Abstract
The aim of this study was to determine the frequency of respiratory viruses responsible for respiratory tract infections in Turkish children during the 2011–2012 influenza season. Nasal swabs were obtained from patients with symptoms suggestive of an influenza‐like illness between December 2011 and April 2012. Samples were analyzed with multiplex real‐time polymerase chain reaction (RT‐PCR) to help identify the causative viral pathogen. A total of 200 patients were enrolled in the study. A respiratory virus was detected successfully in 102 (51%) children; influenza A (H3N2) in 39.2%, influenza B in 23.5%, RSV in 15.6%, rhinovirus in 13.7%, bocavirus in 2.9%, coronavirus in 2.9%, and metapneumovirus in 0.9% of patients. Only one patient was co‐infected with bocavirus and influenza A virus. A statistically significant difference in the mean age of presentation was observed between the various viral pathogens (P < 0.001). Patients with RSV were significantly younger whereas children infected with the influenza viruses were significantly older. Comparison of symptoms revealed that fever and headache occurred more frequently with the influenza viruses than the other viruses combined (P < 0.001, <0.05). Durations of symptoms such as fever, cough, nasal congestion, and rhinorrhea were also significantly longer in the influenza group (P < 0.001, <0.005, <0.001, <0.005, respectively). Demographic analyses revealed that the school/daycare attendance was the only parameter associated with a significantly increased risk for influenza infection. With an overall viral pathogen detection rate of 51%, findings of the present study suggest other respiratory pathogens, whether viral or bacterial, may also lead to hospital visits due to influenza‐like illnesses in children. J. Med. Virol. 86:865–871, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: influenza, respiratory viruses, children
INTRODUCTION
With a wide range of clinical presentations, respiratory tract infections remain a significant cause of morbidity and mortality worldwide, particularly in children [Henrickson et al., 2004; Johansson et al., 2011]. Although many pathogens may cause respiratory tract infections, viruses are the most frequently implicated. Respiratory tract infections have a significant socioeconomic impact due to work and school absenteeism, despite mostly having a mild and self‐limiting clinical course [Bramley et al., 2002; Fendrick et al., 2003]. Annual winter epidemics of severe respiratory infections in infants and young children are caused by a range of respiratory viruses. To date, identification of the causative pathogen in respiratory tract infections has mainly been limited to clinical research studies, and routine testing has yet to become common in general practice.
Respiratory syncytial virus (RSV), human rhinovirus (HRV), and seasonal influenza viruses are responsible for a significant majority of viral respiratory illnesses in humans worldwide, which in turn place a heavy burden on healthcare systems. RSV causes mainly infections in young children and infants, typically as an epidemic during midwinter, particularly in temperate regions. It is estimated that 70% of children are infected before they reach the age of 1 year, with affected individuals usually presenting with bronchiolitis, pneumonitis, bronchitis, or croup [Glezen et al., 1986]. The influenza viruses, on the other hand, are known to cause yearly epidemics, especially during the winter months. They normally give rise to a systemic illness with high fever, myalgia, and a dry cough which may be life‐threatening in high‐risk groups such as the elderly or those with chronic cardio‐respiratory conditions. Although the initial infection itself can be quite debilitating, there is also the risk of acquiring a secondary bacterial superinfection. The common cold, which is the most frequent form respiratory tract infections, is caused mainly by the HRV. Infection may progress rarely to pneumonia or lead to exacerbation of asthma in children [Pierangeli et al., 2007; Matthew et al., 2009]. The human metapneumovirus (HMPV) is also known to cause respiratory tract infection in children and adults, accounting for approximately 5–10% of cases annually [van den Hoogen et al., 2001]. Other viruses such as human parainfluenza viruses (HPIV), human coronaviruses HCoV‐229E and HCoV‐OC43 have also been implicated worldwide. Discovered recently, human coronavirus HCoV‐NL63 [van der Hoek et al., 2004] and human bocavirus (HBoV) [Allander et al., 2007] have joined the family of viruses that cause respiratory tract infections.
