Summary
The aim of the study was to assess the efficacy of BioPlus 2B, a probiotic containing Bacillus licheniformis and B. subtilis spores, on the health status and productivity of pigs, during weaning, growing and finishing stages of growth. On a commercial farrow‐to‐finish farm, five experimental groups were formed, each of 54 weaned piglets. The pigs of the first group (double controls) received normal feed with no probiotic and the pigs of the second group (untreated controls) received BioPlus 2B only during the weaning stage. The pigs of the third, the fourth and the fifth group received the same as the second group feed but, at the growing and at a part of the finishing stages, supplemented with three different doses of Bioplus 2B, a low, medium and high dose, respectively. The results have shown that, compared with the double controls, BioPlus 2B‐treated pigs had a lower morbidity and mortality during the whole trial period, compared with the double controls (range from 9.26 to 14.81% versus 25.93% and from 0.00 to 3.70% versus 11.1%, respectively), as a result of the lower incidence of post‐weaning diarrhoea due mainly to Escherichia coli. Weight gain, feed conversion ratio and carcass quality of the BioPlus 2B‐treated pigs were significantly improved compared with the double controls, whilst the beneficial effects of the probiotic were more pronounced when the medium and high doses were used.
Introduction
During the critical post‐weaning period, pigs are subjected to stressors that reduce their immune response and disturb the equilibrium of the microflora in their gut, making them susceptible to several enteric pathogens. Microorganisms such as enterotoxigenic Escherichia coli (ETEC) strains, Salmonella spp., clostridia, spirochaetes (including Brachyspira hyodysenteriae), Lawsonia intracellularis, Cryptosporidium parvum, rotavirus and coronaviruses are agents potentially causing scours from weaning up to the end of fattening period (Taylor, 1995). To prevent or control post‐weaning diarrhoea, antibiotics in the weaners and growers ration have been used as growth promoters with good results (Kyriakis et al., 1995, 1996, 1997). The antibiotics as growth promoters appear to act by reducing the pathogenic bacteria and modifying the microflora in the gut of the animal (Radostits et al., 1994). However, concern for the presence of drug residues in edible animal products and the potential transfer of antibiotic resistance to human pathogens has increased by such administration (Meng, 2003). For these reasons, alternative solutions, aiming at both the safety of the consumer and the profitability of the farmer, are always welcomed.
Probiotics seem to be a good alternative to the use of antibiotics as growth promoters (Tomasik and Tomasik, 2003). They are live cultures of harmless bacteria or yeast species (e.g. Lactobacillus, Streptococcus, Saccharomyces, etc.) that equilibrate intestinal microflora to the benefit of the animal (Fuller, 1989, 1992; Ferencik et al., 2000). They may have a growth promoting activity by competing with harmful gut flora, and by stimulating the immune system of the animal and therefore increasing the body resistance to infectious agents (Tannock, 1988; Khajarern and Khajarern, 1994; Perdigon et al., 2002; Benyacoub et al., 2003). The positive effect of probiotics on the control of certain pathogens in animals has been shown in only few studies, where they appear to control enteric diseases associated with E. coli or other enteric pathogens, one of which is post‐weaning diarrhoea syndrome (PWDS) in pigs (Kozasa, 1983; Khajarern and Khajarern, 1994; Kyriakis et al., 1999). Therefore, it is reasonable to assume that, because of their property to modulate gut microflora in favour of the animal, probiotics may also exhibit a growth‐enhancing activity (Lyons, 1987).
Since most of the related studies have been carried out in weaners, the aim of the present field study was to assess the effect of a probiotic‐containing Bacillus licheniformis and B. subtilis spores when fed even to growers–finishers, on the health status, the performance, as well as on the carcass quality of pigs.
Materials and Methods
Description of the experimental substance
BioPlus 2B (Chr. Hansen A/S, Denmark) is a probiotic‐containing B. licheniformis (DSM 5749) and B. subtilis (DSM 5750) spores in a 1 : 1 ratio. Bacillus licheniformis of BioPlus 2B has been isolated from soil, while B. subtilis has been isolated from soybean fermentation. Both component microorganisms of BioPlus 2B are registered in Annex II of 70/524 Directive as safe for use as feed additives when used according to the manufacturer's instructions and with the target animal categories specified. Neither of the two main substances is genetically modified nor produces enterotoxins. Both are genetically stable. The carrier substances are sodium aluminium silicate (1%) and whey permeate (98%), free of heavy metals, microorganisms and mycotoxins. In this study, a commercial batch of BioPlus 2B was used with product no. 617136, batch no. 2229166 (approval: 208G859405).
