Abstract
Rationale: The role of FSTL-1 (follistatin-like 1) in lung homeostasis is unknown.
Objectives: We aimed to define the impact of FSTL-1 attenuation on lung structure and function and to identify FSTL-1–regulated transcriptional pathways in the lung. Further, we aimed to analyze the association of FSTL-1 SNPs with lung disease.
Methods: FSTL-1 hypomorphic (FSTL-1 Hypo) mice underwent lung morphometry, pulmonary function testing, and micro–computed tomography. Fstl1 expression was determined in wild-type lung cell populations from three independent research groups. RNA sequencing of wild-type and FSTL-1 Hypo mice identified FSTL-1–regulated gene expression, followed by validation and mechanistic in vitro examination. FSTL1 SNP analysis was performed in the COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) cohort.
Measurements and Main Results: FSTL-1 Hypo mice developed spontaneous emphysema, independent of smoke exposure. Fstl1 is highly expressed in the lung by mesenchymal and endothelial cells but not immune cells. RNA sequencing of whole lung identified 33 FSTL-1–regulated genes, including Nr4a1, an orphan nuclear hormone receptor that negatively regulates NF-κB (nuclear factor-κB) signaling. In vitro, recombinant FSTL-1 treatment of macrophages attenuated NF-κB p65 phosphorylation in an Nr4a1-dependent manner. Within the COPDGene cohort, several SNPs in the FSTL1 region corresponded to chronic obstructive pulmonary disease and lung function.
Conclusions: This work identifies a novel role for FSTL-1 protecting against emphysema development independent of smoke exposure. This FSTL-1–deficient emphysema implicates regulation of immune tolerance in lung macrophages through Nr4a1. Further study of the mechanisms involving FSTL-1 in lung homeostasis, immune regulation, and NF-κB signaling may provide additional insight into the pathophysiology of emphysema and inflammatory lung diseases.
Keywords: chronic obstructive pulmonary disease, SNP, micro–computed tomography, gene expression
At a Glance Commentary
Scientific Knowledge on the Subject
The genetic basis of emphysema is complex, and risk factors for emphysema development remain incompletely defined.
What This Study Adds to the Field
FSTL-1 (follistatin-like 1) hypomorphic mice developed spontaneous emphysema associated with altered immunoregulatory gene expression, including reduced Nr4a1. Exogenous FSTL-1 treatment of macrophages suppressed nuclear factor-κB p65 phosphorylation in an Nr4a1-dependent manner, suggesting that FSTL-1 function in the lung is critical for lung homeostasis. Human SNP analysis of the FSTL1 locus identified a correlation with chronic obstructive pulmonary disease status and lung function.
FSTL-1 (follistatin-like 1) is a 306-amino acid glycoprotein implicated in a myriad of settings, including embryonic development and cardiac and vascular disease and repair, as well as tumorigenesis, metastasis, and inflammation (1–8). A growing body of evidence has illustrated that FSTL-1 plays an important role in lung biology. FSTL-1 is critical for normal lung development by antagonizing BMP4 (bone morphogenetic protein 4) (2), whereas FSTL-1 also promotes pulmonary fibrosis in the bleomycin model (1). FSTL-1 expression has also been implicated in asthma and asthmatic airway remodeling (9–11). Embryonic expression of Fstl1 has identified several lung cell populations producing FSTL-1 (12). However, the role of FSTL-1 in postnatal lung homeostasis is unclear. In vivo models have been limited due to the observation that global germline FSTL-1 knockout mice display a perinatal lethal phenotype associated with multiple lung, skeletal, and urogenital defects (3, 13). Several techniques have been used in murine models to study FSTL-1 function, including neutralizing antibodies, siRNA, adenoviral-gene transfer, and FSTL-1 transgenic models, to begin characterizing in vivo functions of FSTL-1 (1, 2, 4, 5, 7, 14, 15). We had previously used a mouse with reduced Fstl1 gene expression, termed FSTL-1 hypomorphic (FSTL-1 Hypo), to identify that FSTL-1 mediates experimental Lyme arthritis and also influences bone marrow stromal cell transcriptional regulation (16, 17).
In the present study, we sought to characterize the lung phenotype of the FSTL-1 Hypo mouse to better understand the role of FSTL-1 in postnatal lung homeostasis. We identified that FSTL-1 Hypo mice spontaneously developed pulmonary emphysema. To examine the mechanism(s) by which FSTL-1 protects against emphysema, we exposed mice to long-term cigarette smoke and, unexpectedly, found that smoke exposure did not affect FSTL-1–deficient emphysema. FSTL-1–expressing lung cell types were identified by fluorescence-activated cell sorter (FACS)-sorted gene expression and immunohistochemistry. RNA sequencing identified FSTL-1 Hypo mice had reduced antiinflammatory genes and pathways, including Nr4a1, a known inhibitor of LPS-stimulated NF-κB (nuclear factor-κB) signaling in macrophages. After confirming these findings at the RNA and protein expression level in vivo, we identified that exogenous FSTL-1 treatment of macrophages in vitro attenuated NF-κB activation in an Nr4a1-dependent manner. The genetic contribution underlying chronic obstructive pulmonary disease (COPD) and lung disease has been extensively studied in human populations using genome wide association studies (18–21), which enabled analysis of the well-characterized COPDGene (Genetic Epidemiology of COPD) cohort (22). Here, SNPs near the FSTL1 locus were associated with COPD and lung function. Together, our findings reveal a novel role of FSTL-1 in lung homeostasis by identifying FSTL-1–producing and responsive cell types, wherein FSTL-1 suppresses NF-κB signaling in macrophages. This work suggests that FSTL-1 may serve as a therapeutic target in inflammatory lung disease. Some of the results have been previously reported as an abstract (23).
Methods
Animal Experiments
All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine. Animals were housed in a pathogen-free barrier facility with free access to autoclaved water and irradiated pellet food. Female C57Bl/6 (Jackson Laboratories) or FSTL-1 Hypo mice were exposed to room air or cigarette smoke (4 exposures/d, 5 d/wk) beginning at 10 weeks of age, as previously described (24, 25). Briefly, mice were exposed to cigarette smoke with 426 mg of total particulate matter/m3/s for 30 minutes using Kentucky Reference Cigarettes (3R4F) that were obtained from the Tobacco and Health Research Institute of the University of Kentucky. Following smoke exposure, mice were killed by carbon dioxide inhalation, tracheostomized, and lungs were removed and inflated with 10% buffered formalin to a constant pressure of 25 cm H2O for 10 minutes. Lungs were fixed for 24 hours in formalin before embedding in paraffin. Serial midsagittal sections were obtained and stained with modified Gill’s stain. Using Scion Image software (Version 4.0.2; Scion Corp.), mean alveolar linear intercept was calculated using 8 randomly selected 200× fields per slide (24, 26). Airway and vascular structures were manually masked to exclude them from the analysis.
