Abstract
Rationale: Low uptake of low-dose computed tomography (LDCT) lung cancer screening, particularly by current smokers of a low socioeconomic position, compromises effectiveness and equity.
Objectives: To compare the effect of a targeted, low-burden, and stepped invitation strategy versus control on uptake of hospital-based Lung Health Check appointments offering LDCT screening.
Methods: In a two-arm, blinded, between-subjects, randomized controlled trial, 2,012 participants were selected from 16 primary care practices using these criteria: 1) aged 60 to 75 years, 2) recorded as a current smoker within the last 7 years, and 3) no prespecified exclusion criteria contraindicating LDCT screening. Both groups received a stepped sequence of preinvitation, invitation, and reminder letters from their primary care practitioner offering prescheduled appointments. The key manipulation was the accompanying leaflet. The intervention group’s leaflet targeted psychological barriers and provided low-burden information, mimicking the concept of the U.K. Ministry of Transport’s annual vehicle test (“M.O.T. For Your Lungs”).
Measurements and Main Results: Uptake was 52.6%, with no difference between intervention (52.3%) and control (52.9%) groups in unadjusted (odds ratio [OR], 0.98; 95% confidence interval [CI], 0.82–1.16) or adjusted (OR, 0.98; 95% CI, 0.82–1.17) analyses. Current smokers were less likely to attend (adjusted OR, 0.70; 95% CI, 0.56–0.86) than former smokers. Socioeconomic deprivation was significantly associated with lower uptake for the control group only (P < 0.01).
Conclusions: The intervention did not improve uptake. Regardless of trial arm, uptake was considerably higher than previous clinical and real-world studies, particularly given that the samples were predominantly lower socioeconomic position smokers. Strategies common to both groups, including a Lung Health Check approach, could represent a minimum standard.
Clinical trial registered with www.clinicaltrials.gov (NCT02558101) and registered prospectively with the International Standard Registered Clinical/Social Study (N21774741).
Keywords: lung neoplasms, early detection of cancer, behavioral sciences, socioeconomic factors
At a Glance Commentary
Scientific Knowledge on the Subject
Consistently low uptake of low-dose computed tomography lung cancer screening by high-risk groups compromises its effectiveness, limiting the population impact on lung cancer mortality and potentially widening existing inequalities. To date, enrollment in trials has been <5% and only 1.9% of the eligible U.S. population have been screened.
What This Study Adds to the Field
This trial is the first to use behavioral science to design and test a low-cost, primary care practice–based, postal invitation strategy in a real-world demonstration setting. Across both trial arms, uptake was higher than has ever been observed previously at 53%, suggesting the behavioral science strategies common to both trial arms together optimize uptake. These strategies included a “Lung Health Check/M.O.T. For Your Lungs” approach to the screening offer (the latter mimicking the concept of the UK Ministry of Transport annual vehicle test), primary care practitioner endorsement, preinvitations, postal reminders, and scheduled appointments. The targeted, stepped, and low-burden intervention invitation approach did not improve uptake overall but importantly was the more equitable, reducing the social gradient by better engaging those living in areas of highest deprivation and lung cancer incidence.
Lung cancer leads cancer mortality globally (1). Although tobacco control strategies are the primary means to reduce incidence, early diagnosis markedly increases 5-year survival from 6% to 82% (stage IV vs. 1A non-small cell) (2). Currently though, most (66%) diagnoses in the United Kingdom are made at an advanced stage (3). The U.S. National Lung Screening Trial (n = 53,454) demonstrated that screening asymptomatic high-risk adults using low-dose computed tomography (LDCT) reduced the risk of mortality from lung cancer by 20% compared with chest X-ray (4). Consequently, the U.S. Preventive Services Task Force recommended screening for high-risk adults. The U.K. National Screening Committee is awaiting the Dutch-Belgian trial NELSON’s (Nederlands-Leuvens Longkanker Screenings Onderzoek) findings (n = 15,822), but early data suggest a mortality benefit (5).
Engaging those at high risk improves the risk/benefit ratio of screening. However, enrollment into lung screening trials has been low (<5%) (6) and skewed toward those at lower risk. Long-term smokers are overrepresented within lower socioeconomic position (SEP) communities, yet both current smoking status and low SEP are negatively associated with uptake (7, 8) and positively associated with risk (9). Indeed, despite the U.S. Preventive Services Task Force’s recommendation, just 1.9% of eligible, high-risk individuals have been screened in the United States (10). Attendance of the pilot Lung Health Check services in England has been relatively higher at 27% (Nottingham), 26% (Manchester), and 40% (Liverpool). Due to the ineligibility of some attenders, this translated to LDCT uptake by 13%, 14%, and 9%, respectively (11, 12).
Psychological barriers to participation were identified by research (13) that we undertook to inform the present intervention. Together with existing studies, findings suggested smokers (compared with non-smokers) are more fatalistic about lung cancer, perceive treatment efficacy as lower (13–17), feel stigmatized (13, 18), hold higher affective risk perceptions, and fear diagnosis (13, 19). Previous studies in colorectal cancer screening suggest tailoring leaflets to modify attitudinal barriers (20) may improve uptake (20–22). From a translational perspective, leaflets provide a low-cost and scalable intervention.
In addition to targeting psychological barriers, behavioral science theory, such as the Precaution Adoption Process Model (23), proposes that different types of information are needed depending on an individual’s state of engagement, decision-making, and behavior. A first-time invitation might primarily focus on engaging individuals in considering the offer using a low-burden approach, with subsequent communication promoting informed choice and reducing practical barriers. This stepped approach may be particularly important if the offer is anticipated to provoke fear, which can reduce receptivity (24, 25), and for those with lower literacy, because information burden can reduce comprehension and promote distrust (23–26). However, to date, recruitment methods for trials have been cognitively and practically demanding.
Therefore, this trial primarily aimed to test the effect of targeted, stepped, and low-burden invitation materials on uptake of Lung Health Check appointments offered in a real-world context. The secondary aims were to explore whether the intervention materials affected informed decision-making outcomes, to gauge likely uptake of a national program, and to examine the feasibility of invitation via primary care. Some results have been reported as an abstract (26).
Methods
Design
A two-arm, blinded, between-subjects, randomized controlled trial design tested the effect of intervention invitation materials on uptake of a prescheduled Lung Health Check appointment at which LDCT screening might be offered. A protocol has been published (27) with potential overlap. Eligible individuals were identified from primary care practices in London using electronic searches performed between October 2015 and March 2017.
Eligibility Criteria
The searches (n = 147,015) extracted individuals aged 60 to 75 years who had been recorded as smokers since April 2010 (within 7 years of invitation). This was the date smoking status became a Quality and Outcomes Framework indicator to ensure completeness and identify current and recent ex-smokers. The searches excluded individuals who had an active lung cancer diagnosis or metastatic cancer, were on the palliative care register, had undergone a recent computed tomography thorax (≤12 mo), lacked capacity, or had insufficient English or a comorbidity contraindicating screening or treatment. Lists were then screened by primary care practitioners. To avoid contamination, only one eligible individual per household was invited.
