Abstract
BACKGROUND: Acne and rosacea are common chronic inflammatory skin diseases associated with psychosocial impairment, anxiety, depression, and impaired overall quality of life. Timolol maleate is a potent nonselective β-blocker that causes a combination of vasoconstriction and inhibition of inflammatory mediators. OBJECTIVES: We assessed the clinical efficacy and safety of topical timolol maleate 0.5% for the treatment of acne and rosacea. METHODS: A total of 116 patients (58 patients with rosacea and 58 patients with acne) were treated with topical timolol maleate 0.5% every night before bedtime for eight weeks. RESULTS: The severity of both acne and rosacea decreased relative to baseline; however, the improvement in rosacea was not statistically significant. CONCLUSION: In our study, topical timolol maleate 0.5% demonstrated effectiveness in the treatment of acne, especially in noninflammatory lesions, but seems to be more effective in erythematotelangiectatic rosacea than papulopustular rosacea lesions, with insignificant side effects. The addition of topical timolol to the standard treatment protocol for acne and rosacea is expected to be beneficial, especially by way of improving comedones of acne and resistant inflammatory erythema of both acne and rosacea.
Keywords: Acne, rosacea, timolol, erythematotelangiectatic, papulopustular
Acne vulgaris, a pilosebaceous unit disorder, is characterized by noninflammatory lesions, such as comedones, and inflammatory lesions, such as papules, pustules, and nodules.1 Different etiological bases have been implicated in the pathogenesis of acne, including abnormal keratinization of the pilosebaceous duct, androgen-stimulated sebum production, and Propionibacterium acnes proliferation with subsequent inflammation. Genetics, oxidative stress, and nutrition are also involved in the pathogenesis of acne.2
Rosacea is a chronic inflammatory skin condition, the etiopathogenesis of which is not completely understood. Demodex folliculorum, immune dysregulation, and abnormal neurovascular signals are all involved in the pathogenesis of rosacea. Rosacea has main four types: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular. Facial erythema, telangiectasia, and inflammatory papules and pustules are the most commonly reported features.3 Blood vessel abnormality is an important factor in the pathogenesis of rosacea that results in the characteristic flushing, persistent redness, and visible blood vessels. The cause of blood vessel inflammation is still unknown.4 Acne and rosacea can have long-lasting psychosocial sequelae that affect the patient’s quality of life. Moreover, the treatment of both is still a challenge.5,6
Timolol is a nonselective β-blocker that has been used for treating hemangioma. It causes vasoconstriction, induces apoptosis, and inhibits angiogenic factors, such as vascular endothelial growth factor (VEGF), and inflammatory mediators, such as matrix metalloproteinase (MMP)-2, MMP-9, and interleukin (IL)-6.7
Timolol, through its anti-inflammatory properties, might provide a new treatment option for acne and rosacea, because inflammation is known as a fundamental factor in the pathogenesis of both diseases.8,9 In addition, timolol can improve erythema and flushing in rosacea by inhibiting β-adrenergic receptors on the smooth muscles surrounding blood vessels, leading to vasoconstriction of these vessels.9
Accessibility, cost-effectiveness, a simple mode of application, and a low incidence of drug reactions are major advantages of topical timolol.10
METHODS
Patients. This was a multicentric study that included adult and adolescent patients with either acne or rosacea. One hundred forty patients with acne and rosacea were recruited from the outpatient clinics at three locations: Zagazig University Hospital, Al Haud Al Marsaud Dermatology Hospital, and Zagazig General Hospital. Of these, 58 patients with ETR or PPR rosacea and 58 patients with mild or moderate acne were included in the study from December 2016 to October 2017. Participants were excluded if they had received any treatment 30 days prior to the study, had an allergy to β-blockers, were pregnant or lactating, or had hypotension, bradycardia or heart failure, myocardial infarction or arrhythmia, asthma, or bronchospasm. The study was approved by the Zagazig University Hospital Institutional Review Board, and informed consent and photoconsent was acquired from each patient before the start of the study.
A thorough history was recorded for each patient, including family history of acne or rosacea, drug intake, and disease onset, course, and duration. Patients were also asked about any aggravating factors, including food, stress, or associated symptoms. Finally, each patient completed a general examination to detect any other systemic issues or associated disease.
For patients with acne, severity was scored using the Global Acne Grading System (1–18=mild; 19–30=moderate; 31–38=severe; and 39=very severe).11 After a baseline evaluation of acne severity, mild and moderate cases were selected for the study.
