Abstract
The epidemiology of respiratory viruses (RVs) in lung transplant recipients (LTRs) and the relationship of RVs to lung function, acute rejection (AR) and opportunistic infections in these patients are not well known. We performed a prospective cohort study (2009–2014) by collecting nasopharyngeal swabs (NPSs) from asymptomatic LTRs during seasonal changes and from LTRs with upper respiratory tract infectious disease (URTID), lower respiratory tract infectious disease (LRTID) and AR. NPSs were analyzed by multiplex polymerase chain reaction. Overall, 1094 NPSs were collected from 98 patients with a 23.6% positivity rate and mean follow‐up of 3.4 years (interquartile range 2.5–4.0 years). Approximately half of URTIDs (47 of 97, 48.5%) and tracheobronchitis cases (22 of 56, 39.3%) were caused by picornavirus, whereas pneumonia was caused mainly by paramyxovirus (four of nine, 44.4%) and influenza (two of nine, 22.2%). In LTRs with LRTID, lung function changed significantly at 1 mo (p = 0.03) and 3 mo (p = 0.04). In a nested case–control analysis, AR was associated with RVs (hazard ratio [HR] 6.54), Pseudomonas aeruginosa was associated with LRTID (HR 8.54), and cytomegalovirus (CMV) replication or disease was associated with URTID (HR 2.53) in the previous 3 mo. There was no association between RVs and Aspergillus spp. colonization or infection (HR 0.71). In conclusion, we documented a high incidence of RV infections in LTRs. LRTID produced significant lung function abnormalities. Associations were observed between AR and RVs, between P. aeruginosa colonization or infection and LRTID, and between CMV replication or disease and URTID.
Keywords: clinical research/practice, infectious disease, lung transplantation/pulmonology, complication: infectious, infection and infectious agents, viral, infection and infectious agents, viral: influenza, lung disease: infectious, rejection: acute
Short abstract
A large prospective study of the epidemiology of respiratory viruses in lung transplant recipients using molecular assays demonstrates a very high incidence of respiratory viral infection and an association between respiratory virus infectious diseases, immediate allograft dysfunction, and the development of acute rejection and opportunistic infection.
Abbreviations
- AR
acute rejection
- CI
confidence interval
- CMV
cytomegalovirus
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume
- FLU
influenza virus
- HMPV
metapneumovirus
- HR
hazard ratio
- IQR
interquartile range
- LRTID
lower respiratory tract infectious disease
- LTR
lung transplant recipient
- MDIM
microbially determined immune modulation
- NAT
nucleic acid amplification testing
- NPS
nasopharyngeal swab
- PIV
parainfluenza virus
- RSV
respiratory syncytial virus
- RV
respiratory virus
- SD
standard deviation
- URTID
upper respiratory tract infectious disease
Introduction
Respiratory viruses (RVs) are increasingly recognized as a major cause of morbidity and mortality in hematopoietic stem cell transplant and solid organ transplant recipients 1. Lung transplant recipients (LTRs) are a population of patients at constant risk for RV infections because of the continuous exposure of the organ to the external environment and potential respiratory pathogens, the impaired protective mechanisms of the grafted lung, HLA mismatching, and the presence of significant immunosuppression 2, 3.
The sensitivities of contemporary molecular diagnostic techniques have been substantially improved, allowing for the rapid simultaneous detection of a wide variety of conventional and emerging RVs in respiratory samples. At present, these techniques are the preferred diagnostic tools for studying RVs in immunocompromised patients 4.
Previous studies have suggested that in addition to their direct, cytopathic and tissue‐invasive effects, RVs could have immediate impacts on the function of the transplanted lung with functional decline 5. RVs can create an inflammatory environment in the grafted lung that leads to local and systemic microbially determined immune modulation (MDIM) 6, which increases the allo‐ and autoimmune responses that increase susceptibility to other opportunistic infections and results in the development of acute rejection (AR) 7. Although conceptually appealing and supported by experimental animal studies, the clinical link between RVs and these indirect effects has not been clearly assessed. Key limitations have been the low number and incompleteness of events, the heterogeneity of study populations and diagnostic tools, and the frequently retrospective nature of the published reports 8.
The aim of this extensive prospective cohort study based on molecular assays was to characterize the epidemiology of RVs and the associations between RVs and lung function, opportunistic infections and AR beginning at the time of the transplant.
Material and Methods
Study setting and patient population
A prospective cohort study was performed using all consecutive adult patients undergoing lung transplantation at Hospital Univeristari Vall d'Hebron (Barcelona, Spain) from September 2009 to September 2011. We included all patients aged >18 years and began following patients starting from hospital discharge after transplantation. All patients were followed up continuously until September 2014 or until death. Details on immunosuppression and prophylaxis protocols are shown in Table S1.
Data were collected prospectively through the general hospital, microbiology and histopathology databases using a standardized protocol. The study protocol was approved by the Vall d'Hebron Ethics Committee for Clinical Research, and informed consent was obtained from all participants.
