Abstract
A seven‐membered N,N′‐heterocyclic potassium alumanyl nucleophile is introduced and utilised in the metathetical synthesis of Mg−Al and Ca−Al bonded derivatives. Both species have been characterised by experimental and theoretical means, allowing a rationalisation of the greater reactivity of the heavier group 2 species implied by an initial assay of their reactivity.
Keywords: alumanyl, calcium, density functional theory, magnesium, potassium
AlCan Wrap: The Reaction of a seven‐membered cyclic alumanyl anion with a β‐diketiminato calcium tetraphenylborate provides facile access to a stable, but highly reactive, calcium alumanyl.
Alkaline earth (Ae=Be, Mg, Ca, Sr, Ba) compounds continue to provide unprecedented observations for stoichiometric and catalytic bond activation.1 Some of the most notable advances have been provided by kinetically‐stabilized hydride derivatives.2 The β‐diketiminato calcium complex, [(DippBDI)CaH]2 (1, DippBDI=HC{(Me)CNDipp}2; Dipp=2,6‐i‐Pr2C6H3), for example, reacts with alkenes to provide n‐alkyl derivatives, which are sufficiently reactive to effect even the nucleophilic alkylation of benzene.3 These observations also impel the exploration of a broader range of Ae−X bonded compounds. A topical case in point is provided by reports of the reactivity of β‐diketiminato magnesium and calcium complexes with Roesky's similarly coordinated aluminium(I) species, [(DippBDI)Al] (2).4 Compound 2 reacts with [(DippBDI)MgMe], [(DippBDI)MgH]2 or [(TMEDA)MgI2] to provide the Mg‐Al bonded oxidative addition products (e.g., 3 and 4, Scheme 1).5, 6, 7 In contrast, Harder has observed that addition of 2 to (DippBDI)Ca‐based reagents results in arene activation. While reaction with the ionic calcium complex [(DippBDI)Ca]+[B(C6F5)4]− provided two electron reduction of benzene,8 addition of 1 to benzene or toluene solutions of 2 resulted in arene C−H activation and the production of [(DippBDI)Al(H)Ar] complexes (Scheme 1).6 Density functional theory (DFT) calculations of this latter reactivity implicated an initial Ca−Al bonded oxidative addition product, [(DippBDI)Al(H)Ca(DippBDI)] (5), which, although otherwise analogous to compound 4, is unobservable due to its rapid nucleophilic activation of the arene solvent.
Scheme 1.
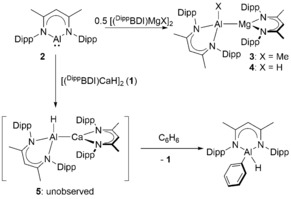
Reactivity of compound 2 with (DippBDI)Ae reagents.
While Scheme 1 highlights how the reactivity of a neutral AlI nucleophile may be augmented by the presence of a calcium center, other recent advances have illuminated a further pathway to the formation of group 2 alumanyl derivatives. Building on the earlier development of boryl and gallyl nucleophiles,9 the groups of Aldridge, Goicoechea and Coles have extended the range of N‐heterocyclic group 13‐centered anions to include the potassium alumanyl species, 6 and 7 (Figure 1).10, 11, 12, 13, 14 The formally monovalent aluminum centers in both compounds can behave as potent reducing agents or as aluminum‐centered nucleophiles. Of most relevance to these studies, the potassium reagent 6 can activate the C−H or C−C bonds of benzene, and reacts with [(MesBDI)MgI(OEt2)] (MesBDI=HC{(Me)CN‐2,4,6‐Me3C6H2}2) to yield a further Mg−Al bonded complex, 8 (Figure 1).10, 12 In contrast to this recent development of magnesium alumanyl chemistry, the gallyl derivatives of Jones and co‐workers, trans‐[Ca{Ga[(DippNCR)2]}2(THF)4] (R=H, Me) and trans‐[Ca{Ga(DippNCH)}2(TMEDA)2],15 provide the only examples of isolable compounds containing calcium‐group 13 σ‐bonds and no calcium alumanyls have been considered beyond the implied intermediacy of species 5.6 In this contribution, therefore, and as an extension to our recent interest in the reactivity of alkaline earth boryls,16 we describe a seven‐membered cyclic alumanyl anion and its use in the synthesis of a stable, yet highly reactive, calcium derivative.
