Asthma is a major public health problem, affecting approximately 235 million people worldwide (1). In the United States, the 2016 National Health Interview Survey estimated the overall prevalence of current asthma as 8.3% in adults and 8.3% in children (2).
In the United States, certain ethnic minority groups, such as Puerto Ricans and African Americans, are heavily affected by asthma and frequently exposed to chronic psychosocial stressors such as violence, poverty, and discrimination (3–5). Moreover, stress-related disorders such as post-traumatic stress disorder (PTSD) are common in certain minority groups at risk for asthma (6, 7). For example, Puerto Rican veterans of the Vietnam War were shown to be at greater risk for PTSD and to have more severe PTSD symptoms than non-Hispanic white Vietnam veterans (7).
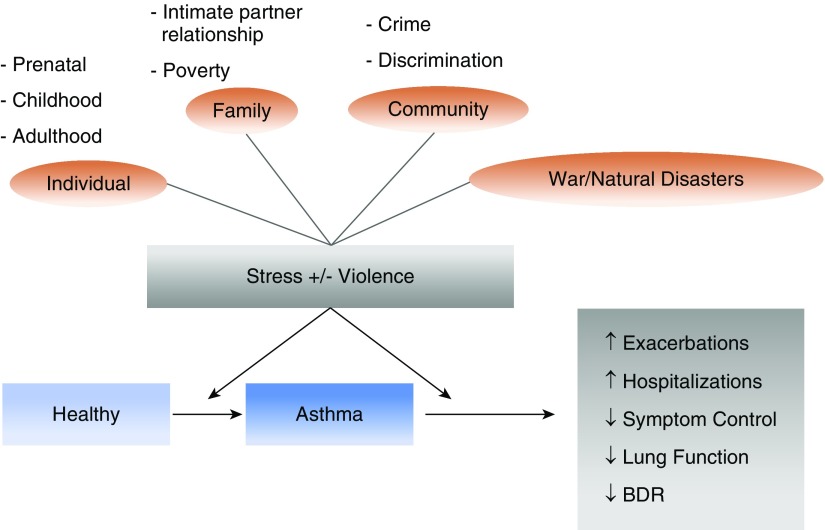
Over the last two decades, an expanding body of evidence has linked chronic psychosocial stress to asthma or morbidity from asthma in children and adults (Figure 1) (3–5, 8–16). Much of this research has focused on exposure to violence and related stressors, such as poverty, although of late studies are considering a broader array of adversities, such as racial or ethnic discrimination (4). Alongside these advances, experimental and population-based studies have yielded novel insights into the potential pathways underlying these connections, such as stress-related changes in epigenetic processes, gene expression, and immune responses (17). Such studies have also begun to examine complex interactions between stressors and other risk factors for asthma, including outdoor air pollution (14, 15, 18–21), diet, and obesity (22–24).
Figure 1.
Exposure to violence, chronic stress, and asthma. BDR = bronchodilator response.
Improving our understanding of whether and how exposure to chronic stressors causes or worsens asthma could help us gain novel insights into disease pathogenesis, design and support public health policies, and develop new interventions. In this review, we assess the evidence linking stress and asthma, describe recent insights on potential mechanisms, and discuss challenges and future directions in this field. We begin by considering the evidence for violence, because it has been the subject of much recent attention in the asthma literature (25, 26) and is a common exposure of significant public health concern.
Violence and Asthma
Children and adults can be exposed to violence in their households, workplaces, and neighborhoods. In some of the most common exposures to violence, a family member is the perpetrator (e.g., child maltreatment and intrapartner violence), but physical and sexual assaults can also be committed by friends, colleagues, acquaintances, and strangers. Besides these direct exposures to victimization, it is also important to consider instances where people are indirectly exposed to violence (e.g., an individual hears about the victimization of someone close to them or a victimization that has occurred in their neighborhood). For example, studies indicate that living in a neighborhood with high murder rates is associated with worse cardiometabolic health, even after accounting for personal victimization and potential confounders such as race or ethnicity, financial security, or availability of nutritious food (27). Recent observational studies have shown that exposure to different types of violence may lead to new-onset asthma or worsen asthma in affected individuals (3, 14–16, 28–42). We summarize this evidence below, according to lifespan stage and type of exposure.