Turkey is a Eurasian country, which is considered a crossroad between Western Asia and Southeastern Europe. Very few studies on the viral etiology of respiratory illness have been reported from Turkey [Hatipoğlu et al., 2011; Ceyhan et al., 2012]. The aim of this prospective clinical study was determine the spectrum of viral etiologies of acute respiratory tract infections in an outpatient Turkish population of children presenting with influenza‐like illnesses. Viral respiratory pathogens were tested using multiplex real‐time reverse transcription polymerase chain reaction (RT‐PCR) assays which allow for the simultaneous detection of a variety of respiratory viruses.
MATERIALS AND METHODS
Patient Selection and Initial Evaluation
This study was conducted at Hacettepe University Medical Faculty Ihsan Dogramacı's Child Hospital, Ankara with the approval of the Ethical Committee. The parent or legal guardians of children aged <18 years presenting between December 2011 and April 2012 with a suspicion of a respiratory tract infection were approached, and following informed consent their children were screened for eligibility before being enrolled in the study. A diagnosis of acute respiratory illness was made in the presence of at least two of the following signs or symptoms for duration of at least one day; (1) temperature ≥37.8°C, (2) cough, (3) headache, (4) sore throat, (5) myalgia, (6) nasal congestion, and (7) rhinorrhea [Cowling et al., 2010]. Patients with influenza‐like illnesses, defined as a temperature ≥37.8°C plus cough or a sore throat by the US Centers for Disease Control and Prevention (CDC), were also included in the study [Babcock et al., 2006]. A surveillance questionnaire was completed for each patient on whom information regarding patient age, onset of symptoms, influenza vaccination status, presence of an underlying chronic disorder, daycare or school attendance, presence of older siblings in daycare or school, family size, and smoking at home was recorded by a pediatrician.
On their initial visit, all patients were subjected to a thorough physical examination during which their body temperatures were recorded. Nasal swab specimens were then collected from all participants according to a standard operating protocol put forth by the CDC. The procedure involves insertion of a sterile cotton swab into a nostril and each specimen obtained at a depth 2–3 cm is then inoculated into a vial containing M4 viral transport medium (Medical Wire & Equipment, Corsham, UK).
Follow‐Up
Patients/legal guardians and older patients were informed in detail regarding the study protocol and were requested to measure and record body temperatures at least three times a day throughout the duration of the study. Standard protocol required participants to attend follow‐up visits on the third, fifth, and tenth day from the onset of symptoms. For patients who were not able to visit the hospital, parents were contacted by phone. All temperature readings were recorded for each participant, and the highest body temperature during the course of the disease was noted. Parents were also questioned regarding the use of medications (antipyretics, antibiotics, antivirals) during the course of the disease. Resolution times of symptoms that were present on first presentation (e.g., fever, cough, sore throat, myalgia, congestion and rhinorrhea, vomiting, and diarrhea) were also noted. A patient was considered afebrile when his/her body temperature remained <37.5°C for more than 24 hr without the use of antipyretics. Patients who developed complications or required hospitalization were identified and relevant information was recorded.
Viral Assays
Specimens obtained from each patient were stored and transported to the Virology Laboratory of Istanbul University at room temperature to be tested for the presence of an influenza virus by RT‐PCR within 72 hr of collection. Specimens were tested for the presence of non‐influenza viruses using a multiplex one‐step real time PCR method following nucleic acid extraction (Purelink viral RNA/DNA Mini Kit, Carlsbad, CA). Testing was done using an available commercially primers‐probe set and enzyme mixtures (Superscript III Platinum One Step qRT‐PCR Kit with rox, Foster City, CA) compatible with an ABI 7500 RT‐PCR device.
Statistical Analysis
Statistical analyses were performed using the commercial package SPSS for Windows version 15.0 (Chicago, IL). Values for numerical variables were provided as mean ± standard deviation or median (minimum–maximum), depending on normality of distribution. Categorical variables were given as numbers and total percentages. For numerical variables, two‐group comparisons were made using the Mann–Whitney U‐test, whereas the Kruskal–Wallis test with Conover post hoc analysis was preferred for multiple comparisons. Comparisons between groups for categorical variables were made using the Chi‐square (χ 2) test. A P‐value of less than 0.05 was considered indicative of statistical significance.
