Abstract
In patients with cardiovascular events, such as myocardial infarction or aortic dissection without known risk factors for cardiovascular disease, neoplastic disease should be considered as a differential diagnosis. In this report, we present a case of a 51-year old man with previously undiagnosed non-small lung cancer leading to fatal cardiovascular complications due to hemovascular spread, diagnosed post-mortem. This case illustrates the value of autopsy in unexpected deaths.
Keywords: Autopsy, cardiovascular complications, cardiovascular disease, non-small cell lung cancer, NSCLC, hemangiosis carcinomatosa.
INTRODUCTION
Primary and secondary malignancies of the aorta are very rare findings. Primary tumors involve the intimal wall of the aorta, sarcoma being the tumor of the aorta detected most often1. Secondary tumors involve the aorta through the adventitia, either by continuity or through secondary infiltration from lymph node metastases, and very rarely through the vasa vasorum of the aorta. There are only a few cases of secondary aortic neoplasms described in the literature2-5.
Secondary involvement of the aorta can occur from a variety of neoplasms. Nevertheless, the most common malignancies to present with aortic invasion are lung cancer, esophageal cancer, and thymoma1.
We report a case of metastatic invasion of the aortic vasa vasorum due to non-small cell lung cancer, diagnosed post-mortem.
CASE REPORT
A 51-year-old Caucasian male, with no significant medical history or chronic ongoing treatment, was found dead in his house. In the months preceding his death, he was complaining of back pain.
At autopsy, acute myocardial infarction of the anterior wall was diagnosed as the immediate cause of death.
Multiple myocardial fibrosis areas were revealed in the anterior wall, suggesting recurrent acute ischemic events. Mild atherosclerosis plaques without significant stenosis were found in the coronary arteries. Neoplastic vascular invasion was present in the coronary vessels and lymphangiosis carcinomatosa with malignant pericardial effusion was detected.
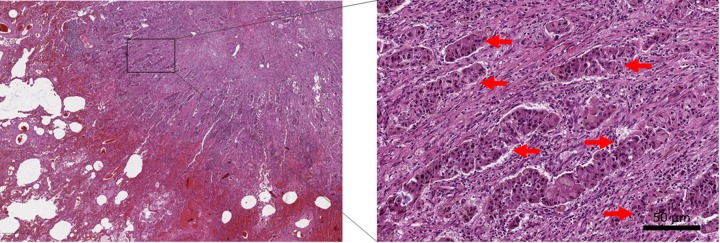
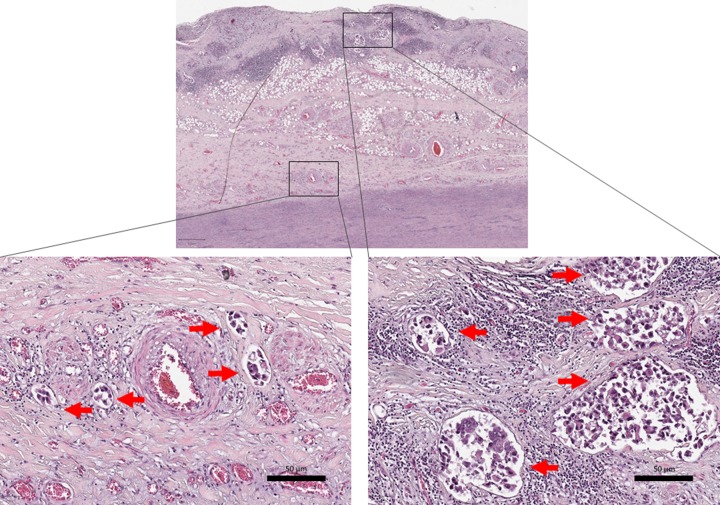
Two tumors were found in the lung, the first of 1.2 cm in diameter in the left inferior lobe, and the second of 0.6 cm in diameter in the right upper lobe. Metastases were found in the intrapulmonary, hilar, jugular, parajugular and paraaortic lymph nodes and invasion was detected within the vasculature, lymphatics and perineural regions. The patient was diagnosed with non-small cell lung cancer with lymphovascular, lymphonodal and hemovascular spread T4N3M1c. The malignancy was identified to be an adenocarcinoma of the lungs (Figure 1). Histological examination of the aorta revealed diffuse infiltration of the adventitia (Figure 2, right panel) and hemovascular spread to the vasa vasorum (Figure 2, left panel).