Figure 1.
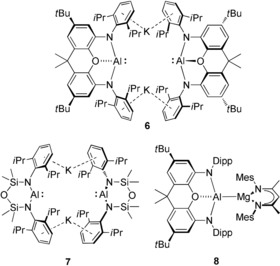
The potassium alumanyl derivatives, 6 and 7, and the Al−Mg bonded species, 8.
Following an analogous procedure to that employed in the synthesis of compound 7,13 addition of the pre‐ligand {SiNDipp}H2 (9, {SiNDipp}={CH2SiMe2N(Dipp)}2) to trimethylaluminum provided [{SiNDipp}AlMe] (10) (Scheme 2). The slow conversion of 10 to [{SiNDipp}AlI] (11) was achieved by reaction with I2, providing >95 % conversion after 5 days at 100 °C. The solid‐state structure of 11 was investigated by single crystal X‐ray diffraction, confirming the formation of a 7‐membered metallacycle via N,N′‐chelation to a planar three‐coordinate aluminum center [Al‐N, 1.782(1) and 1.790(1); Al‐I, 2.4690(5) Å] (Supporting Information, Figure S10).20
Scheme 2.
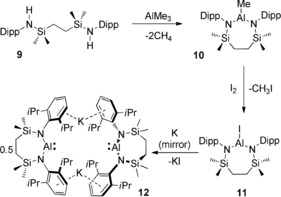
Synthesis of compounds 10–12.
Stirring a solution of 11 in hexane over a potassium mirror resulted in a gradual change from colorless to yellow and the deposition of a grey solid. Filtration of the reaction mixture after 3 days at room temperature and removal of the volatile components gave a yellow powder, which crystallized from Et2O to provide crystals of [Al{SiNDipp}K]2 (12). The resultant 1H and 13C{1H} NMR spectra were consistent with a symmetrical N,N′‐chelated disposition of the diamide ligand about aluminum, a deduction confirmed by a subsequent single crystal X‐ray diffraction analysis (Figure 2). Like both the previously reported derivatives, 6 and 7,10, 13 the asymmetric unit of compound 12 comprises two “Al{SiNDipp}K” entities linked through flanking η6‐K⋅⋅⋅Ar interactions [range 2.945(1)–3.026(1) Å]. As expected for a lower oxidation state aluminum species, the Al−N bond distances [range 1.887(2)–1.892(2) Å] are significantly longer than those of 11. Despite the larger bite angles imposed by chelation of the {SiNDipp} ligand [12: N1−Al1−N2 108.84(9), N3−Al2−N4 108.77(9)° versus 7: 103.89(8), 105.05(8)°], the Al⋅⋅⋅Al distance [5.721(1) Å] and Al⋅⋅⋅K distances [range 3.584(1)–3.625(1) Å] are also comparable to the analogous measurements within compound 7 [Al⋅⋅⋅Al 5.673(1); Al⋅⋅⋅K 3.5916(8) Å].
Figure 2.
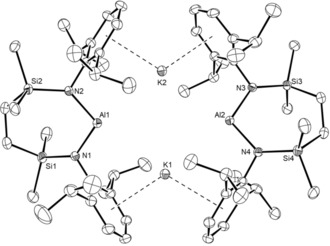
ORTEP representation of compound 12 (30 % probability ellipsoids). Hydrogen atoms and occluded molecule of diethyl ether solvent are omitted for clarity. Selected bond lengths [Å] and angles [°]: Al1−N1 1.887(2), Al1−N2 1.8889(19), Al2−N3 1.890(2), Al2−N4 1.892(2), Si1−N1 1.729(2), Si2−N2 1.7333(19), Si3−N3 1.731(2), Si4−N4 1.731(2); N1−Al1−N2 108.84(9), N3−Al2−N4 108.77(9).
