Summary
In 2015, cholesterol deficiency (CD) was reported for the first time as a new recessive defect in Holstein cattle. After GWAS mapping and identification of a disease‐associated haplotype, a causative loss‐of‐function variant in APOB was identified. CD‐clinically affected APOB homozygotes showed poor development, intermittent diarrhea and hypocholesterolemia and, consequently, a limited life expectation. Herein, we present a collection of 18 cases clinically diagnosed as CD‐affected APOB heterozygotes. CD‐clinically affected heterozygotes show reduced cholesterol and triglyceride blood concentrations. The differences in total blood cholesterol and triglycerides between nine CD‐clinically affected and 36 non‐affected heterozygotes were significant. As only some APOB heterozygotes show the clinical CD phenotype, we assume that the penetrance is reduced in heterozygotes compared to the fully penetrant effect observed in homozygotes. We conclude that APOB‐associated CD represents most likely an incomplete dominant inherited metabolic disease with incomplete penetrance in heterozygotes.
Keywords: apolipoprotein B, calf survival, genetic disorder, incomplete penetrance, rare disease
Recently, numerous calves showing retarded growth and intermittent diarrhea, accompanied by pronounced hypocholesterolemia and low triglyceride concentrations, were observed in the global Holstein population (Kipp et al. 2016; Mock et al. 2016; Schütz et al. 2016). This previously unknown fat metabolism disorder was termed cholesterol deficiency (CD; OMIA 001965‐9913). After a genome‐wide association study uncovering a disease‐associated haplotype on chromosome 11 (Kipp et al. 2016), a 1.3‐kb insertion of a transposable LTR element in the coding sequence of the bovine APOB gene was identified as a disease‐causing variant (Menzi et al. 2016). These findings were independently confirmed (Charlier 2016; Schütz et al. 2016). So far, the defect is clinically identifiable only in the homozygote form: Kipp et al. (2016) reported nine cases homozygous for the disease‐associated haplotype, and Duff et al. (2016), Mock et al. (2016) and Schütz et al. (2016) reported one, six and nine APOB homozygote mutant cases respectively. The disease‐associated haplotype carrying the APOB variant traces back to the North American bull, Maughlin Storm, born in 1991 and used extensively in the Holstein population worldwide (VanRaden & Null 2015). The bovine APOB variant represents a loss of function mutation similar to APOB‐associated familial hypobetalipoproteinemia‐1, showing incomplete penetrance with respect to expression of clinical signs by a particular age of the affected people (OMIM 615558). Therefore, it was assumed that the bovine APOB‐associated CD defect could be explained by an autosomal monogenic recessive mode of inheritance, although clinically normal APOB heterozygotes showed reduced cholesterol and triglyceride blood concentrations, indicating a possible codominant inheritance for cholesterol and triglyceride levels (Gross et al. 2016; Kipp et al. 2016; Saleem et al. 2016).
Since the identification of the causal variant in the APOB gene, we genotyped a total of 91 Holstein calves that appeared much smaller than their contemporaries, had owner‐reported clinical signs of chronic diarrhea and illthrift, and from which blood samples were submitted for diagnostic purposes (S2). In addition, some owners of these 91 calves were suspicious of CD based on the fact that both parents were related to Maughlin Storm. The age of these 91 cases varied from two to 921 days with a mean of 84.8 and a median of 47 (S1). Genomic DNA was extracted from EDTA‐stabilized blood samples using the Maxwell instrument (Promega) and analyzed with the direct test targeting the APOB variant according to Menzi et al. (2016). The three possible APOB genotypes (CDF: non‐carrier; CDC: heterozygous carrier; CDS: homozygous carrier) are presented according to standards of the World Holstein Friesian Federation ([Link]). About 80% of the submitted calves (73/91) were genotyped as homozygous mutant (CDS), confirming the initial suspicion of CD. Interestingly, 20% of clinically affected calves (18/91) were heterozygous mutant (CDC). The mean age of diagnosis of the 73 CDS affected calves was 69 days (SD ± 109.59), and for the 18 CDC clinically affected calves 148 days (SD ± 173.83), which did not differ significantly (S1).
Besides the six APOB‐homozygous mutant CD‐affected animals reported earlier (Mock et al. 2016), we were able to examine an additional 15 CD‐suspect calves clinically (S2). Six of these 15 cases were genotyped as CDS, whereas nine were CDC. The clinical phenotype of all 15 cases included poor development, low weight and intermittent diarrhea, as reported before (Mock et al. 2016). Laboratory examinations for rotavirus, coronavirus, bovine viral diarrhea virus, coccidia, cryptosporidia, E. coli and Salmonella spp. gave negative results, and symptomatic treatments did not lead to clinical improvement. Three affected animals with the CDC genotype were euthanized, and the pathological phenotype of these animals was similar to what we have observed before in CDS‐affected cases (Mock et al. 2016).