RESULTS
Demographic, Clinical, and Laboratory Characteristics of the Study Population
A total of 200 patients (49% male, 51% female, and a median age of 48.5 months) with influenza‐like illnesses were enrolled in this study. With regard to age distribution, 4 patients (2%) were aged ≤3 months, 26 patients (13%) were aged between 4 and 12 months, 90 patients (45%) were aged between 13 and 60 months, and 80 patients (40%) were aged ≥61 months. A history of vaccination with the seasonal influenza vaccine was present in 10 patients (5%). Overall, 89 patients (44.5%) were attending school/daycare. A history of another household member having flu‐like symptoms was present in 106 patients (53%), whereas 101 of participants (50.5%) had a history of regular exposure to second‐hand smoke at home.
In terms of medication use prior to presentation, 168 patients (84%) had used antipyretics, while 47 patients (23.5%) reported antibiotic use. The most commonly used antipyretic was paracetamol (76.1%), and amoxicillin–clavulinic acid was the most frequently used antibiotic (68%). Although six patients (3%) were hospitalized during follow‐up due to breathing difficulties and/or feeding problems, none of them required intensive care.
The median duration between onset of symptoms and acquisition of nasopharyngeal swabs was 3 days (1–15). The presence of at least one respiratory virus was detected by RT‐PCR in 102 (51%) patients, while in 94 patients (46%) samples were negative for viral pathogens. Five specimens (2.5%) were deemed inadequate for evaluation by RT‐PCR. The positivity rate of RT‐PCR for the detection of a respiratory virus was 51% (95% confidence interval [CI] 45.2–59.3).
A comparison of demographic and clinical findings in groups with or without virus detection revealed that virally infected patients had fever more frequently and for a longer mean duration (P < 0.05). Although the duration of cough was longer in the virally infected group, this difference did not reach statistical significance (P = 0.07). In this group, nasopharyngeal hyperemia (55.7%), rhonchi and/or rales on chest auscultation (23%), postnasal serous discharge (15.3%), and hyperemia of the tympanic membrane (5.7%) were the most common physical examination findings documented during the first visit to a physician. A summary of demographic and clinical findings of children with influenza‐like illnesses is provided in Table I.
Table I.
Comparison of the Demographic and Clinical Findings Between Virus Detected and Not Detected Groups Presenting With Influenza‐Like Illnesses
| RT‐PCR positive (n = 102) | RT‐PCR negative (n = 92) | P‐Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, monthsa | 53 (1–205) | 43.5 (2–193) | 0.366 |
| Male genderb | 53 (51.9) | 45 (48.9) | 0.208 |
| Duration between onset of symptoms and nasopharyngeal sampling, daysc | 3 ± 1.95 | 3.4 ± 2.57 | 0.554 |
| School/day care attendanceb | 51 (50) | 38 (41.3) | 0.071 |
| Vaccinated against influenzab | 2 (1.9) | 8 (8.6) | |
| Family sizea | 4.3 ± 1.27 | 3.97 ± 0.84 | 0.092 |
| Family member with reported ILIb | 55 (53.9) | 51 (55.4) | 0.553 |
| Smoking at homeb | 49 (48) | 52 (56.5) | 0.254 |
| Older sibling(s) in day care or schoolb | 47 (46) | 48 (52.1) | 0.432 |
| Clinical symptoms | |||
| Feverb | 86 (84.3) | 66 (71.7) | 0.011 |
| Coughb | 94 (92.1) | 82 (89.1) | 0.117 |
| Nasal congestionb | 85 (83.3) | 90 (97.8) | 0.085 |
| Rhinorrheab | 94 (92.1) | 87 (94.5) | 0.650 |
| Sore throatb | 3 (2.9) | 12 (13) | 0.899 |
| Myalgiab | 25 (24.5) | 21 (22.8) | 0.744 |
| Headacheb | 26 (25.4) | 22 (23.9) | 0.759 |
| Breathing difficultiesb | 13 (12.7) | 17 (18.4) | 0.658 |
| Gastrointestinal symptomsb | 9 (8.8) | 3 (3.2) | 0.220 |
| Positive PE findingb | 52 (50.9) | 42 (45.6) | 0.203 |
| Complicationsb | 25 (24.5) | 27 (29.3) | 0.380 |
| Hospitalizationb | 3 (2.9) | 2 (2.2) | 0.746 |
PE, physical examination.
