Summary
Background
A recent cluster of pneumonia cases in Wuhan, China, was caused by a novel betacoronavirus, the 2019 novel coronavirus (2019-nCoV). We report the epidemiological, clinical, laboratory, and radiological characteristics and treatment and clinical outcomes of these patients.
Methods
All patients with suspected 2019-nCoV were admitted to a designated hospital in Wuhan. We prospectively collected and analysed data on patients with laboratory-confirmed 2019-nCoV infection by real-time RT-PCR and next-generation sequencing. Data were obtained with standardised data collection forms shared by WHO and the International Severe Acute Respiratory and Emerging Infection Consortium from electronic medical records. Researchers also directly communicated with patients or their families to ascertain epidemiological and symptom data. Outcomes were also compared between patients who had been admitted to the intensive care unit (ICU) and those who had not.
Findings
By Jan 2, 2020, 41 admitted hospital patients had been identified as having laboratory-confirmed 2019-nCoV infection. Most of the infected patients were men (30 [73%] of 41); less than half had underlying diseases (13 [32%]), including diabetes (eight [20%]), hypertension (six [15%]), and cardiovascular disease (six [15%]). Median age was 49·0 years (IQR 41·0–58·0). 27 (66%) of 41 patients had been exposed to Huanan seafood market. One family cluster was found. Common symptoms at onset of illness were fever (40 [98%] of 41 patients), cough (31 [76%]), and myalgia or fatigue (18 [44%]); less common symptoms were sputum production (11 [28%] of 39), headache (three [8%] of 38), haemoptysis (two [5%] of 39), and diarrhoea (one [3%] of 38). Dyspnoea developed in 22 (55%) of 40 patients (median time from illness onset to dyspnoea 8·0 days [IQR 5·0–13·0]). 26 (63%) of 41 patients had lymphopenia. All 41 patients had pneumonia with abnormal findings on chest CT. Complications included acute respiratory distress syndrome (12 [29%]), RNAaemia (six [15%]), acute cardiac injury (five [12%]) and secondary infection (four [10%]). 13 (32%) patients were admitted to an ICU and six (15%) died. Compared with non-ICU patients, ICU patients had higher plasma levels of IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα.
Interpretation
The 2019-nCoV infection caused clusters of severe respiratory illness similar to severe acute respiratory syndrome coronavirus and was associated with ICU admission and high mortality. Major gaps in our knowledge of the origin, epidemiology, duration of human transmission, and clinical spectrum of disease need fulfilment by future studies.
Funding
Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission.
Introduction
Coronaviruses are enveloped non-segmented positive-sense RNA viruses belonging to the family Coronaviridae and the order Nidovirales and broadly distributed in humans and other mammals.1 Although most human coronavirus infections are mild, the epidemics of the two betacoronaviruses, severe acute respiratory syndrome coronavirus (SARS-CoV)2, 3, 4 and Middle East respiratory syndrome coronavirus (MERS-CoV),5, 6 have caused more than 10 000 cumulative cases in the past two decades, with mortality rates of 10% for SARS-CoV and 37% for MERS-CoV.7, 8 The coronaviruses already identified might only be the tip of the iceberg, with potentially more novel and severe zoonotic events to be revealed.
In December, 2019, a series of pneumonia cases of unknown cause emerged in Wuhan, Hubei, China, with clinical presentations greatly resembling viral pneumonia.9 Deep sequencing analysis from lower respiratory tract samples indicated a novel coronavirus, which was named 2019 novel coronavirus (2019-nCoV). Thus far, more than 800 confirmed cases, including in health-care workers, have been identified in Wuhan, and several exported cases have been confirmed in other provinces in China, and in Thailand, Japan, South Korea, and the USA.10, 11, 12, 13
Research in context.
Evidence before this study
Human coronaviruses, including hCoV-229E, OC43, NL63, and HKU1, cause mild respiratory diseases. Fatal coronavirus infections that have emerged in the past two decades are severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus. We searched PubMed and the China National Knowledge Infrastructure database for articles published up to Jan 11, 2020, using the keywords “novel coronovirus”, “2019 novel coronavirus”, or “2019-nCoV”. No published work about the human infection caused by the 2019 novel coronavirus (2019-nCoV) could be identified.
Added value of this study
We report the epidemiological, clinical, laboratory, and radiological characteristics, treatment, and clinical outcomes of 41 laboratory-confirmed cases infected with 2019-nCoV. 27 (66%) of 41 patients had a history of direct exposure to the Huanan seafood market. The median age of patients was 49·0 years (IQR 41·0–58·0), and 13 (32%) patients had underlying disease. All patients had pneumonia. A third of patients were admitted to intensive care units, and six died. High concentrations of cytokines were recorded in plasma of critically ill patients infected with 2019-nCoV.
Implications of all the available evidence
2019-nCoV caused clusters of fatal pneumonia with clinical presentation greatly resembling SARS-CoV. Patients infected with 2019-nCoV might develop acute respiratory distress syndrome, have a high likelihood of admission to intensive care, and might die. The cytokine storm could be associated with disease severity. More efforts should be made to know the whole spectrum and pathophysiology of the new disease.
We aim to describe epidemiological, clinical, laboratory, and radiological characteristics, treatment, and outcomes of patients confirmed to have 2019-nCoV infection, and to compare the clinical features between intensive care unit (ICU) and non-ICU patients. We hope our study findings will inform the global community of the emergence of this novel coronavirus and its clinical features.
Methods
Patients
Following the pneumonia cases of unknown cause reported in Wuhan and considering the shared history of exposure to Huanan seafood market across the patients, an epidemiological alert was released by the local health authority on Dec 31, 2019, and the market was shut down on Jan 1, 2020. Meanwhile, 59 suspected cases with fever and dry cough were transferred to a designated hospital starting from Dec 31, 2019. An expert team of physicians, epidemiologists, virologists, and government officials was soon formed after the alert.
