's recommendations on improving the use of essential medicines are:
-
•
Governments and the main public or private payers should establish independent pharmaceutical analytics units (or equivalent) to focus on generating information for action to promote quality use, in conjunction with other objectives.
-
•
Pharmaceutical analytics units must collaborate with multiple stakeholders in all relevant systems to increase their engagement in and accountability for quality use of medicines, and to intervene jointly on medicines use problems.
-
•
Engaged stakeholder groups, led by data produced by the pharmaceutical analytics unit, should identify and prioritise local medicines use problems, identify contributing factors across the system, and develop and implement sustainable, long-term, multi-faceted interventions.
A global research and development (R&D) policy framework is needed to develop missing essential medicines and make them accessible to all
The present system for developing medicines is in crisis, largely failing to produce much needed products that address the health needs of millions of people worldwide. The prices of new essential medicines that are developed are sometimes so high that even high-income countries face financing problems. Pharmaceutical companies and their shareholders are typically reluctant to invest in marketing medicines for patient populations that do not represent a profitable market. These two problems are related, and disproportionately affect people in LMICs.
With the current patent-based innovation system, the feasibility of achieving or maintaining UHC is seriously at risk. Several not-for-profit initiatives, often in collaboration with the pharmaceutical industry, have compensated for some problems with the current system, but they do not represent a long-term solution. A new global policy framework is needed to drastically adapt the current model and to reduce its reliance on market exclusivity as the main driver of innovation. Governments need to define a list of missing essential medicines to be provided under UHC schemes, and governments, non-governmental organisations, and the industry need to make the necessary R&D financing mechanisms available for these identified needs. The price of new essential medicines can then be delinked from development costs and the products can be made widely available and affordable through non-exclusive licensing agreements. The resultant decrease in price can provide the financial space to more directly finance the identified priority R&D.
The Commission's recommendations on developing missing essential medicines are:
-
•
Governments and WHO must take international public leadership for priority setting for essential R&D, with due regard for the public health needs of LMICs.
-
•
Governments must lead the process towards a global research and development policy framework and agreements, which include new financing mechanisms to ensure that missing essential medicines are developed and made affordable.
-
•
The international community must create a general Essential Medicines Patent Pool.
-
•
Governments and national stakeholders must develop and implement comprehensive national action plans to guarantee equitable access to new essential medicines.
-
•
The pharmaceutical industry must better align its R&D priority setting with global health needs, and develop access strategies to make medically important innovations available to all in need.
Measuring progress holds all stakeholders accountable
The Commission's recommendations represent a compilation of proven and promising practices to improve national policies to assure access to quality-assured, affordable essential medicines and their quality use as a central component of UHC. To transform these recommendations into reality will require commitments on the part of governments, policy makers, implementers, the pharmaceutical industry, donors, health-care providers, citizens, and patients, as well as international agencies and civil society organisations. This commitment can be created in part through deliberate steps to document efforts and demonstrate progress. Thus, the Commission proposes a set of 24 core indicators to measure progress in the implementation of comprehensive essential medicines policies.
Together, the proposed indicators can track the progress of countries and the global community in their efforts to advance in the five priority areas for essential medicines policies (financing, affordability, quality and safety, use, and development of new medicines). The Commission intends these indicators to serve as a starting point for the continued development of accountability mechanisms that incorporate independent reviews and corrective actions. Setting appropriate targets for each indicator will be a crucial component of the process, requiring the active involvement of relevant stakeholders. National leadership, and promoting national ownership of results, should be a priority and lead to regional and global data sharing, making local data a global public good.
Accountability will allow governments, global agencies, the pharmaceutical industry, civil society organisations, other institutional stakeholders, and citizens around the world to track progress made on essential medicines policies to support UHC. This tracking will enhance other ongoing processes to measure and document progress towards the Sustainable Development Goals and national targets.
Without essential medicines, health systems cannot truly help people who fall ill, live with chronic disease, and go through various stages of life and death. Without strong health systems, populations cannot realise their right to health. 30 years after the first international conference on medicines policies, essential medicines are still essential. The Commission presents this report in the strong belief that the world can get essential medicines right, promoting improved performance and equity in health systems, while supporting UHC and enabling sustainable development.
Introduction
Essential medicines are central to promoting health and ensuring sustainable development. The Sustainable Development Goals (SDGs) adopted in September, 2015, by the member states of the UN recognise that equitable access to affordable, quality-assured essential medicines is a crucial step in achieving these key development targets (panel 1 ).1
Panel 1. Sustainable Development Goals related to essential medicines.
Sustainable Development Goal (SDG) 3 is: “Ensure healthy lives and promote well-being for all at all ages.” Two targets for Goal 3 specifically mention essential medicines:
-
•
SDG 3.8: “Achieve universal health coverage, including financial risk protection, access to quality essential health-care services, and access to safe, effective, quality and affordable essential medicines and vaccines for all.”
-
•
SDG 3.b: “Support research and development of vaccines and medicines for the communicable and non-communicable diseases that primarily affect developing countries, provide access to affordable essential medicines and vaccines, in accordance with the Doha Declaration…which affirms the right of developing countries to use to the full the provisions in the Agreement on Trade-Related Aspects of Intellectual Property Rights regarding flexibilities to protect public health, and, in particular, provide access to medicines for all.”
Countries have agreed to move towards universal health coverage (UHC).1 The aim of UHC is to “ensure that all people obtain the health services they need without suffering financial hardship when paying for them.”2 SDG 3.8 specifically mentions the importance of “access to safe, effective, quality and affordable essential medicines and vaccines for all.” Throughout this report, the phrase access to essential medicines denotes a broad definition, also used in SDG 3.8, which encompasses the quality, safety, and efficacy of medicines and vaccines, as well as their availability, affordability, and appropriate use.
Assuring access to essential medicines is crucial for moving towards UHC. This report presents the findings of the Lancet Commission on Essential Medicines Policies, which examined five core challenges that every country must address to secure access to essential medicines.
Five core challenges for essential medicines policies
Adequate financing to pay for an appropriate set of essential medicines is the first key challenge. Medicines represent a large proportion of household expenditure on health in low-income and middle-income countries (LMICs).3 According to the World Health Survey, up to 9·5% of the total expenditure of poorer households in LMICs is spent on medicines, far higher than the 3·5% expended by poorer households in high-income countries (HICs).4 This statistic is particularly true in countries where inadequate public financing of health care results in high out-of-pocket expenditure.5 Little evidence exists to indicate how much financing would be required to pay for essential medicines for all.
The focus of the second challenge is affordability of essential medicines, as determined by comparing the price of the product to the amount the buyer can afford. High prices for medicines are often associated with the period of monopoly under patent protection. However, even lower-priced medicines can become unaffordable to most households in low-income countries (LICs).6 Affordability becomes a particularly serious problem when medicines are needed for chronic conditions, including non-communicable diseases (NCDs). Affordability of medicines has become a key issue for governments, as well as public and private payers for health care, regardless of a country's income level. European countries affected by the global financial crisis have reported restricted access to essential medicines.7 In the USA, state-funded health-care institutions that are responsible for prisoners have been sued over the poor access to new high-priced essential medicines for hepatitis C.8
The third key challenge is assuring the quality and safety of essential medicines. Poor-quality medicines seriously undermine the effectiveness of health care, as well as public confidence in the health system. Many incidents of harm from sub-standard and falsified medicines have been recorded.9, 10 For example, poor-quality antimalarial medicines are responsible for an estimated 122 000 deaths per year in children under 5 years in 39 sub-Saharan African countries.11 Contaminated medicinal products were responsible for the deaths of more than 100 children in Panama12 and 230 patients in Pakistan.13
Medicines cannot have a positive impact on health unless they are used appropriately. Nominal health coverage of a population is not sufficient to ensure quality use of medicines. Multiple factors contribute to the problems of overuse, underuse, incorrect use, and unnecessary consumption of expensive medicines. In many countries, injections and antibiotics are heavily overprescribed.14 In surveys of 22 countries outside the Organisation for Economic Co-operation and Development (OECD), no more than 61% of people with hypertension in a given country were taking appropriate medication.15
Finally, certain essential medicines are missing, as noted in SDG 3.b. Patent-driven research and development (R&D) models have not developed many missing essential medicines. Some important unmet public health needs include heat-stable insulin and oxytocin,16 shorter treatments for latent and active tuberculosis, single-day treatments of malaria, and treatments for multidrug-resistant tuberculosis. Beyond neglected diseases, R&D of new medicines has not been aligned with the existing and emergent burden of disease around the world.17
These five core challenges for essential medicines policies are not new. Indeed, over the past few decades the global health community has sought to address them at all levels. However, finding long-lasting sustainable solutions has proved difficult. National and global economic and political interests have strongly influenced the development and implementation of essential medicines policies, which have implications for public health, economic development, and trade. As a result, essential medicines policies are often highly contested, at both national and global levels.
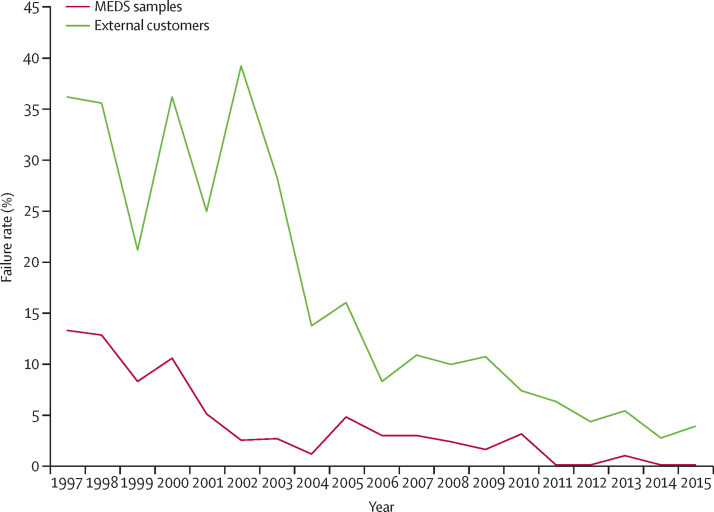
Simultaneously, although these polices affect the prevention and treatment of many diseases, essential medicines are rarely presented at the centre of the global health agenda. Instead, all too often essential medicines policies are incorrectly regarded as a technical side issue for which answers are known and easily applied. In this report, the Commission argues that essential medicines pose a central challenge to the sustainable development agenda, demanding creative and bold action. As an example, the Commission presents the case of new essential medicines for treating hepatitis C (panel 2 ). This case illustrates that essential medicines policies are relevant for all countries regardless of income level, and that the five challenges are closely related.
Panel 2. New essential medicines to treat hepatitis C virus infection.
The marketing of new treatments for hepatitis C virus infection in the past 5 years and the current global debate on equitable access to such treatment have placed effective policies for essential medicines at the centre stage of global health. Details of the case succinctly illustrate each of the five key challenges of essential medicines policies presented in this report: paying for essential medicines, making treatment affordable, assuring quality and safety, promoting quality use, and developing new essential medicines. The case also illustrates how these five challenges are interconnected, and how they are equally relevant for high-income and low-income countries alike.
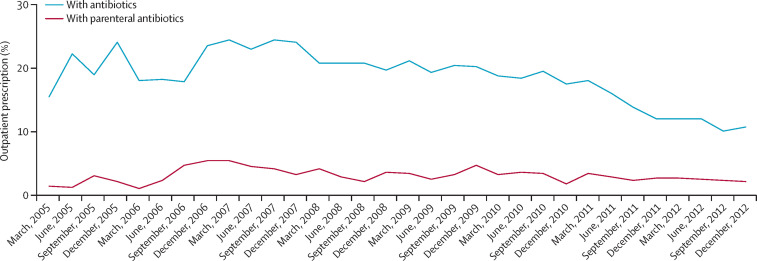
Up to 184 million people globally are living with hepatitis C virus infection.18 The newly developed medicines, known as direct-acting antiviral medicines (DAAs), have dramatically improved the efficacy and safety of hepatitis C treatment, offering substantial improvements in quality of life and longevity. WHO has developed a global strategy for the treatment and elimination of viral hepatitis,19 and added four DAAs—daclatasvir, dasabuvir, simeprevir, and sofosbuvir—to the WHO Model List of Essential Medicines in 2015.20 Overall response rates are substantially higher than with previously used first-line regimens, although the efficacy of DAAs varies with viral genotype.21 National governments and other health-care payers worldwide are now working to scale up access to these medicines to all people living with hepatitis C virus infection.
Paying for DAAs
The most prominent question being debated is how to pay for DAAs, which are extremely costly. Directly related to this question is the need to estimate how much treatment will cost for each specific population. This estimate is in itself a major challenge, since pricing of the new DAAs varies widely among countries and also sometimes within countries.22 For example, it has been estimated that treating all eligible patients in the USA with DAAs would require an additional US$65 billion over the course of 5 years.23 Some US insurance plans offer treatment, but many restrict access to particular subsets of patients with hepatitis C virus infection (based on, for example, severity of illness or likelihood of benefiting from treatment). The high price of sofosbuvir (with a list price of about $84 000 for a full course of treatment) prompted an investigation by the US Senate Committee on Finance; it revealed that even though Medicare spent more than $1 billion on the medicine in 2014, less than 2·4% of patients with hepatitis C virus infection enrolled in Medicare had received treatment.24 The budget implications of paying for DAAs as part of a basic health-care package are tremendous in high-income countries; the budget implications in health systems with far fewer resources are even more daunting.
Making DAAs affordable
Affordability of DAAs is a major global challenge, and is also widely debated. For example, in 2015, sofosbuvir was licensed in Malaysia where hepatitis C virus infection prevalence is estimated at 2·5% of people aged 15–64 years, mostly among men.25 However, sofosbuvir remains unaffordable for patients and the government alike, with a price set at about $87 430 for a 24-week course.26 Malaysia is considered an upper-middle-income country,27 with a gross domestic product per capita of $11 307 in 2014—far less than the cost of a single treatment course.28 Pricing has little to do with production cost; for sofosbuvir, production is estimated to cost between $68 and $136 for a 12-week treatment course.29 The medicine is sold in India for $500,29 and after Egypt introduced local production of the medicine, the price there dropped to about $330.30 Thus, there is ample scope for price reductions in Malaysia and elsewhere, but achieving them requires concerted effort to implement a range of policies to promote affordability. Prices are expected to fall for the production of sofosbuvir, ledipasvir/sofosbuvir combination tablet, and the recently registered tablet sofosbuvir/velpatasvir for sale in 101 low-income and middle-income countries, following Gilead Sciences Inc's signing of voluntary licence agreements with 11 Indian generics companies in 2014.31 The Medicines Patent Pool also offers a licence for daclatasvir for sale in 112 low-income and middle-income countries.32
Assuring quality and safety of DAAs
Mitigating the risk of substandard and falsified DAAs entering supply chains and reaching patients is necessary; quality and safety concerns highlight the need for affordable quality-assured treatments. In March 2016, a non-governmental organisation in Myanmar reported it had identified two falsified products, one claiming to contain 400 mg sofosbuvir + 90 mg ledipasvir, and the other 60 mg daclatasvir.33 The manufacturer listed on the packaging has never produced the combination, nor was it currently producing 60 mg daclatasvir. Lack of access to treatment attracts criminal entities that exploit people's health needs. Other falsified DAAs have been found in Israel,34 and Pakistan's regulatory authorities have identified factories making counterfeit versions.35 Falsified medicines endanger patients' health and undermine trust in legitimate medicines manufacturers. The best way to counter the supply of falsified and substandard medicines is to ensure the availability of affordable, quality-assured essential medicines.
Promoting quality use of DAAs
Substantial risks can also result from inappropriate use of DAAs, leading to operational challenges in expanding access to DAA. Appropriate selection of a DAA-containing regimen requires previous identification of the viral genotype. Inappropriate use of DAAs leads to unnecessary costs; furthermore, the high prices of DAAs might lead to partial courses of treatment or other forms of underuse as patients try to cut expenses. Test-and-treat strategies, short-course fixed-dose combinations, and pan-genotypic regimens can promote quality use.
Developing DAAs
Sofosbuvir, the DAA that forms the backbone of most treatment regimens, was developed initially at an academic institution with US federal research funding. However, because neither universities nor governments have the operational capacity to move a new medicine into production, the discovery was sold first to a small biotech company and then to the pharmaceutical company Gilead Sciences Inc, which bought the biotech company for $11·2 billion. Private investment in the development of the drug is estimated at no more than $200 million.36 Within 1 year of introducing the medicine, Gilead Sciences Inc had recouped the initial expenditure of $11·2 billion; the patent will not expire before 2024. This situation limits the downward pressure on prices created by a competitive generic market, although other DAAs might exert some competitive price pressure.
This report discusses how effective essential medicines policies have been developed and implemented, and describes approaches to contend with remaining and emerging challenges. This introduction places these arguments in the context of the historical evolution of essential medicines within broader movements for global health, and underscores how crucial essential medicines are to every health system. Subsequent sections delve into each of the five key challenges, analysing achievements from the past three decades, identifying lessons learnt, and making actionable, evidence-informed recommendations on best practices and promising new approaches.
Finally, this report proposes a set of indicators for tracking progress on implementation of the recommendations. These indicators provide the scaffolding for building national and global accountability frameworks that can support and propel countries towards effective essential medicines policy implementation.
30 years after Nairobi: The Lancet's Commission on Essential Medicines Policies
The term essential medicines is defined by WHO as “those that satisfy the priority health care needs of the population”. The concept of essential medicines emerged globally in the 1970s as part of the movement for primary health care. WHO published the first Model List of Essential Medicines in 1977, a year before the Alma Ata Conference on Health for All.37
The first international conference on essential medicines policies, the Nairobi Conference on the Rational Use of Drugs (panel 3 ), was held in 1985. The resulting 1986 World Health Assembly resolution on the Revised Drugs Strategy (WHO, unpublished) represented a major milestone. It laid the foundation for many subsequent international policies related to essential medicines, including procurement, supply, prescribing and dispensing of medicines, and the regulation of promotional practices. 30 years after Nairobi, essential medicines has become a widely accepted public policy concept.52
Panel 3. The Nairobi Conference of Experts on the Rational Use of Drugs.
Early initiatives on essential medicines *
Cuba (1963) was probably the first country to introduce a list of basic medicines.38 Maurice King's revolutionary 1966 book, which included the first international checklist of basic medicines,39 was followed by the introduction of national lists in Tanzania in 1970,40 and Peru in 1972.41 The first WHO Model List of 212 essential drugs was published in 1977.37 In 1978, the Declaration of Alma Ata included the provision of essential medicines as the eighth component of primary health care.
The concept of essential medicines proposed by WHO immediately drew mixed reactions. The Lancet called the selection “desert-island drugs” but recommended applying a similar approach in developed countries.42 Consumer activists supported the concept as a way to reduce unbridled promotion of unnecessary and harmful medicines. The pharmaceutical industry argued that restricting prescribers' free choice of medicines would lead to a deterioration of health care. When the WHO Action Programme on Essential Drugs was established in 1982, the pharmaceutical industry feared that WHO, under pressure from consumer groups, would develop an international code on pharmaceutical marketing.43
Conference of Experts on the Rational Use of Drugs 44
The 1984 World Health Assembly (WHA), led by Nordic countries and the Netherlands, asked WHO Director-General Halfdan Mahler to organise a global meeting to discuss the rational use of medicines. The term rational use reflected the view that medicine-related problems went beyond logistics, but were also driven by uncontrolled pharmaceutical markets. WHO kept the participant list secret before the meeting to prevent lobbying, and asked participants not to divulge the background papers. At the opening, Mahler reminded participants that they were invited as experts, not as stakeholder representatives.
A central theme of the conference was the need to restrict marketing of medicines to those that were essential. The meeting stated that any national medicines policy should ensure that medicines of acceptable quality, safety, and efficacy were available at affordable costs to all who needed them. WHO was charged with disseminating guidelines on the development and implementation of national medicines policies. Much discussion focused on making medicine information more objective and accessible; the conference agreed that governments were responsible for regulating pharmaceutical marketing and advertising. There was sharp disagreement over the universality of rationalisation, and whether it should apply to both the public and private sectors, in the interests of public health.
In summing up the conference, Mahler concluded that the experts had invited WHO to take a leadership role without becoming a supranational manipulator of governments, and suggested that WHO establish expert committees to produce guidelines on ethical advertising and developing national medicines policies.
The Revised Drug Strategy
The WHA subsequently adopted a Revised Drug Strategy on the basis of Mahler's summary in 1986. Neither industry nor consumers could oppose it, since their experts had accepted Mahler's conclusions in Nairobi. Yet the WHA meeting was highly politicised, with an industry exhibition and press centre, and a problem drugs pack issued by Health Action International. The USA actively lobbied against WHO's proposed role in regulating the operations of the pharmaceutical industry in developing countries.43
In 1986, the USA failed to pay its assessed contribution to WHO, largely because of its dissatisfaction with WHO's activities in the pharmaceutical area following Nairobi.45 The Revised Drug Strategy has guided WHO's work since, but no further global conference on essential medicines has been held.
Implementation and the long-term impact of the Nairobi recommendations
For over two decades after Nairobi, international donors (particularly the Netherlands and the Nordic countries) gave extensive financial and political support to WHO's Action Programme. Just before Mahler completed his term of office in 1988, WHO issued the Ethical Criteria for Medicinal Drug Promotion.46 Initially seen as a weak compromise, this publication has stood the test of time. That year, WHO also published the first Guidelines for Developing National Drug Policies.47 An updated version has remained in wide use since 2001.48 WHO strengthened the standard format for exchanging regulatory information and intensified its support to national regulatory agencies, ultimately leading to the WHO/UN Prequalification Programme in 2001. Rational use activities were started, such as developing Drug Use Indicators, published in 1992,49 the Guide to Good Prescribing in 1994,50 and many international training courses that have been instrumental in preparing a new generation of international experts.
Following the Nairobi Conference, WHO extensively supported most low-income and middle-income countries in developing and implementing national medicine policies. By 2013, more than 90% of low-income and middle-income countries had formulated a first list of essential medicines and published a national medicines policy (Figure 1, Figure 2).
30 years after Nairobi, essential medicines has become a widely accepted concept against which few can argue. Over the years, WHO has been encouraged and supported by an increasingly professional consumer movement consistently advocating for more decisive action.51
The 30th anniversary of the Nairobi Conference provided an opportune moment to take stock of what has transpired in the intervening years since 1985. The Lancet's Commission on Essential Medicines Policies was established in July, 2014, to explore the progress achieved, the challenges that remain, and the lessons learnt. Details of the Commission's mandate and operations are provided in appendix 1.6.
The evolution of essential medicines policies
The development and implementation of essential medicines policies evolved in three broad eras. The first era was characterised by the establishment of essential medicines as a key element of primary health care. Global interventions in this era focused on providing technical assistance to countries to help them develop essential medicines lists and national medicine policies, predominantly in the public sector. In the second era, essential medicines policies were fundamentally shaped by global investments—financial and political—in expanding access to medicines for selected communicable diseases, in particular AIDS, tuberculosis, and malaria. Public health principles underpinning the concept of essential medicines were established as a central element of the right to health, and the health systems' responses to these three diseases. The third era is characterised by a reframing of essential medicines policies in light of the global SDG of UHC. In this era, the previous focus on major infectious diseases has broadened to encompass chronic NCDs. Increasing equitable and sustainable access to essential medicines, by moving towards UHC, is emphasised.
The first era: a global concept of essential medicines (1970s to 1990s)
The first era of essential medicines policies coincided with the emergence of the primary health-care movement and the Alma Ata Conference. The essential medicines concept was articulated in the first WHO Model List of Essential Medicines published in 1977.37 The first Model List elicited both strong support and strong opposition. Supporters argued that a list of essential medicines established standards that both enabled stakeholders to work toward common aims and provided advocates with a baseline for health-care delivery. However, many health professionals and the pharmaceutical industry were opposed, concerned that the selection of a list of essential medicines would limit health-care delivery, constrain professional autonomy, interfere with pharmaceutical markets, and reduce health benefits for patients. 52
Despite the controversies around the concept of essential medicines, throughout the 1980s governments and health systems around the world—especially, but not only, in LMICs—developed essential medicines lists, largely for the public sector. By the 1990s, many multilateral and bilateral agencies supported national public sector essential medicines programmes. Notable examples included programmes in Bolivia, Ecuador, Kenya, Malawi, Sudan, Tanzania, Uganda, Yemen, Zimbabwe, and later, South Africa. Non-governmental health organisations and faith-based organisations also applied these strategies across Africa via the Ecumenical Pharmaceutical Network among others.53, 54
Most national essential medicines lists in this first era focused on off-patent, lower-priced generic medicines to treat or prevent common acute conditions. Examples include anti-infectives (such as mebendazole, ivermectin, ampicillin, and doxycycline), analgesics (aspirin and paracetamol), antimalarials (chloroquine and primaquine), oral rehydration solution, and childhood vaccines. This emphasis aligned with the movement for selective primary health care that was strongly championed in the 1990s.55 This movement had emerged as a response to the problem of facing enormous unmet medical needs with limited resources. Selective primary health care focused on delivering a restricted range of first-contact services with high cost-effectiveness in LMICs, and emphasised maternal and child health services. It did not directly address health system structures needed for chronic communicable and non-communicable conditions, particularly the emerging burden of HIV. Instead, the emphasis of essential medicines policies in this era was on efficiency (by prioritising low-cost and cost-effective treatments) and equity (by emphasising treatments for diseases associated with poverty).56
The first era also saw a global economic crisis and the imposition of economic structural adjustment programmes on many donor-dependent LMICs. These programmes reduced the fiscal space for public sector primary health care and resulted in huge delivery and access problems.57 Increased user fees for health services and a reliance on revolving drug funds became common features of health policy in such settings. As a result, essential medicines were largely financed by individuals paying out of pocket, often in the private sector.
Two indicators are frequently cited to demonstrate progress during this first era: the number of LMICs that established a national list of essential medicines, and the number of countries that adopted a national medicines policy describing principles for selection, quality, and appropriate use. Although these are useful structural indicators that demonstrate the spread of the concept of essential medicines, the existence of a policy or a list does not in itself guarantee affordable access to quality-assured essential medicines. Neither does it necessarily result in quality use. Furthermore, with reductions in public budgets, some countries have not regularly updated their essential medicines policies or lists, leaving large gaps between policy development and implementation.
Of note, hospitals and health-care organisations in high-income settings also use restricted lists of medicines, or formularies, effectively applying similar principles to determine how to allocate resources.58 Despite this, a general impression developed in the first era that essential medicines were only for LMICs, and that the WHO Model List of Essential Medicines presented a minimum set of medicines relevant only for the most resource-constrained settings. However, this impression is mistaken as this group includes many HICs as well (Figure 1, Figure 2 ). In part, this impression arose from the focus of WHO's guidance on the development of national medicines policies in countries with medicines supply systems dominated by the public sector.
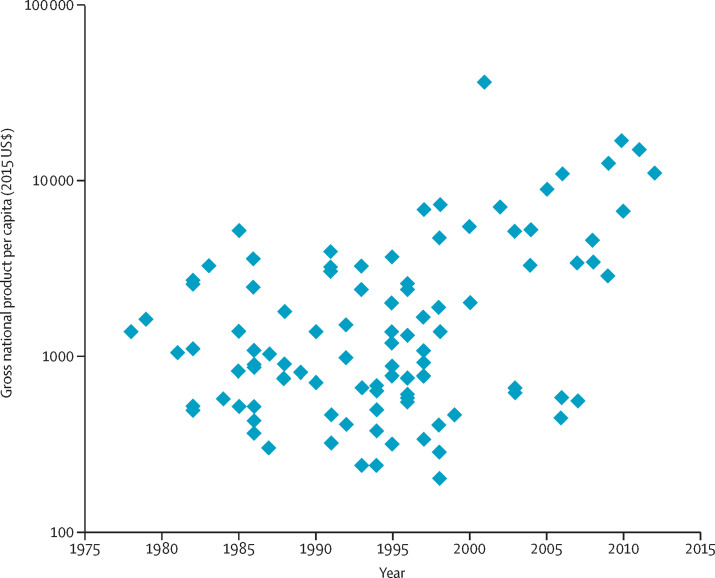
Figure 1.
GDP per capita in the year of a country's first national list of essential medicines
A systematic search of national essential medicines lists was done using the following data repositories: the Documentation Centre of the WHO Department of Essential Medicines and Health Products in Geneva; WHO National Pharmaceutical Profiles of 1997, 2003, 2007, and 2011; literature searches, searches using Google and websites of essential medicine programmes; and a specific call through the E-DRUG listserv. In case of contradictory information, especially with regard to date of publication, the original document was identified and studied when possible. The dataset includes 101 countries for which at least one national essential medicines list could be identified. Excluded were institutional, regional, and national reimbursement lists. For each country, the year of the first national essential medicines list and the GDP per capita in that year, according to the World Bank, were identified. This figure shows the national GDP (expressed in 2015 US$) in the year of publication of the first national essential medicines list. Every dot represents one country with a first national essential medicines list. GDP=gross domestic product.
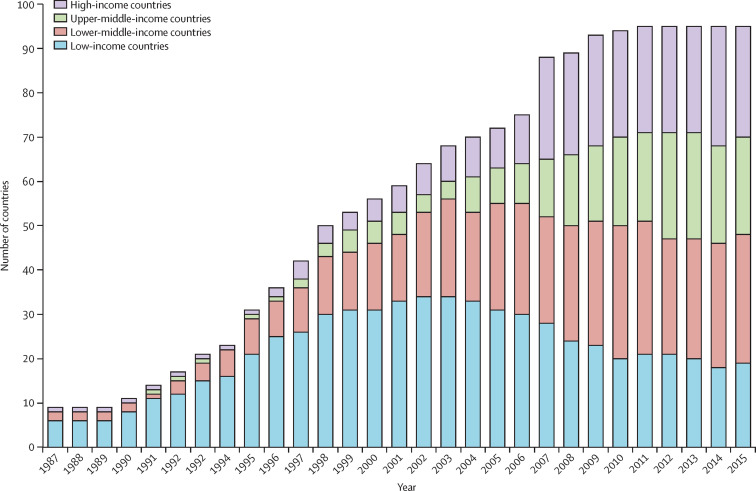
Figure 2.
Number of countries with a first NMP in different economic categories per year
A systematic search of all national medicines policies published was done using the following data repositories: the Documentation Centre of the WHO Department of Essential Medicines and Health Products in Geneva; information from the WHO National Pharmaceutical Profiles of 1997, 2003, 2007, and 2011; literature searches, searches using Google and websites of essential medicine programmes; and a specific call through the E-DRUG listserv. In case of contradictory information, especially with regard to status or date of publication, the original document was identified and studied when possible. The dataset includes 95 countries for which at least one official NMP could be identified. Excluded were draft medicine policy documents and policy documents with unclear status. For each country, the year of the first official NMP and the level of economic development (low, lower-middle, upper-middle, and high) according to the World Bank classification in each year, were identified. While the total number of countries with a first NMP increases over time, the number of countries within an economic category can decrease when a country moves to another category. NMP=national medicine policy.
The second era: expanding access to essential medicines through global programmes (1990s to 2010s)
The second era for essential medicines policies began in the late 1990s with the global moral outrage over the toll of the AIDS epidemic. At the era's outset, effective medicines for the treatment of HIV existed but were unaffordable and unavailable to most people living with the virus. The exceptions were the most privileged people living in HICs. AIDS activists successfully argued, with their allies worldwide, that deaths caused by lack of access to extant medicines, merely because of high prices, were unconscionable. In 2001, the UN's Secretary-General's call to establish the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM), including the provision of medicines, was a landmark event.59
Human rights principles reinforced the arguments used, first by AIDS activists and then by political leaders, to support greater access to treatment for AIDS and to raise awareness about global disparities in access to essential medicines. The concept of a human right to health was first articulated in 1946,60 but it was not until 2002 that the UN appointed the first UN Special Rapporteur on “the right of everyone to the enjoyment of the highest attainable standard of physical and mental health”.61 Subsequently, access to essential medicines was highlighted and elaborated as a concrete element of the right to health.62
To make the process of selecting essential medicines for the WHO Model List more evidence-informed and transparent, major changes were introduced in 2002,63 coinciding with calls by activists and advocacy groups such as Médecins Sans Frontières.64 WHO changed its definition of essential medicines from those “of utmost importance, and are basic, indispensable and necessary for the health and needs of the population”37 to “those that satisfy the priority health care needs of the population”, adding a clarification that “[t]hey are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness.”65, 66 The key change was the new emphasis on cost-effectiveness; high cost of a medicine no longer automatically excluded it from the Model List. With these changes, twelve widely patented antiretroviral medicines, then priced up to US$10 000 per patient per year, were classified as essential in the Model List of 2002.67
This change bolstered the moral argument for policy to progressively realise access to treatment. Previously, affordability had been one condition of selection; selection now became part of the pressure to ensure affordability. In the following decade, due to the concerted global action by patient and activist groups, donor governments, WHO, and generics manufacturers, and the absence of product patents for medicines in India and elsewhere, the cost of antiretroviral medicines fell to less than $100 per person per year, enabling millions of patients to gain access to life-saving treatment.
The second era was also influenced by growing policy attention to health systems, in which medicines are a key component.68 Health systems face profoundly difficult decisions in relation to the allocation of scarce resources.69 New initiatives, such as the Good Governance in Medicines Programme and the Medicines Transparency Alliance, promoted the linking of essential medicines initiatives with other health system components.
The second era also coincided with the setting of the Millennium Development Goals (MDGs). These goals emphasised reducing mortality due to AIDS, tuberculosis, and malaria, and promoting newborn baby, child, and maternal health. Essential medicines were an integral component of interventions to achieve the MDG targets by 2015.70 Reduced mortality from pneumonia, diarrhoea, and measles were responsible for half of the 3·6 million fewer deaths under five years recorded between 2000 and 2013.71 Also by 2013, about 13 million persons living with HIV were receiving life-saving antiretrovirals. Global malaria mortality fell by 42% between 2000 and 2012, with 3·3 million deaths averted because, in part, of increased access to antimalarial medicines.70
Global disease programmes instituted a range of mechanisms to address pricing, selection, quality assurance, and cost-effective procurement of medicines. However, these interventions were rarely identified as essential medicines policies, although the concept supported this public health approach. The GFATM required that only quality assured generic antiretroviral medicines were procured, when possible.72 Pooled procurement of such products was used to exert downward pressure on prices. Additionally, the GFATM required public reporting of procurement prices.73 These policies contributed to improved transparency and set an important precedent for other major donors and procurement programmes.
The second era also generated coordinated advocacy for the development of new essential medicines. In 2004, WHO presented a global survey of therapeutic areas that lacked essential treatments, creating the concept of missing essential medicines and calling for a public health approach to innovation.74 A key group of missing essential medicines were those for children. In parallel with American and European regulatory efforts, the 2007 World Health Assembly Resolution (WHA60.20) on Better Medicines for Children highlighted the need for paediatric dose forms of many essential medicines.75 In response, WHO published the first Model List of Essential Medicines for Children in 2007.76
Although important successes were achieved in relation to the MDGs, the general target related to access to medicines (MDG 8.E: “In cooperation with pharmaceutical companies, provide access to affordable essential drugs in developing countries”) proved difficult to measure with any certainty. A report by the UN pointed out what is obvious to health workers and patients around the world: many poor households remain unable to obtain needed medicines, either because of poor availability, or poor affordability, or both.77 The controversies around essential medicines that characterised the first era continued throughout the second era, and many persist into the current period. For example, in 2005, delegates of the pharmaceutical industry to the UN Millennium Project Task Force refused to sign the assessment report and opted for a statement of dissent.78
However, the second era also brought concerted efforts to mobilise all stakeholders, including the pharmaceutical industry, to work towards improving health by securing access to affordable and quality assured medicines. In 2008, the Human Rights Guidelines for Pharmaceutical Companies in Relation to Access to Medicines were published,79 followed in 2011 by the UN Guiding Principles on Business and Human Rights.80 In the past decade, more than 300 health partnerships in LMICs have been reported wherein the pharmaceutical industry, alone or in collaboration with other stakeholders, is investing to improve health and development.81 Some progress of these initiatives is documented by the Access To Medicines (ATM) Index.82 Rigorous evaluation of the effect of these initiatives remains a key challenge.
Finally, mobilising all implicated stakeholders meant a larger role for other organisations and a declining role for WHO in providing stewardship to global policy making for medicines access. WHO has been confronted by continuing problems of gross underfunding, including of its essential medicines programme.83 Global governance of health and medicines came to involve an expanded set of stakeholders, such as private foundations (eg, the Bill & Melinda Gates Foundation) and public–private initiatives (eg, the Global Vaccine Alliance [GAVI]), and increasingly occurred outside WHO. Plurilateral initiatives, such as the International Council for Harmonisation (ICH), also began expanding their constituencies.84
The third era: UHC demands essential medicines (2010 to present)
The third era for essential medicines policies has been driven by changes in disease burdens and marked by transformations in health systems, in particular the push for UHC.85 The 2010 World Health Report acknowledged that medicines are at the centre of health care.3 However, many countries that have committed to UHC are struggling to fulfil their vision, since medicines represent a substantial proportion of total expenditure on health.86 Every health system is under pressure to increase and maintain appropriate pharmaceutical benefits coverage, while also balancing quality of care, efficiency in spending, and reducing out-of-pocket expenditure.87, 88, 89 Moving towards UHC triggers fundamental changes in how medicines are financed, seeking to shift away from individual out-of-pocket payment and favouring prepayment and pooled financing mechanisms.
The Commission argues that effective policies for essential medicines are central to the financial sustainability of UHC. Policies to support essential medicines must support increasingly comprehensive health services, delivered through pluralistic systems that include both the public and private sectors, and which effectively mobilise and engage with civil society.90
Demographic and epidemiological transitions that accompany social and economic development—namely the ageing of populations and an emerging focus on NCDs—generate major challenges for essential medicines policies. Public financing and provision of essential medicines in the first and second eras traditionally gave priority to medicines to treat or prevent communicable diseases.91 The 2011 UN High Level Meeting on NCDs recognised that addressing non-communicable conditions is necessary in all countries.92 The WHO Global NCD Action Plan 2013–2020 set, as one of its targets, “80% availability of the affordable basic technologies and essential medicines, including generics, required to treat major NCDs in both public and private facilities.”93 All countries need to adopt and implement policies that ensure equitable access to affordable essential medicines.
Many contextual changes affect the evolution of essential medicine policies, including increasingly interdependent pharmaceutical markets, priority setting that is increasingly informed by economic evaluation of new health technologies, faster exchange of large amounts of health-care data (including pharmaceutical usage data), and global regulation of trade (particularly intellectual property rules) that influences competition and the prices of new essential medicines. Climate change and human mobility are reshaping the spread of diseases, as demonstrated by severe acute respiratory syndrome94 and Zika virus.95 Emerging communicable diseases might affect all countries, but the resources to address them vary considerably.
Finally, advances in the development of new medicines include an increase in targeted therapies, particularly for oncology. Of the 225 new molecular entities expected to come to market between 2016 and 2020, most will be cancer medicines and 90% of those will be targeted medicines.96 Targeted therapies, which are effective for a small subgroup of the population only, require sophisticated diagnostic tests97 for which the infrastructure and financial and human resources are often scarce. Many health systems also struggle with trade-offs between investing in targeted therapies for small subgroups and providing treatment for larger population groups.
In 2015, WHO added several high-priced new medicines—for the treatment of hepatitis C, tuberculosis, and cancers—to the Model List of Essential Medicines.20 This move reflects the importance of these products to health systems' ability to meet their populations' needs. It also underscores the need for essential medicines policies in all countries of all income levels, as they confront the limits of their budgetary capacities. Ethical principles, human rights obligations, and the necessary policies, institutions, and stakeholder engagements can contribute to addressing these challenges effectively.
Setting essential medicines goals that promote strong health systems
The Commission recognises that the development of essential medicines policies is difficult, and that effectively implementing them poses substantial challenges to all health systems. While there is much room for improvement in essential medicines policies in countries around the world, there are also many opportunities to implement a range of proven effective strategies, as well as testing innovations.
As emphasised throughout this report, essential medicines are a key component of health systems. The Commission asserts that improving access to quality-assured essential medicines is not an end in itself; rather it is a means to improving the performance and equity of health systems.98
Various frameworks exist for the analysis of health systems,99 including some specifically developed to locate medicines and pharmaceutical policy within health systems.100, 101, 102 Each framework emphasises different aspects of medicines' place within health systems; all show that essential medicines have a key impact at the health system level, with efficiency, quality, and access as intermediate outcomes. Furthermore, essential medicines are indispensable to achieving the ultimate health system goals: improved health status, system responsiveness, and financial protection. The Commission has not selected a single framework to analyse the five main challenges, although several are referred to throughout the report. Instead, each section presents areas of opportunity to strengthen health systems and improve access to essential medicines, identifying policy levers98 such as financing, organisation, regulation, and persuasion, among others.
Each section also describes three cross-cutting themes that are linked to core health system goals and functions, namely: increasing equity, strengthening institutions, and promoting accountability. All policies and implementation efforts must emphasise increasing equity. Strengthening institutions is required to implement and evaluate essential medicines policies. Promoting accountability requires concrete efforts to generate information, increase transparency, and foster the involvement of civil society in decision making about essential medicines selection, quality assurance, improving use, and priority setting for R&D. Accountability also requires independent review of data, and systems for corrective action.
Improving health system performance requires three types of analysis: technical, ethical, and political.98 The implementation of effective essential medicines policies requires understanding the political economy of policy development, implementation, and evaluation. This report suggests concrete steps for countries and health systems to take, while also considering the wider political context.98
Finally, the sixth section introduces an accountability framework comprised of a set of indicators that, when combined, address the five specific challenges and three cross-cutting concerns for advancing essential medicines policies worldwide.
Limitations of the Commission's work
Despite the breadth of the Commission's approach to essential medicines, other relevant issues could not be fully addressed in this report. These include supply chain management, from sourcing raw materials to delivery of final products to consumers; the role of local production of pharmaceuticals; the problem of falsified medicines; promoting adherence; and prevention of medication errors. Since other global efforts address these issues, the Commission decided not to analyse them in depth. However, this decision should not be taken to mean that they deserve less attention, or that all possible remedial actions are already in place. Particularly, achieving UHC will require substantial investment in strengthening supply chains for all health commodities, including essential medicines. An effective, integrated supply chain for medicines will demand good data visibility, a willingness to learn from and leverage the private sector, strong national stewardship, a committed and supported workforce, a focus on continuous improvement, and proactive risk management. Finally, and more generally, the Commission primarily approached essential medicines policies from the policy and academic viewpoints of independent experts on the basis of analysis of the best available evidence. The Commission did not seek to represent the possible viewpoints of all stakeholders.
Conclusion
The Commission firmly believes that incorporating strong and strategic essential medicines policies can enable countries, health systems, and global institutions to take major strides towards achieving the highest attainable standard of health and UHC as part of sustainable development for all. The findings presented in this report seek to renew global debate about effective essential medicines policies, and how to implement them, to advance global welfare in the 21st century.
Section 1: paying for a basket of essential medicines
A patient's experience
Priti, aged 41 years, has been treated for asthma since childhood. Her family does not have health insurance and uses the public hospital, which does not charge for outpatient consultations or medicines. However, when she presents a prescription for a new inhaler, she is told that the hospital has no stock. The pharmacist tells her that stock-outs happen frequently at this time of the year, since the hospital's annual medicine budget from the government is exhausted. She is advised to buy the inhaler from a private pharmacy instead. However, because her family does not have enough money to buy the medicine from the local pharmacy, Priti decides instead to wait until the hospital's stocks are replenished. Within a few days, however, she suffers a major asthma attack and has to be admitted to hospital. Her family must borrow money to pay the in-patient hospital fees.
Introduction
Financing encompasses how funds are raised (by whom and from whom) and how resources are allocated. Health financing is provided by governments (from fiscal revenues), prepaid insurance plans (in the form of employer and employee contributions, or as subscriptions), and as out-of-pocket expenditure by patients and their families at the point of care (either as user fees to pay for services or to cover purchases such as medicines). Donations are also used to finance medicines and other commodities, but represent short-term strategies to address resource gaps locally and are typically used as temporary support in emergencies or in low-resource settings.
A central aspiration of UHC is to protect households from catastrophic health expenditures.98 UHC aims to provide financial risk protection by increasing prepaid coverage, whether from the fiscus or from health insurance funds, thus decreasing reliance on out-of-pocket expenditure.103 The extent to which prepaid benefits include pharmaceutical expenditure is a crucial measure of the adequacy of the benefit package offered under UHC.15 Likewise, the extent to which a health system delivers sufficient quantities of essential medicines is determined largely by its financing capacity, implementation capacity, and system efficiency.
Disease-specific demand forecasts have been developed to solicit funding for priority areas such as HIV,104 tuberculosis,105 and malaria.106 However, evidence-based estimates of how much it would cost to pay for the basket of all essential medicines needed in LMICs are missing, making it difficult to assess the use of resources and effectively advocate for adequate funds.
