To the Editor:
Despite improvements in supportive treatments during the past four decades, females with cystic fibrosis (CF) have worse outcomes than males. A recent registry analysis showed median life expectancy of 38.7 years in males and 36.0 years in females (1). The reasons for the sex gap are not fully understood but may be a result of the effect of estrogen on inflammation (2), infection (3), and/or ion transport (4). Recently, several CFTR (cystic fibrosis transmembrane conductance) modulators have been approved on the basis of their ability to improve percentage of predicted FEV1, body mass index (BMI), sweat chloride, quality of life, and pulmonary exacerbation (PEx) rate (5, 6). The published CFTR modulator clinical trials have reported no significant differences in FEV1 improvement between sexes (5–7). However, studies have not reported on the effect of sex on other important clinical domains including body weight, sweat chloride change, and PEx. Based on the sex gap in CF, it is plausible to consider sex-specific differences in CFTR modulator responses. Thus, we performed a retrospective analysis of the GOAL (G551D Observational) cohort to determine whether females respond as well as males to ivacaftor.
We used data from the GOAL study, which enrolled patients at least 6 years of age with at least one G551D mutation who were beginning ivacaftor. One- and 3-month changes in FEV1, weight, BMI, and sweat chloride were assessed and analyzed for differences based on sex. Although the cohort was followed for 5 years, we chose to analyze short-term differences to avoid confounding of our results by weight changes and hormonal influences from puberty, which may affect outcome measures over time. Linked participant clinical data from the U.S. Cystic Fibrosis Foundation National Patient Registry augmented study data. Annualized PEx rates (treated in hospital or at home with intravenous antibiotics) were calculated for the 2-year period before and the 2.25-year period after ivacaftor initiation. The study was approved by the Institutional Review Board, and written informed consent was obtained. Some of the results of this study have been previously reported in an abstract (8).
Overall, 144 study participants were included; the clinical characteristics for the GOAL cohort have been described in detail previously (9). Participants were 46% female, with mean age of 21.1 years; 49.3% of females and 41.6% of males were between 6 and 17 years of age. Baseline characteristics were similar except that females weighed less than males (48.9 kg vs. 59.1 kg; P = 0.001), and PEx rate before study entry trended greater for females (1.7 PEx/yr vs. 1.1 PEx/yr; P = 0.064). There was no significant difference in Pseudomonas aeruginosa infection, inhaled antibiotics, mucolytics, or oral antibiotics during the study period.
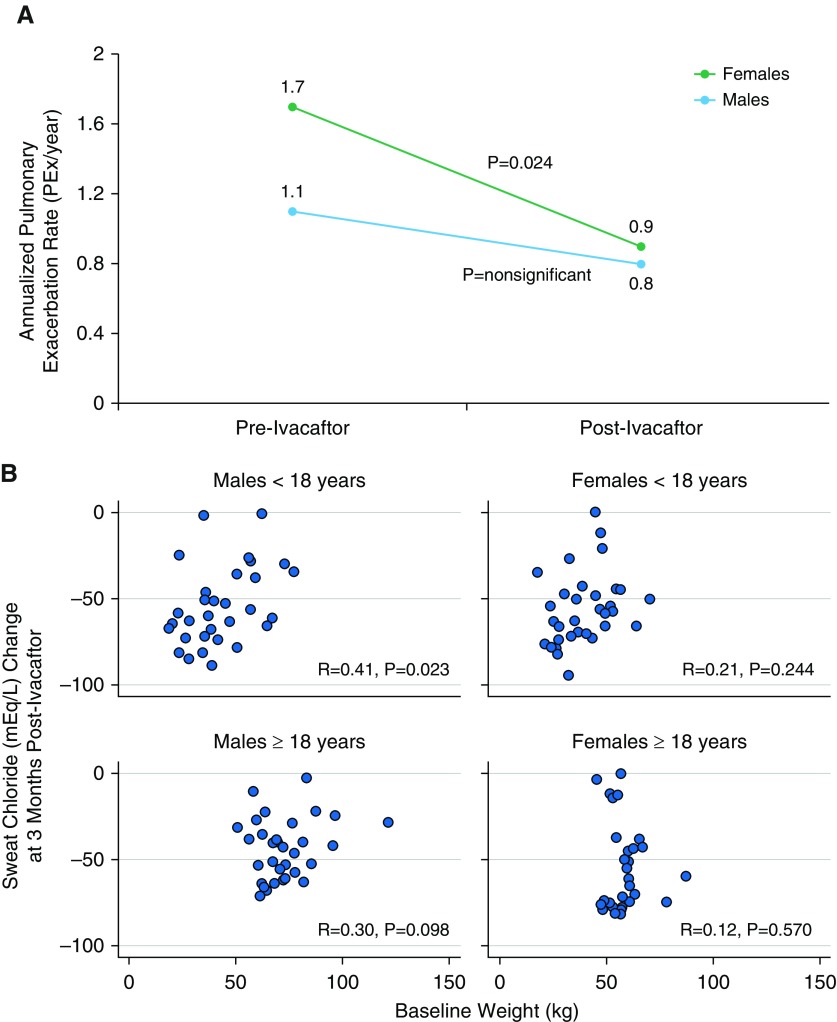
After 1 month of ivacaftor, females and males had similar changes in percentage predicted FEV1 and BMI, although the absolute change in weight was less in females (Table 1). After 3 months, sweat chloride decreased by 55.5 mEq/L in females and 48.8 mEq/L in males (P = 0.045), achieving mean values of 46.8 and 54.7 mEq/L, respectively (Table 1). During the 2.25 years of follow-up, 35.8% of females and 28.6% of males experienced at least 1 pulmonary exacerbation requiring intravenous antibiotics. Despite a greater baseline frequency of exacerbations, females experienced a significant reduction in PEx rate from 1.7 PEx/yr to 0.9 PEx/yr (P = 0.024), whereas the PEx rate in males decreased from 1.1 PEx/yr to 0.8 PEx/yr (P = not significant; Figure 1A). Further analysis showed that 31/67 (46.3%) females compared with 21/77 (27.3%) males had a reduction in PEx rate after ivacaftor (P = 0.024).