Since the cause was unknown at the onset of these emerging infections, the diagnosis of pneumonia of unknown cause in Wuhan was based on clinical characteristics, chest imaging, and the ruling out of common bacterial and viral pathogens that cause pneumonia. Suspected patients were isolated using airborne precautions in the designated hospital, Jin Yin-tan Hospital (Wuhan, China), and fit-tested N95 masks and airborne precautions for aerosol-generating procedures were taken. This study was approved by the National Health Commission of China and Ethics Commission of Jin Yin-tan Hospital (KY-2020-01.01). Written informed consent was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
Procedures
Local centres for disease control and prevention collected respiratory, blood, and faeces specimens, then shipped them to designated authoritative laboratories to detect the pathogen (NHC Key Laboratory of Systems Biology of Pathogens and Christophe Mérieux Laboratory, Beijing, China). A novel coronavirus, which was named 2019-nCoV, was isolated then from lower respiratory tract specimen and a diagnostic test for this virus was developed soon after that.14 Of 59 suspected cases, 41 patients were confirmed to be infected with 2019-nCoV. The presence of 2019-nCoV in respiratory specimens was detected by next-generation sequencing or real-time RT-PCR methods. The primers and probe target to envelope gene of CoV were used and the sequences were as follows: forward primer 5′-ACTTCTTTTTCTTGCTTTCGTGGT-3′; reverse primer 5′-GCAGCAGTACGCACACAATC-3′; and the probe 5′CY5-CTAGTTACACTAGCCATCCTTACTGC-3′BHQ1. Conditions for the amplifications were 50°C for 15 min, 95°C for 3 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s.
Initial investigations included a complete blood count, coagulation profile, and serum biochemical test (including renal and liver function, creatine kinase, lactate dehydrogenase, and electrolytes). Respiratory specimens, including nasal and pharyngeal swabs, bronchoalveolar lavage fluid, sputum, or bronchial aspirates were tested for common viruses, including influenza, avian influenza, respiratory syncytial virus, adenovirus, parainfluenza virus, SARS-CoV and MERS-CoV using real-time RT-PCR assays approved by the China Food and Drug Administration. Routine bacterial and fungal examinations were also performed.
Given the emergence of the 2019-nCoV pneumonia cases during the influenza season, antibiotics (orally and intravenously) and oseltamivir (orally 75 mg twice daily) were empirically administered. Corticosteroid therapy (methylprednisolone 40–120 mg per day) was given as a combined regimen if severe community-acquired pneumonia was diagnosed by physicians at the designated hospital. Oxygen support (eg, nasal cannula and invasive mechanical ventilation) was administered to patients according to the severity of hypoxaemia. Repeated tests for 2019-nCoV were done in patients confirmed to have 2019-nCoV infection to show viral clearance before hospital discharge or discontinuation of isolation.
Data collection
We reviewed clinical charts, nursing records, laboratory findings, and chest x-rays for all patients with laboratory-confirmed 2019-nCoV infection who were reported by the local health authority. The admission data of these patients was from Dec 16, 2019, to Jan 2, 2020. Epidemiological, clinical, laboratory, and radiological characteristics and treatment and outcomes data were obtained with standardised data collection forms (modified case record form for severe acute respiratory infection clinical characterisation shared by WHO and the International Severe Acute Respiratory and Emerging Infection Consortium) from electronic medical records. Two researchers also independently reviewed the data collection forms to double check the data collected. To ascertain the epidemiological and symptom data, which were not available from electronic medical records, the researchers also directly communicated with patients or their families to ascertain epidemiological and symptom data.
Cytokine and chemokine measurement
To characterise the effect of coronavirus on the production of cytokines or chemokines in the acute phase of the illness, plasma cytokines and chemokines (IL1B, IL1RA, IL2, IL4, IL5, IL6, IL7, IL8 (also known as CXCL8), IL9, IL10, IL12p70, IL13, IL15, IL17A, Eotaxin (also known as CCL11), basic FGF2, GCSF (CSF3), GMCSF (CSF2), IFNγ, IP10 (CXCL10), MCP1 (CCL2), MIP1A (CCL3), MIP1B (CCL4), PDGFB, RANTES (CCL5), TNFα, and VEGFA were measured using Human Cytokine Standard 27-Plex Assays panel and the Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA) for all patients according to the manufacturer's instructions. The plasma samples from four healthy adults were used as controls for cross-comparison. The median time from being transferred to a designated hospital to the blood sample collection was 4 days (IQR 2–5).
Detection of coronavirus in plasma
Each 80 μL plasma sample from the patients and contacts was added into 240 μL of Trizol LS (10296028; Thermo Fisher Scientific, Carlsbad, CA, USA) in the Biosafety Level 3 laboratory. Total RNA was extracted by Direct-zol RNA Miniprep kit (R2050; Zymo research, Irvine, CA, USA) according to the manufacturer's instructions and 50 μL elution was obtained for each sample. 5 μL RNA was used for real-time RT-PCR, which targeted the NP gene using AgPath-ID One-Step RT-PCR Reagent (AM1005; Thermo Fisher Scientific). The final reaction mix concentration of the primers was 500 nM and probe was 200 nM. Real-time RT-PCR was performed using the following conditions: 50°C for 15 min and 95°C for 3 min, 50 cycles of amplification at 95°C for 10 s and 60°C for 45 s. Since we did not perform tests for detecting infectious virus in blood, we avoided the term viraemia and used RNAaemia instead. RNAaemia was defined as a positive result for real-time RT-PCR in the plasma sample.
Definitions
Acute respiratory distress syndrome (ARDS) and shock were defined according to the interim guidance of WHO for novel coronavirus.9 Hypoxaemia was defined as arterial oxygen tension (PaO2) over inspiratory oxygen fraction (FIO2) of less than 300 mm Hg.15 Acute kidney injury was identified and classified on the basis of the highest serum creatinine level or urine output criteria according to the kidney disease improving global outcomes classification.16 Secondary infection was diagnosed if the patients had clinical symptoms or signs of nosocomial pneumonia or bacteraemia, and was combined with a positive culture of a new pathogen from a lower respiratory tract specimen (including the sputum, transtracheal aspirates, or bronchoalveolar lavage fluid, or from blood samples taken ≥48 h after admission).17 Cardiac injury followed the definition used in our previous study in H7N9 patients.18 In brief, cardiac injury was diagnosed if serum levels of cardiac biomarkers (eg, troponin I) were above the 99th percentile upper reference limit, or new abnormalities were shown in electrocardiography and echocardiography.
