Abstract
This study investigated changes in dormancy and germination over 8 months for 23 common species (annual and perennial grasses, legumes and other dicotyledons) from herbaceous communities in northern Australia. Seeds were exposed to three storage treatments: relatively constant laboratory conditions, an oven with fluctuating temperatures similar to those found on the soil surface (25/60°C), or exposed on the soil surface at Townsville. There were wide ranges of initial levels of dormancy (9–100%), rates of change of dormancy and response to the different storage conditions showing that species with several types of dormancy characteristics are able to coexist in these communities. The general trend in dormancy levels was a decline with time with the rate of decline greatest for seeds exposed on the soil surface and least for those stored in the laboratory. The species were divided into groups based on dormancy levels in seeds on the soil surface during the late dry and mid wet seasons. The dormancy characteristics of the groups were related to the ecology of the species in the groups. There was an approximately linear increase in germination rate (i.e. a decrease in the number of days to 50% of final germination) over time for all storage treatments; rates for seeds on the soil surface increased more rapidly than those of seeds in laboratory and oven samples.
Keywords: germination rate, grass, legume, seed storage, tropical
INTRODUCTION
The optimum time for seed germination in strongly seasonal climates is as early in the growing season as possible to gain resources for growth and reproduction but not so early that survival is unlikely ( Rathcke & Lacey 1985). Thus a successful species requires some mechanism or mechanisms to prevent germination before, but not at, the optimum time so that the probability of encountering poor growing conditions in the period after germination is reduced ( Angevine & Chabot 1979).
In the seasonally dry tropics most germination occurs early in the wet season ( Torssell & McKeon 1976; Mott 1978, 1980; Marks 1983a, b; McIvor & Gardener 1991). In these environments, species need to avoid germination on small falls of rain during the dry season as mortality can be complete during subsequent dry periods ( McKeon 1985; McKeon et al. 1985 ). Seeds then need to germinate early in the wet season as later‐establishing seedlings are less likely to survive competition from established perennial plants and older seedlings ( Ross & Harper 1972; Cook & Ratcliff 1984, 1985). Surface soil temperatures frequently exceed 50°C during the dry season in tropical areas ( Mott 1978; Mott et al. 1981 ; McKeon et al. 1985 ) and exposure to such high temperatures is an important dormancy‐breaking mechanism in some tropical grasses and Stylosanthes species ( Mott 1978; Mott et al. 1981 ; Hacker 1984; Hacker et al. 1984 ; Hacker & Ratcliff 1989). Herbaceous communities can contain many more species than those assessed already, so this study was undertaken to compare the changes in dormancy and germination of seed of a wide range of native and exotic species (based on Henderson 1997) collected at two sites typical of the seasonally dry tropics. The seed was stored under relatively constant laboratory conditions (control), exposed on the soil surface to simulate field conditions, or kept in an oven with fluctuating temperatures similar to those found on the soil surface (25/60°C). The oven treatment approximates the temperature environment experienced by seed in the field ( Mott 1978; McKeon et al. 1985 ) but excludes other effects such as wetting/drying and fungal infections. Seed size has been related to seed germination and persistence and has been used to predict seed longevity (e.g. Grime 1989; Thompson et al. 1993 ; Bekker et al. 1998 ). Germination rate may control germination in tropical areas ( McKeon et al. 1985 ) so relationships between seed weight and germination rate were also examined to assess the utility of seed size as a predictor.
METHODS
Species and collection sites
Fully mature dispersal units (hereafter referred to as seeds) of 21 species were collected in April–May 1983 from pastures in either the Townsville–Woodstock (indicated by W after the species name) area of north Queensland or from Katherine (K) in the Northern Territory ( Table 1). Seeds of three species (Digitaria ciliaris, Heteropogon contortus and Hyptis suaveolens) were collected from both areas and commercial samples of Stylosanthes hamata and Stylosanthes scabra were also included giving a total of 23 species and 26 seed lots.
Table 1.
Collection site and dispersal unit weight of 26 seed collections tested
| Species | Collection site | Dispersal unit weight (mg) |
|---|---|---|
| Native annual (or short‐lived perennial) grass | ||
| Brachiaria subquadripara (Trin.) Hitchc. | Woodstock | 1.5 |
| Dactyloctenium radulans (R.Br.) P. Beauv. | Townsville | 0.2 |
| Exotic annual (or short‐lived perennial) grass | ||
| Chloris inflata Link | Woodstock | 0.2 |
| Digitaria ciliaris (Retz.) Koeler | Katherine | 0.9 |
| Digitaria ciliaris | Woodstock | 0.9 |
| Echinochloa colona (L.) Link | Woodstock | 1.0 |
| Eleusine indica (L.) Gaertn. | Townsville | 0.4 |
| Pennisetum pedicellatum Brunken | Katherine | 3.3 |
| Themeda quadrivalvis (L.) Kuntze | Woodstock | 1.8 |
| Native perennial grass | ||
| Aristida latifolia Domin | Townsville | 1.0 |
| Bothriochloa decipiens (Domin) C.E. Hubb. | Woodstock | 1.0 |
| Chrysopogon fallax S.T. Blake | Townsville | 4.3 |
| Heteropogon contortus (L.) P. Beauv. ex Roem. & Schult. | Katherine | 1.7 |
| Heteropogon contortus | Woodstock | 1.9 |
| Sporobolus indicus (Buse) Baaijens | Woodstock | 0.1 |
| Themeda triandra Forssk. | Townsville | 2.4 |
| Exotic perennial grass | ||
| Bothriochloa pertusa (L.) A. Camus | Woodstock | 0.7 |
| Cenchrus ciliaris L. | Woodstock | 3.2 |
| Chloris gayana Kunth | Woodstock | 0.4 |
| Urochloa mosambicensis (Hack.) Dandy | Woodstock | 1.8 |
| Exotic annual (or short‐lived perennial) legume | ||
| Stylosanthes hamata (L.) Taub. | Commercial | 2.9 |
| Stylosanthes scabra Vogel | Commercial | 2.0 |
| Other exotic annual (or short‐lived perennial) dicotyledons | ||
| Gomphrena celosioides Mart. | Townsville | 3.0 |
| Hyptis suaveolens (L.) Poit. | Katherine | 5.5 |
| Hyptis suaveolens | Woodstock | 4.2 |
| Sida acuta Burm. f. | Katherine | 3.1 |
The Townsville–Woodstock and Katherine areas are typical of the seasonally dry tropics which are characterized by a hot ‘wet’ season and a warm ‘dry’ season. Average annual rainfall is 1160 mm at Townsville and 860 mm at Woodstock with 84% falling during the wet season (December–April). Maximum temperatures range from 31 to 33°C in December to 25°C in July. Average annual rainfall is 970 mm at Katherine with 92% falling during the wet season (November–March). Maximum temperatures range from 38°C in December to 30°C in July.
