Abstract
The discovery of regulatory RNA has identified an underappreciated area for microbial subversion of the host. There is increasing evidence that RNA can be delivered from bacteria to host cells associated with membrane vesicles or by direct release from intracellular bacteria. Once inside the host cell, RNA can act by activating sequence-independent receptors of the innate immune system, where recent findings suggest this can be more than simple pathogen detection, and may contribute to the subversion of immune responses. Sequence specific effects are also being proposed, with examples from nematode, plant and human models providing support for the proposition that bacteria-to-human RNA signaling and the subversion of host gene expression may occur.
Keywords: Regulatory RNA, cross-kingdom communication, pathogenicity, innate immunity, RNAi, membrane vesicles
SUMMARY
Introduction
Bacterial RNA in the extra- and intra-cellular environment of human cells
Interactions of bacterial RNA with the innate immune system
Sequence-specific action of bacterial RNA in host cells
Conclusions
1. Introduction
Cells do not exist in isolation and so cell-to-cell signaling is important in all biological systems; be it a simple community of single-celled organisms or a multicellular organism coordinating information flow between its own cells and those of its microbiota. Through the course of evolution multiple “languages” have been developed for communications between cells that utilize most, if not all, key biomolecules for intracellular and intercellular signaling pathways: proteins, lipids, carbohydrates, and small organic and inorganic molecules. For humans, an important interface is manifested in the cellular and molecular interactions with microorganisms such as both pathogens and symbionts. It is therefore not surprising that these diverse species have evolved mechanisms for interpreting and manipulating the various signaling systems of the other, giving rise to the phenomenon of cross-kingdom communication.
Perhaps our first appreciation of cross-kingdom communication came from the investigation of the molecular mechanisms of bacterial protein virulence factors. The cholera toxin provides a simple example whereby this protein hijacks key intracellular signaling through the second messenger cAMP1, leading to a profuse acute diarrhea that is hypothesized to help disseminate the pathogen. A more complex “conversation” has been described between Salmonella and its target cells, where the protein effectors delivered by type 3 secretion are primarily responsible for invasion, as well as niche maintenance and dampening of immune responses2.
The discovery that bacterial cells communicated to one another via a range of small molecule signals, such as acylated homoserine lactones, in a process termed “quorum sensing” highlighted the possibility that cross-kingdom communication could use a non-protein language3,4. confirmed by the finding that the quorum sensing signals used by bacteria to co-ordinate their pathogenic activities could also influence immune responses to the pathogen5,6. Moreover, communication is not only from bacterium to target cell, as gene expression is also influenced in bacteria that can intercept intercellular signals deriving from human cells7.
Most recently evidence has emerged for a new “language” for communication between host and bacteria that is based on the identification of regulatory RNAs in both prokaryotes and eukaryotes. Our appreciation of the potential roles of RNA is now going beyond the classical designations of messenger, ribosomal and transfer RNAs in the mechanics of translation. Studies have demonstrated that the manipulation of this “riboregulation” is central to the molecular pathogenicity of some viruses8,9, and highlighted the hitherto underappreciated role that the subversion of regulatory RNAs could play in progressing infections. The discovery of microRNA (miRNA) signals produced by one cell to influence gene expression in another10 has demonstrated RNA as a language of inter-cellular communication identifying them as a potential target for bacterial pathogens.
Today, bacterial RNA is well recognized as an important “pathogen associated molecular pattern” (PAMP) that is involved in human responses to infection. This has been coupled with an appreciation that bacterial RNA is not just a simple uniform trigger of the non-specific immune system, but rather a complex multifaceted signal. For example the differential availability of RNA-recognizing sensors in the host, differences in their subcellular localization and the need to differentiate between ‘self’ and ‘foreign’ RNA define a complex ability of the host to detect intruding RNA and mount a defensive response11. In parallel, bacteria can deliver their RNA to the host, for example, using membrane vesicles (MVs) that may protect their cargo while delivering to specific compartments of the host cell12,13. In this review, we discuss the current understanding of the role of RNA in human/bacteria interactions (as summarized inFigure 1) and provide an outlook for future developments of the field.
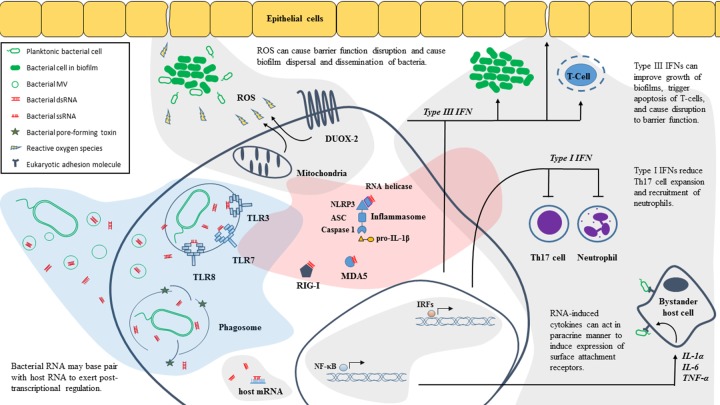
Figure 1. Interactions of bacterial RNA with an eukaryotic host cell.
a)Entry of bacterial RNA (blue shaded area): Bacterial RNA in double and single stranded forms (red) of extracellular bacteria (green) can enter human cells with MVs, whilst intracellular bacteria can secrete RNA into phagosomes and the cytosol. Bacterial RNA can translocate from phagosomes into the cytosol due to the inherent leakiness of phagosome or when bacterial pore-forming toxins (green stars) disrupt integrity of phagosomes. b) Interactions with the innate immune system (red shaded area): In the endosome, bacterial RNA is sensed by TLR3, TLR7, and TLR8, whilst in the cytosol DExD/H motif helicases such as MDA5 and RIG-I and the NLRP3 inflammasome (via a yet unknown intermediate RNA helicase) can interact with bacterial RNA to trigger downstream signaling cascades. c) Modulation of host cell by bacterial RNA (grey-shaded area): Engagement of innate immune system RNA sensors leads to expression and secretion of type I and type III interferons as well as NF-κB-controlled cytokines which can skew the immune system away from antibacterial response and promote bacterial colonization and dissemination. Activation of the NLRP3 inflammasome leads to Caspase 1 mediated cleavage of pro–IL-1β into active IL-1β. It is postulated that bacterial RNA may also exert post-transcriptional control of human gene expression via sequence-specific interactions with host RNAs. Please refer to the main article for more details and definitions of the abbreviations used.
2. Bacterial RNA in the extra- and intra-cellular environment of human cells
RNA is abundant and a lysed bacterial cell will release about tenfold more RNA than DNA14,15. The cells of mucosal barriers and infected tissues will therefore be regularly exposed to RNA from lysed bacteria and other microorganisms. Human tissues and fluids16-19 contain high levels of RNases, in the order of several hundred nanograms per milliliter20,21, that would be expected to degrade this RNA22. However, host miRNA bound to proteins, lipids and lipoproteins is known to be protected from the external RNAses23, with the secondary and tertiary structure of some RNA molecules also likely to provide protection from degradation24. More recently, advances in small RNA sequencing technology have allowed a detailed analysis of circulating RNAs in mammalian blood, identifying a surprisingly large proportion (ranging from 0.31-11%) as microbial RNA25-29. It is therefore probable that some RNA released following bacterial cell death will remain in the immediate environment of the human host.
In addition to the RNA released from lysed bacterial cells, detectable levels of RNA have been reported to be present in supernatants from cultures where most bacteria are viable30-32, suggesting that active secretion of RNA may also be at play. Indeed, extracellular RNA from these bacterial populations has been found associated with MVs12,31,33-35, as well as in a ‘free’ form31. The nano-sized MVs are produced by both gram-positive36 and gram-negative bacteria37 growing in biofilms, planktonic cultures, inside eukaryotic cells and under a variety of other environmental conditions38-40. In many respects, such as size and types of carried molecular cargo, MVs are similar to human exosomes34. Human exosomes have a well-established role in RNA communication41-43, predominantly through their carriage of regulatory miRNAs, and a similar role for bacterial MVs is beginning to emerge in the literature12,13. MVs, like exosomes, protect RNA from degradation as shown by comparisons following RNAse treatment of MVs12. The specific protein or other factors that improve the stability of MV-free bacterial RNA are yet to be determined.
A growing body of work is now available to support various mechanisms of outer membrane vesicle production by Gram-negative bacteria that involve budding from the outer membrane, induction by stress responses and some selectivity in the composition of the MV cargo44-46. An alternative mechanism for the formation of MVs via explosive cell lysis has recently been identified for Pseudomonas aeruginosa35. Strains that encode a prophage endolysin (A0629) can undergo an explosive cell lysis event in response to exogenous stress and produce MVs through vesicularization of membrane fragments. This process allows capture of cellular components released into the extracellular space, including the incorporation of RNA into the MVs. It is yet to be determined if explosive cell lysis under non-stress conditions is a programmed cell death pathway induced by “altruistic suicide”47 to release key nutrients to other bacteria as a “colony public good”, or if it is the result of stochastic expression of the A0629 endolysin or if it could be part of a regulated virulence program. It will be of interest to determine if this process occurs in other species of bacteria, as genes with high similarity to A0629 can be found in the genomes of several other bacterial genera35.
