Abstract
Viruses of lower vertebrates recently became a field of interest to the public due to increasing epizootics and economic losses of poikilothermic animals. These were reported worldwide from both wildlife and collections of aquatic poikilothermic animals. Several RNA and DNA viruses infecting fish, amphibians and reptiles have been studied intensively during the last 20 years. Many of these viruses induce diseases resulting in important economic losses of lower vertebrates, especially in fish aquaculture. In addition, some of the DNA viruses seem to be emerging pathogens involved in the worldwide decline in wildlife. Irido‐, herpes‐ and polyomavirus infections may be involved in the reduction in the numbers of endangered amphibian and reptile species. In this context the knowledge of several important RNA viruses such as orthomyxo‐, paramyxo‐, rhabdo‐, retro‐, corona‐, calici‐, toga‐, picorna‐, noda‐, reo‐ and birnaviruses, and DNA viruses such as parvo‐, irido‐, herpes‐, adeno‐, polyoma‐ and poxviruses, is described in this review.
Introduction
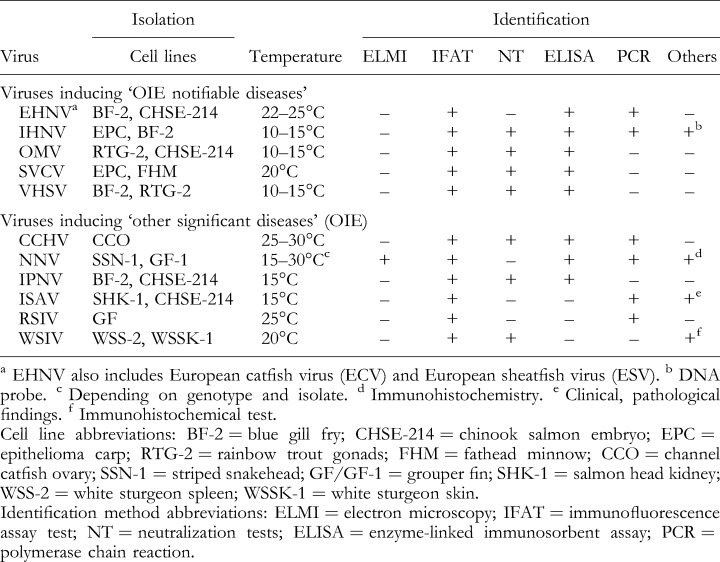
Information on viruses occurring in poikilothermic vertebrates is still behind the knowledge of viruses of homoiothermic vertebrates. About 30 years ago, very little was known about viruses and viral diseases of fish, amphibians and reptiles. However, the maintenance of viruses in lower vertebrates proved to be of veterinarian and public interest, especially RNA viruses causing severe diseases in fish aquaculture that became of worldwide economical importance. Some DNA viruses also induce diseases resulting in important economic losses in fish farms, such as channel catfish herpesvirus disease and epizootic haematopoietic necrosis. In addition, herpesviruses such as green sea turtle fibropapillomatosis virus and frog iridoviruses might be responsible for losses in wildlife affecting endangered species. However, some of these DNA viruses have been used as models for studying disease mechanisms. For example, the Lucké tumour herpesvirus of frogs increased knowledge of the formation of tumours and metastases. Also, adenoviruses of lower vertebrates could be used as vectors for gene therapy or as vaccine vectors in aquaculture. An increased number of studies on viruses of lower vertebrates have been undertaken in recent years, giving greater insight into the biology and characteristics of these viral agents. During the last 20 years understanding of the virology of lower vertebrates has been substantially improved. The following review, divided into virus families, comprises an overview of the present knowledge on RNA (Figs 1 and 2) and DNA viruses (Figs 8 and 9, p. 431, 432) of lower vertebrates. Classification and nomenclature of the viruses described is based on the Seventh Report of the International Committee on Taxonomy of Viruses (van Regenmortel et al., 2000). Target viruses of fish, amphibians and reptiles are isolated and identified according to standard virological procedures. In contrast to the viruses of homoiothermic animals, viruses of poikilothermic animals usually replicate below 30°C in several cell cultures derived from fish (examples in Table 1, p. 406), amphibians or reptiles. Such cell lines are available from the American Type Culture Collection (ATCC), 12301 Parklawn Drive, Rockville, MD 20852–1776, USA (http://www.atcc.org) and from the European Collection of Cell Cultures, Centre for Applied Microbiology and Research, Salisbury, Wiltshire SP4 OJG, UK (http://www.ecacc.org.uk). However, some of the viruses of poikilothermic vertebrates replicate in avian and mammalian cell cultures at temperatures below 30°C. The fish disease commission of the Office International des Epizooties (OIE), 12 rue de Prony, 75017 Paris, France (http://www.oie.int), elaborated for fish diseases the International Aquatic Health Code (OIE, 2001). ‘Diseases notifiable to the OIE’ (previously ‘List B diseases’) are considered to be of socio‐economic and/or public health importance within countries, and significant to the international trade in aquatic animals and aquatic animal products. ‘Other significant diseases’ are of current or potential international significance in aquaculture. The Code includes six notifiable or significant fish pathogenic RNA viruses: Orthomyxoviridae– infectious salmon anaemia virus (ISAV); Rhabdoviridae– infectious haematopoietic necrosis virus (IHNV), spring viraemia of carp virus (SVCV), viral haemorrhagic septicaemia virus (VHSV); Nodaviridae– nervous necrosis virus (NNV), and Birnaviridae– infectious pancreatic necrosis virus (IPNV). The Code includes also five notifiable or significant fish pathogenic DNA viruses: Iridoviridae– epizootic haematopoietic necrosis virus (EHNV), red sea bream iridovirus (RSIV), white sturgeon iridovirus (WSIV); Herpesviridae– Oncorhynchus masou virus (OMV; salmonid herpesvirus 2, SaHV‐2), and channel catfish herpesvirus (CCHV). Table 1 gives an overview of virus isolation and identification of these viruses according to the International Aquatic Animal Health Code and Diagnostic Manual for Aquatic Animal Diseases, copies of which are available from the OIE. Some representative viruses of lower vertebrates are shown in Figs 2 and 9.
Figure 1.

RNA viruses occurring in lower vertebrates. 1 Arthropod‐borne viruses termed ‘arboviruses’ in the review.
Figure 2.
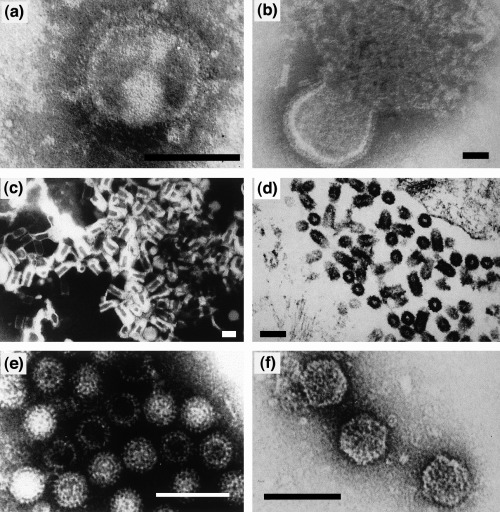
Electron microscopic images of some representative RNA viruses from poikilothermic animals: (a) orthomyxovirus‐like particles from eels (bar: 75 nm); (b) snake paramyxovirus (bar: 60 nm); (c) viral haemorrhagic septicaemia virus, VHSV (a rhabdovirus; bar: 60 nm); (d) spring viraemia of carp virus, SVCV (a rhabdovirus; bar: 150 nm); (f) grass carp reovirus (bar: 150 nm); (g) infectious pancreatic necrosis virus, IPNV (a birnavirus; bar: 80 nm). (a)–(c), (e) and (f) negative staining; (d) ultrathin section.
Figure 8.

DNA viruses occurring in poikilothermic vertebrates.
Figure 9.

Electron microscopy of DNA viruses detected in poikilothermic vertebrates: (a) corn snake parvovirus (bar: 100 nm); (b) tadpole oedema virus, TEV (a frog iridovirus; bar: 200 nm) (kindly provided by K. Wolf, USA); (c) European catfish iridovirus (ECV) budding from infected BF‐2 cells (bar: 250 nm); (d) toad herpesvirus (Pelobates fucus, bar: 75 nm) (F.T. Just, unpublished); (e) corn snake adenovirus (bar: 75 nm).
Table 1.
Isolation and identification of fish viruses inducing Office International des Epizooties (OIE) notifiable diseases, according to the Diagnostic Manual for Aquatic Animal Diseases (OIE, 2001). The OIE notifiable viruses are epizootic haematopoietic necrosis virus (EHNV), infectious haematopoietic necrosis virus (IHNV), Oncorhynchus masou virus (OMV), spring viraemia of carp virus (SVCV), and viral haemorrhagic septicaemia virus (VHSV). The viruses inducing other significant diseases are channel catfish herpesviruses (CCHV), nervous necrosis virus (NNV), infectious pancreatic necrosis virus (IPNV), infectious salmon anaemia virus (ISAV), red sea bream iridovirus (RSIV) and white sturgeon iridovirus (WSIV)
RNA Viruses of Lower Vertebrates
Figure 1 summarizes the RNA viruses occurring in lower vertebrates, and Fig. 2 shows electron microscopic images of some representative RNA viruses occurring in poikilothermic animals.
Single‐stranded (–) RNA viruses
Orthomyxoviridae
The family Orthomyxoviridae contains the genera influenzavirus A, influenzavirus B, influenzavirus C and thogoto‐like viruses. Common features are spherical or pleomorphic virions of 80–120 nm in diameter, including an envelope with surface projections. Virions contain eight (influenzaviruses A and B), seven (influenzavirus C) or six (thogoto‐like virus) segments of linear, negative‐sense ssRNA. Orthomyxovirus‐like particles occurring in lower vertebrates are not included in the present taxonomy of viruses (van Regenmortel et al., 2000).
Orthomyxoviruses occurring in fish
Eel viruses (A1B, EV1 and EV2)
Orthomyxovirus‐like particles have been isolated from European eel (Anguilla anguilla) with stomatopapilloma (‘cauliflower disease’) (Nagabayashi and Wolf, 1979; Neukirch, 1985). The agents designated A1B, EV1 and EV2 represent pleomorphic particles of 80–140 nm in diameter with rod‐shaped projections of 10 nm. EV2 has a buoyant density of 1.19 g/ml sucrose. It haemagglutinates chicken erythrocytes and replicates in fathead minnow (FHM) cells at 10–15°C, inducing syncytia formation. Maximal titres of 105–106 TCID50/ml were obtained in FHM cells at 15°C (Nagabayashi and Wolf, 1979). The pathogenicity of EV isolates and the relationship of the eel orthomyxoviruses to each other and to other orthomyxoviruses are not known (Wolf, 1988).
Infectious salmon anaemia virus (ISAV)
An orthomyxovirus has been isolated from Atlantic salmon (Salmo salar) in Norway suffering from infectious salmon anaemia (ISA) (Falk et al., 1997). ISA, caused by infectious salmon anaemia virus (ISAV), has been recognized as a notifiable disease in Norway since 1988. In 1996, ISA cases were reported in Canada (Mullins et al., 1998). There ISAV was found to also be associated with haemorrhagic kidney syndrome (HKS) in Atlantic salmon (Lovely et al., 1999). Two years later, ISA was detected in Scotland (Rodger et al., 1998). ISAV causes severe economic losses of Atlantic salmon. Sea trout, rainbow trout and Atlantic herring are also susceptible and may function as reservoirs of the virus. The systemic infection can be lethal and is characterized by anaemia, ascites, congestion and enlargement of the liver and spleen. The virus is transmitted by sea lice (Caligus elongatus, Lepeophtheirus salmonis) and via coprophagy (Rolland and Nylund, 1998). It does not seem to be transmitted vertically (Melville and Griffiths, 1999). OIE guidelines and directive 93/53 of the European Community do not include details on confirmation of the virus (IFAT, PCR) and the activities necessary during outbreaks (eradication). ISAV multiplies in salmon head kidney cells (SHK‐1) and in chinook salmon embryo cells (CHSE‐214) at 15°C (Dannevig et al., 1995; Bouchard et al., 1999; Kibenge et al., 2000). Sialic acid residues on the cell surface function as binding sites and fusion of the virus takes place in the acidic endosomes (Eliassen et al., 2000). Actinomycin D inhibits the viral replication in vitro. ISAV is sensitive to chloroform, heat and low pH. The virus has a buoyant density of 1.18 g/ml in sucrose and caesium chloride gradients. ISAV contains four major polypeptides of 71, 53, 43 and 24 kDa (Falk et al., 1997). The genome (14.5 kb) of ISAV consists of eight RNA segments of 1.0–2.3 kb (Mjaaland et al., 1997). Viral RNA termini resemble those of influenzaviruses in both their sequences and secondary structures. These features indicate replication mechanisms similar to other orthomyxoviruses (Sandvik et al., 2000). Genomic comparison of ISAV‐isolates from Europe (Norway, Scotland) and North America (Canada) suggests distinct geographical variants (Blake et al., 1999; Cunningham and Snow, 2000). Phenotypic differences have been found among ISAV strains (Kibenge et al., 2000). Nucleotide sequence of segments 2 and 8 of ISAV demonstrated the separation of European and Canadian ISAV strains and revealed no homogenicity between European strains (Inglis et al., 2000). Analysis of the polymerase (PB1) protein (encoded in segment 2) showed low sequence similarity to other orthomyxoviruses. Phylogenetic analysis revealed significant distance between ISAV and the established genera of Orthomyxoviridae. ISAV possibly represents a species of the proposed genus Aquaorthomyxovirus of Orthomyxoviridae (Krossoy et al., 1999). ISA is an OIE significant disease (OIE, 2001).
Paramyxoviridae
The family Paramyxoviridae contains the genera Respirovirus, Morbillivirus, Rubulavirus, Pneumovirus and Metapneumovirus. Common features are spherical, pleomorphic virions of 150 (or more) nm in diameter including an envelope with spike‐like projections. The nucleocapsid comprises a single molecule of linear, non‐infectious, negative‐sense ssRNA and 10–12 proteins. Fer‐de‐Lance virus (FDLV) is an unassigned virus in the family (van Regenmortel et al., 2000).
Paramyxoviruses occurring in fish
An enveloped, pleomorphic RNA virus of 125–250 nm in diameter with one molecule of helical nucleocapsid (18 nm in diameter, about 1000 nm in length) has been isolated from chinook salmon (Oncorhynchus tshawytscha) in the USA. The chloroform‐sensitive agent has a buoyant density of 1.20 g/ml in caesium chloride gradients and haemagglutinates erythrocytes of fish, birds and mammals. The virus replicates in several fish cell lines at 18°C. The aetiological role of the isolate is not known (Winton et al., 1985). Paramyxovirus‐like particles (100–300 nm) have been isolated from rainbow trout (Oncorhynchus mykiss) and pike (Esox lucius). The agent has proved to be pathogenic to rainbow trout fry (Neumann et al., 1986). Enveloped viral particles displaying features of paramyxovirus could be detected in the cytoplasm of necrotized epithelial cells of black sea bream (Acanthopagrus schlegeli) by electron microscopy (Miyazaki et al., 1989).
Paramyxoviruses occurring in reptiles
Fer‐de‐Lance virus (FDLV)
A disease outbreak in a snake farm in Switzerland was recognized in 1972: 128 American pit vipers (Fer‐de‐Lance, Bothrops atrox) died out of a population of 431 animals. The moribund snakes showed respiratory signs, lethargy and central nervous symptoms (Foelsch and Leloup, 1976). A virus (FDLV) with properties characteristic of Paramyxoviridae was isolated from the lung tissue of a dead Fer‐de‐Lance. The agent replicates in embryonated reptilian and chicken eggs and in a variety of reptilian or mammalian cell cultures at an optimal growth temperature of 30°C. Infected cells show syncytia formation, cytoplasmic inclusion bodies and lysis. The pleomorphic virus (146–321 nm in diameter) exhibits an unsegmented ssRNA genome within an internal nucleocapsid (14–16 nm in diameter). The virus possesses neuraminidase and haemagglutination activities. FDLV (ATCC VR‐895) is antigenically distinct from known paramyxoviruses of birds and mammals (Clark et al., 1979).
Ophidian paramyxoviruses (OPMV)
Paramyxovirus‐like particles (OPMV), physicochemically similar to FDLV, have been detected in different snakes (Boidae, Crotalidae, Colubridae, Elapidae, Viperidae) and lizards (Teidae) (Ahne, 1991). The antigenically related viruses induce syncytia formation in infected cells and haemagglutinate erythrocytes. Affected animals usually show proliferative pneumonia; some snakes exhibit severe pancreatic necrosis and nervous symptoms. Several die‐offs associated with paramyxoviruses involving snakes in collections have been reported (Jacobson, 1986). The pleomorphic virions measure between 30 and 560 nm in diameter, have a helical nucleocapsid and carry an envelope with spikes, e.g. transmembrane glycoproteins with neuraminidase and haemagglutinating activities. The viral nucleocapsid is composed of one molecule of ssRNA associated with proteins. Viral polypeptides have been identified as polymerase (L‐, P‐), haemaglutinin (HN‐), nucleoprotein (NP‐), fusion (F‐), and matrix (M‐) proteins. The viruses are sensitive to ether and to acidic (pH 5) and basic (pH 12) conditions. Virion buoyant density in sucrose is 1.18 g/cm3. No cross‐reactivity of OPMV with the paramyxoviruses of homoiothermic vertebrates has been recognized (Richter et al., 1996). Protein migration patterns of the snake viruses are similar in PAGE, but they are different from those of Sendai virus. Immunoblotting does not show any antigenic relationship to Sendai virus, mumps virus, measles virus, or influenzaviruses A and B (Ahne et al., 1987a; Ahne and Neubert, 1989). However, haemagglutination inhibition tests have revealed an antigenic relationship between the ophidian paramyxoviruses isolated from snakes (Colubridae, Crotalidae, Elapidae, Viperidae) in Germany and avian paramyxoviruses serotypes 1 and 7 (Blahak, 1995). Comparative sequence analysis of partial HN‐ and L‐gene of 15 OPMV and the FDLV has revealed two major genomic subgroups. The subgroups represent distinct virus species (nucleotide divergence values of 20–22%) containing multiple virus strains. The phylogenetic grouping reflects correlation with geographical origin of the viruses (Fig. 3) (Ahne et al., 1999). FDLV is listed as an ‘unassigned virus’ in the family Paramyxoviridae; the other reptilian paramyxoviruses are presently not recognized by the International Committee on Taxonomy of Viruses (ICTV) (van Regenmortel et al., 2000).
Figure 3.

Phenogram showing the genetic relationships between 16 reptilian paramyxoviruses and Sendai virus, based on analysis of part of the L‐gene (nucleotides 9648–10 201). Bootstrap values >700 for the major branches are shown. Place of isolation is indicated by EUR (Europe)/USA, with the numbers following EUR/USA indicating years of isolation. Hosts: Bitis=Bitis sp.; Boa=Boa constrictor; Cro1–3=Crotalus spp. Trim=Trimeresurus spp.; Call=Callopistes maculatus; Both=Bothrops atrox; Ela 1–2=Elaphe guttata; Gono=Gonosoma oxycephala; Lamp=Lampropeltis sp.; Pyth=Python regius; More=Morelia argus (Ahne et al., 1999).
Paramyxoviruses occurring in turtles and lizards
Mediterranean turtles (Testudo graeca, T. hermanni) with dermatitis, apathy and anorexia showed intracytoplasmic inclusion bodies of different sizes in the skin. Electron microscopy has revealed pleomorphic particles (140–500 nm) with nucleocapsids of 12 nm in diameter (Zangger et al., 1991). The authors have recognized three epizootics associated with viral dermatitis in Switzerland. Testudo graeca and T. hermanni suffering from rhinitis have shown high antibody titres against Sendai virus (Jackson and Needham, 1983). Furthermore, a serological survey carried out in Germany has revealed high incidence (44–94%) of Sendai virus antibodies in land tortoise populations (Witte, 1993). A paramyxovirus‐like agent occurring in lizards has been isolated from apparently healthy teju (Callopistes maculatus) (Ahne and Neubert, 1991). Wild healthy iguanas (Ctenosaura bakeri, C. similis, Iguana iguana rhinolopha) on Honduran Islands were tested for the presence of antibodies to reptilian paramyxoviruses. Of the 49 animals tested 41% showed specific antibodies in neutralization and haemagglutination inhibition tests (Gravendyck et al., 1998).
Rhabdoviridae
The family Rhabdoviridae contains the genera Vesiculovirus, Lyssavirus, Ephemerovirus, Cytorhabdovirus, Nucleorhabdovirus and Novirhabdovirus. Rhabdoviruses share some features with members of Filoviridae and Paramyxoviridae (Mononegavirales). Common features of rhabdoviruses are bacilliform (plant rhabdoviruses) or bullet‐shaped (vertebrate rhabdoviruses) morphology. The enveloped virions measure 100–430 × 45–100 nm. The infectious nucleocapsid (30–70 nm in diameter) contains a single molecule of linear, negative‐sense ssRNA. rhabdoviruses generally have five structural polypeptides (polymerase, L; glycoprotein, G; nucleocapsid, N; phosphoprotein, P, and matrix, M). The N‐, L‐ and P‐proteins are associated with the nucleocapsid. The envelope containing the G‐protein is connected with the nucleocapsid by the M‐protein. The piscine rhabdoviruses infectious haematopoietic necrosis virus (IHNV), hirame rhabdovirus (HIRRV), and viral haemorrhagic septicaemia virus (VHSV) comprise species of the genus Novirhabdovirus. Tentative species of the this genus are eel virus B12 (EEV‐B12), eel virus C26 (EEV‐C26), and snakehead rhabdovirus (SHRV). The genus Vesiculovirus includes pike fry rhabdovirus (PFR), spring viraemia of carp virus (SVCV), eel virus American (EVA), and the ulcerative disease rhabdovirus (UDRV) as tentative species of the genus (van Regenmortel et al., 2000).
Rhabdoviruses occurring in fish
Rhabdoviruses constitute one of the largest groups of viruses isolated from teleost fish. The viruses are mostly associated with epizootics and heavy losses in piscine aquaculture. The transmission (Fig. 7, p. 430) of fish pathogenic rhabdoviruses occurs mainly by shedding from infected fish, and the viruses are spread by waterborne contact (Wolf, 1988). The early targets for the viruses are gills, the oesophagus–cardiac stomach region, and mucus‐secreting glands (Fig. 5, p. 416). As shown for VHSV, the primary receptor for fish rhabdoviruses was found to be a cell surface complex where fibronectin acts as an initial cell molecule target (Bearzotti et al., 1999). DNA vaccines encoding the G‐protein of several fish pathogenic rhabdoviruses induce an early interferon‐mediated non‐specific protection followed by a specific immune response (Kim et al., 2000). Several rhabdoviruses have been isolated from a variety of freshwater and marine fish species worldwide, but only some of the agents have been studied in detail. Many of the piscine rhabdoviruses may represent strains or variants of piscine novirhabdovirus or vesiculovirus species. The novirhabdoviruses can be distinguished from the piscine vesiculoviruses (Table 2) by a non‐structural protein (12–14 kDa, 111 amino acids), the non‐virion protein (NV‐protein) (Kurath et al., 1997). The NV protein plays an important role in the viral replication (Nichol et al., 1995). New findings suggest that the IHNV NV protein is not absolutely required for viral replication, but its presence greatly improves viral multiplication (Biacchesi et al., 2000a). The presence of the highly conserved NV genes, located between the G‐ and L‐genes, proved to be unique to the members of the new established genus Novirhabdovirus of Rhabdoviridae (Walker et al., 2000). An RNase protection assay revealed NV gene sequence variations between IHN virus strains (Kurath et al., 1995). Recently it was shown that synthetic cDNA minigenomes of the two salmonid novirhabdoviruses IHNV and VHSV could successfully be recovered following heterologous virus infection as these were replicated, encapsulated and transcribed (Biacchesi et al., 2000b). The molecular biology of fish pathogenic rhabdoviruses has been reviewed by Enzmann (2000). Fish rhabdoviruses such as IHNV and VHSV induce CO2 sensitivity in Drosophila melanogaster in a similar way to the insect rhabdovirus Sigma virus (Bussereau et al., 1975).
Figure 7.
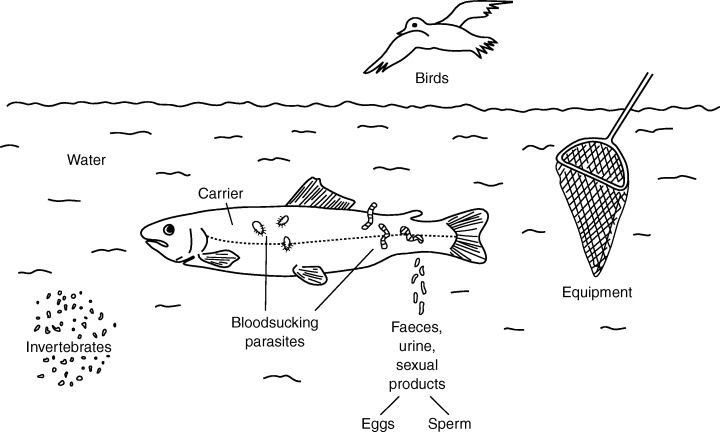
Transmission of fish viruses and their vectors.
Figure 5.

Uptake and spreading of spring viraemia of carp virus (SVCV) in carp fry infected by bath exposure.
Table 2.
Classification of rhabdoviruses occurring in teleost fishes

Infectious haematopoietic necrosis virus (IHNV)
Infectious haematopoietic necrosis virus (IHNV) is responsible for a highly contagious disease (infectious haematopoietic necrosis, IHN) of salmon and trout (Onchorhynchus, Salmo) occurring at water temperatures between 8 and 15°C. The multiplication of virus takes place in endothelial cells of blood capillaries leading to haemorrhages in haematopoietic tissues and nephron cells. Death of fish is finally due to impairment of osmotic balance in connection with oedema. The age of fish influences the course of the infection: the younger the fish the more susceptible they are to the disease. Survivors developed IHNV‐neutralizing antibodies leading to protective immunity. The transmission of IHNV takes place horizontally, vertically, and by biological vectors such as fish parasites (Piscicola salmositica, Salmincola sp.) and carrier fish (Wolf, 1988; Mulcahy et al., 1990). Acutely infected fish release the virus by faeces, urine and external mucus. Carriers shed the agent via sexual products. Truncated IHNV particles, detected in persistently infected rainbow trout, proved to be mediators of persistence of the virus (Kim et al., 1999). IHNV, the type species of the genus Novirhabdovirus, was enzootic in North America for decades, but the agent has been spread to the Far East and continental Europe. IHNV epizootics occur usually in young Salmoniformes, but Acipenseriformes, Pleuronectiformes and Perciformes are also susceptible to the virus (Winton, 1992). A DNA vaccine containing the gene for the glycoprotein (G) induced protective immunity and neutralizing antibodies in rainbow trout fry (Corbeil et al., 2000). IHNV shares physicochemical characteristics of Rhabdoviridae. Like other mononegavirales it has a single molecule of linear, negative‐sense ssRNA genome (11.1 kb). The viral genome contains six genes (N‐, P‐, M‐, G‐, NV‐, and L‐genes) located from 3′ to 5′ (Kurath et al., 1985). The gene junction regions show conserved sequences including transcription‐termination/polyadenylation and transcription‐initiation signals. The IHNV sequence does not contain the transcription start consensus UUGU present in vesiculovirus and rabies virus (Morzunov et al., 1995; Kurath et al., 1997). Forty‐two Alaskan IHNV isolates of different hosts were found to have only low genetic diversity (3–5%) (Emmenegger et al., 2000), but IHNV in rainbow trout aquaculture revealed unusually high genetic diversity as detected by RNase protection assay and nucleotide sequence analysis. Four distinct monophyletic clades were observed during phylogenetic analysis of 84 IHNV isolates (Troyer et al., 2000). IHNV observed in Europe and IHNV strains in North America represent two different genotypes (Arkush et al., 1989). Diagnosis of IHNV is based on direct methods, e.g. isolation (EPC‐cells) and identification (NT, IFAT, ELISA, PCR and DNA probes) of virus or the demonstration of IHNV antigen in infected tissue. Detection of IHNV antibodies is not accepted for assessing the viral status of fish populations (OIE, 2001). Biotinylated oligonucleotide probes are successfully used for identification of IHNV (Deering et al., 1991). At least two functions for the M‐protein in an IHNV infection were suggested: the down‐regulation of host transcription and the induction of programmed cell death (Chiou et al., 2000). IHN is listed as an OIE notifiable disease.
Viral haemorrhagic septicaemia virus (VHSV)
Viral haemorrhagic septicaemia virus (VHSV) is the aetiological agent of viral haemorrhagic septicaemia (VHS), a well‐known important viral disease in European rainbow trout (Oncorhynchus mykiss) aquaculture (Wolf, 1988). VHSV shows the typical morphology and characteristics of Rhabdoviridae. This rhabdovirus is responsible for high mortality rates (90–100%) occurring usually among juvenile fish in trout aquaculture at 4–14°C. The virus possesses a wide host spectrum infecting several freshwater and marine fishes (Anguilliformes, Clupeiformes, Cypriniformes, Gadiformes, Perciformes, Pleuronectiformes and Salmoniformes). Multiplication of the virus takes place in endothelial cells of blood capillaries (leading to haemorrhagic lesions in internal organs and musculature), leucocytes, haematopoietic tissues and nephron cells. Infected fish are lethargic or hyperactive. They may show exophthalmia and bleeding in the skin and fin bases (Fig. 4). Infection of fish is mostly lethal due to impairment of osmotic balance. Clinically infected fish and VHSV carriers are reservoirs of the virus, which is shed by faeces, urine and sexual fluids. VHSV induces interferon in the early stage of infection (de Kinkelin and Dorson, 1973). Survivors are resistant to reinfection due to the development of neutralizing antibodies (Jorgensen, 1971). The transmembrane viral G‐protein functions as target molecule for neutralizing antibodies (Lorenzen et al., 1990). The rainbow trout gene vig‐1 (gene number 1 expressed in spleen and head kidney) is activated during VHSV infection and leads to inactivation of the virus. The vig‐1 gene may be induced by interferon or directly by the viral G‐protein (Boudinot et al., 1999). VHSV occurred exclusively in Europe for decades, but in 1988 VHSV was detected in salmon (Oncorhynchus tshawytscha, O. kisutch) native to the Pacific Northwest returning to hatcheries in Washington located near the open ocean. Molecular analysis proved that North American VHSV strains were not of European origin (Meyers and Winton, 1995). The North American VHSV strain is enzootic in the north‐eastern Pacific Ocean among herring and cod. The virus shows high pathogenicity for Pacific herring (Clupea harengus pallasi) (Kocan et al., 1997). In 1998 thousands of dead Pacific herring, Pacific hake (Merluccius productus) and walleye pollock (Theragra chalcogramma) were found in Alaska, having been infected by the American strain of VHS (Meyers et al., 1999). VHSV was isolated from several marine fishes (Clupea harengus, Sprattus sprattus, Gadus morhua, Rhinonemus cimbrius, Trisopterus esmarkii, Micromesistius poutassou, Merlangius merlangus, Argentina sphyraena) in the Baltic Sea, Kattegat, Skagerrak and North Sea (Mortensen et al., 1999). An RNase protection assay based on the N‐gene sequence data of 39 VHSV isolates from fish in European marine environments identified 10 distinct groups of viruses within three genotypes (Snow et al., 1999). The observations suggest that VHSV isolates of marine origin are usually highly virulent for marine fish, but less virulent or avirulent for freshwater fish (Meyers et al., 1999). VHS outbreaks in farmed turbot (Scophthalmus maximus) in Scotland have been reported (Ross et al., 1994).
Figure 4.
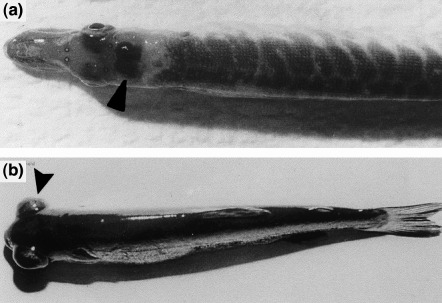
Fish infected with viral haemorrhagic septicaemia virus (VHSV): (a) pike fry showing bleeding in the brain (arrow); (b) rainbow trout fry showing exophthalmia (arrow).
VHSV has a non‐segmented ssRNA genome. Its complete nucleotide sequence (GenBank accession number Y18263) has been determined (Schütze et al., 1999). The genome comprises 11 158 bases containing six open reading frames (3′‐N‐P‐M‐G‐NV‐L‐5′) encoding N‐, P‐, M‐, G‐, NV‐ and L‐proteins (Basurco and Benmansour, 1995; Schütze et al., 1999). The European and American strains of VHSV can be clearly distinguished at the genomic level (Batts et al., 1993; Benmansour et al., 1997). Sequences of the glycoprotein genes of several European and North American VHSV strains show that VHSV displays overall genetic diversity correlating with geographical origin. The phylogeny based on nucleotide sequence data separates the VHSV strains in three genotypes, e.g. genotype I for continental Europe, genotype II for the British Isles, and genotype III for North America. However, neutralizing antibodies directed against the G‐protein are not able to discriminate between the genotypes. The VHSV strains of genotype I have been assigned to four serotypes that correlate partially with the genetic variability of isolates from continental Europe (Benmansour et al., 1997). Diagnosis of VHSV is based on direct methods, e.g. isolation (BF‐2, RTG‐2 cells) and identification (NT, IFAT, ELISA) of the virus. Carriers might be identified by fish serology, but this has yet to be validated (OIE, 2001). VHS is listed as an OIE notifiable disease.
Hirame rhabdovirus (HIRRV)
Hirame rhabdovirus (HIRRV; syn. Rhabdovirus olivaceus), an important pathogen of Japanese flounder, has been isolated from moribund hirame (Paralichthys olivaceus) and ayu (Plecoglossus altivelis) in Japan (Kimura et al., 1986). Several teleost fishes (Perciformes, Pleuronectiformes, Salmoniformes and Scorpaeniformes) have proved to be susceptible to HIRRV. Infected fish exhibit haemorrhages in fins, musculature and internal organs, and necrosis of haematopoietic tissue. Japanese flounder infected with HIRRV exhibited an increased expression of Mx (myxovirus resistance) mRNA in leucocytes and the tissue of internal organs (Lee et al., 2000). Mx is an interferon‐induced protein that prevents replication of viruses in vivo and in vitro. An interferon regulatory factor (fIRF) of HIRRV‐infected flounder has been cloned. The cDNA with 1746 bp (with an open reading frame coding for 297 amino acids) showed 40% identity with mammalian IRF‐1s and IRF‐2s (Yabu et al., 1998). Using cDNAs (up‐regulated in HIRRV‐infected Ig + leucocytes of cloned Japanese flounder), differential hybridization and cloning strategies, several immune‐related genes for biodefence were identified (Aoki et al., 2000). HIRRV is composed of a negative‐sense ssRNA encoding five structural proteins. The structural HIRRV proteins reveal similarity to those of IHNV and VHSV (Nishizawa et al., 1991). Comparison of the N‐, P‐ and M‐gene sequences of HIRRV with the corresponding sequences of IHNV and VHSV indicates a close relationship, but HIRRV is more closely related to IHNV (71.3%) than to VHSV (45.4%) (Nishizawa et al., 1997a).