Lung Imaging
In vivo micro–computed tomography acquisition
Respiration-gated in vivo micro–computed tomography (micro-CT) imaging was performed with Siemens Inveon Multi‐modality micro-CT-SPECT-PET system with the following parameters: full rotation, 720 projections, high magnification, 2 × 2 binning, effective pixel size of 22.48 μm, transaxial field of view 43.89 mm with 3,904 pixels, axial field of view 34.54 mm with 3,072 pixels, 80 kV of voltage, a current of 400 μA, and an exposure time of 1,500 milliseconds. The three-dimensional (3D) micro-CT images were reconstructed using the Feldkamp reconstruction algorithm and were calibrated to read out in Hounsfield units, with distilled and deionized water set to 0 HU, whereas air was set to −1000 HU.
Micro-CT Image Analysis
The 3D micro-CT image stacks were analyzed using the Inveon Research Workplace. The region of interest (ROI) analysis function was used with a thresholding tool to create several ROIs of different Hounsfield units. First, a cylindrical 3D ROI was drawn around the body cavity to encompass the entire chest cavity. Next, all the external air around the mouse was excluded from the ROI. A threshold of −800 HU was applied to the whole stack, and the whole-lung volume and mean lung density were calculated. The mouse lung density typically ranges from −840 to −910 HU.
Lung Gene Expression Analysis and Tabula Murina Data
The Lung Gene Expression Analysis (LGEA) Web Portal was identified as a multiplatform web-based application for gene expression (https://research.cchmc.org/pbge/lunggens/mainportal.html). From the Lung Sorted Cells tool, a single-gene query for Fstl1 of Mouse Major Cell Types from Postnatal Day 28, as well as data from a single-gene query for FSTL1 of Major Cell Types from 20-month-old human lung, was acquired on March 14, 2019.
The Tabula Muris was identified as a web-based application for single-cell gene expression (https://tabula-muris.ds.czbiohub.org). From the Visualization section, we queried the Gene “Fstl1” of “Lung” Tissue by the “FACS” Method on April 16, 2019.
FSTL-1 SNPs in COPDGene
We analyzed genotypic data extracted from the COPDGene dataset. Details on COPDGene study design and methods, including COPD phenotyping and cohort genotyping, have been previously described in detail (19, 22). For our analysis, we focused on 356 SNPs located in a 76.8 kb region in the human FSTL1 locus on chromosome 3 (±10 kb flanks). Our outcomes were COPD affection status (COPD yes/no), post-bronchodilator FEV1% predicted, and post-bronchodilator FEV1/FVC. We analyzed associations between SNPs and outcomes in non-Hispanic white participants, separately for ex-smokers and for current smokers. We used logistic or linear regression as appropriate, under additive coding. All models were adjusted for age, sex, and the genotype principal components (to account for population stratification). Models were additionally adjusted for pack-years (for the analyses in smokers) and for COPD case/control status (for the analyses of FEV1% predicted and FEV1/FVC).
Statistical Analysis
Investigators were not blinded to treatment but were blinded to individual/group during data analysis. Experimental group size is noted in the figure legends, and each experiment was repeated at least once to ensure reproducibility. All in vivo and in vitro statistical analyses were performed using Prism 7 (GraphPad). Briefly, all data are presented with mean ± SEM. Studies comparing two groups were analyzed by two-sided Student’s t tests. Studies comparing more than two groups were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparisons. All statistical analyses considered P < 0.05 significant.
Additional details of the methods employed for this work are described in detail in the online supplement.
Results
FSTL-1 Attenuation Results in Smoke-Independent Emphysema
FSTL-1 Hypo mice were generated as previously described (17) and backcrossed more than 10 generations onto a C57Bl/6 background. We observed that FSTL-1 Hypo mice had increased airspace size consistent with emphysema. We next employed the chronic cigarette smoke exposure model to further characterize the FSTL-1–deficient emphysema phenotype. To our surprise, FSTL-1–dependent emphysema was not exacerbated by cigarette smoke exposure, though smoke-exposed wild-type (WT) C57Bl/6 mice developed increased airspace, as expected (Figures 1A and 1B). Given the histologic evidence of emphysema in FSTL-1 Hypo mice exposed only to room air, we assessed pulmonary function by forced oscillatory measurements (flexiVent; Scireq) in the absence of smoke exposure. Consistent with lung histology, FSTL-1 Hypo mice had increased lung compliance, decreased tissue elastance, decreased hysteresis, and decreased tissue resistance when compared with WT animals (Figures 1C–1F). Representative pressure–volume loops showed a leftward/upward deflection in FSTL-1 Hypo illustrative of emphysematous lung physiology (Figure 1G). To assess lung structure in vivo, FSTL-1 Hypo mice and WT controls underwent micro-CT scan (see Figure E1 in the online supplement). Using respiratory gating, we observed that FSTL-1 Hypo mice had increased lung volumes and decreased lung density (Figures 1H and 1I). Together, these findings suggested that reduced FSTL-1 expression was sufficient to cause mice to develop histologic, functional, and radiographic findings consistent with pulmonary emphysema, independent of chronic cigarette smoke exposure. We additionally queried the Gene Expression Omnibus Database (GSE6591) to determine how aging, a major contributor to emphysema, affected lung Fstl1 expression, which showed that C57Bl/6 and DBA/2 mice have reduced Fstl1 in aged mice (see Figure E2) (27).
Figure 1.
FSTL-1 (follistatin-like 1) attenuation results in smoke-independent emphysema. (A) Representative images from wild-type (WT) and FSTL-1 hypomorphic (FSTL-1 Hypo) mice exposed to room air or smoke for 6 months. (B) Mean linear intercept from WT and FSTL-1 Hypo mice exposed to room air or smoke (n = 7 per group from one of two representative experiments). (C–G) Lung function measurements of WT and FSTL-1 Hypo room air–exposed mice, to determine lung compliance (C), tissue elastance (D), hysteresis (E), tissue resistance (F), and representative pressure–volume loops (G) from WT and FSTL-1 Hypo mice (n = 10–11 per group from two combined experiments). (H and I) Micro–computed tomography scan measurements of mean lung volume (H) and mean lung density (I) in WT and FSTL-1 Hypo mice (n = 7 per group from two combined experiments). *P < 0.05, ***P < 0.001, and ****P < 0.0001.
