Abstract
Wolbachia are being used to reduce dengue transmission by Aedes aegypti mosquitoes around the world. To date releases have mostly involved Wolbachia strains with limited fitness effects but strains with larger fitness costs could be used to suppress mosquito populations. However, such infections are expected to evolve towards decreased deleterious effects. Here we investigate potential evolutionary changes in the wMelPop infection transferred from Drosophila melanogaster to Aedes aegypti more than ten years (~120 generations) ago. We show that most deleterious effects of this infection have persisted despite strong selection to ameliorate them. The wMelPop-PGYP infection is difficult to maintain in laboratory colonies, likely due to the persistent deleterious effects coupled with occasional maternal transmission leakage. Furthermore, female mosquitoes can be scored incorrectly as infected due to transmission of Wolbachia through mating. Infection loss in colonies was not associated with evolutionary changes in the nuclear background. These findings suggest that Wolbachia transinfections with deleterious effects may have stable phenotypes which could ensure their long-term effectiveness if released in natural populations to reduce population size.
Author summary
Mosquitoes infected with Wolbachia bacteria are being deployed into the field where they can suppress mosquito populations and reduce dengue transmission. These programs rely on the use of Wolbachia strains that have desirable phenotypes, which can include deleterious fitness effects, reproductive manipulation and virus blocking. However, theory predicts that Wolbachia will evolve to become less costly to their hosts, reducing the effectiveness of these programs. We investigate the potential for evolutionary changes by performing a comprehensive phenotypic analysis of a deleterious Wolbachia strain, wMelPop-PGYP, that was introduced to Aedes aegypti mosquitoes from Drosophila over ten years ago. In contrast to theoretical expectations and research from Drosophila, our results suggest that Wolbachia strains with deleterious effects may have stable phenotypes, ensuring their long-term effectiveness if released into natural populations.
Introduction
There is increasing interest in using Wolbachia bacterial infections for suppressing dengue transmission by mosquitoes, with field releases aimed at both replacing existing natural mosquito populations with those infected by Wolbachia [1, 2] and suppressing these populations through sterility induced by Wolbachia-infected males [3]. Replacement releases can be effective because the presence of Wolbachia in mosquitoes reduces transmission of arboviruses [4–6]. In addition, Wolbachia decreases the fitness of its mosquito hosts [7]. While this might have a suppressive effect on dengue transmission, for instance, by shortening mosquito lifespan [8], it can make the infections more difficult to introduce into populations because the initial Wolbachia frequency must be higher for the population to be invaded by Wolbachia [9].
The wMelPop infection, which originated from a laboratory strain of Drosophila melanogaster, was one of the first Wolbachia strains successfully introduced into Aedes aegypti [10] where it is very effective at blocking transmission of dengue and other arboviruses [5]. wMelPop in Ae. aegypti represents a variant referred to as wMelPop-PGYP which lacks the Octomom genomic region present in the original strain [11]. The wMelPop strain reduces longevity in D. melanogaster [12] while wMelPop-PGYP in mosquitoes has additional deleterious effects, including reduced viability of eggs maintained in a quiescent state [13, 14]. The wMelPop-PGYP infection was released in field trials in Vietnam and Australia but failed to establish [15], although it successfully invaded semi-field cages [6]. Because of these deleterious effects, wMelPop may represent an effective tool to reduce or even eliminate mosquito populations [16], particularly in isolated populations experiencing seasonal rainfall [17].
One of the challenges in using wMelPop-PGYP is that the strain can be difficult to maintain under laboratory conditions, with the infection occasionally being lost from colonies. For instance, on one occasion we found that 95.5% (43/45) of our colony was infected based on RT-PCR screening but this declined to 6.7% (2/30) four months later. Although the infection causes strong cytoplasmic incompatibility and shows near-complete maternal transmission, which allow Wolbachia infections to invade populations once an unstable equilibrium frequency dictated by deleterious fitness effects is exceeded [6], the infection may still be lost for unknown reasons even when it is detected at a high frequency with molecular assays. Environmental effects might reduce infection frequencies since high temperatures and low levels of antibiotics can clear Wolbachia infections [18, 19]. However, there is normally careful control of temperature and antibiotics in laboratory cultures. Other factors that may contribute to infection loss are inappropriate storage of eggs coupled with sporadic incomplete maternal transmission.
While Wolbachia infections like wMelPop and wAu [4] reduce host fitness, their effects are expected to attenuate over time because any Wolbachia or host alleles that decrease deleterious fitness effects should be favoured by selection [9, 20]. Evidence for such a process has been obtained for the wRi infection of Drosophila simulans where an initially deleterious effect on offspring production has attenuated to the extent that wRi infected D. simulans now have a higher production rate than uninfected females [21]. This could undermine any strategy that relies on maintaining deleterious fitness effects after Wolbachia are established in novel hosts, a process that has been documented for wMelPop after transfer to D. simulans [22, 23]. Evolutionary changes in the nuclear background may also suppress the phenotypic effects of Wolbachia, as demonstrated by the evolution of male-killing suppression in butterflies [24]. Although wMelPop continues to impose deleterious effects in its native host D. melanogaster after many years of laboratory culture [25], it is unclear if deleterious effects and the ability to cause cytoplasmic incompatibility have persisted in the derived wMelPop-PGYP infection of Ae. aegypti.
To investigate these issues, we consider whether there have been evolutionary changes in wMelPop-PGYP or its Ae. aegypti host in the 10-year period since the infection was established by comparing recent and past data on phenotypic effects of the infection. We also investigate factors that may confound monitoring of wMelPop-PGYP and contribute to instability of the infection in laboratory cultures.
Methods
Ethics statement
Blood feeding of female mosquitoes on human volunteers for this research was approved by the University of Melbourne Human Ethics Committee (approval 0723847). All adult subjects provided informed written consent (no children were involved).
Mosquito strains and colony maintenance
We performed experiments with our laboratory populations of wMelPop-PGYP-infected [10], wMel-infected [6], wAlbB-infected [26] and uninfected Ae. aegypti mosquitoes. The wMelPop-PGYP transinfection in Ae. aegypti (which we hereafter refer to simply as wMelPop except where clarification is required) was derived from D. melanogaster [12] and was passaged in a mosquito cell line before being introduced into Ae. aegypti through embryonic microinjection [10]. wMelPop-infected mosquitoes were collected from Babinda, Queensland, Australia in 2012, three months after releases commenced [14] and maintained in the laboratory since collection. All Wolbachia-infected populations were backcrossed to a common Australian nuclear background for at least five generations to ensure that backgrounds were >98% similar [14]. Stock populations were maintained through continued backcrossing to uninfected North Queensland material every six generations. Mosquitoes were reared in a temperature-controlled laboratory environment at 26°C ± 1°C with a 12 hr photoperiod according to methods described previously [27, 28]. Larvae were reared in trays filled with 4 L of reverse osmosis water at a controlled density of 450 larvae per tray. Larvae were fed TetraMin tropical fish food tablets (Tetra, Melle, Germany) ad libitum until pupation. Female mosquitoes from all laboratory colonies and experiments were blood fed on the forearms of human volunteers. For colony maintenance, females were blood fed approximately one week after adult emergence, with eggs normally hatched within one week of collection. Only eggs from the first gonotrophic cycle were used to establish the next generation. An uninfected population (denoted wMelPop-negative) was derived from wMelPop females that had lost their Wolbachia infection in June 2019. The wMelPop-negative population was used in life history experiments and to test for nuclear background evolution. All experiments were performed in 2019 except for the first Wolbachia mating transmission experiment (performed in 2016) and the routine scoring of egg hatch from 2012–2018.
