Abstract
Previous evaluations of the effect of ultraviolet (UV) light on Cryptosporidium parvum oocysts have been limited to a single strain—the Iowa strain. This study investigated the response of five strains of C. parvum to UV. A collimated beam apparatus was used to apply controlled doses of monochromatic (254 nm) UV to oocysts of the Iowa, Moredun, Texas A&M, Maine, and Glasgow strains. Irradiation was measured using a calibrated radiometer and sensor. Inactivation was quantified through animal infectivity by inoculation of cohorts of CD‐1 neonatal mice with UV‐treated and untreated control oocysts of each strain followed by examination of intestinal sections for infection using hemotoxylin and eosin staining. A UV light dose of 10 mJ/cm2 achieved at least 4‐log10 inactivation of all strains evaluated. All five strains of C. parvum were shown to be highly susceptible to low levels of UV light.
Keywords: Cryptosporidium, Regulations, Compliance, Best Available Technology, Ultraviolet Disinfection
Cryptosporidium is recognized as an important and widely distributed enteric pathogen of young livestock and is common in a variety of mammals (Casemore et al, 1997). The life cycle may result in production of resistant oocysts that exhibit long survival times in the aquatic environment. Certain species, including C. parvum (Chappell et al, 1996; Dupont et al, 1995), C. felis (Caccio et al, 2002), C. meleagridis (Pedraza‐Diaz et al, 2001), C. baileyii (Ditrich et al, 1991), C. canis (Fayer et al, 2001), and possibly others, have been classified as zoonotic agents and have been associated with severe diarrheal disease in humans, although C. parvum is the significant human pathogen. Chronic infection may occur in the immunocompromised population, where mortality rates as high as 53% have been observed (Kramer et al, 1996).
This study examined UV's efficacy at doses of 5–40 mJ/cm2 for inactivating five strains of Cryptosporidium known to be infectious to humans.

Under the proposed Long Term 2 Enhanced Surface Water Treatment Rule (LT2ESWTR) (USEPA, 2003a), large surface water treatment facilities will have to perform source water characterization monitoring to determine treatment requirements specific to the system. Treatment options will include, but are not limited to, filtration and chemical disinfection. However, the oocysts are marginally affected by chlorine‐based chemical disinfectants (Finch et al, 1993; Korich et al, 1990) and ozone (AWWARF, 1997) at practicable application levels. In addition, concern over the by‐products of chemical disinfection has prompted investigations of alternative disinfection methodologies. Although utilities will be permitted to implement ultraviolet (UV) disinfection for regulatory compliance, they will be compelled to meet minimum dose requirements based on the known UV dose–response of pathogens such as Cryptosporidium. A draft UV guidance manual (USEPA, 2003b) prepared by the US Environmental Protection Agency (USEPA) in conjunction with industry stakeholders, was made available in June 2003 and provides additional guidance for utilities intent on implementing UV disinfection for control of Cryptosporidium and other waterborne pathogens.
Factors influencing average irradiation to the entire volume included reflection from the water surface, depth of the water, and ultraviolet absorbance of the inoculated test water.
UV Background
UV has long been recognized as an effective disinfectant of bacteria (Zelle & Hollaender, 1955; Sharp, 1939) and viruses (Wilson et al, 1992; Chang et al, 1985; Hill et al, 1970). UV promotes the formation of pyrimidine dimers within the nucleic acids of irradiated cells (Mitchell & Nairn, 1989). The extent of inactivation is a function of characteristics specific to the organism and the UV dose, which is the product of the incident irradiance expressed as mW/cm2 and the time (in seconds) of exposure. This product is expressed as mW·s/cm2 and is equivalent to mJ/cm2. The dose requirements for 4‐log10 (99.99%) inactivation of microorganisms range from 3–10 mJ/cm2 for vegetative bacteria (Wilson et al, 1992; Chang et al, 1985) to 20–50 mJ/cm2 for a number of human enteric viruses (Wilson et al, 1992).
UV and drinking water treatment. UV technology has been applied to drinking water for control of bacteria and viruses in Europe (Sommer et al, 1998) and in wastewater in the United States since the 1950s. Until recently, however, it had not been accepted as an effective disinfectant for control of Cryptosporidium. Early research using in vitro methods (excystation and vital dye stains) to assess oocyst viability following UV treatment suggested that a UV dose of 120 mJ/cm2 would achieve only 2‐log10 inactivation (Ransome et al, 1993) and that doses >8,700 mJ/cm2 were necessary for 3‐log10 inactivation (Campbell et al, 1995).
More recent investigations, using animal infectivity to assess oocyst survival, have shown UV to be an effective disinfectant of C. parvum oocysts, achieving 2‐log10 inactivation at 2 mJ/cm2 (Shin et al, 2001) and 4‐log10 inactivation at 8 mJ/cm2 (Clancy et al, 2000). Several other studies corroborated these findings that C. parvum is susceptible to inactivation by low doses of UV light (Mackey et al, 2002; Craik et al, 2001; Mofidi et al, 2001; Hargy et al, 2000; Bukhari et al, 1999). Each of these studies used Iowa strain oocysts. Any assessment of the applicability of UV to drinking water treatment facilities for disinfection of Cryptosporidium oocysts must characterize the UV dose–response of Cryptosporidium strains (other than the Iowa isolate of C. parvum) that are infectious to humans.