Epizootic ulcerative syndrome rhabdoviruses
Epizootic ulcerative syndrome (EUS) of fish associated with high mortalities of severely affected wild and cultured freshwater and eustarine warm‐water fish species has been reported in 16 countries in South East Asia (Frerichs, 1995). The red spot disease (RSD) of fish in Australia is indistinguishable from EUS. Two rhabdoviruses, the ulcerative disease rhabdovirus (UDRV) isolated from freshwater eel (Fluta alba) (Frerichs et al., 1986), and the snakehead rhabdovirus (SHRV) isolated from snakehead fish (Ophicephalus striatus) (Wattanavavijarn et al., 1986), have been obtained during seasonal EUS in Thailand. UDRV and SHRV can be distinguished by serology and their structural polypeptides. Both viruses are serologically unrelated to the other known fish rhabdoviruses (Ahne et al., 1988). Phylogenetic analysis of the SHRV G‐protein suggests the classification of SHRV into the genus Novirhabdovirus (Johnson et al., 1999). However, the role of UDRV and SHRV as the aetiological agents of EUS is uncertain. During an epizootic less than 5% of examined fish have proved to be positive for the rhabdovirus. Furthermore, experimental infection of snakehead with EUS rhabdoviruses has not induced any lesions indicative of EUS. In conclusion, there is not adequate evidence for the viral aetiology of EUS, but it is now accepted that an invasive fungus (Aphanomyces invaderis, A. piscicida) infection plays the role of main pathogen in EUS (Frerichs, 1995). However, the EUS is listed as an OIE notifiable disease. Its diagnosis is based on the clinical signs, histopathology and evidence of the fungus Aphanomyces (OIE, 2001).
Pike fry rhabdovirus (PFR)
Pike fry rhabdovirus (PFR) was first isolated in 1972 from an acute haemorrhagic epizootic of pike fry (Esox lucius) with significant mortality in a Dutch pike culture (de Kinkelin et al., 1973). The virus reveals bullet‐shaped morphology, measuring 125 × 80 nm. The outer surface of the virion is covered with projections 9 nm long. The RNA genome of PFR has been characterized as a single‐stranded non‐segmented molecule of 4 × 106 Da with a buoyant density of 1.65 g/ml in caesium sulphate. The virus possesses five major polypeptides (L, G, N, P and M). An RNA‐dependent RNA polymerase with a temperature optimum around 20°C has been demonstrated (Roy et al., 1975; Clerx, 1978). The PFR has been isolated from several freshwater fishes (Cypriniformes, Salmoniformes) indicating a wide host spectrum (Ahne et al., 1982; Stone et al., 2001). It has been demonstrated that PFR is spread to predator fish via infected prey (Ahne, 1985b).
Spring viraemia of carp virus (SVCV)
Spring viraemia of carp virus (SVCV) is the causative agent of spring viraemia of carp (SVC), a haemorrhagic disease that predominantly affects common carp (Cyprinus carpio) in European aquaculture. The geographical range of SVC is limited to aquatic environments where temperatures decline during the winter. The outbreaks usually occur in springtime, when water temperature rises (11–17°C). The SVCV is shed via faeces, urine and sexual fluids, and is transmitted by contact and by animate vectors (Argulus foliaceus, Piscicola geometra) (Fig. 7, p. 430) (Ahne, 1985a). After fish have taken up the virus, it is disseminated within a few days in the body of infected carp (Fig. 5, p. 416) (Ahne, 1978a). Multiplication of the virus takes place in endothelial cells of blood capillaries, haematopoietic tissue and nephron cells. Clinical signs of SVCV are external and internal haemorrhages, peritonitis and ascites (Fijan, 1972). An SVCV infection can be lethal because of impairment of the salt‐water balance. The virus has a wide host spectrum, infecting mainly Cypriniformes but also Atheriformes, Salmoniformes and Crustacea (Fijan, 1972; Wolf, 1988; Johnson et al., 1999; Stone et al., 2001). Survivors develop a strong protective immunity associated with circulating antibodies. The SVCV possesses bullet‐shaped morphology, measuring 60–90 × 90–180 nm. The buoyant density of SVCV is 1.195–1.200 g/cm3 caesium chloride. The virion has an inner nucleocapsid consisting of an RNA protein (L, N and P) complex of about 50 nm in diameter, and it is surrounded by a lipid‐containing envelope with spikes. The viral genome consists of one molecule of non‐infectious linear ssRNA, which sediments in a sucrose gradient at 38–40 S (Hill et al., 1975). The viral RNA encodes five structural proteins, the L‐protein (90–190 kDa), the G‐protein (70–88 kDa), the P‐protein (43–53 kDa), the N‐protein (40–52 kDa), and the M‐protein (19–72 kDa) (Clerx, 1978; Roy et al., 1984). The SVCV L‐protein is an RNA‐dependent RNA polymerase, with functions in transcription and replication at its optimal temperature for activity of 20–25°C (Roy and Cleweley, 1978). A comparison of partial nucleotide sequences of the L‐gene of SVCV with other rhabdoviruses of vertebrates shows the closest relationship with vesicular stomatitis virus (VSV), the type species of the genus Vesiculovirus of Rhabdoviridae. The L‐protein (1780 amino acids) has an identity of 57% and a similarity of 72% to that of VSV, but low identity (21%) and similarity (42%) to IHNV (Björklund et al., 1995). The SVCV G‐gene consists of 1588 nucleotides (with a poly‐A tail) encoding 509 amino acids that form the 57 kDa G‐protein. The G‐gene reveals sequence identity of 31–33% and sequence similarity of 51–53% with VSV (Björklund et al., 1996). The G‐gene of a penaeid shrimp SVCV‐like rhabdovirus isolated in Hawaii has been proved to be over 99% identical to the G‐gene nucleotide sequence of SVCV (Johnson et al., 1999). The G‐protein is responsible for the induction of neutralizing antibodies in infected animals and gives rise to the development of antibodies in infected fish (Ahne, 1986). In phylogenetic analysis SVCV is grouped in the genus Vesiculovirus (Björklund et al., 1996). A ribonuclease protection assay using a 32P‐labelled RNA probe made from a cloned copy of the full‐length SVCV glycoprotein gene was able to distinguish between several SVCV isolates. Autoradiography has revealed identical cleavage patterns of RNA in viruses obtained from the same location, whereas a number of mismatches are evident in RNA from viruses from different locations (Ahne et al., 1998a). Seminested PCR (Liu et al., 1998), hybridization with DNA probes and amplification by PCR (Oreshkova et al., 1999) have been used as molecular detection methods for SVCV. SVC is an OIE notifiable disease. The diagnosis of SVCV is based on direct methods; the virus can be isolated by using EPC or FHM cells at 20°C. Virus identification is carried out by neutralization tests, indirect fluorescence antibody tests or enzyme‐linked immunosorbent assays. The detection of fish antibodies is not accepted for assessing the viral status of fish populations (OIE, 2001). SVCV induces apoptosis in EPC‐cells that can be inhibited by human endogenous acid cysteine proteinase inhibitor (Björklund et al., 1997).
PFR and SVCV are listed as tentative species of the genus Vesiculovirus of Rhabdoviridae (van Regenmortel et al., 2000). Both viruses share antigenic determinants and cannot be reliably distinguished by serological approaches such as immunofluorescence (IF) or ELISA (Jorgensen et al., 1989). In contrast to SVCV, PFR is not listed as an OIE notifiable virus. In order to distinguish between PFR and SVCV a ribonuclease protection assay (RPA) using a probe for the full‐length G‐gene of SVCV has been established. The RPA discriminated 13 rhabdoviruses from different freshwater fish cross‐reacting in IF with SVCV/PFR antiserum. Five isolates from common carp and koi carp have been identified as SVCV whereas isolates from coregonus, grass carp, pike, pseudorasbora, river trout, silver bream and tench proved to be PFR (Ahne et al., 1998a). Recent work has shown that nucleotide sequences of the G‐gene of SVCV and PFR reveal enough differences to distinguish PFR from SVCV. Analysis of a 555‐nucleotide region of the G‐gene of 36 putative SVCV and PFR isolates from common carp, grass carp, silver carp, bighead carp, bream, golden ide, false harlekin, crucial carp, brown trout, rainbow trout, roach and tench represented four clusters in phylogenetic analysis, indicating genetic diversity between SVCV and PFR as well as between the PFR isolates (Stone et al., 2001). SVCV and PFR multiply in several fish, avian, mammalian and reptilian cells at 20–25°C (Clark and Soriano, 1974), but also in insects (Drosophila melanogaster) (Bussereau et al., 1975).
Rhabdoviruses occurring in reptiles
‘Arboviruses’ (Marco virus, Chaco virus and Timbo virus) recognized in lizards (Ameiva ameiva, Kentropyx calcaratus) in Brazil (Causey et al., 1966) have been identified as rhabdoviruses by their distinctive morphology of Rhabdoviridae (Monath et al., 1979). Four strains of Marco virus, six of Timbo virus and three of Chaco virus have been recognized. Serological studies have shown that the isolates are not related to 34 rhabdoviruses of insect and mammals origin, but Chaco and Timbo viruses cross‐react (Monath et al., 1979). The optimum replication temperature of 30°C indicates the reptilian origin of Marco, Chaco and Timbo viruses. Chaco and Timbo viruses (Timbo group) are listed as unassigned species in the Rhabdoviridae family. Marco virus and Almpivar virus, which was isolated from skink (Ablepharus boutonii virgatus) in Australia (Berge, 1975), are listed as unassigned animal rhabdoviruses in the seventh report of the ICTV (van Regenmortel et al., 2000). Natural antibodies against the vesicular stomatitis virus have been found in turtles (Trionyx spinifer) (Cook et al., 1965) and in snakes (Natrix eryhtrogaster) (Hoff and Trainer, 1973).
RT‐transcribing viruses
Retroviridae
The family Retroviridae comprises the genera Alpha‐, Beta‐, Gamma‐, Delta‐, and Epsilonretrovirus, Lentivirus and Spumavirus. The genus Gammaretrovirus contains the viper retrovirus (VRV) as species (reptilian virus group). The genus Epsilonretrovirus includes fish retroviruses with walleye dermal sarcoma virus (WDSV) as the type species. Common features of retroviruses are spherical, enveloped virions of 80–100 nm in diameter including glycoprotein surface projections of about 8 nm. The internal, spherical or icosahedral core encapsidates the viral nucleocapsid containing a dimer of linear, positive‐sense ssRNA. There are several polypeptides (about 60% of the virion dry weight) including structural proteins, a protease (PR) encoded by the pro gene, a reverse transcriptase (RT), and an integrase (IN) encoded by the pol gene (van Regenmortel et al., 2000). Significant homology to the human endogenous retrovirus type I (HERV) was found to be present within the genomes of fish, reptiles, birds and mammals. Phylogenetic analysis of nucleotide sequences supports the inclusion of viruses from each of these vertebrate classes into one monophyletic group (Martin et al., 1997).
Retroviruses occurring in fish
Transmission trials have supported the viral aetiology of lymphosarcoma described in muskellunge (Esox masquinongy) and northern pike (Esox lucius) (Sonstegard, 1976). The first evidence for a retrovirus came from detection of reverse transcriptase activity and C‐type virus particles in lymphosarcoma of northern pike. The malignant neoplasm has been found with seasonal periodicity in pike with cutaneous lesions. An investigation of the DNA polymerase from necropsies of tumour‐bearing pike has revealed an optimum temperature profile of 20°C (Papas et al., 1977). The pike lymphosarcoma virus is supposed to induce one of the most frequently found neoplasms (21% frequency) in wild vertebrates, and is epizootic in North America, Sweden and Finland (Papas et al., 1976). Several retrovirus‐like particles associated with proliferative conditions in fish have been reported (Bowser and Casey, 1993). Epidermal papilloma of white sucker (Catostomus commersoni) have carried C‐type particles (100 nm in diameter) associated with transcriptase activity. C‐type particles of about 110–150 nm have been found in Atlantic salmon (Salmo salar) with swimbladder neoplasia and epidermal papilloma. Walleye (Stizostedion vitreum) has for many years shown high incidence (27%) of sarcomatous lesions associated with retroviruses in North America (walleye dermal sarcoma virus, WDSV). The virus does not integrate and tumours do not metastasize (Martineau et al., 1991). Retrovirus particles with RT activity have been found in homogenates of the walleye dermal sarcoma. Isolated viral RNA (12 kb) hybridized with a 13‐kb viral DNA present in DNA of walleye tumours. Lairmore et al. (2000) reported that the D‐cyclin homologue (retroviral (rv) cyclin) is encoded by WDSV and the cyclin mRNA is present in developing tumours. The rv‐cyclin seems to plays an important role in the development of walleye dermal sarcoma. Furthermore, a plasmacytoid leukaemia (PL) of chinook salmon (Oncorhynchus tshawytscha) has been suggested to be of oncovirus origin. Evidence of the retrovirus aetiology of plasmacytoid leukaemia comes from reverse transcriptase activity in affected tissue, visualization of retrovirus‐like particles by electron microscopy, or transmission of the disease. Skin tumours of hooknose (Agonus cataphractus) have contained lentivirus‐like particles of 86–132 nm. C‐type particles have been observed in epidermal papillomatosis of European smelt (Osmerus eperlanus). A dual infection of bluegills (Lepomis macrochirus) with retrovirus and lymphocystis virus has been reported. Cell cultures derived from three species of warm‐water fishes (Ophicephalus striatus, Anabas testudineus, Trichogaster pectoralis) and from neoplastic embryonic tissues of the platyfish (Xiphophorus maculatus) have released spontaneously C‐type retroviruses associated with transcriptase activity (Frerichs et al., 1991; Petry et al., 1992). The complete nucleotide sequence of the snakehead retrovirus (SnRV), exhibiting persistent infection of the striped snakehead (Ophicephalus striatus) cell line (SSN‐1), has been analysed (Hart et al., 1996). The proteins (p70, p65 and p28) of the retrovirus from platyfish cell culture react with antiserum directed against the p27 protein of feline leukaemia virus (FeLV), and RT activity is inhibited by antibodies against the RT of FeLV. In addition, hybridization experiments have revealed sequence homology between FeLV and the genome of platyfish (Petry et al., 1992). Retrovirus‐like particles with RT activity and a RNA of 7.3 kb have been isolated from coho salmon (Oncorhynchus kisutch), masou salmon (O. masou), rainbow trout (O. mykiss), iwana (Salvelinus pluvius) and ayu (Plecoglossus altivelis) (Oh et al., 1995).
Today, piscine retroviruses are grouped within the genus Epsilonretrovirus. The genus is composed of three distinct species of fish retroviruses, i.e. walleye dermal sarcoma virus (WDSV), walleye epidermal hyperplasia virus type 1 (WEHV‐1), and walleye epidermal hyperplasia virus type 2 (WEHV‐2), and two tentative species, i.e. perch hyperplasia virus (PHV), snakehead retrovirus (SnRV) (van Regenmortel et al., 2000).
Retroviruses occurring in amphibians
Three endogenous retroviral fragments, termed DevI, DevII and DevIII, have been isolated from the dart‐poison frog Dendrobates ventrimaculatus. Comparison of the fragments with mammalian and avian isolates reveals significant differences between their nucleotide sequences, suggesting that they are only distantly related to the seven currently recognized retroviral genera. Phylogenetic analysis shows that the amphibian retroviral fragments are approximately equally related to the Moloney leukaemia‐related virus, the spumaviruses, and walleye dermal sarcoma virus (Tristem et al., 1996).
Retroviruses occurring in reptiles
Retroviruses have been detected in tissues of different snakes, turtles, and in the oldest surviving lepidosaurian reptile, the tuatara (Table 3). C‐type‐like viruses (105–110 nm) have been demonstrated in tissue cultures of a Russell’s viper (Vipera russelli) (Zeigel and Clark, 1969). Lunger et al. (1974) have reported the detection of C‐type viruses in corn snakes (Elaphe guttata) with rhabdomyosarcoma. Retrovirus‐like particles have been found in members of the family Boidae showing intracytoplasmic inclusions in neurones, and cutaneous and visceral cells. The enveloped particles present in the tissue measured about 100 nm in diameter and were associated with RT activity. The cytopathogenic virus replicates in cultivated kidney cells of Boa constrictor and Koch’s postulates could be fulfilled using infectious tissue culture medium (Schumacher et al., 1994b). A highly divergent retroviral sequence was detected in blood samples of tuatara (Spenodon punctatus). Sequence analysis of isolated retroviral fragment demonstrated substantially differences concerning protease and reverse transcriptase genes of retroviruses of mammals and birds (Tristem et al., 1995). Tissues of Hawaiian green turtles (Chelonia mydas) with and without fibropapillomas have displayed high levels of RT activity. Sucrose gradient fractions with RT activity have revealed retrovirus‐like particles with an envelope and with spikes in the range of 96–122 nm in size. Seven prominent viral proteins with molecular weights of 116, 83, 51, 43, 40, 20 and 14 kDa could be differentiated by SDS‐PAGE (Casey et al., 1997). Only the viper retrovirus (VRV; reptilian virus group) is characterized as a species of the genus Gammaretrovirus of Retroviridae (van Regenmortel et al., 2000).
Table 3.
Occurrence of retroviruses in reptiles

Single‐stranded (+) RNA viruses
Coronaviridae
Arteriviridae and Coronaviridae are the two families of the order Nidovirales. The family Coronaviridae is composed of the genera Coronavirus and Torovirus. Common features of coronaviruses are pleomorphic, enveloped virions of 120–160 nm in diameter including surface projections. The helical‐tubular nucleocapsid contains a single molecule of linear, positive‐sense ssRNA. Virions consist of five proteins. Coronavirus‐like particles occurring in lower vertebrates are not included in the present ICTV taxonomy of viruses (van Regenmortel et al., 2000).
Coronaviruses occurring in fish
A coronavirus‐like agent has been isolated from common carp (Cyprinus carpio) showing erythematous skin and abdomen. The virus proved to be enveloped, has an RNA genome, and measures 60–100 nm in diameter. The virus‐inducing hepatic, renal and intestinal necrosis in experimentally infected fish was tentatively classified as coronavirus (Sano et al., 1988). Ulcerative dermal lesions associated with high mortality occurred in colour carp (Cyprinus carpio) reared in warm‐water aquacultures in Japan. A round‐shaped virus with surface spikes, measuring 100–180 nm, was isolated from affected fish. The isolate‐induced ulcerative dermal lesions in experimentally infected carp (Miyazaki et al., 2000b).
Caliciviridae
Caliciviruses are non‐enveloped, icosahedral virions measuring 27–40 nm in diameter. The genome consists of one molecule of positive‐sense, linear, ssRNA with a polyadenylated 3′ end. Non‐structural proteins exhibit homology with those of picornaviruses. The family Caliciviridae comprises the genera Lagovirus, ‘Norwalk‐like viruses’, ‘Sapporo‐like viruses’ and Vesivirus. The genus Vesivirus includes feline caliciviruses and the type species vesicular exanthema of swine virus (VESV). The reptile caliciviruses (Cro‐1) and the San Miguel sea lion viruses (SMSV) are listed as strains of VESV in the seventh report of the ICTV (Green et al., 2000; van Regenmortel et al., 2000). More than 15 calicivirus serotypes have been isolated from marine sources (pinnipeds, fish), which are usually referred to San Miguel sea lion viruses (Matson et al., 1996).
Caliciviruses occurring in fish
San Miguel sea lion virus (SMSV)
San Miguel sea lion virus (SMSV), named after San Miguel Island off the coast of Santa Barbara, USA, has been isolated from opaleye fish (Girella nigrigans). The icosahedral virus measures 38 nm in diameter and has one molecule of ssRNA. The role of the virus in inducing disease in opaleye fish is unknown, but the agent proved to be pathogenic to marine pinniped mammals. Opaleyes are associated with California sea lions and serve as intermediated hosts for the lungworm (Parafilaroides decorus) of the sea lion (Smith et al., 1980). Calicivirus (SMSV‐5) isolated from these lungworms has proved to be pathogenic to terrestrial mammals, inducing vesicular exanthema of swine (VES) (Fig. 6). VES epizootics were first recorded in 1932 in California, after which the disease spread through the US via swineherds; it is now eradicated. The origin of the pathogen was not known at that time, but it is now obvious that the virus was transmitted from aquatic to terrestrial animals bridging water–land barriers and phylogenetic distances. Marine caliciviruses seem to continue to emerge from reservoirs in the ocean and be introduced to terrestrial populations. As well as crossing the land–sea interface these viruses cross species and class barriers (Smith et al., 1986a, 1998). Two serotypes (SMSV‐6 and SMSV‐7) detected in opaleye fish are neutralized by sera of California and Steller sea lions (Barlough et al., 1988). It has been shown that the SMSV‐5 serotype, which replicates in African green monkey cells (Vero cells), is able to infect fish, seals, mink, pigs and primates (Smith et al., 1980, 1986b). An unrooted distance tree of caliciviruses established on the RNA polymerase (3D region) sequence grouped SMSV in the genus Vesivirus together with calicivirus of dolphin (Tur‐1), chimpanzee (Pan‐1) and reptiles (Crotalus calicivirus type 1, Cro‐1) (Matson et al., 1996; Nick Knowles, personal communication).
Figure 6.
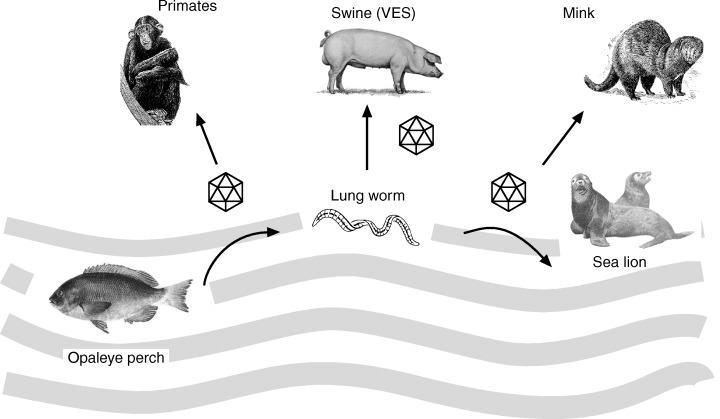
Scheme showing multiple hosts of San Miguel sea lion virus (SMSV5–7), which crosses the water–land environment to affect different animal species.
Caliciviruses occurring in amphibians and reptiles
Sixteen caliciviruses have been isolated from rattlesnakes (Crotalus unicolor, C. lepidus), vipers (Bothrops schlegeli) and frogs (Ceratophrys orata) in a zoological collection. The indistinguishable isolates represent the unique serotype Crotalus calicivirus type 1 (Cro‐1) belonging to the genus Vesivirus (Matson et al., 1996). Cro‐1 viruses are apparently non‐pathogenic to reptiles, frogs and pigs (Smith et al., 1986a).
Togaviridae
The family Togaviridae comprises the genera Alphavirus (arthropod‐borne viruses) and Rubivirus (transplacentar transmission). Common features are spherical virions, 70 nm in diameter, including a lipid envelope with surface projections. The nucleocapsid covers one molecule of linear, positive‐sense ssRNA, which is capped at the 5′ terminus and polyadenylated at the 3′ end. Proteins of the virion are the basic capsid protein and two glycoproteins of the envelope. Non‐structural proteins (ns P1–4), which are detectable in infected cells, are not present in the virion. Togavirus‐like particles occurring in lower vertebrates are not included in the present taxonomy of viruses (van Regenmortel et al., 2000).
Togavirus‐like agents occurring in fish
Salmon pancreatic disease virus (SPDV)
A pancreatic disease causing up to 50% mortality among farmed Atlantic salmon (Salmo salar) has been observed in Europe and North America (Kent and Elston, 1987; Poppe et al., 1989). The causal agent, termed salmon pancreatic disease virus (SPDV), was identified as a toga‐like virus (Nelson et al., 1995). Comparison of experimental transmission of SPDV has shown Atlantic salmon to be highly susceptible, rainbow trout less susceptible, and brown trout least susceptible (Boucher et al., 1995). Analysis of a 5.2‐kb region at the 3′ terminus of the RNA of SPDV genome reveals sequence identity to members of the genus Alphavirus of Togaviridae. SPDV structural proteins encoded by the 5.2‐kb region reveal features unique to alphaviruses. The amino acid sequence of the SPDV structural region and that of alphaviruses revealed 32–33% homology. Based on cleavage site homologies of alphaviruses the sizes of SPDV envelope glycoproteins E2 and E1 were found to be larger than those of other alphaviruses (Weston et al., 1999).
Sleeping disease virus of rainbow trout (SDV)
An enveloped virus of 55–65 nm has been isolated from plasma of rainbow trout lying side up on the bottom of a tank, suffering from sleeping disease (SD) (Castric et al., 1997). The genomic RNA of sleeping disease virus (SDV) consists of 12 kb with appearance of a 26S subgenomic RNA during replication, and has an open reading frame related to the alphavirus E2 glycoprotein. The 26S subgenomic RNA encodes a 1324‐amino acid polypeptide exhibiting typical alphavirus structural protein organization. The SDV proteins show features of alphaviruses. An analysis of structural proteins revealed that the virus is phylogenetically related to the Semiliki Forest virus group of alphaviruses (Villoing et al., 2000a).
Because SDV and SPDV induce similar histopathology and show acquired cross‐protection, it is probable that both agents are related or identical viruses (Weston et al., 1999). Recently it has been shown that a RT‐PCR with RNA of SDV and SPDV, using primers derived from SDV nucleotide sequences, enabled specific DNA amplification of part of the gene encoding the glycoprotein E2 of both viruses (Villoing et al., 2000b).
Erythrocytic inclusion body syndrome virus (EIBSV)
Viral particles (75–100 nm) detected in erythrocytes of salmonid fishes with erythrocytic inclusion body syndrome (EIBS) have been found to be togavirus‐like (Nakajima et al., 1998a). However, the virus has not been sufficient characterized and its pathogenicity is not known.
Picornaviridae
The family Picornaviridae comprises the genera: Aphthovirus, Cardiovirus, Enterovirus, Hepatovirus, Parechovirus and Rhinovirus. Common features are naked, ether‐resistant icosahedral virions measuring 22–30 nm in diameter. The capsid consists of 60 protomers; each subunit has three surface proteins. The core comprises one molecule linear, positive‐sense ssRNA and a small protein (VPg). The viral RNA acts as messenger for protein synthesis. The seventh report of the ICTV includes five piscine picornaviruses, e.g. barramundi virus 1 (BaV), sea bass virus 1 (SBV), smelt virus 1 (SmV‐1), smelt virus 2 (SmV‐2) and turbot virus 1 (TuV‐1) as unassigned viruses in the family (van Regenmortel et al., 2000).
Picornaviruses occurring in fish
Small non‐enveloped, icosahedral RNA viruses measuring <40 nm have been detected in 16 teleost fish species in America, Asia and Europe. Usually hatchery‐reared fish are affected, exhibiting corkscrew‐like swimming often associated with mass mortalities. The victims show a severe encephalomyelitis; the brain and medulla of diseased fish contain numbers of picorna‐like viruses. However, picorna‐like viruses have also been detected in apparently healthy fish. Most of the viruses are demonstrated by electron microscopy. Some picornaviruses have been isolated using fish cell cultures such as CHSE‐214. Sources for isolation of virus are ovarian fluid, brain and internal organs. The viruses replicate in vitro at 10–20°C, inducing syncytia of infected cells (Hetrick and Hedrick, 1993). Picornavirus‐like particles have been detected in several bony fishes, e.g. grass carp (Ctenopharyngodon idella, Mao et al., 1988), sea‐bass (Dicentrarchus labrax, Breuil et al., 1991), barramundi (Lates calcarifer, Glazebrook et al., 1990), smelt (Osmerus eperlanus, Ahne et al., 1990; Osmerus mordax, Moore et al., 1988), turbot (Scophthalmus maximus, Bloch et al., 1991) and salmonids (Salmo trutta fario, Salvelinus fontinalis, Oncorhynchus mykiss, Oncorhynchus clarki, Yun et al., 1989).
Picornaviruses occurring in reptiles
Spherical viruses of 22–27 nm in diameter have been detected by electron microscopy in the cytoplasm of RES‐cells and erythrocytes of the snake Boa constrictor. Aesculapian snake (Elaphe longissima) suffering from gastrointestinal disease have shown 20 nm viruses in the cytoplasm of necrotic cells; these viruses were considered to be picornaviruses (Heldstab and Bestetti, 1984).
Nodaviridae
The family Nodaviridae represents the genera Alphanodavirus (mostly insect hosts, but birds and mammals might also be infected) and Betanodavirus (strictly fish hosts), with the type species striped jack nervous necrosis virus (SJNNV). Common features are non‐enveloped icosahedral virions of about 30 nm in diameter. The capsid is composed of 180 proteins. The viral genome consists of two molecules of positive‐sense ssRNA (van Regenmortel et al., 2000).
Nervous necrosis viruses of fish (NNV)
A viral disease inducing abnormal swimming behaviour, encephalopathy and retinopathy in Japanese parrotfish (Oplegnathus fasceatus) has been defined viral nervous necrosis (VNN) (Yoshikoshi and Inouye, 1990). Since that time, similar disease cases (‘viral encephalopathy and retinopathy’, VER; ‘fish encephalitis’, ‘encephalomyelitis’, ‘striped jack viral nervous necrosis’) occurring with high mortalities among larvae and juveniles of several species out of 10 families of marine fish have been described in the Indo‐Pacific region, the Mediterranean, France and Scandinavia. The causative agents have been previously described as picornavirus‐like. Piscine nodaviruses have a tropism for nerve cells and during NNV‐infection they are spread from the spinal cord to the brain and retina. There are differences regarding the occurrence (age, species) and severity of the disease (OIE, 2001). A vertical transmission of fish nodaviruses has been shown (Munday and Nakai, 1997). The striped jack nervous necrosis virus (SJNNV) isolated from larvae of striped jack (Pseudocaranx dentex) (Mori et al., 1992) is characterized and classified as type species of the genus Betanodavirus of Nodaviridae (van Regenmortel et al., 2000). The non‐enveloped virus particles, measuring 25 nm, contain two molecules of ssRNA with positive‐sense orientation. The RNA does not have poly(A) sequences at the 3′ end. Virions consist of a 40‐kDa and a 42‐kDa protein. The complete RNA2 of SJVNN and that of an isolate from Dicentrarchus labrax with encephalitis have been sequenced (Nishizawa et al., 1995; Delsert et al., 1997). Based on sequence data of the SJNNV‐RNA2 a portion of the protein gene could be amplified by PCR (Nishizawa et al., 1994). A RT‐PCR has been used to detect the presence of piscine nodavirus in a wide range of fish species (Nishizawa et al., 1997b; Thiery et al., 1997). A sequence part of the RNA2 of Atlantic halibut nodavirus (AH95NorA) shows 80% identity to that of SJNNV and comprises features common to all nodaviruses. The RNA2 of isolates from Europe (European sea bass from the Mediterranean) and Japan (redspotted grouper) shows sequence identity of 99.5% (Sideris, 1997). The T2 region of the RNA2 of AH95NorA shares 98% of the nucleotide sequence of barfin flounder nervous necrosis virus, while the nucleotide sequence identity to SJNNV is 76%. Phylogenetic analysis based on the nucleotide sequences of the variable region (T4) of the viral capsid protein gene region of fish nodaviruses reveals a close relationship (Grotmol et al., 2000). Comparison of T4 of 20 piscine nodaviruses shows four major clades, e.g. the striped jack clade, the redspotted grouper clade, the tiger puffer clade and the barfin flounder clade. Interestingly, AH95NorA is grouped in the barfin flounder clade together with strains isolated from Pacific fish, suggesting a transmission of the viruses from the Pacific to the Atlantic Ocean or vice versa (Nishizawa et al., 1997b; Grotmol et al., 2000). However, piscine nodavirus strains of European sea bass from the Atlantic coast of France could not be assigned to any of the four clades (Thiery et al., 1997). Comparison of features of the RNAs, capsid protein processing and the low similarity (e.g. coat protein, 10% amino acid similarity) suggest that piscine nodaviruses are distinct from insect nodaviruses (Delsert et al., 1997; Munday and Nakai, 1997). At present, hosts recognized are several species out of 10 families of fishes. Piscine nodavirus species of the genus Betanodavirus are the type species striped jack nervous necrosis virus (SJNNV), barfin flounder nervous necrosis virus (BFNNV), Dicentrarchus labrax encephalitis virus (DIEV), Japanese flounder nervous necrosis virus (JFNNV), Lates calcarifer encephalitis virus (LcEV), redspotted grouper nervous necrosis virus (RGNNV), and tiger puffer nervous necrosis virus (TPNNV). Tentative species in the genus are the Atlantic halibut nodavirus (AHNV) and the Malabar grouper nervous necrosis virus (MGNNV) (van Regenmortel et al., 2000). Viral encephalopathy and retinopathy or viral nervous necrosis is an OIE significant disease. Piscine nodaviruses can be propagated efficiently in vitro exclusively in the SSN‐1 cell line originating from tissue of striped snakehead (Ophicephalus striatus) (Frerichs et al., 1996; Iwamoto et al., 1999). However, the disadvantage of the use of SSN‐1 cells is that the cell line is persistently infected with the snakehead retrovirus (SnRV) (Frerichs et al., 1991). Optimal growth temperatures differ among the four genotypic variants, e.g. 25–30°C for the RGNNV genotype, 25–30°C for the SJNNV genotype, 20°C for the TPNNV genotype, and 15°C for the BFNNV genotype. Diagnostic procedures recommended are at present electron microscopy, immunohistochemistry, fluorescent antibody techniques, ELISA and PCR (OIE, 2001).
Double‐stranded RNA viruses
Reoviridae
The family Reoviridae contains the genera Aquareovirus, Coltivirus, Cypovirus, Fijivirus, Orbivirus, Orthoreovirus, Oryzavirus, Phytoreovirus and Rotavirus. Members of the family are non‐enveloped, icosahedral to spherical virions with one to three distinct capsid shells and an overall size of 60–80 nm in diameter. The genome consists of 10, 11 or 12 segments of dsRNA. Several polypeptides form one or two outer coats as well as an inner coat. There are at least three internal structural proteins acting as RNA polymerase and an associated enzyme involved in mRNA synthesis. Reoviruses occurring in aquatic animals are grouped in the genus Aquareovirus. Aquareoviruses have a wide host spectrum infecting finfish, shellfish and crustacea. Common features of the aquareoviruses are the double capsid shell, 11 dsRNAs and seven structural proteins (VP1–VP7). Virions, measuring about 80 nm in diameter, have type‐ and group‐specific antigenic determinants located in the outer capsid protein. Aquareoviruses replicate in fish and mammalian cell lines, inducing syncytia formation at 15–25°C (Winton et al., 1987; Lupiani et al., 1995; van Regenmortel et al., 2000).