For rosacea, patients with ETR and PPR types were included in the study. Rosacea severity was assessed using two measures — the Investigator’s Global Assessment (IGA) score and a rosacea clinical scale, where 1=clear and 5=severe.12 Each patient in both groups was instructed to apply 4 to 8 drops of topical timolol 0.5% on the affected areas of the face every night before bedtime for eight weeks.
Assessment of the therapeutic response and follow-up. Patient response to the therapy was assessed every two weeks for eight weeks. For patients with acne, the response to treatment was evaluated by assessing the number of comedones, papules, and pustules and comparing the values of the following two formulations before and after treatment:
-
•
Total lesion count (TLC)=comedones+papules+pustules
-
•
Acne severity index (ASI)=papules+ (2×pustules)+(comedones/4).13
For patients with rosacea, the response to treatment was evaluated by assessing the degree of erythema, telangiectasia, papules, and pustules according to the rosacea clinical scale and by reassessment of the IGA scale scores. Treatment was deemed successful if patients achieved an IGA score of “clear” or “almost clear” (IGA score of 0–1). The percentage of participants with each score at the end of treatment was provided. Patients who achieved a “clear,” “minimal,” or “mild” IGA result after eight weeks of treatment were considered responders, while those with an IGA score of “moderate” or “severe” were considered nonresponders. Adverse effects were evaluated at each follow-up visit.
Statistical analysis. Data analysis was performed using the SPSS Version 21 software program (IBM Corp., Armonk, New York). Quantitative variables were described using means and standard deviations (SDs), while categorical variables were described using absolute frequencies. Kolmogorov-Smirnov (distribution-type) and Levene (homogeneity of variances) tests were used to verify assumptions for use in parametric tests. To compare means, an independent-samples t-test and one-way analysis of variance were used when appropriate. Nonparametric testing (Mann–Whitney U) was used to compare means when data were not normally distributed and to compare medians in categorical data. To evaluate the effect of therapy, a paired-samples t-test (when data were normally distributed) and the Wilcoxon test (when data were not normally distributed) were used. To evaluate the association between categorical variables, Pearson’s chi-squared test was used. The level of statistical significance was set at five percent (p≤0.05).
RESULTS
Acne. The acne group comprised 58 patients with acne vulgaris, including six male patients (10.3%) and 52 female patients (89.7%), with ages ranging from 15 to 28 years (mean±SD age:19.93±2.52 years). The duration of acne ranged from one month to 10 years, with a mean±SD period of 22.34±24.99 months;12 patients (20.6%) had a positive family history for acne. The acne global score ranged from 9 to 24 points, classified into 42 “mild” cases (72.4%) and 16 “moderate” cases (27.6%). After eight weeks of treatment, there was a highly statistically significant reduction in comedones, papules, TLC, and ASI from baseline, as well as a statistically significant reduction in pustules. Mean number of comedones decreased from 32.1 to 24.14, mean number of papules decreased from 4.76 to 3.93, and mean number of pustules decreased from 1.72 to 1.59. Additionally, TLC mean decreased from 38.66 to 29.45 and the ASI mean value decreased from 16.24 to 12.48. Comedones showed the highest mean percentage of improvement at 28.17 percent, while papules and pustules showed mean percentages of improvement of 26.81 percent and 16.05 percent, respectively. Separately, there was a mean percentage of improvement of 25.11 percent in TLC, and a mean percentage of improvement of 23.24 percent in ASI (Figures 1 and 2, Tables 1 and 2). There was a significant negative correlation between TLC score before treatment and the percentage of improvement in TLC and ASI after treatment. There was also a negative correlation between age, ASI score before treatment, and the TLC and ASI percentages of improvement after treatment; however, this finding was not significant. There was no statistically significant correlation between sex, previous therapy, family history, and the percentage of TLC or ASI improvement after treatment.