Although the implications of any metrical adjustments on the electronic structure of compound 12 will be addressed elsewhere, the similarity of its gross features to both compounds 6 and 7 advocates that 12 should display comparable reactivity. Mindful of Evans’ use of tetraphenylborate derivatives of similarly electropositive rare earth elements as hydrocarbon‐soluble reagents in further synthesis,17 we prepared [(DippBDI)AeBPh4] (13, Ae=Mg; 14, Ae=Ca) by the respective reactions of [(DippBDI)Mgn‐Bu] and [(DippBDI)CaN(SiMe3)2] with [HNEt3][BPh4]. Both compounds 13 and 14 were isolated in high yields and their solid‐state structures were confirmed by X‐ray diffraction analysis (Figures S16 and S20) as mononuclear species in which the tetraphenylborate anions interact with the Ae centers via polyhapto Ae⋅⋅μ−Ph−B interactions.
Addition of toluene solutions of compound 12 to either compound 13 or 14 resulted in a colorless suspension of KBPh4 and, after crystallization from n‐hexane and benzene, respectively, the formation of the magnesium and calcium alumanyl species, [{SiNDipp}Al‐Mg(DippBDI)] (15) and [{SiNDipp}Al‐Ca(DippBDI)] (16), as colorless and yellow crystals (Scheme 3). The solid‐state structures of both compounds 15 and 16 were determined by X‐ray diffraction analysis, which confirmed them as magnesium and calcium derivatives comprising aluminum to alkaline earth interactions.
Scheme 3.
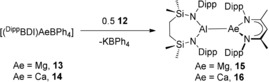
Synthesis of the magnesium and calcium alumanyl compounds, 15 and 16.
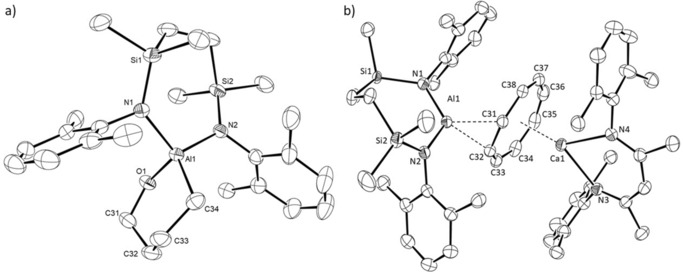
Both the Mg1 and Al1 centers of compound 15 are unambiguously three‐coordinate (closest Al−C separation 4.965 Å) and the two N‐donor ligand systems are almost orthogonal (Figure 3 a), such that the dihedral angle subtended by the N1−Al1−N2 and N3−Mg1−N4 mean planes is 84.1°. Although the Mg1‐Al1 bond in 15 [2.7980(6) Å] is longer than the comparable length in compound 8 [2.696(1) Å],10 it is commensurate with the corresponding distances observed in compounds 3 [2.7687(8) Å] and 4 [2.7687(5) Å],5, 6 which also comprise magnesium coordinated by the DippBDI ligand, and in Jones’ derivative, [(TMEDA)(I)Mg‐Al(I)(DippBDI)] [2.727(2) Å].7
Figure 3.
