Abstract
Aim: To determine whether nasopharyngeal aspirates (NPAs) cytokine response is different according to the causative viruses in children with lower respiratory tract infections (LRTI).
Methods: NPAs from 277 children with LRTI caused by respiratory virus were evaluated. Based on the proven viral agents, LRTI patients were divided into four groups. Levels of IL‐4, IL‐5 and IFN‐γ were determined by ELISA.
Results: Patients with influenza virus infection demonstrated significantly lower IL‐4 and IL‐5 levels than those with other three groups. Patients with respiratory syncytial virus (RSV) infection showed an increase in production of IL‐4 and IL‐5, and a decrease in the IFN‐γ level when compared to patients with influenza virus infection. Interestingly, a similar Th2 response was seen in patients with parainfluenza virus or adenovirus infection.
Conclusion: These results demonstrate that respiratory viruses can induce different local cytokine responses. However, Th2 biased responses are not unique for RSV but seem to be predominant in respiratory viruses of young children.
Keywords: Child, Cytokine, Lower respiratory tract infection, Nasopharyngeal aspirate, Virus
INTRODUCTION
Respiratory viruses are important causes of lower respiratory infections in infants and children. Cytokines are thought to be important in the pathogenesis of inflammation in the respiratory tract (1). Several studies (1, 2, 3) have investigated cytokine profiles in nasopharyngeal aspirates (NPAs) during viral lower respiratory tract infections (LRTI) in children. Previous studies have suggested that respiratory viruses could stimulate both T helper cell type 1 (Th1) cytokines and T helper cell type 2 (Th2) cytokines.
Despite the scanty data available, it is apparent that cytokine responses of LRTI are quite different according to the causative organisms. Previous studies (1, 3) were demonstrated that patients with respiratory syncytial virus (RSV) infection and those with influenza virus infection induce different cytokine profiles. Influenza virus infection leads to a rapid proinflammatory reaction, which may condition the infected host for the subsequent virus antigen‐specific Th1 defense. While, cytokine profiles of RSV infections in children demonstrate variable results. Infants with RSV infection elicit a predominant Th2 response such as interleukin (IL)‐4 or IL‐5 (4, 5). However, it has been shown that in addition to IL‐4 and IL‐5, Th1 cytokines such as interferon (IFN)‐γ are significantly elevated in RSV infection (6). In addition, an in vitro model of respiratory virus infection showed different responses of the cells with infected viruses (7). These results have demonstrated that cytokine responses of the cells infected with different respiratory viruses depend on infectious agents and on the host characteristics.
Cytokines are thought to be important in the pathogenesis of inflammation in the respiratory tract. Lower respiratory tract secretions are usually obtained for cytokine measurement by bronchoalveolar lavage (BAL), which is an invasive technique and requires the use of an anesthetic in young children. On the other hand, NPA is often used for determining cytokine responses of viral respiratory infections in children.
The present study was performed to determine whether clinical responses in children with LRTI would differ according to causative viruses and to examine the differential patterns of IL‐4, IL‐5 and IFN‐γ concentrations in NPA during acute viral lower respiratory infections according to the causative organisms.
MATERIALS AND METHODS
Study subjects
A total of 1137 children with signs and symptoms of acute viral LRTI who were admitted to Korea University Anam Hospital during a period of 12 months (from July 2006 to June 2007) were enrolled in the present study. Characteristic LRTI symptoms included runny nose, coughing, wheezing and difficulty in breathing, with or without fever. Of a total of 303 cases with positive viral culture, two or more viruses were isolated from the same specimen in 26 patients (8.6%). Of these children, 277 cases with a single viral culture were selected as the study group. Based on the proven viral agents, LRTI patients were divided into four groups: 71 patients with influenza virus (the influenza virus group), 56 patients with parainfluenza virus (the parainfluenza virus group), 119 patients with RSV (the RSV group) and 31 patients with adenovirus (the adenovirus group).
Complete history taking, physical examination and routine laboratory tests were performed on all subjects. Patients with bacterial or mycoplasmal infections or those under 12 weeks of age were excluded. Bacterial or mycoplasmal infections were identified as a positive blood culture for bacteria or a 4‐fold or greater increase in the mycoplasma antibody titer. Those who have a past history of chronic lung diseases such as asthma or bronchopulmonary dysplasia were also excluded. Parents gave written informed consent for their children to participate in the study. The study protocol was approved by the Hospital Ethics Committee.