Statistical analysis
Continuous variables were expressed as median (IQR) and compared with the Mann-Whitney U test; categorical variables were expressed as number (%) and compared by χ2 test or Fisher's exact test between ICU care and no ICU care groups. Boxplots were drawn to describe plasma cytokine and chemokine concentrations.
A two-sided α of less than 0·05 was considered statistically significant. Statistical analyses were done using the SAS software, version 9.4, unless otherwise indicated.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
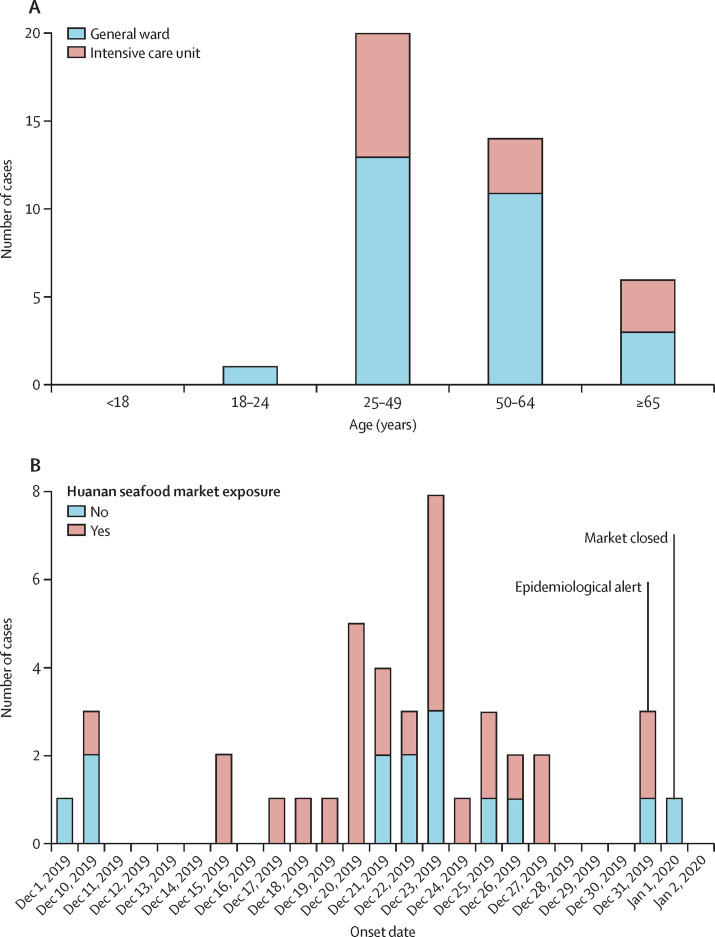
By Jan 2, 2020, 41 admitted hospital patients were identified as laboratory-confirmed 2019-nCoV infection in Wuhan. 20 [49%]) of the 2019-nCoV-infected patients were aged 25–49 years, and 14 (34%) were aged 50–64 years (figure 1 A). The median age of the patients was 49·0 years (IQR 41·0–58·0; table 1 ). In our cohort of the first 41 patients as of Jan 2, no children or adolescents were infected. Of the 41 patients, 13 (32%) were admitted to the ICU because they required high-flow nasal cannula or higher-level oxygen support measures to correct hypoxaemia. Most of the infected patients were men (30 [73%]); less than half had underlying diseases (13 [32%]), including diabetes (eight [20%]), hypertension (six [15%]), and cardiovascular disease (six [15%]).
Figure 1.
Date of illness onset and age distribution of patients with laboratory-confirmed 2019-nCoV infection
(A) Number of hospital admissions by age group. (B) Distribution of symptom onset date for laboratory-confirmed cases. The Wuhan local health authority issued an epidemiological alert on Dec 30, 2019, and closed the Huanan seafood market 2 days later.
Table 1.
Demographics and baseline characteristics of patients infected with 2019-nCoV
| All patients (n=41) | ICU care (n=13) | No ICU care (n=28) | p value | ||
|---|---|---|---|---|---|
| Characteristics | |||||
| Age, years | 49·0 (41·0–58·0) | 49·0 (41·0–61·0) | 49·0 (41·0–57·5) | 0·60 | |
| Sex | .. | .. | .. | 0·24 | |
| Men | 30 (73%) | 11 (85%) | 19 (68%) | .. | |
| Women | 11 (27%) | 2 (15%) | 9 (32%) | .. | |
| Huanan seafood market exposure | 27 (66%) | 9 (69%) | 18 (64%) | 0·75 | |
| Current smoking | 3 (7%) | 0 | 3 (11%) | 0·31 | |
| Any comorbidity | 13 (32%) | 5 (38%) | 8 (29%) | 0·53 | |
| Diabetes | 8 (20%) | 1 (8%) | 7 (25%) | 0·16 | |
| Hypertension | 6 (15%) | 2 (15%) | 4 (14%) | 0·93 | |
| Cardiovascular disease | 6 (15%) | 3 (23%) | 3 (11%) | 0·32 | |
| Chronic obstructive pulmonary disease | 1 (2%) | 1 (8%) | 0 | 0·14 | |
| Malignancy | 1 (2%) | 0 | 1 (4%) | 0·49 | |
| Chronic liver disease | 1 (2%) | 0 | 1 (4%) | 0·68 | |
| Signs and symptoms | |||||
| Fever | 40 (98%) | 13 (100%) | 27 (96%) | 0·68 | |
| Highest temperature, °C | .. | .. | .. | 0·037 | |
| <37·3 | 1 (2%) | 0 | 1 (4%) | .. | |
| 37·3–38·0 | 8 (20%) | 3 (23%) | 5 (18%) | .. | |
| 38·1–39·0 | 18 (44%) | 7 (54%) | 11 (39%) | .. | |
| >39·0 | 14 (34%) | 3 (23%) | 11 (39%) | .. | |
| Cough | 31 (76%) | 11 (85%) | 20 (71%) | 0·35 | |
| Myalgia or fatigue | 18 (44%) | 7 (54%) | 11 (39%) | 0·38 | |
| Sputum production | 11/39 (28%) | 5 (38%) | 6/26 (23%) | 0·32 | |
| Headache | 3/38 (8%) | 0 | 3/25 (12%) | 0·10 | |
| Haemoptysis | 2/39 (5%) | 1 (8%) | 1/26 (4%) | 0·46 | |
| Diarrhoea | 1/38 (3%) | 0 | 1/25 (4%) | 0·66 | |
| Dyspnoea | 22/40 (55%) | 12 (92%) | 10/27 (37%) | 0·0010 | |
| Days from illness onset to dyspnoea | 8·0 (5·0–13·0) | 8·0 (6·0–17·0) | 6·5 (2·0–10·0) | 0·22 | |
| Days from first admission to transfer | 5·0 (1·0–8·0) | 8·0 (5·0–14·0) | 1·0 (1·0–6·5) | 0·0023 | |
| Systolic pressure, mm Hg | 125·0 (119·0–135·0) | 145·0 (123·0–167·0) | 122·0 (118·5–129·5) | 0·018 | |
| Respiratory rate >24 breaths per min | 12 (29%) | 8 (62%) | 4 (14%) | 0·0023 | |
Data are median (IQR), n (%), or n/N (%), where N is the total number of patients with available data. p values comparing ICU care and no ICU care are from χ2 test, Fisher's exact test, or Mann-Whitney U test. 2019-nCoV=2019 novel coronavirus. ICU=intensive care unit.
