Abstract
Practical evidence suggests possible beneficial effects with the combined use of prebiotics and probiotics which can improve production parameters. The objective of the study was to investigate the addition of Saccharomyces cerevisiae (SC) as prebiotic and the combination of Lactobacillus spp. (L), Bacillus spp. (B) as probiotics on productive parameters and economic feasibility. Four hundred male pigs, hybrids of commercial genetic lines (Pietrain), were used: T1 = control group, T2 = 4 kg/tonne SC, T3 = 0.8 kg/tonne feed L and B, T4 = 4 kg/tonne SC + 0.8 kg/tonne L and B. Productive parameters were recorded in the treatment groups for four periods. Then, the viscera of five pigs per treatment were collected after slaughter to evaluate the histological changes and cytokine concentrations in the ileum. The weight gains of groups at 70–100, 100–125 and 125–150 days in the T4 group showed statistically increases (p < .05). Feed intake had a significant difference (p < .05) in T3 versus T1. The feed‐conversion ratio improved for all periods in the T4 group (p < .05). The eosinophil, mononuclear infiltration and cytokines (tumor necrosis factor‐α and interleukin‐6) in the mucosa were lower for treatments with probiotics. In conclusion, there was an economic benefit when using both prebiotics and probiotics in the diet of pigs from weaning to finishing.
Keywords: costs, intestine, performance, pigs, prebiotics, probiotics
1. INTRODUCTION
Pig breeding is promoted by intensive commercial industry, but economic losses due to diseases and compromised well‐being of the animals during the fattening process limit their production. There is a high risk of neonatal diseases, and preventive and prophylactic measures against diarrhea and pneumonia are critical. For the 4–10 days after weaning, diarrhea caused by colibacillosis is very common (Zamora, Reinhardt, Polette, & Macias, 1999); the extrinsic causes are the sudden deprivation of maternal antibodies due to diet changes as well as variations in temperature, humidity and other environmental conditions that affect the immune system of the animal (Bojkovski, Vasiljević, Stojanović, & Rogožarski, 2014). During the fattening phase (after weaning), swine dysentery is another problem, so the use of antibiotics is a common practice to avoid this issue. Antibiotics have been successfully used to control disease spread and to improve growth; however, their use is already prohibited in some countries, and research has been directed toward the study of growth promoters that do not affect human health (Álvarez‐Ordóñez, Martínez‐Lobo, Arguello, Carvajal, & Rubio, 2013).
The use of prebiotics and probiotics in pigs is still being explored to improve productive parameters (Czech, Mokrzycka, Grela, & Pejzak, 2009), but the information available depends on the type, dose and time of administration. In particular, prebiotics act by stimulating several microorganisms that are beneficial to the gastrointestinal tract. For example, the addition of a Saccharomyces cerevisiae fermentation product has been shown to improve the presence of Bacteroides and Lactobacillus (Price et al., 2014), increasing villus height in the small intestine and developing systemic immunity in early‐weaned piglets. Specifically, Saccharomyces cerevisiae CNCM I‐3856 is able to inhibit the enterotoxigenic Escherichia coli induced pro‐inflammatory cytokines and chemokines, associated with an inhibition of the mitogen‐activated protein kinase pathway (Zongyong et al., 2015).
In the case of probiotics, benefits in growth, weight gain improvement, feed conversion (Musa, Wu, Zhu, Seri, & Zhu, 2009), and carcass output and quality (Jukna, Jukna, & Simkus, 2005) have been reported. The physiological action of probiotics depends mainly on the establishment of the beneficial intestinal flora (O′Toole & Cooney, 2008), which decreases the competitive ability of pathogenic bacteria (Robles‐Huaynate et al., 2013). Probiotic bacteria can be viable microorganisms in wet, frozen and lyophilized preparations or as fermented products (Gaggìa, Mattarelli, & Biavati, 2010; Utiyama, Oetting, Giani, Ruiz, & Miada, 2006). Most research published on probiotics and prebiotics has been carried out in newborns with the purpose of decreasing mortality caused by diarrhea; the therapeutic dose varies widely, from 110 to 1,012 viable organisms per animal per day, and the number of organisms must be enough to trigger a beneficial response in the host without inducing digestive disorders (Santos et al., 2016). Information on the combination of prebiotics and probiotics in pigs after weaning is scarce. The purpose of the present research was to evaluate one prebiotic and two probiotics and their combination to assess the productive parameters, intestinal morphological response and economic feasibility in pigs from weaning to 150 days of age.
2. MATERIALS AND METHODS
2.1. Study site and experimental animals
The study was conducted at the Santa Rosa pig farm and in the physiology, pathology and animal nutrition laboratories of the School of Veterinary Medicine at the Benemérita Universidad Autónoma de Puebla (BUAP). These sites are located in the valley of Tecamachalco, Puebla, Mexico at a latitude of 18° 52′ 57′′ north and a longitude of 97° 43′ 49′′ west at 2,055 masl, dominated by a mild climate with a mean temperature of 18°C and rainfall of 700 mm per year (Instituto Nacional de Estadística, Geografía e Informática [INEGI], 2013).
