Abstract
Rationale: Enhancing non–CFTR (cystic fibrosis transmembrane conductance regulator)-mediated anion secretion is an attractive therapeutic approach for the treatment of cystic fibrosis (CF) and other mucoobstructive diseases.
Objectives: To determine the effects of TMEM16A potentiation on epithelial fluid secretion and mucociliary clearance.
Methods: The effects of a novel low-molecular-weight TMEM16A potentiator (ETX001) were evaluated in human cell and animal models of airway epithelial function and mucus transport.
Measurements and Main Results: Potentiating the activity of TMEM16A with ETX001 increased the Ca2+-activated Cl− channel activity and anion secretion in human bronchial epithelial (HBE) cells from patients with CF without impacting calcium signaling. ETX001 rapidly increased fluid secretion and airway surface liquid height in CF-HBE cells under both static conditions and conditions designed to mimic the shear stress associated with tidal breathing. In ovine models of mucus clearance (tracheal mucus velocity and mucociliary clearance), inhaled ETX001 was able to accelerate clearance both when CFTR function was reduced by administration of a pharmacological blocker and when CFTR was fully functional.
Conclusions: Enhancing the activity of TMEM16A increases epithelial fluid secretion and enhances mucus clearance independent of CFTR function. TMEM16A potentiation is a novel approach for the treatment of patients with CF and non-CF mucoobstructive diseases.
Keywords: mucus clearance, mucus hydration, anoctamin-1, calcium-activated chloride channel
At a Glance Commentary
Scientific Knowledge on the Subject
Although increasing anion conductance via CFTR (cystic fibrosis transmembrane conductance regulator) modulation is a clinically validated approach for treating cystic fibrosis (CF), it is only suited to patients with specific mutations.
What This Study Adds to the Field
This report describes a novel positive modulator of the calcium-activated chloride channel, TMEM16A, which enhances anion and fluid secretion in epithelial cells from patients with CF and accelerates mucociliary clearance in vivo. This approach offers the potential to treat all patients with CF as both a standalone therapy and in combination with CFTR modulators.
Cystic fibrosis (CF) is a recessive genetic multiorgan disease that is primarily associated with pulmonary, gastrointestinal, and reproductive tract dysfunction (1). CF is estimated to affect 75,000 patients globally and is caused by loss of function mutations in the anion channel, CFTR (cystic fibrosis transmembrane conductance regulator). Impaired CFTR function results in defective fluid and solute transport across epithelia with consequences for the hydration status and pH of the mucosal environment (2, 3). In the lungs, a major phenotype associated with the loss of CFTR function is a hyperconcentrated mucus gel that is poorly removed from the airways by mucociliary and cough clearance mechanisms (4–6), leading to chronic bacterial infection and a progressive loss of lung function. CFTR modulators, which can partially rescue the function of mutated CFTR, have demonstrated improved clinical outcomes in those patients genetically suited to these therapies and have received recent U.S. Food and Drug Administration approval (7). However, those patients who do not share mutations in CFTR that are susceptible to these drugs will require alternative therapeutics that are mutation independent.
CFTR-independent approaches to treat the ion transport defect in CF include targeting the epithelial sodium channel (ENaC) with blockers to attenuate fluid reabsorption from the airways and increasing the activity of the Ca2+-activated Cl− channel (CaCC) to promote fluid secretion (8). Before understanding the molecular identity of the airway epithelial CaCC, denufosol, an inhaled P2Y2-receptor agonist, was developed as a treatment for CF lung disease (9, 10). In vitro, the denufosol-mediated increase in intracellular Ca2+ concentration ([Ca2+]i) activated the CaCC and attenuated ENaC function in cultured airway epithelial cells, resulting in a transient increase in airway fluid volume (10–12). The subsequent lack of clinical benefit of denufosol has been ascribed to the short pharmacodynamic activity of a compound that depleted intracellular Ca2+ stores and desensitized the receptor. This would render the epithelium refractory to further stimulation by either residual denufosol or the endogenous P2Y2-receptor ligands, ATP and uridine-5′-triphosphate (UTP) (12, 13), potentially slowing mucus transport in the longer term. Furthermore, a global elevation of cellular Ca2+ in the airway epithelium by denufosol also stimulated an acute goblet cell exocytosis (9) that may have negatively impacted changes in lung function subsequent to the increased volume of mucus in the airways. The therapeutic utility of upregulating the CaCC pathway thereby remained an unanswered question.