Eleven of the 12 APOB homozygous mutant CD‐affected animals had cholesterol and triglyceride readings (S2). Mean blood total cholesterol [norm based on Mock et al. (2016): 1.20–3.84 mmol/l] for the 11 CDS cases was 0.21 mmol/l (SD ± 0.09), whereas the mean value for the nine CDC affected calves was 1.34 mmol/l (SD ± 0.76) (S2). The mean measured blood triglyceride concentration [norm based on Mock et al. (2016): 0.19–0.51 mmol/l] was 0.07 mmol/l (SD ± 0.02) for the 11 CDS cases and 0.17 mmol/l (SD ± 0.11) for the nine CDC affected calves (S2). These values for total blood cholesterol and triglycerides were subsequently compared with similar data from adult Holstein males determined in the course of a previous study (Gross et al. 2016). That control cohort of 254 artificial insemination sires included 218 bulls genotyped as CDF and 36 as CDC (S2). A one‐way ANOVA with total cholesterol and triglyceride concentrations as the response variables and the different APOB genotypes (CDS, CDC, CDF) in combination with the clinical status of the animals (CD‐affected, non‐affected) as the explanatory variable resulting in four groups (CDS‐affected, CDC‐affected, CDC‐normal, CDF‐normal), was conducted with the Anova function of the car package (Fox & Weisberg 2011). The significant outcome for total blood cholesterol (F = 96.51, DF = 270, P < 2.2e‐16) and triglyceride concentrations (F = 10.6, DF = 270, P = 1.311e‐06) led to the interpretation that at least both means differed significantly among APOB genotypes (Fig. 1). To further investigate the differences between the genotypes, a post‐hoc Tukey test was performed. The function imple.glht from the mixlm package (Liland 2018) was used for the pairwise comparisons. The pairwise comparison showed a significant difference in blood total cholesterol concentrations between the non‐affected CDC controls and the CD‐affected CDC cases (S3). This effect was not observed in triglyceride concentrations, as there was more variation in the triglyceride levels in the CD‐affected animals than in any other group. The distribution of total cholesterol and triglyceride levels for the four groups investigated in the ANOVA is shown in Fig. 1. In comparing the four groups, a tendency of total cholesterol concentrations increasing with each additional copy of the APOB wild‐type allele (Fig. 1) was observed, indicating an additive (codominant) effect. However, a significant difference was also observed between the two groups of animals with the CDC genotype (affected vs. non‐affected; est = 0.909 with SE ± 0.166, z‐value = 5.465, P < 0.0001) in the pairwise comparison (Fig. 1).
Figure 1.
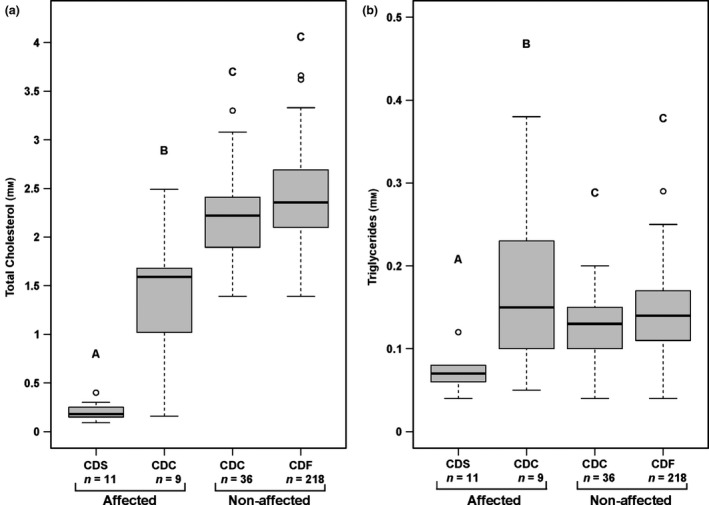
Boxplots showing (a) total blood cholesterol and (b) triglyceride concentrations in clinically CD affected and non‐affected Holstein cattle. APOB genotypes are shown as wild‐type (CDF), heterozygous mutant (CDC) and homozygous mutant (CDS). The number of animals per group are shown below the boxplots (11 CDS affected and nine CDC affected; 36 CDC non‐affected and 218 CDC non‐affected). Different capital letters above the box plots indicate significant differences between the groups (P < 0.025).
Based on these observations, we assume that an animal with a single copy of the APOB variant might show the CD disease due to disturbed lipid homeostasis. Based on our observations, most likely some of these individuals show a fatal inability to maintain a blood cholesterol level sufficient for life. On the other hand, it is known that the cholesterol level is age‐dependent and that the cholesterol level in cattle levels off at about 21 days of age (Shope 1928), and the values of the nine CDC affected calves were determined much later in life (S1). Nonetheless, the CDC‐clinically affected animals were noted much earlier by the owners, at a similar age as the CDS‐clinically affected cases, mostly during the first weeks of life when they were fed milk (S1). Nonetheless, most heterozygous animals develop normally, and therefore the mutation acts most likely as incomplete dominance with reduced penetrance in heterozygotes. As all homozygotes develop the clinical disorder, the penetrance of the APOB variant is fully complete in homozygotes. On the other hand, only some heterozygotes show a clinical phenotype, meaning that penetrance is incomplete in heterozygotes. Recent findings in human genetics indicate that incomplete penetrance for presumed Mendelian diseases is likely more common than previously believed (Chen et al. 2016). Alternatively, possible allelic and/or genetic heterogeneity could also explain the occurrence of the CD disease in some APOB heterozygotes
We conclude that cholesterol deficiency not only affects APOB mutant homozygotes but occurs also in heterozygotes. The CD‐affected heterozygous animals can show clinical signs similar to the homozygous mutants. Furthermore, we showed a significant difference in blood total cholesterol and triglyceride concentrations between clinically CD‐affected and non‐affected APOB heterozygous animals. Therefore, these findings support an incomplete dominance mode of inheritance with incomplete penetrance in heterozygotes.