Randomization
A web-based program individually randomized participants (1:1) using permuted blocks to balance group allocation by practice. Identifiable details were concealed during assignment, which was performed by a blinded researcher. Invited individuals were blind to the research nature at the invitation stage to avoid undermining the primary outcome.
Intervention and Control Invitation Materials
Our invitation methods and evidence are published (13, 27) and appended (see File E1 in the online supplement). Briefly, evidence-based methods were used for both invitation groups, including primary care endorsement (21, 28), prenotification (29), reminders (30, 31), and prescheduled appointments (32, 33). The screening offer was framed within a Lung Health Check. All participants received the same postal invitation letters from their primary care practice: preinvitation letter, invitation letter with scheduled appointment, and reminder reinvitation letter with a second scheduled appointment (sent to nonresponders ≥4 wk after missed appointment). The letters were identical with two exceptions: 1) the intervention group’s letters referred to “ever smokers” whereas the control group’s referred to “current and former smokers” and 2) the intervention group’s invitation letter included a bullet-pointed summary of the Lung Health Check, including LDCT scan offer, on the reverse side.
The key manipulation was the accompanying leaflet. The control group received an information booklet mimicking the facts booklets of NHS (National Health Service) cancer screening programs. The intervention group received an “M.O.T. For Your Lungs” leaflet, designed to target psychological barriers to attendance (fear, fatalism, and stigma), to be low-burden (sufficient for deciding to attend and consider the screening offer), and stepped (full information given at the appointment using the control group’s booklet or available before via a website, phone, or post). An M.O.T. is an annual roadworthy test for vehicles and was a lay concept perceived to be analogous to a medical checkup and preferred by patient and public involvement groups.
Lung Health Check Appointment
The appointments were run by research nurses and clinical trial practitioners at two London hospital outpatient clinics. The appointment included a medical and smoking history to determine risk-based eligibility for the LDCT scan according to one of three criteria: 1) U.S. National Lung Screening Trial ≥30-pack-year smoking history and still smoking or quit ≤15 years; 2) Prostate, Lung, Colorectal, and Ovarian score ≥1.51%; or 3) Liverpool Lung Project score ≥2.5%. Full information about the risks and benefits of screening was provided to all using the control group’s leaflet and supported by the nurse consultation. A spirometry test and a carbon monoxide reading were also performed. Participants self-reporting as current smokers or with a carbon monoxide reading ≥10 ppm were given the accredited “Very Brief Advice” on smoking (National Centre for Smoking Cessation and Training [34]) and randomized to an opt-out or opt-in referral intervention.
Ethics
Approval was granted by an NHS Research Ethics Committee (reference: 15/LO/1186).
Primary Outcome Measure
Attendance of the Lung Health Check appointment (percentage of those invited) was used to measure whether individuals could be engaged in considering a screening offer.
Secondary Outcome Measures
The prespecified secondary endpoints in our statistical analysis plan included comparison of uptake by demographic and smoking status subgroups, uptake of LDCT screening for those eligible (and willingness among those ineligible), and informed decision-making outcomes. Data on participants’ engagement with the invitation materials were also collected. Further prespecified endpoints are LDCT scan results, resource use, and psychological outcomes.
Demographic data
Pseudonymized data on age, sex, ethnicity, and area-level socioeconomic deprivation (Index of Multiple Deprivation [IMD] score and rank) were collected from the primary care records of all those invited and again from attenders using self-report measures. Attenders also reported their education level and marital status. Hospital site of the screening offer was recorded.
Smoking data
Last-recorded smoking status was extracted from primary care records (recoded as current/occasional, former, and never). Self-reported smoking status and smoking history were collected from attenders. Smoking duration and pack-years were calculated by the research nurse in combination with participants’ quit histories. For current smokers, the number of previous serious quit attempts, tobacco dependence (35), and perceived chances of quitting (36) were measured.
Uptake data
Secondary outcomes included uptake of LDCT screening for those eligible and willingness to be screened for those ineligible.
Decision-making outcomes
A self-completed paper questionnaire given at the appointment included adapted items from the Satisfaction with Decision scale (37) and the low-literacy version of the Decisional Conflict scale (38, 39). A further nine items measured conceptual and numerical knowledge of lung cancer screening, including original and adapted items (40). Responses were dichotomized as correct versus incorrect/not sure and summed.
Engagement with the invitation leaflets
Participants were asked whether they remembered, read, and understood their respective leaflet, and whether they had been “useful,” “difficult to understand,” “informative,” “too complicated,” or had “too little information.” Research nurses rated participants’ background knowledge of screening subjectively as “none,” “very little,” “moderate,” “fairly good,” and “very comprehensive/near perfect.”
Statistical Analyses
Sample size
Uptake for the control group was estimated to be 35% based on first-time uptake of the fecal occult blood test (FOBT) colorectal cancer screening program in London within the two most deprived quintiles (41). With a target sample size of 2,000 participants randomized evenly into two arms, the study was statistically powered (at 90%) to detect a 7% increase in uptake using two-sided tests at the 5% significance threshold. The 7% figure was based on studies testing targeted “psycho-educational” invitations in colorectal screening (20, 21) and considered a clinically meaningful benefit.
Primary analyses
Data were analyzed using IBM SPSS (v. 25). Analyses followed a prospectively registered statistical analysis plan (DOI: 10.17605/OSF.IO/HKEMM) and the trial protocol (27). The primary outcome was analyzed using an intention-to-treat approach (N = 2,012). Attendance was compared by invitation group using logistic regression and a deviance chi-squared test for statistical significance.
Secondary analyses
Analyses tested for associations between demographic characteristics, smoking status, and attendance, using bivariate and then multivariable logistic regression models to calculate adjusted odds ratios (aOR) (n = 1,970). Study-specific quintiles for IMD rank were calculated because the sample was skewed toward above-average deprivation.
Logistic regression analyses then explored correlates of LDCT uptake among eligible participants. The decision-making outcomes were compared by invitation group, using chi-squared tests or t tests. For data collected after attendance, “prefer not to say,” “not stated,” or “don’t know” responses were treated as missing.
Results
Characteristics of the Invited Sample
The average age was 66.0 years (SD, 4.3), 53.7% were male, and the majority (79.7%) were from a white ethnic group (Table 1). Overall, there was higher representation of ethnic minority groups compared with the general population (14%) but lower than in London (40%), likely due to the younger age structure and differences in smoking prevalence (42). Nearly all those invited (96.2%) were categorized within the most deprived (60.9%) or second-most deprived (35.3%) IMD quintile. Three quarters (74.5%) were current smokers.
Table 1.