Free RNA added to the medium of cultured cells can induce responses that favour bacterial colonization and immune evasion32. However, the relevance of this role in an infective setting is unclear as RNA released from bacteria into the extracellular environment probably has a long way to travel before it can influence the activity of host cells. Mechanisms of bacterial MV entry into host cells have been widely studied48-56, although it has not yet been shown which mechanism(s) are involved in the uptake of RNA-carrying MVs. Bacterial MVs are a heterogeneous population and we speculate that RNA-carrying MVs may represent only a fraction of the total MV population. At the same time, the mechanism of host entry by a bacterial MV, and consequently the targeting and fate of its cargo, is likely to be different for various MV subpopulations and influenced by MV size, surface molecules etc. In cases where the intracellular delivery of bacterial RNA has been studied, the evidence obtained currently suggests it is necessary for the induction of responses associated with exogenous RNA sensing57,58 (discussed in the section “Interactions of bacterial RNA with the immune system” below).
An alternative route for bacterial RNA to enter human cells is via release from intracellular bacteria. Listeria monocytogenes is a model organism for the study of intracellular bacteria/host interactions59. When the RNA of live L. monocytogenes was labelled with a modified nucleotide, 5-ethynyluridine (5EU), and these bacteria were allowed to infect the human monocytic cell line THP-1 the visualization of bacterial RNA by chemically attaching a fluorophore to the 5EU-RNA revealed it in an extra-bacterial localization in the host cell cytosol60. Further, no evidence of cytoplasmic, extra-bacterial RNA was found for THP-1 cells infected with 5EU-labelled L. monocytogenes lacking the SecA2 auxiliary protein secretion system53, suggesting that the bacterial RNA is actively secreted, possibly in association with a protein chaperone, rather than being a by-product of bacterial lysis.
In a related report, RNA released from bacteria that themselves remain trapped in the phagosome can also exert an effect. In the case of Borrelia burgdorferi, this occurs via RNA interaction with the endosomal TLR8 to induce transcription of IFN-β61. In other instances, RNA from the phagocytosed bacteria can translocate into the cytosol from phagosomes that are intrinsically leaky62, or made leaky by the actions of bacterial pore-forming toxins63.
Overall, the reported findings from the last several years demonstrate that bacterial RNA is a common component in the environment of many human cells. Additionally, current evidence suggests that release of RNA by bacteria is not a simple by-product of bacterial cell death, but an active, and possibly, selective process. The variety of mechanisms involved in the secretion of RNA from bacterial cells, its stability in host extracellular fluids, its association with transport systems for intra- and inter-species signaling such as MVs, and its secretion into host intracellular compartments by live intracellular bacteria suggest that RNA may be used by bacteria as a currently underappreciated virulence factor. Which pathways are engaged by bacterial RNA will likely depend at least in part, on where inside a human cell bacterial RNA is delivered. Evidence to date suggests that bacterial RNA can be delivered into human cytosol30,59,64,65, endosomal and phagosomal compartments66,67 with one study reporting delivery of bacterial MV RNA into human cell nuclei13. Thus, current research has focused on the interactions of bacterial RNA with cytosolic and endosomal receptors of the innate immune system and on the investigation of possibilities for bacterial RNA to affect human gene expression via post-transcriptional mechanisms in a sequence-dependent manner. Key recent discoveries from each of these fields are discussed in the following sections of this review.
3. Interactions of bacterial RNA with the innate immune system
Entry of bacterial RNA into a host eukaryotic cells is sensed as a danger signal by receptors of the innate immune system. In the endosome, RNA can be recognized by TLR3, TLR7, and TLR8. TLR3 senses long (>39bp) double stranded RNA (dsRNA)68, while TLR7 and TLR8 sense degradation products of single stranded RNA (ssRNA). All three TLRs recognize RNA in a sequence-independent manner, although there is some evidence for preferential recognition of RNA rich in some nucleotides or modified nucleotides69,70. It has been proposed this relates to the observation that some pathogens display a greater proportion of certain types of nucleotides in their RNA sequences71. Furthermore, for at least TLR8, the key descriptors for RNA recognition are not yet certain, as THP-1 cells (a cultured monocyte line) challenged with enterococcal RNA only responded (measured as IL-12 production) when 23S and 16S rRNA, but not when mRNA, were applied72.
In the cytosol, some of the key sensors of bacterial RNA are two helicases of the DExD/H motif family, namely RIG-I and MDA5, which detect 5’ triphosphorylated short dsRNA and long dsRNA, respectively73. RIG-I binds blunt ends of dsRNA displaying a 5’ triphosphate moiety whilst MDA5 molecules bind RNA independently of its terminal structures. Additionally, the NLRP3 (NLR family, pyrin domain-containing) inflammasome has recently emerged as an important cytosolic sensor of bacterial RNA57,74,75. It is a signaling complex that consists of the sensor molecule NLRP3, the adaptor protein ASC and caspase 1, which, in addition to bacterial RNA, senses a variety of endogenous and pathogen-associated molecules (pore-forming cytotoxins, ATP, uric acid76).
Induction of the innate immune sensors for bacterial RNA can lead to secretion of type I and type III interferons, and pro-inflammatory cytokines such as TNF-α, IL-1α, IL-1β, IL-6, IL-8, IL-10, and IL-1214,15,32,58,59,61,72,77-82. However, which RNA sensors are engaged and what host effector molecules are secreted is cell type specific. For example, IFN-α secretion is induced in murine plasmacytoid dendritic cells upon DOTAP-mediated transfection of Escherichia coli RNA but not in conventional dendritic cells (cDCs)80. IFN-β is secreted by murine cDCs in response to transfection with RNA from Group B15 and Group A streptococci78. However, in bone marrow-derived macrophages IFN-ß only responded to transfection of DNA, and not RNA, of Group A streptococci. These differences in responses to bacterial RNA reflect differential availability and use of molecular RNA sensors in different cell types. Differences in host cell responses to the type of RNA (tRNA, rRNA, mRNA etc) can also reflect variations in post-transcriptional modifications of a given prokaryotic RNA molecule across various species80,83-85. For example, when polyadenylated, prokaryotic mRNAs have a shorter poly-A tail than their eukaryotic host mRNAs, and as such can be readily detected by TLR769. Additionally, the 2’O-methyl guanosine modification status of tRNA at the conserved G18 residue can determine TLR7 activation. For some pathogenic bacteria, where G18 is unmodified, the induction of type I interferon and pro-inflammatory cytokines is seen in mouse dendritic and human peripheral blood mononuclear cells, whilst modified tRNA from non-pathogenic E. coli Nissle 1917 and Thermus thermophilus does not86. Finally, the cellular localization of the pathogen (cytosolic or phagolysosomal for instance) determines which sensors of bacterial RNA are engaged11,22.
Studies of innate immune responses to bacterial RNA have mostly focused on the identification of host cell receptors for bacterial RNA and downstream signaling cascades. Minimal research has been done to investigate how bacteria could use their RNA to manipulate innate immunity to their advantage. Notably, many of the effector molecules secreted by host cells in response to detection of bacterial RNA are also secreted in response to viral RNA. In fact, the RNA sensors discussed above were originally discovered as sensors of viral infections. In the last decade they have also been validated as sensors of bacterial RNA74,80,87. Studies of secondary bacterial infections that develop as a complication of viral infections, could therefore provide some insights into the relevance of RNA-induced cytokines on the progression of bacterial infections. Interestingly, it has been observed that, although common host mediators are induced, antiviral and antibacterial responses can frequently be at odds with one another88. This provides an opportunity to speculate that bacteria might use interactions of its secreted RNA with host innate immune sensors to its advantage by skewing the immune system towards antiviral responses. In evolutionary terms, it is possible to imagine how such a mechanism for manipulating the human host could have evolved in bacteria. The human microbiome includes not only bacteria but multiple other types of organisms including viruses, fungi and protozoa89. Bacteria/human cross-kingdom interactions are, therefore, a product of dynamic coevolved relationship between the various organism of the microbiome and the immune system. It is therefore possible that bacteria might have evolved to utilize elements of human/virus interaction to their advantage even in the absence of viral co-infection. In this case, we hypothesize that the secretion of bacterial RNA and its detection by human cells could create a beneficial environment for bacteria, similar to that of viral-bacterial co-infection.
The strongest evidence for the beneficial effects of RNA-induced cytokines on a bacterial infection process comes from studies of type I interferons. Whilst induction of type I interferons can lead to host protection and elimination of the pathogen during infections with Chlamydia trachomatis90, Salmonella enterica91, Cryptococcus neoformans92, Group B streptococci, Streptococcus pneumoniae and E. coli93, there is evidence that indicates that type I interferons can also inhibit host defense against other bacteria94,95. For example, pathogen-induced production of type I interferons has been suggested to contribute to the pathogenesis of Mycobacterium tuberculosis infections96, and the dissemination of B. burgdorferi during the early stages of infection97 where TLR7-dependent recognition of B. burgdorferi RNA is necessary for interferon-α production77.