Wolbachia screening
Aedes aegypti females were tested for the presence of Wolbachia DNA using methods previously described with modifications [27, 29]. DNA extraction methods varied between experiments due to our research spanning seven years. Mosquito DNA was extracted using 100–250 μL of 5% Chelex solution (Bio-Rad Laboratories, Gladesville, NSW, Australia) and 2.5–5 μL of Proteinase K (20 mg/mL, Bioline Australia Pty Ltd, Alexandria, NSW, Australia) in either 96-well plates or 1.5 mL tubes. Polymerase chain reactions were carried out with a Roche LightCycler 480 system (384-well format, Roche Applied Science, Indianapolis, IN, USA) using a RT/HRM (real-time PCR/high-resolution melt) assay as described previously [27, 29].
We used mosquito-specific (mRpS6), Aedes aegypti-specific (aRpS6) and Wolbachia-specific primers (w1 primers for the wMelPop and wMel infections and wAlbB primers for the wAlbB infection) to diagnose Wolbachia infections [27](S1 Table). All individuals were expected to have robust and similar amplification of the mRpS6 and aRpS6 primers. An individual was scored as positive for Wolbachia if its w1 or wAlbB Cp (crossing point) value was lower than 35 and its Tm (melting temperature) value was within the expected range based on positive controls (approximately 84.3, but this varied between runs). An individual was negative for Wolbachia when Cp values were 35 or absent and/or Tm values were inconsistent with the controls. For experiments with the wMelPop infection, we assigned infected individuals to two categories: strongly positive (Cp ≤ 23) and weakly positive (Cp > 23). Based on the mating transmission experiments (see below), females that were strongly positive likely represented true infections, while weakly positive females were likely uninfected and had mated with a Wolbachia-infected male. Relative Wolbachia densities were determined by subtracting the Wolbachia Cp from the aRpS6 Cp and then transforming this value by 2n.
Re-evaluation of deleterious effects
The wMelPop-PGYP infection induces a range of deleterious effects, including life shortening, reduced fertility, impaired blood feeding success and reduced quiescent egg viability as outlined below. We re-evaluated these deleterious effects by performing experiments with the wMelPop infection over 10 years after its introduction to Ae. aegypti. Before experiments commenced, the wMelPop-infected colony was purified by pooling the offspring of isolated females that were strongly positive for Wolbachia (see Infection recovery). Female offspring were crossed to uninfected males, and the progeny were used in the following experiments. We compared fitness relative to two uninfected populations; a natively uninfected laboratory population (uninfected) and a population derived from uninfected individuals from the wMelPop colony that had lost their infection (wMelPop-negative). Due to logistical constraints, the fertility experiment included the wMelPop and wMelPop-negative populations only.
Longevity
Previous studies reported that wMelPop shortens adult lifespan by approximately 50% [10, 14]. We performed longevity assays by establishing 8 replicate 3 L cages with 50 adults (25 males and 25 females) for each population. Cages were provided with 10% sucrose and water cups which were replaced weekly. Females were provided with blood meals for 10 minutes once per week and given constant access to an oviposition substrate. Mortality was scored three times per week by removing and counting dead adults from each cage until all adults had died. One replicate of wMelPop was discarded due to a sugar spill early in the experiment which caused high mortality. We used log-rank tests to compare adult longevity between populations. To evaluate Wolbachia density and infection frequencies with adult age, 16 females from separate cages that were 0, 7, 14, 21, 28 and 35 d old were screened for Wolbachia. We used a linear regression to test whether (log) Wolbachia density was affected by adult age. All data were analyzed using SPSS statistics version 24.0 for Windows (SPSS Inc, Chicago, IL).
Fertility
The wMelPop-PGYP infection substantially reduces fertility as females age [13]; we therefore tested the fertility of wMelPop and wMelPop-negative populations over successive gonotrophic cycles. The uninfected population was not included in this experiment. We established two cages of approximately 500 individuals (equal sex ratio) for each population. Five-day old females (starved for 1 d) were blood fed on the forearm of a human volunteer. Thirty-five engorged females were selected randomly from each population and isolated in 70 mL cups with sandpaper strips and larval rearing water to encourage oviposition. Eggs were collected 4 days after blood feeding, partially dried and hatched three days after collection. Fecundity and egg hatch proportions were determined by counting the number of unhatched and hatched eggs (hatched eggs having a clearly detached cap). Following egg collection, females were returned to their respective cages for blood feeding. Successive gonotrophic cycles were initiated every 4–5 days with females selected randomly from cages. Cages were provided with oviposition substrates, however no sugar was provided to isolated females or the population cage during the experiment because sugar feeding influences fecundity [30]. We tested fertility for a total of 9 gonotrophic cycles. Females from the wMelPop population that were still alive after 9 gonotrophic cycles were tested with qPCR to confirm Wolbachia infection. Effects of gonotrophic cycle on egg hatch proportions were compared for the wMelPop and wMelPop-negative populations. Egg hatch proportions were not normally distributed and were therefore analysed with Kruskal-Wallis tests.
Quiescent egg viability
The wMelPop infection reduces the viability of quiescent eggs [13, 14, 16]. For quiescent egg viability assays, eggs were collected from colonies on sandpaper strips and stored in a sealed container with a saturated solution of potassium chloride to maintain ~80% humidity. Nine replicate batches of eggs (40–98 eggs per batch) per population were hatched twice per week by submerging eggs in containers of water with a few grains of yeast. Egg hatch proportions were determined by dividing the number of hatched eggs by the total number of eggs. Larvae that had not completely eclosed and died in the egg were scored as unhatched. This experiment continued until eggs were 31 d old. Effects of egg storage duration on hatch proportions were compared for the wMelPop, wMelPop-negative and uninfected populations Egg hatch proportions were not normally distributed and were therefore analysed with Kruskal-Wallis tests. To test for the potential loss of wMelPop infection with egg storage, we reared larvae hatching from 3, 13, 20, 24, 27 and 31 d old egg to adulthood and scored 16 females (< 24 hr old) for Wolbachia infection and density from each group. We used a linear regression to test whether (log) Wolbachia density was affected by egg storage duration.
Blood feeding success
The wMelPop infection reduces female blood feeding success and affects probing behaviour, particularly in older females [31, 32]. We evaluated blood feeding traits in 5 and 35 d old females according to methods described previously [33]. We recorded pre-probing duration (time from landing to insertion of the proboscis), feeding duration, blood meal weight and proportion feeding. Females that did not feed within 10 minutes were scored as not feeding. The proportion of females exhibiting a bendy or shaky proboscis phenotype [31, 32] was also recorded. Feeding trials were performed on individual females by three experimenters. At least 32 individuals per population and age group were tested across the three experimenters. To confirm the infection status of wMelPop females, we screened all 35 d old females for Wolbachia infection. Pre-probing duration, feeding duration and blood meal weight data were analysed with general linear models, with population (wMelPop, wMelPop-negative and uninfected) and experimenter (the person being fed on by the mosquito) included as factors. Pre-probing and feeding durations were log transformed for normality before analysis. Comparisons of proportional data (proportion feeding and the presence of a bendy or shaky proboscis) with previous studies were performed with two proportions Z-tests.