27 (66%) patients had direct exposure to Huanan seafood market (figure 1B). Market exposure was similar between the patients with ICU care (nine [69%]) and those with non-ICU care (18 [64%]). The symptom onset date of the first patient identified was Dec 1, 2019. None of his family members developed fever or any respiratory symptoms. No epidemiological link was found between the first patient and later cases. The first fatal case, who had continuous exposure to the market, was admitted to hospital because of a 7-day history of fever, cough, and dyspnoea. 5 days after illness onset, his wife, a 53-year-old woman who had no known history of exposure to the market, also presented with pneumonia and was hospitalised in the isolation ward.
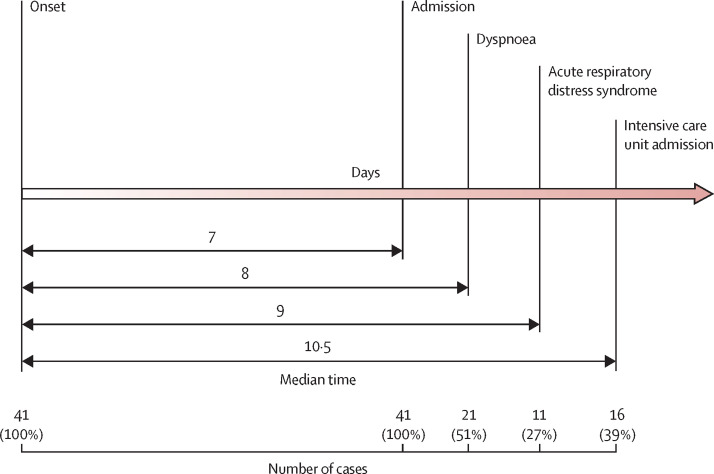
The most common symptoms at onset of illness were fever (40 [98%] of 41 patients), cough (31 [76%]), and myalgia or fatigue (18 [44%]); less common symptoms were sputum production (11 [28%] of 39), headache (three [8%] of 38), haemoptysis (two [5%] of 39), and diarrhoea (one [3%] of 38; table 1). More than half of patients (22 [55%] of 40) developed dyspnoea. The median duration from illness onset to dyspnoea was 8·0 days (IQR 5·0–13·0). The median time from onset of symptoms to first hospital admission was 7·0 days (4·0–8·0), to shortness of breath was 8·0 days (5·0–13·0), to ARDS was 9·0 days (8·0–14·0), to mechanical ventilation was 10·5 days (7·0–14·0), and to ICU admission was 10·5 days (8·0–17·0; figure 2 ).
Figure 2.
Timeline of 2019-nCoV cases after onset of illness
The blood counts of patients on admission showed leucopenia (white blood cell count less than 4 × 109/L; ten [25%] of 40 patients) and lymphopenia (lymphocyte count <1·0 × 109/L; 26 [63%] patients; table 2 ). Prothrombin time and D-dimer level on admission were higher in ICU patients (median prothrombin time 12·2 s [IQR 11·2–13·4]; median D-dimer level 2·4 mg/L [0·6–14·4]) than non-ICU patients (median prothrombin time 10·7 s [9·8–12·1], p=0·012; median D-dimer level 0·5 mg/L [0·3–0·8], p=0·0042). Levels of aspartate aminotransferase were increased in 15 (37%) of 41 patients, including eight (62%) of 13 ICU patients and seven (25%) of 28 non-ICU patients. Hypersensitive troponin I (hs-cTnI) was increased substantially in five patients, in whom the diagnosis of virus-related cardiac injury was made.
Table 2.