An external scientific committee consisted of three investigators, two of whom were lung transplant unit specialists (Hospital Universitario y Politécnico La Fe, Valencia; Hospital Universitario Marqués de Valdecilla, Santander) and one who was an infectious disease specialist (Hospital Universitario 12 de Octubre, Madrid). The members were well‐established independent scientists appointed in their personal capacity with extensive (>10 years) professional and multidisciplinary experience in the field of infections in LTRs. An expert panel was appointed annually for a period of 5 years to evaluate the project, to follow the database and to participate in the interpretation of the results. The panel members evaluated the quality of data collection, looking independently at a random number of cases and focusing on disease definition and patient outcomes. The present study was submitted for publication only after it was approved by the expert panel.
This study adhered to the principles of the Declaration of Helsinki, formulated by the World Medical Association, and the ethical statement of the International Society for Heart and Lung Transplantation.
Respiratory tract infectious disease definitions
An upper respiratory tract infectious disease (URTID) was defined as an illness caused by an acute infection with the onset of sore throat, rhinorrhea or hoarseness. A lower respiratory tract infectious disease (LRTID) was defined as new onset of shortness of breath, cough, sputum, rales, hypoxemia and/or wheezing. When symptoms of LRTID were associated with a new pulmonary infiltrate (on chest radiograph or chest computed tomography), pneumonia was distinguished from tracheobronchitis 9, 10. Nosocomial RV infectious disease refers to any infectious disease contracted by a patient in a hospital at least 48–72 h after being admitted.
Management and follow‐up
Patients were closely followed up frequently by phone calls every week to check their health status. In cases of new‐onset respiratory tract infection symptoms (fever, rhinorrhea, sore throat, cough, dyspnea, sputum, myalgia, fatigue, thoracic pain), patients were instructed to contact the research team to schedule a prompt visit (<24 h) at the outpatient clinic of the lung transplant program or to go to the emergency room.
Systematic collection of nasopharyngeal swabs (NPSs) was performed in all patients in different settings. These patients included asymptomatic patients during seasonal changes (around calendar‐based seasonal changes at spring, summer, autumn and winter) and patients with URTID, LRTID and biopsy‐proven AR. URTID controls were assessed at 1 and 3 mo after the first episode. During the study period, investigators and clinicians who treated the patients were aware of the results of the viral samples collected.
Mulitplex nucleic acid amplification testing for RV
NPSs were collected by specialized medical staff using the standardized procedures of the U.S. Centers for Disease Control and Prevention. NPSs were taken by inserting swabs 2–3 cm into the nostril to access to the posterior nasopharynx and rotating to collect secretions and exfoliated nasopharyngeal cells. The NPSs were immediately placed in tubes containing 2.5 mL of viral transport medium, analyzed and stored at −80°C until further multiplex nucleic acid amplification testing (NAT) was performed at the Department of Microbiology of Basel University Hospital (Switzerland) and Vall d'Hebron University Hospital (Barcelona, Spain). Total nucleic acids were extracted from 200 μL of each sample using the automated easyMAG (bioMérieux, Marcy l'Etoile, France) or QIAsymphony (Qiagen, Hilden, Germany) system, according to the manufacturers’ protocols. Mulitplex NAT was performed using the Seeplex® RV15 OneStep ACE Detection (Seegene, Seoul, Korea) or RespiFinder 22 (PathoFinder, Maastricht, the Netherlands) for the following respiratory viruses: influenza viruses A and B; respiratory syncytial viruses (RSVs) A and B; parainfluenza viruses (PIVs) 1, 2, 3, and 4; rhinoviruses A, B, and C; enterovirus; metapneumovirus (HMPV); coronaviruses OC43, 229E, and NL63; bocavirus; and adenovirus.
Rhinoviruses and enteroviruses are members of the human picornavirus family and are detected by the same assay. We use the term picornavirus when describing these viruses, although the sequence analysis and specific assays used in our study revealed that almost all picornaviruses were rhinoviruses. Human RSV, HMPV and PIV are members of the paramyxoviridae family. Concurrent detection of more than one RV in the same sample was considered to be a single clinical episode.
Data collection, primary and secondary outcomes, and statistical analysis
The first aim of the study was to identify the epidemiology, clinical manifestations and seasonality of RVs in LTRs; therefore, a descriptive analysis was performed. Continuous variables were expressed as medians and ranges. All proportions were calculated as percentages of patients with available data and presented as medians and interquartile ranges (IQRs) for continuous variables. Independent t‐tests were used to compare continuous data, and chi‐square tests were used to compare categorical data.
The second aim was to study the change in allograft function with forced expiratory volume (FEV1) at 1, 3, and 6 mo after a positive RV episode and to evaluate the MDIM effects related to RV infections (biopsy‐proven AR, cytomegalovirus [CMV] replication or disease, Aspergillus spp. colonization or infection, and Pseudomonas aeruginosa colonization or infection).
The surveillance strategy for performing bronchial biopsies to detect AR, respiratory cultures (for P. aeruginosa and Aspergillus spp.) and blood CMV loads were based on routine screening or clinician's judgment for diagnosis of clinical events (Table S1).