Figure 1. Microscopic view of tumor cells in the lung (hematoxylin and eosin staining).
Red arrows point to neoplastic cells (scale bar, right panel: 50 μm).
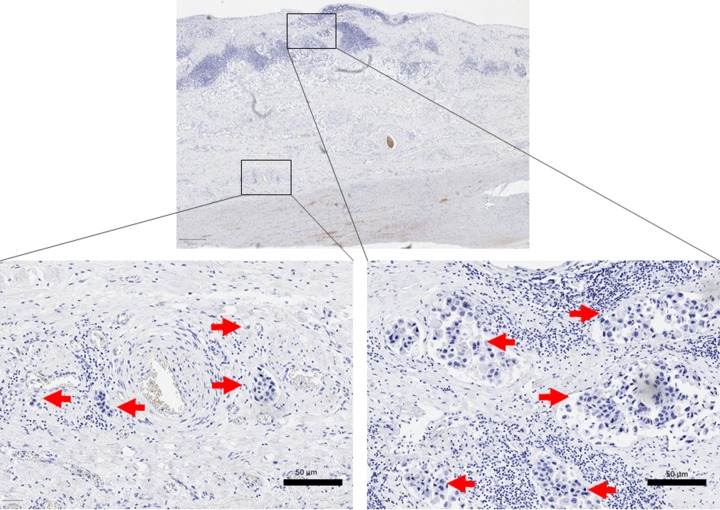
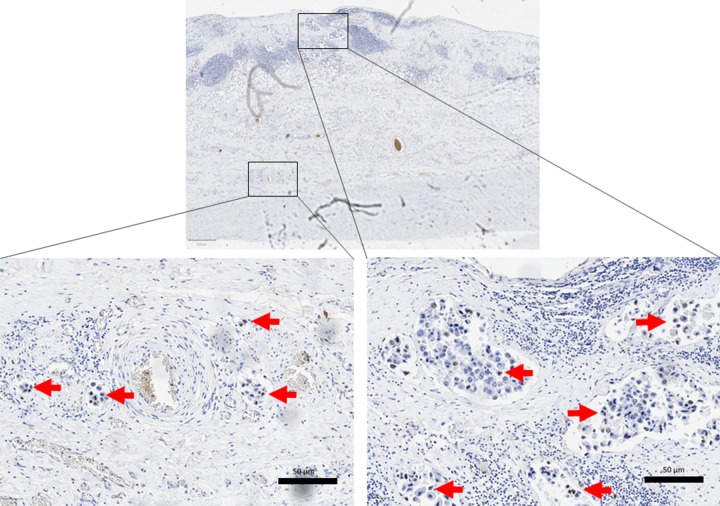
Figure 2. Microscopic view of tumor cells in the aorta.
Left panel: diffuse malignant infiltration of the vasa vasorum (hematoxylin and eosin staining; scale bar: 50 μm). Right panel: tumor cells in the aortic adventitia (hematoxylin and eosin staining; scale bar: 50 μm). Red arrows point to neoplastic cells.