FSTL-1 Expression in Postnatal Mouse Lung
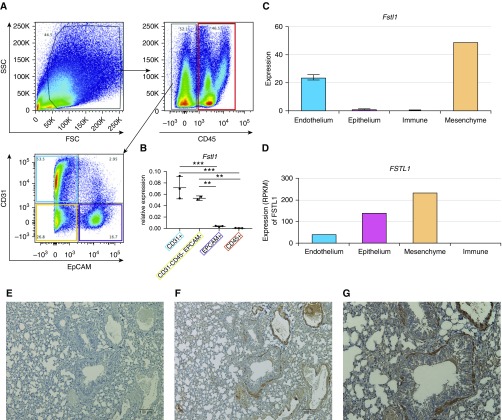
We next sought to investigate the cellular expression of Fstl1 in the lung to identify potential cellular sources of FSTL-1. A single-cell suspension was prepared from the lungs of C57Bl/6 mice and were sorted into four groups based on surface expression of CD45 (cluster of differentiation 45), epithelial cell adhesion molecule (EpCAM), and CD31: immune cells (CD45+EpCAM−CD31−), epithelial cells (CD45−EpCAM+CD31−), endothelial cells (CD45−EpCAM−CD31+), and mesenchymal cells (CD45−EpCAM−CD31−) (Figure 2A). Sorted cell populations were assessed for gene expression of the surface markers used for sorting Cd45, Cd31, and Epcam (see Figure E3), confirming that our sorting strategy isolated the intended populations. Interestingly, Fstl1 was highly expressed in endothelial cells as well as mesenchymal cells, both significantly more so than epithelial cells and immune cells (Figure 2B). To confirm our experimental findings, we analyzed data from the LGEA Web Portal, an interactive web-based tool for lung cell gene expression (28, 29). In sorted murine lung cells at Postnatal Day 28, Fstl1 was most highly expressed in “endothelium” and “mesenchyme” cells (Figure 2C), consistent with our FACS-sorted data. To determine whether murine Fstl1 expression correlated with human lung cell FSTL1 expression, we again analyzed LGEA data from 20-month-old human lung tissue, which identified FSTL1 expression in endothelium, epithelium, and, most highly, in mesenchyme cells (Figure 2D). To additionally verify our findings, we analyzed single-cell transcriptomics from Tabula Muris, a web-based tool for single-cell gene expression from 20 murine organs (30). Upon querying for Fstl1 by the FACS Method for Lung Tissue, we again noted high expression within “stromal cell” and “lung endothelial cell” populations (see Figure E4). Notably, in each analysis, very little, if any, FSTL-1 gene transcript was detected in immune cells. We next assessed FSTL-1 protein expression in murine lung tissue by immunohistochemistry. Compared with isotype control (Figure 2E), anti–FSTL-1 staining was strongly detectable in endothelium, airway smooth muscle, and weakly to moderately detected in interstitial and epithelial cells at 40× (Figure 2F) and 80× (Figure 2G). Together, these observations identify endothelial and mesenchymal cells as the primary producers of FSTL-1 in the postnatal lung.
Figure 2.
FSTL-1 (follistatin-like 1) expression in the lung. (A) Wild-type mice had whole-lung single-cell preparations made, followed by fluorescence-activated cell sorting based on the gating strategy, based on forward scatter (FSC), side scatter (SSC), CD45 (cluster of differentiation 45), epithelial cell adhesion molecule (EpCAM), and CD31. (B) Fstl1 gene expression from lung cell populations sorted by CD31+ (endothelium), CD45+ (leukocytes), EpCAM+ (epithelium), and CD31−CD45−EpCAM− (mesenchyme) (n = 3 mice from one of two representative experiments). (C) Fstl1 gene expression from sorted Postnatal Day 28 murine lung cells. (D) FSTL1 gene expression from sorted 20-month-old human lung cells. (E–G) Immunohistochemistry for FSTL-1 of wild-type paraffin-embedded lung tissue stained with isotype antibody (Ab) at 10× (E), anti–FSTL-1 Ab at 10× (F), and anti–FSTL-1 Ab at 20× (G). **P < 0.01 and ***P < 0.001. RPKM = reads per kilobase of transcript per million mapped reads.
FSTL-1–Dependent Gene Expression in the Lung
To investigate the mechanism(s) involved in leading FSTL-1 Hypo mice to develop emphysema, we performed RNA sequencing on 20-week-old WT and FSTL-1 Hypo mice with and without smoke exposure (Figure 3A). This identified 33 significantly differentially expressed genes between WT and FSTL-1 Hypo mice irrespective of the presence of smoke exposure (controlling the false discovery rate [FDR] at 0.10), including Fstl1 as a positive control (P = 1.41 × 10−13). Notably, several gene regulation and macrophage antiinflammatory genes were differentially expressed in an FSTL-1–dependent manner, including the nuclear orphan receptor Nr4a1, also known as Nur77 (P = 4.45 × 10−5; see Table E1). Confirming our experimental approach, smoke exposure (compared with room air) in WT mice was associated with the differential expression of 354 genes (FDR < 0.10; see Table E1); within the top 50 (FDR < 0.004), there was upregulation of 44 genes, including Cyp1b1, Gpx2, and Aldh3a1, transcripts elicited by smoke exposure, and downregulation of 6 genes, including Hhip, a transcript protective against emphysema development (see Figure E5) (31, 32). Gene set enrichment analysis of the FSTL-1–dependent differentially expressed genes between WT and FSTL-1 Hypo mice identified 41 enriched signature pathways (FDR < 0.2; Figure 3B); these included gene regulatory, protein folding, innate immune, and cellular kinase regulation pathways.
Figure 3.
FSTL-1 (follistatin-like 1)-dependent differential gene expression in the lung. (A and B) Heatmap display of individual genes (A) and gene set enrichment analysis of RNA-sequencing analysis (B) of differential gene expression between wild-type and FSTL-1 Hypo mice with and without cigarette smoke exposure (n = 4 mice per group from one experiment). Hypo = hypomorphic.