Seed storage and preparation
After collection, the seeds were stored at 22–23°C and 55–60% relative humidity in paper envelopes. The seeds were cleaned, and the awns removed from Aristida latifolia, Heteropogon contortus, Themeda quadrivalvis and Themeda triandra. Abnormal and empty seeds were discarded and samples of 50 seeds, all apparently sound, were counted. Seed weights were measured for four of these samples (i.e. 200 seeds).
Storage treatments
There were three storage treatments.
1. Laboratory. The seeds were stored in paper envelopes at 22–23°C and 55–60% relative humidity.
2. Oven. The seeds were stored in paper envelopes in an oven with a diurnal temperature fluctuation of 25/60°C.
3. Soil. The seeds were placed in fine, nylon mesh envelopes which were pegged to the soil surface in a level area, cleared of all vegetation and exposed to full sunlight, at the Davies Laboratory, Townsville.
For each storage treatment, there were four, fully randomised replicates of each seed lot. The seeds were placed in the three locations on 4 July 1983, approximately 2 months after collection. The storage period covered the mid–late dry season (July–November) and early–mid wet season (December–February).
Seed tests
At the time of placement and 4, 8, 12, 16, 20, 24, 28 and 32 weeks later, a sample of each seed lot from each replicate (i.e. 26 seed lots × three storage treatments × four replicates each of 50 seeds) was retrieved and tested for germination and dormancy. For four species (Bothriochloa pertusa, Heteropogon contortus, Themeda triandra and Urochloa mosambicensis), additional samples were retrieved after 36, 44, 64 and 96 weeks.
Any seeds which showed evidence of prior germination were counted and removed and the remainder germinated in Petri dishes in a germination cabinet with a diurnal temperature fluctuation of 30/25°C and a 12‐h day. Optimal conditions for germination are not known for most species but 30/25°C is in the optimum range for many tropical species ( Ellis et al. 1985 ). Germinated seeds were counted and removed daily for 14 days. Any seeds which did not germinate during that period were dissected and classified as dormant if the embryo and endosperm were firm and intact, or as dead if the tissues were pulpy and had begun to decay ( Mott 1978). There was some field germination after 16 weeks and it is possible some seeds in these samples classified as dead had previously germinated. This gave four classes of seed: field‐germinated, germinable, dormant and dead. (For the two legumes, hard seeds were also counted separately but have been included in the dormant class.)
Meteorological data
The seeds exposed on the soil surface were located 10 m from the Davies Laboratory meteorological station where rainfall, and maximum and minimum temperatures were recorded daily.
Data analyses
The data for the proportions of germinable and dormant seeds were analysed using factorial (seed lot × storage treatment × retrieval time) anova after arcsin transformation to normalize the variances. Plots of cumulative germination over the 14‐day test period were all sigmoid shaped so germination rate was estimated by fitting a sigmoid curve to the data, and calculating the number of days required to reach 50% of the final value. These values were analyzed by analysis of variance after logarithmic transformation. When the F statistic in the anova was significant, the treatment means were compared by least significant difference (LSD, P = 0.05). All analyses were performed in GENSTAT ( Payne et al. 1988 ).
RESULTS
Meteorological data
There were only 8 mm of rainfall during the first 12 weeks of storage but 17 mm fell in the 2 weeks prior to the sampling at 16 weeks ( Fig. 1). There were significant falls between all subsequent samplings with 225 mm during weeks 25–28, and 200 mm during weeks 29–32. Maximum temperatures increased from 23 to 25°C at the time of placement to 30–33°C during weeks 17–32. Minimum temperatures showed a similar increase from 10 to 13°C to 20–24°C. For the seeds exposed for a longer period, the second wet season commenced during week 75.
Figure 1.
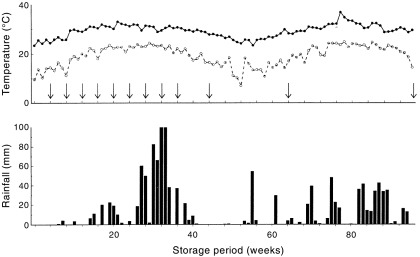
Weekly mean maximum and minimum temperatures and rainfall during the experiment. The arrows indicate when seed samples were retrieved and tested.
Germination and dormancy
Figure 2 shows examples of the changes in the proportions of different seed categories under different storage conditions. To reduce the volume of data presented, six species which cover the range of responses were chosen.
Figure 2.
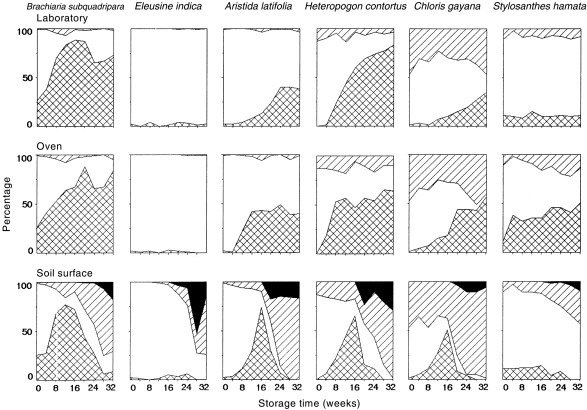
Changes in the percentage germinable (), dormant () (including hard), dead () and apparently field‐germinated () seed of six species stored in a laboratory at 22–23°C, in an oven at 25/60°C, and exposed on the soil surface at Townsville. Weeks 0–16 correspond with the dry season, and weeks 17–32 with the wet season.
