Abstract
We investigated if probiotic supplementation could improve the health and reproductive performance of unvaccinated lactating sows infected with porcine epidemic diarrhea (PED) virus. Twenty unvaccinated pregnant sows were equally allocated to probiotic‐supplemented (P) and control (C) groups. For the experiment, 15 g/day of probiotic compound BIO‐THREE PZ was given to P sows. Reproductive performance was checked daily. The number of neonates fostered by each sow was maintained at eight throughout the experiment. Individual milk production post‐parturition was measured twice. Milk protein and fat ratios were determined by a milk analyzer. Total immunoglobulin (Ig) A and G concentrations were measured by ELISA. At day 7 post‐parturition, the body weight of P sows was 10 kg higher than that of C sows, and at day 3 post‐parturition, P sows produced more milk (+2 kg) and had a higher IgA concentration in whey than did C sows (p < .05). Finally, unlike C sows, P sows tended to return to estrus faster, and had larger piglets at birth with a lower mortality percentage during early days of suckling. In conclusion, probiotic compound BIO‐THREE PZ helped strengthen the immune system of unvaccinated, PED‐infected sows and improved their reproductive performance.
Keywords: milk production, porcine epidemic diarrhea, probiotic compound, reproductive performance, sow
1. INTRODUCTION
Porcine epidemic diarrhea (PED) severely affects pigs reared in commercial farms worldwide, causing serious economic losses (Sasaki et al., 2016). Like in many other Asian countries, farms in Japan have experienced PED epidemics, including one that caused more than 1,000 outbreaks in the period 2013–2014 (Sasaki, Toyomaki, et al., 2017; Suzuki et al., 2015). PED infection can occur not only in farms with unvaccinated animals, but also in those where they have been vaccinated, the former being at a higher risk of causing epidemics. In the case of PED infection in Japan, the reason it spread so rapidly may be due to the fact (Toyomaki, Sekiguchi, Sasaki, Sueyoshi, & Makita, 2018) that suppliers did not distribute the vaccine quickly enough.
Although PED is caused by the same single‐stranded RNA virus of group 1 of the genus Coronavirus, the strain identified in Asia is considered more pernicious than others and affects pigs of all ages (Wentao et al., 2012). Typical PED clinical signs in pigs include watery diarrhea, vomiting, and consequently, loss of bodily fluids, appetite and weight. Reproductive performance of sows is also seriously reduced by PED infection (Sasaki, Kawabata, & Noguchi, 2017). For example, sows in their first month of pregnancy deliver fewer piglets and have higher abortion rates (Olanratmanee, Kunavongkrit, & Tummaruk, 2010). Similarly, the number of stillbirth piglets is high in litters of PED‐infected sows going into their third or fourth month of pregnancy (Olanratmanee et al., 2010). Indeed, mortality can be very high among young pigs, as it can reach nearly 100% in newborns (Jung & Saif, 2015; Sueyoshi et al., 1995) and as much as 80% in suckling pigs (Shibata et al., 2000). Although piglets can survive a PED infection, they experience abnormality and malfunction of the small intestine (Curry, Gibson, et al., 2017), and reduction of fat, lean, protein, and bone mineral gains (Curry, Schwartz, Yoon, Gabler, & Burrough, 2017); hence, subsequent growth performance is greatly affected. In addition, reduced milk production was previously found in PED‐infected sows (Sueyoshi et al., 1995), but it remains unclear to what extent the nutritional level of sow milk is affected by PED infection (Huang et al., 2018).
In recent years, lactic acid bacteria (LAB) supplementation has been used to treat certain viral infections. For example, Zhang, Azevedo, Gonzalez, et al. (2008) demonstrated that lactobacilli induced immune responses in the intestine of neonatal gnotobiotic pigs, which conferred protection against human rotavirus (HRV). Likewise, after Lactobacillus rhamnosus strain GG and Bifidobacterium animalis lactis Bb12 supplementation, Kandasamy, Chattha, Vlasova, Rajashekara, and Saif (2014) found that neonatal gnotobiotic pigs had lower fecal scores and shedding concentrations of HRV, and higher intestinal immunoglobulin A (IgA) antibody‐releasing cell numbers and IgA antibody concentration against the virus. Based on this, giving weaning piglets a cell preparation of Enterococcus faecalis, a LAB species, protected them from rotavirus infection (Tsukahara, Nakanishi, Matsubara, Itoh, & Ushida, 2006). Clearly, LAB supplementation seems to enhance the lactogenic immunity and the efficacy of vaccines administered to pigs. Previously, we reported that a probiotic compound with peptide‐zinc complexes named BIO‐THREE PZ, containing live bacteria Bacillus mesentericus, Clostridium butyricum and Enterococcus faecalis, improved the reproductive performance of sows and prevented post‐weaning diarrhea in piglets (Hayakawa, Masuda, Kurosawa, & Tsukahara, 2016). More recently, we also showed that the above probiotic compound improved the reproductive performance of sows vaccinated against PED and reduced mortality in suckling piglets (Inatomi, Amatatsu, Romero‐Pérez, Inoue, & Tsukahara, 2017).
In the present study, we supplemented BIO‐THREE PZ to unvaccinated, PED‐infected sows and compared them with control sows to assess the improvement of their health and reproductive performance during the lactation period.
2. MATERIALS AND METHODS
2.1. Probiotics
BIO‐THREE PZ (TOA Pharmaceutical Co. Ltd., Tokyo, Japan), a probiotic compound containing Bacillus mesentericus TO‐A (1 × 106 colony‐forming units [cfu]/g), Clostridium butyricum TO‐A (1 × 106 cfu/g) and Enterococcus faecalis T‐110 (1 × 108 cfu/g) in a peptide‐zinc compound (10 mg/g) was used in this study. This compound was the same as the one used in a previous study (Inatomi et al., 2017).
2.2. Farm
The present work was conducted at the same site as the previous study (Inatomi et al., 2017). The site is a commercial swine farm in Kyushu region, Japan, that operates a farrow‐to‐finish business and has a stock of approximately 900 sows (Landrace × Large White). Duroc boars were used to impregnate the sows. Evidence of PED infection resulting from PED outbreaks was found in the feces of sows. Antimicrobial and probiotics‐free commercial feed was given to sows during the gestation and lactation periods, using automatic mixing feeds.