Five strains studied. The current study examined the response to UV doses of 5–40 mJ/cm2 of five strains of C. parvum isolated from different sources across North America and Europe. Cryptosporidium strains were obtained from Scotland (Moredun and Glasgow strains) and the United States (Iowa, Maine, and Texas A&M University [TAMU] strains). Previous genotyping studies of these isolates indicated that they belong to C. parvum genotype 2 and are capable of causing infection in both humans and some animals (Rochelle et al, 1999; Spano et al, 1998; Carraway et al, 1997; Peng et al, 1997; Ortega et al, 1991).
Origin of Iowa strain. The Iowa strain of C. parvum is regularly maintained at the Sterling Parasitology Laboratory at the University of Arizona in Tucson and was originally obtained from Harley Moon of the National Animal Disease Center in Ames, Iowa. It has been used to determine the C. parvum infectious dose–response in neonatal mice (Korich et al, 2000; Finch et al, 1993; Korich et al, 1990) and to characterize the sensitivity of Cryptosporidium to various disinfectants including chlorine, chlorine dioxide, monochloramine (Korich et al, 1990), ozone (Bukhari et al, 2000; AWWARF, 1997; Finch et al, 1993), and UV light (Clancy et al, 2000; Bukhari et al, 1999; Clancy et al, 1998). In addition, the Iowa isolate has been used in various studies examining infection characteristics and the immune response of Cryptosporidium in controlled experiments with healthy human volunteers (Okhuysen et al, 1998; Chappell et al, 1996; Dupont et al, 1995). It has also been used for infectivity studies in gamma interferon knockout mice (Mead & You, 1998).
Origin of Moredun strain. The Moredun strain was obtained from Steve Wright of the Moredun Research Institute in Penicuk, Scotland. It was originally isolated from a red deer calf (Cervus elaphus) (Blewett, 1989) and has been passaged in calves and lambs for approximately 12 years. This isolate has been used in environmental survival studies (Robertson et al, 1992), infectivity studies (Bukhari & Smith, 1997; Bukhari et al, 1995), biochemical studies (Awad‐el‐Karim et al, 1998; Nina et al, 1992), volunteer studies, and immunological characterization studies (Ortega‐Mora & Wright, 1994). This isolate is used in the quality assurance/quality control approach instigated by the United Kingdom Drinking Water Inspectorate for continuous monitoring programs for finished waters (UKDWI, 2003).
Origin of TAMU strain. The TAMU strain was obtained from a veterinary student who was exposed to oocysts during necropsy of an infected foal. The isolate has since been propagated in calves at the Sterling Parasitology Laboratory. Dose–response studies indicate that the ID50 (the dose required to infect 50% of exposed individuals) for the TAMU isolate in healthy volunteers (ID50 = 9) may be approximately 10‐fold lower than the ID50 of the prototype Iowa isolate (ID50 = 87) (Okhuysen et al, 1998) and perhaps 100‐fold lower than the infective dose of the Ungar C. parvum isolate (ID50 = 1,042) (Okhuysen et al, 1999).
Origin of Maine strain. The Maine strain was obtained from Michael Arrowood of the Centers for Disease Control and Prevention in Atlanta, Ga. This isolate was responsible for an outbreak of cryptosporidiosis traced to contaminated apple cider (Millard et al, 1994). Oocysts were isolated directly from the cider, the press used for preparing the cider, and a calf stool specimen from the farm that supplied the apples. Experimental infection studies showed that this isolate is capable of infecting mice, calf, and human hosts; it has been characterized as a genotype 2 isolate using the TRAP‐C2 polymorphic marker (Peng et al, 1997).
Origin of Glasgow strain. The Glasgow strain was received from Huw Smith at the Scottish Parasite Diagnostic Laboratory in Glasgow, Scotland. These oocysts were purified from feces of human patients involved in a waterborne outbreak in Glasgow (Smith, 2001).
An analyst prepares dilutions of coliphage MS2 for enumeration of surviving fraction following ultraviolet irradiation.

Materials and Methods
Microorganism strains and growth conditions. The C. parvum oocysts used in this study were maintained and propagated in the following manner. Within 4–12 h of birth, Holstein calves were fed 2 × 108 oocysts suspended in sterile water. The calves were given four pints of colostrums 1–2 h following inoculation. Calves also received prophylactic doses of oral rotavirus, coronavirus, and Escherichia coli vaccines. Subsequently, the calves were maintained on a diet providing 2,200 kcal/day supplied by a combination of milk replacer, nonfat milk powder, and electrolyte mix.
The total daily fecal output from the infected calves was collected, screened through sieves, and concentrated by centrifugation. Oocysts were isolated from the feces by discontinuous sucrose gradients followed by microcentrifuge‐scale cesium chloride gradients (Arrowood & Donaldson, 1996; Arrowood & Sterling, 1987). The purified oocysts were stored at 4°C in 0.01% polyoxyethylenesorbitan monolaurate solution 1 containing 100 U of penicillin, 100 μg of streptomycin, and 100 μg of gentamicin/mL to retard bacterial growth. These oocysts were used between 10 and 30 days after shedding. Following purification, the excystation rate of each oocyst batch was measured prior to each trial (Robertson et al, 1993). Oocysts were deemed acceptable for use only if the in vitro excystation efficiency was ≥85%.