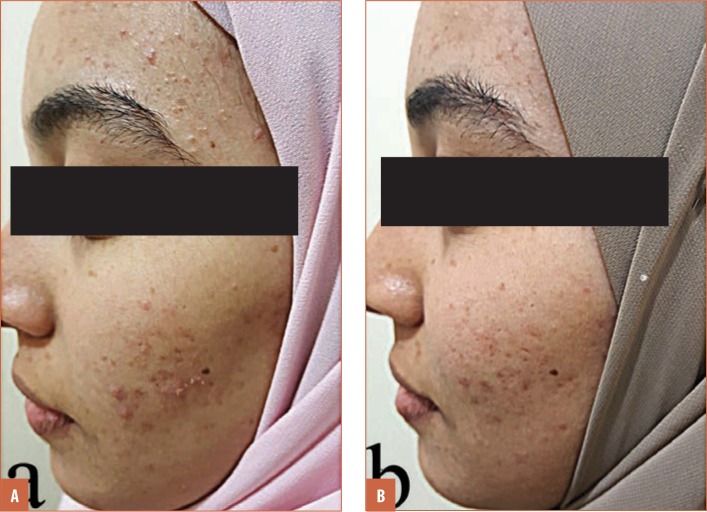
FIGURE 1.
A patient with inflammatory acne that showed good response: A) before treatment and B) after treatment
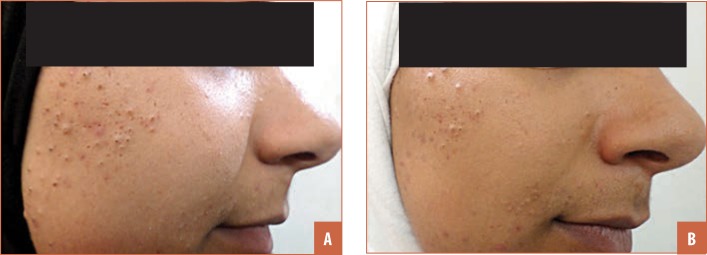
FIGURE 2.
A patient with comedonal acne that showed good response: A) before treatment and B) after treatment
TABLE 1.
Clinical criteria of acne patients before treatment
| Acne severity (Global Acne Grading System) | |
| Mild, n (%) | 42 (72.4) |
| Moderate, n (%) | 16 (27.6) |
| TLC | |
| x±SD | 38.66 ± 16.66 |
| Median (range) | 38 (12–64) |
| ASI | |
| x±SD | 16.24±5.87 |
| Median (range) | 17 (7–30) |
ASI: acne severity index; SD: standard deviation; TLC: total lesion count
TABLE 2.
Response of acne patients to treatment with topical timolol maleate 0.5%
| VARIABLES | BEFORE | AFTER | p-VALUE |
|---|---|---|---|
| Comedones | |||
| Mean±SD | 32.1±15.69 | 24.14±13.69 | <0.001** |
| Median (range) | 28 (8–56) | 24 (4–50) | |
| Papules | |||
| Mean±SD | 4.76±2.14 | 3.93±1.77 | <0.001** |
| Median (range) | 4 (1–8) | 2 (1–6) | |
| Pustules | |||
| Mean±SD | 1.72±1.33 | 1.59±1.21 | 0.043* |
| Median (range) | 2 (0–6) | 1 (0–4) | |
| TLC | |||
| Mean±SD | 38.66±16.66 | 29.45±14.01 | <0.001** |
| Median (range) | 38 (12–64) | 27 (8–55) | |
| ASI | |||
| Mean±SD | 16.24±5.87 | 12.48±4.6 | <0.001** |
| Median (range) | 17 (7–30) | 12 (6–24.5) | |
ASI: acne severity index; SD: standard deviation; TLC: total lesion count
Statistically significant difference (p≤0.05)
Statistically highly significant difference (p≤0.001)
Rosacea. The rosacea group comprised 58 patients with rosacea, including four male patients (6.9%) and 54 female patients (93.1%), with ages ranging from 22 to 73 years and a mean±SD age of 39.45±10.88 years. The duration of rosacea ranged from one month to 15 years, with a mean±SD period of 48.93±45.87 months. Eight patients (13.8%) had a positive family history for rosacea. The IGA score indicated “mild” rosacea in 10 cases (17.2%), “moderate” in 32 cases (55.2%), and “severe” in 16 cases (27.6%). The baseline characteristics of patients with rosacea and their responses to the therapy are shown in Tables 3 and 4.
TABLE 3.