Trial farm
The study was carried out from February to July 2002 on a commercial farrow‐to‐finish pig farm in Karditsa, Greece, with a breeding stock of 230 sows and an annual production of 3700–4000 slaughter pigs [(Belgian Landrace) × (Large White × Landrace)]. The farm had its own feed mill. The piglets in the farm were weaned in weekly batches (every Thursday) of around 100 (95–105) animals each, at the age of 26.5 ± 1 days. In flat decks (rooms of five pens each), pigs were separated in pens of approximately nine animals each. Each room was mechanically ventilated with thermostatic control keeping the temperature between 22 and 27°C. It was daily cleaned and, when vacant, thoroughly washed and disinfected. Entrance of newly weaned piglets into the unit was not earlier than 2–3 days after disinfection. Piglets remained in flat‐deck units until the age of approximately 9 weeks. Then, they were moved into the grower–finisher barns, grouped in pens of nine pigs, and remained until slaughter at the age of 159 ± 1 days (approximately 23 weeks) (body weight of 95–100 kg). Two separate fattening stages were distinguished, depending on the type of feed offered to the fattening pigs, a growing period (61–110 days of age) and a finishing period (111–159 days of age). All pens had slatted floor. Temperature (18–20°C) and air control was kept stable by windows and electrical ventilators.
The farm had a previous history of PWDS caused by an E. coli infection as evidenced by appropriate microbiological and histopathological examinations. Moreover, the farm had a previous history of ileitis caused by L. intracellularis, but was free of Bra. hyodysenteriae and Salmonella spp. Breeding animals were vaccinated against Aujeszky's disease, swine influenza, parvovirus infection, erysipelas and atrophic rhinitis. Fatteners were also vaccinated against Enzootic pneumonia, Aujeszky's disease and swine influenza. For the control of endo/ectoparasites, all adults were treated with ivermectin twice a year.
No castration of male pigs was practiced in the farm. From the seventh day of age until weaning all piglets received creep feed free from any antimicrobials, performance enhancers, probiotics or acidifiers.
Experimental design
From each weekly batch of weaned pigs, 90 pigs (45 males and 45 females) were selected and divided over five experimental groups. The 18 pigs (nine males and nine females) of each group were further allocated in two pens of nine pigs (five males–four females and four males–five females). Homogeneity of the groups was satisfied with regard to sex, average body weight at the start of the trial (P > 0.05), and housing conditions. Each of the pigs was clearly identified (ear‐marked). Three consecutive weekly batches of weaned pigs were obtained until a total of 135 male (M) and 135 female (F) pigs was available. Thus, at the beginning of the trial each of the five experimental groups included: three weaning batches × two pens × nine pigs per pen = 54 pigs (27 M + 27 F) per experimental group. It is important to note that the initial replicate (pen) formed at the beginning of the experiment, in weaners phase, remained the same during the growing and finishing periods (e.g. one pen of the same treatment group in the flat‐deck unit formed one pen in the growing/finishing stable).
From this day, each group was administered different feed inclusions of BioPlus 2B, as follows:
-
1
Control group (C): No addition of BioPlus 2B to feed from weaning up to the age of slaughter.
-
2
BioPlus 2B/weaner‐treated group (BWT): Addition of BioPlus 2B only to the feed for weaners (26–61 days of age) at a dose of 400 g/tonne of feed (=1.28 × 106 viable spores per g of feed).
-
3
BioPlus 2B/weaner‐grower‐finisher low dose‐treated group (BL): Addition of BioPlus 2B to the feed for weaners (26–61 days of age) at a dose of 400 g/tonne of feed (=1.28 × 106 viable spores per g of feed) and to the feed for growers–finishers (62–120 days of age) at a dose of 200 g/tonne of feed (=0.64 × 106 viable spores per g of feed).
-
4
BioPlus 2B/weaner‐grower‐finisher medium dose‐treated group (BM): Addition of BioPlus 2B to the feed for weaners (26–61 days of age) at a dose of 400 g/tonne of feed (=1.28 × 106 viable spores per g of feed) and to the feed for growers–finishers (62–120 days of age) at a dose of 400 g/tonne of feed (=1.28 × 106 viable spores per g of feed).
-
5
BioPlus 2B/weaner‐grower‐finisher high dose‐treated group (BH): Addition of BioPlus 2B to the feed for weaners (26–61 days of age) at a dose of 400 g/tonne of feed (=1.28 × 106 viable spores per g of feed) and to the feed for growers–finishers (62–120 days of age) at a dose of 600 g/tonne of feed (=1.92 × 106 viable spores per g of feed).
From the age of 121 days up to the slaughter age (approximately 159 days of age) the feed for growers–finishers was free of BioPlus 2B in all groups.
The feed provided to the pigs (weaners, growers, finishers) was home‐mixed mash rations based on corn/barley/soya meal and offered ad libitum. During each fattening stage, the feed was common for all groups, and was free of any antimicrobials, performance enhancers, probiotics or acidifiers, except the tested substance (BioPlus 2B) at the recommended dose. Specification of the pig feeds (on dry matter basis) was the following: (a) Weaner feed: ME 3250 kcal/kg, crude protein 21%, fibre 3.5%, lysine 1.4%, calcium 0.85% and available phosphorus 0.55%; (b) grower feed: ME 3150 kcal/kg, crude protein 18.5%, fibre 4%, lysine 1.0%, calcium 0.85% and available phosphorus 0.45%, and (c) finisher feed: ME 3050 kcal/kg, crude protein 17.5%, fibre 4.75%, lysine 0.85%, calcium 0.75% and available phosphorus 0.45%.