In 2008, TMEM16A (anoctamin-1) was identified as the gene encoding the airway CaCC (14–16). Like CFTR, TMEM16A conducts both Cl− and HCO3− across the airway epithelium and is expressed in both the surface epithelium and submucosal glands. In contrast to CFTR, TMEM16A is also abundantly expressed in goblet cells and becomes upregulated under inflammatory conditions (14, 17). Using high-throughput screens of compound libraries together with medicinal chemistry optimization, we have identified novel, selective “potentiators” of TMEM16A. We define a TMEM16A potentiator as a compound that enhances the activity of the channel to an endogenous stimulus, rather than directly activating the channel through an elevation of [Ca2+]i, as was the case with denufosol. Herein, we report that the potentiation of TMEM16A channel function using ETX001 enhances airway epithelial anion and fluid secretion in CF bronchial epithelial cells and promotes mucus clearance in in vivo models of mucociliary clearance (MCC) in sheep. These data support the concept of positive modulation of TMEM16A as a target for respiratory diseases and that compounds such as ETX001 represent therapeutic candidates for clinical development. Some of the results of these studies have been previously reported in the form of abstracts (18–20).
Methods
For full details of methods used, data analysis, and the use of statistical tests, please refer to the online supplement.
Cell Culture
Human bronchial epithelial (HBE) cells were provided by Dr. Scott Randell (University of North Carolina at Chapel Hill) from both the University of North Carolina at Chapel Hill and the Cystic Fibrosis Foundation Therapeutics cell collections. A total of eight individual donor-derived cell codes were used that included both F508del homozygous mutations, as well as F508del heterozygous cells in which the second allele was of minimal function (see online supplement for full details). Cells were cultured at air–liquid interface (ALI), as previously described (21). In some studies, HBE cells were treated with IL-13 (10 ng/ml; 48 h) to increase the TMEM16A assay window (22). FRT-hTMEM16A cells (Fischer rat thyroid cells stably expressing human TMEM16Aabc) were provided by Dr. Luis Galietta (Genoa, Italy) and were cultured, as previously described (14). Chinese hamster ovary cells stably expressing full-length sheep TMEM16A (CHO-sTMEM16A) were obtained from SB Drug Discovery (Glasgow, Scotland).
Whole-Cell Patch-Clamp Assay
Whole-cell voltage-clamp recordings from FRT-hTMEM16A or CHO-sTMEM16A were made using the QPatch planar patch-clamp system, as described previously (23). TMEM16A currents were assessed using chloride-selective solutions with calculated free [Ca2+]i buffered at 260 nM, a value measured to give 20% of full current activation. Current rectification ratios were calculated by dividing the magnitude of the outward current at +90 mV by the magnitude of the inward current at −90 mV.
Short-Circuit Current Measurements
HBE cells mounted in Ussing chambers in symmetrical Ringer’s solutions were voltage clamped, as previously described (21). Following the addition of amiloride to inhibit the ENaC-mediated short-circuit current (ISC), the sarco/endoplasmic reticulum Ca2+-ATPase pump inhibitor cyclopiazonic acid (CPA) or UTP was added to the cells to elevate [Ca2+]i levels to enable the efficacy of ETX001 to be evaluated.
Intracellular Ca2+ Measurements
CF-HBE cells, cultured at ALI for 14 to 21 days and pretreated with IL-13 (10 ng/ml; 48 h), were loaded with the Ca2+-sensitive fluorescent reporter dye Calcium 6 (Molecular Devices) for 120 minutes at 37°C in Hanks’ balanced salt solution buffered with 20 mM N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid (pH 7.4). Calcium 6 was used because its Kd for calcium (320 nM) effectively covers the reported physiological range of [Ca2+]i mobilization by purinoceptors in these cells (0.1–1 μM; 24, 25). The effects of UTP and ETX001 were then tested for direct effects on [Ca2+]i by addition to the apical surface of the epithelia before reading in a PHERAstar plate reader (BMG Lab Tech). Cumulative concentration responses to UTP were also established in the presence or absence of ETX001 to test for the potential for the compound to enhance a UTP-stimulated increase in [Ca2+]i.
Airway Surface Liquid Height Assay
HBE cells were washed to remove accumulated mucus and the airway surface liquid (ASL) was stained with Texas-Red dextran, as previously described (23). XZ-confocal microscopy (SP5; Leica) was used to monitor changes in ASL height both before and after the imposition of shear stress (0.5 dyne/cm2) at 14 cycles per minute to mimic tidal breathing (26). CF-HBE cells used for these studies were not pretreated with IL-13.
In Vivo Studies
All animal studies were approved by the Institutional Animal Care and Use Committee of the Mount Sinai Medical Center (Miami, FL). Tracheal mucus velocity (TMV) was measured in conscious sheep that had been administered aerosolized CFTRInh172, a CFTR blocker, to slow the rate of TMV, as previously described (27). Test compounds were administered to sheep by aerosolization into the lungs and effects on TMV were measured. Alternatively, whole-lung MCC was assessed in conscious sheep following inhalation of technecium-99 (99mTc)-labeled sulfur colloid particles (21). These animals had not been pretreated with CFTRInh172. Test compounds were again administered by aerosolization but at 4 hours before assessing MCC.