Preliminary evidence supports an association between maternal exposure to violence during pregnancy and childhood asthma. In a study of 787 mother–child pairs of predominantly Hispanic or African American ethnicity, prenatal exposure to community violence was associated with nearly twofold increased odds of current wheeze at age 2 years, even after accounting for outdoor pollutants, cockroach allergen levels, and other potential confounders (14). In this cohort, a separate analysis (unadjusted for pollutants) showed that chronic maternal interpersonal trauma (IPT), defined as exposure to IPT during childhood or adolescence and adulthood, was significantly associated with childhood asthma at age 6 years. After stratification by sex, this association was significant in boys but not in girls (28). In that study, a path analysis further showed that chronic maternal IPT was significantly associated with maternal active asthma during pregnancy, and that ∼12% of the estimated effect of chronic maternal IPT on childhood asthma was mediated or explained by maternal active asthma during pregnancy.
Postnatal exposure to violence or traumatic experiences has been linked to asthma at various life stages. At the individual level, a large cross-sectional study of adults in the Americas and Asia found that childhood adversities were associated with adult-onset asthma in a dose–response fashion (29). In that study, early-onset depressive or anxiety disorders were also associated with asthma. Consistent with these findings, childhood abuse—a severe type of adversity—has been associated with asthma in children and young adults. For example, a case–control study of Puerto Rican children reported that a history of physical or sexual abuse in the previous year was associated with asthma, healthcare utilization for asthma, and use of asthma medications, even after accounting for household income, health insurance, and other confounders (30). Physical abuse during childhood was subsequently linked to asthma in a prospective study of African American women (31). Similar results for childhood abuse and asthma were reported in a cross-sectional study of young adults in New Zealand (32), in a large prospective study of Australian children followed from birth until age 21 years (33), and in Puerto Rican children exposed to violence (34). Consistent with findings for childhood abuse, a cross-sectional study of U.S. women with current asthma showed that women who were victims of sexual violence (unwanted touching, attempted unwanted intercourse, or forced unwanted intercourse) were twice as likely to report an asthma attack in the previous year as those not victimized (35).
Exposure to violence in the household has been linked to asthma (36). Among 2,013 children who participated in the Fragile Families and Child Wellbeing Study, maternal report of chronic exposure to intimate partner violence (IPV) and having experienced house disarray were each associated with childhood asthma (15). In that study, children whose mothers had a history of IPV and had experienced house disarray were at highest risk of asthma. In another study that collected data on maternal exposure to IPV and salivary cortisol in children from age 7 to 48 months, children whose mothers experienced IPV and who had increased cortisol levels at ages 7 and 15 months had increased odds of report of physician-diagnosed asthma at age 4 years (37), suggesting that children who are physiologically reactive are particularly vulnerable to the detrimental effects of household violence on asthma.
Community violence has been implicated in asthma. In a longitudinal study of 2,071 children in Chicago, medium and high levels of community violence were associated with 1.6 times increased odds of report of physician-diagnosed asthma, even after accounting for potential confounders at the individual or neighborhood level (3). However, there was no adjustment for indoor or outdoor pollutants, which often colocalize with neighborhood violence and thus could be the agent driving these associations. Similar results were obtained in a cross-sectional study of 1,232 parents (or guardians) in Salvador (Brazil), where wheezing in the prior year was more common in children exposed to violence in the community than in those unexposed (38). In that study, children exposed to high levels of violence were also nearly twice as likely to have asthma symptoms as those who were unexposed. In another cross-sectional study of 92,486 Indian households, women who experienced domestic violence had increased odds of self-reported asthma, and all individuals living in those women’s households were also at increased risk of reported asthma (39).
Other studies have used neighborhood crime rates, instead of participant reports of exposure, to characterize levels of community violence. These crimes rates capture both personal victimization and indirect exposures to violence. In a retrospective study of 4,638 asthma-related emergency department visits and hospitalizations in Cincinnati, both census-level violent crime rates (VCR; r = 0.61) and census-level all-crime rates (r = 0.54) were correlated with asthma utilization rates (16). After adjustment for census-tract poverty, unemployment, and substandard housing, VCR (but not all-crime rates) remained associated with higher asthma utilization rates. Moreover, VCR explained ∼35% of the population-level variability in asthma utilization rates. Similar findings were obtained in a cross-sectional study in Chicago, where living in a neighborhood with high violent criminal activity was significantly associated with higher prevalence of caregiver-reported childhood asthma compared with neighborhoods with lower criminal activity, even after adjusting for individual and sociodemographic characteristics (40).
In a related study that addressed direct exposure, lifetime gun violence (defined as having heard a gunshot more than once) was associated with 1.8 times increased odds of report of physician-diagnosed asthma in 466 Puerto Rican children (41). Of interest, the estimated effect of gun violence on asthma was even stronger among children who were both exposed to gun violence and afraid to leave their home because of violence. A subsequent analysis of the same cohort of Puerto Rican children reported that global African ancestry (measured using genetic markers) interacted with gun violence to further increase asthma risk (42).
