Abstract
Nuclear factor (NF)-κB is a transcription factor that plays significant role in immunity, cellular survival and inhibition of apoptosis, through the induction of genetic networks. Depending on the stimulus and the cell type, the members of NF-κB related family (RelA, c-Rel, RelB, p50, and p52), forms different combinations of homo and hetero-dimers. The activated complexes (Es) translocate into the nucleus and bind to the 10bp κB site of promoter region of target genes in stimulus specific manner. In response to radiation, NF-κB is known to reduce cell death by promoting the expression of anti-apoptotic proteins and activation of cellular antioxidant defense system. Constitutive activation of NF-κB associated genes in tumour cells are known to enhance radiation resistance, whereas deletion in mice results in hypersensitivity to IR-induced GI damage. NF-κB is also known to regulate the production of a wide variety of cytokines and chemokines, which contribute in enhancing cell proliferation and tissue regeneration in various organs, such as the GI crypts stem cells, bone marrow etc., following exposure to IR. Several other cytokines are also known to exert potent pro-inflammatory effects that may contribute to the increase of tissue damage following exposure to ionizing radiation. Till date there are a series of molecules or group of compounds that have been evaluated for their radio-protective potential, and very few have reached clinical trials. The failure or less success of identified agents in humans could be due to their reduced radiation protection efficacy. In this review we have considered activation of NF-κB as a potential marker in screening of radiation countermeasure agents (RCAs) and cellular radiation responses. Moreover, we have also focused on associated mechanisms of activation of NF-κB signaling and their specified family member activation with respect to stimuli. Furthermore, we have categorized their regulated gene expressions and their function in radiation response or modulation. In addition, we have discussed some recently developed radiation countermeasures in relation to NF-κB activation
Keywords: Ionizing radiation, NF-kB, apoptosis, cell proliferation, inflammation, radioprotector
SUMMARY
Introduction
NF-κB/IκB family members & their associated proteins
-
NF-κB activation pathways
3.1 IKKβ dependent (classical) pathway
3.2. IKKα dependent (alternative) pathway
3.3. Atypical pathway
3.4. Oxidative stress-induced pathway
Post translational modifications of NF-κB proteins
NF-κB regulated proteins and their functions in oxidative stress
Radiation Countermeasures in relation to NF-κB activation
Conclusion
1. Introduction
Deleterious effects of ionizing radiation (IR) may lead to significant morbidity and a possible fatal illness that affects various organs of the organism in a dose and time dependent manner1. Exposure of the organism to IR during therapy, or as a result of a radiological/ nuclear incident, or act of terrorism, may symbolize serious health issues. However, this problem remains largely impervious to medical management of IR exposure and therefore, there is a pressing need to develop safe and effective radiation countermeasures agents (RCA) to reduce or mitigate the harmful consequences of IR exposure at cellular, tissue and organism levels. Following exposure of the organism to ionizing radiation, various signaling pathways, such as the mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinases (PI3K), and ataxia telangiectasia mutated (ATM) are activated and all these processes are tightly regulated in relation to changes in expression of various transcription factors (AP, NF-κB, p53, ARE, GADD153 etc) along with changes in the functional status of cell organelles2-4. This may trigger alterations in expression of a large number of genes that are mostly related to cell cycle progression, cell survival, DNA repair and apoptosis4,5.