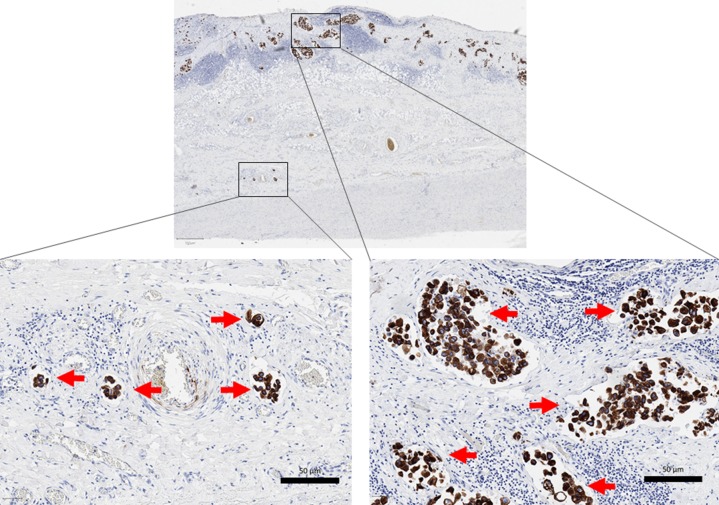
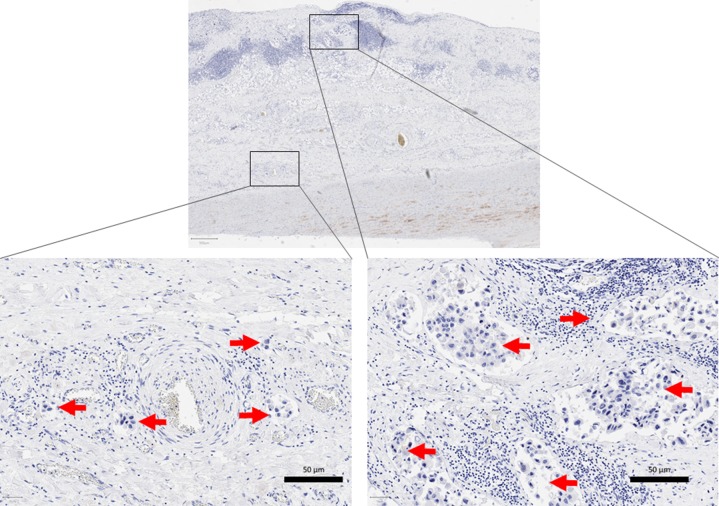
Immunohistochemistry showed positive expression of cytokeratin 7 (CK7) (Figure 3) and negative expression of cytokeratin 20 (CK20) (Figure 4), typical for primary lung adenocarcinoma (CK7+ / CK20−) and ruling out metastatic colonic carcinoma to the lung (CK7−/ CK20+)6. In addition, CDX2, a highly sensitive and specific marker of adenocarcinoma of intestinal origin, was negative (Figure 5). Thyroid transcription factor-1 (TTF1) biomarker was positive, (Figure 6) distinguishing primary (TTF1+) from metastatic lung neoplasms (usually TTF1−).
Figure 3. Immunohistochemistry CK7 positive.
Red arrows point to neoplastic cells; scale bar: 50 μm.
Figure 4. Immunohistochemistry CK20 negative.
Red arrows point to neoplastic cells; scale bar: 50 μm.
Figure 5. Immunohistochemistry CDX2 negative.
Red arrows point to neoplastic cells; scale bar: 50 μm.
Figure 6. Immunohistochemistry TIF1 positive.
Red arrows point to neoplastic cells; scale bar: 50 μm.
DISCUSSION
Although the two most frequent causes of death worldwide, cardiovascular disease and cancer, are often present together, malignant neoplasms leading to fatal cardiovascular events via vascular spread are extremely rare. Nevertheless, cancer and cardiovascular disease share many risk factors, such as tobacco use, poor nutrition and lack of physical activity. Also, chronic inflammation and oxidative stress play central roles in the pathophysiology of both7.
In an observational study, Sturgeon et al. revealed that one in ten cancer patients do not die from malignancy, but due to associated cardiovascular conditions, cancer patients have an on average 2–6 times higher mortality risk due to cardiovascular disease than the general population8.
In this exceptional case, the immediate cause of death was acute myocardial infarction of the anterior wall. Although the coronary arteries had no significant stenosis and he did not have risk factors for cardiovascular diseases, he had multiple recurrent infarctions. Malignant cells were detected in the coronary arteries, potentially due to tumor embolism via pulmonary veins or from aortic metastases. Therefore, the findings suggested coronary embolism from neoplastic cells.
Coronary embolism is the underlying cause of 3% of acute coronary syndromes. However, it is often not considered in the differential diagnosis of acute coronary syndromes9. Previously published papers reported only a few cases of myocardial infarction due to coronary artery tumor embolism, which was diagnosed from coronary artery aspirate10. The major source of cancer embolism to the coronary artery is the lung, apparently via the pulmonary veins, and the diagnosis is often made post-mortem9-11.