A total of 400 male pigs, hybrids of commercial genetic lines (LM 100 × Pietrain), were used. The selected animals were weaned at 21 days old, with a weight range between 5.92 and 6.05 kg live weight. Then, the animals were distributed by weight, with 100 animals per each of the following treatments: T1 = Control Group (without dosing of pre‐probiotics); and Supplemented Groups (with combinations of strains): T2 = 4 kg/tonne Saccharomyces cerevisiae (Diamond® V Mills, Cedar Rapids, IA, USA, containing at least 88.5% of yeast culture with a total microbial activity of 1.6 × 109 colony forming units [cfu]/oz); T3 = 0.8 kg/tonne (a mixture was prepared in equal proportions of Lactobacillus spp. [containing at least 87%, 50 × 106 cfu/g FD‐DVS nu‐trish® LA‐5®] and Bacillus spp. [BioPlus 2B® is manufactured by Chr. Hansen A/S Horsholm, Denmark]). The combination of spray‐dried‐spore‐forming B. subtilis CH201/DSM 5750 and B. licheniformis CH200/DSM 5749 contained at least 3.2 × 109 viable spores/g) and T4 = 4 kg/tonne Saccharomyces cerevisiae + 0.8 kg/tonne Lactobacillus spp. and Bacillus spp. Each treatment was repeated five times with 20 pigs per pen, provided by a feeder and automatic waterer. The care and handling of animals was approved by the ethics committee of the School of Veterinary Medicine and Animal Science at the BUAP and by the regulation of the Mexican Official Standard on Animal Care published by the NOM‐062‐ZOO‐1999 (SAGARPA 1999).
Diets were formulated based on the nutrient requirements of the National Research Council (NRC, 1998) considering the following phases from 21 to 150 days: (i) starter (21–70 days) 19.4% protein (P) and 3.35 Mcal/kg metabolizable energy (ME); (ii) growth (70–100 days) 17.1% P and 3.26 Mcal/kg ME; (iii) development (100–125 days) 16.53% P and 3.23 Mcal/kg ME; (iv) fattening (125–150 days) 15.8% P and 3.2 Mcal/kg ME. Diets were prepared weekly and stored in plastic containers, and samples were obtained periodically to determine the dry matter content using a forced air oven at 60°C. Pigs had free access to feed, considering a daily surplus of 10% in the hopper feeders. Animals were weighed at the beginning of the study, and then the average daily gain (ADG) was recorded for the periods of 21–70, 70–100, 100–125 and 125–150 days. Weighing was performed from 7:00 to 9:00 hours, before providing feed, using an electronic scale with an accuracy of ±10 g (Torrey®, México City, México). The amount of feed offered to each animal based on dry matter coincided with the weighing periods specified (feed intake: FI). The feed‐conversion ratio (FCR) was calculated with the following formula: FCR = FI/ADG.
At the end of the feeding trial, all pigs were weighed (final weight: FW) and slaughtered according to the Mexican Official Standard NOM‐033‐ZOO‐1995 (SAGARPA 1995), using a captive bolt stunner and bleeding by cutting the jugular vein. After slaughter, the animals were eviscerated, and the carcasses were cooled in a chamber maintained at 4°C. The following day, the cold carcass weight (CCW) was recorded, and the cold dressing percentage was calculated as = CCW × 100/FW.
2.2. Viscera collection, intestinal morphology and immunological response
A total of five pigs from each treatment group were randomly selected. The viscera of each pig were collected immediately after slaughter and stored briefly in plastic bags previously identified. Intestinal length was measured, and 1 cm long samples were obtained from the ileum and were fixed with 10% buffered formalin for 24 hours before being embedded in paraffin. Then, 5 μm‐thick sections were cut from each sample, and the sections were stained with hematoxylin‐eosin. No clinical signs of digestive disease were present in the selected pigs; however, an analysis of microscopic lesions at the ileum level was performed to determine morphological changes in the intestinal epithelium mucosa and submucosa, and description of the histological changes in the ileum was performed. The lesions and atrophy related to the villi and crypts were assessed in a scale ranging from normal (Grade 0) to mild (Grade 1) to moderate (Grade 2) to severe (Grade 3). The microscopic variables were quantified with a Likert scale. A photo of the intestine was selected in each treatment, based on the clarity and common findings observed in the microscopy.
2.2.1. Intestinal tissue cytokines concentration
Previously, intestinal tissue samples were thawed from −80°C to room temperature. One hundred milligrams of ileum were homogenized in 1 ml of phosphate‐buffered saline (PBS) solution (0.4 mol/L NaCl and 10 mmol/L NaPO4) containing anti‐proteases (0.1 mmol/L phenylmethanesulfonyl fluoride, 0.1 mmol/L benzethonium chloride, 10 mmol/L ethylenediaminetetraacetic acid and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 15 min at 3,000 × g, and the supernatant was used immediately for ELISA at a third dilution in PBS. The quantity of interleukin 6 (IL‐6) and tumor necrosis factor alpha (TNF‐α) were measured using commercially available kits (ELISA‐Quantikine Kits; R&D Biotechne a Brand Systems Inc. Minneapolis, MN, USA). All samples were analyzed in triplicate within a single assay. IL‐6 and TNF‐α have a sensitivity of 10 and 23 pg/ml, respectively.
2.3. Statistical analysis and model
Data were assessed for normality and homogeneity of variance using the Shapiro–Wilk test and Levene's test, respectively. Then, data were analyzed with a univariate analysis using SPSS® Version 15 software (SPSS Inc., Chicago, IL, USA). Each repetition per pen was considered one experimental unit. The alpha level used for the determination of the significant differences using Tukey's test was set at p ≤ .05. The statistical model used was as follows:
where μ is the general mean, Y ijk = response variable (ADG, FI, FCR) in the different feeding phases, A i = effect of the ith level of inclusion of prebiotic treatment i … n, B j = effect of jth level of inclusion of probiotic treatment i … n, (ABij) = the interaction of treatment i … n, and E = the experimental error ijk.
