To the Editor:
The concept of the “sterile” fetus has been challenged recently (1–4). We previously reported the existence of an airway microbiome at birth in neonates (5) and speculated that this airway microbiome could have fetal origins. The exact source of the infant microbiome is unknown. Other investigators and we have shown that the neonatal microbiome after birth, especially that of the airway, is similar regardless of whether the neonate was delivered vaginally or by cesarean section (5), suggesting that the neonatal microbiome signature could possibly be transplacentally derived and acquired in utero. No studies to date have examined the presence of the fetal lung microbiome in humans from an in utero environment. Therefore, in this study we examined, for the first time, the presence of human fetal and placental microbiomes early in gestation, using state-of-the-art molecular approaches.
Methods
Ethics statement and tissue collection
After informed consent was obtained, deidentified human fetal samples were collected in the United States with institutional review board approval (USC-HS-13-0399 and CHLA-14-2211). The only information collected was regarding gestational age and genetic or structural abnormalities. All human tissues were collected after dilation and curettage or dilation and evacuation via well-established sterile surgical procedures. Lungs and (when available) specimen-matched placentas were collected and snap-frozen for subsequent processing.
Sample collection and workflow
A total of 31 deidentified human fetal tissue samples (18 lungs, 3 intestines, and 10 placentas) from 11 weeks gestation (first trimester) to 20 weeks gestation (second trimester) were collected in the United States via sterile and standardized clinical procedures. The initial microbiome analysis was conducted using the whole genome sequencing (WGS) metagenomic shotgun method. Because these were low-biomass samples, bacteria were not detectable in the samples by WGS (undetectable at an average depth of 13 million reads per sample). Subsequently, we conducted a targeted 16S analysis of the same samples at two independent labs (Lee Kong Chian School of Medicine, Singapore, and the University of Alabama at Birmingham [UAB]). The two labs used different DNA extraction kits and different microbiome analysis pipelines. All 31 samples were analyzed at the UAB, and 26 out of 31 samples (17 lungs, 2 intestines, and 7 placentas) were analyzed in Singapore, owing to a smaller quantity of samples.
DNA extraction and sequencing
Blank DNA extractions from sterile phosphate-buffered saline were performed and served as negative extraction controls. Whole-genome metagenomic shotgun sequencing was performed on a HiSeq 2500 (Illumina) according to previous workflows (6). In parallel, using the same DNA samples, libraries for targeted amplicon sequencing were prepared (7) and sequencing was performed on a MiSeq platform (Illumina).
Bioinformatic analysis
Metagenomic shotgun sequencing reads were aligned to the human reference genome (8). Nonhuman reads were classified using Kraken with default parameters matched (9). Controls from negative PCR and blank DNA extractions were sequenced and assessed using the decontam package to detect potential contaminants (10).
Details regarding the methods used for analyses conducted at the UAB are available in our previous publication (5). Raw sequence data have been deposited in the National Center for Biotechnology Information’s Sequence Read Archive (BioProject Accession No. PRJNA550234).
Results
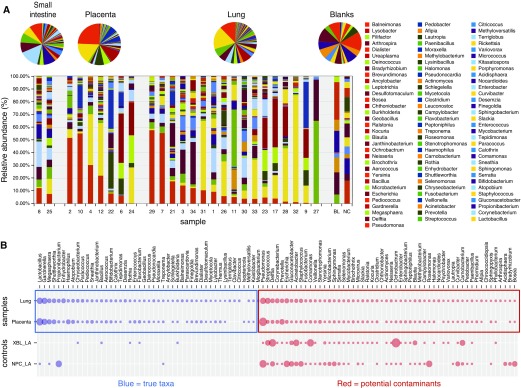
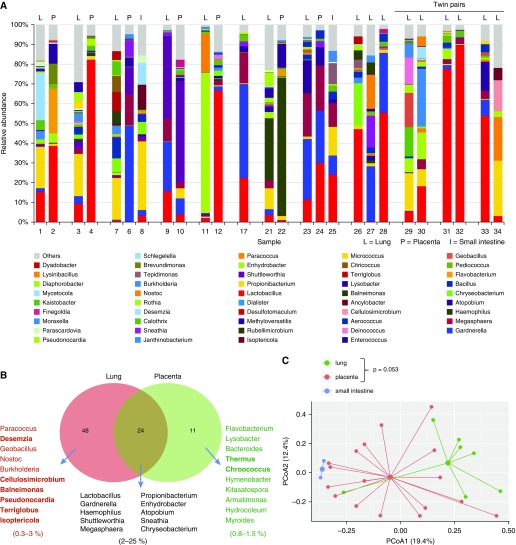
In this targeted analysis, we were able to detect bacterial DNA in all fetal samples at both labs. In the first 16S analysis conducted at Singapore, the lung samples were found to contain 48 unique taxa, whereas in the placenta samples only 11 unique taxa were identified, and 24 taxa were shared (Figure 1A). All data were adjusted for blank sequences, and decontam modules were used to rule out any possible contamination (Figure 1B). In a pairwise lung and placenta analysis, some overlap between lung and placenta microbiome profiles was seen and β-diversity plots suggested some distinct lung and placental microbiome profiles. Although some separation of the microbiome profiles was evident from a principal coordinates analysis (PCoA), the differences in β diversity between the placenta and lung samples did not reach statistical significance (permutational multivariate ANOVA [PERMANOVA], P = 0.053; Figure 2).