Data concerning the clinical courses of the patients were prospectively collected during the patients’ clinical follow‐up. A nested case–control analysis using conditional logistic regression was applied for different MDIM events using time as a matched variable. Each case (patient with an event such as biopsy‐proven AR, CMV replication or disease, Aspergillus spp. colonization or infection, or P. aeruginosa colonization or infection) observed during follow‐up was matched with up to four randomly selected controls (patient without an event) of the cohort that had the same follow‐up time since transplantation. In each case and control, the presence of RV was observed in the 3 mo prior to the event. Secondary outcome measures were evaluated at 3 mo because we considered that adverse clinical events occurring soon after RV infection were more likely to be associated with that infection. A p‐value ≤0.05 was considered significant. Data analyses were performed with Stata 11.2 (StataCorp, College Station, TX).
Results
Epidemiology of RVs after lung transplantation
A total of 98 LTRs were enrolled in this study, including 67 recipients of double‐lung transplants (Table 1). The mean postoperative follow‐up period was 3.4 years (IQR 2.5–4.0 years).
Table 1.
Demographic data and patient characteristics
| Variable | Result |
|---|---|
| Patients, n | 98 |
| Age, years, mean ± SD | 49.9 ±12.6 |
| Sex, n (%) | |
| Male | 62 (63.3) |
| Female | 36 (36.7) |
| Pretransplant diagnosis, n (%) | |
| COPD | 34 (34.7) |
| Idiopathic pulmonary fibrosis | 30 (30.6) |
| Cystic fibrosis | 12 (12.2) |
| Primary pulmonary hypertension | 7 (7.1) |
| Bronchiectasis | 4 (4) |
| Others | 11 (11.2) |
| Transplant type | |
| Double | 67 (68.4) |
| Single | 31 (31.3) |
| Induction regimen, n (%) | |
| Basiliximab | 3 (3.1) |
| Steroids | 98 (100) |
COPD, chronic obstructive pulmonary disease; SD, standard deviation.
The overall detection rate of RVs was 0.76 per patient‐year (95% confidence interval [CI] 0.67–0.87), significantly lower than the rate in patients with symptomatic RV infection (2.08 per patient‐year, 95% CI 1.77–2.43). The RV detection rate was 2.33 per patient‐year (95% CI 1.89–2.84) for URTID, not significantly different from the rate of 1.79 per patient‐year (95% CI 1.38–2.28) for LRTID. The median time from transplant to the first RV infection was 9.2 mo (IQR 4–18 mo).
In total, 1094 NPSs were collected, with a median of 11 samples per patient (minimum 1, maximum 26). The positivity rates for the systematic collection of NPSs in different clinical settings are described in Table 2. Samples collected from LTRs with symptoms of respiratory infections were positive in 55.4% of cases, whereas those from asymptomatic LTRs during seasonal changes were positive in 11.5% of cases (Table 2).
Table 2.
Positivity rates of systematically collected nasopharyngeal swabs in different clinical settings
| Event | Number of positive samples (%) | Patients, n |
|---|---|---|
| Asymptomatic | 68/591 (11.5) | 93 |
| URTID | 97/150 (64.7) | 69 |
| URTID, 1 mo | 16/103 (15.5) | 60 |
| URTID, 3 mo | 8/78 (10.2) | 50 |
| LRTID, tracheobronchitis | 56/108 (51.8) | 61 |
| LRTID, pneumonia | 9/34 (26.4) | 24 |
| AR | 4/30 (13.3) | 25 |
| Total | 258/1094 (23.6) | 98 |
AR, acute rejection; LRTID, lower respiratory tract infectious disease; URTID, upper respiratory tract infectious disease.
Seasonal patterns were observed, and higher incidence rates were found during winter (1.15 RVs per patient‐year, 95% CI 0.92–1.42) and autumn (1.01 RVs per patient‐year, 95% CI 0.80–1.25) (Figure 1).
Figure 1.
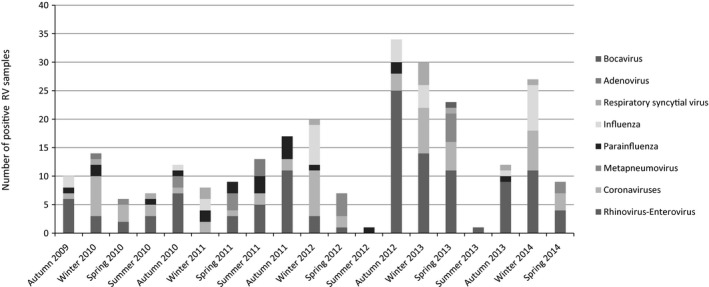
Seasonal patterns of RVs: overall number and type of positive viral tests from September 2009 to September 2014 during autumn (September 21 to December 20), winter (December 21 to March 20), spring (March 21, to June 20), and summer (June 21 to September 20). RV, respiratory virus.
The distribution of RVs in different clinical lung transplant settings is shown in Table 3. Picornaviruses (43.2%) were the most frequently encountered RVs, followed by coronaviruses and influenza (both 16.7%). In fact, half of the URTID episodes (47 of 97, 48.5%) and a third of LRTIDs (23 of 65, 35.3%) were caused by picornaviruses. The second and third most common etiological causes of URTID were coronaviruses (18 of 97, 18.6%) and influenza (16 of 97 16.4%), respectively (Table 3). Among URTIDs, protracted viral shedding was found in 15.5% of cases at 1 mo and 10.2% of cases at 3 mo. In addition, protracted viral shedding was found in cases of infection with coronaviruses and picornaviruses but no cases of persistent influenza shedding.