Evidence that bacteria could benefit from interferon-induced skewing of the innate immunity towards antiviral responses also comes from investigations of the activation of T helper 17 (Th17) cells. The Th17 pathway has a critical role during infection with extracellular bacteria98. IL-17 and IL-22 are hallmark cytokines of Th17 cells, and have been shown to promote clearance of bacteria through the recruitment of phagocytes and the induction of antimicrobial peptides (AMPs)99. Induction of type I interferon production by epithelial cells in response to viral infections skews the immune status towards an antiviral phenotype and attenuates type 17 immunity against such bacterial pathogens as E. coli and P. aeruginosa100, S. aureus101 and S. pneumoniae102. The identification of bacterial communications that direct immune responses away from antibacterial activity have also been a reported feature of the response to some bacterial quorum sensing signals5.
Interactions of bacterial RNA with innate immune sensors could also serve a beneficial function to bacteria via mechanisms not involving interferon signaling. Transfection of bacterial RNA has been shown to induce reactive oxygen species (ROS) production via mechanisms involving NADPH oxidase and the mitochondrial transport chain58. ROS, in turn, can disrupt epithelial barrier function. Specifically, it has been reported that, in polarized airway epithelial cells, poly I:C (a synthetic mimic of dsRNA) signals through a recently discovered cytosolic dsRNA receptor Nod-like receptor X-1 (NLRX-1) and stimulate NADPH oxidase 1 (NOX-1) and mitochondrial ROS production to cause reactive oxygen species (ROS) dependent epithelial barrier function disruption103. A poly I:C challenge also disrupted endothelial barrier function by causing downregulation of the mRNA for claudin-5, a key endothelial tight junction protein. The exact mechanism is unknown but appears to involve a TLR3-TRIF-NF-kB signaling pathway104.
Bacterial RNA engagement with host cell RNA sensors to induce reactive oxygen production can lead to dispersion and dissemination of bacterial biofilm cells. For example, bronchial epithelial cells have been shown to express dual oxidase 2 (Duox2) in response to poly I:C and IFN-γ treatment105. Duox2 is located in the plasma membrane and can secrete H2O2 directly into the extracellular milieu106. Biofilm bacteria, when exposed to oxidative stress, can initiate a dispersal response107 to release free-swimming planktonic bacteria. Whilst induction of H2O2 production as an immune response can be viewed as a negative event for bacteria, both biofilm-associated and planktonic bacteria express antioxidant enzymes to resist H2O2 killing108. Additionally, planktonic bacteria dispersed from biofilms are often as resistant to killing by antimicrobials as their biofilm counterparts109. An example of how this process can lead to worsening of clinical outcomes can be found in cystic fibrosis patients with chronic Pseudomonas aeruginosa infections. It has been reported in this setting that a viral infection causing mild oxidative stress via activation of Duox 2 leads to dispersal of planktonic bacteria from established lung biofilms, increased transmigration of planktonic bacteria from the apical to basolateral surface of mucociliary-differentiated airway epithelial cells, increased planktonic bacterial and therefore the acute symptom burden110.
In an infection, the RNA of pathogenic bacteria may subtly manipulate the host innate immune response to activate inappropriate defense responses that can ultimately favour bacterial survival. In other instances, bacterial mechanisms for disguising their RNA using post-transcriptional modifications can become of importance79,85,86. Bacteria can also use non-RNA virulence factors to interfere with signaling cascades downstream of RNA-sensors. An example of this was recently reported that showed production of IFN-β induced by TLR8-mediated sensing of S. aureus ssRNA, is antagonized by TLR2 signaling activated by S. aureus lipoproteins111. Future detailed investigations of the molecular interactions between bacteria and innate immunity that involve bacterial RNA may eventually provide a better understanding of what stimulates a productive immune response and identify opportunities for developing novel therapeutic strategies. Overall, these findings confirm that detection of bacterial RNA by the innate immune system is not always as simple as an immune surveillance ‘hit’ leading to responses that eliminate bacterial pathogens.
4. Sequence-specific action of bacterial RNA in host cells
Bacterial RNA is seemingly not just a ligand for sequence-independent RNA receptors, but is starting to be appreciated for its potential to act in a sequence-specific manner to regulate gene expression at the post-transcriptional level. It is estimated that ∼60% of the human protein coding genes could be subject to regulation by their regulatory miRNAs112 offering a large target for bacteria to manipulate host cells to their advantage using the same endogenous RNA-inhibition (RNAi) machinery. The use of RNA as an effector molecule by directly targeting host RNA may offer the advantages of suppressing the expression of mediators of immunity before they can exert any antibacterial effects i.e. prior to the production of the protein effector itself.
It is interesting to speculate on how a bacterial pathogen’s RNA might regulate the host in a sequence-specific manner. The first question to ask is whether a bacterial RNA could bind to a host mRNA? There are differences in the specific mechanisms of action of known non-coding RNAs between human and bacterial cells, and RNA regulation in the eukaryotic host is substantially more complex113, but there are several commonalities. Specifically, regulation through direct hybridization between the regulator and the target RNA, mediated by complementary base-pairing, is employed by both kingdoms. For example, both bacterial regulatory RNAs and human miRNA are double-stranded which helps to stabilize the RNA molecule and allows correct orientation of the regulator in relation to the potential target mRNA and accessory proteins114, such as AGO2 in humans and Hfq in bacteria. Both human and bacterial regulatory RNAs tend to have a single stranded ‘seed’ region that is devoid of secondary structure115 to allow perfect antisense binding to mRNA targets. Additionally, both miRNAs and bacterial small RNAs (sRNAs) can have varied levels of complementarity with their target RNAs, which in combination with the short seed sequence allows the binding of a single miRNA/sRNA with multiple target mRNAs116.
The second question to ask is whether naked bacterial RNA could hijack the hosts own protein machinery to allow it to work in a regulatory manner? Interestingly the eukaryotic RNAi machinery appears to have been put together from various prokaryotic sources (the helicase domain of Dicer and AGO from archaea, the RNase domain of Dicer from bacteria, and RNA-dependent RNA polymerase from bacteriophages)117. Furthermore, bacterial sRNA-binding Hfq is an ortholog of eukaryotic Lsm proteins118 which also act as RNA chaperones to aid in splicing and degradation. Preliminary studies also support that exogenous RNA, from viruses118 and bacteria25,119 can bind to host AGO proteins, key proteins in the RNAi machinery.
With laboratory studies being technically challenging and to date limited, the prediction of what targets a bacterial RNA may have relied on computational modelling. These models require many assumptions to be made and as such later wet-lab validation is vital. Most such studies begin by identifying RNA molecules in the non-human organism that could function as a miRNA mimic when transferred into human cells. Such studies have had success in models for cross-kingdom RNA signaling where both organisms have endogenous miRNAs such as the plant Arabidopsis thaliana and humans, and the nematode Heligmosomoides polygyrus and mice120,121. Bacteria, however, do not have eukaryotic-like miRNA and their known non-coding RNAs range from 40 to 500 nucleotides in length122, which is in contrast to the average length of a human miRNA of about 22 nucleotides123. It is possible that long bacteria RNAs are processed into functional fragments. Shmaryahu et al.124 developed a high throughput bioinformatics pipeline, using the assumption of RNA fragmentation, to analyze all genes in a bacterial genome for their potential to produce RNA transcripts with secondary structures containing double-stranded regions that, through processing, could give rise to miRNA-like fragments. An analysis was made of 448 bacterial genomes, identifying on average 15 putative miRNA-like sequences per organism that could bind human mRNA. The authors validated the in silico analyses by synthesizing mimics of three predicted bacterial RNA-derived ‘miRNAs’ and transfected them into the human HEK293 cell line. The mimics represented a sequence derived from Arcobacter butzleri strain RM4018, a close taxonomic relative of Campylobacter jejuni and Helicobacter pylori125, with complementarity for the human DEK oncogene mRNA, a sequence from Burkholderia vietnamiensis G4 with complementarity for the transcript variant 2 mRNA of the human tumour suppressor PTPRJ (protein tyrosine phosphatase receptor type J) gene and a putative sequence from Burkholderia mallei with complementarity for the human NFKBIL1 (nuclear factor nuclear factor kappa-light- chain-enhancer of activated B cells) mRNA. Following transfection, mRNA levels of the predicted target genes were determined by RT-qPCR as reduced in expression. This study provides support for the hypothesis that bacterial RNA sequences could potentially target human mRNA, however, the study did not investigate if these putative sequences exist in nature or if they could be transferred from bacteria into human cells to function in the predicted manner.
Koeppen et al.12 came closer to describing a bacteria-to-human RNA communication system by demonstrating natural transfer of endogenous bacterial short RNA species from Pseudomonas aeruginosa into human host cells via MVs, whilst also demonstrating a reduction in protein levels of several kinases whose mRNAs were predicted to be targeted by one of the bacterial MV sRNAs. Specifically, Koeppen and co-authors identified that sRNA52320, a 24-nucleotide tRNA fragment, is transferred into human cells upon exposure to MVs and that it decreases translation of MAP3K7 and MAP2K4 (kinases in the LPS-simulated MAPK signaling pathway) with subsequent reduction in MV-induced host cell secretion of IL-8.