Values are given as median and range.
Values are given as percentage.
Values are given as mean ± standard deviation.
In terms of types/subtypes of respiratory viruses observed in the virally infected group, 40 patients (39.2%) had influenza A (H3N2), 24 patients (23.5%) had influenza B, 16 patients (15.6%) had RSV, 14 patients (13.7%) had HRV, 3 patients (2.9%) had HBoV, 3 patients (2.9%) had HCoV (2 had OC43, 1 had NL63), 1 patient (0.9%) had MPV, and 1 patient (0.9%) had HBoV plus influenza A H3N2 virus. A significant difference in age distribution according to viral pathogen was observed (P < 0.001), with influenza A patients having a median age of presentation of 55 months (11–168) compared to 72.5 months (15–183) in influenza B patients, 14.5 months (1–67) in RSV patients, and 62 months (11–205) in HRV patients. The median age for the other detected viruses was 39 months (8–74). Patients with RSV were strikingly younger than those affected by other viruses, whereas the influenza viruses affected older children (Fig. 1). The difference in median age between patients with RSV and the other viruses (HRV, influenza A, and influenza B) was statistically significant (P < 0.05).
Figure 1.
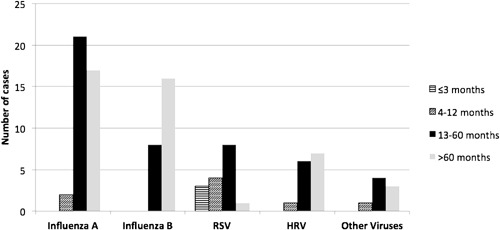
Age distribution according to viral pathogen.
Comparison of symptoms revealed fever and headache to occur more frequently with the influenza viruses compared to the other viruses combined (P < 0.001 and <0.05, respectively). Durations of symptoms such as fever, cough, nasal congestion, and rhinorrhea were also significantly longer in the influenza group (P < 0.001, <0.005, <0.001, and <0.005, respectively). Distribution and frequency of symptoms according to viral pathogen are summarized in Table II. Among the demographic characteristics evaluated, only school/daycare attendance was found to be associated with a significantly increased risk for influenza infection, with no significant difference in association with family size, history of house member with similar symptoms, presence of a smoker in the household (P > 0.05 for all parameters).
Table II.
Distribution and Frequency of Symptoms According to Viral Pathogens
| Influenza viruses (n = 64) | Other viruses (n = 38) | P‐Value | |
|---|---|---|---|
| Age, monthsa | 61 (11–183) | 19 (1–205) | <0.001 |
| Male gender | 32 (50) | 21 (55.2) | 0.599 |
| Clinical symptoms | |||
| Feverb | 62 (96.8) | 24 (63.1) | <0.001 |
| Duration of fever, daysc | 5.50 ± 1.29 | 3.34 ± 1.40 | <0.001 |
| Coughb | 62 (96.8) | 32 (84.2) | 0.135 |
| Duration of cough, daysc | 9.82 ± 2.46 | 8.40 ± 2.10 | <0.005 |
| Nasal congestionb | 54 (84.3) | 31 (81.5) | 1 |
| Duration of congestion, daysc | 8.09 ± 2.05 | 6.25 ± 1.56 | <0.001 |
| Rhinorrheab | 58 (90.6) | 36 (94.7) | 0.253 |
| Duration of rhinorrhea, daysc | 7.94 ± 2.08 | 6.61 ± 1.60 | <0.005 |
| Myalgiab | 18 (28.1) | 7 (18.4) | 0.453 |
| Duration of myalgia, daysc | 3.16 ± 1.04 | 2.57 ± 0.78 | 0.198 |
| Headacheb | 22 (34.3) | 4 (10.5) | 0.020 |
| Duration of headache, daysc | 3.13 ± 1.03 | 3.50 ± 0.57 | 0.429 |
| Breathing difficultiesb | 6 (9.3) | 7 (18.4) | 0.063 |
| Gastrointestinal symptomsb | 8 (12.5) | 1 (2.6) | 0.150 |
| Positive PE findingb | 35 (54.6) | 17 (44.7) | 0.575 |
| Complicationsb | 14 (21.8) | 11 (28.9) | 0.493 |
| Hospitalizationb | 2 (3.1) | 1 (2.6) | — |
PE, physical examination.