The non‐parametric variables observed in the intestinal morphology were analyzed with a non‐parametric statistical analysis using the Kruskal–Wallis test.
2.4. Economic analysis
The economic analysis measured the nutritional feasibility level that was invested during the meat production process, based on a detailed study on the feed investment and the prebiotics and probiotics added to the diets, considering the costs for return on investment.
3. RESULTS
3.1. Productive parameters
Interaction effects between treatments were not found (p > .05). Table 1 shows the productive variables analyzed in pigs. The weight gains of the groups at 70–100, 100–125 and 125–150 days in T4 showed statistically significant increases (p < .05) of 10%, 10.7% and 16%, respectively. There were no significant differences (p > .05) in FI between the study periods, with the exception of T3, which was highest in FI in the 70–100 days period and was increased 9% (p < .05) compared to the other treatments. The FCR improved throughout all periods in T4: 21–70 days, 15.8%; 70–100 days, 13.6%; 100–125 days, 11.3%; and 125–150, 15.1% (p < .05). No significant differences in cold dressing percentages were found between treatments.
Table 1.
Productive parameters of pigs supplemented with pre‐ and probiotics and their combination
| Indicatora | T1 | T2 | T3 | T4 | SEM |
|---|---|---|---|---|---|
| Weight (kg) | |||||
| Initial | 6.05ª | 5.96ª | 6.19ª | 5.92ª | 0.06 |
| Final | 26.63ª | 26.73ª | 27.02ª | 30.07b | 0.06 |
| Days | Average daily gain (kg) | ||||
| 21–70 | 0.42ª | 0.42ª | 0.43ª | 0.49ª | 0.01 |
| 70–100 | 0.67ª | 0.68ª | 0.68ª | 0.75b | 0.01 |
| 100–125 | 0.92ª | 0.93ª | 0.93ª | 1.03b | 0.02 |
| 125–150 | 1.13ª | 1.14ª | 1.15ª | 1.31b | 0.02 |
| Feed intake (kg) | |||||
| 21–70 | 0.67ª | 0.67ª | 0.67ª | 0.67ª | 0.12 |
| 70–100 | 1.55ª | 1.55ª | 1.68b | 1.54ª | 0.10 |
| 100–125 | 2.84ª | 2.83ª | 2.83ª | 2.83ª | 0.11 |
| 125–150 | 4.00a | 4.00a | 4.00a | 4.00a | 0.12 |
| Feed conversion ratio | |||||
| 21–70 | 1.59ª | 1.59a | 1.55a | 1.36b | 0.03 |
| 70–100 | 2.31b | 2.27bc | 2.46c | 2.06ª | 0.03 |
| 100–125 | 3.08b | 3.05b | 3.05b | 2.75ª | 0.05 |
| 125–150 | 3.54b | 3.51b | 3.48b | 3.05ª | 0.08 |
| Dressing (%) | |||||
| 50.20ª | 50.88a | 52.52a | 50.01a | 1.38 | |
T1 = without dosing of pre‐probiotics; T2 = 4 kg/tonne Saccharomyces cerevisiae; T3 = 0.8 kg/tonne: Lactobacillus spp. + Bacillus spp.; T4 = 4 kg/tonne Saccharomyces cerevisiae + 0.8 kg/tonne Lactobacillus spp. and Bacillus spp. Different letters in the same row indicate a significant difference.
One hundred pigs per treatment.
3.2. Intestinal findings and immunological response
Table 2 shows the histopathological lesions and the concentration of two cytokines in the ileum of pigs supplemented with prebiotics, probiotics and their combination. The villi lengths were lower (p < .05) for T1 and T3 versus T2 and T4. The pH in the ileum decreased one unit in the treatment groups supplemented with probiotics. The epithelium status was better for T1 and T4 than for T2 and T3. The eosinophil and mononuclear infiltrations in the mucosa were lower with the probiotic treatments, whereas the mononuclear infiltration in the submucosa increased drastically in T4 compared to the other treatments. The hyperplasia in Peyer's patches and the congestion of the submucosa venules did not show uniformity in the data, and the differences were disproportionate. Figure 1 shows a photographic representation of the histological sections obtained from the ileum of pigs at 150 days of age. The histological examination of the ileum showed mild to moderate lesions for the four experimental treatments. There was a slight colonization of bacteria adhered to enterocytes, and a second pathological finding was mononuclear and eosinophilic infiltrations in the intestinal epithelium, which were also present in the four treatments. Another condition was congestion, which was found with greater intensity in the ileum of T2 and T3. Mixed infiltration was found in the lamina propria of the mucosa. In the submucosa, edema, congestion and cell necrosis in the Peyer's patches were diagnosed, being lowest in T4. The microscopic lesions diagnosed in each treatment were mild, except for T1, which had more severe alterations.
Table 2.