Sample Characteristics of All Those Invited, Overall and by Invitation Group
| All (N = 2,012) | Intervention (n = 1,006) | Control (n = 1,006) | |
|---|---|---|---|
| Sex, % (n) | |||
| F | 46.3 (931) | 44.7 (450) | 47.8 (481) |
| M | 53.7 (1,081) | 55.3 (556) | 52.2 (525) |
| Age, mean (SD) | 66.0 (4.3) | 66.1 (4.3) | 65.9 (4.3) |
| Ethnicity, % (n) | |||
| Asian | 2.1 (42) | 2.3 (23) | 1.9 (19) |
| Black | 9.6 (193) | 9.4 (95) | 9.7 (98) |
| Mixed | 1.7 (34) | 1.4 (14) | 2.0 (20) |
| White | 79.7 (1,604) | 79.6 (801) | 79.8 (803) |
| Other | 2.9 (59) | 3.1 (31) | 2.8 (28) |
| Not stated | 4.0 (80) | 4.2 (42) | 3.8 (38) |
| National Index of Multiple Deprivation quintile, % (n) | |||
| Quintile 1 (1–6,496) most deprived | 60.9 (1,226) | 60.5 (609) | 61.3 (617) |
| Quintile 2 (6,497–12,993) | 35.3 (711) | 35.4 (356) | 35.3 (355) |
| Quintile 3 (12,994–19,489) | 2.3 (47) | 2.5 (25) | 2.2 (22) |
| Quintile 4 (19,490–25,986) | 0.1 (2) | 0.1 (1) | 0.1 (1) |
| Quintile 5 (25,987–32,482) least deprived | — | — | — |
| Missing | 1.3 (26) | 1.5 (15) | 1.1 (11) |
| Smoking status, % (n) | |||
| Current smoker | 74.5 (1,499) | 76.2 (767) | 72.8 (732) |
| Former smoker | 24.7 (497) | 23.0 (231) | 26.4 (266) |
| Never smoked tobacco | 0.6 (13) | 0.8 (8) | 0.5 (5) |
| Refused/not stated | 0.1 (2) | — | 0.2 (2) |
| Missing | 0.0 (1) | — | 0.1 (1) |
| Attendance from all invited, % (n) | |||
| Overall | 52.6 (1,058) | 52.3 (526) | 52.9 (532) |
| Attended first appointment | 40.3 (811) | 39.7 (399) | 41.0 (412) |
| Cancelled first appointment | 5.0 (100) | 4.6 (46) | 5.4 (54) |
| Sent reminder (no response to first invitation) | 54.7 (1,101) | 55.8 (561) | 53.7 (540) |
| Attended second (reminder) appointment | 9.6 (194) | 9.4 (95) | 9.8 (99) |
| Cancelled second (reminder) appointment | 2.9 (59) | 3.4 (34) | 2.5 (25) |
| No response to reminder invitation | 42.1 (848) | 42.9 (432) | 41.4 (416) |
Primary Analyses
Uptake of the Lung Health Check
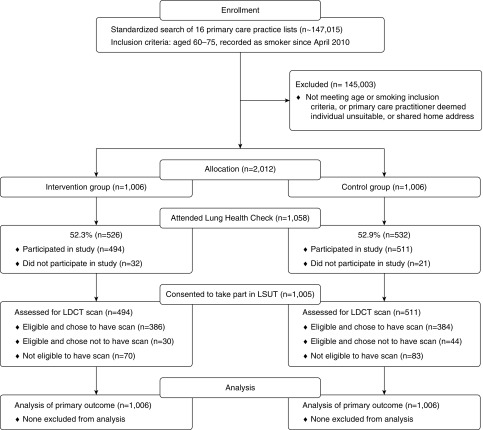
Sixteen primary care practices participated with a combined population of 147,015 patients (Figure 1). Of these, 2,012 individuals were randomized in equal numbers (n = 1,006) to the invitation groups. Over half, 52.6% (n = 1,058), attended their appointment (see Table 1).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) trial flow diagram. LDCT = low-dose computed tomography.
Individuals predominantly attended the first appointment offered (40.3%), but 9.6% attended the second appointment offered with their reminder. There was no response from 42.1%. There was no statistically significant difference in uptake by hospital site (53.0% vs. 50.8%). Most (94.9%) attenders enrolled.
Near equal numbers from the intervention (52.3%) and control groups (52.9%; 526 vs. 532, respectively) attended. In unadjusted analyses, there was no association between invitation group and uptake (OR, 0.98; 95% confidence interval [CI], 0.82–1.16; Table 2).
Table 2.
Frequencies and Logistic Regression Analyses Examining the Correlates of Uptake
| All |
Intervention |
Control |
|||||
|---|---|---|---|---|---|---|---|
| Attended [% (n)] (N = 2,012) | Unadjusted OR (95% CI) (N = 2,012) | Adjusted OR (95% CI) (n = 1,970) | Unadjusted OR (95% CI) (n = 1,006) | Adjusted OR (95% CI) (n = 983) | Unadjusted OR (95% CI) (n = 1,006) | Adjusted OR (95% CI) (n = 987) | |
| Sex | P = 0.557 | P = 0.433 | P = 0.828 | P = 0.944 | P = 0.290 | P = 0.237 | |
| F | 52.0 (479) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| M | 53.4 (574) | 1.05 (0.88–1.26) | 1.08 (0.90–1.29) | 0.97 (0.76–1.25) | 0.99 (0.76–1.29) | 1.14 (0.89–1.47) | 1.17 (0.90–1.52) |
| Age | P = 0.857 | P = 0.879 | P = 0.484 | P = 0.365 | P = 0.331 | P = 0.188 | |
| 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | 0.99 (0.96–1.02) | 0.99 (0.96–1.02) | 1.02 (0.99–1.05) | 1.02 (0.99–1.05) | ||
| Ethnicity | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
| White | 54.1 (864) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Asian | 52.6 (20) | 0.85 (0.46–1.57) | 0.87 (0.45–1.69) | 1.13 (0.49–2.60) | 1.44 (0.56–3.75) | 0.61 (0.24–1.53) | 0.52 (0.20–1.37) |
| Black | 56.0 (107) | 1.11 (0.82–1.49) | 1.11 (0.82–1.51) | 1.09 (0.71–1.68) | 1.06 (0.68–1.65) | 1.12 (0.73–1.71) | 1.17 (0.76–1.81) |
| Mixed | 36.4 (12) | 0.47 (0.23–0.95) | 0.48 (0.24–1.00) | 0.35 (0.11–1.12) | 0.37 (0.11–1.23) | 0.56 (0.23–1.38) | 0.57 (0.23–1.43) |
| Other | 72.9 (43) | 2.29 (1.28–4.10) | 2.34 (1.30–4.20) | 1.82 (0.85–3.92) | 1.92 (0.89–4.15) | 3.07 (1.23–7.66) | 3.23 (1.28–8.14) |
| Not stated* | 8.9 (7) | 0.08 (0.04–0.18) | 0.09 (0.04–0.19) | 0.15 (0.06–0.35) | 0.15 (0.06–0.35) | 0.02 (0.00–0.17) | 0.03 (0.00–0.19) |