It has been reported that the incidence of coronary embolization in patients with myxomas is only 0.06%. Acute myocardial infarction due to lung carcinoma embolization to a coronary artery is even more exceptional12.
Secondary aortic manifestation of neoplastic disease is very rare. Similar to cases involving primary aortic tumors, arterial tumor embolization from secondary aortic involvement of neoplastic disease is very likely to be the cause of myocardial infarction in our case. Our patient had diffuse infiltration of the aortic wall by neoplastic cells that were located primarily in the vasa vasorum.
Many clinical manifestations may accompany aortic malignant neoplasms, regardless of whether the tumor is primary or metastatic. These include but are not limited to embolic events to different organs, aortic aneurysm and dissection.
Ikebe and colleagues described a case of arterial tumor embolization from aortic metastasis in a 67 year-old man with cholangiocarcinoma, with descending aortic wall thickening visualized by positron emission tomography-computed tomography. After 6 months, he had symptoms of mesenteric ischemia. Angiography with thromboaspiration was performed and histopathological analysis of the embolus demonstrated tumor emboli from cholangiocarcinoma metastasis2.
Aortic malignancies affect the structure of the medial layer of the aorta, and primary aortic tumors have previously been associated with aortic dissection13-15. Only few cases of aortic dissection caused by secondary tumors involving the aorta have been reported. Ugurlu et al. present a case of typical ascending aortic dissection associated with metastatic carcinoma originating from the lungs. This metastatic infiltration of the vasa vasorum of the aorta by neoplastic cells may have caused aortic dissection by altering the media3. Another case revealed at autopsy an intramural hematoma of the aorta, as well as systemic metastases of adrenocortical carcinoma with invasion into the aortic wall and formation of a pseudo-lumen accompanied by disruption of the vasa vasorum4.
In the early stages, tumor aneurysms are very similar to atherosclerotic aneurysms on computed tomography scan, often leading to a delay in diagnosis. The atypical localization of the aneurysm, low cardiovascular risk factors, rapidly evolving aneurysms and enhanced tissues around the aorta are suspicious findings for aortic metastasis. PET scan fluorodeoxyglucose uptake around the aneurysm, although not specific, can contribute to the diagnosis5.
CONCLUSION
Cardiovascular events induced by malignant neoplasms are rare and can present with various clinical manifestations. Imaging techniques, such as PET-CT, could improve early diagnosis.
In patients with cardiovascular events, such as myocardial infarction or aortic dissection without known risk factors for cardiovascular disease, neoplastic disease should be considered as a differential diagnosis. This case illustrates the value of autopsy in unexpected deaths, as neither the underlying disease nor the immediate cause of death was suspected ante-mortem.
KEY POINT
◊ Exceptionally, malignant neoplasms can lead to cardiovascular complications and death, via hemangiosis carcinomatosa with secondary infiltration of perivascular connective tissu
Acknowledgments
The first author (RD) received a DAAD Research Grant.
Footnotes
Conflict of interests: The authors declare that there is no conflict of interest.