Viability of C. parvum oocysts. Prior to each exposure series, fresh oocysts were subjected to in vitro excystation assay for viability characterization to determine suitability for disinfection testing. Upon receipt of oocysts in the testing laboratory, the excystation characteristics of each lot of oocysts were determined according to the modified in vitro excystation method described by Robertson and colleagues (1993). According to excystation, the viability prior to UV irradiation ranged from 93 to 98% for the five strains.
Neonatal mouse infectivity assay. CD‐1/ICR dams with four‐to‐five‐day‐old litters were obtained from Charles River Laboratories in Wilmington, Mass. Neonatal mice (five to seven days' old) were inoculated by delivery of a 10‐μL dose of oocysts suspended in sterile water to the back of the throat with a calibrated pipette. Approximately 20 neonatal mice were inoculated at each dose. After a seven‐day incubation, infectivity was determined by microscopic examination of formalin‐fixed longitudinal sections (5 μm × 2–6 cm) of the terminal ileum stained with hemotoxylin and eosin. Infection was defined as the observation of C. parvum parasite development stages in the microvilli of any of the prepared histological sections. Tissues from each mouse were scored plus (infected) or minus (not infected) as determined by microscopic observation.
Oocyst infectivity was assessed using the logit doseresponse mouse model proposed by Finch and co‐workers (1993) and modified by Korich and colleagues (2000). This model relates the proportion of mice infected to the number of oocysts inoculated. Briefly, response logit (RL) was calculated as the natural logarithm of the proportion of animals infected divided by one minus the proportion of animals infected. That is,
| (1) |
in which P is the proportion of animals infected at a given oocyst dose. The untreated oocyst dose–response curves were obtained by performing a least‐squares regression of RL on the logarithm of the number of untreated oocysts in each dose. The regression analysis produced equations of the form
| (2) |
in which y is the RL, x is the logarithm of the oocyst dose, and b is the y intercept. Table 1 lists these equations for the five C. parvum strains investigated in this research.
Table 1.
Logit for the five strains of Cryptosporidium parvum
| Strain | Equation | Infective Dose |
|---|---|---|
| Iowa | y = 2.60 x – 5.23 | ID50 = 103 |
| Moredun | y = 2.65 x – 3.81 | ID50 = 28 |
| TAMU* | y = 4.35 x – 5.90 | ID50 = 23 |
| Maine | y = 1.51 x – 2.02 | ID50 = 22 |
| Glasgow | y = 2.96 x – 4.24 | ID50 = 27 |
Texas A&M University
The equations used in this study were based on dose–response data obtained by the authors as well as data from a concurrent study conducted with Rochelle and co‐workers (2002). These regression equations were used to calculate the number of infective oocysts in each dose of UV‐treated oocysts. This was done by substituting the RL derived from UV‐treated oocysts into the regression equations and solving for dose. The regression equations were also used to determine the ID50 by setting the RL equal to zero and solving for dose. Log‐inactivation levels were determined by subtracting the log of the number of infectious oocysts prior to UV exposure (D 0) from the log of the number of infectious oocysts in the treated dose (D). That is,
| (3) |
For the instances in which no infections were observed in mice fed with UV‐treated oocysts, minimum log10 inactivations were inferred using the endpoint sensitivity of the assay (1 infection per litter) and were reported as “greater than” values. Untreated control oocysts were evaluated for each test by comparing their dose–response to the appropriate dose–response curve. Controls were considered satisfactory as long as the dose–response fell within the 80% prediction bands of each curve.
Male‐specific coliphage. Coliphage MS2 2 was propagated in E. coli F‐amp 3 according to the double agar layer described by Adams (1959). E. coli F‐amp was cultured overnight in trypticase soy broth (TSB) containing ampicillin and streptomycin at 36°C, transferred to fresh TSB, and incubated for an additional 4 h at 36°C. Serial tenfold dilutions of bacteriophage stocks were prepared in phosphate‐buffered water and were added to melted top agar tubes containing 0.7% agar and 0.5% sodium chloride. Then 100 μL each of log‐phase bacteria and coliphage were added to top agar tubes; samples were mixed and poured over nutrient bottom agar plates containing 1.1% nutrient agar and 0.5% sodium chloride. Following overnight incubation at 37°C, the upper soft agar layer from confluent lysis plates was harvested with 5 mL phosphate‐buffered solution and supplemented with an equal volume of chloroform. Samples were vortexed for 60 s and then centrifuged at 5,000 × g for 15 min. Viruses in the aqueous supernatant were recovered, filtered through membranes pretreated with 0.1% polyoxyethylenesorbitan monooleate, 4 and stored at 4°C until day of use. Unused samples were discarded after 30 days.
E. coli. Cultures of E. coli 5 were prepared by inoculating 250 mL sterile TSB and incubating on a shaker apparatus at 35°C for approximately 18 h. This resulted in a late log‐phase culture with an E. coli concentration of approximately 1–3 × 108 cfu/mL. Cultures were harvested and washed one time and resuspended in deionized water for immediate use.
UV irradiation. The UV source was a low‐pressure mercury vapor lamp. 6 Low‐pressure lamps emit nearly monochromatic UV radiation at 254 nm, which can be precisely monitored with a calibrated sensor. The 254‐nm wavelength closely corresponds with peak wavelength of germicidal UV near 260 nm. This lamp was housed above a solenoid‐operated shutter connected to a digital timer. When the shutter was opened, light from the lamp passed through a 33 cm (13 in.) collimating tube to irradiate the test organisms that were suspended in a 6 cm (2.4 in.) diameter petri dish. Prior to irradiations on each day of testing, the lamp was allowed to warm up for at least 30 min.