Loss of Wolbachia during colony maintenance
We carried out a series of experiments and monitoring exercises to understand the loss of the wMelPop infection in colonies during routine maintenance.
Infection recovery
In May 2019 we observed an apparent loss of wMelPop infection from our laboratory colony despite a high level of infection in previous generations. To return the population to a 100% infection frequency, one hundred blood-fed females were isolated for oviposition, screened for Wolbachia, then placed into categories of strongly positive, weakly positive or negative (see Wolbachia screening). We then pooled the offspring of females from each category and screened 30 offspring (15 males and 15 females) for Wolbachia per category. Female offspring from the strongly positive population were crossed to uninfected males before commencing the maternal transmission, nuclear background evolution and life history experiments.
Maternal transmission
We estimated maternal transmission fidelity by crossing wMelPop-infected females to uninfected males, then screening ten offspring (4th instar larvae) from the first gonotrophic cycle of ten females that had been separated individually for oviposition. Maternal transmission fidelity was expressed as the proportion of infected offspring produced by infected mothers, for which 95% binomial confidence intervals were calculated.
Nuclear background evolution
Loss of wMelPop infection in laboratory colonies may be explained by the evolution of resistance to Wolbachia infection by uninfected mosquitoes. We performed crossing experiments to test whether the wMelPop infection was maintained across generations when wMelPop-infected females were crossed to natively uninfected males or uninfected males that had lost their Wolbachia infection (wMelPop-negative). We established two replicate populations for each cross with 200 adults of each sex. Males and females were separated as pupae and then crossed when adults were 3–5 d old. Crosses were performed for four consecutive generations, with each cage maintained according to our regular colony maintenance schedule (females were blood fed approximately one week after emergence and eggs hatched within one week of collection). Thirty individuals from each replicate population per generation were then screened for Wolbachia infection. A wMelPop colony (wMelPop-infected males crossed with wMelPop-infected females) was also monitored across the same time period.
To test for resistance to cytoplasmic incompatibility, we tested the ability of wMelPop-infected males to induce cytoplasmic incompatibility with uninfected and wMelPop-negative females. For each cross, 30 males and 30 females were aspirated into a single 3 L cage. When adults were 5 d old, females were blood fed. Twenty females from each cross were isolated for oviposition and egg hatch proportions were determined according to the fertility experiment (see above).
Wolbachia mating transmission
Although Wolbachia in mosquitoes are maternally transmitted, it is possible that Wolbachia might also be transferred through seminal fluid, leading to the detection of Wolbachia in uninfected females that mate with infected males. To test for Wolbachia transmission through mating, we performed crosses between Wolbachia-infected males and uninfected females. Experiments were performed with the wMelPop, wMel and wAlbB strains. Control crosses were also performed, where both sexes were either infected (positive controls) or uninfected (negative control). Crosses were established with 160 virgin adults of each sex (4–7 d old) in a single cage and left for two days to mate, after which males were removed. Females were blood-fed one week after crosses were established and provided with an oviposition substrate. Thirty females (whole adults) were stored 2, 9, 16 and 23 d after crosses were established and screened for Wolbachia. Females from the positive and negative controls were tested 2 and 23 d after crosses were established. Due to apparent differences in mating transfer between Wolbachia strains, this experiment was repeated with the wAlbB infection, but females were stored 5 d after crosses were established.
We conducted an additional cross between uninfected females and wMelPop-infected males to see if the detection of Wolbachia following transmission through mating was tissue-specific. Females and males were left to mate for five days, after which females were stored in ethanol. Heads and abdomens from 20 uninfected females were dissected and extracted separately for Wolbachia screening.
Relative fitness during laboratory maintenance
We compiled data on egg hatch proportions during our routine maintenance of wMelPop, wMel, wAlbB and uninfected colonies from July 2012 to April 2018. Egg hatch proportions were determined by hatching a subset of eggs collected from each colony during maintenance (>200 eggs per subset), then dividing the number of larvae counted by the number of eggs tested. We then divided the egg hatch proportions of Wolbachia-infected colonies by the egg hatch proportion of the uninfected colony to obtain relative egg hatch proportions. When multiple Wolbachia-infected colonies were maintained simultaneously, we included these as separate estimates. We used sign tests to compare relative hatch proportions of Wolbachia-infected and uninfected colony eggs. We used a general linear model to test for long-term changes in the relative egg hatch proportion of wMelPop-infected colonies.
Results
Re-evaluation of deleterious effects
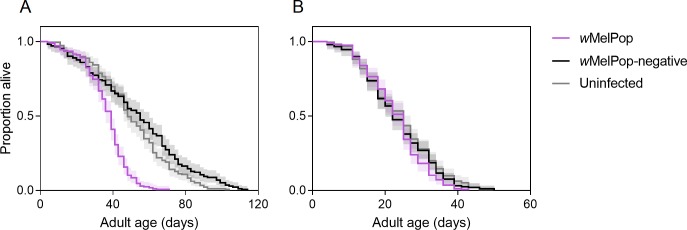
We re-evaluated the deleterious fitness effects induced by wMelPop-PGYP to test for attenuation. In previous experiments conducted more than 10 years ago, the wMelPop-PGYP infection shortened adult male and female lifespan by ~50% relative to uninfected populations [10, 14]. Here, the wMelPop-PGYP infection shortened median female lifespan by 22% compared to the uninfected populations (Log-rank: χ2 = 116.310, df = 2, P < 0.001), while male lifespan was unaffected by population (χ2 = 4.722, df = 2, P = 0.094, Fig 1). These results suggest that the effects of wMelPop on adult lifespan may have attenuated, though direct comparisons with previous studies are difficult since experimental conditions will vary. Although adults from this experiment were not screened for Wolbachia, samples of colony females from the same generation aged 0–35 d (n = 101) all had strongly positive (Cp ≤ 23) infections, suggesting that this result was not influenced by incomplete maternal transmission. (log) Wolbachia density decreased with adult age (linear regression: R2 = 0.186, F1,86 = 20.837, P < 0.001, S1A Fig), in contrast to Drosophila where wMelPop density [34, 35] (and to a lesser extent, wMelPop-CLA density [36]) increases with age.
Fig 1.
Longevity of female (A) and male (B) adult Aedes aegypti from wMelPop (purple lines), wMelPop-negative (black lines) and uninfected (gray lines) populations. Lines represent the proportion of mosquitoes alive, while shaded regions show 95% confidence intervals.