This section presents the first estimate of the total cost of providing a basket of essential medicines for primary and secondary level care to the entire populations of LMICs. The model developed by the Commission is described, along with the resulting estimates. The estimates are contextualised by providing an overview of pharmaceutical expenditures by households, governments, and donors at country level. Finally, actionable recommendations are made to ensure adequate financing of the basket of essential medicines in all countries.
Other topics related to financing essential medicines are discussed in other sections, namely pricing and affordability to payers (section 2), payment of providers to improve the use of essential medicines (section 4), and financing for R&D of medicines (section 5). Strategies that countries can use to raise funds to finance the basket of essential medicines are beyond the scope of this report, but are described elsewhere.103
A model to estimate the cost of paying for a basket of essential medicines
A new model was developed by the Commission for this report to estimate the cost of providing a basket of essential medicines to the populations of LMICs to treat priority diseases at primary and secondary care levels. The estimates are based on disease prevalence, current or projected consumption of medicines, or both, adherence to treatment guidelines, and medicine prices (including procurement, supply chain, and quality assurance costs). The estimates comprise the overall envelope of financing needed to provide universal access to a basic package of essential medicines in LMICs, not the marginal increase over existing expenditure.
These new cost estimates can be used to inform the development of financing strategies and the setting of minimum targets for resource mobilisation as countries implement UHC. One innovation of the model is that it includes a large number of medicines and multiple diseases. Previous costing exercises have covered smaller sets of medicines, focusing on a single therapeutic group107 or disease group.108, 109
More detailed costing at the national level is still necessary, incorporating detailed national data, such as local caseloads, prices, and treatment guidelines. The method presented here could be adapted for use by national governments and organisations. The ideal data source is high-quality, systematically collected information on pharmaceutical utilisation. If data are available, the model could then enable a country to develop estimates of minimum future financing needs for essential medicines. This model also provides an example of how alternative data sources can be used to estimate basic needs for essential medicines when historical local consumption data are not accurate enough.
The parameters of the model
The model includes 201 molecules in 378 unique dose forms and strengths. The list of medicines used in the modelling exercise is presented in appendix 1.1; all are essential medicines that can be administered in health systems with restricted resources and without specialised care. The included medicines are mainly those listed as core in the 2015 WHO Model List of Essential Medicines20 and categorised for use at primary and secondary care levels.
A few additional medicines relevant to LMIC settings were added on the basis of Commissioners' knowledge of the lists of essential medicines in Iran and South Africa. Medicines used only in tertiary care settings were excluded. Because of data limitations, some medicines for the treatment of cancer and for advanced cardiovascular care were also excluded. Similarly, any medicine for which no prevalence or demand data were available, or which was not used in either of the settings used to estimate consumption (namely KwaZulu-Natal and Denmark, as explained below), was excluded from the analysis. A similar approach has been used in other modelling exercises, for example in distinguishing between basic, limited, enhanced, and maximal provision for packages of medicines and care for patients with breast cancer.110
Detailed explanations of the methods used in the modelling exercise are provided in appendices 1.2–1.4. In brief, three methods were used for estimating the quantity of each medicine that is required each year in all LMICs. First, for medicines with a single indication, data on global burden of disease were used to project demand. These data were obtained from the Global Burden of Disease project or from the scientific literature; and were then scaled by an estimate of how many patients with a given condition would receive treatment (known as treatment coverage; appendix 1.4). Additionally, standard treatment guidelines and findings from the literature were used to model how many people on treatment for a condition would receive each medicine (known as medicine coverage; appendix 1.4).
Second, existing demand forecasts were used whenever they were available (such as for HIV, malaria, and to some extent tuberculosis). These forecasts (or in some cases treatment scale-up plans) were developed at specialised agencies, such as the Clinton Health Access Initiative,104 AIDS Medicines and Diagnostics Service,111, 112 UNITAID's ACT forecasting project,106 the Reproductive Health Supplies Coalition,113 and the Stop TB Partnership.105 In most cases, the forecasts include different scenarios of treatment, diagnosis, and other constraints in the cascade of care provision.
Finally, all other estimates (particularly for essential medicines with more than one indication) were based on pharmaceutical consumption data. The ideal data source for demand estimates is high-quality local measures of pharmaceutical consumption under circumstances of good adherence to diagnostic and treatment guidelines. In the case of this model, these data came from Denmark and KwaZulu-Natal province in South Africa. These locations were chosen because both Denmark114 and KwaZulu-Natal115 have implemented policies to promote efficient and safe use of medicines. Therefore, pharmaceutical consumption in their health systems reflects service provision scenarios that would be reasonable for other countries to emulate. The selection of these two locations does not suggest either that their patterns of consumption are representative of other LMICs or that these are absolute ideals; rather, they were selected to represent a reasonably attainable level of consumption that could be applicable to other countries. The Danish dataset covers both public and private sectors, reflecting use by the entire population. The KwaZulu-Natal dataset covers medicines supplied in the public sector, assumed to service most of the population in the province.
The model was run under different scenarios. Scenario 1 incorporated consumption data from Denmark, and Scenario 2 incorporated data from KwaZulu-Natal. As neither Denmark nor KwaZulu-Natal were considered to be fully representative of all LMIC settings, two additional scenarios were tested to assess the robustness of the model results. In Scenarios 3 and 4, on the basis of Scenarios 1 and 2 respectively, consumption parameters for medicines that address diseases with the highest global burden were substituted with data from middle-income countries (MICs) provided by IMS Health. All other inputs were held constant.
Medicine prices were obtained primarily from the International Drug Price Indicator Guide, using median supplier prices whenever possible. Supplemental data on public sector prices were sourced from KwaZulu-Natal and Iran. All prices were converted to US dollars on the basis of the average yearly exchange rates for 2014. The unit prices were then subject to proportional mark-ups to represent additional quality assurance and supply chain costs derived from the literature.116, 117, 118
Sensitivity analyses were done as follows: changing the unit price input data (from the median price to either the highest or lowest price listed in the International Drug Price Indicator Guide) and the price mark-ups for quality assurance and supply chain costs; switching from the midpoint to the limits of demand forecasts' published ranges; and, for prevalence data, using the limits of the confidence intervals, lower and higher forecast ranges, and treatment and medicine coverage estimates when available.
The modelled estimates are based on Global Burden of Diseases 2013 data and do not account either for future epidemiological changes or for successful prevention measures that might change disease burdens. The model only includes direct medicine-related costs, although the Commission recognises that diagnostic tests, other consumables, and wider health system costs are required for delivery of medicines. Importantly, the model does not distinguish between adults and children, which could have resulted in over-estimating needs related to certain diseases (such as diabetes). However, it enabled the model to rely on defined daily doses, which are based on the most common dose for the main indication of a medicine in adults.
Finally, this is a static model that does not account for relationships between supply and demand, such as how increased use might affect prices. Dynamic models are far more complex and would probably require larger datasets. Given the enormous gaps in data availability in the pharmaceutical sector, the number of assumptions in a dynamic model would also have to increase. Construction of such a dynamic model was beyond the scope of this particular analysis, which can be seen as a starting point for future estimates of essential medicine costs in individual LMICs.
Results: the cost of providing a basket of essential medicines to LMIC populations
Using this new model, the Commission estimates the current cost of providing a basket of essential medicines to the total populations of LMICs to be between $77·4 and $151·9 billion per year. The higher estimate (from Scenario 1) is based on past consumption observed in Denmark, and equates to $25·4 per capita per year. The lower estimate (from Scenario 2) of $12·9 per capita per year is based on past consumption observed in KwaZulu-Natal, South Africa.
With the inclusion of the additional information on medicine use in MICs from IMS Health, the overall estimate under Scenario 3 (based on Danish consumption) is $134·1 billion, or $22·4 per capita per year. Under Scenario 4 (based on KwaZulu-Natal consumption), the estimated cost is $97·3 billion, or $16·3 per capita per year. Table 1 shows the results for the full package of medicines under each of these scenarios, as well as for subsets of medicines by clinical area.
Table 1.
Estimated price tag to provide a package of essential medicines in low-income and middle-income countries under four sets of assumptions (scenarios) about levels of consumption
| Scenario 1 (Denmark) | Scenario 2 (KwaZulu-Natal) | Scenario 3 (Denmark + IMS) | Scenario 4 (KwaZulu-Natal + IMS) | ||
|---|---|---|---|---|---|
| Full package of medicines | $151·9 billion | $77·4 billion | $134·1 billion | $97·3 billion | |
| Per capita per year in low-income and middle-income countries | $25·4 | $12·9 | $22·4 | $16·3 | |
| Medicines | |||||
| Antiretroviral for HIV or AIDS* (adult) | $4·9 billion | $4·9 billion | .. | ||
| For tuberculosis* | $0·4 billion | $0·4 billion | .. | ||
| For malaria* | $1·2 billion | $1·2 billion | .. | ||
| For diabetes* | $12·5 billion | $12·5 billion | .. | ||
| For cardiovascular conditions | $44·0 billion | $9·2 billion | .. | ||
| Antimicrobials | $15·6 billion | $15·5 billion | .. | ||
| For respiratory conditions (asthma, chronic obstructive pulmonary disease) | $11·7 billion | $4·9 billion | .. | ||
Data are US$. IMS=IMS Health.
No difference in the result from Scenarios 1 and 2 because all quantities are estimated by demand scenarios and so are unchanged.
Table 2 shows the results of the sensitivity analyses. Overall, the modelled results were fairly robust and not strongly influenced by changes in the data inputs, except in relation to medicine prices. Changing from the median price to the lowest listed price in the International Drug Price Indicator Guide changed the per capita estimate to $17·0 per capita, whereas changing to the highest listed price changed the estimate to $32·7 per capita in Scenario 1. The corresponding estimates in Scenario 2 are $8·8 when changing from median price to lowest listed price and $16·1 per capita when changing to highest listed price.
Table 2.
Results of sensitivity analyses, altering input parameters
|
Scenario 1 (Denmark) |
Scenario 2 (KwaZulu-Natal) |
||||
|---|---|---|---|---|---|
| Lower limit | Upper limit | Lower limit | Upper limit | ||
| Baseline | |||||
| Estimate | $151·9 billion | $151·9 billion | $77·4 billion | $77·4 billion | |
| Per capita per year | $25·4 | $25·4 | $12·9 | $12·9 | |
| Price | |||||
| Switch to lowest or highest IDPIG price* | $101·5 billion | $195·7 billion | $52·4 billion | $96·5 billion | |
| Per capita per year | $17·0 | $32·7 | $8·8 | $16·1 | |
| Change assumptions about mark-ups* | $151·5 billion | $157·5 billion | $77·2 billion | $80·2 billion | |
| Per capita per year | $25·3 | $26·3 | $12·9 | $13·4 | |
| Demand forecasts | |||||
| Use limits of confidence intervals provided by these models | $151·7 billion | $152·3 billion | $77·2 billion | $77·7 billion | |
| Per capita per year | $25·4 | $25·5 | $12·9 | $13·0 | |
| Prevalence data | |||||
| Use limits of confidence intervals for prevalence estimates | $148·2 billion | $155·7 billion | $75·4 billion | $79·3 billion | |
| Per capita per year | $24·8 | $26·0 | $12·6 | $13·3 | |
| Use 50% as lower bound of treatment coverage assumption | $143·9 billion | NA | $70·6 billion | NA | |
| Per capita per year | $24·0 | $24·0 | $11·8 | $11·8 | |
| Use limits of medicine coverage assumption | $150·1 billion | $152·9 billion | $75·6 billion | $78·1 billion | |
| Per capita per year | $25·1 | $25·6 | $12·6 | $13·0 | |
Data are US$. IDPIG=International Drug Price Indicator Guide. NA=not applicable.
For those medicines that had used IDPIG supplier median price for main scenarios.
Comparison of this model with others
Previous estimates of the cost of providing essential medicines have generally focused on medicines for a specific subgroup of patients. For example, a 2011 WHO report estimated that scaling up combination therapy for people with heart disease would cost approximately $70 per person with heart disease in LICs, $85 per person in lower-MICs, and $108 per person in upper-MICs.108
For the management of HIV, the Commission's model for the estimated total cost of antiretrovirals in LMICs is approximately $5 billion annually, far greater than approximately $1 billion, which was reported to have been procured annually by the Global Fund to Fight AIDS, Tuberculosis and Malaria and the President's Emergency Plan for AIDS Relief (PEPFAR).119 However, the combined Global Fund and PEPFAR amount does not include medicines that are funded by countries themselves, nor does it necessarily include distribution costs. The Commission's estimate is comparable, however, with the Clinton Health Access Initiative estimate that meeting the “90–90–90” targets in LMICs would cost $3·8 billion in purchasing costs for antiretrovirals.104
Likewise, for tuberculosis the Commission's modelled estimate for the total cost of medicines is approximately $760 million, with $440 million for first-line treatments and $320 million for second-line treatments. According to UNITAID's 2014 TB Medicines Landscape report, the total value of the global tuberculosis medicines market (combining the public and private sectors) was approximately $700 million, including up to $425 million for first-line treatment in adults and $300 million for treatment of multidrug-resistant tuberculosis in adults.120
The similarities among the Commission's model results and other existing estimates of medicine costs for global treatment of HIV and tuberculosis provide some corroboration of the Commission's estimates.
Assessment of the modelled estimates in the context of pharmaceutical expenditure
The Commission's estimate of between $77·4 and $152·0 billion per year for the total cost of providing a basket of essential medicines for the populations of LMICs needs to be assessed in the context of pharmaceutical expenditure. Total global pharmaceutical sales are projected to reach $1·4 trillion in 2020.121 According to the available data on per capita pharmaceutical expenditure per country by income group reported in 2010,122 mean per capita pharmaceutical expenditure was $8·8 in LICs, $36·9 in lower-MICs, $106·2 in upper-MICs, and $460·1 in HICs (table 3 ).
Table 3.
Mean per capita TPE and THE per country by income group in 2010
|
Mean per capita TPE* |
Mean per capita THE |
|||||||
|---|---|---|---|---|---|---|---|---|
| Public expenditure ($) | Private expenditure ($) | Total $ | Public expenditure ($) | Private expenditure ($) | Total $ | % TPE of THE | ||
| High (n=49) | $279·2 (60·9%) | $179·2 (39·1%) | $458·4 | $2203·0 (73·1%) | $811·7 (26·9%) | $3014·7 | 15·2% | |
| Upper-middle (n=53) | $39·6 (37·2%) | $66·7 (62·8%) | $106·3 | $334·4 (63·9%) | $189·0 (36·1%) | $523·4 | 20·3% | |
| Lower-middle (n=48) | $11·9 (32·4%) | $24·8 (67·6%) | $36·7 | $88·9 (60·1%) | $59·1 (39·9%) | $148·0 | 24·8% | |
| Low (n=32) | $2·0 (22·7%) | $6·8 (77·3%) | $8·8 | $13·9 (40·1%) | $20·8 (59·9%) | $34·7 | 25·4% | |
| Total (n=182) | $90·2 (54·3%) | $75·8 (45·7%) | $166·0 | $716·4 (71·0%) | $292·8 (29·0%) | $1009·2 | 16·4% | |
Data are US$, percentages are in parentheses. TPE= total pharmaceutical expenditure. THE=total health expenditure.
Essential medicines expenditure is a subcomponent of TPE. The data source does not allow differentiation between expenditure on essential and non-essential medicines. Data from National Health Accounts.
In 2010, 28 of 31 LICs and 13 of 47 lower-MICs spent less on pharmaceuticals than the model estimate (figure 3 ). All upper-MICs and HICs spent far more than either the $13 or the $25 per capita estimates.
Figure 3.
Relationship between per capita pharmaceutical expenditure and per capita health expenditure in 2010
Countries are indicated with the ISO Alpha-3 code.*Minimum threshold based on costing model (see section 1). Low-income countries (A). Lower-middle-income countries. †Countries at or below the threshold were: BTN, LAO, GHA, SLV, PNG, STP, SEN, SLB, SDN, TLS, VUT, ZMB (B). Upper-middle-income countries (C). High-income countries (D). TPE=total pharmaceutical expenditure. BTN=Bhutan. LAO=Laos. GHA=Ghana. SLV=El Salvador. PNG=Papua New Guinea. STP=São Tomé and Príncipe. SEN=Senegal. SLB=Solomon Islands. SDN=Sudan. TLS=Timor-Leste. VUT=Vanuatu. ZMB=Zambia. AFG=Afghanistan. AGO=Angola. ALB=Albania. AND=Andorra. ARE=United Arab Emirates. ARG=Argentina. ARM=Armenia. ATG=Antigua and Barbuda. AUS=Australia. AUT=Austria. AZE=Azerbaijan. BDI=Burundi. BEL=Belgium. BEN=Benin. BFA=Burkina Faso. BGD=Bangladesh. BGR=Bulgaria. BHR=Bahrain. BHS=The Bahamas. BIH=Bosnia and Herzegovina. BLR=Belarus. BLZ=Belize. BRA=Brazil. BRB=Barbados. BRN=Brunei. BWA=Botswana. CAF=Central African Republic. CAN=Canada. CHE=Switzerland. CHL=Chile. CHN=China. CMR=Cameroon. COD=Democratic Republic of the Congo. COK=Cook Islands. COL=Colombia. COM=Comoros. CPV=Cape Verde. CRI=Costa Rica. CYP=Cyprus. CZE=Czech Republic. DEU=Germany. DJI=Djibouti. DMA=Dominica. DNK=Denmark. DOM=Dominican Republic. DZA=Algeria. ECU=Ecuador. EGY=Egypt. ERI=Eritrea. ESP=Spain. EST=Estonia. ETH=Ethiopia. FIN=Finland. FJI=Fiji. FRA=France. FSM=Federated States of Micronesia. GAB=Gabon. GBR=Great Britain and Northern Ireland. GEO=Georgia. GIN=Guinea. GMB=The Gambia. GNQ=Equatorial Guinea. GRC=Greece. GRD=Grenada. GTM=Guatemala. GUY=Guyana. HND=Honduras. HRV=Croatia. HTI=Haiti. HUN=Hungary. IND=India. IRL=Ireland. IRQ=Iraq. IRN=Iran. ISL=Iceland. ISR=Israel. ITA=Italy. JAM=Jamaica. JOR=Jordan. JPN=Japan. KAZ=Kazakhstan. KEN=Kenya. KGZ=Kyrgyzstan. KHM=Cambodia. KIR=Kiribati. KNA=Saint Kitts and Nevis. KOR=South Korea. KWT=Kuwait. LBR=Liberia. LBN=Lebanon. LCA=Saint Lucia. LSO=Lesotho. LTU=Lithuania. LUX=Luxembourg. LVA=Latvia. MAR=Morocco. MDA=Moldova. MDG=Madagascar. MDV=Maldives. MEX=Mexico. MHL=Marshall Islands. MKD=Macedonia. MLI=Mali. MLT=Malta. MMR=Myanmar. MNE=Montenegro. MOZ=Mozambique. MRT=Mauritania. MWI=Malawi. MUS= Mauritius. MYS=Malaysia. NAM=Namibia. NER=Niger. NIC=Nicaragua. NLD=Netherlands. NOR=Norway. NPL=Nepal. NRU=Nauru. NZL=New Zealand. OMN=Oman. PAN=Panama. PER=Peru. PHL=Philippines. PLW=Palau. POL=Poland. PRT=Portugal. PRY=Paraguay. QAT=Qatar. ROU=Romania. RUS=Russian. RWA=Rwanda. SAU=Saudi Arabia. SGP=Singapore. SLE=Sierra Leone. SMR=San Marino. SRB=Serbia. SUR=Suriname. SVK=Slovakia. SVN=Slovenia. SWE=Sweden. SWZ=Swaziland. SYC=Seychelles. SYR=Syria. TCD=Chad. TGO=Togo. THA=Thailand. TJK=Tajikistan. TON=Tonga. TTO=Trinidad and Tobago. TUN=Tunisia. TUR=Turkey. TUV=Tuvalu. TZA=Tanzania. UGA=Uganda. UKR=Ukraine. URY=Uruguay. USA=United States of America. VCT=Saint Vincent and the Grenadines. VEN=Venezuela. VNM=Vietnam. WSM=Samoa. ZAF=South Africa.
The OECD reported that total medicines expenditure was $295 per capita in Denmark in 2009.123 This cost is considerably higher than the $25 estimated in Scenario 1, as the model only included a selected set of medicines and applied prices that were likely to be lower than those actually paid in Denmark. Similarly, in KwaZulu-Natal in 2014, the total public sector expenditure on medicines amounted to $28 per capita, which is more than double the $13 estimate from Scenario 2. Here again, the basket of medicines used in Scenario 2 represented only a subset of the medicines actually procured in KwaZulu-Natal, and the global reference prices used in the model may have been lower than those actually paid.
Pharmaceutical expenditure represents a substantial proportion of total health expenditure. According to the Commission analysis using National Health Account data reported in 2010, about 1 in 4 health dollars is spent on medicines in LICs and lower-MICs, and 1 in 5 health dollars is spent on medicines in upper-MICs. Pharmaceutical expenditure ranges from just more than 15% in HICs to 25% in lower-MICs and LICs. By this measure, LMICs pay proportionally more on pharmaceuticals per capita than HICs.
Sources of financing for medicines also vary. National Health Account data show that public expenditure represented most (61%) of the pharmaceutical spending in HICs, while the situation was reversed in LMICs, where more than 62% of pharmaceutical expenditure was in the private sector. In the absence of universal access to health insurance, this implies significantly more out-of-pocket expenditure in LMICs.
The proportion of resources available at national level that is spent on health and pharmaceuticals can indicate a country's potential to increase allocations to pharmaceuticals. Globally, countries spent 7·1% of gross domestic product (GDP) on health in 2010 (appendix 1.5), including 1·5% of GDP on pharmaceuticals. Less than half was expended from public financing sources. The proportion of GDP spent on pharmaceuticals across country income groups was considerably varied. A lower proportion of GDP was spent on pharmaceuticals in HICs (1·4%) than in LMICs (1·6%).
The net effect of higher per capita pharmaceutical expenditure in HICs is that these countries represent about two-thirds of global pharmaceutical expenditure, despite making up only 17% of the world population (figure 4 ). Conversely, LICs represent a fifth of the world population, but account for only 0·5% of total global pharmaceutical expenditure.
Figure 4.
Distribution of world population (A) and total pharmaceutical expenditure (B) in different economic categories in 2010
The disparities are also stark in the case of lower-MICs, where about a third of the world's population reside but accounts for only 5% of pharmaceutical spending. As expenditure is a product of price and volume, both variables contribute to HICs' higher expenditure. In comparison, an analysis showed that the volume of medicines consumption per capita is significantly lower in low-resource settings.121
PEPFAR, the Global Fund, the StopTB Global Drug Facility, GAVI, and other bilateral and multilateral international funding mechanisms have expressed intentions to address disease-specific funding disparities between HICs and LMICs. With a combined total budget of more than $80 billion between inception and 2009, these key donors have provided funding for a wide range of activities including the procurement of pharmaceuticals and other health commodities.124 However, these funding mechanisms are not guaranteed to persist, and their support is frequently predicated on recipient countries gradually shifting from donor support to self-reliance. The feasibility of this transition is evident in several countries that now fund vaccination programmes domestically after previously receiving support from GAVI.125
Financing essential medicines for UHC
The Commission considers the model's estimates to be the bare minimum amount needed for essential medicines in LMICs, assuming efficient procurement and use of pharmaceutical resources, such as prescribing according to standard treatment guidelines and minimal waste. The Commission notes that most LICs spend less on medicines than the estimated absolute minimum of $13 per capita, and more than half of all pharmaceutical expenditure in LMIC is from private sources, namely out of pocket. These findings have important implications for countries moving towards UHC.
First, many LICs and some lower-MICs will need to increase domestic financing to provide a basket of essential medicines as part of the progressive realisation of the right to health. Second, access to insurance funds and public sector health financing must be substantially increased to seek to reduce high levels of out-of-pocket expenditure. Finally, governments that cannot generate sufficient funding for the basic package should be supported in the short term by international funding mechanisms. This support would contribute to ensuring that all people have access to essential medicines, as included in international human rights treaties, and fulfil governments' obligations to realise human rights even beyond their borders.60 Such support would also help LICs to develop health services delivery infrastructure while they work to identify adequate domestic financing.
Many lower-MICs spend more than $13 per capita, so the necessary funds for a basic package do exist. In these cases, promoting equity and fairness in the access to essential medicines is of prime importance, necessitating processes of redistribution within a country. This process might also require redirecting national resources, lowering prices, and eliminating inefficiencies and waste (see section 4). Furthermore, providing access to essential medicines benefit packages that go above and beyond the very basic list included in the model will require additional investments.
The Commission expects that this costing model will be used and adapted by countries to estimate national needs for essential medicines, as governments move towards UHC and guaranteed access to essential medicines. A prerequisite to this application of the model is local data inputs, including medicine prices, distribution costs, and disease prevalence. Modification of the model's inputs would be crucial for individual countries seeking to create their own benchmarks for financing access to essential medicines. An absence of high quality data might substantially hamper accurate estimation of the financial envelope needed. Notably, accurate medicines pricing data are often difficult to obtain (section 2). Distribution costs are frequently not reported either, and detailed local disease prevalence data might also not be easily available.
Similarly, although data on past expenditure on pharmaceuticals are crucial for decision making about future spending, the National Health Account data repository is not adequately updated at the global level, hampering transparency. The comparisons presented in this section were based on the most recent available data, from 2010. With few datapoints over time for comparison, it is difficult to assess the quality of the data submitted by countries, including the relative contributions of the public versus private sectors, and the share of private prepaid and out-of-pocket expenditure.
Finally, the Commission acknowledges that even when national-level analyses have been done, they might provide insufficient evidence on the equity of access to medicines. Just as the aggregate global-level data suggest that pharmaceutical expenditure is adequate to provide essential medicines to all, national per capita expenditure measures might obscure inequities between different regions or subpopulations. Nationally representative data must be collected in ways that allow for disaggregation by key populations.
Recommendations
The Commission's analysis shows that the cost of providing essential medicines to all people in LMICs is a surmountable challenge. The Commission offers the following recommendations in relation to providing sufficient and equitable financing for essential medicines.
-
1
Governments and national health systems must provide adequate financing to ensure the inclusion of essential medicines in benefit packages provided by the public sector and all health insurance schemes. The Commission's modelling exercise can serve as a starting point for determining the financing needs for essential medicines for a particular country. This exercise should be adapted to the national context (including disease burden, standard treatment guidelines, health priorities, and the costs of delivering care in that particular system). In any national costing exercise, particular attention must be paid to the specific needs of underserved communities to promote equity and to assure progressive realisation of the right of access to health-care services. The aim must be to achieve a benefits package that addresses the population health needs in a way that can be sustainably financed from different sources, in a fair and transparent manner.
-
2
Governments and national health systems must implement policies that reduce the amount of out-of-pocket spending on medicines. More than half of all spending on medicines in LMICs comes from out-of-pocket expenditure, which is highly inequitable. Moving towards UHC requires countries to reduce medicines financing via direct payment and to increase financing through required prepayment mechanisms and government allocation.
-
3
The international community must fulfil its human rights obligations to support governments of LICs in financing a basic package of essential medicines for all, if they are unable to do so domestically. This support should come in addition to similar support programmes already in operation for essential medicines for specific diseases such as HIV, tuberculosis, malaria, and neglected diseases. The Commission urges countries to review innovative financing mechanisms to determine whether they can be extended to apply to essential medicines generally.
-
4
Governments and national health systems must invest in the capacity to accurately track expenditure on medicines, especially essential medicines, in both the public and private sectors. Data should be disaggregated between prepaid and out-of-pocket expenditure, and among important key populations. Informed decision making on investments in the purchase of essential medicines requires quality data on current spending. Since national-level data can obscure important inequities, data need to be disaggregated for important key populations, chosen with the particular national situation in mind. In designing monitoring systems, attention must also be paid to enabling maximal involvement of all stakeholders and to the principles of transparency, including access to the data for use by policy analysts and academics. Transparency will facilitate public buy-in and support for the decision-making processes that use such data.
Section 2: making essential medicines affordable
A patient's experience
Adia has worked all her life as a domestic helper in a large city. 10 years ago she was diagnosed with type 2 diabetes, for which she has recently been prescribed insulin. A month's supply of insulin costs the equivalent of 7 days' salary. Additionally, for each visit she must pay for transport to the clinic while losing a day's wages. Adia feels trapped in a vicious cycle of losing more and more of her salary to pay for her treatments.
Introduction
The affordability of essential medicines is a core challenge for any health system working to achieve UHC.126 An appropriate benefit package, including carefully selected essential medicines, is a key component of UHC.127 The affordability of essential medicines is one of the most pressing problems facing health systems, and requires comprehensive policy solutions that promote equity and maintain financial sustainability.
In this section, the Commission argues for concerted efforts, across a range of policy interventions, to ensure the affordability of essential medicines. UHC provides an opportunity to revisit recommended interventions that are underused. Multiple strategies and policies—including pricing policies, pooled funding, leveraging buying power, managing intellectual property for single-source medicines, and careful selection of the medicine benefit package—are needed to address the affordability of both single-source and multi-source medicines.
Defining affordability
The term affordability is used to describe the “ability to purchase a necessary quantity of a product or level of a service without suffering undue financial hardship.”128 No agreement exists on what financial hardship means, nor how best to assess it.128 A common way to measure affordability at an individual or household level is to compare the amount of a payment for a treatment course with the household's available resources.128, 129 Goods that are largely affordable for high-income households could remain out of reach for low-income individuals and households.
At the collective level, such as for public or third-party payers, affordability depends on the price of the product or service, the available budget, and the fiscal space (defined by Heller in 2006 as “the capacity of government to provide additional budgetary resources for a desired purpose without any prejudice to the sustainability of its financial position”126).130, 131 Available financing for essential medicines is relative to, and therefore key to determining, affordability. Affordability is also distinct from the value of a product or service. Thus, an essential medicine might offer a large health benefit or high value (determined, for example, through cost-effectiveness analysis), but still might not be affordable (because of limited resources, high prices, or both), as with new treatments for hepatitis C (panel 3) and cancers. In other cases, medicines might be affordable but offer little additional health value over existing options.
Affordability of essential medicines remains a key challenge to access
Commonly used essential medicines are unaffordable in many settings, despite being available from multiple sources. This is especially true for individuals who are paying out of pocket. When medicines are unavailable in the public sector, patients are forced to purchase them in the private sector using out-of-pocket resources.132 Between 2007 and 2014, generic medicines were available in an average of 58% of public health facilities in LICs and lower-MICs (availability ranged from 17% to 100%).133
Affordability is particularly problematic when medicines must be taken on a continuing basis, such as for the management of chronic communicable or non-communicable conditions. Unlike AIDS, tuberculosis, and malaria, NCDs have not been the target of new global funding facilities. Differences in affordability also exist within the NCDs group: essential medicines for cancer and diabetes are often less affordable than treatments for hypertension.134 Nonetheless, a study of private pharmacy prices for four commonly used cardiovascular medicines in 18 countries showed that they were potentially unaffordable for at least some patients in every country. This result included 0·14% of households in HICs, 25% in upper-MICs, 33% in lower-MICs, as much as 60% in LICs (excluding India), and 59% in India.6
The results of a review132 of WHO/Health Action International (HAI) price data in LMICs showed that a month of treatment for three common chronic NCDs (gastric ulcers, asthma, and type 2 diabetes) was not affordable to large segments of the population when purchased in the private sector. (The WHO/HAI method uses a definition of affordability that is based on the number of days of the minimum public sector wage for unskilled government workers required to purchase 1 month of treatment.)135 Similarly, considerable challenges have been documented with the affordability of insulin for treating diabetes.136 Public sector data from 13 LMICs showed that the mean public sector price of human insulin (100 IU isophane/regular 70/30) represented 0·7 to 6·2 days' minimum public sector wages for 1 month of treatment. In the private sector, across 20 LMICs the same medicine represented about twice the burden, between 1·1 and 13·7 days' wages.137
Routine monitoring of essential medicines prices is needed
The MDG Gap Task Force Report 2015,138 stated that “[a]ccess to essential medicines at affordable prices remains highly problematic, with many households squeezed out of the market due to high prices and limited availability.” It also noted a serious lack of data to adequately track progress. Data on prices need to be analysed and understood to design interventions to improve affordability. However, substantial gaps in the availability and quality of systematically gathered and analysed data on price and affordability of medicines exist at a global level.
The 2015 World Health Statistics report also emphasised that “[d]ata on the availability of medicines are poor in most developing countries.”139 It also emphasised that the only reliable sources of data on medicine prices are the surveys done using the WHO/HAI method. However, these data are limited and rapidly become out of date. In the 2015 World Health Statistics report, only 38 (19·6%) of 194 countries had survey results generated between 2007 and 2013.139 The only datapoints reported were minimum, median, and maximum values for the availability of generic medicines, and the median consumer price ratio (compared with international reference prices) of selected generic medicines in the public and private sectors. Although the WHO/HAI cross-sectional survey method136 has been validated, it remains subject to limitations in terms of sampling, facilities, and the basket of medicines assessed.140 Very few countries have done repeated price surveys, while data on affordability by income strata are not available. Routine data are easier to collect from single-payer and state-operated systems, but data obtained from distributors or retailers have limitations. Such data are often out of date because of price fluctuations, and do not reflect undisclosed discounts, clawbacks, or other types of price reductions. Routine collection of data on the actual prices paid by patients for medicines rarely occurs, especially in settings in which out-of-pocket payments are common and the prices paid are highly variable. The International Drug Price Indicator Guide issued by Management Sciences for Health addresses this gap to a certain extent, by reporting sellers' and buyers' prices from government agencies, pharmaceutical suppliers, and international development organisations. Other price reporting mechanisms have been developed for specific medicines, such as antiretrovirals and tuberculosis treatments.141, 142, 143
The lack of reliable price information is a barrier to cross-national comparisons and impedes the development of responsive pricing policies. Furthermore, because affordability relates to total expenditure, which is determined by both price and volume, data on the use of medicines (see section 4) should be considered as well during the development of evidence-informed policies. The Commission concludes that new systems are needed to routinely collect, analyse, and respond to pricing data in real time, particularly in LMICs. These systems will require investments in strengthening capacity between institutions that generate and analyse information.
A comprehensive set of policies is required to achieve affordable prices
To implement UHC, a benefit package must be designed that includes an evidence-informed list of medicines to be provided or reimbursed. The Commission has identified several strategies beyond the development of an essential medicines list that can improve access to affordable essential medicines at the individual and collective level (panel 4 ). The Commission believes that affordable prices for essential medicines are compatible with the sustainability of the pharmaceutical industry, including, as detailed in section 5, research and innovation to develop missing essential medicines.
Panel 4. A comprehensive suite of essential medicines policies to reduce prices.
Procurement interventions
-
•
Pooled procurement, using limited competitive bidding (tender)
-
•
Pooled procurement (or use of monopsony power), with price negotiation based on volumes procured or inclusion in a reimbursement list
-
•
Parallel importation
Pro-generic policies (note that these policies also rely on an effective medicines regulatory authority, which can assure the quality of all products on the local market)
-
•
International non-proprietary name prescribing
-
•
Mandatory offer of generic substitution or enablement of generic substitution by pharmacists and other dispensers
Pricing interventions
-
•
Reduction or removal of import taxes or sales taxes
-
•
Internal reference pricing (note that this type of pricing is also a pro-generic policy, as it depends on the ability to set a reimbursement limit by reference to the price of a selected generic option)
-
•
External reference pricing
-
•
Regulation of distribution chain mark-ups
-
•
Regulation of professional fees
-
•
Regulation of annual factory-gate price increases
-
•
Patent-related interventions such as encouragement of voluntary licensing and patent pools
Quality use of medicines interventions
-
•
Evidence-informed standard treatment guidelines and essential medicines list or reimbursement list
-
•
Feedback on prescribing behaviour, with peer review and intervention
-
•
Reimbursement based on adherence to guidelines and use of medicines targets
-
•
Reimbursement caps (limit)
-
•
Patient copayments (as a disincentive to overuse)
Trade-Related Aspects of Intellectual Property Rights flexibilities
-
•
No granting or enforcing of medicines patents and test data protection (for least-developed countries)
-
•
Use of parallel import
-
•
Compulsory licensing
-
•
Government use licensing
-
•
Application of strict patentability criteria
Based on existing WHO guidance,144, 145, 146, 147 assessment of the relevance of pharmaceutical policies to low-income and middle-income countries.148, 149
Each country needs to select the policy options appropriate to its particular health system, national priorities, available resources, and human rights considerations. Many factors will affect which policies to apply, particularly how a country's medicine supply chain is structured. For example, health care might be delivered predominantly through the public sector, or involve a wider range of public and private sector actors. Other factors to consider include the degrees to which an established local pharmaceutical market or reliance on imported medicines exists, the extent to which medicines are patented, and the extent of reliance on donor funding for purchasing essential medicines.
WHO offers guidance to countries on a wide range of medicines pricing policy options available to governments and health systems.144 Topics include: internal and external reference pricing,145 reimbursement limits and reasonable co-payments, removing sales taxes and tariffs on medicines,147 regulating increases to factory-gate prices, setting distribution chain price controls, and regulating professional and other fees.146 Although many policy options are relevant to countries where public sector provision of health care predominates, others only become available when countries implement a purchaser–provider split.148
UHC does not imply either complete reliance on public sector service provision or a more pluralistic system involving both public and private providers. However, any system that enables a large purchaser, such as a national health insurance fund or public health sector, to use monopsony (only one buyer) power (through competitive bidding and price negotiations) exerts downward pressure on prices.150 Pooled procurement, when multiple payers within a country or across countries negotiate prices together, can also be used to increase monopsony power.151 Such practices have been widely used, in conjunction with measures to overcome patent barriers, to supply generic antiretrovirals by the global financing initiatives that have transformed the landscape of antiretroviral prices.152, 153 Transparency about prices has been a major feature of these global financing systems, in marked contrast with the situation that pertains to pharmaceutical pricing in other settings. Although little conclusive evidence exists that transparency alone results in price reductions, the possibility has been raised that price transparency could enable collusion or other anticompetitive behaviours between companies154 or in an attempt to limit price reductions. However, as the case of antiretrovirals has shown, transparency can also be accompanied by drastic price reductions.
Ineffective and inefficient national procurement institutions and processes can also contribute to higher than necessary prices for payers, affecting the availability of medicines.124, 155 Over at least the past decade, collective procurement strategies have been promoted globally, particularly between international agencies. For example, manufacturers reduced the prices of etonogestrel and levonorgestrel by roughly 50%, from $18 to $8·50 per unit for etonogestrel and $16·50 to $8·50 per unit for levonorgestrel, thanks to minimum volume guarantees by a consortium of global health partners. The price reductions resulted in a near-doubling of orders from buyers and, ultimately, improved access for women.156, 157, 158
Global donors, technical partners, and manufacturers have also joined forces to help reshape health product markets and reduce the production costs of pharmaceuticals. For example, between 2003 and 2013, GAVI cultivated a competitive marketplace for the pentavalent vaccine, resulting in a price reduction of up to 65% (from $3·56 per dose to as low as $1·19). In 2013, this reduction was projected to save GAVI up to $150 million over the course of 4 years.159
The policies that promote affordability vary depending on the types of essential medicines being considered. Single-source medicines (including those still under patent) and multi-source medicines require different approaches. Pro-generic policies enable considerable cost savings whenever generic alternatives exist.160 For example, it has been estimated that Chinese hospital purchasers could save a total of $1·4 billion (2014 US$) by switching from originator brand antihypertensives and antidiabetics to domestically available generic equivalents.161 Pro-generic policies include: prescription by international nonproprietary name, allowing (or requiring) generic substitution by pharmacists, and using procurement and reimbursement decisions to promote generic use (table 4 ). These policies depend on trust, held by prescribers, dispensers, patients, and carers, in the quality of available generic products (sections 3 and 4).163, 164
Table 4.
Pro-generic policies to increase competition and reduce prices162
| Description or examples | |
|---|---|
| Supply side | |
| Preventing delay in generic entry | Expedited or abbreviated application processes, early working (Bolar) provisions, and biowaivers |
| Incentivising market authorisation | Incentives for manufacturers to file an application for market authorisation of a generic medicine |
| Assuring quality of generic medicines | Requirements for bioequivalence testing and the publication of lists of interchangeable medicines; transparency of reviews of such evidence; reliance on decisions taken by stringent regulators or prequalification |
| Using TRIPS flexibility | Policies that enable the use of TRIPS flexibilities, including undisclosed test data protection that does not prohibit the registration of a generic |
| Increasing competition between manufacturers | Patent pools, improving transparency of patent information, and publishing information on the prices of medicines |
| Pricing for affordability | Internal reference pricing, external reference pricing, pricing controls, the regulation of distribution chain mark-ups, and charges; pooled procurement and tenders |
| Demand side | |
| Promoting generic prescribing | Prescribing medicines by the international non-proprietary (generic) name |
| Enabling substitutions | Mandate or enable the dispensing of generic equivalents instead of branded products by pharmacists and other dispensers |
| Adapting medicines reimbursement policies | Promoting generic medicines via waiver of copayments or the application of internal reference pricing |
| Promoting independent medicines information | Banning the provision of free medicine samples, banning direct-to-consumer advertising of prescription medicines |
| Monitoring consumption | Monitor and report the consumption pattern of generic medicines |
TRIPS=Trade-Related Aspects of Intellectual Property Rights.
For the past 5 years, price increases of up to 1250% for medicines available as generic products have come under intense scrutiny in North America165 and Europe.166 Manufacturers of older generic medicines point out that the larger companies have little interest in these products, because of low profitability. Little or no competition between the few remaining producers removes downward pressure on prices, enabling inordinate price hikes. The manufacturers argue that pricing reflects the value of the medication, but others interpret it as unscrupulous price gouging and call for policy solutions on moral grounds.
Interventions to increase use of generics are difficult when the medicine in question is patented, or when generic equivalents cannot be produced. The World Trade Organization's Agreement on Trade-related Aspects of Intellectual Property Rights (TRIPS) obliges all member countries to provide patents for all technologies, including pharmaceuticals, with a minimum duration of 20 years. (The exemption for least developed countries [LDCs] is time limited, to 2033.) TRIPS also includes a range of flexibilities that provide governments with options that allow for the protection of public health, including access to affordable medicines (section 5). Widespread use of these flexibilities has been key, for example, in the supply of generic antiretrovirals.167 However, these flexibilities are under continual threat from the TRIPS-plus obligations included in bilateral and regional trade agreements. For example, data exclusivity provisions require each manufacturer of generic or biosimilar medicines to generate its own data for applications to regulatory authorities for market authorisation—for many, this requirement becomes an insurmountable barrier.168 Furthermore, it raises ethical concerns when a standard of care has already been established. Countries that use TRIPS flexibilities also risk trade pressures, such as being included in annual watch lists like the Special 301 Report issued annually by the US Trade Representative.
A systemic response to the challenges of intellectual property barriers is provided by the Medicines Patent Pool (MPP),169 which is described in more detail in section 5. Patent licences, such as those available from the MPP or certain patent-holding companies, provide a legal means to reduce the negative effects of a patent monopoly on the availability of a generic medicine and lead to greater availability at more affordable prices. Use of a licence might require the payment of a royalty, and is subject to restrictions in terms of geographical scope. Companies making generic medicines under an MPP licence and certain company licences can, nevertheless, supply countries outside of the scope of the licence, provided those countries have issued a compulsory licence or enabled government use of a patent, or such supply does not otherwise infringe on a granted patent in that country.170
Local production has been promoted as a strategy to expand access to affordable generic medicines. For example, Mozambique received support from the government of Brazil to promote domestic pharmaceutical production,171 whereas the European Union funds a large project to foster technology transfer between regions.172 In the early 1990s, the national HIV treatment programmes of Brazil and Thailand were both largely dependent on locally produced low-cost antiretrovirals.173 Two earlier literature reviews174, 175 concluded that, while promoting domestic production is often politically or economically motivated, meaningful assessment of the impact is missing. What evidence exists on the impact of domestic production on prices is contradictory,175 perhaps partly caused by conflicting public health and industrial policy agendas.176 Weak regulatory authorities and a dearth of human resources are among the economic and institutional barriers that have impeded domestic production in many settings.176, 177
The problem of affordability of single-source medicines under patent protection might well be most acute for MICs excluded from voluntary licensing agreements. These countries are obliged to grant patent protection for pharmaceuticals and are vulnerable to political pressures when they attempt to apply TRIPS flexibilities. They might also be excluded from receiving donor support. In this regard, South Africa is an exceptional case. On the basis of its high HIV prevalence, it is included in many voluntary licences and non-enforcement agreements for antiretroviral drugs. However, South Africa is subject to the full force of the TRIPS Agreement and has yet to amend its patent laws to take full advantage of available flexibilities.
The Commission argues that all countries need to use the full range of pricing policies, including all TRIPS flexibilities, to promote affordability of essential medicines at both the individual and collective levels.