Table 1.
Differences in Clinical Responses in Ivacaftor-treated Males and Females with CF
| Variable | Males |
Females |
P Value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | 95% CI | n | Mean (SD) | 95% CI | ||
| ppFEV1 | |||||||
| Baseline | 77 | 80.4 (24.7) | 74.8 to 86.0 | 67 | 81.9 (25.8) | 75.6 to 88.2 | 0.644 |
| 1 mo | 77 | 87.0 (25.1) | 81.3 to 92.7 | 67 | 88.5 (24.4) | 82.6 to 94.5 | 0.642 |
| Absolute change at 1 mo | — | 6.6 (8.5) | 4.7 to 8.6 | — | 6.6 (10.3) | 4.1 to 9.1 | 0.487 |
| 3 mo | 72 | 86.4 (25.6) | 80.5 to 92.3 | 65 | 88.1 (24.1) | 82.2 to 93.9 | 0.691 |
| Absolute change at 3 mo | — | 5.5 (6.9) | 3.9 to 7.1 | — | 5.0 (8.8) | 2.9 to 7.2 | 0.711 |
| BMI, kg/m2 | |||||||
| Baseline | 77 | 21.6 (4.4) | 20.6 to 22.6 | 67 | 20.5 (4.1) | 19.5 to 21.5 | 0.059 |
| 1 mo | 77 | 22.0 (4.4) | 21.0 to 23.0 | 67 | 20.8 (4.0) | 19.8 to 21.8 | 0.039 |
| Absolute change at 1 mo | — | 0.5 (0.6) | 0.3 to 0.6 | — | 0.3 (0.5) | 0.2 to 0.4 | 0.078 |
| 3 mo | 74 | 22.2 (4.5) | 21.1 to 23.3 | 65 | 20.9 (4.2) | 19.9 to 22.0 | 0.043 |
| Absolute change at 3 mo | — | 0.7 (0.8) | 0.5 to 0.8 | 0.5 (1.0) | 0.2 to 0.7 | 0.099 | |
| Sweat chloride, mEq/L | |||||||
| Baseline | 74 | 104.0 (13.5) | 100.9 to 107.1 | 66 | 101.9 (14.6) | 97.7 to 104.9 | 0.13 |
| 1 mo | 69 | 57.4 (23.6) | 51.7 to 63.0 | 65 | 52.6 (23.5) | 46.8 to 58.4 | 0.123 |
| Absolute change at 1 mo | — | −45.8 (19.0) | −50.5 to −41.2 | — | −49.1 (22.6) | −54.8 to −43.5 | 0.18 |
| 3 mo | 65 | 54.7 (22.5) | 49.1 to 60.2 | 62 | 46.8 (23.8) | 40.7 to 52.8 | 0.029 |
| Absolute change at 3 mo | — | −48.8 (20.7) | −54.0 to −43.7 | — | −55.5 (23.0) | −61.4 to −49.7 | 0.045 |
| Annualized PEx rate per year* | 77 | 0.8 (2.0) | 0.4 to 1.3 | 67 | 0.9 (1.6) | 0.5 to 1.3 | 0.421 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; CF = cystic fibrosis; PEx = pulmonary exacerbation; pp = percentage predicted via Global Lung Function Initiative equations.
Bold emphasizes statistically significant P values.
Annualized pulmonary exacerbation rate was calculated for the 2.25 years after ivacaftor initiation.
Figure 1.