The UV incident to the surface of the petri dish was measured using a radiometer 7 and detector 8 calibrated at 254 nm. The incident irradiation across the surface of the petri dish was measured at 5 mm (0.2 in.) intervals along an x–y grid originating at the center of the dish. The overall irradiance distribution, or petri factor, was determined relative to the center reading. This value was then used in the calculation of average irradiation incident to the water surface (Bolton & Linden, 2003). Factors influencing average irradiation to the entire volume included reflection from the water surface, depth of the water, and UV absorbance of the inoculated test water. The last was measured at 254 nm by spectrophotometry. Table 2 shows the UV254 absorbance in 1 cm (0.4 in.) and petri factor for each test. UV dosage was defined as the average irradiation in the exposure solution multiplied by the exposure time.
Table 2.
Irradiance calculation factors for Escheria coli, MS2, and Cryptosporidium parvum
| Organism Type and Identification | UV254 * Absorbance/1 cm | Petri Factor |
|---|---|---|
| E. coli 1 | 0.204 | 0.991 |
| E. coli 2 | 0.305 | 0.981 |
| E. coli 3 | 0.180 | 0.997 |
| E. coli 4 | 0.316 | 0.975 |
| MS2 1 | 0.012 | 0.991 |
| MS2 2 | 0.026 | 0.981 |
| MS2 3 | 0.215 | 0.971 |
| MS2 4 | 0.212 | 0.975 |
| C. parvum Iowa | 0.091 | 0.976 |
| C. parvum Moredun | 0.175 | 0.971 |
| C. parvum TAMU† | 0.386 | 0.981 |
| C. parvum Maine | 0.211 | 0.991 |
| C. parvum Glasgow | 0.154 | 0.982 |
UV254—ultraviolet light at 254 nm
TAMU—Texas A&M University
Irradiations of suspensions of each surrogate organism were made in duplicate across a range of exposure times. E. coli 5 was exposed to UV doses of 0, 2.5, 5.0, 7.5, and 10 mJ/cm2 and MS2 to doses of 0, 10, 20, 30, 40, and 50 mJ/cm2. Test organisms were suspended in 15 mL of deionized water in 6 cm (2.4 in.) diameter petri dishes with a 12 mm (0.5 in.) stir bar. A preenumerated stock suspension containing 5 × 107 live Cryptosporidium oocysts of the appropriate strain in 15 mL of deionized water was vortexed for 30 s and added to the continuously stirred petri dish. After 1 min, the UV lamp shutter was opened and the suspension was irradiated for the predetermined length of time. A control dose (0 mJ/cm2) was run simultaneously with the irradiation test for the highest dose level for each strain. Controls were dishes containing oocysts suspended and stirred in the test water in the absence of UV. Other research has found no photoreactivation with Cryptosporidium (Shin et al, 2001; Rochelle et al, 1999), and no precautions were taken to avoid white light exposure.
Microscopist quantifies excystation of Cryptosporidium oocysts before exposure to UV light.

Results
Confirmation of UV dose. The dose‐response curves generated for E. coli 5 and coliphage MS2 are shown in Figures 1 and 2, respectively, along with the UV dose‐responses of these organisms derived from the literature. The inactivation of E. coli 5 by 2.5 and 5 mJ/cm2 exceeded that predicted by Sommer and colleagues (1998) on two test dates. Tests using exposure times expected to provide 5 mJ/cm2 achieved inactivation levels predicted for 7.5 mJ/cm2 in the reference. Although this constitutes a 50% difference, the results do indicate that the lower target doses were not overapplied by orders of magnitude. Notably, the response of the MS2 to UV doses of 10–50 mJ/cm2 fell within the range specified in the USEPA draft UV guidance manual (USEPA, 2003b); the response to UV doses of 10–40 mJ/cm2 fell within the range suggested by the National Water Research Institute and AWWA Research Foundation guidelines (NWRI/ AWWARF, 2003). These dose comparisons support the reliability of the radiometer irradiance readings and offer confidence that the reported UV doses were applied to the Cryptosporidium oocysts.
Figure 1.
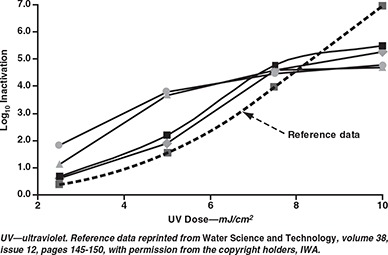
Experimental dose responses of Escherichia coli 5 compared with reference data of Sommer et al (1998)
Figure 2.