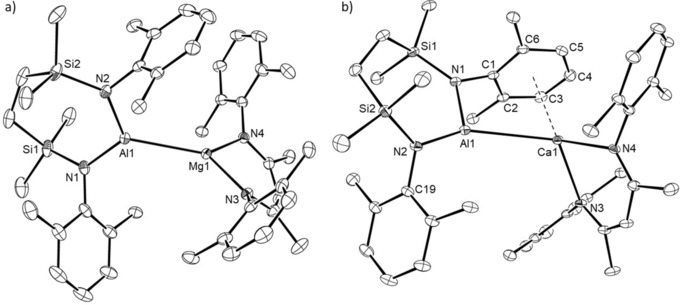
ORTEP representations of a) compound 15 and b) compound 16 (30 % probability ellipsoids). Hydrogen atoms, iso‐propyl carbon atoms and disordered molecules of solvent are omitted for clarity. Selected bond lengths [Å] and angles [°]: (15) Al1−Mg1 2.7980(6), Al1−N1 1.8482(13), Al1−N2 1.8402(13), Mg1−N3 2.0528(12), Mg1−N4 2.0721(12); N1−Al1−Mg1 125.94(5), N2−Al1−Mg1 121.91(4), N2−Al1−N1 111.87(6), N3−Mg1−Al1 132.59(4), N3−Mg1−N4 93.77(5), N4−Mg1−Al1 132.94(4); (16) Ca1−Al1 3.1229(5), Al1−N1 1.8973(13), Al1−N2 1.8544(14), Ca1−N3 2.3538(12), Ca1−N4 2.3682(13), Ca1−C1 3.1735(15), Ca1−C2 3.1333(16), Ca1−C3 3.0213(17), Ca1−C4 2.9790(17), Ca1−C5 3.0884(16), Ca1−C6 3.2215(15); N2−Al1−N1 111.08(6), N3−Ca1−N4 81.10(4), N1−Al1−Ca1 98.14(4), N2−Al1−Ca1 150.30(5), N3−Ca1−Al1 118.99(3), N4−Ca1−Al1 123.45(3).
Like 15, compound 16 contains a direct alkaline earth to aluminum bond (Figure 3 b). Although the Al−Ca bond length [3.1664(4) Å] is 6.6 % longer than the sum of covalent radii of the metal centers, this distance is closely comparable with that calculated for the analogous intermetallic separation [3.120 Å] in the unobservable intermediate 5 (Scheme 1).
The coordination sphere of Ca1 is augmented by a pronounced η6‐interaction with the C1‐C6‐containing Dipp substituent [Ca1−C range; 2.9790(17)–3.2215(15) Å] of the aluminum‐coordinated diamide ligand. The consequent asymmetry in the relative orientation of the {SiNDipp}Al and (DippBDI)Ca units is particularly reflected by the N1−Al−Ca1 [98.14(4)°] and N2−Al1−Ca1 [150.30(5)°] angles.
Insight into the contrasting structures of 15 and 16 was provided by DFT calculations.18 Both structures optimized to geometries close to those in the solid state, albeit with slightly overestimated metal‐metal bond lengths (15, Mg−Al 2.85; 16, Ca‐Al 3.25 Å). Inspection of the localised Pipek–Mezey orbitals for compounds 15 and 16 (Figures 4 a,b) emphasizes the polarization intrinsic to both the Al−Mg and Al−Ca σ‐bonds. Although contributions from the aluminum centers dominate both orbitals, the charge distributions clearly reflect the greater ionic character of the Al−Ca interaction, an observation further underscored by the calculated NBO charges (15, Mg +1.45 Al +0.83; 16 Ca +1.65, Al +0.72 a.u.). Similarly, QTAIM plots of 15 and 16 identify bond critical points (BCPs) on both Ae−Al bond paths (Figures 4 c,d). The attributes of the BCP associated with the Al−Mg bond path [higher ρ(r), 0.028 a.u. (15) versus 0.020 (16) and more negative H(r), −0.008 a.u. (15) versus −0.002 a.u. (16)], however, are indicative of a marginally higher covalency between magnesium and aluminum than for the equivalent Al−Ca interaction. Although compound 16 features a further bond path between the Ca center and a Dipp substituent of the {SiNDipp}Al unit, this BCP is characterized by a very small ρ(r) and small positive H(r). Similarly, analysis of the relevant NBOs of 16 (HOMO‐8 and HOMO‐10, Figure S37) and visualization of the analogous non covalent interaction (NCI) index (Figure S39) indictate that this interaction is non‐covalent in origin and only weakly stabilizing of the structure as a whole.