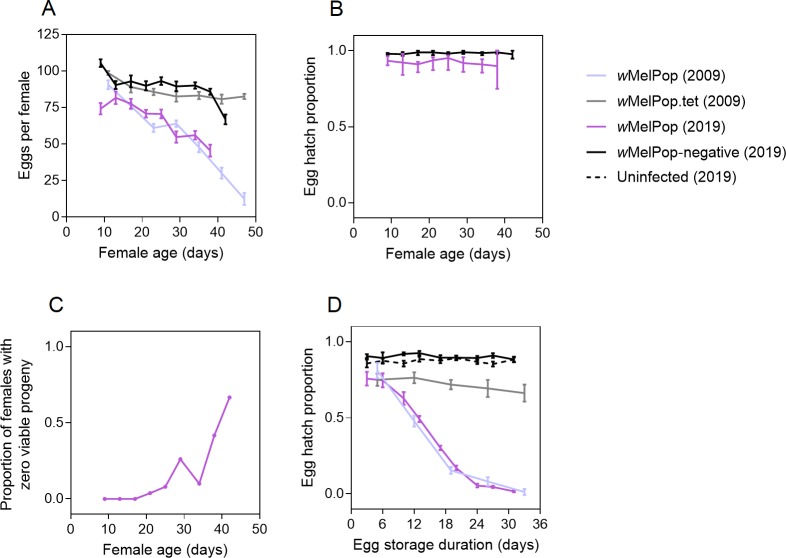
In previous studies, wMelPop infection reduced fertility with increasing female age [13] and egg storage duration [13, 14]. In the current experiment, wMelPop infection reduced fecundity by 22.54% and egg hatch by 11.44% overall, indicating that deleterious effects have persisted for over 10 years after transinfection. The viability of wMelPop-infected eggs declined rapidly with increasing storage duration (Kruskal-Wallis: χ2 = 69.307, df = 8, P < 0.001, Fig 2D) but hatch proportions for wMelPop-negative (χ2 = 7.199, df = 8, P = 0.515) and uninfected (χ2 = 5.503, df = 8, P = 0.703) eggs were stable across the same duration. Patterns of fecundity (Fig 2A) and quiescent egg viability (Fig 2D) observed here were similar to a previous study [13] although experimental conditions would have differed somewhat. Loss of female fertility with age was due to declining fecundity rather than egg hatch, which was stable across gonotrophic cycles for both wMelPop (Kruskal-Wallis: χ2 = 4.654, df = 7, P = 0.702) and wMelPop-negative (χ2 = 7.580, df = 8, P = 0.476) females (Fig 2B).
Fig 2. Fertility of wMelPop-infected and uninfected Aedes aegypti populations with increasing female age and egg storage duration.
(A) Fecundity across gonotrophic cycles. (B) Egg hatch proportion across gonotrophic cycles. (C) Proportion of wMelPop-infected females with zero viable progeny across gonotrophic cycles. (D) Egg hatch proportion with different durations of egg storage. Data for 2009 (pale lines) were manually extracted from McMeniman and O'Neill [13] using ScanIt software (https://www.amsterchem.com/scanit.html). Lines and error bars are means and standard errors respectively, consistent with the original study.
As adult age increased, we observed an increasing proportion of wMelPop females that had a high egg production but had zero eggs hatching (Fig 2C). We excluded these individuals from the results since they may represent uninfected mosquitoes that mated with wMelPop-infected males. Uninfected individuals may result from incomplete maternal transmission and become increasingly represented throughout the experiment due to having a longer lifespan (Fig 1A). Only two of the seven wMelPop females surviving to the ninth gonotrophic cycle had a strongly positive (Cp ≤ 23) Wolbachia infection, indicating maternal transmission leakage. In contrast, all individuals hatching from quiescent eggs (storage durations of 3–31 d, n = 96) were strongly positive for Wolbachia (Fisher’s exact test: P < 0.001), although adult Wolbachia density decreased with increasing egg storage duration (linear regression: R2 = 0.108, F1,83 = 10.087, P = 0.002, S1B Fig).
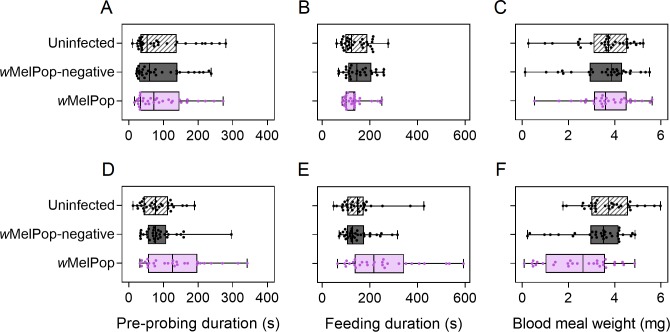
The wMelPop infection reduces female blood feeding success and affects probing behaviour, particularly in older females [31, 32]. Here we found no effect of population on pre-probing and feeding duration or blood meal weight in 5 d old females (GLM: all P > 0.05, Fig 3). Conversely, in 35 d old females we observed costs of wMelPop infection for all traits, with significant effects of population for pre-probing duration (F2,82 = 26.135, P < 0.001), feeding duration (F2,82 = 7.988, P = 0.001) and blood meal weight (F2,82 = 14.338, P < 0.001, Fig 3). Substantial effects of experimenter were also observed for all three traits tested (all P < 0.01), leading to differences of up to 0.37 mg (10.27%) in blood meal weight, 39.5 s (27.96%) in feeding duration and 100 s (113.64%) in pre-probing duration.
Fig 3.
Pre-probing duration (A,D), feeding duration (B,E) and blood meal weight (C,F) of uninfected, wMelPop-negative and wMelPop Aedes aegypti females aged 5 (A-C) or 35 d (D-F). Box plots show medians and interquartile ranges, with error bars representing minimum and maximum values. Data for individual females are shown by dots.
Effects of wMelPop infection on blood feeding traits may have been weaker in comparison to previous studies with similar methods. For instance, Turley et al. [31] observed a 50.3% (95% confidence interval: 37.5–63.1%) reduction in blood meal weight in 35 d old females due to wMelPop infection, while we observed a 29.5% (95% confidence interval: 12.1–46.7%) reduction relative to the two uninfected populations. Aged wMelPop females had reduced feeding success (65% feeding compared to 91% for uninfected populations) and also displayed a bendy/shaky proboscis phenotype as characterized previously [31, 32]. However, these phenotypes occurred at a significantly lower frequency than previously reported [32] (proportion feeding: two proportions Z-test: Z = 3.431, P < 0.001, bendy/shaky proboscis: Z = 4.288, P < 0.001). Weaker effects relative to previous studies may result from methodological differences, human experimenter effects, effects of laboratory rearing, attenuation or incomplete maternal transmission. Wolbachia screening of 35 d old females showed that 6 females (20%) had a weakly positive (Cp > 23) infection which may indicate maternal transmission leakage.
Loss of Wolbachia during colony maintenance
Infection recovery
Due to an apparent loss of Wolbachia from our wMelPop-PGYP colony, we isolated females to restore the wMelPop infection in the population. Of the females that produced viable offspring, 20 were negative, 17 were strongly positive (median Cp 16.3, range 3.33) and 41 were weakly positive (median Cp 31.38, range 8.37). These results point to a polymorphic colony despite the colony having been scored as 100% infected prior to this time (all Cp values ≤ 23). All offspring tested from strongly positive females were strongly positive (females: median Cp 19.19, range 0.61), males: median Cp 19.12, range 5.83). No offspring from the weakly positive or negative females were infected (n = 30 each), thus females scored as weakly positive were unable to transmit wMelPop to the next generation.
Maternal transmission
We tested ten offspring from ten wMelPop-infected females and found that a single female produced two uninfected offspring, with an overall maternal transmission fidelity of 98% (binomial confidence interval: 92.96–99.76%). These results are consistent with previous studies that indicate a low level of maternal transmission failure [14, 18].