The diets were manufactured to prevent cross‐contamination with BioPlus 2B, with production of batches running in treatment sequence from C to BH. Every batch of feed was prepared about 4 weeks prior to its use and of every feed prepared, five equal aliquots from five different places were randomly sampled and mixed. The mixture was then divided into two parts. The first part was assayed for its spore content at Chr. Hansen A/S, Denmark. For this purpose, the feed samples were extracted according to procedure AM20015 (collection and dispatch of feed samples) and analysed for B. licheniformis and B. subtilis according to QAM‐124 (enumeration of germinating spores) and 205‐10 (determination of spores of growth promoting bacilli strains from BioPlus 2B). The calculation of total count was carried out according to QAM‐022. The second part of the feed was subjected to chemical analysis for the determination of its content in protein, oil, NDF, ash and moisture, according to the official methods of the Association of Official Analytical Chemists (AOAC, 1990). Each batch of feed was not released for use except results of the laboratory examinations were acceptable (see Results ).
Moreover, in an attempt to confirm that the correct treatments were applied to the trial groups, approximately at the mid of the growing stage (age of 85 days), a total of 30 grab manure samples were taken (one per pen at random = six per experimental group). Each grab‐sample (approximately 20 g) was placed in a small container (supplied by Chr. Hansen) and filled completely to prevent aerobic conditions. Samples were taken around the same time of day from the annulus by using a speculum. Each container was labelled with date, piglet ID and treatment group and immediately stored in a freezer at −18°C. The frozen samples were analysed for the quantitative viable cell count of B. licheniformis and B. subtilis by Chr. Hansen A/S, according to the protocol no. 20001059 and journal no. 3383 (see Results ).
Biosecurity measures
The risk of cross‐contamination of control pigs with the organisms contained in BioPlus 2B was minimized through the following biosecurity measures:
-
1
During the weaner phase (flat‐decks) the double control pigs (group C), were housed in a separate building to those on pigs of BW, BL, BM and BH treatment groups. This facilitated independent cleaning of the main accommodation without the need to clean and disinfect the scraper between runs.
-
2
During the weaners phase pigs in groups BW, BL, BM and BH were penned so that the muck scrapper always shifted manure down the dunging passage in the direction of lowest to highest BioPlus 2B dose rate (i.e. treatment BW–BL to BM–BH).
-
3
During the growing/finishing phases the trial pigs were housed in three separate buildings (one for C group, one for BW group and one for BL, BM and BH groups).
-
4
At all times, pigs were maintained in their respective treatment groups (i.e. there were no mixing).
-
5
Control pigs were allocated a separate set of cleaning (clothing, boots and shovels) and feeding (scoops, barrows) equipment. These were clearly colour‐coded to differentiate them from equipment used in the management of pigs in treatment groups.
-
6
Moreover, risk was reduced by employing a different member of staff to manage the control animals.
Records
All trial pigs were monitored daily for disease signs and all incidences were recorded. In case of a disease problem, only individual injectable therapy was performed. The exact treatment, the time and the duration of application were recorded. For every dead pig, its weight and the date of death were recorded; autopsy was performed and if needed the diagnosis was confirmed by proper microbiological and pathological examinations. The morbidity and in mortality in general, as well as only those related to diarrhoea were calculated per fattening subperiod (weaners, growers, finishers) and for the total trial period.
Weighing of the trial pigs had been performed at the start of trial (at weaning) and at the end of each fattening subperiod (61, 110 and 159 days of age). Moreover, the feed consumption per pen was recorded on weekly basis. The total feed intake per pen and per period was determined by the weigh‐back method. This means that every day the quantity of feed added to the feeder was recorded and at the end of each week the remaining feed in the feeder was weighed and then the total consumption of feed per pen and per week was calculated.
The average daily gain (ADG), the average daily feed intake (ADFI) and the feed conversion ratio (FCR) were calculated per pen for each subperiod and for the entire trial duration. In the case an animal was removed, the feed intake was corrected using the animal‐day method. In this way, the above performance parameters were calculated per subperiod or the total observation period on the basis of the number of animals remaining in the trial during the defined period.
Finally, the carcasses of a subtotal of the pigs involved in the study were evaluated in the abattoir. For this purpose, four pigs (two M + two F) from each pen were randomly selected. Thus, three weaning batches × two pens × four pigs per pen = 24 pigs (12 M + 12 F) per experimental group (a total of 120 pigs) were included in the evaluation. The EUROP (SEUROP) system was followed for carcass quality classification, according to the relative EU regulations as follows: S = >60% lean, E = 55–60% lean, U = 50–54% lean, R = 45–49% lean, O = 40–44% lean and P = <40% lean.