Collection and preparation of specimens
NPAs were collected usually within the first 3 h of admission as part of the routine hospital procedure. In a small number of cases, which were admitted at nighttime or on a Sunday, NPAs were collected sometime after but within 48 h of admission. A polyethylene suction catheter was placed into the nasopharyngeal cavity via the nostril. Aspiration was then conducted using a suction pump. NPAs collected from each patient were used for both virus culture and cytokine analysis. The specimen for culture was immediately placed in 2 mL of HH medium after collection, transported to the virus laboratory on ice and kept at −20°C until cultures were performed.
Process of virus culture
NPAs from patients were cultured by using the cryopreserved R‐Mix cultures (Diagnostic Hybrids Inc., Athens, OH, USA) for four respiratory viruses (influenza virus, parainfluenza virus, RSV and adenovirus). Identification of rhinovirus, coronavirus, bocavirus and metapneumovirus was not performed, as these were not a part of the routine service offered by the hospital virology department.
Analysis of viral culture positive cases
We analysed characteristics including age, sex and the clinical diagnosis of the culture proven cases. The clinical diagnosis of acute LRTI included tracheobronchitis, bronchiolitis and pneumonia.
Measurement of the total serum IgE, serum eosinophil cationic protein (ECP) and blood eosinophil counts
Total serum IgE levels were measured using Coat‐A‐Count Total IgE IRMA (Diagnostic Products Co., Los Angeles, CA, USA). The serum ECP level was measured using a commercially available fluoroimmunoassay kit (Pharmacia ECP UniCAP System FEIA; Pharmacia Diagnostics, Uppsala, Sweden) which had a detection limit of less than 2.0 μg/L. Blood sample collection, serum preparation and serum ECP analyses were performed according to the manufacturer's instructions. The number of peripheral blood eosinophils was counted with blood samples containing ethylenediaminetetraacetic acid (EDTA) using an automated haematology analyzer (Coulter Counter STKS; Beckman Coulter, Fullerton, CA, USA).
Measurement of IL‐4, IL‐5 and IFN‐γ in NPAs
Before cytokine measurement, each specimen was weighed and diluted to a volume of 2 mL using phosphate‐buffered saline (PBS). The final volume of NPA/PBS solution was recorded and the exact dilution of NPA was calculated. IL‐4, IL‐5 and IFN‐γ levels in NPAs were measured using a commercial ELISA kit (BD Inc., San Diego, CA, USA) according to the manufacturer's instructions. The detection limit was 2 pg/mL for IL‐4, IL‐5 and IFN‐γ, respectively. All assays were run in duplicate, and the mean value was used for statistical analysis.
Statistical analysis
Cytokine levels in NPAs were expressed per milliliter and were presented as means ± SD. We assigned cytokine values that were below the limit of detection a value of zero for facilitating statistical analyses (8). The values for serum total IgE, serum ECP and blood eosinophil counts were logarithmically transformed before analysis and expressed as geometric means (range of 1 SD). The variables were compared between the four groups using one‐way ANOVA and were followed by the Tukey multiple comparison test. The frequencies were examined using the chi‐square test. All statistical analyses were performed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). A p‐value of <0.05 was considered statistically significant.
RESULTS
The clinical characteristics of the four study groups are summarized in Table 1. The mean age of RSV group was significantly lower than that of the other three groups. There was no significant difference in gender between the four groups. However, there were more male patients in all age groups.
Table 1.
Clinical characteristics of four study groups
| Influenza virus group (n = 71) | Parainfluenza virus group (n = 56) | RSV group (n = 119) | Adenovirus group (n = 31) | |
|---|---|---|---|---|
| Age (years)* | 2.8 ± 2.4 | 2.2 ± 1.6 | 1.2 ± 0.7 | 2.3 ± 2.2 |
| Sex (male/female) | 40/31 | 32/24 | 81/38 | 23/8 |
| Total IgE (IU/mL)† | 52.5 (17.0–162.2) | 40.7 (9.12–182.0) | 40.7 (7.76–213.8) | 89.1 (28.8–275.4) |
| Blood eosinophil count (/μL)† | 38.0 (10.7–134.9) | 44.7 (12.3–162.2) | 93.3 (30.2–288.4)‡ | 95.5 (33.9–269.2)‡ |
| Eosinophil cationic protein (μg/mL)† | 6.92 (4.37–11.0) | 4.27 (2.09–8.71) | 19.6 (7.46–53.7)‡ | 9.12 (5.89–14.1) |
*Means ± SD.