Apart from porcine reproductive and respiratory virus (PRRSV) and porcine circovirus type2 (PCV2) (which were positive but not active), a preliminary survey of the farm was negative for the following pathogens in sows and suckling piglets: rotavirus, transmissible gastroenteritis virus (TGEV), Clostridium perfringens, enterotoxigenic Escherichia coli (ETEC), Salmonella sp., Brachyspira hyodysenteriae, Lawsonia intracellularis and classical swine fever virus (CSF). All pathogens except for the CSF virus were tested for by PCR methods as described elsewhere (PRRSV, Inoue, Tsukahara, Sunaba, Itoh, & Ushida, 2007; PCV2, Sasaki et al., 2010; rotavirus, Ushida et al., 2009; TGEV, Kim, Song, & Park, 2001; C. perfringens, Takahashi, Yoshida, Nakanishi, Tsukahara, & Ushida, 2008; ETEC and Salmonella spp., Fukushima, Tsunomori, & Seki, 2003; B. hyodysenteriae, Piao et al., 2007; L. intracellularis, Suto et al., 2004). CSF virus has been already eradicated in Japan. As expected, diagnosis by typical clinical signs (Le Potier, Mesplede, & Vannier, 2006) confirmed as negative the presence of CSF virus.
2.3. Experimental design
Twenty unvaccinated pregnant sows with similar mean parity (3.3) were equally divided and allocated to probiotic‐supplemented (P) and control (C) groups. Commercial feed was given twice a day at 9:00 and 17:00 hours. Individual intake was measured by subtracting the weight of leftovers from the initial feed ration weight. Sows had water ad libitum throughout this study. During gestation, all sows were individually housed in stalls and fed a gestation period diet (Shuton‐B; Minami Nihon Kumiai Siryo, Kagoshima, Japan). Approximately 1 week before parturition, sows were transferred to farrowing pens. During their stay in the farrowing pens, sows were given a lactation stage diet (Shuton‐Lactation; Minami Nihon Kumiai Siryo, Kagoshima).
Four weeks prior to parturition to 1 week post‐parturition, 15 g/day of the probiotic compound was orally administered to sows in group P, whereas 15 g/day of a standard, probiotic‐free diet was given to sows in group C via top‐dressing. Individual body weight was measured 28 days pre‐parturition and 0 and 7 days post‐parturition. Reproductive performance was checked daily. Due to sows used in this study delivering at least eight neonates in their past farrowing, the number of neonates was also maintained at eight heads per sow throughout this experiment. If piglets of the experimental sows died during lactation, healthy, same‐age foster piglets with a similar body weight were introduced from unrelated, spare sows either supplemented with probiotics or not. This experiment was approved by the ethics committee of Inatomi Animal Hospital.
Throughout this study, experimental sows sometimes showed typical symptoms of PED, such as constipation, diarrhea and vomiting. Moreover, some piglets died during the experiment. Clinical signs included watery yellowish diarrhea and dehydration. An autopsy showed that their small intestine was thin and flaccid. Our diagnosis confirmed that piglets died from PED infection (Pensaert & Yeo, 2006).
2.4. Sample collection and analysis
Blood was collected from the jugular vein of sows 14 days prior to parturition and 0 and 7 days post‐parturition.
Milk secretion was determined by a general method described in the Standard Methods of Evaluation of Reproductive Performance compiled by Japan's Pork Producers Association (http://www.jppa.biz). This method is the same as “the weigh‐suckle‐weigh technique,” which has been previously reported (Speer & Cox, 1984). Milk production of each sow was measured at days 3 and 7 post‐parturition. Portions of milk samples were collected at days 0, 3 and 7 post‐parturition. In addition, the total body weight of neonates was repeatedly measured immediately prior to and after daily suckling. When piglets of the experimental sows died during lactation, they were substituted by healthy, similar‐age foster piglets from other sows, so that all piglets ingested a similar amount of maternal milk.
2.5. Analyses of milk composition
Protein and fat ratio in milk at days 0 and 7 post‐parturition were determined by a milk analyzer (MilkoScan™ FT1; FOSS, Eden Prairie, MN, USA). Whey was collected from milk after centrifugation (13,000 × g, 30 min, 4°C). Serum was also collected from blood after centrifugation (1,200 × g, 20 min, room temperature). Total IgA and IgG concentrations were measured by a commercial ELISA kit (Porcine IgA or IgG ELISA Quantitation Set; Bethyl, Montogomery, TX, USA). The determination method was as previously described elsewhere (Ogawa et al., 2014). A portion of whey was sent to the Nansatsu Livestock Hygiene Center (Kagoshima, Japan), and neutralized antibody titer (NAT) against PED in whey and serum was determined by a general method as previously described elsewhere (Kusanagi et al., 1992; Shibata et al., 2000).
2.6. Statistical analyses
Either the Student's or Welch's t test was used to analyze differences between means in all parameters. Values are shown as the means ± SE. Differences between means in all statistical analyses were considered significant at p < .05 and with tendency to be significant at p < .1. All calculations were made using Statcel3 (OMS, Tokyo, Japan) as an add‐in application for Microsoft Excel® (Microsoft, Seattle, WA, USA).
3. RESULTS
3.1. Feed intake
The mean feed intake of sows is shown in Table 1 and Figure 1. Sows supplemented with dietary probiotic compound had a significantly higher feed intake compared with that of control sows, both pre‐ (2.62 ± 0.07 kg/day vs. 2.90 ± 0.03 kg/day) and post‐parturition (2.92 ± 0.04 kg/day vs. 3.25 ± 0.03 kg/day).
Table 1.
