Abstract
Obesity and type 2 diabetes disproportionately impact U.S. racial and ethnic minority communities and low-income populations. Improvements in implementing efficacious interventions to reduce the incidence of type 2 diabetes are underway (i.e., National Diabetes Prevention Program), but challenges in effectively scaling-up successful interventions and reaching at-risk populations remain. In October 2017, the National Institutes of Health convened a workshop to understand how to (1) address socioeconomic and other environmental conditions that perpetuate disparities in the burden of obesity and type 2 diabetes; (2) design effective prevention and treatment strategies that are accessible, feasible, culturally relevant, and acceptable to diverse population groups; and (3) achieve sustainable health improvement approaches in communities with the greatest burden of these diseases. Common features of guiding frameworks to understand and address disparities and promote health equity were described. Promising research directions were identified in numerous areas, including study design, methodology, and core metrics; program implementation and scalability; the integration of medical care and social services; strategies to enhance patient empowerment; and understanding and addressing the impact of psychosocial stress on disease onset and progression in addition to factors that support resiliency and health.
Introduction
Obesity and type 2 diabetes are national epidemics that disproportionately impact certain populations in the United States (i.e., disparity populations). Specifically, Alaska Native, American Indian, Asian American, Native Hawaiian and Pacific Islander, non-Hispanic Black,1 and Hispanic adults bear a disproportionate burden of illness related to these conditions compared to non-Hispanic Whites,1, 2 as do those with low socioeconomic status, living in rural areas, and identifying as LGBTQ.3 Large efficacy trials have demonstrated that lifestyle change and/or medication (i.e., metformin) can prevent or delay progression of prediabetes to type 2 diabetes.4
Efforts to scale-up and spread efficacious interventions are underway (e.g., National Diabetes Prevention Program),5 but our knowledge of evidence-based strategies that specifically reduce diabetes-related disparities is limited. Innovative approaches, including strategies to improve available interventions and promote their long-term, wide-spread implementation among those at greatest risk are needed. A central challenge in improving population health is translating research conducted under the best case scenarios of well-resourced randomized controlled trials into real world scenarios, which requires addressing environmental, economic, and social factors that affect individuals’ engagement in and response to these interventions.6
Workshop overview
The workshop entitled Enhancing Opportunities in Addressing Obesity and Type 2 Diabetes Disparities, was convened at the National Institutes of Health (NIH) in Bethesda, Maryland on October 24–25, 2017 to inform research opportunities for reducing disparities in these two conditions. The workshop was co-sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Cancer Institute (NCI), and the NIH Office of Disease Prevention (ODP), and organized in coordination with representatives of six NIH Institutes/Offices.2 Opening remarks by Dr. Griffin Rodgers, the NIDDK Director, and Dr. Eliseo Pérez-Stable, Director of the National Institute on Minority Health and Health Disparities (NIMHD), emphasized the importance of the workshop in identifying focal points for the next generation of high impact studies designed to reduce disparities in the burden of obesity and diabetes through elucidating the social contextual mechanisms of disease etiology, and facilitating lifestyle behavior changes, healthcare system interventions, and partnered community-based programs. Many questions remain, including how best to (1) address the socioeconomic and other environmental influences that have historically and currently affected the same minority populations and under-resourced and rural communities that bear a disproportionate burden of illness; (2) design prevention and treatment strategies to be accessible, feasible, culturally-relevant, and acceptable to at-risk communities; and (3) achieve sustainable health improvement strategies in communities that have the greatest burden of these chronic diseases.
More than 80 participants attended the workshop, including academic researchers and healthcare leaders with expertise in epidemiology, healthcare systems, primary care, behavioral interventions, public health, cultural adaptation of interventions, behavioral economics, health policy and administration, and implementation science. During the 2-day workshop, expert presentations facilitated rigorous discussion and helped identify promising research directions.
Epidemiologic overview
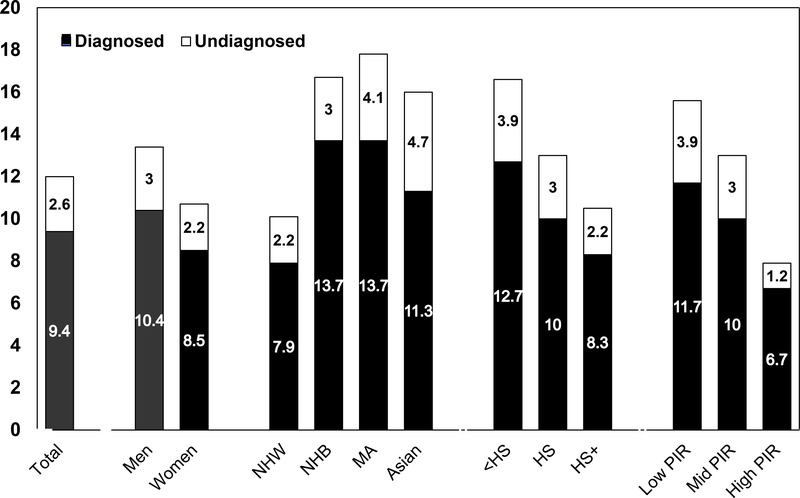
Epidemiological trends illustrate how the obesity and diabetes epidemics have grown in recent decades and the consequent adverse impact on population health. Figure 1 shows marked disparities in diabetes prevalence by race/ethnicity, education, and income.7 Prevalence of diagnosed and undiagnosed diabetes is highest in non-Hispanic Black and Mexican Adults and notably higher in all three ethnic minority groups when compared with Whites. Based on Indian Health Service data, the prevalence of diagnosed diabetes among American Indians/Alaska Natives is 15%, higher than in the other ethnic minority populations.8
Figure 1.
Prevalence of total diabetes (diagnosed and undiagnosed diabetes) in the U.S. adult population, aged ≥20 years, 2011–2016. NHW, non-Hispanic White; NHB, non-Hispanic Black; MA, Mexican American; HS, high school education; PIR, poverty income ratio. Source: Unpublished data, National Health and Nutrition Examination Survey.7
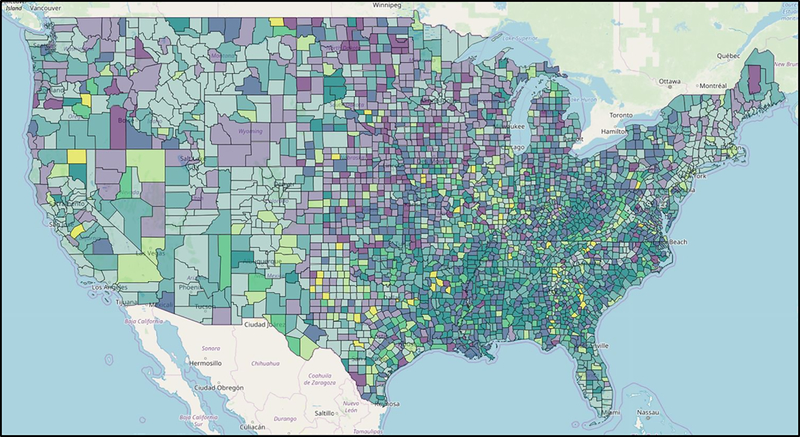
Figure 1 also shows the inverse gradients in diabetes prevalence with education and poverty. Figure 2 depicts striking geographic variations in diabetes and obesity prevalence. Evidence indicates that area-level poverty is the strongest single predictor of being a high-risk county.9 The specific factors explaining why high poverty counties are at such excess risk, and what works to reduce this risk, need to be elucidated. In under-resourced communities, the importance of neighborhood context as a constraint on access to resources and options for healthy eating and active living has been well-documented,10,11, 12 yet we lack sufficient surveillance data to adequately identify modifiable risk factors in the highest risk neighborhoods.
Figure 2.
Diagnosed Diabetes (%): Low (<9.0), Mid (9.0–13.9), High (>13.9); Obesity (%): Low (<29.1), Mid (29.1–36.0), High (>36.0). Estimates are percentages at the county-level; natural breaks were used to create categories using 2016 data.
The effects of education, income, and other indices of SES among people with or at risk for diabetes are often mediated by behavioral risk factors, including dietary patterns, levels of physical activity, and smoking.13 For example, Siegel et al.10 reported that, in a nationally-representative survey, higher education was associated with meeting diet-related diabetes prevention goals for intake of vegetables, whole grains, meats, and healthy oils. Lower SES has historically been associated with worse glycemic control among adults with type 2 diabetes, particularly younger adults.14, 15 Quality of diabetes care and preventive care practices to forestall diabetes-related complications vary according to disparities in access to care.16 For example, even among insured populations, Latinos are less likely to receive regular care and less likely to meet HbA1c targets.17, 18 Lack of access to care in non-Hispanic Blacks is associated with not meeting blood pressure targets.19
Although there have been encouraging reductions in most diabetes complications in the United States, with some improvements across all affected groups, disparities remain. They are observed most clearly in non-Hispanic Blacks, who have substantially higher rates of end-stage renal disease (ESRD), amputation, and stroke;20 and in Hispanics and Asian Americans who have elevated ESRD complications.21, 22 Within these groups, men have notably higher rates of lower extremity amputation and myocardial infarction than women. The pattern of disparities in complications according to markers of social class and education does not appear to be consistent.