Stress and Asthma
A growing body of evidence supports a link between other chronic stressors and asthma risks at both the pre- and postnatal stages (17). Prenatal stress may increase the risk of childhood wheeze or asthma, through mechanisms including altered Th1/Th2 cytokine balance with persistent Th2 immune responses in early life (43), changes in DNA methylation and/or gene expression, abnormal regulation of neuroendocrine or neurotransmitter receptor interactions (44), altered glucocorticoid receptors in the fetus with postnatal abnormalities in the hypothalamic–pituitary–adrenal axis (45), and abnormal lung organogenesis due to altered information trafficking between the feto-placental and maternal compartments (46).
In two separate meta-analyses, children born to mothers exposed to any stressor during pregnancy had increased risks of early-onset wheeze, persistent wheeze, and asthma (8, 47). However, preliminary evidence suggests that ethnicity, sex, and outdoor air pollutants may modify potential effects of prenatal stress on asthma. In a birth cohort study, the risk of lifetime wheeze in the offspring of mothers who experienced anxiety during pregnancy, two or more negative life events, and low paternal support was modestly higher in the offspring of Latinas than in those of non-Hispanic white women, independent of income level (48). This finding may be explained by an increased perception of stress among Latinas and inadequate access to mental health resources or practices that would help develop coping mechanisms. In a separate birth cohort study of predominantly minority women–child pairs, prenatal stress was more significantly and strongly associated with asthma in boys, whereas postnatal stress was more significantly and strongly associated with asthma in girls (49). Similar results were obtained in a separate prospective study of Mexican children (50). With regard to air pollution, prenatal exposure to particulate matter with a diameter < 2.5 μm or nitrate has been associated with current wheeze or asthma in children whose mothers were highly stressed during pregnancy, with one study reporting an association in boys but not in girls (19, 20). Pre- and early postnatal stress may affect lung function growth, as one prospective study showed that high levels of stress (five or more negative life events) during pregnancy and within the first 2 years of life were associated with decrements in FEV1 and FVC by age 7 years, even after adjustment for confounders (51).
Preliminary evidence supports an association between postnatal stress and new-onset asthma. In a prospective study, children without an asthma diagnosis by age 16 years but who had a high life events score had fourfold significantly increased odds of developing asthma by age 29 years (9). Moreover, adolescents who lose a close relative have been shown to have a modestly increased risk of hospitalization due to asthma and are less likely to use asthma medications (52). This could be partly explained by a transient increase in the production of cytokines (IL-4, IL-5, and IFN-Y) associated with acute stressful events, particularly in children with high chronic stress (53).
There is also a growing literature asking whether chronic stress affects the course of asthma. Preliminary evidence suggests that distinct stressful situations may have a different impact on asthma outcomes. In a 2-year study of young subjects with asthma, a history of targeted rejections (intentional rejections of a person by another person or by a group of people) were associated with decreased expression of mRNA for the glucocorticoid receptor and β2-adrenergic receptor genes in white blood cells (10). Racial or ethnic discrimination can be considered a type of targeted rejection, and perception of such discrimination has been associated with asthma and worse asthma control in African American children (4). In that study, Mexican Americans who perceived racial or ethnic discrimination had increased odds of asthma only if they were of low socioeconomic status (SES). In a small randomized two-period crossover study among college students with mild asthma, perceived stress during final exams was associated with eosinophilia and eosinophil-derived neurotoxin in sputum after inhaled antigen challenge, as well as with a decline in FEV1 (54).
In adults with asthma, stress at home or at work can lead to worse asthma control and decreased medication adherence (55). Although a cross-sectional study showed that overcommitment at work was associated with worse asthma control (56), a meta-analysis of prospective data from 102,175 adults in 11 European studies found no significant association between job strain and severe asthma exacerbations (57). Thus, personal characteristics such as resiliency and available coping mechanisms may be more influential than actual work conditions in subjects with asthma. Consistent with this hypothesis, children with asthma of low SES who reinterpret stressors in a more positive light and remain optimistic about the future (shift and persist) have been shown to have less airway inflammation at baseline and lower use of rescue inhaler and fewer missed school days after 6 months of follow-up (58).