Overall, these studies have provided early indications that bacterial RNA could act in human cells in a sequence-specific manner to exert post-transcriptional effects on gene expression. Studies of two model systems Caenorhabditis elegans and Arabidopsis thaliana have begun to generate interesting hypotheses and scientific debate based on the biology of RNA in the interactions of C. elegans with dietary E. coli and A. thaliana with the mould Botrytis cinerea. However, some caution in the interpretation is required here as these two hosts have systems to allow signal amplification of exogenous RNA via RNA-dependent polymerases126,127. This mechanism, overcomes a potentially contentious issue in the bacteria-to-human signaling field, concerning whetherthe amount of bacterial RNA transferred is enough to have a function in the host cells.
Alterations in physiological functions were observed in C. elegans fed with E. coli overexpressing the non-coding RNAs OxyS (an oxidative stress response regulator) and DsrA (an acid stress response regulator)128. In bacteria over-expressing OxyS, a negative foraging effect was observed in the nematodes, which preferred to feed on E. coli not over-expressing OxyS when given a choice. The nematodes did not exhibit the repulsion effect when only fed with the OxyS over-expressing strain. Computational analysis of the C. elegans genome identified the che-2 chemosensory gene as a possible target for a sequence-specific interaction with OxyS. Down regulation of CHE-2-GFP fusion expression was visibly seen in C. elegans fed on the OxyS over-expressing strain when compared to wild type K12 E. coli controls.
Interpretation of these results above led the authors to a hypothesis of environmental RNAi, with OxyS from the diet bacteria suppressing the ability of C. elegans to find them. It was shown that the transferred OxyS required the host RNAi pathway, specifically proteins ALG-1 and RDE-4, to function to repress the target CHE-2 protein128. The fact that other RNAi genes were described as dispensable128 raised questions regarding the exact biological mechanism eliciting the gene expression changes and behavioural responses observed129 and this second, independent study, was unable to support the findings of the original study. By using small RNA-sequencing they were unable to find evidence of OxyS RNAs in fed C. elegans that could capably bind to the 17nt interaction site on the che-2 target mRNA and were unable to validate regulation of the target mRNA itself129. Differences between these two study’s findings128,129 could in part be explained by the use of different strains of dietary bacteria, and differences in feeding experiment protocols, such that the role of OxyS RNA might only manifest when the nematode has to make a choice about its food. Further investigations are needed to explain the action of OxyS RNA in C. elegans, and the story to date highlights the power we now have with RNA sequencing to test complex sequence-specific cross-kingdom communication hypotheses.
RNA sequencing was also a key technique used to determine the potential role of fungal small RNAs in the infective processes of B. cinerea using the model plants A. thaliana and Solanum lycopersicum130. B. cinerea sRNAs involved in pathogenicity were identified in infected plants, and a simple bioinformatic approach identified sRNAs with miRNA-like structures that were predicted to suppress 4 genes, by perfect antisense binding, all with roles in plant immunity. Ectopic expression of three of these sRNAs in A. thaliana left the plant with an increased susceptibility to infection. Importantly, and unlike the situation with C. elegans129, the microbial sRNAs were shown to bind to host AGO1 within the RNAi machinery, and A. thaliana strains in which AGO1 is mutated exhibit reduced susceptibility to Botrytis infection. Finally, they demonstrated that B. cinerea Dicer mutants lacking the ability to process sRNAs from longer transposon RNAs were less infective. The challenge now is to identify and explain sequence-specific subversion of a bacterial mammalian host by its prokaryotic pathogen.
5. CONCLUSIONS
Over the last several years the nascent field of cross-kingdom RNA signaling has undergone significant growth. Immune stimulatory effects of bacterial RNA and the role that they play in eliciting and/or suppressing host protective responses are becoming better understood. The sequence-specific effects of regulatory RNAs add an extra dimension of possibilities for RNA signaling. The corresponding development of RNA sequencing and accompanying bioinformatic pipelines now give us powerful tools to investigate this area further. As a consequence, an appreciation that bacterial RNAs are not just a simple uniform trigger of the non-specific immune system, but rather act as complex multifaceted signals is beginning to emerge. On one side is the differential availability of RNA-recognizing sensors within cells, including in their subcellular localization and the need to distinguish between self and foreign RNA. These define the ability of the host to detect intruding RNA and mount a defensive response. On the other side, bacteria appear to utilize multiple methods for protecting and delivering RNA to the host, ranging from MVs which can deliver their cargo over distance, to the intracellular transfer of pathogen’s RNA between host cell compartments. The further coexistence of bacterial RNA with other virulence factors such as lipopolysaccharide53, with which they could simultaneously travel, serves to add a potential extra level of complexity to unraveling host/pathogen RNA interactions and effects on inflammatory responses128,129.
To advance knowledge, careful experimental design and data interpretation is required to physiologically model relevant amounts and modes of RNA delivery into human cells. Overall, many important technical and biological questions await answers in the coming years to decipher the messages conveyed to human cells by bacterial RNA. Identification of the human cellular, molecular, and genetic networks that can both interact with, and be manipulated by, bacterial RNA signals offers exciting new research directions in the study of bacterial pathogenesis.
Bullet Points
◊ Bacterial RNA can be delivered to human cells during infection
◊ Bacterial RNA can have effects beyond simple sensing as a pathogen danger signal
◊ Bacterial RNA can act in sequence-independent and sequence-dependent mechanisms
Open Questions
◊ What are the key bacterial RNAs?
◊ How important is the effect of RNA when compared to protein virulence factors?
◊ Do bacterial RNAs exert an effect in the same way as miRNA?
Acknowledgments
The authors are grateful to Priscila Dauros-Singorenko, Jiwon Hong, Anita Muthukaruppan, Vanessa Chang, Kathryn Askelund, Peter Tsai and Cris Print for stimulating discussions that helped form the ideas expressed in this review. We apologize to the authors whose work we were not able to directly cite due to space limitations. Research by the authors was funded by grants received from the following: Lottery Health Research (NZ), Health Research Council (NZ), Maurice Wilkins Centre for Biodiscovery (NZ), Maurice and Phyllis Paykel Trust (NZ), Ministry of Business, Innovation and Employment (NZ), Johnson and Johnson Surgical Research Fellowship (NZ). These funding entities had no role in the decision to publish or preparation of the manuscript.
Footnotes
Conflict of interests: The authors declare that there are no conflicts of interest.
Adenosine triphosphate (ATP); apoptosis-associated speck-like protein containing a CARD (ASC); argonaute (AGO); caspase recruitment domain (CARD); conventional dendritic cell (cDC); cyclic adenosine monophosphate (cAMP); deoxyribonucleic acid (DNA); double stranded RNA (dsRNA); N-[1-(2,3-dioleoyloxy)propyl]-N,N,N- trimethylammonium methyl sulfate (DOTAP); dual specificity mitogen-activated protein kinase kinase 4 (MAP2K4); dual oxidase 2 (Duox2); 5-ethynyluridine (5EU); interferon (IFN); Interferon regulatory factors (IRFs); interleukin (IL); lipopolysaccharide (LPS); Melanoma Differentiation-Associated protein 5 (MDA5); membrane vesicle (MV); messenger RNA (mRNA); microRNA (miRNA); mitogen activated protein kinase (MAPK); Mitogen-activated protein kinase kinase kinase 7 (MAP3K7); NADPH oxidase 1 (NOX-1); nicotinamide adenine dinucleotide phosphate (NADPH); NLR family, pyrin domain containing (NLRP3); Nod-like receptor X-1 (NLRX-1); nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); NF-κB inhibitor-like protein 1 (NFKBIL1); pathogen associated molecular pattern (PAMP); polyinosinic:polycytidylic acid (poly I:C); reactive oxygen species (ROS); Receptor-type tyrosine-protein phosphatase eta (PTPRJ); retinoic acid-inducible gene I (RIG-I); ribonuclease (RNase); ribonucleic acid (RNA); RNA interference (RNAi); ribosomal RNA (rRNA); single stranded RNA (ssRNA); small RNA (sRNA); T-helper (Th); TIR-domain-containing adapter-inducing interferon-β (TRIF); toll-like receptor (TLR); transfer RNA (tRNA); tumour necrosis factor (TNF).