Nuclear background evolution
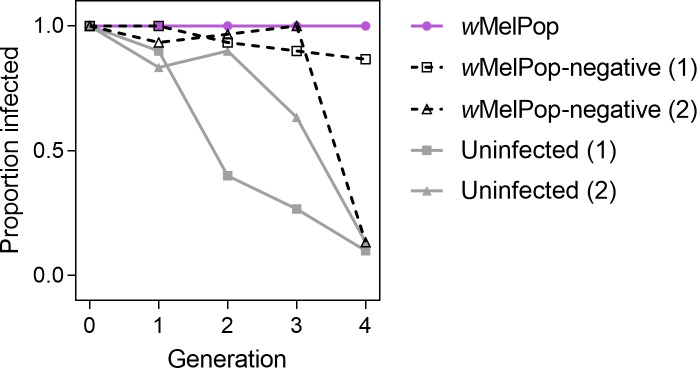
We crossed wMelPop-infected females to wMelPop-negative or uninfected males for four generations to see if the loss of wMelPop infection was associated with changes in the nuclear background. The wMelPop infection frequency declined in all four populations (Fig 4). In contrast, when wMelPop-infected females were crossed to wMelPop-infected males the infection frequency remained at 100%, likely due to cytoplasmic incompatibility. Loss of wMelPop infection does not appear to be strongly related to nuclear background since the infection declined in both sets of crosses. Rather, declines in infection frequency are likely due to a combination of incomplete maternal transmission and fitness costs.
Fig 4. Loss of wMelPop infection in Aedes aegypti in the absence of cytoplasmic incompatibility.
wMelPop-infected females were crossed to wMelPop-negative (gray), uninfected (gray) or wMelPop (purple) males each generation for four generations. Infection frequencies were determined for 30 individuals per population, per generation.
wMelPop-infected males induced complete cytoplasmic incompatibility with uninfected females (no eggs hatching, Table 1), suggesting that this phenotype has remained stable since transinfection over 10 years ago [10]. Compatible crosses exhibited high hatch proportions, showing that the wMelPop infection is self-compatible. wMelPop-infected males also induced complete cytoplasmic incompatibility with wMelPop-negative females, indicating that this population has not evolved resistance to cytoplasmic incompatibility.
Table 1. Egg hatch proportions resulting from crosses between wMelPop, wMelPop-negative and uninfected Aedes aegypti populations.
| Male | ||||
|---|---|---|---|---|
| wMelPop | Uninfected | wMelPop-negative | ||
| Female | wMelPop | 0.933 (0.903, 0.964) | 0.988 (0.970, 1) | Not tested |
| Uninfected | 0 (0, 0) | 0.936 (0.893, 0.969) | Not tested | |
| wMelPop-negative | 0 (0, 0) | Not tested | 0.980 (0.972, 0.984) | |
Data are medians followed by 95% confidence intervals (lower, upper).
Wolbachia mating transmission
We crossed Wolbachia-infected males with uninfected females to test the potential for Wolbachia to be transferred through mating. In control crosses, Wolbachia-infected females had a 100% infection frequency and high densities (Fig 5), while Wolbachia were not detected when uninfected females were crossed to uninfected males. We detected Wolbachia in uninfected females that were crossed to wMelPop- (Fig 5A) and wMel-infected (Fig 5B) males for up to 23 d post-mating, with the proportion scored as positive decreasing with time after mating. Wolbachia densities in uninfected females were distinctly lower than in females with a maternally-inherited Wolbachia infection. In an additional cross, we specifically tested for transfer of seminal fluid by crossing uninfected females to wMelPop-infected males and testing the heads and abdomens of females separately. All heads were negative for Wolbachia, while 19/20 abdomens were positive with a median Cp of 28.78 (range 4.44). Uninfected females can therefore be incorrectly scored as infected if they have mated with a wMelPop or wMel-infected male.
Fig 5. Detection of Wolbachia in uninfected Aedes aegypti females via seminal fluid from Wolbachia-infected males.
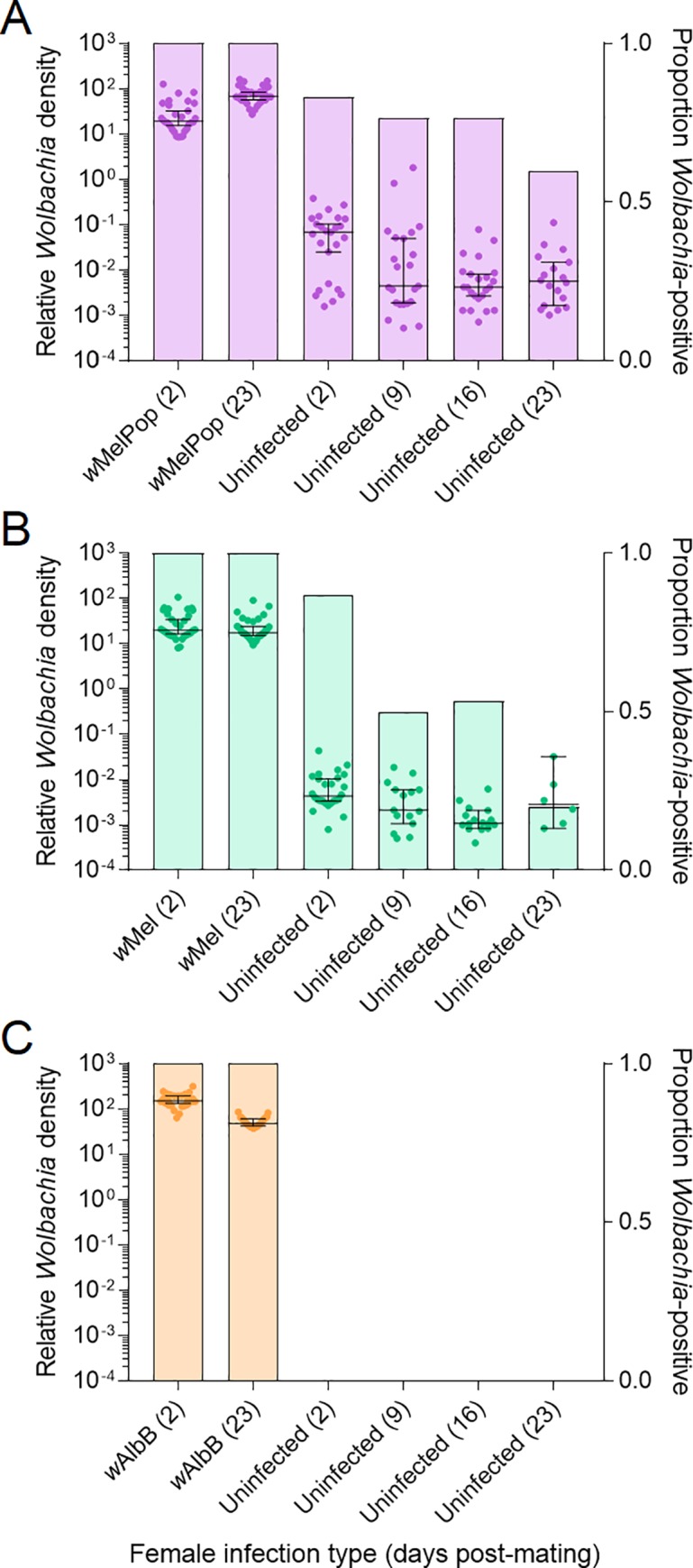
Males were infected with the (A) wMelPop, (B) wMel or (C) wAlbB Wolbachia strains. Dots show Wolbachia densities of individual females (left y-axis), while horizontal lines and error bars are medians and 95% confidence intervals respectively. Shaded bars show proportions of females (n = 30) from each group that tested positive for Wolbachia (right y-axis).