Differences in clinical response to ivacaftor, by sex. (A) Reduction in annualized pulmonary exacerbation rate after ivacaftor treatment, by sex. Females experienced a greater reduction in annualized pulmonary exacerbation rate than males while receiving ivacaftor. “Pre-Ivacaftor” represents the 2-year period before ivacaftor initiation. “Post-Ivacaftor” represents the 2.25-year period after ivacaftor initiation. (B) Correlation between sweat chloride change and baseline weight after ivacaftor treatment, by sex and age. The strongest correlation between sweat chloride change and baseline weight was seen in males younger than 18 years. PEx = pulmonary exacerbation.
We then assessed the relationships among age, weight, and sex on sweat chloride change. Among subjects aged 18 years and older, women had a lower mean baseline weight (58.6 vs. 72.7 kg; P < 0.0001) and a greater sweat chloride decrease (−55.2 vs. −44.1 mEq/L; P = 0.025) than men. In contrast, in subjects younger than 18 years, girls and boys had similar baseline weights (39.5 vs. 42.9 kg) and similar changes in sweat chloride (−55.8 vs. −53.9 mEq/L). There was a small but significant correlation (r = 0.29; P = 0.001) between weight and sweat chloride decrease in the overall cohort. When stratified by age and sex, the strongest correlation between weight and sweat chloride decrease was in boys younger than 18 years (r = 0.41; P = 0.023; Figure 1B).
In this letter, we report three novel findings. The first is that ivacaftor-treated females with CF had a greater reduction in PEx than males with CF, noting that the baseline rate was higher in females. Second, females had a greater reduction in sweat chloride in response to ivacaftor than males. Finally, the sweat chloride response to ivacaftor is correlated with baseline weight. These data suggest that although females and males with CF showed similar salutary responses to ivacaftor in FEV1 and BMI, there may be important differential responses based on both sex and body weight.
The greater reduction in sweat chloride and PEx in females after ivacaftor was unexpected. Our data that females have a higher baseline PEx is consistent with previous reports (1, 3). The greater reduction in pulmonary exacerbation frequency in ivacaftor-treated females is consistent with the idea that estrogen may mediate its detrimental effects by modulating CFTR function. The differential sweat chloride response may in part be explained by greater ivacaftor exposure resulting from differences in baseline weight (10). Estrogen has been shown to affect drug pharmacokinetics (11), and it may also affect ivacaftor metabolism, which may partly explain the smaller correlation between sweat chloride and weight in females. Finally, it should be noted that ivacaftor-mediated reductions in sweat chloride have recently been shown to correlate with attenuation of FEV1 decline (10, 12), which suggests that decreases in sweat chloride may also correlate with reduced lung transplantation and mortality risk. Thus, one potential implication of our data is that optimization of CFTR modulator dosing based on maximal sweat chloride reduction may lead to reduced long-term risk for lung transplantation and mortality. Further studies are needed to determine whether CFTR modulation will lead to equivalent long-term outcomes in females compared with males and whether our observations apply to other CFTR modulators.
Supplementary Material
Footnotes
Supported by a grant from the Cystic Fibrosis Foundation (GOAL13K2) and by NIH/National Center for Advancing Translational Sciences Colorado CTSA grant number UL1 TR002535 and NIH/National Institute of Diabetes and Digestive and Kidney Diseases P30 DK089507.
Author Contributions: All authors contributed to the study design and data interpretation for this manuscript; GOAL Site Investigators performed data collection; K.E.S. and M.J. drafted the manuscript; J.S.G., S.L.H., S.D.S., and S.M.R. contributed significantly to the manuscript; B.J. and S.L.H. performed analyses; and all authors reviewed the manuscript and approved of the final version of the manuscript before submission.
Originally Published in Press as DOI: 10.1164/rccm.201909-1845LE on December 16, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R. Gender differences in outcomes of patients with cystic fibrosis. J Womens Health (Larchmt) 2014;23:1012–1020. doi: 10.1089/jwh.2014.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Cela E, Gagnon S, Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res. 2010;11:166. doi: 10.1186/1465-9921-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chotirmall SH, Smith SG, Gunaratnam C, Cosgrove S, Dimitrov BD, O’Neill SJ, et al. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med. 2012;366:1978–1986. doi: 10.1056/NEJMoa1106126. [DOI] [PubMed] [Google Scholar]
- 4.Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, et al. 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest. 2008;118:4025–4035. doi: 10.1172/JCI33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377:2013–2023. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 8.Secunda KE, Jovanovic BD, Rowe SM, Heltshe SL, Jain M. Is there a “gender gap” in the response to ivacaftor in cystic fibrosis patients? [abstract] Am J Respir Crit Care Med. 2019;199:A4329. [Google Scholar]
- 9.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guimbellot JS, Baines A, Khan U, Heltshe SL, VanDalfsen J, Jain M, et al. Long term effects of ivacaftor in G551D patients: five year follow-up data in GOAL-E2; Presented at the North American Cystic Fibrosis Conference. October 18, 2018, Denver, Colorado. [Google Scholar]
- 11.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler MC, Beusmans J, Panorchan P, Van Goor F. Correlation of sweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017;16:41–44. doi: 10.1016/j.jcf.2016.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.