For seeds stored in the laboratory or in the oven, there was generally little change in the proportion of dead seed with storage time. Under these conditions there were three patterns of change in the proportion of germinable seeds: little or no change (e.g. Stylosanthes hamata in the laboratory and Eleusine indica in both regimes), a gradual increase (e.g. Chloris gayana in both laboratory and oven) or a rapid initial increase followed by a period with little or no change (e.g. Brachiaria subquadripara, Aristida latifolia and Heteropogon contortus in both regimes). As there was little change in the proportion of dead seed, the change in the proportion of dormant seeds was the reverse of that in germinable seeds.
The changes for seeds on the soil surface were markedly different to those of seeds in the laboratory or oven. The proportion of dead seeds generally increased slowly during the dry season and then more rapidly after the wet season commenced. For the proportions of dormant and germinable seed there were three general patterns. For one group of species, there was little change in the numbers of germinable or dormant seed until rain fell between weeks 14 and 16. After this, both components declined but some seeds remained dormant at the final sampling (e.g. Stylosanthes hamata). In a second group, the proportion of germinable seeds increased (and dormant decreased) during the dry season and the numbers of germinable seeds declined rapidly during the wet season. Within this group, the species could be divided into those with some dormant seed at the final sampling (e.g. Brachiaria subquadripara) and those with no seeds remaining dormant (e.g. Aristida latifolia, Heteropogon contortus, Chloris gayana). In the third group, high initial dormancy rates declined sharply with the onset of the wet season but germinable seed percentages remained low (e.g. Eleusine indica).
To enable a comparison of all species, the percentages of germinable and dormant seeds in the initial and final samples, plus those on the soil surface at the end of the dry season (week 16) are shown in Table 2.
Table 2.
Changes in the proportions of germinable and dormant (including hard) seeds in samples stored under different regimes for varying periods
| Germinable seeds | Dormant seeds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Init. week | Lab | Oven | Soil | Soil | Init. | Lab | Oven | Soil | Soil | |
| 0 | 32 | 32 | 16 | 32 | 0 | 32 | 32 | 16 | 32 | |
| Species | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| Native annual grass | ||||||||||
| Brachiaria subquadripara | 26b | 73ab | 84a | 72ab | 8c | 74a | 25b | 12b | 18b | 21b |
| Dactyloctenium radulans | 0a | 1a | 4a | 0a | 0a | 100a | 98ab | 96ab | 100a | 84b |
| Exotic annual grass | ||||||||||
| Chloris inflata | 2b | 47a | 44a | 2b | 0b | 98a | 2c | 1c | 64b | 0c |
| Digitaria ciliaris (K) | 8c | 43b | 78a | 45b | 0c | 92a | 56b | 19c | 49b | 0d |
| Digitaria ciliaris (W) | 4b | 76a | 64a | 79a | 0b | 95a | 23b | 30b | 19b | 2c |
| Echinochloa colona | 1c | 8bc | 49a | 17ab | 0c | 99a | 92ab | 51c | 74b | 6d |
| Eleusine indica | 2a | 2a | 0a | 5a | 0a | 98a | 98a | 99a | 93a | 26b |
| Pennisetum pedicellatum | 0b | 22a | 33a | 4ab | 0b | 96a | 42b | 36b | 85a | 1c |
| Themeda quadrivalvis | 32b | 71a | 69a | 89a | 0 c | 68a | 26bc | 29b | 7c | 0d |
| Native perennial grass | ||||||||||
| Aristida latifolia | 2b | 38a | 71a | 74a | 0b | 98a | 58b | 27c | 16c | 0d |
| Bothriochloa decipiens | 2c | 74a | 70ab | 54b | 0c | 94a | 14bc | 15b | 13bc | 2c |
| Chrysopogon fallax | 41a | 62a | 69a | 52a | 0b | 47a | 23b | 23b | 22b | 1c |
| Heteropogon contortus (K) | 0c | 83a | 64b | 65b | 0c | 88a | 13cd | 24b | 17bc | 1d |
| Heteropogon contortus (W) | 0b | 50a | 47a | 27a | 1b | 76a | 32bc | 39bc | 42b | 17c |
| Sporobolus indicus | 2b | 47a | 73a | 73a | 0b | 98a | 53b | 28b | 9c | 0c |
| Themeda triandra | 9ab | 22a | 16a | 22a | 0b | 76a | 67ab | 73a | 50b | 9c |
| Exotic perennial grass | ||||||||||
| Bothriochloa pertusa | 0b | 7ab | 5ab | 22a | 0b | 76a | 53b | 27b | 53b | 4c |
| Cenchrus ciliaris | 5c | 16bc | 32b | 55a | 6c | 89a | 56b | 41b | 13c | 14c |
| Chloris gayana | 0b | 35a | 57a | 51a | 0b | 53a | 29ab | 3c | 12bc | 1c |
| Urochloa mosambicensis | 0b | 2ab | 8ab | 15a | 0b | 100a | 97ab | 92ab | 85b | 34c |
| Exotic legume | ||||||||||
| Stylosanthes hamata | 11b | 11b | 51a | 14b | 0b | 79a | 81a | 35b | 74a | 66a |
| Stylosanthes scabra | 18b | 14b | 44a | 14b | 0c | 47a | 43a | 3b | 33a | 9b |
| Other exotic dicotyledons | ||||||||||
| Gomphrena celusoides | 47a | 66a | 72a | 55a | 0b | 52a | 33a | 36a | 44a | 4b |
| Hyptis suaveolens (K) | 86a | 95a | 77a | 98a | 1b | 9ab | 5ab | 22a | 1b | 0b |
| Hyptis suaveolens (W) | 7bc | 26ab | 17ab | 41a | 0c | 54a | 32ab | 23b | 0c | 1c |
| Sida acuta | 0b | 1b | 36a | 0b | 0b | 99a | 96a | 61b | 100a | 47b |
Values for germinable or dormant seeds of a species followed by the same letter are not significantly different (P > 0.05). K, collection from Katherine; W, collection from Woodstock.