In contrast to the other two infections, we did not detect Wolbachia in any uninfected females that were crossed to wAlbB-infected males (Fig 4C). We detected no Wolbachia in a second independent experiment, indicating that this Wolbachia strain is not transferred through mating. Furthermore, we found no evidence for Wolbachia transfer through mating in two Drosophila species, even for the wMel infection in D. melanogaster (S1 Appendix).
Relative fitness during laboratory maintenance
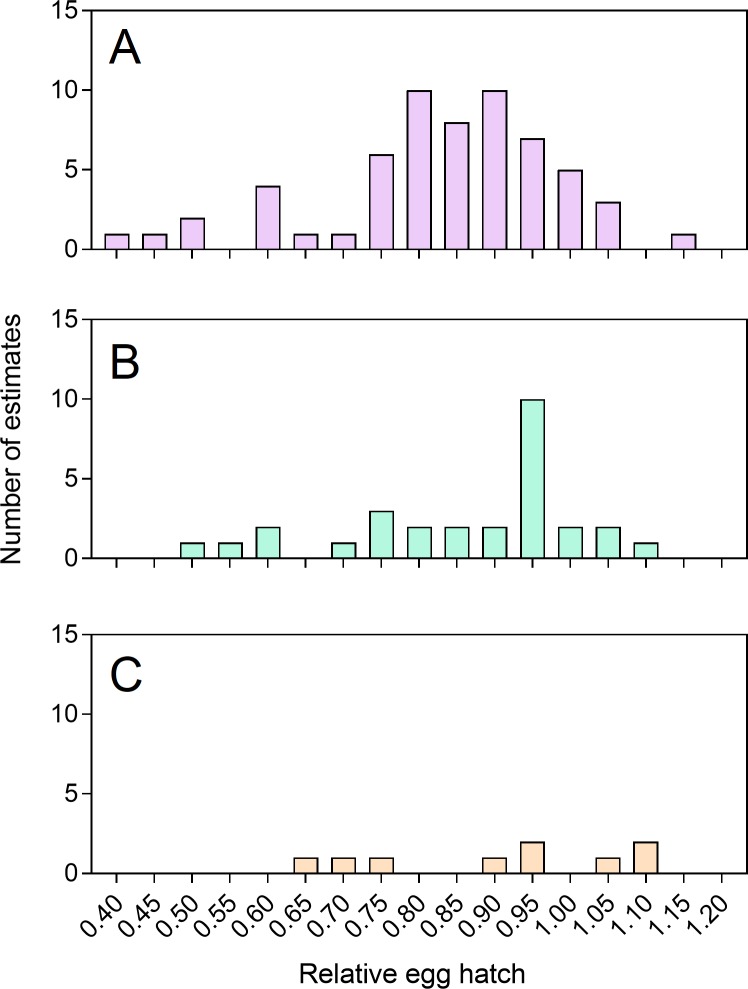
We monitored egg hatch proportions of our Wolbachia-infected laboratory colonies across multiple generations to assess variance in fitness costs. wMelPop-infected (Sign test: Z = 6.197, P < 0.001) and wMel-infected (Z = 3.900, P < 0.001) colonies tended to have lower egg hatch proportions relative to uninfected colonies (Fig 6). wAlbB-infected colonies had similar hatch proportions to uninfected colonies overall (Z = 1.000, P = 0.317), though the sample size for this infection was much lower. For the wMelPop infection, relative egg hatch proportions were as low as 40% which may contribute to the loss of infection from colonies. Because data were collected over nearly a 6-year period, we could test for changes in egg hatch across time. For wMelPop, where the most data were available, there was no temporal difference in relative egg hatch (General linear model: F17,42 = 1.727, P = 0.076), suggesting that there has been no major change in relative fitness during this period. These results are consistent with a compilation of fitness estimates from previous studies showing that wMelPop consistently induces fertility costs while effects of other Wolbachia infections are weaker (S2 Fig, [7]).
Fig 6.
Histograms of egg hatch proportions of (A) wMelPop, (B) wMel and (C) wAlbB colonies relative to uninfected colonies during routine laboratory maintenance. Each estimate was undertaken on a different laboratory generation or colony from at least 200 eggs.
Discussion
Here we provide data that suggests limited evolutionary attenuation of deleterious effects in wMelPop-PGYP cultures, either through changes in the host nuclear genome or the Wolbachia genome. This is despite an elapsed period of more than ten years or ~120 generations of rearing in the laboratory (and with an additional short period in the field). This contrasts sharply with the attenuation of wMelPop seen in D. simulans following its transfer from D. melanogaster, although the wMelPop-PGYP strain in Ae. aegypti differs genomically from the Drosophila strain, particularly for the Octomom region associated with Wolbachia virulence [25]. As in its native host, wMelPop reduced longevity when transferred to D. simulans [37], Ae. aegypti [10] and Aedes albopictus [38]. Other deleterious effects in D. simulans were also detected; however, many of these attenuated after around 20 generations, including effects on egg hatch [34]. Moreover, after around 200 generations, wMelPop-infected D. simulans lines no longer showed a decrease in longevity in some genetic backgrounds [22].
It is unclear why most deleterious effects in Ae. aegypti appear to have persisted. Although our laboratory maintenance schedule should reduce the potential for selection, fitness costs are apparent even under benign conditions (such as during the first gonotrophic cycle in the laboratory). Compared to studies performed over ten years ago, some deleterious effects of wMelPop appear weaker, particularly blood feeding traits [31, 32] and male longevity [10, 14]. Although this may indicate attenuation, direct comparisons with previous studies are difficult due to methodological differences and potential confounding effects of inbreeding, drift and laboratory adaptation that can occur during colony maintenance [39]. Our observations could in part be explained by the fact that the wMelPop line tested here experienced past selection for attenuation. wMelPop went through substantial genetic adaptation to the mosquito cell line [36] with reduced virulence, but then experienced no genomic changes after four years within Ae. aegypti mosquitoes [11]. Our line also experienced a brief period in the field, which is likely to have imposed strong selection for attenuation. Selection experiments for increased quiescent egg viability in wMelPop-infected Ae. aegypti found evidence for attenuation, however this involved nuclear background evolution rather than Wolbachia evolution [16].
Because Wolbachia are maternally inherited, selection acts to increase maternal transmission fidelity and not the ability of males to induce cytoplasmic incompatibility [20]. Novel Wolbachia infections tend to induce much stronger cytoplasmic incompatibility than natural infections, suggesting that these effects can attenuate [40]. Furthermore, theory predicts that resistance to cytoplasmic incompatibility may evolve if maternal transmission is incomplete [41]. Although hosts may evolve resistance to the effects of Wolbachia on reproduction, such as male killing in Hypolimnas bolina [24] and cytoplasmic incompatibility in D. melanogaster [9], effects can also remain stable despite intense selection pressure [42, 43]. Over ten years after wMelPop was introduced to Ae. aegypti, the infection still induces complete cytoplasmic incompatibility. We therefore find no evidence to suggest that cytoplasmic incompatibility has attenuated or that Ae. aegypti has evolved to suppress cytoplasmic incompatibility. In crossing experiments, the wMelPop infection was lost from colonies regardless of whether infected females were crossed to uninfected males or males that had lost the wMelPop infection, suggesting that loss of wMelPop was not due to paternal factors that affect Wolbachia maternal transmission.