Histopathological lesions and cytokine concentrations in the ileum of pigs supplemented with pre‐ and probiotics and their combination
| Indicatora | T1 | T2 | T3 | T4 | SEM |
|---|---|---|---|---|---|
| Length, m | 3.6 | 4.2 | 3.7 | 4.0 | |
| pH in ileum | 8.33a | 7.33b | 7.33b | 7.33b | 0.89 |
| Epithelium status | 1.67ª | 2.33b | 2.00c | 1.67a | 0.55 |
| Eosinophil infiltration | 2.33ª | 1.33b | 1.33c | 1.33c | 0.60 |
| Mononuclear infiltration | 2.00ª | 2.00a | 1.33b | 1.33b | 0.33 |
| Capillary congestion | 1.00ª | 1.33b | 1.33b | 1.33b | 0.33 |
| Hyperplasia in crypts | 1.33ª | 1.00b | 1.00b | 1.33a | 0.33 |
| Mononuclear infiltration | 1.33a | 1.00b | 1.33a | 1.67c | 0.33 |
| Hyperplasia of Peyer's patches | 2.00ª | 1.33b | 1.00c | 2.00ª | 0.12 |
| Venule congestion | 1.33ª | 1.00b | 1.00b | 1.33a | 0.33 |
| Cytokines, pg/ml | |||||
| Tumor necrosis factor (TNF‐α) | 139.75a | 152.71a | 116.32b | 90.35b | 8.21 |
| Interleukin 6 (IL‐6) | 76.91ab | 83.78a | 68.51b | 55.25c | 7.16 |
T1 = without dosing of pre‐probiotics; T2 = 4 kg/tonne Saccharomyces cerevisiae; T3 = 0.8 kg/tonne: Lactobacillus spp. + Bacillus spp.; T4 = 4 kg/tonne Saccharomyces cerevisiae + 0.8 kg/tonne Lactobacillus spp. and Bacillus spp. Different letters in the same row indicate a significant difference.
Five pigs from each treatment.
Figure 1.
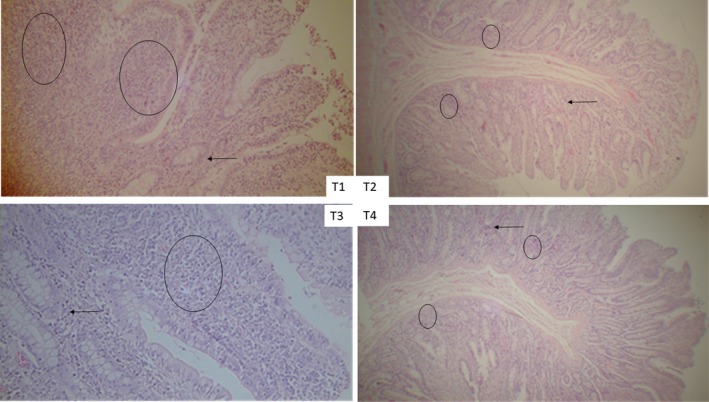
Microscopic lesions in the ileum. T1: ileum, 40×. A moderate inflammatory response with a predominance of macrophages, lymphocytes and eosinophils in the lamina propria was observed (indicated with the circles). There is slight congestion in the mucosa (indicated with the arrow). T2: with prebiotics, ileum, 10×. The lamina propria of the ileum shows moderate mononuclear infiltration (indicated with the circles) and congestion of the mucosal and submucosal capillaries (indicated with the arrow). T3: with probiotics, ileum, 40×. The mucosa shows moderate mononuclear infiltration and mild eosinophilic infiltration (indicated with the circle). The capillaries are congested (indicated with the arrow). T4: with interaction between prebiotics + probiotics. 10x. Lymphoid tissue associated with the intestines (Peyer's Patch) (indicated with the circles). Hyperplasia of germinal centers is found (indicated with the arrow).
TNF‐α concentration was similar (p = .21) between T1 versus T2 and T3 versus T4 (p = .33). T3 and T4 had lower (p < .01) concentrations than T1 and T2. IL‐6 concentration was similar (p = .13) between T1 versus T2 and T1 versus T3. T4 was lower (p < .02) than T1, T2 and T3. However, pigs in the T4 showed lower (p < .02) intestinal proinflammatory cytokine concentrations.
3.3. Economic feasibility
Table 3 shows the costs (in dollars) generated from feeding and production during all phases of the fattening. The net income was greater than $2.5 in T4 versus T1, followed by T2 which had a net income of $0.50 versus T1.
Table 3.
Costs (dollars) and economic feasibility for pigs fattened with prebiotics and probiotics in the diet
| Concept | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Percentage of mortality | 5 | 2 | 2 | 0 |
| Mean cost per kg of feed | 0.3 | 0.3 | 0.3 | 0.3 |
| Operating production costs (fixed costs)/kg live weight produced | 0.3 | 0.3 | 0.3 | 0.3 |
| Invested cost of feed/pig | 71.6 | 72.6 | 73.8 | 73.7 |
| Production cost per feed/kg live weight produced | 0.7 | 0.7 | 0.7 | 0.7 |
| Production cost/kg live weight produced | 1.1 | 1.1 | 1.1 | 1.1 |
| Price of the pig for sale | 1.3 | 1.3 | 1.3 | 1.3 |
| Net income/kg sold | 0.2 | 0.2 | 0.2 | 0.2 |
| Net income/weight gain/treatment/pig | 19.6 | 19.5 | 18.8 | 21.1 |
| Net income/weight at sale/treatment/pig | 20.9 | 20.8 | 20.1 | 22.4 |
| Net income, taking mortality into account (variable costs) | 18.6 | 19.1 | 18.4 | 21.1 |
T1 = without dosing of pre‐probiotics; T2 = 4 kg/tonne Saccharomyces cerevisiae; T3 = 0.8 kg/tonne: Lactobacillus spp. + Bacillus spp.; T4 = 4 kg/tonne Saccharomyces cerevisiae + 0.8 kg/tonne Lactobacillus spp. and Bacillus spp.
