Abstract
This review focuses on relevant scientific information regarding the current knowledge of the yellow head complex viruses, yellow head virus and gill‐associated virus. The yellow head complex viruses have been problematic within the aquaculture industry for over 10 years and still retain their research topicality. Presently, there are numerous research papers from different journals covering the identification, disease expression and spread, pathogenesis, detection, morphology, genomic sequence and protein profiles of the yellow head complex viruses. Indeed, there has been no extensive review to compare these studies, and as a corollary, to assess flaws in contemporary research and knowledge. Additionally, the yellow head complex viruses rank within the top four prawn viruses with respect to disease impact and economic loss. This review collectively reports on all the findings and current methods of research and aims to identify weak areas of research where conclusions have been unjustifiably drawn and furthermore to elucidate areas that have a gap of knowledge.
Keywords: yellow head virus (YHV), gill‐associated virus (GAV), mid‐crop mortality syndrome (MCMS), Penaeus monodon
Introduction
Penaeid aquaculture was originally performed as ‘catch and hold’ culture systems. For centuries, Southeast Asian farms have been producing incidental crops of wild prawns in tidal ponds. With the advent of everadvancing technology and the increasing requirement for low‐cost protein as a food source, penaeid culture has advanced from its experimental nascent beginnings to major industries generating hundreds of thousands of jobs, billions of dollars in revenue and an expansion of the world's food supply with a high‐value crop (Lightner & Redman 1998). The importance of disease within this area has increased proportionally with the growth of the prawn industry.
Until the early 1990s, prawn aquaculture exhibited an astonishing growth of 16.8% per annum between 1984 and 1995 (Subasinghe, Bartley, McGladdery & Barg 1998). Since then, however, diseases have had devastating impacts on the industry. An economic impact assessment from Lundin (1997) reported that in 1994, just over 2 billion US dollars were lost due to disease. Disease outbreaks continue to cause major losses. The purpose of this review is to focus on the yellow head complex viruses, gill‐associated virus (GAV) and yellow head virus (YHV) with respect to the present body of literature on these viruses.
An overview of the yellow head complex viruses
Currently, the literature categorizes the yellow head complex viruses into two distinct viruses: the GAV and the YHV. Gill‐associated virus is the junior synonym of lymphoid organ virus (LOV), which was reported in 1995 by Spann, Vickers and Lester. Lymphoid organ virus was reported to be found only in the lymphoid organ, bearing a similarity to YHV with respect to ultrastructural and cytopathological features. However, LOV was reported as having no association with disease and mortality (Spann, Vickers & Lester 1995; Spann, Cowley, Walker & Lester 1997). Gill‐associated virus was subsequently reported as a pathogenic relative of LOV, found both in the lymphoid organ and the gills of infected Penaeus monodon (Spann et al. 1997). With the use of sequencing analysis on the genome of LOV and GAV, research identified that in fact, LOV had a 98.9% nucleotide identity to the GAV sequence, indicating that they are the same virus (Cowley, Dimmock, Spann & Walker 2000b). However, the method to determine the level of nucleotide similarity used only two clones to determine the 1.1% (3/274) nucleotide variation.
Juxtaposing this, GAV had an 85.1% nucleotide identity to YHV from a 577 base pair (bp) region and an 83% nucleotide identity to YHV from a 135 bp sequence of a cDNA clone. From this, YHV was reported to be a closely related geographic topotype of GAV. However, this study only performed sequence analysis on three YHV clones (Cowley, Dimmock, Wongteerasupaya, Boonsaeng, Panyim & Walker 1999).
Even though GAV and YHV are currently classified as distinct viruses, research applied to form these conclusions was both constrictive and limited. Owing to the extremely small number of clones that were sequenced and the small sequence region, the research obviously did not acknowledge the possibility of the thousands of mutants within the clones that can constitute so‐called quasi‐species within a population (Van Regenmortel 2000), in addition to the highly possible natural genomic variation within the so‐called two distinct viruses. Therefore, due to the small sample size, the sequence variation was not representative of the actual population and the actual nucleotide variation could greatly deviate from the reported variation, resulting in either the viruses being the same virus or distinctly different.
Since 1991, the International Committee on Taxonomy of Viruses (ICTV) has accepted the definition that ‘a virus species is a polythetic class of viruses that can constitute a replicating lineage and occupy a particular ecological niche’. Van Regenmortel (2000) lists the following characteristics for discriminating between virus species:
-
•
Relatedness of genome sequence.
-
•
Natural host range.
-
•
Cell and tissue tropism.
-
•
Pathogenicity and cytopathology.
-
•
Mode of transmission.
-
•
Physicochemical properties of virions.
-
•
Antigenic properties of viral proteins.
Owing to GAV and YHV sharing these same above characteristics and with the genome matching 491 bp out of a compared 577 bp, combined with the fact that the viruses are morphologically indistinguishable and cause the same gross disease, in this article, GAV and YHV will be referred to as the same virus: the ‘yellow head‐like virus’ (YHLV).
Taxonomic classification of YHLV
There has been considerable confusion with respect to the taxonomic classification of YHLV. Initially, YHLV was proposed to be a baculovirus due to its size and enveloped rod‐shaped appearance (Chantanachookin, Boonyaratpalin, Kasornchandra, Direkbusarakom, Ekpanithanpong, Supamataya, Sriurairatana & Flegel 1993). However, upon the discovery that the genome consisted of ssRNA, it was reported that the virus was either a rhabdovirus or a coronavirus (Wongteerasupaya, Sriurairatana, Vickers, Akrajamorn, Boonsaeng, Panyim, Tassanakajon, Withyachumnarnkul & Flegel 1995). Loh, Tapay, Lu and Nadala (1997) reported the genome as negative in polarity, resulting in the virus being classified as Rhabdoviridae. However, it was subsequently reported that YHLV was a plus‐strand RNA virus via in situ hybridization and sequence analysis (Tang & Lightner 1999). These latest results placed YHLV into the corona‐like viruses. In 2000, Cowley, Dimmock, Spann & Walker (2000a) stated that the YHLV genome contains an open reading frame (ORF) 1a polyprotein containing a 3C‐like Cys protease, an ORF1b coding sequence with replicase functions including an SDD polyprotein, and a helicase domain, an efficient – one ribosomal frameshift site at the ORF1a/1b overlap that facilitates translation of a 759 kDa ORF1ab polyprotein. From this, it was concluded that the YHLV was a unique member of the Nidovirales. The YHLV have subsequently been placed as members of a new genus Okavirus of a new family Roniviridae, within the order Nidovirales (Mayo 2002). There are two other families within the order Nidovirales. These are Coronaviridae and Arteriviridae (Fig. 1).
Figure 1.
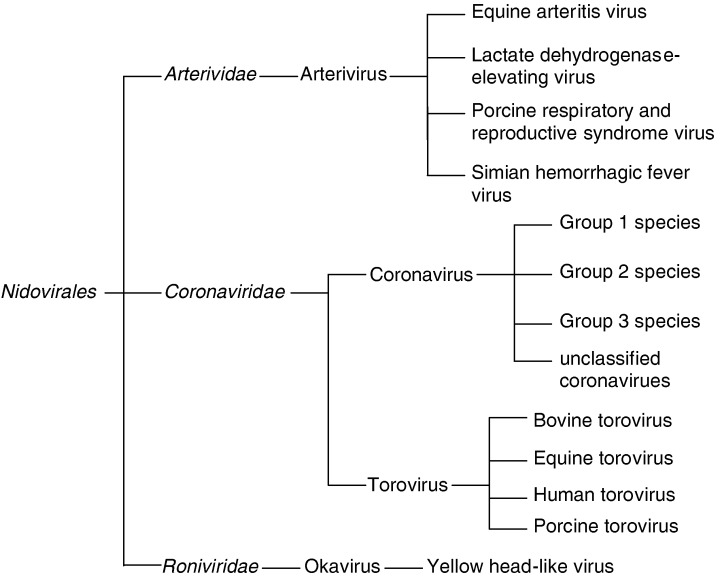
Family tree of the genera and families within the order Nidovirales.