| Study-specific deprivation quintile† | P < 0.01‡ | P < 0.01 | P = 0.154‡ | P = 0.100 | P < 0.01‡ | P < 0.05 | |
| Quintile 1 (most deprived) | 45.2 (179) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quintile 2 | 51.6 (205) | 1.29 (0.97–1.70) | 1.28 (0.96–1.71) | 1.25 (0.84–1.86) | 1.28 (0.85–1.92) | 1.31 (0.89–1.93) | 1.31 (0.87–1.96) |
| Quintile 3 | 57.5 (234) | 1.63 (1.23–2.15) | 1.62 (1.21–2.15) | 1.49 (1.00–2.21) | 1.49 (0.99–2.24) | 1.77 (1.20–2.62) | 1.74 (1.16–2.61) |
| Quintile 4 | 51.3 (195) | 1.27 (0.96–1.68) | 1.23 (0.92–1.64) | 0.98 (0.66–1.47) | 0.96 (0.64–1.45) | 1.63 (1.10–2.42) | 1.60 (1.06–2.41) |
| Quintile 5 (least deprived) | 58.2 (227) | 1.65 (1.25–2.19) | 1.68 (1.26–2.25) | 1.36 (0.91–2.02) | 1.44 (0.96–2.17) | 2.01 (1.35–2.99) | 1.93 (1.28–2.93) |
| Smoking status | P < 0.001§ | P < 0.01 | P < 0.05§ | P < 0.05 | P < 0.01§ | P < 0.05 | |
| Former smoker | 60.2 (299) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Current smoker | 50.3 (754) | 0.67 (0.55–0.82) | 0.70 (0.56–0.86) | 0.70 (0.52–0.94) | 0.72–0.53–0.97) | 0.65 (0.49–0.86) | 0.68 (0.51–0.92) |
| Invitation group | P = 0.789 | P = 0.843 | — | — | — | — | |
| Control | 53.0 (529) | 1.00 | 1.00 | — | — | — | — |
| Intervention | 52.5 (524) | 0.98 (0.82–1.16) | 0.98 (0.82–1.18) | — | — | — | — |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Bold indicates ORs that are significant at P < 0.01.
No record of ethnic group in primary care.
2010 Index of Multiple Deprivation rank quintile with cutoffs based on distribution in LSUT sample.
Cases with no Index of Multiple Deprivation rank and/or score were excluded (n = 26 in full sample).
Never smokers (n = 13 in full sample) and refused/missing smoking status (n = 3 in full sample) were excluded.
Secondary Analyses
Correlates of uptake of the Lung Health Check
Neither sex nor age were associated with uptake (see Table 2). Ethnicity was associated with uptake across groups (P < 0.001). Compared with those of a white ethnic background, individuals of other ethnic background were more likely to attend (aOR, 2.34; 95% CI, 1.30–4.20) and those with no recorded ethnic group were less likely to attend (aOR, 0.09; 95% CI, 0.04–0.19). Higher deprivation was associated with lower uptake across study-specific IMD quintiles (P < 0.01). Individuals categorized within the three least deprived study-specific quintiles had higher odds of attendance compared with those in the most deprived quintile (aOR, 1.62; 95% CI, 1.21–2.15 and aOR, 1.68; 95% CI, 1.26–2.25, respectively). Current smokers were significantly less likely to attend than former smokers (aOR, 0.70; 95% CI, 0.56–0.86).
When analyses of uptake were stratified by invitation group, there were again no associations with sex, age, or hospital site. For the control group, the same associations with other (vs. white) ethnicity (aOR, 3.23; 95% CI, 1.28–8.14) and not-stated ethnicity (aOR, 0.03; 95% CI, 0.00–0.19) were observed. Deprivation was significantly associated with increasingly lower odds of attendance across quintiles (P < 0.05). For example, the odds of uptake for the least deprived quintile were nearly twice as high as those for the most deprived (aOR, 1.93; 95% CI, 1.28–2.93). Ethnicity was also associated with uptake for the intervention group (P < 0.001), with lower odds of uptake for those with no stated ethnic group (aOR, 0.15; 95% CI, 0.06–0.35). Conversely, deprivation did not significantly differentiate uptake in the intervention invitation group.
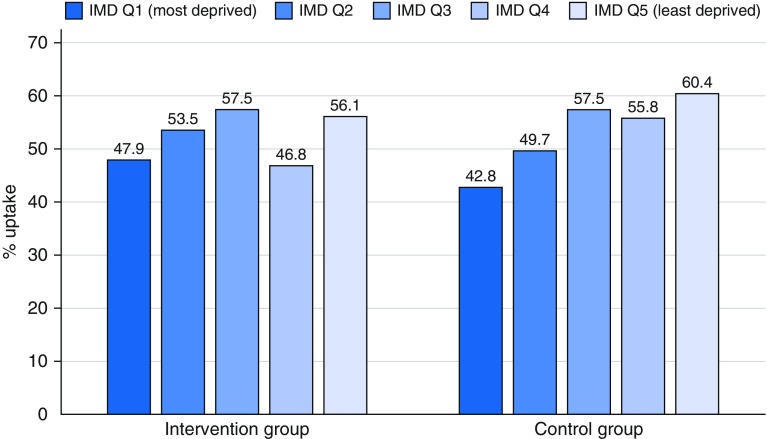
Figure 2 presents the absolute percent uptake by study-specific IMD quintile and invitation group. The gradient appears relatively less steep in the intervention group, with uptake relatively higher for the two most deprived quintiles in the intervention group (47.9% and 53.5%, respectively) compared with the control group (42.8% and 49.7%, respectively) and relatively lower for the two least deprived quintiles (46.8% and 56.1% vs. 55.8% and 60.4%, respectively).
Figure 2.
Uptake by study-specific deprivation quintile (Q) Index of Multiple Deprivation (IMD) for each invitation group (N = 2,012). Note that 2010 IMD rank quintile with cutoffs were based on distribution in the LSUT sample.
Smoking characteristics and eligibility for screening
On average, attenders reported beginning smoking at age 17.9 years (SD, 5.8) and accumulated a 39.4 (SD, 25.0)-pack-year history (Table 3). Most current smokers had tried to quit previously (78.7%) and had low confidence in their chances of quitting (58.7%). The majority (84.5%) were eligible for LDCT screening. Among those ineligible (n = 160), willingness to be screened was high (81.9%).
Table 3.