4. DISCUSSION
4.1. Productive parameters
One of the problems attributed to the early weaning of pigs is the reduction of feed intake, in particular during the first week after weaning, which likely causes intestinal villous atrophy and poor nutrient absorption, affecting weight gain (Utiyama et al., 2006). Therefore, the use of prebiotic oligosaccharides incorporated into the diet, at a dose of 5–40 g/kg, induces a beneficial population of microorganisms in different intestinal segments, maintaining the health of the animals (Mikkelsen, Jakobsen, & Jensen, 2003). However, the post‐weaning use of prebiotics and probiotics is very questionable; several authors (Fedalto, Tkacz, & Ader, 2002; Mikkelsen et al., 2003; Sanches et al., 2006; Santos et al., 2003) reported a lack of improvement in weight gain and feed conversion from 22 to 54 days of age. In theory, prebiotics can improve the digested volume as a result of the increase in microbial biomass, and consequently, the action of a probiotic is suitable (Lan, Tran, & Kim, 2017; Utiyama et al., 2006). In our study, the ADG response was greatest in T4 (combined use of prebiotic and probiotics), and the FCR improved throughout all the fattening periods. Another study reported an increase in weight gain with a combination of probiotics (Taras, Vahjen, & Simon, 2007), and the use of anaerobic fermentable products of Saccharomyces cerevisiae has also been reported to improve weight gain in weaned piglets due to the increase in beneficial microorganisms in the intestine (Price et al., 2014). Therefore, the inconsistencies among the above‐mentioned authors can be explained by the type of prebiotic and probiotics used for each experiment. The inclusion levels of efficient prebiotics in feed improve the concentration of bifidobacteria and lactobacilli in the intestine, although high concentrations might affect the digestibility of nutrients (Cross, 2002). Prebiotics improve energy efficiency, allowing the animal to generate muscle mass and a desired body weight for the market (Branner & Roth‐Maier, 2006).
4.2. Intestinal findings and immunological response
Atrophy constituted one of the most important pathologies in T1, as demonstrated by an alteration in the relationship between villus length and crypt depth, limiting the normal processes of water and electrolyte absorption, excretion (Acres, 1985; Arruda, Madson, Ramirez, Rowe, & Songer, 2016), and higher concentration of cytokines such as TNF‐α and IL‐6 (Nyachoti, Kiarie, Bhandari, Zhang, & Krause, 2012). The intestinal villous atrophy in T1 was mild in the ileum compared with the other treatments. Hornich, Salajka, Sarmanova, Ulmann, and Sedlacek (1975) observed the importance of atrophy of the intestinal villi in pig ilea in diets that did not include any additive or growth promoter. They concluded that atrophy not only affects the normal mechanisms of absorption but also allows development of pathogenic microorganisms such as Rotavirus and Coronavirus, which are important agents responsible for lesions of the intestinal epithelium. On the other hand, in other studies, reporting that enterotoxigenic E. coli strains do not cause significant villi atrophy, and when atrophy is produced in newborn piglets, it is associated with enteroinvasive E. coli. (Barker & Van Dreumel, 1991; Castillo, Martín‐Orúe, Nofrarías, Manzanilla, & Gasa, 2007). Microscopically, the ileum of T1 pigs was the most affected segment, showing congestion, bacterial adherence, epithelial vacuolization of intestinal villi, atrophy, eosinophilic infiltration, mononuclear infiltration (greater inflammation) and lymphoid necrosis in Peyer's patches, with the T1 pigs showing the most severe lesions compared to the other treatments. Lemos et al. (2005) studied the effect of probiotics and prebiotics added to the diet of pigs on the structure of small intestine villi and found atrophy when these additives were not included. On the other hand, the concentration of cytokines was essential and corroborated the presence of an inflammatory reaction in the ileum. TNF‐α and IL‐6 are proinflammatory cytokines that play a critical role in normal host resistance to infection, serving as immmunomodulators of inflammatory responses (Nyachoti et al., 2012). Both are primarily created by activated macrophages through many signals, such as ionophores, lipopolysaccharide, and antigenic stimulation by pathogenic microorganisms (MohanKumar et al., 2017). TNF‐α and IL‐6 are mostly derived from lymphocytes and macrophages, but it was produced at a lower concentration with pre‐probiotics; perhaps this type of cytokines is also produced by endothelial cells.
Probiotics should resist the low pH and proteolytic enzymes in the digestive tract (Hou, Zeng, Yang, Liu, & Qiao, 2015). The retention time, the mixture of material ingested with the gastric juices and previously digested food influence the survival of the probiotic strains administered. In the anterior part of the small intestine, the most important defense is the rapid flow velocity, which prevents the excessive growth of microbes when the microorganisms do not adhere to the epithelium. The presence of bile in this region also represses the survival and activity of microorganisms. In the cecum, probiotics have to compete with the native microflora already established in the healthy animal, but the rate of passage is slower, and the microorganisms establish more easily (Morais, Berto, Hauptli, Wechsler, & Trindade, 2010). In this area, the epithelial cells are continuously released and are covered by intestinal bacterial cells, including lactobacilli (Chiquieri et al., 2006; Yang, Hou, Zeng, & Qiao, 2015). In our study, the continuous administration of probiotics throughout the entire fattening phase maintained a constant proliferation of lactic acid bacteria; physiologically, the food particles take approximately 2.5 hours to pass through the small intestine (Choudhari, Shinde, & Ramteke, 2008), and during this time, it is difficult for the pathogenic bacteria to multiply quickly; consequently, the probiotics predominate (Estienne, Hartsock, & Harper, 2005). Lactic acid bacteria (Lactobacillus and Streptococcus spp.) generally predominate in the small intestine and help reduce the pH of the stomach because of the production of lactic acid and organic acids (Utiyama et al., 2006).