Tissue distribution of YHLV
A study by Lu, Tapay, Loh, Brock and Gose (1995) reported that YHLV particles were detected in the gill, lymphoid organ, head soft tissue, heart, midgut, hepatopancreas, abdominal muscle, eyestalk and nerve cord of an experimentally infected Penaeus vannamei. Lu et al. (1995) reported that the lymphoid organ, gill and head muscle had a 50% tissue culture infectious dose assay (TCID50) titre (mL−1) of 106, while the midgut, abdominal muscle and heart had a TCID50 titre (mL−1) of 105 and the nerve cord, hepatopancreas and the eyestalk had a TCID50 titre (mL−1) of 104. These findings suggest that the lymphoid organ, gill and head muscle contained the highest number of infectious virions compared with the other tested tissue/organs. Cowley, Hall, Cadogan, Spann and Walker (2002) tested gonads from P. monodon for signs of YHLV and reported that the reverse transcription nested polymerase chain reaction (RT‐nPCR) products had a greater intensity from the spermatophores than those amplified from the lymphoid organ. These results indicate that the viral infection was systemic. Virions with a morphological appearance similar to YHLV have also been reported in the optic nerve fibres and in the nerve cord of P. monodon (Smith 2000; Callinan, Jiang, Smith & Soowannayan 2003). To confirm the entire viral distribution in prawn tissues, a more comprehensive examination must be conducted in other organs and tissues such as haematopoietic tissue, Y‐organ, stomach, antennal gland, periopods, pleopods and uropods.
Morphology and properties of YHLV
Yellow head‐like virus replication occurs in the cell cytoplasm, primarily in the prawn lymphoid organ, gills, haemocytes and connective tissues (Cowley, Dimmock, Spann & Walker 2001). The YHLV virions are rod‐shaped, enveloped particles containing helical nucleocapsids that mature by the process of budding through intracytoplasmic membranes (1993, 1997). The nucleocapsids exhibit striations with a periodicity of approximately 7 nm and are often observed in association with the distended endoplasmic reticulum (Spann et al. 1997).
The virions vary as 160–200 by 34–63 nm in size and are often packed densely into vesicles, resembling paracrystalline arrays (Fig. 2). Free virions are also observed in intercellular spaces probably via release from disintegrating cells (1993, 1997). Within all stages of YHLV‐infected cells, virogenic stroma and filamentous YHLV nucleocapsids, 116–435 by 16–18 nm in size, are often observed scattered randomly within the cytoplasm (Spann & Lester 1997). The nucleocapsid of YHLV becomes enveloped by passage through the endoplasmic reticulum or the virions have occasionally been observed invading the interstitial spaces of the lymphoid organ and gain their envelope by passage through the plasma membrane (Spann & Lester 1997).
Figure 2.
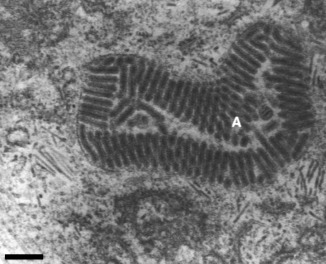
Yellow head‐like virus‐infected Penaeus monodon lymphoid organ cell showing paracrystalline arrays of enveloped virions (a). Scales bar=200 nm (Spann et al. 1995).
The genome of YHLV consists of 26 235 nucleotides organized into four ORFs (Fig. 3) (Cowley & Walker 2002). Initially, YHLV was considered to consist of four structural proteins with the following estimated molecular weights: 170, 135, 67 and 22 kDa (Nadala, Tapay & Loh 1997). The proteins were believed to represent the L (RNA transcriptase), G (spike), N (nucleocapsid) and M (matrix) respectively. However, Wang and Chang (2000) indicated only three major YHLV proteins, being 110, 63 and 20 kDa in size and suggested that the larger protein (170 kDa) reported by Nadala et al. (1997) was cellular in origin. However, it is feasible to suggest that due to the techniques used to purify the virus by Wang and Chang (2000), the 170 kDa polyprotein may have been cleaved to produce the reported 110 and 63 kDa proteins.
Figure 3.
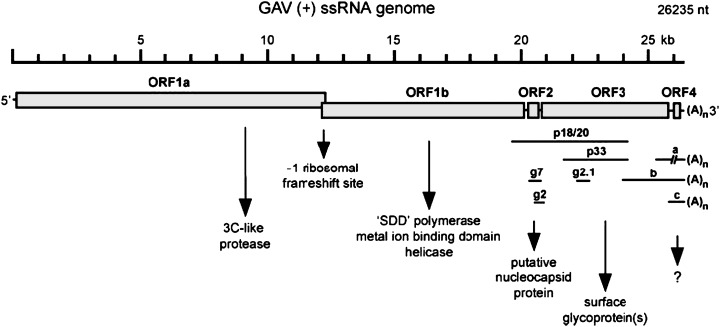
Organization of the 26235 nt (+) ssRNA yellow head‐like virus genome indicating translation features and deduced open reading frame (ORF) functions (Cowley & Walker 2002).
Serological activity from YHLV has been reported by Nadala et al. (1997). They reported that purified YHLV agglutinated chicken erythrocytes yielding a haemagglutination (HA) end‐point titre of 1:256 and the virus was not eluted after 24 h, suggesting that the reaction was stable and that the virus lacked receptor‐destroying enzymes. Haemagglutination activity from YHLV‐infected prawns was confirmed by Munro and Owens (2005), while YHLV‐free prawns demonstrated negligible HA activity. The protein responsible for the HA is thought to be similar to the HA protein on the outside of some Coronaviruses (Fig. 4).
Figure 4.
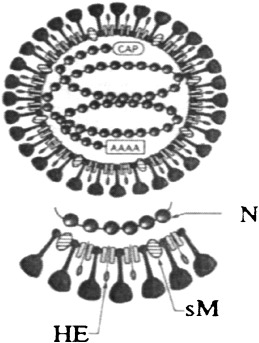
Diagrammatic representation of the structural components and schematic morphology of coronavirus virions. Note: N, nucleocapsid protein; sM, small‐membrane protein; HE, haemagglutinin‐esterase protein (Siddell 1995).
Epidemiology of YHLV
Geographical distribution
The YHLV throughout most of Southeast Asia is reported to be a highly pathogenic agent for cultured P. monodon, causing significant mortalities and adversely affecting the mariculture prawns in Thailand (Chantanachookin et al. 1993). The YHLV was first described in Thailand in 1990 by Limsuwan (Chantanachookin et al. 1993). Limsuwan named the new syndrome based on the light yellow colouration of the dorsal cephalothorax area and the general pale appearance of the infected prawn. This yellow appearance was a result of the underlying enlarged yellow hepatopancreas. Since this time, the disease has been associated with epizootic mortalities in Thailand. Evidence suggests that YHLV is one of the most highly virulent viruses of causative agents in Thailand, as it is associated with the massive mortality of P. monodon, until recently the principal penaeid species cultured in Thailand (Sithigorngul, Chauychuwong, Sithigorngul, Longyant, Chaivisuthangkura & Menasveta 2000). Since the identification of the causative agent as YHLV in Thailand in 1990 and the resulting epizootic mortalities it was associated with, the virus has been associated with mortalities in penaeid prawns in Taiwan, Indonesia, Malaysia, China, Philippines, India, Australia and the Americas (1996, 1996, 1997).
There have been several reported occurrences of YHLV in the Americas, the first at a Penaeus setiferus farm in Texas. The farm was in close proximity to a prawn processing plant and it was suggested that the virus was imported from Asia (Lightner, Redman, Poulos, Nunan, Mari & Hasson 1997). However, research by Pantoja and Lightner (2003) demonstrated that the diagnosis of the YHLV was most probably due to misinterpretation of the lymphoid organ necrosis in the prawns infected with acute white spot syndrome virus (WSSV) infection. Pantoja and Lightner (2003) demonstrated that acute WSSV infection can cause necrosis of the lymphoid organ and other tissues and display histological characteristics very similar to those observed from YHLV infection. Yellow head‐like virus has also been detected in frozen prawns that had been imported into the United States from Asia. At present, there is no evidence to show that YHLV is now present in wild or farmed prawns in the Americas.
Initially, YHLV principally infected pond‐reared juveniles to sub‐adult prawns 5–15 g in size, especially at 50–70 days of pond culture (Lightner 1996), although prawns up to 40 g have exhibited signs of disease (Spann & Lester 1997). Since the initial epizootic, it has been suggested that the disease is now less severe than when YHLV was first isolated in Thailand. Yellow head‐like virus infection is now common in healthy prawns. This disease resistance is proposed to be from ‘active accommodation’ using a tolerance mechanism involving the binding of viral antigens to cellular receptors during the early life stages of the prawn (Flegel & Pasharawipas 1998).
This theory of ‘active accommodation’ is amply supported in the literature. For example, transmission electron microscopy (TEM) of broodstock collected in Thailand before the initial reports of the virus indicated that YHLV was present in one out of seven healthy broodstock sampled. During the peak of the YHLV epidemic in Thailand, YHLV was detected by TEM in gill samples from at least one prawn from 15 ponds with gross signs and a pond history that indicated YHLV was present. In the three ponds with no signs of YHLV disease, six out of six prawns sampled negative for YHLV by TEM. However, later in the YHLV epidemic, YHLV could be detected by TEM in gill samples from 33 out of 44 healthy prawns sampled from 11 ponds without signs of YHLV (Walker, Cowley, Spann, Hodgson, Hall & Withyachumnarnkul 2001).