Laboratory findings of patients infected with 2019-nCoV on admission to hospital
| All patients (n=41) | ICU care (n=13) | No ICU care (n=28) | p value | ||
|---|---|---|---|---|---|
| White blood cell count, × 109/L | 6·2 (4·1–10·5) | 11·3 (5·8–12·1) | 5·7 (3·1–7·6) | 0·011 | |
| <4 | 10/40 (25%) | 1/13 (8%) | 9/27 (33%) | 0·041 | |
| 4–10 | 18/40 (45%) | 5/13 (38%) | 13/27 (48%) | .. | |
| >10 | 12/40 (30%) | 7/13 (54%) | 5/27 (19%) | .. | |
| Neutrophil count, × 109/L | 5·0 (3·3–8·9) | 10·6 (5·0–11·8) | 4·4 (2·0–6·1) | 0·00069 | |
| Lymphocyte count, × 109/L | 0·8 (0·6–1·1) | 0·4 (0·2–0·8) | 1·0 (0·7–1·1) | 0·0041 | |
| <1·0 | 26/41 (63%) | 11/13 (85%) | 15/28 (54%) | 0·045 | |
| ≥1·0 | 15/41 (37%) | 2/13 (15%) | 13/28 (46%) | .. | |
| Haemoglobin, g/L | 126·0 (118·0–140·0) | 122·0 (111·0–128·0) | 130·5 (120·0–140·0) | 0·20 | |
| Platelet count, × 109/L | 164·5 (131·5–263·0) | 196·0 (165·0–263·0) | 149·0 (131·0–263·0) | 0·45 | |
| <100 | 2/40 (5%) | 1/13 (8%) | 1/27 (4%) | 0·45 | |
| ≥100 | 38/40 (95%) | 12/13 (92%) | 26/27 (96%) | .. | |
| Prothrombin time, s | 11·1 (10·1–12·4) | 12·2 (11·2–13·4) | 10·7 (9·8–12·1) | 0·012 | |
| Activated partial thromboplastin time, s | 27·0 (24·2–34·1) | 26·2 (22·5–33·9) | 27·7 (24·8–34·1) | 0·57 | |
| D-dimer, mg/L | 0·5 (0·3–1·3) | 2·4 (0·6–14·4) | 0·5 (0·3–0·8) | 0·0042 | |
| Albumin, g/L | 31·4 (28·9–36·0) | 27·9 (26·3–30·9) | 34·7 (30·2–36·5) | 0·00066 | |
| Alanine aminotransferase, U/L | 32·0 (21·0–50·0) | 49·0 (29·0–115·0) | 27·0 (19·5–40·0) | 0·038 | |
| Aspartate aminotransferase, U/L | 34·0 (26·0–48·0) | 44·0 (30·0–70·0) | 34·0 (24·0–40·5) | 0·10 | |
| ≤40 | 26/41 (63%) | 5/13 (38%) | 21/28 (75%) | 0·025 | |
| >40 | 15/41 (37%) | 8/13 (62%) | 7/28 (25%) | .. | |
| Total bilirubin, mmol/L | 11·7 (9·5–13·9) | 14·0 (11·9–32·9) | 10·8 (9·4–12·3) | 0·011 | |
| Potassium, mmol/L | 4·2 (3·8–4·8) | 4·6 (4·0–5·0) | 4·1 (3·8–4·6) | 0·27 | |
| Sodium, mmol/L | 139·0 (137·0–140·0) | 138·0 (137·0–139·0) | 139·0 (137·5–140·5) | 0·26 | |
| Creatinine, μmol/L | 74·2 (57·5–85·7) | 79·0 (53·1–92·7) | 73·3 (57·5–84·7) | 0·84 | |
| ≤133 | 37/41 (90%) | 11/13 (85%) | 26/28 (93%) | 0·42 | |
| >133 | 4/41 (10%) | 2/13 (15%) | 2/28 (7%) | .. | |
| Creatine kinase, U/L | 132·5 (62·0–219·0) | 132·0 (82·0–493·0) | 133·0 (61·0–189·0) | 0·31 | |
| ≤185 | 27/40 (68%) | 7/13 (54%) | 20/27 (74%) | 0·21 | |
| >185 | 13/40 (33%) | 6/13 (46%) | 7/27 (26%) | .. | |
| Lactate dehydrogenase, U/L | 286·0 (242·0–408·0) | 400·0 (323·0–578·0) | 281·0 (233·0–357·0) | 0·0044 | |
| ≤245 | 11/40 (28%) | 1/13 (8%) | 10/27 (37%) | 0·036 | |
| >245 | 29/40 (73%) | 12/13 (92%) | 17/27 (63%) | .. | |
| Hypersensitive troponin I, pg/mL | 3·4 (1·1–9·1) | 3·3 (3·0–163·0) | 3·5 (0·7–5·4) | 0·075 | |
| >28 (99th percentile) | 5/41 (12%) | 4/13 (31%) | 1/28 (4%) | 0·017 | |
| Procalcitonin, ng/mL | 0·1 (0·1–0·1) | 0·1 (0·1–0·4) | 0·1 (0·1–0·1) | 0·031 | |
| <0·1 | 27/39 (69%) | 6/12 (50%) | 21/27 (78%) | 0·029 | |
| ≥0·1 to <0·25 | 7/39 (18%) | 3/12 (25%) | 4/27 (15%) | .. | |
| ≥0·25 to <0·5 | 2/39 (5%) | 0/12 | 2/27 (7%) | .. | |
| ≥0·5 | 3/39 (8%) | 3/12 (25%)* | 0/27 | .. | |
| Bilateral involvement of chest radiographs | 40/41 (98%) | 13/13 (100%) | 27/28 (96%) | 0·68 | |
| Cycle threshold of respiratory tract | 32·2 (31·0–34·5) | 31·1 (30·0–33·5) | 32·2 (31·1–34·7) | 0·39 | |
Data are median (IQR) or n/N (%), where N is the total number of patients with available data. p values comparing ICU care and no ICU care are from χ2, Fisher's exact test, or Mann-Whitney U test. 2019-nCoV=2019 novel coronavirus. ICU=intensive care unit.
Complicated typical secondary infection during the first hospitalisation.
Most patients had normal serum levels of procalcitonin on admission (procalcitonin <0·1 ng/mL; 27 [69%] patients; table 2). Four ICU patients developed secondary infections. Three of the four patients with secondary infection had procalcitonin greater than 0·5 ng/mL (0·69 ng/mL, 1·46 ng/mL, and 6·48 ng/mL).