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Stimulation of Intestinal Adenyl Cyclase by Cholera Toxin. SHARP GEOFFREY W. G., HYNIE SIXTUS. Nature. 1971;229(5282):266-269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- 2.Salmonella effectors: important players modulating host cell function during infection. Agbor Terence A., McCormick Beth A. Cellular Microbiology. 2011;13(12):1858-1869. doi: 10.1111/j.1462-5822.2011.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. Fuqua W C, Winans S C, Greenberg E P. Journal of Bacteriology. 1994;176(2):269-275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SigMol: repertoire of quorum sensing signaling molecules in prokaryotes. Rajput Akanksha, Kaur Karambir, Kumar Manoj. Nucleic Acids Research. 2015;44(D1):D634-D639. doi: 10.1093/nar/gkv1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone enhances keratinocyte migration and induces Mmp13 gene expression in vitro. Paes Camila, Nakagami Gojiro, Minematsu Takeo, Nagase Takashi, Huang Lijuan, Sari Yunita, Sanada Hiromi. Biochemical and Biophysical Research Communications. 2012;427(2):273-279. doi: 10.1016/j.bbrc.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Williams Paul. Microbiology (Reading, England) 2007;153(Pt 12):3923–38. doi: 10.1099/mic.0.2007/012856-0. [DOI] [PubMed] [Google Scholar]
- 7.Inter-kingdom signalling: communication between bacteria and their hosts. Hughes David T, Sperandio Vanessa. Nature reviews. Microbiology. 2008;6(2):111–20. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Identification of Virus-Encoded MicroRNAs. Pfeffer S. Science. 2004;304(5671):734-736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 9.Viruses and microRNAs: RISCy interactions with serious consequences. Cullen B. R. Genes & Development. 2011;25(18):1881-1894. doi: 10.1101/gad.17352611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Valadi Hadi, Ekström Karin, Bossios Apostolos, Sjöstrand Margareta, Lee James J, Lötvall Jan O. Nature cell biology. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 11.Bacterial RNA: An Underestimated Stimulus for Innate Immune Responses. Eigenbrod Tatjana, Dalpke Alexander H. Journal of immunology (Baltimore, Md. : 1950) 2015;195(2):411–8. doi: 10.4049/jimmunol.1500530. [DOI] [PubMed] [Google Scholar]
- 12.A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. Koeppen Katja, Hampton Thomas H, Jarek Michael, Scharfe Maren, Gerber Scott A, Mielcarz Daniel W, Demers Elora G, Dolben Emily L, Hammond John H, Hogan Deborah A, Stanton Bruce A. PLoS pathogens. 2016;12(6):e1005672. doi: 10.1371/journal.ppat.1005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uropathogenic Escherichia coli Releases Extracellular Vesicles That Are Associated with RNA. Blenkiron Cherie, Simonov Denis, Muthukaruppan Anita, Tsai Peter, Dauros Priscila, Green Sasha, Hong Jiwon, Print Cristin G, Swift Simon, Phillips Anthony R. PloS one. 2016;11(8):e0160440. doi: 10.1371/journal.pone.0160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macrophages recognize streptococci through bacterial single-stranded RNA. Deshmukh Sachin D, Kremer Bernhard, Freudenberg Marina, Bauer Stefan, Golenbock Douglas T, Henneke Philipp. EMBO reports. 2011;12(1):71–6. doi: 10.1038/embor.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Mancuso Giuseppe, Gambuzza Maria, Midiri Angelina, Biondo Carmelo, Papasergi Salvatore, Akira Shizuo, Teti Giuseppe, Beninati Concetta. Nature immunology. 2009;10(6):587–94. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 16.Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Spencer John David, Schwaderer Andrew L, Wang Huanyu, Bartz Julianne, Kline Jennifer, Eichler Tad, DeSouza Kristin R, Sims-Lucas Sunder, Baker Peter, Hains David S. Kidney international. 2013;83(4):615–25. doi: 10.1038/ki.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Becknell Brian, Eichler Tad E, Beceiro Susana, Li Birong, Easterling Robert S, Carpenter Ashley R, James Cindy L, McHugh Kirk M, Hains David S, Partida-Sanchez Santiago, Spencer John D. Kidney international. 2015;87(1):151–61. doi: 10.1038/ki.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. Harder Jurgen, Schroder Jens-Michael. The Journal of biological chemistry. 2002;277(48):46779–84. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 19.Human hair follicle epithelium has an antimicrobial defence system that includes the inducible antimicrobial peptide psoriasin (S100A7) and RNase 7. Reithmayer K, Meyer K C, Kleditzsch P, Tiede S, Uppalapati S K, Gläser R, Harder J, Schröder J-M, Paus R. The British journal of dermatology. 2009;161(1):78–89. doi: 10.1111/j.1365-2133.2009.09154.x. [DOI] [PubMed] [Google Scholar]
- 20.Ribonuclease activity in human plasma. Kamm R.C., Smith A.G. Clinical Biochemistry. 1972;5(1-4):198-200. doi: 10.1016/s0009-9120(72)80033-x. [DOI] [PubMed] [Google Scholar]
- 21.Ribonucleases of human serum, urine, cerebrospinal fluid, and leukocytes. Activity staining following electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. Blank A., Dekker Charles A. Biochemistry. 1981;20(8):2261-2267. doi: 10.1021/bi00511a030. [DOI] [PubMed] [Google Scholar]
- 22.Nucleic acids and endosomal pattern recognition: how to tell friend from foe? Brencicova Eva, Diebold Sandra S. Frontiers in Cellular and Infection Microbiology. 2013;3 doi: 10.3389/fcimb.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Creemers Esther E, Tijsen Anke J, Pinto Yigal M. Circulation research. 2012;110(3):483–95. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 24.New hypotheses derived from the structure of a flaviviral Xrn1-resistant RNA: Conservation, folding, and host adaptation. Kieft Jeffrey S, Rabe Jennifer L, Chapman Erich G. RNA biology. 2015;12(11):1169–77. doi: 10.1080/15476286.2015.1094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The complex exogenous RNA spectra in human plasma: an interface with human gut biota? Wang Kai, Li Hong, Yuan Yue, Etheridge Alton, Zhou Yong, Huang David, Wilmes Paul, Galas David. PloS one. 2012;7(12):e51009. doi: 10.1371/journal.pone.0051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unbiased approach to profile the variety of small non-coding RNA of human blood plasma with massively parallel sequencing technology. Semenov Dmitry V, Baryakin Dmitry N, Brenner Evgeny V, Kurilshikov Alexander M, Vasiliev Gennady V, Bryzgalov Leonid A, Chikova Elena D, Filippova Julia A, Kuligina Elena V, Richter Vladimir A. Expert opinion on biological therapy. 2012;12 Suppl 1:S43–51. doi: 10.1517/14712598.2012.679653. [DOI] [PubMed] [Google Scholar]
- 27.Small RNAs from plants, bacteria and fungi within the order Hypocreales are ubiquitous in human plasma. Beatty Meabh, Guduric-Fuchs Jasenka, Brown Eoin, Bridgett Stephen, Chakravarthy Usha, Hogg Ruth Esther, Simpson David Arthur. BMC genomics. 2014;15:933. doi: 10.1186/1471-2164-15-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Circulating microbial RNA and health. Leung Ross Ka-Kit, Wu Ying-Kit. Scientific Reports. 2015;5(1) doi: 10.1038/srep16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diverse human extracellular RNAs are widely detected in human plasma. Freedman Jane E., Gerstein Mark, Mick Eric, Rozowsky Joel, Levy Daniel, Kitchen Robert, Das Saumya, Shah Ravi, Danielson Kirsty, Beaulieu Lea, Navarro Fabio C. P., Wang Yaoyu, Galeev Timur R., Holman Alex, Kwong Raymond Y., Murthy Venkatesh, Tanriverdi Selim E., Koupenova-Zamor Milka, Mikhalev Ekaterina, Tanriverdi Kahraman. Nature Communications. 2016;7:11106. doi: 10.1038/ncomms11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fusobacterium nucleatum activates the immune response through retinoic acid-inducible gene I. Lee P, Tan K S. Journal of dental research. 2014;93(2):162–8. doi: 10.1177/0022034513516346. [DOI] [PubMed] [Google Scholar]