A study of 19 P. monodon broodstock collected from hatcheries in Thailand was conducted using RT‐PCR on total nucleic acid extracted from gill tissue (Wongteerasupaya, Tongchuea, Boonsaeng, Panyim, Tassanakajon, Withyachumnarnkul & Flegel 1997). All 19 prawns tested negative for YHLV. However, using a two‐step RT‐PCR on the same prawns resulted in 15 out of the 19 prawns (78.9%) testing positive for YHLV (Wongteerasupaya et al. 1997). A survey conducted in the Philippines on 219 healthy prawns with Western blot analysis also indicated a relatively high prevalence (24.2%) of YHLV infection (Natividad, Magbanua, Migo, Alfafara, Albaladejo, Nadala, Loh & Tapay 1999). The prevalence varied between 0% and 66.7% depending upon which districts were sampled. There was also evidence of a higher prevalence of infection in postlarvae (54.5%) than in broodstock (16.9%). With respect to the limited sensitivity of Western blot methods compared with two‐step PCR, the true level of chronic YHLV infection in the Philippines may be much higher. Yang, Shariff, Lee and Hassan (2000) also reported that YHLV has a high prevalence in Malaysia but it does not appear to have been associated with significant mortalities.
These studies imply or offer the notion that a high proportion of apparently healthy P. monodon broodstock from some areas of Asia carry chronic infections of YHLV. It is probable that the previous TEM studies of broodstock and farmed prawns in Thailand would not have detected this low level of infection that is often only evident in two‐step PCR. Therefore, chronic YHLV infection may have been highly prevalent in healthy prawns before the appearance of the disease. The increase in the prevalence of infection detected by TEM could have been due to a general increase in viral load in the farmed prawns. The relationship between viral load and susceptibility to disease is the subject of ongoing research.
Within Australia, YHLV has been associated with significant mortalities that have adversely affected the prawn farm industry since at least 1996 (Spann, Donaldson, Cowley & Walker 2000). A chronically infected P. monodon with YHLV displays no gross signs of disease or tissue necrosis, while acute infections result in necrosis, disease and mortalities. Under experimental conditions, YHLV is reportedly highly pathogenic, causing mortalities from 4 to 5 days post‐infection (Walker et al. 2001).
The reported prevalence of YHLV within Australia in P. monodon broodstock from a sample size of 148 prawns captured in north‐eastern Queensland was 97.3%. The prevalence of YHLV in postlarvae from a sample size of 50 was 100%, and the prevalence of YHLV in juveniles from a sample size of 56 was 98.2% (Walker et al. 2001). Unfortunately, no information was released as to the methodology of obtaining the samples, and so it is unknown whether they are from the same hatchery and/or the same broodstock.
Mode of infection of yellow head‐like viruses
The modes of infection of YHLV have been placed into two general groups: horizontal transmission and vertical transmission (Fig. 5).
Figure 5.
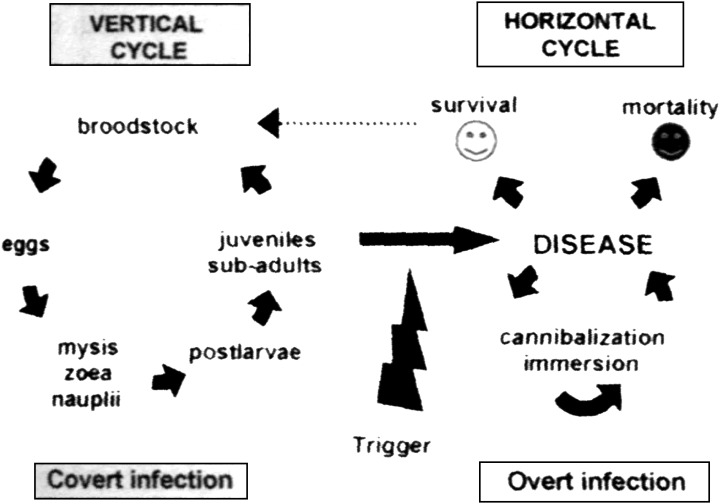
Model for the infection and disease cycle of the yellow head‐like virus (Walker et al. 2001).
Horizontal transmission can occur when YHLV‐free P. monodon either feed on infected carcasses, experience bath exposure to membrane‐filtered tissue extracts, by cohabitation with infected prawns, or by direct experimental injection of the viral inoculum (Walker et al. 2001). An experiment to determine the susceptibility of postlarval (PL) P. monodon to YHLV by ingestion showed that PL20 died 7–10 days post‐infection but PL15 survived the exposure (Walker et al. 2001). The available data suggested that disease was associated with viral loading. Walker et al. (2001) reported that YHLV from a diseased P. monodon caused mortalities after ingestion or immersion exposure but extracts from YHLV‐infected healthy P. monodon caused infection without mortality. However, after the viral concentration of the healthy prawns had an equivalent titre to the diseased prawn, YHLV extracts from chronically infected, healthy P. monodon also induced disease, indicating that the disease is associated with viral loading. However, in these studies there was no referral to the possibility of multiple viral infections.
The potential for vertical transmission was first reported by Chantanachookin et al. (1993). They reported that YHLV infection in larval offspring could occur from latent, asymptomatic, infected, broodstock prawns. This theory was originally dismissed for YHLV because TEM screening of P. monodon in Thailand suggested that the prevalence of YHLV was low, and therefore, vertical transmission was unlikely to contribute significantly to the occurrence of infection and disease on farms (Flegel, Boonyaratpalin & Withyachumnarnkul 1997). With more recent screening of broodstock with RT‐nPCR, the prevalence of YHLV infection in Thailand may be significantly higher than originally indicated using TEM (Walker et al. 2001).
To determine whether vertical transmission of YHLV contributes to the high prevalence of chronic infections in wild and farmed P. monodon in eastern Australia, Cowley et al. (2002) tested gonads and lymphoid organs for signs of YHLV from healthy male and female P. monodon broodstock and in fertilized eggs in addition to nauplii spawned from wild‐fertilized females using RT‐nPCR. The results indicated that the level of YHLV in wild P. monodon was generally low. However, high levels of YHLV were detected in moribund male broodstock reared in captivity for more than 12 months. The RT‐nPCR products from spermatophores in these prawns were also significantly greater than those amplified from the lymphoid organ, which had previously been identified as the primary site of YHLV replication in chronically infected P. monodon (Spann et al. 1995). It was also reported that in one out of three spermatophores examined by TEM, mature YHLV virions were detected in the seminal fluid but not in the sperm cells. The RT‐nPCR for YHLV in eggs were positive; however, nauplii and protozoea were generally negative. This implies that YHLV is associated with the egg surface and the majority of the virus is lost when the nauplii hatch and that the infection levels in the protozoea remain low. Cowley et al. (2002) argued that this could be due to the lack of development of the lymphoid organ in larval and early postlarval life stages, which is likely to limit potential infection levels. Reverse transcription nested polymerase chain reaction has detected YHLV in PL5 to PL15 both from hatcheries and experimental spawnings of P. monodon (Walker et al. 2001), suggesting that viral replication in postlarvae is occurring at sufficient levels to be detected (Walker et al. 2001). Cowley et al. (2002) reported that the identification of lymphoid organ spheroid bodies and YHLV particles in ∼1.2 g juvenile P. monodon grown from hatchery stocks (PL6 and PL20) suggests that at least some of the postlarvae were infected with YHLV. However, as individual postlarvae were not grown in isolation, it strongly raises the distinct possibility that some juvenile infections occurred during the course of the grow‐out through cannibalism or water‐borne transmission. Clearly, this horizontal transmission could promote translocation of YHLV and the potential infection of wild P. monodon in the vicinity of farms via water or through the escape of infected farmed prawns. The authors concluded that the high prevalence of chronic YHLV infection in P. monodon broodstock from northeastern Queensland and farmed prawns produced from these broodstock promotes the idea that this is perpetuated primarily by vertical transmission both in the wild and in hatcheries. Unfortunately, however, in that paper, the authors failed to comment on the likely survival of the YHLV‐infected progeny. They only tested eggs and nauplii. It is only a speculation that these postlarvae survive through to adulthood.
Pathogenicity
Despite the high prevalence of YHLV, not all YHLV‐infected P. monodon express disease. For YHLV‐related disease to be expressed, there appear to be other factors involved. These are hypothesized to be the viral load of parental broodstock, the initial viral load of postlarvae, co‐infecting viruses or unknown environmental factors acting as a stressing agent.