The dormancy level in the initial samples varied from low in the Katherine sample of Hyptis suaveolens to complete in Dactyloctenium radulans and Urochloa mosambicensis. No seeds germinated in the initial samples of eight species.
For seeds stored in the laboratory, over 90% of the seeds of Dactyloctenium radulans, Echinochloa colona, Eleusine indica, Urochloa mosambicensis and Sida acuta remained dormant after 32 weeks. In all other species except the two Stylosanthes, dormancy levels decreased and germination increased although the effects were not always significant.
Oven storage did not lead to significant changes in the percentage of dormant seeds in samples of Dactyloctenium radulans, Eleusine indica, Themeda triandra and Urochloa mosambicensis but dormancy levels decreased and germination increased (relative to initial levels) in other species. The percentage germination at 32 weeks was significantly higher in oven stored than laboratory stored seed for five species (Digitaria ciliaris (K), Echinochloa colona, Stylosanthes hamata, Stylosanthes scabra, Sida acuta (K)) and values were similar in the two storage regimes for the remainder of the species except Heteropogon contortus (K) which showed the reverse pattern.
In the field, after 16 weeks there was almost complete breakdown of dormancy in Themeda quadrivalvis, Sporobolus indicus and Hyptis suaveolens but dormancy remained at the initial levels in Dactyloctenium radulans, Eleusine indica, Stylosanthes hamata and Sida acuta. After 32 weeks in the field, 22 species had no germinable seeds remaining and germination exceeded 1% in Brachiaria subquadripara and Cenchrus ciliaris only. Some species had considerable dormant seed in the field at this time with more than 40% of the seeds of Dactyloctenium radulans, Stylosanthes hamata and Sida acuta in this category.
For the species studied over the longer period, seed of Heteropogon contortus and Urochloa mosambicensis exposed on the soil surface had a second, smaller peak of germination around week 64 ( Table 3). After 96 weeks exposure, there was negligible germination and little dormant seed of any species stored on the soil.
Table 3.
Changes in the proportions of germinable and dormant seeds in samples of four perennial grasses stored under different regimes for varying periods
| Germinable | Dormant | |||||
|---|---|---|---|---|---|---|
| seeds | seeds | |||||
| Week | Lab. | Oven | Soil | Lab. | Oven | Soil |
| Bothriochloa pertusa | ||||||
| 36 | 56a | 46a | 0a | 7c | 10c | 5b |
| 44 | 7b | 12b | 0a | 50ab | 50b | 20a |
| 64 | 2b | 0b | 1a | 57a | 57ab | 8b |
| 96 | 6b | 0b | 0a | 41b | 72a | 1b |
| Heteropogon contortus | ||||||
| 36 | 68a | 47a | 0b | 10c | 14c | 3a |
| 44 | 67a | 31b | 5a | 8c | 56b | 1b |
| 64 | 33b | 0c | 6a | 24b | 56b | 1b |
| 96 | 23b | 0c | 1b | 38a | 72a | 1b |
| Themeda triandra | ||||||
| 36 | 16a | 11a | 0a | 70a | 81a | 4a |
| 44 | 9ab | 8ab | 0a | 70a | 73a | 1b |
| 64 | 17a | 0b | 0a | 71a | 86a | 1b |
| 96 | 5b | 0b | 0a | 81a | 76a | 0b |
| Urochloa mosambicensis | ||||||
| 36 | 1a | 2a | 0b | 98a | 97a | 35a |
| 44 | 2a | 2a | 1b | 97a | 98a | 28b |
| 64 | 1a | 0b | 15a | 99a | 100a | 2c |
| 96 | 0a | 0b | 0b | 99a | 100a | 2c |
Values for germinable or dormant seeds of a species within a treatment followed by the same letter are not significantly different (P > 0.05).
Germination rate
There was an approximately linear increase in germination rate (i.e. a decrease in the number of days to 50% of final germination) with time for all storage treatments ( Fig. 3). The laboratory and oven samples changed slowly (0.044 units/week) but the seeds on the soil surface changed more rapidly (0.104 units/week).
Figure 3.
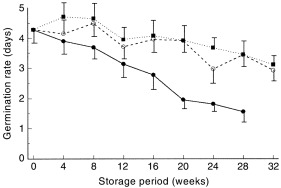
Mean (±SE) germination rates (days to reach 50% of final germination) of seeds stored for different periods of time in a laboratory at 22–23°C (▪), in an oven at 25/60°C (○), and exposed on the soil surface at Townsville (●).
To illustrate the differences between species, Table 4 shows the values for seeds on the soil surface at the commencement of the wet season after 20 weeks’ exposure. There were large differences between species with half the seeds of Stylosanthes hamata, Themeda quadrivalvis and Aristida latifolia germinating within 16 h compared with over 4 days for Sida acuta and Hyptis suaveolens (K). Apart from Digitaria ciliaris (W) and Hyptis suaveolens (K), seeds exposed on the soil surface germinated faster than seeds stored in the laboratory.
Table 4.