New essential medicines threaten the financial sustainability of health systems
High prices of medicines pose problems for LMICs and HICs alike, threatening the sustainability of health systems and raising serious ethical questions about equity and coverage. The price of a new medicine is typically set by the manufacturer to maximise its profits during the period of monopoly supply under patent. Tiered pricing schemes have been promoted as a strategy to protect affordability. However, this practice can artificially segment a market, or result in only short-term price reductions, particularly when compared with the long-term impact of competition.178
Large volumes, high prices, or a combination of both can contribute to making essential medicines unaffordable. Whenever a large amount of medicine is required, such as when a large patient pool exists, a health system's financial capacities can be stressed. If the price of a widely used medicine, or an entire class of medicines, is increased, the budgetary impact can be devastating. Antiretroviral drugs exemplified these challenges as access to treatment was expanded.179 An example is the new direct-acting antiviral medicines to treat hepatitis C.22 As described in panel 2, these expensive medicines are urgently needed by millions of people worldwide, and the expiration of primary patents for these products is only expected from 2024.180
Biological medicines (those based on large, complex molecules, such as proteins and antibodies, as opposed to the simpler, smaller molecules that are incorporated in chemical medicines), such as monoclonal antibodies used to treat cancers, are another example of medicines whose prices present affordability challenges to all countries, regardless of income level. In the USA, the average acquisition price (the price charged to patients, insurers, or the health system) of newer cancer treatments now ranges from $10 000 to $30 000 per month. Biological medicines are projected to comprise approximately 20% of the value of the global pharmaceutical market in 2017.181 They contributed to the largest percentage increase in pharmaceutical expenditure in HICs between 2005 and 2015 (figure 5 ).
Figure 5.
Percentage contribution to change in market share by type of product in middle-income and high-income countries in 2005–15
This figure is a new elaboration of IMS Health data (for more information on the classification and countries included see appendix 2.3).
High prices have led to calls to place more emphasis on assessing the value of new medicines to “help physicians, payers, and patients…make better choices about their use.”182 As UHC is extended, there is a crucial need for tools to assess the value of new medicines, particularly those that are highly priced. The American Society of Clinical Oncology, for example, has proposed a value analysis framework.183 Assessing the value of new medicines only, however, does not necessarily lead to affordable prices. In the USA, where a largely laissez-faire approach to medicine pricing has been in effect for many years, there are now calls for a change to the “system in which prices are linked to the value of products.”184 In 2016, US President Barack Obama issued a call for transparency in reporting production and development costs, increasing rebates by manufacturers to pay for medicines received by certain groups of beneficiaries of Medicare and Medicaid, and granting government the authority to negotiate prices for certain high-priced medicines.185 These developments represent a complete change in attitudes about new medicines and their costs in the USA, which pays, on average, the highest prices for medicines globally. The terms newer is better and newer is needed are no longer considered appropriate or defensible justifications. Similar challenges face any health system that is deciding whether to include a new medicine on its essential medicines or reimbursement lists.
Strengthen national capacity to assess value
The health technology assessment (HTA) method is one approach to assessing the value of a new medicine. HTA goes far beyond cost-effectiveness analysis; it is “a multidisciplinary activity that systematically examines the safety, clinical efficacy and effectiveness, cost, cost-effectiveness, organisational implications, social consequences, legal and ethical considerations of the application of a health technology—usually a drug, medical device or clinical/surgical procedure.”186 A similar process, described as priority setting, has been cited as essential to achieving UHC.187
HTA programmes have been established in a number of HICs with national health insurance systems.188 Several transitional and MICs, such as Poland, Colombia, and Malaysia, have also established HTA agencies. A 2014 survey189 of 17 countries in Latin America and the Caribbean and 22 countries in Central and Eastern Europe showed that numerous countries were setting up institutional frameworks, developing procedures and standards, and establishing policies to support HTA.
A 2014 World Health Assembly Resolution (WHA67.23)190 noted the prerequisites necessary to implement HTA begin with an independent body, free from political pressure and other vested interests in medicines policy. Engagement with stakeholders, such as academic institutions and professional associations, is also needed. HTA often requires the creation of new capacity at the national level. Finally, substantial and sustained financial investment is needed to provide for the resources needed to undertake HTA. For example, in 2008 the Korean National Evidence-based Healthcare Collaborating Agency had around 120 staff, a budget of $10 million, and completed 90 HTAs (of which 59 were done in-house).191 Panel 5 reviews these prerequisites using the example of the Health Intervention and Technology Assessment Program, the national agency performing and supporting HTAs in Thailand.
Panel 5. The Health Intervention and Technology Assessment Program (HITAP) of Thailand.
Thailand's experience demonstrates how health technology assessment (HTA) can be successfully used as a tool for developing and implementing policies that reduce the prices of essential medicines and technologies.
In 2007, the Ministry of Public Health in Thailand established HITAP to generate evidence to inform decision making about which medicines and health technologies would be covered by the public health system.192 By 2015, HITAP had 50 staff, and a budget of approximately US$2 million per year. The assessment process used by HITAP is presented in appendix 2.2. HITAP does between 20 and 25 assessments per year.
Four crucial features have enabled Thailand to effectively use HTA to make medicines more affordable:
-
•
Independence from decision making about reimbursement: HITAP does not make decisions on the inclusion of a medicine in the National List of Essential Medicines (NLEM), the Thai public health system's reimbursement list. Instead, its expert assessments are provided to relevant government authorities. The link between technical assessment and reimbursement decisions should be transparent and clearly defined.193
-
•
Compensating for a shortage of in-house HTA capacity: in Thailand, most HTAs are done either by HITAP or by other public research institutes. On lower priority topics, the NLEM Subcommittee allows HTA submissions from industry, provided they follow national guidelines. This action allows HITAP to allocate scarce technical resources to high priority assessments.
-
•
Using price quotations as an input for HTA and a trigger for pricing interventions: HTAs in Thailand go beyond consideration of the existing price of a product in the local market. Manufacturers and marketers of medicines under HTA consideration might submit price quotations that reflect the economies of scale that would follow if the product is deemed reimbursable. Additionally, if the HTA shows that the incremental cost effectiveness ratio exceeds a certain threshold—approximately US$5000 (160 000 Thai Baht) per quality-adjusted life-year or disability-adjusted life-year—then price negotiations ensue to reach a price that is acceptable for all parties. Thus, the Thai HTA process has led to the activation of price interventions aimed at ensuring the affordability of essential medicines (see also panel 6).
-
•
Using HTA as an input for budgetary impact consideration: no health system has an unlimited budget; there will always be tensions between ensuring availability of cost-effective essential medicines and securing the means to meet the cost of such medicines. Demonstrating cost-effectiveness, even on the basis of locally-relevant thresholds, values, and preferences, is not sufficient to ensure that a medicine is affordable for either the health system or patients. For example, a Thai HTA concluded that reimbursing screening-plus-treatment for osteoporosis at any age was not cost-effective.195 Even if the acquisition price of the medicine being assessed, alendronate, were to be reduced by 40%, it was projected that it would comprise almost 20% of the UHC scheme's budget if included.
The Commission acknowledges that HTA alone cannot make essential medicines affordable. However, HTA can substantially contribute to the evidence base for selection and reimbursement decisions related to medicines.196 Moreover, results from HTAs have been used by government agencies as an input in price negotiations over new essential medicines.197, 198 Examples from Thailand (panel 6 ) illustrate how HTA has been used in supporting reimbursement decisions and triggering pricing interventions for medicines.
Panel 6. Examples of price interventions triggered by Thai health technology assessments (HTAs).
Thai HTAs have triggered interventions to reduce the price of a medicine under consideration for inclusion in the National List of Essential Medicines (NLEM) such as:
-
•
Price negotiation: in 2012, oxaliplatin was added to the Thai NLEM for the treatment of metastatic colorectal cancer, as part of the FOLFOX adjuvant chemotherapy regimen (with folinic acid [also known as leucovorin] and 5-fluorouracil). An initial HTA had shown that FOLFOX would be considered cost-effective compared with the alternative (adjuvant therapy with folinic acid, 5-fluoruoracil, and capecitabine), but only if the price of oxaliplatin were reduced by at least 40%. A final price reduction of 70% was negotiated, which has saved the Thai health system approximately US$4·75 million (152 million Thai Baht) per year.
-
•
Off-label use: in 2012, the Thai NLEM confirmed a decision to include intravitreal bevacizumab, rather than ranibizumab, for the treatment of age-related macular degeneration. Although bevcizumab had been used off-label, concerns were raised after such use was challenged in the UK.194 An HTA done by the Health Intervention and Technology Assessment Program concluded that the two medicines were equivalent in terms of effectiveness, but noted that safety data were insufficiently robust. A multi-stakeholder process, including ophthalmologists, academics, and representatives from the Thai Food and Drug Administration and pharmaceutical industry, recommended that negotiations commence with the producer of ranibizumab to reduce the price, but in the event that these price negotiations failed, bevacizumab will be included in the NLEM (with development of a system for monitoring serious adverse effects).
-
•
Cost-sharing arrangement: although an HTA of imiglucerase for the treatment of type 1 Gaucher disease showed the product not to be cost-effective, a cost-sharing model was negotiated, which allowed the product to be included on the NLEM in 2012. For the first five patients newly identified each year, costs were to be shared equally by the manufacturer and government (an effective price reduction of 50%), and for subsequent patients identified in the same year, the manufacturer would cover the entire cost of treatment.
Several examples have shown that ad hoc solutions, such as creating specific earmarked funds for a particular medicine, disease, or patient group, are often tempting because of political expediencies. However, ad hoc solutions are rarely sustainable, and risk undermining a well designed and effective decision-making process for a health system overall (panel 7 ).
Panel 7. The trastuzumab example: the limits of ad-hoc or short-term solutions.
When the outcome of a health technology assessment and a decision to deny coverage of a medicine is socially unacceptable, politicians might be asked to seek short-term solutions. Trastuzumab posed an existential challenge to the health technology assessment process in England and Wales.
In the face of an initial rejection by the National Institute for Health and Care Excellence, the UK health secretary intervened to force reimbursement by a primary care trust in 2005.199 In 2010, the Cancer Drugs Fund was formed specifically to “help patients get access to innovative new cancer drugs.”200 A follow-on formulation, trastuzumab emtansine (sold as Kadcyla by Roche), for the treatment of HER2-positive locally advanced or unresectable, or metastatic (stage IV) breast cancer, was initially funded by the Cancer Drugs Fund but was scheduled to be withdrawn in November 2015. This reversal provoked fierce responses from cancer groups, including a request to the UK Government to issue a compulsory licence to allow a lower priced version of the product.201 The product remains available through the Cancer Drugs Fund after an agreement between the manufacturer and National Health Service in England was reached.202 However, the Cancer Drugs Fund was not considered to be a long-term solution, and it was replaced by the Managed Access Fund.203, 204
Sustainability lies in lower pricing of medicines considered essential. Political response to the Pharmaceutical Benefits Advisory Committee's rejection of trastuzumab also resulted in the creation of a unique funding programme in Australia in 2001.205 In 2015, trastuzumab was added to the WHO Model List of Essential Medicines, together with several other expensive cancer treatments and all of the currently available direct-acting antivirals for hepatitis C virus infection.20 That step was only the first in the process of ensuring equitable access to all who need these essential medicines.206
HTA requires investing in capacity to assess clinical evidence, consider local costs of services and inputs, and project potential budget impacts of competing options. Budget impact assessment should then trigger urgent discussions about the acceptability of prices for new essential medicines, and the activation of other essential medicines policies that could improve affordability, expand access, and ensure sustainable health gains. Budget impact assessment is already a formal requirement in reimbursement decision making in numerous countries, including Australia, Norway, Canada, and Italy.207 As Bulfone and colleagues208 commented, “The role of economic evaluation in decision-making…remains as a part of the whole and not the ‘end game'.”
The Commission recognises that HTA is but one tool, and still raises many unresolved challenges.209 Among these are: the types of technologies to be considered, the values assigned to health status, the selection of the metrics used for cost-effectiveness comparisons, the costs of doing HTA, the risk of duplication of efforts, the impact of disease-specific approaches, and the risk of gaming the system. Commentators have argued that the HTA method is promoted by donor agencies but inadequately adapted or used by national governments or decision makers. Therefore, they warn that HTA should be applied only with great caution.210 Given these limitations and challenges, it seems crucial to identify the areas where HTA can make the greatest contribution to decision making and where there is need for other existing or innovative tools.
Transparency is essential to effective data analysis and decision making
HTA requires a commitment to transparency between all stakeholders. The data used in assessments should be available for review by both health professionals and consumers. This kind of transparency could have implications for agencies that use commercial in-confidence evidence provided by pharmaceutical companies. However, as with medicines regulatory structures, a deliberate policy of maximal transparency helps to engender trust in the procedure and the outcomes of assessments.
Regional and global collaborations between HTA agencies can promote efficiency in doing the analyses, especially when capacity is poor. Such cooperation relies on transferability, the extent to which HTAs done in one setting can be used in, or adapted to, another setting.197, 211 Cooperation requires a commitment to share information on evidence of comparative effectiveness, estimates of cost-effectiveness, results of budget impact assessments, and outcomes of pricing interventions. However, when relying on an assessment done elsewhere to inform a local decision-making process, an HTA should include a crucial examination of the applicability of the evidence used to local conditions, societal values, and the prices offered for medicines under consideration. Several networks have established procedures for information sharing, such as the HTA Core Model developed by EUnetHTA.212 Existing networks in this field are listed in appendix 2.1.
HTA—or any other assessment process—also requires transparency with respect to the process and values applied. Otherwise it becomes impossible to assess, for example, how priorities are set for specific subpopulations.208, 213 Fulfilment of the right to the highest attainable standard of health is not an event, but a process of progressive realisation that can take resource limitations into account.214 For example, the mere fact that a disease is rare does not provide a human-rights-based justification for the immediate reimbursement of a treatment with a less-favourable marginal cost-effectiveness than other treatments for other diseases.215 It has been proposed that new expensive treatments should be added to reimbursement packages on the basis of the marginal cost-effectiveness criteria (cost per quality adjusted life-year gained) as other medicines.216 However, the Commission notes that societies might choose to apply different norms in particular circumstances, such as in relation to end-of-life care. For instance, a review of reimbursement decisions in 14 OECD countries found that both the severity and the rarity of a condition were used to justify higher incremental cost-effectiveness ratios or higher prices than would be the norm.217 Balancing the various considerations and justifications that could be used in such decisions highlights the necessity of explicitly defining ethical principles to guide the use of economic analyses in the selection of medicines.
Finally, HTA—or any similar approach—requires a commitment to meaningful involvement of the public and other stakeholders throughout the assessment process. Any analysis of value involves the application of so-called value judgments that reflect social values and preferences of patients and carers, in addition to data on costs and relative effectiveness.218, 219 Just as patient and public participation is increasingly recognised as important for the validity of medicines regulatory decisions, so too is their involvement in medicines selection and, especially, decisions to restrict access to medicines and other health technologies.
Conclusion
The affordability of essential medicines, from those that have been on the market for years to those that are new or have newly attained essential status, is a fundamental challenge in both LMICs and HICs. Affordability is a high priority in countries moving towards UHC. Designing and then equitably implementing effective policies can only be achieved through concerted efforts by governments and health systems, in concert with the pharmaceutical industry.
The Commission maintains that every available regulatory and management intervention that could improve the affordability of medicines must be considered. The suite of policy instruments includes: pricing interventions, pro-generic policies, use of TRIPS flexibilities when patent barriers prevent access to lower-priced alternatives, and avoidance of TRIPS-plus provisions in bilateral trade agreements. Considerable evidence supports the use of these policies to support the affordability of essential medicines.220
No option should be regarded as off the table for political reasons. For example, removing taxes and tariffs is well within the power of governments, even if it might be unpopular with certain stakeholders. Moreover, committing to UHC provides countries with new opportunities to effectively implement policies, such as leveraging newly created buyer power and financial pooling, to address high prices of medicines. The ultimate objective is not merely cost-containment. Instead, as previously noted, UHC aims for the long-term sustainability of the health system; the ability to develop and provide proven effective medicines to all people who need them, including disadvantaged groups; and to improve their health status and personal satisfaction, and the financial protection offered to citizens.
HTA is one method that generates evidence to support decision making about the selection of essential medicines for procurement and reimbursement, and can be an input in price negotiations, especially for new essential medicines. But HTA is not an end in itself, nor is it necessarily the best method of priority-setting in every context. HTA and other methods to assess value, such as budget impact assessments, should be used in combination with other policy instruments that can deliver more affordable medicines. It has been noted that “[p]riority-setting cannot solve all of the challenges and barriers associated with health resource allocation.” However, “it can support transparency and accountability and other such factors that enhance good governance.”187
Recommendations
The Commission offers the following key recommendations to promote the affordability of essential medicines:
-
1
Governments and health systems must create and maintain information systems for routine monitoring of data on the affordability of essential medicines, as well as price and availability, in both the public and private sectors. Countries moving towards UHC have an opportunity to respond to the needs of payers for quality price information for procurement and reimbursement decisions. Simultaneously, countries should measure their progress on providing affordable medicines. Monitoring systems should inform decision making about the need for interventions on medicines price and affordability. Monitoring systems also need to include regular surveys to obtain data disaggregated by economic status, rural or urban setting, sex, and other key population groups.
-
2
Governments must implement a comprehensive set of policies to achieve affordable prices for essential medicines. Countries moving towards UHC should consider using pricing policies that leverage large buyer power, setting a reimbursement limit by reference, and creating incentives for prescribers and patients such as reimbursement caps or copayments when appropriate. Policies to address high prices of generic essential medicines might require a different set of policies from essential medicines under patent protection. The full range of medicines pricing policy interventions must be employed to ensure the affordability of essential medicines for individuals and populations, including exempting medicines from taxes and tariffs, pro-generic policies in the case of multi-source products, and the use of TRIPS flexibilities in the case of single-source products. To protect a wide range of policy options, countries should abstain from demanding or agreeing to TRIPS-plus provisions in trade agreements.
-
3
Governments and health systems must develop national capacity to create medicines benefit packages that guide procurement and reimbursement for affordable essential medicines. This development requires building capacity to identify where health technology assessments can make the greatest contribution to decision making, translate findings of these assessments to the local context, and use the findings as inputs in decision making (including to help identify instances for governments to intervene in relation to medicine pricing).
-
4
Governments, national health systems, and the pharmaceutical industry must promote transparency by sharing health and medicines information. Globally and regionally, countries and health systems must participate in transparent sharing of information on pricing, evidence for comparative effectiveness, cost-effectiveness estimates, or other economic assessment of technologies, budget impact assessments, and the outcomes of pricing interventions. Sharing promotes efficiency by avoiding duplication, enabling countries with lower resources to use and adapt assessment of medicines (and other health technologies) according to their needs.
Section 3: assuring the quality and safety of essential medicines
A patient's experience
Adwoa, a girl aged 2 years from a rural village, had been feverish for 3 days. Her mother, Grace, feared that her daughter had malaria. As the district hospital was far away, Grace went to a local shop where she was sold 3 loose tablets. The shopkeeper told her to give Adwoa a half-tablet immediately and the rest divided over 2 days. 2 days later Adwoa developed a high fever and experienced seizures. Her family borrowed money to take her to hospital. She was admitted and immediately treated for cerebral malaria. Although Adwoa survived, she might have suffered permanent brain damage. Grace worried that she had done something wrong with the medication. In fact, the medicine was poorly manufactured and did not contain enough active ingredient.
Introduction
Medicines are complex products with powerful effects, which can be enormously helpful or disastrously harmful. Likewise, medicine safety is complex, covering three dimensions: molecule-based (linked to the active ingredient), product-based (linked to the quality of the product), and use-based (linked to the prescription—eg, right dosage for right disease). This section focuses on product-based quality and safety, while use-based safety is discussed in section 4. Medical devices and in-vitro diagnostics are not specifically considered.
A medicine's quality and safety cannot reliably be assessed by a consumer; even professionals need specialised training, equipment, and information. Governments, therefore, have a positive obligation to protect the health of the public by assuring the quality and safety of medicines60 through the regulation of R&D, manufacture, marketing, distribution, and use. These government actions enable health professionals and patients to trust the quality and safety of products on the market. This multipronged process becomes even more challenging for biological medicines with complex structures and manufacturing processes, including biosimilars.
Poor-quality medicines (also referred to as substandard, spurious, falsely labelled, falsified, and counterfeit; panel 8 ) can cause serious, even fatal, harm to patients. Money spent on poor-quality medicines is wasted, at the least; often, additional costs are incurred to counteract harm. At the population level, poor-quality medicines reduce health outcomes and endanger public health, for example, by contributing to the development of antimicrobial resistance and loss of public trust in the health system.
Panel 8. Substandard, spurious, falsely labelled, falsified and counterfeit medicines.
No international agreement exists on exact definitions for the various types of poor quality products.221 Following the approach used by the Institute of Medicine in 2013,222 in this report a distinction is made between substandard medicines (genuine products that fail to meet national standards set for them) and falsified products (spurious, falsely labelled, and falsified medicines; these medicines are intentionally fake products, and the identity of the actual maker cannot be established). The term counterfeit is only used “to describe trademark infringement, which is not a problem of primary concern to public health organizations.” 222 Products may fall in more than one category at the same time. For example, all falsified products are by definition also substandard.
The Commission believes that achieving sustainable development requires concerted efforts to improve the quality and safety of essential medicines, through building appropriate regulatory systems as part of health systems. New concepts and approaches have emerged in recent decades.223, 224
This section identifies five crucial areas of opportunity for improving the quality and safety of essential medicines: regulatory harmonisation, prequalification, improved procurement, enhanced surveillance, and accountability.
The breadth and depth of the medicine quality and safety problem
Low quality of some medicines continues to be a pervasive and poorly understood problem. For example, a 2008 survey225 found that 76 (28%) of 267 antimalarial medicine samples in Cameroon, Ethiopia, Ghana, Kenya, Nigeria, and Tanzania were substandard. Considerable differences existed between countries, with the lowest failure rates in Ethiopia (0%) and Kenya (5%) and the highest in Nigeria (64%).225 In 2009, 33 (11%) of 291 antituberculosis medicines from Armenia, Azerbaijan, Belarus, Kazakhstan, Ukraine, and Uzbekistan did not meet quality specifications; for rifampicin capsules the failure rate was 28%.226 Results of a systematic review227 of 44 studies in 25 countries (primarily LICs and lower-MICs) showed a median prevalence of substandard medicines of 28·5% (range 11–48%).
The true extent of the problem, however, remains unknown. Most studies on substandard and falsified medicines (57/66; 86%) focus on infectious disease treatments, especially for malaria.228 Much less information is available about medicines for NCDs. Most studies are cross-sectional surveys providing information at one point in time; other study designs are necessary to enable the examination of longitudinal changes.229
The impact of poor-quality products can be devastating. At their most benign, poor-quality medicines have no treatment effect; at their worst, they cause human disasters. An estimated 122 350 deaths in children under 5 years in 39 sub-Saharan African countries in 2013 were attributed to the consumption of poor-quality antimalarial medicines.11 The true cause of these deaths is rarely noted, as the children are assumed to have died from malaria.
Dramatic incidents can generate public outcry. In 2006, the inclusion of toxic diethylene glycol in a paracetamol oral liquid dosage form led to the deaths of more than 100 children in Panama.12 The ingredient was imported from China via a European broker that had not disclosed its origin or true contents.230 In a similar case in Haiti, the product came from a Dutch broker.230 Addition of an incorrect active ingredient to a tablet for cardiovascular disease resulted in an estimated 230 deaths in Pakistan (appendix 3.4). In all cases the cause was serious negligence in manufacturing, with subsequent failures of the quality assurance process, including failure of proper regulatory oversight.
A 2011 survey (Hall P, Concept Foundation, unpublished) assessed the capacity of manufacturers of oral contraceptives to produce good-quality products. All 44 generic manufacturers in the LMICs surveyed were declared to be in compliance with national good manufacturing practice (GMP) standards. Yet less than a third of the manufacturing plants met global GMP requirements set by either WHO or the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Cooperation Scheme (jointly abbreviated as PIC/S). Local GMP requirements might be distinct from WHO or PIC/S requirements; non-global GMP requirements does not mean, by default, that the products are poor quality. However, WHO and PIC/S set global standards that aim to ensure quality and safety. Another survey,231 on the quality of misoprostol in 15 MICs, showed that only 119 (55·3%) of 215 products contained the correct quantity of active ingredient; 14 (7%) of 215 did not contain any and were probably falsified (panel 9 ).
Panel 9. Widespread quality problems with misoprostol, a life-saving medicine for prevention and treatment of post-partum haemorrhage.
The active ingredient in misoprostol tablets tends to degrade rapidly, making it technically demanding to produce a quality-assured product. Between 2011 and 2015, the Concept Foundation tested 215 samples of misoprostol acquired from licensed sellers in Argentina, Bangladesh, Egypt, Cambodia, Kenya, India, Indonesia, Kazakhstan, Mexico, Nigeria, Nepal, Pakistan, Peru, the Philippines, and Vietnam (figure 6 ).
Figure 6.
Quality of misoprostol tablets in 15 low-income and middle-income countries
Figure was adapted from Hall and Tagontong.231 A date of production could not be established for two samples. Those samples were each assigned zero days. LA=labelled amount. pl/alu=blister packaging consisting of a plastic and an aluminium component. alu/alu=blister packaging consisting of all aluminium components. SRA=stringent regulatory authority. ERP=Global Fund Expert Review Panel. PQd=WHO Prequalification Programme. Falsified/no miso=falsified product that contained no misoprostol.
119 (55·3%) of 215 products had a level of active ingredient within 90–110% of specifications (between the horizontal lines in the figure), while 85 (39·5%) had less than 90%. More products failed the longer they were stored. In 11 (5·1%) products, more than 110% of stated content was found, perhaps to compensate in advance for degradation with time. Most importantly, 14 products (including 10 from a single manufacturer) contained neither misoprostol nor its principal degradation product. These were classified as falsified (diamond symbol in the figure); some were disguised as branded products and were therefore counterfeits as well.
Most generic misoprostol products claim a shelf life of 2 years, while the innovator product has a shelf life of 3 years. Figure 6 clearly shows that after 1 year all 29 products packaged in plastic/aluminium blisters (red circles in figure 6) failed. Failure rates of full aluminium blisters (green circles in figure 6) were lower but still unacceptable (58/164, or 35·4%). Aluminium blisters by themselves do not guarantee good quality, because the manufacturing environment also needs to be controlled.
Despite the manufacturing challenges, quality-assured misoprostol products—all packed in plastic/aluminium blisters (green square boxes in figure 6)—can be manufactured. Only 1 (2·0%) of 51 of the products approved by stringent regulatory authorities (SRAs), WHO prequalification, or the Global Fund Expert Review Panel (square boxes in figure 6) failed the test; this outcome is in contrast with non-SRA approved generic products (circles in figure 6). Regulatory oversight by an SRA is essential for this life-saving product.
Falsified medicines are deliberately and fraudulently mislabelled with respect to their identity or source. Falsified products often carry fake logos to purport to be recognised as innovator or generic brands. They might contain the correct ingredients in inappropriate amounts, the wrong ingredients, or no active ingredients at all. A key factor is an absence of documentation through which they can be traced back to a legitimate manufacturer. The supply of falsified medicines is a criminal act. The problem of falsification is distinct from other quality problems, such as production that does not meet relevant quality standards, and addressing it involves a wide array of stakeholders, such as politicians, customs, and other law enforcement, and the judiciary. The sophistication of falsified medicines seems to be increasing (appendix 3.1), as does their number, although it is not clear to what extent this is because of better reporting (figure 7 ).
Figure 7.
Reports of substandard/spurious/falsely-labelled/falsified/counterfeit medical products to WHO Rapid Alert database
Total reports=1166. Data from WHO Global Rapid Alert System, between July, 2013, and August, 2016.
Since 2011, WHO has encouraged the reporting of substandard or falsified medicines by National Medicine Regulatory Agencies (NMRAs) and large procurement agencies, using a standard form.232 The data are analysed by WHO's Rapid Alert System. The initial identification of a problem relies on voluntary reporting by health-care professionals, non-governmental organisations, the pharmaceutical industry, customs, police, patients, or caregivers. By August, 2016, the Rapid Alert System database contained more than 1000 reports (figure 7). Anti-infectives and antiparasitics were the most frequently reported classes of falsified medicines. Although this outcome might reflect the substantial resources available or the higher volume of medicines for HIV, TB, and malaria, it remains a worrying finding because of the potential for developing antimicrobial resistance.
The WHO database reveals that, contrary to common belief, generic medicines are also frequently falsified (figure 8 ). Falsified vaccines and diagnostics are also emerging. However, the individual case reports and aggregated data are not publicly available, hampering assessment of the extent of the problem.
Figure 8.
Confirmed falsified products reported from the six WHO regions
The numbers on the bars represent the number of reports of confirmed falsified products received between July, 2013, and August, 2016. Total reports=604. Data from WHO Global Rapid Alert System.
The number of falsified medicines as a proportion of all substandard products is difficult to estimate. Published studies might not make a distinction with other quality problems, use different definitions, or suffer from sampling bias (eg, checking the quality of suspect samples only, or depending on spontaneous reporting). A 2008 review by Médecins Sans Frontières233 suggested that falsified products only constitute a small proportion of substandard products. They concluded that national and international action should therefore focus on preventing substandard products without a specific focus on falsified products. Notably, the problem of falsified and counterfeit medicines222 is currently being addressed by a member–state mechanism coordinated by WHO,10, 232 replacing an earlier programme that was perceived by some parties as industry driven.
This review of the available evidence shows that many problems exist with medicine quality and product safety worldwide, and that action is urgently needed. Substandard medicines are a symptom of underlying structural problems in ensuring compliance with regulatory standards such as GMP. Despite limited capacity between regulatory systems in many countries, and a growing number of production facilities and products on the market, some progress has been made—but more data and more effective regulatory agencies are needed to assure the quality and safety of essential medicines in all settings. The Commission believes that important opportunities to ensure further progress exist on all levels.
Opportunities to improve medicine quality and safety
The following section identifies opportunities to promote more effective regulatory oversight, and to involve multiple stakeholders in the supply chain to improve medicine quality and safety. The final responsibility for quality production rests with manufacturers, which are liable in cases of non-compliance; however, the topic of quality production is not further addressed in this report. It is the governments' responsibility to create and enforce quality and safety standards for medicines and other health technologies. Key areas discussed in the following are international regulatory harmonisation, broadening the prequalification programme, good procurement practices, quality and safety surveillance, and commitment to advance accountability.
Expand international regulatory convergence and harmonisation
NMRAs vary in their ability to do effective regulatory activities, depending on access to funding and available technical capacity, among other factors. Over the past 20 years there has been a clear trend towards increased regulatory harmonisation, creating new opportunities to bolster national efforts with international resources.
In the 1990s, the ICH (previously known as the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use [also abbreviated as ICH]), brought together regulators and pharmaceutical industry associations in Europe, USA, and Japan. The ICH developed harmonised guidelines aimed at eliminating duplication in registration, including a Common Technical Document for regulatory applications. Although initially perceived as industry driven and focused on HICs, the ICH has opened up to broader global perspectives, including additional NMRAs, regional regulatory harmonisation initiatives, and industry groupings. Thus, ICH has the potential to become more relevant to LMICs.
The International Conference of Drug Regulatory Authorities (ICDRA) is the largest global regulators' forum, with participants from more than 100 nations.234 The 16th ICDRA, hosted by Brazil in 2014, dedicated a separate meeting to regulating similar biotherapeutic products (or biosimilars), issuing useful recommendations to countries and WHO.235 In view of the progress by African regulatory systems, in 2016 the ICDRA will, for the first time, be hosted by an African nation, South Africa. The ICDRA forum has been especially valuable for small and medium size regulators, as other forums (such as ICH) have in the past rarely addressed their specific needs.
PIC/S has provided an active and constructive forum for cooperation in the field of GMP inspection. The mission of PIC/S is to promote cooperation between NMRA inspectorates, and lead the development of harmonised GMP standards. PIC/S has established a rigorous assessment process for inspectorates, and only those meeting the standard are allowed to join the scheme. 46 NMRAs are members of PIC/S. Most members are from HICs and upper-MICs, such as Argentina, Indonesia, Malaysia, South Africa, and Ukraine; China and Brazil are applying for membership.
Regional harmonisation programmes in the Americas, Asia, and in Africa, provide opportunities to simplify regulatory processes and focus on NMRA activities that add value. A harmonised medicines registration system is being set up in the East African Community (EAC). The first joint WHO/EAC product assessment exercise, organised in 2010 by the WHO/UN Prequalification Programme, resulted in prompt national registrations of several needed essential medicines. In 2014, joint WHO/EAC assessments of misoprostol tablets, levonorgestrel tablets and artesunate-amodiaquine fixed-dose combination tablets yielded similar results.236 EAC working groups are focused on Common Technical Document use, GMP, and information management.
The Association of Southeast Asian Nations (ASEAN) has developed an ASEAN Common Technical Dossier and ASEAN Common Technical Requirements, allowing for the implementation of common standards and the elimination of unnecessary country-specific regulatory requirements. Acceptance of bioequivalence reports from ASEAN members, for instance, can facilitate faster market entry for generic products.237 A mutual recognition arrangement for GMP inspection reports is also in preparation.
Information is increasingly available from the most stringent regulatory agencies such as those from the USA, the EU, Canada, Switzerland, Japan, and Australia; and from regional authorities recognised as reference authorities. NMRAs, especially in resource-constrained settings, should expand their use of this information instead of emphasising national sovereignty in all regulatory decisions.
By 2016, 27 countries had joined WHO's collaborative registration procedure; 100 products have now been approved this way with very short timelines. Increased sharing of electronic applications for marketing authorisation in the standard electronic Common Technical Document format provides another opportunity for speeding up the approval processes and creating efficiencies for the pharmaceutical industry, allowing for wider marketing of essential medicines in smaller markets.
Harmonisation at the international level needs clear decisions on whether dossiers that have already been reviewed by stringent regulatory authorities following internationally agreed-upon standards require reevaluation.238 Several HICs, such as Canada, Switzerland, Singapore, and New Zealand, have decided not to repeat such assessments. Resource-constrained NMRAs should also adopt this approach, rather than investing in repeat assessments and other activities that do not add considerable value. Continued reliance on premarketing quality control testing by NMRAs also needs reconsideration. These actions could improve the efficiency of NMRA activities and enable faster market entry for important new essential medicines and quality-assured generics.
Broaden the WHO/UN Prequalification Programme
In the face of weak national regulatory systems, many countries rely on WHO to verify whether specific selected medicines, vaccines, and in-vitro diagnostic products meet international quality standards. There is substantial opportunity to strengthen and expand this existing programme.
WHO's process of prequalification for participating in international tenders for UN procurement began in 1987 with childhood vaccines.239 Prequalification of medicines started in 2001, initially because of the very small number of affordable products available for procurement by large-scale HIV treatment programmes such as the Global Fund. By August, 2015, 420 products had been prequalified by WHO, including 262 for HIV, 76 for tuberculosis, 41 for malaria, and 41 for other conditions.240 WHO prequalification of diagnostic products has also expanded, focusing mainly on in-vitro diagnostics for HIV, malaria, and hepatitis C.
As of August, 2015, 38 medicine quality control laboratories were prequalified by WHO. Procedures have been established for rapidly assessing products that have already been approved by a stringent regulatory agency, medicines urgently needed when prequalified products are not available, and products for emergency use, such as diagnostic tests and investigational products for the Ebola virus.
The effect of the prequalification programme is far-reaching; for example, prequalified vaccines now immunise 64% of infants worldwide.241 Without prequalified products, largely from Indian generics manufacturers, widespread affordable access to antiretrovirals would not have been possible. Quality standards used in prequalification have also been adopted by other institutions, including NMRAs, donors such as the Global Fund and UNITAID, and the MPP.
The prequalification programme is a concrete application of WHO's global norms and standards for medicines quality and safety (panel 10 ). It has positioned WHO as a global regulatory agency and has greatly shaped the world's generic markets, driving down costs while ensuring the quality of products.244 It has also become an important training ground for regulators and inspectors, paving the way for regional harmonisation.245
Panel 10. Progress in international norms and standards for quality and safety.
Since its founding in 1946, WHO has established global norms and standards for quality assurance to cover all stages of the product life cycle of essential medicines. Using global expertise from both well resourced and resource-constrained countries, the WHO Expert Committee reports, as published in the WHO Technical Report Series, set general and product-specific quality, safety, and efficacy standards for all medicines, including vaccines, blood products, and biosimilars. The norms and standards developed by WHO are widely used as essential tools for standardising quality control of pharmaceuticals. They include the assignment of international non-proprietary (generic) names, WHO standards for Good Manufacturing Practices, WHO manufacturing guidelines for blood products, regulatory guidelines for the assessment of similar biotherapeutic products, and the International Pharmacopoeia.242 In recent years all major national or regional pharmacopoeias have worked towards convergence and harmonisation.243
The Commission believes that the WHO/UN Prequalification Programme can and should evolve to address a wider range of essential medicines, at least until international standards have been established and the global generic market has developed sufficient capacity to produce and distribute adequate supplies of quality, safe medicines. For example, assessments of new generic versions of first-line antiretrovirals can now be left to stringent national authorities or regional networks. The focus of WHO's programme should then shift to other priority medicines, such as generic insulin, other biosimilars, and newly developed essential medicines that still pose challenges to NMRAs. The programme's quality standards and public reports should be used as the basis for regulatory convergence and reciprocal recognition; its Public Assessment Reports and Public Inspection Reports can serve as examples for all NMRAs. The transparency initiatives of the European and Australian regulators can also serve as exemplars in this regard.246 A multilateral financing mechanism would enable the prequalification programme to evolve in this way—not merely increasing its existing activities but moving towards the analysis of selected new essential medicines independently from individual donors and manufacturers alike.
Establish good procurement practices at all levels
Good procurement practices are a crucial tool to assure product quality and safety, protecting against various problems, including corruption.247 Many international organisations (such as the Global Fund, UNITAID, the US PEPFAR, the UN Population Fund, the StopTB Global Drug Facility, Médecins Sans Frontières, and the International Committee of the Red Cross) have implemented strict guidelines that require procurement only of products with stringent regulatory agencies' approval, WHO prequalification, or interim approval (eg, by a WHO Expert Review Panel).248
Many LMICs have centralised medicines procurement agencies, which provide the opportunity to apply good procurement practice.124 An increasing number of national procurement agencies are applying similar policies, insisting on products that meet prespecified quality standards. The Mission for Essential Drugs and Supplies in Kenya has been a pioneer in this regard. Panel 11 describes how strict quality requirements and frequent controls have reduced the percentage of quality failures.
Panel 11. Reduction in quality failures after introduction of a strict quality assurance policy in Kenya.
Mission for Essential Drugs and Supplies (MEDS) is a collective medicine procurement agency of faith-based health organisations in Kenya, established in 1986. For many years, MEDS has had a strict quality assurance policy, operating its own medicine quality control laboratory, which is prequalified by WHO. About 4% of the buyer price charged is invested into quality assurance. Figure 9 shows the reduction in quality failures between 1997 and 2013.
Figure 9.
Percentage of quality failures reported by MEDS, Nairobi (1997–2015)
Data from MEDS. Figure adapted from MEDS, Nairobi, Kenya, 2015, with permission. The red line in the graph presents the reduction in quality failures of medicines procured by MEDS, showing that a well published and enforced quality assurance programme can lead to better quality products. The green line presents the quality results in external samples, which were submitted to the MEDS laboratory for testing, where a similar trend is visible. A failure rate below 3–5% of samples tested indicates a well functioning quality assurance system. MEDS=Mission for Essential Medicines and Supplies.
Many procurement guidelines exist, but major challenges remain in implementation, as quality procurement is complex and costly.249 Corruption in procurement represents a major obstacle for both national and international agencies and contributes to problems in quality and safety as well as efficiency.250 The Commission believes that countries moving towards UHC must invest in improving procurement processes for quality-assured essential medicines. The use of pooled procurement can contribute to improved affordability. Quality assurance mechanisms as described previously can help to attain the ultimate goal. Coordinated international and national efforts are required to achieve system-wide and sustainable improvement in procurement practices.251
Promote surveillance of product quality and safety
Pharmacovigilance, the continual monitoring of medicines following their release, embraces an overall concept of patient safety, including documentation of adverse reactions, substandard quality, and inadequate use. A large number of newly established national pharmacovigilance programmes in LMICs have joined the WHO Programme for International Drug Monitoring over the past 20 years (appendix 3.2).252
LMICs represent 75 of the 122 countries contributing to the global programme for pharmacovigilance. Although participation in the programme is growing, the frequency and quality of reporting needs substantial improvement. The analysis of so-called safety signals—indications of an emergent problem with medicines safety—requires sophisticated algorithms, which might be beyond the capacity of LMICs. However, a global database, VigiBase (Uppsala Monitoring Centre), was established to allow for the analysis of aggregated data from countries participating in the global programme. By 2015, VigiBase contained over 10 million individual case safety reports (figure 10 ). Although only 9·4% of those reports had been submitted from LMICs, these contributions are increasing. By contributing to the Uppsala Monitoring Centre database, LMIC regulators and pharmacovigilance programmes can generate safety signals to detect potential medication-related safety problems relevant to their settings.
Figure 10.
Number of adverse drug reaction reports in HIC and LMIC
Data from Uppsala Monitoring Centre. HIC=high-income countries. LMIC=low-income and middle-income countries.
When use of a particular medicine is concentrated in LMICs only, the contribution of safety reports can be substantial. For example, extrapyramidal disorders from artesunate-amodiaquine malaria treatments were identified on the basis of VigiBase case reports submitted from eight nations in Africa.253 In LMICs, new medicines for tropical diseases, often developed through public health initiatives, are being widely introduced without previous experience in countries with well developed pharmacovigilance systems.254 Rather than waiting for spontaneous reporting, such launches should be accompanied by active surveillance of cohorts of exposed subjects.255 For tropical diseases, such active surveillance is the only mechanism to establish the safety profile of new products under typical use conditions. Regional regulatory networks are also harmonising pharmacovigilance systems, adapting ICH pharmacovigilance requirements to the situations of their members.256
New mobile telephone technologies create opportunities to make spontaneous reporting easier for health-care professionals and patients. Methods are currently being developed to ethically analyse social media information for early identification of possible problems. The widespread introduction of electronic health records can also enable rapid access to information on patient outcomes in exposed populations. Early experiences suggest that data mining technology can also be used to identify substandard and falsified products—eg, if national centres can handle reports quickly, can transmit detailed information on the product and its distribution channels, can contact primary reporters for further information, and have the laboratory capacity to test suspect products.257 NMRAs should invest in developing these capabilities.
Medicine quality surveillance could also be advanced by new tools that do not require analysis in a laboratory. Thus, new technologies could also allow other stakeholders and the general public to be involved in quality assurance of medicines. In January, 2016, only six countries in Africa and six countries in Asia have WHO prequalified medicine quality control laboratories.258 In 1997, an inexpensive mobile thin-layer chromatography technology became available, known as Minilab (Global Pharma Health Fund). The Minilab259 can detect markedly substandard or falsified pharmaceutical products outside of a laboratory environment—for example, in customs offices—and does not require specialised skills to interpret the results.260 More than 700 Minilabs are now operational in at least 70 LMICs. In a study on antimalarials in six African countries, 935 samples were tested in Minilabs and 305 samples in laboratories.261 Minilab results for co-trimoxazole were largely comparable with those of classical tests.262 Diagnostic accuracy for other medicines has not been evaluated. The major drawback is that the outcome depends on the visual acuity of the observer,263 but this can now be resolved with a smartphone app. This method is especially useful for detecting a gross lack of active ingredient.
In the past decade a variety of portable, battery-powered, non-destructive chemical analysers that require no sample preparation or consumables have also been developed. Some techniques can be done through blister packs and bottles.264, 265 Although relatively capital expensive, they are inexpensive to run.266 A suitcase-sized microfluidics device for rapid chemical analysis of medicines has been developed.267 The US Food and Drug Administration has developed CD3, a handheld tool for the visual examination of packaging in comparison with reference images in the device.268 Laboratory-based evaluations of single systems for a few medicines have been done, but diagnostic accuracy, ease of use, or cost-effectiveness, especially for co-formulated medicines, have not been assessed.264, 269 Hand-held tools are not always accurate in detecting substandard medicines, but can be used to detect falsified medicines that contain no active ingredients.