Mean feed intake (kg/day) of sows with or without probiotic supplementation during the pre‐ (day 28 to day 1 pre‐parturition) and post‐ (delivery to day 7 post‐parturition) parturition periods
| Period (days) | C | P | t test p value |
|---|---|---|---|
| Pre‐parturition (−28 to −1) | 2.62 ± 0.07 | 2.90 ± 0.03 | .003 |
| Post‐parturition (0–7) | 2.92 ± 0.04 | 3.25 ± 0.03 | <.001 |
The sows in group P were fed daily 15 g of probiotic compound consisting of 1.5 × 107 colony‐forming units (cfu) of Bacillus mesentericus TO‐A, 1.5 × 107 cfu of Clostridium butyricum TO‐A, 1.5 × 109 cfu/g of Enterococcus faecalis T‐110 and 150 mg of the peptide‐zinc compound.
Figure 1.
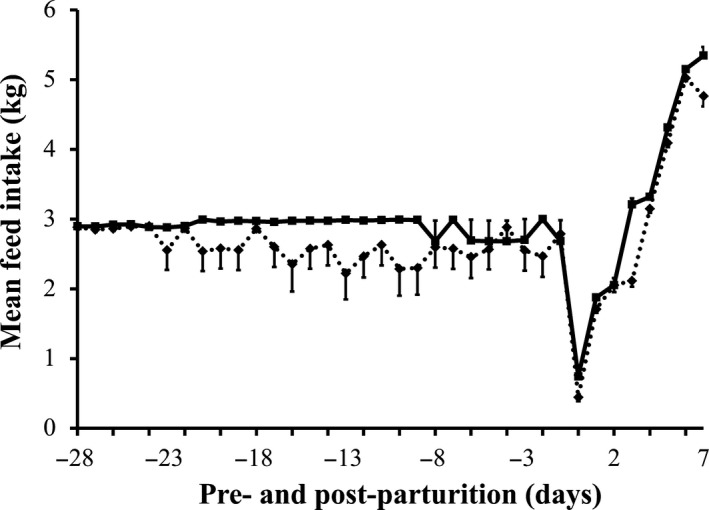
Mean daily feed intake of sows with or without probiotic compound supplementation from day 28 pre‐parturition to day 7 post‐parturition. Solid line: sows supplemented with the probiotic compound (group P). Dashed line: control sows (group C)
3.2. Body condition
At day 28 pre‐parturition, the mean body weight of P sows tended to be greater than that recorded for C sows (Figure 2). As sows approached parturition, the mean body weight of C sows increased from 183 ± 1 kg to almost 186 ± 1 kg at parturition day, that is, a gain of only approximately 3 kg, whereas the mean body weight of P sows remarkably increased from 185 ± 1 kg to more than 193 ± 2 kg at parturition day, a gain of approximately 8 kg. This significant difference between the mean body weight of C and P sows at delivery was even greater after parturition, as the body weight of C sows at day 7 post‐parturition drastically decreased to less than 180 ± 1 kg, whereas that of P sows decreased only to 190 ± 1 kg.
Figure 2.
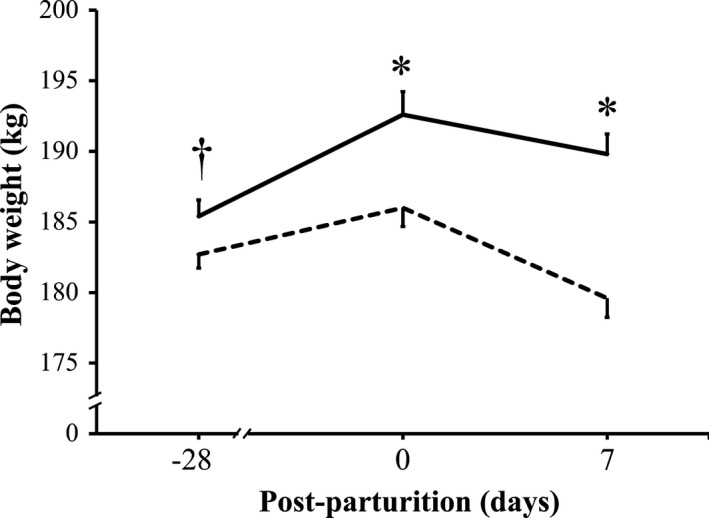
Mean body weight of sows with or without probiotic compound supplementation. Solid line: sows supplemented with probiotic compound (group P). Dashed line: control sows (group C). *Significant differences between sow groups (p < .05). †Tendency of significance between sow groups. Values are means ± SE
3.3. Milk production and product quality
With respect to milk production, at day 3 post‐parturition P sows produced more than 2.5 ± 0.1 kg of milk, which was significantly higher than the almost 1.9 ± 0.1 kg of milk produced by C sows (Figure 3). When milk production was measured at day 7 post‐parturition, P sows still produced more than C sows did (4.0 ± 0.1 kg vs. 4.3 ± 0.1 kg), an observation that tended to be significant. However, although the protein ratio was significantly greater in milk of P sows than it was in milk of C sows at day 0 (10.0 ± 0.3% vs. 12.6 ± 0.5%), by day 7 post‐parturition, no difference in protein percentage was detected between milk samples of P and C sows (4.5 ± 0.5% vs. 5.6 ± 0.6%) (Figure 4). Likewise, fat percentage in milk did not differ between groups at days 0 and 7 post‐parturition (data not shown).
Figure 3.
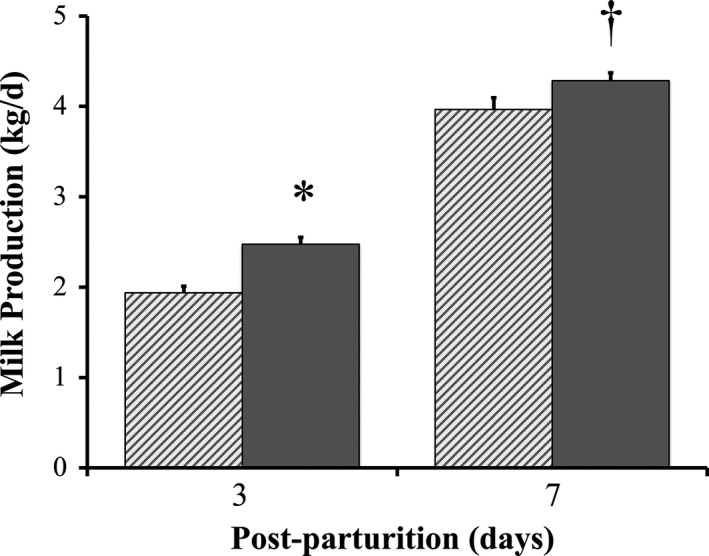
Milk production of sows with or without probiotic compound supplementation. Solid bars: sows supplemented with probiotic compound (group P). Hashed bars: control sows (group C). *Significant differences between sow groups (p < .05). †Tendency of significance between sow groups. Values are means ± SE
Figure 4.