The immune system of piglets is fully functional at weaning, but it is possible that it needs to be stimulated to prevent diarrhea. Probiotics can stimulate the immune system, and hypersensitivity responses in early weaned piglets can be induced by components of the diet. The performance and health can be assessed by growth rates, the use of feed, the number of deaths and the occurrence of diarrhea (Rai, Yadav, & Lakhani, 2013). Other benefits that have been reported for the combination of prebiotics and probiotics in pigs are the stimulation of intestinal motility, mineral absorption, the elimination of ammonium and the stimulation of the immune system (Cross, 2002; Yang et al., 2015). In our study, the prebiotic influenced the majority of non‐digestible oligosaccharides, which can control or manipulate the microbial composition and/or activity and help maintain a beneficial microflora that in turn inhibits the growth of pathogens. The combination of prebiotics and probiotics improves the stabilization and survival of probiotics during their passage through the digestive tract (Zimmermann et al., 2016). Mainly, the ingestion of prebiotics can stimulate the activity of pre‐existing species, and probiotic strains, such as lactobacilli, bacilli and bifidobacteria, compete for adhesive access to attachment sites on epithelial cells, provided by mannose‐specific interactions which prevent colonization by pathogenic bacteria (Solis, de los Reyes‐Gavilan, Fernández, Margolles, & Gueimonde, 2010). The combination of prebiotics and probiotics exert effects on mucosal barrier function and the responses of the underlying immune tissue of the gut‐associated lymphoid tissue. They can induce antimicrobial peptides against pathogens as bacteriocins and probiotics also produce the expression of immunoglobulin (Ig) receptors on the basolateral surface of intestinal epithelial cells to enhance transcytosis of IgA through the epithelial cell of the gut (Cross, 2002; Hou et al., 2015), demonstrating the ability of probiotics to improve the intestinal epithelial barrier function.
4.3. Economic feasibility
There were no dead pigs in T4. Choudhari et al. (2008) found a positive relationship between reduced mortality and the addition of a growth promoter based on prebiotics and probiotics to pig feed. The investment return rate (IRR) was determined, considering the variable costs attributable to treatments and the benefits derived from the sale of live pigs. T4 had the highest economic benefits, followed by T1, T2 and T3. T4 had a better IRR than that of T1: 7.5:1. Similarly, Modesto et al. (2009) found that piglets fed with growth promoters based on prebiotics and probiotics had better economic benefits.
5. CONCLUSION
The post‐weaning mortality risk decreased in T4 due to the additive effect of the prebiotic and probiotics. The intake in T4 was better than in the other treatments. The intestinal cytokines concentrations were lower and the pH in the ileum was neutral, which inhibited the growth of pathogenic enterobacteria in the intestinal tract. Economically, greater benefit was obtained when using 4 kg/tonne prebiotics + 0.800 kg/tonne probiotics in the food, and a marginal return of 7.5:1 was found. The administration of 4 kg/tonne of growth promoter based on prebiotics and 0.800 kg/tonne based on probiotics in the diet of pigs from weaning to finishing is recommended. This addition achieves an increase in weight gain and improves the feed conversion, and there is a net economic benefit of 1:7.5.
Méndez‐Palacios N, Méndez‐Mendoza M, Vázquez‐Flores F, Castro‐Colombres JG, Ramírez‐Bribiesca JE. Productive and economic parameters of pigs supplemented from weaning to finishing with prebiotic and probiotic feed additives. Anim Sci J. 2018;89:994–1001. 10.1111/asj.13008
REFERENCES
- Acres, S. D. (1985). Enterotoxigenic Escherichia coli infection in new borne calves. Review. Journal of Dairy Science, 68, 229–252. 10.3168/jds.s0022-0302(85)80814-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez‐Ordóñez, A. , Martínez‐Lobo, F. J. , Arguello, H. , Carvajal, A. , & Rubio, P. (2013). Swine dysentery: Aetiology, pathogenicity, determinants of transmission and the fight against the disease (Review). International Journal of Environment Research Public Health, 10, 1927–1947. 10.3390/ijerph10051927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda, P. H. , Madson, D. M. , Ramirez, A. , Rowe, E. W. , & Songer, J. G. (2016). Bacterial probiotics as an aid in the control of Clostridium difficile disease in neonatal pigs. Canadian Vetetinary Journal, 57, 183–188. [PMC free article] [PubMed] [Google Scholar]
- Barker, I. K. , & Van Dreumel, A. A. (1991). El sistema digestivo In Jubb K. V. F., PC K. & Palmer N. (Eds.), Patología de los Animales Domésticos (Vol. 2, 3rd ed., p. 80–84). Hemisferio Sur, Montevideo: Montevideo, Uruguay. [Google Scholar]