Results
ETX001 Potentiates Recombinant TMEM16A Currents
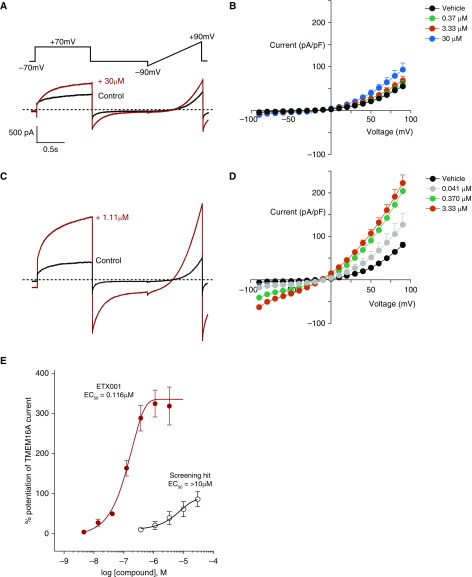
To identify positive modulators of TMEM16A, parallel fluorescence and electrophysiological screening campaigns were used to interrogate a large (>150 K compounds) low-molecular-weight chemical library. After hit triage and confirmation, a low potency primary hit was identified that weakly enhanced TMEM16A currents produced by 260 nM [Ca2+]i in the whole-cell patch-clamp assay at a concentration of 30 μM (Figures 1A and 1B). Subsequent rounds of medicinal chemistry optimization improved potency and efficacy and delivered ETX001. ETX001 robustly potentiated TMEM16A currents in both the inward and outward directions, preserving the characteristic biophysical properties of the channel of outward rectification and time-dependent activation/inactivation (14–16). However, the outward rectification was significantly reduced by ETX001, consistent with a more pronounced effect on the inward (outward chloride ion flow) than the outward currents (inward chloride ion flow). The rectification ratio of 20.3 ± 4.5 in the absence of compound was reduced to 3.7 ± 0.2 by 3.33 μM ETX001 (n = 10; P < 0.002). The effects of ETX001 were concentration dependent with a half-maximal effective concentration (EC50) of 116 ± 16 nM up to a maximum magnitude of 345 ± 36% (n = 14) (Figures 1C–1E). When [Ca2+]i was reduced to 0 nM, below the threshold for TMEM16A activity, ETX001 was without effect, consistent with its effect being channel potentiation rather than activation (see Figure E1 in the online supplement). Using the identical FRT-hTMEM16A cell line cultured as a monolayer on Snapwell inserts, ETX001 also potentiated UTP-stimulated current changes in ion transport experiments (see Figure E2).
Figure 1.
ETX001 is a potent and efficacious potentiator of human recombinant TMEM16A (anoctamin-1). (A and C) Whole-cell patch-clamp electrophysiological recordings from Fischer rat thyroid cells stably expressing hTMEM16A in the absence (black lines) and presence (red lines) of primary screening hit (A) and the optimized compound ETX001 (C) at indicated concentrations. Dashed lines indicate the zero-current level. (B and D) Current–voltage relationships for vehicle and indicated concentrations of compounds are shown. (E) Concentration–response curves for the primary screening hit and ETX001. Mean data (±SEM) (screening hit n = 5; ETX001 n = 14) are shown. EC50 = half-maximal effective concentration.
Potentiation of Calcium-activated Anion Secretory Currents in Primary HBE Cells from Patients with CF by ETX001
The effects of ETX001 on the anion secretory currents in fully differentiated HBE cells from patients with CF grown at ALI and pretreated with IL-13 for 48 hours was assessed in two formats of the ISC assay. They first used a concentration required to give 20% of the maximal effect (EC20) of the sarco/endoplasmic reticulum Ca2+-ATPase pump inhibitor CPA to elevate [Ca2+]i, providing a stable baseline to assess the cumulative concentration response to ETX001. In this format, ETX001 effectively enhanced the anion secretory currents, an effect that was fully inhibited by the selective TMEM16A blocker, Ani9 (Figure 2A). The EC50 for ETX001 was 177 ± 23 nM with a 262 ± 22% potentiation of baseline current (n = 52 inserts from eight different CF donor codes). Potency and efficacy values from the primary cell assay were found to correlate well with the recombinant system used in the QPatch assay for the ETX series of TMEM16A potentiators (Figure 2C).
Figure 2.