FSTL-1 Attenuation Is Associated with Decreased Nr4a1/Nur77 Expression in Distinct Lung Immune Cells
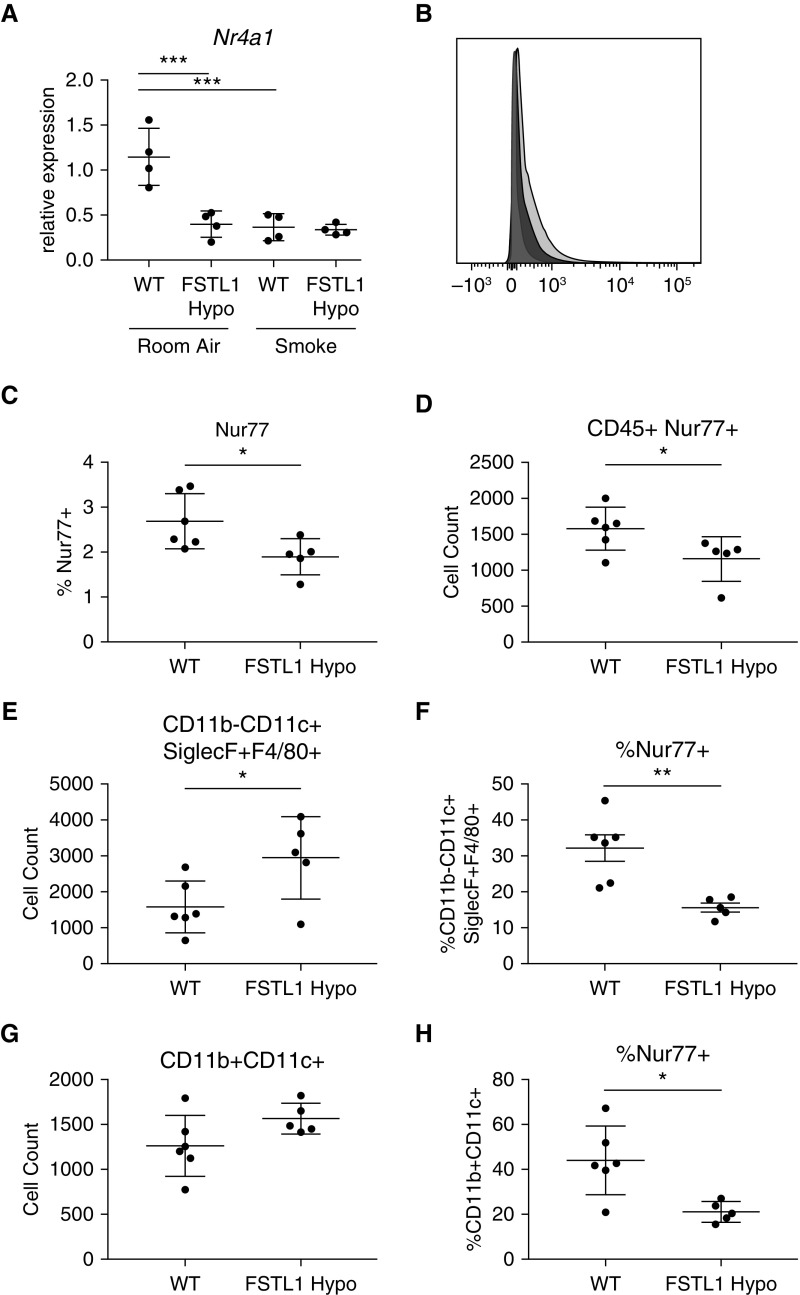
We next evaluated gene expression by qRT-PCR and found significantly decreased Nr4a1 gene expression in FSTL-1 Hypo mice, as well as smoke-exposed WT mice, when compared with room air WT mice (Figure 4A). We next sought to identify lung cell populations differentially expressing Nr4a1 in room air. By FACS, FSTL-1 Hypo mice had decreased total Nur77+ expression in the lung of FSTL-1 Hypo mice and specifically within the CD45+ population (Figures 4B–4D). Within the CD45+ population, FSTL-1 Hypo mice had increased CD11c+F4/80+SiglecF+CD11b− cells (alveolar macrophages), wherein these cells had reduced Nur77 positivity (Figures 4E and 4F). Additionally, the CD45+ population had increased CD11b+CD11c+ cells, which also revealed reduced Nur77+ status (Figures 4G and 4H). Together, this illustrated that reduced FSTL-1 expression is associated with decreased Nr4a1 expression and Nur77+ staining within the lung, which corresponded with increases in myeloid cell abundance and reduced myeloid Nur77 positivity, suggesting that FSTL-1 may act directly on macrophages to influence Nr4a1/Nur77 function.
Figure 4.
FSTL-1 (follistatin-like 1) attenuation is associated with decreased Nr4a1/Nur77 expression in distinct lung populations. (A) Nr4a1 expression in murine lung of wild-type (WT) and FSTL-1 hypomorphic (FSTL-1 Hypo) mice exposed to room air or smoke (n = 4 per group from one representative experiment). (B) Representative histogram of murine lung staining by fluorescence-activated cell sorter using anti–Nur77 antibody in WT (light gray) or FSTL-1 Hypo (dark gray) compared with isotype antibody (silhouette gray). (C and D) Fluorescence-activated cell sorter staining of WT and FSTL-1 Hypo mice from selected populations identified decreased total Nur77+ lung cells (C) and CD45+ (cluster of differentiation 45+) Nur77c+ (D) in FSTL-1 Hypo mice. Both (E and F) CD11b−CD11c+SiglecF+F4/80+ (alveolar) macrophages and (G and H) CD11b+CD11c+ cells revealed (F and H) decreased Nur77 positivity (n = 5–6 per group from one representative experiment). *P < 0.05, **P < 0.01, and ***P < 0.001.