†Geometric mean (range of 1 SD).
‡p < 0.05 versus influenza virus group.
RSV = respiratory syncytial virus.
The geometric mean (range of 1 SD) of the total IgE level in the influenza virus group [52.5 (17.0–162.2) IU/mL] was not significantly different from those of the other three groups: the parainfluenza virus group [40.7 (9.12–182.0) IU/mL], the RSV group [40.7 (7.76–213.8) IU/mL] and the adenovirus group [89.1 (28.8–275.4) IU/mL]. The geometric mean (range of 1 SD) peripheral blood eosinophil counts of the parainfluenza virus, RSV and the adenovirus groups were 44.7/μL (12.3–162.2/μL), 93.3/μL (30.2–288.4/μL) and 95.5/μL (33.9–269.2/μL), respectively, which were significantly higher than that of the influenza virus group [38.0/μL (10.7–134.9/μL); p < 0.05]. The serum ECP level was significantly higher in the RSV group [geometric mean (range of 1 SD); 19.6 (7.46–53.7 μg/mL)] than in the influenza virus group [6.92 (4.37–11.0 μg/mL)], the parainfluenza virus group [4.27 (2.09–8.71 μg/mL)] and the adenovirus group [9.12 (5.89–14.1 μg/mL); p < 0.05].
Monthly distributions of the isolated viruses are shown in Figure 1. LRTI by influenza virus, parainfluenza virus and RSV occurred in epidemics and LRTI by adenovirus occurred during all seasons of the year. The distribution of principal viral agents identified from patients with different respiratory syndromes is depicted in Figure 2. Influenza virus mainly accounted for pneumonia and tracheobronchitis, and parainfluenza virus caused pneumonia. RSV was most frequently associated with bronchiolitis, and adenovirus was associated with tracheobronchitis and pneumonia.
Figure 1.
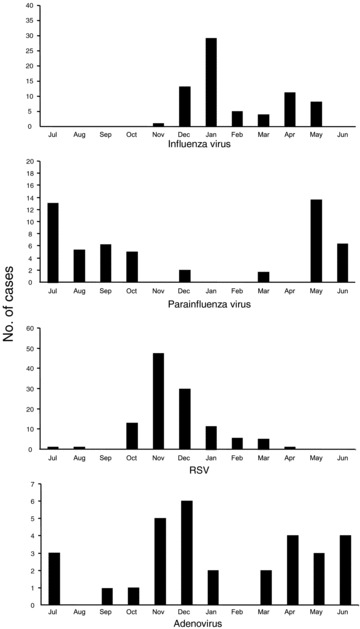
Monthly isolation of influenza virus, parainfluenza virus, respiratory syncytial virus (RSV) and adenovirus.
Figure 2.
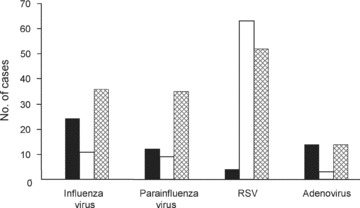
Proportion of specific viral agents associated with three clinical syndromes of acute viral lower respiratory infection; (▪) tracheobronchitis; (□) bronchiolitis;  pneumonia.
pneumonia.
The mean (±SD) IL‐4 level was 2.8 (±2.2) pg/mL in the influenza virus group, which was significantly lower than that of the parainfluenza virus group (15.4 ± 5.2 pg/mL), the RSV group (14.3 ± 3.5 pg/mL) and the adenovirus group (17.5 ± 7.8 pg/mL) (p < 0.05 for each). The mean (±SD) IL‐5 level was 6.5 (±3.1) pg/mL in the influenza virus group, which was also significantly lower than that of the parainfluenza virus group (15.1 ± 4.9 pg/mL), the RSV group (11.5 ± 3.2 pg/mL) and the adenovirus group (14.5 ± 5.5 pg/mL) (p < 0.05 for each). In contrast, the mean (±SD) IFN‐γ level was 19.4 ± 13.5 pg/mL in the RSV group, which was significantly lower than that of the other three groups (the influenza virus group, 38.5 ± 14.3 pg/mL; the parainfluenza virus group, 30.1 ± 19.1 pg/mL; the adenovirus group, 37.5 ± 13.3 pg/mL) (Table 2).
Table 2.