Table 3.
Etiology of RV infections in different clinical settings
| Event | Etiology of RV infection, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Picornavirus | Coronavirus | FLU | HMPV | PIV | RSV | Adenovirus | Bocavirus | Total | |
| Asymptomatic | 37 (54.4) | 18 (26.4) | 1 (1.5) | 2 (2.9) | 5 (7.4) | 3 (4.4) | 1 (1.5) | 1 (1.5) | 68 |
| Symptomatic | 70 (43.2) | 27 (16.7) | 27 (16.7) | 16 (9.9) | 13 (8.0) | 7 (4.3) | 2 (1.2) | 0 | 162 |
| URTID | 47 (48.5) | 18 (18.6) | 16 (16.4) | 8 (8.2) | 4 (4.1) | 2 (2.1) | 2 (2.1) | 0 | 97 |
| LRTID | 23 (35.3) | 9 (13.9) | 11 (16.9) | 8 (12.3) | 9 (13.9) | 5 (7.9) | 0 | 0 | 65 |
| Tracheobronchitis | 22 (39.3) | 8 (14.2) | 9 (16.1) | 8 (14.3) | 7 (12.5) | 2 (3.6) | 0 | 0 | 56 |
| Pneumonia | 1 (11.1) | 1 (11.1) | 2 (22.2) | 0 | 2 (22.2) | 3 (33.3) | 0 | 0 | 9 |
| AR | 1 (25) | 1 (25) | 0 | 0 | 2 (50) | 0 | 0 | 0 | 4 |
| Total | 108 (41.8) | 46 (17.8) | 28 (10.8) | 18 (6.8) | 20 (7.7) | 10 (3.9) | 3 (1.1) | 1 (0.4) | 234 |
AR, acute rejection; FLU, influenza virus; HMPV, metapneumovirus; LRTID, lower respiratory tract infectious disease; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, respiratory virus; URTID, upper respiratory tract infection.
Overall, 40.1% (65 of 162) of symptomatic RV infections progressed to LRTID. In addition to picornaviruses, multiple different RVs were detected as causes of tracheobronchitis, including paramyxovirus and coronavirus, as shown in Table 3. Paramyxovirus (PIV and RSV, five of nine, 55.5%) and influenza (two of nine, 22.2%) were the most frequently isolated viruses in LTRs with pneumonia (nine of 162, 5.5% incidence); these viruses had a statistically significantly higher association with pneumonia compared with picornavirus (one of nine, 11.1%, p = 0.02) (Table 3). Asymptomatic infection was associated mainly with picornavirus (37 of 68, 54.4%) and coronavirus (18 of 68, 26.4%). RV coinfection was uncommon (12 of 1094; 1.1%) and mostly involved influenza and picornaviruses (five of 12, 41.6%). Higher tacrolimus serum trough levels were not observed in patients with different viral syndromes (Table 4).
Table 4.
Tacrolimus serum trough levels in patients with different viral syndromes
| Time period | Tacrolimus level, μg/L, median (IQR) | p‐value | ||||
|---|---|---|---|---|---|---|
| Asymptomatic | URTID | LRTID, tracheobronchitis | LRTID, pneumonia | Total | ||
| 2 weeks before RV | 7.70 (6.00–10.70)a | 8.65 (7.30–9.40) | 9.30 (6.55–11.30) | 10.10 (8.10–12.10) | 8.45 (6.25–9.90) | 0.371 |
| 1 mo before RV | 8.05 (5.80–13.00)b | 9.40 (8.40–10.70) | 9.20 (6.45–11.50) | 8.10 (7.10–8.70) | 9.00 (7.20–11.00) | 0.190 |
IQR, interquartile range; LRTID, lower respiratory tract infectious disease; RV, respiratory virus; URTID, upper respiratory tract infection.
Asymptomatic patients compared with symptomatic patients (URTID, LRTID; p < 0.24).
Asymptomatic patients compared with symptomatic patients (URTID, LRTID; p < 0.21).
Clinical outcome and mortality
The majority of patients with symptomatic RV infections could be managed as outpatients (117 of 162, 72.2%) (Table S1). Paramyxovirus (11 of 35, 31.4%) and influenza (10 of 27, 37.0%) infections presented higher risks of hospitalization compared with picornavirus infection (10 of 70, 14.2%; p < 0.001). Nosocomial acquisition of RV infection was diagnosed in 11 of 162 (6.8%) episodes (Table S1). Severe disease occurred in four patients (4.1%) and required intensive care unit admission (two RSV and two influenza). During the study period, 27 of 98 LTRs (27.5%) died, including five (5.1%) with RV‐attributable deaths (two influenza, two rhinovirus and one RSV).
Spirometric outcomes
Patients with URTID had a significant loss of FEV1 compared with preinfection lung function, with a mean loss of 90 mL (95% CI 20–160 mL, p = 0.03). This difference was significant only at the 1‐mo time point and disappeared subsequently. In patients with LRTID, lung function changed significantly compared with the preinfection values at both 1 mo (140 mL, 95% CI −20 to 300 mL, p = 0.03) and 3 mo (130 mL, 95% CI 10–260 mL, p = 0.04) after RV infection (Table 5). No significant spirometric changes were observed in asymptomatic patients at comparable time points (Table 5). Specific RVs and RV coinfection could not be attributed to more pronounced clinical effects on allograft function.