Clinical criteria of patients with rosacea before treatment
| VARIABLES | n (%) |
|---|---|
| Erythema | |
| Absent | 0 (0) |
| Mild | 18 (31) |
| Moderate | 40 (69) |
| Papules and pustules | |
| Absent | 22 (37.9) |
| Mild | 22 (37.9) |
| Moderate | 12 (20.7) |
| Severe | 2 (3.4) |
| Telangectasia | |
| Absent | 0 (0) |
| Mild | 30 (51.7) |
| Moderate | 28 (48.3) |
| IGA score | |
| Mild | 10 (17.2) |
| Moderate | 32 (55.2) |
| Severe | 16 (27.6) |
| Rosacea clinical score severity | |
| x±SD | 11.55 ± 4.1 |
| Median (range) | 12 (6–20) |
IGA: Investigator's Global Assessment; SD: standard deviation
TABLE 4.
Response of rosacea patients to treatment with topical timolol maleate 0.5%
| VARIABLES | BEFORE, n (%) | AFTER, n (%) | P-VALUE |
|---|---|---|---|
| Erythema | |||
| Absent | 0 (0) | 8 (13.8) | 0.09* |
| Mild | 18 (31) | 20 (34.5) | |
| Moderate | 40 (69) | 30 (51.7) | |
| Papules/pustules | |||
| Absent | 22 (37.9) | 28 (48.3) | 0.62* |
| Mild | 22 (37.9) | 22 (37.9) | |
| Moderate | 12 (20.7) | 8 (13.8) | |
| Severe | 2 (3.4) | 0 (0) | |
| Telangiectasia | |||
| Absent | 0 (0) | 6 (10.3) | 0.16* |
| Mild | 30 (51.7) | 32 (55.2) | |
| Moderate | 28 (48.3) | 20 (34.5) | |
| IGA | |||
| Almost clear | 0 (0) | 6 (10.4) | 0.09* |
| Mild | 10 (17.2) | 20 (34.5) | |
| Moderate | 32 (55.2) | 20 (34.5) | |
| Severe | 16 (27.6) | 12 (20.6) | |
Not significant (P>0.05)
In this group, 10.4 percent of patients achieved an IGA result of “clear” or “almost clear” after treatment. By the end of the study, 34.2 percent of the acne cases were considered “mild,” 34.5 percent “moderate,” and 20.6 percent “severe,” compared to baseline (17.2%, 55.2% and 27.6 percent, respectively) (Figures 3 and 4). According to IGA results, 44.9 percent of patients were considered responders to treatment, while 55.1 percent were considered nonresponders.
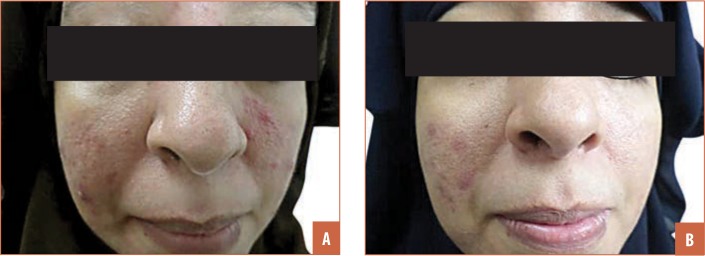
FIGURE 3.
A patient with papulopustular rosacea that showed good response; A) before treatment and B) after treatment
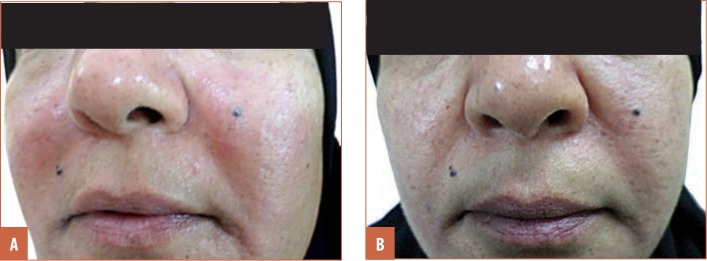
FIGURE 4.
A patient with erythematotelangiectatic rosacea that showed good response; A) before treatment and B) after treatment
Side effects. All reported side effects in both groups were mild and tolerable. Dryness was the most frequent complaint; other reported side effects included a burning sensation, mild irritation, scaly skin, and itching.