Values are given as median (minimum–maximum).
Values are given as percentage.
Values are given as mean ± standard deviation.
Seasonal Variability
Between December 2011 and April 2012, all the cases in whom influenza A was detected presented in December and January, whereas influenza B cases started presenting in January, peaking in March, with cases also presenting in April. The influenza viruses were the most frequently detected viruses (62.7%) during the influenza season, while the frequency of other viral pathogens was 37.2% (Fig. 2).
Figure 2.
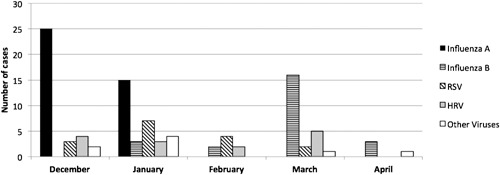
Distribution of viral pathogen according to month.
DISCUSSION
The aim of this study was to determine the prevalence of respiratory viruses and epidemiological characteristics of patients with influenza‐like illnesses during the 2011–2012 influenza season. The overall detection rate of a respiratory virus in our study population was 52%, a value that is slightly higher than previously reported rates of 37% and 48% [Druce et al., 2005; Brittain‐Long et al., 2008]. This difference could be attributed to variations in age, seasonality, environmental factors, and socio‐economic status. Patients enrolled in this study were indeed younger, and nasopharyngeal swabs were obtained during the peak season for respiratory tract infections, both of which are factors known to increase diagnostic yield. Furthermore, the PCR assays performed in this study allowed for the detection of a wider variety of viruses, including HBoV, HCoV‐OC43, NL63, and HMP, which improves detection rate. Underdeveloped immunity and prolonged viral shedding are believed to be the main contributing factors for the higher incidence of viral respiratory tract infections in children [Jartti et al., 2004; Naghipour et al., 2009; To et al., 2010]. Furthermore, unlike adults, young children tend to be brought to medical attention earlier in the course of disease, and it has been established that the detection rate with RT‐PCR is correlated inversely with the duration of symptoms [Brittain‐Long et al., 2010].
In this study, 60% of children presenting to the outpatient clinic with influenza‐like illnesses were younger than 5 years of age. A comparison of presenting age between viral pathogens revealed patients with RSV to be significantly younger than patients with HRV, influenza A, and influenza B (P < 0.05 for all comparisons). Khamis et al. [2012] observed in community‐based study that RSV infection occurred solely in children aged less than 2 years of age, with 57% of infections occurring in infants younger than 6 months. The oldest patient with RSV in the present study was a 67‐month‐old child, and only 4 (25%) out of 16 patients with documented RSV were older than 2 years of age. Additionally, an influenza virus was detected in nearly two‐thirds of patients who were included in the virally infected group, with an overall frequency in children with influenza‐like illnesses of 32%. In this research group's study from last influenza season, influenza viruses were detected in 129 of 300 patients (43%) with influenza‐like illnesses [Ceyhan et al., 2012]. While the H1N1 strain of influenza A was predominant last season, it has been replaced this season by the H3N2 strain as well as influenza B. Other viruses that were detected this season were RSV, HRV, HBoV, HCoV, and MPV. The present study shows that it may not be possible to distinguish between influenza viruses and other respiratory viruses in patients with influenza‐like illnesses based on symptoms alone, since all the respiratory pathogens evaluated have the potential to cause a febrile illness in affected children during the influenza season.