GCP and GLP statement
All work during this study was carried out according to the Good Clinical Practice for the Conduct of Clinical Trials Guidelines (GCP, July 2001) and Good Laboratory Practice Guidelines (GLP, Council Directive 87/18/EEC and Commission Directive 1999/11/EC). The specifications of the trial satisfied all welfare needs of the animals with regard to feed, water, space, treatments, etc. (Good Farm Practice guidelines, GFP).
Statistical evaluation
In this study individual piglet was the experimental unit. Each recorded parameter was subjected to a one‐way anova using the general linear models (GLM) procedure. Duncan's multiple range test was used to determine differences among the different groups. Parameters were also subjected to anova using pen, sex and experimental week as covariates. The parameters expressed as percentages were subjected to Pearson's chi‐square analysis for the determination of differences among the different groups. The statistical software used was the sas Package (‘The SAS® System’ release 8.1 for WINDOWS – 2002; SAS Institute, Inc., Cary, NC, USA) which is installed in the main PC of the Clinic of Medicine/Pig Diseases and Reproduction section, with Site Code No. 0084912001.
Results
According to the laboratory results, the contents of bacilli in the feed for weaners from the control group, as well as in the feed for growers and finishers from the control and the BW groups, were <1 × 105 cfu/g, as expected. The mean recovery of bacilli in the feed for weaners from the control, the low, medium and high dose of BioPlus 2B groups, were 1.70, 1.80, 2.00 and 2.00 × 106 cfu/g, respectively, where the expected result was 1.28 × 106 cfu/g for all groups. The above mean values were not found to be significantly different among groups (P > 0.05). The mean contents of bacilli in the feed for growers from the low, medium and high dose of BioPlus 2B groups, were 0.67, 1.50 and 2.40 × 106 cfu/g, respectively, where the expected results were 0.64, 1.28 and 1.92 × 106 cfu/g. Moreover, the mean contents of bacilli in the feed of finishers for the low, medium and high dose of BioPlus 2B groups, were 0.64, 1.30 and 1.90 × 106 cfu/g, respectively, where the expected results were 0.64, 1.28 and 1.92 × 106 cfu/g. Finally, the mean (±SD) recovery of the bacilli in faeces from grab manure samples, were <4.00, <4.00, 5.22 ± 0.29, 5.49 ± 0.33 and 5.82 ± 0.12 log10 cfu/g of faeces, respectively, in the five groups, verifying the correct use of feed to the different groups.
The mean (±SD) weaning age was 26.33 ± 0.95, 26.17 ± 1.08, 26.50 ± 0.97, 26.33 ± 0.95, 26.17 ± 1.08, respectively, in control, BW, low, medium and high BioPlus 2B dose groups. No significant differences existed among the different experimental groups for weaning age (P > 0.05). Since all pigs were introduced to the next growth stage exactly after a definite interval (i.e. they remained 5 weeks in the weaners stage, 7 weeks in the growing stage and 7 weeks in finishing stage), there were also no differences among the different groups as regard the mean age at the end of each subperiod of growth.
The morbidity rates in the different experimental groups for each stage of growth and for the total observation period are shown in Fig. 1. The morbidity in the control group was almost twice as that in the remaining four treatment groups, but this difference, although tended to be, was not statistically significant. The vast majority of morbidity was referred to the weaners stage. Figure 2 further distinguishes the morbidity associated with diarrhoea from the general morbidity. It is evident that all cases of sick animals during the weaners stage were associated with diarrhoea that was apparent 6–10 days post‐weaning, lasting 1–3 days. In pigs of all groups’ stools were watery, pasty or rarely mucoid in consistency and yellowish or greenish in colour, without blood but with a characteristic smell. From the disease history of the trial farm, the diarrhoea characteristics, as well as from the necropsy findings and the microbiology of the dead piglets, this diarrhoeic syndrome was attributed to ETEC strains. Moreover, it was shown that during the growing and the finishing periods, abnormal clinical signs were recorded only to a small proportion of animals. In the vast majority of sick pigs respiratory disease was evident (cough), which was attributed to enzootic pneumonia caused by Mycoplasma hyopneumoniae. Only in one pig of the double control group non‐mucoid diarrhoea was recorded, attributed to ileitis caused by L. intracellularis.
Figure 1.
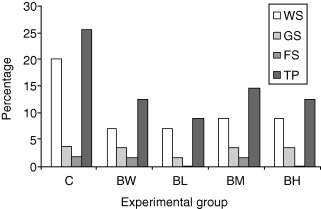
Morbidity rates in general (%) in the different experimental groups (weaner stage; GS, growing stage; FS, finishing stage; TP, total period).
Figure 2.

Morbidity rates associated with diarrhoea (%) in the different experimental groups (weaner stage; GS, growing stage; FS, finishing stage; TP, total period).