Figure 4.
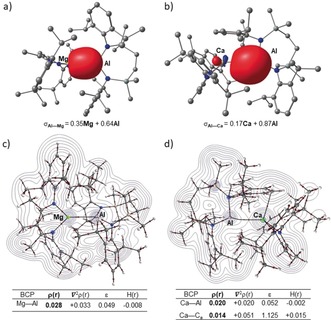
Localized Pipek–Mezey orbitals of a) 15 and b) 16 showing Al−Ca and Al−Mg σ‐bonding orbitals, respectively. QTAIM molecular graphs of c) 15 and d) 16. The electron density contours are computed in the {Mg/Al/Si} planes with bond critical points (BCPs) shown as small red spheres. BCP electron densities (ρ(r) in e Å−3), values of the Laplacian of the electron density (∇2 ρ(r) in e Å−5), ellipticities (ϵ) and total energy densities (H(r) in a.u.) are tabulated beneath the relevant figures.
Preliminary investigations indicate that compound 16 provides a significantly enhanced source of reactivity in comparison to its lighter analogue. While neither compound displays any observable reaction with benzene or toluene, and compound 15 is stable in ether solvents, addition of THF to a solution of compound 16 in methylcyclohexane resulted in the immediate decoloration of the yellow solution and the formation of a single new compound (17). The ionic complex 17 comprises a charge separated [(DippBDI)Ca(THF)3]+ cation and an aluminate anion (Figure 5 a), which may be considered as the formal product of oxidative addition of a THF C−O bond to the aluminum(I) center of the [{SiNDipp}Al]− anion.19 Similarly, compound 15 is unreactive toward 1,3,5,7‐cyclooctatetraene (COT), whilst addition of COT to compound 16 results in its two‐electron aromatization and production of the asymmetric and heterobimetallic inverse sandwich species, compound 18 (Figure 5 b). Although the chemistry of compounds 17, 18 and related derivatives will be discussed in detail elsewhere, these initial observations confirm that such heavier alkaline earth alumanyls, like group 2 organo‐derivatives, may provide a source of striking and unprecedented reactivity. We are continuing to elaborate these possibilities.
Figure 5.
ORTEP representations of (a) the aluminate component of compound 17 and (b) compound 18 (30 % probability ellipsoids). Hydrogen atoms and iso‐propyl carbon atoms, in both structures, and a disordered molecule of benzene solvent in 18 are omitted for clarity. Selected bond lengths [Å] and angles [°]: (17) Al1−O1 1.7637(16), Al1−N1 1.8997(18), Al1−N2 1.889(2), Al1−34 1.984(3); O1−Al1−N1 109.88(8), O1−Al1−N2 106.51(8), O1−Al1−C34 102.23(10), N1−Al1−C34 113.76(10), N2−Al1−N1 110.75(8), N2−Al1−C34 113.10(10); (18) Al1−N1 1.8085(17), Al1−N2 1.8200(16), Al1−C31 2.125(2), Al1−C32 2.123(2), Ca1−N3 2.3437(17), Ca1−N4 2.3831(18), Ca1−C31 2.7107(19), Ca1−C32 2.804(2), Ca1−C33 2.818(2), Ca1−C34 2.753(2), Ca1−35 2.693(2), Ca1−C36 2.695(2), Ca1−C37 2.706(2), Ca1−C38 2.698(2); N1−Al1−N2 119.27(8), N3−Ca1−N4 79.34(6).