- Bojkovski, J. , Vasiljević, T. , Stojanović, D. , & Rogožarski, D. (2014). Health control of pig herds on commercial farms. Arhiv Veterinarske Medicine, 7, 59–69. [Google Scholar]
- Branner, G. R. , & Roth‐Maier, D. A. (2006). Influence of pre‐, pro‐, and synbiotics on the intestinal availability of different B‐vitamins. Archives of Animal Nutrition, 60, 191–204. 10.1080/17450390600678985 [DOI] [PubMed] [Google Scholar]
- Castillo, M. , Martín‐Orúe, S. M. , Nofrarías, M. , Manzanilla, E. G. , & Gasa, J. (2007). Changes in caecal microbiota and mucosal morphology of weaned pigs. Veterinary Microbiology, 124, 239–247. 10.1016/j.vetmic.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Chiquieri, J. M. S. , Soares, R. T. , Hurtado, R. N. , Nery, V. L. , Ferreira, R. A. , & Ventura, B. G. (2006). Probiotic and prebiotic in swine feeding in growing and finishing. Archives of Zootecnie, 55, 305–308. [Google Scholar]
- Choudhari, A. , Shinde, S. , & Ramteke, B. N. (2008). Prebiotics and probiotics as health promoter. Veterinary World, 1, 59–61. [Google Scholar]
- Cross, M. L. (2002). Microbes versus microbes: Immune signals generated by probiotic lactobacilli and their role inprotection against microbial pathogens. FEMS Immunology and Medical Microbiology, 34, 245–253. 10.1111/j.1574-695x.2002.tb00632.x [DOI] [PubMed] [Google Scholar]
- Czech, A. , Mokrzycka, A. , Grela, E. R. , & Pejzak, Z. (2009). Influence of mannanoligossacharides additive to sow diets on blood parameters of sows and their piglets. Bulletin of the Veterinary Institute in Pulawy, 53, 89–95. [Google Scholar]
- Estienne, M. J. , Hartsock, T. G. , & Harper, A. F. (2005). Effects of antibiotics and probiotics on suckling pig and weaned pig performance. International Journal of Applied Research in Veterinary Medicine, 3, 303–308. [Google Scholar]
- Fedalto, L. M. , Tkacz, M. , & Ader, L. P. (2002). Probioticos na alimentação de leitões do desmame ao 63 dias de idade. Archives of Veterinary Science, 7, 83–88. [Google Scholar]
- Gaggìa, F. , Mattarelli, P. , & Biavati, B. (2010). Probiotics and prebiotics in animal feeding for safe food production. International Journal of Food Microbiology, 141, 15–28. 10.1016/j.ijfoodmicro.2010.02.031 [DOI] [PubMed] [Google Scholar]
- Hornich, M. , Salajka, E. , Sarmanova, Z. , Ulmann, L. , & Sedlacek, M. (1975). Histopathological changes produced by two enteropathogenic strains of Escherichia coli in gnotobiotic piglets. Journal of Comparative Pathology, 85, 277–283. 10.1016/0021-9975(75)90069-9 [DOI] [PubMed] [Google Scholar]
- Hou, C. , Zeng, X. , Yang, F. , Liu, H. , & Qiao, S. (2015). Study and use of the probiotic Lactobacillus reuteri in pigs: A review. Journal of Animal Science and Biotechnology, 6, 14 10.1186/s40104-015-0014-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Estadística, Geografía e Informática (INEGI) . (2013). Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Tecamachalco, Puebla. Clave geoestadística 21154. [Google Scholar]
- Jukna, C. , Jukna, V. , & Simkus, A. (2005). The effect of probiotics and phytobiotics on meat properties and quality in pigs. Veterinarija ir zootechnika, 29, 80–84. [Google Scholar]
- Lan, R. , Tran, H. , & Kim, I. (2017). Effects of probiotic supplementation in different nutrient density diets on growth performance, nutrient digestibility, blood profiles, fecal microflora and noxious gas emission in weaning pig. Journal of Science Food Agriculture, 97, 1335–1341. 10.1002/jsfa.7871 [DOI] [PubMed] [Google Scholar]
- Lemos, B. F. E. , Thomaz, M. C. , Kronka, R. N. , Okada, N. L. S. , Marcussi, T. F. , Fraga, A. L. , … Robles, H. R. A. (2005). Effect of probiotics and prebiotics inclusion in weaned piglet diets on structure of small intestine. Brazilian Archives of Biology and Technology, 48, 921–929. [Google Scholar]
- Mikkelsen, L. L. , Jakobsen, M. , & Jensen, B. B. (2003). Effects of dietary oligosaccharides on microbial diversity and fructo‐oligosaccharide degrading bacteria in faeces of pigs post‐weaning. Animal Feed Science and Technology, 109, 144–150. [Google Scholar]
- Modesto, M. , D'Aimmo, M. R. , Stefanini, I. , Trevisi, P. , De Filippi, S. , Casini, L. , … Biay, B. (2009). A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livestock Science, 122, 248–258. 10.1016/j.livsci.2008.08.017 [DOI] [Google Scholar]
- MohanKumar, K. , Namachivayam, K. , Ho, T. , Torres, B. A. , Ohls, R. K. , & Maheshwari, A. (2017). Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Seminars in Perinatology, 41, 52–60. 10.1053/j.semperi.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais, K. M. , Berto, D. A. , Hauptli, L. , Wechsler, F. S. , & Trindade, M. A. (2010). Probióticos para leitões lactentes na fase de creche. Vetérinaria e Zootecnia, 17, 519–527. [Google Scholar]