Exogenous FSTL-1 Treatment of Macrophages Reduces NF-κB p-p65 via Nr4a1
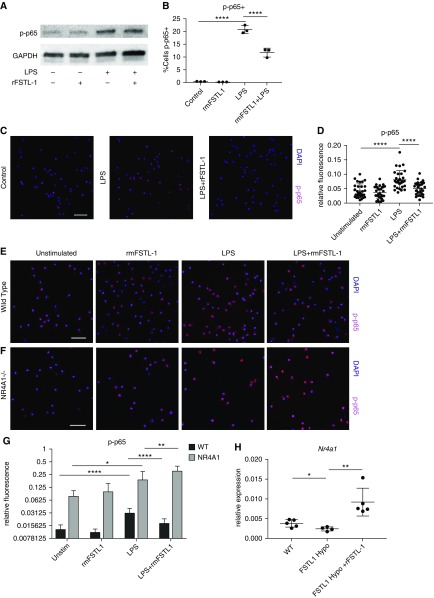
Nr4a1/Nur77 has been shown to suppress proinflammatory cytokine production in macrophages via decreased NF-κB activity in the context of LPS stimulation by associating with the p65 subunit, preventing its phosphorylation, nuclear translocation, and interaction with the κB element (33, 34). Thus, to examine a causal relationship between FSTL-1 and Nur77, we used the RAW 264.7 murine macrophage cell line to assess NF-κB activity as determined by LPS responsive NF-κB p65 phosphorylation (p-p65). Here we found that recombinant FSTL-1 (rFSTL-1) protein treatment of RAW cells attenuated LPS-stimulated p-p65, as shown by Western blot (Figure 5A). FACS analysis of RAW 264.7 cells showed that rFSTL-1 reduced the percentage of LPS-stimulated p-p65+ cells (Figure 5B). Further, confocal immunofluorescence microscopy also found that LPS treatment increased p-p65 in RAW 264.7 cells but that rFSTL-1 reduced LPS-stimulated p-p65 intensity (Figures 5C and 5D). In comparison, cigarette smoke extract failed to stimulate p-p65 except at very high concentrations (100 μg/ml) (see Figures E6A and E6B), in contrast to prior studies using cigarette smoke extract in macrophages (35). At this high dose, rFSTL-1 did not attenuate p-p65 (see Figure E6C), which is consistent with our in vivo observation that FSTL-1 Hypo emphysema is not impacted by cigarette smoke exposure.
Figure 5.
Exogenous FSTL-1 (follistatin-like 1) treatment of macrophages reduces nuclear factor-κB p65 phosphorylation (p-p65) via Nr4a1. Exogenous FSTL-1–treated RAW 264.7 macrophages had decreased nuclear factor-κB p-p65 following LPS stimulation as assessed by (A) Western blot and (B) fluorescence-activated cell sorter (n = 3 per treatment). Confocal immunofluorescence of RAW 264.7 for p-p65 at 40× magnification shows (C) representative images and (D) quantification of p-p65 fluorescence (per cell) following no treatment (control), LPS-treatment (LPS), and LPS +rFSTL-1. (E–G) Confocal immunofluorescence at 40× magnification for p-p65 of primary alveolar macrophages from wild-type (WT) (E) and Nr4a1−/− (F) mice with fluorescence quantification (G) (from one representative experiment). (H) Nr4a1 gene expression of WT, FSTL-1 hypomorphic (Hypo), and rFSTL-1–treated FSTL-1 Hypo lung (n = 4–5 per group from one representative experiment). (C, E, and F) Scale bars, 50 μm. *P < 0.05, **P < 0.01, and ****P < 0.0001. rFSTL-1 = recombinant FSTL-1; rm = recombinant murine; Unstim = unstimulated.
To determine if Nr4a1 is required for FSTL-1–attenuated p-p65 following LPS stimulation, we next studied primary murine alveolar macrophages from WT and Nr4a1−/− mice (Nr4a1 knockout) by confocal immunofluorescence. WT alveolar macrophages had very little p-p65 positivity under baseline or rFSTL-1–treated conditions. However, following LPS-stimulation, p-p65 staining was significantly increased, whereas this increase was significantly attenuated under LPS + rFSTL-1 conditions (Figures 5E and 5G). In Nr4a1−/− alveolar macrophages, some p-p65 was detectable in control and rFSTL-1–only conditions, which LPS stimulation predictably increased. Remarkably, rFSTL-1 treatment of Nr4a1−/− alveolar macrophages failed to reduce p-p65 levels (Figures 5F and 5G). Together, these observations suggested that rFSTL-1 attenuates NF-κB p-p65 in macrophages via Nr4a1/Nur77. To determine if exogenous FSTL-1 could increase Nr4a1 expression in vivo, we administered rFSTL-1 daily to FSTL-1 Hypo mice, wherein we observed that rFSTL-1 significantly increased Nr4a1 expression (Figure 5H), suggesting that extracellular FSTL-1 acts within the lung microenvironment to regulate Nr4a1.
To explore a functional consequence of this Nr4a1-mediated protective mechanism requiring FSTL-1, we noted that in unique cell populations both Nr4a1 and FSTL-1 have been shown to influence cell survival via apoptosis (36–41). Therefore, we reasoned that lung cell apoptosis, a known cause of emphysema development (42–47), could explain the observed FSTL-1 Hypo phenotype. We tested the hypothesis that endothelial or epithelial cell apoptosis would explain the FSTL-1 Hypo phenotype by performing FACS analysis of murine lung tissue to identify cell-specific apoptosis in the lung, using the markers CD45, EpCAM, and CD31: immune cells (CD45+EpCAM−CD31−), epithelial cells (CD45−EpCAM+CD31−), endothelial cells (CD45−EpCAM−CD31+), and mesenchymal cells (CD45−EpCAM−CD31−), paired with Annexin V/7−AAD staining to identify apoptotic cells (Annexin V+/7−AAD−). We observed that FSTL-1 Hypo mice had no difference in the percentage of apoptosis in endothelial, epithelial, or mesenchymal cells but did have greater apoptosis in CD45+ cells (see Figure E7). We then determined that, within the lung, distinct lung immune cell populations had increased apoptosis in FSTL-1 Hypo mice, though notably not alveolar macrophages (CD11b−/CD11c+/SiglecF+) cells. However, CD11b+/CD11c+ and CD11b+/CD11c− cells showed increased apoptosis in the FSTL-1 Hypo lung (see Figure E8).
SNPs of the Human FSTL-1 Locus Are Associated with COPD and Lung Function
We next sought to explore the role of FSTL-1 in the clinical context of human emphysema. As shown in Figure 2G, FSTL1 is expressed within the human lung in distinct cell populations. Noting that Fstl1 germline deletion is incompatible with survival in the mouse, we suspected that, in humans, nonsynonymous or nonsense FSTL1 mutations would be uncommon. Thus, we queried the Exome Aggregation Consortium database, a catalog of protein-coding gene variants for 60,706 (mostly healthy) individuals. Using Exome Aggregation Consortium, Lek and colleagues (48) determined the probability of loss-of-function intolerance (pLI) for each gene (pLI > 0.9 denotes haploinsufficiency). FSTL1 had a pLI = 0.96, identifying strong evolutionary selection against FSTL1 haploinsufficiency.