Table 5.
Mean loss of FEV1 between pre‐ and postinfectious disease periods in patients with URTID, LRTID, or asymptomatic status
| Disease status | 1 mo | 3 mo | 6 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FEV1 (95% CI) | na | p‐value | FEV1 (95% CI) | na | p‐value | FEV1 (95% CI) | na | p‐value | |
| URTID | 90 (20–160) | 21 | 0.03 | 0 (−80 to 60) | 37 | 0.7 | 40 (−60 to 140) | 43 | 0.9 |
| LRTID | 140 (−20 to 300) | 17 | 0.03 | 130 (10–260) | 21 | 0.04 | 100 (−90 to 290) | 24 | 0.7 |
| Asymptomatic | 110 (−110 to 330) | 9 | 0.21 | 10 (−150 to 170) | 16 | 0.9 | 5 (−100 to 30) | 23 | 0.2 |
FEV1 is given in milliliters. CI, confidence interval; FEV1, forced expiratory volume; LRTID, lower respiratory tract infectious disease; URTID, upper respiratory tract infectious disease;
Number of lung transplant recipients who received spirometric tests.
MDIM effects
A total of 33 biopsy‐proven cases of AR were diagnosed (30.6%): 30 cases were cellular AR (two graded A1, 16 graded A2, nine graded A3, three graded A4), two were humoral and one was mixed (graded A4). In the nested case–control study, the appearance of AR was associated with the presence of an RV in the previous 3 mo (hazard ratio [HR] 6.54, 95% CI 1.47–29.08, p = 0.01) (Table 6). The diagnosis of AR was based on histology and could not be associated with a predominant clinical presentation. Moreover, the appearance of P. aeruginosa was associated with the presence of an RV in the previous 3 mo (HR 3.35, 95% CI 0.93–5.46, p = 0.07), mainly in the form of LRTID (HR 8.54, 95% CI 1.54–47.4, p = 0.01). Aspergillus spp. colonization and/or infection, however, was not associated with the presence of an RV in the previous 3 mo (HR 0.71, 95% CI 0.19–2.69, p = 0.61) (Table 6). Interestingly, we observed a trend toward an association between CMV replication or disease (odds ratio 1.60, 95% CI 0.94–2.75, p = 0.08) and the presence of an RV in the previous 3 mo, mainly in the form of URTID (HR 2.53, 95% CI 1.22–5.2, p = 0.01) (Table 6). Specific RVs and RV coinfection could not be attributed to different clinical effects on MDIM effects.
Table 6.
Nested case–control analysis for RVs associated with acute rejection, Pseudomonas aeruginosa colonization or infection, Aspergillus spp. colonization or infection, and CMV replication or disease
| Variable | Case | Control | HR (95% CI) | p‐value |
|---|---|---|---|---|
| Acute rejection | ||||
| RV negative | 27 (81.85%) | 124 (96.9%) | – | – |
| RV positive | 6 (18.8%) | 4 (3.1%) | 6.54 (1.47–29.08) | 0.01 |
| URTID | 1 (3%) | 2 (1.6%) | 2.14 (0.19–23.79) | 0.53 |
| LRTID | 1 (3%) | 1 (0.8%) | 3.65 (0.22–59.60) | 0.37 |
| Asymptomatic | 0 | 1 (0.8%) | 0.00 (0–0) | – |
| AR | 4 (12.1%) | 0 (0%) | 0.00 (0–0) | – |
| P. aeruginosa colonization/infection | ||||
| RV negative | 43 (84.31%) | 190 (93.6%) | – | – |
| RV positive | 9 (17.3%) | 18 (8.6%) | 3.35 (0.93–5.46) | 0.07 |
| URTID | 3 (5.8%) | 4 (2.0%) | 3.35 (0.73–15.4) | 0.12 |
| LRTID | 4 (7.8%) | 2 (1.09%) | 8.54 (1.54–47.4) | 0.01 |
| Asymptomatic | 1 (1.9%) | 7 (3.5%) | 0.70 (0.08–5.78) | 0.74 |
| AR | 4 (12.1%) | 0 (0%) | 0.90 (0.09–8.7) | 0.93 |
| Aspergillus spp. colonization/infection | ||||
| RV negative | 21 (87.5%) | 80 (83.3%) | – | – |
| RV positive | 3 (12.5%) | 16 (16.7%) | 0.71 (0.19–2.69) | 0.61 |
| URTID | 0 | 6 (6.3%) | 0.00 (0–0) | – |
| LRTID | 1 (4.17%) | 2 (2.1%) | 2.05 (0.19–22.68) | 0.56 |
| Asymptomatic | 2 (8.33%) | 4 (4.2%) | 1.81 (0.28–11.56) | 0.53 |
| AR | 0 (0%) | 4 (4.2%) | 0.00 (0–0) | – |
| CMV replication/disease | ||||
| RV negative | 83 (79.1%) | 358 (86.1%) | – | – |
| RV positive | 22 (21.0%) | 58 (13.9%) | 1.5 (0.89–2.67) | 0.12 |
| URTID | 13 (12.4%) | 22 (5.2%) | 2.53 (1.22–5.2) | 0.01 |
| LRTID | 6 (5.7%) | 17 (4.1%) | 1.52 (0.59–3.92) | 0.59 |
| Asymptomatic | 0 (0%) | 15 (3.6%) | 0.00 (0–0) | – |
| AR | 3 (2.9%) | 4 (0.96%) | 2.22 (0.40–12.21) | 0.359 |
AR, acute rejection; CI, confidence interval; CMV, cytomegalovirus; HR, hazard ratio; LRTID, lower respiratory tract infectious disease; RV, respiratory virus; URTID, upper respiratory tract infectious disease.