Prevailing research currently dictates YHLV to be highly pathogenic to P. monodon within Australia (1997, 2004). The pathogenicity of YHLV was determined by inoculation of filtered homogenates of the lymphoid organ, gills and whole cephalothoraces from P. monodon that were positive for YHLV, resulting in mortality from 7 to 8 days post‐inoculation (Spann et al. 1997). However, at the time of the pathogenicity trial, there were at least five concomitant viruses infecting Australian P. monodon. These viruses consisted of monodon baculovirus (MBV) (Doubrovsky, Paynter, Sambhi, Atherton & Lester 1988), lymphoid parvo‐like virus (LPV) (Owens, De Beer & Smith 1991), infectious hypodermal and haematopoietic necrosis virus (IHHNV) (Owens, Anderson, Kenway, Trott & Benzie 1992), spawner‐isolated mortality virus (SMV) (Fraser & Owens 1996) and Mourilyan virus (MoV) (Cowley, McCulloch, Spann, Cadogan & Walker 2005), which all could have possibly influenced the mortality of the P. monodon in this trial. This infection trial was repeated in 2004 by Vega et al. In that study, the prawns were again inoculated with filtered prawn homogenate containing levels of YHLV (not purified virus). They reported that 100% (15/15) of the YHLV‐injected prawns died compared with 40% (2/5) of the controls. While there was a significant increase (P=0.010) in YHLV for both the infected prawns and the controls during the trial, the YHLV increase was significantly higher (P=0.047) in the YHLV‐injected prawns than the control prawns. Again, no other viruses were tested for in that study or referred to as potential pathogens. There are many papers that report YHLV to be pathogenic. However, there is no substantial evidence to support this. Currently, there is only an association with disease. Many papers report that severe necrosis of the lymphoid organ is a typical lesion caused by YHLV (1993, 1993, 1994, 1996). However, penaeid prawns with severe WSSV exhibit the same marked lymphoid organ necrosis (Pantoja & Lightner 2003). The method of injecting YHLV‐infected crude homogenate of prawn tissue to determine pathogenicity gives no information as to the virulence of that virus to a species of prawn with respect to our current knowledge of possible multiple viral infections within the same prawn or homogenate sample. To demonstrate a direct pathogenic effect of YHLV, either pathogen‐free stock would be required or a viable penaeid cell line would be needed with purified viable YHLV. Until these requirements are available, YHLV can only be said to be associated with disease.
Interactions with other viruses
At present, there are approximately 18 viruses that have been reported in penaeids. Not all of these viruses have been shown to cause disease or to interact with each other once they have infected the prawn. Within the YHLV, there are two main groups of viruses that have been indicated to cause disease when a dual infection occurs. The two groups of viruses that interact with each other are the YHLV with WSSV and YHLV with SMV.
White spot syndrome virus was previously classed as a baculo‐like virus (Nadala, Tapay & Loh 1998). However, it has recently been placed into a new family and genus: family Nimaviridae, genus Whispovirus (Mayo 2002). It was first seen as a dual infection with YHLV in Thailand in P. monodon in 1993 and was originally named systemic ectodermal and mesodermal baculovirus (SEMBV) (Wongteerasupaya, Vickers, Sriurairatana, Nash, Akarajamorn, Boonsaeng, Panyim, Tassanakajon, Withyachumnarnkul & Flegel 1995). The virus was first characterized as WSSV from an outbreak in a Penaeus japonicus in Japan in 1993 (Flegel 1997). The naming of the virus derived from the gross examination displaying white spots. However, Chou, Huang, Wang, Chiang and Lo (1995) reported that the first epizootic of WSSV occurred in Taiwan in 1992. The dual infection of WSSV and YHLV has since been reported in India and Taiwan (Mohan, Shankar, Kulkarni & Sudha, 1998; Wang & Chang 2000).
Both WSSV and YHLV can cause significant mortalities in penaeid prawns (1993, 2002) and were once the most serious diseases threatening the P. monodon industry in Thailand (Flegel et al. 1997). At present, there is no further information on the interaction between YHLV and WSSV in cultured prawns. Wang and Chang (2000) suggested that the reason for mass loss in the prawn culture industry in Taiwan between 1996 and 1999 was not only WSSV, but a dual infection of WSSV and YHLV. Prawns with dual infection generally exhibit only typical signs of white spot syndrome, with the YHLV symptoms being less obvious. This would result in the mixed disease being diagnosed as only WSSV infection.
In 1994, prawn farms in northern Australia experienced increased mortality rates in 12–15 g prawns, with mortality reaching as high as 80% in some ponds (Owens, Haqshenas, McElnea & Coelen 1998). This disease outbreak was named the mid‐crop mortality syndrome (MCMS). An investigation into the syndrome revealed two distinct viral types using TEM (Owens et al. 1998).The two viruses that were implicated as being involved in MCMS were YHLV and SMV (Anderson & Owens 2001). Spawner‐isolated mortality virus is a parvo‐like virus that was first identified by Fraser and Owens (1996). This virus was isolated from prawns affected by MCMS by Owens et al. (1998). Using a bioassay, Owens et al. (1998) reported that this parvo‐like virus was capable of causing mortality. The gross symptoms of SMV were lethargy, reduced feeding and redness of the carapace and pleopods (Fraser & Owens 1996). Owens et al. (1998) reported that SMV virulence in MCMS‐affected prawns was enhanced by the presence of other co‐infecting viruses such as an enveloped, filiform virus. The co‐infecting virus was YHLV, which had first been identified by Spann et al. (1995). The enhanced virulence from YHLV was demonstrated when Owens et al. (1998) treated a prawn extract with ether before injecting it into P. monodon. As SMV is unenveloped, the ether should not have harmed the parvovirus. The extract killed at a slower rate than untreated extract, suggesting that the SMV (or another non‐enveloped virus) was capable of causing mortality but that its virulence was enhanced by the presence of other co‐infecting viruses such as an enveloped, filiform virus like YHLV. Spann et al. (1997) reported that YHLV was also isolated from prawns affected by MCMS. They reported that diseased prawns were observed swimming at the surface and edge of ponds and displayed varying degrees of red body colouration. Symptoms from both these viruses were apparent in MCMS‐affected prawns.
In recent years, two other viruses have been reported as being present during the MCMS. These viruses are IHHNV (Krabsetsve, Cullen & Owens 2004) which is similar to SMV with respect to being a non‐enveloped DNA virus, and MoV (Cowley et al. 2005), which is similar to YHLV with respect to being an enveloped RNA virus. These two viruses could both have influenced the mortality of the infected prawns.
In 2000, lesions were found in a P. monodon displaying non‐specific signs of disease (Smith 2000). The causative agents of the lesions appeared to be Vibrio spp. and a rod‐shaped virus similar to YHLV. Being TEM, it was observed that the nerve cells in the fasciculated zone contained cytoplasmic vesicles with particles and rod‐shaped nucleocapsids. These rods were similar to YHLV and were 130–260 nm long × 10–16 nm in diameter and had a helical symmetry with a screw‐like thread. Also, an unidentified enveloped virus, ranging from 50 to 96 nm in diameter, was observed in cytoplasmic vesicles in the fasciculated zone (Fig. 6). Smith (2000) reported that the unidentified enveloped virus was a possible aetiological agent in one of the disease outbreaks. In the paper, Smith (2000) did not suggest what the virus was. From what is reported on the present penaeid viruses, it is likely that this virus was MoV, which is an enveloped virus, averaging 85–100 nm in diameter. From the TEM photograph (Fig. 7), Smith (2000) also reports particles 20 nm in diameter. However, Smith (2000) does not suggest what these could be. It is feasible that these particles were the parvovirus SMV, which is reported to be non‐enveloped, averaging 20 nm in diameter. If this is correct, it would suggest triple viral infection between YHLV, and another two viruses, that based on their morphology, might be SMV and MoV causing disease.
Figure 6.
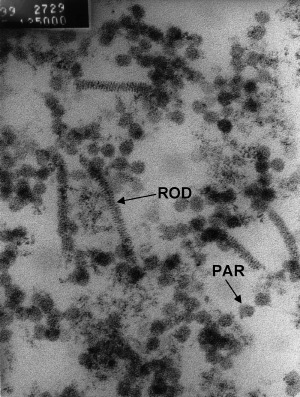
Transmission electron microscopy of vesicle showing particles (PAR) of 50–96 nm in diameter and rod‐shaped structures (ROD) 155–207 nm long (Smith 2000).
Figure 7.
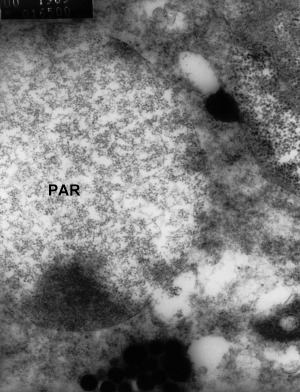
Transmission electron microscopy of vesicles within nerve cells of the fasciculated zone of the eye of moribund Penaeus monodon. The vesicles are 3 μm in diameter and contain unidentified particles (PAR) 20 nm in diameter. Some particles also appear to be free in the cytoplasm (Smith 2000).