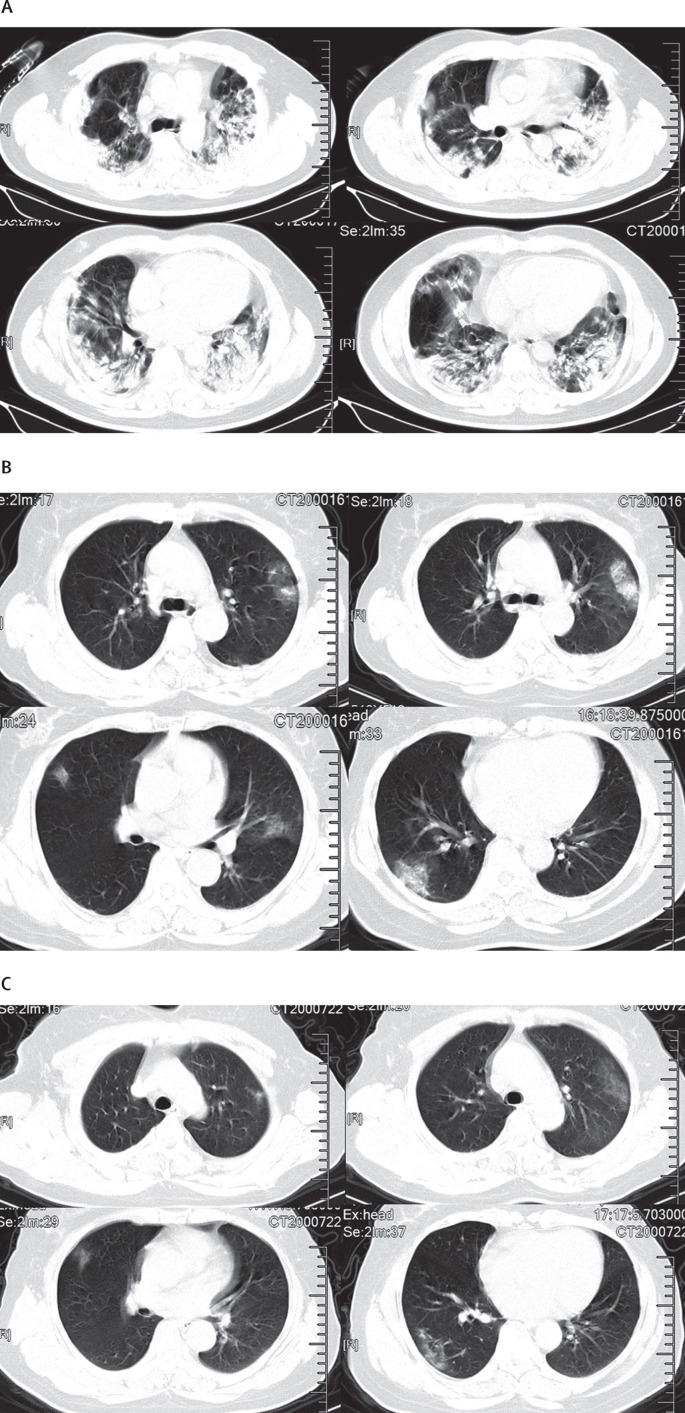
On admission, abnormalities in chest CT images were detected among all patients. Of the 41 patients, 40 (98%) had bilateral involvement (table 2). The typical findings of chest CT images of ICU patients on admission were bilateral multiple lobular and subsegmental areas of consolidation (figure 3 A). The representative chest CT findings of non-ICU patients showed bilateral ground-glass opacity and subsegmental areas of consolidation (figure 3B). Later chest CT images showed bilateral ground-glass opacity, whereas the consolidation had been resolved (figure 3C).
Figure 3.
Chest CT images
(A) Transverse chest CT images from a 40-year-old man showing bilateral multiple lobular and subsegmental areas of consolidation on day 15 after symptom onset. Transverse chest CT images from a 53-year-old woman showing bilateral ground-glass opacity and subsegmental areas of consolidation on day 8 after symptom onset (B), and bilateral ground-glass opacity on day 12 after symptom onset (C).
Initial plasma IL1B, IL1RA, IL7, IL8, IL9, IL10, basic FGF, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1A, MIP1B, PDGF, TNFα, and VEGF concentrations were higher in both ICU patients and non-ICU patients than in healthy adults (appendix pp 6–7). Plasma levels of IL5, IL12p70, IL15, Eotaxin, and RANTES were similar between healthy adults and patients infected with 2019-nCoV. Further comparison between ICU and non-ICU patients showed that plasma concentrations of IL2, IL7, IL10, GCSF, IP10, MCP1, MIP1A, and TNFα were higher in ICU patients than non-ICU patients.
All patients had pneumonia. Common complications included ARDS (12 [29%] of 41 patients), followed by RNAaemia (six [15%] patients), acute cardiac injury (five [12%] patients), and secondary infection (four [10%] patients; table 3 ). Invasive mechanical ventilation was required in four (10%) patients, with two of them (5%) had refractory hypoxaemia and received extracorporeal membrane oxygenation as salvage therapy. All patients were administered with empirical antibiotic treatment, and 38 (93%) patients received antiviral therapy (oseltamivir). Additionally, nine (22%) patients were given systematic corticosteroids. A comparison of clinical features between patients who received and did not receive systematic corticosteroids is in the appendix (pp 1–5).
Table 3.
Treatments and outcomes of patients infected with 2019-nCoV
| All patients (n=41) | ICU care (n=13) | No ICU care (n=28) | p value | ||
|---|---|---|---|---|---|
| Duration from illness onset to first admission | 7·0 (4·0–8·0) | 7·0 (4·0–8·0) | 7·0 (4·0–8·5) | 0·87 | |
| Complications | |||||
| Acute respiratory distress syndrome | 12 (29%) | 11 (85%) | 1 (4%) | <0·0001 | |
| RNAaemia | 6 (15%) | 2 (15%) | 4 (14%) | 0·93 | |
| Cycle threshold of RNAaemia | 35·1 (34·7–35·1) | 35·1 (35·1–35·1) | 34·8 (34·1–35·4) | 0·35 | |
| Acute cardiac injury* | 5 (12%) | 4 (31%) | 1 (4%) | 0·017 | |
| Acute kidney injury | 3 (7%) | 3 (23%) | 0 | 0·027 | |
| Secondary infection | 4 (10%) | 4 (31%) | 0 | 0·0014 | |
| Shock | 3 (7%) | 3 (23%) | 0 | 0·027 | |
| Treatment | |||||
| Antiviral therapy | 38 (93%) | 12 (92%) | 26 (93%) | 0·46 | |
| Antibiotic therapy | 41 (100%) | 13 (100%) | 28 (100%) | NA | |
| Use of corticosteroid | 9 (22%) | 6 (46%) | 3 (11%) | 0·013 | |
| Continuous renal replacement therapy | 3 (7%) | 3 (23%) | 0 | 0·027 | |
| Oxygen support | .. | .. | .. | <0·0001 | |
| Nasal cannula | 27 (66%) | 1 (8%) | 26 (93%) | .. | |
| Non-invasive ventilation or high-flow nasal cannula | 10 (24%) | 8 (62%) | 2 (7%) | .. | |
| Invasive mechanical ventilation | 2 (5%) | 2 (15%) | 0 | .. | |
| Invasive mechanical ventilation and ECMO | 2 (5%) | 2 (15%) | 0 | .. | |
| Prognosis | .. | .. | .. | 0·014 | |
| Hospitalisation | 7 (17%) | 1 (8%) | 6 (21%) | .. | |
| Discharge | 28 (68%) | 7 (54%) | 21 (75%) | .. | |
| Death | 6 (15%) | 5 (38%) | 1 (4%) | .. | |
Data are median (IQR) or n (%). p values are comparing ICU care and no ICU care. 2019-nCoV=2019 novel coronavirus. ICU=intensive care unit. NA=not applicable. ECMO=extracorporeal membrane oxygenation.