There have been successes in reducing diabetes-related complications through improvements in medical technology and care, cardiovascular risk factor management and glycemic control, self-management, and policy approaches (e.g., policy changes that have decreased smoking rates or improved access to health insurance and care).23, 24 Yet, there has been little success in reducing disparities. Reducing the disparities gap in diabetes and obesity incidence and outcomes requires tackling the social and environmental influences (e.g., neighborhood poverty, access to quality care, psychosocial stressors) known to affect disease etiology and exacerbate disparities. Diverse methods for assessing the effectiveness of interventions to reduce disparities and increase knowledge regarding the pathways and mechanisms through which social disadvantage translates into increased risk of disease are also needed.
Definitions and guiding frameworks
The concepts of health equity and social determinants of health (SDoH) were central to the workshop dialogue. According to the World Health Organization, “‘Health equity’ or ‘equity in health’ implies that ideally everyone should have a fair opportunity to attain their full health potential and that no one should be disadvantaged from achieving this potential.”25 Improving health equity is a stated U.S. national priority and is inextricably linked to the goal of eliminating health disparities.26 The concept of equity involves “the absence of avoidable, unfair, or remediable differences among groups of people, whether those groups are defined socially, economically, demographically or geographically or by other means of stratification”.25 A large body of research demonstrates that such public health goals cannot be realized without addressing the underlying SDoH, which include environmental, economic, and social factors that significantly contribute to disparities and thus warrant much more attention.27
Several frameworks useful for understanding and addressing health disparities and health equity issues in obesity and diabetes prevention and care were presented. These included a novel healthcare and community systems-oriented model for assessing policy and social/environmental factors influencing health equity, informed by joint analyses of health equity issues affecting ethnic minority populations in the United States and Aotearoa/New Zealand.28 This model depicts the way government and private policies impact the healthcare system, the integration of healthcare system and social services, and the relevant SDoH, and consequently health equity (e.g., related to race/ethnicity, SES or socioeconomic deprivation)—all set within a larger context of history, culture, and values. Other notable models discussed for conceptualizing health equity issues included: the Robert Wood Johnson Foundation’s “Finding Answers” framework;29 the Getting to Equity in Obesity Prevention research and action framework;30 the Three-Axis Model of Health Inequity;31 the Consolidated Framework for Implementation Research; 32 and behavioral change models involving beliefs, knowledge, social norms, environmental factors, and self-efficacy, and intrinsic and extrinsic motivation.33, 34 The NIMHD Research Framework35 along with the Patient-Centered Outcomes Research Institute (PCORI) perspectives were also featured as valuable resources that illustrate funding agencies’ strategic priorities.
A theme that emerged from these presentations is that, despite sharing common features among health equity frameworks, there is value in having different frameworks for guidance within the policy, practice, and community contexts relevant to prevention and treatment. Some frameworks are designed to explain causes of disparities while others are designed to show where and how solutions to disparities could and should focus. Most frameworks—including those that focus primarily on healthcare delivery systems—acknowledge the importance of community contexts as key health determinants. Other common features among the frameworks were:
Prominent recognition of the fundamental roles of “race,” ethnicity, SES, gender, and geography in determining health.
Emphasis on the need to tailor conceptual frameworks according to different health domains and contextual levels.
For example, with respect to the latter, causes rooted in inequitable social structures or inadequate social protections suggest high-level policy solutions, whereas causes related to risky behaviors may point to policy-oriented and individually or family-oriented interventions and the proximal contextual factors influencing these behaviors. Causes of inequities rooted in healthcare system processes could trigger solutions involving regulatory or financing agencies, institutions involved in provider training, and system-level policy mandates addressing ongoing provider training and quality improvement. Regardless, virtually all frameworks emphasize the need for mutually reinforcing interventions at multiple levels, through socioecological models using the traditional concentric circles or other formats, to represent interrelationships among individual, community, neighborhood and/or healthcare- and policy-level influences.
Bridging interventions in healthcare settings to broader community contexts
Interventions in healthcare settings to address obesity and type 2 diabetes-related disparities involve complex considerations at the patient-, provider-, healthcare system and policy-levels. Novel implementation approaches that take account of individuals’ social contexts are necessary for full and sustained achievement of healthy lifestyle behaviors. Although a clinical perspective is considered foundational for diabetes treatment, the traditional clinical context is too narrow to accommodate broader influences on health disparities.
Perspectives and pragmatic lessons
Research agencies, such as PCORI, have shown a growing interest in simultaneously improving healthcare systems and addressing health disparities. Healthcare system interventions designed primarily for populations who face relatively few barriers to accessing care or adhering to medical recommendations may be less effective or even totally ineffective when implemented for patient populations whose access to care and barriers to participation are more challenging. Barriers to quality care and better outcomes that have been documented in the literature include: high and increasing out-of-pocket costs; time or distance factors or lack of transportation which limit access to care; absence of zoning laws and other policies that prevent exposure to adverse neighborhood conditions; unmet social needs; lack of language access, low health literacy, or cultural factors that influence communication; and implicit or explicit racial/ethnic or other biases among healthcare providers, other staff, or other healthcare system issues.36–40
The many ways that unmet social needs influence the effectiveness of treatments was a key workshop theme. As healthcare systems move toward value-based purchasing, models such as accountable care organizations and sharing of costs and savings with payors have increased incentives to address patients’ health-related social needs, or unmet basic needs, to improve patient outcomes. Several of the frameworks discussed suggest ways to remove barriers and mitigate the adverse effects of social needs on treatment effectiveness. Medical interventions that consider social context and patients’ social risk profiles to inform care or directly intervene on SDoH should also consider patient empowerment strategies. Shared-decision making (SDM) or informed care, wherein patients participate as full partners in the medical encounter and select a medical option that suits their values and priorities, was deemed critical. SDM improves outcomes such as patient satisfaction and maintaining treatment regimens; however, certain at-risk groups, such as African Americans, experience SDM less often than Whites,41 which may exacerbate health disparities. A workshop presentation exemplifies how the integration of medical and social care can improve patient outcomes (see Box 1).34, 42–54
Box 1. Promising approaches for medical and social care integration.
The Improving Diabetes Care and Outcomes on the South Side of Chicago program (known as the South Side Diabetes Project) is a multi-site, multi-targeted intervention designed to address the multiple factors that drive diabetes disparities among low-income racial minorities. The University of Chicago research team works with four federally qualified health centers (FQHCs), each of which is part of a large network of health centers, an academic internal medicine/primary care practice, and an academic endocrinology/diabetes clinic. The intervention is built on the Chronic Care Model and has four key pillars: patient education and empowerment (e.g., culturally tailored skills training in patient/provider communication and shared decision-making), healthcare provider training, quality improvement (QI)/health systems change, and community connections to local resources for diabetes self-management. 34, 42–54 The research team utilized evidence-based strategies to develop the programs, and the intervention has improved patient experience, patient skills and health behaviors, processes within health systems, and diabetes-related health outcomes, including hemoglobin HbA1c, HDL cholesterol, and foot care.43–45, 50–54
The health system and community components of the intervention integrate to provide seamless support for patients’ diabetes management. For example, physicians can write ‘prescriptions’ for healthy food (with an accompanying voucher or coupon) at a neighborhood Farmer’s Market or a participating Walgreens’ store.47 Once there, patients receive tours of the healthy food items, participate in cooking demonstrations, and are exposed to other hands-on skills training to support healthy lifestyles. Patients who completed the diabetes education classes were more likely to participate in the community-based programs the team has created (e.g., grocery store tours, community exercise programs) than other patients in the health centers. Thus, there may be a greater opportunity to promote sustained behavioral changes among diabetes patients when health system changes (including patient education) are combined with community-based support programs.49
The South Side Diabetes program has been able to meet people where they are and provide the education, skills and tools they need when they are ready for it, utilizing the infrastructure of the health system and community to support the process. The project continues to expose patients to the various clinical and community components of the intervention and evaluates long-term outcomes as guided by the project’s multi-level/multi-sectoral framework. (See Fig. 1 in Ref. 126).