Susceptibility to the infection
A range of crustaceans can be infected with YHLV (Table 1). Penaeus monodon is the only crustacean that is commonly affected by YHLV (Walker et al. 2001). However, several other penaeid prawns and other crustaceans have been reported to be susceptible by either natural or experimental infection. Unlike WSSV, YHLV has not been seen to infect crabs or freshwater prawns (Flegel 1997; Longyant, Sattaman, Chaivisuthangkura, Rukpratanporn, Sithigorngul & Sithigorgul 2006). Natural infection from YHLV has only been detected in Penaeus esculentus that were co‐cultivated with P. monodon (Table 1). However, only 14 prawns from one location were tested, with eight being positive. For a more substantial conclusion to be drawn, larger sample numbers are needed. The YHLV infection in P. esculentus was reported to be chronic and there were no signs of gross disease. In Thailand, it has been reported that co‐cultivation of Penaeus merguiensis and Penaeus indicus with P. monodon during early outbreaks of YHLV resulted in no disease in the P. merguiensis and P. indicus (1991, 1998). However, it was not reported whether they were tested to determine infection.
Table 1.
Natural and experimental host range for YHLV (Lu et al. 1994;, Flegel 1997;, Walker et al. 2001;, Longyant et al. 2006)
| Species | Evidence of infection |
|---|---|
| Penaeus monodon | N, E |
| Penaeus esculentus | N, E |
| Penaeus duorarum | E |
| Penaeus japonicus | N, E |
| Penaeus merguiensis | E |
| Penaeus aztecus | E |
| Penaeus setiferus | E |
| Penaeus stylirostris | E |
| Penaeus vannamei | E |
| Metapenaeus brevicornis | E |
| Metapenaeus enis | N |
| Metapenaeus affinis | E |
| Metapenaeus bennettae | E |
| Palaemon styliferus | N |
| Euphasia superba | N |
N, natural infection; E, experimental infection.
Different penaeids have different susceptibility to YHLV infection. It has been demonstrated that P. monodon, P. japonicus, P. esculentus and P. merguiensis were susceptible to experimental infection (Spann et al. 2000), while P. monodon were the most susceptible out of the four different penaeids and P. japonicus were the least susceptible and displayed a size‐related response to the disease with an increased size resulting in increased resistance. The difference in susceptibility between the penaeids may be related to both the dose and their relative susceptibility to the disease, rather than an indication of resistance to infection (Spann et al. 2000). This experiment demonstrated that the four penaeids mentioned were susceptible to infection by YHLV via intra‐muscular injection. However, this does not demonstrate that they can be naturally infected. For more substantial results, the prawns should have been infected via bath inoculum or fed infected carcasses. This would give a stronger indication as to whether the three species of prawns are naturally susceptible to YHLV infection because the viral agent would not be by‐passing the primary defences of the prawn, i.e. the cuticle or gut. This difference in susceptibility by either experimental or natural pathways is demonstrated by Lu et al. (1994). This research demonstrated that an intra‐muscular injection of YHLV into P. vannamei resulted in 100% mortality, despite the fact that P. vannamei now being the dominant species cultured in Asia, there has been no disease report due to YHLV where YHLV is highly enzootic in this region. Other possible reasons for this phenomenon are that YHLV may have mutated from its highly pathogenic variant; multiple viruses are involved in the disease expression or biosecurity and specific pathogen‐free stocks may be limiting the chance of infection. However, YHLV is still causing disease in P. monodon within this region and other viruses including the Taura syndrome virus and WSSV still cause disease losses in P. vannamei in this region, resulting in the most probable reason for YHLV not causing disease in P. vannamei even though there is a high chance of infection being the low susceptibility demonstrated by P. vannamei. there is also the possibility that the intra‐muscular injection of YHLV by Lu et al. (1994) contained multiple viruses, resulting in high mortality.
Disease signs and diagnosis
Clinical signs
Initially, infection of YHLV in P. monodon of cultured populations and experimental trials affected juvenile to sub‐adult prawns (5–15 g) and often induced 100% mortality within 3–5 days from the infection date (Chantanachookin et al. 1993). However, natural disease outbreaks have been reported in P. monodon up to 40 g (Spann et al. 1997).
Gross lesions
The first gross sign of infection is an increase in feeding at an abnormally high rate for several days, followed by a sudden decline in appetite (Chantanachookin et al. 1993). Within 1 day of the prawns ceasing to feed, they begin either slowly or erratically swimming near the edge of the pond, and mortalities soon follow. Dead prawns are found at the edge of the pond and scattered evenly over the entire bottom of the pond. The disease is usually characterized by a pale to yellowish colouration of the cephalothorax and gills due to the underlying yellow hepatopancreas showing through the translucent carapace of the prawn and also a generally pale or bleached appearance of affected prawns (1993, 1999). It has also been reported that YHLV from Australia causes the colour of the prawn to change to a degree of pink to red, with primarily the appendages, tail fan and mouth parts being most noticeable; however, this characteristic can often be attributed to general stress and is not necessarily caused only by YHLV infection. Spann et al. (1997) also reported that the gills changed from the normal clear/yellow to pink and the prawns exhibited fouling of the gills and shell and tail rot.
Histopathology
Spann et al. (1997) reported that being a light microscope, diseased prawns display disorganization and loss of a normal, defined tubule structure in the lymphoid organ. The gills of diseased prawns displayed structural damage such as fusion of gill filament tips, general necrosis and loss of cuticle from primary and secondary lamellae. However, this detection method and signs of disease only indicate some form of disease and as mentioned previously, these symptoms are also characteristic of penaeid prawns infected with other viruses.
Flegel et al. (1997) reported that YHLV can be diagnosed histologically in moribund prawns by the presence of intensely basophilic inclusions in many different tissues and that these inclusions can be seen best with haematoxylin and eosin (H&E) staining of sectioned stomach and gill tissue. However, the cytoplasmic, virus‐associated inclusions stain deeply basophilic in the same manner as pyknotic nuclei and it is difficult to differentiate between the two without using an electron microscope, suggesting that there is no clear way to characterize YHLV infection using standard histological examination with H&E‐stained preparations (Chantanachookin et al. 1993). The lymphoid organ is distinctly abnormal in YHLV infections, showing nuclear abnormalities, cytoplasmic abnormalities and necrotic cells, also being characteristic of penaeid prawns infected with other viruses (Chantanachookin et al. 1993).
Electron microscopy
Transmission electron microscopy, which gives an image of the virus, is the gold standard test for the visualization and confirmation of YHLV infection. However, this method is not useful for detecting early stage infections or for on‐farm application. It is time consuming, requires expensive equipment and necessitates that the penaeid be killed, and therefore, it is not applicable for on‐farm use or for screening hatchery broodstock.
PCR
In 1997, Wongteerasupaya et al. (1997) developed an RT‐PCR for the detection of YHLV in Thailand. This test was specific and sensitive for the selected region of the YHLV genome, with other nucleic acid templates (WSSV and HPV) giving no amplification signal. The RT‐PCR was able to amplify as little as 0.01 pg of YHLV‐RNA and showed evidence of infection in P. monodon at 6–12 h after experimental exposure to the virus. However, it is more likely that the RT‐PCR detected the YHLV genome that was injected into the prawn and circulated in the haemolymph, than it was to be detecting infection at such an early time after injection. In 2000, Cowley et al. (2000b) developed an RT‐nPCR for the detection of YHLV within Australia. The specific genome amplification with the one‐step PCR is able to detect 0.01 pg cDNA while using the two‐step PCR technique 0.01 fg cDNA was detectable. The YHLV genome could be detected within 6 h of experimental infection of a P. japonicus. However, again it is probable that the test was detecting genome that was injected into the prawns and does not necessarily indicate viral replication. Gill biopsies can be used as a tissue sample (Cowley et al. 2000b) or more recently, it has been reported that dried haemolymph can be used for the detection of YHLV with RT‐PCR (Kiatpathomchai, Jitrapakdee, Panyim & Boonsaeng 2004), resulting in the ability to sample broodstock. A multiplex RT‐nPCR has been developed for the differentiation of YHLV from Australia and YHLV from Thailand (Cowley, Cadogan, Wongteerasupaya, Hodgson, Boonsaeng & Walker 2004). The test detected the YHLV in approximately 10 fg of lymphoid organ total RNA. The YHLV from Australia produced a 406 bp product, while the YHLV from Thailand produced a 277 bp product.
Real‐time PCR has been developed for the detection of YHLV (2002, 2004). Both these tests have the same sensitivity as the RT‐PCRs, but give the quantitative load of infection in the sample.