As shown in 3, 4, the mortality in the control group was almost three times as high compared with the remaining four treatment groups, but also this difference, although tended to be, was not statistically significant. The mortality pattern was similar with that of morbidity. So, the vast majority of mortality was referred to the weaners stage, and according to necropsy findings and microbiology, it was due to acute colibacillosis. The rate of mortality was low during the growing and finishing stages. The cause of death was either a complicated enzootic pneumonia or an acute ileitis.
Figure 3.
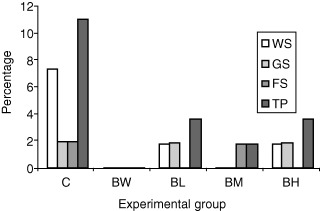
Mortality rates in general (%) in the different experimental groups (WS, weaner stage; GS, growing stage; FS, finishing stage; TP, total period).
Figure 4.
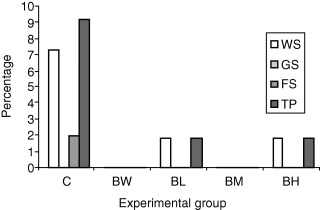
Mortality rates associated with diarrhoea (%) in the different experimental groups (WS, weaner stage; GS, growing stage; FS, finishing stage; TP, total period).
Growth performance parameters such as ADG and FCR were significantly improved after the administration of BioPlus 2B (Table 1). The ADFI was similar in all groups during all trial subperiods and in total (P > 0.05).
Table 1.
Growth performance parameters (mean ± SD) of pigs during each stage in the different experimental groups
| Stage | Experimental groups | ||||
|---|---|---|---|---|---|
| C | BW | BL | BM | BH | |
| Average daily gain (kg) | |||||
| WS | 0.430b ± 0.065 | 0.460a ± 0.053 | 0.464a ± 0.050 | 0.464a ± 0.060 | 0.457a ± 0.047 |
| GS | 0.578d ± 0.048 | 0.585cd ± 0.050 | 0.598bc ± 0.042 | 0.615ab ± 0.053 | 0.624a ± 0.040 |
| FS | 0.929a ± 0.045 | 0.923a ± 0.058 | 0.928a ± 0.054 | 0.933a ± 0.052 | 0.930a ± 0.060 |
| TP | 0.669d ± 0.017 | 0.677c ± 0.019 | 0.684b ± 0.016 | 0.693a ± 0.020 | 0.693a ± 0.020 |
| Average daily feed intake (kg) | |||||
| WS | 0.648a ± 0.031 | 0.623a ± 0.025 | 0.631a ± 0.022 | 0.635a ± 0.035 | 0.620a ± 0.013 |
| GS | 1.572a ± 0.037 | 1.572a ± 0.038 | 1.544a ± 0.017 | 1.535a ± 0.020 | 1.538a ± 0.034 |
| FS | 2.967a ± 0.029 | 2.986a ± 0.021 | 2.951a ± 0.036 | 2.961a ± 0.036 | 2.975a ± 0.034 |
| TP | 1.876a ± 0.045 | 1.843a ± 0.025 | 1.838a ± 0.052 | 1.840a ± 0.055 | 1.843a ± 0.042 |
| Feed conversion ratio | |||||
| WS | 1.51a ± 0.13 | 1.36b ± 0.07 | 1.37b ± 0.07 | 1.37b ± 0.11 | 1.36b ± 0.06 |
| GS | 2.72a ± 0.13 | 2.69ab ± 0.11 | 2.58bc ± 0.06 | 2.48c ± 0.08 | 2.47c ± 0.09 |
| FS | 3.20a ± 0.03 | 3.24a ± 0.03 | 3.18a ± 0.08 | 3.17a ± 0.04 | 3.20a ± 0.04 |
| TP | 2.80a ± 0.08 | 2.72b ± 0.04 | 2.68b ± 0.09 | 2.65b ± 0.07 | 2.66b ± 0.05 |
WS, weaners stage; GS, growing stage; FS, finishing stage; TP, total period.
a–dMean values within each row with different superscripts differ significantly (P < 0.05).
During the weaners stage the ADG of the BioPlus 2B‐treated groups was significantly higher than that of the controls (P < 0.05). During the growing stage a gradual improvement of ADG was noticed in BioPlus 2B‐treated animals related to the dose tested. In contrast, during the finishing stage there were no differences among the different experimental groups. When ADG was calculated for the entire observation period, the pigs of all BioPlus 2B‐treated groups performed significantly better than those of the control group. Additionally, pigs receiving BioPlus 2B also during the growing stage and a part of the finishing stage performed better than those of the controls and the BW group (P < 0.05). However, the beneficial effect was more pronounced in pigs treated with medium and high doses of BioPlus 2B, compared with those treated with the low dose (P < 0.05).
When compared with the control group, the FCR was significantly improved in all BioPlus 2B‐treated groups during the weaners stage (P < 0.05). However, during the growing stage FCR was significantly improved only in the three groups treated during this period, compared with the controls, while it did not differ between controls and BW group. Conversely, during the finishing stage there were no differences among the different experimental groups. When FCR was calculated for the entire observation period, the pigs of all BioPlus 2B‐treated groups had a better‐feed utilization than those of the control group.