To investigate whether FSTL1 SNPs were associated with COPD-related phenotypes, we analyzed genotype data from non-Hispanic white participants in the COPDGene project, focusing on 356 SNPs within 10 kb flanking the FSTL1 locus (see Table E1). Notably, among non-current smokers, we identified 71 FSTL1 SNPs associated with COPD disease status at P < 0.05, with the lowest P = 0.0012 for reference SNP (rs)2488 (Figure 6A). Likewise, we found 49 SNPs associated with FEV1/FVC (lowest P = 0.0037 for rs13326852; Figure 6B) and 44 SNPs associated with FEV1% predicted (lowest P = 0.0092 for rs11712887; Figure 6C). In contrast, the same analyses among smokers yielded 27 SNPs for disease status (lowest P = 0.012), 1 for FEV1 (lowest P = 0.04), and 10 for FEV1/FVC (lowest P = 0.01) (see Table E1). Recently, two large meta genome-wide association studies for COPD and lung function were published (20, 21). Here we found that rs2488, (the most significant SNP for COPD disease status in COPDGene) showed nominal significance for COPD (P = 0.009548) and FEV1 (P = 0.01), though not FEV1/FVC (P = 0.49). Together, these data suggest that FSTL-1 loss-of-function mutations are poorly tolerated in humans and that genetic polymorphisms in the FSTL1 locus may influence COPD and lung function in a subset of individuals.
Figure 6.
SNPs in the FSTL1 locus are associated with emphysema risk status. COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) analysis of 356 SNPs in proximity to the FSTL1 gene locus identified that within non-smoking, non-Hispanic whites there were (A) 71 SNPs associated with chronic obstructive pulmonary disease affectation status, (B) 49 SNPs associated with FEV1/FVC, and (C) 44 SNPs associated with FEV1% predicted. Horizontal line represents P < 0.05.
Discussion
Several studies have identified FSTL-1 to be essential in mammalian biology, from embryonic development, tissue repair, and paracrine signaling to maintaining tissue homeostasis. FSTL-1 been shown to both mediate inflammation and to protect against inflammation in different models of disease, including systemic infection and autoimmunity. Within the lung, little is known about the homeostatic function of FSTL-1, though its expression during development is essential for normal branching morphogenesis. In an FSTL-1 haplosufficient mouse model, FSTL-1 was shown to promote a fibrotic response to bleomycin that was driven by myofibroblast expression of FSTL-1, whereas a chronic ovalbumin-driven asthma model demonstrated that FSTL-1 mediates eosinophilic airway remodeling in part through macrophages. The current study examined the postnatal consequences of reduced FSTL-1 expression in the absence of experimental insult. Surprisingly, FSTL-1 Hypo mice developed spontaneous pulmonary emphysema as determined using histologic, functional, and radiographic approaches, standard clinical metrics of emphysema, thus confirming a critical role for FSTL-1 in maintaining lung homeostasis. Previous studies have identified several specific lung cell types that can produce FSTL-1 during embryonic development, including mesenchymal cells, vascular endothelial cells, and smooth muscle (12). However, postnatal mammalian lung cell expression of Fstl1 has not been characterized. The current study identified that, by FACS sorting whole lung, several cell types express Fstl1, including high levels in endothelial and mesenchymal cells, whereas epithelial cells also express Fstl1. Contrasting the ovalbumin–asthma model, wherein macrophage-specific deletion of Fstl1 attenuated airway remodeling (10), in the present study, CD45+ cells recovered from the naïve lung had no detectable Fstl1 transcript. Our findings were confirmed by data from the LungMAP investigators, Tabula Muris investigators, and the embryonic lung as determined by FSTL-1–reporter mice, wherein FSTL-1 was found to be expressed most highly in endothelial cells and smooth muscle cells and to a select population of CCSP+ cells and Type II alveolar sac cells but not PDGFRa+ (platelet-derived growth factor α–positive) fibroblasts (12). These results suggest that finer resolution of FSTL-1 expression in the lung, specifically in the dynamic lung macrophage populations that are recruited during chronic inflammation, may help refine the understanding of cell-intrinsic and extrinsic expression of FSTL-1.
The observation that FSTL-1 Hypo mice developed spontaneous pulmonary emphysema suggested that novel pathways mediating pulmonary emphysema could be identified. Given the global impact of smoke exposure in emphysema and COPD, we employed the gold-standard model of cigarette smoke–induced emphysema and were surprised to find that smoke exposure did not impact the severity of emphysema in FSTL-1 Hypo mice. Although this model has been invaluable to studying mediators of emphysema development, our findings suggested that FSTL-1–dependent emphysema and smoke-dependent emphysema may have divergent mechanisms. Our unbiased gene expression analysis identified a limited set of genes that were associated with FSTL-1 expression, none of which have previously been shown to mediate pulmonary emphysema development. Though annotated pathways associated with FSTL-1–dependent gene sets were limited, it was notable that several macrophage antiinflammatory genes were differentially expressed, suggesting that FSTL-1 may influence macrophage tissue homeostasis or signaling to constrain emphysema development, including Nr4a1.
The orphaned nuclear receptor Nr4a1/Nur77 is expressed in several cell types, including macrophages, lymphocytes, and lung epithelial cells. In macrophages and lung epithelial cells population, Nur77 limits proinflammatory signaling by binding to the NF-κB p65 subunit to inhibit its phosphorylation, subsequent nuclear translocation, and proinflammatory transcriptional program activation (33, 49). The finding that FSTL-1 Hypo mice have reduced Nr4a1 expression and reduced Nur77 positive cells, coupled with the lack of Fstl1 expression in CD45+ cells, suggested that FSTL-1 could act on macrophages to maintain Nur77 inhibition of proinflammatory pathways. Indeed, our findings identify that FSTL-1 acts on lung macrophages, and the finding that exogenous rFSTL-1 treatment of macrophages attenuated LPS-induced NF-κB p-p65 was Nr4a1-dependent identified a causal relationship between FSTL-1, Nr4a1, and NF-κB activation. Although the role of Nr4a1 in pulmonary emphysema is unknown, Nr4a1 has previously been shown to mediate noninflammatory (tolerogenic) macrophage handling of apoptotic lung cells. Several studies have identified Nr4a1 as an essential transcriptional and metabolic regulator in immune cells, including macrophages, that represses inflammation (50–52). The precise nature of an FSTL-1/Nr4a1/NF-κB pathway in emphysema/COPD remains to be defined, and further investigation is needed to determine what role Nr4a1 plays in emphysema development, including if enhanced Nr4a1 function/expression can rescue the FSTL-1 Hypo phenotype or other models of emphysema, such as chronic cigarette smoke exposure.