- Musa, H. H. , Wu, S. L. , Zhu, C. H. , Seri, H. I. , & Zhu, G. Q. (2009). The potential benefits of probiotics in animal production and health. Journal of Animal and Veterinary Advances., 8, 313–321. [Google Scholar]
- National Research Council (NRC) (1998). Nutrient requirement of swine (p. 189). Washington: National Academy. [Google Scholar]
- Nyachoti, C. M. , Kiarie, E. , Bhandari, S. K. , Zhang, G. , & Krause, D. O. (2012). Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. Journal of Animal Science, 90, 252–260. 10.2527/jas.2010-3596 [DOI] [PubMed] [Google Scholar]
- O′Toole, P. W. , & Cooney, J. C. (2008). Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiciplinary perspectiveson infectious diseases, 2008, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, K. L. , Totty, H. R. , Lee, H. B. , Utt, M. D. , Fitzner, G. E. , Yoon, I. , … Escobar, J. (2014). Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. Journal of Animal Science, 88, 3896–3908. [DOI] [PubMed] [Google Scholar]
- Rai, V. , Yadav, B. , & Lakhani, G. P. (2013). Applications of probiotic and prebiotic in animals production: A review. Enviroment and Ecology, 31, 873–876. [Google Scholar]
- Robles‐Huaynate, R. A. , Thomaz, M. C. , Santana, A. E. , Masson, G. C. I. H. , Amorim, A. B. , Silva, S. Z. , … Budiño, F. E. L. (2013). Efeito da adição de probiótico em dietas de leitõesdesmamados sobre as características do sistema digestório e do desempenho. Revista Brasileira de Saúde e Produção Animal, 14, 248–258. 10.1590/s1519-99402013000100009 [DOI] [Google Scholar]
- SAGARPA . (1995). Official Mexican Standard. Humanitarian slaughter of domestic and wild animals ‐ Official Gazette of the Federation, Mexico. [Google Scholar]
- SAGARPA . (1999). Official Mexican Standard. NOM‐062‐ZOO‐1999. Specifications for animal care. Official Journal of the Federation, Mexico. [Google Scholar]
- Sanches, A. L. , Lima, J. A. F. , Fialho, E. T. , Murgas, L. D. S. , Almeida, E. C. , Vieira Neto, J. , & Freitas, R. T. F. (2006). Utilização de probiótico, prebiótico e simbiótico em rações de leitões ao desmame. Ciência e Agrotecnologia, 30, 774–777. 10.1590/s1413-70542006000400026 [DOI] [Google Scholar]
- Santos, W. G. , Filgueiras, E. P. , Bertechini, A. G. , Fialho, E. T. , Lima, J. A. F. , & Brito, M. A. V. P. (2003). Manose na alimentação de leitões na fase de creche: Desempenho, pH do tratogastrintestinal e peso de órgãos. Ciência e Agrotecnologia, 27, 696–702. 10.1590/s1413-70542003000300027 [DOI] [Google Scholar]
- Santos, A. V. , Tadeu, F. E. , Zangerônimo, M. G. , de Souza, C. , da Silva, T. T. , & Pelição, M. T. J. (2016). Additive antibiotic, probiotic and prebiotic for early weaned piglets. Ciencia Animal Brasileira, 17, 1–10. [Google Scholar]
- Solis, G. , de los Reyes‐Gavilan, C. G. , Fernández, N. , Margolles, A. , & Gueimonde, M. (2010). Establishment and development of lactic acid bacteria and bibidobacteria microbiota in breast‐milk and the infant gut. Anaerobe, 16, 307–310. 10.1016/j.anaerobe.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Taras, D. , Vahjen, M. M. , & Simon, O. (2007). Probiotics in pigs: Modulation of their intestinal distribution and of their impact on health and performance. Livestock Science, 108, 229–231. 10.1016/j.livsci.2007.01.075 [DOI] [Google Scholar]
- Utiyama, C. A. , Oetting, L. L. , Giani, P. A. , Ruiz, U. S. , & Miada, V. S. (2006). Efeitos de antimicrobianos, prebióticos, probióticos e extratos vegetais sobre a microbiota intestinal, a freqüência de diarréia e o desempenho de leitões recém desmamados. Revista Brasileira Zootecnia, 35, 2359–2367. 10.1590/s1516-35982006000800023 [DOI] [Google Scholar]
- Yang, F. , Hou, C. , Zeng, X. , & Qiao, S. (2015). The use of lactic acid bacteria as a probiotic in swine diets. Review. Pathogens, 4, 34–45. 10.3390/pathogens4010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora, J. , Reinhardt, G. , Polette, M. , & Macias, P. (1999). Diarrea neonatal porcina. Aislamiento de cepas de Escherichia coli toxigénicas productoras de STa, LT y VT. Archivos de medicina veterinaria, 31, 237–242. [Google Scholar]
- Zimmermann, J. A. , Fusari, M. L. , Rossler, E. , Blajman, J. E. , Romero‐Scharpen, E. , Astesana, D. M. , … Berisvil, A. P. (2016). Effects of probiotics in swines growth performance: A meta‐analysis of randomised controlled trials. Animal Feed Science and Technology, 219, 280–293. 10.1016/j.anifeedsci.2016.06.021 [DOI] [Google Scholar]
- Zongyong, J. , Shaoyong, W. , Zhilin, W. , Cui, Z. , Shenglan, H. , Chuntian, Z. , … Xuefen, Y. (2015). Effects of different forms of yeast Saccharomyces cerevisiae on growth performance, intestinal development, and systemic immunity in early‐weaned piglets. Journal of Animal Science and Biotechnology, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