The results of the carcass classification, presented in Table 2, show a positive effect of the administration of BioPlus 2B on carcass quality. Indeed, carcasses of the BioPlus 2B‐treated pigs during the growing and a part of finishing stages were of a better quality compared with those of the two control groups. Additionally, the 58.33% and 58.34% of the carcasses of the medium and high doses of BioPlus 2B groups were classified in the top two categories of the SEUROP scale (S and E), whilst the respective figures were 25.00%, 8.33% and 4.17% for the low BioPlus 2B, control and BW groups, respectively (P < 0.05).
Table 2.
Carcass scoring according to SEUROP system (cases in category/total examined: percentages in parentheses)
| Class | Lean (%) | Experimental groups | ||||
|---|---|---|---|---|---|---|
| C | BW | BL | BM | BH | ||
| S | >60 | 0/24 (0.00) | 0/24 (0.00) | 1/24 (4.17) | 2/24 (8.33) | 4/24 (16.67) |
| E | 55–60 | 1/24 (4.17) | 2/24 (8.33) | 5/24 (20.83) | 12/24 (50.00) | 10/24 (41.67) |
| U | 50–54 | 9/24 (37.50) | 13/24 (54.17) | 10/24 (41.67) | 9/24 (37.50) | 8/24 (33.33) |
| R | 45–49 | 10/24 (41.67) | 8/24 (33.33) | 8/24 (33.33) | 1/24 (4.17) | 2/24 (8.33) |
| O | 40–44 | 3/24 (12.50) | 1/24 (4.17) | 0/24 (0.00) | 0/24 (0.00) | 0/24 (0.00) |
| P | <40 | 1/24 (4.17) | 0/24 (0.00) | 0/24 (0.00) | 0/24 (0.00) | 0/24 (0.00) |
Finally, from the statistical analysis, no sex, pen and experimental week effect was found regarding all parameters examined.
Discussion
The post‐weaning problem seen in the present farm had epidemiological, clinical and postmortem characteristics indicative for ETEC strains, known to contribute to PWDS of pigs (Kyriakis, 1989). Additional support of this diagnosis was provided by microbiology. From the results of the present study it was shown that in‐feed B. licheniformis and B. subtilis, strongly tended to reduce the morbidity, as well as the mortality of pigs associated with E. coli diarrhoea in weaners. This finding is in agreement with previous reports shown that probiotics have a positive effect on PWDS (Kyriakis et al., 1999), as well as on other diarrhoeas (Breton and Munoz, 1998; Candy et al., 2000; Marcin et al., 2000). The beneficial effect of the probiotic used in the present study can be explained by the decrease of pathogens locally in the gut, brought about by the ingestion of the probiotics, which did not allow increased proliferation of harmful bacteria (Vandevoorde et al., 1992; Alvarez et al., 1996). However, the exact mechanism by which B. licheniformis and B. subtilis acted in the gut still remains unclear, e.g. whether this was due to competition for receptors on gut mucosa, competition for nutrients, production of antibacterial substances, stimulation of immunity or/and other mechanisms (Fuller, 1989; Perdigon et al., 1990; Rodriguez‐Ropon et al., 1998).
According to the results of our study the beneficial effect of the probiotic has also been extended to the growing and finishing stages. An important index for evaluating a product, and one, which is of utmost importance for the farmer, is the animal performance. In the present study, several efficacy parameters of treated animals (ADG and FCR), particularly those of the groups that received the medium and the high dose of the probiotic, were superior to those seen in double controls. For example, during the growing stage, ADG in the medium and the high dose groups was 6.4% and 7.2% higher than that of the double controls and for the total fattening period they both were 3.6% higher. Additionally, during the growing stage, FCR in the medium and the high dose groups was 8.8% and 9.2% lower than that of the double controls and for the total fattening period they were 5.4% and 5.0%, lower, respectively. These figures are of particular significance for the farmer considering that, the continuous use of growth promoters during weaning‐to‐slaughter period is not expected to confer improvement in efficacy parameters higher than 5% (Buttery, 1993). Furthermore, the significantly higher proportions of carcasses classified in the top two categories of the SEUROP scale (S and E) that were obtained in pigs receiving the medium and high dose of the probiotic, gives an additional benefit to the farmer. A possible explanation for the benefit after the administration of the probiotic during the growing stage and a short‐time at the beginning of the finishing period could be that microflora balance in the gut of these animals is optimized – as is the case in weaners – and a better utilization of nutrients is taking place, thereby leading to faster metabolism and transformation of feed into body mass (ADG, FCR) and transformation into lean meat. However, such an explanation is just a hypothesis, as there is no data available from the current literature to correlate necessarily the high growth rates of pigs during the nursery and growing periods with high lean yield at the end of finishing period.