Defined as blood levels of hypersensitive troponin I above the 99th percentile upper reference limit (>28 pg/mL) or new abnormalities shown on electrocardiography and echocardiography.
As of Jan 22, 2020, 28 (68%) of 41 patients have been discharged and six (15%) patients have died. Fitness for discharge was based on abatement of fever for at least 10 days, with improvement of chest radiographic evidence and viral clearance in respiratory samples from upper respiratory tract.
Discussion
We report here a cohort of 41 patients with laboratory-confirmed 2019-nCoV infection. Patients had serious, sometimes fatal, pneumonia and were admitted to the designated hospital in Wuhan, China, by Jan 2, 2020. Clinical presentations greatly resemble SARS-CoV. Patients with severe illness developed ARDS and required ICU admission and oxygen therapy. The time between hospital admission and ARDS was as short as 2 days. At this stage, the mortality rate is high for 2019-nCoV, because six (15%) of 41 patients in this cohort died.
The number of deaths is rising quickly. As of Jan 24, 2020, 835 laboratory-confirmed 2019-nCoV infections were reported in China, with 25 fatal cases. Reports have been released of exported cases in many provinces in China, and in other countries; some health-care workers have also been infected in Wuhan. Taken together, evidence so far indicates human transmission for 2019-nCoV. We are concerned that 2019-nCoV could have acquired the ability for efficient human transmission.19 Airborne precautions, such as a fit-tested N95 respirator, and other personal protective equipment are strongly recommended. To prevent further spread of the disease in health-care settings that are caring for patients infected with 2019-nCoV, onset of fever and respiratory symptoms should be closely monitored among health-care workers. Testing of respiratory specimens should be done immediately once a diagnosis is suspected. Serum antibodies should be tested among health-care workers before and after their exposure to 2019-nCoV for identification of asymptomatic infections.
Similarities of clinical features between 2019-nCoV and previous betacoronavirus infections have been noted. In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections.20, 21 However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (eg, rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (eg, diarrhoea), whereas about 20–25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.21 Faecal and urine samples should be tested to exclude a potential alternative route of transmission that is unknown at this stage.
The pathophysiology of unusually high pathogenicity for SARS-CoV or MERS-CoV has not been completely understood. Early studies have shown that increased amounts of proinflammatory cytokines in serum (eg, IL1B, IL6, IL12, IFNγ, IP10, and MCP1) were associated with pulmonary inflammation and extensive lung damage in SARS patients.22 MERS-CoV infection was also reported to induce increased concentrations of proinflammatory cytokines (IFNγ, TNFα, IL15, and IL17).23 We noted that patients infected with 2019-nCoV also had high amounts of IL1B, IFNγ, IP10, and MCP1, probably leading to activated T-helper-1 (Th1) cell responses. Moreover, patients requiring ICU admission had higher concentrations of GCSF, IP10, MCP1, MIP1A, and TNFα than did those not requiring ICU admission, suggesting that the cytokine storm was associated with disease severity. However, 2019-nCoV infection also initiated increased secretion of T-helper-2 (Th2) cytokines (eg, IL4 and IL10) that suppress inflammation, which differs from SARS-CoV infection.22 Further studies are necessary to characterise the Th1 and Th2 responses in 2019-nCoV infection and to elucidate the pathogenesis. Autopsy or biopsy studies would be the key to understand the disease.
In view of the high amount of cytokines induced by SARS-CoV,22, 24 MERS-CoV,25, 26 and 2019-nCoV infections, corticosteroids were used frequently for treatment of patients with severe illness, for possible benefit by reducing inflammatory-induced lung injury. However, current evidence in patients with SARS and MERS suggests that receiving corticosteroids did not have an effect on mortality, but rather delayed viral clearance.27, 28, 29 Therefore, corticosteroids should not be routinely given systemically, according to WHO interim guidance.30 Among our cohort of 41 laboratory-confirmed patients with 2019-nCoV infection, corticosteroids were given to very few non-ICU cases, and low-to-moderate dose of corticosteroids were given to less than half of severely ill patients with ARDS. Further evidence is urgently needed to assess whether systematic corticosteroid treatment is beneficial or harmful for patients infected with 2019-nCoV.
No antiviral treatment for coronavirus infection has been proven to be effective. In a historical control study,31 the combination of lopinavir and ritonavir among SARS-CoV patients was associated with substantial clinical benefit (fewer adverse clinical outcomes). Arabi and colleagues initiated a placebo-controlled trial of interferon beta-1b, lopinavir, and ritonavir among patients with MERS infection in Saudi Arabia.32 Preclinical evidence showed the potent efficacy of remdesivir (a broad-spectrum antiviral nucleotide prodrug) to treat MERS-CoV and SARS-CoV infections.33, 34 As 2019-nCoV is an emerging virus, an effective treatment has not been developed for disease resulting from this virus. Since the combination of lopinavir and ritonavir was already available in the designated hospital, a randomised controlled trial has been initiated quickly to assess the efficacy and safety of combined use of lopinavir and ritonavir in patients hospitalised with 2019-nCoV infection.