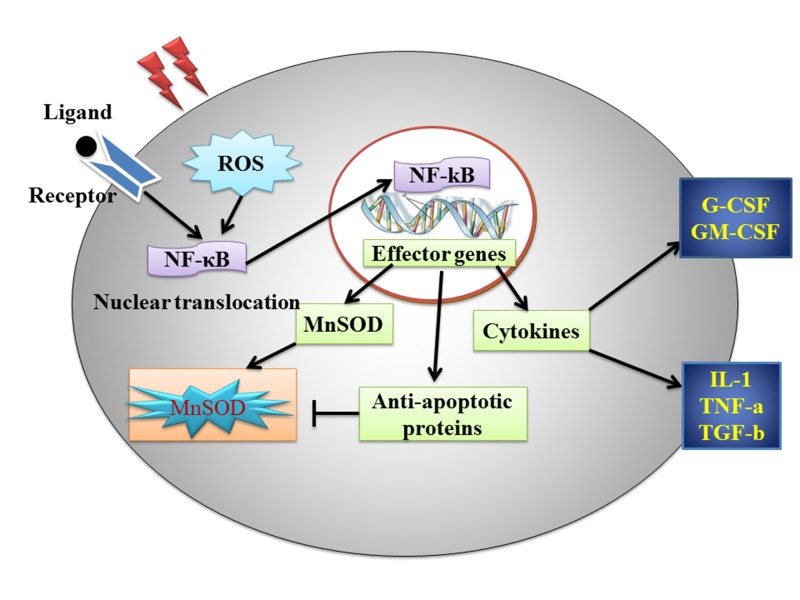
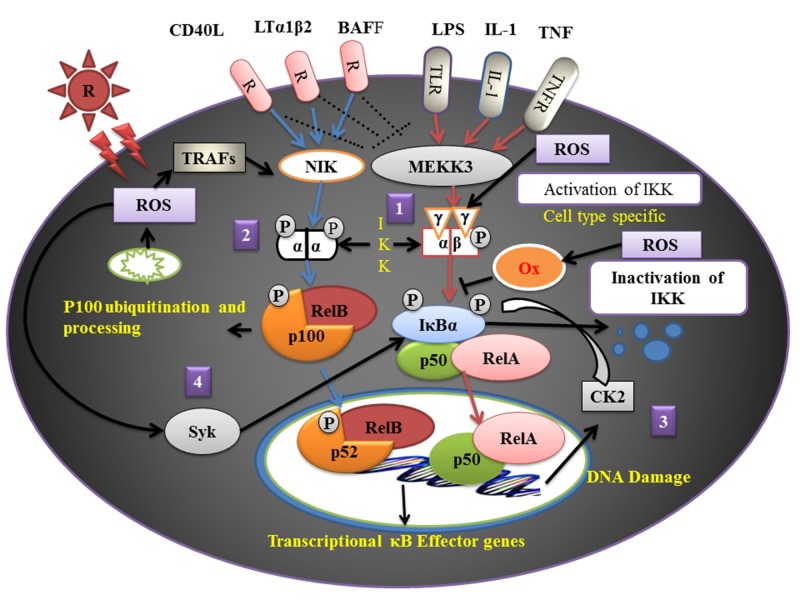
NF-κB was first discovered by Baltimore & Sen as a B cell specific nuclear protein that binds to a site in the immunoglobulin kappa (Igk) light chain gene enhancer6. NF-κB is basically a highly conserved and inducible transcription factor, which regulates the expression of over 200 genes involved in a broad range of events, including the immune response7, inflammation8, differentiation, proliferation, cell survival, apoptosis9,10. The role of NF-κB in protection of cells from the complement dependent cytotoxicity has been recently reported by Gancz et al11. Although there are few exceptions where NF-κB contributes to cell death12, in most cases, the expression of NF-κB target genes promotes cellular survival. Normally, NF-κB transcription factor is bound to the Inhibitor(s) of kappa B (IκB) and is located in the cytoplasm. The NF-κB is activated by numerous stimuli through a variety of receptors or other intrinsic activation pathways. This recruits unique combinations of scaffolding and signaling proteins, that ultimately converge to the IκB kinase (IKK) complex. There are over 150 different stimuli that can activate NF-κB13. Most of the disparate ligands act upon similar cell surface and intracellular receptors including the cytokines (TNF-α, IL-1α/β and TRAIL)14, bacterial molecules (LPS, flagellin, and non-methylated dsDNA)15, viral components (dsDNA, dsRNA and ssRNA), DNA damaging agents (ionizing radiation or oxidative stress and chemotherapeutic drugs)16,17. A majority of NF-κB activators are functionally related to either pathogenic cellular invasion or a cellular insult that initiates an immune response. Overall, NF-κB is activated in parallel with other mitogenic pathways, through induction of its genetic network (Figure 1).
Figure 1. Schematic picture of nuclear factor (NF)-κB signaling events that influence the cellular responses to IR.
Abnormal activation of NF-κB subsidizes in many human diseases, such as in cancer and inflammatory diseases. Hence, elucidating how NF-κB signaling is regulated in different contexts is important for the identification and development of therapeutics for various ailments, such as atherosclerosis, asthma, arthritis and cancer18,19. NF-κB is one of the major targets for the screening and identification of promising radiation countermeasure agents (RCAs). In this review, we have mainly focused on NF-kB modulation following IR exposure and associated target genes for NF-kB in relation to identification of RCAs. We have also discussed the current status of RCAs, specifically their role in NF-κB activation.