Germination rates (number of days required to achieve 50% of final germination value) of seeds of herbaceous tropical species at the commencement of the growing season following exposure on the soil surface for 20 weeks
| Germination rate | ||
|---|---|---|
| Species | Soil | Lab. |
| Stylosanthes hamata | 0.60 (0.21) | 1.3 |
| Themeda quadrivalvis | 0.65 (0.22) | 1.9 |
| Aristida latifolia | 0.68 (0.22) | 3.5 |
| Heteropogon contortus (K) | 0.83 (0.26) | 2.4 |
| Echinochloa colona | 0.88 (0.27) | 4.6 |
| Urochloa mosambicensis | 0.93 (0.28) | 5.5 |
| Bothriochloa decipiens | 1.18 (0.34) | 3.0 |
| Sporobolus indicus | 1.20 (0.34) | 2.5 |
| Brachiaria subquadripara | 1.20 (0.34) | 3.0 |
| Themeda triandra | 1.40 (0.38) | 2.3 |
| Heteropogon contortus (W) | 1.45 (0.38) | 3.0 |
| Digitaria ciliaris (K) | 1.45 (0.39) | 3.0 |
| Chloris gayana | 1.50 (0.39) | 3.4 |
| Chloris inflata | 1.50 (0.40) | 3.5 |
| Chrysopogon fallax | 1.53 (0.40) | 3.2 |
| Cenchrus ciliaris | 1.63 (0.42) | 4.9 |
| Digitaria ciliaris (W) | 1.68 (0.43) | 1.7 |
| Eleusine indica | 1.90 (0.46) | 7.0 |
| Stylosanthes scabra | 1.93 (0.45) | 5.4 |
| Bothriochloa pertusa | 2.50 (0.51) | 4.5 |
| Gomphrena celosioides | 2.80 (0.57) | 4.4 |
| Hyptis suaveolens (W) | 3.53 (0.65) | 6.8 |
| Pennisetum pedicellatum | 4.00 (0.69) | 5.9 |
| Sida acuta | 4.50 (0.74) | 7.8 |
| Hyptis suaveolens (K) | 4.53 (0.74) | 4.6 |
| Dactyloctenium radulans* | ||
The transformed (log [x + 1]) values are shown in parentheses; the least significant difference for the transformed values = 0.11 (P = 0.05). For comparison, germination rates of seeds stored in the laboratory for the same period are also shown. *No seeds of Dactyloctenium radulans germinated.
There was a weak negative correlation (r 2 = 0.35, P = 0.002) between germination rate and seed size.
DISCUSSION
There were wide ranges of both initial levels of dormancy and rates of change of dormancy in these species under different storage conditions. Thus species with several types of dormancy characteristics coexist in these communities as previously described for temperate and desert communities ( Angevine & Chabot 1979; Baskin & Baskin 1988).
The general trend in dormancy levels was a decline with time with the rate of decline greatest for seeds exposed on the soil surface and least for those stored in the laboratory. The 26 seed lots have been divided into four groups based on dormancy levels (expressed as a percentage of the initial values) after 16 (late dry season) and 32 weeks (mid wet season) exposure on the soil surface. On each occasion the species were divided into groups of high (above median value) and low (below median value) dormancy. The median values were 50% for week 16 (dry season) and 4% for week 32 (wet season).
Group 1
Group 1: species with high dormancy during both dry (mean = 81%) and wet (mean = 32%) seasons: Dactyloctenium radulans, Echinochloa colona, Eleusine indica, Bothriochloa pertusa, Heteropogon contortus (W), Themeda triandra, Urochloa mosambicensis, Stylosanthes hamata, Stylosanthes scabra, Gomphrena celosioides and Sida acuta.
Apart from Themeda triandra, this mixed group of annual and perennial grasses, legumes and dicots are all good colonizers of bare areas and a number are major weeds in Australia and other tropical areas –Echinochloa colona, Eleusine indica and Sida acuta ( Holm et al. 1977 ; Kleinschmidt & Johnson 1977; Mott 1980; Akobundu & Agyakwa 1987; Martin 1996). Dactyloctenium radulans is an annual grass which increases under heavy stocking ( Anderson 1993; Roberts & Silcock 1993) and can be a weed of cultivated areas and roadsides ( Tothill & Hacker 1983). Urochloa mosambicensis is a perennial grass sown in pastures but which is naturalized in northern Australia and can be a problem in cultivation ( Kleinschmidt & Johnson 1977). Stylosanthes hamata and S. scabra are important sown legumes in semiarid tropical areas and the high hard seed level is an important adaptation to the variable climate experienced in these areas. Gomphrena celosioides is a common weed of disturbed areas and often invades overgrazed pastures ( Kleinschmidt & Johnson 1977; Anderson 1993). Although all species in this group had high dormancy levels during the wet season and some may survive in the soil for a number of years (e.g. Eleusine indica; Hawton & Drennan 1980), very few seeds of Urochloa mosambicensis and Themeda triandra exposed on the soil surface for a further 64 weeks were dormant at the end of the period.
Group 2
Group 2: species with high dormancy in dry season (mean = 77%) and low dormancy in wet season (mean = 1%): Chloris inflata, Pennisetum pedicellatum.
These two annual grasses are common colonizers of waste land and can be weeds in cropping areas ( van Rijn 1968; Kleinschmidt & Johnson 1977; Mott 1980; Martin 1996). This dormancy pattern results in the retention of the seed bank until the start of the wet season when most of the seed becomes germinable thus providing for mass germination events due to the high seed production of these species ( McIvor et al. 1996 ).
Group 3
Group 3: species with relatively low dormancy in dry season (mean = 20%) but relatively high dormancy in wet season (mean = 22%): Brachiaria subquadripara, Cenchrus ciliaris.
Brachiaria subquadripara is an annual or short‐lived perennial which invades legume‐based pastures ( Gillard & Fisher 1978; Gillard et al. 1980 ; Tothill et al. 1982 ). Cenchrus ciliaris is a strongly perennial introduced grass widely sown in Australia and now naturalized in central inland Queensland ( Kleinschmidt & Johnson 1977) and invading along river banks into mesic habitats in the arid zone ( Humphries et al. 1991 ). The retention of some dormancy during the wet season suggests some seeds of these species would persist in the soil, although Silcock & Smith (1990) showed Cenchrus ciliaris seed on or in the soil lost most of its viability within two years.