Our study has some limitations. First, for most of the 41 patients, the diagnosis was confirmed with lower respiratory tract specimens and no paired nasopharyngeal swabs were obtained to investigate the difference in the viral RNA detection rate between upper and lower respiratory tract specimens. Serological detection was not done to look for 2019-nCoV antibody rises in 18 patients with undetectable viral RNA. Second, with the limited number of cases, it is difficult to assess host risk factors for disease severity and mortality with multivariable-adjusted methods. This is a modest-sized case series of patients admitted to hospital; collection of standardised data for a larger cohort would help to further define the clinical presentation, natural history, and risk factors. Further studies in outpatient, primary care, or community settings are needed to get a full picture of the spectrum of clinical severity. At the same time, finding of statistical tests and p values should be interpreted with caution, and non-significant p values do not necessarily rule out difference between ICU and non-ICU patients. Third, since the causative pathogen has just been identified, kinetics of viral load and antibody titres were not available. Finally, the potential exposure bias in our study might account for why no paediatric or adolescent patients were reported in this cohort. More effort should be made to answer these questions in future studies.
Both SARS-CoV and MERS-CoV were believed to originate in bats, and these infections were transmitted directly to humans from market civets and dromedary camels, respectively.35 Extensive research on SARS-CoV and MERS-CoV has driven the discovery of many SARS-like and MERS-like coronaviruses in bats. In 2013, Ge and colleagues36 reported the whole genome sequence of a SARS-like coronavirus in bats with that ability to use human ACE2 as a receptor, thus having replication potentials in human cells.37 2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity.
This online publication has been corrected. The corrected version first appeared at thelancet.com on January 30, 2020
Data sharing
The data that support the findings of this study are available from the corresponding author on reasonable request. Participant data without names and identifiers will be made available after approval from the corresponding author and National Health Commission. After publication of study findings, the data will be available for others to request. The research team will provide an email address for communication once the data are approved to be shared with others. The proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability to request for our data. The corresponding author and National Health Commission will make a decision based on these materials. Additional materials may also be required during the process.
Acknowledgments
Acknowledgments
This work is funded by the Special Project for Emergency of the Ministry of Science and Technology (2020YFC0841300) Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003), a National Science Grant for Distinguished Young Scholars (81425001/H0104), the National Key Research and Development Program of China (2018YFC1200102), The Beijing Science and Technology Project (Z19110700660000), CAMS Innovation Fund for Medical Sciences (2016-I2M-1-014), and National Mega-projects for Infectious Diseases in China (2017ZX10103004 and 2018ZX10305409). We acknowledge all health-care workers involved in the diagnosis and treatment of patients in Wuhan; we thank the Chinese National Health Commission for coordinating data collection for patients with 2019-nCoV infection; we thank WHO and the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) for sharing data collection templates publicly on the website; and we thank Prof Chen Wang and Prof George F Gao for guidance in study design and interpretation of results.
Contributors
BC and JW had the idea for and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YWa, GF, XG, JiXu, HL, and BC contributed to writing of the report. BC contributed to critical revision of the report. YWa, GF, XG, JiXu, and HL contributed to the statistical analysis. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
Declaration of interests
All authors declare no competing interests.
Contributor Information
Jianwei Wang, Email: wangjw28@163.com.
Bin Cao, Email: caobin_ben@163.com.
Supplementary Material
References
- 1.Richman DD, Whitley RJ, Hayden FG. 4th edn. ASM Press; Washington: 2016. Clinical virology. [Google Scholar]
- 2.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 3.Kuiken T, Fouchier RAM, Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drosten C, Günther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 5.de Groot RJ, Baker SC, Baric RS. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 7.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Dec 31, 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/
- 8.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) November, 2019. http://www.who.int/emergencies/mers-cov/en/
- 9.WHO Novel coronavirus – China. Jan 12, 2020. http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 10.WHO Novel coronavirus – Thailand (ex-China) Jan 14, 2020. http://www.who.int/csr/don/14-january-2020-novel-coronavirus-thailand/en/
- 11.WHO Novel coronavirus – Japan (ex-China) Jan 17, 2020. http://www.who.int/csr/don/17-january-2020-novel-coronavirus-japan-ex-china/en/
- 12.WHO Novel coronavirus – Republic of Korea (ex-China) Jan 21, 2020. http://www.who.int/csr/don/21-january-2020-novel-coronavirus-republic-of-korea-ex-china/en/
- 13.CDC First travel-related case of 2019 novel coronavirus detected in United States. Jan 21, 2020. https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html
- 14.Tan W, Zhao X, Ma X. A novel coronavirus genome identified in a cluster of pneumonia cases — Wuhan, China 2019−2020. http://weekly.chinacdc.cn/en/article/id/a3907201-f64f-4154-a19e-4253b453d10c [PMC free article] [PubMed]
- 15.Sanz F, Gimeno C, Lloret T. Relationship between the presence of hypoxemia and the inflammatory response measured by C-reactive protein in bacteremic pneumococcal pneumonia. Eur Respir J. 2011;38(suppl 55):2492. [Google Scholar]
- 16.Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group KDIGO clinical practice guideline for acute kidney injury. March, 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf
- 17.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 18.Gao C, Wang Y, Gu X. Association between cardiac injury and mortality in hospitalized patients infected with avian influenza A (H7N9) virus. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004207. published online Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 21.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong CK, Lam CWK, Wu AKL. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, Ding Y, Zhang Q. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faure E, Poissy J, Goffard A. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9 doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falzarano D, de Wit E, Rasmussen AL. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansbury L, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2019;2:CD010406. doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arabi YM, Mandourah Y, Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 30.WHO Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Jan 11, 2020. https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 31.Chu CM. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arabi YM, Alothman A, Balkhy HH. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheahan TP, Sims AC, Graham RL. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheahan TP, Sims AC, Leist SR. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge X-Y, Li J-L, Yang X-L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Hu Z. Bats as animal reservoirs for the SARS coronavirus: hypothesis proved after 10 years of virus hunting. Virol Sin. 2013;28:315–317. doi: 10.1007/s12250-013-3402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request. Participant data without names and identifiers will be made available after approval from the corresponding author and National Health Commission. After publication of study findings, the data will be available for others to request. The research team will provide an email address for communication once the data are approved to be shared with others. The proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability to request for our data. The corresponding author and National Health Commission will make a decision based on these materials. Additional materials may also be required during the process.