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the Royal Commission for the Exhibition of 1851 for the provision of a Postdoctoral Fellowship (RJS) and the EPSRC (EP/R020752/1) for support of this research. This research made use of the Balena High Performance Computing (HPC) Service at the University of Bath.
R. J. Schwamm, M. P. Coles, M. S. Hill, M. F. Mahon, C. L. McMullin, N. A. Rajabi, A. S. S. Wilson, Angew. Chem. Int. Ed. 2020, 59, 3928.
Contributor Information
Prof. Michael S. Hill, Email: msh27@bath.ac.uk.
Dr. Claire L. McMullin, Email: cm2025@bath.ac.uk.
References
- 1.
- 1a. Hill M. S., Liptrot D. J., Weetman C., Chem. Soc. Rev. 2016, 45, 972–988; [DOI] [PubMed] [Google Scholar]
- 1b. Sarazin Y., Carpentier J. F., Chem. Rec. 2016, 16, 2482–2505; [DOI] [PubMed] [Google Scholar]
- 1c. Crimmin M. R., Hill M. S. in Alkaline-Earth Metal Compounds: Oddities and Applications, Vol. 45 (Ed.: S. Harder), Springer, Heibelberg, 2013, pp. 191–241; [Google Scholar]
- 1d. Harder S., Chem. Rev. 2010, 110, 3852–3876; [DOI] [PubMed] [Google Scholar]
- 1e. Barrett A. G. M., Crimmin M. R., Hill M. S., Procopiou P. A., Proc. R. Soc. London Ser. A 2010, 466, 927–963. [Google Scholar]
- 2.
- 2a. Mukherjee D., Okuda J., Angew. Chem. Int. Ed. 2018, 57, 1458–1473; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 1472–1488; [Google Scholar]
- 2b. Mukherjee D., Schuhknecht D., Okuda J., Angew. Chem. Int. Ed. 2018, 57, 9590–9602; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 9736–9749; [Google Scholar]
- 2c. Harder S., Chem. Commun. 2012, 48, 11165–11177. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Wilson A. S. S., Hill M. S., Mahon M. F., Dinoi C., Maron L., Science 2017, 358, 1168–1171; [DOI] [PubMed] [Google Scholar]
- 3b. Wilson A. S. S., Dinoi C., Hill M. S., Mahon M. F., Maron L., Angew. Chem. Int. Ed. 2018, 57, 15500–15504; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 15726–15730; [Google Scholar]
- 3c. Wilson A. S. S., Hill M. S., Mahon M. F., Organometallics 2019, 38, 351–360. [Google Scholar]
- 4. Cui C. M., Roesky H. W., Schmidt H. G., Noltemeyer M., Hao H. J., Cimpoesu F., Angew. Chem. Int. Ed. 2000, 39, 4274–4276; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 4444–4446. [Google Scholar]
- 5. Bakewell C., Ward B. J., White A. J. P., Crimmin M. R., Chem. Sci. 2018, 9, 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brand S., Elsen H., Langer J., Grams S., Harder S., Angew. Chem. Int. Ed. 2019, 58, 15496–15503; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 15642–15649. [Google Scholar]
- 7. Paparo A., Smith C. D., Jones C., Angew. Chem. Int. Ed. 2019, 58, 11459–11463; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 11581–11585. [Google Scholar]
- 8. Brand S., Elsen H., Langer J., Donaubauer W. A., Hampel F., Harder S., Angew. Chem. Int. Ed. 2018, 57, 14169–14173; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 14365–14369. [Google Scholar]
- 9.