Stress may worsen asthma control and asthma outcomes by reducing response to short-acting β2-agonists and corticosteroids (59). As noted above, targeted rejection has been associated with downregulation of mRNA for the receptors through which these agents signal (43). Consistent with this possibility, a cross-sectional study of Puerto Rican children with asthma showed that children with high levels of chronic stress had lower bronchodilator response (BDR) than those with lower levels of chronic stress (11). In that study, anxiety or anxiety disorders were associated with reduced BDR in two independent cohorts of racially or ethnically diverse children with or at risk for asthma (11). Of interest, the estimated effect of chronic stress on reduced BDR in Puerto Rican children was enhanced when both children and their mothers had high chronic stress. Moreover, a SNP in the gene ADCYAP1R1 (adenylate cyclase activating polypeptide 1 receptor type 1) was shown to be associated with reduced BDR in a meta-analysis of data from seven cohorts of children with asthma, with reduced expression of the gene ADRB2 (β2-adrenergic receptor) in CD4+ lymphocytes from children and adults with asthma and with reduced functional connectivity of the amygdala and the insula (a marker of anxiety) in functional magnetic resonance imaging studies of the brain of inner-city women with asthma (11). Taken together, these findings suggest that high chronic stress leads to downregulation of the β2-adrenergic receptor in genetically susceptible children, perhaps due to persistent secretion of catecholamines.
Specific neuronal pathways may mediate the effects of stress on airway inflammation in subjects with asthma. In a study of 30 subjects with mild allergic asthma, those with chronic stress had a larger hypothalamic–pituitary–adrenal axis response to an acute social stressor than subjects without chronic stress (60). Moreover, positron emission tomography scans showed that increased metabolism in the anterior insula correlated with increased fractional exhaled nitric oxide and that greater activity in the mid-cingulate cortex during acute stress was associated with increased IL-23A mRNA expression in subjects with chronic stress. In another study of six subjects with mild allergic asthma, activity in the anterior cingulate complex and the insula was associated with inflammatory markers and airflow obstruction after antigen inhalation challenge (61).
PTSD and Asthma
PTSD and PTSD symptoms have been linked to asthma. In a study conducted using the Vietnam Era Twin Registry, twins with PTSD symptoms in the upper quartile had twice the odds of having asthma when compared with twins with PTSD symptoms in the lower quartile. This association persisted even after a within-pair analysis was conducted (62). After the terrorist attack on New York’s World Trade Center (WTC) on September 11, 2001, a large 9-year study of rescue and recovery workers reported that the cumulative incidences of asthma and PTSD were 27.6% and 31.9%, respectively (12). In a separate cross-sectional study of 71,473 participants in the WTC Health Registry (including lower Manhattan residents, office workers, and passersby), probable PTSD (a score ≥44 points in the PTSD Checklist questionnaire) at a baseline visit in 2003 to 2004 was associated with 1.65 times increased odds of newly diagnosed asthma, even after accounting for SES, dust exposure, and smoking (63). A follow-up study of the WTC Health Registry was conducted in 2006 to 2007, when 46,322 (68%) of participants agreed to participate (64). In that follow-up study, intense cloud exposure around the WTC attack was a significant contributor to a new asthma diagnosis within 5 to 6 years, with the highest rate of PTSD symptoms in passersby.
In a study of 11,481 WTC rescue and recovery workers, probable PTSD at the baseline visit (after September 11, 2001) was associated with 1.43 times increased odds of BDR (defined as an increment of ≥12% and ≥200 ml in FEV1 after bronchodilator administration) at the same visit, even after accounting for SES, smoking status, pack-years of smoking, body mass index, and WTC occupational exposure (65). Similar results were obtained when the analysis was restricted to 6,133 never-smokers without asthma. In that study, probable PTSD at the baseline visit was associated with 2.4 times increased odds of incident asthma (3.7–5.9 yr later) among 3,757 never-smokers who had not been diagnosed with asthma at or before the baseline visit, even after accounting for baseline BDR, WTC occupational exposure, body mass index, and other confounders (65). In a separate cross-sectional study of 1,772 German adults, PTSD was associated with reduced FEV1 and FEV1/FVC and airflow limitation (66). Little is known about PTSD and asthma or lung function in children, with one cross-sectional study linking PTSD to asthma in adolescents (67).
PTSD is present in in 5% to 24% of subjects exposed to a natural disaster (68). Studying PTSD and asthma after natural disasters is challenging, as such events can also increase exposure to allergens such as mold and pollen and limit access to health care. In a study of victims of Hurricane Katrina (13), post-traumatic stress symptoms were not associated with asthma, but each 1-point increment in the Impact of Event Scale–Revised avoidance score (which correlates with behaviors and avoidant coping mechanisms) was associated with twofold increased odds of an asthma attack or episode since the hurricane. Although that study did not account for confounders, it highlights the importance of preparing to care for mental illness and asthma after natural disasters. For example, the population of Puerto Rico is commonly affected by both PTSD and asthma, and local healthcare providers have been alarmed by an increased occurrence of asthma attacks after Hurricane Maria (69).