ETX001 potentiates the calcium-activated anion secretory current in primary cystic fibrosis (CF)–human bronchial epithelial (HBE) cells. (A) Sample raw data trace illustrating the experimental protocol for assessing the effects of TMEM16A (anoctamin-1) potentiator compounds. Increasing concentrations of ETX001 were added to CF-HBE cells following stimulation of the short-circuit current (ISC) with a submaximal (EC20) concentration of cyclopiazonic acid (CPA). Ani9 (10 μM; downward arrow) was added at the completion of each experiment to confirm the sensitivity of the ISC response to the TMEM16A blocker. (B) Sample concentration–response data derived from the raw data in A. (C) Correlation of data from the CPA ISC assay to the QPatch whole-cell patch-clamp assay. ETX001 is shown as a solid red circle. (D) Sample raw data trace illustrating the effects of ETX001 (1 μM) on the UTP-stimulated anion current response. After inhibition of the ENaC current with amiloride (10 μM), inserts were treated with either DMSO (gray dashed trace) or 1 μM ETX001 (red line) before the cumulative addition of increasing concentrations of UTP. (E) Mean data illustrating the potentiation of the UTP concentration response by ETX001. Data are expressed as the mean of 6 to 8 inserts of CF-HBE cells (donor: KK036H). See online supplement for additional donor-derived CF-HBE cell data. All CF-HBE cells in A to E were IL-13 treated (10 ng/ml) for 48 hours before ion transport assay to optimize the TMEM16A assay window. (F and G) CF-HBE cells that had not been treated with IL-13 also showed a UTP-stimulated (10 μM) anion secretory response that was potentiated by ETX001. *P < 0.005 and **P < 0.001 using a two-way ANOVA with Siadak’s multiple comparison test; #P < 0.05 using two-tailed Student’s t test. AUC = area under the curve; EC50 = half-maximal effective concentration; UTP = uridine-5′-triphosphate.
The second assay format assessed the effect of ETX001 on anion currents elicited in HBE cells using a physiologically relevant stimulus, the P2Y2-receptor agonist UTP. As shown in Figure 2D, ETX001 (1 μM) significantly potentiated the magnitude of the anion current response to increasing concentrations of UTP, enhancing both the peak current and the duration of the current response as evidenced by the residual current level at the end of each UTP response. Integrating the area under the curve for each addition, the potentiating effect of ETX001 could be quantified (Figures 2E and E4). ETX001 also potentiated the UTP-stimulated anion current in non–IL-13 treated CF-HBE cells (Figures 2F and 2G).
The ion transport effect of ETX001 in HBE cells was selective and limited to the calcium-activated chloride conductance. Pretreatment of HBE cells with ETX001 revealed no effects on either the amiloride-sensitive ISC response (ENaC-dependent) or the forskolin-stimulated anion secretion (CFTR-dependent) (see Figure E5).
To build confidence in a direct effect of ETX001 on TMEM16A function, we evaluated whether there was any impact of ETX001 on [Ca2+]i in CF-HBE cells. It has been suggested that TMEM16A activity can regulate the levels of [Ca2+]i in epithelial cells (28) and activation of TMEM16A can elevate [Ca2+]i. ETX001 was without effect on the baseline levels of [Ca2+]i per se (Figure 3B), or the magnitude and sensitivity of the response to the calcium-mobilizing P2Y2-receptor agonist UTP (Figures 3B and 3C).
Figure 3.
ETX001 does not affect calcium handling in differentiated cystic fibrosis–human bronchial epithelial (HBE) cells. (A) A cartoon illustrating the experimental protocol for assessing effects of compounds on [Ca2+]i in air–liquid interface cultures of HBE cells. (B and C) ETX001 (1 μM) had no direct effect on [Ca2+]i levels (B) and also had no effect on the UTP-stimulated increase in [Ca2+]i (C). Mean data ± SD (n = 6 inserts per group) are shown from a single donor. addn/incubn = addition/incubation; ALI = air–liquid interface; AM = acetoxymethyl ester; RFU = relative fluorescence units; UTP = uridine-5′-triphosphate.
ETX001 Increases Fluid Secretion and ASL Height in CF-HBE Cells
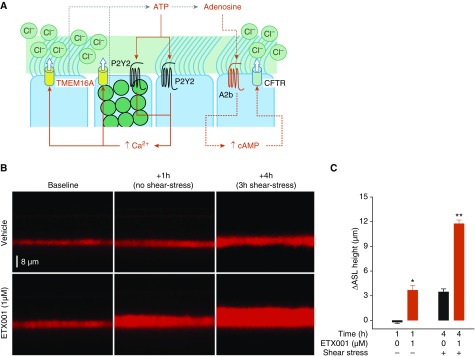
To establish whether potentiation of TMEM16A-mediated anion currents could ultimately enhance airway fluid secretion, a model of shear stress–induced mucosal hydration was used (26). In this system, CF-HBE cells were exposed to conditions designed to mimic the shear stress associated with tidal breathing. These forces stimulate the endogenous release of ATP and adenosine, which regulate both [Ca2+]i and [cAMP]i, respectively, thereby controlling TMEM16A and CFTR function (29, 30) (Figure 4A). Fluid secretion could then be quantified by measuring the change in height of the ASL using confocal microscopy. ETX001 (1 μM) was able to significantly increase fluid secretion and ASL height in CF-HBE cells from multiple donors. Following exposure to ETX001 for 60 minutes under static conditions (no shear stress), ASL height increased compared with the vehicle control (Figures 4B and 4C). The subsequent imposition of cyclical shear stress induced an increase in ASL height in the vehicle-treated CF-HBE cells that was enhanced in the ETX001-treated epithelia (Figures 4B and 4C).