In situ hybridization has been developed (Tang & Lightner 1999) for the detection of YHLV infection in P. vannamei using a cDNA fragment labelled with digoxigenin that resulted in a highly sensitive test. This same probe was subsequently used to detect YHLV from Australia (Tang, Spann, Owens & Lightner 2002). Spann, McCulloch, Cowley, East and Walker (2003) have also reported the development of an in situ hybridization probe for the detection of YHLV in P. monodon and P. esculentus within Australia. However, these molecular techniques have practical limitations for widespread commercial application. This includes the need for special equipment and highly trained personnel, which result in expensive assay costs for small sample numbers that can limit RT‐PCR and in situ hybridization use.
Serology
Polyclonal antisera have been produced for the detection of YHLV (Nadala et al. 1997). However, the assay (Western blot analysis) was not highly sensitive and was not applicable to on‐farm field examinations (Sithigorngul, Rukpratanporn, Longyant, Chaivisuthangkura, Sithigorngul & Menasveta 2002). Reported in 2000 and again in 2002, Sithigorngul et al. produced monoclonal antibodies (MAbs) specific to YHLV‐enveloped protein. In both experiments, IgG‐MAbs were produced. The first experiment resulted in a low yield of hybridomas with MAbs specific to YHLV due to the usage of crude YHLV extract from the gills of an infected P. monodon. The second experiment resulted in higher success of hybridomas specific to YHLV. Most of the YHLV‐specific MAbs were specific to the 67 kDa protein and only a few were specific to the 135 and 22 kDa protein. Several of these antibodies against YHLV did bind to haemolymph and tissues from uninfected prawns (Sithigorngul et al. 2002). They did not elaborate as to why this may have occurred. There could be three possibilities for this occurrence; non‐specific binding to similar epitopes from the prawn cells, the hybridomas may not have sufficiently screened or even possibly, the reported uninfected prawns had an undetected low level of infection.
Yellow head‐like virus has been reported to haemagglutinate chicken erythrocytes (Nadala et al. 1997). Haemagglutination activity was determined via a qualitative and quantitative detection method. Munro and Owens (2005) used this HA activity of the YHLV to develop a low‐cost detection method for YHLV in P. monodon, while they demonstrated that YHLV RT‐nPCR‐negative prawns caused negligible HA.
Conclusion
Prawn aquaculture is a rapidly increasing global industry, being one of the fastest‐growing aquaculture sectors in Asia and Latin America. In 2004, the prawn aquaculture industry produced 2.47 million tons valued at US$ 9735 million (Fig. 8), compared with 1.65 million tonnes produced in 2000, equating to a 50% increase in production within 4 years.
Figure 8.
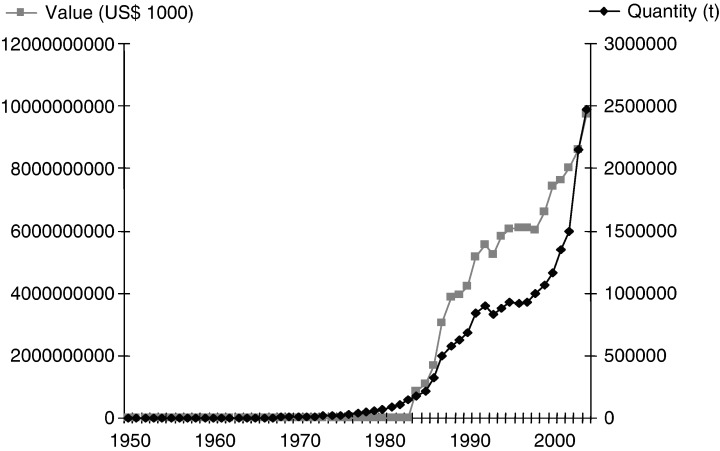
World prawn aquaculture production and value up to 2004 (Food and Agriculture Organization of the United Nations).
Unarguably, infectious diseases, particularly viral diseases, are recognized as a threat to the long‐term viability of the prawn farming industry worldwide. The major taxonomic groups are the families Nimaviridae, Parvoviridae, Picornaviridae and the order Nidovirales. Over the last 10 years, widespread epidemics from viruses have affected all aspects of prawn farming, from intensive farms in Thailand to extensive systems in Bangladesh.
The emergence of ‘new’ viruses is rapidly increasing. For example, in 1989 Lightner et al. (1989) reported six viruses affecting penaeid prawns. By 1992, the list of known viruses affecting penaeid prawns had increased to 12 (Lightner 1996) and presently, approximately 18 viruses have been reported in penaeid prawns.
At present, the most common methods for the detection of prawn viruses use PCR or specific probes. These diagnostic methods are sensitive and specific for the detection of viruses; however, they require special equipment/reagents and highly trained personnel, which result in expensive assays and thus a limited number of samples being tested, which mitigates widespread stock assessment in developing countries. This precipitates an enhanced spread of disease and increased mortalities on the farm primarily due to unknown viral infection or viral load of the prawns being produced or grown in hatcheries and farms. It is inconceivable to ignore the necessity of cheaper detection methods such as the antibody‐based tests or HA assays that need to be available for on‐farm application, allowing farmers to detect the presence of the virus and the loading of the virus in their farm stock/broodstock.
As mentioned previously, when a viral epidemic occurs, one virus is usually assigned as the aetiological agent. This is because either only the most probable virus was tested for or, alternatively, if other viruses were detected but were in low numbers, then they are usually ignored, with the virus in the greatest abundance designated as the disease‐causing agent. With the high level of prawn viral infection throughout the world, contemporaneous reports appear to ignore the potentially profound effects of dual infection of viruses in prawns with regards to disease. Unquestionably, for a greater understanding of how prawn viruses are interacting with each other to cause disease, there is a requirement for further research to infect prawns with individual viruses drawing comparison with prawns dually infected with known viruses. This would enable farmers to predict adequately the likelihood of the occurrence of disease outbreak. For example, it has been reported previously that YHLV infection is present in approximately 97% of P. monodon in Australia. It would be advantageous to determine the effect of dual infection of SMV, MBV, IHHNV, MoV, etc. in the same prawns to ascertain the likelihood occurrence of disease and what mixture of viruses results in disease expression.
The process by which viruses cause disease is not yet understood as not all prawns with a specific virus will exhibit disease symptoms or suffer mortality. Disease appears to occur when the prawns are not able to control the covert form of infection. There is no evidence to indicate that a genetic change (i.e. mutation) in the virus is required to cause disease. There are many possible triggers for diseases to appear, including external factors such as environmental stress (i.e. poor water quality). In some viruses, the size and age of the prawn seem to determine the likelihood of disease outbreak (i.e. IHHNV), or a secondary, complicating infection.
References
- Anderson I.G. & Owens L. (2001) The diagnosis and prevention of the mid‐crop mortality syndrome of pond‐reared black tiger prawns (Penaeus monodon). Fisheries Research and Development Corporation Project 96/301 ISBN: 0 7345 01455.