Conclusions, Challenges, and Future Directions
Available evidence from experimental and observational studies supports a causal association between chronic stress and worse asthma control, and a growing body of literature suggests that pre- or postnatal chronic stress may lead to new-onset asthma. Such evidence supports conducting randomized controlled trials of stress-reduction interventions to improve asthma control in subjects with high chronic stress. On the other hand, the evidence of a causal link between exposure to violence and asthma is weaker than that for chronic stress and asthma, because of the paucity of experimental or longitudinal studies.
Subjects exposed to violence or experiencing chronic stress are often coexposed to risk factors for asthma or worse asthma control, including poverty, smoking, second-hand smoke, indoor and outdoor pollutants, limited access to health care or medications, reduced adherence to controller medications, an unhealthy diet, and obesity. Thus, longitudinal studies with assessment of coexposures are key not only to better understand the independent effects of violence or stress on asthma but also to identify factors that could ameliorate or worsen such effects. In particular, we need to improve our understanding of whether and how sex, pollutants, diet, and obesity affect asthma risk in individuals exposed to violence or experiencing chronic stress, while also characterizing the complex interactions between violence exposure and chronic stress on asthma. Moreover, future studies should address the role of coping mechanisms in mitigating the detrimental effects of stress on asthma. In this context, the unfortunate recent increase in natural disasters should motivate both observational and interventional studies to improve our preparation and response to such events.
Identification and improved understanding of the mechanisms underlying the link between stress and asthma are needed to move this field forward. Preliminary evidence suggests that chronic stress affects expression of genes conferring susceptibility to asthma, either directly or through epigenetic regulation. As is the case with epidemiologic studies, future studies should pay attention to co-exposures that may affect DNA methylation or gene expression (e.g., pollutants and smoking) and carefully choose the cells to be studied, as epigenetics is cell specific.
Supplementary Material
Footnotes
Supported by NIH grants HL117191, HL119952, HL122328, and MD011764; and NIH training grant T32 HL129949 (J.L.-G.).
Originally Published in Press as DOI: 10.1164/rccm.201905-1073PP on December 4, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Fact sheet: asthma. 2017 [accessed 2018 Sept 13]. Available from: http://www.who.int/news-room/fact-sheets/detail/asthma.
- 2.Centers for Disease Control and Prevention. Asthma: most recent asthma data [accessed 2018 Sept 13]. Available from: https://www.cdc.gov/asthma/most_recent_data.htm.
- 3.Sternthal MJ, Jun HJ, Earls F, Wright RJ. Community violence and urban childhood asthma: a multilevel analysis. Eur Respir J. 2010;36:1400–1409. doi: 10.1183/09031936.00003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur N, Barcelo NE, Borrell LN, Singh S, Eng C, Davis A, et al. Perceived discrimination associated with asthma and related outcomes in minority youth: the GALA II and SAGE II studies. Chest. 2017;151:804–812. doi: 10.1016/j.chest.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonas MA, Lange NE, Celedón JC. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol. 2012;12:202–210. doi: 10.1097/ACI.0b013e32835090c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galea S, Vlahov D, Tracy M, Hoover DR, Resnick H, Kilpatrick D. Hispanic ethnicity and post-traumatic stress disorder after a disaster: evidence from a general population survey after September 11, 2001. Ann Epidemiol. 2004;14:520–531. doi: 10.1016/j.annepidem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Ortega AN, Rosenheck R. Posttraumatic stress disorder among Hispanic Vietnam veterans. Am J Psychiatry. 2000;157:615–619. doi: 10.1176/appi.ajp.157.4.615. [DOI] [PubMed] [Google Scholar]
- 8.van de Loo KFE, van Gelder MMHJ, Roukema J, Roeleveld N, Merkus PJFM, Verhaak CM. Prenatal maternal psychological stress and childhood asthma and wheezing: a meta-analysis. Eur Respir J. 2016;47:133–146. doi: 10.1183/13993003.00299-2015. [DOI] [PubMed] [Google Scholar]