In conclusion, administration of the probiotic BioPlus 2B at the dose of 400 g/tonne of feed, that is equal to 1.28 × 106 viable spores of B. Licheniformis and B. subtilis per g of feed from weaning up to the age of 120 days, can (a) prophylactically reduce the morbidity and the mortality in recently weaned piglets, (b) improve the performance parameters of the fattening pigs and (c) improve the carcass quality, to the economic benefit of the farmer. Finally, as probiotics are generally considered to be harmless, the findings of this study further support the view that the use of antimicrobials both for competing scours problems in pigs and for promoting growth of the animals can be reduced, in this way minimizing the risks for public health (drug residues and antibiotic‐resistant strains of bacteria).
However, the results from the use of probiotics are not always consistent (Stavric et al., 1995; Kritas and Morrison, 2003). Personal experience and mainly unpublished communication with scientists and farmers shows that probiotic preparates do not always provide benefits in pigs. It is true that systematic knowledge on these immunomodulators is rather shallow. Therefore, wide variations in protocol designs, in tested commensal microorganisms and pathogens, in target animal species, and in health status of farms may account for significant variability in results. However, inconsistency of results is a characteristic of every new development. Our opinion is that probiotics are not a panacea and should be used after critical thinking. They can be introduced in cases similar to that described as successful in literature. If novel applications are sought, limited number of pigs should be used and the product should be tested. Failure does not necessarily mean that probiotics do not work. Their effect is dependent on several factors as previously mentioned. A trial‐and‐error thinking should be practiced at least in the first years of investigating their effect. Their potential to substitute for antibiotics in simple illnesses and to produce ‘ecological’ pork may also guide practitioners’ decisions.
References
- Alvarez, A. I. , Rojas A., Lara V., and Rodriguez A., 1996: The effect of Lactobacillus xilosus and Streptococcus faecalis in the activity of enterotoxigenic Escherichia coli in piglets In: Monetti P. G., and Vignola G. (eds), Proc. 14th International Pig Veterinary Society Congress, 453 pp. Press Point, Abbiategrasso (MI), Italy. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 1990: Official Methods of Analysis of the Association of Official Analytical Chemists. 15th edn. Association of Official Analytical Chemists, Arlington, VA, USA. [Google Scholar]
- Benyacoub, J. , Czarnecki‐Maulden G. L., and Cavadini C., 2003: Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J. Nutr. 133, 1158–1162. [DOI] [PubMed] [Google Scholar]
- Breton, J. , and Munoz A., 1998: Effects of probiotics in the incidence and treatment of neonatal diarrhoea In: Done S., Thomson J., and Varley M. (eds), Proc. 15th International Pig Veterinary Society Congress, p. 3, 207 Nottingham University Press, Nottingham GN11 OAX, England. [Google Scholar]
- Buttery, P. , 1993: Growth promotion in animals – an overview In: Buttery P. (ed.), Livestock Productivity Enhancers: An Economic Assessment, pp. 7–23. CAB Int., UK. [Google Scholar]
- Candy, D. C. A. , Lamont L. S., Greig M., Lewis J., Bennett H., and Griffiths M., 2000: Effect of administration of Lactobacillus caseishirota on sodium balance in an infant with short bowel syndrome In: Bomba A. (ed.), Proc. International Probiotic Conference on ‘The Prospects of Probiotics in Prevention and Therapy of Diseases of Young’, 35 pp. Research Institute of Veterinary Medicine, Department of Gnotobiology and Diseases of Young, 040 01 Kosice, Slovac Republic, High Tatras, Slovac Republic. [Google Scholar]
- Ferencik, M. , Mikes Z., Seman M., and Ebringer L., 2000: Beneficial modification of the human intestinal microflora using orally administered enterococci In: Bomba A. (ed.), Proc. International Probiotic Conference on ‘The Prospects of Probiotics in Prevention and Therapy of Diseases of Young’, 46 pp. Research Institute of Veterinary Medicine, Department of Gnotobiology and Diseases of Young, 040 01 Kosice, Slovac Republic, High Tatras, Slovac Republic. [Google Scholar]
- Fuller, R. , 1989: Probiotics in man and animals. J. Appl. Bacteriol. 66, 365–368. [PubMed] [Google Scholar]
- Fuller, R. , 1992: History and development of probiotics In: Fuller R. (ed.), Probiotics – the Scientific Basis, pp. 1–8. Chapman and Hall, London, UK. [Google Scholar]