Making the business case for promising interventions
A sound business case is a critical step for supporting the adoption, dissemination, and spread of promising interventions, yet this aspect of interventions is rarely addressed in research. Analyses of costs, who bears them, and who benefits from the investment are recommended to promote sustained investments by payors and avoid the discontinuation of high-value effective interventions, as observed in previous prevention efforts.55 Without an equity lens, most current payment mechanisms do not support or incentivize the provision of tailored care approaches necessary to reduce disparities. A key factor is the period in which the return on investment (ROI) is expected. Private insurers or agencies with clients that incur high costs may fear losing them before ROI occurs. For example, there is potential for loss of ROI because of relocations, job and insurance changes, or temporal gaps in coverage due to lack of affordability or strict health insurance eligibility criteria.
Addressing social determinants in community and neighborhood contexts
Workshop participants discussed research on three types of interventions to address social determinants of health in community and neighborhood contexts. Two types represent compensatory interventions that provide supports that enable individuals to fill gaps and access otherwise inaccessible or unavailable resources to overcome influences of negative SDoH.6 The third type concerns root cause27, 56 oriented interventions designed to change underlying structures/systems rather than compensate for them.
Community Health Worker (CHW) programs
Community Health Worker (CHW) interventions represent a key compensatory strategy to address gaps in healthcare system access, communication and navigation, and the integration of social and healthcare needs.6 CHWs are trained, frontline public health workers or extended healthcare team members, who often share characteristics (community, culture, and language) with their clients (individuals or families).3 They typically garner trust and provide cultural mediation among community members, healthcare systems, and social services; and deliver culturally relevant and accessible program content, informal counseling, coaching, and advocacy for clients to ensure their needs are met.57–59 Models of care vary, as CHWs may work alone or as part of delivery teams to conduct a range of activities effective for preventing and managing chronic diseases, promoting the use of primary care and follow-up care, reducing unnecessary utilization, and providing outreach and navigation to social and community services.60 CHWs and lay persons who complete training as Diabetes Prevention Program (DPP) lifestyle coaches are being tested on a national scale for effectiveness in achieving DPP-related lifestyle change and behavioral outcomes among people at increased risk for type 2 diabetes.61 Interventions engaging CHWs can improve glycemic control and weight-related outcomes among people at increased risk for type 2 diabetes, be cost effective, and thus are deemed to play an important role in reducing health disparities, improving minority health, and enhancing health equity when implemented in under-resourced communities.60
Remotely delivered Intervention formats
The increasing use of internet, mobile phones/smart phones, and social media in the highly digitized economy of the 21st century has enabled tests of remotely delivered approaches to expand reach of and access to effective prevention and treatment programs. The potential convenience and enhanced options for people with limited access, including some in racial/ethnic minority populations, low-income populations and rural populations,62 foretell substantial gains for prevention and treatment. The literature on effectiveness of remotely delivered approaches to treat obesity is promising, but the effects specific to minority populations are understudied. A systematic review of eHealth interventions for weight management shows interventions for the prevention and treatment of adult obesity have generally been effective compared to usual care or controls but with modest weight losses.63 Few studies had 50% or more participants from racial or ethnic minority groups or outcomes reported by race.63 Subsequent studies have demonstrated the acceptability and feasibility of remotely delivered obesity programs and suggest strategies to enhance recruitment of African Americans and Hispanics.64 A trial with predominantly Hispanic women participating in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) program found that an internet-based weight loss program in addition to WIC resulted in significantly greater weight loss over 12 months compared with the WIC program alone.65
Intervening on neighborhood contexts
Physical, or built, environments can profoundly shape health and health behaviors related to eating and physical activity. Relevant interventions include: making improvements to physical infrastructures (safe/walkable neighborhoods, recreational facilities, convenient transportation choices, access to healthy foods), and complementary policy and messaging strategies. Such strategies require a systems approach, with coordinated action by multiple sectors and disciplines (e.g., community stakeholders, economists, urban and regional planners, social scientists) within and outside of the biomedical-behavioral fields. Moving to Opportunity, a randomized social experiment offers convincing evidence of the impact of one’s neighborhood environment on diabetes and obesity.66 Neighborhood change was effected by affording women with children the opportunity to move from a neighborhood with high poverty rates to a neighborhood with lower poverty rates. Moving was associated with lower obesity and diabetes biomarkers and reductions in prevalence of obesity and diabetes.66
Improving community engagement and cultural relevance
Efforts to maximize effectiveness of interventions targeting obesity and diabetes in racial/ethnic minority, rural or other under-resourced communities further underscore the significance of social context and cultural relevance. Presenters discussed their experiences working with Black Americans, Hispanic Americans, Asian Americans, and American Indians and Alaska Natives (AI/AN).
Theoretical and conceptual frameworks
Two complementary cultural adaptation frameworks are widely used in this arena. Resnicow et al. differentiate between adaptations of surface or deep structure.67 Surface structure adaptions are relatively superficial, such as, matching intervention components to observable characteristics of the population group of interest, while deep structure adaptations incorporate elements of the relevant core values and key cultural practices of community members.67 Kreuter et al. describe five specific types of cultural tailoring: peripheral tailoring is similar to Resnicow’s surface structure; evidential tailoring refers to using data showing relevance of the problem; linguistic tailoring refers to using the preferred language(s); constituent tailoring involving approaches base adaptations on information obtained through direct engagement with members of the population of interest; and sociocultural tailoring—similar to deep structure—incorporates relevant core values and sociocultural perspectives and other health determinants.68 Examples from The Special Diabetes Program for Indians (SDPI) underscores the anthropological perspective on culture, that is, deep structure. Culturally influenced explanatory models of illness and how symptoms are interpreted may differ markedly from the views of health providers,69 which was an implicit or explicit theme across many workshop presentations.
Definitions of culture in practice
Counseling for obesity and diabetes-related behavior changes addresses similar variables for all populations: for example, dietary patterns and food preferences, body image, physical activity, and sedentary behavior. What is referred to as “cultural” encompasses a broad array of social and environmental contexts: the nature and level of desired and available family and social support; natural and built physical environments that affect food access and options for physical activity; economic factors; and various logistical challenges that influence whether people can achieve the intended level of intervention attendance. At any given level of motivation, these factors shape or interact with health-related knowledge, norms, values, and beliefs to influence behavior or behavior change. Thus, cultural adaptations must consider ways to help people navigate challenges they encounter in attempting to following recommendations for weight loss or diabetes self-management during and after a program. A common theme was that effective interventions must be grounded in a deep understanding of both culture and contextual variables, both how these variables interact with each other and how they affect individuals and communities. Intersections among various influences were also stressed. For example, understanding how obesity and diabetes management in Black women in the Deep South (e.g., rural Alabama and Mississippi) may be influenced not only by race and gender, but also by regional, rural, and economic factors.70, 71
For Asian Americans, the definition of obesity itself is problematic. Current guidelines and practice lead to underdiagnosis of obesity among Asian Americans by clinicians and national surveys. Obesity as assessed by body mass index (BMI) may lead Asian Americans to also underestimate their obesity-related risks. Diabetes prevalence in Asian Americans is higher than would be expected based on their average BMI levels, and is more similar to that in Black and Hispanic Americans than in Whites (Fig. 1).72
BMI is particularly inadequate for reflecting risks related to body fatness and body fat distribution in diverse Asian American populations. If risk is not recognized, there is insufficient triggering of preventive and treatment interventions. In 2015, the American Diabetes Association revised the BMI cut point for diabetes screening among Asian Americans to BMI≥ 23 kg/m2.73 Awareness and implementation of these revised guidelines among clinicians and Asian American communities have not been evaluated, but reinforces the need to adapt health messaging to the culture of Asian Americans.73, 74
For example, Filipino Americans with normal BMI have significantly more visceral adipose tissue (by computed tomography) compared to clinically obese African Americans,75 while South Asians have excess hepatic fat accumulation.76 Differences in diabetes prevalence among Asian American subgroups emphasize the importance of disaggregating Asian American subgroups; diabetes prevalence in California was highest among Pacific Islanders, Filipinos, and South Asians (from India, Pakistan, Bangladesh, Sri Lanka) compared to groups often perceived to be at highest risk for diabetes, including non-Hispanic Blacks, Hispanics and Native Americans.77 Diabetes risk was 50% higher among Southeast Asians, Japanese, Vietnamese, Koreans, and Chinese Americans compared with White populations, with onset of diabetes occurring at lower BMI levels.77 Understanding the unique pathophysiology of type 2 diabetes, including regional fat distribution, in specific Asian American communities, is urgently needed to inform effective interventions in this rapidly growing population. Raising awareness of body composition and metabolic profiles in subgroups of Asian Americans is also needed to improve interventions aimed at reducing diabetes within these communities.