Reoviruses occurring in fish
The first finfish reovirus (golden shiner virus, GSRV) was isolated from golden shiner (Notemigonus crysoleucas) in the USA (Plumb et al., 1979). Later, several reovirus‐like agents were found to be present in a variety of aquatic animals (finfish, shellfish and crustaceans). The aquatic reoviruses did not fit within any of the established genera of Reoviridae. Apart from the 11 segments of dsRNA of which species of the genus Rotavirus are composed, aquatic reoviruses differ from other reoviruses in terms of host range, optimal growth temperature, serology, RNA profiles and polypeptide profile (Winton et al., 1987). Consequently, reoviruses of aquatic animals were grouped in the genus Aquareovirus of Reoviridae (van Regenmortel et al., 2000). Aquareoviruses measure about 80 nm; their viral density in caesium chloride is 1.36 g/cm3. Virions contain seven structural proteins (VP1: 130 kDa; VP2: 127 kDa; VP3: 126 kDa; VP4: 73 kDa; VP5: 71 kDa; VP6: 46 kDa; and VP7: 35 kDa) and five non‐structural proteins (NS97: 97 kDa; NS39: 39 kDa; NS29: 29 kDa; NS28: 28 kDa; and NS15: 15 kDa). The inner capsid is encoded by segments 1, 2, 3, 5, 6 and 8, the major outer capsid by segment 10, and the non‐structural proteins by segments 4, 7, 9 and 11. Segments 1–10 encode one protein, while segment 11 encodes two proteins, the non‐structural proteins NS29 and NS15. Comparison of these two non‐structural proteins by partial protease digestion indicated that NS15 is a truncated form of NS29, being initiated at a downstream initiation codon (Subramanian et al., 1994). Nucleotide sequence analysis of segment 11 of the genome of striped bass reovirus (SBRV) revealed a 780‐nucleotide open reading frame (ORF) coding for 236 amino acids and a 480‐nucleotide ORF coding for 145 amino acids. The genome segment 11 contains 24 non‐translated nucleotides at the 5′ end and 48 at the 3′ end. The gene codes for NS29 and NS15 showed no sequence relatedness to gene sequences of the other members of Reoviridae (Subramanian and Samal, 1997). On the basis of RNA–RNA hybridization, several genotypes of aquareoviruses among more than 40 strains have been recognized (Rangel et al., 1999). The genus Aquareovirus includes at present 26 reoviruses of fish and two of shellfish. The species in the genus are divided into six genotypes (Aquareovirus A–F) (Table 4). Genogroup A is the most heterogeneous, consisting of members of cold‐ and warm‐water fishes of several continents. Only group A and B show serologic cross‐reactivity. RNA–RNA hybridization between genogroups A and B revealed segment 10 (encoding the major outer capsid protein) to be the most variable gene (Lupiani et al., 1995). The VP7, the glycosylated outer capsid protein, being responsible for virulence, stability and neutralization of the reoviruses is different between the genotypes as determined via Western blots (Lupiani et al., 1997; McPhillips et al., 1998). Three‐dimensional cryomicroscopy showed that trypsin treatment removes VP7 completely and induces conformational changes in the VP5 trimer (Nason et al., 2000). The viruses replicate in several fish and mammalian cells at 15–25°C forming syncytia and lysis of infected cells (Winton et al., 1987; Samal et al., 1998). The infectivity of the viruses can be increased by treatment with proteases (McPhillips et al., 1998). Several reoviruses have been isolated during surveillance of cultured and feral fish worldwide. Most of the isolates are non‐pathogenic or of low pathogenicity in their host species (Hetrick et al., 1992). However, the grass carp reovirus (GCRV), infecting cyprinids in China seems to be highly pathogenic in grass carp (Ctenopharyngodon idella) (Chen and Jiang, 1984). A specific selected peptide from a nonapeptides library inhibited grass carp haemorrhage virus (GCHV) by 10 000 times in vitro (Wang et al., 2000). The GCRV segments 1, 2 and 3 have been sequenced recently and revealed conserved terminal sequences comparable with mammalian reoviruses. Segment 1 (3939 bp) has amino acid homology (41% similarity) to orthoreovirus segment 2 and encodes a putative guanylyl/methyl transferase. The segment 2 of GCRV resembles segment 1 of orthoreoviruses (RNA‐dependent RNA polymerase) (57% similarity), segment 3 encodes for a dsRNA binding protein (NTPase, helicase) (50% similarity) as orthoreovirus segment 3 (Fang et al., 2000).
Table 4.
Classification of species and tentative species in the genus Aquareovirus (van Regenmortel et al., 2000)

Reoviruses occurring in reptiles
Several reovirus‐like agents have been detected in reptiles, but they have not been conclusively demonstrated to be responsible for disease in their hosts. Jacobson (1986) reported that an imported Chinese viper (Azemiops feyi) died shortly after acquisition. Viper heart cells (VH2) exhibited giant cell formation after inoculation with suspensions of the liver and spleen of the animal. Electron microscopy of intestinal epithelial cells and infected cell cultures have shown non‐enveloped intracytoplasmic viruses of 66 nm in diameter most closely resembling the morphology of reoviruses. In addition, syncytia‐forming virus particles have been isolated from internal organs of a moribund royal python (Python regius). Electron microscopy of negatively stained virions has revealed spherical to icosahedral particles of 70–75 nm in diameter with a double capsid layer. The viral genome was found to be composed of 10 segments of dsRNA. Migration patterns of the RNA differed from those of the mammalian reovirus 3. Antiserum against mammalian reoviruses types 1, 2 and 3 did not neutralize the python reovirus. The virus was not able to haemagglutinate human type 0 erythrocytes (Ahne et al., 1987b). Reo‐like viruses have been isolated from the brain of a moribund rattlesnake (Crotalus viridis) with central nervous system symptoms (loss of coordination, loss of general proprioception). Infected Vero cells formed syncytial giant cells 4 days after infection at 30°C. Electron microscopy of negatively stained virus preparations from infected cell cultures has revealed icosahedral particles with a double capsid layer measuring 75 nm in diameter. Electrophoretic analysis of the genome separated 10 segments of dsRNA similar to those of the python‐reovirus, but different from those of avian and mammalian reoviruses. The rattlesnake reovirus did not haemagglutinate pig erythrocytes and was not neutralized by antisera against the avian reovirus S1133 and the mammalian reovirus type 3 (Vieler et al., 1994). Four reoviruses isolated from emerald tree boa (Corallus caninus), Aesculapian snake (Elaphe longissima) and green iguana (Iguana iguana) replicate in reptilian cells at 28°C, forming giant syncytia. 5‐iododeoxyuridine (IUdR) and chloroform did not inactivate the viral infectivity. Electron microscopy of infected tissue culture fluid revealed reovirus particles that did not haemagglutinate avian or human erythrocytes. In cross‐neutralization tests some serotype‐like differences between snake reoviruses can be observed, but no serological reaction with the mammalian reovirus type 3 occurred. However, one‐way reaction between antisera against the reptilian and avian reoviruses has been observed. Electrophoretic analysis showed minor differences between the migration patterns of the 10 dsRNAs of the reptilian reoviruses, but they resembled the pattern of chicken reovirus (Blahak et al., 1995). Elaphe reovirus isolated from juvenile Moellendorff’s ratsnakes (Elaphe moellendorffi) and beauty snakes (Elaphe taenuris) produced large syncytia formation in VH2 cells at 30°C. The viruses (70–85 nm) exhibiting a double capsid layer did not haemagglutinate guinea pig and chicken erythrocytes. The genome composed of 10 segments of dsRNA showed different migration patterns to those of mammalian and avian reoviruses. Experimental infection of black ratsnake (Elaphe obsoleta obsoleta) with the Elaphe reovirus has induced diffuse subacute interstitial pneumonia with respiratory epithelial cell hyperplasia and syncytia (Lamirande et al., 1999). Recently, reoviral infection of iguanas on Honduran islands has been reported; 47% of 49 sera from wild healthy spiny‐tailed iguanas tested positive for reptilian reoviruses in neutralization tests (Gravendyck et al., 1998).
Birnaviridae
The family Birnaviridae contains the genera Aquabirnavirus, Avibirnavirus and Entomobirnavirus. Common features are single‐shelled, non‐enveloped icosahedral virions of 60 nm in diameter. The virions contain five polypeptides and two segments of dsRNA. The genus Aquabirnavirus comprises birnaviruses infecting fish, molluscs and crustacea. Species in the genus Aquabirnavirus are infectious pancreatic necrosis virus (IPNV) and yellowtail ascites virus (van Regenmortel et al., 2000).
Birnaviruses occurring in fish
The aquatic birnaviruses are the largest group of viruses within the Birnaviridae, including several strains from numerous fish species and marine invertebrates.
Infectious pancreatic necrosis virus (IPNV)
The first fish pathogenic birnavirus was isolated from brook trout (Salvelinus fontinalis) at the National Fish Hatchery, Leetown, West Virginia, USA (Wolf et al., 1959). The agent was obtained during an epizootic of rainbow trout fingerlings suffering from infectious pancreatic necrosis (IPN). This infectious pancreatis necrosis virus (IPNV) was deposited in the ATCC as ATCC VR299. IPNV had been detected for years in several places in North America associated with high mortality rates of trout fry at water temperatures of 8–12°C. In the following years, IPNV strains were recognized in many countries around the world among individuals of more than 20 families of teleost fish. The agent is probably present in all major trout‐farming countries. Recently, an IPNV closely related to IPNV fr21 and N1 was first detected in Australia (Crane et al., 2000). IPNV is transmitted by faeces, urine and sexual products of infected fish and it is easily spread by shipment of contaminated fish eggs from one country to another. Studies on factors affecting the transmission and outbreaks of IPN indicated that iodophores used as a disinfectant during the artificial egg‐fertilization process did not completely eradicate IPN infectivity (Ahne et al., 1989). Survivors of an IPNV outbreak become IPNV carriers and can shed the virus for the whole lifetime (Hill, 1982; Wolf, 1988). IPNV can be transmitted by faeces of piscivorous birds, e.g. heron, crow, grackle, kingfisher, sparrows, mallards, egrets and ospreys (McAllister and Owens, 1992). The majority of aquatic birnaviruses are antigenically related, representing one large serogroup (A) with 10 serotypes. Only a few antigenically unrelated aquatic birnaviruses form a second, minor serogroup (B) (Table 5). Most IPNV isolates from USA belong to the A1 (West Buxton) serotype, the Canadian isolates (C1, C2, C3, Jasper) to serotypes A6–A9, and the European isolates (Sp, Ab, He, Te) to the A2–A5 and A10 serotypes. The IPNV serotypes A1, A2, and A3 have been detected in Asia (Hill and Way, 1995).
Table 5.
Serological classification of aquatic birnaviruses (Hill and Way, 1995)
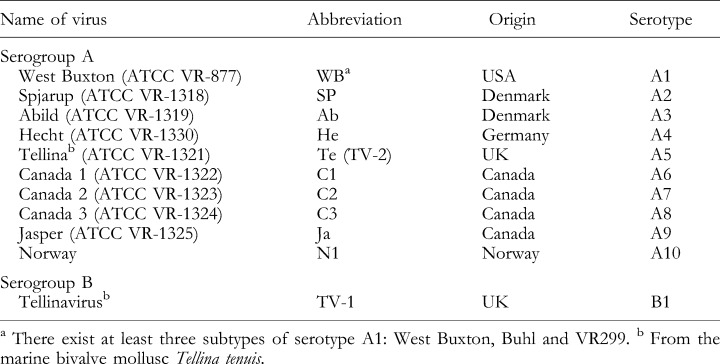
The aquatic birnaviruses share similarities in morphology and biochemical/biophysical properties. The non‐enveloped, single‐shelled IPNV virions have an icosahedral morphology and measure 60 nm in diameter. They possess a bisegmented dsRNA genome. The larger genome segment A (3 kb) contains an ORF encoding a 100‐kDa protein that is subsequently cleaved to pVP2 (63 kDa), NS (29 kDa) and VP3 (29–31 kDa) proteins by the protease activity associated with the VP4 (NS) protein. The VP4 protease is a unusual type of serine protease sharing properties with prokaryotic leader peptidases and other bacterial proteases, and cannot cleave heterologous substrates (e.g. infectious bursal disease virus, IBDV, proteins) or vice versa (Lejal et al., 2000; Petit et al., 2000). The A segment contains a small ORF partly overlapping the 5′ end of the ORF encoding VP5 (17 kDa) (Heppell et al., 1995a). The smaller genome segment B encodes the transcriptase (VP1, 90–110 kDa), with 41% homology to the IBDV VP1. It is present as free and genome‐linked (5′ terminus) protein VP1 in the virion. The virion VP1 primes viral RNA synthesis in vitro, a VP1–pN structure acts as a primer for viral RNA synthesis (Magyar et al., 1998). The pVP2 protein is processed to the major capsid protein VP2 (50–55 kDa) (Duncan and Dobos, 1986; Harvarstein et al., 1990; Manning and Leong, 1990). Investigation of the subcellular localization of IPNV structural proteins suggests that VP2 is glycosylated freely in the cytoplasm of infected cells (Espinoza et al., 2000). The central variable domain and 20 adjacent amino acids of the conserved C‐terminal part of the major immunogenic polypeptide VP2 induce the animal’s protective immunogenic response (Heppell et al., 1995b). A reverse genetic system for IPNV was established by Yao and Vakharia (1998) using plus‐stranded RNA transcripts derived from cloned cDNA. Quasispecies heterogeneity and rapid mutation characteristics of IPNV have been studied with RNA fingerprinting. It has been found that three clusters exist and that the IPNV Sp represents a different evolutionary route from those of the IPNV Ab and the VR299 clusters. Molecular relationship could be correlated with biological characteristics (e.g. cell line, adaptive replication at high temperature) (Hsu et al., 1995). Phylogenetic comparison of the deduced amino acid sequences of the VP2 coding region of 28 aquatic birnaviruses, including the type strains of the serogroup A, reveals six genogroups with several genotypes. The most divergent viruses exhibit a similarity of 81.2%. The genogroups based on the entire VP2–ORF generally correlate with geographical origin and serological classification (B. L. Nicholson, personal communication). Investigations of the VP2 gene by PCR and RFLP cluster analysis grouped 17 Asian isolates (all serotype A3) in four major genogroups. The groups based on the variation of the VP2 gene correlated with the serological classification based on VP2‐specific monoclonal antibody reaction pattern (Lee et al., 1996). However, characterization of the small ORF on the genome segment A of 20 IPNV strains reveals three major genotypes. According to correlation between serological and genomic classification, some of the 10 serotypes of serogroup A seem in fact to be subtypes. Serotype A4 (IPNV isolated from pike (Hecht) in Germany) (Ahne, 1978b) appears different from all serotypes of Serogroup A (Heppell et al., 1995b). IPNV induces apoptosis preceding necrosis in fish cell lines by down‐regulating the survival factor Mcl‐1, a Bcl‐2 homologue (Hong et al., 1998, 1999a). A variant of the green fluorescent protein gene (EGFP) expressed in a fish cell line showed dynamic non‐typical apoptotic cell morphological changes during IPNV infection (Hong et al., 1999b). Induction of the antiviral Mx protein by IFN protects against IPNV in CHSE cells (Nygaard et al., 2000). Different IPNV vaccines have been established: an inactivated vaccine used in Norway, an attenuated vaccine, and a recombinant vaccine available in Norway based on the VP2 protein of strain N1 (Frost and Ness, 1997; Pettersen, 1997; Frost et al., 1998). IPN is an OIE significant disease. Recommended screening procedures for IPNV are direct methods such as isolation, neutralization and immune fluorescence assay test (OIE, 2001).
Yellowtail ascites virus and marine fish birnaviruses
Investigation of the amino acids of the variable VP2–NS junction showed that several marine birnaviruses (MABV, e.g. yellowtail ascites virus) comprise a new genogroup distinguishable from IPNV (Hosono et al., 1996). A cell line derived from a snakehead fish (Channa lucius) was found to be persistently infected with a birnavirus showing distinctness in serology, biochemistry and phenotype from the type species of the genus Aquabirnavirus, IPNV (John and Richards, 1999).
Figure 7 summarizes transmission routes of fish viruses.
Arthropod‐borne Viruses (‘Arboviruses’)
The term ‘arboviruses’ is not taxonomically accurate. It comprises several distinct RNA viruses transmitted by arthropods. Arboviruses usually have more than one host and can spread between different host species. Most of them cause viral zoonoses. A variety of animal reservoir hosts and arthropod vectors play an important role in the maintenance of arboviruses in nature. Some viruses are maintained within populations of lower vertebrates.
Arthropod‐borne viruses of reptiles and amphibians
Several turtles, snakes and lizards have been found to be positive for arthropod‐borne viruses or antibodies, e.g. bhanjavirus, Crimean‐Congo haemorrhagic fever virus (Bunyaviridae), Japanese encephalitis virus, St Louis encephalitis virus, Powassan virus (Flaviviridae), western equine encephalitis virus, and Venezuelan equine encephalitis virus (Togaviridae) (Thomas and Eklund, 1960; Burton et al., 1966; Whitney et al., 1968; Shortridge et al., 1974; Shortridge and Oya, 1984). It is probable that reptiles are involved in the infection cycles of these viruses and play an important role as reservoir hosts of arthropod‐borne viruses (Ahne, 1993). Experimental infection of lower vertebrates suggests that arboviruses may persist in such animals, allowing these viruses to be maintained in nature, e.g. overwintering (Shortridge, 1989).
DNA Viruses of Lower Vertebrates
Figure 8 summarizes the DNA viruses occurring in lower vertebrates, and Fig. 9 (p. 432) shows electron microscopic images of some representative DNA viruses occurring in poikilothermic animals.
Single‐stranded DNA viruses
Parvoviridae
The family Parvoviridae contains six genera: Parvovirus, Erythrovirus, Dependovirus, Densovirus, Iteravirus and Brevidensovirus. Common features are non‐enveloped, icosahedral virions of 18–26 nm in diameter composed of 60 copies of the capsid protein. The viral genome consists of one linear molecule of ssDNA. Certain parvoviruses need for replication helper viruses such as adenoviruses or herpesviruses. Parvoviruses of lower vertebrates are not included in the present taxonomy of viruses (van Regenmortel et al., 2000).
Parvoviruses occurring in reptiles
Heldstab and Bestetti (1984) have reported parvovirus‐like particles in the necrotic duodenum of an Aesculapian snake (Elaphe longissima) and a four‐lined snake (Elaphe quatuorlineata) that had a concomitant infection with other viruses. A parvovirus‐like particle has been isolated from corn snake (Elaphe guttata) (Ahne and Scheinert, 1989). The non‐enveloped virions (30 nm) replicate in iguana heart (IgH2) cell cultures, inducing nuclear inclusion bodies and reaching titres up to 106,5 TCID50/ml. In bearded dragon lizards (Pogona vitticeps) parvovirus‐like particles have been found to appear beneath an adenovirus infection (Jacobson et al., 1986a).
Double‐stranded DNA viruses
Iridoviridae
The family Iridoviridae includes the genera Iridovirus, Chloriridovirus, Ranavirus and Lymphocystivirus. The genera Lymphocystivirus and Ranavirus comprise iridoviruses of poikilothermic vertebrates. Common features are icosahedral virions of 120–350 nm in diameter. Most of the iridoviruses have an envelope derived from the host‐cell membrane released by a budding process. The viral genome consists of one molecule of linear dsDNA; virions contain up to 36 polypeptides (van Regenmortel et al., 2000). The main structural component of the iridoviruses is the major capsid protein (MCP), which seems to be highly conserved among members of the families Iridoviridae, Phycodnaviridae and Asfarviridae (Tidona et al., 1998).
Iridoviruses occurring in lower vertebrates
Members of the genus Lymphocystivirus cause chronic and benign infections in more than 100 species of fish, resulting in hypertrophied epidermal cells. Iridoviruses of the genus Ranavirus infect amphibians, but related agents have been recognized as cause of serious systemic diseases among feral, cultured and ornamental fish, leading to severe necrosis of the renal and spleenic haematopoietic tissue (derivation of name: epizootic haematopoietic necrosis, EHN) (Langdon and Humphrey, 1987; Ahne et al., 1997). It has been shown that the amino acid sequence of the MCP contains highly conserved domains within many of the newly described iridoviruses of lower vertebrates. About 30 iridovirus‐like isolates from different species of lower vertebrates exhibiting systemic EHN disease syndromes have been placed in the genus Ranavirus. MCP sequences and comparison of nucleic acid profiles (restriction profile length patterns, RFLP) revealed six groups of viruses according to geographical origin and hosts: group 1: isolates from Australian fish; group 2: isolates from Australian frogs; group 3: isolates from South East Asian fish; group 4: isolates from European fish; group 5: isolates from South American frogs; and group 6: isolates from European and North American frogs (Hyatt et al., 2000). The seventh report of the ICTV lists the frog virus 3 as type species (with four strains) and five tentative species (with several strains) of the genus Ranavirus. The genus Lymphocystivirus comprises the type species lymphocystis disease virus 1 (with two strains), and lymphocystis disease virus 2 (with one strain) as tentative species and the goldfish virus 1 as unassigned species in the family Iridoviridae (van Regenmortel et al., 2000) (Table 6, p. 434).
Table 6.
Classification of species, tentative species and unassigned species in the genera Ranavirus and Lymphocystivirus (van Regenmortel et al., 2000)

Iridoviruses occurring in amphibians
Frog virus 3 (FV‐3)
Frog virus 3 (FV‐3, ATCC VR‐567), the type species of the genus Ranavirus, isolated in 1965 from a leopard frog (Rana pipiens) with renal adenocarcinoma, has been studied extensively (Granoff, 1989). FV‐3 is pathogenic for tailbuds, hatching stage embryos and tadpoles of frogs and Fowler’s toads (Bufo woodhousei fowleri). The virus causes severe oedema, dermal haemorrhages and high mortalities. FV‐3 replicates in mammalian, reptilian and fish cell lines, and in primary chicken embryo cells (Essani and Granoff, 1989) between 12 and 30°C. Virions measure 130–160 nm in diameter, and contain 29 structural and 61 non‐structural proteins (Elliott and Kelly, 1980). The core consists of 10–15 phosphoproteins (M r 10–114 × 103). The dsDNA genome is highly methylated at CpG sequences by a virus‐encoded methyl‐transferase. It is circularly permuted and terminally redundant (Goorha and Murti, 1982; Essani and Granoff, 1989). The methylation of DNA takes place in the cytoplasm and is required for the production of genomic‐size DNA during FV‐3 replication. The FV‐3 DNA methyl‐transferase has been identified and analysed (Kaur et al., 1995). The viral life cycle is characterized by two‐stage DNA synthesis cycles (Fig. 10, p. 435). In the first stage, less than the full genome of viral DNA is synthesized in the nucleus. In the second stage, the DNA is replicated in the cytoplasm as concatemers, in a similar way to bacteriophage genomes. Finally, the concatemers are cleaved to produce mature viral DNA (Goorha, 1982; Granoff, 1989). The virions assemble in the cytoplasm and are released by budding (Goorha, 1995). An ATPase is associated with viral particles and possibly integrated in virions (Aubertin et al., 1971). Virion proteins contain a peptide rapidly inhibiting cellular macromolecular synthesis (Willis and Granoff, 1976). Cellular RNA and DNA synthesis are shut down upon infection. Three temporal classes of mRNAs without poly‐A tails have been identified as undergoing no post‐transcriptional cleavage. RNA synthesis is a sequentially controlled process starting in the nucleus of the infected cells requiring modified cellular RNA polymerase II (Goorha, 1981). Protein translation is determined by the availability of transcripts. Virus proteins are regulated at the transcriptional and post‐transcriptional level (Willis et al., 1985). FV‐3 seems to have evolved unique signals for the inhibition and termination of transcription as it has no regulatory sequences resembling those of other eukaryotic genes. Highly methylated promoters that are normally refractory to transcription are used from the host polymerase. A few ORFs of FV‐3 have been sequenced and their regulating regions studied (Thompson et al., 1986; Munnes et al., 1995). FV‐3 induces changes in the cellular cytoskeleton. Microtubules are reduced and intermediate filaments are reorganized around virus assembly sites and anchor the virus particles beneath the nucleus. Late in infection many ‘microvillus‐like’ projections containing microfilaments and virus particles appear on the cell surface, suggesting a role for these cytoskeleton elements in virus release. FV‐3 inhibits selectively tubulin synthesis, phosphorylates vimentin and modifies actin. The changes are relevant to FV‐3 replication as heat‐inactivated or mutants of FV‐3 do not induce these alterations (Murti and Goorha, 1990). Parenteral inoculation of mice or rats with FV‐3, inactivated FV‐3 or its solubilized proteins induces an acute degenerative hepatitis leading to death in less than 24 h. The pathogenic mechanism seems to be a toxic one because FV‐3 does not multiply at temperatures above 30°C (Kirn et al., 1989). Frog virus 1 (FV‐1), frog virus 2 (FV‐2), and Lucké tumour‐associated viruses (LT, T) are antigenically related and probably represent various strains of FV‐3. All of these viruses induce oedema and mortalities in tadpoles of toads and frogs. The tadpole oedema virus (TEV; ATCC VR‐678) causes oedema and death in adult anurans such as bullfrogs and toads (Scaphiosus hammondii americanus, Bufo americanus, B. woodhousii fowleri) (Granoff, 1989). Analysis of the MCP and RFLP of TEV revealed high homology to FV‐3 (Hyatt et al., 2000).
Figure 10.
Replication scheme for frog virus 3, FV‐3 (after Goorha, 1982). C=cytoplasm; N=nucleus.
Rana esculenta iridovirus (REIR)
Rana esculenta iridovirus (REIR) was isolated from necrotic tissue of moribund green frogs (Rana esculenta L.) in eastern Europe (Fijan et al., 1991). Frogs developed no clinical symptoms after experimental infection with REIR by parenteral inoculation (i.m., i.p., s.c.) or exposure (water bath). REIR is antigenically related to FV‐3 and the systemic piscine iridoviruses comprising the epizootic haematopoietic necrosis virus (EHNV) group (Ahne et al., 1998b).
Bohle iridovirus (BIV)
Bohle iridovirus (BIV) has been isolated in Australia from the ornate burrowing frog (Limnodynastes ornatus) exhibiting mortalities during metamorphosis (Speare and Smith, 1992). BIV is highly pathogenic to tadpoles and juvenile amphibians (Limnodynastes ornatus, L. terrareginae, Bufo marinus, Litoria latopalmata) causing haematopoietic necrosis (Moody and Owens, 1994; Cullen et al., 1995). BIV (120–136 nm) shares characteristics of the piscine EHN iridoviruses, e.g. morphogenesis, ultrastructure, serology, susceptibility to cell lines, pathogenicity and genomic features. The DNA of EHNV and BIV cross‐hybridize to a high degree. However, BIV differs in molecular size, polypeptide profiles, cytopathogenic effects, RE profile and host susceptibility (Hengstberger et al., 1993). Most important is the fact that BIV crosses the species barrier, being pathogenic to fish, e.g. barramundi (Lates calcarifer) and tilapia (Oreochromis mossambicus) (Moody and Owens, 1994). Crossing of the class barrier has also been reported in the case of an iridovirus (Redwood Park virus, RPV) infecting stickleback fish (Gasterostelus aculeatus) and red‐legged frog (Rana aurora) in the USA (Mao et al., 1999).
Salamander iridoviruses
An epizootic associated with red leg disease in populations of tiger salamander (Ambystoma tigrinum stebbensi) has been recognized in South Arizona, USA. A highly contagious iridovirus (160–180 nm), the Ambystoma tigrinum stebbensi virus (ATV), has been isolated from affected salamanders. The disease is characterized by inclusion bodies in cells of skin and liver. Die‐offs of salamander (A. maculatum) associated with iridoviruses have been reported from several places in the USA (Daszak et al., 1999). Similar epizootics in salamander (A. tigrinum diaboli) with the presence of an iridovirus (Regina ranavirus, RRV) have been recognized in Canada. The isolated agent proved to be highly pathogenic for larvae but it does not induce skin alterations such as those induced by ATV. An MCP analysis showed that the RRV has 93% amino acid identity with FV‐3 (Bollinger et al., 1999).
Other ranavirus‐like iridoviruses of amphibians
Venezuelan toads (Bufo marinus) carry a ranavirus‐like agent: gutapovirus (GV). The virus (about 160 nm in diameter) is highly pathogenic to tadpoles of toads and to other amphibians (Daszak et al., 1999). Furthermore, it has been demonstrated that Bufo marinus sera contain antibodies to EHNV. It is possible that the toad, which has been imported to Australia for insect control, could play an important role in transmission of the iridoviruses. Bufo marinus might be a reservoir for the ranaviruses (Whittington et al., 1997; Zupanovic et al., 1998).
Mass mortality and decline induced by iridovirus infections have been reported from Rana temporaria exhibiting ulcerative lesions of skin and systemic haemorrhages in the UK (Cunningham et al., 1996). Phylogenetic analysis of the MCP gene revealed a grouping of this virus with North American strains as FV‐3 (Hyatt et al., 2000).
An iridovirus (Rana grylio virus, RGV) has been isolated from pig frogs (Rana grylio) exhibiting ulcerative disease in China. An MCP sequence analysis revealed high identity with FV‐3 (Zhang et al., 1999). Furthermore, reports have come from Thailand of iridovirus infections of Rana tigrina associated with similar ulcerative skin lesions and mortalities up to 95% (Kanchanakhan, 1998). This virus has not yet been classified.
Erythrocytic necrosis virus (ENV)
Intra‐erythrocytic inclusions of several species of lower vertebrates have been described for more than 30 years. The causative agent was believed to be a protozoan parasite, named ‘Toddia’ or ‘Pirhemocyton’. However, electron microscopy studies have shown that the inclusion bodies consist of arrays of iridovirus‐like particles of different sizes (up to 450 nm) depending on the hosts. Viral features such as size, formation of crystalline arrays of particles and membrane arrangement in mature erythrocytes differ within the diverse anuran host species (Alves de Matos and Paperna, 1993a, b; Alves de Matos et al., 1995). Erythrocytic necrosis virus (ENV) appears to acquire its envelope from lamellar membranes which surround the particles. The virions contain at least 16 proteins (19–91 kDa) and two major proteins of 32 and 43 kDa (Gruia‐Gray et al., 1989). In situ hybridization with an ENV probe has been used in order to detect viral‐specific DNA. Positive signals are analysed in the cytoplasm of erythrocytes, suggesting that ENV does not (in contrast to FV‐3) require host nuclear enzymes for viral replication, resembling the replication strategy of poxviruses (Gruia‐Gray et al., 1992). Prevalence of ENV is three times higher in juvenile Rana catesbeiana than in adults, while no infections are encountered in tadpoles (Gruia‐Gray et al., 1992). The viruses could not be grown in any cell cultures investigated.
Table 7 summarizes the iridoviruses described in species of amphibia.
Table 7.
Iridoviruses occurring in amphibia
Iridoviruses occurring in fish
Some of the iridoviruses occurring in teleost fish were placed by the ICTV in the genera Ranavirus and Lymphocystivirus of Iridoviridae. The goldfish virus 1 (isolated from cell cultures of goldfish) is an unassigned species of the family. However, most of the iridovirus‐like isolates of different countries and fish species origin (Table 8) are not listed in the seventh report of the ICTV (van Regenmortel et al., 2000). Three fish diseases caused by iridoviruses (EHNV, RSIV, WSIV) are notifiable or significant diseases according to the OIE.
Table 8.
Iridoviruses occurring in teleost fishes

Lymphocystis disease viruses (LCDV)
For about the last 100 years, giant cells in connective tissue and benign nodules (Fig. 11, p. 439) of the skin of centrarchid fishes, plaice and flounder have been known as lymphocystis disease (LCD). The causative agent has been identified as an iridovirus termed lymphocystis disease virus (LCDV). Strains of LCDV appear to be heterologous in size, measuring 198–227 nm and sometimes up to 380 nm. LCDV has been detected in more than 140 species of freshwater, eustarine and marine fishes (Wolf, 1988). LCDV‐1 affects flounder (Platichthys flesus) and plaice (Pleuronectes platessa). LCDV‐2 is found in dab (Limanda limanda) (Anders, 1989a). The viruses cannot be sufficiently cultivated in cell cultures. The icosahedral virions are covered by two protein layers and have filaments on the surface. LDCV DNA exhibits low homology with DNA of FV‐3 (Darai et al., 1983, 1985). The LCDV‐1 genome contains 195 potential ORFs of 102.7 kbp (GenBank L63545) (Tidona and Darai, 1997). By sequence analysis, e.g. a DNA‐dependent RNA polymerase II subunit, zinc‐finger proteins, a helicase and a guanosine triphosphate (GTP) phosphohydrolase have been detected in LCDV‐1 (van Regenmortel et al., 2000). The LCDV‐2 genome consists of 98 kbp, as shown by restriction endonuclease analysis (van Regenmortel et al., 2000). About 33 LCDV polypeptides (M r 14–220 × 103) can be separated by SDS‐PAGE. A thymidine kinase, an ATPase, a DNase and an RNase have been demonstrated in purified LCDV particles (Flügel et al., 1982). ORF 167L of LCDV encodes a protein with homology to the TNFR superfamily. The ORF is conserved in the genome of LCDV affecting flounder, plaice and dab. A monoclonal antibody (mAb) has been produced against the 29‐kDa protein of plaice LCDV expressed in E. coli. The mAb binds specifically to LCDV proteins present in the cytoplasm of infected cells around the lymphocystis nodules of dab and plaice (S. Essbauer, unpublished data). Infection with LCDV leads to transformation and enlargement of cells of the skin and in the connective tissue of internal organs. Infected cells undergo massive hypertrophy and encapsidation by an extracellular hyaline matrix. LCD is characterized as a chronic benign disease with rare mortality. Infection rates are increased by stress factors (Wolf, 1988). LCDV infections are diagnosed by electron microscopy, by PCR specific for the MCP of LCDV (Tidona et al., 1998), or by serology (ELISA, Lorenzen and Dixon, 1991).
Figure 11.
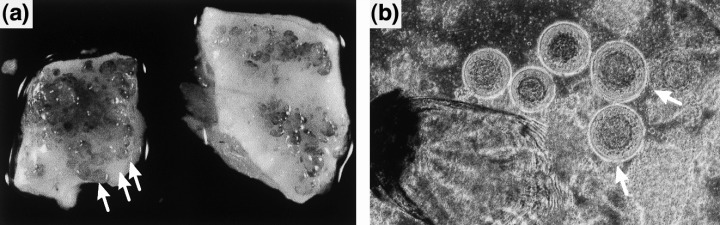
Lymphocystis disease virus (LCDV) infection in flounder. (a) Skin of LCDV‐infected flounder exhibiting multiple lymphocystis nodules (see arrows). (b) Explantats of LCDV‐infected flounder skin: lymphocystis cells are round and hypertrophied (see arrow), and 1000 times the size of uninfected cells (× 22). (The fish was kindly provided by Dr Steinhagen, Faculty of Veterinary Medicine, Hannover, Germany.)