- 9a. Schmidt E. S., Jockisch A., Schmidbaur H., J. Am. Chem. Soc. 1999, 121, 9758–9759; [Google Scholar]
- 9b. Baker R. J., Farley R. D., Jones C., Kloth M., Murphy D. M., J. Chem. Soc. Dalton Trans. 2002, 3844–3850; [Google Scholar]
- 9c. Segawa Y., Yamashita M., Nozaki K., Science 2006, 314, 113–115; [DOI] [PubMed] [Google Scholar]
- 9d. Yamashita M., Nozaki K. in Synthesis and Application of Organoboron Compounds, Vol. 49 (Eds.: E. Fernandez, A. Whiting), Springer, Heidelberg, 2015, pp. 1–37. [Google Scholar]
- 10. Hicks J., Vasko P., Goicoechea J. M., Aldridge S., Nature 2018, 557, 92–95. [DOI] [PubMed] [Google Scholar]
- 11. Hicks J., Mansikkamaki A., Vasko P., Goicoechea J. M., Aldridge S., Nat. Chem. 2019, 11, 237–241. [DOI] [PubMed] [Google Scholar]
- 12. Hicks J., Vasko P., Goicoechea J. M., Aldridge S., J. Am. Chem. Soc. 2019, 141, 11000–11003. [DOI] [PubMed] [Google Scholar]
- 13. Schwamm R. J., Anker M. D., Lein M., Coles M. P., Angew. Chem. Int. Ed. 2019, 58, 1489–1493; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 1503–1507. [Google Scholar]
- 14.A dialkyl-substituted alumanyl anion has also very recently been described; Kurumada S., Takamori S., Yamashita M., Nat. Chem. 2019, 12, 36–39.. [DOI] [PubMed] [Google Scholar]
- 15. Jones C., Mills D. P., Platts J. A., Rose R. P., Inorg. Chem. 2006, 45, 3146–3148; [DOI] [PubMed] [Google Scholar]; Bonello O., Jones C., Stasch A., Woodul W. D., Organometallics 2010, 29, 4914–4922. [Google Scholar]
- 16.
- 16a. Pécharman A. F., Colebatch A. L., Hill M. S., McMullin C. L., Mahon M. F., Weetman C., Nat. Commun. 2017, 8, 15022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Pécharman A. F., Hill M. S., McMullin C. L., Mahon M. F., Angew. Chem. Int. Ed. 2017, 56, 16363–16366; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16581–16584; [Google Scholar]
- 16c. Pécharman A. F., Rajabi N. A., Hill M. S., McMullin C. L., Mahon M. F., Chem. Commun. 2019, 55, 9035–9038; [DOI] [PubMed] [Google Scholar]
- 16d. Pécharman A. F., Hill M. S., McMullon G., McMullin C. L., Mahon M. F., Chem. Sci. 2019, 10, 6672–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans W. J., Seibel C. A., Ziller J. W., J. Am. Chem. Soc. 1998, 120, 6745–6752. [Google Scholar]
- 18.The BP86 functional was used for optimizations, with Mg, Al, Si and Ca centres described with the Stuttgart RECPs and 6-31G** basis set for all other atoms. Further and more detailed information regarding the methodology can be found in the Supporting Information.
- 19.Analogous reactivity with THF at either the isolated AlI center of compound 2 or during attempted reduction of AlIII has previously been observed in
- 19a. Chu T., Boyoko Y., Korobkov I., Nikonov G., Organometallics 2015, 34, 5363–5365; [Google Scholar]
- 19b. Schnitter C., Roesky H. W., Röpken C., Herbst-Irmer R., Schmidt H.-G., Noltemeyer M., Angew. Chem. Int. Ed. 1998, 37, 1952–1955; [Google Scholar]; Angew. Chem. 1998, 110, 2059–2062; [Google Scholar]
- 19c. Schormann M., Klimek K. S., Hatop H., Varkey S. P., Roesky H. W., Lehmann C., Röpken C., Herbst-Irmer R., Schmidt H.-G., Noltemeyer M., J. Solid State Chem. 2001, 162, 225–236. [Google Scholar]
- 20.CCDC https://www.ccdc.cam.ac.uk/services/structures?id=doi:10.1002/anie.201914986 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from http://www.ccdc.cam.ac.uk/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