Supporting information
Figure S1. Boxplots showing the age distribution in 91 CD‐affected and 254 non‐affected Holstein cattle.
Table S1. List of animals used in the study.
Table S2. Output of the Tukey test showing the pairwise comparisons in detail.
Acknowledgements
The authors are grateful to Swissgenetics and the Swiss Cattle Breeding Associations (http://www.swissherdbook.ch and http://www.holstein.ch) for financial support. The authors thank J.J. Gross and R.M. Bruckmaier for providing the blood total cholesterol and triglyceride data of the control cattle. Furthermore, we thank all farmers and veterinarians for sending blood samples of suspicious cases for genotyping. Finally, we thank Nathalie Besuchet‐Schmutz, Muriel Fragnière and Sabrina Schenk for expert technical assistance. This study was partially funded by the Swiss National Science Foundation.
References
- Charlier C. (2016) The role of mobile genetic elements in the bovine genome, Plant and Animal Genome Conference XXIV, January 9–13, 2016 San Diego Abstract W636.
- Chen R., Shi L., Hakenberg J. et al (2016) Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood diseases. Nature Biotechnology 34, 531–8. [DOI] [PubMed] [Google Scholar]
- Duff J.P., Passant S., Wessels M., Charlier C., Hateley G. & Irvine R.M. (2016) Cholesterol deficiency causing calf illthrift and diarrhoea. Veterinary Record 178, 424–5. [DOI] [PubMed] [Google Scholar]
- Fox J. & Weisberg S. (2011) An R Companion to Applied Regression, 2nd edn Sage, Thousand Oaks, CA: (http://socserv.socsci.mcmaster.ca/jfox/Books/Companion). [Google Scholar]
- Gross J.J., Schwinn A.‐C., Schmitz‐Hsu F., Menzi F., Drögemüller C., Albrecht C. & Bruckmaier R.M. (2016) Rapid communication: cholesterol deficiency–associated APOB mutation impacts lipid metabolism in Holstein calves and breeding bulls. Journal of Animal Science 94, 1761–6. [DOI] [PubMed] [Google Scholar]
- Kipp S., Segelke D., Schierenbeck S. et al (2016) Identification of a haplotype associated with cholesterol deficiency and increased juvenile mortality in Holstein cattle. Journal of Dairy Science 99, 1–17. [DOI] [PubMed] [Google Scholar]
- Liland K.H. (2018) mixlm: mixed model anova and statistics for education (R package version 1.2.3). (https://CRAN.R-project.org/package=mixlm)
- Menzi F., Besuchet‐Schmutz N., Fragnière M. et al (2016) A transposable element insertion in APOB causes cholesterol deficiency in Holstein cattle. Animal Genetics 47, 253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock T., Mehinagic K., Menzi F., Studer E., Oevermann A., Stoffel M.H., Drögemüller C., Meylan M. & Regenscheit N. (2016) Clinicopathological phenotype of autosomal recessive cholesterol deficiency in Holstein cattle. Journal of Veterinary Internal Medicine 30, 1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S., Heuer C., Sun C., Kendall D., Moreno J. & Vishwanath R. (2016) Technical note: the role of circulating low‐density lipoprotein levels as a phenotypic marker for Holstein cholesterol deficiency in dairy cattle. Journal of Dairy Science 99, 5545–50. [DOI] [PubMed] [Google Scholar]
- Schütz E., Wehrhahn C., Wanjek M., Bortfeld R., Wemheuer W.E., Beck J. & Brenig B. (2016) The Holstein Friesian lethal haplotype 5 (HH5) results from a complete deletion of TBF1M and cholesterol deficiency (CDH) from an ERV‐(LTR) insertion into the coding region of APOB . PLoS ONE 11, e0154602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope R.E. (1928) The effect of age on the total and combined cholesterol of the blood serum. Journal of Biological Chemistry 80, 141–8. [Google Scholar]
- VanRaden P. & Null D. (2015) Holstein Haplotype for Cholesterol Deficiency (HCD). (https://queries.uscdcb.com/reference/changes/HCD_inheritance.pdf).
- World Holstein Friesian Federation (n.d.) Standardized Labelling for Genetic Trait Coding. (http://www.whff.info/documentation/documents/WHFFStandardizeLabellingofGeneticTraitCodingv4.pdf)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Boxplots showing the age distribution in 91 CD‐affected and 254 non‐affected Holstein cattle.
Table S1. List of animals used in the study.
Table S2. Output of the Tukey test showing the pairwise comparisons in detail.