Epizootic haematopoietic necrosis virus group (EHNV)
Iridoviruses causing severe necrosis of the haematopoietic tissue of infected fish have been recognized in perch and rainbow trout (epizootic haematopoietic necrosis virus, EHNV), in sheatfish (European sheatfish virus, ESV) and in catfish (European catfish virus, ECV) so far. The viruses inducing high mortality in affected fish share features with FV‐3 and are considered as tentative members (EHNV group) of the genus Ranavirus of Iridoviridae (Ahne et al., 1997, 1998b). EHNV has been isolated from redfin perch (Perca fluviatilis) (redfin perch virus, RFPV) and from rainbow trout (Oncorhynchus mykiss) (rainbow trout virus, RTV) in Australia (Langdon et al., 1986; Langdon and Humphrey, 1987; Whittington and Reddacliff, 1995; van Regenmortel et al., 2000). The virus was experimentally transmitted to fry and young fish of Percichthyidae, Teraponidae, Salmonidae and Galaxiidae, inducing high mortalities within few days (Whittington and Reddacliff, 1995). ESV has been isolated from an epizootic in sheatfish (Silurus glanis) in Germany (Ahne et al., 1997). ECV was isolated from disease outbreaks in catfish (Ictalurus melas) in France and in Italy (Pozet et al., 1992; Bovo et al., 1993). The highly pathogenic viruses EHNV, ESV and ECV cause systemic infections leading to severe haematopoietic necrosis (EHN) in fish. Viruses of the EHN group replicate in several fish cell lines at 15–28°C, inducing cytoplasmic inclusion bodies and lysis of infected cells. The viruses are released from infected cells by budding. EHN is notifiable to the OIE. The EHN viruses can be identified by IFAT, ELISA, immunoelectron microscopy and PCR, but not by neutralization tests, as the agents do not induce neutralizing antibodies (Hedrick et al., 1992a; Ahne et al., 1997; OIE, 2001). EHNV, ESV, ECV have been compared with iridovirus isolates from amphibians, e.g. REIR and FV‐3. The viruses share similarities in morphology and size (160 ± 10 nm in diameter), they cross‐react in IFAT, but the viruses can be distinguished by SDS‐PAGE, Western blotting and RE fragment patterns. More than 20 polypeptides can be recognized by SDS‐PAGE. The MCP of EHNV is slightly larger (51 kDa) compared with that of ESV and ECV (49 kDa). The polypeptides of 120, 55, 40 and 21 kDa of the piscine and amphibian iridoviruses associated with systemic diseases cross‐react to a high degree with homologous and heterologous antisera in Western blot. Electrophoresis patterns of the restriction enzyme fragments are similar in the case of the European piscine iridoviruses (ESV, ECV), but they are different from EHNV (Ahne et al., 1998b). According to sequence analysis of two cosmids from an EHNV library, the virus encodes a DNA polymerase, a helicase and an eIF‐2α‐like protein (Yu et al., 1999). In order to investigate the function of eIF‐2α proteins of different iridoviruses (EHNV, ESV, ECV, FV‐3 and REIR), the ORF of eIF‐2α has been expressed in prokaryotic and eukaryotic systems. This viral protein acts as a translation initiation factor homologue during the infection of cells with the iridoviruses. The protein has been found to be of 29 kDa, its sequence is highly conserved in EHNV, ECV, ESV and in the frog viruses FV‐3 and REIR (Fig. 12) (S. Essbauer, unpublished data). Analysis of proteins by SDS‐PAGE and Western blots with different antisera and MCP sequencing of a pike‐perch (Stizostedion lucioperca), a cod (Gadus morhua) and a turbot (Scopthalmus maximus) iridovirus revealed a close relationship between the investigated viruses and the EHNV group (Ariel et al., 2000; Tapiovaara et al., 2000).
Figure 12.
Phylogenetic tree of piscine and amphibian iridoviruses based on eIF‐2α homologous sequences. Three hundred and fifty nucleotides of frog virus 3 (FV‐3), European sheatfish virus (ESV), European catfish virus (ECV), epizootic haematopoietic necrosis virus (EHNV), and Rana esculenta iridovirus (REIR) eIF‐2α genes were compared with partial eIF‐2α genes of humans, Drosophila, and yeast, and complete K3L genes of Variola major virus, Vaccinia strain Copenhagen, and swine poxvirus. Figures on each branch represent percentage bootstrap support for maximum parsimony (S. Essbauer unpublished data).
Santee‐Cooper ranavirus (SCRV)
Three iridoviruses, i.e. largemouth bass iridovirus (LMBV), doctor fish virus (DFV‐16) and guppy virus (GV‐6), have proved to be very similar and were designated ‘Santee‐Cooper ranavirus (SCRV)’, after their place of origin in South Carolina, USA (Mao et al., 1997). LMBV (174 nm), which causes mortality in wild largemouth bass (Micropterus salmoides), has been isolated from the swimbladder and liver of infected fish (Piaskoski et al., 1999). Juvenile largemouth bass and striped bass (Morone saxatilis) are susceptible to LMBV. To determine the prevalence of LMBV, wild largemouth bass were investigated during 1997–1998 in south‐eastern USA. LMBV was detected by isolation in dead or healthy fish in seven natural reservoirs. The only pathological sign seen was an enlarged, inflamed swimbladder in infected fish. In the summer of 2000 recent outbreaks of LMBV in five Texan reservoirs and on lakes in four other southern US states have been reported. Investigations on what triggers its deadly effect on largemouth bass, or whether it might have any impact on other species are under progress (http://www.promedmail.org; release from the Texas Parks and Wildlife Department). Horizontal transmission of LMBV has been shown; vertical transmission is suspected as high concentrations of virus are maintained in gonad tissue (Plumb et al., 1996). Sequence analysis of the MCP and the methyl‐transferase genes reveals high identity of LMBV with iridoviruses isolated from doctor fish (Labroides dimidatus, DFV‐16) and guppy (Poecilia reticulata, GV‐6) (Mao et al., 1997; Plumb et al., 1999). The MCPs of DFV‐16 and GV‐6, which are obtained from fish imported from South East Asia, have proved to be identical (Hedrick and McDowell, 1995; Mao et al., 1997). Both viruses possess low pathogenicity to rainbow trout and chinook salmon (Hedrick and McDowell, 1995). Polypeptide pattern comparison and RFLP analysis of the SCRV‐like viruses reveal a few differences (Mao et al., 1997). All three isolates cross‐react with the EHN viruses and FV‐3 (Hedrick and McDowell, 1995; Mao et al., 1997; Plumb et al., 1999), but the MCP of LMBV has only 80% amino acid identity with FV‐3. DFV and GV‐6 DNA do not hybridize with FV‐3 DNA. These results confirm that FV‐3 and SCRV are distinct Ranavirus species (Mao et al., 1997; Plumb et al., 1999; Hyatt et al., 2000).
White sturgeon iridovirus group (WSIV)
White sturgeon iridovirus (WSIV), which measures 283–314 nm in diameter, was first described among hatchery‐raised white sturgeon (Acipenser transmontanus) in North America. The internal nucleid surrounds a circular to bar‐shaped electron‐dense core and has a diameter of 208 nm (Hedrick et al., 1990a; Adkison et al., 1998). The virus is potentially enzootic in wild white sturgeon populations throughout the Pacific Northwest of North America. The disease caused by WSIV is most severe in juvenile sturgeon younger than one year old (LaPatra et al., 1996). WSIV is an epitheliotropic virus infecting the skin, gills and the upper alimentary tract, inducing mortality (Hedrick et al., 1990a). Parts of the integument and skin are hypertrophied, with enlarged Malpighian cells, usually full of virus particles. The WSIV has been isolated in sturgeon cell lines. In contrast to other fish iridoviruses, WSIV is suspected to be horizontally and vertically transmitted from broodstock. Antigenic relationship of WSIV to the EHN‐like virus group or RSIV is low. WSIV is epitheliotropic, morphologically different from other iridoviruses, and it can also be distinguished by host‐cell susceptibility, type of CPE, and the location of the target host cells. An iridovirus similar to WSIV has been identified in Russian sturgeon (Acipenser guldenstadi) in Northern Europe, where it may be enzootic among cultured populations of several species of sturgeon. Losses of Russian sturgeon due to the viral infection reach 50%. Siberian sturgeon (A. beari) are also susceptible to the virus (Adkison et al., 1998). Lake sturgeon (A. fulvescens) can be infected by WSIV but do not suffer mortality (Hedrick et al., 1992b). The WSIV disease is a significant disease according to the OIE (OIE, 2001). WSIV is diagnosed by immunohistochemistry, IFAT, or neutralization tests.
Red sea bream iridoviruses group (RSIV)
Red sea bream iridovirus (RSIV), which measures 200–240 nm in diameter, causes mortality in cultured juvenile red sea bream (Pagrus major) and was first isolated in 1990 in Japan (Nakajima and Sorimachi, 1994; Matsuoka et al., 1996). Twenty‐five fish species of the orders Perciformes, Pleuronectiformes and Tetradontiformes have been reported to be susceptible to RSIV. Infected fish exhibit severe anaemia, petechia of the gills and enlargement of the spleen (Jung et al., 1997). An iridovirus serologically and genetically related to RSIV has been isolated from brown‐spotted grouper (Epinephelus tauvina) in Thailand. The isolate does not induce disease in experimentally infected red sea bream (Miyata et al., 1997). RSIV‐like viruses can be distinguished by genetic variability (Matsuoka et al., 1996). Polyclonal rabbit antiserum, but not monoclonal antibodies against RSIV, cross‐react weakly in IFAT with ranaviruses of the EHNV group, FV‐3, and an iridovirus isolated from Japanese eel (Anguilla japonica) (Nakajima et al., 1998b). RSIV itself induces minor levels of neutralizing antibodies in immunized rabbits or infected fish. A partial genomic library of RSIV has been constructed and sequenced. ORFs coding for FV‐3‐homologous ATPase, LCDV‐homologous DNA‐dependent RNA polymerase, and DNA polymerase have been analysed and the small subunit of the ribonucleotide reductase (RNRS) has been identified (Oshima et al., 1998). The RSIV can be isolated from the kidney and spleen of infected fish using fish cell cultures at 20–25°C (Nakajima and Sorimachi, 1994). The agent is identified by IFAT or PCR amplification (specific for RNRS fragment of RSIV) and sequencing (Nakajima et al., 1995; Kurita et al., 1998; Oshima et al., 1998; OIE, 2001). A formalin‐killed RSIV vaccine has proved to be effective in a field trial with red sea bream (Nakajima et al., 1999).
Goldfish virus 1‐like iridovirus (GFV‐1)
Two iridoviruses of about 180 nm in diameter, goldfish viruses 1 and 2 (GFV‐1, GFV‐2) have been isolated from swimbladder tissue culture of healthy goldfish (Carassius auratus). The role of the viruses in disease has not been determined (Berry et al., 1983; Wolf, 1988). CpG and CpT sequences of the DNA of GFV are highly methylated (Goorha, 1995). The DNA of GFV is not cleaved by HindIII (Essani and Granoff, 1989).
Erythrocytic necrosis virus (ENV)
Iridoviruses in the cytoplasm of erythrocytes (erythrocytic necrosis virus, ENV, or viral erythrocytic necrosis, VEN) have been detected in cyclostoms, elasmobranchs and in 14 families of marine and andromous bony fish (Wolf, 1988). Affected erythrocytes contain a single, round cytoplasmic inclusion body which is pathognomic for ENV infection. Viral particles within the inclusions have varying sizes of 150–360 nm probably depending on the host. Antisera against ENV react specifically with the inclusion bodies, cellular membranes and the nuclei of infected erythrocytes in IFAT, immunoperoxidase assays and in immunoelectron microscopy (Pinto et al., 1991).
Iridoviruses occurring in reptiles
Several iridoviruses have been reported in reptiles (Table 9). Spontaneous viral hepatitis, spleenitis and enterocolitis developed in Mediterranean land tortoise (Testudo hermanni) were found to be associated with iridovirus‐like particles measuring 140–160 nm in diameter (Heldstab and Bestetti, 1982). Müller et al. (1988) report iridoviruses associated with an spontaneous nasopharyngeal and pneumonal epidemic disease in Mediterranean land tortoises (T. hermanni hermanni). Iridoviruses of 150–220 nm in diameter have been detected by electron microscopy in the tracheal lumen and epithelial lung cells of a free‐living gopher tortoise (Gopherus polyphemus) suffering from respiratory and pharyngo‐oesophageal disease (Daszak et al., 1999). Furthermore, iridoviruses have been detected in different turtles (Testudo horsfieldii, T. hermanni, Terrapene carolina). The iridovirus occurring in T. hermanni with hyperaemic mucosas and liver necrosis could be isolated. It replicates in different reptilian cell lines with an optimum at 28°C (Marschang et al., 1999). The MCP of the turtle iridoviruses reveals high homology to that of FV‐3. Phylogenetic analysis of the MCP sequences suggests that the turtle viruses are members of the genus Ranavirus of Iridoviridae (Mao et al., 1997; Marschang et al., 1999). Recently, an iridovirus has been isolated from juvenile soft‐shelled turtle (Trionyx sinensis) with ‘red neck disease’ in China (Daszak et al., 1999). The virus multiplying in different fish cell lines at 15–30°C with an optimum at 25°C showed iridovirus‐like morphology and measured 120–160 nm in diameter. The virus proved to be pathogenic to turtles, inducing mortality.
Table 9.
Iridoviruses occurring in reptiles

DNA from fixed tissue of chondra pythons (Chondrapython viridis) has been extracted. MCP‐specific PCR and sequence analysis showed that the animals were infected with iridoviruses of the genus Ranavirus (Daszak et al., 1999).
Electron microscopy studies in North Africa and Australia have shown that many reptile species carry erythrocytic inclusions that resemble iridoviruses (Alves de Matos and Paperna, 1993b). Intra‐erythrocytic inclusions associated with iridoviruses have been observed in the erythrocytes of the snake Bothrops moojeni (Johnsrude et al., 1997).
Poxviridae
The family Poxviridae comprises 11 genera within the two subfamilies Chordopoxvirinae and Entomopoxvirinae. Common features are pleomorphic to brick‐shaped virions measuring 220–450 × 140–260 × 140–260 nm. The biconcave core contains a single, linear molecule of covalently closed dsDNA encoding 150–300 proteins, of which 100 are present in the virion (van Regenmortel et al., 2000).
Poxviruses occurring in amphibia
Poxvirus‐like particles have been found to be associated with mortalities of the frog Rana temporaria in the UK. Animals have exhibited lesions and ulcers of the skin, haemorrhagic gastritis, haemorrhages in muscle, and necrosis of extremities (Cunningham et al., 1993, 1996). The viruses have not yet been characterized.
Poxviridae occurring in reptiles
Crocodiles (Caiman sclerops), exhibiting grey‐white skin lesions over the whole body and oral lesions on the tongue, showed intracytoplasmic inclusions within hypertrophied epithelial cells. Electron microscopy revealed in the inclusions poxvirus‐like particles of 220 × 100 nm (Jacobson, 1978; Jacobson et al., 1979; Vetesi et al., 1988). Similar cases of hatched Nile crocodiles (Crocodylus niloticus) (Horner, 1988; Pandey et al., 1990) and of South African crocodiles (Caiman crocodilus fuscus) (Penrith et al., 1991) have been reported. Poxviruses have been found in monocytes of a flap‐necked chameleon (Chamaeleo dilepis) in Tanzania (Jacobson and Telford, 1990). The seventh report of the ICTV lists the Nile crocodile poxvirus (CRV) and the spectacled caiman poxvirus (SPV) as unassigned viruses in the family Poxviridae (van Regenmortel et al., 2000).
Herpesviridae
The family Herpesviridae contains nine genera within the three subfamilies: Alphaherpesvirinae, Betaherpesvirinae, Gammaherpesvirinae and the unassigned genus ‘Ictalurid herpes‐like viruses’. Common features are enveloped virions of quasispherical morphology, measuring 100–200 nm in diameter. An icosahedral capsid of 100–110 nm in diameter is composed of 162 capsomeres and a linear dsDNA. The seventh report of the ICTV lists several herpesviruses of fish, amphibians and reptiles as unassigned viruses in the family (van Regenmortel et al., 2000).
Herpesviruses occurring in fish
Several herpes‐like viruses (Table 10) have been detected in different fish species but only few are sufficiently characterized. Channel catfish herpesvirus (CCHV) and salmonid herpesvirus (SaHV‐2) induce OIE notifiable or significant diseases.
Table 10.
Herpesviruses occurring in fish
Herpesvirus occurring in shark
Herpesvirus‐like particles (150–180 nm) were detected by electron microscopy in the necrotic skin of fins and trunk of smooth dogfish (Mustelus canis) (Leibovitz and Lebouitz, 1985).
Ictalurid herpesvirus 1 (IcHV‐1), syn. channel catfish herpesvirus (CCHV)
Ictalurid herpesvirus 1 (IcHV‐1; Herpesvirus ictaluri; ATCC VR‐665) is responsible for economic losses of channel catfish (Ictalurus punctatus) fry and fingerlings in North America, Russia and Honduras. The virus has been cultivated in different fish cell lines at 25°C, reaching titres of 107,5 PFU/ml. Virions, measuring 175–200 nm in diameter, are sensitive to lipid solvents and heat treatment (1 h, 60°C) and UV (20–40 min). The viral replication is inhibited by acyclovir and IUdR (Plumb, 1989). The virus has a buoyant density of 1.29 g/cm3 caesium chloride. Thirty‐two polypeptides are encoded in the virus genome, of which are 18 structural proteins. The IcHV‐1 genome is 134.2 kbp long and has 77 ORFs; 14 of these are in direct repeated regions (DR, each 18.5 kbp) flanking a 97.1 kbp unique long (UL) region. The IcHV‐1 seems to be unrelated to mammalian herpesviruses (Davison, 1992; Kucuktas and Brady, 1999; Pringle, 1999). According to genetic comparisons and the low similarity to other herpesviruses a separate evolutionary origin for IcHV‐1 must be considered (Davison, 1992; Davison and Davison, 1995). IcHV‐1 gene expression in the direct repeat region is temporally regulated: immediate‐early transcripts encoding viral transactivators are of ORFs 1 and 3, early RNAs are encoded by ORFs 2–9 and 11–14, immediate to late RNAs by ORFs 4, 7 and 10–13. For several transcripts (e.g. of ORFs 5 and 6) bicistronic transcripts are reported (Stingley and Gray, 2000). ORF 59 of IcHV‐1 encodes an abundant hydrophobic membrane glycoprotein, the major envelope glycoprotein of the virus (Kucuktas et al., 1998). ORF 50 encodes a secreted, mucin‐like glycoprotein (gp250) revealing different size in several isolates of IcHV‐1. This is a characteristic of mucin genes caused by crossing‐over events between the internal repeat sequences (Vanderheijden et al., 1999). IcHV‐1 thymidine kinase (TK, encoded by ORF 5 in DR) is highly divergent and biochemically distinguishable from TKs of host cells or other herpesviruses, as it is inhibited by deoxypurines and shows less dTTP‐mediated feedback inhibition. The thymidine kinase of IcHV‐1 has the unique property to use CTP as a donor of phosphate and is related to human deoxycytidine kinase (dCK) (Harrison et al., 1991; Hanson and Thune, 1993). Experimental infection of channel catfish fingerlings with IcHV‐1 leads to a haemorrhagic, oedematous and anaemic disease and mortality (Plumb, 1989). Fish produce neutralizing antibodies against IcHV‐1 maintaining for 2 years (Hedrick et al., 1987). Different genetic strains of channel catfish vary in susceptibility to IcHV‐1, and hybrids especially have proved to be less susceptible (Plumb, 1989). IcHV‐1 virulence is reduced at temperatures below 20°C (Wolf, 1988). Blue catfish (Ictalurus furcatus) and white catfish (I. catus) are susceptible to IcHV‐1 (Wolf and Darlington, 1971; Plumb, 1989). Horizontal transmission has been shown for IcHV‐1. Nucleic acid‐probing techniques give evidence for vertical transmission of IcHV‐1 (Wise et al., 1988). Diagnosis of IcHV‐1 infection that according to the OIE induces a significant disease is carried out by IFAT, neutralization tests (Wolf, 1988), ELISA (Crawford et al., 1999), or PCR (Gray et al., 1999a). Less than 0.1 pg of IcHV‐1 viral DNA can be specifically detected by PCR or nucleic acid‐probing technique in acutely or latently infected catfish (Plumb, 1989; Tham and Moon, 1996; Gray et al., 1999a, b). During latency the IcHV‐1 genome may exist as circular or concatemeric DNA in several tissues in the leucocytes (Gray et al., 1999b). Identification of carrier fish can also be carried out by screening for neutralizing antibodies that vary seasonally (OIE, 2001). A recombinant TK gene deletion mutant of IcHV‐1 has been constructed. Exposure of fish to the IcHV‐1 TK− mutant induces protective immunity against challenge with a lethal dose of wild‐type IcHV‐1 (Zhang and Hanson, 1995). IcHV‐1 TK− infections do not persist as long as IcHV‐1 infection and have reduced shedding ability (Kancharla and Hanson, 1996). An E. coli lacZ gene inserted into the IcHV‐1 genome is expressed by IcHV‐1 (Zhang and Hanson, 1996). The potential use of IcHV‐1 as a vaccine vector for the channel catfish industry is in discussion. An epizootic associated with herpesvirus infection has been recognized in pilchards (Sardinops sagax neopilchardus) off the Australian and New Zealand coastline (Hyatt et al., 1997). The herpesvirus‐specific TK has been detected in infected fish by using a PCR technique developed for the IcHV‐1 TK (Tham and Moon, 1996). The IcHV‐1 is listed as species in the unassigned genus ‘Ictalurid herpes‐like viruses’ in the seventh report of the ICTV (van Regenmortel et al., 2000).
Herpesviruses of cyprinid fishes
Herpesvirus‐like particles have been isolated from papillomas of skin and fin (‘carp pox’) of fancy carp (Cyprinus carpio L.) (Sano et al., 1985). Infected cells have shown intranuclear inclusions of Cowdry type A and the karyoplasm exhibited many virus particles. The isolated virus (150–240 nm) termed Herpesvirus cyprini is now known as cyprinid herpesvirus 1 (CyHV‐1) (van Regenmortel et al., 2000). CyHV‐1 is ether‐, pH 3‐ and heat‐labile. It has proved to be pathogenic to two‐week‐old carp, inducing necrosis in internal organs and mortality. Oncogenicity has been shown as surviving carp fry developed rapid papillomatosis and neoplasms (Sano et al., 1991, 1993a). In situ hybridization with biotinylated probes of CyHV‐1 genome in infected carp shows positive signals in cranial nerve ganglia, subcutaneous tissue and spinal nerves (Sano et al., 1993b).
An epizootic with severe mortality has occurred among cultured goldfish, Carassius auratus (L.), in Japan. A herpesvirus, termed goldfish haematopoietic necrosis virus (GFHNHV) but presently assigned as cyprinid herpesvirus 2 (CyHV‐2) (van Regenmortel et al., 2000) has been isolated from moribund goldfish. Electron microscopy has revealed virions of 170–220 nm in diameter with hexagonal nucleocapsids. CyHV‐2 has proved to be non‐pathogenic to fancy carp (Jung and Miyazaki, 1995). A comparable herpesvirus infection has been reported from juvenile goldfish in the USA. During epizooties in springtime mortalities of goldfish reach up to 100%. Herpesvirus‐like particles have appeared in hyperplastic branchial and necrotic renal cells (Groff et al., 1998). Similar herpesvirus‐like particles have been found during an epizootic with high mortality in goldfish fry in Taiwan (Chang et al., 1999).
Koi carp (Cyprinus carpio) have shown a systemic disease with skin lesions associated with high mortality in many countries during the last years. Diseased fish exhibit papillomas on the caudal regions, especially in the fins. Enlarged nuclei of affected cells contain numerous herpesvirus‐like particles measuring 157 nm (Hedrick et al., 1990b). Herpesviruses associated with mass mortality of juvenile and adult koi carp can be isolated using koi fin cells (KF‐1 cells) (Hedrick et al., 2000). Herpesviruses causing mortalities in Koi carp have been reported from Israel (Ariav et al., 1999) and Germany (Bretzinger et al., 1999). The koi carp herpesviruses (KHV) from Israel and North America seem to be identical but can be distinguished serologically from CyHV‐1 (Hedrick et al., 2000).
Electron microscopy of golden ide (Leuciscus idus melanotus) with skin lesions has exhibited herpesvirus‐like particles (115 nm) in epidermal hyperplasia (McAllister et al., 1985; Steinhagen et al., 1992).
Herpesviruses occurring in eels
Herpesvirus‐like particles have been detected in skin lesions of eel (Anguilla anguilla) in Hungary (Bekesi et al., 1986). A herpesvirus isolated from eels without showing any signs of a disease has induced syncytia in rainbow trout gonad (RTG‐2) and epithelioma carp (EPC) cell lines (Jorgensen et al., 1994). Anguilla herpesvirus 1 (AngHV‐1) has been isolated in EK‐1 (eel kidney) cells from Japanese eel (Anguilla japonica) showing skin lesions. Electron microscopy has revealed virions of 200 nm with nucleocapsids measuring about 115 nm. In experimental infections of Japanese eel cutaneous lesions have been induced (Kobayashi and Miyazaki, 1997). Later, an infectious gill disease among Japanese eel associated with mass mortalities was found to be of herpesvirus origin. The isolated virus is neutralized by AngHV‐1 antiserum, is pathogenic to eels, and induces dermal lesions such as those reported for AngHV‐1 (Lee et al., 1999). European eel (Anguilla anguilla) that exhibited haemorrhagic septicaemia and skin lesions have carried herpesvirus‐like particles. The isolated viruses have nucleocapsids of 110 nm surrounded by an envelope with spikes. The isolates are serologically related to AngHV‐1 and ‘eel herpesvirus Formosa’ (EHVF) (Davidse et al., 1999), a herpesvirus‐like agent isolated from Japanese eel with varicella on the surface of the skin (Ueno et al., 1992). In a caesium chloride gradient two virus bands (1.25–1.28 and 1.30–1.33 g/ml) are obtained. The guanine–cytosine (GC) content of the viral DNA is 32.2% (Shih et al., 1993). EHFV and AngHV‐1 are tightly clustered, as shown by comparison studies. Both viruses develop syncytia, have the same size and show similar polypeptide patterns to the major polypeptides in SDS‐PAGE. Cross‐reactivity has been shown by neutralization and Western blot. However, the viruses do obviously not have the same host range (Ueno et al., 1996). European eel infected with EHVF have exhibited severe necrotic hepatitis, haemorrhage and tubular nephritis (Shih, 1997).
Salmonid herpesvirus 1 (SalHV‐1)
Salmonid herpesvirus 1 (SalHV‐1; syn. Herpesvirus salmonis‐1; ATCC VR‐868) has been isolated from the ovarian fluid of rainbow trout (Oncorhynchus mykiss) showing high post‐spawning mortality. Infected rainbow trout cell lines exhibit pycnosis, syncytia and Cowdry type A intranuclear inclusions carrying hexagonal nucleocapsids of 90–95 nm. The enveloped virus (150 nm) is labile to treatment with acid, heat, ether and chloroform; acyclovir inhibits the replication of SalHV‐1 (Hedrick and Sano, 1989). The viral DNA (GC content of 50%) has a buoyant density of 1.709 g/cm3 (Wolf, 1988). The viral genome (174.4 kbp) consists of a long unique region (133.4 kbp) linked to a short one (25.6 kbp) flanked by an inverted repeat (7.7 kbp). This genomic organization resembles that of members of the genus Varicellovirus, subfamily Alphaherpesvirinae, of Herpesviridae. The SaHV‐1 shares at least 18 genes with IcHV‐1. The nucleic acid probe technique has shown that conserved SalHV‐1 genes are located in the long unique region in at least five rearranged blocks. The junction between two gene blocks contains a dUTPase gene and a part of a putative spliced DNA polymerase gene (Davison, 1998). The SalHV‐1 has proved to be lethal to fry and fingerlings of rainbow trout. Affected fish exhibit exophthalmia, oedema and necrosis in gill, heart and haematopoietic tissues (Wolf, 1988). Horizontal infection occurs in rainbow trout, brown trout and brook trout (Hedrick and Sano, 1989). Kokanee salmon (O. nerka) seems to be resistant to SalHV‐1 (Wolf and Smith, 1981). The SalHV‐1 proved to be not oncogenic (Hedrick et al., 1986).
Salmonid herpesvirus 2 (SalHV‐2)
Salmonid herpesvirus 2 (SalHV‐2; syn. Herpesvirus salmonis‐2, syn. Oncorhynchus masou virus) was isolated in Japan from ovarian fluid of masou salmon, Oncorhynchus masou, inducing syncytia formation and lysis of infected cells. Electron microscopy revealed virions of 200–220 nm with a nucleocapsid of 115 nm in diameter. The SalHV‐2 is labile to ether, heat, acid, and UV treatment. Replication of SalHV‐2 is inhibited by acyclovir and IUdR. In virus‐infected cells 34 polypeptides appear, but the polypeptide pattern is distinct from that of IcHV‐1 (Kimura and Yoshimizu, 1989). The DNA of SalHV‐2 has a molecular weight of about 100 × 106 Da without terminal repeats (Guo et al., 1991a). The SaHV‐2 genome shows a relationship to the IcHV‐1 genome (Bernard and Mercier, 1993). The temperature optimum of the viral DNA polymerase is 25°C (Suzuki et al., 1986). Experimentally infected salmon fry exhibit necrosis and hyperplasia of haematopoietic tissues (Kimura and Yoshimizu, 1989). Horizontal and vertical transmission of SalHV‐2 have been shown (Wolf, 1988; Kumagai et al., 1997). Survivors of the acute infection developed epidermal papillomas (Kimura and Yoshimizu, 1989). Viral DNA polymerase activity is found in tumours of masou salmon, indicating replication of SalHV‐2 DNA in the transformed cells (Suzuki et al., 1992). Jodophore treatment of the eggs of masou salmon reduces the transmission of SalHV‐2 (Yoshimizu et al., 1988). Viral DNA (10 copies/cell) has been detected by DNA hybridization two weeks earlier compared with isolation of virus (Guo et al., 1991b). The SalHV‐2 related herpesviruses – the Yamame tumour virus (YTV) isolated from a mandibular tumour of masou salmon (O. masou) and the Nerka tumour virus (NeVTA) obtained from kokanee salmon (O. kisutch) – are two herpesviruses of salmonid fishes in Japan that have been described in detail (Kimura and Yoshimizu, 1989). NeVTA, SalHV‐2 and YTV are of high virulence to masou salmon but of lower virulence to coho salmon and rainbow trout (Kumagai et al., 1995). The viruses exhibit close serological and genetic relationships with SalHV‐2. Differences in migration, of two polypeptides in particular, led to the classification of 12 SalHV‐2 strains in six groups (Kimura et al., 1984). All Japanese salmonid herpesviruses can be clearly distinguished from the North American herpesvirus strains (Hedrick and Sano, 1989; Kimura and Yoshimizu, 1989; Guo et al., 1991c; Yoshimizu et al., 1995). The SalHV‐2 strains induce diseases notifiable to the OIE. Viruses are identified by direct methods as neutralization tests, IFAT or ELISA (OIE, 2001).
Other herpesviruses of salmonids
Cumulative mortality of lake trout (Salvelinus namaycush) in US hatcheries was induced by a herpesvirus, termed epizootic epitheliotropic disease virus (EEDV) or salmonid herpesvirus 3. Affected fish exhibited hyperplasia, hypertrophy, necrosis of epidermal cells and oedema in the gills. Infected cells contained intranuclear inclusion bodies and herpesvirus‐like particles of about 100 nm have been detected. Infection trials with the isolated agent induced mortality in lake trout but not in rainbow, brook and brown trout, or Atlantic and chinook salmon (Bradley et al., 1989). Observations of epizootics in fingerling and yearling of lake trout caused by EEDV were made in the Great Lakes area between 1983 and 1986 in the USA (McAllister and Herman, 1989). The steelhead herpesvirus (SHV, 231 nm) was isolated from ovarian fluid of steelhead trout (O. mykiss) in the USA. Low virulence appears in rainbow trout and chinook salmon. Infected fish show hepatocytic syncytia and Cowdry type A inclusion bodies. Vertical transmission of the virus is believed to occur, as SHV was detected in semen samples of steelhead trout. Serum cross‐neutralization tests, polypeptide composition and DNA sequence comparisons show that SHV is related to SaHV‐1, but it differs in pathogenicity, transmission and host spectrum. SHV has proved not to be oncogenic (Eaton et al., 1989). Papillomatosis of cultured Atlantic salmon (Salmo salar) observed in Russia and Scandinavia have been associated with herpesvirus‐like particles (200–240 nm) (Wolf, 1988; Shchelkunov et al., 1992).
Esocid herpesvirus 1 (EsHV‐1)
Esocid herpesvirus 1 of about 200 nm in diameter has been observed in populations of northern pike (Esox lucius) and muskellunge (E. masquinongy) with hyperplastic epidermal lesions (blue spot disease) in the USA during the spring spawning periods, with a prevalence of around 30%. The virus could not be cultivated using esocid or percid cultures (Yamamoto et al., 1983; Wolf, 1988).
Acipenserid herpesviruses (1 and 2)
Serious losses of hatchery‐reared fry and fingerlings of white sturgeon (Acipenser transmontanus) in the USA have been found to be associated with herpesvirus infections of the integument and oropharyngal mucosa. The acipenserid herpesvirus 1 (AciHV‐1; syn. white sturgeon herpesvirus, WSHV‐1) measures about 230 nm and has a capsid of about 110 nm in diameter. The virus was isolated using sturgeon cell lines. Fibrillar material and nucleocapsids are present in the cytoplasm of infected cells. Bath exposure of white sturgeon with the isolated virus induced mortality. A second acipenserid herpesvirus (AciHV‐2) was isolated from the ovarian fluid of white sturgeon. The particles measure 177 nm in diameter. AciHV‐2 induces cumulative mortality of 80% in juvenile white sturgeon. Sera from naturally infected juveniles and from adults hyperimmunized with AciHV‐2 fail to neutralize AciHV‐1 (Hedrick et al., 1991; Watson et al., 1995).
Pleuronectid herpesvirus 1 (PlHV‐1)
Pleuronectid herpesvirus (PlHV‐1; syn. Herpesvirus scopthalmi) of 200–220 nm in diameter was detected in turbot (Scopthalmus maximus) in Scotland and Denmark. Turbot fry showing moderate mortality suffered from a severe general oedema (Wolf, 1988; Bloch and Larsen, 1993).
Percid herpesvirus 1 (PeHV‐1)
Percid herpesvirus 1 (PeHV‐1; syn. Herpesvirus vitreum) was isolated during spring spawning from walleye (Stizostedion vitreum vitreum) showing epidermal hyperplasia. The viral particles measuring 190–230 nm are labile to treatment with ether and heat. Viral replication in walleye cell cultures can be inhibited by IUdR and acyclovir (Wolf, 1988).
Additional herpesviruses of fish
A papillomatosis of European smelt (Osmerus eperlanus) reported in the Baltic Sea and the USA was shown to be of herpesvirus origin (Anders, 1989b; Morrison et al., 1996). Similar herpesvirus infection associated with epithelial skin tumours occurred in rainbow smelt (Osmerus mordax) in several lakes in the USA during spawning time, with a prevalence of 30% (Burke et al., 1989; Herman et al., 1997). Mass mortality of Japanese flounder (Paralichtys olivaceus) was found to be caused by a herpesvirus. The so‐called viral epidermal necrosis of flounders (VENF) is characterized by papillate cell aggregates in the epidermis and acidophilic cytoplasmic inclusion bodies filled up with herpesvirions (167–190 nm) (Miyazaki et al., 1989). Another herpesvirus (167–230 nm) of Japanese flounder was found, associated with epidermal hyperplasia of fin and skin (Iida et al., 1989). In contrast to VENF no cytoplasmic inclusion bodies, giant cells or syncytia were observed in infected flounder. Infection trials showed that both viruses induce mass mortality in the larvae of flounders (Miyazaki et al., 1989; Iida et al., 1989, 1991). Herpesvirus‐like particles (135 nm) were found in spleen macrophages and monocytes of angelfish (Pterophyllum altum) imported from the Amazon area to Denmark. The fish have shown disseminated skin haemorrhages and ulcerations (Mellergard and Bloch, 1988). Redstriped rockfish (Sebastes proriger) suffering from prominent hepatic lesions showed inclusion bodies of Cowdry type A and herpesvirus‐like particles of 100 nm in hepatic cells (Kent and Meyers, 2000).