- 31.The extracellular RNA complement of Escherichia coli. Ghosal Anubrata, Upadhyaya Bimal Babu, Fritz Joëlle V, Heintz-Buschart Anna, Desai Mahesh S, Yusuf Dilmurat, Huang David, Baumuratov Aidos, Wang Kai, Galas David, Wilmes Paul. MicrobiologyOpen. 2015 doi: 10.1002/mbo3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stable Extracellular RNA Fragments of Mycobacterium tuberculosis Induce Early Apoptosis in Human Monocytes via a Caspase-8 Dependent Mechanism. Obregón-Henao Andrés, Duque-Correa María A., Rojas Mauricio, García Luis F., Brennan Patrick J., Ortiz Blanca L., Belisle John T. PLoS ONE. 2012;7(1):e29970. doi: 10.1371/journal.pone.0029970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Functional Advantages of Porphyromonas gingivalis Vesicles. Ho Meng-Hsuan, Chen Chin-Ho, Goodwin J. Shawn, Wang Bing-Yan, Xie Hua. PLOS ONE. 2015;10(4):e0123448. doi: 10.1371/journal.pone.0123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Membrane vesicle-mediated release of bacterial RNA. Sjöström Annika E., Sandblad Linda, Uhlin Bernt Eric, Wai Sun Nyunt. Scientific Reports. 2015;5(1) doi: 10.1038/srep15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Turnbull Lynne, Toyofuku Masanori, Hynen Amelia L., Kurosawa Masaharu, Pessi Gabriella, Petty Nicola K., Osvath Sarah R., Cárcamo-Oyarce Gerardo, Gloag Erin S., Shimoni Raz, Omasits Ulrich, Ito Satoshi, Yap Xinhui, Monahan Leigh G., Cavaliere Rosalia, Ahrens Christian H., Charles Ian G., Nomura Nobuhiko, Eberl Leo, Whitchurch Cynthia B. Nature Communications. 2016;7:11220. doi: 10.1038/ncomms11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Brown Lisa, Wolf Julie M., Prados-Rosales Rafael, Casadevall Arturo. Nature Reviews Microbiology. 2015;13(10):620-630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Schwechheimer Carmen, Kuehn Meta J. Nature Reviews Microbiology. 2015;13(10):605-619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gram-negative and Gram-positive bacterial extracellular vesicles. Kim Ji Hyun, Lee Jaewook, Park Jaesung, Gho Yong Song. Seminars in Cell & Developmental Biology. 2015;40:97-104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Pathirana Rishi D., Kaparakis-Liaskos Maria. Cellular Microbiology. 2016;18(11):1518-1524. doi: 10.1111/cmi.12658. [DOI] [PubMed] [Google Scholar]
- 40.Outer membrane vesicles in service as protein shuttles, biotic defenders, and immunological doppelgängers. Laughlin Richard C., Alaniz Robert C. Gut Microbes. 2016;7(5):450-454. doi: 10.1080/19490976.2016.1222345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J., Lötvall J. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 42.Exosomes: Fit to deliver small RNA. Zomer Anoek, Vendrig Tineke, Hopmans Erik S, van Eijndhoven Monique, Middeldorp Jaap M, Pegtel D Michiel. Communicative & integrative biology. 2010;3(5):447–50. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Functional delivery of viral miRNAs via exosomes. Pegtel D Michiel, Cosmopoulos Katherine, Thorley-Lawson David A, van Eijndhoven Monique A J, Hopmans Erik S, Lindenberg Jelle L, de Gruijl Tanja D, Würdinger Thomas, Middeldorp Jaap M. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Kulp Adam, Kuehn Meta J. Annual review of microbiology. 2010;64:163–84. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A bilayer-couple model of bacterial outer membrane vesicle biogenesis. Schertzer Jeffrey W, Whiteley Marvin. mBio. 2012;3(2) doi: 10.1128/mBio.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Roier Sandro, Zingl Franz G, Cakar Fatih, Durakovic Sanel, Kohl Paul, Eichmann Thomas O, Klug Lisa, Gadermaier Bernhard, Weinzerl Katharina, Prassl Ruth, Lass Achim, Daum Günther, Reidl Joachim, Feldman Mario F, Schild Stefan. Nature communications. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacterial programmed cell death: making sense of a paradox. Bayles Kenneth W. Nature reviews. Microbiology. 2014;12(1):63–9. doi: 10.1038/nrmicro3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. Bomberger Jennifer M, Maceachran Daniel P, Coutermarsh Bonita A, Ye Siying, O'Toole George A, Stanton Bruce A. PLoS pathogens. 2009;5(4):e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. Bielaszewska Martina, Rüter Christian, Kunsmann Lisa, Greune Lilo, Bauwens Andreas, Zhang Wenlan, Kuczius Thorsten, Kim Kwang Sik, Mellmann Alexander, Schmidt M Alexander, Karch Helge. PLoS pathogens. 2013;9(12):e1003797. doi: 10.1371/journal.ppat.1003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Kaparakis Maria, Turnbull Lynne, Carneiro Leticia, Firth Stephen, Coleman Harold A, Parkington Helena C, Le Bourhis Lionel, Karrar Abdulgader, Viala Jérôme, Mak Johnson, Hutton Melanie L, Davies John K, Crack Peter J, Hertzog Paul J, Philpott Dana J, Girardin Stephen E, Whitchurch Cynthia B, Ferrero Richard L. Cellular microbiology. 2010;12(3):372–85. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 51.Cytotoxic and Inflammatory Responses Induced by Outer Membrane Vesicle-Associated Biologically Active Proteases from Vibrio cholerae. Mondal Ayan, Tapader Rima, Chatterjee Nabendu Sekhar, Ghosh Amit, Sinha Ritam, Koley Hemanta, Saha Dhira Rani, Chakrabarti Manoj K., Wai Sun Nyunt, Pal Amit. Infection and Immunity. 2016;84(5):1478-1490. doi: 10.1128/IAI.01365-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uptake of Helicobacter pylori Vesicles Is Facilitated by Clathrin-Dependent and Clathrin-Independent Endocytic Pathways. Olofsson A., Nygard Skalman L., Obi I., Lundmark R., Arnqvist A. mBio. 2014;5(3):e00979-14-e00979-14. doi: 10.1128/mBio.00979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uptake of Helicobacter pylori Outer Membrane Vesicles by Gastric Epithelial Cells. Parker H., Chitcholtan K., Hampton M. B., Keenan J. I. Infection and Immunity. 2010;78(12):5054-5061. doi: 10.1128/IAI.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Outer Membrane Vesicles from Brucella abortus Promote Bacterial Internalization by Human Monocytes and Modulate Their Innate Immune Response. Pollak Cora N., Delpino M. Victoria, Fossati Carlos A., Baldi Pablo C. PLoS ONE. 2012;7(11):e50214. doi: 10.1371/journal.pone.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aggregatibacter actinomycetemcomitans Outer Membrane Vesicles Are Internalized in Human Host Cells and Trigger NOD1- and NOD2-Dependent NF-κB Activation. Thay Bernard, Damm Anna, Kufer Thomas A., Wai Sun Nyunt, Oscarsson Jan. Infection and Immunity. 2014;82(10):4034-4046. doi: 10.1128/IAI.01980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Kunsmann Lisa, Rüter Christian, Bauwens Andreas, Greune Lilo, Glüder Malte, Kemper Björn, Fruth Angelika, Wai Sun Nyunt, He Xiaohua, Lloubes Roland, Schmidt M. Alexander, Dobrindt Ulrich, Mellmann Alexander, Karch Helge, Bielaszewska Martina. Scientific Reports. 2015;5(1) doi: 10.1038/srep13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Sha Wenwen, Mitoma Hiroki, Hanabuchi Shino, Bao Musheng, Weng Leiyun, Sugimoto Naoshi, Liu Ying, Zhang Zhiqiang, Zhong Jin, Sun Bing, Liu Yong-Jun. Proceedings of the National Academy of Sciences. 2014;111(45):16059-16064. doi: 10.1073/pnas.1412487111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bacterial RNA Mediates Activation of Caspase-1 and IL-1 Release Independently of TLRs 3, 7, 9 and TRIF but Is Dependent on UNC93B. Eigenbrod T., Franchi L., Munoz-Planillo R., Kirschning C. J., Freudenberg M. A., Nunez G., Dalpke A. The Journal of Immunology. 2012;189(1):328-336. doi: 10.4049/jimmunol.1103258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.RIG-I Detects Triphosphorylated RNA of Listeria monocytogenes during Infection in Non-Immune Cells. Hagmann Cristina Amparo, Herzner Anna Maria, Abdullah Zeinab, Zillinger Thomas, Jakobs Christopher, Schuberth Christine, Coch Christoph, Higgins Paul G., Wisplinghoff Hilmar, Barchet Winfried, Hornung Veit, Hartmann Gunther, Schlee Martin. PLoS ONE. 2013;8(4):e62872. doi: 10.1371/journal.pone.0062872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.RIG-I Detects Triphosphorylated RNA of Listeria monocytogenes during Infection in Non-Immune Cells. Hagmann C., Herzner A., Abdullah Z. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0062872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes. Cervantes Jorge L, La Vake Carson J, Weinerman Bennett, Luu Stephanie, O'Connell Caitlin, Verardi Paulo H, Salazar Juan C. Journal of leukocyte biology. 2013;94(6):1231–41. doi: 10.1189/jlb.0413206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Sander Leif E, Davis Michael J, Boekschoten Mark V, Amsen Derk, Dascher Christopher C, Ryffel Bernard, Swanson Joel A, Müller Michael, Blander J Magarian. Nature. 2011;474(7351):385–9. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.RNA and β-hemolysin of group B Streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. Gupta Rahul, Ghosh Shubhendu, Monks Brian, DeOliveira Rosane B, Tzeng Te-Chen, Kalantari Parisa, Nandy Anubhab, Bhattacharjee Bornali, Chan Jennie, Ferreira Fabianno, Rathinam Vijay, Sharma Shruti, Lien Egil, Silverman Neal, Fitzgerald Katherine, Firon Arnaud, Trieu-Cuot Patrick, Henneke Philipp, Golenbock Douglas T. The Journal of biological chemistry. 2014;289(20):13701–5. doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.RNA and β-hemolysin of Group B streptococcus induce IL-1β by activating NLRP3 inflammasomes in mouse macrophages. Gupta R., Ghosh S., Monks B. J Biol Chem. 2014 doi: 10.1074/jbc.C114.548982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Kailasan Vanaja Sivapriya, Rathinam Vijay A K, Atianand Maninjay K, Kalantari Parisa, Skehan Brian, Fitzgerald Katherine A, Leong John M. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(21):7765–70. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes. Cervantes J., La Vake C., Weinerman B. J Leukoc Biol. 2013;94:1231–1241. doi: 10.1189/jlb.0413206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.TLR8 Senses Bacterial RNA in Human Monocytes and Plays a Nonredundant Role for Recognition of Streptococcus pyogenes. Eigenbrod Tatjana, Pelka Karin, Latz Eicke, Kreikemeyer Bernd, Dalpke Alexander H. Journal of immunology (Baltimore, Md. : 1950) 2015;195(3):1092–9. doi: 10.4049/jimmunol.1403173. [DOI] [PubMed] [Google Scholar]