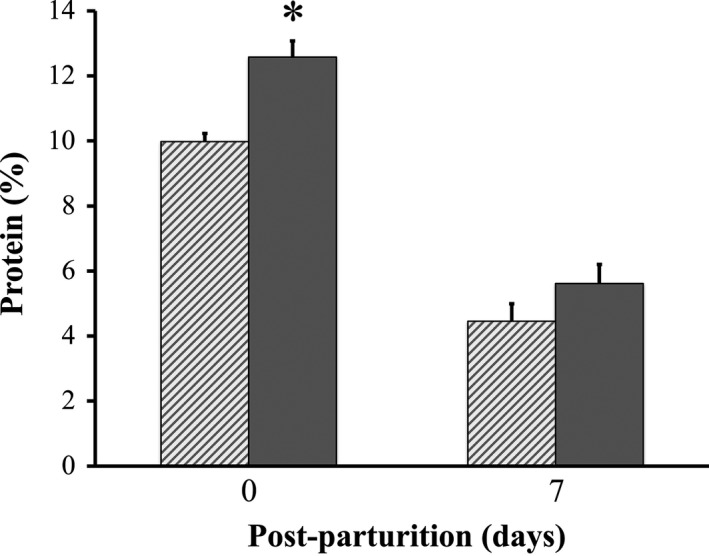
Total protein percentage in milk of sows with or without probiotic compound supplementation. Solid bars: sows supplemented with probiotic compound (group P). Hashed bars: control sows (group C). *Significant differences between sow groups (p < .05). Values are means ± SE
3.4. Immunity parameters
Regarding the lactogenic immunity parameters, although total IgA concentration in whey remained unchanged between C and P sows immediately after parturition (1.8 ± 0.1 g/dl vs. 2.0 ± 0.1 g/dl), it was significantly higher at day 3 post‐parturition in whey of P sows in comparison with that of C sows (1.8 ± 0.0 g/dl vs. 1.9 ± 0.0 g/dl), a trend that was still detected at day 7 post‐parturition (1.1 ± 0.0 g/dl vs. 1.2 ± 0.0 g/dl) (Figure 5a). Nonetheless, total IgG concentration in whey of C and P sows differed very little, and it showed only a tendency to be significantly higher in whey of P sows immediately after parturition (6.1 ± 0.2 g/dl vs. 6.6 ± 0.2 g/dl) (Figure 5b). There were differences detected in the antibody titer against PED virus in the blood and milk of C and P sows. For example, from day 0 to day 7 post‐parturition the antibody titer was significantly higher in serum of P sows in comparison with that of C sows (day 0, 14.9 ± 1.1 vs. 21.1 ± 1.1; day 7, 10.6 ± 1.1 vs. 16.0 ± 1.2 in geometric means) (Figure 5c), but differences were barely detectable in whey of both C and P sows during the same period (day 0, 42.2 ± 1.2 vs. 64.0 ± 1.2; day 7, 21.1 ± 1.2 vs. 29.9 ± 1.2 in geometric means) (Figure 5d).
Figure 5.
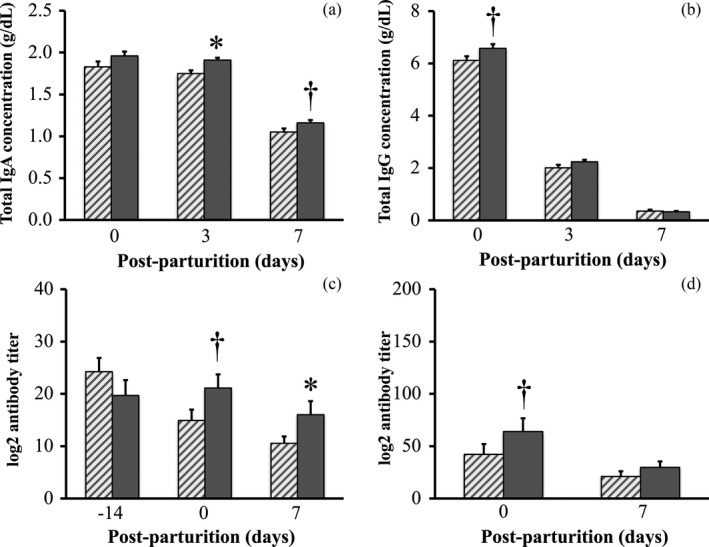
Immunology parameters measured in serum and whey of sows with or without probiotic compound supplementation. (a) Total immunoglobulin A (IgA) concentration in whey. (b) Total IgG concentration in whey. (c) Porcine epidemic diarrhea (PED) virus‐specific antibody titer in serum. (d) PED virus‐specific antibody titer in whey. Hashed bars: control sows (group C). Solid bars: sows supplemented with probiotic compound (group P). *Significant differences between sow groups (p < .05). †Tendency of significance between sow groups. Values are means ± SE
3.5. Reproductive performance
Supplementation of the probiotic compound improved the reproductive performance of sows. For example, P sows tended to have fewer days between weaning and estrus than did C sows (9.4 ± 1.6 day vs. 15.7 ± 2.8 day) (Figure 6). In addition, piglets of P sows were larger at birth (7,804 ± 165 g vs. 8,320 ± 165 g) (Figure 7a) and with a lower mortality percentage during the first 21 days of suckling than those born to C sows (16.3 ± 3.8% vs. 28.8 ± 4.9%) (Figure 7b).
Figure 6.