Success stories and promising approaches
While culturally and contextually adapted interventions constitute a relatively small portion of the evidence base regarding obesity and diabetes interventions, all presenters provided evidence of successful approaches. For example, the potential value and practicality of using individually tailored, small changes approaches to prevent excess weight gain in Black women was noted.78 Among Hispanic women, preintervention educational approaches that provide basic information on diabetes, food measurement or nutrition facilitate intervention uptake and improve success in behavioral weight loss programs.79
Because of its scope and special features, the SDPI was highlighted as an exemplar of cultural and contextual adaptations. The successes of this program attest to the value of: having a year-long process for strategic planning and increasing community readiness; building upon cultural strengths and traditions; incorporating family in the intervention process; emphasizing collective as well as individual support; and ongoing reinforcement of core principles for success among families, providers, and in the community at large.80, 81
Psychosocial and socioecological stress as an emerging theme
Increasingly, epidemiological research investigating factors associated with risk of chronic diseases such as diabetes and obesity is focusing on understanding the underlying biological pathways or mechanisms through which social disadvantage “gets under the skin” to increase risk of disease, thus potentially identifying new leverage points for intervention. The importance of identifying biomarkers to elucidate mechanisms through which stressors increase risk of disease was also presented. Psychosocial stress, which includes diverse stressors at the individual and community levels (e.g., physical and sexual abuse, neighborhood-level poverty, work stress, discrimination), has been shown to increase individuals’ risk of many chronic diseases. For example, self-reported experiences of discrimination has been associated with increased visceral fat in women,82 and increased risk of type 2 diabetes independent of obesity or behavioral and psychosocial factors.83 Stress at work and home, financial stress, depression, and perceived ability to control life circumstances have been associated with increased risk of acute myocardial infarction.84 Because certain psychosocial stressors (e.g., exposure to violence, social position, trauma) are disproportionately experienced by poor and minority communities in the United States,85 investigating the underlying mechanisms through which such stressors operate to increase risk of disease is a critical piece of the puzzle in eliminating disparities in the burden of illness.86
Dysregulation of the stress pathway is one way in which adverse psychosocial exposures becomes embodied. Human experiments show that both emotional and physical stressors trigger the central stress response and neuroendocrine systems, which can result in a cascade of hormonal changes linked to increased risk of obesity, metabolic syndrome, and poor glycemic control.87 While animal models have illuminated some of the key mechanisms at play, it is impossible to use these to model the diverse stressors faced by humans. Key variables for future studies include inherited and acquired personal characteristics (e.g., physiology/genetic, personality type, past trauma, perceptions of the stress) and characteristics of the stressor (e.g., severity, duration, frequency). DNA methylation provides a valuable platform for investigating the impact of various psychosocial stressors on risk of disease. Altered DNA methylation in hypothalamic–pituitary–adrenal axis (i.e., stress related) genes has been associated with increased risk of hypertension, certain cancers, and post-traumatic stress disorders.88 Telomere length is another valuable biomarker for exploring the role of psychosocial stress in disease. Telomeres contribute to cell senescence and longevity, and measures of psychosocial stress have been associated with accelerated telomere shortening.89
Research is also beginning to focus on positive psychosocial factors thought to support resiliency and health. Mind-body stress-reduction interventions such as the Relaxation Response Resiliency (3RP) Program or meditation, for example, have been shown to enhance expression of genes associated with favorable energy metabolism, insulin secretion, and telomere maintenance;90, 91 and suggest a positive benefit for cardiovascular health and reducing blood glucose levels.92,93 Religion and spirituality have emerged as potentially important sources of resiliency for minority and low-income communities,94 and may be particularly important for African American and Hispanic/Latino communities who report higher levels of religious and spiritual beliefs and practices than White and Asian American populations.95 In a recent national study of African American women in the United States; for example, those who used religion or spirituality to cope with stress were significantly less likely to develop hypertension, and this protective effect was greater among those with the highest levels of perceived stress.96 The same impact of religious coping on risk of hypertension was not found in a national sample of White women.97 Social support has also been shown to buffer the adverse effects of stress on one’s health, with evidence from randomized controlled trials and experimental studies showing that various facets of social support improve diabetes control (HbA1c) and diabetes-related physical activity, weight loss, and quality of life.98–100
Evidence in the areas of socioecological and psychosocial stress is long-standing and provides emerging opportunities to improve obesity and diabetes prevention and treatment. Future challenges include modeling the complexity of these interactions as well as determining any differences in patterns of stress across and within different racial/ethnic and socioeconomic groups. Equally important are studies investigating positive resources for resiliency, which may help identify and prioritize additional areas for intervention. Ultimately, there is a need to identify and better understand effective strategies to minimize the adverse effects of psychosocial stress on diabetes and obesity outcomes and remove, where possible, the adverse stressors that disproportionately impact minority and other underserved populations.
Frameworks for understanding mental health and diabetes distress
Mental health conditions such as depression and diabetes-related distress are known risk factors for obesity and type 2 diabetes.101 Depression can induce neuroendocrine reactivity and metabolic consequences resulting in obesity and type 2 diabetes.87, 102 Evidence from epidemiological studies show that depression is both a risk factor for diabetes as well as a comorbid condition of diabetes. Depression is up to twice as common among individuals with diabetes compared to those without the condition;103, 104 and has consistently been associated with higher risk of diabetes complications,105 poorer quality of life,106 and increased risk of mortality.107, 108 Pharmacological treatments, such as antidepressant use, are associated with the risk of incident type 2 diabetes among adults102, 109, 110 and youth;111 and the use of antipsychotic drugs is associated with high fasting blood glucose and diabetes-related complications.109 Additionally, cultural misunderstandings and clinician bias have resulted in prescribing more and higher doses of antipsychotic medications to African Americans compared with Whites, possibly without awareness of the potential higher sensitivity of some African Americans to certain psychotropic medication, causing more severe side effects (e.g., delirium).112 Clinicians are cautioned to avoid under or overtreatment for mental health conditions by examining patient-specific drug sensitivities and by taking cultural factors into account. Given the potential for adverse side effects of drugs used to treat mental illness and evidence that culture and ethnic factors influence provider bias,112 studies of appropriate prescribing and diagnostic accuracy are urgent research needs.
Diabetes-related distress, another psychosocial condition, refers to unique often hidden “emotional distress in diabetes that emphasizes the demanding experience of diabetes and requires diabetes-specific measurement and treatment approaches” (p. 236) 113 and “the spectrum of patient experience when managing a severe, demanding chronic disease like diabetes” (p. 259).114 Diabetes distress is considered common and persistent over time,114 with higher rates among ethnic diverse patients than non-Hispanic Whites.115 Diabetes-related distress is associated with diabetes self-care and elevated HbA1c, which in turn increases the risk for the development of diabetes complications,116, 117 but this consideration may not be reflected in current healthcare practice.
Research translation: challenges and opportunities
A cross-cutting discussion focused on the central challenges of moving from efficacy studies (i.e., the best-case scenarios that provide convincing evidence of what can work) to demonstrating effectiveness in terms of what works in diverse and particularly under-resourced communities. A concern—and frustration—related to repeated observations that research findings from efficacy studies are not reaching populations at large, especially higher risk populations, in ways that fulfill the promise inherent in this research118 was frequently voiced by workshop participants. The relevance of models typically used in efficacy studies to effectiveness research in real-world settings was questioned based on differences in both participant and intervention characteristics. Workshop participants’ views on this problem echoed several themes from the discussion about community engagement and cultural relevance as well as other sessions, framing the issues as contrasts between efficacy and effectiveness research. Overall, workshop participants emphasized that better translation science and efforts are needed, and this reflects a broader concern in the field.118
Population characteristics and circumstances
Even when participants from high-risk populations are included in efficacy studies, the screening and selection into these studies achieve a certain level of homogeneity on variables related to the ability to participate. Because unbiased interpretation of efficacy trials requires achievement of the intended intervention dose and high participant retention, extraordinary measures may be taken to mitigate circumstances that constrain the necessary level and quality of participation. By contract, recognizing and allowing for heterogeneity on variables such as cultural perspectives, attitudes and behaviors, and socioeconomic circumstances, neighborhoods, built environments and resources (transportation, etc.) among these population groups become critical in community-based research if the research findings are to be meaningful in practice. Factors related to healthcare access, delivery patterns and out-of-pocket costs must be considered inasmuch as they determine the context for adoption and maintenance of health behavior changes. The rise of high deductible health plans and limited benefits have decreased the affordability of healthcare especially for employed persons with limited incomes.119 For people who are not U.S. citizens, immigration-related factors related to employment or fear of deportation may be an important overlay influencing program participation or use of healthcare.120 These factors, if not recognized or understood, can lead to inappropriate assumptions, e.g., that low motivation, rather than practical issues or preferences, is the main reason for lower participation rates or suboptimal behavioral outcomes.