Figure 4.
ETX001 enhances fluid secretion in cystic fibrosis–human bronchial epithelial (HBE) cells under static and shear stress–stimulated conditions. (A) A cartoon illustrating the physiological regulation of anion secretion in the human airway epithelium. (B) Sample XZ confocal images of airway surface liquid (ASL) visualized using Texas red staining in the absence and presence of ETX001 (1 μM). (C) The change in ASL height from baseline was measured following a 1-hour treatment with ETX001 or vehicle under static conditions (i.e., no shear stress) and after 3 hours of continual shear stress. Data are expressed as the mean change in ASL height (±SEM) for seven to eight inserts from a F508del/F508del HBE cells donor and is representative of three independent experiments. Cystic fibrosis–HBE cells had not been pretreated with IL-13. *P < 0.001 and **P < 0.00001 for ETX001-treated versus time-matched vehicle control using two-tailed Student’s t test. TMEM16A = anoctamin-1.
Potentiation of TMEM16A Enhances MCC In Vivo
In vitro, ETX001 potentiated recombinant sheep TMEM16A with an EC50 of 127 ± 21 nM and a magnitude of 554 ± 104% (n = 7; see Figure E6). We also demonstrated the expression of TMEM16A in primary cultures of ovine tracheal epithelial cells and the presence of a robust UTP-stimulated anion secretory current in these cells (see Figure E6). The ability of inhaled ETX001 to affect the rate and magnitude of mucus clearance in vivo was assessed using two sheep models. Dosing sheep with inhaled CFTRInh172 slowed the rate of TMV over a 4-hour period after administration (Figure 5A). TMV remained attenuated for the duration of the study. Dosing with inhaled ETX001 after previously slowing TMV with CFTRInh172 restored the rate of TMV to 90% to 95% of the healthy baseline rate (Figure 5A). The second model assessed whole-lung MCC rates by measuring the clearance of aerosolized 99mTc sulfur colloid in naive sheep (i.e., with fully functional CFTR). Clearance was assessed 4 hours after inhaled dosing of either vehicle or ETX001. As shown in Figure 5B, ETX001 gave a robust, durable, and dose-dependent enhancement of MCC in this model.
Figure 5.
Inhaled ETX001 accelerates mucociliary clearance in conscious sheep models. (A) Dosing of conscious sheep with CFTRInh172 slowed the rate of tracheal mucus velocity (TMV). At 4 hours after dosing with CFTRinh172, ETX001 or vehicle was administered as an inhaled, nebulized, DMSO-based solution. TMV values are expressed as the mean (±SEM) % of the initial (pre-CFTRInh172) rate (n = 3 sheep per group). (B) To assess whole-lung clearance, ETX001 or vehicle were dosed as an inhaled, nebulized suspension 4 hours before the inhaled administration of technetium-labeled sulfur colloid (99mTc-SC). Mean (±SEM) clearance of 99mTc-SC in sheep is expressed as the percentage of the initial deposited lung dose (n = 3–4 sheep per group). Symbols denote a significant difference from the time-matched vehicle control animals using two-way ANOVA with a post hoc Dunnett’s test. #P < 0.01, ##P < 0.001, and *P < 0.0001.
Discussion
In this study we present data that validates the concept that potentiating the activity of the Ca2+-activated chloride conductance TMEM16A in airway epithelia can enhance MCC, thus offering a novel therapeutic approach for the treatment of patients with CF and other diseases characterized by mucus obstruction. Importantly, because TMEM16A expression and function is independent of the disease-causing mutations in CFTR, this therapeutic approach is predicted to be suitable for all patients with CF (8).