DISCUSSION
Acne, one of the most common skin diseases, affects approximately 85 percent of the global population at some point in life and occurs most prominently on skin sites with a high density of sebaceous glands, such as the face, back, and chest. However, acne can affect all areas of the body, except the palms and soles, due to the lack of sebaceous glands on these regions.14
Rosacea is a chronic facial inflammatory dermatosis that poses a therapeutic challenge due to its multifactorial pathogenesis.15
Timolol maleate is a potent nonselective β-blocker, that causes a combination of vasoconstriction, inhibition of angiogenic factors, such as VEGF, inhibition of the inflammatory mediators, and induction of apoptosis.7 The anti-inflammatory action of timolol could be useful in the treatment of acne and rosacea, due to the inflammatory pathogenesis of both skin conditions. Also, nonselective β-blockers like timolol are thought to decrease erythema and flushing in rosacea by inhibiting β-adrenergic receptors on the smooth muscles surrounding blood vessels, leading to vasoconstriction of these vessels, and by inhibition of the angiogenic factors, such as VEGF.11
To the best of our knowledge, there are few studies that assess the efficacy of topical timolol in the treatment of acne and rosacea. Thus, the present study was carried out to assess the efficacy and safety of topical timolol maleate 0.5% in the treatment of mild-to-moderate acne and rosacea (ETR and PPR types). After eight weeks of treatment, the severity of acne and rosacea decreased from baseline; however, the improvement in rosacea was not statistically significant. More specifically, in the patients with acne, there was a highly statistically significant reduction in comedones, papules, TLC, and ASI from baseline, with a significant reduction in pustules. The best clinical improvement was observed in noninflammatory lesions (i.e., comedones). Theoretically, this finding might be attributed to timolol's vasoconstrictive effect, which could possibly decrease the blood flow to sebaceous glands, causing a subsequent decrease in sebum production. In this study, timolol was associated with minimal, tolerable side effects, including dryness, burning, stinging, erythema, and mild irritation. These side effects were regressed by the use of emollients.
When comparing our results to those of previous studies of acne treatments, retinoids and benzoyl peroxide (BPO) appear to be more effective than timolol, especially in reducing inflammatory lesions; the available literature indicates that retinoids reduce the number of comedones and inflammatory lesions between 40 and 70 percent, while BPO reduces TLC between 72.7 and 75.0 percent.16,17 However, timolol appears to be better tolerated and associated with milder side effects than retinoids.
Emerging studies indicate a decrease in effectiveness of topical antibiotics for the treatment of inflammatory acne lesions, likely due to the emergence of antibiotic-resistant bacteria.16 Timolol maleate has the advantage of avoiding bacterial resistance, and the percentage of improvement in TLC with timolol is close to that currently achieved with topical antibiotics.
Among the patients with rosacea in our study clinical parameters of rosacea were improved by timolol administration; however, this improvement was not statistically significant. Cases of telangiectasia had the highest mean percentage of improvement (50%), followed by erythema (41.38%), while the mean percentage of improvement for papules and pustules was 7.41 percent and that for severity according to rosacea score was 27.99 percent. The superior therapeutic response seen among cases of telangiectasia and erythema might be explained by the inhibitory action of timolol on β-adrenergic receptors on the smooth muscles surrounding blood vessels, leading to the vasoconstriction of these vessels.
Commonly used treatments for rosacea, such as ivermectin, permethrin, topical calcineurin inhibitors, and metronidazole are all effective mainly on inflammatory papules and pustules, with unsatisfactory, poor effects on persistent erythema and telangiectasia,18,19 while timolol affects mainly telangiectasia and erythema. Thus, the combination of timolol and one of these other options might yield an even better result on all rosacea manifestations.
Separately, α-receptor agonists, such as brimonidine gel and oxymetazoline, can effectively decrease the persistent erythema of rosacea.20 However, they have not been shown to be effective in improving telangiectasia. Also, some patients treated with brimonidine gel have been shown to develop a paradoxical increase in facial erythema either during treatment or after treatment. This phenomenon might result from the stimulation of endothelial cell α-2 receptors with subsequent release of NO that invokes vasodilation.21,22
Laser and light-based therapies, such as pulsed dye laser and potassium titanyl phosphate, have shown effectiveness in reducing telangiectasia, with a limited effect on erythema, in patients with rosacea and posttreatment purpura and postinflammatory hyperpigmentation.23 Timolol, however, is less expensive than laser and light-based therapies, which is an important advantage, especially in developing countries.
Limitations. This is not a controlled study. More studies are needed to investigate the ideal period of treatments, determine the expected time until remission from the treatment, and determine the ideal maintenance schedule of this treatment to maintain improvements in acne and rosacea.
CONCLUSION
Timolol was effective in reducing noninflammatory lesions of acne and symptoms of erythematotelangiectatic rosacea. Timolol was less effective in treating papulopustular rosacea. Minimal side effects were observed. We believe timolol is a safe, effective adjunctive therapy for acne and rosacea.