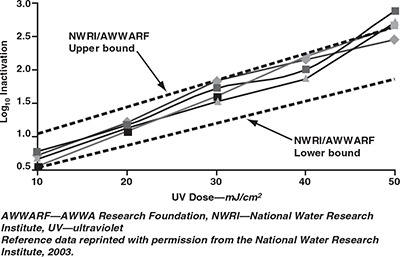
Dose–response curves of MS2 coliphage with reference to UV guidelines published by NWRI/AWWARF (2003)
UV dose‐response. Table 3 and Figure 3 show the results of UV treatment on the oocyst strains tested. UV doses of 10 and 40 mJ/cm2 were selected originally for all strains. Because other research has provided a large body of data for the Iowa strain in this UV dose range (Craik et al, 2001; Clancy et al, 2000; AWWARF, 2000; Bukhari et al, 1999; Finch & Belosevic, 1999), a reduced dosage range was evaluated in this study (2–4 mJ/cm2) to assess the effect of low UV dose for that strain. Shin and co‐workers (2001) showed that a low‐pressure UV dose of 3 mJ/cm2 resulted in 2.6‐log10 inactivation of the Iowa strain of C. parvum using cell culture to measure inactivation. The results in the current study showed good agreement with those data, with 3.2‐log10 inactivation at a UV dose of 2 mJ/cm2 and 4.1‐log10 inactivation at 4 mJ/cm2. The inactivation of the Moredun strain could not be precisely measured; UV doses of 10 and 40 mJ/cm2 resulted in complete loss of oocyst infectivity, yielding a value of at least 5.6‐log10 inactivation at 10 mJ/cm2. The TAMU strain showed >5.5‐log10 inactivation at 40 mJ/cm2 with a measured 5.2‐log10 inactivation at 10 mJ/cm2.
Table 3.
Log inactivation of five strains of Cryptosporidium parvum to UV* light
| C. parvum Oocyst Strain | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Iowa | Moredun | TAMU† | Maine | Glasgow | ||||||||||
| UV dose—mJ/cm2 | 0 | 2 | 4 | 0 | 10 | 40 | 0 | 10 | 40 | 0 | 5 | 20 | 0 | 5 | 20 |
| Number of oocysts/mouse | 1.5 × 102 | 1.1 × 104 | 9.4 × 104 | 1.3 × 102 | 9.0 × 105 | 1.5 × 105 | 1.2 × 102 | 1.4 × 106 | 1.1 × 106 | 8.1 × 101 | 1.1 × 105 | 9.6 × 105 | 1.1 × 102 | 1.0 × 106 | 1.0 × 106 |
| Number of mice | 12 | 23 | 22 | 20 | 21 | 23 | 23 | 26 | 35 | 23 | 24 | 24 | 24 | 23 | 24 |
| Number of mice infected | 7 | 1 | 1 | 8 | 0 | 0 | 22 | 4 | 0 | 20 | 7 | 3 | 22 | 1 | 0 |
| Percent of mice infected | 58.3 | 4.4 | 4.6 | 40 | <4.8 | <4.4 | 95.7 | 15.4 | <2.9 | 87.0 | 29.2 | 12.5 | 91.7 | 4.4 | <4.2 |
| Number of infective ocysts | 92 | 7 | 7 | 19 | <2 | <2 | 117 | 9 | <4 | 414 | 6 | 1 | 174 | 2 | <2 |
| Log10 inactivation | 0.0 | 3.2 | 4.1 | 0.8 | >5.6 | >4.9 | 0.0 | 5.2 | >5.5 | 0.0 | 4.3 | 5.9 | 0.0 | 5.6 | >5.7 |
UV—ultraviolet
TAMU—Texas A&M University
Figure 3.
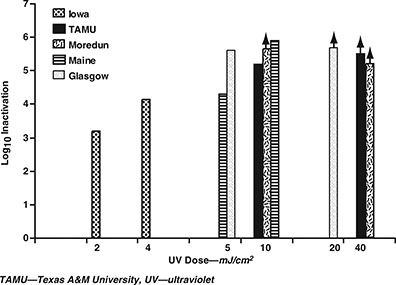
Response of five Cryptosporidium parvum oocyst strains to UV inactivation
After noting the consistent high susceptibility of the Iowa, TAMU, and Moredun strains, the authors decided to use lower UV doses (5 and 20 mJ/cm2) for the two remaining strains tested in an effort to avoid the use of assay detection limits. The Glasgow strain showed 5.6‐log10 inactivation at 5 mJ/cm2, whereas the Maine strain showed 5.9‐log10 inactivation at 20 mJ/cm2 and 4.3‐log10 inactivation at 5 mJ/cm2. A UV light dose of 10 mJ/cm2 achieved at least 4‐log10 inactivation of all strains evaluated.
Discussion
This study evaluated the response of five strains of C. parvum oocysts to UV light. Prior to this research, only the Iowa strain had been investigated for its response to UV light. Because the effectiveness of UV technologies for the control of Cryptosporidium in drinking water has been recognized only recently (AWWARF, 2000; Clancy et al, 2000; Bukhari et al, 1999; Clancy et al, 1998), questions have arisen regarding the universality of the effect of UV on other oocyst strains infective to humans. Although these questions have not been answered for other chemical disinfectants such as ozone or chorine dioxide, this study was designed to answer these questions with regard to UV.
Results showed that infectivity of all five strains tested was reduced by at least 4 log10 or 99.99% at a UV dose ≤10 mJ/cm2. In each trial, the UV irradiance measurements obtained by radiometry were verified by examining the response of organisms of known UV response—E. coli and coliphage MS2—with the test apparatus and methods and comparing the inactivation achieved against reference data.
Iowa strain oocysts were subjected to lower UV doses of 2 and 4 mJ/cm2 in order to better characterize that strain's response to low UV dose. Inactivations of 3.2 and 4.2 log10 were achieved at these doses, indicating that Cryptosporidium is more susceptible to UV inactivation than are many human enteric viruses and some enteric bacteria. These results apply only to the UV response of genotype 2 strains of C. parvum, which are capable of infecting both humans and some animals, and do not rule out the existence of some strain(s) exhibiting greater resistance to UV. However, the results do expand the under‐standing of the UV dose–response of Cryptosporidium oocysts from a single strain to five and bolster confidence in UV technology as an effective treatment of drinking water for disinfection of Cryptosporidium. Future research targeting the characterization of the UV response of Cryptosporidium should consider genotype 1 isolates as well.