The identification of low-molecular-weight TMEM16A potentiator compounds such as ETX001 has enabled the physiological relevance of enhancing CaCC activity, with respect to anion and fluid secretion in the airway epithelium, to be evaluated. ETX001 was identified as a potent potentiator of TMEM16A function in a patch-clamp assay using recombinantly expressed TMEM16A (Figure 1). The pharmacology of ETX001 translated from the whole-cell electrophysiology format into epithelial ion transport studies using either the same FRT-hTMEM16A cell line (see Figure E2) or CF-HBE cells (Figure 2). The correlation between the patch-clamp and the CF-HBE cell ion transport assays that was observed with more than 50 compounds from the ETX001 series (Figure 2C) builds confidence that the Ca2+-dependent chloride secretory current in the native CF cells is dominated by a TMEM16A-mediated mechanism. This contrasts to a report that TMEM16A plays only a minor role in airway CaCC function (31). This study suggested that only a minor component of the CF-HBE cell CaCC-mediated response was sensitive to the TMEM16A inhibitor, T16A(inh)-A01. The reason for this inconsistency is likely due to only a partial inhibition of TMEM16A by T16A(inh)-A01 in patch-clamp studies (unpublished observations), as well as questions relating to the real target of this compound (32). In terms of selectivity versus other ion channels expressed in the airway epithelium, ETX001 was without effect on either CFTR-mediated secretory responses in non–CF-HBE cells or on ENaC-mediated currents. In HBE cells, both CFTR and ENaC currents depend on accessory ion channels and transporters (e.g., NKCC1, ATP1A1, and Kv1.7) to maintain ion transport function (33), which are presumably likewise unaffected by ETX001 treatment at pharmacologically relevant concentrations.
Having established that ETX001 and related compounds could potentiate TMEM16A function under conditions in which [Ca2+]i was predicted to be static (i.e., buffered at 260 nM in patch-clamp or stable following stimulation with an EC20 concentration of CPA in CF-HBE cells), it was important to establish whether the pharmacology was observed over a physiologically relevant range of [Ca2+]i. To address this, the P2Y2-receptor agonist UTP was added to IL-13 pretreated CF-HBE cells at increasing concentrations in the presence or absence of ETX001. ETX001 enhanced the exogenous UTP-induced secretory current response of CF-HBE cells (Figure 2D), causing both a leftward shift in the UTP concentration response and enhancing the maximum efficacy (see Figure 2E and additional donors in Figure E4), consistent with the ability of ETX001 to potentiate TMEM16A function across a physiologically relevant range of [Ca2+]i. In these studies, CF-HBE cells had been pretreated with IL-13 to increase the expression and function of TMEM16A and thereby maximize the assay window (17). The upregulation of TMEM16A function by IL-4 and IL-13 has been proposed to be a regulatory mechanism to ensure that mucin and fluid production by the airway remain in balance and the present data therefore support a potentiator activity of ETX001 under these inflammatory conditions. However, the potentiator activity of ETX001 was independent of an inflamed environment because efficacy was also observed in IL-13–naive CF-HBE cells (Figures 2F and 2G).
The use of P2Y2-receptor ligands in these studies is particularly relevant because the local release of ATP and UTP into the airway mucosa represents a key regulatory mechanism in the control of ion transport and fluid homeostasis (29, 30) (Figure 4). The release of these mediators is a kinetic process that is stimulated by shear and compressive forces during normal breathing (26, 34, 35) and is proposed to adapt to maintain mucosal hydration as the respiration rate changes following the impaction of inhaled particulates on the epithelium and during cough (36, 37). Tarran and colleagues (26) illustrated that imposing cyclical shear stress to CF-HBE cells to mimic tidal breathing induced an ATP-dependent increase in fluid secretion, measured as an increase in height of ASL. Using a similar model system, ETX001 enhanced the fluid secretory response of CF-HBE cells (Figure 4). The ETX001-enhanced increase in fluid secretion was observed both before and after the initiation of shear stress, further supporting the conclusion that potentiation of TMEM16A could be achieved over the range of resting to physiologically stimulated [Ca2+]i.
Two in vivo sheep models were used to evaluate the potential for ETX001 to increase mucus clearance. Inhaled dosing of ETX001 to conscious sheep enhanced the rates of airway mucus clearance in the CFTR-inhibited TMV model (27) and in the whole-lung clearance model in which CFTR function is normal (21). Nominal doses in the range of 30 to 100 μg/kg provided a long-lasting enhancement of mucus clearance in both models. The efficacy observed in the whole-lung MCC model in which CFTR function is normal provides early data to support the concept that a TMEM16A potentiator may show benefit in patients with CF who are treated with CFTR potentiator and corrector therapies, as well as in patients without CF who have alternative mucoobstructive lung disease.