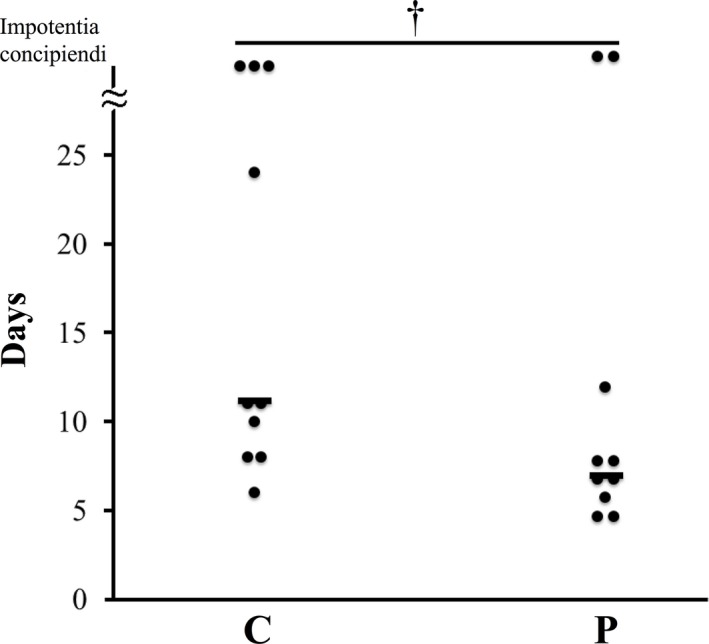
Recurrence of estrus in sows with or without probiotic compound supplementation C: control sows (no probiotics). P: sows supplemented with probiotic compound. †Tendency of significance between sow groups. Bars represent the mean number of days between weaning and return to estrus
Figure 7.
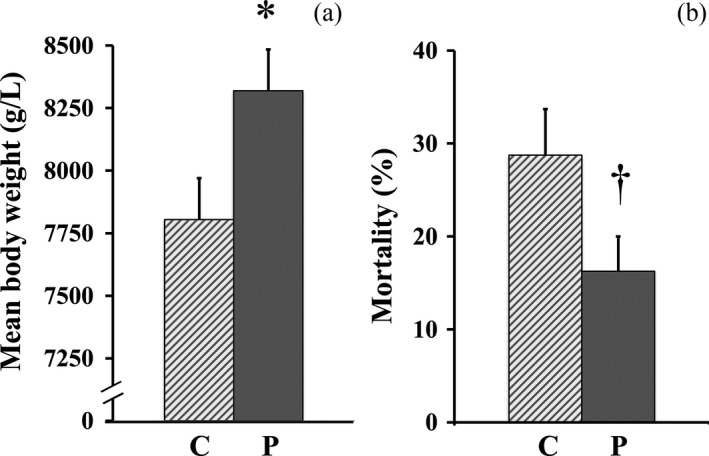
Reproductive parameters measured for sows with or without probiotic compound supplementation. (a) Mean litter weight at birth in groups C and P. (b) Mortality percentage of piglets during suckling (0–21 days post‐parturition). Solid bars: sows supplemented with probiotic compound (group P). Hashed bars: control sows (group C). *Significant differences between sow groups (p < .05). †Tendency of significance between sow groups. Values are means ± SE
4. DISCUSSION
Porcine epidemic diarrhea is a very aggressive disease and in spite of continuous effort to eradicate it, including vaccination programs (Kadoi, Sugioka, Satoh, & Kadoi, 2002; Kweon, Kwon, Lee, Kwon, & Kang, 1999), outbreaks have continued in Japan (Suzuki et al., 2015). As probiotics have been touted as promising alternatives to antibiotics for treating viral infections (Canning et al., 2017; Kandasamy et al., 2014; Zhang, Azevedo, Wen, et al., 2008), in the present work we investigated whether supplementation of a dietary probiotic compound could help improve the reproductive performance of unvaccinated PED‐infected sows.
In our previous study, we showed that the probiotic compound improved the reproductive performance of sows vaccinated against PED by stimulating PED‐specific antibodies (Inatomi et al., 2017). In the present study, we supplemented this probiotic compound to unvaccinated sows. When compared with control sows, unvaccinated sows supplemented with the probiotic compound ate more feed before and after parturition (Table 1 and Figure 1) and gained more body weight from the beginning of the experiment to until parturition, but had only a minimal weight loss by day 7 post‐parturition (Figure 2). Moreover, milk production of probiotic‐supplemented sows was greater than that of control sows when analyzed at days 3 and 7 post‐parturition (Figure 3).
Previously, a probiotic compound containing B. licheniformis and B. subtilis spores was supplemented to sows for two reproductive cycles (Kritas et al., 2015). Kritas et al. observed several benefits conferred by probiotic supplementation, including increased feed consumption and reduced weight loss during lactation in sows. Due to our previous study also partly proving that supplementation with the probiotic (BIO‐THREE) improved the feed intake of sows during lactation (Hayakawa et al., 2016), we believe that similar benefits were conferred to sows in the present study. Although no phylogenetic screening of gut microbes was performed in the present work, we can cautiously but positively speculate that probiotics likely competed with pathogens in the gut and stimulated the immune system of sows, which equipped the animals with more resistance to infections (Kritas & Morrison, 2005). Our previous study also suggested that probiotics supplementation modified the gut microbiota by inducing an increase in lactobacilli and a decrease in E. coli (Hayakawa et al., 2016). Peptide‐zinc complexes in the probiotic compound used in this study also likely contributed to enhance the immune system of sows, as addition of zinc‐oxide to feed was previously shown to help reduce diarrhea incidence and assist in weight gain in pigs (Chai et al., 2014). Thus, a supply of beneficial bacterial strains and zinc to PED‐infected sows likely enhanced their overall health, which permitted them to eat more, better utilizing nutrients from feed, and gaining more weight. Consequently, healthier sows produced more milk (Figure 3) and had higher protein concentrations in milk that improved its quality (Figure 4). Therefore, it can be considered that a greater weight gain and a higher milk production were indirect benefits of probiotic compound supplementation (Böhmer, Kramer, & Roth‐Maier, 2006).