Figure 1.
(A) Microbiome analysis of fetal lung, placenta, and small intestine conducted at Singapore. (B) True taxa versus potential contaminants.
Figure 2.
(A) Matched microbiome profiles of lung (L), placenta (P), and intestines (I) from the same patients. Lung microbiome profile of triplets (LLL) and twins (LL). (B) Shared microbiota in fetal lungs and placentas. (C) Principal coordinate plot showing the β diversity of the fetal and placental microbiomes. PCoA = principal coordinates analysis.
The 16S analysis conducted at the UAB on the same samples (total n = 31; 18 lungs, 3 intestines, and 10 placentas) also identified microbiome DNA signature in all samples with the PCoA plot analysis of the human fetal lung and placental microbiomes showing overlap, and no statistical differences (P > 0.1, PERMANOVA). A PCoA plot analysis of the human fetal lung microbiomes split into two separate groups (11–15 wk gestation [n = 3] and 16–20 wk gestation [n = 11]) demonstrated increasing β diversity (P < 0.01, PERMANOVA). Analysis of the distance and clustering (with closer clustering signifying a shared larger proportion of the phylogenetic tree) indicated a significant difference in microbiome diversity between the two gestational age groups. Overall, at both sites, analysis of the bacterial taxa distribution and diversity showed some overlap in the microbiome signatures of fetal lungs and matched placentas.
Discussion
Recent studies have confirmed the presence of a diverse microbiome in humans, including neonates, but the fetal presence of the lung microbiome remains questionable. Herein, we present the first microbiome study conducted on human fetal tissues, in which we demonstrate the presence of microbial DNA in human fetal lungs and placentas as early as 11 weeks gestation.
In summary, our major novel finding is the confirmation of the presence of a human fetal microbiome DNA signature, as early as the first trimester. Although it was not detected by WGS metagenomic analysis owing to low biomass, we were able to detect a microbiome DNA signature on a targeted 16S analysis in two independent analyses. In addition, we identified temporal changes in fetal lung microbiome diversity during development, suggesting maturational changes with advancing gestational age. Although the reason for these maturational changes is unknown, it is possible that they could be related to maternal or intrauterine factors. Our analysis also confirms the existence of a placental microbiome that shows some overlap with the corresponding human fetal lung microbiome, based on the overall microbiome analysis, as well as α and β diversities. We speculate that materno–fetal transfer of microbial DNA (and perhaps of other microbial products and whole live or dead bacteria) is a realistic possibility and may serve to “prime” the developing innate immune system of the fetus and help to establish a normal host–commensal relationship.
Supplementary Material
Footnotes
Supported by grant 17SDG32720009 (C.V.L.) and NIH grant K08HL141652 (C.V.L.).
Author Contributions: D.A.A., S.D., B.G., and D.W. collected the samples. N.A.B.M.A., M.M., S.H.C., D.W., A.G., N.A., and C.V.L. contributed to microbiome analysis. C.V.L. conceived the project and drafted the manuscript. D.A.A., S.D., B.G., N.A.B.M.A., M.M., S.H.C., D.W., A.G., N.A., and C.V.L. edited and approved the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201911-2127LE on January 3, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willyard C. Could baby’s first bacteria take root before birth? Nature. 2018;553:264–266. doi: 10.1038/d41586-018-00664-8. [DOI] [PubMed] [Google Scholar]
- 3.Younge N, McCann JR, Ballard J, Plunkett C, Akhtar S, Araújo-Pérez F, et al. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight. 2019;4:127806. doi: 10.1172/jci.insight.127806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, et al. Human placenta has no microbiome but can contain potential pathogens. Nature. 2019;572:329–334. doi: 10.1038/s41586-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B, et al. The airway microbiome at birth. Sci Rep. 2016;6:31023. doi: 10.1038/srep31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junqueira ACM, Ratan A, Acerbi E, Drautz-Moses DI, Premkrishnan BNV, Costea PI, et al. The microbiomes of blowflies and houseflies as bacterial transmission reservoirs. Sci Rep. 2017;7:16324. doi: 10.1038/s41598-017-16353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Illumina. 16s metagenomic sequencing library preparation: preparing 16S ribosomal RNA gene amplicons for the Illumina MiSeq system; 2013 [accessed 2019 Jun]. Available from: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf.
- 8.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6:226. doi: 10.1186/s40168-018-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.