Herpesviruses occurring in amphibia
Ranid herpesvirus 1 (RaHV‐1) syn. Lucké tumour herpesvirus (LTHV)
Lucké (1934) illustrated intranuclear inclusion bodies in renal adenocarcinoma of northern leopard frogs (Rana pipiens). Later a herpesvirus, the Lucké tumour herpesvirus (LTHV, a syn. of ranid herpesvirus 1, RaHV‐1), was isolated from the tumour‐bearing frogs (Lunger et al., 1965). The icosahedral virus has 162 capsomeres and measures about 100 nm in diameter. The viral aetiology of the frog adenocarcinoma was demonstrated and the agent was isolated using frog pronephric cells. Nuclear inclusions and development of virus were shown in explantats of inclusion‐free primary tumours kept at temperatures lower than 8°C. The RaHV‐1 genome consists of a linear dsDNA (217 kbp) with GC content of 45–47% (Twedell, 1989). A 39.8‐kbp insert of a cosmid containing 12 genes homologous to ictalurid herpesvirus 1 genes was sequenced. Genes are present in two blocks, one of which is repeated, indicating similar organization to other fish herpesvirus genomes. Genes without counterparts in other herpesviruses, e.g. DNA (cytosine‐5) methyltransferase, which plays a role in the replication and methylation of cellular DNA, were detected in RaHV‐1. RaHV‐1 is a distant relative of the fish herpesvirus lineage. It represents a third subdivision of this lineage, additional to those containing IcHV‐1 and SaHV‐1/2 (Davison et al., 1999). The virus induces primary tumours, malignancies by transformation of parenchymal cells of the kidney, forming an unencapsulated, locally invasive adenocarcinoma (Duryee, 1956). Tumours are present in frogs throughout the year, but the inclusion bodies and therefore virus replication are found only in animals kept at temperatures below 12°C. Metastatic nodules containing viral particles develop in the liver, lung, pancreas, fat body and urine bladder at 4°C (Twedell, 1989). Tumour cells still produce membrane antigen at higher temperatures, as shown by immunofluorescence. Transcripts of viral DNA are present in these tumours (Collard et al., 1973). A PCR for analysing a 1.2‐kbp HindII fragment (JH12) of RaHV‐1 DNA has been established. RaHV‐1‐specific DNA is detected in a significant percentage of normal kidneys as well as in virus‐free ‘warm’ tumours and virus‐containing ‘cold’ tumours. No viruses could be detected by electron microscopy in spontaneous tumours of frogs maintained under ‘warm conditions’ even though the ‘virus‐free’ (warm) tumours contain latent RaHV‐1 (Carlson et al., 1994b, c;, McKinnell et al., 1995; ">Williams et al., 1996). Genome sequences required for tumourgenesis are unknown (Carlson et al., 1994a). RaHV‐1 is able to rescue amphibian pronephros cells from apoptosis (Carlson et al., 1995).
Ranid herpesvirus 2 (RaHV‐2) syn. frog virus 4 (FV‐4)
Ranid herpesvirus 2 (RaHV‐2; FV‐4, ATCC VR‐568) isolated from the urine of adenocarcinoma‐bearing leopard frogs (Rana pipiens) is clearly distinguishable from Lucké tumour herpesvirus as it fails to induce tumours in frogs. RaHV‐2, which measures 100 nm in diameter, replicates in frog cell lines, inducing intranuclear inclusions and vacuolization. The molecular weight of viral DNA is 77.2 × 106 Da, the GC content is 54–56%. Experimental infection of developing leopard frogs leads to mortality (Twedell, 1989).
Herpesvirus of Rana dalmatina
Rana dalmatina with cutaneous alterations were found in Italy. Electron microscopy of skin lesions exhibited herpesvirus particles. Mortality of affected frogs reached up to 80% (Bennati et al., 1994).
Herpesviruses occurring in reptiles
Several herpesvirus‐like particles have been detected in snakes, lizards and turtles (Table 11). However, most of the agents have not been fully characterized so far.
Table 11.
Herpesviruses occurring in reptiles

Boid herpesvirus 1 (BoiHV‐1)
Herpesvirus‐like particles of 125–200 nm in diameter were detected by electron microscopy in young Boa constrictor showing intranuclear inclusion bodies in hepatocytes and necrosis of the liver (Hauser et al., 1983). Organ samples of juvenile boas contained herpesvirus‐like particles of 115–125 nm (Jacobson, 1986).
Elapid herpesvirus 1 (EpHV‐1)
Elapid herpesvirus 1 (EpHV‐1) was detected in the venom of Indian cobra (Naja naja) and banded krait (Bungarus fasciatus) by electron microscopy. The virus exhibits an electron‐dense core and a capsid with diameters of 100–125 nm. Siamese cobra snakes (Naja naja kaouthia) producing poor‐quality venom carried herpesvirus‐like particles in necrotic and ruptured columnar epithelial cells of the venom glands (Jacobson, 1986).
Iguanid herpesvirus 1 (IgHV‐1)
A herpesvirus (165–300 nm) was isolated from spontaneous lesions of primary monolayer cultures of healthy green iguana (Iguana iguana). The agent is sensitive to treatment with ether. BUdR inhibits viral replication. The isolated virus, termed iguanid herpesvirus 1 (IgHV‐1), replicates in several cell lines of reptilian origin, forming syncytia of infected cells. The iguanid herpesvirus has been shown to be serologically distinct from herpesviruses of birds and mammals. Another report of herpesvirus in green iguana has come from an animal with severe lymphocytosis. The virus could not be isolated using reptilian cell culture (Jacobson, 1986).
Lacertid herpesvirus 1 (LaHV‐1)
Herpesvirus‐like particles have been present in cutaneous papillomas of the base tail and trunk of Italian green lizard (Lacerta viridis) females. The virus has proved to be non‐pathogenic to ring snake (Tropidonotus tessellata) (Raynaud and Adrian, 1976).
Agama herpesvirus 1 (AgHV‐1)
Two agamas (Agama agama) exhibited herpesvirus‐like particles measuring 100 nm in diameter in nuclear inclusion bodies of necrotic liver and lung cells (Watson, 1993).
Chelonian herpesviruses (CnHV)
Herpesviruses were identified in freshwater, marine and terrestrial tortoises as pathogens inducing different diseases and leading to mortalities up to 100%. Thirty‐one recombinant HindIII DNA fragments of a herpesvirus isolated from a Herman’s tortoise (Testudo hermanni) were partially sequenced; a large and small subunit of the ribonucleotide reductase (RR) gene are identified. Serological and molecular techniques such as ELISA and RR‐specific PCR for different chelonian herpesviruses have been established to diagnose herpesvirus diseases in chelonias (Origgi and Jacobson, 1999; Origgi et al., 2000).
Chelonid herpesvirus 1 (CnHV‐1) syn Grey patch disease herpesvirus (GPDHV)
The grey patch disease herpesvirus (CnHV‐1) is an important pathogen of green sea turtles (Chelonia mydas). The viruses (160–180 nm) are present in epizootics associated with benign pustules on the skin of neck and flippers, inducing grey patches and mortality among the turtles. Transmission of the virus is established by the introduction of infectious material to scratched skin. Stress, and a water temperature of around 25°C favour the outbreak of grey patch disease. Animals between 8 and 15 weeks old were found to be most affected. Neutralizing antibody developed in yearlings protect the turtles from subsequent challenge with CnHV‐1 (Jacobson, 1986).
Chelonid herpesvirus 2 (CnHV‐2)
Herpesvirus‐like particles were detected in internal organs of pacific pond turtle (Clemmys marmorata) with acute hepatic necrosis and intranuclear inclusions in hepatocytes, as well as in the spleen and renal tubules (Frye et al., 1977; Jacobson, 1986). In addition, similar herpesvirus‐like particles (140 nm) were detected in several turtles associated with liver necrosis and lung oedema (Jacobson, 1986).
Chelonid herpesvirus 3 (CnHV‐3)
An adult painted turtle (Chrysemys picta) showing intranuclear inclusions in liver and epithelial lung cells carried the chelonid herpesvirus 3 (ChHV‐3). After introduction of infected painted turtle to map turtles a die‐off of Graptemys pseudogeographica and G. barbouri was observed. Affected turtles had eosinophilic intranuclear inclusions in liver, kidney and pancreas (Jacobson et al., 1986b).
Chelonid herpesvirus 4 (CnHV‐4)
Evidence for a herpesviral agent involved in the stomatitis–rhinitis disease complex (mouth rot) were reported from a desert tortoise (Gopherus agassizii) (Harper et al., 1982). The causative agent (chelonid herpesvirus 4, ChHV‐4) of the severe necrosis of the oral mucosa and glossitis was found in Argentinean tortoises (Geochelone chilensis) (Jacobson, 1986). Further reports have shown that the herpesvirus infection is common in Mediterranean tortoises (Posthaus et al., 1997). Mortality of 50% was recognized in a population of tortoises, consisting mainly of Hermann’s tortoises (Testudo hermanni) and four‐toed tortoises (Agrionemys horsfieldii) (Cooper et al., 1988; Lange et al., 1989). During a spontaneous outbreak of stomatitis–rhinitis complex with 50% mortality among T. hermanni in a collection of Mediterranean land tortoises (T. hermanni, T. graeca) in southern Germany, herpesviruses were isolated from dead T. hermanni. Neutralizing antibodies to chelonian herpesviruses were present in T. graeca. Testudo hermanni and T. horsfieldii seem to be more sensitive to the herpesvirus infection than spur‐thighed tortoises (T. graeca) and T. marginata (Kabisch and Frost, 1994; Marschang et al., 1997). There is evidence for the existence of two serotypes among herpesviruses of land tortoises (Biermann and Blahak, 1993; Kabisch and Frost, 1994). Acyclovir and gancyclovir inhibit virus replication in vitro (Marschang et al., 1997). The UL5 gene from a German CnHV‐4 was sequenced. A digoxigenin‐labelled 307‐bp UL5 probe was used for in situ hybridization in tortoise tissue. Hybridization signals were detected in epithelial cells of lingual mucosa and glands, in the tracheal and renal epithelium, pneumocytes, hepatocytes, cerebral glial cells, neurones, and intramural intestinal ganglia. Systemic infection was found in tortoises of different geographical origin, indicating high conservation of the UL5 fragment (Teifke et al., 2000).
Green sea turtle fibropapillomatosis herpesvirus (GTFPHV)
Green sea turtles (Chelonia mydas) suffering from cutaneous fibropapillomatosis (green turtle fibropapillomatosis, GTFP) were first reported in Florida (USA) over 60 years ago (Jacobson, 1986). GTFP emerged as a significant worldwide epizootic caused by green sea turtle fibropapillomatosis herpesvirus (GTFPHV) with high prevalence in green turtle populations. Lesions similar to the GTFP were observed in olive ridleys (Lepidochelys olivacea), flatbacks (Natator depressus), and loggerheads (Caretta caretta) (Herbst, 1994). Cases appeared in this species in Florida and at the Pacific coasts of Mexico and Costa Rica (Herbst, 1994; Aguirre et al., 1999). GTFPHV present in spontaneous and experimentally induced tumours measures 110–125 nm. The virus resists cultivation in chelonian cell lines. The GTFP can be experimentally transmitted to young captive‐reared green turtles using cell‐free fibropapilloma extracts prepared from free‐ranging turtles (Herbst et al., 1995). Treatment of the inoculum with lipid solvents prior to inoculation destroys infectivity (Herbst et al., 1996). Serodiagnostics show a strong correlation between GTFP status in captive‐reared and free‐ranging turtles (Herbst et al., 1998). Consensus primer PCR is used to analyse the association of a chelonian herpesvirus with fibropapillomatosis. Degenerate PCR primers that target highly conserved regions of genes encoding herpesvirus DNA polymerases are used to amplify a DNA sequence of fibropapillomatosis cases from Hawaiian and Florida green turtles, loggerhead and olive ridley turtles. The herpesvirus appears to be generally limited to tumours (2–5 copies viral DNA/cell). Sequencing of the turtle herpesvirus DNA polymerase gene fragments (483 bp) shows that the GTFP agents are closely related and are intimately involved in the genesis of fibropapillomatosis (Quackenbush et al., 1998; Lackovich et al., 1999). A larger fragment (1632 bp) flanking the DNA polymerase gene of GTFPHV showed a high degree of homology with other mammalian and avian herpesviruses. Phylogenetic analysis confirmed that this herpesvirus belongs to the Alphaherpesvirinae subfamily and not to the fish herpesvirus lineage (Yu et al., 2000).
Lung–eye–trachea disease‐associated herpesvirus (LETDHV)
Lung–eye–trachea disease (LETD) of green sea turtles was found to be associated with a herpesvirus termed lung–eye–trachea disease herpesvirus (LETDHV). The agent (132–147 nm) present in epizootics is spread within hatched turtles. The virus has been isolated, but not characterized until now (Jacobson et al., 1986b).
Adenoviridae
The family Adenoviridae comprises the genera Mastadenovirus and Aviadenovirus. Common features are non‐enveloped, icosahedral virions measuring 70–90 nm in diameter. Virions contain one single linear molecule of dsDNA and about 40 proteins. Adeno‐like viruses of lower vertebrates such as frog adenovirus (FrAdV‐1) and snake adenovirus (SnAdv‐1) are unassigned viruses in the family (van Regenmortel et al., 2000).
Adenoviruses occurring in fish
Adenovirus‐like particles have been found in cod (Gadus morhua) with epidermal hyperplasia in Norway. The non‐enveloped virus, measuring 77 nm in diameter, has an inner core surrounded by a capsid with fibres (Wolf, 1988). Mortality rates up to 50% in white sturgeon (Acipenser transmontanus) populations were associated with an adenovirus. Enlarged nuclei found in the epithelium of the intestine and liver parenchyma contained double‐shelled particles of 74 nm diameter (Ghittino and Ghittino, 1985; Wolf, 1988). Adeno‐like viruses were isolated from wild Columbia River white sturgeon (S. E. LaPatra, personal communication).
Adenovirus infection was recognized in dabs (Limanda limanda) of the North Sea exhibiting epithelial hyperplasia or papillomas. Up to 200 particles of 80 nm in diameter were suited in the nuclei of cells near the skin surface of infected fish (Anders, 1988).
In Japanese red sea bream (Pagrus major), adenovirus‐like particles (78–83 nm) were found to be associated with lympholeukaemia. The disease reached an epizootic extent and fish displayed severe anaemia and markedly increased numbers of neoplastic lymphoid cells in the blood (Miyazaki et al., 2000a).
Adenoviruses occurring in amphibians
The frog adenovirus 1 (FrAdV‐1; ATCC VR‐896) was isolated from granuloma‐bearing kidneys of leopard frogs (Rana pipiens). The virus, which measures 73–83 nm in diameter, replicates in turtle cell lines (TH‐1) at 30°C. Viral particles were found to be arranged in crystalline‐like arrays in the nuclei of infected cells (Granoff, 1989). FrADV‐1 is the first adenovirus of lower vertebrates being sequenced. The linear molecule of dsDNA has a size of 26 kbp. The FrAdV‐1 genome (which, at 45 kbp, is smaller as other adenovirus genomes) contains direct repeats on the right terminus that might be a result of a recombination event of a parenteral genome. FrAdV‐1 is genomically most closely related to turkey adenovirus 3 (TAdV‐3). Both viruses have very similar genome sizes, comparable GC contents, short inverted terminal repeat (ITR) lengths, analogous gene organizations and both encode a sialidase. Only 16 genes are conserved in comparison with all three other adenovirus genera. Phylogenetic analysis suggests that FrAdV‐1 and TAdV‐3 represent a new genus of Adenoviridae (Davison et al., 2000).
Adenoviruses occurring in reptiles
Liver necrosis of a bearded dragon lizard (Amphibolurus barbatus) was found to be associated with adenovirus‐like particles. The viruses, which measure 65–70 nm in diameter, were detected in the nuclei of liver and kidney epithelial cells (Julian and Durham, 1982; Jacobson, 1986). Similar cases have been reported from dragon lizards (Pogona vitticeps, P. henrylawson) (Jacobson et al., 1986a; Frye et al., 1994).
A Jackson’s chameleon (Chamaeleo jacksoni) has shown adenovirus‐like particles (60–65 nm) in the oesophageal and tracheal mucosa (Jacobson and Gardiner, 1990). An adult, wild‐caught male mountain chameleon (Chamaeleo montium) died of anorexia and its intestine revealed numbers of enterocytes containing 2–15‐μm basophilic, intranuclear inclusions. The inclusions consisted of crystalline arrays of hexagonal viral particles (67–76 nm) (Kinsel et al., 1997). Similar viral particles were detected in Savannah monitor (Varanus exanthemicus) (Jacobson and Kollias, 1986) and in two Nile crocodiles (Crocodylus niloticus) (Jacobson, 1986).
A boa (Boa constrictor) with liver necrosis has shown intranuclear inclusion bodies in the hepatocytes, associated with adenoviruses (70–79 nm). The isolated virus was pathogenic to neonatal boa snakes, inducing hepatic necrosis, and the virus could be reisolated using VH‐2 cells at 25°C (Jacobson, 1986). Heldstab and Bestetti (1984) report adenovirus‐like particles in internal organs of a boa constrictor, an Aesculapian snake (Elaphe longissima), a four‐lined rat snake (E. quatourlineata), and a Gabbon viper (Bitis gabonica). Adult rosy boas (Lichanura trivirgata) died due to infection with adenoviruses. Basophilic intranuclear inclusion bodies were found in hepatocytes, heterophils within sinusoids of the liver, renal tubular epithelial cells, and the endocardium. Electron microscopic examination demonstrated nuclear inclusions containing adenovirus‐like particles with a diameter of 65–70 nm (Schumacher et al., 1994a).
Symmetric hexagonal adenovirus‐like particles measuring 67–79 nm in diameter were isolated from a moribund royal python (Phyton regius). The DNA containing virus replicates in reptilian cell cultures at 25°C forming eosinophilic intranuclear inclusion bodies. The virus is stable to treatment with chloroform, pH 3 and pH 12 but it is labile to heat (56°C) (Ogawa et al., 1992).
An adenovirus has been isolated from the internal organs of a moribund corn snake (Elaphe guttata) showing pneumonia. The virus replicates in reptilian cell cultures, but not in fish cell lines. Electron microscopy revealed non‐enveloped icosahedral particles (65–70 nm). The DNA‐containing agent is stable at pH 3 (and more or less stable at pH 12) and to treatment with chloroform, but it is rapidly inactivated at 56°C (Juhasz and Ahne, 1993). The morphological alterations of infected IgH2 cell cultures was investigated during one cycle replication of the corn snake adenovirus. As shown by electron microscopy, virus particles entering the cells 1 h after infection are present in cytoplasmic receptosomes. About 20 h later, viral nucleocapsids invade the nuclei of the cells. Eosinophilic granulation of the nucleus and intranuclear inclusion bodies appear 30–60 h after infection. Infected cells develop three morphologically different intranuclear inclusion bodies. Typical crystalline aggregates of virions appear at the periphery of the nucleus 48–60 h after infection. About 64 h after infection progressive disintegration of the nuclear membrane is evident. The release of virions into the cytoplasm is followed by lysis of the infected cells. Complete destruction of cell monolayer is evident 96 h after infection (Ahne and Juhasz, 1995).
Polyomaviridae
Viruses of the family Polyomaviridae (previously termed Papovaviridae) comprise the genus Papillomavirus. Common features are non‐enveloped viruses with an icosahedral capsid composed of 72 capsomeres. Virions, measuring 40 nm in diameter, contain one molecule of circular dsDNA and 5–10 proteins. Polyoma‐like viruses of lower vertebrates are not included in the present taxonomy of viruses (van Regenmortel et al., 2000).
Polyoma‐like viruses occurring in fish
Polyoma‐like viruses were recognized in hybrids of swordtail fish (Xiphophorus sp.) associated with melanomas (Kollinger et al., 1979) and in winter flounder (Pseudopleuronectes americanus) with epidermal hyperplasia (Wolf, 1988). An icosahedral virus measuring 55–65 nm was isolated during epidemiological surveys of white sturgeon (Acipenser transmontanus) in the USA (LaPatra et al., 1998).
Polyoma‐like viruses occurring in amphibia
Polyoma‐like viruses of 45 nm in diameter have been isolated from kidney tumours of leopard frog (Rana pipiens) using embryo cell cultures of leopard frog. Similar particles have been found in thin sections of Lucké tumour cells (Granoff, 1989).
Polyoma‐like viruses occurring in reptiles
Electron microscopical investigation of papillomas in green lizards (Lacerta viridis) revealed polyoma‐like viruses beside herpesvirus‐ and reovirus‐like particles (Raynaud and Adrian, 1976; Cooper et al., 1982). Similar viruses measuring 42–53 nm in diameter have been detected in Bolivian side‐neck turtles (Platemys platycephala) carrying benign skin lesions with hyperkeratosis and hyperplasia (Jacobson et al., 1982).
Outlook
The first studies on the viruses of lower vertebrates began about 30 years ago. Since that time RNA and DNA viruses have been detected as pathogenic agents in many fish, amphibian and reptile species. However, most of the agents have not been fully characterized until now. For several viruses molecular data have been elaborated, but the knowledge of the viruses of lower vertebrates is still behind that of the viruses of homoiothermic vertebrates. The sequences of two herpesviruses, one iridovirus (LCDV), and an adenovirus originating from lower vertebrates have recently been published, but none of the systemic iridoviruses (genus Ranavirus), the parvo‐, pox‐ or polyomaviruses genomes have been analysed. Investigations on the molecular biology of severe viral pathogens in aquaculture, such as piscine rhabdoviruses, orthomyxoviruses, togaviruses and nodaviruses, certainly represent new tasks for the future. For example, information is needed to clarify the origin and evolution of viruses of lower vertebrates, to determine their taxonomic position, to analyse their viral biology, to prevent virus transmission, and to establish immune prophylaxis. RNA viruses usually undergo high mutation rates, therefore molecular data are needed in order to distinguish between virulent and avirulent strains. One of the most important tasks will be to establish proper vaccination trials. In addition, further investigations on virus transmission and vectors, viral receptors, and the viral host’s immune modulation will open a new field in the established animal virology and give an insight in the complexity of viruses of lower vertebrates.
References
- 1. Adkison, A. M. , Cambre M., Hedrick R. P., 1998: Identification of an iridovirus in Russian sturgeon (Acipenser guldenstadi) from northern Europe. Bull. Eur. Assoc. Fish Pathol. 18 , 29–32. [Google Scholar]
- 2. Aguirre, A. A. , Spraker T. R., Chaves A., DuToit L., Eure W., Balazs G. H., 1999: Pathology of fibropapillomatosis in olive ridley turtles Lepidochelys olivacea nesting in Costa Rica. J. Aquat. Anim. Health 11 , 283–289. [Google Scholar]
- 3. Ahne, W. , 1978a: Uptake and multiplication of spring viremia of carp virus in carp (Cyprinus carpio). J. Fish Dis. 1 , 265–268. [Google Scholar]
- 4. Ahne, W. , 1978b: Isolation and characterization of infectious pancreatic necrosis virus from pike (Esox lucius). Arch. Virol. 58 , 65–69. [DOI] [PubMed] [Google Scholar]
- 5. Ahne, W. , 1985a: Argulus foliaceus L., and Piscicola geometra L. as mechanical vectors of spring viraemia of carp virus (SVCV). J. Fish Dis. 8 , 241–242. [Google Scholar]
- 6. Ahne, W. , 1985b: Viral infection cycles in pike (Esox lucius L.). J. Appl. Ichthyol. 1 , 90–91. [Google Scholar]
- 7. Ahne, W. , 1986: The influence of environmental temperature and infection route on the immune response of carp (Cyprinus carpio) to SVCV. Vet. Immunol. Immunopathol. 12 , 383–386. [DOI] [PubMed] [Google Scholar]
- 8. Ahne, W. , 1991: Viral infections of reptiles. Proceedings of 4th International Colloquium on Pathology and Medicine of Reptiles and Amphibians, 27–29 September, German Veterinary Association, Bad Nauheim, Germany, pp. 1–12.
- 9. Ahne, W. , 1993: Viruses of chelonia. J. Vet. Med. 40 , 35–45. [DOI] [PubMed] [Google Scholar]
- 10. Ahne, W. & Juhasz A., 1995: In vitro replication of a reptilian adenovirus. Vet. Res. 26 , 443–448. [PubMed] [Google Scholar]
- 11. Ahne, W. & Neubert W. J., 1989: Antigenic relationship between three pleomorphic RNA viruses isolated from different snakes. Herpetopathologia 1 , 65–72. [Google Scholar]
- 12. Ahne, W. & Neubert W. J., 1991: Isolation of paramyxovirus‐like agents from teju (Callopistes maculatus) and python (Python regius). Proceedings of the 2nd International Symposium on Viruses of Lower Vertebrates, 29–31 July, Oregon State University, Corrallis, USA, pp. 203–210.
- 13. Ahne, W. & Scheinert P., 1989: Reptilian viruses: isolation of parvo‐virus‐like particles from corn snake Elaphe guttata (Colubridae). J. Vet. Med. B 36 , 409–412. [DOI] [PubMed] [Google Scholar]
- 14. Ahne, W. , Mahnel H., Steinhagen P., 1982: Isolation of pike fry rhabdovirus from tench, Tinca tinca L., and white bream, Blicca bjoerkna L. J. Fish Dis. 5 , 535–537. [Google Scholar]
- 15. Ahne, W. , Neubert W. J., Thomsen I., 1987a: Reptilian viruses, isolation of myxovirus‐like particles from the snake Elaphe oxycephala . J. Vet. Med. B 34 , 607–612. [DOI] [PubMed] [Google Scholar]
- 16. Ahne, W. , Thomsen I., Winton J., 1987b: Isolation of a reovirus from the snake, Python regius . Arch. Virol. 94 , 135–139. [DOI] [PubMed] [Google Scholar]
- 17. Ahne, W. , Jorgensen P. E. V., Olesen N. J., Wattanavijarn W., 1988: Serological examination of a rhabdovirus isolated from snakehead (Ophicephalus striatus) in Thailand with ulcerative syndrome. J. Appl. Ichthyol. 4 , 194–196. [Google Scholar]
- 18. Ahne, W. , Kelly R. K., Schlotfeldt H. J., 1989: Factors affecting the transmission and outbreak of infectious pancreatic necrosis (IPN). In: Lillelund, K., and H. Rosenthal (eds), Fish Health Protection Strategies, pp. 19–67. Specific Publishers German Federal Ministry for Research and Technology, Bonn.
- 19. Ahne, W. , Anders K., Halder M., Yoshimizu M., 1990: Isolation of picorna‐like virus particles from the European smelt, Osmerus eperlanus (L.). J. Fish Dis. 13 , 167–168. [Google Scholar]
- 20. Ahne, W. , Bremont M., Hedrick R. P., Hyatt A. D., Whittington R. J., 1997: Special topic review: iridoviruses associated with EHN in aquaculture. World J. Microbiol. Biotechnol. 13 , 367–373. [Google Scholar]
- 21. Ahne, W. , Kurath G., Winton J. R., 1998a: A ribonuclease protection assay can distinguish spring viremia of carp virus from pike fry rhabdovirus. Bull. Eur. Assoc. Fish Pathol. 18 , 220–224. [Google Scholar]
- 22. Ahne, W. , Bearzotti M., Bremont M., Essbauer S., 1998b: Comparison of European systemic piscine and amphibian iridoviruses with EHNV and FV‐3. J. Vet. Med. B 45 , 373–383. [DOI] [PubMed] [Google Scholar]
- 23. Ahne, W. , Batts W. N., Kurath G., Winton J. R., 1999: Comparative sequence analysis of sixteen reptilian paramyxoviruses. Virus Res. 63 , 65–74. [DOI] [PubMed] [Google Scholar]
- 24. Alves de Matos, A. P. & Paperna I., 1993a: Ultrastructure of erythrocytic virus of the South African anuran Ptychadena anchietae . Dis. Aquat. Org. 16 , 105–109. [Google Scholar]
- 25. Alves de Matos, A. P. & Paperna I., 1993b: Ultrastructural study of Pirhemocyton virus in lizard erythrocytes. Ann. Parasitol. Hum. Comp. 68 , 24–33. [Google Scholar]
- 26. Alves de Matos, A. P. , Paperna I., Lainson R., 1995: An erythrocytic virus of the Brazilian tree‐frog, Phrynohyas venulosa . Mem. Inst. Oswaldo Cruz 90 , 653–655. [DOI] [PubMed] [Google Scholar]
- 27. Amend, D. F. , Yasutake W. T., Mead R. W., 1969: A haematopoietic virus disease of rainbow trout and sockeye salmon. Trans. Am. Fish Soc. 98 , 796–804. [Google Scholar]
- 28. Anders, K. , 1988: Biology of Tumour‐ and Tumour‐like Diseases of Fish from the Lower Elbe River. Möller Publications, Kiel.
- 29. Anders, K. , 1989a: LCD of fishes. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 141–160. Springer‐Verlag, Heidelberg.
- 30. Anders, K. , 1989b: A herpesvirus associated with an epizootic epidermal papillomatosis in European smelt, Osmerus eperlanus L. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 184–197. Springer‐Verlag, Heidelberg.
- 31. Anderson, I. G. , Prior H. C., Rodwell B. J., Harris G. O., 1993: Iridovirus‐like virions in imported dwarf gourami (Colisa lalia) with systemic amoebiasis. Aust. Vet. J. 70 , 66–67. [DOI] [PubMed] [Google Scholar]
- 32. Aoki, T. , Hirono I., Kim M. G., Katagiri T., Tokuda Y., Toyohara H., Yamamoto E., 2000: Identification of viral induced genes in Ig+ leucocytes of Japanese flounder Paralichthys olivaceus, by differential hybridisation with substracted and un‐substracted cDNA probes. Fish Shellfish Immunol. 10 , 623–630. [DOI] [PubMed] [Google Scholar]
- 33. Ariav, R. , Tinman S., Bejerano I., 1999: First report of newly emerging viral disease of Cyprinus carpio species in Israel. Proceedings of 9th International Conference on Diseases of Fish and Shellfish, 19–24 September, Rhodes, Greece, p. P151.
- 34. Ariel, E. , Tapiovaara H., Olesen N.‐J., 2000: Serological comparison of the Finnish and Danish iridovirus isolates with reference to other piscine and amphibian iridovirus isolates. XIIth International Poxvirus and Iridovirus Symposium, 2–6 September, Montpellier, France, p. 31.
- 35. Arkush, K. D. , Bovo G., De Kinkelin P., Winton J. R., Wingfield W. H., Hedrick R. P., 1989: Biochemical and antigenic properties of the first isolates of infectious haematopoietic necrosis virus from salmonid fish in Europe. J. Aquat. Health 1 , 148–153. [Google Scholar]
- 36. Armstrong, R. D. & Ferguson H. W., 1989: Systemic viral disease of the chromide cichlid Etroplus maculatus . Dis. Aquat. Org. 7 , 155–157. [Google Scholar]
- 37. Aubertin, A. M. , Plaese P., Tan K. B., Vilagines R., McAuslan B. R., 1971: Proteins of a polyhedral cytoplasmic deoxyvirus. III. Structure of FV‐3 and location of virus‐associated ATP phosphohydrolase. J. Virol. 8 , 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barlough, J. E. , Berry E. S., Skilling D. E., Smith A. W., 1988: Prevalence and distribution of serum neutralizing antibodies to San Miguel sea lion virus types 6 and 7 in selected populations of marine mammals. Dis. Aquat. Org. 5 , 75–80. [Google Scholar]
- 39. Basurco, B. & Benmansour A., 1995: Distant strains of the fish rhabdovirus VHSV maintain a sixth functional cistron which codes for a nonstructural protein of unknown function. Virology 212 , 741–745. [DOI] [PubMed] [Google Scholar]
- 40. Batts, W. N. , Arakawa C. K., Bernard J., Winton J. R., 1993: Isolates of viral hemorrhagic septicemia virus from North America and Europe can be detected and distinguished by DNA probes. Dis. Aquat. Org. 17 , 67–71. [Google Scholar]
- 41. Bearzotti, M. , Delmas B., Lamoureux A., Loustau A. M., Chilmonczyk S., Bremont M., 1999: Fish rhabdovirus cell entry is mediated by fibronectin. J. Virol. 73 , 7703–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bekesi, L. , Horvath I., Kovacs‐Gayer E., Csaba G., 1986: Demonstration of herpesvirus‐like particles in skin lesions of European eel (Anguilla anguilla). J. Appl. Ichthyol. 2 , 190–192. [Google Scholar]
- 43. Benmansour, A. , Basurco B., Monnier A. F., Vende P., Winton J. R., De Kinkelin P., 1997: Sequence variation of the glycoprotein gene identifies three distinct lineages within field isolates of viral haemorrhagic septicaemia virus, a fish rhabdovirus. J. Gen. Virol. 78 , 2837–2846. [DOI] [PubMed] [Google Scholar]
- 44. Bennati, R. , Bonetti M., Lavazza A., Gelmetti D., 1994: Skin lesions associated with herpesvirus‐like particles in frogs (Rana dalmatina). Vet. Rec. 135 , 625–626. [PubMed] [Google Scholar]
- 45. Berge, T. O. , 1975: International Catalogue of Arboviruses. Publication no. CDC 75–8301, US Department of Health Educucation and Welfare, Atlanta, GA, USA, p. 92f.
- 46. Bernard, G. W. , Cooper E. L., Mandell M. L., 1968: Lamellar membrane encircled viruses in the erythrocytes of Rana pipiens . J. Ultrastruct. Res. 26 , 8–16. [DOI] [PubMed] [Google Scholar]
- 47. Bernard, J. & Mercier A., 1993: Sequence of two Eco RI fragments from salmonis herpesvirus 2 and comparison with ictalurid herpesvirus 1. Arch. Virol. 132 , 437–442. [DOI] [PubMed] [Google Scholar]
- 48. Berry, E. S. , Shea T. B., Gabliks J., 1983: Two iridoviruses isolates from Carassius carassius (L.). J. Fish Dis. 6 , 501–510. [Google Scholar]
- 49. Biacchesi, S. , Thoulouze M. I., Bearzotti M., Yu Y. X., Bremont M., 2000a: Recovery of NV knockout infectious haematopoietic necrosis virus expressing foreign genes. J. Virol. 74 , 11247–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Biacchesi, S. , Yu Y. X., Bearzotti M., Tafalla C., Fernandez‐Alonso M., Bremont M., 2000b: Rescue of synthetic salmonid rhabdovirus minigenomes. J. Gen. Virol. 81 , 1941–1945. [DOI] [PubMed] [Google Scholar]
- 51. Biermann, R. & Blahak S., 1993: First isolation of a herpesvirus from tortoises with diphtheroid‐necrotizing stomatitis. Abstracts of the 2nd World Congress of Herpetology, 29 December–6 January, Adelaide, Australia, p. 27.