In each trial, the UV irradiance measurements obtained by radiometry were verified by examining the response of organisms of known UV response with the test apparatus and methods and comparing the inactivation achieved against reference data.

UV doses as high as 40 mJ/cm2 were evaluated in preliminary experiments because this dose was originally considered as a potential requirement for treating drinking water for Cryptosporidium control in the United States. Currently, UV dose requirements for drinking water disinfection have not been mandated by the federal government. However, USEPA is considering doses that may be <12 mJ/cm2 to achieve 3‐log credit for control of Cryptosporidium (Schmelling, 2002). In Austria (Austrian Standards Institute, 2001) and Germany (DVGW, 1997), doses of 40 mJ/cm2 are required when UV disinfection of drinking water is practiced. USEPA is expected to promulgate the LT2ESWTR sometime in 2004, requiring up to 2.5‐log10 inactivation of Cryptosporidium in addition to the physical removal achieved by coagulation and filtration processes. The findings in this study support UV as a technology capable of achieving that level of inactivation. Economic analyses suggest that the application of UV at doses as high as 40 mJ/cm2 would be cost‐effective relative to ozone disinfection in small (1 mgd [3.8 ML/d]) to large (>100 mgd [379 ML/d]) drinking water utilities (Cotton et al, 2001; Dyksen et al, 1998).
Acknowledgment
The authors gratefully acknowledge the financial support of the AWWA Research Foundation.
Biography
Jennifer L. Clancy 9 is president of Clancy Environmental Consultants, Inc., PO Box 314, St. Albans, VT 05478; e‐mail jclancy@clancyenv.com. She has a BS degree in microbiology from Cornell University in Ithaca, N.Y., an MS degree in microbiology and biochemistry from the University of Vermont in Burlington, a PhD in microbiology and immunology from McGill University in Montreal, Que., and an MS degree in environmental law from Vermont Law School in South Royalton. A winner of the 2000 AWWA Best Paper Award, Clancy has more than 25 years of experience

Footnotes
Tween 20, Fisher Scientific, Pittsburgh, Pa.
No. 15597‐B1, American Type Culture Collection, Rockville, Md.
No. 700891, American Type Culture Collection, Rockville, Md.
Tween 80, Fisher Scientific, Pittsburgh, Pa.
No. 29522, American Type Culture Collection, Rockville, Md.
Atlantic Ultraviolet G12T6L, Happauge, N.Y.
1400 A, International Light, Newburyport, Mass.
SEL 240, International Light, Newburyport, Mass.
To whom correspondence should be addressed
References
- Adams, M.H. , 1959. Bacteriophages. Interscience Publishers, New York. [Google Scholar]
- Arrowood, M.J. & Donaldson, K. , 1996. Improved Purification Methods for Calf‐Derived Cryptosporidium parvum Oocysts Using Discontinuous Sucrose and Cesium Gradients. Jour. Euk. Microbiol., 43:89S. [DOI] [PubMed] [Google Scholar]
- Arrowood, M.J. & Sterling, C.R. , 1987. Isolation of Cryptosporidium Oocysts and Sporozoites Using Discontinuous Sucrose and Isopycnic Percoll Gradients. Jour. Parasitol., 73:314. [PubMed] [Google Scholar]
- Austrian Standards Institute , 2001. Austrian National Standard ÖNORM M 5873‐1. Plants for the Disinfection of Water Using Ultraviolet Radiation, Requirements and Testing. Part 1: Low‐pressure Mercury Lamps. Austrian Standards Institute, Vienna, Austria. [Google Scholar]
- Awad‐el‐Karim, F.M. et al, 1998. Differentiation Between Human and Animal Isolates of Cryptosporidium parvum Using Molecular and Biological Markers. Parasitol. Res., 84:297. [DOI] [PubMed] [Google Scholar]
- AWWARF (AWWA Research Foundation) , 2000. Viability and Infectivity of Cryptosporidium. AWWA, Denver. [Google Scholar]
- AWWARF , 1997. Effects of Various Disinfection Methods on the Inactivation of Cryptosporidium. AWWA, Denver. [Google Scholar]
- Blewett, D.A. , 1989. Disinfection and Oocysts. Cryptosporidiosis (Angus K.W. and Blewett D.A., editors) Proc. First International Workshop. Animal Diseases Research Assn., Edinburgh, Scotland. [Google Scholar]
- Bolton, J.R. & Linden, K.G. , 2003. Standardization of Methods for Fluence (UV Dose) Determination in Bench‐scale UV Experiments. ASCE Jour. Envir. Engrg., 129:3:20. [Google Scholar]
- Bukhari, Z. & Smith, H.V. , 1997. Cryptosporidium parvum: Oocyst Excretion and Viability Patterns in Experimentally Infected Lambs. Epidemiol. Infect., 119:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari, Z. et al, 2000. Cryptosporidium parvum Viability and Infectivity: Interlaboratory Comparisons to Determine Correlation Between Assays. Appl. & Envir. Microbiol., 66:7:2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari, Z. et al, 1999. Medium‐pressure UV Light for Oocyst Inactivation. Jour AWWA, 91:3:86. [Google Scholar]