To our knowledge, this is the first report of the discovery and efficacy of TMEM16A potentiator compounds. Compounds initially reported to be direct modulators of the channel (e.g., Eact) (38) have subsequently been shown to act indirectly via elevation of [Ca2+]i (39, 40), negating their utility for validating CaCC function due to the numerous other cellular effects elevation of [Ca2+]i will trigger (e.g., goblet cell exocytosis and bronchospasm). In contrast, ETX001 potentiates the opening of the TMEM16A channel (i.e., it enhances the magnitude of the current in the presence of physiologically regulated [Ca2+]i), but ETX001 does not cause increases in [Ca2+]i per se. This conclusion is supported by two pieces of data. First, ETX001 enhances current in whole-cell patch-clamp studies when [Ca2+]i is clamped and heavily buffered with a combination of both fast (BAPTA) and slow (EGTA) chelators. Second, ETX001 was shown to have no effect on [Ca2+]i levels in fully differentiated CF-HBE cells per se nor any effect on the response to a calcium-mobilizing agonist, UTP (Figure 3). These findings are in contrast to others that have suggested positive TMEM16A modulation will lead to increases in [Ca2+]i, albeit those studies have used extrapolations from knockdown approaches and nonselective compounds that are proposed to be TMEM16A blockers (28). It has recently been suggested that inhibiting the function of TMEM16A may be of therapeutic benefit for the treatment of CF (41). This proposal is based on reports that nonselective regulators of TMEM16A function may have effects on airway smooth muscle and mucin release from goblet cells (42, 43). To date, we have observed no effects of TMEM16A potentiator compounds on airway smooth muscle function (in vitro) or lung function (in vivo) (unpublished observations). Likewise, there were no effects of ETX001 or related compounds on the central to peripheral ratios of 99mTc in the lung scans from the sheep MCC model, changes that would have been expected were there a bronchospasm associated with TMEM16A potentiator administration (unpublished observations). Selective potentiators and blockers of TMEM16A will enable a thorough evaluation of the physiological role of this channel in a variety of biological systems.
Conclusions
The concept that enhancing airway anion secretion will provide clinical benefit to many patients with CF is now well established with CFTR potentiator and corrector therapies (1). Whether clinical efficacy can be established by enhancing the anion secretory function of TMEM16A in the airways remains to be established. The positive effects of ETX001 on both airway fluid secretion in CF-HBE cells and mucus clearance in vivo, together with the population of patients with CF who are not genetically matched to existing CFTR repair therapies, build a case for testing the hypothesis in the clinic.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Drs. Luis Galietta (Telethon Institute of Genetics and Medicine, Italy) and Scott Randell (University of North Carolina at Chapel Hill) for the provision of the FRT (hTMEM16Aabc and CFTR) and HBE cells, respectively. They acknowledge the long-standing support of the late Dr. William Abraham (Mount Sinai Medical Center) to the authors and this work.
Footnotes
Supported by Enterprise Therapeutics Limited and a Cystic Fibrosis Foundation Therapeutics Development Award to Enterprise Therapeutics Limited.
Author Contributions: Performed experiments: H.L.D., S.L., R.F., H.C., J.S., and B.B. Compounds design and synthesis: C.M. and S.P.C. Study concept and design: H.L.D., J.S., B.B., and M.G. Analysis and interpretation: H.L.D., S.L., R.F., H.C., J.S., B.B., and M.G. Drafting the manuscript for important intellectual contribution: H.L.D. and M.G.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201908-1641OC on January 3, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher RC. Muco-obstructive lung diseases. N Engl J Med. 2019;380:1941–1953. doi: 10.1056/NEJMra1813799. [DOI] [PubMed] [Google Scholar]
- 4.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill DB, Long RF, Kissner WJ, Atieh E, Garbarine IC, Markovetz MR, et al. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur Respir J. 2018;52:1801297. doi: 10.1183/13993003.01297-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Button B, Goodell HP, Atieh E, Chen YC, Williams R, Shenoy S, et al. Roles of mucus adhesion and cohesion in cough clearance. Proc Natl Acad Sci USA. 2018;115:12501–12506. doi: 10.1073/pnas.1811787115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med. 2013;1:158–163. doi: 10.1016/S2213-2600(12)70057-7. [DOI] [PubMed] [Google Scholar]
- 8.Mall MA, Danahay H, Boucher RC. Emerging concepts and therapies for mucoobstructive lung disease. Ann Am Thorac Soc. 2018;15(Suppl_3):S216–S226. doi: 10.1513/AnnalsATS.201806-368AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, et al. Pharmacology of INS37217 [P(1)-(uridine 5′)-P(4)- (2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- 10.Kellerman D, Rossi Mospan A, Engels J, Schaberg A, Gorden J, Smiley L. Denufosol: a review of studies with inhaled P2Y(2) agonists that led to Phase 3. Pulm Pharmacol Ther. 2008;21:600–607. doi: 10.1016/j.pupt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- 12.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal. 2013;6:ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss RB. Pitfalls of drug development: lessons learned from trials of denufosol in cystic fibrosis. J Pediatr. 2013;162:676–680. doi: 10.1016/j.jpeds.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 17.Scudieri P, Caci E, Bruno S, Ferrera L, Schiavon M, Sondo E, et al. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol. 2012;590:6141–6155. doi: 10.1113/jphysiol.2012.240838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danahay H, Lilley S, Charlton H, Fox R, Button B, Gosling M. The pharmacology of novel TMEM16A potentiator compounds. Presented at the 16th European Cystic Fibrosis Society Basic Science Conference. March 27–30, 2019, Dubrovnik, Croatia. Abstract P102, p. 77.