REFERENCES
- Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582–585. [PubMed] [Google Scholar]
- Suh DH, Kwon HH. What’s new in the physiopathology of acne?. Br J Dermatol. 2015;172:13–19. doi: 10.1111/bjd.13634. 1. [DOI] [PubMed] [Google Scholar]
- Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):749–758. doi: 10.1016/j.jaad.2014.08.028. quiz 759–760. [DOI] [PubMed] [Google Scholar]
- Nordqvist C. “What is rosacea?”. https://www.medicalnewstoday.com/articles/160281 Available at. php. 2 Mar 2020.
- Tanghetti EA, Kawata AK, Daniels SR et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7(2):22–30. [PMC free article] [PubMed] [Google Scholar]
- Baldwin H. Psychosocial implications of rosacea. Dermatologist. 2012. pp. 2–4. Suppl.
- Krakowski AC, Nguyen TA. Inhibition of angiofibromas in a tuberous sclerosis patient using topical timolol 0.5% gel. Pediatrics. 136(2015)(3):e709–e713. doi: 10.1542/peds.2015-0025. [DOI] [PubMed] [Google Scholar]
- Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10. doi: 10.1186/1471-5945-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghallaigh SN, Powell FC. Berlin, Germany: Springer; 2015. Rosacea. In: European Handbook of Dermatological Treatments. pp. 835–843. [Google Scholar]
- Arora P, Meena N, Sharma PK, Bhardwaj M. Angiolymphoid hyperplasia with eosinophilia and its response to the combination of radiofrequency ablation and topical timolol. Indian Dermatol Online J. 2017;8(4):267–270. doi: 10.4103/idoj.IDOJ_293_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adityan B1, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75(3):323–326. doi: 10.4103/0378-6323.51258. [DOI] [PubMed] [Google Scholar]
- Wilkin J, Dahl M, Detmar M et al. Standard grading system for rosacea: report of the National Society Expert Committeeon the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Enshaieh S., Jooya A., Siadat A. H. et al. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: a randomized, double-blind placebo-controlled study. Indian J Dermatol Venereol Leprol. 2007;73(1):22–25. doi: 10.4103/0378-6323.30646. [DOI] [PubMed] [Google Scholar]
- Picardo M, Eichenfield LF, Tan J. Acne and rosacea. Dermatol Ther (Heidelb) 7(2017)(1):43–52. doi: 10.1007/s13555-016-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz M., Ruzicka T., Steinhoff M. et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14:4–15. doi: 10.1111/ddg.13139. 6. [DOI] [PubMed] [Google Scholar]
- Degitz K, Ochsendorf F. Pharmacotherapy of acne. Expert Opin Pharmacother. 2008;9(6):955–971. doi: 10.1517/14656566.9.6.955. [DOI] [PubMed] [Google Scholar]
- Haider A, Shaw JC. Treatment of acne vulgaris. JAMA. 2004;292(6):726–735. doi: 10.1001/jama.292.6.726. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20(6):623–629. doi: 10.18553/jmcp.2014.20.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa FF, El Harras MA, Gomaa SM et al. Comparative study of some treatment modalities of rosacea. J Eur Acad Dermatol Venereol. 2009;23(1):22–28. doi: 10.1111/j.1468-3083.2008.02940.x. [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ. Topical α-agonist therapy for persistent facial erythema of rosacea and the addition of oxmetazoline to the treatment armamentarium: where are we now?. J Clin Aesthet Dermatol. 2017;10(7):28–32. [PMC free article] [PubMed] [Google Scholar]
- 21- Werner K., Kobayashi T. T. Dermatitis medicamentosa: severe rebound erythema secondary to topical brimonidine in rosacea. Dermatol Online J. 2015;21(3) pii: 13030/qt93n0n7pp. [PubMed] [Google Scholar]
- Moustafa FA, Sandoval LF, Feldman SR. Rosacea: new and emerging treatments. Drugs. 2014;74(13):1457–1465. doi: 10.1007/s40265-014-0281-x. [DOI] [PubMed] [Google Scholar]
- Micali G, Gerber PA, Lacarrubba F. Schäfer G3. Improving treatment of erythematotelangiectatic rosacea with laser and/or topical therapy through enhanced discrimination of its clinical features. J Clin Aesthet Dermatol. 2016;9(7):30–39. [PMC free article] [PubMed] [Google Scholar]