Serum IL‐4, IL‐5 and IFN‐γ levels of four study groups
| Influenza virus group | Parainfluenza virus group | RSV group | Adenovirus group | |
|---|---|---|---|---|
| IL‐4 (pg/mL) | 2.8 ± 2.2 | 15.4 ± 5.2† | 14.3 ± 3.5† | 17.5 ± 7.8† |
| IL‐5 (pg/mL) | 6.5 ± 3.1 | 15.1 ± 4.9† | 11.5 ± 3.2† | 14.5 ± 5.5† |
| IFN‐γ (pg/mL) | 38.5 ± 14.3 | 30.1 ± 19.1 | 19.4 ± 13.5† | 37.5 ± 13.3 |
| IL‐4/IFN‐γ | 0.29 ± 0.16 | 0.71 ± 0.25 | 3.35 ± 1.91† | 3.32 ± 1.43† |
*Means ± SD.
†p < 0.05 versus influenza virus group.
DISCUSSION
The present study of viral LRTI children indicated that as seen in NPAs local inflammatory cytokine responses differed according to the causative organisms. Patients with influenza virus infection have shown the lowest levels of IL‐4 and IL‐5, and the highest level of IFN‐γ. Patients with RSV infection showed an increase in production of IL‐4 and IL‐5, and a decrease in the IFN‐γ level when compared to patients with influenza virus infection. Interestingly, a similar Th2 response was also seen in patients with parainfluenza virus or adenovirus infection. However, these two groups showed significantly higher IFN‐γ production than the RSV group. These results suggest that respiratory viruses can induce different local cytokine responses and that Th2 biased responses are not unique for RSV but seem to be predominant in young children.
Previous studies have shown variable relationships between viral respiratory infection and cytokine responses. However, because of the small number of cases involved (3) and a relatively short study period (9), a larger group of patients and a longer study period are necessary in order to better delineate the clinical and immunological behaviour of respiratory viruses. Furthermore, since different monthly distributions of respiratory virus infection cases have been reported in our country (10), we have examined cytokine responses involving a large series of LRTI patients during a period of 12 months.
Lower respiratory tract secretions are usually obtained for cytokine measurement by BAL, which is an invasive technique that makes it very difficult to obtain appropriate samples for analysis. In the present study, we used NPAs instead of BAL for cytokine analysis. However, this factor is unlikely to affect our results because a previous study (11) has demonstrated that cytokines in NPAs are comparable to those in the lower respiratory tract of infants with respiratory virus infections.
A few studies have investigated cytokine profiles in NPAs of patients with RSV LRTI. Infants with RSV infection have significantly elevated local production of Th2 cytokine, IL‐4 and a lower level of IFN‐γ (12). In addition, more severely infected patients are associated with less local productions of IFN‐γ (13, 14). Conversely, other studies have shown that levels of both IFN‐γ and IL‐4 are significantly elevated in the nasal secretions of RSV bronchiolitis (6, 15). These data suggest that host‐response factors, such as age, atopy or severity of infection, as well as causative viruses are important for determining local cytokine responses. Allergic disease was caused by stimulation of a Th2 response with consequent secretion of cytokines such as IL‐4 and IL‐5 (16). Since IL‐4 stimulates IgE synthesis to induce type 1 hypersensitivity, it is indicated that an allergic mechanism may contribute to the pathogenesis of wheezing in acute bronchiolitis during RSV infection (3).
Compared to RSV infection, cell‐mediated immunological responses of influenza virus infection have attracted less attention. While a predominant Th2 cytokine and its related immunological responses are observed in infants with RSV infection, a predominant Th1 cytokine response is observed in infants with influenza virus infection (17). This may explain different clinical manifestations between the two viral infections in infants.
The role of IFN‐γ in the pathogenesis of viral respiratory infections has been relatively well elucidated. Hall et al. (17) have already speculated that the direct antiviral activity of IFN‐γ as well as cell‐mediated immunity may be important in the pathogenesis of RSV LTRI. IFN‐γ is considered to be a key cytokine in inducing protective responses against viral pathogens (7). We have found that RSV LTRI is associated with the reduced local level of IFN‐γ. Owing to the lower IFN‐γ level, patients with RSV infection were most frequently associated with severe bronchiolitis and a longer duration of hospitalization. Although the mean age of patients with RSV infections are significantly lower than that of the other three groups, it is unlikely that the difference in age could affect the difference in the IFN‐γ level between this study groups. We excluded patients younger than three months of age from the study because T‐cell mediated responses are delayed during the first 4–8 weeks of postnatal age and are accompanied by an impaired capacity to produce IFN‐γ (18).