- 52. Björklund, H. V. , Olesen N. J., Jorgensen P. E. V., 1994: Biophysical and serological characterization of rhabdovirus 903/87 isolated from European lake trout Salmo trutta lacustris . Dis. Aquat. Org. 19 , 21–26. [Google Scholar]
- 53. Björklund, H. V. , Emmenegger E. J., Kurath G., 1995: Comparison of the polymerases (L genes) of SVCV and IHNV. Vet. Res. 26 , 394–398. [PubMed] [Google Scholar]
- 54. Björklund, H. V. , Higmann K. H., Kurath G., 1996: The glycoprotein genes and gene junctions of the fish rhabdoviruses SVCV and HIRRV, analysis of relationships with other rhabdoviruses. Virus Res. 42 , 65–80. [DOI] [PubMed] [Google Scholar]
- 55. Björklund, H. V. , Johannson T. R., Rinne A., 1997: Rhabdovirus‐induced apoptosis in a fish cell line is inhibited by a human endogenous acid cysteine proteinase inhibitor. J. Virol. 71 , 5658–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blahak, S. , 1995: Isolation and characterization of paramyxoviruses from snakes and their relationship to avian paramyxoviruses. J. Vet. Med. 42 , 216–224. [DOI] [PubMed] [Google Scholar]
- 57. Blahak, S. , Ott I., Vieler E., 1995: Comparison of 6 different reoviruses of various reptiles. Vet. Res. 26 , 470–476. [PubMed] [Google Scholar]
- 58. Blake, S. , Bouchard D., Keleher M., Opitz M., Nicholson B. L., 1999: Genomic relationships of the North American isolate of infectious salmon anemia virus (ISAV) to the Norwegian strain of ISAV. Dis. Aquat. Org. 35 , 139–144. [DOI] [PubMed] [Google Scholar]
- 59. Bloch, B. & Larsen J. L., 1993: A case of severe general edema in young farmed turbot associated with a herpes virus infection. Bull. Eur. Assoc. Fish Pathol. 14 , 130–131. [Google Scholar]
- 60. Bloch, B. , Gravningen K., Larsen J. L., 1991: Encephalomyelitis among turbot associated with a picornavirus‐like agent. Dis. Aquat. Org. 10 , 65–70. [Google Scholar]
- 61. Bollinger, T. K. , Mao J., Schock D., Brigham R. M., Chinchar V. G., 1999: Pathology, isolation, and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. J. Wildl. Dis. 35 , 413–429. [DOI] [PubMed] [Google Scholar]
- 62. Booker, K. A. & Younge H., 1982: Cytotodia (=Toddia) infection of serpentes and its incidence in two geographical areas. Virg. J. Sci. 33 , 11–21. [Google Scholar]
- 63. Bouchard, D. , Keleher W., Opitz H. M., Blake S., Edwards K. C., Nicholson B. L., 1999: Isolation of infectious salmon anemia virus (ISAV) from Atlantic salmon in New Brunswick. Can. Dis. Aquat. Org. 35 , 131–137. [DOI] [PubMed] [Google Scholar]
- 64. Boucher, P. , Raynard R. S., Houghton G., Baudin Laurencin F., 1995: Comparative experimental transmission of pancreas disease in Atlantic salmon, rainbow trout and brown trout. Dis. Aquat. Org. 22 , 19–24. [Google Scholar]
- 65. Boudinot, P. , Massin P., Blanco M., Riffault S., Benmansour A., 1999: Vig‐1, a new fish gene induced by the rhabdovirus glycoprotein, has a virus‐induced homologue in humans and shares conserved motifs with the MoaA family. J. Virol. 73 , 1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bovo, G. , Comuzi M., DeMas S., Ceschia G., Giorgetti G., Giacometti P., Cappellozza E., 1993: Isolamento di un agente virale irido‐like da pesce gatto (Ictalurus melas) dàllevamento. Boll. Soc. Ital. Patol. Ittica. 11 , 3–10. [Google Scholar]
- 67. Bovo, G. , Olesen N. J., Jorgensen P. E. V., Ahne W., Winton J. R., 1995: Characterization of a rhabdovirus isolated from carpione Salmo trutta carpio in Italy. Dis. Aquat. Org. 21 , 115–122. [Google Scholar]
- 68. Bowser, P. R. & Casey J. W., 1993: Retroviruses of fish. Annu. Rev. Fish Dis. 3 , 209–224. [Google Scholar]
- 69. Bradley, T. M. , Medina D. J., Chang P. W., McClain J., 1989: Epizootic epitheliotropic disease of lake trout (Salvelinus namaycush): history and viral etiology. Dis. Aquat. Org. 7 , 195–201. [Google Scholar]
- 70. Bretzinger, A. , Fischer‐Scherl T., Oumona M., Hoffmann R., Truyen U., 1999: Mass mortalities in koi carp, Cyprinus carpio, associated with gill and skin disease. Bull. Eur. Assoc. Fish Pathol. 19 , 182–185. [Google Scholar]
- 71. Breuil, G. , Bonami J. R., Pepin J. F., Pichot Y., 1991: Viral infection (picorna‐like virus) associated with mass mortalities in hatchery reared sea bass (Dicentrarchus labrax) larvae and juveniles. Aquaculture 97 , 109–116. [Google Scholar]
- 72. Burke, C. N. , Herman R. L., Perry S., 1989: Virus infection of squamous cell carcinoma in rainbow smelt (Osmerus mordax). Proceedings of the 14th Annual Eastern Fish Health Workshop, 17–20 July, Annapolis, USA, p. 15.
- 73. Burton, A. N. , McLinstock J., Rempel J. G., 1966: Western equine encephalitis virus in Saskatchewan garter snakes and leopard frogs. Science 154 , 1029–1032. [DOI] [PubMed] [Google Scholar]
- 74. Bussereau, F. , De Kinkelin P., Le Berre M., 1975: Infectivity of fish rhabdovirus for Drosophila melanogaster . Ann. Microbiol. Sci. 126a , 389–395. [PubMed] [Google Scholar]
- 75. Carlson, D. L. , Sauerbier W., McKinnell R. G., 1994a: PCR amplification of Lucké herpesvirus genomic sequences in normal kidney, renal carcinoma, and cloned tumor nuclear transplant tissues. Proc. Am. Assoc. Cancer Res. Annu. Meeting 35 , 590–590. [Google Scholar]
- 76. Carlson, D. L. , Sauerbier W., Rollins Smith L. A., McKinnell R. G., 1994b: Fate of herpesvirus DNA in embryos and tadpoles cloned from Lucké renal carcinoma nuclei. J. Comp. Pathol. 111 , 197–204. [DOI] [PubMed] [Google Scholar]
- 77. Carlson, D. L. , Sauerbier W., Rollins Smith L. A., McKinnell R. G., 1994c: The presence of DNA sequences of the Lucké herpesvirus in normal and neoplastic kidney tissue of Rana pipiens . J. Comp. Pathol. 110 , 349–355. [DOI] [PubMed] [Google Scholar]
- 78. Carlson, D. L. , Williams J. W., Rollins Smith L. A., Christ C. G., John J. C., Williams C. S., McKinnell R. G., 1995: Pronephric carcinoma: chromosomes of cells rescued from apoptosis by an oncogenic herpesvirus detected with a PCR. J. Comp. Pathol. 113 , 277–286. [DOI] [PubMed] [Google Scholar]
- 79. Carneiro, S. M. , Tanaka H., Kisielius J. J., 1992: Occurrence of retrovirus‐like particles in various cellular and intercellular compartments of the venom gland from Bothrops jararacussu . Res. Vet. Sci. 53 , 299–301. [DOI] [PubMed] [Google Scholar]
- 80. Casey, R. N. , Quackenbush S. L., Work T. M., Balazs G. H., Bowser P. R., Casey J. W., 1997: Evidence for the retrovirus infections in green turtles Chelonia mydas from the Hawaiian islands. Dis. Aquat. Org. 31 , 1–7. [Google Scholar]
- 81. Castric, J. , Rasschaert D., Bernard J., 1984: Evidence of lyssavirus isolates from European eel Anguilla anguilla . Ann. Virol. (Inst. Pasteur) 135E , 35–55. [Google Scholar]
- 82. Castric, J. , Baudin Laurencin F., Bremont M., Jeffroy J., Le Ven A., Bearzotti M., 1997: Isolation of the virus responsible for sleeping disease in experimentally infected rainbow trout (Oncorhynchus mykiss). Bull. Eur. Assoc. Fish Pathol. 17 , 27–30. [Google Scholar]
- 83. Causey, O. R. , Shope R. E., Bensabath G., 1966: Marco, Timbo, and Chaco, newly recognized arboviruses from lizards in Brazil. Am. J. Trop. Med. Hyg. 15 , 239–243. [DOI] [PubMed] [Google Scholar]
- 84. Chang, P. H. , Lee S. H., Chiang H. C., Jong M. H., 1999: Epizootic of herpes‐like virus infection in goldfish, Carassius auratus, in Taiwan. Fish Pathol. 34 , 209–210. [Google Scholar]
- 85. Chen, Y. & Jiang Y., 1984: Morphological and physico‐chemical characterization of the hemorrhagic virus of grass carp. Kexue Tongboa 29 , 832–835. [Google Scholar]
- 86. Chiou, P. P. , Kim C. H., Ormonde P., Leong J. A., 2000: Infectious haematopoietic necrosis virus matrix protein inhibits host‐directed gene expression and induces morphological changes of apoptosis in cell cultures. J. Virol. 74 , 7619–7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clark, H. F. & Soriano E. Z., 1974: Fish rhabdovirus replication in non‐piscine cell culture, new system for the study of rhabdovirus–cell interaction in which the virus and cell have different temperature optima. Infect. Immun. 10 , 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Clark, H. F. , Lief F. S., Lunger D. P., Waters D., Leloup P., Foelsch D. W., Wyler R. W., 1979: Fer‐de‐Lance virus (FDLV), a probable paramyxovirus isolated from a reptile. J. Gen. Virol. 44 , 405–418. [DOI] [PubMed] [Google Scholar]
- 89. Clerx, J. P. M. , 1978: Studies on pike fry rhabdovirus and the immunoglobulin of pike (Esox lucius). PhD dissertation, Rijksuniversiteit, Utrecht, pp. 1–102.
- 90. Collard, W. , Thornton H., Mizell M., Green M., 1973: Virus‐free adenocarcinoma of the frog (summer phase tumor) transcribes LTHV‐specific RNA. Science 181 , 448–449. [DOI] [PubMed] [Google Scholar]
- 91. Cook, R. S. , Trainer D. O., Glazener W. C., Nassif B. B., 1965: A serologic study of infectious diseases of wild populations in South Texas. Trans. North Am. Wildl. Nat. Res. Conference 30 , 142–155. [Google Scholar]
- 92. Cooper, J. E. , Geschmeissner S., Holt P. E., 1982: Viral particles in a papilloma from a green lizard (Lacerta viridis). Lab. Anim. 16 , 12–13. [DOI] [PubMed] [Google Scholar]
- 93. Cooper, J. E. , Geschmeissner S., Bone R. D., 1988: Herpes‐like virus particles in necrotic stomatitis of tortoises. Vet. Rec. 123 , 554–554. [DOI] [PubMed] [Google Scholar]
- 94. Corbeil, S. , Kurath G., LaPatra S. E., 2000: Fish DNA vaccine against infectious haematopoietic necrosis virus: efficacy of various routes of immunization. Fish Shellfish Immunol. 10 , 711–723. [DOI] [PubMed] [Google Scholar]
- 95. Crane, M. S. T. J. , Hardy‐Smith P., Williams L. M., Hyatt A. D., Eaton L. M., Gould A., Handlinger J., Kattenbelt J., Gudkovs N., 2000: First isolation of an aquatic birnavirus from farmed and wild fish species in Australia. Dis. Aquat. Org. 43 , 1–4. [DOI] [PubMed] [Google Scholar]
- 96. Crawford, S. A. , Gardner I. A., Hedrick R. P., 1999: An ELISA for detection of antibodies to channel catfish virus in channel catfish. J. Aquat. Anim. Health 11 , 148–153. [Google Scholar]
- 97. Cullen, B. R. , Owens L., Whittington R. J., 1995: Experimental infection of Australian anurans (Limnodynastes terrareginae and Litoria latopalmata) with Bohle iridovirus. Dis. Aquat. Org. 23 , 83–92. [Google Scholar]
- 98. Cunningham, A. A. , Langton T. E. S., Bennett P. M., Drury S. E. N., Gough R. E., Kirkwood J. K., 1993: Unusual mortalities associated with poxvirus‐like particles in frogs (Rana temporaria). Vet. Rec. 133 , 141–142. [DOI] [PubMed] [Google Scholar]
- 99. Cunningham, A. A. , Langton T. E., Bennet P. M., Lewin J. F., Drury S. E., Gough R. E., Macgregor S. K., 1996: Pathological and microbiological findings from incidents of unusual mortality of the common frog (Rana temporaria). Philos. Trans. R. Soc. Lond. B. Biol. Sci. 351 , 1539–1557. [DOI] [PubMed] [Google Scholar]
- 100. Cunningham, C. O. & Snow M., 2000: Genetic analysis of infectious anaemia virus (ISAV) from Scotland. Dis. Aquat. Org. 41 , 1–8. [DOI] [PubMed] [Google Scholar]
- 101. Dannevig, B. H. , Falk N., Namork E., 1995: Isolation of the causal virus of infectious salmon anemia (ISA) in a long term cell line from Atlantic salmon head kidney. J. Gen. Virol. 76 , 1353–1359. [DOI] [PubMed] [Google Scholar]
- 102. Darai, G. , Anders K., Koch H., Delius H., Gelderblom H., Samalecos C., Flügel R. M., 1983: Analysis of the genome of FLDV isolated directly from epidermal tumours of Pleuronectes . Virology 126 , 466–479. [DOI] [PubMed] [Google Scholar]
- 103. Darai, G. , Delius H., Clarke J., Apfel H., Schnitzler P., Flügel R. M., 1985: Molecular cloning and physical mapping of the genome of the genome of FLDV. Virology 146 , 292–301. [DOI] [PubMed] [Google Scholar]
- 104. Daszak, P. , Berger L., Cunningham A. A., Hyatt A. D., Green D. E., Speare R., 1999: Emerging infectious diseases and amphibian population declines. Emerging Infect. Dis. 5 , 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Davidse, A. , Haenen O. L. M., Dijkstra S. G., Van Niuwstadt A. P., Van der Vorst T. J. K., Wagenaar F. W., Wellenberg G. J., 1999: First isolation of herpesvirus of eel (Herpesvirus anguillae) in diseased european eel (Anguilla anguilla L.) in Europe. Bull. Eur. Assoc. Fish Pathol. 19 , 137–141. [Google Scholar]
- 106. Davison, A. J. , 1992: Channel catfish virus: a new type of herpesvirus. Virology 186 , 9–14. [DOI] [PubMed] [Google Scholar]
- 107. Davison, A. J. , 1998: The genome of salmonid herpesvirus 1. J. Virol. 72 , 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Davison, A. J. & Davison M. D., 1995: Identification of structural proteins of CCV by mass spectrometry. Virology 206 , 1035–1043. [DOI] [PubMed] [Google Scholar]
- 109. Davison, A. J. , Sauerbier W., Dolan A., Addison C., McKinnell R. G., 1999: Genomic studies of the LTHV. J. Cancer Res. Clin. Oncol. 125 , 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Davison, A. J. , Wright K. M., Harrach B., 2000: DNA sequence of frog adenovirus. J. Gen. Virol. 81 , 2431–2439. [DOI] [PubMed] [Google Scholar]
- 111. Deering, R. E. , Arakawa C. K., Oshima K. H., O'Hara P. J., Landolt M. L., Winton J. R., 1991: Development of a biotinylated DNA probe for detection and identification of infectious haematopoietic necrosis virus. Dis. Aquat. Org. 11 , 57–65. [Google Scholar]
- 112. Delsert, C. , Morin N., Comps M., 1997: Fish nodavirus lytic cycle and semipermissive expression in mammalian and fish cell cultures. J. Virol. 71 , 5673–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dorson, M. , Torchy C., Chilmonczyk S., De Kinkelin P., Michel C., 1984: A rhabdovirus pathogenic for perch, Perca fluviatilis L.: isolation and preliminary study. J. Fish Dis. 7 , 241–245. [Google Scholar]
- 114. Drury, S. E. N. , Gough R. E., Cunningham A. A., 1995: Isolation of an iridovirus‐like agent from common frog (Rana temporaria). Vet. Rec. 127 , 72–73. [DOI] [PubMed] [Google Scholar]
- 115. Duncan, R. & Dobos P., 1986: The nucleotide sequence of infectious pancreatic necrosis virus (IPNV) dsRNA A segment reveals one large ORF encoding a precursor protein. Nucleic Acids Res. 14 , 5934–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Duryee, W. R. , 1956: Precancer cells in amphibian adenocarcinoma. Ann. NY Acad. Sci. 63 , 1280–1302. [DOI] [PubMed] [Google Scholar]
- 117. Eaton, W. D. , Wingfield W. H., Hedrick R. P., 1989: Prevalence and experimental transmission of the steelhead herpesvirus in salmonid fishes. Dis. Aquat. Org. 7 , 23–39. [Google Scholar]
- 118. Eliassen, T. M. , Froystad M. K., Dannevig B. H., Jakowska M., Brech A., Falk K., Romoren K., Gjoen T., 2000: Initial events in infectious salmon anemia virus infection: evidence for the requirement of a low‐pH step. J. Virol. 74 , 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Elliott, R. M. & Kelly D. C., 1980: FV‐3 replication: induction and intracellular distribution of polypeptides in infected cells. J. Virol. 33 , 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Emmenegger, E. J. , Meyers T. R., Burton T. O., Kurath G., 2000: Genetic diversity and epidemiology of infectious haematopoietic necrosis virus in Alaska. Dis. Aquat. Org. 40 , 163–170. [DOI] [PubMed] [Google Scholar]
- 121. Enzmann, P. E. , 2000: Molecular biology of fishpathogenic rhabdoviruses. In: Fingerman, M., and R. Nagabhushanam (eds), Recent Advances in Marine Biotechnology, Vol. 5. Immunology and Pathology, pp. 269–293. Science Publishers Inc., Enfield, IL.
- 122. Espinoza, J. C. , Hjalmersson A., Everitt E., Kuznar J., 2000: Temporal and subcellular localization of infectious pancreatic necrosis virus structural proteins. Arch. Virol. 145 , 739–748. [DOI] [PubMed] [Google Scholar]
- 123. Essani, K. & Granoff A., 1989: Properties of amphibian and piscine iridoviruses: a comparison. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 79–85. Springer‐Verlag, Heidelberg.
- 124. Falk, K. , Namork E., Rimstad E., Mjaaland S., Dannevig B. H., 1997: Characterization of infectious salmon anemia virus, an orthomyxo‐like virus isolated from Atlantic salmon (Salmo salar L.). J. Virol. 71 , 9016–9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fang, Q. , Attoui H., Biagini J. F. P., Zhu Z., DeMicco P., DeLamballerie X., 2000: Sequence of genome segments 1, 2, 3 of the grass carp reovirus (genus Aquareovirus, family Reoviridae). Biochem. Biophys. Res. Commun. 274 , 762–766. [DOI] [PubMed] [Google Scholar]
- 126. Fijan, N. , 1972: Infectious dropsy in carp – a disease complex. Symp. Zool. Soc. Lond. 30 , 39–51. [Google Scholar]
- 127. Fijan, N. , Petrinec Z., Sulimanovic D., Zwillenberg L. O., 1971: Isolation of the viral causative agent from the acute form of infectious dropsy of carp. Vet. Arh. 41 , 125–138. [Google Scholar]
- 128. Fijan, N. , Matasin Z., Petrinec Z., Valpotic I., Zwillenberg L. O., 1991: Isolation of an iridovirus‐like agent from the green frog (Rana esculenta L.). Veterinarski Arhiv Zagreb 61 , 151–158. [Google Scholar]
- 129. Flügel, R. M. , Darai G., Gelderblom H., 1982: Viral proteins and adenosine triphosphate phosphohydrolase activity of fish lymphocystis disease virus. Virology 122 , 48–55. [DOI] [PubMed] [Google Scholar]
- 130. Foelsch, D. W. & Leloup P., 1976: Fatale endemische Infektion in einem Serpentarium. Tierärztl. Prax. 4 , 527–536. [PubMed] [Google Scholar]
- 131. Fraser, W. A. , Keefe T. J., Bolon B., 1993: Isolation of an iridovirus from farm‐raised gouramis (Trichogaster trichopterus) with fatal disease. J.Vet. Diagn. Invest. 5 , 250–253. [DOI] [PubMed] [Google Scholar]
- 132. Frerichs, G. N. , 1995: Viruses associated with the epizootic ulcerative syndrome (EUS) of fish in South‐East Asia. Vet. Res. 26 , 449–454. [PubMed] [Google Scholar]
- 133. Frerichs, G. N. , Millar S. D., Roberts R. J., 1986: Ulcerative rhabdovirus in fish in South‐East‐Asia. Nature 322 , 216–216. [DOI] [PubMed] [Google Scholar]
- 134. Frerichs, G. N. , Morgan D., Hart D., Skerrow C., Roberts R. J., Onions D. E., 1991: Spontaneously productive C‐type retrovirus infection of fish cell lines. J. Gen. Virol. 72 , 2537–2539. [DOI] [PubMed] [Google Scholar]
- 135. Frerichs, G. N. , Rodger H. D., Peric Z., 1996: Cell culture isolation of piscine neuropathy nodavirus from juvenile sea bass Dicentrarchus labrax . J. Gen. Virol. 77 , 2067–2071. [DOI] [PubMed] [Google Scholar]
- 136. Frost, P. & Ness A., 1997: Vaccination of Atlantic salmon with recombinant VP2 of infectious pancreatic necrosis virus (IPNV), added to a multivalent vaccine, suppresses viral replication following IPNV challenge. Fish Shellfish Immunol. 7 , 609–619. [Google Scholar]
- 137. Frost, P. , Borsheim K., Endresen C., 1998: Analysis of the antibody response in Atlantic salmon against recombinant VP2 of infectious pancreatic necrosis virus. Fish Shellfish Immunol. 8 , 447–456. [Google Scholar]
- 138. Frye, F. L. , Oshiro L. O., Dutra F. R., Carney J. D., 1977: Herpesvirus‐like infection in two Pacific pond turtles. J. Am. Vet. Med. Assoc. 171 , 882–884. [PubMed] [Google Scholar]
- 139. Frye, F. L. , Munn R. J., Gardner M., Barten S. L., Hadfy L. B., 1994: Adenovirus‐like hepatitis in a group of related Rankin’s dragon lizards (Pogona henrylawsoni). J. Zoo Wildl. Med. 25 , 167–171. [Google Scholar]
- 140. Ghittino, P. & Ghittino C., 1985: Probalie adenovirosi nel giovane storione dàllevamento (Acipenser transmontanus). Riv. Ital. Piscic. Ittiop. 10 , 137–139. [Google Scholar]
- 141. Glazebrook, J. S. , Heasman M. P., DeBeer S. W., 1990: Picorna‐like viral particles associates with mass mortalities in larval barramundi, Lates calcarifer Bloch. J. Fish Dis. 13 , 245–249. [Google Scholar]
- 142. Goorha, R. , 1981: FV‐3 requires RNA polymerase II for its replication. J. Virol. 37 , 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Goorha, R. , 1982: FV‐3 DNA replication occurs in two stages. J. Virol. 43 , 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Goorha, R. , 1995: Iridoviridae . In: Murphy, F. A., C. M. Fauquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Jarvis, G. P. Mertelli, M. A. Mayo, and M. D. Summers (eds), Virus Taxonomy. Sixth Report of the International Committee on Taxonomy of Viruses, pp. 95–99. Springer‐Verlag, Heidelberg.
- 145. Goorha, R. & Murti K. G., 1982: The genome of FV‐3, an animal DNA virus, is circularly permuted and terminally redundant. Proc. Natl Acad. Sci. USA 79 , 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Granoff, A. , 1989: Viruses of amphibia: an historical perspective. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 3–12. Springer‐Verlag, Heidelberg.
- 147. Gravendyck, M. , Ammermann P., Marschang R. E., Kaleta E. F., 1998: Paramyxoviral and reoviral infections of iguanas on Honduran islands. J. Wildl. Dis. 34 , 33–38. [DOI] [PubMed] [Google Scholar]
- 148. Gray, W. L. , Williams R. J., Griffin B. R., 1999a: Detection of CCV DNA in acutely infected channel catfish, Ictalurus punctatus (Rafinesque), using the PCR. J. Fish Dis. 22 , 111–116. [Google Scholar]
- 149. Gray, W. L. , Williams R. J., Jordan R. L., Griffin B. R., 1999b: Detection of CCV DNA in latently infected catfish. J. Gen. Virol. 80 , 181–182. [DOI] [PubMed] [Google Scholar]
- 150. Green, K. Y. , Ando T., Balayan M. S., Berke T., Clarke I. N., Estes M. K., Matson D. O., Nakata S., Neill J. D., Studdert M. J., Thiel H. J., 2000: Taxonomy of the caliciviruses. J. Infect. Dis. 181 , 322–330. [DOI] [PubMed] [Google Scholar]
- 151. Groff, J. M. , LaPatra S. E., Munn R. J., Zinkl J. G., 1998: A viral epizootic in cultured populations of juvenile goldfish due to a putative herpesvirus etiology. J. Vet. Diagn. Invest. 10 , 375–378. [DOI] [PubMed] [Google Scholar]
- 152. Grotmol, S. , Nerland A. H., Biering E., Totland G. K., Nishizawa T., 2000: Characterisation of the capsid protein gene from a nodavirus strain affecting the Atlantic halibut Hippogossus hippogossus and design of an optimal RT‐PCR detection assay. Dis. Aquat. Org. 39 , 79–88. [DOI] [PubMed] [Google Scholar]
- 153. Gruia‐Gray, J. , Petric M., Desser S. S., 1989: Ultrastructural, biochemical, and biological properties of an erythrocytic virus of frogs from Ontario, Canada. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 69–78. Springer‐Verlag, Heidelberg. [DOI] [PubMed]
- 154. Gruia‐Gray, J. , Ringuette M., Desser S. S., 1992: Cytoplasmic localization of the DNA virus frog erythrocytic virus. Intervirology 33 , 159–164. [DOI] [PubMed] [Google Scholar]
- 155. Guo, D. F. , Kodama H., Onuma M., 1991a: Partial restriction map of OMV DNA for three restriction enzymes BamHI, EcoRI and XhoI. J. Vet. Med. Sci. 53 , 213–218. [DOI] [PubMed] [Google Scholar]
- 156. Guo, D. F. , Kubota H., Onuma M., Kodama H., 1991b: Detection of OMV in fish by Southern‐blot technique. J. Vet. Med. Sci. 53 , 43–48. [DOI] [PubMed] [Google Scholar]
- 157. Guo, D. F. , Kodama H., Onuma M., Kimura T., Yoshimizu M., 1991c: Comparison of different OMV strains by DNA restriction endonuclease cleavage analysis. Jpn J. Vet. Res. 39 , 27–37. [PubMed] [Google Scholar]
- 158. Hanson, L. A. & Thune R. L., 1993: Characterization of thymidine kinase encoded by CCV. J. Aquat. Anim Health 5 , 199–204. [Google Scholar]
- 159. Harper, P. A. W. , Hammond D. C., Heuschele W. P., 1982: A herpesvirus‐like agent associated with a pharyngal abscess in desert tortoise. J. Wildl. Dis. 18 , 113–120. [DOI] [PubMed] [Google Scholar]
- 160. Harrison, P. T. , Thompson R., Davison A. J., 1991: Evolution of herpesvirus thymidine kinases from cellular deoxycytidine kinase. J. Gen. Virol. 72 , 2583–2586. [DOI] [PubMed] [Google Scholar]
- 161. Hart, D. , Frerichs G. N., Rambaut A., Onions D. E., 1996: Complete nucleotide sequence and transcriptional analysis of the snakehead fish retrovirus. J. Virol. 70 , 3606–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Harvarstein, L. S. , Kalland K. H., Christie K. E., Endresen C., 1990: Sequence of the large double‐stranded RNA segment of the N1 strain of infectious pancreatic necrosis virus, a comparison with other Birnaviridae. J. Gen. Virol. 73 , 2863–2870. [DOI] [PubMed] [Google Scholar]
- 163. Hauser, B. , Mettler F., Rübel A., 1983: Herpesvirus‐like infection in two young boas. J. Comp. Pathol. 93 , 5515–5519. [DOI] [PubMed] [Google Scholar]
- 164. Hedrick, R. P. & McDowell T. S., 1995: Properties of iridoviruses from ornamental fish. Vet. Res. 26 , 423–427. [PubMed] [Google Scholar]
- 165. Hedrick, R. P. & Sano T., 1989: Herpesvirus of fishes. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 161–170. Springer‐Verlag, Heidelberg.
- 166. Hedrick, R. P. , McDowell T., Eaton W. D., Chan L., Wingfield W. H., 1986: Herpesvirus salmonis: first occurence in anadromous salmonids. Bull. Eur. Assoc. Fish Pathol. 6 , 66–67. [Google Scholar]
- 167. Hedrick, R. P. , Groff J. M., McDowell T., 1987: Response of adult channel catfish to waterborne exposures of CCV. Progr. Fish Culturist 49 , 181–187. [Google Scholar]
- 168. Hedrick, R. P. , Groff J., McDowell T. S., Wingfield W. H., 1990a: An iridovirus infection of the integument of the white sturgeon (Acipenser transmontanus). Dis. Aquat. Org. 8 , 39–44. [Google Scholar]
- 169. Hedrick, R. P. , Groff J. M., Okihiro M. S., McDowell T. S., 1990b: Herpesviruses detected in papillomatous skin growth of Koi carp Cyprinus carpio . J. Wildl. Dis. 26 , 578–581. [DOI] [PubMed] [Google Scholar]
- 170. Hedrick, R. P. , McDowell T. S., Groff J. M., Yun S., Wingfield W. H., 1991: Isolation of an epitheliotropic herpesvirus from white sturgeon, Acipenser transmontanus . Dis. Aquat. Org. 11 , 49–55. [Google Scholar]
- 171. Hedrick, R. P. , McDowell T. S., Ahne W., Torhy C., De Kinkelin P., 1992a: Properties of three iridovirus‐like agents associated with systemic infections of fish. Dis. Aquat. Org. 13 , 203–209. [Google Scholar]
- 172. Hedrick, R. P. , McDowell T. S., Groff J. M., Yun S., Wingfield W. H., 1992b: Isolation and properties of an iridovirus‐like agent from white sturgeon Acipenser transmontanus . Dis. Aquat. Org. 12 , 75–81. [Google Scholar]
- 173. Hedrick, R. P. , Gilard O., Yun S., Spangenberg J. V., Nordhausen R. W., Kebus M. J., Bercovier H., Eldar A., 2000: A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of common carp. J. Aquat. Anim. Health 12 , 44–57. [DOI] [PubMed] [Google Scholar]
- 174. Heldstab, A. & Bestetti G., 1982: Spontaneous viral hepatitis in a spur‐tailed Mediterranean land tortoise (Testudo hermanni). J. Zoo Anim. Med. 13 , 113–120. [Google Scholar]
- 175. Heldstab, A. & Bestetti G., 1984: Virus associated with gastrointestinal diseases in snakes. J. Zoo Anim. Med. 15 , 118–128. [Google Scholar]
- 176. Hengstberger, S. H. , Hyatt A. D., Speare R., Coupar B. E. H., 1993: Comparison of EHNV and BIV, recently isolated Australian iridoviruses. Dis. Aquat. Org. 15 , 93–107. [Google Scholar]
- 177. Heppell, J. , Tarrab E., Berthiaume L., Lecomte J., Arella M., 1995a: Characterization of the small open reading frame on genome A of infectious pancreatic necrosis virus. J. Gen. Virol. 76 , 2091–2096. [DOI] [PubMed] [Google Scholar]
- 178. Heppell, J. , Tarrab E., Lecomte J., Berthiaume L., Arella M., 1995b: Strain variability and localization of important epitopes on the major structural protein (VP2) of infectious pancreatic necrosis virus. Virology 214 , 40–49. [DOI] [PubMed] [Google Scholar]
- 179. Herbst, L. H. , 1994: Fibropapillomatosis of marine turtles. Annu. Rev. Fish Dis. 4 , 389–425. [Google Scholar]
- 180. Herbst, L. H. , Jacobson E. R., Moretti R., Brown T., Sundberg J. P., Klein P. A., 1995: Experimental transmission of green turtle fibropapillomatosis using cell‐free tumor extracts. Dis. Aquat. Org. 22 , 1–12. [Google Scholar]
- 181. Herbst, L. H. , Moretti R., Brown T., Klein P. A., 1996: Sensitivity of the transmissible green turtle fibropapillomatosis agent to chloroform and ultracentrifugation conditions. Dis. Aquat. Org. 25 , 225–228. [Google Scholar]
- 182. Herbst, L. H. , Greiner E. C., Ehrhart L. M., Bagley D. A., Klein P. A., 1998: Serological association between spirorchidiasis, herpesvirus infection, and fibropapillomatosis in green turtles from Florida. J. Wildl. Dis. 34 , 496–507. [DOI] [PubMed] [Google Scholar]
- 183. Herman, R. L. , Burke C. N., Perry S., 1997: Epidermal tumors of rainbow smelt in associated virus. J. Wildl. Dis. 33 , 925–929. [DOI] [PubMed] [Google Scholar]
- 184. Hetrick, F. M. & Hedrick R. P., 1993: New viruses described in finfish from 1988 to 1992. Annu. Rev. Fish Dis. 3 , 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hetrick, F. M. , Samal S., Lupiani B., Dopazo C., Subramanian K., Mohanty S., 1992: Members of the family Reoviridae found in aquatic animals. In: Kimura, T., (ed), Proceedings of the OIJ International Symposium on Salmonid Diseases, pp. 33–40. Hokkaido University Press, Sapporo.
- 186. Hill, B. J. , 1982: Infectious pancreatic necrosis virus and its virulence. In: Roberts, R. J. (ed.), Microbial Diseases of Fish, pp. 91–114. Academic Press, New York, NY.