The persistence of deleterious fitness effects may contribute to the occasional loss of the wMelPop-PGYP infection from Ae. aegypti laboratory populations. Following Hoffmann et al. [44] the change in frequency of the infection (pf) in a population is given by
where u is the fraction of uninfected progeny produced by infected females, sf is the fecundity deficit (representing a combination of the number of eggs laid and that hatch) and sh is the incompatibility between infected and uninfected strains. In the presence of strong maternal transmission (u = 0) the unstable point for invasion versus loss of the infection is given by the ratio of sf/sh [9]. This means that if incompatibility is very strong (sh near 1) as is the case with wMelPop, it is normally very unlikely for a deleterious fitness effect to result in a loss of infection in a population.
However, we have observed a low level of maternal transmission failure in our wMelPop colony of 2%, with an upper estimate of 7%. When coupled with large deleterious effects, this level of leakage may be sufficient to trigger a loss of the wMelPop infection. Based on the variance in egg hatch proportions and costs to fecundity, we estimate that the relative fitness of wMelPop-infected mosquitoes compared to uninfected mosquitoes may fall to as low as 28% during routine maintenance, or even lower if adults are aged or eggs are stored before hatching. This will produce a situation where p(t+1) is less than p, and the infection will continue to drop out unless relative fitness is increased.
Our detection of Wolbachia at low densities in uninfected females that had mated with Wolbachia-infected males was unexpected, given that Wolbachia are absent from mature sperm in other insects [45–47]. However, a recent report in Hylyphantes graminicola spiders demonstrated sexual transmission of Wolbachia, both from males to females and from females to males [48]. Our results have implications for Wolbachia monitoring in laboratory and field populations because uninfected females might be incorrectly scored as infected. Assuming random mating, the incidence of false positive detections is equivalent to the frequency of infected individuals in the population. If a loss in infection occurs, it may not be detected immediately when an infection is monitored only by screening adult females. Although false positive individuals in the laboratory can be identified with quantitative assays, determining infection status based on a threshold Wolbachia density may be unreliable under field conditions because environmental conditions can affect Wolbachia density [18, 19]. We therefore advise that during laboratory maintenance and field monitoring, infection frequencies are determined by screening immature stages, unmated adults or dissected heads. This issue appears to be specific to certain Wolbachia strains given that we found no evidence for the transmission through mating of wAlbB.
Our findings have implications for the long-term effectiveness of Wolbachia releases and for the maintenance of wMelPop stocks in the laboratory. The apparent relative stability of deleterious effects shown here suggests that wMelPop-PGYP can suppress populations for a long time once established. However, field trials with this infection suggest that long-term persistence in natural populations is unlikely [15]. wMelPop-PGYP is difficult to maintain even under benign laboratory conditions due to a combination of incomplete maternal transmission, deleterious effects due to infection, and monitoring issues (false positive detections due to transmission of Wolbachia through mating), but a strict rearing schedule and regular Wolbachia screening will help to ensure its persistence in a colony.
Due to its fitness costs, wMelPop may be suitable for temporary suppression or elimination of populations rather than population replacement which is now taking place in field populations with the wMel and wAlbB strains [1, 49]. Suppression through the release of wMelPop was proposed as a way of tackling mosquito incursions in isolated areas [17]; as long as such areas are sufficiently isolated to reduce the likelihood of a subsequent invasion by uninfected mosquitoes, this approach could suppress or eliminate mosquito populations without the extensive use of pesticides. Establishing wMelPop in large semi-field cages and then imposing a dry period that required the persistence of quiescent eggs led to population elimination [16]. Due to cytoplasmic incompatibility and the deleterious effects of infection, releases of wMelPop-infected males and females into the field could result in population suppression once high infection frequencies are reached. This approach to suppression does not require sex separation unlike strategies that rely on cytoplasmic incompatibility [50] and could be effective even if the infection does not persist in the long-term. Although research has shifted away from this deleterious Wolbachia infection, wMelPop may still prove to be useful when seasonal population suppression is desirable.
Supporting information
(PDF)
Relative Wolbachia density of wMelPop-infected females with increasing (A) adult age or (B) egg storage duration. Each dot represents the Wolbachia density of a single female, while solid lines join the median densities for each time point.
(TIF)
Relative fitness is expressed in terms of effect sizes (Hedges’ g), where values below zero indicate a fitness cost. Each dot represents a single fitness estimate. Box plots show medians and interquartile ranges, with error bars representing minimum and maximum values.
(TIF)
(DOCX)
Acknowledgments
We thank Chengjun Li, Tiana Rey and Véronique Paris for assistance with experimental work. We also thank Ewa Chrostek and two anonymous reviewers for their constructive feedback on the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Health and Medical Research Council (1132412 and 1118640 to AAH, www.nhmrc.gov.au) and the Australian Research Council (DP190101877 to AAH, www.arc.gov.au). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–7. http://www.nature.com/nature/journal/v476/n7361/abs/nature10356.html#supplementary-information. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 2.O'Neill S, Ryan P, Turley A, Wilson G, Retzki K, Iturbe-Ormaetxe I, et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2018;2(36). 10.12688/gatesopenres.12844.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, Zhang D, Li Y, Yang C, Wu Y, Liang X, et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019;572:56–61. 10.1038/s41586-019-1407-9 [DOI] [PubMed] [Google Scholar]
- 4.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14(1):19 10.1371/journal.ppat.1006815 WOS:000424003200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–78. 10.1016/j.cell.2009.11.042 ISI:000273048700015. [DOI] [PubMed] [Google Scholar]
- 6.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476(7361):450–3. http://www.nature.com/nature/journal/v476/n7361/abs/nature10355.html#supplementary-information. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 7.Ross PA, Turelli M, Hoffmann AA. Evolutionary ecology of Wolbachia releases for disease control. Annu Rev Genet. 2019;53:1–11.24. 10.1146/annurev-genet-112618-043708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook PE, McMeniman CJ, O'Neill SL. Modifying insect population age structure to control vector-borne disease. In: Aksoy S, editor. Transgenesis and the Management of Vector-Borne Disease. Advances in Experimental Medicine and Biology. 6272008. p. 126–40. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann AA, Turelli M. Cytoplasmic incompatibility in insects. In: O'Neill S, Hoffmann AA, Werren JH, editors. Influential Passengers: Microorganisms and Invertebrate Reproduction Oxford: Oxford University Press; 1997. [Google Scholar]
- 10.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323(5910):141–4. 10.1126/science.1165326 ISI:000262104100061. [DOI] [PubMed] [Google Scholar]