In our study, it was found that levels of IL‐4 and IL‐5 were higher in the parainfluenza virus group or the adenovirus group than in the influenza virus group. There have been quite a few reports regarding these two viruses. Kristjansson et al. (19) have demonstrated that there is no significant difference in the Th2 cytokine level between infants with parainfluenza virus infection and those with RSV infection or influenza virus infection. They concluded that infections with parainfluenza virus or RSV in early infancy preferentially promote a Th2‐like response with local production of IL‐4 and IL‐5.
Adenovirus is also a well‐known cause of LRTI in children, and most cases of infections are self‐limited, whereas several cases of this infection may be associated with severe infections or chronic sequelae such as hyperlucent lung and bronchiolitis obliterans. Cytokines are partly responsible for chronic sequelae of these infections. Since authors have proposed that adenovirus produces a significantly higher IFN‐γ level and induces a Th1 immune response (7, 20), whereas other authors have shown no difference when compared to RSV (21, 22). In concordance with the study by Fernandez et al. study (20), we observed that the IFN‐γ level was higher in the adenovirus group than in the RSV group. These results indicate that adenovirus and RSV infections in children differ significantly with regard to the magnitude of production of IFN‐γ.
We found significant increases of eosinophil counts and serum ECP concentrations in the RSV group. Our findings were in concordance with those reported by Oymar et al. (23) in that the serum ECP concentration was significantly higher in patients with RSV LRTI than in patients with non‐RSV LRTI. This potentially explained the tendency of eosinophilic inflammation in patients with RSV infection.
The cytokine responses to acute respiratory viral infections are known to vary according to the duration of infection (22, 24). However, the duration of the symptoms before admission was not significantly different between the four study groups. In this study, laboratory diagnoses of LRTI have been made by virus culture instead of PCR. Given that all patients included in the study proven with positive virus culture, it is possible that subject with negative culture might have had a viral infection that was not detected. We were unable to detect any viral etiology other than four kinds of respiratory viruses from NPAs in children recruited for this study. This may be attributed to the limitation of viral cultures in NPA samples, which was not a part of the routine service offered by our hospital virology department. Nevertheless, we were able to obtain significant results from patients with proven viral etiology.
The present study was performed in patients with lower respiratory infection over a period of one year. We have obtained results from a relatively large number of patients. Finally, we now prepared prospective study to determine how long after respiratory virus infection the cytokine imbalance may persist.
In summary, we found a predominant Th2 cytokine response in children with RSV infection and a predominant Th1 cytokine response in children with influenza virus infection. Children with parainfluenza virus infection and those with adenovirus infection elicited both Th1 and Th2 cytokine responses. These suggest that as seen in NPAs, local inflammatory cytokine responses may differ in children with acute viral respiratory infections according to causative viruses and that a Th2 biased response may be not unique for RSV infection but it seems to be predominant in respiratory viral infections in young children. Investigation of local cytokine responses evolved by common respiratory viruses is essential for understanding of the pathogenesis of distinct respiratory viral infections and development of their proper treatment modalities.
References
- 1. Oh JW, Lee HB, Park IK, Kang JO. Interleukin‐6, interleukin‐8, interleukin‐11, and interferon‐gamma levels in nasopharyngeal aspirates from wheezing children with respiratory syncytial virus or influenza A virus infection. Pediatr Allergy Immunol 2002; 13: 350–6. [DOI] [PubMed] [Google Scholar]
- 2. Joshi P, Shaw A, Kakakios A, Isaacs D. Interferon‐gamma levels in nasopharyngeal secretions of infants with respiratory syncytial virus and other respiratory viral infections. Clin Exp Immunol 2003; 131: 143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sung RY, Hui SH, Wong CK, Lam CW, Yin J. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr 2001; 160: 117–22. [DOI] [PubMed] [Google Scholar]