- 187. Hill, B. J. & Way K., 1995: Serological classification of IPNV and other aquatic birnaviruses. Annu. Rev. Fish Dis. 5 , 55–77. [Google Scholar]
- 188. Hill, B. J. , Underwood B. O., Smale C. J., Brown F., 1975: Physico‐chemical and serological characterization of five rhabdoviruses infecting fish. J. Gen. Virol. 27 , 369–378. [DOI] [PubMed] [Google Scholar]
- 189. Hoff, G. & Trainer D. O., 1973: Arboviruses in reptiles: isolation of a Bunyamwera group virus from a naturally infected turtle. J. Herpetol. 7 , 55–62. [Google Scholar]
- 190. Hong, J. R. , Lin T. L., Hsu Y. L., Wu J. L., 1998: Apoptosis precedes necrosis of fish cell line with infectious pancreatic necrosis virus infection. Virology 250 , 76–84. [DOI] [PubMed] [Google Scholar]
- 191. Hong, J. R. , Hsu Y. L., Wu J. L., 1999a: Infectious pancreatic necrosis virus induces apoptosis due to down‐regulation of survival factor MCL‐1protein. Virus Res. 63 , 75–83. [DOI] [PubMed] [Google Scholar]
- 192. Hong, J. R. , Lin T. T., Yang J. Y., Hsu Y. L., Wu J. L., 1999b: Dynamics of nontypical apoptotic morphological changes visualized by green fluorescent protein in living cells with infectious pancreatic necrosis virus infection. J. Virol. 73 , 5056–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Horner, R. E. , 1988: Poxvirus in farmed Nile crocodiles. Vet. Rec. 122 , 459–461. [DOI] [PubMed] [Google Scholar]
- 194. Hosono, N. , Suzuki S., Kusuda R., 1996: Genogrouping of birnaviruses isolated from marine fish: a comparison of VP2/NS junction regions on genome segment A. J. Fish Dis. 19 , 295–302. [Google Scholar]
- 195. Hsu, Y. L. , Cheng C. C., Wu J. L., 1995: Molecular relationships in infectious pancreatic necrosis virus. Virus Res. 37 , 239–252. [DOI] [PubMed] [Google Scholar]
- 196. Hyatt, A. D. , Hine P. M., Jones H. B., Whittington R. J., Kearns C., Wise T. G., Crane M. S., Williams L. M., 1997: Epizootic mortality in the pilchard Sardinops sagax neopilchardus in Australia and New Zealand in 1995: II. Identification of a herpesvirus within the gill epithelium. Dis. Aquat. Org. 28 , 17–29. [Google Scholar]
- 197. Hyatt, A. D. , Gould A. R., Zupanovic Z., Cunningham A. A., Hengstberger S., Whittington R. J., Kattenbelt J., Coupar B. E. H., 2000: Comparative studies of piscine and amphibian iridoviruses. Arch. Virol. 145 , 301–331. [DOI] [PubMed] [Google Scholar]
- 198. Iida, Y. , Masumura K., Nakai T., Sorimachi M., Matsuda H., 1989: A viral disease occurred in larvae and juveniles of Japanese flounder, Paralichthys olivaceus . J. Aquat. Anim. Health 1 , 7–12. [Google Scholar]
- 199. Iida, Y. , Nakai T., Sorimachi M., Masumura K., 1991: Histopathology of a herpesvirus infection in larvae of Japanese flounder, Paralichthys olivaceus . Dis. Aquat. Org. 10 , 59–63. [Google Scholar]
- 200. Inglis, J. A. , Bruce J., Cunningham C. O., 2000: Nucleotide sequence variation in isolates of infectious salmon anaemia virus (ISAV) from Atlantic salmon Salmo salar in Scotland and Norway. Dis. Aquat. Org. 43 , 71–76. [DOI] [PubMed] [Google Scholar]
- 201. Ippen, R. , Mladenov Z., Konstantinov A., 1978: Leukosis with viral presence proven by means of an electron microscope in two boa constrictors. Schweiz. Arch. Tierheilkd. 120 , 357–368. [PubMed] [Google Scholar]
- 202. Iwamoto, T. , Mori K., Arimoto M., Nakai T., 1999: High permissivity of the fish cell line SSN‐1 for piscine nodaviruses. Dis. Aquat. Org. 39 , 37–47. [DOI] [PubMed] [Google Scholar]
- 203. Jackson, O. F. & Needham J. R., 1983: Rhinitis and virus antibody titres in chelonians. J. Small. Anim. Pract. 24 , 31–36. [Google Scholar]
- 204. Jacobson, E. R. , 1978: Diseases of the respiratory system of reptiles. Vet. Med. Sm. Clin. 73 , 1169–1175. [PubMed] [Google Scholar]
- 205. Jacobson, E. R. , 1986: Viruses and viral associated diseases of reptiles. Acta Zool. Pathol. Antiv. Maint. Reprod. Rept. Capt. 79 , 73–90. [Google Scholar]
- 206. Jacobson, E. R. & Gardiner C. H., 1990: Adeno‐like virus in esophageal and tracheal mucosa of a Jackson’s chameleon (Chamaeleo jacksoni). Vet. Pathol. 27 , 210–212. [DOI] [PubMed] [Google Scholar]
- 207. Jacobson, E. R. & Kollias G. V., 1986: Adenovirus‐like infection in a Savannah monitor. J. Zoo An. Med. 17 , 149–151. [Google Scholar]
- 208. Jacobson, E. R. & Telford S. R., 1990: Chlamydial and poxvirus infections of circulating monocytes of a flap‐necked chamaeleon (Chamaeleo dilepis). J. Wildl. Dis. 6 , 72–577. [DOI] [PubMed] [Google Scholar]
- 209. Jacobson, E. R. , Popp R. P., Shields R. P., 1979: Pox‐like virus associated with skin lesions in captive caimans. J. Am. Vet. Med. Assoc. 175 , 937–940. [PubMed] [Google Scholar]
- 210. Jacobson, E. R. , Gaskin J. M., Clubb S. L., 1982: Papilloma‐like virus infection in Bolivian side‐neck turtles. J. Am. Vet. Med. Assoc. 181 , 1325–1328. [PubMed] [Google Scholar]
- 211. Jacobson, E. R. , Kopit W., Kennedy F. A., Funk R. S., 1986a: Coinfection of a bearded dragon, Pogona vitticeps, with adenovirus‐ and dependovirus‐like viruses. Vet. Pathol. 33 , 343–346. [DOI] [PubMed] [Google Scholar]
- 212. Jacobson, E. R. , Gaskin J. M., Roelke M., Greimer E. C., Allen J., 1986b: Conjunctivitis, tracheitis, and pneumonia associated with herpesvirus infection in green sea turtles. J. Am. Vet. Med. Assoc. 189 , 1020–1023. [PubMed] [Google Scholar]
- 213. Jensen, M. H. , 1963: Preparation of fish tissue cultures for virus research. Bull. Off. Inst. Epizoot. 59 , 131–134. [Google Scholar]
- 214. Jensen, N. J. , Bloch B., Larsen J. L., 1979: The ulcus‐syndrome in cod (Garhus morhua). 3. A preliminary virological report. Nordisk Veterinaer Med. 31 , 436–442. [PubMed] [Google Scholar]
- 215. John, R. K. & Richards R. H., 1999: Characteristics of a new birnavirus associated with a warm‐water fish cell line. J. Gen. Virol. 80 , 2061–2065. [DOI] [PubMed] [Google Scholar]
- 216. Johnson, M. C. , Maxwell J. M., Loh P. C., Leong J. C., 1999: Molecular characterization of the glycoproteins from two warm water rhabdoviruses, snakehead rhabdovirus (SHRV) and rhabdovirus of penaeid shrimp (RPS), spring viremia of carp virus (SVCV). Virus Res. 64 , 95–106. [DOI] [PubMed] [Google Scholar]
- 217. Johnsrude, J. D. , Raskin R. E., Hoge A. Y., Erdos G. W., 1997: Intraerythrocytic inclusions associated with iridoviral infection in a fer de lance (Bothrops moojeni) snake. Vet. Pathol. 34 , 235–238. [DOI] [PubMed] [Google Scholar]
- 218. Johnston, M. R. L. , 1975: Distribution of Pirhemocyton Chatton & Blanc and other possibly related, infections of poikilotherms. J. Protozool. 22 , 529–535. [Google Scholar]
- 219. Jorgensen, P. E. V. , 1971: Egtved virus, demonstration of neutralizing antibodies in serum from artificially infected rainbow trout (Salmo gairdneri). J. Fish. Res. Board Can. 28 , 875–877. [Google Scholar]
- 220. Jorgensen, P. E. V. , Olesen N. J., Ahne W., Lorenzen N., 1989: SVCV and PFR viruses, serological examination of 22 isolates indicates close relationship between the two fish rhabdoviruses. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 349–366. Springer‐Verlag, Heidelberg.
- 221. Jorgensen, P. E. V. , Castric J., Hill B., Ljungberg O., De Kinkelin P., 1994: The occurrence of virus infection in elvers and eels (Anguilla anguilla) in Europe with particular reference to VHSV and IHNV. Aquaculture 123 , 11–19. [Google Scholar]
- 222. Juhasz, A. & Ahne W., 1993: Physicochemical properties and cytopathogenicity of an adenovirus‐like agent isolated from corn snake (Elaphe guttata). Arch. Virol. 130 , 429–439. [DOI] [PubMed] [Google Scholar]
- 223. Julian, A. F. & Durham P. J. K., 1982: Adenoviral hepatitis in a female bearded dragon (Amphibolurus barbatus). NZ Vet. J. 30 , 59–60. [DOI] [PubMed] [Google Scholar]
- 224. Jung, S. J. & Miyazaki T., 1995: Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.). J. Fish Dis. 18 , 211–220. [Google Scholar]
- 225. Jung, S. J. , Miyazaki T., Miyata M., Danayadol Y., Tanaka S., 1997: Pathogenicity of iridovirus from Japan and Thailand for the red sea bream Pagrus major in Japan, and histopathology of experimentally infected fish. Fish Sci. 63 , 735–740. [Google Scholar]
- 226. Kabisch, D. & Frost J. W., 1994: Isolation of a herpesvirus from T., and Agrionemys horsfieldii . Verh. Berichte Erkrankungen Zootiere 36 , 241–245. [Google Scholar]
- 227. Kanchanakhan, S. , 1998: An ulcerative disease of the cultured tiger frog, Rana tigrina, in Thailand: virological examination. Aahri Newsletter 7 , 1–2. [Google Scholar]
- 228. Kancharla, S. R. & Hanson L. A., 1996: Production and shedding of CCV and TK− CCV in immersion exposed channel catfish. Dis. Aquat. Org. 27 , 25–34. [Google Scholar]
- 229. Kaur, K. , Rohozinski J., Goorha R., 1995: Identification and characterization of the FV‐3 DNA MTase gene. J. Gen. Virol. 76 , 1937–1943. [DOI] [PubMed] [Google Scholar]
- 230. Kent, M. L. & Elston R. A., 1987: Pancreas disease in pen‐reared Atlantic salmon in North America. Bull. Eur. Assoc. Fish Pathol. 7 , 9–31. [Google Scholar]
- 231. Kent, M. L. & Meyers M. S., 2000: Hepatic lesions in a redstriped rockfish (Sebastes proriger) suggestive of a herpesvirus infection. Dis. Aquat. Org. 41 , 237–239. [DOI] [PubMed] [Google Scholar]
- 232. Kibenge, F. S. B. , Lyaku J. R., Rainnie D., Hammell K. L., 2000: Growth of infectious salmon anaemia virus in CHSE‐214 cells and evidence for phenotypic differences between virus strains. J. Gen. Virol. 81 , 143–150. [DOI] [PubMed] [Google Scholar]
- 233. Kim, C. H. , Dummer D. M., Chiou P. P., Leong J. A., 1999: Truncated particles produced in fish surviving infectious haematopoietic necrosis virus infection: mediators of persistence? J. Virol. 73 , 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kim, C. H. , Johnson M. C., Drennan J. D., Simon B. E., Thomann E., Leong J. A., 2000: DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J. Virol. 74 , 7048–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Kimura, T. & Yoshimizu M., 1989: Salmon herpesvirus: OMV, Oncorhynchus masou virus. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 171–183. Springer‐Verlag, Heidelberg.
- 236. Kimura, T. , Yoshimizu M., Gorie S., 1986: A new rhabdovirus isolated in Japan from cultured hirame (Japanese flounder) Paralichthys olivaceus and ayu Plecoglossus altivelis . Dis. Aquat. Org. 1 , 209–217. [Google Scholar]
- 237. Kimura, T. , Yoshimizu M., Yano Y., 1984: Comparison of virus‐induced polypeptides from fish herpesvirus‐OMV, H. salmonis, and CCV. Abstracts of the 6th International Congress of Virology, Japan. p. 347.
- 238. De Kinkelin, P. & Dorson M., 1973: Interferon production in rainbow trout (Salmo gairdneri) experimentally infected with Egtved virus. J. Gen. Virol. 19 , 125–127. [DOI] [PubMed] [Google Scholar]
- 239. De Kinkelin, P. , Galimard B., Bootsma R., 1973: Isolation and identification of the causative agent of ‘red disease’ of pike (Esox lucius). Nature 241 , 465–467. [Google Scholar]
- 240. Kinsel, M. J. , Barbiers R. B., Manharth A., Murnane R. D., 1997: Small intestinal adeno‐like virus in a mountain chameleon (Chameleo montium). J. Zoo Wildl. Med. 28 , 498–500. [PubMed] [Google Scholar]
- 241. Kirn, A. , Gendrault J. L., Steffan A. M., Gut J. P., Bingen A., 1989: Murine hepatitis induced by FV‐3. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 60–68. Springer‐Verlag, Heidelberg.
- 242. Kobayashi, T. & Miyazaki T., 1997: Characterization and pathogenicity of a herpesvirus isolated from cutaneous lesion in Japanese eel, Anguilla japonica . Fish Pathol. 32 , 89–95. [Google Scholar]
- 243. Kocan, R. , Bradley M., Elder N., Meyers T., Batts W., Winton J. R., 1997: North American strain of viral hemorrhagic septicemia virus is highly pathogenic for laboratory reared pacific herring. J. Aquat. Anim. Health 9 , 279–290. [Google Scholar]
- 244. Kollinger, G. , Schwab M., Anders F., 1979: Virus‐like particles induced by BdUR in melanoma and neuroblastoma of Xiphophorus. J. Cancer Res. Clin. Oncol. 95 , 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Koski, P. , Hill B. J., Way K., Neuvonen E., Rintamäki P., 1992: A rhabdovirus isolated from brown trout (Salmo trutta m. lacustris L.) with lesions in parenchymatous organs. Bull. Eur. Assoc. Fish Pathol. 12 , 177–180. [Google Scholar]
- 246. Krossoy, B. , Hordvik I., Nilsen F., Nylund A., Endresen C., 1999: The putative polymerase sequence of infectious salmon anemia virus suggests a new genus within the Orthomyxoviridae . J. Virol. 73 , 2136–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Kucuktas, H. & Brady Y. J., 1999: Molecular biology of CCV. Aquaculture 172 , 147–161. [Google Scholar]
- 248. Kucuktas, H. , Brady Y. J., Tuzun S., 1998: Cloning and expression of a putative glycoprotein gene of CCV using baculovirus expression system. Dis. Aquat. Org. 34 , 231–237. [DOI] [PubMed] [Google Scholar]
- 249. Kumagai, A. , Takahashi K., Fukuda H., 1995: Pathogenicity of SalHV‐2 isolated from marine‐cultured coho salmon to salmonids. Fish Pathol. 30 , 215–220. [Google Scholar]
- 250. Kumagai, A. , Takahashi K., Fukuda H., 1997: Infection source of herpesvirus disease in coho salmon culture and its control. Fish Pathol. 32 , 103–108. [Google Scholar]
- 251. Kurath, G. , Ahern K. G., Pearson G. D., Leong J. C., 1985: Molecular cloning of the six mRNA species of infectious haematopoietic necrosis virus, a fish rhabdovirus, and gene order determination by R‐loop mapping. J. Virol. 53 , 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kurath, G. , Higman K. H., Björklund H. V., 1995: The NV genes of fish rhabdoviruses: development of RNase protection assays for rapid assessment of genetic variation. Vet. Res. 26 , 477–485. [PubMed] [Google Scholar]
- 253. Kurath, G. , Higman K. H., Björklund H. V., 1997: Distribution and variation of NV genes in fish rhabdoviruses. J. Gen. Virol. 78 , 113–117. [DOI] [PubMed] [Google Scholar]
- 254. Kurita, J. , Nakajima K., Hirono I., Aoki T., 1998: PCR amplification of DNA of RSIV. Fish Pathol. 33 , 17–23. [Google Scholar]
- 255. Lackovich, J. K. , Brown D. R., Homer B. L., Garber R. L., Mader D. R., Moretti R. H., Patterson A. D., Herbst L. H., Oros J., Jacobson E. R., Curry S. S., Klein P. A., 1999: Association of herpesvirus with fibropapillomatosis of the green turtle Chelonia mydas and the loggerhead turtle Caretta caretta in Florida. Dis. Aquat. Org. 37 , 89–97. [DOI] [PubMed] [Google Scholar]
- 256. Lairmore, M. D. , Stanley J. R., Weber S. A., Holzshu D. L., 2000: Squamous epithelial proliferation induced by walleye dermal sarcoma retrovirus cyclin in transgenic mice. Proc. Natl Acad. Sci. USA 87 , 6114–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lamirande, E. W. , Nichols D. K., Owens J. W., Gaskin J. M., Jacobson E. R., 1999: Isolation and experimental transmission of a reovirus pathogenic in ratsnakes (Elaphe species). Virus Res. 63 , 135–141. [DOI] [PubMed] [Google Scholar]
- 258. Langdon, J. S. & Humphrey J. D., 1987: Epizootic haematopoietic necrosis, a new viral disease in redfin perch, Perca fluviatilis L., in Australia. J. Fish Dis. 10 , 289–297. [Google Scholar]
- 259. Langdon, J. S. , Humphrey J. D., Williams L. M., Hyatt A. D., Westbury H. A., 1986: First virus isolation from Australian fish: an iridovirus‐like pathogen from redfin perch, Perca fluviatilis L. J. Fish Dis. 9 , 263–268. [Google Scholar]
- 260. Lange, H. , Herbst W., Wiechert J. M., Schliesser T., 1989: Electron microscopic detection of herpesviruses in a mass death of Greek tortoises (Testudo hermanni) and four‐toed turtles (Agrionemys horsfieldii). Tierärztl. Prax. 17 , 319–321. [PubMed] [Google Scholar]
- 261. LaPatra, S. E. , Groff J. M., Patterson T. L., Shewmaker W. D., Casten M., Siple J., Hauck A. K., 1996: Preliminary evidence of sturgeon density and other stressors on manifestation of WSIV. J. Appl. Aquaculture 6 , 51–58. [Google Scholar]
- 262. LaPatra, S. E. , Parker B. L., Groff J. M., Engelking H. M., Kaufman J., Munn R. J., 1998: Epidemiology of viral infections in white sturgeon from the Pacific Northwest. Proceedings of the 49th Pacific Northwest Fish Culture Conference, 1–3 December, Idaho, USA, pp. 27–31.
- 263. Lee, J. Y. , Hirono I., Aoki T., 2000: Cloning and analysis of Mx cDNA in Japanese flounder, Paralichthys olivaceus . Dev. Comp. Immunol. 24 , 407–415. [DOI] [PubMed] [Google Scholar]
- 264. Lee, M.‐K. , Blake S. L., Singer J. T., Nicholson B. L., 1996: Genomic variation of aquatic birnaviruses analyzed with restriction fragment length polymorphism. Appl. Environ. Microbiol. 62 , 2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Lee, N.‐S. , Kobayashi J., Miyazaki T., 1999: Gill filament necrosis in farmed Japanese eels, Anguilla japonica (Temminck & Schlegel), infected with Herpesvirus anguillae . J. Fish Dis. 22 , 457–463. [Google Scholar]
- 266. Leibovitz, L. & Lebouitz S. S., 1985: A viral dermatitis of the smooth dogfish, Mustelus canis (Mitchill). J. Fish Dis. 21 , 199–204. [Google Scholar]
- 267. Leibovitz, L. & Riis R. C., 1980: A viral disease of a aquarium fish. J. Am. Vet. Med. Assoc. 177 , 414–416. [PubMed] [Google Scholar]
- 268. Lejal, N. , DaCosta B., Huet J. C., Delmas B., 2000: Role of the Ser‐652 and Lys‐692 in the protease activity of infectious bursal disease virus VP4 and identification of its substrate cleavage sites. J. Gen. Virol. 81 , 983–992. [DOI] [PubMed] [Google Scholar]
- 269. Liu, C. T. , Schmidt N. T., Stone D. M., 1998: Detection of SVCV in fish tissues by semi‐nested PCR. Proceedings of the 4th Symposium on Viruses of Lower Vertebrates, 29–31 July, Weymouth, UK. p. PP‐05.
- 270. Lorenzen, K. & Dixon P. F., 1991: Prevalence of antibodies to LD in eustarine flounder Platichthys flesus . Dis. Aquat. Org. 11 , 99–103. [Google Scholar]
- 271. Lorenzen, N. , Olesen N. J., Jorgensen P. E. V., 1990: Neutralization of Egtved virus pathogenicity to cell cultures and fish by monoclonal antibodies to the viral G protein. J. Gen. Virol. 71 , 561–567. [DOI] [PubMed] [Google Scholar]
- 272. Lovely, J. E. , Dannevig B. H., Falk K., Hutchin L., MacKinnon A. M., Melville K. J., Rimstad E., Griffiths S. G., 1999: First identification of infectious salmon anemia virus in North america with haemorrhagic kidney syndrome. Dis. Aquat. Org. 35 , 145–148. [DOI] [PubMed] [Google Scholar]
- 273. Lucké, B. , 1934: A neoplastic disease of the kidneys of the frog, Rana pipiens . Am. J. Cancer 20 , 352–379. [Google Scholar]
- 274. Lunger, P. D. , Darlington R. W., Granoff A., 1965: Cell‐virus relationships in the Lucké renal adenocarcinoma: an ultrastructure study. Ann. NY Acad. Sci. 186 , 289–289. [DOI] [PubMed] [Google Scholar]
- 275. Lunger, P. D. , Hardy W. D., Clark H. F., 1974: C‐type particles in a reptilian tumor. J. Natl Cancer Inst. 52 , 1231–1235. [DOI] [PubMed] [Google Scholar]
- 276. Lupiani, B. , Subramanian K., Samal S. K., 1995: Aquareoviruses. Annu. Rev. Fish Dis. 5 , 175–208. [Google Scholar]
- 277. Lupiani, B. , Reddy S. M., Subramanian K., Samal S. K., 1997: Cloning, sequencing analysis and expression of the major outer capsid protein gene of an aquareovirus. J. Gen. Virol. 78 , 1379–1383. [DOI] [PubMed] [Google Scholar]
- 278. Magyar, G. , Chung H. K., Dobos P., 1998: Conversion of VP1 to VPg in cells infected by infectious pancreatic necrosis virus. Virology 245 , 142–150. [DOI] [PubMed] [Google Scholar]
- 279. Malsberger, R. G. & Lautenslager G., 1980: Fish viruses: rhabdovirus isolated from a species of the family Cichlidae. Fish Health News 9 , i–ii. [Google Scholar]
- 280. Manning, D. S. & Leong J. C., 1990: Expression in Escherichia coli of the large genomic segment of infectious pancreatic necrosis virus. Virology 179 , 16–25. [DOI] [PubMed] [Google Scholar]
- 281. Mao, J. , Hedrick R. P., Chinchar V. G., 1997: Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses. Virology 229 , 212–220. [DOI] [PubMed] [Google Scholar]
- 282. Mao, J. , Wang J., Chinchar G. D., Chinchar V. G., 1999: Molecular characterization of a ranavirus isolated from largemouth bass Micropterus salmoides . Dis. Aquat. Org. 37 , 107–114. [DOI] [PubMed] [Google Scholar]
- 283. Mao, S. , Hang Q., Chen H., Zhang N., Yang G., 1988: Study on cytopathology of two pathogenic viruses of hemorrhage in grass carp, Ctenopharyngodon idellus . Oceanol. Limnol. Sin. 19 , 435–438. [Google Scholar]
- 284. Marschang, R. E. , Gravendyck M., Kaleta E. F., 1997: Herpesviruses in tortoises: investigations into virus isolation and the treatment of viral stomatitis in Testudo hermanni, and T. graeca . J. Vet. Med. B 44 , 385–394. [DOI] [PubMed] [Google Scholar]
- 285. Marschang, R. E. , Becher P., Posthaus H., Wild P., Thiel H.‐J., Müller‐Doblies U., Kaleta E. F., Bacciarini L. N., 1999: Isolation and characterization of an iridovirus from Hermann’s tortoises (T. hermanni). Arch. Virol. 144 , 1909–1922. [DOI] [PubMed] [Google Scholar]
- 286. Martin, J. , Herniou E., Cook J., Waugh‐O'Neill R., Tristem M., 1997: Human endogenous retrovirus type I‐related viruses have an apparently widespread distribution within vertebrates. J. Virol. 71 , 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Martineau, D. , Renshaw R., Williams J. R., Casey J. W., Bowser P. R., 1991: A large unintegrated retrovirus DNA species present in adermal tumor of walleye Stizostedion vitreum . Dis. Aquat. Org. 10 , 153–158. [Google Scholar]
- 288. Matson, D. O. , Berke T., Dinulos M. B., Poet E., Zhong W.‐M., Dai X. M., Jiang X., Golding B., Smith W. A., 1996: Partial characterization of the genome of nine animal caliciviruses. Arch. Virol. 141 , 2443–2456. [DOI] [PubMed] [Google Scholar]
- 289. Matsuoka, S. , Inouye K., Nakajima K., 1996: Cultured fish species affected by RSIVD from 1992 to 1995. Fish Pathol. 31 , 233–234. [Google Scholar]
- 290. McAllister, P. E. & Herman R. L., 1989: Epizootic mortality in hatchery‐reared lake trout Salvelinus namaycush caused by a putative virus possibly of the herpesvirus group. Dis. Aquat. Org. 6 , 113–119. [Google Scholar]
- 291. McAllister, P. E. & Owens W. J., 1992: Recovery of infectious pancreatic necrosis virus from the faeces of wild piscivorous birds. Aquaculture 106 , 227–232. [Google Scholar]
- 292. McAllister, P. E. , Lidgerding B. C., Herman R. L., Hoyer L. C., Hankins J., 1985: Viral diseases of fish: first report of carp pox in golden ide (Leuciscus idus) in North America. J. Wildl. Dis. 21 , 199–204. [DOI] [PubMed] [Google Scholar]
- 293. McKinnell, R. G. , Carlson D. L., Christ C. G., John J. C., 1995: Detection of LTHV DNA sequences in normal and neoplastic tissue obtained from the northern leopard frog, Rana pipiens . In: Zwart, P., and G. Matz (eds), Comptes Rendus Cinquieme International de Pathologie des Reptiles and des Amphibiens, pp. 13–16. NRG Repro Facility BV’s‐Hertogenbosch.
- 294. McPhillips, T. H. , Dinan D., Subramanian K., Samal S. K., 1998: Enhancement of aquareovirus infectivity by treatment with proteases: mechanism of action. J. Virol. 72 , 3387–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Mellergard, S. & Bloch B., 1988: Herpesvirus‐like particles in angelfish (Pterophyllum altum). Dis. Aquat. Org. 5 , 151–155. [Google Scholar]
- 296. Melville, K. J. & Griffiths S. G., 1999: Absence of vertical transmission of infectious salmon anemia virus (ISAV) from individually infected Atlantic salmon Samo salar . Dis. Aquat. Org. 38 , 231–234. [DOI] [PubMed] [Google Scholar]
- 297. Meyers, T. R. & Winton J. R., 1995: Viral hemorrhagic septicemia virus in North America. Annu. Rev. Fish Dis. 5 , 3–24. [Google Scholar]
- 298. Meyers, T. R. , Short S., Lipson K., 1999: Isolation of the North American strain of viral hemorrhagic septicemia virus (VHSV) associated with epizootic mortality in two new host species of Alaskan marine fish. Dis. Aquat. Org. 38 , 81–86. [DOI] [PubMed] [Google Scholar]
- 299. Miyata, M. , Matsuno K., Jung S. J., Danayadol Y., Miyazaki T., 1997: Genetic similarity of iridoviruses from Japan and Thailand. J. Fish Dis. 20 , 127–134. [Google Scholar]
- 300. Miyazaki, T. , Fujiwara K., Kobara J., Matsumoto M., Abe M., Nagano T., 1989: Histopathology with two viral diseases of larval and juvenile fishes, epidermal necrosis of the Japanese flounder Paralichtys olivaceus and epithelial necrosis of black sea bream Acanthopagrus schlegeli . J. Aquat. Anim. Health 1 , 85–93. [Google Scholar]
- 301. Miyazaki, T. , Asai Y., Kobayashi T., Miyata M., 2000a: Lympholeukemia in madai Pagrus major in Japan. Dis. Aquat. Org. 40 , 147–155. [DOI] [PubMed] [Google Scholar]
- 302. Miyazaki, T. , Okamoto H., Kageyama T., Kobayashi T., 2000b: Viremia‐associated ana‐aki‐byo, a new viral disease in color carp Cyprinus carpio in Japan. Dis. Aquat. Org. 39 , 183–192. [DOI] [PubMed] [Google Scholar]
- 303. Mjaaland, S. , Rimstad E., Falk K., Dannevig B. H., 1997: Genomic characterization of the virus causing infectious salmon anemia in Atlantic salmon (Salmo salar L.): an orthomyxo‐like virus in a teleost. J. Virol. 71 , 7681–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Monath, T. P. , Gropp C. B., Frazier C. L., Murphy F. A., Whitfield S. G., 1979: Viruses isolated from reptiles, identification of three new members of the Family Rhabdoviridae . Arch. Virol. 60 , 1–12. [DOI] [PubMed] [Google Scholar]
- 305. Moody, N. J. G. & Owens L., 1994: Experimental demonstration of the pathogenity of a frog virus, BIV for a fish species, barramundi Lates calcarifer . Dis. Aquat. Org. 18 , 95–102. [Google Scholar]
- 306. Moore, A. R. , Li M. F., McMenemy M., 1988: Isolation of a picorna‐like virus from smelt, Osmerus mordax (Mitchill). J. Fish Dis. 11 , 179–184. [Google Scholar]
- 307. Mori, K. I. , Nakai T., Muroga K., Arimoto M., Mushiake K., Furusawa I., 1992: Properties of a new virus belonging to Nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology 187 , 368–337. [DOI] [PubMed] [Google Scholar]
- 308. Morrison, C. M. , Leggiadro C. T., Martell D. J., 1996: Visualization of viruses in tumors of rainbow smelt Osmerus mordax . Dis. Aquat. Org. 26 , 19–23. [Google Scholar]
- 309. Mortensen, H. F. , Heuer O. E., Lorenzen N., Otte L., Olesen N. J., 1999: Isolation of viral haemorrhagic septicaemia virus (VHSV) from wild marine fish species in the Baltic Sea, Kattegat and the North Sea. Virus Res. 63 , 95–106. [DOI] [PubMed] [Google Scholar]
- 310. Morzunov, S. , Winton J. R., Nichol S. T., 1995: The complete genome structure and phylogenetic relationship of infectious haematopoietic necrosis virus. Virus Res. 38 , 175–192. [DOI] [PubMed] [Google Scholar]
- 311. Mulcahy, D. , Klaybor D., Batts W. N., 1990: Isolation of infectious haematopoietic necrosis virus from a leech (Piscicola salmositica) and a copepod (Salmincola sp.), ectoparasites of sockeye salmon Oncorhynchus nerka . Dis. Aquat. Org. 8 , 29–34. [Google Scholar]
- 312. Müller, M. , Zanger N., Denzler T., 1988: Iridovirusepedemie bei der griechischen Landschildkröte (Testudo hermanni hermanni). In: Iffen, H., and H.‐D. Schröder (eds), Erkrankungen der Zootiere. Proceedings of the International Symposium on Zoo and Wild Animals, Berlin, Germany, pp. 271–274. Akademie‐Verlag, Berlin.
- 313. Mullins, J. E. , Groman D., Wadowska D., 1998: Infectious salmon anemia in salt water Atlantic salmon (Salmon salar L.) in New Brunswick. Can. Bull. Eur. Assoc. Fish Pathol. 18 , 110–114. [Google Scholar]
- 314. Munday, B. L. & Nakai T., 1997: Special topic review: nodaviruses as pathogens in larval and juvenile marine fish. World. J. Microbiol. Biotechnol. 13 , 375–381. [Google Scholar]
- 315. Munnes, M. , Schetter C., Hölker I., Doerfler W., 1995: A fully 5′‐CG‐3′ but not a 5′‐CCGG‐3′ methylated late FV‐3 promoter retains activity. J. Virol. 69 , 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Murti, K. G. & Goorha R., 1990: Virus–cytoskeleton interaction during replication of FV‐3. In: Darai, G. (ed.), Molecular Biology of Iridoviruses, Developments in Molecular Virology, pp. 137–161. Kluwer Academic Publishers, Dordrecht.
- 317. Nagabayashi, T. & Wolf K., 1979: Characterization of EV‐2, a virus isolated from European eels (Anguilla anguilla) with stomatopapilloma. J. Virol. 30 , 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Nakajima, K. & Sorimachi M., 1994: Biological and physico‐chemical properties of the iridovirus isolated from cultured seam bream, Pagrus major . Fish Pathol. 29 , 29–33. [Google Scholar]
- 319. Nakajima, K. , Maeno Y., Fukudome M., Fukuda Y., Tanaka S., Matsuoka S., Sorimachi M., 1995: Immunofluorescense test for the rapid diagnosis of red sea bream iridovirus infection using monoclonal antibodies. Fish Pathol. 30 , 115–119. [Google Scholar]
- 320. Nakajima, K. , Inouye K., Sorimachi M., 1998a: Viral diseases in cultured marine fish in Japan. Fish Pathol. 33 , 181–188. [Google Scholar]
- 321. Nakajima, K. , Maeno Y., Yokoyama K., Kaji C., Manabe S., 1998b: Antigen analysis of RSIV and comparison with other fish iridoviruses. Fish Pathol. 33 , 73–78. [Google Scholar]
- 322. Nakajima, K. , Maeno Y., Honda A., Yokoyama K., Tooriyama T., Manabe S., 1999: Effectiveness of a vaccine against RSIV disease in a field trial test. Dis. Aquat. Org. 36 , 73–75. [DOI] [PubMed] [Google Scholar]
- 323. Nason, E. L. , Samla S. K., Venkataram P. B. V., 2000: Trypsin‐induced structural transformatio in aquareovirus. J. Virol. 74 , 6546–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Nelson, R. T. , McLoughlin M. F., Rowley H. M., Platten M., McCormick J. I., 1995: Isolation of a toga‐like virus from farmed Atlantic salmon (Salmo salar) with pancreas disease. Dis. Aquat. Org. 22 , 25–32. [Google Scholar]
- 325. Neukirch, M. , 1985: Isolation of an orthomyxovirus‐like agent from European eel. Bull. Eur. Assoc. Fish Pathol. 5 , 12–13. [Google Scholar]
- 326. Neumann, W. , Wilke I., Friedrich D., Böhme R., 1986: Electron microscopy for detection of paramyxovirus‐like particles in coarse fish. Mh. Vet. Med. 41 , 164–166. [Google Scholar]
- 327. Nichol, S. T. , Rowe J. E., Winton J. R., 1995: Molecular epizootiology and evolution of the glycoprotein and non‐virion protein genes of infectious haematopoietic necrosis virus, a fish rhabdovirus. Virus Res. 38 , 159–173. [DOI] [PubMed] [Google Scholar]
- 328. Nishizawa, T. , Yoshimizu M., Winton J., Ahne W., Kimura T., 1991: Characterization of structural proteins of hirame rhabdovirus, HRV. Dis. Aquat. Org. 10 , 167–172. [Google Scholar]
- 329. Nishizawa, T. , Mori K., Nakai T., Furusawa I., Muroga K., 1994: PCR amplification of RNA of striped jack nervous necrosis virus (SJNNV). Dis. Aquat. Org. 18 , 103–107. [Google Scholar]
- 330. Nishizawa, T. , Mori K., Furuhashi M., Nakai T., Furusawa I., Muroga K., 1995: Comparison of the coat protein genes of five fish nodaviruses, the causative agents of viral nervous necrosis in marine fish. J. Gen. Virol. 76 , 1563–1569. [DOI] [PubMed] [Google Scholar]
- 331. Nishizawa, T. , Kurath G., Winton J. R., 1997a: Sequence analysis and expression of the M1 and M2 matrix protein genes of hirame rhabdovirus (HIRRV). Dis. Aquat. Org. 31 , 9–17. [Google Scholar]
- 332. Nishizawa, T. , Furuhashi M., Nagai T., Muroga T., 1997b: Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl. Environ. Microbiol. 63 , 1633–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Nougayrede, P. , De Kinkelin P., Chilmonczyk S., Vuillanme A., 1992: Isolation of a rhabdovirus from the pike‐perch Stizostedion lucioperca (L.1758). Bull. Eur. Assoc. Fish Pathol. 12 , 5–7. [Google Scholar]
- 334. Nygaard, R. , Husgard S., Sommer A. I., Leong J. A. C., Robertson B., 2000: Induction of Mx protein by interferon and double‐stranded RNA in salmonid cells. Fish Shellfish Immunol. 10 , 435–450. [DOI] [PubMed] [Google Scholar]
- 335. Office International des Epizooties , 2001: International Aquatic Animal Health Code and Diagnostic Manual for Aquatic Animal Diseases. OIE, Paris. [PubMed]
- 336. Ogawa, M. , Ahne W., Essbauer S., 1992: Reptilian viruses: adenovirus‐like agent isolated from royal python (Python regius). J. Vet. Med. B 39 , 732–736. [DOI] [PubMed] [Google Scholar]
- 337. Oh, M. J. , Yoshimizu M., Kimura T., Ezura Y., 1995: A new virus isolated from salmonid fish. Fish Pathol. 30 , 23–32. [Google Scholar]
- 338. Oreshkova, S. F. , Shchelkunov I. S., Tikunova N. V., Shchelkunova T. I., Puzyrev A. T., Ilyichev A. A., 1999: Detection of SVCV isolates by hybridization with non‐radioactive probes and amplification by PCR. Virus Res. 63 , 3–10. [DOI] [PubMed] [Google Scholar]
- 339. Origgi, F. C. & Jacobson E. R., 1999: Development of an ELISA and an immunoperoxidase based test for herpesvirus exposure detection in tortoises. Proc. American Reptilian and Amphibian Viralogists, 65–67.