Track-and-trace technologies are also being developed to enable supply chain operators and patients to check the true identity of a product. A wide array of technologies have been proposed, from unique serial numbers to radio frequency identification tags, miniature edible tablet tags, or short message service verification.270 In the USA, medicine packages are now required to bear a unique serial number for tracking the product through the supply chain. An EU directive is requiring all facilities that import, buy, or sell medical products to track many products through two-dimensional barcodes by 2019.271 The Ministry of Health Malaysia has invested in the Meditag hologram system. After criminals faked registration numbers, the Ministry supplied decoders to all licensed pharmacies and pharmaceutical enforcement branches.272
Mobile telephone technologies can empower consumers to check the authenticity of a product before purchase—eg, through a scratch panel on the packet revealing a unique single-use code. These techniques are increasingly being used in wealthier countries in Asia and Africa. Implementation of these systems, however, can face substantial obstacles. For example, attempts to falsify this system have already been uncovered in Nigeria.273
In conclusion, new technologies have a great potential to create systems to prevent falsified products from entering the legal supply chain, but require constant upgrades and strengthening to avoid infiltration by falsifiers. Such verification systems are likely to expand and become the norm, although they will be more difficult to establish and operate effectively in many LMICs.
Leverage political attention and commitment to advance accountability
The evidence-informed policies on quality and safety of medicines proposed by the Commission will only be successful with concerted and consistent political pressure. In 2014, two World Health Assembly resolutions274, 275 called on member states and WHO to strengthen regulatory systems for medical products and biotherapeutics, through political leadership, legal and policy actions, adequate funding, transparent decision making, collaboration, and information sharing. The resolutions also charged WHO with supporting and assessing the performance of NMRAs.
Few in-depth and up-to-date studies exist on the performance of NMRAs. Two WHO assessments276, 277 done several years ago found that regulatory capacities varied greatly worldwide, and that a considerable number of NMRAs fell far short of desired standards. The second survey from 2010,278 showed some progress: the number of regulatory websites with defined types and quality of information more than doubled, from 53 in 2001, to 116 in 2009. Another study identified 118 functional regulatory websites in 2015.279 Updates of pharmacovigilance information and guidance for applications had also become more frequent. However, many NMRAs remained unable to do the minimum range of regulatory functions, as defined by WHO (appendix 3.3).
The Commission notes that not all performance data are public; this lack of transparency hinders efforts at creating greater accountability and effectiveness of NMRAs. For example, the 2010 WHO study277 on 26 African regulatory authorities did not identify the countries discussed. In Latin America, WHO has identified six national authorities of reference, but neither the criteria nor assessment results are public. WHO should be more proactive in championing and supporting public assessments of the performance of NMRAs. If WHO cannot make the data and country names from its assessments available to the public, because of procedural or other limitations, then a more independent entity should be established to carry out the crucial task of assuring public accountability of NMRAs.
Several conditions must be in place to ensure effectiveness of an NMRA: a clear mission statement, adequate medicines legislation and regulations, appropriate organisational structure and facilities with clearly defined roles and responsibilities, adequate financial resources to develop and retain staff and ensure operational efficiency, effective guidelines and procedures, and internal quality assurance systems.280 The Commission believes that political attention can only deliver results if specific targets are defined for improving the performance of the NMRA, with timelines, process and outcome measures, and the means to do and then make such assessments publicly accessible.
The Commission has identified a number of key areas in which NMRAs have great potential for progress (panel 12 ). These key areas have been formulated so that they can also serve as indicators of regulatory performance for use in national and international assessments. Effective mechanisms for continuous monitoring, reporting, and corrective action need to be developed (section 6).
Panel 12. Priority areas for the development of new performance indicators of national medicine regulatory agencies.
A need exists for independent public assessment of the performance of national medicine regulatory agencies (NMRAs) in the following key areas using these suggested indicators.
A public regulatory website which is continuously updated
This website should present full information on applicable legislation, registered products, public assessment reports with approved product information, licensed facilities, public inspection reports, results of risk-based sampling with quality tests performed, and a module for safety reporting. A public website is an essential condition for transparency and accountability.
Product application dossiers and assessment reports published
This information can be further supplemented with information on the mean processing time of an application. Consumers might not be aware that some products are not regulated at all. In many low-income and middle-income countries the quality and safety of medical devices (including in-vitro diagnostics) and certain biological products (particularly blood and blood products) are subject to very weak, or even no, regulatory oversight. This information will also contribute to enhanced transparency.
Regulatory committees with one or more patient representatives
In many countries the general public and patients are not involved in regulatory assessments and decisions, although civil society involvement supports adherence to human rights principles.
Inspections performed and inspection reports published
Even when policies exist on paper, many countries struggle to enforce them with inspections. Many agencies therefore de facto do not control their markets or hold the various stakeholders (manufacturers, importers, wholesalers, and consumers such as hospitals and retail pharmacies) accountable. Inspections at points of sale can also be inconsistent, allowing unregistered and untraceable products through the supply chain and into the market. In several countries, including the USA, China, India, and Pakistan, some regulatory powers regarding the supply chain have been delegated to states or provinces, leading to discrepancies in enforcement and lack of central oversight (appendix 3.4). More data will promote accountability.
National manufacturers supported in achieving and maintaining good manufacturing practice (GMP)
The basic responsibility for quality and safety of a product lies with its manufacturer. Governments are only responsible to ensure that manufacturing standards exist and are enforced. Yet collaboration between NMRAs and manufacturers in promoting GMP presents an important opportunity. In 2012, a step-wise approach towards obtaining GMP was developed in Nigeria. Small incremental steps incentivised companies to aspire to the goal of manufacturing quality-assured medicines. In Ghana and Ethiopia, similar phase-in approaches towards full GMP compliance are in place. GMP qualification of a manufacturing facility unit can motivate working towards product-based WHO prequalification.
Risk-based surveys and samples tested to monitor the quality of marketed products and analysis reports published per year
With the costs of new rapid quality assessment tools dropping, there is the potential to increase the number of quality assessment studies, facilitating more accurate determinations of the prevalence of substandard and falsified products and comparisons across settings. A particular need exists for studies on the quality of medicines for non-communicable diseases and biological products.
Quality pharmacovigilance reports received and submitted to international databases
The number of reports from low-income and middle-income countries is still insufficient; tracking this information could motivate NMRAs to collaborate internationally. This might include spontaneous reporting and cohort event monitoring with national disease control programmes, especially for medicines for neglected tropical diseases after their release.
Regulation of products for export
Most countries, including high-income countries, have serious gaps in regulating the quality and safety of products for export.
Absence of legal obligation of patent linkage and extended data exclusivity
Some bilateral or regional trade agreements have imposed patent linkage, and test data exclusivity norms that are not required under the Trade-Related Aspects of Intellectual Property Right Agreement (so-called TRIPS-plus requirements; see also section 5 and appendix 5.1).
Conclusion
The Commission concludes that, despite impressive progress in several areas, serious problems remain with medicine quality and safety, particularly in LMICs. Many manufacturers produce substandard products, and the current global supply chain allows for many unsafe and dishonest practices. Complex products, such as biological medicines, also pose challenges for all regulators. In 2016, regulatory capacity and enforcement are insufficient in many countries, especially LMICs. This limitation threatens the health of people, as described in the case of Adwoa, and results in wasted resources. Global and national regulatory systems require considerable and urgent reform and strengthening to assure the quality and safety of medicines and contribute to more sustainable health systems and the achievement of UHC.
The Commission argues that the quality assurance strategies established by large donor programmes for AIDS, tuberculosis, and malaria should be leveraged to ensure future progress. The implementation of the recommendations requires the involvement of multiple stakeholders at all levels, including manufacturers, governments, procurers, and end users.
Recommendations
The Commission's recommendations, specifying the main actors involved in implementation, are:
-
1
Global efforts must be made to promote the harmonisation of quality assurance efforts through the use of an international standard regulatory dossier that covers both format and content. The implementation of e-CTD globally should be promoted to facilitate rapid exchanges of product assessments and site inspection reports among agencies. More intensive international collaboration and electronic exchange of information could simplify processes, prevent unnecessary duplication of effort in dossier assessments and site inspections, facilitate innovation, and shorten approval times.
-
2
WHO should evolve the WHO/UN Prequalification Programme to maintain a moving focus on new essential medicines. This evolution should move attention from mature products towards priority essential medicines that pose special challenges to regulators, such as human insulin and other biosimilars, and newly developed essential medicines. Its standards and public assessment reports should form the basis for regulatory convergence and mutual recognition, leading to rapid regulatory approval. A sustainable financial base must be created to maintain its full independence from donors and manufacturers.
-
3
Payers and procurement agencies must adopt good procurement practices that incorporate effective and transparent quality assurance. Quality assurance mechanisms must exist at all points in the supply chain. Appropriate quality assurance systems require investment. Sharing test results and findings of inspections can avoid duplication and increase efficiency.
-
4
Governments must redirect the activities of national regulatory agencies towards those that add value and reduce duplication of effort, and engage with a system for independent and public assessment of the performance of NMRAs. Activities aiming to address efficiency and effectiveness should cover all the basic components of a national medicines regulatory authority, with special focus on international harmonisation, prevention of duplicative efforts, maintenance of a single or central NMRA within a country, inspections and enforcement of regulations, assessments of new essential medicines for neglected diseases in their jurisdiction, regulation of medicine promotion, transparent reporting on the prevalence of substandard medicines in the market, pharmacovigilance, collaborating with domestic manufacturers in promoting GMP, and abstention from patent linkage and extended periods of data exclusivity.
-
5
Regulatory agencies must encourage the involvement of other stakeholders and the general public in promoting the quality and safety of essential medicines. This action can be achieved through, for example, the involvement of stakeholder representatives before regulatory decisions, the use of product quality verification testing at the point of sale, involving unique barcodes, portable low-cost quality-control equipment, or other technical devices linked via smartphones and the internet.
-
6
WHO and national governments must establish concrete targets and a public accountability mechanism for assessing the performance of national regulatory authorities. The goals should encompass all the basic components of a national regulatory authority, as listed in appendix 3.3, recommendations 4 and 5, and panel 12.
Section 4: promoting quality use of essential medicines
A patient's experience
Jomkwan, an obese man aged 65 years who attends a primary care clinic affiliated with the national health insurance scheme, is presenting with symptoms of uncontrolled diabetes. In consulting the patient's medical record, the provider on duty sees that the patient was prescribed glibenclamide (5 mg daily) when he last came to the clinic, about 3 months ago. He has also been diagnosed with hypertension and hypercholesterolaemia. Upon questioning, Jomkwan mentions that lately he has not been feeling well; he stated that he was somewhat shaky and his heart was pounding. He did not like that he was gaining more weight, despite skipping meals, and had decided to stop taking the glibenclamide since he had heard that it can make you gain weight. The doctor explains that diabetes medicines must be taken every day, and that it is important to have regular meals and physical activity. He adds a new antidiabetes medicine, sitagliptin, a medicine not included in the national insurance benefit package, to Jomkwan's regimen and instructs him to come back for a check-up in 3 months. When Jomkwan returns after 1 month, with worsening symptoms, he sees a different doctor, who tells him that according to standard treatment guidelines metformin, not sitagliptin and glibenclamide, should be used for his condition. She changes his prescription to metformin, and at his next check-up he reports feeling much better.
Quality use of medicines and UHC
Medicines have enormous potential to prevent premature deaths, alleviate suffering, and contribute to human wellbeing—but only when they are used appropriately. Medicines are beneficial when patients are prescribed clinically correct and affordable medicines to treat their conditions, and the medicines are taken in a timely way for the recommended duration. Yet inappropriate use of medicines continues, despite decades of efforts to improve it.281
For clinical, public health, social, economic, and ethical reasons, quality use of medicines must become an explicit intermediate performance goal of all health systems working toward UHC. Indeed, the Commission contends that UHC both necessitates and can facilitate a change toward quality use of medicines. If health-care delivery and financing systems do not focus on quality use of medicines—including using less expensive equivalent products when they are available—as a core system objective, they will waste resources on inappropriate use of medicines. In short, if measures are not taken, moving towards UHC can also increase the inappropriate use of medicines. Ghana's early experiences in expanding coverage showed that failure to address medicines use can threaten system sustainability.282 In the USA between 1999 and 2010, substituting generic products for their brand-name counterparts saved the health system more than $1 trillion.283 WHO has estimated that, if 18 common medicines were sold as lowest-price generics rather than originator brands, between 9% and 89% of costs could be saved across 17 countries, mostly MICs.284
Inappropriate use of medicines, which is a longstanding challenge, becomes increasingly problematic as pharmacotherapy evolves.96 Prescribing second-line and third-line treatments with higher prices when older, safer, first-line therapies with lower prices are indicated and available, is inappropriate. For example, the use of insulin analogues increased from 19% in 2000, to 92% in 2010, among privately insured patients with type 2 diabetes treated with insulin in the USA. This increase was associated with an increase in patients' out-of-pocket costs, but with no clear evidence of clinical benefit.285 Since poverty is associated with poorer health and the need for more medicines, inappropriate use of medicines by the poor could exacerbate health disparities. Expanding coverage without addressing how medicines are used can harm patients, waste resources, and impede reaching the goals of UHC.
Increasingly available targeted therapies—very highly priced and highly effective for certain patient subgroups—represent another challenge, as their use requires extensive diagnostic testing and careful monitoring in patients whose genomic, molecular, or cellular disease markers they target. Inappropriate use of targeted therapies medicines will also waste substantial resources.
Reasons for limited progress in promoting quality use of medicines
The Commission recognises three main reasons for the little progress in improving use of medicines. The first is that access to medicines has dominated the global discourse about, and funding for, medicines since HIV treatments became available in the 1990s.286, 287, 288, 289 This intense focus on access has limited the focus on the issue of appropriate use, through which the potential benefits of accessible medicines might not be realised.
The second reason is that the problem of inappropriate use of medicines has had no clear owner. Medicines use is determined by the combined behaviours of many actors in local and national health systems (panel 13 ). The health, direct, and indirect economic costs of inappropriate use are often borne by individual patients and households paying out of pocket for medicines. Efforts to quantify the system-wide and individual health and economic consequences of inappropriate use are largely speculative. Reliable data on medicines expenditures and use by individual patients are scarce, and models linking clinician and patient behaviours with long-term health and financial outcomes are underdeveloped, especially in LMICs. The system-wide effects of inappropriate use on population health and economic development are therefore not widely recognised.
Panel 13. Appropriate use of medicines depends on behaviours of many stakeholders.
-
•
Patients must take the medicines that are clinically appropriate for their illnesses, in the right doses and dosage forms, at the right time, and for the recommended duration. Patients and their caregivers require: knowledge about symptoms and information to decide when and where to seek care; convenient access to quality medicines at affordable costs; and knowledge, motivation, and skills to use the recommended medicines as directed.
-
•
Prescribers must prescribe clinically appropriate, cost-effective products. They require: diagnostic and therapeutic decision-making skills; up-to-date, evidence-based treatment guidelines that are consistent with medicines available and reimbursed in their systems; reliable, valid diagnostic tools in facilities; professionalism, training, time, and appropriate incentives to act in the interests of patients and caregivers.
-
•
Dispensers must provide high-quality products and sound advice at affordable prices. They require: knowledge to correctly order, purchase, store, and sell high-quality products from essential medicines and reimbursement lists that are consistent with up-to-date, evidence-based treatment guidelines; facilities, tools, processes to correctly order, purchase, receive, store, and sell needed, high-quality products; professionalism, training, time, and appropriate incentives to act in the interests of patients and customers.
-
•
Professional boards are responsible for setting standards for training and licensing care providers. They need: licensing and continuing education requirements that promote competent clinicians (doctors, pharmacists, nurses, and others); regulatory oversight and power to enforce professional standards.
-
•
Consumer organisations and pharmaceutical manufacturers provide information to health professionals, and in some settings directly to the public. They require: regulatory oversight to provide unbiased, evidence-based information.
-
•
The public sector and the private sector must meet demand for medicines with efficient supply systems. This requires: governance and management structures of public facilities, which must function accountably and efficiently to maximise the effective and efficient use of public resources; government standards and oversight of private sector providers from whom most medicines in low-income and middle-income countries are purchased.
-
•
Third-party payers who are increasingly covering care costs in systems moving towards universal health coverage must make pharmaceutical coverage decisions and reimbursement arrangements with public and private sector providers that incentivise appropriate use of medicines. They require: financial resources, technical know-how, fair processes, and management tools to ensure that they pay for the right medicines at costs they can sustainably afford, considering population and individual patient needs and up-to-date clinical evidence; routinely collected information to monitor medicines use and spending; negotiating skills to engage in value-focused contracts with providers and pharmaceutical manufacturers.
-
•
Regulators must guarantee that only safe, efficacious, high-quality products are available on the market, and should regulate promotional activities by industry. They require: capacity and resources to review and decide on licensing of originator, generic, and biosimilar products in a timely manner; capacity and resources to ensure licensed product quality; independence, transparency, and accountability of regulatory processes.
-
•
Manufacturers and importers must produce and sell quality medicines that are needed, working with public procurement systems, wholesalers and distributors to establish efficient supply chains. They need: regulatory oversight, technology, and incentives to ensure manufacture, import, and distribution of needed quality products.
-
•
Manufacturer associations establish and monitor industry codes of conduct. Effective regulatory environments within which high standard codes of conduct are enforced.
-
•
Scientists in universities and companies must invent new molecules and formulations that meet population needs. They require: society, industry, and philanthropy funding and incentives to conduct needed research and development.
Finally, intervening to improve medicines use is challenging. A wide array of health-system stakeholders with legitimately different objectives, functions, and incentives influence medicines use.68, 290 One driver of inappropriate use is the economic profits for vendors and service providers, whose incomes depend on selling medicines.291 Fragmentation in health systems hinders concerted, system-focused efforts to improve medicines use; these problems have been further exacerbated by vertical programmes for access to medicines that focus only on specific health problems. Coordinated and sustained attention to priority medicine use problems is also undermined by a range of factors: the almost singular focus on the part of many international donors, non-governmental organisations, and development agencies on access to medicines for AIDS, tuberculosis, and malaria; fragmented and frequently competing priorities across stakeholders; and operating environments with weak legal and regulatory structures, lack of awareness of the problem, or inadequate political will to tackle it.
The Commission suggests that barriers to quality use of medicines could be addressed by an explicit, system-wide, evidence-based emphasis on medicines use by all relevant stakeholders. As countries take concrete steps towards achieving universal coverage, the time is right to design and implement novel approaches to promote quality use of medicines, building on lessons from the past, and taking advantage of current and future system opportunities and technology innovations. The processes entailed in developing UHC offer unique opportunities through: engaging different stakeholders;292 generating new laws, regulations, and institutions; cultivating information-driven organisations to manage and coordinate benefit packages; and focusing investment, policy, and delivery system strategies towards achieving population health, value-for-money, and sustainability of systems.
In the remainder of this section, the Commission first proposes a taxonomy for inappropriate use, then summarises what is known about interventions to promote quality use of medicines, and describes national strategies in three countries to improve use of medicines. Lastly, it offers actionable recommendations to promote the quality use of medicines.
A taxonomy of inappropriate use of medicines
Since the 1985 Nairobi Conference on the Rational Use of Drugs,44 initiatives that focused on medicines use have used various terminology, including rational,281, 293 quality,294 and responsible,295 to convey the concept of appropriateness. The Commission uses appropriate use of medicines to refer to use of medicines that is both consistent with clinical evidence and economically wise (that is, generating health value for money spent within a given budget). The phrase quality use of medicines is used interchangeably, and should not be confused with a focus on the product quality of medicines.
Inappropriate use of medicines can happen through using too much, too little, or the wrong kind of medicine. To facilitate exploration of medicines use problems, the Commission classifies inappropriate use into four categories: unnecessary use (overuse), failure to use needed medicines (underuse), incorrect use (misuse), and unnecessary use of highly priced medicines (table 5 ). All types of inappropriate use can harm individual and population health directly and indirectly, waste scarce resources, and undermine public trust in providers and the health system.
Table 5.
Categories and examples of use of medicines problems
| Definition | Examples | |
|---|---|---|
| Unnecessary medicines use (overuse) | Use of a medicine that is not effective or needed for the target indication according to clinical evidence | Antibiotic* use for viral illnesses; vitamins, corticosteroids without appropriate indications; malaria treatment without proper diagnosis; fixed-dose combination products when one drug would suffice (eg, cough and cold remedies) |
| Failure to use needed medicines (underuse) | Lack of use of a medicine that is standard of care to effectively treat a target indication | Lack of treatment for patients with non-communicable diseases, including lack of treatment of mental illness; lack of secondary prevention combination therapy for patients with a history of cardiovascular events; underuse of opioids for cancer or other severe pain; lack of use of oral rehydration solution for patients with diarrhoea; poor adherence to treatment |
| Incorrect medicines use (misuse) | Use of the wrong medicine for the target indication and patient, or wrong use of the right medicine | Broad-spectrum antibiotics when narrow-spectrum antibiotics would suffice; teratogenic medicines used in pregnant women; coprescribing of absolutely contraindicated medicines; prescribing contraindicated medicines based on patient characteristics (eg, aspirin in children and adolescents for the treatment of fever); medicine dose not adjusted to patient age, weight, organ function; injections for patients who can swallow oral products; targeted cancer therapy use without confirming presence of targets |
| Unnecessary use of highly priced medicines | Use of a medicine that is more costly than a possibly equally effective and safe medicine | Use of originator brand products and branded generics when lower-priced quality international non-proprietary name generic products could be used; use of second-line and third-line medicines when first-line medicines should appropriately be tried first; use of new and highly priced medicines of questionable added value, when an older, better-characterised medicine would suffice (eg, new oral and injectable options for type 2 diabetes, new analogue insulins for type 1 diabetes) |
For some therapeutic groups, such as antibiotics, addressing inappropriate use requires key interventions outside the health sector (eg, agriculture), which have been described elsewhere.296
Medicines use depends on the behaviours of many stakeholders in health systems (panel 13), particularly the diagnosis, prescribing, and dispensing practices of providers, and the care-seeking and medicines-taking practices of patients. The patient, Jomkwan, was prescribed an antidiabetic medicine that was not appropriate given his other conditions, and suffered from adverse effects such as gaining weight.
Other stakeholders involved in the regulation, financing, payment, and organisation of health-care delivery services98 influence the behaviours of providers and patients (panel 14 ). Promotion is particularly relevant in this regard (panel 15 ). As a result, each type of inappropriate use can have multiple contributing factors along the complex chain of developing, licensing, manufacturing, procuring, distributing, prescribing, dispensing, buying, reimbursing, and taking medicines (panel 17 ). In Jomkwan's story, the providers are salaried employees and prescribing is less subject to influence by financing incentives, either to underprescribe (to stay within a limited budget) or overprescribe (to generate revenue), than might be the case in other provider payment systems.306
Panel 14. Influences of health systems' functions**Selected system functions as defined by Roberts and colleagues.98 on provider and patient behaviours.
Regulation
-
•
Limited provider competencies
-
•
Lack of low-cost, quality-assured generic products on markets
-
•
Actual or perceived low quality of generic product
-
•
Actual or perceived low quality of care in public sector
Financing
-
•
Prevention prioritised, at the exclusion of financing treatment
-
•
Lack of functioning chronic care-delivery systems
-
•
Lack of diagnostic and monitoring tools
-
•
Lack of effective treatments in needed forms
Payment
-
•
Incentives for low-volume or high-volume prescribing or dispensing
-
•
Lack of incentives for therapeutic drug monitoring
-
•
Reimbursement restrictions to medicines in inpatient settings
-
•
Out-of-pocket payment
Organisation
-
•
Lack of care systems for continued ambulatory care for chronic conditions
-
•
Lack of qualified diagnosticians, prescribers, dispensers, other care givers
-
•
Supply chain problems leading to stock-outs of tests, medicines
Panel 15. Better regulatory control of pharmaceutical promotion is necessary.
A key driver of inappropriate use of medicines is pharmaceutical promotion,297 when companies deliberately seek to influence sales by targeting health professionals and patients. Although data remain poor, promotion appears to be growing in middle-income countries because of growing markets, increasing numbers of local manufacturers, more direct-to-consumer advertising, lack of local codes of marketing practice, weaker regulation, and less-developed consumer movements when compared with high-income countries.
Most new pharmaceutical promotions are subtle—they might not even be immediately recognisable as product advertisement. Some instances have received considerable attention, such as direct payments to medical practitioners in China.298 Globally, the problem is increasingly hard to control as companies transition to digital methods, including the use of social media (panel 16 ). The number and severity of breaches of national legislation and industry codes are not related to the size of the company.82
Panel 16. Digital promotion of medicine goes beyond web sites.
Companies increasingly use a wide range of digital marketing approaches, such as online events, emailed product updates, and webinars. Health-care professionals use websites to read medical news, connect with peers, and obtain continuing education credits—these often incorporate advertisements, sponsored discussion forums, and marketing games. For example, Sermo's Alzheimer's Challenge invited site users to earn cash by answering questions about clinical trial data for a branded product.299
Companies also target consumers through apps, search engine optimisation, and social media campaigns. In 2015, an Instagram posting featuring Kim Kardashian promoted a morning sickness medicine to her 42 million social media followers (appendix 4.3). The US Food and Drug Administration ordered the manufacturer to remove the posting on the grounds that it was “false or misleading”.300 By the time the decision was reached the post had received nearly half a million likes and 11 000 comments.
An extensive literature review301 by WHO and HAI found that promotion strongly influences prescribing, and that prescribers underestimate the influence of company funding, educational events, and research. Effective interventions to counter this effect were: government regulation, training of students, media exposure of abusive promotion, and free provision of non-commercial therapeutic information to professionals and the public.
Yet most regulatory authorities struggle to control promotion. Many governments do not consider it a priority, and enforcement is often poor. Preapproval of advertisements is scarce, so breaches are identified after exposure and weak penalties are not a deterrent. Some governments rely on industry self-regulation,302 but this strategy is often insufficiently effective, as voluntary codes are created and monitored by the companies themselves and are not necessarily legally enforceable.
The WHO Ethical Criteria for Medicinal Drug Promotion46 remain the gold standard for controlling promotion. They advise, among other strategies, against direct-to-consumer advertising of prescription medicines to the public. So, while they did not explicitly anticipate internet advertising or social media, a ban on direct-to-consumer advertising is enough to set the baselines for the regulation of both methods. Regulators can then adapt the criteria to the contemporary context. The US Sunshine Act,303 France's Loi Bertrand,304 and the Dutch Transparency Register, which mandate disclosure of financial links between pharmaceutical companies and health-care professionals, also reflect the ethical criteria. This transparency now needs to be followed by an independent review and, when necessary, corrective action.
Stricter regulation of pharmaceutical promotion is one of the core functions of national regulatory authorities. Lack of funding for adequate monitoring and enforcement remains a key barrier that needs to be removed.305 Governments should also ensure access to unbiased and free information on medicines, which should be treated as a public good.
Panel 17. Examples of system factors contributing to various forms of inappropriate use of medicines.
Overuse
-
•
Availability of products that lack efficacy, safety, or comparative effectiveness
-
•
Business models incentivising aggressive marketing of products
-
•
Lack of effective regulation to limit licensing of ineffective products, aggressive marketing
-
•
Payment models incentivising high-volume prescribing, dispensing, and use
-
•
Lack of qualified prescribers, dispensers, or other care givers
-
•
Patient or public expectations, perceptions, and preferences
Underuse
-
•
Lack of practical, affordable, reliable, and valid diagnostic tools
-
•
Lack of effective treatments in needed forms for certain conditions
-
•
Supply chain problems leading to stock outs of tests and medicines
-
•
Lack of qualified diagnosticians, prescribers, dispensers, and other care givers
-
•
Lack of patient knowledge and resources to seek care, purchase, and take medicines
-
•
Payment models incentivising low-volume prescribing, dispensing
-
•
Out-of-pocket payment resulting in underuse
-
•
Patient or public expectations, perceptions, and preferences
Misuse
-
•
Lack of needed dosage forms and strengths
-
•
Lack of practical, affordable, reliable, valid diagnostic tools, and therapeutic monitoring tests
-
•
Supply chain problems leading to stock-outs of medicines and test materials
-
•
Payment models do not incentivise use for therapeutic drug monitoring
-
•
Lack of qualified prescribers, dispensers, and other care givers
-
•
Lack of resources for therapeutic drug monitoring
-
•
Limited time and knowledge of prescribers and dispensers
-
•
Patient or public expectations, perceptions, and preferences
Unnecessary use of highly priced medicines
-
•
Ineffective regulation to guarantee low-cost, quality-assured generic products on markets
-
•
Ineffective policy processes to encourage price and quality competition for generic products
-
•
Ineffective policy and negotiation processes to lower prices of medicines
-
•
Payment models incentivising use of high-cost products
-
•
Payment models do not incentivise use of low-cost, quality-assured generic products
-
•
Lack of effective communication on quality and value of lower cost, high-quality generic products
-
•
Lack of communication on evidence or guideline-based care algorithms
-
•
Provider, patient, public expectations, perceptions, and preferences
Effective approaches to promote quality use and reduce inappropriate use depend on the type of medicines use problem targeted, the system factors and actors, and the specific context, including the health system and economic, legal, societal, and political environments.
Promoting the quality use of medicines is challenging but possible
The need to promote the quality use of medicines to optimise health outcomes and increase the efficiency of health and medicines expenditures has long been recognised. The 2014 Alliance for Health Policy and Systems Research Flagship Report on Medicines in Health Systems68 traced the evolution of thinking about the rational use of medicines (originally drugs), beginning with the pivotal role of the 1985 Nairobi Conference.44
Since the Nairobi Conference, WHO member states have endorsed a series of World Health Assembly resolutions related to improving use of essential medicines (appendix 4.1). The resolutions cover a wide range of related topics, offering approaches to different pieces of a complex puzzle. Among other strategies, they urge member states to “invest sufficiently in human resources and provide adequate financing to strengthening institutional capacity”.307 In May 2015, WHO member states also committed to a Global Action Plan to tackle antimicrobial resistance, which covers the use of antimicrobials in human health, animal health, and agriculture.308
Targeted approaches: effective under the right conditions
Many studies have assessed strategies intended to improve use of medicines by health-care providers and users in a wide range of settings, including public and private sector health-care facilities, pharmacies and drug shops, and communities. The various interventions have targeted a broad array of health workers, most commonly physicians, but also paramedics (clinical officers, nurses, and midwives), pharmacists and other dispensers, shop attendants, community health workers, and patients and community members. Interventions have focused on problem practices (eg, antibiotic use, injection use, or polypharmacy), care for specific conditions (eg, respiratory infections, malaria, diarrhoea, hypertension, or diabetes), or on processes of care (eg, diagnosis, laboratory testing, communication, treatment decision making, or explanations about medicines). However, key contextual factors and details about implementation are often poorly described in published reports. Summarising research on the effectiveness of many heterogeneous interventions done in diverse settings is therefore challenging.
The Rx for Change database, maintained by the Canadian Agency for Drugs and Technologies in Health, is the most comprehensive source of information about the effectiveness of interventions targeting medicines use. Rx for Change identifies systematic reviews of interventional approaches and summarises results of high-quality reviews that used rigorous study designs endorsed by the Cochrane Collaboration Effective Practice and Organization of Care309 group.310 Using the Rx for Change intervention classification, the Commission summarised evidence about the effectiveness of different types of interventions targeting health professionals (figure 11 ; appendix 4, table 1) and patients or consumers (figure 12 ; appendix 4, table 2).
Figure 11.
Evidence from high-quality systematic reviews about effectiveness interventions targeting use of medicines by health professionals
Figure 12.
Evidence from high-quality systematic reviews about effectiveness of different types of interventions targeting patients and consumers
No large-scale systematic reviews have been done of interventions to improve consumer and patient behaviour in LMICs. Many studies on how to improve medicines use in LMICs focused on strategies that target relatively small groups (of clinicians, health facilities, or patients) in geographically limited programmes. Very little system-wide work has been done to bring successful pilot interventions to scale and assess the effect. Two systematic reviews311, 312 of evidence on interventions to improve health-worker behaviour have used the Effective Practice and Organization of Care criteria313 for study quality and restricted their analyses to studies from LMICs only. In the first, Holloway and colleagues311 summarised the studies on the effects of well designed interventions to improve treatment of paediatric infections (n=44) and general outpatient prescribing (n=110). The median improvement in practice across all types of interventions in LMIC settings was modest (about 16% for the treatment of paediatric infections and 7% for general outpatient prescribing).14, 311 Larger median effects tended to occur when multifaceted strategies combined several components (for example, education directed at both providers and consumers about the same medicine use issue), as compared with single strategies. Community case management programmes (in which community members are trained to recognise and treat common illnesses such as respiratory infections and diarrhoea, provided with medicines, and supervised in care delivery) had consistently positive effects that were notably higher than those of other strategies.
The second systematic review is the Health Care Provider Performance Review,312 a methodologically rigorous, large-scale systematic review of 497 studies of interventions to improve health-worker performance (including diagnosis, prescribing, and dispensing) in LMICs. Its preliminary results indicate that:
-
•
High-intensity training (more than 5 days and with an interactive training modality) combined with post-training supervision is particularly effective, with a median improvement of 28% in LICs and 17% in MICs.
-
•
Interventions that engage health-worker teams in group problem solving, such as quality improvement collaboratives, combined with low-intensity training (less than 5 days or no interactive training modality), also have sizeable effects in LICs (median 45% improvement). However, most studies of these interventions had methodological limitations.
-
•
In MICs, supportive interventions for patients or community members combined with low-intensity training for health workers tended to have large effects (median 24% improvement); effectiveness increased when the intervention was combined with other management techniques and strengthening supervision, infrastructure, or governance (median 30% improvement).
System-wide approaches: change is difficult to scale up
Evaluation results from a small number of large-scale programmes to improve the quality use of medicines have demonstrated that the complexity of the many factors that influence medicines use necessitates multifaceted interventions. Furthermore, it is challenging to effectively adapt these approaches to local circumstances. Two examples are instructive:
-
•
The Integrated Management of Child Health (IMCI) approach was promoted by WHO, implemented in many countries in the late 1990s, and assessed at scale in five countries.314 IMCI involves algorithm-based and symptom-based treatment of common childhood illnesses, extensive health-worker training and supervision, and community sensitisation to improve illness recognition and care seeking. IMCI is the type of multi-component intervention that literature reviews suggest should be effective. However, national evaluation results were sobering.315 Most countries found it challenging to scale up the strategy while maintaining fidelity to the approach. Community engagement was generally weak, and essential programme messages were not effectively communicated. Furthermore, programme reach suffered because implementation exclusively relied on public sector delivery systems and failed to involve other sources of care. The assessments suggested that successful implementation of large national programmes to improve use of medicines requires more than specific technical design elements. Political commitment, dedicated human and financial resources, coordinated policies and programmes, and meaningful engagement of various stakeholders are equally important.315
-
•
The tuberculosis Directly Observed Therapy (DOT) programme is one of the core elements at the heart of WHO's Stop TB Strategy.316 Daily supervision of therapy, often in specialised tuberculosis treatment centres, is burdensome; nevertheless, the importance of DOT has frequently been defended as essential to programme success.317 However, a systematic review318 concluded that DOT did not provide a solution to low levels of adherence. Given that substantial resources are required to implement DOT, the review concludes that tailoring treatment models to local circumstances might better address financial and logistical barriers to care, as well as patient and staff motivation and other issues.
Examples of effective system-focused approaches
Despite the sobering assessment results described, there are also promising interventions. Comparison of three long-term national programmes—from Australia, Brazil, and China—illustrates different priorities of medicines use across countries, as well as multifaceted and context-driven efforts to achieve improvements through coordinated policy implementation.
Australia: NPS MedicineWise is committed to ensure quality use of medicines
NPS MedicineWise was established in 1998 to implement a pillar of Australia's National Medicines Policy that commits all stakeholders to ensuring quality use of medicines.319 An independent organisation, NPS MedicineWise does a range of multifaceted, evidence-based activities and interventions, including therapeutic behavioural change programmes, data-driven quality improvement reports and interventions, consumer awareness campaigns, and decision-support tools for health professionals and consumers. NPS MedicineWise also provides professional development activities, including online learning, online case studies, clinical e-audits, and educational visiting. Its in-depth assessment strategy incorporates strong qualitative elements, such as regular stakeholder surveys, as well as time-series analyses of routine prescribing data, to document changes stimulated by its programmes (panel 18 , table 6 ). Funded by the Australian Government, NPS MedicineWise reports annually on its achievements in quality gains and cost savings. In 2013–14, in addition to savings in other areas, NPS MedicineWise programmes across seven therapeutic areas resulted in savings of AU$ 69·2 million for the Pharmaceutical Benefit Scheme.321 Its revenue reported in 2014–15 was about $45 million,320 compared with total spending of just over $9 billion in 2013–14.322
Panel 18. Improving quality use of medicines by Australian providers and consumers.
NPS MedicineWise is an independent, not-for-profit, evidence-based organisation that works across the Australian health sector and broader community to deliver improved medicines use, better health outcomes, and more efficient health care.
NPS MedicineWise involves stakeholders, develops key messages, and produces a mix of publications, products, and interventions designed to achieve these specific outcomes.
An in-house evaluation team assesses NPS MedicineWise and its activities. As part of its evaluation process, NPS MedicineWise conducts regular general practitioner, pharmacist, and consumer surveys of knowledge, attitudes, awareness, and behaviours around medicine use and NPS programmes.
Table 6.
NPS MedicineWise operating results reported in the 2015 Director's Report320
|
2014 |
2015 |
|||
|---|---|---|---|---|
| Target | Actual | Target | Actual | |
| Reported Pharmaceutical Benefit Scheme savings (AU$ million)* | 69·26 | 70·44 | 69·28 | 69·24 |
| Reported Medical Benefit Scheme savings (AU$ million)† | 5·0 | N/A | 4·5 | 33·05 |
| Number unique general practitioner participants | 14 000 | 13 129 | 14 000 | 14 447 |
| Number consumer interactions | 200 000 | 942 436 | 200 000 | 1 732 635 |
Pharmaceutical Benefit Scheme savings reported for a particular year are on the basis of the evaluation report completed during the year, based on the previous year's data.
Medical Benefit Scheme savings reported in 2015 covers savings for both 2014 and 2015.
NPS MedicineWise reaches a broad range of health professionals and consumers. A total of 21 715 health professionals participated in NPS MedicineWise programmes in 2013–14, including 65% of registered general practitioners. NPS MedicineWise also has a prominent internet presence with 3·3 million visits to its website and 2·3 million Twitter and 1·5 million Facebook views in the 2013–14 financial year.
With funding of $48·6 million from the Department of Health (Australia) in 2015, NPS MedicineWise is estimated to have saved the Pharmaceutical Benefit Scheme more than $69·2 million, with more than $15 million in additional savings to the Medical Benefit Scheme in that year, for an annual Return on Investment of more than $1·7 billion.319 Since its inception, NPS MedicineWise is estimated to have delivered savings to the Australian Government of more than $900 million.
Key features that contribute to the success of NPS MedicineWise include independence from government, a coordinated range of evidence-based programmes influencing both prescribers and consumers, the security of long-term funding against the agreed upon benchmarks, and using both ad hoc and routine data to evaluate success.
China: improving the quality of antibiotics use
Increasing rates of antimicrobial resistance are a major public health issue in China and a substantial challenge to global health. Overprescription of antimicrobials and the use of antibiotic infusions for outpatients are widespread. Primary health care is still emerging in China—only 57% of outpatient visits took place in primary care facilities in 2014.323 Public tertiary care hospitals are the main health-care providers, and most patients access these hospitals even for common illnesses.
Spurred to action by the 2002 severe acute respiratory syndrome outbreak,324 the Chinese Government has adopted a number of policies to address antimicrobial use (figure 13 ). In 2011, the Ministry of Health in China launched a campaign to promote appropriate use of antimicrobials in hospitals.326
Figure 13.
Total and parenteral outpatient antibiotic use in tertiary hospitals in China (2005–12)
Data adapted with permission from Sun and colleagues. 325 The comprehensive antibiotic stewardship programme in hospitals targeted outpatient antibiotic prescribing rates of <20% of patients and inpatient antibiotic prescribing rates of <60% of patients; antibiotic prophylaxis to be given before incision and not continued for more than 24 h; and total inpatient antibiotic consumption <400 defined daily doses per 1000 inpatient days. By December, 2012, antibiotic consumption had dropped to half of its highest level and use of parenteral antibiotics in outpatients had begun to decline.
Following consultations with stakeholders, the government announced a multifaceted package of policy reforms.325 Hospitals that did not meet established targets risked being downgraded and dismissal of their leadership; medical staff violating the regulations could have professional qualifications revoked. The 2011 interventions were followed by immediate and substantial reductions in both inpatient and outpatient antimicrobial use.325, 326 Although the proportions of outpatient and inpatient antibiotic use achieved national targets following the 2011 interventions, achieving targets for quality of antibiotic prophylaxis and practice requires additional policies and long-term implementation efforts.
Key features that contributed to the success in changing antibiotic use patterns included: sequential policies aimed at different aspects of the problem; availability of data from the antibiotic clinical use and resistance monitoring network; establishment of clear performance targets; and threat of individual and institutional sanctions for hospitals that failed to meet targets.
Brazil: Improving—and tracking—access to medicines through Farmacia Popular
Brazil, like many LMICs, is experiencing demographic and health transitions that require an increasing focus on strategies to enable patients to manage chronic illnesses, especially diabetes and hypertension. Interventions aim to prevent negative clinical consequences and reduce the incidence of costly downstream health events. To this end, Brazil launched the Farmacia Popular (FP) programme in 2004 (figure 14 ). FP was designed to alleviate two barriers to quality use of medicines: shortages of medicines in public sector health facilities and high medicine prices in the private sector.327
Figure 14.
Farmacia Popular participation and proportion of days covered
This data is after policies first increased patient cost sharing and then made medicines available for free to patients. In 2011, essential medicines for hypertension, diabetes, and asthma began to be provided in both public and private pharmacies with zero copayment from patients. This change led to a dramatic rise in participation in the programme by patients with diabetes, from 400 000 to more than 1·4 million prescriptions filled per month. Proportion of days covered (a measure of adherence) increased to about 80%, which is considered close to the optimum value for chronic illness care. Participation in Farmacia Popular (A). Proportion of days covered (B).
In 2006, the programme extended coverage to selected private sector pharmacies licensed by the government. FP then reduced reference prices paid for covered medicines, resulting in increased patient out-of-pocket costs. In 2011, FP made medicines for diabetes and hypertension available for free to patients who fill their prescriptions in either public or affiliated private sector pharmacies.
Expanding the programme to include private retail pharmacies stimulated programme growth and allowed patients to access medicines more conveniently. Brazilian Ministry of Health data indicate that the number of private pharmacies participating in FP increased substantially, from 2955 in 2006, to 25 220 in 2013. The number of public FP pharmacies also grew, but only from 259 to 558 during the same period.328 However, participating pharmacies were unequally distributed, with higher coverage in the wealthy areas in the south and southeast, and lower coverage mostly in relatively poorer areas with greater need for improved access to medicines, such as the north and northeast.
The government took the additional step of making specific medicines free to patients in the private sector (as they already were in public clinics). As a consequence, the government's expanded market share gave it greater leverage to drive prescribing toward the guideline-recommended medicines covered by the programme, and to negotiate lower prices to help contain costs.
Additionally, as a purchaser, Brazil's Government now has access to the private sector dispensing data, which are usually inaccessible to governments. Overall, by 2012, a total of 6 032 380 patients received oral hypoglycaemic and 14 392 076 patients received antihypertensive medicines. The Commission analysis shows that the proportion of days covered reached 80·7% overall, indicating good adherence to the programme after the medicines were offered free-of-charge. The government has integrated use of medicines data from these patients into its Unified Data System, although outcomes data are not yet linked. Consumer profiles and pharmaceutical practices differ between FP and regular private pharmacies.329 Patients using FP pharmacies were more likely to receive information regarding dispensed medicines, and prescription-only status was respected in all cases. By contrast, 63·5% of medicine purchases in private pharmacies occurred without a prescription.
The FP experience suggests that a government and the private sector can partner in creative ways to make care for chronic illness more convenient and affordable to patients. However, the programme comes at a cost. Government expenditure on diabetes medicines reached US$10·3 million per month in 2014 (this figure was calculated by the Commission on the basis of data from the Brazilian Ministry of Health Strategic Management using conversion rates to US$ by Brazil's Central Bank). Nevertheless, the government sees the private sector's partnership in the FP programme as a key component of its current strategy to reduce underuse of medicines.
Key lessons for interventions on quality use of medicines
From these three examples, several themes emerge to inform the development of other programmes:
-
•
Sustained implementation and resources are needed to achieve long-term quality use goals through incremental improvement. Governments must commit to coordinated policies implemented over time.
-
•
Medicines use problems are multifactorial, so multiple stakeholders in systems should be engaged, including patients and consumers.
-
•
Independence from government or any other stakeholder could be advantageous. Australia's NPS MedicineWise has an established brand identity, an ability to work effectively across stakeholders, and a stability across successive political administrations.
-
•
Policies and programmes need to address economic incentives, including the financial barriers faced by patients who need medicines, and the financial incentives driving prescribers, dispensers, health-care institutions, and the pharmaceutical industry.