Discussion
In this prospective study, we performed a complete examination using molecular assays of the epidemiology of RVs in LTRs and demonstrated very high incidence of RV infections. RVs were associated not only with direct significant clinical impacts but also with immediate allograft dysfunction and MDIM effects, contributing significantly to the development of AR and opportunistic infections. The strengths of the present study reside in the large lung transplant population analyzed and the lengthy follow‐up throughout all seasons compared with previous literature in this area.
RVs have been increasingly recognized as common pathogens in LTRs. Previous cohorts with different types of viral screening had reported incidence of RV infections in LTRs in the range of 7.7–64.0% 2, 5. Our results can be compared with data published by Bridevaux et al 11, who performed a large prospective study among LTRs. The elevated incidence described in both studies (0.83 and 2.03 RVs per patient‐year) is probably associated with the adoption of molecular diagnostics that provide high sensitivity and detection of emerging viruses 12.
All RVs cause a variety of direct effects, and no one virus is exclusively associated with one clinical syndrome, although some RVs show varying tropism for the respiratory tract 2. In our study, picornaviruses (mainly rhinovirus), coronaviruses and influenza virus were the most common etiological agents; together, these RVs accounted for 76.6% of the microbiologically confirmed symptomatic infections. Rhinoviruses were the leading cause of RV infectious diseases in LTRs (43.3%), and that finding is in agreement with the knowledge that this RV is the primary cause of acute viral respiratory illnesses 5, 11. Our study also confirmed the tropism of rhinovirus for the lower respiratory tract, as it has been associated with LRTIDs (mainly tracheobronchitis) 11, 13. Interestingly, certain viruses, namely influenza and those from the paramyxoviridae family (which includes RSV, HMPV and PIVs), were more likely to be associated with LRTID (pneumonia) and higher hospitalization rates 14, 15, 16.
The distribution of RV infections throughout the year suggests that seasonal patterns of RV circulation in LTRs are similar to those circulating in the community 11. Most RV infections in our study were caused by picornaviruses, which are known to circulate throughout the year. Our data suggest, however, that the clinically more severe manifestations and their subsequent impact on lung allograft function is driven mostly by those viruses circulating preferentially during the winter seasons. Consequently, we suggest that clinicians be aware of circulating community RV infections to vigilantly maintain knowledge of the epidemiology among LTRs 11. Moreover, effective prophylaxis and treatment involving vaccination and antiviral therapies would be needed most urgently for RVs such as influenza and paramyxoviruses.
Careful collection of clinical data demonstrates that RV infections are present in 11.5% of asymptomatic LTRs and consisted mostly of picornaviruses and coronaviruses 11. Of note, asymptomatic RV infections were not associated with a significant decline in lung function as measured by FEV1 at 1, 3, and 6 mo of follow‐up. In contrast, RVs were detectable in a significantly higher proportion of symptomatic patients with URTID and LRTID, included a broader range of agents, and probably highlighted the potential role of inflammation for transient FEV1 decline. Importantly, with the increasing severity of the clinical presentation from the upper to lower respiratory tract, an increasing impact on lung function became apparent, with significant decline at 1 and 3 mo for patients with LRTID. In contrast to previous reports 5, in our study, we could not demonstrate that different RVs exerted different effects on allograft function; this was probably due to the sample size.
RVs are an increasingly recognized cause of community‐acquired infections, but different hospital outbreaks have been described, and the exact role of RVs in nosocomial acquisition is not well known 17. Notably, in our study, we found that 6.8% of RV infections were acquired after hospital admission. In immunosuppressed patients, RVs have also been shown to cause protracted clinical disease and viral shedding 18. In keeping with previous reports 18, our study demonstrates prolonged shedding for picornaviruses and coronaviruses, which may be a source of nosocomial transmission. These concerning data emphasize the need for guidelines for managing nosocomial RVs 19.
The relationship between RV infections and AR has not been clearly established 7, 8, 11, 20. The present study included the acute phase of the viral infection (with no relation to clinical presentation) and a follow‐up period of 3 mo. Despite a relatively small sample size of AR, this is the first prospective study that suggested a trend (HR 6.54, 95% CI 1.47–29.08, p = 0.01) toward a significant clinical link between RV infection and biopsy‐proven AR in lung. Although the pathogenesis of this link is not well understood, it is probably related to the response of circulating leukocytes, which are initiated by acute viral replication or induced by various inflammatory signals, including Th1‐ and Th2‐type alloreactive cytokines released by the damaged parenchyma, to infiltrate the transplanted organ 6.