Respiratory virus infections of early childhood are confined mostly to the upper respiratory tract resulting in common cold‐like symptoms such as coryza, cough and sore throat. On the contrary, nearly one‐third of infants with respiratory viral infections present with symptoms such as tachypnea, wheezing, severe cough, breathlessness, and respiratory distress, suggestive of lower respiratory tract involvement [Pavia, 2011]. In this study, 23% of patients who had a positive RT‐PCR result had lower respiratory symptoms, whereas the most frequently encountered symptoms were cough (92.1%), rhinorrhea (92.1%), fever (84.3%), nasal congestion (83.3%), headache (25.3%), and myalgia (24.5%). The presence of a significant difference between viral pathogens in terms of frequency and duration of symptoms is also demonstrated. Fever and headache were observed more commonly in patients with infections due to the influenza viruses compared to other viruses, while the duration of symptoms such as fever, cough, rhinorrhea and nasal congestion was also significantly longer with influenza virus infections. In this group's recent study during the last influenza season [Ceyhan et al., 2012], it was also observed that the duration of symptoms such as cough, nasal congestion, and rhinorrhea in influenza positive patients were longer than those reported in the previous season, with no significant difference in the duration of fever. The conclusion shared by different studies is that on its own, clinical presentation should not be considered a reliable indicator of the causative viral pathogen.
The rate of detecting multiple pathogens, the clinical significance of which remains disputed, was much lower in this study (0.9%) compared to previous community‐based studies [Brittain‐Long et al., 2012; Khamis et al., 2012]. However, it has been postulated that multiple viral infections may be associated with higher fever, longer durations of hospital stay, more frequent use of antibiotics and increased risk of requiring intensive care in a hospital‐based study [Semple et al., 2005]. Current evidence suggests that co‐infections such as RSV + HRV or RSV + HMPV may lead to a more severe clinical course of disease in infants with bronchiolitis [Semple et al., 2005; Paranhos‐Baccala et al., 2008; Richard et al., 2008]. Only one of the patients in the study was coinfected with HBoV and the H3N2 strain of influenza A, which was not found to be associated with a more severe clinical course than the other virally infected patients. No clear consensus on the effect of co‐infection on disease severity is remained.
Risk factors for acquiring a viral respiratory tract infection have been investigated extensively in numerous community and hospital‐based studies [Esposito et al., 2011; Khamis et al., 2012; Papenburg et al., 2012]. Male gender, low socioeconomic status, exposure to cigarette smoke or animals, history of breastfeeding, presence of a co‐morbidity such as asthma, chronic heart disease or iron deficiency anemia have all been linked with an increased risk of RSV infection. Data on risk factors for other viral pathogens are limited. In a recent study on an adult population, cigarette smoking was found to be associated with higher mortality in patients with influenza [Wong et al., 2013]. In the present study, younger age and daycare/school attendance were linked with an increased likelihood of having a viral pathogen (P < 0.005). In contrast, a history of household smoking, another house member with similar symptoms, and siblings attending school/daycare did not seem to be significantly associated with an increased risk for respiratory infection with a viral etiology (P > 0.05, for all).
Common respiratory viruses usually show typical seasonal distribution in temperate climates. The best examples are RSV, influenza A, and occasionally HMPV, which usually peak during the winter months [Stensballe et al., 2003; Monto, 2004; du Prel et al., 2009]. In a previous study from India, a similar pattern for influenza A and RSV has been observed [Agrawal et al., 2009]. An age distribution leaning toward a young age for RSV infections during winter may be attributed to an actual high incidence as well as increased parental awareness. It has long been established through several observational studies that the seasonal incidence of many respiratory viruses is influenced by weather conditions. The incidence of RSV infections has been found to be correlated inversely with average temperature, while a significant positive association between the incidence of HRV infections and increased humidity has also been reported [du Prel et al., 2009]. During the 5‐month follow‐up period in this study, influenza A cases were seen mostly in the months of December and January, with no further cases after February. On the other hand, the first case of influenza B was detected in January, with the number of new cases peaking in March. Cases of RSV and HRV presented in each month of the influenza season studied.