Smoking Characteristics of Attenders Consenting to LSUT and Eligibility for LDCT
| All (n = 1,000)* | Intervention (n = 492) | Control (n = 508) | |
|---|---|---|---|
| Age started smoking, mean (SD, range) | 17.9 (5.8, 6–55) | 17.9 (5.5, 7–55) | 17.9 (6.1, 6–55) |
| Age stopped smoking†, mean (SD, range) | 59.4 (10.7, 0–75) | 59.8 (10.4, 21–75) | 59.1 (11.0, 0–75) |
| Number of years smoked, mean (SD, range) | 45.5 (9.5, 2–64) | 45.6 (9.1, 2–64) | 45.4 (9.9, 3–63) |
| Pack-years, mean (SD, range) | 39.4 (25.0, 1–171) | 38.0 (22.2, 1–128) | 40.7 (27.5, 1–171) |
| Usual daily cigarette consumption‡,§, % (n) | |||
| 1–10 | 55.7 (395) | 55.3 (199) | 56.2 (196) |
| 11–20 | 33.3 (236) | 34.7 (125) | 31.8 (111) |
| 21–30 | 5.9 (42) | 5.3 (19) | 6.6 (23) |
| ≥31 | 2.3 (16) | 2.2 (8) | 2.3 (8) |
| Missing | 2.8 (20) | 2.5 (9) | 3.2 (11) |
| Time to first cigarette§, % (n) | |||
| Within 5 min | 16.5 (117) | 16.9 (61) | 16.0 (56) |
| 6–30 min | 33.4 (237) | 33.9 (122) | 33.0 (115) |
| 31–60 min | 16.8 (119) | 17.2 (62) | 16.3 (57) |
| >60 min | 31.5 (223) | 31.1 (112) | 31.8 (111) |
| Missing | 1.8 (13) | 0.8 (3) | 2.9 (10) |
| Nicotine dependence (HSI score)§, % (n) | |||
| Low dependence | 38.9 (276) | 38.6 (139) | 39.3 (137) |
| Moderate dependence | 42.9 (304) | 43.1 (155) | 42.7 (149) |
| High dependence | 14.5 (103) | 15.3 (55) | 13.8 (48) |
| Missing | 3.7 (26) | 3.1 (11) | 4.3 (15) |
| Perceived chance of quitting§, % (n) | |||
| Very low/low/not very high | 58.7 (416) | 56.9 (205) | 60.5 (211) |
| Quite high/very high/extremely high | 38.5 (273) | 41.4 (149) | 35.5 (124) |
| Missing | 2.8 (20) | 1.7 (6) | 4.0 (14) |
| Previous quit attempts§, % (n) | |||
| None | 20.3 (144) | 21.7 (78) | 18.9 (66) |
| 1–5 | 59.7 (423) | 57.5 (207) | 61.9 (216) |
| >5 | 19.0 (135) | 20.0 (72) | 18.1 (63) |
| Missing | 1.0 (7) | 0.8 (3) | 1.1 (4) |
| Eligibility for LDCT scan, % (n) | 84.5 (845) | 84.6 (416) | 83.4 (429) |
| LDCT scan willingness (of ineligible), % (n) | |||
| Yes, definitely | 66.9 (107) | 71.8 (56) | 62.2 (51) |
| Yes, probably | 15.0 (24) | 10.3 (8) | 19.5 (16) |
| Probably not | 3.8 (6) | 1.3 (1) | 6.1 (5) |
| Definitely not | 3.8 (6) | 5.1 (4) | 2.4 (2) |
| Missing | 10.3 (17) | 11.5 (9) | 9.8 (8) |
Definition of abbreviations: LDCT = low-dose computed tomography; HSI = Heaviness of Smoking Index.
Never smokers (n = 4) and missing smokers (n = 1) were excluded.
Former smokers only (n = 269).
For participants reporting grams of tobacco per week, these were converted to number of cigarettes per day.
Current smokers only (n = 709).
Uptake of the LDCT scan
Most (91.2%) of those eligible chose to have the scan (Table 4). Sex, age, and marital status were not associated with LDCT uptake. For ethnicity, Asian ethnicity predicted lower odds of uptake compared with white ethnicity (aOR, 0.09; 95% CI, 0.02–0.31), but there were few Asian participants (n = 13). There was no association with black ethnicity and too few noncases within the other ethnic groups. Deprivation was not associated with LDCT uptake. In unadjusted analyses, current smokers were less likely to opt for the LDCT scan than former smokers, but the association was not statistically significant in adjusted analyses (aOR, 0.52; 95% CI, 0.27–1.01). Invitation group did not affect the likelihood of LDCT uptake.
Table 4.
Frequencies and Logistic Regression Analyses Examining the Correlates of Uptake of the LDCT Scan among LDCT-Eligible Attenders
| Attenders Eligible for LDCT (n = 845) |
|||
|---|---|---|---|
| LDCT Uptake [% (n)] | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Overall | 91.2 (770) | — | — |
| Sex | P = 0.846 | P = 0.979 | |
| F | 91.4 (342) | 1.00 | 1.00 |
| M | 91.1 (428) | 0.95 (0.59–1.54) | 1.01 (0.60–1.68) |
| Age | — | P = 0.275 | P = 0.267 |
| 0.97 (0.92–1.03) | 0.97 (0.91–1.03) | ||
| Marital status | P = 0.443 | P = 0.394 | |
| Married/cohabiting | 92.2 (320) | 1.00 | 1.00 |
| Single/separated/divorced/widowed | 90.7 (449) | 0.82 (0.50–1.35) | 0.79 (0.46–1.36) |
| Ethnicity | P < 0.01 | P < 0.01 | |
| White | 91.3 (642) | 1.00 | 1.00 |
| Asian | 53.8 (7) | 0.11 (0.04–0.34) | 0.09 (0.02–0.31) |
| Black | 92.7 (76) | 1.20 (0.50–2.88) | 1.28 (0.52–3.14) |
| Mixed | 100.0 (8) | — | — |
| Other | 97.1 (34) | — | — |
| Not stated | 100.0 (3) | — | — |
| Study-specific deprivation quintile* | P = 0.074 | P = 0.072 | |
| Quintile 1 (most deprived) | 88.2 (134) | 1.00 | 1.00 |
| Quintile 2 | 91.7 (154) | 1.48 (0.71–3.08) | 1.82 (0.75–3.49) |
| Quintile 3 | 95.6 (172) | 2.89 (1.22–6.85) | 2.82 (1.18–6.78) |
| Quintile 4 | 87.7 (136) | 0.96 (0.48–1.91) | 0.94 (0.46–1.91) |
| Quintile 5 (least deprived) | 92.7 (165) | 1.71 (0.81–3.61) | 1.74 (0.80–3.77) |
| Smoking status | P < 0.05 | P = 0.052 | |
| Former | 94.6 (211) | 1.00 | 1.00 |
| Current (incl. occasional) | 90.0 (559) | 0.51 (0.27–0.97) | 0.52 (0.27–1.01) |
| Invitation group | P = 0.177 | P = 0.075 | |
| Control | 89.7 (384) | 1.00 | 1.00 |
| Intervention | 92.8 (386) | 1.47 (0.91–2.40) | 0.63 (0.37–1.05) |
Definition of abbreviations: CI = confidence interval; LDCT = low-dose computed tomography; OR = odds ratio.