- 9.Oren E, Gerald L, Stern DA, Martinez FD, Wright AL. Self-reported stressful life events during adolescence and subsequent asthma: a longitudinal study. J Allergy Clin Immunol Pract. 2017;5:427–434, e2. doi: 10.1016/j.jaip.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy MLM, Slavich GM, Chen E, Miller GE. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma. Psychol Sci. 2015;26:111–121. doi: 10.1177/0956797614556320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brehm JM, Ramratnam SK, Tse SM, Croteau-Chonka DC, Pino-Yanes M, Rosas-Salazar C, et al. Stress and bronchodilator response in children with asthma. Am J Respir Crit Care Med. 2015;192:47–56. doi: 10.1164/rccm.201501-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisnivesky JP, Teitelbaum SL, Todd AC, Boffetta P, Crane M, Crowley L, et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: a cohort study. Lancet. 2011;378:888–897. doi: 10.1016/S0140-6736(11)61180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcaya MC, Lowe SR, Rhodes JE, Waters MC, Subramanian SV. Association of PTSD symptoms with asthma attacks among hurricane Katrina survivors. J Trauma Stress. 2014;27:725–729. doi: 10.1002/jts.21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu Y-HM, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133:713–22, e4. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suglia SF, Duarte CS, Sandel MT, Wright RJ. Social and environmental stressors in the home and childhood asthma. J Epidemiol Community Health. 2010;64:636–642. doi: 10.1136/jech.2008.082842. [Published erratum appears in J Epidemiol Community Health 64:1105.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AF, Huang B, Ryan PH, Sandel MT, Chen C, Kahn RS. Areas with high rates of police-reported violent crime have higher rates of childhood asthma morbidity. J Pediatr. 2016;173:175–182, e1. doi: 10.1016/j.jpeds.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SL, Miller GE, Brehm JM, Celedón JC. Stress and asthma: novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol. 2014;134:1009–1015. doi: 10.1016/j.jaci.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen E, Miller GE, Shalowitz MU, Story RE, Levine CS, Hayen R, et al. Difficult family relationships, residential greenspace, and childhood asthma. Pediatrics. 2017;139:e20163056. doi: 10.1542/peds.2016-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bose S, Chiu YM, Hsu HL, Di Q, Rosa MJ, Lee A, et al. Prenatal nitrate exposure and childhood asthma. influence of maternal prenatal stress and fetal sex. Am J Respir Crit Care Med. 2017;196:1396–1403. doi: 10.1164/rccm.201702-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, et al. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119:232–237, e1. doi: 10.1016/j.anai.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwerling C, Peek-Asa C, Whitten PS, Choi SW, Sprince NL, Jones MP. Fatal motor vehicle crashes in rural and urban areas: decomposing rates into contributing factors. Inj Prev. 2005;11:24–28. doi: 10.1136/ip.2004.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreier HMC, Chen E, Miller GE. Child maltreatment and pediatric asthma: a review of the literature. Asthma Res Pract. 2016;2:7. doi: 10.1186/s40733-016-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp LK, Curtis LM, Mosnaim G, Shalowitz MU, Catrambone C, Sadowski LS. The influence of caregiver’s psychosocial status on childhood asthma and obesity. Ann Allergy Asthma Immunol. 2009;103:386–394. doi: 10.1016/S1081-1206(10)60357-2. [DOI] [PubMed] [Google Scholar]
- 24.Szentpetery SS, Gruzieva O, Forno E, Han Y-Y, Bergström A, Kull I, et al. Combined effects of multiple risk factors on asthma in school-aged children. Respir Med. 2017;133:16–21. doi: 10.1016/j.rmed.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin KA, Basu A, Walsh K, Slopen N, Sumner JA, Koenen KC, et al. Childhood exposure to violence and chronic physical conditions in a national sample of US adolescents. Psychosom Med. 2016;78:1072–1083. doi: 10.1097/PSY.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen RT, Celedón JC. Community violence and health disparities in asthma. J Pediatr. 2016;173:13–15. doi: 10.1016/j.jpeds.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Miller GE, Chen E, Armstrong CC, Carroll AL, Ozturk S, Rydland KJ, et al. Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proc Natl Acad Sci USA. 2018;115:12063–12068. doi: 10.1073/pnas.1810067115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunst KJ, Rosa MJ, Jara C, Lipton LR, Lee A, Coull BA, et al. Impact of maternal lifetime interpersonal trauma on children’s asthma: mediation through maternal active asthma during pregnancy. Psychosom Med. 2017;79:91–100. doi: 10.1097/PSY.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott KM, Von Korff M, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, et al. Childhood adversity, early-onset depressive/anxiety disorders, and adult-onset asthma. Psychosom Med. 2008;70:1035–1043. doi: 10.1097/PSY.0b013e318187a2fb. [DOI] [PubMed] [Google Scholar]