-
•
Reliable data are essential; building a national data system on use of medicines takes substantial resources and concerted long-term effort.
-
•
To maximise clinical value, evaluation metrics need to focus not only on access to medicines and their prices, but specifically on appropriate, quality use and the achievement of clinical endpoints.
-
•
The three examples are from upper-MICs and HICs with more resources and longer-standing pharmaceutical system development than LICs or lower-MICs. The latter countries might face great challenges when trying to mobilise sufficient resources and implement similar programmes.
Opportunities to advance quality use of medicines along the path to UHC
Appropriate use of medicines is key to providing high-quality, high-value care while using scarce resources wisely—thus, quality use of medicines is key to achieving the goals of UHC. The Commission identifies three immediate opportunities to advance quality use of medicines and UHC.
Improve the quality and quantity of information available about medicines use
A health system will encounter major problems in performance if it does not know which medicines are used, for whom, and at what cost. Detailed information and analysis about how medicines are used is therefore essential. Information relevant for assessing medicines use can be constructed from data collected de novo (from the DHS Program Stat Compiler, Health Action International, and ACTWatch)—for example, via surveys of household members, patients, and providers—and from data that routinely exist in systems, such as procurement, prescribing, dispensing, and payment records.
Generating information from routine data has enormous benefits. Routinely collected data can allow for assessments of past and current practice patterns, without the costs and time constraints of de-novo prospective data collection. Also, they reflect actual practices in the system. Routine data relevant for assessments of medicines use can be characterised across seven information dimensions (appendix 4.2): pharmaceutical, longitudinal, geographic, organisational, socio-demographic, clinical, and financial.
Indicators based on pharmaceutical data alone help focus attention on key issues, including highlighting variation among providers or from an expected norm (table 7 ). The more that data across dimensions can be linked, the more detailed the questions addressed and conclusions can be.343, 344, 345 Indicator validity is important, but limitations in measurability should not deter from focusing on important issues. If validity limits a system to focusing—and acting—only on indicators that are easily measurable, it could skew resource allocation to the detriment of either priority medicines use issues or patient groups with less data.346
Table 7.
Indicators used to monitor inappropriate use of medicines in different settings
| Setting | Example indicator* | Data source | Pharmaceutical | Longitudinal | Geographical | Organisational | Sociodemographic | Clinical | Financial | |
|---|---|---|---|---|---|---|---|---|---|---|
| Unnecessary medicines use (overuse) | ||||||||||
| Antibiotic use330, 331, 332 | Primary care | Percentage encounters with an antibiotic prescribed | Sample of paper prescriptions | Yes | Yes | No | No | No | No | No |
| Medication safety333 | Hospital | Number of patients prescribed warfarin or clopidogrel concomitantly with a NSAID | Prescription charts or prescription database | Yes | Yes | No | No | No | No | No |
| Inequality330, 331, 332, 333 | Primary care | Percentage encounters with an injection prescribed | Sample of paper prescriptions | Yes | Yes | NA | Yes | No | No | No |
| Failure to use needed medicines (underuse) | ||||||||||
| Cardiovascular disease334 | Residential care | Number of patients who were given angiotensin-converting enzyme inhibitor/angiotensin receptor blocker for hypertension or congestive heart failure | Audit of patient notes or electronic patient record | Yes | Yes | No | Yes | No | Yes | NA |
| Asthma335 | Primary care | >1 defined daily dose beta agonist and <1 defined daily dose of inhaled steroids and beta agonists in patients aged 15–44 years | Prescribing database | Yes | Yes | No | No | Yes | No | NA |
| Chronic kidney disease336 | Primary care | Percentage of patients aged 18–80 years with chronic kidney disease stages 4–5 and hypertension in the absence of low diastolic blood pressure | Electronic medical record | Yes | Yes | No | No | Yes | Yes | NA |
| Medication safety337 | Primary care | Number of patients with history of peptic ulceration or gastrointestinal bleed, not prescribed gastroprotection and prescribed NSAID | Electronic medical record | Yes | Yes | No | No | Yes | Yes | NA |
| Incorrect medicines use (misuse) | ||||||||||
| Drug abuse338 | General | Volume of psychotropic drug volume in one area exceeds average by factor of two or more | Electronic prescription database | Yes | No | Yes | No | No | No | No |
| Cardiovascular disease334, 339 | Residential care | Number of patients at risk of falling or with a history of falls given a medicine to avoid falling (eg, sedating antihistamines, tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antipsychotics) | Administrative and prescribing databases | Yes | Yes | No | Yes | NA | Yes | NA |
| Chronic kidney disease336 | Primary care | Percentage of patients ≥18 years of age with estimated glomerular filtration rate of <30 mL/min/1·73 m2 and diabetes who are prescribed metformin | Electronic medical record | Yes | Yes | No | No | Yes | Yes | NA |
| Unnecessary use of highly priced medicines | ||||||||||
| Low cost prescribing330 | General | Percentage of drugs prescribed by generic name; percentage of drugs prescribed from National Essential Medicines List or Formulary | Sample of paper prescriptions | Yes | NA | No | No | No | No | No |
| Budget impact340, 341, 342 | General | Relative expenditure per year survived of different treatment regimens for lung cancer | Health insurance claims database | Yes | Yes | No | Yes | Yes | Yes | Yes |
NSAID=non-steroidal anti-inflammatory drug. NA=not applicable.
These indicators are sometimes constructed as the volume of a product (or products) sold per head of population. Examples include: total volumes of antibiotic or antidiabetic medicines, or patterns of use of critical antibiotics. Appendix 4.2 gives a suggestion of the range of indicators that have been used to monitor inappropriate use of medicines across several countries, settings, and therapeutic areas. The indicators listed in appendix 4.2 have generally been shown to have face and content validity; however, experts caution that there could be justifiable reasons for outlying prescribing patterns.328, 340
Engage multiple stakeholders to encourage collaboration and coordinated action
Stakeholders whose behaviours influence use of medicines include government ministries of health and finance, social security offices, private payers, non-governmental organisations, civil society organisations, local and international pharmaceutical manufacturers, pharmaceutical distributors and retailers; as well as medical, nursing, pharmacy and other health-care professionals, academics, patients, and citizens (panel 13).
Stakeholder relationships in the pharmaceutical sector have frequently been characterised by misunderstanding and conflict. Different stakeholders have legitimately different perspectives and own different data that could be used to generate better quality information about medicines use. Identifying patterns of medicines use by integrating data from regulatory, procurement, delivery, or reimbursement systems, as well as industry sales, would lead to the creation of increasingly sophisticated data systems and metrics for measuring quality of care and health outcomes to meet the information needs of different stakeholders.
As health systems move towards UHC, opportunities will emerge to establish new stakeholder collaborations to act on the analyses of medicines information. Changing the use of medicines at a systems level will require dialogues about the role of quality use of medicines in achieving different stakeholder objectives and enabling a sustainable and quality health care and financing system. The Medicines Transparency Alliance piloted meaningful stakeholder engagement in seven countries. However, the project was not as successful as many had hoped. Key lessons learned from Medicines Transparency Alliance were: engaging stakeholders requires building trust and confidence over time and creating a neutral environment for the stakeholders to work together; multi-stakeholder engagement alone was insufficient to create change; and careful disclosure of data was essential.347, 348
Undertake concerted action to establish priorities and implement national interventions
Appropriate use of medicines might be an individual behaviour, but it must be enabled by core system functions. Pharmaceutical systems are continuously changing as new products enter markets, evidence about best practices evolves, and information moves through medical and social networks. Clinician and patient choices change in response to these and other factors. These changes, in turn, determine the quality of care, efficiency of health spending, achievement of positive health outcomes, and ultimately whether people are able to engage in the global development agenda.349
A systematic and coherent process involving multiple stakeholders to promote quality use of medicines could be developed through the following concrete activities:
-
1
Prioritising medicines use problems based on estimated health and economic harm. Examples are as follows.
-
•Assessing trends in value and volume in the top 20 medicines provided in the public and private sectors, with a focus on those that treat the most prevalent and the most severe acute and chronic health problems.
-
•Determining the appropriateness of the use of medicines that are responsible for the highest cost and volume, especially those used to treat the most important health problems.
-
•Determining the appropriateness of the use of high-alert medicines350 that have narrow therapeutic spectra and thus a high potential for fatal outcomes and other harm.
-
•
-
2
Reviewing the—probably interrelated—contributions of each system actor (Panel 13, Panel 14) and identifying key stakeholders for the priority problems identified.
-
3
Developing complementary, multifaceted strategies to engage different stakeholders in addressing prioritised medicines use problems, reaching different levels of the system and building on existing evidence on intervention impacts (Figure 11, Figure 12; appendix 4.4, Table 1, Table 2).
-
4
Defining target outcomes for change and agree on monitoring and assessment indicators. Implementing complementary programmes, implementation research,351 and monitoring strategies.
-
5
Assessing the effects (both desired and any undesired) of the strategies, using routine and other data and soliciting different stakeholders' perspectives.
-
6
Investing in developing high-impact programmes and systems that have the potential to promote quality use of medicines by both providers and consumers over the long term.
Conclusion
The Commission concludes that promoting the appropriate and quality use of medicines should be an explicit and key objective of all national and institutional pharmaceutical, clinical, educational, and health financing policies and programmes in all countries. On the basis of existing information and experiences, the Commission suggests that accelerating progress toward appropriate, quality use of medicines requires an explicit, system-wide, evidence-informed focus among all relevant stakeholders.
Recommendations
Therefore, the Commission proposes three recommendations to governments and the main public or private payers to operationalise this focus while implementing health system reforms toward UHC.
-
1
Governments and the main public or private payers should establish independent pharmaceutical analytics units (or equivalent) to focus on generating information for action to promote quality use, in conjunction with other objectives. Systems working toward UHC should invest in an independent local institution that can produce policy-relevant pharmaceutical analytics. The country context will influence both the appropriate structure and resource base to support an independent medicines-focused analytics unit.
-
2
Pharmaceutical analytics units must collaborate with multiple stakeholders in all relevant systems to increase their engagement in and accountability for quality use of medicines, and to intervene jointly on use of medicines problems. An analytics unit independent from public and private sector stakeholders is positioned to interact with multiple stakeholders, whose diverging objectives and behaviours might contribute to appropriate and inappropriate use of medicines, engaging them in information generation and analysis, intervention design, implementation, and monitoring and assessment.
-
3
Engaged stakeholder groups, led by data produced by the pharmaceutical analytics unit, should identify and prioritise local medicines use problems, identify contributing factors across the system, and develop and implement sustainable, long-term, multifaceted interventions. The process of multi-stakeholder engagement in a priority setting for national activities to promote quality use of medicines can result in better understanding of each stakeholder's underlying assumptions, motivations, and objectives. To succeed, there must be tangible benefits of such constructive engagement for all stakeholders.
Section 5: developing missing essential medicines
A patient's experience
Bina is a single mother with three children. Just after the youngest was born, Bina tested positive for pulmonary tuberculosis. After a few months of treatment she felt better and stopped the treatment. A year later she began coughing again, and she was diagnosed with drug-resistant tuberculosis. Following this she needed daily injections for at least 6 months, as well as many pills. Bina was terrified: she was not sure how to keep up with the treatment and continue supporting her children at the same time. She begged the doctors for another medicine that was easier to use and less toxic. They told her that this medicine did not exist, and that she should consider herself lucky to live in an area with a hospital that could treat drug-resistant tuberculosis.
Introduction
The present system of developing new medicines is in crisis, as it largely fails to produce much-needed products to address the health needs of millions of people.352 When new essential medicines are developed, market exclusivity, through patents or other mechanisms, allows for pricing that potentially makes them unaffordable, even in HICs.353, 354
In many cases missing essential medicines are not even developed at all. Even though the early stages of R&D of medicines has large public investment, the process of taking them to market is largely carried out by for-profit companies. Pharmaceutical companies and their shareholders are typically reluctant to invest in developing medicines for patient populations that do not represent a profitable market or for diseases predominantly affecting LMICs.355
The problems of high prices (section 2) and missing essential medicines are related, and both disproportionately affect people in LMICs. This section presents a summary of the complex and political problems ingrained in the current patent-based innovation system.356, 357 It examines the initiatives to address the system's deficiencies, and proposes concerted global actions and public policy interventions to lay the foundation for sustainable approaches to essential medicines development.
Key problems of the current innovation system
WHO,74, 358 The Lancet's Commission on Global Health 2035,359 and the UN16 have all offered lists of missing essential medicines. Some important unmet public health needs include heat-stable insulin and oxytocin,16 shorter treatments for latent and active tuberculosis, single-day treatments of malaria, and treatments for multidrug-resistant tuberculosis. Essential diagnostics are also needed, such as a point-of-care test to distinguish between bacterial and viral infections of the upper respiratory tract.360 Some essential medicines do exist but have been abandoned—these are no longer produced in volumes that meet global demand because they are not sufficiently profitable. Examples include snake antivenoms and benzathine benzylpenicillin.
A major category of missing essential medicines reflects a historic lack of attention to the specific needs of children. Between 1995 and 2005, 107 (44%) of the 243 medicines authorised in Europe by the European Medicines Agency (EMA) had a potential paediatric use, but no data on use in children were available at the time of authorisation.361 In 2007, WHO published the first Model List of Essential Medicines for Children76 and launched the Make Medicines Child Size campaign.362 A key example is the gap in paediatric treatments for HIV—2·6 million children are living with HIV (88% of them in sub-Saharan Africa),363 but this statistic has not attracted sufficient commercial R&D investments.364
The alarming crisis in antimicrobial development is another example.308 A market-driven R&D system will not invest in new life-saving antimicrobials if their use will have to be rationed from the start to prevent resistance.17 The failure to respond to the 2014 Ebola virus outbreak showcases another example.365 Clinical testing of an Ebola virus vaccine has shown promising results,366 but it took 11 000 deaths and extensive political mobilisation to take the vaccine candidate off a shelf, where it had been sitting for 10 years after initial development by the Public Health Agency of Canada.367 Extensive R&D activity only started when the outbreak threatened richer populations. By October 2015, 31 molecules for Ebola virus treatment were under commercial development.
The issue of missing essential medicines has been discussed for decades. In 1990, only $1·6 billion (5·3%) of $30 billion spent annually on health research was oriented to the needs of LMICs.368 In a widely quoted study by Médecins Sans Frontières, only 15 (1·1%) of 1393 new medicines developed between 1975 and 1999 were for tropical diseases and tuberculosis, which account for 12% of the global disease burden.369 Between 2000 and 2011, only 37 (4·4%) of 850 newly approved products were for neglected diseases, most of which were new formulations or combinations of existing medicines.370 Similarly, in December, 2011, of nearly 150 000 registered clinical trials, only 1·0% were for neglected diseases. By October, 2015, only 167 (2·3%) of 7217 products in active development were for 19 of 64 listed neglected diseases (Commission's analysis of the data). Some work is being done, but it covers only part of the need.
The failures of market-driven R&D go beyond neglected diseases. An analysis of 1345 new medicine approvals in Europe revealed that no real breakthroughs occurred between 2000 and 2014; only 9% of new medicines offered an advance, and 20% were possibly helpful.371 51% of newly marketed medicines were modified versions of existing medicines, adding little to the treatment armamentarium. Nowadays, the R&D efforts therefore yield very few truly innovative products that respond to essential public health needs.
New essential medicines that are unaffordable to most people can also be considered as missing. High prices are a direct result of the reliance on the market monopoly granted by the patent system for the financing of R&D. High prices of new pharmaceutical products have long affected LMICs, but are increasingly being felt in HICs as well, and medical specialists in the USA372 and the UK373, 374, 375, 376 have started to protest (section 2).
Lessons learnt from initiatives to promote R&D of missing essential medicines
Not-for-profit R&D initiatives start to bear fruit
Several not-for profit Product Development Partnerships for neglected diseases have been established in recent years. In the Product Development Partnerships approach, R&D investments are funded up-front through philanthropic and public financing, so companies do not need to recoup the full costs of R&D afterwards through high medicine prices. Examples include the Drugs for Neglected Diseases initiative (DNDi), the Medicines for Malaria Venture, the Global Alliance for TB Drug Development, the International AIDS Vaccine Initiative, the Foundation for Innovative New Diagnostics, Aeras Global TB Vaccine Foundation, and the Program for Appropriate Technology in Health. Some governments and major philanthropic actors, such as the Bill & Melinda Gates Foundation, have committed substantial funding to these initiatives. New industry R&D platforms have been created, and new incentives for industry involvement developed.377, 378
These initiatives are starting to bear fruit (table 8 ). For example, DNDi has developed six new treatments since 2003, and expects to complete 10–12 additional new treatments by 2023. DNDi is expanding its scope from neglected diseases to HIV, hepatitis C, and antimicrobial resistance.379 These initiatives have also provided important insights into the true cost of R&D (panel 19 ). Yet the research agenda of these initiatives largely follows the priorities of donor governments and foundations. A transparent priority-setting process is missing. As a result, some important therapeutic areas are hardly covered, such as diabetes, cancers and other NCDs, and mental disorders.
Table 8.
Key achievements of not-for-profit Product Development Partnerships and their partners
| Disease | Medicine | |
|---|---|---|
| DNDi with Sanofi | Malaria | Artesunate-amodiaquine |
| DNDi with Farmanguinhos/Cipla | Malaria | Artesunate-mefloquine |
| DNDi with Laboratório Farmacêutico do Estado de Pernambuco (LAFEPE) | American trypanosomiasis (Chagas Disease) | Paediatric benznidazole |
| Institute for OneWorld Health (iOWH) | Leishmaniasis | Paromomycin |
| MMV with Novartis | Malaria | Artemether-lumefantrine dispersible tablets |
| MMV with Guilin | Malaria | Injectable artesunate |
| MMV with Sigma-Tau | Malaria | Dihydroartemisinin-piperaquine |
| MMV with Shin Poong | Malaria | Pyronaridine-artesunate |
| MMV with Guilin | Paediatric malaria | Sulfadoxine-pyrimethamine and amodiaquine |
| DNDi | African trypanosomiasis (sleeping sickness) | Nifurtimox and eflornithine combination therapy |
| DNDi | Visceral leishmaniasis (East Africa) | Sodium stibogluconate and paromomycin combination therapy |
| DNDi | Visceral leishmaniasis (Asia) | Liposomal amphotericin B, miltefosine, and paromomycin combination therapy |
Data from European Union Product Development Partnership Coalition. May 7, 2015. DNDi=Drugs for Neglected Diseases initiative. MMV=Medicines for Malaria Venture.
Panel 19. Developing a new medicine: how much does it cost?
The real costs of pharmaceutical research and development (R&D) are often kept as trade secrets. In 2016, industry-supported estimates set the average cost for medicines developed between 1995 and 2007 at US$2·5 billion per new product. In 2012, an industry-funded study by the Office of Health Economics came to an estimate of $1·506 billion for development cost per new product. These figures are used by the pharmaceutical industry to justify high medicine prices, but have been challenged by others.380, 381 Even some in the industry have expressed scepticism. GlaxoSmithKline's chief executive officer, Sir Andrew Witty, called the $1 billion figure “one of the great myths of the industry.”382 Light and Warburton380 estimated that the net investment by the industry to discover important new medicines amounts to 1·2% of sales. Table 9 summarises the R&D cost estimates published since 1991.
In 2001, the Global Alliance for Tuberculosis Drug Development estimated the costs of successfully developing a new chemical entity to treat tuberculosis to be approximately $37–40 million (excluding the costs of failure). This cost covers preclinical development ($4·9–5·3 million), pharmaceutical development (>$5·3 million), and phases 1 to 3 of clinical development ($26·6 million). Including the costs of unsuccessful projects would increase the total costs to $76–115 million.388
The Drugs for Neglected Diseases initiative estimated that R&D expenditure for an improved treatment (ie, a combination product using existing molecules) would be between $10 and $40 million. The Drugs for Neglected Diseases initiative's cost for the development of a new chemical entity is estimated at €100–150 million, on the basis of the real cost for products developed by the Product Development Partnership and including cost of failures.389 These estimates do not include in-kind contributions by the industry.
The Commission concludes that international agreement should be sought on a global list of missing essential medicines with due regard of the needs of LMICs. R&D on the listed diseases should be supported by dedicated funds, and the list should be regularly updated.
Alternative incentives signal interest for change
In the past decade and a half, new push and pull incentive mechanisms have been established. Some new donors, such as UNITAID and the Japanese Global Health Innovative Technology Fund (which includes private companies, among others), have increased funding for R&D of missing essential medicines. The Longitude Prize established a prize fund of £10 million in 2014 for the development of a point-of-care diagnostic test to determine whether (and which) antibiotics are appropriate in a given case.390 These initiatives are too new to show definitive results yet, but they signal public and private interest in new ways to incentivise innovation. The Commission supports the assessment of these alternate incentives.
Regulatory incentives show mixed results
New initiatives such as the UN Prequalification Programme managed by the WHO (section 3) and the EMA's Article 58391 adapt regulatory activities to global health purposes. Under Article 58, the EMA provides scientific assessments, in coordination with WHO, of medicinal products for human use in markets outside the EU.392
Since 2007, US federal legislation has allowed for priority review vouchers (PRVs). However, PRVs have been criticised because there is no provision that the product should be made available and affordable, and PRVs can also be used for products already registered outside of the USA or by a company that did not invest in the R&D.378, 393 A marketed antituberculosis medicine, bedaquiline, was offered for prices of around $3000 in MICs and $900 in LICs. Yet in the USA it was marketed for $30 000 per treatment, despite having received a PRV and fast-track approval by the US Food and Drug Administration. Efforts are underway to include access and novelty requirements into the legislation.394
New regulations to encourage paediatric medicine development have been introduced by the USA395 and the EU.396 As of 2008, all new applications in the EU must include data for children (0–17 years) unless a specific waiver is approved. An increase in new paediatric formulations is possible,397 yet the costs to society might become higher than the actual R&D investment. Whether these innovations meet priority needs or are primarily used to extend the market exclusivity of products with predominantly adult indications remains unclear.398, 399 In 2016, the EU initiated a review of R&D incentive mechanisms, including those for paediatric R&D, to strengthen the balance of pharmaceutical systems in Europe.400
Regulatory approval of new essential medicines poses great challenges, for example with onerous studies needed for new paediatric formulations401 or assessments of new medicines for neglected diseases not prevalent in countries with stringent regulatory authorities. The Commission asserts that assessments of new medicines for neglected diseases should be led by regulatory authorities in the affected areas. These institutions will probably need further strengthening to do such reviews, through enhanced collaboration with stringent regulatory authorities and the WHO/UN Prequalification Programme (section 3). Regional regulatory initiatives within zones with similar disease patterns should also be supported.
The costs of R&D are not transparent
High prices for medicines are justified by the pharmaceutical industry as compensation for the costs of R&D and the high failure rate. However, the real costs of R&D are not well known (panel 19). In 2014, industry-supported estimates set the average cost for medicines developed between 1995 and 2007 at $2·5 billion per new product (table 9 ).402 Although direct comparisons are not possible because of the lack of comparative datapoints, R&D cost data from not-for-profit developers show that substantial innovations are possible for much less, especially for small molecules. For example, DNDi's real cost for the development of a new chemical entity including the cost of failures is estimated at €100–150 million, or about 7% of the industry figure.389 The Commission argues for transparency in the costs of R&D to enable effective dialogue and decision making on affordable pricing of new essential medicines, and a fair return on R&D investments.
Table 9.
Estimates of R&D cost from different sources and years
| Estimates of R&D costs in US$ | |
|---|---|
| DiMasi et al (1991)383 | $231 million (expressed in 1987 dollars) |
| Office of Technology Assessment, US Congress (1993)384 | $140–194 million (expressed in 1990 dollars) |
| DiMasi et al (2003)385 | $802 million |
| Office of Health Economics (2012)386 | $1.5 billion |
| DiMasi et al (2016)387 | $2.5 billion |
R&D=research and development.
Public funding of R&D: the public often pays twice
Initial pharmaceutical research is often largely funded from public funds, such as the US National Institutes of Health or the European Horizon 2020 programme. For childhood cancers, virtually all research funding comes from the National Cancer Institute, private foundations, and philanthropic sources.403 However, the final commercialisation steps of the development process are usually done by for-profit pharmaceutical companies, which obtain the intellectual property rights from publicly funded research institutes, thus controlling the technology, including decisions about commercialisation and pricing.404
Medicines should be priced such that the public does not pay twice for innovation: first through government-funded scientific research and then through high medicine prices. UN Special Rapporteur on the Right to Health Paul Hunt has noted that, “[b]ecause of its critical social function, a patent on a life-saving medicine places important right-to-health responsibilities on the patent holder. These responsibilities are reinforced when the patented life-saving medicine benefited from R&D undertaken in publicly funded laboratories.”405 The student movement Universities Allied for Essential Medicines lobbies for responsible licensing by universities. The Commission recognises the need to actively manage and protect the public interest in the proceeds of state-funded research.
Patent pooling supports generic manufacturing
As a direct result of the Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property of 2008,355 UNITAID established an MPP for HIV medicines in 2010. The MPP initially focused on patents related to HIV medicines to promote low-cost generic production and the development of fixed-dose combinations and paediatric formulations. The MPP has expanded its mandate to cover hepatitis C and tuberculosis. In November, 2015, the MPP signed an agreement with Bristol-Myers Squibb that allows supply of generic daclatasvir in 112 LMICs.32 Separate from the MPP, Gilead Sciences Inc has licensed patents for its hepatitis C virus medicines for use in 101 LMICs.31 Unfortunately, some MICs are excluded from these licences and must continue to rely on TRIPS flexibilities to access low-priced generics (appendix 5). Generics companies that produce hepatitis C virus and HIV medicines under a licence agreement with the MPP and Gilead Sciences Inc are mostly allowed to supply generic product to a country that makes use of TRIPS flexibilities.170
After 5 years of operation of the MPP, millions of people have benefited and impressive financial savings have been achieved (panel 20 ). The Commission concludes that there is great potential for expanding access to other new essential medicines through licensing of patents through patent pooling.
Panel 20. Achievements of the Medicines Patent Pool (MPP) between 2010 and 2015.
Patent licences and agreements
-
•
Patent licences signed on 12 priority antiretroviral medicines with six patent holders, and 59 sub-licences with 14 generic manufacturers
-
•
One licence on a treatment for hepatitis C virus infection for 112 low-income and middle-income countries
-
•
One agreement to increase access to treatment of cytomegalovirus retinitis
-
•
One agreement for antiretroviral medicines as nanomedicines, for all 135 low-income and middle-income countries and two high-income countries in Africa
Effect on production and supply
-
•
Generic companies with MPP licences have supplied more than 7 million patient-years of WHO-recommended antiretroviral drugs in 117 countries, including 41 countries that were previously unable to benefit from generic competition for such medicines
-
•
MPP licences enable manufacturing and sale of generic adult antiretroviral medicines to 87–93% of people with HIV in the developing world, which includes all 34 low-income countries and 55–80% of middle-income countries
-
•
MPP sublicensees supplied 4·3 million patient-years of tenofovir disoproxil fumarate in the first 6 months of 2012, shortly after the agreement was reached
Financial savings
-
•
In 2011–12, in Azerbaijan, Belarus, Egypt, El Salvador, Georgia, Iran, Iraq, and Tunisia the price of tenofovir-containing products dropped to a median of 13% of the price before the agreement (2010–11)406
-
•
MPP agreements have led to antiretroviral medicines procurement savings of US$119·6 million between 2010 and 2015
-
•
The total direct global savings generated by the MPP406 are estimated at $2·2 billion by 2028, implying that for every dollar spent, the global community gains $40407
TRIPS flexibilities have been used widely but are under threat
Patents present substantial challenges to medicines availability. However, flexibilities in patent law have been used by a number of countries to secure access to generic medicines. The most frequently deployed flexibilities are compulsory licensing of medicines, government use of patents, and the waiver that allows LDCs to postpone granting or enforcing medicines patents and test data protection until 2033.
These options have been used more widely than is usually assumed. New figures167 show that since 2001, there have been 34 instances of compulsory licensing (CL) of medicines by 24 countries, 51 instances of government use of patents by 35 countries, and 32 of non-enforcement of patents by 24 World Trade Organization LDC Members. The peak of these instances falls between 2004 and 2008, coinciding with increased global funding for HIV. Although originally focused on HIV, 23 out of 85 total instances of CL and government use have concerned non-HIV medicines, including seven instances for cancer medicines between 2008 and 2014, of which five were granted. These measures have improved access to medicines. For example, in Thailand, CLs for erlotinib, docetaxel, letrozole, and clopidogrel save the health-care system $142 million per year.408
In the past decade and a half, some countries have amended their patent laws to reflect health concerns. For example, India rewards innovation409 but prevents trivial patents and so-called ever-greening of patents.410 South Africa has proposed introducing patent examination to limit the number of inappropriate patents.411 Rwanda, Uganda, and Cambodia have all excluded medicines from patentability, pursuant to Decisions of the Council for TRIPS of June 27, 2002 (IP/C/25), and of June 11, 2013 (IP/C/64).412 In December, 2015, the Organisation Africaine De La Propriété Intellectuelle amended the Bangui Agreement to allow its LDC members to postpone granting of patents and protection of regulatory test data until 2033.413
However, the plethora of trade agreements with TRIPS-plus provisions is a serious threat to the policy and legal space that TRIPS provides. Examples of such provisions are patent linkage, data exclusivity, extension of the patent terms and scope, and restrictions on grounds for compulsory licensing and parallel importation. Some or all of these provisions appear in various trade agreements,414, 415, 416 in World Trade Organization accession agreements such as those with China and Cambodia, and in the Trans Pacific Partnership Agreement. It's intellectual property chapter is promoted as the new standard for global trade rules.417, 418 More information about the patent system, TRIPS flexibilities, and TRIPS-plus provisions is given in appendix 5.
The Commission believes that governments must make full use of all available TRIPS flexibilities and enable their efficient use through national legislation. Governments should stop making TRIPS-plus demands in trade agreements and resist any pressure to include TRIPS-plus provisions in their national laws. The Commission believes that the drive for ever-higher levels of intellectual-property protection through trade agreements should be stemmed and will probably require intervention at the multilateral level.
Many pharmaceutical companies neglect their social responsibility
Globalised norms for patent protection and very high prices for new products make for a very successful pharmaceutical business model, thus satisfying the needs of investors. However, it is increasingly clear that this approach endangers the progressive realisation of global health equity objectives and human rights. The global community has laid out a vision of health care as a human right in treaties such as the 1966 International Covenant on Economic, Social and Cultural Rights, which enshrined the right to health and was ratified by more than 160 countries. The right to essential medicines is a key component of the right to health,60, 62 and this also implies certain human rights obligations for pharmaceutical companies.79
Some pharmaceutical companies fail to acknowledge their unique role in society as the providers of life-saving medicines. One assessment of five large pharmaceutical companies showed that their corporate social responsibility approaches were inconsistently applied.419 In some cases, official company credos are not in fact reflected in the company's actions. For example, Johnson & Johnson publicly commits to striving to reduce costs and maintain reasonable prices, yet the company does not license its HIV medicines patents to the MPP;420 and one HIV medicine, darunavir, was priced at $810 per patient per year in certain LMIC markets for both a 600 mg dose and only declined to $663 by 2015.421 In the USA, the price of Novartis' imatinib for the treatment of chronic myeloid leukaemia has tripled since 2001,422 to $92 000 per year, although the company received orphan drug incentives for its development and the number of users continues to rise.423 It also vigorously defends its patents in LMICs that strive to have access to imatinib.424, 425 AbbVie charges MICs $740 per patient per year for lopinavir/ritonavir (more than twice the price of $231 per patient per year in LDCs); this price has not changed since 2012. A price of more than $3500 per patient per year was quoted for lopinavir/ritonavir in 2014 in Malaysia. Investors' profit-seeking has been blamed when companies fail to arrange for access pricing.426
Pharmaceutical manufacturers in LMICs are also expected to contribute to public health needs. However, many fail to produce essential medicines, or to produce them according to acceptable quality standards (panel 9).174 Academic institutions, when seeking to increase the commercial value of their research, also have an insufficient focus on developing missing essential medicines.427
Towards a global R&D framework that assures access and innovation
The initial focus on R&D for neglected diseases in developing countries has driven many international policy developments in this area.428 However, a simplistic dichotomy between developed and developing countries is no longer appropriate. LMICs are experiencing an epidemiological transition, with increasing prevalence of NCDs. Certain neglected tropical diseases and emerging diseases also pose a threat to HICs, due to climate change and international travel.429, 430, 431 Therefore, high prices of patented newly developed essential medicines affect everyone in all settings.
Market failure or public policy failure?
The lack of private sector investment in developing medicines for diseases affecting people without purchasing power or for small patient populations is often described as market failure. The Commission disagrees. Relying on a profit-driven R&D model to respond to public health needs represents a public policy failure. As Nobel laureate Sir John Sulston said, “We have to recognize that the free market, as good a servant as it is, is a bad master. We cannot take important global decisions on the basis of the free market alone.”432
Inadequate regulation of the business sector to protect and promote human rights is also a public policy failure.433 The Commission concludes that government intervention, including at the international level, is needed to ensure markets respond to public health needs, and to hold private sector partners accountable, including with regards to their responsibility to protect and promote human rights.
Public spending, public policy—the urgent need for global action
The imperative for governments to act is pressing. The global market of pharmaceutical products was almost $1 trillion in 2013, and is expected to have reached $1·2 trillion by 2017.434 The market share of LMICs, particularly those in Asia and Latin America, is growing at a rapid pace.96 The global medicines market represents money the public spends, either out of pocket, or through health insurance, social security schemes, or tax-based government-provided health care. Yet as previously described, industrial investment in R&D for neglected diseases remains very low. In 2013, public and private investment for R&D in 34 neglected diseases was $3·2 billion, of which pharmaceutical corporations only contributed $401 million. The latter amount represents only 0·8% of total industrial R&D spending of $51·2 billion in 2014.435
Not-for-profit R&D initiatives have compensated for some deficiencies of the current system, but they cannot provide a permanent solution to the underlying fundamental problem of an innovation system relying on market exclusivity. The Commission believes that governments need to proactively set public health-based research priorities for so-called essential R&D and not leave these priorities to pharmaceutical manufacturers. Governments also need to finance new models of biomedical innovation that address access from the early stages of development, such as the Global Health Innovative Technology based in Japan. The massive spending on pharmaceuticals through increasingly higher pricing of medicines can be repurposed to shape a new R&D framework. As countries cannot do this on their own, it will require international agreement and regulation.
Delinking R&D costs from the price of medicines
The concept of delinking costs from prices is based on the premise that costs and risks associated with R&D should be rewarded, and incentives for R&D provided by means other than through the price of the product.436 If the R&D cost of new medicines did not have to be recouped through high prices, those medicines would be free of market exclusivity and could be made more widely available and more affordably priced through better competition.
The Commission supports proposals to progressively delink the cost of R&D for priority medicines from the price of the products, and to develop new ways of sharing the cost burden of innovation internationally. As James Love suggested at the hearing of the UN High Level Panel on Access to Medicines in March 2016: “Let's outcompete the patent-based innovation.” For example, countries could contribute to the development of missing essential medicines in amounts proportionate to their economic development. This contribution would reflect that R&D of essential medicines is a global public good, and would help to ensuring that the fruits of R&D efforts are accessible to all.
Public policy must be expressed in a global R&D framework
In 2006, WHO stated that “access to drugs cannot depend on the decisions of private companies, but is also a government responsibility.”356 In 2008, after intense negotiations, WHO members adopted the Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property (GSPA).437 The GSPA encourages needs-driven research rather than purely market-driven research and contains many practical recommendations.
Several proposals have been made for new policy frameworks and, in particular, new international agreements on medical R&D to achieve the two objectives of innovation and access.438 The first proposal was made by Hubbard and Love in 2004.439 Over the years, their proposal has received support from an increasing number of governments, scientists, Nobel laureates, civil society organisations, and other experts.440, 441, 442 In 2015, representatives of research and international organisations also called for a Global Biomedical R&D Fund and Mechanism for Innovations of Public Health Importance.443 Separate global financing mechanisms for innovation have been discussed for neglected diseases, antimicrobials, and Ebola virus, which all lack sufficient commercial market opportunities. As these are priorities for LICs, MICs, and HICs alike, the medical tools to address them should be considered as global public goods. All R&D needs should be reconciled within a global umbrella framework for funding and coordinating R&D that not only emphasises innovation but also secures access.
The need for new global approaches was reinforced by UN Secretary-General Ban Ki-moon's call for a new deal at the establishment of the High-Level Panel for Access to Medicines in November 2015.444 The scope of this panel's investigation was “to review and assess proposals and recommend solutions for remedying the policy incoherence between the justifiable rights of inventors, international human rights law, trade rules and public health in the context of health technologies.”444
WHO member states will continue to discuss the monitoring, coordination, and financing of health R&D, taking into account the report of the UN Panel445 and that of the WHO Consultative Expert Working Group on Research and Development: Financing and Coordination,446 which recommended the establishment of a biomedical R&D treaty (panel 21 ). The talks about a new R&D framework are likely to be intensely political, as were the negotiations for the GSPA. It will be important for clear R&D priorities to inform this process.451
Panel 21. An international agreement on research and development (R&D)447.
Several proposals have been made for an international agreement on medical R&D to achieve the two objectives of financing needed innovations, and equitable access to those innovations. Key features of such an agreement include:
-
•
R&D priorities driven by health needs rather than commercial potential
-
•
Binding obligations on governments to invest in R&D
-
•
Equitable distribution of contributions across countries
-
•
Measures to improve the regulatory environment
-
•
Measures to ensure affordability of the end product
-
•
Access-maximising licensing practices to deal with intellectual property issues
-
•
Innovative approaches to promote R&D while delinking its cost from the ultimate sale price
Such an agreement could be crafted under the auspices of WHO, whose constitution allows for its 194 member states to negotiate formal international law.448 While both formal and informal norms (such as guidelines or global strategies) can influence the behaviour of states and other actors, binding international law offers several potential advantages. An important precedent was set with the 2005 Framework Convention on Tobacco Control, the first public health agreement negotiated within WHO, which has contributed significantly to global tobacco control efforts.449, 450
The necessary practical details of a new medical R&D framework will need to be negotiated. These global discussions on R&D priorities provide opportunities for national governments, WHO, and the UN to fulfil their obligations to present a bold new global framework for achieving the dual objectives of health-need driven R&D and equitable access to its products.452, 453
Pooling patents of new essential medicines promotes universal access to innovation
On the basis of the positive outcomes of the MPP, the Commission concludes that there is a wide scope for patent pooling for other essential medicines (as defined by WHO or national committees). To this end, the current MPP could be expanded into an Essential MPP (or EMPP). This expansion would create an opportunity for companies to license patents for the purpose of creating a competitive generic market of essential medicines, in line with their responsibility to protect and promote human rights.454 Patents of medicines developed under the new research agreement or new financing mechanisms could also be licensed. The EMPP should use a tiered royalty system to remunerate patent holders and to contribute to R&D expenditure at levels proportionate to the economies of the countries where the medicines are used.455
The Commission notes that a patent owner's refusal to license an essential medicine to the EMPP would satisfy the condition for granting a compulsory licence under TRIPS Article 31, which requires the grantee to have made efforts to obtain authorisation from the right holder on reasonable commercial terms and conditions.456 There is no such requirement in cases of national emergency, extreme urgency, or public non-commercial use.456 Governments should also ensure that national patent legislation allows for easy deployment of TRIPS flexibilities, effective automatic licensing of essential medicines in the absence of voluntary agreements, and regulatory rules for protection of test data that provide the necessary flexibility to register products submitted by licensees (see also section 2).457
The pharmaceutical industry should live up to its special responsibilities
Instances of important achievements when industry is open to collaboration are apparent. Examples have included the MPP, collaborative research for vaccines,458 and neglected diseases research. In recent years, some firms have made listings of their patents available. In 2016, GlaxoSmithKline announced that it will not file or enforce patents in LICs, license its patents in LMICs, make its patent landscape more transparent, and commit its future oncology medicine patents to patent pooling.459 These hopeful developments might set important precedents. Yet the deep changes implied by a new global R&D framework will also require a general culture change in the industry and among its investors.
Detailed descriptions of what would be expected from the industry have been formulated since 2001. For example, the UN Special Rapporteur454 and the Human Rights Council460 have defined the human rights responsibilities of pharmaceutical companies. These responsibilities include refraining from actions that limit accessibility, such as pursuing stronger intellectual property protection, and also taking all reasonable steps to make new medicines accessible to all those in need, within a viable business model. Company violations of these human rights principles give national governments a strong justification to impose corrective measures, such as compulsory licences for domestic production or importation.
The ATM Index is an independent review mechanism by which the policies and practices of large pharmaceutical companies with regard to LMICs are assessed every 2 years. The ATM Index is strongly based on human rights principles and has been refined over time in collaboration with the industry.
The Commission believes that moving away from an exclusively profit-oriented approach, towards a more patient-centred and public-centred, socially-responsive, open, and collaborative enterprise, would improve global health and the reputation of the pharmaceutical industry. As a result of the special nature of its products, the pharmaceutical industry has a unique role in society. It should now live up to this special responsibility, and be seen to do so.
Conclusion
Access to new essential medicines is a key component of UHC and of the progressive realisation of the right to health. Some of the developments described in this section represent real progress and will help bring new essential medicines to the market; and for certain diseases they will bring medicine prices down. Yet the recommended policies are often restricted to certain therapeutic areas (eg, HIV, neglected diseases, or paediatric formulations), and they are not sustainable when largely dependent on charitable contributions. While repairing some of its excesses, these partial solutions leave the existing system in place.
With the current patent-based innovation system, the feasibility of achieving or maintaining UHC is seriously at risk. The Commission therefore believes that business as usual will not resolve the problems with R&D, and that concerted global action is the only way forward. A new global R&D policy framework is needed to drastically adapt the current model and to reduce its reliance on market exclusivity as the main driver of innovation. The Commission concludes that a more public health-oriented R&D system is needed, but recognises that no country can tackle this issue on its own. International public policy should play a much greater role in setting R&D priorities and financing, and in coordinating new approaches to promote access to new essential medicines.
Practically, the Commission concludes that governments need to define a list of missing essential medicines to be provided under UHC schemes. Governments and non-governmental organisations need to make the necessary R&D financing mechanisms available for these identified needs. The price of new essential medicines can then be delinked from development costs and the products can be made widely available and affordable through non-exclusive licensing agreements. The resultant decrease in price can provide the financial space to more directly finance the identified priority R&D.
Recommendations
The Commission's analysis shows that challenges of access to new essential medicines are directly associated to the failure of the current R&D system to develop much needed new medicines. The Commission makes the following recommendations for stronger public policies on R&D, including at the international level.
-
1
Governments and WHO must take international public leadership for priority setting for essential R&D, with due regard for the public health needs of LMICs. This should include developing a list of missing essential medicines, within the context of the WHO Global Health R&D observatory and in close connection with the WHO Model List of Essential Medicines. The WHO mechanism to identify missing essential medicines should be further developed, with the involvement of all relevant stakeholders.
-
2
Governments must lead the process towards a global R&D policy framework and agreements, which include new financing mechanisms to ensure that missing essential medicines are developed and made affordable. Such mechanisms should be based on transparent estimates of the real cost of R&D; they might include a pooled fund for global health R&D, prize funds, targeted research partnerships and advance market agreements, and licensing of related patents, leading to an increasing number of new priority products with an affordable price which is delinked from R&D costs (known as progressive delinking).
-
3
The international community must create a general EMPP. Such a pool could be hosted and managed by the current MPP. Companies should license their patents related to essential medicines to the EMPP under a set of conditions. Patents of medicines developed under the new research agreement or any other new financing mechanism could also be licensed through the EMPP. The EMPP should use a tiered royalty system to remunerate the patent holder and to contribute to R&D expenditure.
-
4
Governments and national stakeholders must develop and implement comprehensive national action plans to guarantee equitable access to new essential medicines, including open knowledge innovation, fair licensing practices, support for a patent pool for essential medicines, full use of TRIPS flexibilities when needed, and rejecting TRIPS-plus provisions.
-
5
The pharmaceutical industry must better align its R&D priority setting with global health needs, and develop access strategies to make medically important innovations available to all in need. To this end the industry could determine a certain percentage of its profits to reinvest in R&D for medicines that are not commercially attractive, but are deemed essential from a public health perspective. Equitable access strategies should include broad licensing of patents and technology transfer to enable generic medicines production; and equitable pricing mechanisms. The policies and practices of pharmaceutical companies should be independently assessed by international mechanisms, such as the ATM Index.
Section 6: measuring progress on essential medicines policies
Indicators for measuring progress on essential medicines policies
A persistent and prominent gap exists between the current situation of access to affordable and quality-assured essential medicines and the ideal of equitable access to essential medicines for all. In the previous sections, the Commission made a series of recommendations on the basis of evidence and crucial analysis, intended to guide countries towards progressively closing this gap (panel 22 ). The Commission recognises that implementation of these recommendations, positioning essential medicines as an integral part of UHC and a major contribution to sustainable development, requires adaptations to local circumstances.