Cytokeratin 7 (CK7); cytokeratin 20 (CK20); thyroid transcription factor-1 (TTF-1); positron emission tomography – computed tomography (PET/CT).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Aortic Tumors. Restrepo Carlos S., Betancourt Sonia L., Martinez-Jimenez Santiago, Gutierrez Fernando R. Seminars in Ultrasound, CT and MRI. 2012;33(3):265-272. doi: 10.1053/j.sult.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Tumor Emboli from Aortic Metastasis. Ikebe Yohei, Sueyoshi Eijun, Ishimaru Hideki, Hidaka Masaaki. Internal Medicine. 2018;57(6):907-908. doi: 10.2169/internalmedicine.9696-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dissection of the ascending aorta due to metastatic carcinoma. Ugurlu B S, Hazan E, Badak O, Yörükoğlu K, Oto O. The Annals of thoracic surgery. 2001;72(2):614–5. doi: 10.1016/s0003-4975(00)02260-8. [DOI] [PubMed] [Google Scholar]
- 4.Aortic intramural hematoma associated with metastatic carcinoma. Tsuchida Rikuhei, Kasahara Naoto, Inobe Megumi, Terado Yuichi, Horita Ayako, Yokoyama Kenichi, Sakamoto Atsushiko, Fujioka Yasunori, Kurata Atsushi. Pathology, research and practice. 2010;206(12):839–45. doi: 10.1016/j.prp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Descending thoracic aortic aneurysm revealing metastasis of a soft tissue fibrosarcoma: a case report and review of the literature. Delerce Clotilde, Bailly Olivia, Bouhamama Amine, Couchon Sophie, Pilleul Frank, Thivolet Arnaud, Mastier Charles. Clinical sarcoma research. 2018;8:22. doi: 10.1186/s13569-018-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combined immunohistochemistry of beta-catenin, cytokeratin 7, and cytokeratin 20 is useful in discriminating primary lung adenocarcinomas from metastatic colorectal cancer. Ikeda Satoshi, Fujimori Masahiko, Shibata Satoshi, Okajima Masazumi, Ishizaki Yasuyo, Kurihara Takeshi, Miyata Yoshihiro, Iseki Masahiko, Shimizu Yosuke, Tokumoto Noriaki, Ozaki Shinji, Asahara Toshimasa. BMC cancer. 2006;6:31. doi: 10.1186/1471-2407-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heart Disease and Cancer: Are the Two Killers Colluding? Kitsis Richard N, Riquelme Jaime A, Lavandero Sergio. Circulation. 2018;138(7):692–695. doi: 10.1161/CIRCULATIONAHA.118.033907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A population-based study of cardiovascular disease mortality risk in US cancer patients. Sturgeon Kathleen M, Deng Lei, Bluethmann Shirley M, Zhou Shouhao, Trifiletti Daniel M, Jiang Changchuan, Kelly Scott P, Zaorsky Nicholas G. European heart journal. 2019;40(48):3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronary Embolus: An Underappreciated Cause of Acute Coronary Syndromes. Raphael Claire E, Heit John A, Reeder Guy S, Bois Melanie C, Maleszewski Joseph J, Tilbury R Thomas, Holmes David R. JACC. Cardiovascular interventions. 2018;11(2):172–180. doi: 10.1016/j.jcin.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 10.Carcinoma embolization in coronary artery causing myocardial infarction. Šteiner Ivo, Vojáček Jan. Pathology - Research and Practice. 2014;210(3):198-200. doi: 10.1016/j.prp.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Acute myocardial infarction caused by coronary tumour embolism. Kushiyama Shuhei, Ikura Yoshihiro, Iwai Yasuhiro. European heart journal. 2013;34(48):3690. doi: 10.1093/eurheartj/eht413. [DOI] [PubMed] [Google Scholar]
- 12.Acute myocardial infarction due to malignant neoplastic coronary embolus. Kumagai Naoko, Miura Shin-Ichiro, Toyoshima Hideo, Koga Kaori, Takeda Satoshi, Sato Susumu, Kodama Shiho, Ogawa Masahiro, Matsuo Kunihiro, Nabeshima Kazuki, Ishikura Hiroyasu, Watanabe Kentaro, Saku Keijiro. Journal of cardiology cases. 2010;2(3):e123–e127. doi: 10.1016/j.jccase.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Primary aortic sarcoma mimicking aortic dissection. Borislow D S, Floyd W L, Sane D C. The American journal of cardiology. 1989;64(8):549–51. doi: 10.1016/0002-9149(89)90443-8. [DOI] [PubMed] [Google Scholar]
- 14.Multicentric granular cell tumor of the heart presenting with aortic dissection. Fujise K, Sacchi T J, Williams R J, DiCostanzo D P, Tranbaugh R F. The Annals of thoracic surgery. 1994;57(6):1653–5. doi: 10.1016/0003-4975(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 15.Primary malignant fibrous histiocytoma of the aorta associated with aortic dissection. Chen W J, Chen C L, Liau C S, Chu S H, Lee Y T. Chest. 1991;99(4):1049–50. doi: 10.1378/chest.99.4.1049. [DOI] [PubMed] [Google Scholar]