- 30.Cohen RT, Canino GJ, Bird HR, Celedón JC. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008;178:453–459. doi: 10.1164/rccm.200711-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coogan PF, Wise LA, O’Connor GT, Brown TA, Palmer JR, Rosenberg L. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J Allergy Clin Immunol. 2013;131:1058–1063. doi: 10.1016/j.jaci.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott KM, Smith DAR, Ellis PM. A population study of childhood maltreatment and asthma diagnosis: differential associations between child protection database versus retrospective self-reported data. Psychosom Med. 2012;74:817–823. doi: 10.1097/PSY.0b013e3182648de4. [DOI] [PubMed] [Google Scholar]
- 33.Abajobir AA, Kisely S, Williams G, Strathearn L, Suresh S, Najman JM. The association between substantiated childhood maltreatment, asthma and lung function: a prospective investigation. J Psychosom Res. 2017;101:58–65. doi: 10.1016/j.jpsychores.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Boutaoui N, Brehm JM, Han Y-Y, Schmitz C, Cressley A, et al. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2013;187:584–588. doi: 10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossarte RM, Swahn MH, Choudhary E. The associations between area of residence, sexual violence victimization, and asthma episodes among US adult women in 14 states and territories, 2005-2007. J Urban Health. 2009;86:242–249. doi: 10.1007/s11524-008-9340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bair-Merritt M, Zuckerman B, Augustyn M, Cronholm PF. Silent victims—an epidemic of childhood exposure to domestic violence. N Engl J Med. 2013;369:1673–1675. doi: 10.1056/NEJMp1307643. [DOI] [PubMed] [Google Scholar]
- 37.Bair-Merritt MH, Voegtline K, Ghazarian SR, Granger DA, Blair C, Johnson SB Family Life Project Investigators. Maternal intimate partner violence exposure, child cortisol reactivity and child asthma. Child Abuse Negl. 2015;48:50–57. doi: 10.1016/j.chiabu.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alves G da C, Santos DN, Feitosa CA, Barreto ML. Community violence and childhood asthma prevalence in peripheral neighborhoods in Salvador, Bahia State, Brazil. Cad Saude Publica. 2012;28:86–94. doi: 10.1590/s0102-311x2012000100009. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian SV, Ackerson LK, Subramanyam MA, Wright RJ. Domestic violence is associated with adult and childhood asthma prevalence in India. Int J Epidemiol. 2007;36:569–579. doi: 10.1093/ije/dym007. [DOI] [PubMed] [Google Scholar]
- 40.Gupta RS, Zhang X, Springston EE, Sharp LK, Curtis LM, Shalowitz M, et al. The association between community crime and childhood asthma prevalence in Chicago. Ann Allergy Asthma Immunol. 2010;104:299–306. doi: 10.1016/j.anai.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 41.Ramratnam SK, Han Y-Y, Rosas-Salazar C, Forno E, Brehm JM, Rosser F, et al. Exposure to gun violence and asthma among children in Puerto Rico. Respir Med. 2015;109:975–981. doi: 10.1016/j.rmed.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas-Salazar C, Han Y-Y, Brehm JM, Forno E, Acosta-Pérez E, Cloutier MM, et al. Gun violence, African ancestry, and asthma: a case-control study in Puerto Rican children. Chest. 2016;149:1436–1444. doi: 10.1016/j.chest.2016.02.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trump S, Bieg M, Gu Z, Thürmann L, Bauer T, Bauer M, et al. Prenatal maternal stress and wheeze in children: novel insights into epigenetic regulation. Sci Rep. 2016;6:28616. doi: 10.1038/srep28616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 46.Rosa MJ, Lee AG, Wright RJ. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr Opin Allergy Clin Immunol. 2018;18:148–158. doi: 10.1097/ACI.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flanigan C, Sheikh A, DunnGalvin A, Brew BK, Almqvist C, Nwaru BI. Prenatal maternal psychosocial stress and offspring’s asthma and allergic disease: a systematic review and meta-analysis. Clin Exp Allergy. 2018;48:403–414. doi: 10.1111/cea.13091. [DOI] [PubMed] [Google Scholar]