- 340. Origgi, F. C. , Jacobson E. R., Romero C. H., Klein P. A., 2000: Serological and molecular diagnostic techniques for chelonian herpesviruses. Proceedings of the 5th International Congress of the European Society for Veterinary Virology, pp. 113–114. Breschia, Italy.
- 341. Osadschaya, Y. F. & Nakonechnaya M. G., 1981: Rhabdovirus salmonis, the cause of a new disease in rainbow trout, Salmo gairdneri . J. Ichthyol. 21 , 113–121. [Google Scholar]
- 342. Oshima, S. , Hata J., Hirasawa N., Ohtaka T., Hirono T., Aoki T., Yamashita S., 1998: Rapid diagnosis of RSIV infection using the PCR. Dis. Aquat. Org. 32 , 87–90. [DOI] [PubMed] [Google Scholar]
- 343. Pandey, G. S. , Inoue N., Ohshima K., Okada K., Chihaya Y., Fujimoto Y., 1990: Poxvirus infection in Nile crocodiles (Crocodylus niloticus). Res. Vet. Sci. 49 , 171–176. [PubMed] [Google Scholar]
- 344. Papas, T. S. , Dahlberg J. E., Sonstegard R. A., 1976: Type C virus in lymphosarcoma in northern pike (Esox lucius). Nature 261 , 506–508. [DOI] [PubMed] [Google Scholar]
- 345. Papas, T. S. , Pry T. W., Schafer M. P., Sonstegard R. A., 1977: Presence of DNA polymerase in lymphosarcoma in northern pike (Esox lucius). Cancer Res. 37 , 3214–3217. [PubMed] [Google Scholar]
- 346. Paperna, I. & Alves de Matos A. P., 1993: Erythrocytic viral infections of lizards and frogs: new hosts, geographical locations and description of the infection process. Ann. Parasitol. Hum. Comp. 68 , 11–23. [Google Scholar]
- 347. Penrith, M.‐L. , Nesbit J. W., Huchzermeyer F. W., 1991: Poxvirus infection in captive juvenile caimans (Caiman crocodilus fuscus) in South Africa. J. S. Afr. Vet. Assoc. 62 , 137–139. [PubMed] [Google Scholar]
- 348. Petit, S. , Lejal N., Huet J. C., Delmas B., 2000: Active residues and viral substrate cleavage sites of the protease of the birnavirus infectious pancreatic necrosis virus. J. Virol. 74 , 2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Petry, H. , Petry K., Schmidt M., Hunsmann G., Anders F., Lücke W., 1992: Isolation and characterization of a retrovirus from the fish genus Xiphophorus . Virology 188 , 785–792. [DOI] [PubMed] [Google Scholar]
- 350. Pettersen, E. F. , 1997: Infectious pancreatic necrosis (IPNV) and the development of a viral vaccine. Norsk Veterinaer 109 , 499–505. [Google Scholar]
- 351. Piaskoski, T. O. , Plumb J. A., Roberts S., 1999: Characterization of LMBV in cell culture. J. Aquat. Anim. Health 11 , 45–51. [Google Scholar]
- 352. Pinto, R. M. , Jofre J., Bosch A., 1991: Viral erythrocytic infection in sea bass: virus purification and confirmative diagnosis. Arch. Virol. 120 , 83–96. [DOI] [PubMed] [Google Scholar]
- 353. Plumb, J. A. , 1989: Channel catfish virus. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 198–216. Springer‐Verlag, Heidelberg.
- 354. Plumb, J. A. , Bowser P. R., Grizzle J. M., Mitchell A. J., 1979: Fish viruses, a double‐stranded RNA icosahedral virus from a North American cyprinid. J. Fish Res. Board Can. 36 , 1390–1394. [Google Scholar]
- 355. Plumb, J. A. , Grizzle M., Young H. E., Noyes A. D., Lamprecht S., 1996: An iridovirus isolated from wild largemouth bass. J. Aquat. Anim Health 8 , 265–270. [Google Scholar]
- 356. Plumb, J. A. , Noyes A. D., Graziano S., Wang J., Mao J., Chinchar V. G., 1999: Isolation and identification of viruses from adult largemouth bass during 1997–98 survey in the Southeastern USA. J. Aquat. Anim. Health 11 , 391–399. [Google Scholar]
- 357. Poppe, T. , Rimstad E., Hyllseth B., 1989: Pancreas disease in Atlantic salmon (Salmo salar) post smolts infected with infectious pancreatic necrosis virus (IPNV). Bull. Eur. Assoc. Fish Pathol. 9 , 83–85. [Google Scholar]
- 358. Posthaus, H. , Marschang R. E., Gravendyck M., Bacciarini L. N., 1997: Study on herpesvirus infections in land tortoises in Switzerland. Proceedings of the American Association of Zoo Veterinarians, Houston, Texas, pp. 17–18.
- 359. Pozet, F. , Morand M., Moussa A., Torhy C., De Kinkelin P., 1992: Isolation and preliminary characterization of a pathogenic icosahedral deoxyribovirus from the catfish (Ictalurus melas). Dis. Aquat. Org. 14 , 35–42. [Google Scholar]
- 360. Pringle, C. R. , 1999: Virus taxonomy – 1999. Arch. Virol. 144 , 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Quackenbush, S. L. , Work T. M., Balazs G. H., Casey R. N., Rovnak J., Chaves A., DuToit L., Baines J. D., Parrish C. R., Bowser P. R., Casey J. W., 1998: Three closely related herpesviruses are associated with fibropapillomatosis in marine turtles. Virology 246 , 392–399. [DOI] [PubMed] [Google Scholar]
- 362. Rangel, A. A. , Rockemann D. D., Hetrick F. M., Samal S. K., 1999: Identification of grass carp haemorrhage virus as a new genogoup of aquareovirus. J. Gen. Virol. 80 , 2399–2402. [DOI] [PubMed] [Google Scholar]
- 363. Raynaud, A. & Adrian M., 1976: Lesions cutanees a structure papillomateuse associees a des virus chez le Lezard vert (Lacerta viridis Laur.). C. R. Acad. Sci. D 283 , 845–847. [PubMed] [Google Scholar]
- 364. Regenmortel, M. H. V. Van, Fauquet C. M., Bishop D. H. L., Carstens E. B., Estes M. K., Lemon S. M., Maniloff J., Mayo M. A., McGeoch D. J., Pringle C. R., Wickner R. B., 2000: Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, New York, NY.
- 365. Richter, G. A. , Homer B. L., Moyer S. A., Williams D. S., Scherba G., Tucker S. J., Hall B. J., Pedersen J. C., Jacobson E. R., 1996: Characterization of paramyxoviruses isolated from three snakes. Virus Res. 43 , 77–83. [DOI] [PubMed] [Google Scholar]
- 366. Rodger, H. D. , Kobs M., Macartney A., Frerichs G. N., 1997: Systemic iridovirus infection in freshwater angelfish, Pterophyllum scalare (Lichtenstein). J. Fish Dis. 20 , 69–72. [Google Scholar]
- 367. Rodger, H. D. , Turubull T., Muir F., Millan S., Richards R. H., 1998: Infectious salmon anaemia (ISA) in the United Kingdom. Bull. Eur. Assoc. Fish Pathol. 18 , 115–116. [Google Scholar]
- 368. Rolland, J. B. & Nylund A., 1998: Infectiousness of organic material originating in ISA‐infected fish and transmission of the disease via salmon lice (Lepeophtheirus salmonis). Bull. Eur. Assoc. Fish Pathol. 18 , 173–180. [Google Scholar]
- 369. Ross, K. , McCarthy U., Huntly P. J., Wood B. P., Stuart D., Rough E. I., Smail D. A., Bruno D. W., 1994: An outbreak of viral haemorrhagic septicaemia (VHS) in turbot (Scophthalmus maximus) in Scotland. Bull. Eur. Assoc. Fish Pathol. 14 , 213–213. [Google Scholar]
- 370. Roy, P. & Cleweley J. P., 1978: SVCV RNA and virion‐associated transcriptase activity. J. Virol. 25 , 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Roy, P. , Clark F., Madore H. P., Bishop L., 1975: RNA polymerase associated with virions of pike fry rhabdovirus. J. Virol. 15 , 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Roy, P. , Gupta K. C., Kiuchi A., 1984: Characterization of spring viremia of carp virus mRNA species and the 3′ sequence of the viral RNA. Virus. Res. 1 , 189–202. [Google Scholar]
- 373. Samal, S. K. , McPhillips T. H., Dinan D., Rockemann D. D., 1998: Lack of restriction of growth for aquareovirus in mammalian cells. Arch. Virol. 143 , 571–579. [DOI] [PubMed] [Google Scholar]
- 374. Sandvik, T. , Rimstad E., Mjaaland S., 2000: The viral RNA 3′ and 5′‐end structure and mRNA transcription of infectious salmon anaemia virus resemble those of influenza viruses. Arch. Virol. 145 , 1659–1669. [DOI] [PubMed] [Google Scholar]
- 375. Sano, N. , Moriwake M., Sano T., 1993a: Herpesvirus cyprini: thermal effects on pathogenicity and oncogenicity. Fish Pathol. 28 , 171–175. [Google Scholar]
- 376. Sano, N. , Moriwake M., Hondo R., Sano T., 1993b: Herpesvirus cyprini: a search for viral genome in infected fish by in situ hybridization. J. Fish Dis. 16 , 495–499. [Google Scholar]
- 377. Sano, T. , 1976: Viral diseases of cultured fishes in Japan. Fish Pathol. 10 , 221–226. [Google Scholar]
- 378. Sano, T. , Nishimura T., Okamoto N., Fukuda H., 1976: Isolation of rhabdovirus from European eels (Anguilla anguilla) at Japanese port of entry. Fish Health News 5 , 5–6. [Google Scholar]
- 379. Sano, T. , Fukuda H., Furukawa M., 1985: Herpesvirus cyprini: biological and oncogenic properties. Fish Pathol. 20 , 381–388. [Google Scholar]
- 380. Sano, T. , Yamaki T., Fukuda H., 1988: A novel carp coronavirus, characterization and pathogenicity. International Fish Health Conference, Vancouver, Canada, p. 160.
- 381. Sano, T. , Morita N., Shima N., Akimoto M., 1991: Herpesvirus cyprini: lethality and oncogenicity. J. Fish Dis. 14 , 533–543. [Google Scholar]
- 382. Schumacher, J. , Jacobson E. R., Burns R., Tramontin R. R., 1994a: Adenovirus‐like infection in two rosy boas (Lichanura trivirgata). J. Zoo Wildl. Med. 25 , 461–465. [Google Scholar]
- 383. Schumacher, J. , Jacobson E. R., Homer B. L., Gaskin J. M., 1994b: Inclusion body disease in boid snakes. J. Zoo. Wildl. Med. 25 , 511–524. [Google Scholar]
- 384. Schütze, H. , Mundt E., Mettenleiter T. C., 1999: Complete genomic sequence of viral hemorrhagic septicemia virus, a fish rhabdovirus. Virus Genes 19 , 59–65. [DOI] [PubMed] [Google Scholar]
- 385. Shchelkunov, I. S. & Shchelkunova T. I., 1990: Infectivity experiments with Cyprinus carpio iridovirus (CCIV), a virus unassociated with gill necrosis. J. Fish Dis. 13 , 475–484. [Google Scholar]
- 386. Shchelkunov, I. S. , Skurat E. K., Sivolotskaya V. A., Sapotko K. V., Shimko V. V., Linnik V. Y., 1989: Rhabdovirus anguilla in eel in the USSR and its pathogenicity. All Union Res. Fish. Prod. Soc. 1 , 81–84. [PubMed] [Google Scholar]
- 387. Shchelkunov, I. S. , Karaseva T. A., Kadoshnikov Y. U. P., 1992: Atlantic salmon papillomatosis: visualization of herpesvirus‐like particles in skin growth of affected fish. Bull. Eur. Assoc. Fish Pathol. 12 , 28–29. [Google Scholar]
- 388. Shih, H. H. , 1997: Lethality and pathogenicity of eel herpesvirus in Formosa (EHVF) in eel, Anguilla anguilla . Rep. Fish Dis. Res. 18 , 9–15. [Google Scholar]
- 389. Shih, H. H. , Lu C. C., Chen S. N., 1993: Eel herpesvirus in Formosa: a herpesvirus from cultured Japanese eel. Rep. Fish Dis. Res. 13 , 86–96. [Google Scholar]
- 390. Shortridge, K. F. , 1989: Viruses of reptilia. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 89–104. Springer‐Verlag, Heidelberg.
- 391. Shortridge, K. F. & Oya A., 1984: Arboviruses. In: Hoff, G. L., F. L. Frye, and E. R. Jacobson (eds), Diseases of Amphibians and Reptiles, pp. 107–148. Plenum Press, New York, NY.
- 392. Shortridge, K. F. , Ng M. H., Oya A., Kobayashi M., Munro R., Wong F., Lance V., 1974: Arbovirus infections in reptiles: immunological evidence for a high incidence of Japanese encephalitis virus in the cobra Naja naja . Trans. R. Soc. Trop. Med. Hyg. 68 , 454–460. [DOI] [PubMed] [Google Scholar]
- 393. Sideris, D. C. , 1997: Cloning, expression and purification of the coat protein of encephalitis virus (DIEV) infecting Dicentrarchus labrax . Biochem. Mol. Biol. Int. 42 , 409–417. [DOI] [PubMed] [Google Scholar]
- 394. Smith, A. W. , Skilling D. E., Dardiri A. H., Latham A. B., 1980: Calicivirus pathogenic for swine, a new serotype isolated from opaleye Girella nigricans, an ocean fish. Science 209 , 940–941. [DOI] [PubMed] [Google Scholar]
- 395. Smith, A. W. , Skilling D. E., Barlough J. E., Berry E. S., 1986a: Distribution in the North Pacific Ocean, Bering Sea, and Arctic Ocean of animal populations known to carry pathogenic caliciviruses. Dis. Aquat. Org. 2 , 73–80. [Google Scholar]
- 396. Smith, A. W. , Anderson M. P., Skilling D. E., Barlough J. E., Ensley P. K., 1986b: First isolation of calicivirus from reptiles and amphibians. Am. J. Vet. Res. 47 , 1718–1721. [PubMed] [Google Scholar]
- 397. Smith, A. W. , Skilling D. E., Cherry N., Mead J. H., Matson D. O., 1998: Calicivirus emergence from ocean reservoirs: zoonotic and interspecies movements. Emerging Infect. Dis. 4 , 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Smith, T. G. , Desser S. S., Hong H., 1994: Morphology, ultrastructure and taxonomic status of Toddia sp. in Northern water snakes (Nerodia sipedon sipedon) from Ontario. Can. J. Wildl. Dis. 30 , 169–175. [DOI] [PubMed] [Google Scholar]
- 399. Snow, M. , Cunningham C. O., Melvin T. W., Kurath G., 1999: Analysis of the nucleoprotein gene identifies distinct lineages of viral haemorrhagic septicaemia virus within the European marine environment. Virus Res. 63 , 35–44. [DOI] [PubMed] [Google Scholar]
- 400. Sonstegard, R. A. , 1976: Studies of the etiology and epizootiology of lymphosarcoma in Esox (Esox lucius L., and Esox masquinongy). Prog. Exp. Tumor Res. 20 , 141–155. [DOI] [PubMed] [Google Scholar]
- 401. Sousa, M. A. & Weigl D. R., 1976: The viral nature of Toddia Franca, 1912. Mem. Inst. Oswaldo Cruz 74 , 213–230. [Google Scholar]
- 402. Speare, R. & Smith J. R., 1992: An iridovirus‐like agent isolated from the ornate burrowing frog Limnodynastes ornatus in northern Australia. Dis. Aquat. Org. 14 , 51–57. [Google Scholar]
- 403. Speare, R. , Freeland W. J., Bolton S. J., 1991: A possible iridovirus in erythrocytes of Bufo marinus in Costa Rica. J. Wildl. Dis. 27 , 457–482. [DOI] [PubMed] [Google Scholar]
- 404. Stehbens, W. E. & Johnston M. R. L., 1966: The viral nature of Pirhemocyton tarentolae . J. Ultrastruct. Res. 15 , 543–554. [DOI] [PubMed] [Google Scholar]
- 405. Steinhagen, D. , Kruse P., Neukirch M., 1992: Virus‐associated epidermal hyperplasia in golden ide Leuciscus idus melanotus . Dis. Aquat. Org. 13 , 225–229. [Google Scholar]
- 406. Stingley, R. L. & Gray W. L., 2000: Transcriptional regulation of the channel catfish virus genome direct repeat region. J. Gen. Virol. 81 , 2005–2010. [DOI] [PubMed] [Google Scholar]
- 407. Stone, D. M. , Ahne W., Denham K. L., Dixon P. F., Liu C. T. Y., Sheppard M., Taylor G. R., Way K., 2001: Nucleotide sequence analysis of the glycoprotein gene of putative spring viraemia of carp virus and pike fry rhabdovirus isolates reveals four distinct piscine vesiculovirus species. J. Gen. Virol. in press. [DOI] [PubMed]
- 408. Subramanian, K. & Samal S. K., 1997: Cloning and sequence analysis of a non‐strucutral gene of an aquareovirus. Virus Genes 15 , 83–86. [DOI] [PubMed] [Google Scholar]
- 409. Subramanian, K. , McPhilips T. H., Samal S. K., 1994: Characterization of the polypeptides and determination of genome coding assignments of an aquareovirus. Virology 205 , 75–81. [DOI] [PubMed] [Google Scholar]
- 410. Suzuki, S. , Kimura T., Saneyoshi M., 1986: Characterization of DNA polymerase induced by salmon herpesvirus, OMV. J. Gen. Virol. 67 , 405–408. [DOI] [PubMed] [Google Scholar]
- 411. Suzuki, S. , Yoshimizu M., Saneyoshi M., 1992: Detection of viral DNA polymerase activity in salmon tumour tissue induced by herpes virus, OMV. Acta Virol. 36 , 326–328. [PubMed] [Google Scholar]
- 412. Tapiovaara, H. , Olesen N.‐J., Linden J., Rimaila‐Pärnären E., Von Bonsdorff C.‐H., 1998: Isolation from an iridovirus from pike‐perch Stizostediun lucioperca . Dis. Aquat. Org. 32 , 185–193. [DOI] [PubMed] [Google Scholar]
- 413. Tapiovaara, H. , Paulin L., Ariel E., Huovilainen A., Olesen N.‐J., 2000: Comparison of nucleotide sequences for the major capsid protein of pike‐perch (Stizostedion luciperca), cod (Gadus morhua) and other piscine or amphibian iridovirus isolates. Abstracts of the XIIth International Poxvirus and Iridovirus Symposium, 2–6 September, Montpellier, France, p. 35.
- 414. Teifke, J. P. , Lohr C. V., Marschang R. E., Osterrieder N., Posthaus H., 2000: Detection of chelonid herpesvirus DNA by nonradioactive in situ hybridization in tissues from tortoises suffering from stomatitis‐rhinitis complex in Europe and North America. Vet. Pathol. 37 , 377–385. [DOI] [PubMed] [Google Scholar]
- 415. Telford, S. R. & Jacobson E. R., 1993: Lizard erythrocytic virus in East African chameleons. J. Wildl. Dis. 29 , 57–63. [DOI] [PubMed] [Google Scholar]
- 416. Tham, K. M. & Moon C. D., 1996: PCR amplification of the TK and protein kinase‐related genes of CCV and a putative pilchard herpesvirus. J. Virol. Methods 61 , 65–72. [DOI] [PubMed] [Google Scholar]
- 417. Thiery, R. , Arnault C., Delsert C., 1997: Two isolates of the sea bass, Dicentrarchus labrax L., nervous necrosis virus with distinct genomes. J. Fish Dis. 22 , 201–207. [Google Scholar]
- 418. Thomas, L. A. & Eklund C. M., 1960: Overwintering of Western equine encephalomyelitis virus in experimentally infected garter snakes and transmission to mosquitoes. Proc. Soc. Exp. Biol. 105 , 52–55. [DOI] [PubMed] [Google Scholar]
- 419. Thompson, J. P. , Granoff A., Willis D. B., 1986: Trans‐activation of a methylated adenovirus promoter by a FV‐3 protein. Proc. Natl Acad. Sci. USA 83 , 7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Tidona, C. A. & Darai G., 1997: The complete DNA sequence of LCDV. Virology 230 , 207–216. [DOI] [PubMed] [Google Scholar]
- 421. Tidona, C. A. , Schnitzler P., Kehm R., Darai G., 1998: Is the major capsid protein of iridoviruses a suitable target for the study of viral evolution? Virus Genes 16 , 59–66. [DOI] [PubMed] [Google Scholar]
- 422. Tristem, M. , Myles T., Hill F., 1995: A highly divergent retroviral sequence in the tuatura (Sphenodon). Virology 210 , 206–211. [DOI] [PubMed] [Google Scholar]
- 423. Tristem, M. , Herniou E., Summers K., Cook J., 1996: Three retroviral sequences in amphibians are distinct from those in mammals and birds. J. Virol. 70 , 4864–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Troyer, R. M. , LaPatra S. E., Kurath G., 2000: Gentic analysis reveal unusually high diversity of infectious haematopoietic necrosis virus in rainbow trout aquaculture. J. Gen. Virol. 81 , 2823–2832. [DOI] [PubMed] [Google Scholar]
- 425. Twedell, K. S. , 1989: Herpesviruses: interaction with frog renal cells. In: Ahne, W., and E. Kurstak (eds), Viruses of Lower Vertebrates, pp. 13–29. Springer‐Verlag, Heidelberg.
- 426. Ueno, Y. , Kitao T., Chen S.‐N., Aoki T., Kou G. H., 1992: Characterization of a herpes‐like virus isolated from cultured Japanese eel in Taiwan. Gyobyo Kenkyu 27 , 7–17. [Google Scholar]
- 427. Ueno, Y. , Shi J.‐W., Yoshida T., Kitao T., Sakai M., Chen S.‐N., Kou G. H., 1996: Biological and serological comparison of eel herpesvirus in Formosa (EHVF) and Herpesvirus anguillae (HVA). J. Appl. Ichthyol. 12 , 49–51. [Google Scholar]
- 428. Vanderheijden, N. , Hanson L. A., Thiry E., Martial J. A., 1999: Channel catfish virus gene 50 encodes a secreted, mucin‐like glycoprotein. Virology 257 , 220–227. [DOI] [PubMed] [Google Scholar]
- 429. Vetesi, F. , Dobos‐Kovacs M., Ujhelyi I., Janisch M., Horvath L., 1988: Pockenartiger Ausschlag bei Kaimanen (Caiman sclerops). Int. Symp. Erk. Zoo- Wildtiere 30 , 359–364. [Google Scholar]
- 430. Vieler, E. , Baumgärtner W., Herbst W., Köhler G., 1994: Characterization of a reovirus isolate from a rattle snake, Crotalus viridis, with neurological dysfunction. Arch. Virol. 138 , 341–344. [DOI] [PubMed] [Google Scholar]
- 431. Villoing, S. , Bearzotti M., Chilmonczyk S., Castric J., Bremont M., 2000a: Rainbow trout sleeping disease virus is an atypical alphavirus. J. Virol. 74 , 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Villoing, S. , Castric J., Jeffroy J., Le Ven A., Thiery R., Bremont M., 2000b: An RT‐PCR‐based method for the diagnosis of the sleeping disease virus in experimentally and naturally infected salmonids. Dis. Aquat. Org. 40 , 19–27. [DOI] [PubMed] [Google Scholar]
- 433. Walker, P. J. , Benmansour A., Calisher C. H., Dietzgen R., Fang R. X., Jackson A. O., Kurath G., Leong J. C., Nadin‐Davies S., Tesh R. B., Tordo N., 2000: Family Rhabdoviridae . In: van Regenmortel, M. H. V., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (eds), Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses, pp. 563–583. Academic Press, New York, NY.
- 434. Wang, B. , Ke L. H., Jiang H., Li C. Z., Tien P., 2000: Selection of a specific peptide from a nona‐peptide library for in vitro inhibition of grass carp hemorrhage virus replication. Virus Res. 67 , 119–127. [DOI] [PubMed] [Google Scholar]
- 435. Watson, G. L. , 1993: Herpesvirus in red‐headed (common) agamas (Agama agama). J. Vet. Diagn. Invest. 5 , 444–445. [DOI] [PubMed] [Google Scholar]
- 436. Watson, L. R. , Yun S. C., Groff J. M., Hedrick R. P., 1995: Characteristics and pathogenicity of a novel herpesvirus isolated from adult and subadult white sturgeon Acipenser transmontanus . Dis. Aquat. Org. 22 , 199–210. [Google Scholar]
- 437. Wattanavavijarn, W. , Wattanodorn S., Hunnak P., Tangtrongpiros J., Rattanaphani R., 1986: Viruses of ulcerative diseased fish. Electron Microsc. Soc. Thailand News 3 , 20–23. [Google Scholar]
- 438. Weston, J. H. , Welsh M. D., McLoughlin F., Todd D., 1999: Salmon pancreas disease virus, an alphavirus infecting farmed Atlantic salmon, Salmo salar . Virology 256 , 188–195. [DOI] [PubMed] [Google Scholar]
- 439. Whitney, L. , Jamnback H., Means R. G., Watthews T. H., 1968: Arthropod‐borne‐virus survey in St Lawrence county, New York. Am. J. Trop. Med. Hyg. 17 , 645–650. [DOI] [PubMed] [Google Scholar]
- 440. Whittington, R. J. & Reddacliff L. A., 1995: Influence of environmental temperature on experimental infection of redfin perch, Perca fluviatilis L., and rainbow trout, Oncorhynchus mykiss (Walbaum) with experimental infected epizootic haematopoietic necrosis virus, an Australian iridovirus. Aust. Vet. J. 72 , 421–424. [DOI] [PubMed] [Google Scholar]
- 441. Whittington, R. J. , Kearns C., Speare R., 1997: Detection of antibodies against iridoviruses in the serum of the amphibian Bufo marinus . J. Virol. Methods 68 , 105–108. [DOI] [PubMed] [Google Scholar]
- 442. Williams, J. W. , Tweedell K. S., Sterling D., Marshall N., Christ C., Carlson D. L., McKinnell R. G., 1996: Oncogenic herpesvirus DNA absence in kidney cell lines established from the northern leopard frog Rana pipiens . Dis. Aquat. Org. 27 , 1–4. [Google Scholar]
- 443. Willis, D. B. & Granoff A., 1976: Macromolecular synthesis in cells infected by FV‐3 IV. Regulation of virus‐specific RNA synthesis. Virology 70 , 397–410. [DOI] [PubMed] [Google Scholar]
- 444. Willis, D. B. , Goorha R., Chinchar V. G., 1985: Macromolecular synthesis in cells infected by FV‐3. Curr. Top. Microbiol. Immunol. 116 , 77–106. [DOI] [PubMed] [Google Scholar]
- 445. Winton, J. R. , 1992: Evolution of fish rhabdoviruses. Kimura, T. (ed), Proceedings of the OIJ International Symposium on Salmonid Diseases, Sapporo, Japan, 22–25 October 1991. Hokkaido University Press, Sapporo, Japan.
- 446. Winton, J. R. , Lannan C. N., Ranson D. P., Freyer J. L., 1985: Isolation of a new virus from chinook salmon (Oncorhynchus tshawytscha) in Oregon USA Fish Pathol. 20 , 373–380. [Google Scholar]
- 447. Winton, J. R. , Lannan C. N., Fryer J. L., Hedrick R. P., Meyers T. R., Plumb J. A., Yamamoto T., 1987: Morphological and biochemical properties of four members of a novel group of reoviruses isolated from aquatic animals. J. Gen. Virol. 68 , 353–364. [DOI] [PubMed] [Google Scholar]
- 448. Wise, J. A. , Harrell S. F., Busch R. L., Boyle J. A., 1988: Vertical transmission of CCV. Am. J. Vet. Res. 49 , 1506–1509. [PubMed] [Google Scholar]
- 449. Witte, A. , 1993: Ein Beitrag zum Nachweis von Antikörpern gegen Herpes‐ und Paramyxoviren bei verschiedenen Landschildkröten. PhD dissertation, Gießen Universität, Germany.
- 450. Wolf, K. , 1988: Fish Viruses and Fish Viral Diseases. Cornell University Press, Ithaca, NY.
- 451. Wolf, K. & Darlington R. W., 1971: CCV: a new herpesvirus of ictalurid fish. J. Virol. 8 , 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Wolf, K. & Smith C. E., 1981: Herpesvirus salmonis: pathological changes in parenterally‐infected rainbow trout, Salmo gairdneri Richardson, fry. J. Fish Dis. 4 , 445–457. [Google Scholar]
- 453. Wolf, K. , Snieszko S. F., Dunbar E., 1959: Infectious pancreatic necrosis, a virus‐caused disease of fish. Excerpta. Med. 13 , 228–228. [Google Scholar]
- 454. Yabu T., Hirose H., Hirono I., Katagiri T., Aoki T., Yamamoto E., 1998: Molecular cloning of a novel interferon regulatory factor in Japanese flounder, Paralichthys olivaceus . Mol. Mar. Biol. Biotechnol. 7 , 138–144. [PubMed] [Google Scholar]
- 455. Yamamoto, T. , Kelly R. K., Nielsen O., 1983: Epidermal hyperplasia of northern pike (Esox lucius) associated with herpesvirus and C‐type particles. Arch. Virol. 9 , 255–272. [DOI] [PubMed] [Google Scholar]
- 456. Yao, K. & Vakharia V. N., 1998: Generation of infectious pancreatic necrosis virus from cloned cDNA. J. Virol. 72 , 8913–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Yoshikoshi, K. & Inouye K., 1990: Viral nervous necrosis in hatchery‐reared larvae and juveniles of Japanese parrotfish, Oplegnathus fasciatus (Temminck & Schlegel). J. Fish Dis. 13 , 69–77. [Google Scholar]
- 458. Yoshimizu, M. , Tanaka M., Kimura T., 1988: Histopathological study of tumors induced by OMV infection. Fish Pathol. 23 , 133–138. [Google Scholar]
- 459. Yoshimizu, M. , Fukuda H., Sano T., Kimura T., 1995: SalHV‐2. Epizootiology and serological relationship. Vet. Res. 26 , 486–492. [PubMed] [Google Scholar]
- 460. Yu, Q. , Lu Y., Nerurkar V. R., Yanagihara R., 2000: Amplification and analysis of DNA flanking known sequences of a novel herpesvirus from green turtles with fibropapilloma. Arch. Virol. 145 , 2669–2676. [DOI] [PubMed] [Google Scholar]
- 461. Yu, Y. , Bearzotti M., Vende P., Ahne W., Bremont M., 1999: Partial mapping and sequencing of a fish iridovirus genome. Virus Res. 63 , 53–63. [DOI] [PubMed] [Google Scholar]
- 462. Yun, S. , Hedrick R. P., Wingfield W. H., 1989: A picorna‐like virus from salmonids in California. Am. Fish. Soc. Newsletter 17 , 6–6. [Google Scholar]
- 463. Zangger, N. , Müller M., Pagan O., 1991: Viral dermatitis in the spur‐tailed (Testudo graeca) and the spur‐thighed tortoise (Testudo hermanni) in Switzerland. 4th International Colloquium on Pathology and Medicine of Reptiles and Amphibians, 27–29 September, German Veterinary Association, Gießen, Germany, pp. 17–24.
- 464. Zeigel, R. F. & Clark H. F., 1969: Electron microscopic observations on a ‘C’‐type virus in cell cultures derived from a tumor‐bearing viper. J. Natl Cancer Inst. 43 , 1097–1102. [PubMed] [Google Scholar]
- 465. Zhang, H. G. & Hanson L. A., 1995: Deletion of TK gene attenuates CCV while maintaining infectivity. Virology 209 , 658–663. [DOI] [PubMed] [Google Scholar]
- 466. Zhang, H. G. & Hanson L. A., 1996: Recombinant CCV can express foreign genes and induce antibody production against the gene product. J. Fish Dis. 19 , 121–128. [Google Scholar]
- 467. Zhang, Q. Y. , Li Z. Q., Gui J. F., 1999: Studies on morphogenesis and cellular interactions of Rana grylio virus in an infected fish cell line. Aquaculture 175 , 185–197. [Google Scholar]
- 468. Zhang, Q. Y. , Li Z. Q., Gui J. F., 2000: Isolation of a lethal rhabdovirus from the cultured Chinese sucker Myxocyprinus asiaticus . Dis. Aquat. Org. 10 , 1–9. [DOI] [PubMed] [Google Scholar]
- 469. Zschiesche, W. , Konstantinov A., Ippen R., Mladenov Z., 1988: Lymphoid leukemia with the presence of type C particles in a four‐lined chicken snake (Elaphe obsoleta quadrivittata). Proc. XIII Int. Symp. Erk. Zootiere 30 , 275–277. [Google Scholar]
- 470. Zupanovic, Z. , Lopez G., Hyatt A. D., Shiell B. J., Robinson A. J., 1998: An improved ELISA for detection of anti‐ranavirus antibodies in the serum of giant toad (Bufo marinus). Dev. Comp. Immunol. 22 , 573–585. [DOI] [PubMed] [Google Scholar]