- Bukhari, Z. et al, 1995. Comparison of Excretion and Viability Patterns in Experimentally Infected Animals: Potential for Release of Viable C. parvum Oocysts Into the Environment. Protozoan Parasites and Water (Betts W.B. et al, editors) Royal Society of Chemistry, Cambridge, England. [Google Scholar]
- Caccio, S. et al, 2002. Human Infection With Cryptosporidium felis: Case Report and Literature Review. Jour. Emerg. Infect. Dis., 81:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, A.T. et al, 1995. Inactivation of Oocysts of Cryptosporidium parvum by Ultraviolet Irradiation. Water Res., 29:2583. [Google Scholar]
- Carraway, M. et al, 1997. A New Restriction Fragment Length Polymorphism From Cryptosporidium parvumIdentifies Genetically Heterogeneous Parasite Populations and Genotypic Changes Following Transmission From Bovine to Human Hosts. Jour. Infect. Immun., 65:9:3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casemore, D.P. et al, 1997. Cryptosporidiosis—Human and Animal Epidemiology. Cryptosporidium and Cryptosporidiosis (Fayer R., editor). CRC Press, Boca Raton, Fla. [Google Scholar]
- Chang, J.C.H. et al, 1985. UV Inactivation of Pathogenic and Indicator Microorganisms. Appl. & Envir. Microbiol., 49:6:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell, C.L. et al, 1996. Cryptosporidium parvum: Intensity of Infection and Oocyst Excretion Patterns in Healthy Volunteers. Jour. Infect. Dis., 173:232. [DOI] [PubMed] [Google Scholar]
- Clancy, J.L. et al, 2000. Using UV to Inactivate Cryptosporidium. Jour. AWWA, 92:9:97. [Google Scholar]
- Clancy, J.L. et al, 1998. Inactivation of Cryptosporidium parvum Oocysts in Water Using Ultraviolet Light. Jour. AWWA, 90:9:92. [Google Scholar]
- Cotton, C.A. et al, 2001. UV Disinfection Costs for Inactivating Cryptosporidium. Jour. AWWA, 93:6:82. [Google Scholar]
- Craik, S.A. et al, 2001. Inactivation of Cryptosporidium parvum Oocysts Using Medium‐ and Low‐pressure Ultraviolet Radiation. Water Res., 35:6:1387. [DOI] [PubMed] [Google Scholar]
- Ditrich, O. et al, 1991. First Finding of Cryptosporidium baileyi in Man. Parasitol. Res., 77:44. [DOI] [PubMed] [Google Scholar]
- Dupont, H.L. et al, 1995. Infectivity of Cryptosporidium parvum in Healthy Volunteers. New England Jour. Med., 332:885. [DOI] [PubMed] [Google Scholar]
- DVGW (Deutsche Vereinigung des Gas‐ und Wasserfaches) , 1997. Arbeitsblatt W294 UV‐Desinfektionsanlagen für die Trinkwasserversorrgung—Anforderungen und Prüfung, Bonn, Germany. [Google Scholar]
- Dyksen, J.E et al, 1998. Cost of Advanced UV for Inactivating Cryptosporidium. Jour. AWWA, 90:9:103. [Google Scholar]
- Fayer, R. et al, 2001. Cryptosporidium canis n. sp. From Domestic Dogs. Jour. Parasitol., 87:6:1415. [DOI] [PubMed] [Google Scholar]
- Finch, G.R. & Belosevic, M. , 1999. Inactivation of Cryptosporidium parvum and Giardia muris With Medium‐pressure Ultraviolet Radiation. Proc. USEPA Workshop on UV Disinfection of Drinking Water, Arlington, Va. [Google Scholar]
- Finch, G.R. et al, 1993. Dose Response of Cryptosporidium parvum in Outbred Neonatal CD‐1 Mice. Appl. & Envir. Microbiol., 59:11:3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargy, T.M. et al, 2000. Shedding UV Light on the Cryptosporidium Threat. Proc. Small Drinking Water & Wastewater Systems Conf., Phoenix, Ariz. [Google Scholar]
- Hill, W.F. et al, 1970. Ultraviolet Devitalization of Eight Selected Enteric Viruses in Estuarine Water. Jour. Appl. Microbiol., 19:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korich, D.G. et al, 2000. Interlaboratory Comparison of the CD‐1 Neonatal Mouse Dose Response Model for Cryptosporidium parvum Oocysts. Jour. Euk. Microbiol., 47:3:294. [DOI] [PubMed] [Google Scholar]
- Korich, D. G. et al, 1990. Effects of Ozone, Chlorine Dioxide, Chlorine, and Monochloramine on Cryptosporidium parvum Oocyst Viability. Appl. & Envir. Microbiol., 56:5:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M.H. , 1996. Waterborne Disease: 1993–1994. Jour. AWWA, 88:3:66. [Google Scholar]
- Mackey, E.D. et al, 2002. Comparing Cryptosporidium and MS‐2 Bioassays—Implications for Comparing UV Reactor Validation. Jour. AWWA, 94:2:62. [Google Scholar]
- Mead, J.R. & You, X.D. , 1998. Susceptibility Differences to Cryptosporidium parvum Infections in Two Strains of Gamma Interferon Mice. Jour. Parasitol., 84:1045. [PubMed] [Google Scholar]
- Millard, P.S. et al, 1994. An Outbreak of Cryptosporidiosis From Freshpressed Apple Cider. Jour. Amer. Med. Assoc., 272:20:1592. [PubMed] [Google Scholar]