- Boonyaratpalin S., Supamattaya K., Kasornchandra J., Direkbusaracom S., Aekpanithanpong U. & Cantanachookin A. (1993) Non‐occluded baculo‐like virus, the causative agent of yellow‐head disease in the black tiger shrimp (Penaeus monodon). Fish Pathology 28, 103–109. [Google Scholar]
- Callinan R.B., Jiang L., Smith P.T. & Soowannayan C. (2003) Fatal, virus‐associated peripheral neuropathy and retinopathy in farmed Penaeus monodon in eastern Australia. I. Pathology. Diseases of Aquatic Organisms 53, 181–193. [DOI] [PubMed] [Google Scholar]
- Chantanachookin C., Boonyaratpalin S., Kasornchandra J., Direkbusarakom S., Ekpanithanpong U., Supamataya K., Sriurairatana S. & Flegel T.W. (1993) Histology and ultrastructure reveal a new granulosis‐like virus in Penaeus monodon affected by yellow‐head disease. Diseases of Aquatic Organisms 17, 145–157. [Google Scholar]
- Chou H.Y., Huang C.H., Wang C.H., Chiang H.C. & Lo C.F. (1995) Pathogenicity of a baculovirus infection causing White Spot Syndrome in cultured penaeid shrimp in Taiwan. Diseases of Aquatic Organisms 23, 165–173. [Google Scholar]
- Cowley J.A. & Walker P.J. (2002) The complete genome sequence of gill‐associated virus of Penaeus monodon prawns indicate a genome organisation unique among nidoviruses. Archives of Virology 147, 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Wongteerasupaya C., Boonsaeng V., Panyim S. & Walker P.J. (1999) Yellow head virus from Thailand and gill‐associated virus from Australia are closely related but distinct prawn viruses. Diseases of Aquatic Organisms 36, 153–157. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Spann K.M. & Walker P.J. (2000a) Gill‐associated virus of Penaeus monodon prawns: an invertebrate virus with ORF 1a and ORF 1b genes related to arteri‐ and coronaviruses. Journal of General Virology 81, 1473–1484. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock C.M., Spann K.M. & Walker P.J. (2000b) Detection of Australian gill‐associated virus (GAV) and lymphoid organ virus (LOV) of Penaeus monodon by RT‐nested PCR. Diseases of Aquatic Organisms 39, 159–167. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Dimmock CM., Spann K.M. & Walker P.J. (2001) Gill‐associated virus of Penaeus monodon prawns; molecular evidence for the first invertebrate nidovirus. Advances in Experimental Medicine and Biology 498, 43–48. [PubMed] [Google Scholar]
- Cowley J.A., Hall M.R., Cadogan L.C., Spann K.M. & Walker P.J. (2002) Vertical transmission of gill‐associated virus (GAV) in the black tiger prawn Penaeus monodon . Diseases of Aquatic Organisms 50, 95–104. [DOI] [PubMed] [Google Scholar]
- Cowley J.A., Cadogan L.C., Wongteerasupaya C., Hodgson R.A.J., Boonsaeng V. & Walker P.J. (2004) Multiplex RT‐nested PCR differentiation of gill‐associated virus (Australia) from yellow head virus (Thailand) of Penaeus monodon . Journal of Virological Methods 117, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley J.A., McCulloch R., Spann K., Cadogan L. & Walker P. (2005) Preliminary molecular and biological characterisation of Mourilyan virus (MoV): a new bunya‐related virus of penaeid prawns. In: Diseases in Asian Aquaculture V (ed. by Walker P.J., Lester R.G. & Bondad‐Reantaso M.G.), pp. 113–124. Fish Health Section, Asian Fisheries Society, Manila, Philippines. [Google Scholar]
- Dhar A.K., Roux M. & Klimpel K.R. (2002) Quantitative assay for measuring the Taura syndrome virus and yellow head virus load in shrimp by real‐time RT‐PCR using SYBR Green chemistry. Journal of Virological Methods 104, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovsky A., Paynter J.L., Sambhi S.K., Atherton J.G. & Lester R.J.G. (1988) Observations on the ultrastructure of baculovirus in Australian Penaeus monodon and Penaeus merguiensis . Australian Journal of Freshwater Resources 39, 743–749. [Google Scholar]
- Flegel T.W. (1997) Special topic review: major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World Journal of Microbiology and Biotechnology 13, 433–442. [Google Scholar]
- Flegel T.W. & Pasharawipas T. (1998) Active viral accommodation: a new concept for crustaceans response to viral pathogens. In: Advances in Shrimp Biotechnology. Proceedings of the Special Session on Shrimp Biotechnology, 5th Asian Fisheries Forum, Chiengmai, Thailand, 11–14 November 1998, BIOTEC (ed. by Flegel T.W.), pp. 245–250.Bangkok, Thailand. [Google Scholar]
- Flegel T.W., Boonyaratpalin S. & Withyachumnarnkul B. (1997) Progress in research on yellow‐head virus and white‐spot virus in Thailand In: Disease in Asian Aquaculture III (ed. by Flegel T.W. & MacRae I.H.), pp. 285–295. Fish Health Section, Asian Fisheries Society, Manila, Philippines. [Google Scholar]
- Food and Agriculture Organization of the United Nations: Fisheries Global Information System . (http://www.fao.org/figis/servlet/static?dom=root&xml=aquaculture/index.xml). In: Global Aquaculture Production 1950–2004.
- Fraser C.A. & Owens L. (1996) Spawner‐isolated mortality virus from Australian Penaeus monodon . Diseases of Aquatic Organisms 27, 141–148. [Google Scholar]
- Kiatpathomchai W., Jitrapakdee S., Panyim S. & Boonsaeng V. (2004) RT‐PCR detection of yellow head virus (YHV) infection in Penaeus monodon using dried haemolymph spots. Journal of Virological Methods 119, 1–5. [DOI] [PubMed] [Google Scholar]
- Krabsetsve K., Cullen B.R. & Owens L. (2004) Rediscovery of the Australian strain of infectious hypodermal and haematopoietic necrosis virus. Disease of Aquatic Organisms 61, 153–158. [DOI] [PubMed] [Google Scholar]
- Lightner D.V. (1996) A handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp. World Aquaculture Society, Baton Rouge, Louisiana, USA. [Google Scholar]
- Lightner D.V., Bell T.A. & Redman R.M. (1989) A review of the known hosts, geographical range and current diagnostic proceedures for the virus diseases of cultured penaid shrimp. Advances in Tropical Aquaculture, Actes de Colloques 9 IFREMER, 113–126. [Google Scholar]
- Lightner D.V. & Redman R.M. (1998) Shrimp diseases and current diagnostic methods. Aquaculture 164, 201–220. [Google Scholar]
- Lightner D.V., Redman R.M., Poulos B.T., Nunan L.M., Mari J.L. & Hasson K.W. (1997) Risk of spread of penaeid shrimp viruses in the Americas by the international movement of live and frozen shrimp Scientific and Technical Review of the Office International des Epizooties, 16, 146–160. In: Walker P.J., Cowely J.A., Spann K.M., Hodgson R.A.J., Hall M. & Withyachumnarnkul B. (2001) Yellow head complex viruses: Transmission cycles and topographical distribution in the Asia‐Pacific region. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture (ed. by C.L. Browdy & D.E. Jory), pp. 292–302. The World Aquaculture Society, Baton Rouge, LA, USA. [DOI] [PubMed] [Google Scholar]
- Limsuwan C. (1991) Handbook for Cultivation of Black Tiger Prawns. Tansetakit, Bangkok, Thailand (in Thai), 202pp. Paper unavailable, In: Walker P.J., Cowely J.A., Spann K.M., Hodgson R.A.J., Hall M. & Withyachumnarnkul B. (2001) Yellow head complex viruses: Transmission cycles and topographical distribution in the Asia‐Pacific region. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture (ed. by C.L. Browdy & D.E. Jory), pp. 292–302. The World Aquaculture Society, Baton Rouge, LA, USA. [Google Scholar]
- Liu W., Wang Y.T., Tian D.S., Yin Z.C. & Kwang J. (2002) Detection of white spot syndrome virus (WSSV) of shrimp by means of monoclonal antibodies (MAbs) specific to an enveloped protein (28 kDa). Diseases of Aquatic Organisms 49, 11–18. [DOI] [PubMed] [Google Scholar]
- Loh P.C., Tapay L.M., Lu Y. & Nadala E.C.B. Jr. (1997) Viral pathogens of penaeid shrimp. Advances in Viral Research 48, 263–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longyant S., Sattaman S., Chaivisuthangkura P., Rukpratanporn S., Sithigorngul W. & Sithigorgul P. (2006) Experimental infection of some penaeid shrimps and crabs by yellow head virus (YHV). Aquaculture 257, 83–91. [Google Scholar]
- Lu Y., Tapay L.M., Brock J.A. & Loh P.C. (1994) Infection of the yellow head baculo‐like virus (YBV) in two species of penaeid shrimp, Penaeus stylirostris (Stimpson) and Penaeus vannamei (Boone). Journal of Fish Diseases 17, 649–656. [Google Scholar]
- Lu Y., Tapay L.M., Loh P.C., Brock J.A. & Gose R.B. (1995) Distribution of yellow‐head virus in selected tissues and organs of penaeid shrimp Penaeus vannamei . Diseases of Aquatic Organisms 23, 67–70. [Google Scholar]
- Lundin C.G. (1997) Global attempt to address shrimp disease Second Asian‐Pacific Marine Biotechnology Conference and third Asia‐Pacific Conference on Algal Biotechnology. Paper unavailable, In: Fegan D.F. & Clifford H.C. III (2001) Health management for viral diseases in shrimp farms. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture (ed. by C.L. Browdy & D.E. Jory), pp. 168–198. The World Aquaculture Society, Baton Rouge, LA, USA