Panel 22. Summary of recommendations and responsible parties.
Paying for a basket of essential medicines
-
•
Governments and national health systems must provide adequate financing to ensure inclusion of essential medicines in the benefit packages provided by the public sector and all health insurance schemes
-
•
Governments and national health systems must implement policies that reduce the amount of out-of-pocket spending on medicines
-
•
The international community must fulfil its human rights obligations to support governments of low-income countries in financing a basic package of essential medicines for all, if they are unable to do so domestically
-
•
Governments and national health systems must invest in the capacity to accurately track expenditure on medicines, especially essential medicines, in both the public and private sectors, disaggregated between prepaid and out-of-pocket expenditure, and among important key populations
Making essential medicines affordable
-
•
Governments and health systems must create and maintain information systems for routine monitoring of data on the affordability of essential medicines, as well as price and availability, in the public and private sectors
-
•
Governments must implement a comprehensive set of policies to achieve affordable prices for essential medicines
-
•
Governments and health systems must develop national capacity to create medicines benefit packages that guide procurement and reimbursement for affordable essential medicines
-
•
Governments, national health systems, and the pharmaceutical industry must promote transparency by sharing health and medicines information
Assuring quality and safety of essential medicines
-
•
Global efforts must be made to promote the harmonisation of quality assurance efforts through the use of an international standard regulatory dossier that covers both format and content
-
•
WHO should evolve the WHO/UN Prequalification Programme to maintain a moving focus on new essential medicines
-
•
Payers and procurement agencies must adopt good procurement practices that incorporate effective and transparent quality assurance mechanisms
-
•
Governments must redirect the activities of national regulatory agencies towards those that add value and reduce duplication of effort, and engage with a system for independent and public assessment of the performance of NMRAs
-
•
Regulatory agencies must encourage the involvement of other stakeholders and the general public in promoting the quality and safety of essential medicines
-
•
WHO and national governments must establish concrete targets and a public accountability mechanism for the performance of national regulatory authorities
Promoting quality use of essential medicines
-
•
Governments and the main public or private payers should establish independent pharmaceutical analytics units (or equivalent) to focus on generating information for action to promote quality use, in conjunction with other objectives
-
•
Pharmaceutical analytics units must collaborate with multiple stakeholders in all relevant systems to increase their engagement in and accountability for quality use of medicines, and to intervene jointly on use of medicines problems
-
•
Engaged stakeholder groups, led by data produced by the pharmaceutical analytics unit, should identify and prioritise local medicines use problems, identify contributing factors across the system, and develop and implement sustainable, long-term, multi-faceted interventions
Developing missing essential medicines
-
•
Governments and WHO must take international public leadership for priority setting for essential R&D, with due regard for the public health needs of low-income and middle-income countries
-
•
Governments must lead the process towards a global research & development policy framework and agreements, which include new financing mechanisms to ensure that missing essential medicines are developed and made affordable
-
•
The international community must create a general Essential Medicines Patent Pool
-
•
Governments and national stakeholders must develop and implement comprehensive national action plans to guarantee equitable access to new essential medicines
-
•
The pharmaceutical industry must better align its research and development priority setting with global health needs, and develop access strategies to make medically important innovations available to all in need
This final section addresses the challenge of accountability, proposing a set of indicators (panel 23 ) for measuring implementation of the recommendations and describing the criteria used to select the indicators. The indicators are tied to the strategies outlined in the recommendations, as well as to the cross-cutting themes.
Panel 23. Proposed core indicators measuring progress associated with the Commission's recommendations.
The Commission proposes the following 24 indicators to measure progress on its recommendations in the 5 key areas.
Paying for essential medicines
-
•
Total pharmaceutical expenditure as a percentage of total health expenditure
-
•
Per capita total pharmaceutical expenditure
-
•
Public sector expenditure on pharmaceuticals as a percentage of total pharmaceutical expenditure
-
•
Household expenditure on pharmaceuticals as a percentage of total household expenditure*
-
•
Out-of-pocket expenditure on pharmaceuticals as a percentage of total pharmaceutical expenditure*
Affordability of essential medicines
-
•
Median availability of a basket of essential medicines in the public and private sectors (percentage)
-
•
Median consumer price ratio of a basket of essential medicines in the public and private sectors
-
•
Median public sector procurement or reimbursement price of essential medicines as a percentage of international reference price
-
•
Market share of multi-source medicines† (branded and unbranded generic products) by volume and value in public and private sector
Quality and safety of essential medicines
-
•
Number of national approvals of new chemical entities and generic products based on a Common Technical Document without any additional national requirements for quality, efficacy, and safety, as a percentage of total new chemical entities and generic approvals‡
-
•
Current and accumulated total number of medicines included in the WHO/UN Prequalification Programme (disaggregated by unique strength or dosage and pharmaceutical classes)‡
-
•
Number of failed quality control samples of essential medicines procured as a percentage of total number of samples of procured products tested per year (per procurement agency)
-
•
Number of pharmacovigilance reports for medicines submitted to the Uppsala Monitoring Centre per million population per year
-
•
Results of quality testing are publicly available‡
-
•
Number of core National Medicine Regulatory Agency performance indicators (listed in panel 12) that are independently assessed and publicly reported‡
Use of medicines
-
•
Existence of an independent national programme or institute promoting scientifically sound and cost-effective use of medicines (yes/no)‡
-
•
Stakeholder representation including civil society and patient representatives in the independent programme or institute is specifically provided for (yes/no)‡
-
•
Quality of prescribing in public and private sector§
-
•
Adherence to national standard treatment guidelines for common conditions in public and private sectors*
-
•
A legally enforceable code of marketing practice is in place and implemented (yes/no)‡
Developing missing essential medicines
-
•
Number of licence agreements concerning essential medicines concluded through patent pooling, stratified by in-licence and out-licence‡ ¶
-
•
Number of products produced under an Essential Medicines Patent Pool licence that are authorised by at least one of the following: International Council for Harmonisation or Pharmaceutical Inspection Convention and Pharmaceutical Inspection Cooperation Scheme member, or WHO/UN Prequalification Programme‡
-
•
National laws, including patent and medicines regulation laws, contain effective provisions for the application of all Trade-Related Aspects of Intellectual Property Rights -compatible flexibilities (yes/no)
-
•
Share of the research pipeline reflecting new molecules for diseases within the scope of the ATM Index461 (per company)
This set of indicators is intended to help national governments and the international community to establish systems for review and corrective action on essential medicines policies, moving the world towards accountability for equitable access to essential medicines. These systems would hold all stakeholders, including governments and multilateral agencies, accountable for steady progress towards effective implementation of essential medicines policy as a component of UHC.
Finally, the section proposes next steps towards creating stakeholder agreement on indicators, defining global or national targets as appropriate, and establishing an independent review mechanism to enable measurement of progress and ultimately corrective action.
Cross-cutting themes
Three themes cut across all of the priority areas for action on essential medicines policies discussed by the Commission:
-
1
Prioritising equity: access to appropriate, available, affordable, and quality essential medicines is important for all people, but is most difficult to achieve among poor and otherwise marginalised populations, both between and within nations. Promoting equity in access to essential medicines must be a key priority. Throughout the report, the Commission noted the special importance of essential medicines policies that contribute to achieving equity. Disaggregating data ensures that different strata of society are well represented and can be compared when measuring the effect of such policies.
-
2
Strengthening institutions: throughout the report, the Commission identified a range of specialised knowledge and skills required to implement essential medicines policies, as well as to collect and analyse data for decision making and accountability. These capacities cannot rest solely with a single person in a country. For sustainability and accountability, institutional structures and processes must be created and supported to develop, administer, implement, and assess policies. One key structure and process is an independent review mechanism that can identify when corrective action is necessary. The capacities of various institutions to carry out these tasks must be strengthened through the establishment of explicit mandates, the assurance of independence, and the provision of dedicated funding.
-
3
Promoting accountability: accountability refers to a responsibility to meet specific obligations, and for actors to answer to one another in a public and transparent process.462 The ultimate aims of UHC—improving health status, protecting against financial catastrophe due to illness, and achieving patient satisfaction—require commitment and concerted action by governments and other partners. Governments must demonstrate to citizens that their policies, and the institutions and administrative mechanisms that implement policies, are accomplishing these goals. Increasingly, governments are also agreeing to demonstrate progress to the international community, as with the SDGs. To track change, data must be collected, analysed, and shared with stakeholders. Accountability requires transparency in decision making and about the results of independent assessments of interventions.463 Stakeholder participation in the processes of decision making also promotes accountability; beneficiaries must be represented whenever decisions are made on their behalf. Civil society participation is key to representing the public at large, as well as to protecting particular subgroups.464
These cross-cutting themes align with broader global agendas for the SDGs, UHC, and strengthening of health systems, and the progressive realisation of the human right to health. The 24 core indicators the Commission recommends are intended to complement those being developed for the SDGs, including the WHO 100 Core Health Indicators.465
Initiatives such as the Medicines Transparency Alliance348, 466 and the WHO Good Governance for Medicines467 approach have made substantial, but insufficient, contributions to increasing transparency related to medicines; efforts need to be expanded. Information on medicine prices, availability, quality, and use generated at both national and international levels can contribute to other countries' and health systems' policies, and should be considered as global public goods, similar to the pricing information of medicines made available by Management Sciences for Health, shared HTA results, and clinical trials registries. The Commission acknowledges that generating information requires upfront investments, as with any public good; in this respect, the costs can in some cases be recouped over the longer term. The Commission further understands that access to good quality information is a necessary, but not sufficient, condition to improving access to good-quality essential medicines. Many other factors need to be mobilised to assure effective adoption and implementation of the necessary essential medicines policies. The Commission recognises that not enough has been done to establish a coordinated global approach to strengthening essential medicines-related institutions and holding them accountable—this is a key aim of the Commission's recommended indicators.
Towards a system of accountability for progress on essential medicines
The pharmaceutical sector has long been characterised by a lack of transparency, exacerbating the lack of accountability of meeting global health and essential medicines goals. Demonstrating and communicating progress is crucial to increasing transparency; it also enables the identification of good practices in effective implementation of essential medicines policies. Progress should be tracked by independent agencies, as governments and multilateral organisations might have conflicts of interest.
The Commission proposes 24 core indicators (panel 23) with the expectation that governments, health authorities, and other stakeholders will use them to create baseline measurements for assessing essential medicines policy development and implementation. Sharing data between countries would help to refine the instruments; repeating measurements over time would reveal progress and demonstrate the effectiveness of medicine policies and corrective actions. Documented progress on essential medicines policies would help direct resources to effective programmes and health institutions.
Setting appropriate targets for each indicator—a crucial component of their continued development process—remains to be done. This action will require the active involvement of all relevant stakeholders.462 Context-specific needs and past performance must be taken into consideration when defining targets and priority areas for improvement.
Since 16 of the 24 proposed core indicators are already well established and validated, countries can begin immediately to use them to assess their current performance and formulate their targets. When existing data are not appropriately disaggregated, additional efforts will be needed to address this deficit.462 The proposed new indicators urgently need to be validated.
Setting targets and measuring indicators alone cannot drive effective change. This proposed set of indicators is meant as a stepping-stone towards an accountability system for effective essential medicines policy implementation.462 To continue the process, mechanisms must be established to incentivise improvement and to implement corrective action.468 It is desirable to have multiple independent institutions, including academic centres, studying essential medicines availability, prices, and consumption. Key non-governmental organisations have long played important roles in collecting and disseminating information on health systems' performance in relation to essential medicines, and in holding different actors accountable. For example, Health Action International has taken the lead in measuring availability and price of medicines.140 Transparency International has assessed governance and transparency in the pharmaceutical sector, identifying corruption and how to implement preventive strategies.469 Likewise, the private business and non-governmental organisation sectors undertake medicines quality measurements (such as Mission for Essential Drugs and Supplies in Kenya)470 and gathers intelligence on market dynamics (as with IMS Health).96 These data sources have limitations—eg, market intelligence information might not include information on small countries or specific geographical regions—but in many settings they might be the only sources of this type of information.
Non-governmental actors need to continue to play a part in measuring progress, but cannot substitute for national and global governmental leadership and stewardship. Dedicated funds are necessary, such as a stated percentage of the medicines procurement or reimbursement budget. Australia's NPS MedicinesWise has demonstrated that it is possible to obtain a return on a small investment (0·54% of the medicine budget in the 2013–14 financial year) through savings in medicines expenditure.471 The Commission recommends that each country establish and support independent institutions or programmes to fulfil key functions, including: collecting, analysing, and disseminating information on prices of medicines, availability, affordability, quality, and use; coordinating HTA or other value-based analysis of new and existing essential medicines; and improving the use of medicines.
Selecting indicators for assessing progress
The Commission used several criteria to select its proposed indicators. First, the Commission gave preference to using existing validated indicators, rather than creating new ones. Using existing validated indicators means historical data are available as baseline benchmarks. Furthermore, these indicators have the advantage of proven feasibility of data collection.
However, existing indicators do not cover all recommendations made by the Commission. Thus, the Commission also includes new indicators that will require further validation.
Second, instead of input indicators (eg, physical resources such as staff and materials, or financial resources) the Commission prioritised output indicators (such as the availability of essential medicines in the public sector).472 It is important to note that the proposed indicators are not measuring the ultimate goals of the health system, such as health status or patient satisfaction. Each indicator does, however, contribute information to measure progress on the five core areas addressed in this report: paying for essential medicines, guaranteeing affordability, quality and safety assurance, improving use, and developing missing essential medicines.
Rather than assigning specific indicators to each recommendation or attempting to measure each recommendation comprehensively, indicators were selected to serve as sensitive flags of progress. For example, the indicator related to the market share of generic medicines can gauge the extent to which pro-generic policies have been implemented to promote affordability. However, the indicator cannot measure the extent to which the full range of pricing policies has been implemented.
Third, emphasis was placed on selecting indicators that measure progress on the three themes: increasing equity, strengthening institutions, and promoting accountability.
Finally, the effect of most of the Commission's recommendations can only be measured comprehensively by using a set of multiple indicators. However, at this stage the Commission elected to focus on a small set of core indicators to be indicative of one or two specific aspects. For example, it would take several indicators to demonstrate progress made in relation to establishing an MPP. However, one core indicator and two complementary indicators were chosen to represent several others.
In many instances, measurement disaggregated by essential versus non-essential medicines is not feasible (eg, a country's pharmaceutical expenditure). Although the Commission has focused on essential medicines, measuring all medicines for many of the indicators is desirable, since which medicines are considered essential will change over time. Likewise, product quality improvement should also focus on all medicines, not only on essential medicines.
Continuing to develop indicators will require an ongoing consultation process with the full range of relevant stakeholders. These indicators are proposed as an intermediate step in a longer process of developing a global consensus on a final set of key indicators. Particularly, the Commission recognises the need for setting targets at the national level. In addition to the overarching targets related to essential medicines in the SDGs, the international community has already settled on some targets in disease-specific areas. For example, countries have agreed to aim for 80% availability of affordable basic technologies and essential medicines, including generics, required to treat major NCDs.473
Panel 23 summarises the suggested core indicators for each section. An additional list of proposed complementary indicators is presented in appendix 6.
Overview of proposed indicators
Paying for essential medicines
The five indicators selected for financing of essential medicines (panel 23) are well established,474 but there is still a lack of comparative and comprehensive data and analysis of pharmaceutical expenditure between LMICs. The National Health Account information collected by WHO does not provide the most recent information on pharmaceutical spending by country.475 Likewise, the World Health Statistics provide information on overall health expenditure, but not on pharmaceutical expenditure.139 The latest comprehensive analysis of global pharmaceutical expenditure presents data from 2006, already at least 10 years old.476 The OECD has published expenditure on pharmaceutical expenditures, but only for selected countries.477
Given the general difficulty of measuring pharmaceutical expenditures, countries are unlikely to be able to further disaggregate expenditure on essential medicines from spending on other medicines. The percentage of public expenditure on pharmaceuticals could be used as a proxy indicator, assuming that public financing for medicines prioritises items on the national essential medicines list.
Financing of essential medicines is influenced by organisational arrangements and ultimately affects financial protection, one of the key goals of a health system and a cardinal feature of UHC. One of the indicators included in this set—household expenditure on pharmaceuticals as a percentage of total household expenditure—is a measure of financial protection. To measure progress on reducing disparities related to financing essential medicines, household expenditure on pharmaceuticals as a percentage of total household expenditure should be disaggregated by income, ethnicity, education, geography, and other relevant characteristics (such as households with a member living with NCDs). Since household data on expenditure is often separate from data on the health status of household members, it can be difficult to meaningfully measure equity with existing datasets.15 Indicators for financing depend on household surveys to obtain data on household expenditures on pharmaceuticals, including out-of-pocket expenditure. Yet many countries still lack routinely collected and nationally representative household data.15 Measuring progress towards UHC provide an opportunity to integrate the indicators into a wider SDG-monitoring framework.
Making essential medicines affordable
To estimate affordability in a given setting, the aggregated medicines price per time period is divided by the household income per equivalent time period (eg, wage of the lowest-paid government worker).135 These indicators require information on the prices of pharmaceuticals, data that have been notoriously difficult to capture because of a lack of transparency and investment in monitoring. Three of the indicators to measure comparable information on prices of medicines and availability have already been used extensively, including two that are standard indicators in the World Health Statistics.139
Ensuring that information on price is available depends on several prerequisites. The prices of essential medicines must be monitored regularly at several points along the supply chain, from procurement prices in the public and private sectors (including hospitals), to retail consumption. The prices of individual products should be collected and reported to provide a mean price ratio that can be combined with maximal and minimal values for individual products.135 It is rare for hospital procurement prices to be openly available.478 Health insurance funds can play a key part in collecting and publicising reimbursement price information.
No current indicator exists to measure the transparency of decision making regarding the inclusion of essential medicines into reimbursement or procurement lists. More work needs to be done in this area to develop and validate indicators.
Complementary indicators (appendix 6) include those related to transparency (such as the existence of mechanisms to reveal conflicts of interest of members involved in reimbursement decisions). Several others measure the degree to which information (results of clinical trials informing reimbursement decisions) is made publicly available. Greater information sharing across institutions would allow greater efficiency in avoiding duplication of assessments of safety and efficacy. However, this action would require globally and nationally standardised formats for reporting the information.479
Assuring the quality and safety of essential medicines
Two of the core indicators on quality and safety are well established, since they are included in the WHO Pharmaceutical Country Profiles.480
The first and third indicator in the Quality and safety of essential medicines section (panel 23) depend in part on the transparency of the NMRA in providing data on performance. Measuring performance of NMRAs, and holding them accountable, has often proved challenging.481 Increasing transparency of NMRA data should include providing access to data used by NMRAs for decision making on safety (eg, market intelligence on consumption which is often proprietary and not publicly accessible).482, 483 Additional indicators that should be used to bolster accountability systems for NMRAs are included in the appendix.
The second indicator in the Quality and safety of essential medicines section (panel 23) measures the evolution of the WHO/UN Prequalification Programme, and the third indicator in this section provides information on promoting the quality of products procured. Ideally, a composite indicator comprising different dimensions of procurement performance would be used to measure performance.
Improving the use of essential medicines
Measures such as the extent of adherence to standard treatment guidelines for common conditions are well established as indicators of the quality use of essential medicines.484 Additionally, the widely used set of indicators to measure quality of prescribing in primary care has been included.330 One of the standard indicators included in this set measures the proportion of patients who were prescribed an antimicrobial,484 which is a key global priority.485 In the past, the latter indicator emphasised acute conditions. The conditions taken into consideration in future should be expanded to include chronic non-communicable conditions (such as hypertension, diabetes, and cancer) and palliative care. Data on medicines consumption should be disaggregated by sex, age, education, income, and insurance benefits, among other locally relevant variables, to capture disparities in access.486 Some experts have suggested using a composite indicator to measure quality of prescribing, which could be used in the future.330
The first indicator under the Use of medicines section (panel 23) relates to the cross-cutting theme of institutional strengthening by measuring a structural component, the existence of an independent national programme or institute to promote scientifically sound and cost-effective use of medicines. The second indicator in this section measures an aspect related to accountability: stakeholder representation. The first two indicators in this section are new and would need to be validated.
Additional complementary indicators are included in the appendix, which measure commitment to transparency (eg, the existence of a policy for conflicts of interest). This measure aims to protect public knowledge of, and if necessary to restrain the influence of, any private-sector actor with a major interest in the development of public policies related to changing the use of medicines.
Developing missing essential medicines
The common goal of all five recommendations in this section is to promote and accelerate the development of essential medicines that address crucial unmet health needs. Two indicators suggested for measuring progress on developing missing essential medicines are new. The first two recommendations do not have corresponding indicators; these would need to be developed once instituted. However, some institutions, such as the ATM Foundation,461 already use indicators on development of missing essential medicines (eg, similar to the fourth indicator under the Developing missing essential medicines section in panel 23). The ATM Index is published every 2 years by the ATM Foundation and covers 20 leading pharmaceutical companies. To measure the performance of other companies not yet included in the ATM Index, additional resources are required to expand the scope of the Index or create new organisations with similar missions and methods. However, securing long-term sustainable financing of such independent organisations must be assured by the international health community.
A future of accountability for essential medicines policies
The Commission is confident that new endeavours to create an independent accountability system, supported by the global community, will ensure that crucial actions are taken to protect investments made in essential medicines, and that these investments translate into health and development for all.
Without essential medicines, no health system can ensure that the population it serves progressively realises its right to health. Yet essential medicines policies have received insufficient attention since the Nairobi conference in 1985. In this report the Commission presents practical recommendations that will enable a new era of equity, strengthened institutions, and accountability to ensure that essential medicines policies support UHC and sustainable development in the 21st century.
Acknowledgments
Acknowledgments
We are very grateful to the many experts who contributed to the production of this report. We thank The Lancet, in particular Pam Das, Senior Executive Editor, for her guidance in the process. We are also extremely grateful to Anya Levy Guyer for her editorial assistance throughout the process. Many researchers have generously contributed their time, efforts, and unpublished data to the work of the Commission. We thank Amey N Shroff (University of Michigan), Nattha Tritasavit (Health Intervention and Technology Assessment Program, Thai Ministry of Public Health), Tessa Hermens (NPS MedicineWise), Michael Deats (World Health Organization), Peter Hall (Concept Foundation), Nikita V de Jong (University Medical Centre Groningen), Thomas Layloff (Management Sciences for Health), Patrick H Lukulay (United States Pharmacopeial Convention), Zafar Mirza (World Health Organization), Paul Newton (Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit), Sten Olsson (Uppsala Monitoring Centre), Michaella Latkovic (Boston University School of Public Health), Steven Crimaldi (Boston University School of Public Health), Jaime Espin (Escuela Andaluza de Salud Publica), Jean-Pierre Sallet (Axios International), Vusi C Dlamini (KwaZulu-Natal Department of Health) is thanked for providing medicines consumption data, and all external reviewers for their constructive comments. Finally, we thank the University Medical Centre Groningen of the University of Groningen, Netherlands, and the Health Foundation, UK, for providing the Lancet Commission with meeting venues. The work of the Commission was funded by the Bill & Melinda Gates Foundation, World Health Organization, the University Medical Centre Groningen, Boston University, and by all the academic institutions and other organisations that have generously allowed their staff to devote time to the work of the Commission.
Contributors
VJW, HVH, and ALG developed the proposal for the Commission and drew up its objectives. The Commission met on three occasions: in Groningen (2014), London (2015), and Amsterdam (2015). All Commissioners and coauthors contributed to the identification of key problems and the selection of five main topics for review. As co-chairs of the Commission, HVH, VJW, and ALG planned and coordinated all activities of the Commission, the development and review of the various drafts of the report, and preparation for external peer review. VJW, HVH, ALG, and MRR reviewed and edited all sections of the report. All Commissioners reviewed the various stages of the report and approved the final version. Introduction section: HVH, ALG, and VJW wrote the first and subsequent drafts of the introduction with input from MRR. Section 1: ALG and VJW wrote the first and subsequent drafts of the section on financing of essential medicines with input from HVH and MRR. The costing model was developed by PY, AR, CM, and VJW. The section on the costing model was drafted by CM with input by PY, VJW, and PNS. Section 2: YT wrote the first draft of the section on affordability of essential medicines, with input from ALG, EFM'tH, MAE, and MRR. ALG substantially revised the following drafts with additional input from VJW, HVH, EFM'tH and MRR. Section 3: LR and HVH wrote the first and subsequent drafts of the section on quality of essential medicines with input from VJW, ALG, MAE, and MRR. Section 4: AKW and DR-D wrote the first and subsequent drafts of the section on promoting quality use of medicines, with contributions from SJ, VLL, and PNS. HVH, ALG, VJW, and MRR provided input on the drafts. The panel on promotion was drafted by HVH with substantial input by MAE. Section 5: EFM'tH and HVH wrote the first and subsequent drafts of the section on R&D of essential medicines with inputs from BP, ALG, MRR, and VJW. Section 6: this section was written by VJW with substantial input from MB, MRR, ALG, and HVH. VJW, HVH, and ALG formulated the first version of the proposed indicators for the accountability framework with substantial input by other Commissioners.
Declaration of interests
CPdJ was director of Essential Medicines and Health Products at WHO until April 30, 2016. ALG is a member of the WHO Expert Panel on Drug Policies and Management, the South African Medicines Control Council, and the South African National Essential Medicines List Committee. HVH reports personal fees from WHO, Health Action International, and Access to Medicine Index 2016, outside the submitted work. DRD reports personal fees from WHO Access to Medicines Research Network/Oswaldo Cruz during the study; grants from IMS Institute (using funds from Novartis), Indonesia Poverty Commission (using funds from AusAID), University of Indonesia and Hacettepe University (using funds from Novartis), FSG, and German International Development Organization (GIZ), in collaboration with the World Bank Institute; and personal fees from the National University of Singapore, all outside the submitted work. PNS reports other from pharmaceutical company, governments, and academic institutions; and grants from donors/multilateral organisations, outside the submitted work. EFM'tH reports personal fees from Drugs for Neglected Diseases initiative, during the conduct of the study. AKW reports personal fees and other from WHO Geneva and Myanmar country office; grants and other from University of Indonesia, Hacettepe University, and Acibadem University (using funds from Novartis); grants from IMS Institute (using funds from Novartis), Indonesia Poverty Commission (using funds from AusAid), FSG, National University of Singapore, GIZ with World Bank, Alliance for Health Policy and Systems Research, and Global Task Force for Cancer Care and Control, Harvard School of Public Health (using funds from Pfizer), all outside the submitted work. Additionally, AKW has a patent Tufts Medical Center License with Johnson and Johnson, topiramate, with royalties paid to Tufts Medical Center. VJW reports grants from the Bill & Melinda Gates Foundation during the conduct of the study, and grants from Sandoz International GmbH and from Management Sciences for Health, outside the submitted work. PY reports grants from the Bill & Melinda Gates Foundation during the conduct of the study, and non-financial support from Merck For Mothers and Merck Serono, outside the submitted work. All other authors declare no competing interests. The thoughts and opinions expressed here are those of the individual contributors alone and do not necessarily reflect the views of their employers or affiliated organisations.
Since 2002, WHO has used the term essential medicines rather than essential drugs to prevent confusion with drugs of abuse. In this report, the term medicines is used unless citing an original document or structure that used the former terminology.
Selected system functions as defined by Roberts and colleagues.98
Equity: data should be stratified by the following variables: gender, ethnicity, education, place of residence, and wealth quintile.
Ideally, market share of essential medicines by volume should be measured in addition to medicines in general.
New indicator that requires validation.
Five individual indicators: (1) prescriptions including an antimicrobial (%; desired threshold of <30%); (2) polypharmacy prescription (%; desired threshold of 0%); (3) prescriptions including injection (%; desired threshold of <10%); (4) medicines prescribed by generic name (%; desired threshold of 100%); (5) medicines prescribed from essential medicines list or reimbursement list (%; desired threshold of 100%).49
In-licensing refers to the granting of a licence to the patent pool by the patent holder; out-licensing refers to the licensing of generic producers by the patent pool, in accordance with the provisions of the original licence to the pool (eg, geographical scope or limitations to specific types of dosage forms such as paediatric dosage forms).
Supplementary Material
References
- 1.UN Sustainable development goals. 2015. https://sustainabledevelopment.un.org/sdgs (accessed March 4, 2016).
- 2.WHO What is universal health coverage? http://www.who.int/universal_health_coverage/en/index.html (accessed April 9, 2016).
- 3.WHO . The world health report—health systems financing: the path to universal coverage. World Health Organization; Geneva: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner AK, Graves AJ, Reiss SK, Lecates R, Zhang F, Ross-Degnan D. Access to care and medicines, burden of health care expenditures, and risk protection: results from the World Health Survey. Health Policy. 2011;100:151–158. doi: 10.1016/j.healthpol.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. Lancet. 2011;377:505–515. doi: 10.1016/S0140-6736(10)61894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatib R, McKee M, Shannon H, for the PURE study investigators Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387:61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 7.Suhrcke M, Stuckler D, Suk JE. The impact of economic crises on communicable disease transmission and control: a systematic review of the evidence. PLoS One. 2011;6:e20724. doi: 10.1371/journal.pone.0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftus P. Prisoners sue Massachusetts for withholding hepatitis C drugs [Blog post] Wall Street Journal (Washington, DC) June 11, 2015 http://blogs.wsj.com/pharmalot/2015/06/11/prisoners-sue-massachusetts-for-withholding-hepatitis-c-drugs/ (accessed June 30, 2016). [Google Scholar]
- 9.Johnston A, Holt DW. Substandard drugs: a potential crisis for public health. Br J Clin Pharmacol. 2014;78:218–243. doi: 10.1111/bcp.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton WL, Doyle C, Halliwell-Ewen M, Lambert G. Public health interventions to protect against falsified medicines: a systematic review of international, national and local policies. Health Policy Plan. 2016 doi: 10.1093/heapol/czw062. published online June 16. [DOI] [PubMed] [Google Scholar]
- 11.Renschler JP, Walters KM, Newton PN, Laxminarayan R. Estimated under-five deaths associated with poor-quality antimalarials in sub-Saharan Africa. Am J Trop Med Hyg. 2015;92(suppl):119–126. doi: 10.4269/ajtmh.14-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rentz ED, Lewis L, Mujica OJ. Outbreak of acute renal failure in Panama in 2006: a case-control study. Bull World Health Organ. 2008;86:749–756. doi: 10.2471/BLT.07.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Alert No. 125. Contaminated Isotab (isosorbide mononitrate) incident in Lahore Pakistan. 2012. http://www.who.int/medicines/publications/drugalerts/DrugSafetyAlert125.pdf (accessed Dec 18, 2013).
- 14.Holloway KA, Ivanovska V, Wagner AK, Vialle-Valentin C, Ross-Degnan D. Have we improved use of medicines in developing and transitional countries and do we know how to? Two decades of evidence. Trop Med Int Health. 2013;18:656–664. doi: 10.1111/tmi.12123. [DOI] [PubMed] [Google Scholar]
- 15.WHO . World Bank. Tracking Universal Health Coverage. First global monitoring report. World Health Organization; Geneva: 2015. [Google Scholar]
- 16.UN Commission on Life-Saving Commodities for Women and Children Commissioners' Report. Aug 31, 2012. http://www.unfpa.org/sites/default/files/pub-pdf/Final%20UN%20Commission%20Report_14sept2012.pdf (accessed Feb 28, 2016).
- 17.Outterson K. New Business Models for Sustainable Antibiotics. The Royal Institute of International Affairs; London: 2014. http://www.chathamhouse.org/sites/files/chathamhouse/public/Research/Global%20Health/0214SustainableAntibiotics.pdf (accessed Feb 28, 2016). [Google Scholar]
- 18.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 19.WHO . Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. World Health Organization; Geneva: 2016. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf (accessed July 31, 2016). [Google Scholar]
- 20.WHO The Selection and Use of Essential Medicines. Report of the WHO Expert Committee, 2015 (including the 19th WHO Model List of Essential Medicines and the 5th WHO Model List of Essential Medicines for Children). WHO Technical Report Series number 994. http://apps.who.int/iris/bitstream/10665/189763/1/9789241209946_eng.pdf (accessed July 30, 2016).
- 21.Zopf S, Kremer AE, Neurath MF, Siebler J. Advances in hepatitis C therapy: What is the current state - what come's next? World J Hepatol. 2016;8:139–147. doi: 10.4254/wjh.v8.i3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyengar S, Tay-Teo K, Vogler S. Prices, costs, and affordability of new medicines for hepatitis C in 30 countries: an economic analysis. PLoS Med. 2016;13:e1002032. doi: 10.1371/journal.pmed.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Senate Committee on Finance Wyden-Grassley Sovaldi Investigation Finds Revenue-Driven Pricing Strategy Behind $84,000 Hepatitis Drug. http://www.finance.senate.gov/ranking-members-news/wyden-grassley-sovaldi-investigation-finds-revenue-driven-pricing-strategy-behind-84-000-hepatitis-drug (accessed July 11, 2016).
- 25.McDonald SA, Mohamed R, Dahlui M, Naning H, Kamarulzaman A. Bridging the data gaps in the epidemiology of hepatitis C virus infection in Malaysia using multi-parameter evidence synthesis. BMC Infect Dis. 2014;14:564. doi: 10.1186/s12879-014-0564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achariam T. Hepatitis C cure too expensive in Malaysia. The Sun Daily (Malaysia), 20 October 2015. http://www.thesundaily.my/news/1586956 (accessed July 11, 2016).
- 27.World Bank Country and Lending Groups. http://data.worldbank.org/about/country-and-lending-groups#Lower_middle_income (accessed June 3, 2016).
- 28.World Bank GDP per capita (current US$). Data. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD/countries (accessed May 27, 2016).
- 29.Hill A, Pozniak A, Freeman J. Contribution to the United Nations Secretary-General's High-Level Panel on Access to Medicines. Feb 25, 2016. http://www.unsgaccessmeds.org/inbox/2016/2/25/andrew-hill-dr-anton-pozniak-and-dr-james-freeman (accessed July 31, 2016).
- 30.McNeil DG. Curing Hepatitis C, in an Experiment the Size of Egypt. The New York Times Online (USA) Dec 15, 2015. http://www.nytimes.com/2015/12/16/health/hepatitis-c-treatment-egypt.html (accessed July 31, 2016).
- 31.Gilead Chronic Hepatitis C Treatment Expansion – Generic Manufacturing for Developing Countries. August 2015. https://www.gilead.com/∼/media/files/pdfs/other/hcv%20generic%20agreement%20fast%20facts%20021616.pdf (accessed Aug 8, 2016).
- 32.Medicines Patent Pool . The Medicines Patent Pool signs licence with Bristol-Myers Squibb to increase access to hepatitis C medicine Daclatasvir. Medicines Patent Pool; Geneva: Nov 23, 2015. http://www.medicinespatentpool.org/the-medicines-patent-pool-signs-licence-with-bristol-myers-squibb-to-increase-access-to-hepatitis-c-medicine-daclatasvir/ (accessed Aug 8, 2016). [Google Scholar]
- 33.WHO . Medical Product Alert number 3/2016: Falsified Hepatitis C medicines circulating in South East Asia [Ref. RHT/SAV/Alert 3.2016] World Health Organization; Geneva: 2016. [Google Scholar]
- 34.Brennan Z. Counterfeit Versions of Gilead's Blockbuster Hepatitis C Drug Found in Israel. 2016. http://raps.org/Regulatory-Focus/News/2016/03/07/24487/Counterfeit-Versions-of-Gilead%E2%80%99s-Blockbuster-Hepatitis-C-Drug-Found-in-Israel/ (accessed July 13, 2016).
- 35.Maqbool S. Factory making fake hepatitis C drug raided. The News (Islamabad) March 1, 2015. https://www.thenews.com.pk/print/26710-factory-making-fake-hepatitis-c-drug-raided (accessed July 13, 2016).
- 36.Sachs J. Gilead's greed that kills. Huffington Post. July 27, 2015. http://www.huffingtonpost.com/jeffrey-sachs/gileads-greed-that-kills_b_7878102.html (accessed July 11, 2016).
- 37.WHO The selection of essential drugs: report of a WHO expert committee [Technical Report Series no. 615] 1977. http://apps.who.int/iris/bitstream/10665/41272/1/WHO_TRS_615.pdf?ua=1) (accessed July 4, 2016). [PubMed]
- 38.United Nations . Technology policies in the pharmaceutical sector in Cuba [UNCTAD/TT/33] United Nations; New York: 1980. [Google Scholar]
- 39.King M, editor. Medical care in developing countries. Oxford University Press; Nairobi: 1966. [Google Scholar]
- 40.Van Amelsvoort V. Standard notes for Tanzanian dispensaries (experimental edition) Dar es Salaam University, Department of Preventive and Social Medicine; Dar es Salaam: 1970. [Google Scholar]
- 41.Ministerio de Salud . Vademécum official de medicamentos básicos del sector salud 1972. Ministerio de Salud; Lima: 1972. [Google Scholar]
- 42.The Lancet Desert-island drugs. Lancet. 1978;I:423–424. [PubMed] [Google Scholar]
- 43.Hardon A. Consumers versus Producers – powerplay behind the scenes. In: Kanji N, Hardon A, Harnmeijer JW, Mamdani M, Walt G, editors. Drugs policy in developing countries. Zed Books; London and New Jersey: 1992. [Google Scholar]
- 44.WHO The rational use of drugs. Report of the conference of experts: Nairobi, 25-29 November 1985. http://apps.who.int/medicinedocs/documents/s17054e/s17054e.pdf (accessed April 10, 2016).
- 45.Kanji N, Hardon A, Harnmeijer JW, Mamdani M, Walt G. Drugs policy in developing countries. Zed Books; London and New Jersey: 1992. [Google Scholar]
- 46.WHO Ethical criteria for medicinal drug promotion. 1988. http://apps.who.int/medicinedocs/documents/whozip08e/whozip08e.pdf (accessed April 9, 2016).
- 47.WHO . Guidelines for developing and implementing national drug policies. World Health Organization; Geneva: 1988. [Google Scholar]
- 48.WHO How to develop and implement a national drug policy. Updates and replaces Guidelines for Developing National Drug Policies, 1988. Second edn. 2001. http://apps.who.int/medicinedocs/pdf/s2283e/s2283e.pdf (accessed July 11, 2016).
- 49.WHO How to investigate drug use in health facilities: selected drug use indicators, EDM Research Series No. 007, WHO/DAP/93.1. 1993. http://www.who.int/medicines/publications/how-to-investigate_drug-use/en/ (accessed July 11, 2016).
- 50.DeVries TPGM, Fresle DA, Henning RH, Hogerzeil HV. Guide to Good Prescribing – A Practical Manual. 1994. http://apps.who.int/medicinedocs/en/d/Jwhozip23e/ (accessed July 11, 2016).
- 51.Kanji N, Hardon A. What has been achieved and where are we now? In: Kanji N, Hardon A, Harnmeijer JW, Mamdani M, Walt G, editors. Drugs policy in developing countries. Zed Books; London and New Jersey: 1992. [Google Scholar]
- 52.Greene JA. Making medicines essential: The emergent centrality of pharmaceuticals in global health. Biosocieties. 2011;6:10–33. [Google Scholar]
- 53.WHO/Ecumenical Pharmaceutical Network Multi-country study of medicine supply and distribution activities of faith-based organizations in sub-Saharan African countries (WHO/PSM/PAR/2006.2) 2006. http://www.who.int/medicines/areas/access/EN_EPNstudy.pdf (accessed July 4, 2016).
- 54.Olivier J, Tsimpo C, Gemignani R. Understanding the roles of faith-based health-care providers in Africa: review of the evidence with a focus on magnitude, reach, cost, and satisfaction. Lancet. 2015;386:1765–1775. doi: 10.1016/S0140-6736(15)60251-3. [DOI] [PubMed] [Google Scholar]
- 55.Walsh J, Warren K. Selective PHC – an interim strategy for disease control in developing countries. N Engl J Med. 1979;301:967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- 56.Reich MR. Essential Drugs: Economics and Politics in International Health. Health Policy. 1987;8:39–57. [Google Scholar]
- 57.Oyugui E, Kigozi D, On'gwen O. Structural Adjustment and Public Social Spending - Kenya. Social Watch. http://www.socialwatch.org/node/10596 (accessed Aug 13, 2016).
- 58.Gustafsson LL, Wettermark B, Godman B, for the Regional Drug Expert Consortium The ‘wise list'- a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108:224–233. doi: 10.1111/j.1742-7843.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 59.UN Secretary-General proposes Global Fund Fight against HIV/AIDS and other infectious diseases at African Leader Summit (SG/SM/777.9/Rev.1) April 26, 2001. http://www.un.org/press/en/2001/SGSM7779R1.doc.htm (accessed July 4, 2016).
- 60.United Nations Economic and Social Council . General Comment No. 14: The right to the highest attainable standard of health (article 12 of the International Covenant on Economic, Social and Cultural Rights) United Nations; Geneva: 2000. [Google Scholar]
- 61.United Nations General Assembly . Report of the Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health (A/61/338) United Nations; New York: 2006. [Google Scholar]
- 62.Hogerzeil HV. Essential medicines and human rights: what can they learn from each other? Bull World Health Organ. 2006;84:371–375. doi: 10.2471/blt.06.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO Essential Medicines – WHO Model List (revised April 2002) Core List (12th edn) 2002. http://apps.who.int/iris/bitstream/10665/67335/1/a76618.pdf (accessed July 30, 2016).
- 64.Consumers International HAI, MSF, Oxfam. NGO Statement for the WHO Executive Board. Jan 14, 2002. http://www.msfaccess.org/content/ngo-statement-who-executive-board (accessed July 30, 2016).
- 65.WHO Essential medicines. http://www.who.int/topics/essential_medicines/en/ (accessed Sept 29, 2016).
- 66.Laing R, Waning B, Gray A, Ford N, 't Hoen E. 25 years of the WHO essential medicines lists: progress and challenges. Lancet. 2003;361:1723–1729. doi: 10.1016/S0140-6736(03)13375-2. [DOI] [PubMed] [Google Scholar]
- 67.WHO The selection and use of essential medicines. Report of the WHO Expert Committee, 2002 (including the Model List of Essential Medicines) (Technical Report Series 914) 2002. http://apps.who.int/iris/bitstream/10665/42620/1/WHO_TRS_914_eng.pdf (accessed July 30, 2016). [PubMed]
- 68.Bigdeli M, Peters D, Wagner A, eds. Medicines in Health Systems: Advancing access, affordability and appropriate use. 2014. http://www.who.int/alliance-hpsr/resources/publications/9789241507622/en/ (accessed April 10, 2016).
- 69.WHO . Everybody's business. Strengthening health systems to improve health outcomes. World Health Organization; Geneva: 2007. [Google Scholar]
- 70.UN . Millennium Development Goal Report 2014. United Nations; New York: 2014. [Google Scholar]
- 71.Liu L, Oza S, Hogan D. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 72.Piot P, Abdool Karim SS, Hecht R, the UNAIDS–Lancet Commission Defeating AIDS—advancing global health. Lancet. 2015;386:171–218. doi: 10.1016/S0140-6736(15)60658-4. [DOI] [PubMed] [Google Scholar]
- 73.The Global Fund to Fight AIDS . Tuberculosis and Malaria. Guide to Global Fund Policies on Procurement and Supply Management of Health Products. Global Fund; Geneva: 2012. http://apps.who.int/medicinedocs/documents/s19721en/s19721en.pdf (accessed July 4, 2016). [Google Scholar]
- 74.Kaplan W, Laing R. Priority Medicines for Europe and the World (WHO/EDM/PAR//2004.7) 2004. http://apps.who.int/iris/bitstream/10665/68769/1/WHO_EDM_PAR_2004.7.pdf (accessed July 4, 2016).
- 75.WHO Better Medicines for Children (WHA60.20 – Agenda item 12.18) 2007. http://apps.who.int/medicinedocs/documents/s21455en/s21455en.pdf (accessed July 4, 2016).
- 76.WHO The selection and use of essential medicines. Report of the WHO Expert Committee, October 2007 (including the Model List of Essential Medicines for Children) (Technical Report Series 950) 2007. http://apps.who.int/iris/bitstream/10665/43887/1/WHO_TRS_950_eng.pdf (accessed July 4, 2016).
- 77.UN . Millennium Development Goal 8. Taking stock of global partnership for development. MDG Taskforce Report 2015. United Nations; New York: 2015. [Google Scholar]
- 78.Leach B, Paluzzi JE, Munderi P. Prescription for healthy development: increasing access to medicines. United Nations Development Programme, Earthscan; London: 2005. http://www.unmillenniumproject.org/documents/TF5-medicines-Complete.pdf (accessed July 20, 2016). [Google Scholar]
- 79.Hunt P. The right of everyone to the highest attainable standard of physical and mental health. A/61/338, 2006, 19–21. http://www.who.int/medicines/areas/human_rights/A61_338.pdf (accessed July 6, 2016).