Intervention characteristics
The time and logistical demands of attending a series of classes or counseling sessions can be prohibitive, particularly given competing demands on time or other practical constraints as noted above. Possible ways to address this include data collection to better understand these constraints, combined with testing more flexible ways of delivering interventions. A distinction was made between achieving flexibility versus reducing participant burden by limiting the dose (e.g., minimal models for lifestyle intervention). The content or frequency needed to achieve the optimal effect of interventions is not always clear. One approach that might increase the feasibility and sustainability of interventions would be linking them to ongoing, community services (e.g., linking to commercial weight loss programs which are more consumer or client oriented, and sustainable, than researcher-designed approaches). One example is the aforementioned remote intervention linked to WIC 65 that embeds interventions in federal or state-funded programs which reach low-income populations to expand dissemination efforts across diverse populations in settings that are integral to people’s daily lives. Box 2 highlights an example of linking nutrition and physical activity counseling to services provided by a national parent support organization.121–123 Such approaches may allow for the needed dose of interventions to be achieved over a longer period, or intermittently, compared to the typical approach of providing a high, front-end dose within a concentrated period.
Box 2. Promising approaches for delivering and scaling-up obesity prevention programs: translations in underserved communities nationwide.
Two studies conducted in St. Louis Missouri, which embedded weight loss counseling based on the principles of the Diabetes Prevention Program (DPP) within a national home visiting program, showed substantial promise for obesity prevention with widespread reach. The home visiting program, Parents as Teachers National Center, Inc. (hereafter referred to as parents as teachers or PAT), trains and coordinates the services of parent-educators who promote early development, learning, and school readiness through ongoing support to families with children from prenatal through kindergarten (https://parentsasteachers.org/). Families can receive up to 25 home visits annually, depending on need. Importantly, PAT uses a resource network to provide comprehensive services to families and children (e.g., unmet basic needs such as housing, food) to ensure optimal early development, health and children’s school readiness and success. PAT is located in all 50 states and reaches over 225,000 children annually.
The Healthy Eating and Active Living Taught at Home (HEALTH) Study was designed as a 2-year randomized study to assess the impact of a DPP-derived lifestyle weight loss intervention embedded within the PAT curriculum.121 PAT + HEALTH was compared with PAT only (usual care) in a cohort of 179 ethnically and socioeconomically diverse women with overweight or obesity (BMI ≥25) and a pre-school child at home. Women in PAT + HEALTH were more likely than those in usual care to achieve 5% weight loss at 24 months (11% vs 26%, p=0.01), with a 4.7-kg weight difference (p=0.002). Notably, the weight difference between groups was attributed to the intervention group’s maintenance of a modest loss of weight versus the control group continuing to gain weight, indicating the value of this strategy for reversing obesity trends by preventing weight gain overtime.
Similarly, the LifeMoms–Washington University Program compared the PAT curriculum to the PAT + Lifestyle intervention, conducted with pregnant and post-partum African American women with overweight or obesity, living with significant socioeconomic disadvantage (e.g., Medicaid recipients or living in low-income neighborhoods, 90 percent reporting household incomes of less than $25,000 annually, and approximately half being single parents). By 12 months postpartum, the PAT + Lifestyle group had gained less weight (2.5 kg vs. 5.7 kg; P = 0.01) and were more likely to return to their baseline weight (38.0% vs. 21.5%; P = 0.01) than those receiving the PAT curriculum.122,123
The scalability of these embedded lifestyle interventions offers the potential to partner with existing national programs like PAT and leverage infrastructure to reach underserved mothers who have extensive barriers to care for widespread intervention dissemination, reach, and impact.
Additionally, greater use of telephone or digital technology to deliver or tailor interventions 64 was discussed as having a high potential because many high-risk populations are heavy users of web- or cell-technologies.124 However, limitations on broadband access were noted as a potential issue to be resolved for rural populations.125
Conclusion
Promising research directions to address obesity and type 2 diabetes disparities consider at the person-, community/neighborhood-, and system-levels, and are guided by frameworks to promote health equity (see Table 1). Translating lifestyle interventions for diverse communities requires research to demonstrate the effectiveness of interventions that are affordable, accessible, convenient, and sensitive to socioecological contexts, and offer equitable access to these interventions. The adoption of health equity approaches in intervention design (e.g., engagement and recruitment, implementation strategies) are needed. The ultimate goal of investments in this research would be to promote individuals’ engagement in evidenced-based interventions and help population groups reduce exposure to or overcome the effects of practical and stress-related challenges in their physical, sociocultural, and economic environments.
Table 1:
Promising research directions for obesity and type 2 diabetes
| Study Designs | • Novel designs/methods for evaluating
multi-level interventions including mixed methods
research • Study designs other than the traditional RCTs to allow for flexible recruitment approaches and account for dynamic, pragmatic issues • Natural experiments to assess the impacts of population-level health programs and policies • Life course approaches to understand the interactions of various determinants of health and influences on disease onset and progression across the lifespan • Study designs to understand where or how human behavior may overcome the influence of environmental barriers (i.e., resilience factors) on health • Cohort studies (existing and new) and new analytic methods to better understand mechanisms driving obesity and type 2 diabetes (T2D) disparities • Engagement of diverse stakeholders (patients, medical staff, community, healthcare systems) in the full spectrum of science to promote appropriate research questions, approaches, interpretation and dissemination of findings • Prospective studies and simulation modeling techniques to increase understanding of comprehensive environmental change to reduce community-level risk of obesity and T2D • Approaches to systematically address the pervasive nature of culture in the experience of illness, in context, and demonstrating the value added by cultural and contextual adaptations from theoretical and programmatic perspectives |
| Metrics and Methods | • Core metrics of health equity and
aspects of built environments and culture that may be key drivers of
outcomes in obesity and T2D • Criteria for establishing novel partnership models in research and assessing commitment to sustainability of successful interventions beyond grant funding • Standard methods to examine the effects of socioecological stress on stress reactivity in various contexts (laboratory experimental, naturalistic settings) • Measures for business case analyses (e.g., alignment of financial incentives and supports for ongoing organizational investments) |
| Behavioral, Metabolic, and Environmental Phenotyping | • Deep environmental and behavioral
phenotypes (environment and social factors, epigenetics, metabolic
correlates) to characterize high-risk populations and develop effective
interventions • Multi-level population datasets and systems to characterize elements of society (housing, education, food resources, activity space, stress levels, etc.) that affect health equity • Characterization of specific elements of ‘neighborhood deprivation’ that influence diabetes prevention, treatment, and control • Characterization of the unique pathophysiology of T2D among Asian Americans to facilitate the design of adequately powered studies to evaluate weight control approaches designed to address this pathophysiology • Phenotypes of specific characteristics within the historically highest risk sub-groups and the socioecological contexts related to poorer outcomes (e.g., Which African American men with T2D are at increased risk for amputations?) |
| Considerations for Stress and Resilience Research | • Strategies for preventing the over-
and under-treatment of serious mental health conditions that address
realities and perceptions about drug sensitivities, physician bias, and
cultural preferences • Interactive roles of medications on stress-related disorders (e.g., depression) and diabetes • Culturally relevant sources of resilience and coping, and non-traditional interventions (e.g., mind-body interventions) to improve obesity and T2D outcomes in populations at high risk of various exposures to stress • Evaluation of intervention models to address DNA methylation to reduce risk for obesity and diabetes • Genetic-based research with sufficient racial/ethnic minority representation to study diverse characteristics (e.g., histories) and diverse settings to understand gene expression, stress, and chronic conditions |
| Innovative Partnership Models | • Biomedical research that is expanded
to include the multi-sectoral and multidisciplinary nature (e.g.,
economists, architects, urban/regional planners) of built environment
and health research • Novel partnerships to pursue research questions and designs relevant for diverse groups and health equity • Strategies for testing and supporting sustainable partnerships and collaboration practices |
| Intervention Approach and Delivery | • Multi-sectoral (e.g., academic
institutions, government, community organization, public health
entities) partnerships and designs to test the effectiveness of
intervening on or compensating for the influence of systemic barriers
(SDoH) on health • Linkages of neighborhood-level characteristics, health systems, and outcomes data • Cohort studies with design and planning of frequent, rapid interventions (e.g., cohorts as platform for interventions) to allow for adaptive trials • Different combinations of intervention delivery in various contexts to include use of CHWs and peer support, healthcare and social service team members, e-Health, and community support and mobilization • Remotely delivered interventions with sufficient racial/ethnic minority representation to allow for reporting research findings by sub-populations • Study designs that allow exploration of how intersections of various identities (race/ethnicity, gender, income, place, unemployment, low educational attainment, difficulty with access to care, etc.) influence intervention effectiveness • Effects of routine screening of SDoH in the medical encounter to inform care and/or facilitate referrals to address unmet health-related social or basic needs, and testing of different models to address these • Longitudinal studies to capture impacts of scaling-up efficacious obesity interventions through national and state programs to address unmet health-related social needs • Effective use of technology for interventions in rural areas with incomplete access to broadband • Patient-centered (i.e., patient/relative/friend/caregiver) communication such as informed decision-making processes to promote patients’ ability to participate equally in medical decisions and effects on outcomes (QoL, medical choice, maintaining medical regimen/patient’s medical choice; clinician-patient communication) in high-risk populations • Intervention approaches to improve treatment effect for non-responders and/or those who are less likely to engage fully in treatment |
| Implementation, Scalability, and Sustainability | • Understanding of the “active
ingredients” of obesity prevention and treatment programs that
can be delivered efficiently and disseminated broadly at relatively low
cost • Approaches to sustain positive program outcomes after grant funding, including strategies for scaling-up programs whose initial success may be tied to non-reproducible features (charismatic leadership, etc.) and adapt them for diverse population groups with the community • Ways to support sustainable reimbursement models for CHWs and peer support in clinical and community contexts • Implementation issues that prevent adoption, achieved dose, and sustainability of effective programs • Broad dissemination of the “Screen at 23” campaign in Asian American subgroups; and systematic approaches to estimate risk of overweight and obesity in specific Asian subgroups |
Intervening on the SDoH can improve health inequity by removing systemic barriers, thereby addressing root causes of obesity and diabetes-related disparities, and helping individuals overcome contextual challenges related to prevention and self-care. Novel research approaches could account for community realities and resources and treat neighborhoods as focal points for intervening on the compelling geographic variations in health. Implementation efforts can also leverage national and state-wide programs to expand reach of evidence-based interventions to diverse communities and intergenerational households. Importantly, high-impact research opportunities that leverage health equity approaches may identify ways to interrupt the intergenerational consequences of obesity and diabetes; and more effectively treat individual, families and communities that are currently affected to support reaching their highest health potential.