Group 4
Group 4: species with low dormancy during both dry (mean = 20%) and wet seasons (mean = 1%): Digitaria ciliaris (K) and (W), Themeda quadrivalvis, Aristida latifolia, Bothriochloa decipiens, Chrysopogon fallax, Heteropogon contortus (K), Sporobolus indicus, Chloris gayana, Hyptis suaveolens (K) and (W).
This group contains predominantly perennial grasses, five native and one sown (Chloris gayana, which is now naturalised in central and southern Queensland), with two annual grasses and the dicot, Hyptis suaveolens. Mott (1978) showed seeds of a number of perennial native grasses were dormant at maturity but that dormancy was broken by the beginning of the following wet season. The low dormancy levels in the three weedy species in this group (Digitaria ciliaris, Themeda quadrivalvis and Hyptis suaveolens) is surprising although Marks & Nwachuku (1986) found there were few viable seeds of Digitaria ciliaris after storage in the soil for a wet season. These results suggest persistent seed banks will not be important for species in this group which rely on long‐lived plants (perennial grasses) or frequent seed input (annual species) for long‐term survival.
Only one seed lot was used for most species but for the three species with collections from both Katherine and Woodstock there was some variability between species in the grouping of the collections from the two sites. Seeds for both collections of Hyptis suaveolens and Digitaria ciliaris were in the same group but the dormancy patterns for the two Heteropogon contortus collections were different. The sample of Heteropogon contortus collected from Katherine had lower dormancy than the Woodstock collection in both the late dry (17 vs 42%) and mid wet seasons (1 vs 17%). Such variation between collections could be expected as Heteropogon contortus is a variable species ( Tothill 1966; Tothill & Hacker 1976) and a higher level of dormancy would be expected in a collection from an area with more variable climate (e.g. Woodstock) than one from an area with more reliable climate such as Katherine ( McCown 1981).
In tropical areas where high evaporation rates mean that the soil surface may remain moist for only a short period following rain, germination rate may be the major attribute controlling subsequent establishment ( McKeon et al. 1985 ). Almost all species germinated faster after exposure on the soil surface than after storage for the same time in the laboratory. McKeon (1984) found a similar result with Stylosanthes hamata, Stylosanthes humilis and Digitaria ciliaris which all germinated faster after exposure on the soil surface to rainfall. The wide range in germination rates between species means there could be large differences in establishment after small falls of rain; some species had almost complete germination within 1 day whereas others required more than 4 days to reach 50% germination. Rapid germination may particularly increase the likelihood of establishment of smaller seeds in seasonally dry environments ( Murali 1997) and in this experiment there was a weak trend for germination rate to decline as seed size increased. Similar relationships have been found with other suites of species or genotypes from the seasonally dry tropics ( Krishnasamy 1986; Agboola 1996; Murali 1997) but not necessarily in temperate (e.g. Counts & Lee 1991) or cultivated species (e.g. Imrie 1972; Newell & Bludau 1993).
All but seven of the 26 seed lots had either low initial dormancy or significantly reduced dormancy after 16 weeks exposure on the soil surface. This is just prior to the median start of the wet season at Townsville ( McCown 1981). For three species (Dactyloctenium radulans, Eleusine indica and Gomphrena celosioides), dormancy level did not alter during storage in the oven also, suggesting dormancy‐breaking is complex and not simply the result of high temperatures. Silcock et al. (1990) found virtually no germination of Dactyloctenium radulans even after 8 years’ storage in a laboratory unless the seed was scarified; treatment with concentrated sulphuric acid can also increase germination of this species ( Silcock & Williams 1975). Dormancy of Eleusine indica is decreased by gibberellic acid and potassium nitrate and can be broken by mechanical or acid scarification ( Hawton & Drennan 1980; Kanzler & van Staden 1984). Dormancy in the other four species (two Stylosanthes species, Pennisetum pedicillatum and Sida acuta) responds to high temperatures ( Mott 1980; Mott et al. 1981 ) and it is possible the temperatures in the field were not sufficiently high to affect them; Mott (1980) found Pennisetum pedicillatum seed in the hotter environment at Katherine was nondormant by the end of the dry season and in this study there was a significant decline after 32 weeks in the oven.
For the four perennial grasses stored for 96 weeks, there was negligible germinable or dormant seed for any species after two wet seasons exposure on the soil surface. This result is consistent with previous studies which have shown these species and a number of other common perennial grasses, usually have small soil seed banks ( McIvor 1987; Howden 1988; McIvor & Gardener 1991, 1994). Removal of a species from the community for more than 1 or 2 years is likely to leave no seed available for re‐establishment and could result in permanent changes to the community unless the species is able to recolonize from elsewhere or is deliberately reintroduced.
CONCLUSIONS
We draw three major conclusions from this study. First, the dormancy and germination characteristics of the species depend on storage conditions with the changes for seeds stored on the soil surface markedly different to the changes for seeds stored in the laboratory or oven. Other authors working both with temperate ( Grime et al. 1981 ; Baskin & Baskin 1988; Washitani & Masuda 1990) and with tropical species ( Elberse & Breman 1989) have concluded that field behaviour can be related to laboratory germination and response to storage conditions. From this study, the most useful treatments will be those where the storage conditions are as similar as possible to the conditions actually experienced by seeds in the field, for example the soil storage treatment in this experiment. Second, there are a range of dormancy responses in species from these communities. This is true even within a plant type, for example all four dormancy groups were represented in the annual grasses. The major native perennial grasses had very low to low levels of dormancy after exposure on the soil surface during the dry and wet seasons but a number of weedy annual grasses and dicots (except Hyptis suaveolens) had medium to high dormancy levels at the end of the dry season which were maintained to different degrees during the following wet season. Third, provenance or seed collection site (they were confounded in this experiment) may affect seed dormancy patterns.
Acknowledgements
We thank the late Dr C. J. Gardener for his contributions to experimental design and data collection.