One of the main limitations of this study is that subjects were not randomized which could have led to a certain degree of bias. However, it is believed that potential bias was minimized since the goal was to enroll all patients presenting to the outpatient clinic during the 5‐month study period, provided consent was given. Another limitation is that children with respiratory tract infections due to bacterial pathogens such as Streptococcus pneumoniae and Streptococcus pyogenes were not excluded from the study, which may have affected the calculated detection rates.
The findings of this study suggest that almost all of the major groups of respiratory viruses may be responsible for influenza‐like illnesses in children from Ankara, Turkey. Among the patients, the influenza viruses were the most frequently detected viruses (62.8%), with non‐influenza viruses responsible for the remaining 37.2% of cases. Taking the overall viral pathogen detection rate of 51% into consideration, it is obvious to conclude that other respiratory pathogens, whether viral or bacterial, also lead to respiratory tract infections related hospital visits in children.
ACKNOWLEDGMENT
We are grateful to Sevilay Karahan, Ph.D., Department of Biostatistics, Hacettepe University for her help in the statistical analysis.
REFERENCES
- Agrawal AS, Sarkar M, Chakrabarti S, Rajendran K, Kaur H, Mishra AC, Chatterjee MK, Naik TN, Chadha MS, Chawla‐Sarkar M. 2009. Comparative evaluation of real‐time PCR and conventional RT‐PCR during a 2 year surveillance for influenza and respiratory syncytial virus among children with acute respiratory infections in Kolkata, India, reveals a distinct seasonality of infection. J Med Microbiol 58:1616–1622. [DOI] [PubMed] [Google Scholar]
- Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung‐Lindell A, van den Hoogen BG, Hyypiä T, Ruuskanen O. 2007. Human bocavirus and acute wheezing in children. Clin Infect Dis 44:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock HM, Merz LR, Fraser VJ. 2006. Is influenza an influenza‐like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol 27:266–270. [DOI] [PubMed] [Google Scholar]
- Bramley TJ, Lerner D, Sames M. 2002. Productivity losses related to the common cold. J Occup Environ Med 44:822–829. [DOI] [PubMed] [Google Scholar]
- Brittain‐Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M. 2008. Multiplex real‐time PCR for detection of respiratory tract infections. J Clin Virol 41:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain‐Long R, Westin J, Olofsson S, Lindh M, Andersson LM. 2010. Prospective evaluation of a novel multiplex real‐time PCR assay for detection of fifteen respiratory pathogens‐duration of symptoms significantly affects detection rate. J Clin Virol 47:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain‐Long R, Andersson LM, Olofsson S, Lindh M, Westin J. 2012. Seasonal variations of 15 respiratory agents illustrated by the application of a multiplex polymerase chain reaction assay. Scand J Infect Dis 44:9–17. [DOI] [PubMed] [Google Scholar]
- Ceyhan M, Karadag‐Oncel E, Badur S, Ciblak MA, Alhan E, Celik US, Kurugol Z, Saz EU, Ozsurekci Y, Celik M, Parlakay AO. 2012. Effectiveness of a new bioequivalent formulation of oseltamivir (Enfluvir®) on 2010–2011 seasonal influenza viruses: An open phase IV study. Int J Infect Dis 16:273–278. [DOI] [PubMed] [Google Scholar]
- Cowling BJ, Chan KH, Fang VJ, Lau LL, So HC, Fung RO, Ma ES, Kwong AS, Chan CW, Tsui WW, Ngai HY, Chu DW, Lee PW, Chiu MC, Leung GM, Peiris JS. 2010. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med 362:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce J, Tran T, Kelly H, Kaye M, Chibo D, Kostecki R, Amiri A, Catton M, Birch C. 2005. Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002–2003. J Med Virol 75:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Prel JB, Puppe W, Grondahl B, Knuf M, Weigl JA, Schaaff F, Schmitt HJ. 2009. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis 49:861–868. [DOI] [PubMed] [Google Scholar]