Acknowledgements
Additional substantial contributions to the development of this manuscript were made by Dr. Joseph Selby who provided a scientific presentation and contributed to rigorous participant deliberations at the workshop that informed Table 1. M. Austin Argentieri affiliated with the Harvard/MGH Center on Genomics, Vulnerable Populations, and Health Disparities assisted with coediting the psychosocial/socioecological stress section and technical editing. The authors wish to acknowledge the contributions of the NIDDK for initiating and co-funding the workshop effort, the trans-NIH workshop organizing committee for substantial contributions to the workshop program; and the National Cancer Institute Division of Cancer Control and Population Sciences, and the NIH Office of Disease Prevention for cosponsoring the workshop with travel support for presenters. The authors also acknowledge and appreciate contributions of Dr. Griffin Rodgers, director of the NIDDK, and Dr. Eliseo Pérez-Stable, director of the NIMHD, for their presentations to set the tone of the workshop deliberations.
The workshop was co-funded/co-sponsored by the NIDDK, NCI, and ODP.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, or any other institution mentioned in the manuscript.
Competing interests
M.H.C. is a consultant to the Patient-Centered Outcomes Research Institute and a member of the National Advisory Council to the National Institute on Minority Health and Health Disparities. M.D.G. is a faculty consultant to the Lifescan Diabetes Institute and Eli Lilly, Co. C.M.M. is a member of the U.S. Preventive Services Task Force. This article does not necessarily represent the views and policies of the U.S. Preventive Services Task Force. D.F.T. is a member of the WW Scientific Advisory Board.
Also referred to in this manuscript as African American or non-Hispanic Black based on the data source.
National Institute of Diabetes and Digestive and Kidney Diseases; the National Cancer Institute; the NIH Office of Disease Prevention; National Heart, Lung, and Blood Institute; National Institute on Minority Health and Health Disparities; and NIH Office of Behavioral and Social Sciences Research.
Community Health Workers are also known by a variety of names, including community health aide, promotora/promotores de salud, and patient navigator.
References
- 1.Bullard KM, Cowie CC, Lessem SE, et al. 2018. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep. 67: 359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Fakhouri TH, Carroll MD, et al. 2017. Prevalence of Obesity Among Adults, by Household Income and Education - United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 66: 1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dilley JA, Simmons KW, Boysun MJ, et al. 2010. Demonstrating the importance and feasibility of including sexual orientation in public health surveys: health disparities in the Pacific Northwest. Am J Public Health. 100: 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. 2018. National Diabetes Prevention Program; Accessed January 10, 2018 https://www.cdc.gov/diabetes/prevention/index.html. [Google Scholar]
- 6.Haire-Joshu D & Hill-Briggs F 2019. The Next Generation of Diabetes Translation: A Path to Health Equity. Annu Rev Public Health. [DOI] [PubMed] [Google Scholar]
- 7.Gregg E 2019. ‘Prevalence of Total Diabetes (Diagnosed Diabetes and Undiagnosed Diabetes) in the U.S. Adult Population, Age > 20, 2011–2016 (PIR=Poverty Income Ratio)’ unpublished data, National Health and Nutrition Examination Survey. [Google Scholar]
- 8.Centers for Disease Control and Prevention. (Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.). National Diabetes Statistics Report, 2017. Atlanta, GA. [Google Scholar]
- 9.Cunningham SA, Patel SA, Beckles GL, et al. 2018. County-level contextual factors associated with diabetes incidence in the United States. Ann Epidemiol. 28: 20–25 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel KR, Bullard KM, Imperatore G, et al. 2018. Prevalence of Major Behavioral Risk Factors for Type 2 Diabetes. Diabetes Care. 41: 1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovasi GS, Hutson MA, Guerra M, et al. 2009. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 31: 7–20. [DOI] [PubMed] [Google Scholar]
- 12.Nelson MC, Larson NI, Barr-Anderson D, et al. 2009. Disparities in dietary intake, meal patterning, and home food environments among young adult nonstudents and 2- and 4-year college students. Am J Public Health. 99: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrovic D, de Mestral C, Bochud M, et al. 2018. The contribution of health behaviors to socioeconomic inequalities in health: A systematic review. Prev Med. 113: 15–31. [DOI] [PubMed] [Google Scholar]
- 14.Bijlsma-Rutte A, Rutters F, Elders PJM, et al. 2018. Socio-economic status and HbA1c in type 2 diabetes: A systematic review and meta-analysis. Diabetes/metabolism research and reviews. 34: e3008. [DOI] [PubMed] [Google Scholar]
- 15.Ali MK, McKeever Bullard K, Imperatore G, et al. 2012. Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes--National Health and Nutrition Examination Survey, United States, 2007–2010. MMWR Suppl. 61: 32–37. [PubMed] [Google Scholar]
- 16.Mayberry LS, Bergner EM, Chakkalakal RJ, et al. 2016. Self-Care Disparities Among Adults with Type 2 Diabetes in the USA. Curr Diab Rep. 16: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown AF, Gerzoff RB, Karter AJ, et al. 2003. Health behaviors and quality of care among Latinos with diabetes in managed care. Am J Public Health. 93: 1694–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosen DM, Feldstein A & Borin A 2015. Disparities in Glycemic Control Among Hispanic Adults with Diabetes Journal of Patient-Centered Research and Reviews. 2: 114–. [Google Scholar]
- 19.Muntner P, Abdalla M, Correa A, et al. 2017. Hypertension in Blacks: Unanswered Questions and Future Directions for the JHS (Jackson Heart Study). Hypertension. 69: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregg EW, Li Y, Wang J, et al. 2014. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 370: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 21.Desai N, Lora CM, Lash JP, et al. 2019. CKD and ESRD in US Hispanics. Am J Kidney Dis. 73: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Ley SH & Hu FB 2018. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 14: 88–98. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Ajani UA, Croft JB, et al. 2007. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 356: 2388–2398. [DOI] [PubMed] [Google Scholar]
- 24.Ali MK, Bullard KM & Gregg EW 2013. Achievement of goals in U.S. Diabetes Care, 1999–2010. N Engl J Med. 369: 287–288. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2019. Health Equity. Accessed May 27, 2019 https://www.who.int/topics/health_equity/en/.