- 80.Ruggie JG. Guiding Principles on Business and Human Rights: Implementing the United Nations “Protect, Respect and Remedy” Framework, Doc No A/HRC/17/31. United Nations; New York: 2011. [Google Scholar]
- 81.International Federation of Pharmaceutical Manufacturers and Associations Developing World Health Partnership Directory. http://partnerships.ifpma.org/partnerships/by-company (accessed July 4, 2016).
- 82.Access to Medicines Foundation . Access to Medicines Index 2014. ATM Foundation; Haarlem: 2014. [Google Scholar]
- 83.Kamal-Yanni MM. Action to preserve WHO's core functions cannot wait for organisational reform. Lancet. 2012;379:309. doi: 10.1016/S0140-6736(12)60040-3. [DOI] [PubMed] [Google Scholar]
- 84.International Council for Harmonisation Organisational Changes. http://www.ich.org/about/organisational-changes.html (accessed June 24, 2016).
- 85.Evans DB, Marten R, Etienne C. Universal health coverage is a development issue. Lancet. 2012;380:864–865. doi: 10.1016/S0140-6736(12)61483-4. [DOI] [PubMed] [Google Scholar]
- 86.Reich MR, Harris J, Ikegami N. Moving towards universal health coverage: lessons from 11 country studies. Lancet. 2016;387:811–816. doi: 10.1016/S0140-6736(15)60002-2. [DOI] [PubMed] [Google Scholar]
- 87.Garabedian LF, Ross-Degnan D, Ratanawijitrasin S, Stephens P, Wagner AK. Impact of universal health insurance coverage in Thailand on sales and market share of medicines for non-communicable diseases: an interrupted time series study. BMJ Open. 2012;2:001686. doi: 10.1136/bmjopen-2012-001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katrein F, Tejada CA, Restrepo-Méndez MC, Bertoldi AD. Inequality in Brazilian women's access to medicines for chronic diseases. Cad Saude Publica. 2015;31:1416–1426. doi: 10.1590/0102-311X00083614. (in Portuguese). [DOI] [PubMed] [Google Scholar]
- 89.Lee JT, Hamid F, Pati S, Atun R, Millett C. Impact of noncommunicable disease multimorbidity on healthcare utilisation and out-of-pocket expenditures in middle-income countries: cross sectional analysis. PLoS One. 2015;10:e0127199. doi: 10.1371/journal.pone.0127199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bigdeli M, Laing R, Tomson G, Babar ZU. Medicines and universal health coverage: challenges and opportunities. J Pharm Policy Pract. 2015;8:8. doi: 10.1186/s40545-015-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cameron A, Roubos I, Ewen M, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Differences in the availability of medicines for chronic and acute conditions in the public and private sectors of developing countries. Bull World Health Organ. 2011;89:412–421. doi: 10.2471/BLT.10.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.UN 2011 High-level meeting on the prevention and control of non-communicable diseases. http://www.un.org/en/ga/ncdmeeting2011/ (accessed Aug 14, 2016).
- 93.WHO WHO's Global NCD Action Plan 2013–2020. http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf?ua=1 (accessed Aug 14, 2016).
- 94.Heymann DL, Mackenzie JS, Peiris M. SARS legacy: outbreak reporting is expected and respected. Lancet. 2013;381:779–781. doi: 10.1016/S0140-6736(13)60185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.The Lancet Zika virus resource centre. http://www.thelancet.com/campaigns/zika (accessed July 30, 2016).
- 96.IMS Health Institute for Healthcare Informatics Global medicines use in 2020: outlook and Implications. 2015. http://www.imshealth.com/en/thought-leadership/ims-institute/reports/global-medicines-use-in-2020 (accessed April 10, 2016).
- 97.Food and Drug Administration FDA's role in precision medicine. http://www.fda.gov/ScienceResearch/SpecialTopics/PrecisionMedicine/default.htm (accessed July 6, 2016).
- 98.Roberts MJ, Hsiao W, Berman P, Reich MR. Getting Health Reform Right: a guide to improving performance and equity. Oxford University Press; New York: 2004. [Google Scholar]
- 99.Shakarishvili G, Atun R, Berman P, Hsiao W, Burgess C, Lansang MA. Converging Health Systems Frameworks: towards a concepts-to-actions roadmap for health systems strengthening in low and middle income countries. Glob Health Gov. 2010;3:1–7. [Google Scholar]
- 100.Management Sciences for Health Systems for Improved Access to Pharmaceuticals and Services (SIAPS). SIAPS fact sheet. 2013. http://siapsprogram.org/wp-content/uploads/2013/09/SIAPS-Fact-Sheet_2013.pdf (accessed July 4, 2016).
- 101.Bigdeli M, Jacobs B, Tomson G. Access to medicines from a health system perspective. Health Policy Plan. 2013;28:692–704. doi: 10.1093/heapol/czs108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frost LJ, Reich MR. Access: how do good health technologies get to poor people in poor countries? Harvard Center for Population and Development Studies, Harvard University Press; Cambridge: 2008. [Google Scholar]
- 103.WHO . World health report. Health systems financing: the path to universal health coverage. World Health Organization; Geneva: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clinton Health Access Initiative (CHAI) The state of the antiretroviral drug market in low- and middle-income countries, 2014–2019. 2015. http://www.clintonhealthaccess.org/content/uploads/2015/11/CHAI-ARV-Market-Report-2015_FINAL.pdf (accessed July 11, 2016).
- 105.Stop TB. Roadmap for MDR-Tb Scale up; the Global Drug Facility (GDF). Increasing access to MDR-Tb drug through innovation and action. The Past, Present and Future of GDF's activities to meet increased scale up demand. http://www.stoptb.org/assets/documents/resources/publications/plan_strategy/GDF%20ROADMAP%20FOR%20MDR%20TB%202010%20Final.pdf (accessed Oct 1, 2016).
- 106.UNITAID . Global malaria diagnostic and artemisinin treatment commodities demand forecast: 2015–2018. UNITAID; Geneva: 2016. [Google Scholar]
- 107.William Davidson Institute . ACT forecast for malaria case management—technical report. William Davidson Institute; Ann Arbor, MI: 2015. [Google Scholar]
- 108.WHO Scaling up action against noncommunicable diseases: how much will it cost? 2011. http://apps.who.int/iris/bitstream/10665/44706/1/9789241502313_eng.pdf (accessed March 5, 2015).
- 109.WHO . Constraints to scaling up health related MDGs: costing and financial gap analysis. Background to the Working Group 1 report to the Taskforce on Innovative International Financing for Health Systems. World Health Organization; Geneva: 2009. [Google Scholar]
- 110.Anderson BO, Yip CH, Smith RA. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(suppl):2221–2243. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 111.Paediatric ARV Procurement Working Group Consolidated pediatric ARV forecast of the procurement. Presented at WHO/AMDS meeting with pharmaceutical companies and stakeholders; Geneva, Switzerland; March 20, 2015. www.who.int/hiv/amds/11_PAPWG-PC-Forecast.pdf (accessed June 15, 2016).
- 112.Stover J, Adesina A, for the Forecasting Technical Working Group. WHO. UNICEF. CHAI. SCMS. Global Fund. UNAIDS. Avenir Health . Consolidated forecast of global ARV demand for adults: scenarios, data and forecasts 2014–2018. Futures Institute; Glastonbury, CT: 2015. [Google Scholar]
- 113.Reproductive Health Supplies Coalition . DMPA IM demand forecasting: methodologies and results, April 2016. WDI, Concept Foundation and RHSC; Brussels: 2015. http://www.rhsupplies.org/uploads/tx_rhscpublications/DMPA_IM_Demand_Forecasting_-_Methodologies_and_Results.pdf (accessed June 15, 2016). [Google Scholar]
- 114.Vogler S, Schmickl B. Rational use of medicines in Europe. Austrian Federal Ministry of Health; Vienna: 2010. [Google Scholar]
- 115.Gray A, Suleman F, Patel A, Bannenberg W. South Africa: implementation of reforms under the National Drug Policy (WHO/HIS/HGF/CaseStudy/15.9). Health systems governance & financing. World Health Organization; Geneva: 2015. [Google Scholar]
- 116.Sarley D, Allain L, Akkihal A. Estimating the global in-country supply chain costs of meeting the MDGs by 2015. Deliver Project, Task Order. USAID; Arlington: 2009. [Google Scholar]
- 117.Baruwa E, Islam M. Literature review of costing supply chains and logistics. Abt Associates for the USAID, Deliver Project; Washington, DC: 2008. [Google Scholar]
- 118.Shretta R, Johnson B, Smith L. Costing the supply chain for delivery of ACT and RDTs in the public sector in Benin and Kenya. Malar J. 2015;14:57. doi: 10.1186/s12936-014-0530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.The Global Fund and PEPFAR. The Global Fund-PEPFAR ARV Supplier Conference. Dubai, United Arab Emirates; June 24–25, 2014.
- 120.UNITAID . Tuberculosis medicines technology and market landscape. 2nd edn. World Health Organization; Geneva: 2014. [Google Scholar]
- 121.Health IMS. Global use of medicines in 2020. IMS Institute for Healthcare Informatics; Parsippany: 2015. [Google Scholar]
- 122.WHO . Health Accounts. Pharmaceutical expenditure 2010. World Health Organization; Geneva: 2010. [Google Scholar]
- 123.OECD . Pharmaceutical expenditure. In: Health at a glance 2015. Organisation for Economic Co-operation and Development; Paris: 2015. [Google Scholar]
- 124.Dickens T. Procurement of medicines. In: WHO. World medicines situation report 2011. World Health Organization; Geneva: 2011. [Google Scholar]
- 125.Saxenian H, Hecht R, Kaddar M, Schmitt S, Ryckman T, Cornejo S. Overcoming challenges to sustainable immunization financing: early experiences from GAVI graduating countries. Health Policy Plan. 2015;30:197–205. doi: 10.1093/heapol/czu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.UN . The right to the highest attainable standard of health. General Comment number 14. Document E/C.12/2000/4. Economic and Social Council; New York: 2000. [Google Scholar]
- 127.WHO Universal health coverage, factsheet number 395. December, 2015. http://www.who.int/mediacentre/factsheets/fs395/en/ (accessed July 25, 2016).
- 128.World Bank Development Research Group Environment and Energy Team: Kessides I. Raffaele Miniaci. Scarpa C, Valbones P. Toward defining and measuring the affordability of public utility services; Policy Research Working Paper WPS4915. World Bank; Washington, DC: 2009. [Google Scholar]
- 129.Niëns LM, Van de Poel E, Cameron A, Ewen M, Laing R, Brouwer WB. Practical measurement of affordability: an application to medicines. Bull World Health Organ. 2012;90:219–227. doi: 10.2471/BLT.10.084087. http://www.who.int/bulletin/volumes/90/3/10-084087/en/ (accessed July 6, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Durairaj V, Evans DB. Fiscal space for health in resource poor countries. World Health Report, 2010. Background paper, 41. World Health Organization; Geneva: 2010. [Google Scholar]
- 131.Heller PS. The prospects of creating ‘fiscal space' for the health sector. Health Policy Plan. 2006;21:75–79. doi: 10.1093/heapol/czj013. [DOI] [PubMed] [Google Scholar]
- 132.Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373:240–249. doi: 10.1016/S0140-6736(08)61762-6. [DOI] [PubMed] [Google Scholar]
- 133.UN We can end poverty: Millennium Development Goals and beyond 2015. 2015. http://www.un.org/millenniumgoals/global.shtml (accessed April 9, 2015).
- 134.Global Task Force on Expanded Access to Cancer Care and Control in Developing Countries Cancer medicine prices in low- and middle-income countries. 2011. http://apps.who.int/medicinedocs/documents/s21671en/s21671en.pdf (accessed April 9, 2015).
- 135.WHO/Health Action International . Measuring medicine prices, availability, affordability and price components. 2nd edn. WHO/PSM/PAR/2008.3. World Health Organization; Geneva: 2008. [Google Scholar]
- 136.Beran D, Ewen M, Laing R. Constraints and challenges in access to insulin: a global perspective. Lancet Diabet Endocrinol4: 275–85. [DOI] [PubMed]
- 137.Health Action International Insulin prices profile. April 2016. http://haiweb.org/what-we-do/acciss/ (accessed Oct 1, 2016).
- 138.MDG Gap Task Force . Report 2015—Millennium Development Goal 8. Taking stock of the Global Partnership for Development. United Nations; New York, NY: 2015. [Google Scholar]
- 139.WHO World Health Statistics. 2015. http://apps.who.int/iris/bitstream/10665/170250/1/9789240694439_eng.pdf?ua=1&ua=1 (accessed Oct 1, 2016).
- 140.Medicine Prices HAI. Availability, affordability & price components. http://www.haiweb.org/medicineprices/manual/documents.html (accessed June 4, 2016).
- 141.WHO Global price reporting mechanism. http://apps.who.int/hiv/amds/price/hdd/ (accessed July 23, 2016).
- 142.Médecins Sans Frontières Untangling the web of antiretroviral price reductions. 18th edn. http://www.msfaccess.org/content/untangling-web-antiretroviral-price-reductions (accessed July 24, 2016).
- 143.Médecins Sans Frontières (MSF) DR-TB drugs under the microscope: Sources and prices for drug-resistant tuberculosis medicines. 4th edition. MSF; Geneva: 2016. https://www.msfaccess.org/sites/default/files/TB_report__DR-TB_DRUGS_UTM__4th_edition_2016.pdf (accessed July 25, 2016). [Google Scholar]
- 144.WHO WHO guideline on country pharmaceuticals pricing policies. 2015. http://apps.who.int/medicinedocs/documents/s21016en/s21016en.pdf (accessed July 11, 2016).
- 145.WHO/Health Action International Review series on pharmaceutical pricing policies and interventions. Working Paper 1: External reference pricing. 2011. http://haiweb.org/wp-content/uploads/2015/08/ERP-final-May2011a1.pdf (accessed Oct 1, 2016).
- 146.WHO/Health Action International Review series on pharmaceutical pricing policies and interventions. Working Paper 3: The regulation of mark-ups in the pharmaceutical supply chain. 2011. http://haiweb.org/medicineprices/05062011/Mark-ups%20final%20May2011.pdf (accessed Oct 1, 2016).
- 147.WHO/Health Action International Review series on pharmaceutical pricing policies and interventions. Working Paper 5: Sales taxes on medicines. 2011. http://haiweb.org/wp-content/uploads/2015/08/Taxes-final-May2011a1.pdf (accessed Oct 1, 2016).
- 148.Gray AL, Suleman F. The relevance of systematic reviews on pharmaceutical policy to low- and middle-income countries. Int J Clin Pharm. 2015;37:717–725. doi: 10.1007/s11096-015-0156-6. [DOI] [PubMed] [Google Scholar]
- 149.Kaplan WA, Ritz LS, Vitello M, Wirtz VJ. Policies to promote use of generic medicines in low and middle income countries: a review of published literature, 2000–2010. Health Policy. 2012;106:211–224. doi: 10.1016/j.healthpol.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 150.World Health Organization/Health Action International Review series on pharmaceutical pricing policies and interventions. Working Paper 2: The role of health insurance in the cost-effective use of medicines. 2011. http://haiweb.org/wp-content/uploads/2015/08/2_WHOHAI_Policy-Brief_Insurance_2015.pdf (accessed Oct 1, 2016).
- 151.Gómez-Dantés O, Wirtz VJ, Reich MR, Terrazas P, Ortiz M. A new entity for the negotiation of public procurement prices for patented medicines in Mexico. Bull World Health Organ. 2012;90:788–792. doi: 10.2471/BLT.12.106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Waning B, Kaplan W, King AC, Lawrence DA, Leufkens HG, Fox MP. Global strategies to reduce the price of antiretroviral medicines: evidence from transactional databases. Bull World Health Organ. 2009;87:520–528. doi: 10.2471/BLT.08.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Waning B, Kyle M, Diedrichsen E. Intervening in global markets to improve access to HIV/AIDS treatment: an analysis of international policies and the dynamics of global antiretroviral medicines markets. Global Health. 2010;6:9. doi: 10.1186/1744-8603-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Organisation for Economic Co-operation and Development Information exchanges between competitors under competition law. OECD roundtable. http://www.oecd.org/competition/cartels/48379006.pdf (accessed July 6, 2016).
- 155.Organisation for Economic Co-operation and Development . Progress made in implementing the OECD recommendation on enhancing integrity in public procurement. OECD; Paris: 2012. [Google Scholar]
- 156.Bill & Melinda Gates Foundation . Partnership reduces cost of Bayer's long-acting reversible contraceptive implant by more than 50 percent. Bill & Melinda Gates Foundation; Leverkusen: 2013. [Google Scholar]
- 157.Reproductive Health Supplies Coalition Coalition-supported initiative triggers more than $15 million in savings. Nov 19, 2012. http://www.rhsupplies.org/news-events/news/article/coalition-supported-initiative-triggers-more-than-15-million-in-savings-1399/ (accessed April 10, 2016).
- 158.Reproductive Health Supplies Coalition Single-rod implant now more affordable to the world's poorest countries. May 20, 2013. http://www.rhsupplies.org/news-events/news/article/single-rod-implant-now-more-affordable-to-the-worlds-poorest-countries-1415/ (accessed April 10, 2016).
- 159.Alliance GAVI. The GAVI Alliance 2013 Annual Progress Report. 2014. http://gaviprogressreport.org/2013/ (accessed April 10, 2016).
- 160.WHO . Cost savings of switching private sector consumption from originator brands to generic equivalents. World Health Report, background paper 35. World Health Organization; Geneva: 2010. [Google Scholar]
- 161.Sun J, Ren L, Wirtz V. How much could be saved in Chinese hospitals in procurement of anti-hypertensives and anti-diabetics? J Med Econ. 2016;19:881–888. doi: 10.1080/13696998.2016.1181641. [DOI] [PubMed] [Google Scholar]
- 162.WHO/Health Action International . Working Paper 7: Policy options for promoting the use of generic medicines in low- and middle-income countries, 2015. Boston University School of Public Health; Boston: 2016. [Google Scholar]
- 163.Patel A, Gauld R, Norris P, Rades T. Quality of generic medicines in South Africa: perceptions versus reality—a qualitative study. BMC Health Serv Res. 2012;12:297. doi: 10.1186/1472-6963-12-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patel A, Gauld R, Norris P, Rades T. “This body does not want free medicines”: South African consumer perceptions of drug quality. Health Policy Plan. 2010;25:61–69. doi: 10.1093/heapol/czp039. [DOI] [PubMed] [Google Scholar]
- 165.Alpern JD, Song J, Stauffer WM. Essential medicines in the United States—why access is diminishing. N Engl J Med. 2016;374:1904–1907. doi: 10.1056/NEJMp1601559. [DOI] [PubMed] [Google Scholar]
- 166.Kenber J. ‘Extortionate' prices add £260m to NHS drug bill. Times Investigation. The Times (London) June 3 2016 http://www.thetimes.co.uk/article/extortionate-prices-add-260m-to-nhs-drug-bill-8mwtttwdk (accessed June 13, 2016). [Google Scholar]
- 167.'t Hoen E. Private Patents and Public Health: Changing intellectual property rules for access to medicines. http://accesstomedicines.org/resources/ (accessed July 24, 2016).
- 168.WHO. World Intellectual Property Organization and World Trade Organization . Promoting Access to Medical Technologies and Innovation. Intersections between public health, intellectual property and trade. World Health Organization, World Intellectual Property Organization, World Trade Organization; Geneva: 2012. [Google Scholar]
- 169.Medicines Patent Pool The Medicines Patent Pool expands mandate to hepatitis c and tuberculosis treatment. Nov 6, 2015. http://www.medicinespatentpool.org/the-medicines-patent-pool-expands-mandate-to-hepatitis-c-and-tuberculosis-treatment/ (accessed July 24, 2016).
- 170.'t Hoen EF. Indian hepatitis C drug patent decision shakes public health community. Lancet. 2016;387:2272–2273. doi: 10.1016/S0140-6736(16)30656-0. [DOI] [PubMed] [Google Scholar]
- 171.Russo G, de Oliveira L, Shankland A, Sitoe T. On the margins of aid orthodoxy: the Brazil-Mozambique collaboration to produce essential medicines in Africa. Global Health. 2014;10:70. doi: 10.1186/s12992-014-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.WHO Public health, innovation, intellectual property and trade. Technology transfer. http://www.who.int/phi/implementation/tech_transfer/en/ (accessed May 22, 2016).
- 173.Ford N, Wilson D, Costa Chaves G, Lotrowska M, Kijtiwatchakul K. Sustaining access to antiretroviral therapy in the less-developed world: lessons from Brazil and Thailand. AIDS. 2007;21(suppl 4):S21–S29. doi: 10.1097/01.aids.0000279703.78685.a6. [DOI] [PubMed] [Google Scholar]
- 174.Kaplan WA. Local production and access to medicines in low- and middle-income countries. A literature review and critical analysis. World Health Organization; Geneva: 2011. [Google Scholar]
- 175.Kaplan WA, Laing RO. Local production of pharmaceuticals: industrial policy and access to medicines - an overview of key concepts, issues and opportunities for future research. World Bank; Washington, DC: 2005. [Google Scholar]
- 176.Wilson KR, Kohler JC, Ovtcharenko N. The make or buy debate: considering the limitations of domestic production in Tanzania. Global Health. 2012;8:20. doi: 10.1186/1744-8603-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pinheiro ES, Brüning K, Macedo MF, Siani AC. Production of antiretroviral drugs in middle- and low-income countries. Antivir Ther. 2014;19(suppl 3):49–55. doi: 10.3851/IMP2900. [DOI] [PubMed] [Google Scholar]
- 178.Moon S, Jambert E, Childs M, von Schoen-Angerer T. A win-win solution?: A critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health. 2011;7:39. doi: 10.1186/1744-8603-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Joint United Nations Programme on HIV/AIDS . How AIDS changed everything. MDG 6: 15 years, 15 lesson of hope from the AIDS response. UNAIDS; Geneva: 2015. [Google Scholar]
- 180.WHO Patent situation of key products for treatment of hepatitis C: sofosbuvir. 2015. http://www.who.int/phi/implementation/ip_trade/sofosbuvir_report_updated.pdf?ua=1 (accessed July 6, 2016).
- 181.IMS Institute for Healthcare Informatics The Global Use of Medicines: Outlook through 2017. 2015. http://www.imshealth.com/files/web/IMSH%20Institute/Reports/The_Global_Use_of_Medicines_2017/global%20use%20of%20med%202017%20right6%20Biologics_Market.pdf (accessed Aug 14, 2016).
- 182.Neumann PJ, Cohen JT. Measuring the Value of Prescription Drugs. N Engl J Med. 2015;373:2595–2597. doi: 10.1056/NEJMp1512009. [DOI] [PubMed] [Google Scholar]
- 183.Schnipper LE, Davidson NE, Wollins DS, for the American Society of Clinical Oncology American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bach PB, Pearson SD. Payer and policy maker steps to support value-based pricing for drugs. JAMA. 2015;314:2503–2504. doi: 10.1001/jama.2015.16843. [DOI] [PubMed] [Google Scholar]
- 185.Obama B. United States Health Care Reform: Progress to Date and Next Steps. JAMA. 2016;316:525–532. doi: 10.1001/jama.2016.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Taylor R, Taylor R. What is health technology assessment? NPR09/1114. April, 2009. http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/What_is_health_tech.pdf (accessed April 9, 2016).
- 187.Chalkidou K, Glassman A, Marten R. Priority-setting for achieving universal health coverage. Bull World Health Organ. 2016;94:462–467. doi: 10.2471/BLT.15.155721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.International Network of Agencies for Health Technology Assessment (INAHTA) INAHTA member list. http://www.inahta.org/members/ (accessed July 24, 2016).
- 189.Espin J. HTA in emerging countries: Examples for Eastern Europe and Latin America. http://www.advance-hta.eu/PDF/Conference_presentations/WP6_Pharmaccess.pdf (accessed July 6, 2016).
- 190.World Health Assembly Health intervention and technology assessment in support of universal health coverage. WHA67. 2014. http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R23-en.pdf (accessed April 9, 2016).
- 191.Chootipongchaivat S, Tritasavit N, Luz A, Teerawattananon Y, Tantivess S. Factors conducive to the development of health technology assessment in Asia: impacts and policy options. World Health Organization, Asia Pacific Observatory on Health Systems and Policies; Geneva: 2015. [Google Scholar]
- 192.Bredenkamp C, Evans T, Lagrada L, Langenbrunner J, Nachuk S, Palu T. Emerging challenges in implementing universal health coverage in Asia. Soc Sci Med. 2015;145:243–248. doi: 10.1016/j.socscimed.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 193.Drummond M, Neumann P, Jönsson B. Can we reliably benchmark health technology assessment organizations? Int J Technol Assess Health Care. 2012;28:159–165. doi: 10.1017/S0266462312000098. [DOI] [PubMed] [Google Scholar]
- 194.Torjesen I. Why using Avastin for eye disease is so difficult. BMJ. 2012;344:e3012. doi: 10.1136/bmj.e3012. [DOI] [PubMed] [Google Scholar]
- 195.Kingkaew P, Maleewong U, Ngarmukos C, Teerawattananon Y. Evidence to inform decision makers in Thailand: a cost-effectiveness analysis of screening and treatment strategies for postmenopausal osteoporosis. Value Health. 2012;15(suppl):S20–S28. doi: 10.1016/j.jval.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 196.Li R, Hernandez-Villafuerte K, Towse A, Vlad I, Chalkidou K. Mapping Priority Setting in Health in 17 Countries Across Asia, Latin America, and sub-Saharan Africa. Health Systems Reform. 2016;2:71–83. doi: 10.1080/23288604.2015.1123338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Whyte P, Hall C. The Role of Health Technology Assessment in Medicine Pricing and Reimbursement. HAI/WHO. Review Series on Pharmaceutical Pricing Policies and Interventions. Working Paper 6. WHO/HAI Project on Medicine Prices and Availability. June 2013. www.haiweb.org/wp-content/uploads/2015/08/HTA-final-Aug2013a1.pdf (accessed April 9, 2016).
- 198.Teerawattananon Y, Tritasavit N. A learning experience from price negotiations for vaccines. Vaccine. 2015;33(suppl 1):A11–A12. doi: 10.1016/j.vaccine.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 199.Meikle J. Hewitt steps in as trust refuses Herceptin to cancer patient. The Guardian (London). Nov 9. 2005. http://www.theguardian.com/society/2005/nov/09/health (accessed July 11, 2016).
- 200.Department of Health £50M additional funding for cancer drugs. July 27. 2010. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/MediaCentre/Pressreleases/DH_117970 (accessed July 11, 2016).
- 201.Hirschler B. Call for Britain to over-ride patents on Roche cancer drug. Reuters. Oct 1, 2015. http://www.reuters.com/article/roche-cancer-britain-idUSL5N1211VA20151001 (accessed July 11, 2016).
- 202.England NHS. Cancer Drug Fund decision summaries. V5.1. 11 September 2015. https://www.england.nhs.uk/wp-content/uploads/2015/09/ncdf-list-sept15.pdf (accessed July 11, 2016).
- 203.Mayor S. New “managed access” process for Cancer Drugs Fund to go ahead, NHS England confirms. BMJ. 2016;352:i1208. doi: 10.1136/bmj.i1208. [DOI] [PubMed] [Google Scholar]
- 204.Ainger J. Make Drugmakers Pay: England's Strategy for Cancer Medicines. Bloomberg. http://www.bloomberg.com/news/articles/2016-07-28/cancer-drugs-fund-revamp-means-drugmakers-shoulder-overspending (accessed July 28, 2016).
- 205.Mackenzie R, Chapman S, Salkeld G, Holding S. Media influence on Herceptin subsidization in Australia: application of the rule of rescue? J R Soc Med. 2008;101:305–312. doi: 10.1258/jrsm.2008.070289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gray AL, Wirtz VJ, 't Hoen EF, Reich MR, Hogerzeil HV. Essential medicines are still essential. Lancet. 2015;386:1601–1603. doi: 10.1016/S0140-6736(15)00514-0. [DOI] [PubMed] [Google Scholar]
- 207.Paris V, Belloni A. Value in pharmaceutical pricing. Organisation for Economic Co-operation and Development Health Working Paper No 63 (DELSA/HEA/WD/HWP(2013)4) Organisation for Economic Co-operation and Development; 2013. [Google Scholar]
- 208.Bulfone L, Younie S, Carter R. Health technology assessment: reflections from the Antipodes. Value Health. 2009;12(suppl 2):S28–S38. doi: 10.1111/j.1524-4733.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 209.Mills A. The Challenges of Prioritization. Health Systems Reform. 2016;2:20. doi: 10.1080/23288604.2016.1124173. [DOI] [PubMed] [Google Scholar]
- 210.Yadzeck AS. The Newest Three-Letter Fad in Health: Can HTA escape the fate of NHA, CEA, GDB? Health Systems Reform. 2016;2:102–105. doi: 10.1080/23288604.2016.1164278. [DOI] [PubMed] [Google Scholar]
- 211.O'Sullivan A, Thompson D, Bekker D. Country-to-country adaptation of pharmacoeconomic research: methodologic challenges and potential solutions. ISPOR Connect. 2009;15:6–8. [Google Scholar]
- 212.EUnetHTA Joint Action WP4 – Policy for the HTA Core Model and core HTA information. 12 Dec 2012. http://www.eunethta.eu/outputs/policy-hta-core-model-and-core-hta-information (accessed Oct 2, 2016).
- 213.Drummond M, Tarricone R, Torbica A. Assessing the added value of health technologies: reconciling different perspectives. Value Health. 2013;16(suppl):S7–13. doi: 10.1016/j.jval.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 214.WHO/UN High Commissioner for Human Rights The Right to Health. Fact Sheet 31. http://www.ohchr.org/Documents/Publications/Factsheet31.pdf (accessed June 26, 2016).
- 215.Perehudoff SK, Toebes BC, Hogerzeil HV. A human-rights based approach to reimbursing expensive medicines. Bull World Health Organ (in print). [DOI] [PMC free article] [PubMed]
- 216.McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ. 2005;331:1016–1019. doi: 10.1136/bmj.331.7523.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.OECD . Value in pharmaceutical pricing. Health Working Paper. DELSA/HEA/WD/HWP(2013)4. Organization for Economic Cooperation and Development; Paris: 2013. [Google Scholar]
- 218.Levy AR, Mitton C, Johnston KM, Harrigan B, Briggs AH. International comparison of comparative effectiveness research in five jurisdictions: insights for the US. Pharmacoeconomics. 2010;28:813–830. doi: 10.2165/11536150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 219.Oh J, Ko Y, Baer Alley A, Kwon S. Participation of the Lay Public in Decision-Making for Benefit Coverage of National Health Insurance in South Korea. Health Systems Reform. 2015;1:62–71. doi: 10.4161/23288604.2014.991218. [DOI] [PubMed] [Google Scholar]
- 220.Niessen LW, Khan JAM. Universal access to medicines. Lancet. 2016;387:9–11. doi: 10.1016/S0140-6736(15)00552-8. [DOI] [PubMed] [Google Scholar]
- 221.WHO Definitions of SSFFC medical products. 2016. http://www.who.int/medicines/regulation/ssffc/definitions/en/ (accessed Aug 13, 2016).
- 222.Institute of Medicine . In: Countering the problem of falsified and substandard drugs. Gostin LO, Buckley GJ, editors. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 223.Rägo L, Santoso B. Chapter 6: Drug Regulation: History, Present and Future. In: van Boxtel CJ, Santoso B, Edwards IR, editors. Drug Benefits and Risks: International Textbook of Clinical Pharmacology. revised 2nd edn. IOS Press and Uppsala Monitoring Centre; Amsterdam: 2008. [Google Scholar]
- 224.Tominaga T, Ando Y, Kondo T. International vision and strategy for drug regulatory authority: the PMDA's international vision. Clin Pharmacol Ther. 2012;92:349–351. doi: 10.1038/clpt.2012.90. [DOI] [PubMed] [Google Scholar]
- 225.WHO Survey of the quality of selected antimalarial medicines circulating in six countries of sub-Saharan Africa. 2011. http://www.who.int/prequal/info_applicants/qclabs/monitoring_documents/WHO_QAMSA_report.pdf (accessed Jan 15, 2015).
- 226.WHO Survey of the quality of anti-tuberculosis medicines circulating in selected newly independent states of the former Soviet. 2011. http://www.who.int/prequal/info_applicants/qclabs/monitoring_documents/TBQuality-Survey_Nov2011.pdf (accessed Jan 15, 2015).
- 227.Almuzaini T, Choonara I, Sammons H. Substandard and counterfeit medicines: a systematic review of the literature. BMJ Open. 2013;3:e002923. doi: 10.1136/bmjopen-2013-002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Alghannan AFA, Aslanpour Z, Evans S, Schifano F. A systematic review of counterfeit and substandard medicines in field quality surveys. Integr Pharm Res Pract. 2014;3:71–88. [Google Scholar]
- 229.Newton PN, Lee SJ, Goodman C. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med. 2009;6:e52. doi: 10.1371/journal.pmed.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bogdanich WFDA. Tracked Poisoned Drugs, but Trail Went Cold in China. New York Times (New York) June 17, 2007 http://www.nytimes.com/2007/06/17/health/17poison.html?_r=0 (accessed Feb 26, 2016). [Google Scholar]
- 231.Hall PE, Tagontong N. Quality of misoprostol products. WHO Drug Information. 2016;30:35–39. [Google Scholar]
- 232.Burns W, WHO WHO launches taskforce to fight counterfeit drugs. Bull World Health Organ. 2006;84:689–690. [PMC free article] [PubMed] [Google Scholar]
- 233.Caudron JM, Ford N, Henkens M, Macé C, Kiddle-Monroe R, Pinel J. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Trop Med Int Health. 2008;13:1062–1072. doi: 10.1111/j.1365-3156.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 234.WHO 16th ICDRA recommendations. WHO Drug Information. 2014;28:303–305. [Google Scholar]
- 235.WHO International Conference of Drug Regulatory Authorities (ICDRA) http://www.who.int/medicines/icdra (accessed Feb 26, 2016).
- 236.WHO support for medicines regulatory harmonization in Africa: focus on East African Community. WHO Drug Information. 2014;28:11–15. [Google Scholar]
- 237.Association of Southeastern Nations ASEAN Develops Mutual Recognition Arrangement for Bioequivalence Study Reports. ASEAN Secretariat News. 22 October 2014 http://www.asean.org/asean-develops-mutual-recognition-arrangement-for-bioequivalence-study-reports/ (accessed April 10, 2016). [Google Scholar]
- 238.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH guidelines. http://www.ich.org/products/guidelines.html (accessed April 10, 2016).
- 239.WHO . Global vaccine safety blueprint. The landscape analysis. WHO/IVB/12/04. World Health Organization; Geneva: 2012. [Google Scholar]
- 240.WHO WHO list of prequalified medicinal products. http://apps.who.int/prequal/info_general/notes.htm (accessed April 10, 2016).
- 241.Dellepiane N, Wood D. Twenty-five years of the WHO vaccines prequalification programme (1987-2012): lessons learned and future perspectives. Vaccine. 2015;33:52–61. doi: 10.1016/j.vaccine.2013.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.WHO The International Pharmacopoeia. Fifth Edition. 2015. http://apps.who.int/phint/en/p/about/ (accessed Sept 2, 2015).
- 243.WHO . Good pharmacopoeial practices. Revised draft for comments. Working document QAS/13.526/Rev.6. World Health Organization; Geneva: 2015. [Google Scholar]
- 244.Waning B, Diedrichsen E, Moon S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc. 2010;13:35. doi: 10.1186/1758-2652-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.'t Hoen EF, Hogerzeil HV, Quick JD, Sillo HB. A quiet revolution in global public health: the World Health Organization's prequalification of medicines programme. J Public Health Policy. 2014;35:137–161. doi: 10.1057/jphp.2013.53. [DOI] [PubMed] [Google Scholar]
- 246.Papathanasiou P, Brassart L, Blake P. Transparency in drug regulation: public assessment reports in Europe and Australia. Drug Discov Today. 2016 doi: 10.1016/j.drudis.2016.06.025. published online June 29. [DOI] [PubMed] [Google Scholar]
- 247.Bannenberg W, Roberts MJ. Case study E: drug procurement in East Africania. In: Roberts MJ, Reich MRR, eds. Pharmaceutical reform: a guide to improving performance and equity. 2011. http://apps.who.int/medicinedocs/documents/s18825en/s18825en.pdf (accessed Oct 1, 2016).
- 248.WHO Expert Review Panel. http://apps.who.int/prequal/info_press/documents/ERP_article.pdf (accessed Oct 1, 2016).
- 249.WHO . A model quality assurance system for procurement agencies. WHO/Interagency Publication; Geneva: 2007. [Google Scholar]
- 250.Vian T. Review of corruption in the health sector: theory, methods and interventions. Health Policy Plan. 2008;23:83–94. doi: 10.1093/heapol/czm048. [DOI] [PubMed] [Google Scholar]
- 251.WHO The world medicines situation report. 2011. http://www.who.int/medicines/areas/policy/world_medicines_situation/en/ (accessed April 10, 2016).
- 252.WHO The importance of pharmacovigilance–safety monitoring of medicinal products. 2002. http://apps.who.int/medicinedocs/pdf/s4893e/s4893e.pdf (accessed Feb 26, 2016).
- 253.McEwen J. Artesunate- and amodiaquine-associated extrapyramidal reactions: a series of 49 cases in VigiBase™. Drug Saf. 2012;35:667–675. doi: 10.1007/BF03261963. [DOI] [PubMed] [Google Scholar]
- 254.Bollyky TJ, Stergachis A, SSWG A report of the Safety and Surveillance Working Group. 2014. https://docs.gatesfoundation.org/documents/SSWG%20Final%20Report%2011%2019%2013_designed.pdf (accessed April 10, 2016).
- 255.Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36:75–81. doi: 10.1007/s40264-012-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Uppsala Monitoring Centre National pharmacovigilance guidelines. Nov 19, 2015. http://www.who-umc.org/DynPage.aspx?id=127878&mn1=7347&mn2=7252&mn3=7253&mn4=7745 (accessed Feb 26, 2016).
- 257.Juhlin K, Karimi G, Andér M. Using VigiBase to Identify substandard medicines: detection capacity and key prerequisites. Drug Saf. 2015;38:373–382. doi: 10.1007/s40264-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.WHO WHO list of prequalified quality control laboratories. 2015. http://apps.who.int/prequal/lists/PQ_QCLabsList.pdf (accessed Jan 23, 2016).
- 259.Hajjou M, Krech L, Lane-Barlow C. Monitoring the quality of medicines: results from Africa, Asia, and South America. Am J Trop Med Hyg. 2015;92(suppl):68–74. doi: 10.4269/ajtmh.14-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yu H, Le HM, Kaale E. Characterization of drug authenticity using thin-layer chromatography imaging with a mobile phone. J Pharm Biomed Anal. 2016;125:85–93. doi: 10.1016/j.jpba.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 261.Jahnke RW, Kusters G, Fleischer K. Low-cost quality assurance of medicines using the GPHF Minilab. Ther Innov Regul Sci. 2001;35:941–945. [Google Scholar]
- 262.Kaale E, Manyanga V, Makori N, Jenkins D, Michael Hope S, Layloff T. High-performance thin layer chromatography to assess pharmaceutical product quality. Trop Med Int Health. 2014;19:747–751. doi: 10.1111/tmi.12303. [DOI] [PubMed] [Google Scholar]
- 263.Risha P, Msuya Z, Ndomondo-Sigonda M, Layloff T. Proficiency testing as a tool to assess the performance of visual TLC quantitation estimates. J AOAC Int. 2006;89:1300–1304. [PubMed] [Google Scholar]
- 264.Kovacs S, Hawes SE, Maley SN, Mosites E, Wong L, Stergachis A. Technologies for detecting falsified and substandard drugs in low and middle-income countries. PLoS One. 2014;9:e90601. doi: 10.1371/journal.pone.0090601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mukhopadhyay R. The hunt for counterfeit medicine. Drugs manufactured by counterfeiters are infiltrating markets worldwide. Investigators are harnessing a variety of analytical techniques to catch as many of the fakes as they can. Anal Chem. 2007;79:2622–2627. doi: 10.1021/ac071892p. [DOI] [PubMed] [Google Scholar]
- 266.Ricci C, Nyadong L, Yang F. Assessment of hand-held Raman instrumentation for in situ screening for potentially counterfeit artesunate antimalarial tablets by FT-Raman spectroscopy and direct ionization mass spectrometry. Anal Chim Acta. 2008;623:178–186. doi: 10.1016/j.aca.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 267.Ho NT, Desai D, Zaman MH. Rapid and specific drug quality testing assay for artemisinin and its derivatives using a luminescent reaction and novel microfluidic technology. Am J Trop Med Hyg. 2015;92(suppl):24–30. doi: 10.4269/ajtmh.14-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ranieri N, Tabernero P, Green MD. Evaluation of a new handheld instrument for the detection of counterfeit artesunate by visual fluorescence comparison. Am J Trop Med Hyg. 2014;91:920–924. doi: 10.4269/ajtmh.13-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hajjou M, Qin Y, Bradby S, Bempong D, Lukulay P. Assessment of the performance of a handheld Raman device for potential use as a screening tool in evaluating medicines quality. J Pharm Biomed Anal. 2013;74:47–55. doi: 10.1016/j.jpba.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 270.Hall C. Technology for combating counterfeit medicine. Pathog Glob Health. 2012;106:73–76. doi: 10.1179/204777312X13419245939485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.European Medicines Agency Falsified medicines. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000186.jsp (accessed June 18, 2016).
- 272.Pharmaceutical Services. Ministry of Health. Malaysia New hologram meditag. April 8, 2015. http://www.pharmacy.gov.my/v2/en/content/new-hologram-meditag.html (accessed July 31, 2016).
- 273.Onukwugha A. NAFDAC's war against fake drugs, foods in south-south. Leadership Newspaper Group (Abuja). Oct 26, 2015.
- 274.World Health Assembly . Regulatory systems strengthening for medical products (resolution WHA67.20). In: Sixty-seventh World Health Assembly, Geneva, May 19-24, 2014. Resolutions and decisions. Annexes. World Health Organization; Geneva: 2014. [Google Scholar]
- 275.World Health Assembly . Access to biotherapeutic products, including similar biotherapeutic products, and ensuring their quality, safety and efficacy (resolution WHA67.21). In: Sixty-seventh World Health Assembly, Geneva, May 19-24, 2014. Resolutions and decisions. Annexes. World Health Organization; Geneva: 2014. [Google Scholar]
- 276.Ratanawijitrasin S, Wondemagegnehu E. Effective drug regulation: a multicountry study. 2002. www.who.int/medicinedocs/pdf/s2300e/s2300e.pdf (accessed Sept 4, 2015).
- 277.WHO Assessment of medicines regulatory systems in sub-Saharan African countries. An overview of findings from 26 assessment reports. 2010. http://apps.who.int/medicinedocs/en/d/Js17577en/ (accessed Sept 4, 2015).
- 278.Cornips C, Rägo L, Azatyan S, Laing R. Medicines regulatory authority websites: review of progress made since 2001. Int J Risk Saf Med. 2010;22:77–88. [Google Scholar]
- 279.Wirtz V, Knox R, Cao C, Mehrtash H, Posner N, McClenathan J. Global market situation for insulin – the ACCISS study. Health Action International; Amsterdam: 2016. [Google Scholar]
- 280.WHO . Effective medicines regulation: ensuring safety, efficacy and quality. WHO policy perspective on medicines no. 7, 2003. World Health Organization; Geneva: 2003. [Google Scholar]
- 281.INRUD International Conferences for Improving Use of Medicines 1997, 2004. 2011. http://www.inrud.org/ICIUM/ (accessed Jan 14, 2016).
- 282.Roberts MJ, Reich MRR. Case study J: drug coverage in Ghana's national health insurance scheme. In: Pharmaceutical reform: a guide to improving performance and equity. World Bank; Washington, DC: 2011. http://apps.who.int/medicinedocs/documents/s18825en/s18825en.pdf (accessed Oct 1, 2016). [Google Scholar]
- 283.US Government Accountability Office Drug pricing: research on savings from generic drug use. GAO-12-371R. Jan 31, 2012. http://www.gao.gov/assets/590/588064.pdf (accessed April 10, 2016).
- 284.Cameron A, Laing R. Cost savings of switching private sector consumption from originator brand medicines to generic equivalents. World Health report 2010, background paper 35. http://www.who.int/healthsystems/topics/financing/healthreport/35MedicineCostSavings.pdf (accessed April 10, 2016).
- 285.Lipska KJ, Ross JS, Van Houten HK, Beran D, Yudkin JS, Shah ND. Use and out-of-pocket costs of insulin for type 2 diabetes mellitus from 2000 through 2010. JAMA. 2014;311:2331–2333. doi: 10.1001/jama.2014.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.WHO Essential medicines and health products. http://www.who.int/medicines/about/en/ (accessed April 10, 2016).