Vaccines generally cause an immunoprophylactic effect in pregnant sows known as lactogenic immunity (Song et al., 2007). In an elegant review, Song and Park (2012) described lactogenic immunity as protection against infection given by vaccinated sows to suckling piglets via colostrum and milk. Unexpectedly, in the present study probiotic supplementation to sows significantly increased the concentration of IgA and IgG in milk despite the fact that they were unvaccinated. Nonetheless, it has been previously reported that probiotic Lactobacillus acidophilus given to pigs acted as adjuvant to vaccination which resulted in enhanced immune cells producing antibodies such as IgA, IgG and IgM (Zhang, Azevedo, Wen, et al., 2008). Thus, a possible scenario in the present study can be described as follows: probiotic bacteria used re‐colonized the gut of sows, which likely stimulated early maturation of gut immunity and fended off infection (Chattha, Roth, & Saif, 2015), resulting in an increase in the concentration of PED‐specific antibodies in whey. This plausible scenario is strongly supported by our results (Figure 5c,d).
PED infection causes impaired reproductive performance in sows which results in negative productive performance (e.g., mortality) during the development of suckling piglets. It is believed that the lower body weight of sows caused by a decrease in appetite and a lower efficiency of nutrient utilization during the course of a viral infection prevents sows from returning to estrus in a timely manner (Tantasuparuk, Dalin, Lundeheim, Kunavongkrit, & Einarsson, 2001). However, Kritas et al. (2015) showed that giving B. subtilis C‐3102 to sows helped them return to estrus more rapidly. In the present study, probiotic supplementation to PED‐infected sows significantly reduced the number of days to recurrence of estrus (Figure 6). As discussed above, when compared to control sows, better nutrition in probiotic‐supplemented sows was likely the cause for a shorter period between weaning and estrus (Tantasuparuk et al., 2001). In contrast, decreased appetite likely caused reduction of milk production. Indeed, while producing milk for eight piglets, suckling healthy sows produced more than 6.6 kg milk/day for 4–7 days post‐parturition (Toner, King, Dunshea, Dove, & Atwood, 1996); milk supply to neonates of suckling PED‐infected sows was no more than 4.0 kg/day 7 days post‐parturition in same conditions (Figure 3). This result is in agreement with a previous report (Sueyoshi et al., 1995).
PED infection was previously reported as the cause of reduction in the body weight of piglets at birth in a commercial swine farm, after a PED outbreak (Olanratmanee et al., 2010). Moreover, Kritas et al. (2015) reported that piglets of sows infected with pathogenic E. coli did not benefit from B. subtilis C‐3102 supplementation, as the body weight at birth and weaning of piglets from probiotic‐supplemented and control sows were similar. In contrast, in the present study piglets farrowed by probiotic‐supplemented sows had a significantly lower mortality percentage and tended to have a greater weight at birth than did those farrowed by control sows (Figure 7). A plausible explanation for this data discrepancy may be that the probiotic compound used in the present study was not a single stain probiotic compound but rather a combination of B. mesentericus, C. butyricum and E. faecalis. Indeed, multi‐strain probiotic compounds have been found to be more effective at fending off pathogens (Chapman, Gibson, & Rowland, 2011, 2012) than single stain probiotics such as the one used by Kritas et al. (2015).
To summarize, in the present study it was proven that a probiotic compound strengthened the immune system of PED‐infected sows and improved their reproductive performance. As the effect of this probiotic compound may exert on PED‐infected piglets is of great interest, work to evaluate it is currently in progress.
Tsukahara T, Inatomi T, Otomaru K, Amatatsu M, Romero‐Pérez GA, Inoue R. Probiotic supplementation improves reproductive performance of unvaccinated farmed sows infected with porcine epidemic diarrhea virus. Anim Sci J. 2018;89:1144–1151. 10.1111/asj.13040
REFERENCES
- Böhmer, B. M. , Kramer, W. , & Roth‐Maier, D. A. (2006). Dietary probiotic supplementation and resulting effects on performance, health status, and microbial characteristics of primiparous sows. Journal of Animal Physiology and Animal Nutrition, 90, 309–315. 10.1111/j.1439-0396.2005.00601.x [DOI] [PubMed] [Google Scholar]
- Canning, P. , Ruston, C. , Madson, D. , Bates, J. , Skoland, K. , Davenport, J. , … Karriker, L. (2017). Effect of direct‐fed microbial Bacillus subtilis C‐3102 on enteric health in nursery pigs afer challenge with porcine epidemic diarrhea virus. Journal of Swine Health and Production, 25, 129–137. [Google Scholar]
- Chai, W. , Zakrzewski, S. S. , Günzel, D. , Pieper, R. , Wang, Z. , Twardziok, S. , … Burwinkel, M. (2014). High‐dose dietary zinc oxide mitigates infection with transmissible gastroenteritis virus in piglets. BMC Veterinary Research, 10, 75 10.1186/1746-6148-10-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, C. M. C. , Gibson, G. R. , & Rowland, I. (2011). Health benefits of probiotics: Are mixtures more effective than single strains? European Journal of Nutrition, 50, 1–17. 10.1007/s00394-010-0166-z [DOI] [PubMed] [Google Scholar]
- Chapman, C. M. C. , Gibson, G. R. , & Rowland, I. (2012). In vitro evaluation of single‐ and multi‐strain probiotics: Inter‐species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe, 18, 405–413. 10.1016/j.anaerobe.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Chattha, K. S. , Roth, J. A. , & Saif, L. J. (2015). Strategies for design and application of enteric viral vaccines. Annual Review of Animal Biosciences, 3, 375–395. 10.1146/annurev-animal-022114-111038 [DOI] [PubMed] [Google Scholar]