Missing data were excluded. Bold indicates ORs that are significant at P < 0.05.
2010 Index of Multiple Deprivation rank quintile with cutoffs based on distribution in LSUT sample.
Engagement with the invitation leaflets
A higher number of control participants (81.3%) remembered receiving their respective leaflet compared with the intervention group (64.1%; P < 0.001). Intervention participants understood more of their leaflet (P < 0.05) but there were no differences in background knowledge. File E2 presents further analyses.
Decision-making outcomes
There was no difference in mean scores for conceptual and numerical knowledge by invitation group (see File E2). Across both groups, endorsement of the Decisional Conflict Scale was high (≥76.2%), indicating low conflict. Most participants reported awareness of the benefits of screening, knew which they valued, felt supported, and were clear about their choice (all ≥89.6%). The risks were less well-understood. Fewer control participants reported that they knew what the risks were compared with intervention participants (76.2% vs. 83.2%; P < 0.05), but similar numbers knew which they valued (84.6% and 84.2%, respectively). Decisional satisfaction was high across groups, both self-reported and nurse-rated (all ≥97.3%).
Discussion
Uptake of the Lung Health Check was 53%, which is an important finding in itself, considerably higher than previously observed. The population was high-risk, with the majority eligible for LDCT screening. The intervention made no difference to uptake overall or by smoking status, with uptake biased in favor of former (compared with current) smokers. However, there was evidence that the targeted, stepped, and low-burden materials were relatively more effective at engaging the most deprived individuals.
A major strength of this study is its ecological validity. The design simulated a real-world service using practically feasible invitation methods via primary care, with the invited sample unaware their attendance was under study. Collecting individual-level demographic and smoking data provided a comprehensive understanding of nonresponders. A census-derived, area-based measure of deprivation allows national comparison but is less sensitive to individual variation. Moreover, the generalizability of these findings to affluent high-risk groups, a wider age range, and ethnic minority groups may be limited. We had complete data on most variables but there were 26 (1.3%) missing deprivation scores. Sensitivity analysis using multiple imputation made no difference to the findings.
Fifty-three percent uptake is an encouraging figure compared with trials and pilot services to-date (11, 12); especially given the invited sample was predominantly comprised of lower SEP current smokers. In UKLS (UK Lung Cancer Screening trial), interest from the most deprived quintile did not reach 20% (9). Indeed, attenders were high-risk, with 84% eligible for LDCT screening. Furthermore, this was a first-time invitation with no wider publicity or community engagement (11, 12). Uptake also compares favorably with first-time uptake of colorectal screening by FOBT in London (41%) and is on a par with national FOBT uptake (54%) when launched in 2006 (41). However, uptake is lower than current national figures for breast (71%) and cervical (72%) cancer but seemingly not because men were less likely to attend.
Finding a reduced socioeconomic gradient in uptake for the intervention group suggests that targeted and low-burden invitation materials show promise for better engaging high-risk individuals living in the most socioeconomically deprived areas. Nevertheless, it was the control invitation strategy that achieved the highest uptake for the least deprived quintile. These results suggest that the intervention invitation approach may be the more equitable, holding potential for reducing inequalities and achieving a greater reduction in lung cancer mortality by engaging those at highest risk. Future research should examine the feasibility and acceptability of stratifying invitation materials by area-level deprivation.
Related to this, intervention and control participants achieved similar decision-making outcomes, suggesting the low information burden component did not compromise decision-making. In fact, it was control participants who less frequently felt informed about the risks of screening despite receiving this information in advance. Our low-burden component was informed by evidence that information burden can deter individuals with low literacy (43–45) and that a third of nonparticipants in colorectal screening have not read the information booklet (46). Moreover, information receptivity and comprehension may be adversely affected by a fearful emotional state (24, 25), which a first-time lung screening invitation could provoke (13). Perhaps the appointment was a better environment to achieve comprehension, with the research nurse’s support and time to mentally adjust to the offer. Alternatively, control participants may have paid less attention to the booklet at their appointment because the information was not novel. Nevertheless, these findings suggest that providing detailed information with screening invitations may neither be sufficient for supporting informed choice nor an equitable invitation approach. A low-burden approach that builds up information in steps to full information provision during the appointment could be further tested for decision-making and inequalities in participation.
The intervention had no effect on smoking-related inequalities, with uptake skewed in favor of former smokers, as in previous trials (7–9) and screening programs for other cancer types (47–50). Research suggests that fatalism, fear, and stigma are deep-rooted attitudes (13, 17), which may be particularly resistant to change among current smokers. Alternatively, perhaps addiction-specific factors are more instrumental. Because this was a multifactorial intervention with no process evaluation, we cannot draw conclusions about individual components. It does, however, highlight there are both independent and shared barriers to participation associated with lower SEP and current smoking status.
A simple primary care record search effectively identified a largely screening-eligible population, suggesting invitation through primary care is feasible for a population-based program, as well as a strategy likely to improve uptake. Indeed, adopting the invitation methods common to both groups may optimize participation. This includes a Lung Health Check approach, primary care endorsement (21, 23), preinvitations (29), postal reminders (30), and scheduled appointments (32, 34). The reminder reinvitations offering a second scheduled appointment prompted uptake by a further 10%, suggesting that lowering practical demands helps nonresponders overcome nonintentional barriers. Although offering scheduled appointments appears to have been effective, 47% of invited individuals did not attend, which has resource implications. We mitigated the impact by overbooking appointments, and other strategies might include asking invitees to confirm attendance. Lessons could be learned from the United Kingdom’s NHS Breast Cancer Screening Program, which sends timed appointments (30). Overall, the likely effectiveness of the methods shared by both trial arms suggests that translating intention into action may be easier to achieve than changing attitudes.
There remains a gap in knowledge of the most effective means of modifying psychological barriers to participation. More foundational and experimental research is needed to isolate and test different approaches. It is likely that a multipronged screening communication strategy would be needed, as well as interventions at the wider healthcare system level, to ensure that the screening pathway optimizes individuals’ screening experience.