- Mitchell, D.L. & Nairn, R.S. , 1989. Biology of the (6‐4) Photoproduct. Photochem. Photobiol., 49:805. [DOI] [PubMed] [Google Scholar]
- Mofidi, A.A. et al, 2001. Disinfection of Cryptosporidium parvum With Polychromatic UV Light. Jour. AWWA, 93:6:95. [Google Scholar]
- Nina, J.M. et al, 1992. Comparative Study of Antigenic Composition of Oocyst Isolates of Cryptosporidium parvum From Different Isolates. Parasite Immunol., 14:227. [DOI] [PubMed] [Google Scholar]
- NWRI/AWWARF (National Water Research Institute/AWWA Research Foundation) , 2003. Ultraviolet Disinfection: Guidelines for Drinking Water and Water Reuse. NWRI, Fountain Valley, Calif. [Google Scholar]
- Okhuysen, P.C. et al, 1999. Virulence of Three Distinct Cryptosporidium parvum Isolates for Healthy Adults. Jour. Infect. Dis., 180:4:1275. [DOI] [PubMed] [Google Scholar]
- Okhuysen, P.C. et al, 1998. Susceptibility and Serologic Response of Healthy Adults to Reinfection With Cryptosporidium parvum. Infect. & Immun., 66:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, Y.R. et al, 1991. Restriction Fragment Length Polymorphism Analysis of Cryptosporidium parvum Isolates of Bovine and Human Origin. Jour. Protozool., 38:40S. [PubMed] [Google Scholar]
- Ortega‐Mora, L.M. & Wright, S.E. , 1994. Age‐related Resistance in Ovine Cryptosporidiosis: Patterns of Infection and Humoral Immune Response. Infect. & Immun., 62:5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza‐Diaz, S. et al, 2001. Nested Polymerase Chain Reaction for Amplification of the Cryptosporidium Oocyst Wall Protein Gene. Emerg. Infect. Dis., 7:1:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, M.M. et al, 1997. Genetic Polymorphism Among Cryptosporidium parvum Isolates: Evidence of Two Distinct Human Transmission Cycles. Emerg. Infect. Dis., 3:4:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransome, M.E. et al, 1993. Effects of Disinfectants on Viability of Cryptosporidium parvum Oocysts. Water Supply (Amsterdam), 11:75. [Google Scholar]
- Robertson, L.J. et al, 1993. In vitro Excystation of Cryptosporidium parvum . Parasitol., 106:13. [DOI] [PubMed] [Google Scholar]
- Robertson, L.J. et al, 1992. Survival of Oocysts of Cryptosporidium parvum Under Various Environmental Pressures. Appl. & Envir. Microbiol., 58: 3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle, P.A. et al, 2002. Comparison of in vitro Cell Culture and a Mouse Assay for Measuring Infectivity of Cryptosporidium parvum . Appl. & Envir. Microbiol., 68:8:3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle, P.A. et al, 1999. Polymorphisms in the Beta‐tubulin Gene of Cryptosporidium parvum Differentiate Between Isolates Based on Animal Host But Not Geographic Origin. Jour. Parasitol., 85:5:986. [PubMed] [Google Scholar]
- Schmelling, D. 2002. Draft EPA UV Disinfection and Guidance. Proc. AWWA WQTC UV Workshop, Seattle.
- Sharp, D.G. , 1939. Lethal Action of Short Ultraviolet Rays on Several Common Pathogenic Bacteria. Jour. Bacteriol., 37:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, G‐A , et al, 2001. Low‐pressure UV Inactivation and Subsequent DNA Repair Potential of Cryptosporidium parvum Oocysts. Appl. & Envir. Microbiol., 67:7:3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, H.V. , 2001. Personal communication.
- Sommer, R. et al, 1998. Time Dose Reciprocity in UV Disinfection of Water. Water Sci. & Technol., 38:12:145. [Google Scholar]
- Spano, F. et al, 1998. Multilocus Genotypic Analysis of Cryptosporidium parvum Isolates From Different Hosts and Geographical Origins. Jour. Clin. Microbiol., 36:11:3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKDWI (United Kingdom Drinking Water Inspectorate) , 2003. Cryptosporidium Monitoring Programme. http://www.dwi.gov.uk/regs/crypto/index.htm (accessed January 2002).
- USEPA (US Environmental Protection Agency) , 2003a. National Primary Drinking Water Regulations: Long‐Term 2 Enhanced Surface Water Treatment Rule; Proposed Rule. Fed. Reg., 68:154:47640. [Google Scholar]
- USEPA , 2003b. Ultraviolet Light Guidance Manual, June 2003 Draft. Prepared by the Cadmus Group, Malcolm Pirnie, and Carollo Engineers. for the USEPA Office of Ground Water and Drinking Water.
- Wilson, B.R. et al, 1992. Coliphage MS‐2 as a UV Water Disinfection Efficacy Test Surrogate for Bacterial and Viral Pathogens. Proc. AWWA WQTC, Toronto. [Google Scholar]
- Zelle, M.R. & Hollaender, A. , 1955. Effects of Radiation on Bacteria. Radiation Biology, Vol. II (Hollaender A., editor). McGraw‐Hill, New York. [Google Scholar]