Bacterial and fungal superinfections are a dangerous complication of RV infections 21. In this study, we aimed to analyze the relationship between RVs and P. aeruginosa because the latter is an especially problematic pathogen in LTRs. Epidemiological data collected in vitro and in animal models suggest a role for RV infections in facilitating colonization and infection with P. aeruginosa 22. Nevertheless, this is the first prospective study to reveal that RV infections involving the lower respiratory tract in LTRs predispose these patients to P. aeruginosa isolation (HR 8.54, 95% CI 1.54–47.4, p = 0.01). Although we did not statistically control for other effects, the results of our investigation support this notion. The immunomodulatory effects of CMV are well known and may predispose patients to several opportunistic infections 23. In contrast, we observed that having a URTID may favor CMV replication or disease (HR 2.53, 95% CI 1.22–5.2, p = 0.01). These results have not been reported before and may have different interpretations. The patients’ epidemiological exposures to RVs could trigger CMV replication or an RV could be a surrogate for the immunosuppressive state of a host who is more susceptible to both CMV and RV infections 6, 24.
Previous studies have suggested that an RV infection may be a risk factor for subsequent Aspergillus spp. infection 25. In our study, no statistical association was found, probably because of a statistical limitation related to a low incidence of aspergillosis secondary to an effective antifungal prophylaxis regimen 26.
The interpretation of a positive test in asymptomatic patients is difficult, and there are no published data on long‐term consequences 3, 27. Interestingly, in our study, no association was observed between an asymptomatic status and indirect effects (AR, subsequent bacterial or fungal superinfection). It is probable that asymptomatic detection in LTRs is associated with the use of molecular techniques that have increased viral diagnostic rates that can detect minimal amounts of copy numbers of RVs 23. Moreover, the asymptomatic detection in LTRs could be the result of the complex balance that exists between immunosuppression and the inflammatory response against an RV manifesting with an asymptomatic status 24.
Calcineurin inhibitors weaken the lymphocyte‐mediated immune response, and previous studies that used both quantitative evaluation of immunosuppression and concomitant viral screening in LTRs found elevated tacrolimus levels to be a risk factor for RV infections 11. In our cohort, however, no association was observed between median calcineurin inhibitor levels and clinical presentation.
Our study has some limitations. First, we collected only NPSs; however, they are painless, practical for widespread use and comparable in sensitivity to nasopharyngeal aspirates or bronchoalveolar lavage for the detection of all major RVs 28, 29. Second, there was the possibility of missing clinically significant viral infections. Our methodological strategy has been used in other prospective studies 1, 11, and the probability of this error is very unlikely because the infection rates are the highest described in the medical literature until now 7, 8, 11. Third, the spirometric outcomes were evaluated according to clinical lung allograft surveillance without a protocol after RV sampling; therefore, we missed functional tests, and that likely biased the observed decline in FEV1. Fourth, because of the small sample size of patients (although large for an LTR population) and MDIM events, the statistical analyses showed wide CIs but nonetheless strongly suggested the existence of a real association in the LTR population. Last, the median incubation period of the RV is 4–5 days, but statements are based on limited evidence 30; therefore, we preferred to use the standardized definition of hospital‐acquired infections 31, although we could have overestimated our rates of RV nosocomial acquisition (2‐ to 3‐day window).
In conclusion, in LTRs, the incidence of RV infections is very high, and RVs are associated with a significant clinical impact. Picornaviruses were the most frequently encountered RVs and were the main cause of URTID, LRTID tracheobronchitis and asymptomatic infections, whereas most LRTID pneumonias were caused by paramyxovirus and influenza virus and resulted in higher hospitalization rates. RV infections were associated with immediate allograft dysfunction, as assessed by the spirometry outcomes. RVs produce local and systemic MDIM, which contributes significantly to AR, P. aeruginosa colonization and infection, and CMV replication and disease. These data highlight the importance of infection control and mitigation against nosocomial infections in this high‐risk patient population.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Table S1: Management of lung transplant recipients with symptomatic, confirmed respiratory virus infectious diseases (upper and lower respiratory tract infectious diseases).
Acknowledgments
The authors are grateful to all patients for their perseverance, generosity and complete collaboration. The authors thank Santi Perez‐Hoyos for excellent statistical support and Lidia Garcia‐Losada for providing assistance throughout the study. The authors thank the Nature Journal Publishing Group for English editing. This study was supported by research grants from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (FIS 80554) and was cofinanced by the Spanish Network for Research in Infectious Diseases (REIPI).