- 19.Danahay H, Lilley S, Charlton H, Fox R, Button B, Gosling M. WS03-6 TMEM16A potentiators: a new therapeutic opportunity for treating cystic fibrosis-related lung disease. J Cyst Fibros. 2019;18:S6. [Google Scholar]
- 20.Danahay H, Lilley S, Charlton H, Fox R, Button B, Sabater J, et al. The in vitro and in vivo pharmacology of novel TMEM16A potentiator compounds. Pediatr Pulmonol. 2019;54:532P. [Google Scholar]
- 21.Coote KJ, Paisley D, Czarnecki S, Tweed M, Watson H, Young A, et al. NVP-QBE170: an inhaled blocker of the epithelial sodium channel with a reduced potential to induce hyperkalaemia. Br J Pharmacol. 2015;172:2814–2826. doi: 10.1111/bph.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L226–L236. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- 23.Bill A, Popa MO, van Diepen MT, Gutierrez A, Lilley S, Velkova M, et al. Variomics screen identifies the re-entrant loop of the calcium-activated chloride channel ANO1 that facilitates channel activation. J Biol Chem. 2015;290:889–903. doi: 10.1074/jbc.M114.618140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradiso AM, Ribeiro CMP, Boucher RC. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol. 2001;117:53–67. doi: 10.1085/jgp.117.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro CMP, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- 26.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott DW, Walker MP, Sesma J, Wu B, Stuhlmiller TJ, Sabater JR, et al. SPX-101 is a novel epithelial sodium channel-targeted therapeutic for cystic fibrosis that restores mucus transport. Am J Respir Crit Care Med. 2017;196:734–744. doi: 10.1164/rccm.201612-2445OC. [DOI] [PubMed] [Google Scholar]
- 28.Cabrita I, Benedetto R, Fonseca A, Wanitchakool P, Sirianant L, Skryabin BV, et al. Differential effects of anoctamins on intracellular calcium signals. FASEB J. 2017;31:2123–2134. doi: 10.1096/fj.201600797RR. [DOI] [PubMed] [Google Scholar]
- 29.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boedtkjer DM, Kim S, Jensen AB, Matchkov VM, Andersson KE. New selective inhibitors of calcium-activated chloride channels - T16A(inh) -A01, CaCC(inh) -A01 and MONNA - what do they inhibit? Br J Pharmacol. 2015;172:4158–4172. doi: 10.1111/bph.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boucher RC. Human airway ion transport: part one. Am J Respir Crit Care Med. 1994;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- 34.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 35.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodd ME, Prasad SA. Physiotherapy management of cystic fibrosis. Chron Respir Dis. 2005;2:139–149. doi: 10.1191/1479972305cd078ra. [DOI] [PubMed] [Google Scholar]
- 37.Wheatley CM, Baker SE, Morgan MA, Martinez MG, Liu B, Rowe SM, et al. Moderate intensity exercise mediates comparable increases in exhaled chloride as albuterol in individuals with cystic fibrosis. Respir Med. 2015;109:1001–1011. doi: 10.1016/j.rmed.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 2011;25:4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Feng J, Luo J, Yang P, Brett TJ, Hu H. Eact, a small molecule activator of TMEM16A, activates TRPV1 and elicits pain- and itch-related behaviours. Br J Pharmacol. 2016;173:1208–1218. doi: 10.1111/bph.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genovese M, Borrelli A, Venturini A, Guidone D, Caci E, Viscido G, et al. TRPV4 and purinergic receptor signalling pathways are separately linked in airway epithelia to CFTR and TMEM16A chloride channels. J Physiol. 2019;597:5859–5878. doi: 10.1113/JP278784. [DOI] [PubMed] [Google Scholar]
- 41.Cabrita I, Benedetto R, Schreiber R, Kunzelmann K. Niclosamide repurposed for the treatment of inflammatory airway disease. JCI Insight. 2019;4:128414. doi: 10.1172/jci.insight.128414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA. 2012;109:16354–16359. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miner K, Labitzke K, Liu B, Wang P, Henckels K, Gaida K, et al. Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways. Front Pharmacol. 2019;10:51. doi: 10.3389/fphar.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.