- Curry, S. M. , Gibson, K. A. , Burrough, E. R. , Schwartz, K. J. , Yoon, K. J. , & Gabler, N. K. (2017). Nursery pig growth performance and tissue accretion modulation due to porcine epidemic diarrhea virus or porcine deltacoronavirus challenge. Journal of Animal Science, 95, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry, S. M. , Schwartz, K. J. , Yoon, K. J. , Gabler, N. K. , & Burrough, E. R. (2017). Effects of porcine epidemic diarrhea virus infection on nursery pig intestinal function and barrier integrity. Veterinary Microbiology, 211, 58–66. 10.1016/j.vetmic.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Fukushima, H. , Tsunomori, Y. , & Seki, R. (2003). Duplex real‐time PCR SYBR green PCR assays for detection of 17 species of food‐ or waterborne pathogens in stools. Journal of Clinical Microbiology, 41, 5134–5146. 10.1128/jcm.41.11.5134-5146.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa, T. , Masuda, T. , Kurosawa, D. , & Tsukahara, T. (2016). Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of postweaning diarrhea, and histopathological parameters in the intestine of weaned piglets. Animal Science Journal, 87, 1501–1510. 10.1111/asj.12565 [DOI] [PubMed] [Google Scholar]
- Huang, M.‐Z. , Wang, S.‐Y. , Wang, H. , Cui, D.‐A. , Yang, Y.‐J. , Liu, X.‐W. , … Li, J.‐Y. (2018). Differences in the intestinal microbiota between uninfected piglets and piglets infected with porcine epidemic diarrhea virus. PLoS ONE, 13, e0192992 10.1371/journal.pone.0192992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatomi, T. , Amatatsu, M. , Romero‐Pérez, G. A. , Inoue, R. , & Tsukahara, T. (2017). Dietary probiotic compound improves reproductive performance of porcine epidemic diarrhoea virus‐infected sows reared in a Japanese commercial swine farm under vaccine control condition. Frontiers in Immunology, 8, 1877 10.3389/fimmu.2017.01877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, R. , Tsukahara, T. , Sunaba, C. , Itoh, M. , & Ushida, K. (2007). Simple and rapid detection of the porcine reproductive and respiratory syndrome virus from pig whole blood using filter paper. Journal of Virological Methods, 141, 102–106. 10.1016/j.jviromet.2006.11.030 [DOI] [PubMed] [Google Scholar]
- Jung, K. , & Saif, L. J. (2015). Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. The Veterinary Journal, 204, 134–143. 10.1016/j.tvjl.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoi, K. , Sugioka, H. , Satoh, T. , & Kadoi, B. K. (2002). The propagation of a porcine epidemic diarrhea virus in swine cell lines. New Microbiologica, 25, 285–290. [PubMed] [Google Scholar]
- Kandasamy, S. , Chattha, K. S. , Vlasova, A. N. , Rajashekara, G. , & Saif, L. J. (2014). Lactobacilli and bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes, 5, 639–651. 10.4161/19490976.2014.969972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. Y. , Song, D. S. , & Park, B. K. (2001). Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT‐PCR. Journal of Veterinary Diagnostic Investigation, 13, 516–520. 10.1177/104063870101300611 [DOI] [PubMed] [Google Scholar]
- Kritas, S. K. , Marubashi, T. , Filioussis, G. , Petridou, E. , Christodoulopoulos, G. , Burriel, A. R. , … Pískoriková, M. (2015). Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C‐3102). Journal of Animal Science, 93, 405–413. 10.2527/jas.2014-7651 [DOI] [PubMed] [Google Scholar]
- Kritas, S. K. , & Morrison, R. B. (2005). Evaluation of probiotics as a substitute for antibiotics in a large pig nursery. Veterinary Record, 156, 447–448. 10.1136/vr.156.14.447 [DOI] [PubMed] [Google Scholar]
- Kusanagi, K. , Kuwahara, H. , Katoh, T. , Nunoya, T. , Ishikawa, Y. , Saejima, T. , & Tajima, M. (1992). Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate. Journal of Veterinary Medical Science, 54, 313–318. 10.1292/jvms.54.313 [DOI] [PubMed] [Google Scholar]
- Kweon, C.‐H. , Kwon, B.‐J. , Lee, J.‐G. , Kwon, G.‐O. , & Kang, Y.‐B. (1999). Derivation of attenuated porcine epidemic diarrhea virus (PEDV) as vaccine candidate. Vaccine, 17, 2546–2553. 10.1016/s0264-410x(99)00059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Potier, M.‐F. , Mesplede, A. , & Vannier, P. (2006). Classical swine fever and other pestiviruses In Straw B. E., Zimmerman J. J., D'Allaire S., & Taylor D. J. (Eds.), Diseases of swine (9th ed., pp. 309–322). Ames, IA: Blackwell Publishing. [Google Scholar]
- Ogawa, S. , Tsukahara, T. , Tsuruta, T. , Nishibayashi, R. , Okutani, M. , Nakatani, M. , … Inoue, R. (2014). The evaluation of secretion volume and immunoglobulin A and G concentrations in sow colostrum from anterior to posterior teats. Animal Science Journal, 85, 678–682. 10.1111/asj.12211 [DOI] [PubMed] [Google Scholar]
- Olanratmanee, E.‐O. , Kunavongkrit, A. , & Tummaruk, P. (2010). Impact of porcine epidemic diarrhea virus infection at different periods of pregnancy on subsequent reproductive performance in gilts and sows. Animal Reproduction Science, 122, 42–51. 10.1016/j.anireprosci.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Pensaert, M. B. , & Yeo, S.‐G. (2006). Porcine epidemic diarrhea In Straw B. E., Zimmerman J. J., D'Allaire S., & Taylor D. J. (Eds.), Diseases of swine (9th ed., pp. 367–372). Ames, IA: Blackwell Publishing. [Google Scholar]
- Piao, S.‐J. , Tsukahara, T. , Itoh, M. , Shiga, A. , Adachi, Y. , & Ushida, K. (2007). The organic acid profiles in the feces of pigs in Brachyspira hyodysenteriae‐ or B. pilosicoli‐positive farms. Journal of Veterinary Medical Science, 69, 425–428. 10.1292/jvms.69.425 [DOI] [PubMed] [Google Scholar]