- 26.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. 2019, March 20 Healthy People 2020; https://www.healthypeople.gov/. [Google Scholar]
- 27.Marmot M 2005. Social determinants of health inequalities. Lancet. 365: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 28.Chin MH, King PT, Jones RG, et al. 2018. Lessons for achieving health equity comparing Aotearoa/New Zealand and the United States. Health Policy. 122: 837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin MH, Clarke AR, Nocon RS, et al. 2012. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 27: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumanyika S 2017. Getting to Equity in Obesity Prevention: A New Framework. 2017, January 18 Accessed 2018, January 3, 2018 https://nam.edu/getting-to-equity-in-obesity-prevention-a-new-framework/.
- 31.Tung EL, Cagney KA, Peek ME, et al. 2017. Spatial Context and Health Inequity: Reconfiguring Race, Place, and Poverty. J Urban Health. 94: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damschroder LJ, Aron DC, Keith RE, et al. 2009. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao AC 2015. Driven to Care: Aligning External Motivators with Intrinsic Motivation. Health Serv Res. 50 Suppl 2: 2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peek ME, Ferguson MJ, Roberson TP, et al. 2014. Putting theory into practice: a case study of diabetes-related behavioral change interventions on Chicago’s South Side. Health Promot Pract. 15: 40S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services, N.I.o.H., National Institute on Minority Health and Health Disparities,. 2018. NIMHD Minority Health and Health Disparities Research Framework; Accessed January 3, 2018 https://www.nimhd.nih.gov/about/overview/research-framework/. [Google Scholar]
- 36.Alley DE, Asomugha CN, Conway PH, et al. 2016. Accountable Health Communities--Addressing Social Needs through Medicare and Medicaid. N Engl J Med. 374: 8–11. [DOI] [PubMed] [Google Scholar]
- 37.Cooper LA, Roter DL, Carson KA, et al. 2012. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 102: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben J, Cormack D, Harris R, et al. 2017. Racism and health service utilisation: A systematic review and meta-analysis. PLoS One. 12: e0189900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berwick DM, Nolan TW & Whittington J 2008. The triple aim: care, health, and cost. Health Aff (Millwood). 27: 759–769. [DOI] [PubMed] [Google Scholar]
- 40.Chen AH, Youdelman MK & Brooks J 2007. The legal framework for language access in healthcare settings: Title VI and beyond. J Gen Intern Med. 22 Suppl 2: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peek ME, Odoms-Young A, Quinn MT, et al. 2010. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 71: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkes AE, Bordenave K, Vinci L, et al. 2011. Addressing diabetes racial and ethnic disparities: lessons learned from quality improvement collaboratives. Diabetes Manag (Lond). 1: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peek ME, Wilkes AE, Roberson TS, et al. 2012. Early lessons from an initiative on Chicago’s South Side to reduce disparities in diabetes care and outcomes. Health Aff (Millwood). 31: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peek ME, Harmon SA, Scott SJ, et al. 2012. Culturally tailoring patient education and communication skills training to empower African-Americans with diabetes. Transl Behav Med. 2: 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nundy S, Dick JJ, Solomon MC, et al. 2013. Developing a behavioral model for mobile phone-based diabetes interventions. Patient Educ Couns. 90: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nundy S, Lu CY, Hogan P, et al. 2014. Using Patient-Generated Health Data From Mobile Technologies for Diabetes Self-Management Support: Provider Perspectives From an Academic Medical Center. J Diabetes Sci Technol. 8: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goddu AP, Roberson TS, Raffel KE, et al. 2015. Food Rx: a community-university partnership to prescribe healthy eating on the South Side of Chicago. J Prev Interv Community. 43: 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nundy S, Dick JJ, Goddu AP, et al. 2012. Using mobile health to support the chronic care model: developing an institutional initiative. Int J Telemed Appl. 2012: 871925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peek ME, Ferguson M, Bergeron N, et al. 2014. Integrated community-healthcare diabetes interventions to reduce disparities. Curr Diab Rep. 14: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin MH, Goddu AP, Ferguson MJ, et al. 2014. Expanding and sustaining integrated health care-community efforts to reduce diabetes disparities. Health Promot Pract. 15: 29S–39S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goddu AP, Raffel KE & Peek ME 2015. A story of change: The influence of narrative on African-Americans with diabetes. Patient Educ Couns. 98: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nundy S, Mishra A, Hogan P, et al. 2014. How do mobile phone diabetes programs drive behavior change? Evidence from a mixed methods observational cohort study. Diabetes Educ. 40: 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nundy S, Dick JJ, Chou CH, et al. 2014. Mobile phone diabetes project led to improved glycemic control and net savings for Chicago plan participants. Health Aff (Millwood). 33: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chin MH & Goldmann D 2011. Meaningful disparities reduction through research and translation programs. JAMA. 305: 404–405. [DOI] [PubMed] [Google Scholar]
- 55.Leatherman S, Berwick D, Iles D, et al. 2003. The business case for quality: case studies and an analysis. Health Aff (Millwood). 22: 17–30. [DOI] [PubMed] [Google Scholar]
- 56.Marmot M & H. Commission on Social Determinants of. 2007. Achieving health equity: from root causes to fair outcomes. Lancet. 370: 1153–1163. [DOI] [PubMed] [Google Scholar]
- 57.Kim K, Choi JS, Choi E, et al. 2016. Effects of Community-Based Health Worker Interventions to Improve Chronic Disease Management and Care Among Vulnerable Populations: A Systematic Review. Am J Public Health. 106: e3–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gary TL, Batts-Turner M, Bone LR, et al. 2004. A randomized controlled trial of the effects of nurse case manager and community health worker team interventions in urban African-Americans with type 2 diabetes. Control Clin Trials. 25: 53–66. [DOI] [PubMed] [Google Scholar]
- 59.Gary TL, Batts-Turner M, Yeh HC, et al. 2009. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 169: 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Community Preventive Services Task Force. 2017. Diabetes Prevention: Interventions Engaging Community Health Workers: A Systematic Review; Accessed January 10, 2018 https://www.thecommunityguide.org/findings/diabetes-prevention-interventions-engaging-community-health-workers. [Google Scholar]
- 61.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. 2018, March 1. Centers for Disease Control and Prevention Diabetes Prevention Recognition Program: Standards and Operating Procedures; Accessed January 20, 2019, 2018. https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf. [Google Scholar]
- 62.Cho YM, Lee S, Islam SMS, et al. 2018. Theories Applied to m-Health Interventions for Behavior Change in Low- and Middle-Income Countries: A Systematic Review. Telemed J E Health. 24: 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennett GG, Steinberg DM, Stoute C, et al. 2014. Electronic health (eHealth) interventions for weight management among racial/ethnic minority adults: a systematic review. Obes Rev. 15 Suppl 4: 146–158. [DOI] [PubMed] [Google Scholar]
- 64.Tate DF, Valle CG, Crane MM, et al. 2017. Randomized trial comparing group size of periodic in-person sessions in a remotely delivered weight loss intervention. Int J Behav Nutr Phys Act. 14: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phelan S, Hagobian T, Brannen A, et al. 2017. Effect of an Internet-Based Program on Weight Loss for Low-Income Postpartum Women: A Randomized Clinical Trial. JAMA. 317: 2381–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ludwig J, Sanbonmatsu L, Gennetian L, et al. 2011. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med. 365: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Resnicow K, Baranowski T, Ahluwalia JS, et al. 1999. Cultural sensitivity in public health: defined and demystified. Ethn Dis. 9: 10–21. [PubMed] [Google Scholar]
- 68.Kreuter MW, Lukwago SN, Bucholtz RD, et al. 2003. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav. 30: 133–146. [DOI] [PubMed] [Google Scholar]
- 69.Allan D & Cornes D 1998. The impact of management of change projects on practice: a description of the contribution that one educational programme made to the quality of health care. J Adv Nurs. 27: 865–869. [DOI] [PubMed] [Google Scholar]
- 70.Shikany JM, Carson TL, Hardy CM, et al. 2018. Assessment of the nutrition environment in rural counties in the Deep South. J Nutr Sci. 7: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ard JD, Carson TL, Shikany JM, et al. 2017. Weight loss and improved metabolic outcomes amongst rural African American women in the Deep South: six-month outcomes from a community-based randomized trial. J Intern Med. 282: 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menke A, Casagrande S, Geiss L, et al. 2015. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 314: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 73.American Diabetes A 2015. (2) Classification and diagnosis of diabetes. Diabetes Care. 38 Suppl: S8–S16. [DOI] [PubMed] [Google Scholar]
- 74.Hsu WC, Araneta MR, Kanaya AM, et al. 2015. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 38: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Araneta MR & Barrett-Connor E 2005. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 13: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 76.Shah AD, Kandula NR, Lin F, et al. 2016. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes (Lond). 40: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karter AJ, Schillinger D, Adams AS, et al. 2013. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care. 36: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phillips EG, Wells MT, Winston G, et al. 2017. Innovative approaches to weight loss in a high-risk population: The small changes and lasting effects (SCALE) trial. Obesity (Silver Spring). 25: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lindberg NM, Stevens VJ, Vega-Lopez S, et al. 2012. A weight-loss intervention program designed for Mexican-American women: cultural adaptations and results. J Immigr Minor Health. 14: 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang L, Manson SM, Beals J, et al. 2013. Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care. 36: 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang L, Johnson A, Pratte K, et al. 2018. Long-term Outcomes of Lifestyle Intervention to Prevent Diabetes in American Indian and Alaska Native Communities: The Special Diabetes Program for Indians Diabetes Prevention Program. Diabetes Care. 41: 1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis TT, Kravitz HM, Janssen I, et al. 2011. Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. Am J Epidemiol. 173: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitaker KM, Everson-Rose SA, Pankow JS, et al. 2017. Experiences of Discrimination and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol. 186: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosengren A, Hawken S, Ounpuu S, et al. 2004. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 364: 953–962. [DOI] [PubMed] [Google Scholar]
- 85.American Psychological Association APA Working Group on Stress and Health Disparities. 2017. Stress and Health Disparities Report: Contexts, Mechanisms, and Interventions Among Racial/Ethnic Minority and Low Socioeconomic Status Populations. https://www.apa.org/pi/health-disparities/resources/stress-report.