REFERENCES
- Agboola D. A. (1996) The effect of seed size on germination and seedling growth of three tropical tree species. J. Trop. For. Sci. 9, 44 51. [Google Scholar]
- Akobundu I. O. & Agyakwa C. W. (1987) A Handbook of West African Weeds. International Institute of Tropical Agriculture, Ibadan, Nigeria.
- Anderson E. R. (1993) Plants of Central Queensland. Queensland Department of Primary Industries Information Series QI92037. Queensland Department of Primary Industries, Brisbane.
- Angevine M. W. & Chabot B. F. (1979) Seed germination syndromes in higher plants. In: Topics in Plant Population Biology (Eds O. T. Solbrig, S. Jain, G. B. Johnson & P. H. Raven) pp. 188–206. Columbia University Press, New York.
- Baskin C. C. & Baskin J. M. (1988) Germination ecophysiology of herbaceous plant species in a temperate region. Amer. J. Bot. 75, 286 305. [Google Scholar]
- Bekker R. M., Bakker J. P., Grandin U. et al. (1998) Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Funct. Ecol. 12, 834 42. [Google Scholar]
- Cook S. J. & Ratcliff D. (1984) A study of the effects of root and shoot competition on the growth of green panic (Panicum maximum var. trichoglume) seedlings in an existing grassland using root exclusion tubes. J. Appl. Ecol. 21, 971 82. [Google Scholar]
- Cook S. J. & Ratcliff D. (1985) Effect of fertilizer, root and shoot competition on the growth of Siratro (Macroptilium atropurpureum) and green panic (Panicum maximum var. trichoglume) seedlings in a native speargrass (Heteropogon contortus) sward. Aust. J. Agric. Res. 36, 233 45. [Google Scholar]
- Counts R. L. & Lee P. F. (1991) Germination and early seedling growth in some northern wild rice (Zizania palustris) populations differing in seed size. Can. J. Bot. 69, 689 96. [Google Scholar]
- Elberse W. TH. & Breman H. (1989) Germination and establishment of Sahelian rangeland species. I. Seed properties. Oecologia 80, 477 84. [DOI] [PubMed] [Google Scholar]
- Ellis R. H., Hong T. D., Roberts E. H. (1985) Handbook of Seed Technology for Genebanks 2. Compendium of Specific Germination Information and Test Recommendations. International Board for Plant Genetic Resources, Rome.
- Gillard P., Edye L. A., Hall R. L. (1980) Comparison of Stylosanthes humilis with S. hamata and S. subsericea in the Queensland dry tropics: effects on pasture composition and cattle liveweight gain. Aust. J. Agric. Res. 31, 205 20. [Google Scholar]
- Gillard P. & Fisher M. J. (1978) The ecology of Townsville stylo‐based pastures in northern Australia. In: Plant Relations in Pastures (Ed. J. R. Wilson) pp. 340–52. CSIRO, Melbourne.
- Grime J. P. (1989) Seed banks in ecological perspective. In: Ecology of Soil Seed Banks (Eds M. A. Leck V. T. Parker & R. L. Simpson) pp. xv–xxii. Academic Press, London.
- Grime J. P., Mason G., Curtis A. V. et al. (1981) A comparative study of germination characteristics in a local flora. J. Ecol. 69, 1017 59. [Google Scholar]
- Hacker J. B. (1984) Genetic variation in seed dormancy in Digitaria milanjiana and its correlation with rainfall at the collection site. J. Appl. Ecol. 21, 947 59. [Google Scholar]
- Hacker J. B., Andrew M. H., McIvor J. G., Mott J. J. (1984) Evaluation in contrasting climates of dormancy characteristics of seed of Digitaria milanjiana. J. Appl. Ecol. 21, 961 9. [Google Scholar]
- Hacker J. B. & Ratcliff D. (1989) Seed dormancy and factors controlling dormancy breakdown in buffel grass accessions from contrasting provenances. J. Appl. Ecol. 26, 201 12. [Google Scholar]
- Hawton D. & Drennan D. S. H. (1980) Studies on the longevity and germination of seed of Eleusine indica and Crotalaria goreenensis. Weed Res. 20, 217 23. [Google Scholar]
- Henderson R. J. F. (ed.) (1997) Queensland Plants: Names and Distribution. Queensland Herbarium, Queensland Department of Environment, Brisbane.
- Holm L. G., Plucknett D. L., Pancho J. V., Herbenger J. P. (1977) The World’s Worst Weeds. East–West Centre, University of Hawaii, Honolulu.
- Howden S. M. (1988) Some aspects of the ecology of four tropical grasses with special emphasis on Bothriochloa pertusa. PhD Thesis, Griffith University, Brisbane.
- Humphries S. E., Groves R. H., Mitchell D. S. (1991) Plant invasions: The incidence of environmental weeds in Australia. Kowari 2, 1 134. [Google Scholar]
- Imrie B. C. (1972) Effect of seed size on germination and seedling yield of Desmodium. SABRAO Newsl. 4, 85 9. [Google Scholar]
- Kanzler A. & van Staden J. (1984) Seed germination in goose grass (Eleusine indica). S. Afr. J. Bot. 3, 108 10. [Google Scholar]
- Kleinschmidt H. E. & Johnson R. W. (1977) Weeds of Queensland. Government Printer, Brisbane.