Because haploinsufficient variants in FSTL-1 are selected against in the human population, protein-coding FSTL1 SNPs may be under-represented in the COPD study populations. How intolerance of FSTL1 loss-of-function in the human population impacts SNP abundance in distinct disease states remains unclear. Our analysis identifies SNPs surrounding the FSTL1 locus that were associated with COPD affectation status and lung function among non-smokers in the well-characterized COPDGene cohort, further supported by the nominal significance of our top SNP in larger genome-wide association study data. This work represents the first description of FSTL1 gene polymorphisms associated with human lung disease, supporting our in vivo observations.
Conclusions
The present study identifies FSTL-1 as a previously unrecognized monogenic cause of spontaneous pulmonary emphysema in a mouse model. Further, by identifying Fstl1-expressing lung cell populations and FSTL-1–dependent genes, we observed a role for FSTL-1 in promoting immune cell tolerance. Mechanistically, FSTL-1 directly influences NF-κB signal transduction in macrophages via modulation of the inflammation repressor Nr4a1/Nur77, providing insight into candidate molecular mediators of FSTL-1 signaling. Further exploration into the role of FSTL-1 and Nr4a1 in lung homeostasis, inflammation, and immune function is needed to enhance understanding of the therapeutic potential of FSTL-1 in lung diseases.
Supplementary Material
Footnotes
Supported by NIH grants F30AI114146 (T.E.), R01HL107380 (J.F.A.), R01AI073556 and T32AR052282 (R.H), R35HL139930 (J.K.K.), and K08HL128809 (B.T.C.); by the Flight Attendant Medical Research Institute (A.D.G); and in part by the Children’s Hospital of Pittsburgh of UPMC Health System (B.T.C).
Author Contributions: M.H., J.P., T.E., and B.T.C. conceived, designed, and executed studies; analyzed and interpreted results; and drafted and revised the manuscript globally. A.D.G. and S.D.S. designed, executed, and interpreted smoke and lung morphometry studies. E.F., M.H.C., and J.C.C. designed, executed, and interpreted SNP analysis studies. A.R.T. executed in vitro macrophage studies. W.H. and D.K. performed and analyzed RNA sequencing studies. N.S. and Y.W. conducted live animal imaging studies. J.F.A. executed and interpreted pulmonary function testing. K.S.R. executed and interpreted confocal microscopy. R.H. conceived and designed the follistatin-like 1 hypomorphic mouse and interpreted in vivo studies. J.K.K. conceived studies, interpreted results, and revised the manuscript. All authors have given final approval for publication and agree to be accountable for the integrity of the information contained in this manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201905-0973OC on December 13, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med. 2015;212:235–252. doi: 10.1084/jem.20121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci USA. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sylva M, Li VS, Buffing AA, van Es JH, van den Born M, van der Velden S, et al. The BMP antagonist follistatin-like 1 is required for skeletal and lung organogenesis. PLoS One. 2011;6:e22616. doi: 10.1371/journal.pone.0022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylva M, Moorman AF, van den Hoff MJ. Follistatin-like 1 in vertebrate development. Birth Defects Res C Embryo Today. 2013;99:61–69. doi: 10.1002/bdrc.21030. [DOI] [PubMed] [Google Scholar]
- 5.Umezu T, Yamanouchi H, Iida Y, Miura M, Tomooka Y. Follistatin-like-1, a diffusible mesenchymal factor determines the fate of epithelium. Proc Natl Acad Sci USA. 2010;107:4601–4606. doi: 10.1073/pnas.0909501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams D, Larman B, Oxburgh L. Developmental expression of mouse Follistatin-like 1 (Fstl1): dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns. 2007;7:491–500. doi: 10.1016/j.modgep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–2516. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller M, Esnault S, Kurten RC, Kelly EA, Beppu A, Das S, et al. Segmental allergen challenge increases levels of airway follistatin-like 1 in patients with asthma. J Allergy Clin Immunol. 2016;138:596–599, e4. doi: 10.1016/j.jaci.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller M, Beppu A, Rosenthal P, Pham A, Das S, Karta M, et al. Fstl1 promotes asthmatic airway remodeling by inducing oncostatin M. J Immunol. 2015;195:3546–3556. doi: 10.4049/jimmunol.1501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Liu Y, Miller M, Cao L, Zhao J, Wu J, et al. Autophagy plays a role in FSTL1-induced epithelial mesenchymal transition and airway remodeling in asthma. Am J Physiol Lung Cell Mol Physiol. 2017;313:L27–L40. doi: 10.1152/ajplung.00510.2016. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Liu Y, Yang Z, Ning W. Cell type specific expression of Follistatin-like 1 (Fstl1) in mouse embryonic lung development. J Mol Histol. 2018;49:399–409. doi: 10.1007/s10735-018-9780-5. [DOI] [PubMed] [Google Scholar]
- 13.Chaly Y, Blair HC, Smith SM, Bushnell DS, Marinov AD, Campfield BT, et al. Follistatin-like protein 1 regulates chondrocyte proliferation and chondrogenic differentiation of mesenchymal stem cells. Ann Rheum Dis. 2015;74:1467–1473. doi: 10.1136/annrheumdis-2013-204822. [DOI] [PubMed] [Google Scholar]
- 14.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234–239. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang X, Hu Q, Li B, McBride D, Bian H, Spagnoli P, et al. Follistatin-like 1 attenuates apoptosis via disco-interacting protein 2 homolog A/Akt pathway after middle cerebral artery occlusion in rats. Stroke. 2014;45:3048–3054. doi: 10.1161/STROKEAHA.114.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campfield BT, Eddens T, Henkel M, Majewski M, Horne W, Chaly Y, et al. Follistatin-like protein 1 modulates IL-17 signaling via IL-17RC regulation in stromal cells. Immunol Cell Biol. 2017;95:656–665. doi: 10.1038/icb.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campfield BT, Nolder CL, Marinov A, Bushnell D, Davis A, Spychala C, et al. Follistatin-like protein 1 is a critical mediator of experimental Lyme arthritis and the humoral response to Borrelia burgdorferi infection. Microb Pathog. 2014;73:70–79. doi: 10.1016/j.micpath.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oelsner EC, Ortega VE, Smith BM, Nguyen JN, Manichaikul AW, Hoffman EA, et al. A genetic risk score associated with chronic obstructive pulmonary disease susceptibility and lung structure on computed tomography. Am J Respir Crit Care Med. 2019;200:721–731. doi: 10.1164/rccm.201812-2355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho MH, McDonald ML, Zhou X, Mattheisen M, Castaldi PJ, Hersh CP, et al. NETT Genetics, ICGN, ECLIPSE and COPDGene Investigators. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med. 2014;2:214–225. doi: 10.1016/S2213-2600(14)70002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakornsakolpat P, Prokopenko D, Lamontagne M, Reeve NF, Guyatt AL, Jackson VE, et al. SpiroMeta Consortium; International COPD Genetics Consortium. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet. 2019;51:494–505. doi: 10.1038/s41588-018-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrine N, Guyatt AL, Erzurumluoglu AM, Jackson VE, Hobbs BD, Melbourne CA, et al. Understanding Society Scientific Group. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet. 2019;51:481–493. doi: 10.1038/s41588-018-0321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campfield BT, Kolls JK. Loss of follistatin-like protein 1 causes spontaneous emphysema in a murine model [abstract] Am J Respir Crit Care Med. 2015;191:A5561. [Google Scholar]
- 24.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17:320–328. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra V, Lee H, Singh A, Huang K, Thimmulappa RK, Mitzner W, et al. Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol Genomics. 2007;31:429–440. doi: 10.1152/physiolgenomics.00060.2007. [DOI] [PubMed] [Google Scholar]
- 28.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, et al. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax. 2017;72:481–484. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y, Guo M, Whitsett JA, Xu Y. ‘LungGENS’: a web-based tool for mapping single-cell gene expression in the developing lung. Thorax. 2015;70:1092–1094. doi: 10.1136/thoraxjnl-2015-207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabula Muris Consortium; Overall Coordination; Logistical Coordination; Organ Collection and Processing; Library Preparation and Sequencing; Computational Data Analysis; Cell Type Annotation; Writing Group; Supplemental Text Writing Group; Principal Investigators. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562:367–372. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lao T, Jiang Z, Yun J, Qiu W, Guo F, Huang C, et al. Hhip haploinsufficiency sensitizes mice to age-related emphysema. Proc Natl Acad Sci USA. 2016;113:E4681–E4687. doi: 10.1073/pnas.1602342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yun JH, Morrow J, Owen CA, Qiu W, Glass K, Lao T, et al. Transcriptomic analysis of lung tissue from cigarette smoke-induced emphysema murine models and human chronic obstructive pulmonary disease show shared and distinct pathways. Am J Respir Cell Mol Biol. 2017;57:47–58. doi: 10.1165/rcmb.2016-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol. 2015;11:339–346. doi: 10.1038/nchembio.1788. [DOI] [PubMed] [Google Scholar]
- 34.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Xu F, Lin Y. Cigarette smoke synergizes lipopolysaccharide-induced interleukin-1β and tumor necrosis factor-α secretion from macrophages via substance P-mediated nuclear factor-κB activation. Am J Respir Cell Mol Biol. 2011;44:302–308. doi: 10.1165/rcmb.2009-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ipseiz N, Uderhardt S, Scholtysek C, Steffen M, Schabbauer G, Bozec A, et al. The nuclear receptor Nr4a1 mediates anti-inflammatory effects of apoptotic cells. J Immunol. 2014;192:4852–4858. doi: 10.4049/jimmunol.1303377. [DOI] [PubMed] [Google Scholar]
- 37.Shin HJ, Lee BH, Yeo MG, Oh SH, Park JD, Park KK, et al. Induction of orphan nuclear receptor Nur77 gene expression and its role in cadmium-induced apoptosis in lung. Carcinogenesis. 2004;25:1467–1475. doi: 10.1093/carcin/bgh135. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Shen H, Xu M, Liu O, Zhao L, Liu S, et al. FRP inhibits ox-LDL-induced endothelial cell apoptosis through an Akt-NF-kappaB-Bcl-2 pathway and inhibits endothelial cell apoptosis in an apoE-knockout mouse model. Am J Physiol Endocrinol Metab. 2010;299:E351–E363. doi: 10.1152/ajpendo.00005.2010. [DOI] [PubMed] [Google Scholar]
- 39.Ni X, Cao X, Wu Y, Wu J. FSTL1 suppresses tumor cell proliferation, invasion and survival in non-small cell lung cancer. Oncol Rep. 2018;39:13–20. doi: 10.3892/or.2017.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, et al. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010;285:7127–7134. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tacke R, Hilgendorf I, Garner H, Waterborg C, Park K, Nowyhed H, et al. The transcription factor NR4A1 is essential for the development of a novel macrophage subset in the thymus. Sci Rep. 2015;5:10055. doi: 10.1038/srep10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou HH, Cheng SL, Liu HT, Yang FZ, Wang HC, Yu CJ. Elastase induced lung epithelial cell apoptosis and emphysema through placenta growth factor. Cell Death Dis. 2013;4:e793. doi: 10.1038/cddis.2013.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike K, Berdyshev EV, Mikosz AM, Bronova IA, Bronoff AS, Jung JP, et al. Role of glucosylceramide in lung endothelial cell fate and emphysema. Am J Respir Crit Care Med. 2019;200:1113–1125. doi: 10.1164/rccm.201812-2311OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. 2010;181:344–352. doi: 10.1164/rccm.200906-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueno M, Maeno T, Nishimura S, Ogata F, Masubuchi H, Hara K, et al. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat Commun. 2015;6:6332. doi: 10.1038/ncomms7332. [DOI] [PubMed] [Google Scholar]
- 48.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurakula K, Vos M, Logiantara A, Roelofs JJ, Nieuwenhuis MA, Koppelman GH, et al. Nuclear receptor Nur77 attenuates airway inflammation in mice by suppressing NF-κB activity in lung epithelial cells. J Immunol. 2015;195:1388–1398. doi: 10.4049/jimmunol.1401714. [DOI] [PubMed] [Google Scholar]
- 50.Koenis DS, Medzikovic L, van Loenen PB, van Weeghel M, Huveneers S, Vos M, et al. Nuclear receptor Nur77 limits the macrophage inflammatory response through transcriptional reprogramming of mitochondrial metabolism. Cell Rep. 2018;24:2127–2140, e7. doi: 10.1016/j.celrep.2018.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature. 2019;567:525–529. doi: 10.1038/s41586-019-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liebmann M, Hucke S, Koch K, Eschborn M, Ghelman J, Chasan AI, et al. Nur77 serves as a molecular brake of the metabolic switch during T cell activation to restrict autoimmunity. Proc Natl Acad Sci USA. 2018;115:E8017–E8026. doi: 10.1073/pnas.1721049115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.