- Khajarern, S. , and Khajarern J., 1994: Effects of a probiotics (Toyocerin) in sow and creep feeds on resistance of diarrhoea in piglets In: Chulalongkorn University, Faculty of Veterinary Science, Bangkok (ed.), Proc. 13th IPVS Congress, 294 pp. Chulalongkorn University, Faculty of Veterinary Science, Bangkok, Thailand. [Google Scholar]
- Kozasa, M. , 1983: Toyocerin (Bacillus toyoi) as growth promoter for animal feeding. Microbiologie-Aliments-Nutrition 4, 121–124. [Google Scholar]
- Kritas, S. K. , and Morrison R. B., 2003: A critical review of feeding probiotics to pigs In: Proceedings of A.D. Leman Swine Conference, , MN, USA, 30, 252–255. [Google Scholar]
- Kyriakis, S. C. , 1989: New aspects of the prevention and/or treatment of the major stress induced diseases of early weaned piglet. Pigs News Info. 2, 176–181. [Google Scholar]
- Kyriakis, S. C. , Sarris K., Kritas S. K., Saoulidis K., Tsinas A. C., and Tsiloyiannis V. K., 1995: The effect of salinomycin on the control of Clostridium perfringens type‐A infection in growing pigs. J. Vet. Med. B 42, 355–359. [DOI] [PubMed] [Google Scholar]
- Kyriakis, S. C. , Sarris K., Kritas S. K., Tsinas A. C., and Giannakopoulos C., 1996: Effect of salinomycin in the control of Clostridium perfringens type‐C infection in suckling pigs. Vet. Rec. 138, 281–283. [DOI] [PubMed] [Google Scholar]
- Kyriakis, S. C. , Tsiloyiannis V. K., Lekkas S., Petridou E., Vlemmas J., and Sarris K., 1997: The efficacy of enrofloxacin in‐feed medication, by applying different programmes for the control of post‐weaning diarrhoea syndrome of piglets. J. Vet. Med. B 44, 513–521. [DOI] [PubMed] [Google Scholar]
- Kyriakis, S. C. , Tsiloyiannis V. K., Vlemmas J., Sarris K., Tsinas A. C., Alexopoulos C., and Jansegers L., 1999: The effect of probiotic LSP 122 on the control of post‐weaning diarrhoea syndrome of piglets. Res. Vet. Sci. 67, 223–228. [DOI] [PubMed] [Google Scholar]
- Lyons, T. P. , 1987: Probiotics: an alternative to antibiotics. Pigs News Info. 8, 157–164. [Google Scholar]
- Marcin, A. , Mican P., and Falat M., 2000: Imuguard P – protection of gastrointestinal tract of suckling piglets and weaned pigs against the invasion of enteropathogens In: Bomba A. (ed.), Proc. International Probiotic Conference on ‘The Prospects of Probiotics in Prevention and Therapy of Diseases of Young’, 73 pp. Research Institute of Veterinary Medicine, Department of Gnotobiology and Diseases of Young, 040 01 Kosice, Slovac Republic, High Tatras, Slovac Republic. [Google Scholar]
- Meng, J. , 2003: Emerging antimicrobial resistance in foodborne pathogens In: Leontides L. (ed.), Proc. 5th International Symposium on the Epidemiology and Control of Foodborne Pathogens in Pork, 27 pp. ERGO M&P Graphics Arts, Heraklion, Crete, Greece. [Google Scholar]
- Perdigon, G. , Alvarez S., Nader De Macias M. E., Roux M. E., and Pesche De Ruiz Holdago A. A., 1990: The oral administration of lactic acid bacteria increase the mucosal immunity in response to enteropathogens. J. Food Prot. 53, 404. [DOI] [PubMed] [Google Scholar]
- Perdigon, G. , Galdeano C. M., and Valdez J. C., 2002: Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56 (Suppl. ), 21–26. [DOI] [PubMed] [Google Scholar]
- Radostits, O. M. , Leslie K. E., and Fetrow J., 1994: Planned animal health and production in swine herds In: Radostits O. M., Leslie K. E., and Fetrow J. (eds), Herd Health. Food Animal Production Medicine. 2nd edn. pp. 435–526. W.B. Saunders Co., London. [Google Scholar]
- Rodriguez‐Ropon, A. , Alvarez‐Manrique C. I., Estrada‐Parra S., and Serrano‐Miranda E., 1998: Effects of the administration of a probiotic on the immune parameters of piglets In: Done S., Thomson J., and Varley M. (eds), Proc. 15th International Pig Veterinary Society Congress, p. 3, 37 Nottingham University Press, Nottingham GN11 OAX, England. [Google Scholar]
- Stavric, S. , Kornegay E. T., Wallace R. J., and Chesson A., 1995: Microbial probiotics for pigs and poultry In: Wallace R. J. (ed.), Biotechnology in Animal Feeds and Animal Feeding, pp. 205–231. VCH Verlagsgesellschaft mbH, Weinheim Germany. [Google Scholar]
- Tannock, G.W. , 1988: The normal microflora: new concepts in health promotion. Microbiol. Sci. 5, 4–8. [PubMed] [Google Scholar]
- Taylor, D. J. , 1995: Pig diseases, 6th edn. St Edmundsbury Press, Suffolk, UK. [Google Scholar]
- Tomasik, P. J. , and Tomasik P., 2003: Probiotics and prebiotics. Cereal Chem. 80, 113–117. [Google Scholar]
- Vandevoorde, L. , Vandewoestyne M., Bruyneel B., Christieaens H., and Verstraete W., 1992: The Lactic Acid Bacteria. p. 1, 447 Chapman and Hall, London. [Google Scholar]