Conclusions
Uptake of LDCT screening is likely to increase if offered as an organized Lung Health Check program and individuals are invited via primary care. It is possible to engage a high-risk, screening-eligible sample of lower SEP current smokers using feasible, population-based, and low-cost methods. A targeted, stepped, and low-burden invitation approach shows promise for reducing the social gradient in uptake by engaging individuals living in areas of highest deprivation, without compromised decision-making. Further research is critical to understand how to further reduce inequalities, especially for current smokers.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Professor Jane Wardle (1950–2015) who first conceived of this study, was the Principal Investigator together with S.M.J., and who made a substantial intellectual contribution to every aspect. The authors dedicate this work to Jane. They also thank all of those who were so dedicated in helping to deliver the study, which includes all staff at the participating primary care and secondary care sites. More specifically, they thank the Research Nurses and Clinical Trial Practitioners who carried out the Lung Health Check appointments (Claire Whipp, Juancho Salgado, Nilabhra Dutta, Amy Smith, Krishna Patel, Nivea Douglas, Gemma Hector, Derya Ovayolu, Agnieszka Zielonka, Celia Simeon, and Adelaide Austin), the radiologists and radiographers who carried out and interpreted the low-dose computed tomography scans (Penny Shaw, Stephen Burke, Magali Taylor, Asia Ahmed, May Jan Soo, Arjun Nair, Carolyn Horst, Nicholas Woznitza, and James Batty), and the primary care cancer leads who helped recruit primary care practices (Eleanor Hitchman and Lucia Grun). The authors are also very grateful to Anand Devraj for helping to develop the radiology protocol and training, as well as the Picture Archiving and Communication System managers at each hospital site (Junaid Chowdhury and Mohmed Patel). They really appreciate all of Kylie Gyertson’s and Christine Inwang’s work in helping us to plan, set up, and run the study at the hospital sites, as well as Badar Alavi’s efforts in administrating participants’ results letters. Thanks also to external members of our Trial Steering Committee (Thomas Newsom-Davies, Matthew Callister, Nicholas Counsell, and Judith Cass) and Independent Data Monitoring Committee (Michael Peake and Gianluca Baio). Finally, the authors would like to thank all of the participants who gave up their time to help with this research study.
Footnotes
Supported by National Awareness and Early Diagnosis Initiative (NAEDI) project grant C1418/A17976 awarded by Cancer Research UK (CRUK) and a consortium of funders (Department of Health [England]; Economic and Social Research Council; Health and Social Care R&D Division, Public Health Agency, Northern Ireland; National Institute for Social Care and Health Research, Wales; and the Scottish government) (S.M.J., S.L.Q., M.R., S.W.D., and J. Waller); Wellcome Trust Senior Fellowship in Clinical Science grant WT107963AIA, the Rosetrees Trust, the Roy Castle Lung Cancer Foundation, the Stoneygate Trust, the Welton Trust, the Garfield Weston Trust, and the University College London Hospital Charitable Foundation (S.M.J.); the Department of Health’s NIHR Biomedical Research Centre’s funding scheme (N.N. and S.M.J.); CRUK postdoctoral fellowship C50664/A24460 and the Roy Castle Lung Cancer Foundation (S.L.Q.); CRUK career development fellowship C7492/A17219 (J. Waller); and Yorkshire Cancer Research Academic Fellowship funding L389RB (R.J.B.). The funders had no role in the study design, data collection, data analysis and interpretation, the writing or the manuscript, or in the decision to submit the manuscript for publication. All authors and researchers are independent of the study funders.
Data sharing statement: Relevant individual deidentified participant data (including data dictionaries) will be made available upon reasonable request to S.M.J. Data will be available to share after the publication of the study primary and secondary endpoints. The study protocol and statistical analysis plan are openly available online and referenced in this manuscript.
Author Contributions: J. Wardle, S.L.Q., A.M., D.R.B., S.W.D., and S.M.J. conceived the study design and wrote the funding application. J. Wardle, S.L.Q., M.R., R.J.B., A.M., D.R.B., A.B., N.N., K.S., J. Waller, and S.M.J. developed the protocol and measures. S.L.Q., M.R., and J.L.D. led the management and execution of the study. S.L.Q. performed the analyses with oversight from S.W.D. All authors contributed to the drafting of the manuscript. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication and attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201905-0946OC on December 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Eastern Cancer Registration and Information Centre (ECRIC) Stage distribution of cancers diagnosed in 2009 in the East of England by cancer site and area of residence. 2009 [accessed 2019 Mar 8] Available from: http://www.ecric.nhs.uk/docs/ECRIC_incidenceXstage_2009.pdf.
- 4.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Koning HJ, van der Aalst CM, Ten Haaf KE, Oudkerk M. Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population based trial. J Thorac Oncol. 2018;13:S185. [Google Scholar]
- 6.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, et al. National Lung Screening Trial Research Team. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [Published erratum appears in J Natl Cancer Inst 103:1560.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousaf-Khan U, Horeweg N, van der Aalst C, Ten Haaf K, Oudkerk M, de Koning H. Baseline characteristics and mortality outcomes of control group participants and eligible non-responders in the NELSON lung cancer screening study. J Thorac Oncol. 2015;10:747–753. doi: 10.1097/JTO.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 9.McRonald FE, Yadegarfar G, Baldwin DR, Devaraj A, Brain KE, Eisen T, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res (Phila) 2014;7:362–371. doi: 10.1158/1940-6207.CAPR-13-0206. [DOI] [PubMed] [Google Scholar]
- 10.Pham D, Bhandari S, Oechsli M, Pinkston CM, Kloecker GH. Lung cancer screening rates: data from the lung cancer screening registry. J Clin Oncol. 2018;36:6504. [Google Scholar]
- 11.Accelerate C Evaluate (ACE) Programme. Proactive approaches to individuals at high risk of lung cancer. 2018 [accessed 2019 Mar 8] Available from: https://www.cancerresearchuk.org/sites/default/files/ace_proactive_lung_report_with_economic_evaluation_final_version_1.1a.pdf.