- 287.Doctors without Borders Campaigns: access to medicines. http://www.doctorswithoutborders.org/issue/access-medicines (accessed April 10, 2016).
- 288.WHO Access to affordable essential medicines. http://www.who.int/medicines/mdg/MDG08ChapterEMedsEn.pdf (accessed April 10, 2016).
- 289.UN Goal 3: ensure health lives and promote well-being for all at all ages. http://www.un.org/sustainabledevelopment/health/ (accessed April 10, 2016).
- 290.Wagner AK, Quick JD, Ross-Degnan D. Quality use of medicines within universal health coverage: challenges and opportunities. BMC Health Serv Res. 2014;14:357. doi: 10.1186/1472-6963-14-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Management Sciences for Health . Managing for rational medicine use. Chapter 27. In: Managing access to medicines and health technologies. Management Sciences for Health; Arlington, VA: 2012. [Google Scholar]
- 292.Gilson L, Erasmus E, Borghi J, Macha J, Kamuzora P, Mtei G. Using stakeholder analysis to support moves towards universal coverage: lessons from the SHIELD project. Health Policy Plan. 2012;27(suppl 1):i64–i76. doi: 10.1093/heapol/czs007. [DOI] [PubMed] [Google Scholar]
- 293.Ross-Degnan D, Laing R, Quick J. A strategy for promoting improved pharmaceutical use: the International Network for Rational Use of Drugs. Soc Sci Med. 1992;35:1329–1341. doi: 10.1016/0277-9536(92)90037-q. [DOI] [PubMed] [Google Scholar]
- 294.Australian Government. Department of Health Quality use of medicines. http://www.health.gov.au/internet/main/publishing.nsf/content/nmp-quality.htm (accessed April 10, 2016).
- 295.Government of the Netherlands. Ministry of Health. Welfare and Sport The benefits of responsible use of medicines. https://www.government.nl/documents/reports/2012/11/16/the-benefits-of-responsible-use-of-medicines (accessed April 10, 2016).
- 296.WHO Call for participation: Global Antimicrobial Resistance Surveillance System (GLASS) www.who.int/drugresistance/surveillance/glass-enrolment/en/ (accessed Oct 1, 2016).
- 297.Spurling GK, Mansfield PR, Montgomery BD. Information from pharmaceutical companies and the quality, quantity, and cost of physicians' prescribing: a systematic review. PLoS Med. 2010;7:e1000352. doi: 10.1371/journal.pmed.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fletcher N. GlaxoSmithKline to pay £297m fine over China bribery network. The Guardian (London) Sept 19, 2015 http://www.theguardian.com/business/2014/sep/19/glaxosmithkline-pays-297m-fine-china-bribery (accessed April 9, 2016). [Google Scholar]
- 299.Health Action International . Fact or fiction? What healthcare professionals need to know about pharmaceutical marketing in the European Union. Health Action International; Amsterdam: 2016. [Google Scholar]
- 300.Department of Health & Human Services Warning Letter. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies/UCM457961.pdf (accessed Oct 5, 2016).
- 301.Norris P, Herxheimer A, Lexchin A, Mansfield P. Drug promotion. What we know, what we have yet to learn. 2005. http://www.who.int/medicines/areas/rational_use/drugPromodhai.pdf (accessed April 9, 2016).
- 302.World Health Organization and Health Action International Understanding and responding to pharmaceutical promotion. A practical guide. 2009. http://haiweb.org/what-we-do/pharmaceutical-marketing/guide-to-understanding-and-responding-to-pharmaceutical-promotion/ (accessed April 9, 2016).
- 303.Agrawal S, Brennan N, Budetti P. The Sunshine Act—effects on physicians. N Engl J Med. 2013;368:2054–2057. doi: 10.1056/NEJMp1303523. [DOI] [PubMed] [Google Scholar]
- 304.Government of France LOI n° 2011–2012 du 29 décembre 2011 relative au renforcement de la sécurité sanitaire du médicament et des produits de santé. https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000025053440&categorieLien=id (accessed July 11, 2016).
- 305.Lexchin J. Models for financing the regulation of pharmaceutical promotion. Global Health. 2012;8:24. doi: 10.1186/1744-8603-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Gosden T, Forland F, Kristiansen IS. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev. 2000;3 doi: 10.1002/14651858.CD002215. CD002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.World Health Assembly Progress in the rational use of medicines (WHA60.16). In: World Health Assembly. 60th World Health Assembly Resolutions. 2007. http://apps.who.int/gb/ebwha/pdf_files/WHASSA_WHA60-Rec1/E/reso-60-en.pdf?ua=1#page=27 (accessed July 31, 2016).
- 308.WHO Global action plan on antimicrobial resistance. 2015. http://www.who.int/antimicrobial-resistance/global-action-plan/en (accessed Oct 1, 2016).
- 309.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2011. http://community.cochrane.org/handbook (accessed Jan 24, 2016). [Google Scholar]
- 310.Effective Practice and Organisation of Care (EPOC) What study designs should be included in an EPOC review? EPOC Resources for review authors. 2013. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/05%20What%20study%20designs%20should%20be%20included%20in%20an%20EPOC%20review%202013%2008%2012_2.pdf (accessed Jan 24, 2016).
- 311.Holloway KA, Ivanovska V, Wagner AK, Vialle-Valentin C, Ross-Degnan D. Prescribing for acute childhood infections in developing and transitional countries, 1990–2009. Paediatr Int Child Health. 2015;35:5–13. doi: 10.1179/2046905514Y.0000000115. [DOI] [PubMed] [Google Scholar]
- 312.Rowe A, Rowe S, Peters D, Holloway K, Chalker J, Ross-Degnan D. Health care provider performance review [presentation for the Institute of Medicine] Jan 27, 2015. https://iom.nationalacademies.org/∼/media/Files/Activity%20Files/Global/USAIDstandingcomm/Jan%2028-29/8%20Rowe.pdf (accessed April 10, 2016).
- 313.Effective Practice and Organisation of Care (EPOC) Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Norwegian Knowledge Centre for the Health Services; Oslo: 2015. [Google Scholar]
- 314.Bryce J, Victora CG, Habicht JP, Vaughan JP, Black RE. The multi-country evaluation of the integrated management of childhood illness strategy: lessons for the evaluation of public health interventions. Am J Public Health. 2004;94:406–415. doi: 10.2105/ajph.94.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bryce J, Victora CG, Habicht J-P, Black RE, Scherpbier RW, the MCE-IMCI Technical Advisors Programmatic pathways to child survival: results of a multi-country evaluation of integrated management of childhood illness. Health Policy Plan. 2005;20(suppl 1):i5–17. doi: 10.1093/heapol/czi055. [DOI] [PubMed] [Google Scholar]
- 316.WHO Pursue high-quality DOTS expansion and enhancement. http://www.who.int/tb/dots/en/ (accessed Jan 25, 2016).
- 317.Frieden TR, Sbarbaro JA. Promoting adherence to treatment for tuberculosis: the importance of direct observation. World Hosp Health Serv. 2007;43:30–33. [PubMed] [Google Scholar]
- 318.Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2015;5 doi: 10.1002/14651858.CD003343.pub4. CD003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Weekes LM, Mackson JM, Fitzgerald M, Phillips SR. National prescribing service: creating an implementation arm for national medicines policy. Br J Clin Pharmacol. 2005;59:112–116. doi: 10.1111/j.1365-2125.2005.02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.NPS NPS MedicineWise annual report. 2015. http://www.nps.org.au/__data/assets/pdf_file/0019/311662/NPS-Annual-Report-2014-15.pdf (accessed July 4, 2016).
- 321.NPS NPS MedicineWise annual evaluation report 2015. http://www.nps.org.au/__data/assets/pdf_file/0006/317418/Annual_Eval_Report_2015.pdf (accessed July 4, 2016).
- 322.PBS Information Management Section Pharmaceutical Policy Branch Expenditure and prescriptions 12 months to 30 June 2014. http://www.pbs.gov.au/statistics/2013-2014-files/expenditure-and-prescriptions-12-months-to-30-june-2014.pdf (accessed July 4, 2016).
- 323.National Health and Family Planning Commission. China Health and family planning development statistics bulletin 2014. http://en.nhfpc.gov.cn/reports.html (accessed Oct 1, 2016).
- 324.Huang Y. The SARS Epidemic and its aftermath in China: a political perspective. 2004. http://www.ncbi.nlm.nih.gov/books/NBK92479/ (accessed Jan 25, 2016).
- 325.Sun J, Shen X, Li M. Changes in patterns of antibiotic use in Chinese public hospitals (2005–2012) and a benchmark comparison with Sweden in 2012. J Glob Antimicrob Resist. 2015;3:95–102. doi: 10.1016/j.jgar.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 326.Xiao Y, Zhang J, Zheng B, Zhao L, Li S, Li L. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Med. 2013;10:e1001556. doi: 10.1371/journal.pmed.1001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Pinto CB, Miranda ES, Emmerick IC, Costa NR, Castro CG. Medicine prices and availability in the Brazilian Popular Pharmacy Program [Preços e disponibilidade de medicamentos no Programa Farmácia Popular do Brasil] Rev Saude Publica. 2010;44:611–619. doi: 10.1590/s0034-89102010005000021. [DOI] [PubMed] [Google Scholar]
- 328.da Silva RM, Caetano R. “Farmácia Popular do Brasil” program: characterization and evolution between 2004 and 2012. Cien Saude Colet. 2015;20:2943–2956. doi: 10.1590/1413-812320152010.17352014. [DOI] [PubMed] [Google Scholar]
- 329.Ruppenthal LR, Petrovick PR. Comparison of user's profile and of dispensed drugs from a community pharmacy and a commercial drugstore at Porto Alegre, Brazil. [Comparação do perfil dos usuários e dos medicamentos dispensados na farmácia popular do Brasil e em drogaria privada em Porto Alegre, Brasil.] Lat Am J Pharm. 2010;29:22–29. [Google Scholar]
- 330.Dong L, Yan H, Wang D. Drug prescribing indicators in village health clinics across 10 provinces of Western China. Fam Pract. 2011;28:63–67. doi: 10.1093/fampra/cmq077. [DOI] [PubMed] [Google Scholar]
- 331.Nguyen HT, Wirtz VJ, Haaijer-Ruskamp FM, Taxis K. Indicators of quality use of medicines in South-East Asian countries: a systematic review. Trop Med Int Health. 2012;17:1552–1566. doi: 10.1111/j.1365-3156.2012.03081.x. [DOI] [PubMed] [Google Scholar]
- 332.Awad AI, Ball DE, Eltayeb IB. Improving rational drug use in Africa: the example of Sudan. East Mediterr Health J. 2007;13:1202–1211. doi: 10.26719/2007.13.5.1202. [DOI] [PubMed] [Google Scholar]
- 333.Thomas SK, McDowell SE, Hodson J. Developing consensus on hospital prescribing indicators of potential harms amenable to decision support. Br J Clin Pharmacol. 2013;76:797–809. doi: 10.1111/bcp.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hillen JB, Vitry A, Caughey GE. Evaluating medication-related quality of care in residential aged care: a systematic review. Springerplus. 2015;4:220. doi: 10.1186/s40064-015-0984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lagerløv P, Hjortdahl P, Saxegaard L, Andrew M, Matheson I. Structuring prescribing data into traffic-light categories; a tool for evaluating treatment quality in primary care. Fam Pract. 2001;18:528–533. doi: 10.1093/fampra/18.5.528. [DOI] [PubMed] [Google Scholar]
- 336.Smits KPJ, Sidorenkov G, Bilo HJG, et al. Development and initial validation of prescribing quality indicators for patients with chronic kidney disease. Nephrol Dial Transplant 2016; PII:gfv420. [DOI] [PubMed]
- 337.Stocks SJ, Kontopantelis E, Akbarov A, Rodgers S, Avery AJ, Ashcroft DM. Examining variations in prescribing safety in UK general practice: cross sectional study using the clinical practice research datalink. BMJ. 2015;351:h5501. doi: 10.1136/bmj.h5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rossow I, Bramness JG. The total sale of prescription drugs with an abuse potential predicts the number of excessive users: a national prescription database study. BMC Public Health. 2015;15:288. doi: 10.1186/s12889-015-1615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lund BC, Carrel M, Gellad WF, Chrischilles EA, Kaboli PJ. Incidence- versus prevalence-based measures of inappropriate prescribing in the Veterans health administration. J Am Geriatr Soc. 2015;63:1601–1607. doi: 10.1111/jgs.13560. [DOI] [PubMed] [Google Scholar]
- 340.Purmonen TT, Auvinen PK, Martikainen JA. Budget impact analysis of trastuzumab in early breast cancer: a hospital district perspective. Int J Technol Assess Health Care. 2010;26:163–169. doi: 10.1017/S0266462310000103. [DOI] [PubMed] [Google Scholar]
- 341.Sullivan SD, Mauskopf JA, Augustovski F. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17:5–14. doi: 10.1016/j.jval.2013.08.2291. [DOI] [PubMed] [Google Scholar]
- 342.Schwarzkopf L, Wacker M, Holle R. Cost-components of lung cancer care within the first three years after initial diagnosis in context of different treatment regimens. Lung Cancer. 2015;90:274–280. doi: 10.1016/j.lungcan.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 343.Belfrage B, Koldestam A, Sjöberg C, Wallerstedt SM. Number of drugs in the medication list as an indicator of prescribing quality: a validation study of polypharmacy indicators in older hip fracture patients. Eur J Clin Pharmacol. 2015;71:363–368. doi: 10.1007/s00228-014-1792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.The Dartmouth Institute for Health Policy and Clinical Practice The Dartmouth Atlas of Health Care. 2015. http://www.dartmouthatlas.org/ (accessed Oct 1, 2016). [PubMed]
- 345.Fromm MF, Maas R, Tümena T, Gaßmann KG. Potentially inappropriate medications in a large cohort of patients in geriatric units: association with clinical and functional characteristics. Eur J Clin Pharmacol. 2013;69:975–984. doi: 10.1007/s00228-012-1425-0. [DOI] [PubMed] [Google Scholar]
- 346.Grant R, Parle J. How to assess quality in primary care. BMJ. 2015;351:h5950. doi: 10.1136/bmj.h5950. [DOI] [PubMed] [Google Scholar]
- 347.Medicines Transparency Alliance A review of the pilot. http://www.medicinestransparency.org/fileadmin/uploads/Documents/MeTA_review_pilot.pdf (accessed Oct 1, 2016).
- 348.Stedman-Bryce G, Schatz F, Hodgkin C, Balogun P. Medicines Transparency Alliance (MeTA) evaluation. Testing MeTA's underlying intervention logic. 2015. http://itad.com/wp-content/uploads/2016/05/MeTA-Evaluation-Final-Report.pdf (accessed May 27, 2016).
- 349.NPS MedicineWise NPS MedicineWise 2014 annual report. 2014. http://www.nps.org.au/__data/assets/pdf_file/0003/264801/NPS-MedicineWise-Annual-Report-2013-2014.pdf (accessed July 4, 2016).
- 350.Institute for Safe Medication Practices ISMP high-alert medications. http://www.ismp.org/tools/highalertmedicationLists.asp (accessed June 18, 2016).
- 351.Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ. 2013;347:f6753. doi: 10.1136/bmj.f6753. [DOI] [PubMed] [Google Scholar]
- 352.WHO . Research and development to meet health needs in developing countries: strengthening global financing and coordination. World Health Organization; Geneva: 2012. [Google Scholar]
- 353.Ward JW, Mermin JH. Simple, effective, but out of reach? Public health implications of HCV drugs. N Engl J Med. 2015;373:2678–2680. doi: 10.1056/NEJMe1513245. [DOI] [PubMed] [Google Scholar]
- 354.Hollande F. Towards a global agenda on health security. Lancet. 2016;387:2173–2174. doi: 10.1016/S0140-6736(16)30393-2. [DOI] [PubMed] [Google Scholar]
- 355.WHO . Global strategy and plan of action on public health, innovation and intellectual property. World Health Organization; Geneva: 2011. [Google Scholar]
- 356.Commission on Intellectual Property Rights. Innovation and Public Health (CIPIH) Public health, innovation and intellectual property rights. 2006. http://www.who.int/intellectualproperty/report/en/index.html (accessed Feb 28, 2016).
- 357.WHO Consultative Expert Working Group on Research and Development (CEWG) Financing and coordination. Research and development to meet health needs in developing countries: Strengthening global financing and coordination. April 2012. http://www.who.int/phi/cewg_report/en/index.html (accessed Feb 28, 2016).
- 358.Kaplan W, Wirtz VJ, Mantel-Teeuwisse A, Stolk P, Duthey B, Laing R. Priority medicines for Europe and the World 2013 update. 2013. http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf?ua=1 (accessed Feb 28, 2016).
- 359.Jamison DT, Summers LH, Alleyne G. Global health 2035: a world converging within a generation. Lancet. 2013;382:1898–1955. doi: 10.1016/S0140-6736(13)62105-4. [DOI] [PubMed] [Google Scholar]
- 360.Mendelson M, Røttingen JA, Gopinathan U. Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet. 2016;387:188–198. doi: 10.1016/S0140-6736(15)00547-4. [DOI] [PubMed] [Google Scholar]
- 361.Saint Raymond A. Regulatory aspects of drug development in children: change and resistance to change. Expert Rev Clin Pharmacol. 2010;3:593–595. doi: 10.1586/ecp.10.38. [DOI] [PubMed] [Google Scholar]
- 362.Finney E. Children's medicines: A situational analysis. 2011. http://www.who.int/childmedicines/progress/CM_analysis.pdf?ua=1 (accessed Feb 28, 2016).
- 363.Elizabeth Glaser Pediatric AIDS Foundation About Pediatric AIDS. http://www.pedaids.org/pages/about-pediatric-aids (accessed Feb 28, 2016).
- 364.Access to Medicine Foundation The Access to Medicine index. 2014. http://www.accesstomedicineindex.org/sites/2015.atmindex.org/files/2014_accesstomedicineindex_fullreport_clickablepdf.pdf (accessed April 9, 2016).
- 365.Fox M. “No Market”: scientists struggle to make Ebola vaccines, treatments. NBC News (New York, NY) July 29, 2014 http://www.nbcnews.com/storyline/ebola-virus-outbreak/no-market-scientists-struggle-make-ebola-vaccines-treatments-n167871 (accessed Feb 28, 2016). [Google Scholar]
- 366.Henao-Restrepo AM, Longini IM, Egger M. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–866. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 367.Jones SM, Feldmann H, Ströher U. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 368.The Commission on Health Research for Development Health research: essential link to equity in development. 1990. http://www.cohred.org/downloads/open_archive/ComReports_0.pdf (accessed July 19, 2015).
- 369.Trouiller P, Torreele E, Olliaro P. Drugs for neglected diseases: a failure of the market and a public health failure? Trop Med Int Health. 2001;6:945–951. doi: 10.1046/j.1365-3156.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 370.Pedrique B, Strub-Wourgaft N, Some C. The drug and vaccine landscape for neglected diseases (2000–11): a systematic assessment. Lancet Glob Health. 2013;1:e371–e379. doi: 10.1016/S2214-109X(13)70078-0. [DOI] [PubMed] [Google Scholar]
- 371.Année du medicament Rev Prescrire 35 (376):132-136, 2015. English summary available online. http://english.prescrire.org/en/109B561E03CAD2313B7046521B310752/Download.aspx (accessed April 10, 2016).
- 372.Bach PB, Saltz LB, Wittes RE. In cancer care, cost matters. New York Times (New York, NY), pA25, Oct 15, 2012. http://www.nytimes.com/2012/10/15/opinion/a-hospital-says-no-to-an-11000-a-month-cancer-drug.html?_r=0 (accessed April 10, 2016).
- 373.Moberly T. Exclusive: PCTs ‘blacklist' drugs backed by NICE. Nov 24, 2011. http://www.gponline.com/News/article/1105156/Exclusive-PCTs-blacklist-drugs-backed-NICE/ (accessed April 10, 2016).
- 374.Rawlins M. Michael Rawlins: playing fair on treatments. Health Serv J. 2012;122:18. [PubMed] [Google Scholar]
- 375.Lyons J. Sue NHS to stop drug rationing: Watchdog urges patients to take action against health trusts which deny them expensive medicine. Mirror (London) Aug 3, 2012 http://www.mirror.co.uk/news/uk-news/nice-urges-patients-to-take-action-1214102 (accessed April 10, 2016). [Google Scholar]
- 376.McKee S. NHS trusts unlawfully denying patients NICE drugs. PharmaTimes (London) Aug 7, 2012 http://www.pharmatimes.com/Article/12-08-07/NHS_trusts_unlawfully_denying_patients_NICE_drugs.aspx (accessed April 10, 2016). [Google Scholar]
- 377.Gaffney A, Mezher M. Regulatory explainer: everything you need to know about FDA's priority review vouchers. July 2, 2015, updated March 2, 2016. http://www.raps.org/Regulatory-Focus/News/2015/07/02/21722/Regulatory-Explainer-Everything-You-Need-to-Know-About-FDA%E2%80%99s-Priority-Review-Vouchers/ (accessed April 10, 2016).
- 378.Pécoul B, Balasegaram M. FDA voucher for leishmaniasis treatment: can both patients and companies win? http://blogs.plos.org/speakingofmedicine/2015/01/20/fda-voucher-leishmaniasis-treatment-can-patients-companies-win/ (accessed April 10, 2016).
- 379.Drugs for Neglected Diseases initiative Business plan 2015—2023. http://www.dndi.org/wp-content/uploads/2009/03/DNDi_Business_Plan_2015-2023.pdf (accessed Feb 28, 2016).
- 380.Light DW, Warburton R. Demythologizing the high costs of pharmaceutical research. Biosocieties. 2011;6:34–50. [Google Scholar]
- 381.Love J. KEI comment on the new Tufts Study on Drug Development Costs. Nov 18, 2014. http://keionline.org/node/2127 (accessed Feb 28, 2016).
- 382.Hirschler B. GlaxoSmithKline boss says new drugs can be cheaper. http://www.reuters.com/article/2013/03/14/us-glaxosmithkline-prices-idUSBRE92D0RM20130314 (accessed July 11, 2016).
- 383.DiMasi JA, Hansen RW, Grabowski HG, Lasagna L. Cost of innovation in the pharmaceutical industry. J Health Econ. 1991;10:107–142. doi: 10.1016/0167-6296(91)90001-4. [DOI] [PubMed] [Google Scholar]
- 384.US Congress. Office of Technology Assessment Pharmaceutical R&D: costs, risks, and rewards. OTA-H-522. February 1993. http://govinfo.library.unt.edu/ota/Ota_1/DATA/1993/9336.PDF (accessed Feb 28, 2016).
- 385.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 386.Mestre-Ferrandiz J, Sussex J, Towse A. The R&D cost of a new medicine. December 2012. https://www.ohe.org/publications/rd-cost-new-medicine (accessed Feb 28, 2016).
- 387.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 388.Global Alliance for TB Drug Development Executive summary for the economics of TB drug development. October 2001. http://www.tballiance.org/downloads/publications/TBA_Economics_Report_Exec.pdf (accessed Feb 28, 2016).
- 389.Drugs for Neglected Diseases initiative An innovative approach to R&D for neglected patients. Ten years of experience and lessons learned by DNDi. 2013. http://www.dndi.org/2014/advocacy/ten-years-of-experience-and-lessons-learned-by-dndi/ (accessed July 22, 2016).
- 390.Kiddell-Monroe R, Greenberg E, Basey M. Re:Route: a map of the alternative biomedical R&D. 2015. http://altreroute.com/ (accessed July 6, 2016).
- 391.European Parliament. Council of the European Union Regulation (EC) No. 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. March 31, 2004. http://ec.europa.eu/health/files/eudralex/vol-1/reg_2004_726/reg_2004_726_en.pdf (accessed Sept 29, 2016).
- 392.European Medicines Agency Article 58 applications: regulatory and procedural guidance. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000157.jsp&mid=WC0b01ac05800240d1 (accessed Feb 28, 2016).
- 393.Doshi P. US incentive scheme for neglected diseases: a good idea gone wrong? BMJ. 2014;349:g4665. doi: 10.1136/bmj.g4665. [DOI] [PubMed] [Google Scholar]
- 394.Médecins Sans Frontières Health groups ask senate for changes to FDA priority review voucher program for neglected diseases. Nov 19, 2015. http://www.doctorswithoutborders.org/article/health-groups-ask-senate-changes-fda-priority-review-voucher-program-neglected-diseases (accessed Feb 28, 2016).
- 395.United States Government Publishing Office One hundred twelfth congress of the United States of America at the second session. https://www.gpo.gov/fdsys/pkg/BILLS-112s3187enr/pdf/BILLS-112s3187enr.pdf (accessed April 10, 2016).
- 396.European Medicines Agency Paediatric regulation EU. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000068.jsp (accessed April 10, 2016).
- 397.Olski TM, Lampus SF, Gherarducci G, Saint Raymond A. Three years of paediatric regulation in the European Union. Eur J Clin Pharmacol. 2011;67:245–252. doi: 10.1007/s00228-011-0997-4. [DOI] [PubMed] [Google Scholar]
- 398.Prescrire Who benefits from the European Paediatric Regulation? Nov 28, 2012. http://english.prescrire.org/Docu/DOCSEUROPE/20121128_EN_ResponsePediatricRegul.pdf (accessed Feb 28, 2016). [PubMed]
- 399.Hoppu K, Hogerzeil HV. Global aspects of drug development. In: Seyberth HW, Rane A, Schwab M, editors. Pediatric clinical pharmacology. Springer; Berlin, Heidelberg: 2011. pp. 353–372. [Google Scholar]
- 400.Council of the European Union Council conclusions on strengthening the balance in the pharmaceutical systems in the EU and its member states. June 17, 2016. http://www.consilium.europa.eu/en/press/press-releases/2016/06/17-epsco-conclusions-balance-pharmaceutical-system/?utm_source=dsms-auto&utm_medium=email&utm_campaign=Council+conclusions+on+strengthening+the+balance+in+the+pharmaceutical+systems+in+the+EU+and+its+Member+States (accessed June 21, 2016).
- 401.Sam T, Stevens R. R&D pharmaceutical industry perspective on specifications for pediatric medicines. http://www.unicef.org/supply/files/8_Tom_Sam_-_IFPMA_-_R_and_D_Pharmaceutical_Industry_Perspective.pdf (accessed Feb 28, 2016).
- 402.The Economist The price of failure. A startling new cost estimate for new medicines is met with scepticism. Economist (London) Nov 29, 2014 http://www.economist.com/news/business/21635005-startling-new-cost-estimate-new-medicines-met-scepticism-price-failure (accessed July 31, 2016). [Google Scholar]
- 403.Adamson PC, Houghton PJ, Perilongo G, Pritchard-Jones K. Drug discovery in paediatric oncology: roadblocks to progress. Nat Rev Clin Oncol. 2014;11:732–739. doi: 10.1038/nrclinonc.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Reichman J, So A, Sampat B, Cook-Deegan R, Weissman R, Kapczynski A. Is Bayh-Dole good for developing countries? Lessons from the U.S. experience. PLoS Biol. 2008;6:e262. doi: 10.1371/journal.pbio.0060262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hunt P. Report of the Special Rapporteur on the right of everyone to the enjoyment of the highest attainable standard of health, annex: mission to GlaxoSmithKline, UN Doc. No. A/HRC/11/12/Add.2. May 5, 2009. United Nations; New York, NY: 2009. [Google Scholar]
- 406.Medicines Patent Pool Progress and achievements of the medicines patent pool 2010–2015. http://www.medicinespatentpool.org/wp-content/uploads/WEB_Progress_Report_2015_EN.pdf (accessed Feb 28, 2016).
- 407.Burrone E, Perry G. Ensuring new medicines reach those in most need. Lancet HIV, 2: e362–63. [DOI] [PubMed]
- 408.Mohara A, Yamabhai I, Chaisiri K, Tantivess S, Teerawattananon Y. Impact of the introduction of government use licenses on the drug expenditure on seven medicines in Thailand. Value Health. 2012;15(suppl):S95–S99. doi: 10.1016/j.jval.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 409.Hogerzeil HV, Liberman J, Wirtz VJ, for the Lancet NCD Action Group Promotion of access to essential medicines for non-communicable diseases: practical implications of the UN political declaration. Lancet. 2013;381:680–689. doi: 10.1016/S0140-6736(12)62128-X. [DOI] [PubMed] [Google Scholar]
- 410.Government of India The patents act, section 3: what are not inventions. Part (d) 1970. http://ipindia.nic.in/IPActs_Rules/updated_Version/sections/ps3.html (accessed July 9, 2016).
- 411.Malebona PM. Intervention at the 134th session of the World Health Organization's Executive Board under agenda item 9.7. Jan 23, 2014. World Health Organization; Geneva: 2014. [Google Scholar]
- 412.Boulet P, 't Hoen E. Procurement of patented medicines by SADC Member States: a report for SADC Member States and the future SADC Pharmaceutical Procurement Services (SPPS) based on the lessons learned during the Trade, TRIPS and Access to Medicines project 2012–2014. https://www.researchgate.net/publication/286925982_Procurement_of_Patented_Medicines_by_SADC_Member_States (accessed March 3, 2016).
- 413.Article 46 Organisation Africaine De La Propriete Intellectuelle (Oapi) Accord De Bangui Instituant Une Organisation Africaine De La Propriete Intellectuelle, Acte Du 14 Decembre 2015. http://www.oapi.int/index.php/fr/toute-lactualite/579-le-benin-signe-laccord-de-bangui-revise (accessed Sept 29, 2016).
- 414.Office of the United States Trade Representative CAFTA-DR (Dominican Republic-Central America FTA) https://ustr.gov/trade-agreements/free-trade-agreements/cafta-dr-dominican-republic-central-america-fta (accessed July 6, 2016).
- 415.Malpani R. All costs, no benefits: how the US-Jordan free trade agreement affects access to medicines. J Generic Med. 2009;6:206–217. [Google Scholar]
- 416.Secretariat NAFTA. North American Free Trade Agreement (NAFTA) 2014. https://www.nafta-sec-alena.org/Home/Legal-Texts/North-American-Free-Trade-Agreement (accessed July 6, 2016).
- 417.UNITAID The Trans-Pacific Partnership Agreement: implications for access to medicines and public health. 2014. http://www.unitaid.eu/images/marketdynamics/publications/TPPA-Report_Final.pdf (accessed Feb 28, 2016).
- 418.van Onselen L. TPP IP chapter “a disaster for global health”. http://www.macrobusiness.com.au/2015/10/tpp-ip-chapter-a-disaster-for-global-health/ (accessed Feb 28, 2016).
- 419.Salton R, Jones S. The corporate social responsibility reports of global pharmaceutical firms. BJHCM. 2015;21:21–25. [Google Scholar]
- 420.Johnson & Johnson Our Credo values. http://www.jnj.com/about-jnj/jnj-credo (accessed July 6, 2016).
- 421.Access Campaign MSF. Untangling the web of antiretroviral price reductions. 18th edn. July 2016. http://www.msfaccess.org/content/untangling-web-antiretroviral-price-reductions (accessed July 6, 2016).
- 422.Experts in Chronic Myeloid Leukemia The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121:4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Gagnon MA. New drug pricing: does it make any sense? Prescrire Int. 2015;24:192–195. [PubMed] [Google Scholar]
- 424.Silverman E. Novartis, Colombia face off over cancer drug cost. STATNews (Boston, MA) June 14, 2016. https://www.statnews.com/pharmalot/2016/06/14/novartis-colombia-drug-costs/ (accessed Aug 13, 2016).
- 425.'t Hoen E. A victory for global public health in the Indian Supreme Court. J Public Health Policy. 2013;34:370–374. doi: 10.1057/jphp.2013.21. [DOI] [PubMed] [Google Scholar]
- 426.Crow D. Valeant: the harder they fall. Financial Times (London), March 28, 2016. http://www.ft.com/cms/s/0/dbc52fa8-f0d6-11e5-9f20-c3a047354386.html#axzz4Dj7y9Xms (accessed July 6, 2016).
- 427.Stephens P, Leufkens HGM. Research and Development. In: World medicines situation, 2011. World Health Organization; Geneva: 2011. [Google Scholar]
- 428.World Health Organization Macroeconomics and health: investing in health for economic development: report of the Commission on Macroeconomics and Health. 2001. http://www.who.int/macrohealth/infocentre/advocacy/en/investinginhealth02052003.pdf (accessed Feb 28, 2016).
- 429.Jennings G. Emerging infection diseases: global risks, global strategy. A global village. Oct 5, 2011. http://www.aglobalvillage.org/journal/issue5/the-right-to-health/emerging-infectious-disease/ (accessed Feb 28, 2016).
- 430.Hotez P. The disease next door: how the world's nastiest and least-known outbreaks are afflicting some of the world's wealthiest countries. March 25, 2013. http://foreignpolicy.com/2013/03/25/the-disease-next-door/ (accessed Feb 28, 2016).
- 431.Kelly JC. First Zika virus in continental United States confirmed in Texas. Medscape Medical News (New York, NY), Jan 11, 2016.
- 432.Sulston J. Presentation. Neglected Diseases Group Meeting; Penang, Malaysia; Feb 6–7, 2004.
- 433.United Nations . General Comment nr 14, paragraph 42. In: New York: Economic and Social Council, 2001. Official records of 22nd, 23rd and 24th sessions. Document e/2001/22; E/C.12/2000/22. United Nations; New York, NY: 2001. pp. 128–148. [Google Scholar]
- 434.Pharmaceutical Commerce IMS Institute projects global pharmaceutical market of $1·17–1·20 trillion in 2017. Jan 20, 2014. http://pharmaceuticalcommerce.com/business-and-finance/ims-institute-projects-global-pharma-market-of-1-17-1-20-trillion-in-2017/ (accessed July 31, 2016).
- 435.PhRMA Biopharmaceutical research industry 2015 profile. April 2015. http://www.phrma.org/sites/default/files/pdf/2014_PhRMA_PROFILE.pdf (accessed Feb 28, 2016).
- 436.WHO Trilateral study: Promoting Access to Medical Technologies and Innovation. Intersections between public health, intellectual property and trade.'. http://www.wto.org/english/res_e/booksp_e/pamtiwhowipowtoweb13_e.pdf (accessed Feb 28, 2016).
- 437.WHA . Global strategy and plan of action on public health, innovation and intellectual property. Resolution 61.21. World Health Assembly; Geneva: 2008. [Google Scholar]
- 438.Moon S, 't Hoen E. Medicines for the World: a global R&D treaty could boost innovation and improve the health of the world's poor—and rich. The Scientist (Midland, ON) Oct 1, 2012 http://www.the-scientist.com/?articles.view/articleNo/32664/title/Medicines-for-the-World/ (accessed July 7, 2016). [Google Scholar]
- 439.Hubbard T, Love J. A new trade framework for global healthcare R&D. PLoS Biol. 2004;2:E52. doi: 10.1371/journal.pbio.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Governments of Bangladesh. Barbados. Bolivia. Suriname Proposal for WHO discussions on a biomedical R&D treaty. 2015. http://www.who.int/phi/Bangladesh_Barbados_Bolivia_Suriname_R_DTreaty.pdf (accessed July 7, 2016).
- 441.Dentico N, Ford N. The courage to change the rules: a proposal for an essential health R&D treaty. PLoS Med. 2005;2:e14. doi: 10.1371/journal.pmed.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Health Action International Global. Initiative for Health and Equity in Society. Knowledge Ecology International. Medecins Sans Frontières. Third World Network An essential health & biomedical treaty. 2011. http://www.who.int/phi/news/phi_1_joint_submission_en.pdf (accessed July 11, 2016).
- 443.Balasegaram M, Bréchot C, Farrar J. A global biomedical R&D fund and mechanism for innovations of public health importance. PLoS Med. 2015;12:e1001831. doi: 10.1371/journal.pmed.1001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.UNDP Secretary-General Ban Ki-moon issues call for new deal on medicines. Dec 11, 2015. http://www.undp.org/content/undp/en/home/presscenter/pressreleases/2015/12/11/secretary-general-ban-ki-moon-issues-call-for-new-deal-on-medicines.html (accessed Feb 28, 2016).
- 445.UN The United Nations Secretary-General's high-level panel on access to medicines. Final report. 2016. http://www.unsgaccessmeds.org/final-report/ (accessed Oct 8, 2016).
- 446.WHA . Follow up of the report of the Consultative Expert Working Group on Research and Development: Financing and Coordination (resolution WHA 66.22) World Health Organization; Geneva: 2013. [Google Scholar]
- 447.Moon S, Bermudez J, 't Hoen E. Innovation and access to medicines for neglected populations: could a treaty address a broken pharmaceutical R&D system? PLoS Med. 2012;9:e1001218. doi: 10.1371/journal.pmed.1001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.WHA Constitution of the World Health Organization. Basic Documents, forty-fifth edition. October 2006. http://www.who.int/governance/eb/who_constitution_en.pdf (accessed July 7, 2016).
- 449.Nikogosian H. WHO framework convention on tobacco control: a key milestone. Bull World Health Organ. 2010;88:83. doi: 10.2471/BLT.10.075895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Velasquez G, Seuba X. Rethinking global health: a binding convention for R&D for pharmaceutical products (research paper 42) 2011. http://www.southcentre.int/wp-content/uploads/2013/04/RP42_Rethinking-global-health_EN.pdf (accessed July 7, 2016).
- 451.Agitha TG. Alternative incentive models delinking R&D costs from pharmaceutical product price. JIPR. 2013;18:491–498. [Google Scholar]
- 452.El Said M, Kapczynski A. Access to Medicines: the role of intellectual property law and policy. Working paper prepared for the 3rd Meeting of the Technical Advisory Group of the Global Commission on HIV and the Law, July 7–9, 2011. http://www.unsgaccessmeds.org/the-process/ (accessed Feb 28, 2016).
- 453.Seuba X. Global Health Law Committee of the International Law Association Submission to the UN High Level Panel. Feb 22, 2016. http://www.unsgaccessmeds.org/inbox/2016/2/22/contributionxavier-seubaon-behalf-of-global-health-law-committee-of-the-international-law-association (accessed April 10, 2016).
- 454.UN . Guidelines for pharmaceutical companies. Document A/63/263; p. 6-12. UN General Assembly; New York, NY: 2008. [Google Scholar]
- 455.Love J. Remuneration guidelines for non-voluntary use of a patent on medial technologies. Health Economics and Drugs TCM Series no. 18 (WHO/TCM/2005.1) 2005. http://www.who.int/hiv/amds/WHOTCM2005.1_OMS.pdf (accessed Feb 28, 2016).
- 456.WHO Trade Related Intellectual Property Rights Agreement, part II – standards concerning the availability, scope and use of intellectual property rights, article 31. https://www.wto.org/english/docs_e/legal_e/27-trips_04c_e.htm (accessed July 7, 2016).
- 457.Global Health Law Committee of the International Law Association Submission to the UN High-Level on Access to Medicines by the Global Health Law Committee. 2016. http://www.rug.nl/research/groningen-centre-for-law-and-governance/onderzoekscentra/ghlg/ila-ghlc-proposal1-hlp-process-update.pdf (accessed July 6, 2016).
- 458.IDRI IDRI and Sanofi Pasteur team with philanthropy to develop new model for vaccine development. Oct 15, 2015. http://idri.org/press-10-15-15.php (accessed July 6, 2016).
- 459.GSK GSK expands graduated approach to patents and intellectual property to widen access to medicines in the world's poorest countries. March 31, 2016. http://www.gsk.com/en-gb/media/press-releases/2016/gsk-expands-graduated-approach-to-patents-and-intellectual-property-to-widen-access-to-medicines-in-the-world-s-poorest-countries/ (accessed April 1, 2016).
- 460.Office of the High Commissioner on Human Rights . Guiding principles on business and human rights. United Nations; New York and Geneva: 2011. [Google Scholar]
- 461.Access to Medicines Foundation 2015 methodology for the 2016 Access to Medicine index. http://www.accesstomedicineindex.org/2015-methodology-2016-access-medicine-index (accessed May 22, 2016).
- 462.Swinburn B, Kraak V, Rutter H. Strengthening of accountability systems to create healthy food environments and reduce global obesity. Lancet. 2015;385:2534–2545. doi: 10.1016/S0140-6736(14)61747-5. [DOI] [PubMed] [Google Scholar]
- 463.Kosack S, Fung A. Does transparency improve governance? Annu Rev Polit Sci. 2014;17:65–87. [Google Scholar]
- 464.Smith J, Buse K, Gordon C. Civil society: the catalyst for ensuring health in the age of sustainable development. Global Health. 2016;12:40. doi: 10.1186/s12992-016-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.WHO . Global reference list of 100 core health indicators. World Health Organization; Geneva: 2015. [Google Scholar]
- 466.Medicine Transparency Alliance Pharmaceutical sector scan. http://www.medicinestransparency.org/resources/meta-resources/meta-toolkits/ (accessed April 10, 2016).
- 467.WHO Evaluation of the good governance in medicines programme 2004–2012. http://www.who.int/medicines/areas/policy/goodgovernance/ggm_evaluation_report/en/ (accessed May 30, 2016).
- 468.Bovens M. Analysing and assessing public accountability. A conceptual framework. European Governance Papers (EUROGOV) No. C-06-01. http://edoc.vifapol.de/opus/volltexte/2011/2459/pdf/egp_connex_C_06_01.pdf (accessed April 10, 2016).
- 469.Transparency International. Kohler JC, Martinez MG, Petkov M, Sale J. Corruption in the pharmaceutical sector. Diagnosing the challenges. Transparency International; London: 2016. [Google Scholar]
- 470.Mission for Essential Drugs and Supplies Quality assurance–overview. http://www.meds.or.ke/QA_Overview.html (accessed April 10, 2016).
- 471.NPS MedicineWise Australia Seventeenth annual evaluation report 2013/2014. 2015. http://www.nps.org.au/media-centre/media-releases/repository/new-nps-medicinewise-annual-evaluation-report (accessed April 10, 2016).
- 472.Berman P, Bitran R. Health system strengthening for better health system analysis. World Bank; Washington, DC: 2011. [Google Scholar]
- 473.WHO Target 9: provide essential medicines to treat NCDs. http://www.who.int/nmh/ncd-tools/target9/en/ (accessed April 10, 2016).
- 474.WHO Manual for the household survey to measure access and use of medicines. 2008. http://www.who.int/medicines/areas/coordination/household_manual_february_2008.pdf (accessed April 10, 2016).
- 475.WHO Global health expenditure database. http://www.who.int/health-accounts/ghed/en/ (accessed April 10, 2016).
- 476.Lu Y, Hernandez P, Abegunde D, Edejer T. Medicine expenditures [WHO/EMP/MIE/2011.2.6]. In: WHO. World medicines situation report. World Health Organization; Geneva: 2011. [Google Scholar]
- 477.Organization for Economic Corporation and Development . Pharmaceutical spending trends and future challenges [chapter 2]. Health at Glance 2015. OECD; Paris: 2015. [Google Scholar]
- 478.Vogler S, Hable C, Leopold C, Mazag J, Morak S, Zimmermann N. Pharmaceutical health information report. Hospital Pharma Report 2010. Austrian Federal Ministry of Health; Vienna: 2010. [Google Scholar]
- 479.Viergever RF, Li K. Trends in global clinical trial registration: an analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015;5:e008932. doi: 10.1136/bmjopen-2015-008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.WHO. The Global Fund Pharmaceutical sector country profile questionnaire. 2012. http://www.who.int/medicines/areas/coordination/Empty_English_Questionnaire.pdf?ua=1 (accessed April 10, 2016).
- 481.WHO . 14th International Conference of Drug Regulatory Authorities. World Health Organization; Geneva: 2010. [Google Scholar]
- 482.Bauschke R. Regulatory agencies, pharmaceutical information and the internet: a European perspective. Health Policy. 2012;104:12–18. doi: 10.1016/j.healthpol.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 483.Ferrario A, Sautenkova N, Bezverhni Z. An in-depth analysis of pharmaceutical regulation in the Republic of Moldova. J Pharm Policy Pract. 2014;7:4. doi: 10.1186/2052-3211-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Harvard Medical School and Harvard Pilgrim Healthcare/WHO . Using indicators to measure pharmaceutical country situations. Fact book level I and II monitoring indicators. WHO/TCM/2006.2. World Health Organization; Geneva: 2006. [Google Scholar]
- 485.Årdal C, Outterson K, Hoffman SJ. International cooperation to improve access to and sustain effectiveness of antimicrobials. Lancet. 2016;387:296–307. doi: 10.1016/S0140-6736(15)00470-5. [DOI] [PubMed] [Google Scholar]
- 486.Sun J, Zhang X, Zhang Z, Wagner AK, Ross-Degnan D, Hogerzeil HV. Impacts of a new insurance benefit with capitated provider payment on healthcare utilization, expenditure and quality of medication prescribing in China. Trop Med Int Health. 2016;21:263–274. doi: 10.1111/tmi.12636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.