- 86.Shields AE 2017. Epigenetic signals of how social disadvantage “gets under the skin”: a challenge to the public health community. Epigenomics. 9: 223–229. [DOI] [PubMed] [Google Scholar]
- 87.Brotman DJ, Golden SH & Wittstein IS 2007. The cardiovascular toll of stress. Lancet. 370: 1089–1100. [DOI] [PubMed] [Google Scholar]
- 88.Argentieri MA, Nagarajan S, Seddighzadeh B, et al. 2017. Epigenetic Pathways in Human Disease: The Impact of DNA Methylation on Stress-Related Pathogenesis and Current Challenges in Biomarker Development. EBioMedicine. 18: 327–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Epel ES, Blackburn EH, Lin J, et al. 2004. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 101: 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhasin MK, Dusek JA, Chang BH, et al. 2013. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One. 8: e62817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dusek JA, Otu HH, Wohlhueter AL, et al. 2008. Genomic counter-stress changes induced by the relaxation response. PLoS One. 3: e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levine GN, Lange RA, Bairey-Merz CN, et al. 2017. Meditation and Cardiovascular Risk Reduction: A Scientific Statement From the American Heart Association. J Am Heart Assoc. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray IB, Menezes AR, Malur P, et al. 2014. Meditation and coronary heart disease: a review of the current clinical evidence. Ochsner J. 14: 696–703. [PMC free article] [PubMed] [Google Scholar]
- 94.VanderWeele TJ, Yu J, Cozier YC, et al. 2017. Attendance at Religious Services, Prayer, Religious Coping, and Religious/Spiritual Identity as Predictors of All-Cause Mortality in the Black Women’s Health Study. Am J Epidemiol. 185: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pew Research Center. 2014. Religious Landscape Study. https://www.pewforum.org/religious-landscape-study/.
- 96.Cozier YC, Yu J, Wise LA, et al. 2018. Religious and Spiritual Coping and Risk of Incident Hypertension in the Black Women’s Health Study. Ann Behav Med. 52: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spence N, Farvid M, Warner E, et al. 2019. [in press]. Religious Service Attendance, Religious Coping, and Risk of Hypertension in Women Participating in the Nurses’ Health Study II. American Journal of Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Dam HA, van der Horst FG, Knoops L, et al. 2005. Social support in diabetes: a systematic review of controlled intervention studies. Patient Educ Couns. 59: 1–12. [DOI] [PubMed] [Google Scholar]
- 99.Cohen S 2004. Social relationships and health. Am Psychol. 59: 676–684. [DOI] [PubMed] [Google Scholar]
- 100.Berkman LF, Glass T, Brissette I, et al. 2000. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 51: 843–857. [DOI] [PubMed] [Google Scholar]
- 101.Young-Hyman D, de Groot M, Hill-Briggs F, et al. 2016. Psychosocial Care for People With Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 39: 2126–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Noordam R, Aarts N, Peeters RP, et al. 2016. Selective Serotonin Reuptake Inhibitors Decrease Pancreatic Insulin Secretion in Older Adults and Increase the Risk of Insulin Dependence in Type 2 Diabetes Patients. J Clin Psychiatry. 77: e1124–e1129. [DOI] [PubMed] [Google Scholar]
- 103.Anderson RJ, Freedland KE, Clouse RE, et al. 2001. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 24: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 104.Ali S, Stone MA, Peters JL, et al. 2006. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 23: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 105.de Groot M, Anderson R, Freedland KE, et al. 2001. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 63: 619–630. [DOI] [PubMed] [Google Scholar]
- 106.Goldney RD, Phillips PJ, Fisher LJ, et al. 2004. Diabetes, depression, and quality of life: a population study. Diabetes Care. 27: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 107.Katon WJ, Rutter C, Simon G, et al. 2005. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 28: 2668–2672. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Norris SL, Gregg EW, et al. 2005. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol. 161: 652–660. [DOI] [PubMed] [Google Scholar]
- 109.Rubin RR, Ma Y, Peyrot M, et al. 2010. Antidepressant medicine use and risk of developing diabetes during the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 33: 2549–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andersohn F, Schade R, Suissa S, et al. 2009. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 166: 591–598. [DOI] [PubMed] [Google Scholar]
- 111.Burcu M, Zito JM, Safer DJ, et al. 2017. Concomitant Use of Atypical Antipsychotics With Other Psychotropic Medication Classes and the Risk of Type 2 Diabetes Mellitus. J Am Acad Child Adolesc Psychiatry. 56: 642–651. [DOI] [PubMed] [Google Scholar]
- 112.News and Notes. 2001. US Surgeon General releases report on mental health: culture, race, and ethnicity. Public Health Rep. 116: 376. [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzalez JS, Fisher L & Polonsky WH 2011. Depression in diabetes: have we been missing something important? Diabetes Care. 34: 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fisher L, Hessler DM, Polonsky WH, et al. 2012. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 35: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Naranjo D, Hessler DM, Deol R, et al. 2012. Health and psychosocial outcomes in U.S. adult patients with diabetes from diverse ethnicities. Curr Diab Rep. 12: 729–738. [DOI] [PubMed] [Google Scholar]
- 116.Hilliard ME, Yi-Frazier JP, Hessler D, et al. 2016. Stress and A1c Among People with Diabetes Across the Lifespan. Curr Diab Rep. 16: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fisher L, Skaff MM, Mullan JT, et al. 2007. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 30: 542–548. [DOI] [PubMed] [Google Scholar]
- 118.Koh HK, Oppenheimer SC, Massin-Short SB, et al. 2010. Translating research evidence into practice to reduce health disparities: a social determinants approach. Am J Public Health. 100 Suppl 1: S72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wharam JF, Zhang F, Eggleston EM, et al. 2018. Effect of High-Deductible Insurance on High-Acuity Outcomes in Diabetes: A Natural Experiment for Translation in Diabetes (NEXT-D) Study. Diabetes Care. 41: 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.National Academies of Sciences, E. & Medicine. 2018. Immigration as a Social Determinant of Health: Proceedings of a Workshop. The National Academies Press; Washington, DC. [PubMed] [Google Scholar]
- 121.Haire-Joshu D, Schwarz CD, Steger-May K, et al. 2018. A Randomized Trial of Weight Change in a National Home Visiting Program. Am J Prev Med. 54: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cahill AG, Haire-Joshu D, Cade WT, et al. 2018. Weight Control Program and Gestational Weight Gain in Disadvantaged Women with Overweight or Obesity: A Randomized Clinical Trial. Obesity (Silver Spring). 26: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haire-Joshu D, Cahill AG, Stein RI, et al. 2019. Randomized Controlled Trial of Home-Based Lifestyle Therapy on Postpartum Weight in Underserved Women with Overweight or Obesity. Obesity (Silver Spring). 27: 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pew Research Center. 2019, June 12. Mobile Fact Sheet Accessed July 30, 2019 https://www.pewinternet.org/fact-sheet/mobile/.
- 125.Pew Research Center. 2019, May 31. Digital Gap Between Rural and Nonrural America Persists Accessed July 30, 2019 https://www.pewresearch.org/fact-tank/2019/05/31/digital-gap-between-rural-and-nonrural-america-persists/.
- 126.Chin et al. 2014. Components of the Intervention to Improve Diabetes Care and Outcomes on the South Side. Health Promot Pract. 15:29S–39S [DOI] [PMC free article] [PubMed] [Google Scholar]