- 68.The TLR3 signaling complex forms by cooperative receptor dimerization. Leonard Joshua N, Ghirlando Rodolfo, Askins Janine, Bell Jessica K, Margulies David H, Davies David R, Segal David M. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):258–63. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Diebold Sandra S, Kaisho Tsuneyasu, Hemmi Hiroaki, Akira Shizuo, Reis e Sousa Caetano. Science (New York, N.Y.) 2004;303(5663):1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 70.Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Heil Florian, Hemmi Hiroaki, Hochrein Hubertus, Ampenberger Franziska, Kirschning Carsten, Akira Shizuo, Lipford Grayson, Wagner Hermann, Bauer Stefan. Science (New York, N.Y.) 2004;303(5663):1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 71.Synergistic activation of Toll-like receptor 8 by two RNA degradation products. Geyer Matthias, Pelka Karin, Latz Eicke. Nature structural & molecular biology. 2015;22(2):99–101. doi: 10.1038/nsmb.2967. [DOI] [PubMed] [Google Scholar]
- 72.RNA of Enterococcus faecalis Strain EC-12 Is a Major Component Inducing Interleukin-12 Production from Human Monocytic Cells. Nishibayashi Ryoichiro, Inoue Ryo, Harada Yuri, Watanabe Takumi, Makioka Yuko, Ushida Kazunari. PloS one. 2015;10(6):e0129806. doi: 10.1371/journal.pone.0129806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Viral RNA detection by RIG-I-like receptors. Yoneyama Mitsutoshi, Onomoto Koji, Jogi Michihiko, Akaboshi Teppei, Fujita Takashi. Current opinion in immunology. 2015;32:48–53. doi: 10.1016/j.coi.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Kanneganti Thirumala-Devi, Ozören Nesrin, Body-Malapel Mathilde, Amer Amal, Park Jong-Hwan, Franchi Luigi, Whitfield Joel, Barchet Winfried, Colonna Marco, Vandenabeele Peter, Bertin John, Coyle Anthony, Grant Ethan P, Akira Shizuo, Núñez Gabriel. Nature. 2006;440(7081):233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 75.Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Kailasan Vanaja S., Rathinam V., Atianand M. Proc Natl Acad Sci U S A. 2014;111:7765–7770. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molecular mechanisms regulating NLRP3 inflammasome activation. Jo Eun-Kyeong, Kim Jin Kyung, Shin Dong-Min, Sasakawa Chihiro. Cellular & molecular immunology. 2016;13(2):148–59. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borrelia burgdorferi RNA Induces Type I and III Interferons via Toll-Like Receptor 7 and Contributes to Production of NF- B-Dependent Cytokines. Love A. C., Schwartz I., Petzke M. M. Infection and Immunity. 2014;82(6):2405-2416. doi: 10.1128/IAI.01617-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Type I Interferon Production Induced by Streptococcus pyogenes-Derived Nucleic Acids Is Required for Host Protection. Gratz Nina, Hartweger Harald, Matt Ulrich, Kratochvill Franz, Janos Marton, Sigel Stefanie, Drobits Barbara, Li Xiao-Dong, Knapp Sylvia, Kovarik Pavel. PLoS Pathogens. 2011;7(5):e1001345. doi: 10.1371/journal.ppat.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Identification of modifications in microbial, native tRNA that suppress immunostimulatory activity. Gehrig Stefanie, Eberle Mariel-Esther, Botschen Flavia, Rimbach Katharina, Eberle Florian, Eigenbrod Tatjana, Kaiser Steffen, Holmes Walter M., Erdmann Volker A., Sprinzl Mathias, Bec Guillaume, Keith Gérard, Dalpke Alexander H., Helm Mark. The Journal of Experimental Medicine. 2012;209(2):225-233. doi: 10.1084/jem.20111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bacterial RNA is recognized by different sets of immunoreceptors. Eberle Florian, Sirin Mehtap, Binder Marco, Dalpke Alexander H. European journal of immunology. 2009;39(9):2537–47. doi: 10.1002/eji.200838978. [DOI] [PubMed] [Google Scholar]
- 81.Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Karikó Katalin, Buckstein Michael, Ni Houping, Weissman Drew. Immunity. 2005;23(2):165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 82.Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. Koski Gary K, Karikó Katalin, Xu Shuwen, Weissman Drew, Cohen Peter A, Czerniecki Brian J. Journal of immunology (Baltimore, Md. : 1950) 2004;172(7):3989–93. doi: 10.4049/jimmunol.172.7.3989. [DOI] [PubMed] [Google Scholar]
- 83.Bacterial RNA: An Underestimated Stimulus for Innate Immune Responses. Eigenbrod T., Dalpke A. J Immunol. 2015;195:411–418. doi: 10.4049/jimmunol.1500530. [DOI] [PubMed] [Google Scholar]
- 84.A modified dinucleotide motif specifies tRNA recognition by TLR7. Kaiser Steffen, Rimbach Katharina, Eigenbrod Tatjana, Dalpke Alexander H, Helm Mark. RNA (New York, N.Y.) 2014;20(9):1351–5. doi: 10.1261/rna.044024.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.A single naturally occurring 2'-O-methylation converts a TLR7- and TLR8-activating RNA into a TLR8-specific ligand. Jung Stephanie, von Thülen Tina, Laukemper Viktoria, Pigisch Stephanie, Hangel Doris, Wagner Hermann, Kaufmann Andreas, Bauer Stefan. PloS one. 2015;10(3):e0120498. doi: 10.1371/journal.pone.0120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.The 2′-O-methylation status of a single guanosine controls transfer RNA–mediated Toll-like receptor 7 activation or inhibition. Jöckel Stefanie, Nees Gernot, Sommer Romy, Zhao Yang, Cherkasov Dmitry, Hori Hiroyuki, Ehm Gundi, Schnare Markus, Nain Marianne, Kaufmann Andreas, Bauer Stefan. The Journal of Experimental Medicine. 2012;209(2):235-241. doi: 10.1084/jem.20111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.RIG-I detects infection with liveListeriaby sensing secreted bacterial nucleic acids. Abdullah Zeinab, Schlee Martin, Roth Susanne, Mraheil Mobarak Abu, Barchet Winfried, Böttcher Jan, Hain Torsten, Geiger Sergej, Hayakawa Yoshihiro, Fritz Jörg H, Civril Filiz, Hopfner Karl-Peter, Kurts Christian, Ruland Jürgen, Hartmann Gunther, Chakraborty Trinad, Knolle Percy A. The EMBO Journal. 2012;31(21):4153-4164. doi: 10.1038/emboj.2012.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. Shahangian Arash, Chow Edward K., Tian Xiaoli, Kang Jason R., Ghaffari Amir, Liu Su Y., Belperio John A., Cheng Genhong, Deng Jane C. Journal of Clinical Investigation. 2009;119(7):1910-1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Transkingdom control of viral infection and immunity in the mammalian intestine. Pfeiffer J. K., Virgin H. W. Science. 2016;351(6270):aad5872-aad5872. doi: 10.1126/science.aad5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.cPLA2Regulates the Expression of Type I Interferons and Intracellular Immunity toChlamydia trachomatis. Vignola Mark J., Kashatus David F., Taylor Gregory A., Counter Christopher M., Valdivia Raphael H. Journal of Biological Chemistry. 2010;285(28):21625-21635. doi: 10.1074/jbc.M110.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.SalmonellaSuppresses the TRIF-Dependent Type I Interferon Response in Macrophages. Owen Katherine A., Anderson C. J., Casanova James E. mBio. 2016;7(1):e02051-15. doi: 10.1128/mBio.02051-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis. Sionov Edward, Mayer-Barber Katrin D., Chang Yun C., Kauffman Keith D., Eckhaus Michael A., Salazar Andres M., Barber Daniel L., Kwon-Chung Kyung J. PLOS Pathogens. 2015;11(8):e1005040. doi: 10.1371/journal.ppat.1005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Type I IFN Signaling Is Crucial for Host Resistance against Different Species of Pathogenic Bacteria. Mancuso G., Midiri A., Biondo C., Beninati C., Zummo S., Galbo R., Tomasello F., Gambuzza M., Macri G., Ruggeri A., Leanderson T., Teti G. The Journal of Immunology. 2007;178(5):3126-3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 94.Role of type I interferons in inflammasome activation, cell death, and disease during microbial infection. Malireddi R. K. Subbarao, Kanneganti Thirumala-Devi. Frontiers in Cellular and Infection Microbiology. 2013;3 doi: 10.3389/fcimb.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Rayamajhi Manira, Humann Jessica, Kearney Staci, Hill Krista K., Lenz Laurel L. Virulence. 2010;1(5):418-422. doi: 10.4161/viru.1.5.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The Mechanism for Type I Interferon Induction by Mycobacterium tuberculosis is Bacterial Strain-Dependent. Wiens Kirsten E., Ernst Joel D. PLOS Pathogens. 2016;12(8):e1005809. doi: 10.1371/journal.ppat.1005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borrelia burgdorferi induces a type I interferon response during early stages of disseminated infection in mice. Petzke Mary M., Iyer Radha, Love Andrea C., Spieler Zoe, Brooks Andrew, Schwartz Ira. BMC Microbiology. 2016;16(1) doi: 10.1186/s12866-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.T-cell response to bacterial agents. D'Elios Mario Milco, Benagiano Marisa, Della Bella Chiara, Amedei Amedeo. The Journal of Infection in Developing Countries. 2011;5(09) doi: 10.3855/jidc.2019. [DOI] [PubMed] [Google Scholar]