- 11.Woolfit M, Iturbe-Ormaetxe I, Brownlie JC, Walker T, Riegler M, Seleznev A, et al. Genomic evolution of the pathogenic Wolbachia strain, wMelPop. Genome Biol Evol. 2013;5(11):2189–2204. 10.1093/gbe/evt169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min KT, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A. 1997;94(20):10792–6. 10.1073/pnas.94.20.10792 WOS:A1997XY99800056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMeniman CJ, O'Neill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. Plos Neglect Trop Dis. 2010;4(7):e748 10.1371/journal.pntd.0000748 WOS:000280412300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeap HL, Mee P, Walker T, Weeks AR, O'Neill SL, Johnson P, et al. Dynamics of the "popcorn" Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 2011;187(2):583–95. 10.1534/genetics.110.122390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TH, Le Nguyen H, Nguyen TY, Vu SN, Tran ND, Le TN, et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015;8:14 10.1186/s13071-014-0609-0 WOS:000363511600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. Plos Neglect Trop Dis. 2015;9(7):17 10.1371/journal.pntd.0003930 WOS:000359079700036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rašić G, Endersby EM, Williams C, Hoffmann AA. Using Wolbachia-based releases for suppression of Aedes mosquitoes: insights from genetic data and population simulations. Ecol Appl. 2014;24:1226–34. 10.1890/13-1305.1 [DOI] [PubMed] [Google Scholar]
- 18.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2017;13(1):e1006006 10.1371/journal.ppat.1006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endersby-Harshman NM, Axford JK, Hoffmann AA. Environmental concentrations of antibiotics may diminish Wolbachia infections in Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2019;56(4):1078–86. 10.1093/jme/tjz023 [DOI] [PubMed] [Google Scholar]
- 20.Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48(5):1500–13. 10.1111/j.1558-5646.1994.tb02192.x WOS:A1994QQ65900008. [DOI] [PubMed] [Google Scholar]
- 21.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007;5(5):997–1005. 10.1371/journal.pbio.0050114 WOS:000246716700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrington LB, Hoffmann AA, Weeks AR. Monitoring long-term evolutionary changes following Wolbachia introduction into a novel host: the Wolbachia popcorn infection in Drosophila simulans. Proc R Soc B-Biol Sci. 2010;277(1690):2059–68. 10.1098/rspb.2010.0166 WOS:000278056400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrington LB, Leslie J, Weeks AR, Hoffmann AA. The popcorn Wolbachia infection of Drosophila melanogaster: can selection alter Wolbachia longevity effects? Evolution. 2009;63(10):2648–57. 10.1111/j.1558-5646.2009.00745.x CABI:20093310226. [DOI] [PubMed] [Google Scholar]
- 24.Hornett EA, Charlat S, Duplouy AMR, Davies N, Roderick GK, Wedell N, et al. Evolution of male-killer suppression in a natural population. PLoS Biol. 2006;4(9):1643–8. 10.1371/journal.pbio.0040283 WOS:000240740900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chrostek E, Teixeira L. Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol. 2015;13(2):e1002065 10.1371/journal.pbio.1002065 MEDLINE:25668031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi ZY, Khoo CCH, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310(5746):326–8. 10.1126/science.1117607 WOS:000232670100056. [DOI] [PubMed] [Google Scholar]
- 27.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA. Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg. 2016;94(3):507–16. 10.4269/ajtmh.15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross PA, Axford JK, Richardson KM, Endersby-Harshman NM, Hoffmann AA. Maintaining Aedes aegypti mosquitoes infected with Wolbachia. J Vis Exp. 2017;(126):e56124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SF, White VL, Wee70(8ks AR, Hoffmann AA, Endersby NM. High-throughput PCR Assays To monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol. 2012;78(13):4740–3. 10.1128/AEM.00069-12 WOS:000305376600024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naksathit AT, Scott TW. Effect of female size on fecundity and survivorship of Aedes aegypti fed only human blood versus human blood plus sugar. J Am Mosq Control Assoc. 1998;14(2):148–52. [PubMed] [Google Scholar]
- 31.Turley AP, Moreira LA, O'Neill SL, McGraw EA. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. Plos Neglect Trop Dis. 2009;3(9):e516 e516 10.1371/journal.pntd.0000516 ISI:000270818900012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira LA, Saig E, Turley AP, Ribeiro JM, O'Neill SL, McGraw EA. Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl Trop Dis. 2009;3(12):e568 10.1371/journal.pntd.0000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross PA, Lau M-J, Hoffmann AA. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS One. 2019;14(11):e0224268 10.1371/journal.pone.0224268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGraw EA, Merritt DJ, Droller JN, O'Neill SL. Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A. 2002;99(5):2918–23. 10.1073/pnas.052466499 WOS:000174284600060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, et al. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: A phenotypic and phylogenomic analysis. PLoS Genet. 2013;9(12):22 10.1371/journal.pgen.1003896 WOS:000330533300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMeniman CJ, Lane AM, Fong AWC, Voronin DA, Iturbe-Ormaetxe I, Yamada R, et al. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol. 2008;74(22):6963–6969. 10.1128/AEM.01038-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGraw EA, Merritt DJ, Droller JN, O'Neill SL. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc R Soc B. 2001;268(1485):2565–70. 10.1098/rspb.2001.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suh E, Mercer DR, Fu Y, Dobson SL. Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl Environ Microbiol. 2009;75(24):7783–8. 10.1128/AEM.01331-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross PA, Endersby-Harshman NM, Hoffmann AA. A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evol Appl. 2019;12(3):572–86. 10.1111/eva.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann AA, Ross PA, Rašić G. Wolbachia strains for disease control: ecological and evolutionary considerations. Evol Appl. 2015;8(8):751–68. 10.1111/eva.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bull JJ, Turelli M. Wolbachia versus dengue: evolutionary forecasts. Evol Med Public Health. 2013;1:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrington LB, Lipkowitz JR, Hoffmann AA, Turelli M. A re-examination of Wolbachia-Induced cytoplasmic incompatibility in California Drosophila simulans. Plos One. 2011;6(7):12 10.1371/journal.pone.0022565 WOS:000293172900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaenike J, Dyer KA. No resistance to male-killing Wolbachia after thousands of years of infection. J Evol Biol. 2008;21(6):1570–7. 10.1111/j.1420-9101.2008.01607.x [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann AA, Turelli M, Harshman LC. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics. 1990;126:933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen JH, Barr AR. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature. 1971;232(5313):657–8. 10.1038/232657a0 [DOI] [PubMed] [Google Scholar]
- 46.Binnington KC, Hoffmann AA. Wolbachia like organisms and cytoplasmic incompatability in Drosophila simulans. J Invertebr Pathol. 1989;54(3):344–52. 10.1016/0022-2011(89)90118-3 WOS:A1989AW90600007. [DOI] [Google Scholar]
- 47.Clark ME, Bailey-Jourdain C, Ferree PM, England SJ, Sullivan W, Windsor DM, et al. Wolbachia modification of sperm does not always require residence within developing sperm. Heredity. 2008;101(5):420–8. 10.1038/hdy.2008.71 [DOI] [PubMed] [Google Scholar]
- 48.Su Q, Hu G, Yun Y, Peng Y. Horizontal transmission of Wolbachia in Hylyphantes graminicola is more likely via intraspecies than interspecies transfer. Symbiosis. 2019;79(2):123–128. [Google Scholar]
- 49.Nazni W, Hoffmann A, Afizah AN, Cheong Y, Mancini M, Golding N, et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol. 2019;29:1–8. 10.1016/j.cub.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mains JW, Kelly PH, Dobson KL, Petrie WD, Dobson SL. Localized control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via inundative releases of Wolbachia-infected male mosquitoes. J Med Entomol. 2019;56(5):1296–1303. 10.1093/jme/tjz051 [DOI] [PubMed] [Google Scholar]