- Sasaki, Y. , Alvarez, J. , Sekiguchi, S. , Sueyoshi, M. , Otake, S. , & Perez, A. (2016). Epidemiological factors associated to spread of porcine epidemic diarrhea in Japan. Preventive Veterinary Medicine, 123, 161–167. 10.1016/j.prevetmed.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Sasaki, Y. , Kawabata, T. , & Noguchi, M. (2017). The effect of porcine epidemic diarrhea (PED) on ovarian function and reproductive performance after weaning in Berkshire sows. Tropical Animal Health and Production, 49, 879–882. 10.1007/s11250-017-1257-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, Y. , Toyomaki, H. , Sekiguchi, S. , Sueyoshi, M. , Makita, K. , Otake, S. , … Alvarez, J. (2017). Spatial dynamics of porcine epidemic diarrhea (PED) spread in the southern Kyushu, Japan. Preventive Veterinary Medicine, 144, 81–88. 10.1016/j.prevetmed.2017.05.025 [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Tsukahara, T. , Taira, O. , Tsuchiya, K. , Itoh, M. , & Ushida, K. (2010). Prevalence of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 in piglets after weaning on a commercial pig farm in Japan. Animal Science Journal, 81, 135–141. 10.1111/j.1740-0929.2009.00706.x [DOI] [PubMed] [Google Scholar]
- Shibata, I. , Tsuda, T. , Mori, M. , Ono, M. , Sueyoshi, M. , & Uruno, K. (2000). Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Veterinary Microbiology, 72, 173–182. 10.1016/s0378-1135(99)00199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, D. S. , Oh, J. S. , Kang, B. K. , Yang, J. S. , Moon, H. J. , Yoo, H. S. , … Park, B. K. (2007). Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Research in Veterinary Science, 82, 134–140. 10.1016/j.rvsc.2006.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, D. , & Park, B. (2012). Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes, 44, 167–175. 10.1007/s11262-012-0713-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer, V. C. , & Cox, D. F. (1984). Estimating milk yield of sows. Journal of Animal Science, 59, 1281–1285. 10.2527/jas1984.5951281x [DOI] [PubMed] [Google Scholar]
- Sueyoshi, M. , Tsuda, T. , Yamazaki, K. , Yoshida, K. , Nakazawa, M. , Sato, K. , … Mori, M. (1995). An immunohistochemical investigation of porcine epidemic diarrhoea. Journal of Comparative Pathology, 113, 59–67. 10.1016/s0021-9975(05)80069-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto, A. , Asano, S. , Goto, Y. , Murata, J. , Mori, T. , & Adachi, M. (2004). Survey of porcine proliferative enteritis in Tohoku district of Japan. Journal of Veterinary Medical Science, 66, 547–549. 10.1292/jvms.66.547 [DOI] [PubMed] [Google Scholar]
- Suzuki, T. , Murakami, S. , Takahashi, O. , Kodera, A. , Masuda, T. , Itoh, S. , … Tsutsui, T. (2015). Molecular characterization of pig epidemic diarrhoea viruses isolated in Japan from 2013 to 2014. Infection, Genetics and Evolution, 36, 363–368. 10.1016/j.meegid.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Takahashi, S. , Yoshida, Y. , Nakanishi, N. , Tsukahara, T. , & Ushida, K. (2008). Quantitative real‐time PCR monitoring of Escherichia coli and Clostridium perfringens with oral administration of Lactobacillus plantarum strain Lq80 to weaning piglets. Animal Science Journal, 79, 737–744. 10.1111/j.1740-0929.2008.00588.x [DOI] [Google Scholar]
- Tantasuparuk, W. , Dalin, A. M. , Lundeheim, N. , Kunavongkrit, A. , & Einarsson, S. (2001). Body weight loss during lactation and its influence on weaning‐to‐service interval and ovulation rate in Landrace and Yorkshire sows in the tropical environment of Thailand. Animal Reproduction Science, 65, 273–281. 10.1016/s0378-4320(00)00218-9 [DOI] [PubMed] [Google Scholar]
- Toner, M. S. , King, R. H. , Dunshea, F. R. , Dove, H. , & Atwood, C. S. (1996). The effect of exogenous somatotropin on lactation performance of first‐litter sows. Journal of Animal Science, 74, 167–172. 10.2527/1996.741167x [DOI] [PubMed] [Google Scholar]
- Toyomaki, H. , Sekiguchi, S. , Sasaki, Y. , Sueyoshi, M. , & Makita, K. (2018). Factors associated with farm‐level infection of porcine epidemic diarrhea during the early phase of the epidemic in Japan in 2013 and 2014. Preventive Veterinary Medicine, 150, 77–85. 10.1016/j.prevetmed.2017.12.008 [DOI] [PubMed] [Google Scholar]
- Tsukahara, T. , Nakanishi, N. , Matsubara, N. , Itoh, M. , & Ushida, K. (2006). The effect of Enterococcus faecalis cell preparation (EC‐12) against the diarrhea in the nursing and weaning piglets under the clinical condition. Proceedings of the Japan Pig Veterinary Society, 48, 19–23. [Google Scholar]
- Ushida, K. , Kishimoto, A. , Piao, S.‐J. , Itoh, M. , Shiga, A. , Nakanishi, N. , & Tsukahara, T. (2009). An epidemiological survey on pigs showing symptoms of infectious enteric diseases and dyspepsia in Japan. Animal Science Journal, 80, 556–561. 10.1111/j.1740-0929.2009.00671.x [DOI] [PubMed] [Google Scholar]
- Wentao, L. , Heng, L. , Yunbo, L. , Yongfei, P. , Feng, D. , Yanhua, S. , … Qigai, H. (2012). New variants of porcine epidemic diarrhea virus, China, 2011. Emerging Infectious Diseases, 18, 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Azevedo, M. S. P. , Gonzalez, A. M. , Saif, L. J. , Van Nguyen, T. , Wen, K. , … Yuan, L. (2008). Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Veterinary Immunology and Immunopathology, 122, 175–181. 10.1016/j.vetimm.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Azevedo, M. S. P. , Wen, K. , Gonzalez, A. , Saif, L. J. , Li, G. , … Yuan, L. (2008). Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine, 26, 3655–3661. 10.1016/j.vaccine.2008.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]