- 99.Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. Liang Spencer C., Tan Xiang-Yang, Luxenberg Deborah P., Karim Riyez, Dunussi-Joannopoulos Kyriaki, Collins Mary, Fouser Lynette A. The Journal of Experimental Medicine. 2006;203(10):2271-2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Lee Benjamin, Robinson Keven M., McHugh Kevin J., Scheller Erich V., Mandalapu Sivanarayana, Chen Chen, Di Y. Peter, Clay Michelle E., Enelow Richard I., Dubin Patricia J., Alcorn John F. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;309(2):L158-L167. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Influenza A Inhibits Th17-Mediated Host Defense against Bacterial Pneumonia in Mice. Kudva A., Scheller E. V., Robinson K. M., Crowe C. R., Choi S. M., Slight S. R., Khader S. A., Dubin P. J., Enelow R. I., Kolls J. K., Alcorn J. F. The Journal of Immunology. 2010;186(3):1666-1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Type I Interferon Induction during Influenza Virus Infection Increases Susceptibility to Secondary Streptococcus pneumoniae Infection by Negative Regulation of T Cells. Li W., Moltedo B., Moran T. M. Journal of Virology. 2012;86(22):12304-12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nod-Like Receptor X-1 Is Required for Rhinovirus-Induced Barrier Dysfunction in Airway Epithelial Cells. Unger B. L., Ganesan S., Comstock A. T., Faris A. N., Hershenson M. B., Sajjan U. S. Journal of Virology. 2014;88(7):3705-3718. doi: 10.1128/JVI.03039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poly(I:C) Induces Human Lung Endothelial Barrier Dysfunction by Disrupting Tight Junction Expression of Claudin-5. Huang Li-Yun, Stuart Christine, Takeda Kazuyo, D’Agnillo Felice, Golding Basil. PLOS ONE. 2016;11(8):e0160875. doi: 10.1371/journal.pone.0160875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. Harper Richart W, Xu Changhong, Eiserich Jason P, Chen Yin, Kao Cheng-Yuan, Thai Philip, Setiadi Henny, Wu Reen. FEBS letters. 2005;579(21):4911–7. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. Geiszt Miklós, Witta Jassir, Baffi Judit, Lekstrom Kristen, Leto Thomas L. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(11):1502–4. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 107.Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. Barraud Nicolas, Hassett Daniel J, Hwang Sung-Hei, Rice Scott A, Kjelleberg Staffan, Webb Jeremy S. Journal of bacteriology. 2006;188(21):7344–53. doi: 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Differential roles of OxyR-controlled antioxidant enzymes alkyl hydroperoxide reductase (AhpCF) and catalase (KatB) in the protection of Pseudomonas aeruginosa against hydrogen peroxide in biofilm vs. planktonic culture. Panmanee Warunya, Hassett Daniel J. FEMS microbiology letters. 2009;295(2):238–44. doi: 10.1111/j.1574-6968.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. Spoering A L, Lewis K. Journal of bacteriology. 2001;183(23):6746–51. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Chattoraj Sangbrita S, Ganesan Shyamala, Jones Andrew M, Helm Jennifer M, Comstock Adam T, Bright-Thomas Rowland, LiPuma John J, Hershenson Marc B, Sajjan Umadevi S. Thorax. 2011;66(4):333–9. doi: 10.1136/thx.2010.151431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.TLR8 Senses Staphylococcus aureus RNA in Human Primary Monocytes and Macrophages and Induces IFN-β Production via a TAK1-IKKβ-IRF5 Signaling Pathway. Bergstrøm Bjarte, Aune Marie H, Awuh Jane A, Kojen June F, Blix Kjetil J, Ryan Liv, Flo Trude H, Mollnes Tom E, Espevik Terje, Stenvik Jørgen. Journal of immunology (Baltimore, Md. : 1950) 2015;195(3):1100–11. doi: 10.4049/jimmunol.1403176. [DOI] [PubMed] [Google Scholar]
- 112.Most mammalian mRNAs are conserved targets of microRNAs. Friedman R. C., Farh K. K.-H., Burge C. B., Bartel D. P. Genome Research. 2008;19(1):92-105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.An overview of microRNAs. Hammond Scott M. Advanced drug delivery reviews. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.The functional Hfq-binding module of bacterial sRNAs consists of a double or single hairpin preceded by a U-rich sequence and followed by a 3' poly(U) tail. Ishikawa Hirokazu, Otaka Hironori, Maki Kimika, Morita Teppei, Aiba Hiroji. RNA (New York, N.Y.) 2012;18(5):1062–74. doi: 10.1261/rna.031575.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mature miRNAs form secondary structure, which suggests their function beyond RISC. Belter Agnieszka, Gudanis Dorota, Rolle Katarzyna, Piwecka Monika, Gdaniec Zofia, Naskręt-Barciszewska Mirosława Z, Barciszewski Jan. PloS one. 2014;9(11):e113848. doi: 10.1371/journal.pone.0113848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sounds of silence: synonymous nucleotides as a key to biological regulation and complexity. Shabalina Svetlana A, Spiridonov Nikolay A, Kashina Anna. Nucleic acids research. 2013;41(4):2073–94. doi: 10.1093/nar/gks1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Origins and evolution of eukaryotic RNA interference. Shabalina Svetlana A, Koonin Eugene V. Trends in ecology & evolution. 2008;23(10):578–87. doi: 10.1016/j.tree.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lsm proteins and Hfq: Life at the 3' end. Wilusz Carol J, Wilusz Jeffrey. RNA biology. 2013;10(4):592–601. doi: 10.4161/rna.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Isolation and profiling of protein-associated small RNAs. Zhao Hongwei, Lii Yifan, Zhu Pei, Jin Hailing. Methods in molecular biology (Clifton, N.J.) 2012;883:165–76. doi: 10.1007/978-1-61779-839-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Role of plant MicroRNA in cross-species regulatory networks of humans. Zhang Hao, Li Yanpu, Liu Yuanning, Liu Haiming, Wang Hongyu, Jin Wen, Zhang Yanmei, Zhang Chao, Xu Dong. BMC systems biology. 2016;10(1):60. doi: 10.1186/s12918-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Buck Amy H, Coakley Gillian, Simbari Fabio, McSorley Henry J, Quintana Juan F, Le Bihan Thierry, Kumar Sujai, Abreu-Goodger Cei, Lear Marissa, Harcus Yvonne, Ceroni Alessandro, Babayan Simon A, Blaxter Mark, Ivens Alasdair, Maizels Rick M. Nature communications. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Regulatory RNAs and target mRNA decay in prokaryotes. Lalaouna David, Simoneau-Roy Maxime, Lafontaine Daniel, Massé Eric. Biochimica et biophysica acta. 2013;1829(6-7):742–7. doi: 10.1016/j.bbagrm.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 123.The Long and Short of MicroRNA. Yates Luke A., Norbury Chris J., Gilbert Robert J.C. Cell. 2013;153(3):516-519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 124.Prediction of Bacterial microRNAs and possible targets in human cell transcriptome. Shmaryahu Amir, Carrasco Margarita, Valenzuela Pablo D.T. Journal of Microbiology. 2014;52(6):482-489. doi: 10.1007/s12275-014-3658-3. [DOI] [PubMed] [Google Scholar]
- 125.The Complete Genome Sequence and Analysis of the Epsilonproteobacterium Arcobacter butzleri. Miller William G., Parker Craig T., Rubenfield Marc, Mendz George L., Wösten Marc M. S. M., Ussery David W., Stolz John F., Binnewies Tim T., Hallin Peter F., Wang Guilin, Malek Joel A., Rogosin Andrea, Stanker Larry H., Mandrell Robert E. PLoS ONE. 2007;2(12):e1358. doi: 10.1371/journal.pone.0001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Biology and Mechanisms of Short RNAs in Caenorhabditis elegans. Grishok Alla. Advances in Genetics. 2013:1-69. doi: 10.1016/B978-0-12-407675-4.00001-8. [DOI] [PubMed] [Google Scholar]
- 127.A biochemical framework for RNA silencing in plants. Tang G. Genes & Development. 2003;17(1):49-63. doi: 10.1101/gad.1048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caenorhabditis elegans SID-2 is required for environmental RNA interference. Winston William M, Sutherlin Marie, Wright Amanda J, Feinberg Evan H, Hunter Craig P. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10565–70. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.E. coli OxyS non-coding RNA does not trigger RNAi in C. elegans. Akay Alper, Sarkies Peter, Miska Eric A. Scientific reports. 2015;5:9597. doi: 10.1038/srep09597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Weiberg Arne, Wang Ming, Lin Feng-Mao, Zhao Hongwei, Zhang Zhihong, Kaloshian Isgouhi, Huang Hsien-Da, Jin Hailing. Science (New York, N.Y.) 2013;342(6154):118–23. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]