- Esposito S, Molteni CG, Daleno C, Valzano A, Fossali E, Da Dalt L, Cecinati V, Bruzzese E, Giacchino R, Giaquinto C, Lackenby A, Principi N. 2011. Clinical and socioeconomic impact of different types and subtypes of seasonal influenza viruses in children during influenza seasons 2007/2008 and 2008/2009. BMC Infect Dis 11:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. 2003. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Arch Intern Med 24:487–494. [DOI] [PubMed] [Google Scholar]
- Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140:543–546. [DOI] [PubMed] [Google Scholar]
- Hatipoğlu N, Somer A, Badur S, Unüvar E, Akçay‐Ciblak M, Yekeler E, Salman N, Keser M, Hatipoğlu H, Siraneci R. 2011. Viral etiology in hospitalized children with acute lower respiratory tract infection. Turk J Pediatr 53:508–516. [PubMed] [Google Scholar]
- Henrickson KJ, Hoover S, Kehl KS, Hua W. 2004. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J 23:11–18. [DOI] [PubMed] [Google Scholar]
- Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. 2004. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol 72:695–699. [DOI] [PubMed] [Google Scholar]
- Johansson N, Kalin M, Hedlund J. 2011. Clinical impact of combined viral and bacterial infection in patients with community‐acquired pneumonia. Scand J Infect Dis 43:609–615. [DOI] [PubMed] [Google Scholar]
- Khamis FA, Al‐Kobaisi MF, Al‐Areimi WS, Al‐Kindi H, Al‐Zakwani I. 2012. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J Med Virol 84:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew J, Pinto Pereira LM, Pappas TE, Swenson CA, Grindle KA, Roberg KA, Lemanske RF, Lee WM, Gern JE. 2009. Distribution and seasonality of rhinovirus and other respiratory viruses in a cross‐section of asthmatic children in Trinidad, West Indies. Ital J Pediatr 35:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS. 2004. Occurrence of respiratory virus: Time, place and person. Pediatr Infect Dis J 23:58–64. [DOI] [PubMed] [Google Scholar]
- Naghipour M, Hart CA, Dove W, Leatherbarrow AJ, Cuevas LE. 2009. Adenovirus infections within a family cohort in Iran. Pediatr Pulmonol 44:749–753. [DOI] [PubMed] [Google Scholar]
- Papenburg J, Hamelin MÈ, Ouhoummane N, Carbonneau J, Ouakki M, Raymond F, Robitaille L, Corbeil J, Caouette G, Frenette L, De Serres G, Boivin G. 2012. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis 206:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranhos‐Baccala G, Komurian‐Pradel F, Richard N, Vernet G, Lina B, Floret D. 2008. Mixed respiratory virus infections. J Clin Virol 43:407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia AT. 2011. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 52:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierangeli A, Gentile M, Di Marco P, Pagnotti P, Scagnolari C, Trombetti S, Lo Russo L, Tromba V, Moretti C, Midulla F, Antonelli G. 2007. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol 79:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard N, Komurian‐Pradel F, Javouhey E, Perret M, Rajoharison A, Bagnaud A, Billaud G, Vernet G, Lina B, Floret D, Paranhos‐Baccalà G. 2008. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J 27:213–217. [DOI] [PubMed] [Google Scholar]
- Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA. 2005. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis 191:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensballe LG, Devasundaram JK, Simoes EA. 2003. Respiratory syncytial virus epidemics: The ups and downs of a seasonal virus. Pediatr Infect Dis J 22:21–32. [DOI] [PubMed] [Google Scholar]
- To KK, Chan KH, Li IW, Tsang TY, Tse H, Chan JF, Hung IF, Lai ST, Leung CW, Kwan YW, Lau YL, Ng TK, Cheng VC, Peiris JS, Yuen KY. 2010. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol 82:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 7:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L, Pyrc K, Jebbink MF, Vermeulen‐Oost W, Berkhout RJ, Wolthers KC, Wertheim‐van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. 2004. Identification of a new human coronavirus. Nat Med 10:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Yang L, Chan KP, Chan WM, Song L, Lai HK, Thach TQ, Ho LM, Chan KH, Lam TH, Peiris JS. 2013. Cigarette smoking as a risk factor for influenza‐associated mortality: Evidence from an elderly cohort. Influenza Other Respi Viruses 7:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]