- 4. Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, et al Respiratory syncytial virus infection in infants is associated with predominant Th2‐like response. Am J Respir Crit Care Med 1997; 156: 190–5. [DOI] [PubMed] [Google Scholar]
- 5. Welliver RC, Duffy L. The relationship of RSV‐specific immunoglobulin E antibody responses in infancy, recurrent wheezing and pulmonary function at age 7–8 years. Pediatr Pulmonol 1993; 15: 19–27. [DOI] [PubMed] [Google Scholar]
- 6. Chen ZM, Mao JH, Du LZ, Tang YM. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr 2002; 91: 914–22. [DOI] [PubMed] [Google Scholar]
- 7. Diaz PV, Calhoun WJ, Hinton KL, Avendano LF, Gaggero A, Simon V, et al Differential effects of respiratory syncytial virus and adenovirus on mononuclear cell cytokine responses. Am J Respir Crit Care Med 1999; 160: 1157–64. [DOI] [PubMed] [Google Scholar]
- 8. Taniguchi H, Katoh S, Kadota J, Matsubara Y, Fukushima K, Mukae H, et al Interleukin 5 and granulocyte‐macrophage colony‐stimulating factor levels in bronchoalveolar lavage fluid in interstitial lung disease. Eur Respir J 2000; 16: 959–64. [DOI] [PubMed] [Google Scholar]
- 9. Pitrez PM, Brennan S, Sly PD. Inflammatory profile in nasal secretions of infants hospitalized with acute lower airway tract infections. Respirology 2005; 10: 365–70. [DOI] [PubMed] [Google Scholar]
- 10. Kim MR, Lee HR, Lee GM. Epidemiology of acute viral respiratory tract infections in Korean children. J Infect 2000; 41: 152–8. [DOI] [PubMed] [Google Scholar]
- 11. Joshi P, Kakakios A, Jayasekera J, Isaacs D. A comparison of IL‐2 levels in nasopharyngeal and endotracheal aspirates of babies with respiratory syncytial viral bronchiolitis. J Allergy Clin Immunol 1998; 102: 618–20. [DOI] [PubMed] [Google Scholar]
- 12. Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2003; 158: 633–9. [DOI] [PubMed] [Google Scholar]
- 13. Bont L, Heijnen CJ, Kavelaars A, Van Aalderen WM, Brus F, Draaisma JM, et al Local interferon‐γ levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 2001; 184: 355–8. [DOI] [PubMed] [Google Scholar]
- 14. Semple MG, Dankert HM, Ebrahimi B, Correia JB, Booth JA, Stewart JP, et al Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS ONE 2007; 17: e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan MA, Richards D, Kemedy DM, Milner AD. A balanced Th‐1 and Th‐2 type cytokine response in nasal secretions of respiratory syncytial virus positive bronchiolitis. Thorax 1998; 53(Suppl 4S): 56A. [Google Scholar]
- 16. Poulsen LK, Bindslev‐Jensen C, Diamant M, Hansen MB, Jepsen KF, Reimert CM, et al Biomolecular regulation of IgE immune response III. Cytokine profiles in atopic dermatitis, inhalant allergy and non‐allergic donors. Cytokine 1996; 8: 651–7. [DOI] [PubMed] [Google Scholar]
- 17. Hall CB, Douglas RG Jr, Simons RL, Geiman JM. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr 1987; 93: 28–32. [DOI] [PubMed] [Google Scholar]
- 18. Chandwani S, Borkowsky W, Krasinski K, Lawrence R, Welliver R. Respiratory syncytial virus infection in human immunodeficiency virus‐infected children. J Pediatr 1990; 117: 251–4. [DOI] [PubMed] [Google Scholar]
- 19. Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, et al Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local Th2‐like response. J Allergy Clin Immunol 2005; 116: 805–11. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez JA, Tapia L, Palomino MA, Larranaga C, Pena M, Jaramillo H. Plasma interferon‐γ, interleukin‐10 and soluble markers of immune activation in infants with primary adenovirus (ADV) and respiratory syncytial virus (RSV) infection. Eur Cytokine Netw 2005; 16: 35–40. [PubMed] [Google Scholar]
- 21. Kawasaki Y, Hosoya M, Katayose M, Suzuki H. Correlation between serum interleukin 6 and C‐reactive protein concentrations in patients with adenoviral respiratory infection. Pediatr Infect Dis J 2002; 21: 370–4. [DOI] [PubMed] [Google Scholar]
- 22. Oda K, Yamamoto Y. Serum interferon‐γ, interleukin‐4, and interleukin‐6 in infants with adenovirus and respiratory syncytial virus infection. Pediatric Int 2008; 50: 92–4. [DOI] [PubMed] [Google Scholar]
- 23. Oymar K, Elsayed S, Bjerknes R. Serum eosinophil cationic protein and interleukin‐5 in children with bronchial asthma and acute bronchiolitis. Pediatr Allergy Immunol 1996; 7: 180–6. [DOI] [PubMed] [Google Scholar]
- 24. Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. J Clin Invest 1998; 101: 643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