- Krishnasamy V. (1986) Study of seed quality factors among sorghum (Sorghum bicolor (L.) Moench) genotypes. Seed Sci. Tech. 14, 577 83. [Google Scholar]
- Marks M. K. (1983a) Periodicity of seedling emergence in six monocotyledonous weeds in south eastern Nigeria. Oecologia Applicata 4, 75 85. [Google Scholar]
- Marks M. K. (1983b) Timing of seedling emergence and reproduction in some tropical dicotyledonous weeds. Weed Res. 23, 325 32. [Google Scholar]
- Marks M. K. & Nwachuku A. C. (1986) Seed‐bank characteristics in a group of tropical weeds. Weed Res. 26, 151 7. [Google Scholar]
- Martin C. C. (1996) Weed control in tropical ley farming systems: a review. Aust. J. Exp. Agric. 36, 1013 23. [Google Scholar]
- McCown R. L. (1981) The climatic potential for beef cattle production in tropical Australia: Part III – Variation in the commencement, cessation and duration of the green season. Agric. Syst. 7, 163 78. [Google Scholar]
- McIvor J. G. (1987) Changes in the germinable seed levels in the soil beneath pastures in the seasonally dry tropics of north Queensland. Aust. J. Exp. Agric. 27, 283 9. [Google Scholar]
- McIvor J. G. & Gardener C. J. (1991) Soil seed densities and emergence patterns in pastures in the seasonally dry tropics of north‐eastern Australia. Aust. J. Ecol. 16, 159 69. [Google Scholar]
- McIvor J. G. & Gardener C. J. (1994) Soil seed banks in native pasture communities in northeastern Australia. Aust. J. Exp. Agric. 34, 1113 9. [Google Scholar]
- McIvor J. G., Singh V., Corfield J. P., Jones R. J. (1996) Seed production of native grasses: effects of stocking rate and season. Trop. Grassl. 33, 262 9. [Google Scholar]
- McKeon G. M. (1984) Field changes in germination requirements: effect of natural rainfall on potential germination speed and light requirement of Stylosanthes humilis, Stylosanthes hamata and Digitaria ciliaris. Aust. J. Agric. Res. 35, 807 19. [Google Scholar]
- McKeon G. M. (1985) Pasture seed dynamics in a dry monsoonal climate. II. The effect of water availability, light and temperature on germination speed and seedling survival of Stylosanthes humilis and Digitaria ciliaris. Aust. J. Ecol. 10, 149 63. [Google Scholar]
- McKeon G. M., Rose C. W., Kalma J. D., Torssell B. W. R. (1985) Pasture seed dynamics in a dry monsoonal climate. 1. Germination and seedbed environment of Stylosanthes humilis and Digitaria ciliaris. Aust. J. Ecol. 10, 135 47. [Google Scholar]
- Mott J. J. (1978) Dormancy and germination in five native grass species from savannah woodland communities of the Northern Territory. Aust. J. Bot. 26, 621 31. [Google Scholar]
- Mott J. J. (1980) Germination and establishment of the weeds Sida acuta and Pennisetum pedicellatum in the Northern Territory. Aust. J. Exp. Agric. Anim. Husb. 20, 463 9. [Google Scholar]
- Mott J. J., McKeon G. M., Gardener C. J., Mannetje L. T. (1981) Geographic variation in the reduction of hard seed content of Stylosanthes seed in the tropics and subtropics of northern Australia. Aust. J. Agric. Res. 32, 861 9. [Google Scholar]
- Murali K. S. (1997) Patterns of seed size, germination and seed viability of tropical tree species in southern India. Biotropica 29, 271 9. [Google Scholar]
- Newell A. J. & Bludau N. K. (1993) Variation in total germination, rate of germination and seed weight among cultivars of Poa pratensis. J. Sports Turf Res. Inst. 69, 83 9. [Google Scholar]
- Payne R. W., Lane P. W., Ainsley A. E. et al. (1988) GENSTAT 5 Reference Manual. Clarendon Press, Oxford.
- Rathcke B. & Lacey E. P. (1985) Phenological patterns of terrestrial plants. Ann. Rev. Ecol. System. 16, 179 214. [Google Scholar]
- van Rijn P. J. (1968) Ecological aspects of weed control in cotton in the Ord River valley, W.A. 1. Conditions affecting germination of weeds. Aust. J. Exp. Agric. Anim. Husb. 8, 620 24. [Google Scholar]
- Roberts B. R. & Silcock R. G. (1993) Western Grasses: a Grazier’s Guide to the Grasses of South‐West Queensland. USQ Press, Toowoomba.
- Ross M. A. & Harper J. L. (1972) Occupation of biological space during seedling establishment. J. Ecol. 60, 77 88. [Google Scholar]
- Silcock R. G. & Smith F. T. (1990) Viable seed retention under field conditions by western Queensland pasture species. Trop. Grassl. 24, 65 74. [Google Scholar]
- Silcock R. G. & Williams L. M. (1975) Methods for improving the laboratory germination of Dactyloctenium radulans seed. Aust. Seed Sci. Newsl. 1, 59 61. [Google Scholar]
- Silcock R. G., Williams L. M., Smith F. T. (1990) Quality and storage characteristics of the seeds of important native pasture species in south‐west Queensland. Aust. Rangeland J. 12, 14 20. [Google Scholar]
- Thompson K., Band S. R., Hodgson J. G. (1993) Seed size and shape predict persistence in soil. Funct. Ecol. 7, 236 41. [Google Scholar]
- Torssell B. W. R. & McKeon G. M. (1976) Germination effects on pasture composition in a dry monsoonal climate. J. Appl. Ecol. 13, 593 603. [Google Scholar]
- Tothill J. C. (1966) Phenological variation in Heteropogon contortus and its relation to climate. Aust. J. Bot. 14, 35 47. [Google Scholar]
- Tothill J. C. & Hacker J. B. (1976) Polyploidy, flowering phenology and climatic adaption in Heteropogon contortus (Gramineae). Aust. J. Ecol. 1, 213 22. [Google Scholar]
- Tothill J. C. & Hacker J. B. (1983) The Grasses of Southern Queensland. University of Queensland Press, Brisbane.
- Tothill J. C., Mott J. J., Gillard P. (1982) Pasture weeds of the tropics and subtropics with special reference to Australia. In: Biology and Ecology of Weeds (Eds W. Holzner & M. Numata) pp. 403–27. Dr W. Junk, The Hague.
- Washitani I. & Masuda M. (1990) A comparative study of the germination characteristics of seeds from a moist tall grassland community. Funct. Ecol. 4, 543 57. [Google Scholar]