Peghin M, Hirsch HH, Len Ó, Codina G, Berastegui C, Sáez B, Solé J, Cabral E, Solé A, Zurbano F, López‐Medrano F, Román A & Gavaldá J. Epidemiology and Immediate Indirect Effects of Respiratory Viruses in Lung Transplant Recipients: A 5‐Year Prospective Study. Am J Transplant 2017; 17: 1304–1312
References
- 1. Lopez‐Medrano F, Aguado JM, Lizasoain M, et al. Clinical implications of respiratory virus infections in solid organ transplant recipients: A prospective study. Transplantation 2007; 84: 851–856. [DOI] [PubMed] [Google Scholar]
- 2. Manuel O, Estabrook M; Practice ASTIDCo . RNA respiratory viruses in solid organ transplantation. Am J Transplant 2013; 13 Suppl 4: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glanville AR. Community‐acquired respiratory viruses after lung transplantation: Common, sometimes silent, potentially lethal. Thorax 2014; 69: 1–2. [DOI] [PubMed] [Google Scholar]
- 4. Hammond SP, Gagne LS, Stock SR, et al. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J Clin Microbiol 2012; 50: 3216–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sayah DM, Koff JL, Leard LE, Hays SR, Golden JA, Singer JP. Rhinovirus and other respiratory viruses exert different effects on lung allograft function that are not mediated through acute rejection. Clin Transplant 2013; 27: E64–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fishman JA. From the classic concepts to modern practice. Clin Microbiol Infect 2014; 20(Suppl 7): 4–9. [DOI] [PubMed] [Google Scholar]
- 7. Soccal PM, Aubert JD, Bridevaux PO, et al. Upper and lower respiratory tract viral infections and acute graft rejection in lung transplant recipients. Clin Infect Dis 2010; 51: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. Respiratory viruses in lung transplant recipients: A critical review and pooled analysis of clinical studies. Am J Transplant 2011; 11: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Husain S, Mooney ML, Danziger‐Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011; 30: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL‐4): Guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bridevaux PO, Aubert JD, Soccal PM, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: A prospective study. Thorax 2014; 69: 32–38. [DOI] [PubMed] [Google Scholar]
- 12. Al‐Tawfiq JA, Zumla A, Gautret P, et al. Surveillance for emerging respiratory viruses. Lancet Infect Dis 2014; 14: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis 2000; 181: 1875–1884. [DOI] [PubMed] [Google Scholar]
- 14. Weinberg A, Lyu DM, Li S, Marquesen J, Zamora MR. Incidence and morbidity of human metapneumovirus and other community‐acquired respiratory viruses in lung transplant recipients. Transpl Infect Dis 2010; 12: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCurdy LH, Milstone A, Dummer S. Clinical features and outcomes of paramyxoviral infection in lung transplant recipients treated with ribavirin. J Heart Lung Transplant 2003; 22: 745–753. [DOI] [PubMed] [Google Scholar]
- 16. Ng BJ, Glanville AR, Snell G, et al. The impact of pandemic influenza A H1N1 2009 on Australian lung transplant recipients. Am J Transplant 2011; 11: 568–574. [DOI] [PubMed] [Google Scholar]
- 17. Aitken C, Jeffries DJ. Nosocomial spread of viral disease. Clin Microbiol Rev 2001; 14: 528–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med 2006; 174: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 19. Lopez‐Medrano F, Cordero E, Gavalda J, et al. Management of influenza infection in solid‐organ transplant recipients: Consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI). Enferm Infecc Microbiol Clin 2013; 31: 526.e1–526.e20. [DOI] [PubMed] [Google Scholar]
- 20. Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community‐acquired respiratory viruses in lung transplant recipients. Transplantation 2010; 89: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 21. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011; 377: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vrankrijker AM, Wolfs TF, Ciofu O, et al. Respiratory syncytial virus infection facilitates acute colonization of Pseudomonas aeruginosa in mice. J Med Virol 2009; 81: 2096–2103. [DOI] [PubMed] [Google Scholar]
- 23. Fishman JA. Overview: Cytomegalovirus and the herpesviruses in transplantation. Am J Transplant 2013; 13 (Suppl 3): 1–8; quiz 8. [DOI] [PubMed] [Google Scholar]
- 24. Solidoro P, Balestro E, Boffini M. Viral Infections in lung transplant recipients: Devils or trolls? Minerva Med. 2014; 105 (3 Suppl 2): 15–21. [PubMed] [Google Scholar]
- 25. Garcia‐Vidal C, Royo‐Cebrecos C, Peghin M, et al. Environmental variables associated with an increased risk of invasive aspergillosis. Clin Microbiol Infect 2014; 20: O939–O945. [DOI] [PubMed] [Google Scholar]
- 26. Peghin M, Monforte V, Martin‐Gomez MT, et al. 10 years of prophylaxis with nebulized liposomal amphotericin B and the changing epidemiology of Aspergillus spp. infection in lung transplantation. Transpl Int 2016; 29: 51–62. [DOI] [PubMed] [Google Scholar]
- 27. Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: Toward defining clinically relevant cutoff values. J Clin Microbiol 2011; 49: 2631–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heikkinen T, Marttila J, Salmi AA, Ruuskanen O. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J Clin Microbiol 2002; 40: 4337–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hakki M, Strasfeld LM, Townes JM. Predictive value of testing nasopharyngeal samples for respiratory viruses in the setting of lower respiratory tract disease. J Clin Microbiol 2014; 52: 4020–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lessler J, Brookmeyer R, Reich NG, Nelson KE, Cummings DA, Perl TM. Identifying the probable timing and setting of respiratory virus infections. Infect Control Hosp Epidemiol 2010; 31: 809–815. [DOI] [PubMed] [Google Scholar]
- 31. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16: 128–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Management of lung transplant recipients with symptomatic, confirmed respiratory virus infectious diseases (upper and lower respiratory tract infectious diseases).