- 12.Crosbie PA, Balata H, Evison M, Atack M, Bayliss-Brideaux V, Colligan D, et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax. 2019;74:405–409. doi: 10.1136/thoraxjnl-2017-211377. [DOI] [PubMed] [Google Scholar]
- 13.Quaife SL, Vrinten C, Ruparel M, Janes SM, Beeken RJ, Waller J, et al. Smokers’ interest in a lung cancer screening programme: a national survey in England. BMC Cancer. 2018;18:497. doi: 10.1186/s12885-018-4430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quaife SL, Marlow LAV, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect. 2017;20:563–573. doi: 10.1111/hex.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smits SE, McCutchan GM, Hanson JA, Brain KE. Attitudes towards lung cancer screening in a population sample. Health Expect. 2018;21:1150–1158. doi: 10.1111/hex.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62:126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter-Harris L, Brandzel S, Wernli KJ, Roth JA, Buist DSM. A qualitative study exploring why individuals opt out of lung cancer screening. Fam Pract. 2017;34:239–244. doi: 10.1093/fampra/cmw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter-Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: what do long-term smokers know and believe? Health Expect. 2017;20:59–68. doi: 10.1111/hex.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali N, Lifford KJ, Carter B, McRonald F, Yadegarfar G, Baldwin DR, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open. 2015;5:e008254. doi: 10.1136/bmjopen-2015-008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wardle J, Williamson S, McCaffery K, Sutton S, Taylor T, Edwards R, et al. Increasing attendance at colorectal cancer screening: testing the efficacy of a mailed, psychoeducational intervention in a community sample of older adults. Health Psychol. 2003;22:99–105. doi: 10.1037//0278-6133.22.1.99. [DOI] [PubMed] [Google Scholar]
- 21.Hewitson P, Ward AM, Heneghan C, Halloran SP, Mant D. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br J Cancer. 2011;105:475–480. doi: 10.1038/bjc.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerrison RS, McGregor LM, Counsell N, Marshall S, Prentice A, Isitt J, et al. Use of two self-referral reminders and a theory-based leaflet to increase the uptake of flexible sigmoidoscopy in the English bowel scope screening program: results from a randomized controlled trial in London. Ann Behav Med. 2018;52:941–951. doi: 10.1093/abm/kax068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein ND, Sandman PM, Blalock SJ. Health behaviour and health education. San Francisco, CA: Jossey-Bass; 2008. The precaution adoption process model; pp. 123–147. [Google Scholar]
- 24.Miles A, Voorwinden S, Chapman S, Wardle J. Psychologic predictors of cancer information avoidance among older adults: the role of cancer fear and fatalism. Cancer Epidemiol Biomarkers Prev. 2008;17:1872–1879. doi: 10.1158/1055-9965.EPI-08-0074. [DOI] [PubMed] [Google Scholar]
- 25.Brown S, Locker E. Defensive responses to an emotive anti-alcohol message. Psychol Health. 2009;24:517–528. doi: 10.1080/08870440801911130. [DOI] [PubMed] [Google Scholar]
- 26.Quaife S, Ruparel M, Dickson J, Beeken RJ, McEwen A, Baldwin D, et al. The Lung Screen Uptake Trial (LSUT): testing targeted materials to optimise informed uptake among high-risk groups. Ann Behav Med. 2019;53:S21. [Google Scholar]
- 27.Quaife SL, Ruparel M, Beeken RJ, McEwen A, Isitt J, Nolan G, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients. BMC Cancer. 2016;16:281. doi: 10.1186/s12885-016-2316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wardle J, von Wagner C, Kralj-Hans I, Halloran SP, Smith SG, McGregor LM, et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet. 2016;387:751–759. doi: 10.1016/S0140-6736(15)01154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, et al. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen. 2011;18:24–29. doi: 10.1258/jms.2011.011002. [DOI] [PubMed] [Google Scholar]
- 30.Allgood PC, Maxwell AJ, Hudson S, Offman J, Hutchison G, Beattie C, et al. A randomised trial of the effect of postal reminders on attendance for breast screening. Br J Cancer. 2016;114:171–176. doi: 10.1038/bjc.2015.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch EA, New ML, Brown SP, Barón AE, Malkoski SP. Patient reminders and longitudinal adherence to lung cancer screening in an academic setting. Ann Am Thorac Soc. 2019;16:1329–1332. doi: 10.1513/AnnalsATS.201902-152RL. [DOI] [PubMed] [Google Scholar]
- 32.Bevan R, Rubin G, Sofianopoulou E, Patnick J, Rees CJ. Implementing a national flexible sigmoidoscopy screening program: results of the English early pilot. Endoscopy. 2015;47:225–231. doi: 10.1055/s-0034-1378119. [DOI] [PubMed] [Google Scholar]
- 33.Hudson S, Brazil D, Teh W, Duffy SW, Myles JP. Effectiveness of timed and non-timed second appointments in improving uptake in breast cancer screening. J Med Screen. 2016;23:160–163. doi: 10.1177/0969141315624937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Centre for Smoking Cessation and Training (NCSCT) Very brief advice training module. 2014 [accessed 2019 Mar 8] Available from: https://www.ncsct.co.uk/publication_very-brief-advice.php.
- 35.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 36.Kotz D, Brown J, West R. Predictive validity of the Motivation To Stop Scale (MTSS): a single-item measure of motivation to stop smoking. Drug Alcohol Depend. 2013;128:15–19. doi: 10.1016/j.drugalcdep.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making. 1996;16:58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 38.Linder SK, Swank PR, Vernon SW, Mullen PD, Morgan RO, Volk RJ. Validity of a low literacy version of the decisional conflict scale. Patient Educ Couns. 2011;85:521–524. doi: 10.1016/j.pec.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connor AM. User manual - decisional conflict scale [Internet] 2010 [accessed 2019 Mar 8] Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.
- 40.Hersch J, Barratt A, Jansen J, Irwig L, McGeechan K, Jacklyn G, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet. 2015;385:1642–1652. doi: 10.1016/S0140-6736(15)60123-4. [DOI] [PubMed] [Google Scholar]
- 41.von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011;40:712–718. doi: 10.1093/ije/dyr008. [DOI] [PubMed] [Google Scholar]
- 42.Office for National Statistics (ONS) Ethnicity and national identity in England and Wales. 2011 Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11.
- 43.Shaw A, Ibrahim S, Reid F, Ussher M, Rowlands G. Patients’ perspectives of the doctor-patient relationship and information giving across a range of literacy levels. Patient Educ Couns. 2009;75:114–120. doi: 10.1016/j.pec.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 44.von Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: the role of information processing. Patient Educ Couns. 2009;75:352–357. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Kahneman D. Thinking, fast and slow. London: Penguin; 2011. [Google Scholar]
- 46.Kobayashi LC, Waller J, von Wagner C, Wardlê J. A lack of information engagement among colorectal cancer screening non-attenders: cross-sectional survey. BMC Public Health. 2016;16:659. doi: 10.1186/s12889-016-3374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fredman L, Sexton M, Cui Y, Althuis M, Wehren L, Hornbeck P, et al. Cigarette smoking, alcohol consumption, and screening mammography among women ages 50 and older. Prev Med. 1999;28:407–417. doi: 10.1006/pmed.1998.0445. [DOI] [PubMed] [Google Scholar]
- 48.Sutton S, Wardle J, Taylor T, McCaffery K, Williamson S, Edwards R, et al. Predictors of attendance in the United Kingdom flexible sigmoidoscopy screening trial. J Med Screen. 2000;7:99–104. doi: 10.1136/jms.7.2.99. [DOI] [PubMed] [Google Scholar]
- 49.Byrne MM, Davila EP, Zhao W, Parker D, Hooper MW, Caban-Martinez A, et al. Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol. 2010;34:611–617. doi: 10.1016/j.canep.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Vander Weg MW, Howren MB, Cai X. Use of routine clinical preventive services among daily smokers, non-daily smokers, former smokers, and never-smokers. Nicotine Tob Res. 2012;14:123–130. doi: 10.1093/ntr/ntr141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.