- Mayo M.A. (2002) A summary of taxonomic changes recently approved by the ICTV. Archives of Virology 147, 1655–1656. [DOI] [PubMed] [Google Scholar]
- Mohan C.V. (1996) Health management strategy for a rapidly developing shrimp industry ‐ an Indian perspective In: Health Management in Asian Aquaculture. Proceedings of the Regional Expert Consultation on Aquaculture Health Management in Asia and the Pacific. FAO Fisheries Technical Paper 360 (ed. by Subasinghe R.P., Arthur J.R. & Shariff M.), pp. 75–87. FAO, Rome, Italy. Paper unavailable, In: Mohan C.V., Shankar K.M., Kulkarni S. & Sudha P.M. (1998) Histopathology of cultured shrimp of yellow head syndrome and white spot syndrome during 1994 Indian epizootics. Diseases of Aquatic Organisms 34, 9–12. [DOI] [PubMed] [Google Scholar]
- Mohan C.V., Shankar K.M., Kulkarni S. & Sudha P.M. (1998) Histopathology of cultured shrimp showing gross signs of yellow head syndrome and white spot syndrome during 1994 Indian epizootics. Diseases of Aquatic Organisms 34, 9–12. [DOI] [PubMed] [Google Scholar]
- Munro J. & Owens L. (2005) Haemagglutination as a low‐cost detection method for gill‐associated virus and by inference, yellowhead virus in Penaeus monodon Fabricius, 1798. Aquaculture Research 36, 1369–1373. [Google Scholar]
- Nadala E.C.B., Tapay L.M. & Loh P.C. (1997) Yellow‐head virus: a rhabdovirus‐like pathogen of penaeid shrimp. Diseases of Aquatic Organisms 31, 141–146. [Google Scholar]
- Nadala E.C.B., Tapay L.M. & Loh P.C. (1998) Characterization of a non‐occluded baculovirus‐like agent pathogenic to penaeid shrimp. Diseases of Aquatic Organisms 33, 221–229. [DOI] [PubMed] [Google Scholar]
- Natividad K.D.T., Magbanua F.O., Migo V.P., Alfafara C.G., Albaladejo J.D., Nadala E.C.B., Loh P.C. & Tapay L.D. (1999) Detection of yellow head virus in cultured black tiger shrimp (Penaeus monodon Fabricius) from selected shrimp farms in the Philippines Paper unavailable, In: Walker P.J., Cowely J.A., Spann K.M., Hodgson R.A.J., Hall M. & Withyachumnarnkul B. (2001) Yellow head complex viruses: Transmission cycles and topographical distribution in the Asia‐Pacific region. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture (ed. by C.L. Browdy & D.E. Jory), pp. 292–302. The World Aquaculture Society, Baton Rouge, LA, USA
- Owens L., De Beer S. & Smith J. (1991) Lymphoidal parvovirus‐like particles in Australian penaeid prawns. Diseases of Aquatic Organisms 11, 129–134. [Google Scholar]
- Owens L., Anderson I.G., Kenway M., Trott L. & Benzie A.H. (1992) Infectious hypodermal and haematopoietic necrosis virus (IHHNV) in a hybrid penaeid prawn from tropical Australia. Diseases of Aquatic Organisms 14, 219–228. [Google Scholar]
- Owens L., Haqshenas G., McElnea C. & Coelen R. (1998) Putative spawner‐isolated mortality virus associated with mid‐crop mortality syndrome in farmed Penaeus monodon from northern Australia. Diseases of Aquatic Organisms 34, 177–185. [DOI] [PubMed] [Google Scholar]
- Pantoja C.R. & Lightner D.V. (2003) Similarity between the histopathology of white spot syndrome virus and yellow head syndrome virus and its relevance to diagnosis of YHV disease in the Americas. Aquaculture 218, 47–54. [Google Scholar]
- Siddell S.G. (1995) The Coronaviridae: an introduction. In: The Coronaviridae (ed. by Siddell S.G.), pp 1–176. Plenum Press, New York, USA. [Google Scholar]
- Sithigorngul P., Chauychuwong P., Sithigorngul W., Longyant S., Chaivisuthangkura P. & Menasveta P. (2000) Development of a monoclonal antibody specific to yellow head virus (YHV) from Penaeus monodon . Diseases of Aquatic Organisms 42, 27–34. [DOI] [PubMed] [Google Scholar]
- Sithigorngul P., Rukpratanporn S., Longyant S., Chaivisuthangkura P., Sithigorngul W. & Menasveta P. (2002) Monoclonal antibodies specific to yellow‐head virus (YHV) of Penaeus monodon . Diseases of Aquatic Organisms 49, 71–76. [DOI] [PubMed] [Google Scholar]
- Smith P.T. (2000) Diseases of the eye of farmed shrimp Penaeus monodon . Diseases of Aquatic Organisms 43, 159–173. [DOI] [PubMed] [Google Scholar]
- Spann K.M. & Lester R.J.G. (1997) Special topic review: viral disease of penaeid shrimp with particular reference to four viruses recently found in shrimp from Queensland. World Journal of Microbiology and Biotechnology 13, 419–426. [Google Scholar]
- Spann K.M., Vickers J.E. & Lester R.J.G. (1995) Lymphoid organ virus of Penaeus monodon from Australia. Diseases of Aquatic Organisms 23, 127–134. [Google Scholar]
- Spann K.M., Cowley J.A., Walker P.J. & Lester R.J.G. (1997) A yellow‐head‐like virus from Penaeus monodon cultured in Australia. Diseases of Aquatic Organisms 31, 169–179. [Google Scholar]
- Spann K.M., Donaldson R.A., Cowley J.A. & Walker P.J. (2000) Differences in the susceptibility of some penaeid prawn species to gill‐associated virus (GAV) infection. Diseases of Aquatic Organisms 42, 221–225. [DOI] [PubMed] [Google Scholar]
- Spann K.M., McCulloch R.J., Cowley J.A., East I.J. & Walker P.J. (2003) Detection of gill‐associated virus (GAV) by in situ hybridization during acute and chronic infections of Penaeus monodon and P. esculentus . Diseases of Aquatic Organisms 56, 1–10. [DOI] [PubMed] [Google Scholar]
- Subasinghe R.P., Bartley D.M., McGladdery S. & Barg U. (1998) Sustainable shrimp culture development: biotechnologies issues and challenges. In: Advances in Shrimp Biotechnology (ed. by T.W. Flegel), pp. 13–18. BIOTEC, Bangkok, Thailand. Paper unavailable, In Fegan D.F. & Clifford H.C. III (2001) Health management for viral diseases in shrimp farms. In: The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture (ed. by C.L. Browdy & D.E. Jory), pp. 168–198. The World Aquaculture Society, Baton Rouge, LA, USA
- Tang K.F.J. & Lightner D.V. (1999) A yellow head virus gene probe: nucleotide sequence and application for in situ hybridisation. Diseases of Aquatic Organisms 35, 165–173. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Spann K.M., Owens L. & Lightner D.V. (2002) In situ detection of Australian gill‐associated virus with a yellow head virus gene probe. Aquaculture 205, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel M.H.V. (2000) Introduction to the species concept in virus taxonomy In: Virus Taxonomy, Seventh Report of the International Committee on Taxonomy of Viruses (ed. by Van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R. & Wickner R.B.), pp. 3–16. Academic Press, London, UK. [Google Scholar]
- Vega E.D.L., Degnan B.M., Hall M.R., Cowley J.A. & Wilson K. (2004) Quantitative real‐time RT‐PCR demonstrates that handling stress can lead to rapid increases of gill‐associated virus (GAV) infections levels in Penaeus monodon . Diseases of Aquatic Organisms 59, 195–203. [DOI] [PubMed] [Google Scholar]
- Walker P.J., Cowley J.A., Spann K.M., Hodgson R.A.J., Hall M. & Withyachumnarnkul B. (2001) Yellow head complex viruses: transmission cycles and topographical distribution in the Asia‐Pacific region. In: The new wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture (ed. by Browdy C.L. & Jory D.E.), pp. 227–237. The World Aquaculture Society, Baton Rouge, LA, USA. [Google Scholar]
- Wang Y.C. & Chang P.S. (2000) Yellow head virus infection in the giant tiger prawns Penaeus monodon cultured in Taiwan. Fish Pathology 35, 1–10. [Google Scholar]
- Wang C.S., Tang K.F.J., Kou G.H. & Chen S.N. (1996) Yellow head disease‐like virus infection in the Kuruma shrimp Penaeus japonicus cultured in Taiwan. Fish Pathology 31, 177–182. [Google Scholar]
- Wongteerasupaya C., Sriurairatana S., Vickers J.E., Akrajamorn A., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnkul B. & Flegel T.W. (1995) Yellow‐head virus of Penaeus monodon is an RNA virus. Diseases of Aquatic Organisms 22, 45–50. [Google Scholar]
- Wongteerasupaya C., Vickers J.E., Sriurairatana S., Nash G.L., Akarajamorn A., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnkul B. & Flegel T.W. (1995) A non‐occluder, systemic baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn Penaeus monodon . Diseases of Aquatic Organisms 21, 69–77. [Google Scholar]
- Wongteerasupaya C., Tongchuea W., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnkul B. & Flegel T.W. (1997) Detection of yellow‐head virus (YHV) of Penaeus monodon by RT‐PCR amplification. Diseases of Aquatic Organisms 31, 181–186. [Google Scholar]
- Yang Y.G., Shariff M., Lee L.K. & Hassan M.D. (2000) Malaysia In: Thematic Review on Management Strategies for Major Diseases in Shrimp Aquaculture. (ed. by Subasingh R.P., Arthur J.R., Phillips M.J. & Reantaso M.B.), Proceedings of World Bank/NACA/World Wildlife Fund/FAO Workshop. Cebu City, Philippines. (1999) . FAO, Rome, Italy. [Google Scholar]