- 48.Bandoli G, von Ehrenstein O, Ghosh JKC, Flores MES, Dunkel Schetter C, Ritz B. Prenatal maternal stress and the risk of lifetime wheeze in young offspring: an examination by stressor and maternal ethnicity. J Immigr Minor Health. 2016;18:987–995. doi: 10.1007/s10903-015-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee A, Mathilda Chiu Y-H, Rosa MJ, Jara C, Wright RO, Coull BA, et al. Prenatal and postnatal stress and asthma in children: temporal- and sex-specific associations. J Allergy Clin Immunol. 2016;138:740–747, e3. doi: 10.1016/j.jaci.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosa MJ, Just AC, Tamayo Y Ortiz M, Schnaas L, Svensson K, Wright RO, et al. Prenatal and postnatal stress and wheeze in Mexican children: sex-specific differences. Ann Allergy Asthma Immunol. 2016;116:306–312, e1. doi: 10.1016/j.anai.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee AG, Chiu YM, Rosa MJ, Cohen S, Coull BA, Wright RO, et al. Association of prenatal and early childhood stress with reduced lung function in 7-year-olds. Ann Allergy Asthma Immunol. 2017;119:153–159. doi: 10.1016/j.anai.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Olsen J, Agerbo E, Yuan W, Cnattingius S, Gissler M, et al. Psychological stress and hospitalization for childhood asthma-a nationwide cohort study in two Nordic countries. PLoS One. 2013;8:e78816. doi: 10.1371/journal.pone.0078816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosom Med. 2009;71:378–384. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 55.Wisnivesky JP, Lorenzo J, Feldman JM, Leventhal H, Halm EA. The relationship between perceived stress and morbidity among adult inner-city asthmatics. J Asthma. 2010;47:100–104. doi: 10.3109/02770900903426989. [DOI] [PubMed] [Google Scholar]
- 56.Hartmann B, Leucht V, Loerbroks A. Work stress, asthma control and asthma-specific quality of life: initial evidence from a cross-sectional study. J Asthma. 2017;54:210–216. doi: 10.1080/02770903.2016.1201836. [DOI] [PubMed] [Google Scholar]
- 57.Heikkilä K, Madsen IEH, Nyberg ST, Fransson EI, Westerlund H, Westerholm PJM, et al. IPD-Work Consortium. Job strain and the risk of severe asthma exacerbations: a meta-analysis of individual-participant data from 100 000 European men and women. Allergy. 2014;69:775–783. doi: 10.1111/all.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen E, Strunk RC, Trethewey A, Schreier HMC, Maharaj N, Miller GE. Resilience in low-socioeconomic-status children with asthma: adaptations to stress. J Allergy Clin Immunol. 2011;128:970–976. doi: 10.1016/j.jaci.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci USA. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenkranz MA, Esnault S, Christian BT, Crisafi G, Gresham LK, Higgins AT, et al. Mind-body interactions in the regulation of airway inflammation in asthma: a PET study of acute and chronic stress. Brain Behav Immun. 2016;58:18–30. doi: 10.1016/j.bbi.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, et al. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci USA. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodwin RD, Fischer ME, Goldberg J. A twin study of post-traumatic stress disorder symptoms and asthma. Am J Respir Crit Care Med. 2007;176:983–987. doi: 10.1164/rccm.200610-1467OC. [DOI] [PubMed] [Google Scholar]
- 63.Shiratori Y, Samuelson KW. Relationship between posttraumatic stress disorder and asthma among New York area residents exposed to the World Trade Center disaster. J Psychosom Res. 2012;73:122–125. doi: 10.1016/j.jpsychores.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Brackbill RM, Hadler JL, DiGrande L, Ekenga CC, Farfel MR, Friedman S, et al. Asthma and posttraumatic stress symptoms 5 to 6 years following exposure to the World Trade Center terrorist attack. JAMA. 2009;302:502–516. doi: 10.1001/jama.2009.1121. [DOI] [PubMed] [Google Scholar]
- 65.de la Hoz RE, Jeon Y, Miller GE, Wisnivesky JP, Celedón JC. Post-traumatic stress disorder, bronchodilator response, and incident asthma in world trade center rescue and recovery workers. Am J Respir Crit Care Med. 2016;194:1383–1391. doi: 10.1164/rccm.201605-1067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spitzer C, Koch B, Grabe HJ, Ewert R, Barnow S, Felix SB, et al. Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J. 2011;37:1068–1075. doi: 10.1183/09031936.00028010. [DOI] [PubMed] [Google Scholar]
- 67.Kean EM, Kelsay K, Wamboldt F, Wamboldt MZ. Posttraumatic stress in adolescents with asthma and their parents. J Am Acad Child Adolesc Psychiatry. 2006;45:78–86. doi: 10.1097/01.chi.0000186400.67346.02. [DOI] [PubMed] [Google Scholar]
- 68.Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38:467–480. doi: 10.1017/S0033291707001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coto D. Puerto Rico struggles with jump in asthma cases post-Maria. US News & World Report. 2018 Jun 18 [accessed 2018 Sept 14]. Available from: https://www.usnews.com/news/healthiest-communities/articles/2018-06-18/puerto-rico-struggles-with-jump-in-asthma-cases-post-maria.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.