2. NF-κB/IκB family members & associated proteins
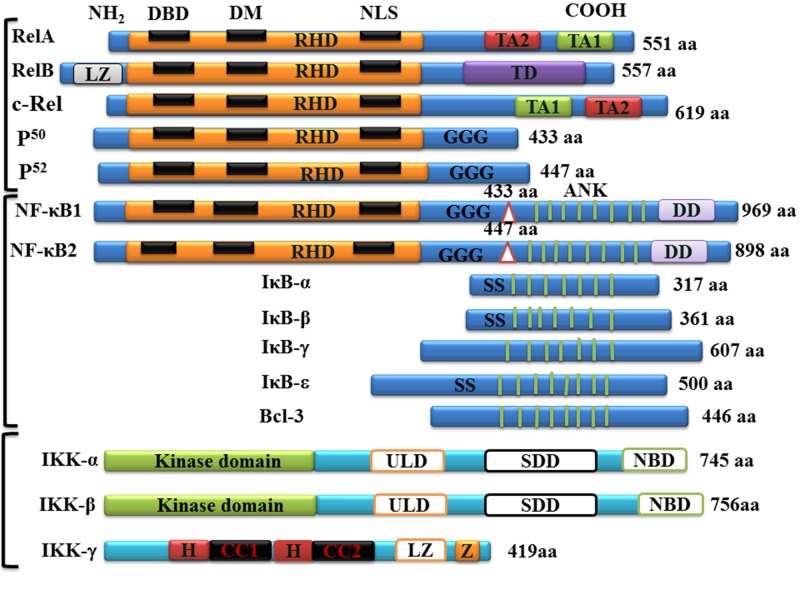
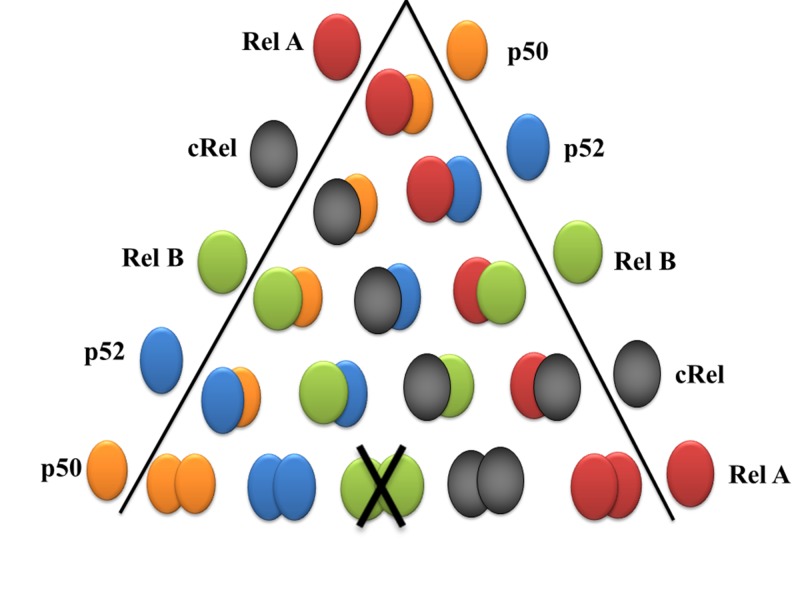
The mammalian NF-κB/Rel family possesses five different related monomers (RelA (p65), c-Rel, RelB, NF-κB1 (p50; p105), and NF-κB2 (p52; p100)) that form homo- and hetero-dimers, and bind to 10-base pair kappa B site of promoter region of target genes20. The N-terminus of these proteins contains the structurally conserved 300 amino acid sequence called the RHD region, which possesses the dimerization domain (DM), nuclear localization sequence (NLS), DNA-binding domain (DBD) and interaction site with IκBs21,22. Three of the family members, RelA, c-Rel, and RelB, have a C-terminal transactivation domain (TAD) that regulates expression of genes. RelA and RelB have two subdomains (TA1/2) of C-terminal transactivation domain23. NF-κB1/p105 and NF-κB2/p100 are the inactive precursors of the p50 and p52 proteins, respectively (Figure 2)14. All monomers of Rel family are capable to form 14 types of homo- or heterodimers and thereby determine the intrinsic NF-κB specificity and its regulation24-27, with the exception of RelB, which can only form heterodimers (Figure 3).
Figure 2. Schematic drawings of NF-κB/Rel proteins. Structures of the mammalian NF-κB, IκB, and IKK proteins.
The number of amino acids in each protein is indicated on the right. Presumed sites of cleavage for p105/NF-κB1 (amino acid 433) and p100/NF-κB2 (amino acid 447) are shown on the top of each protein. The positions of functional domains are indicated, including the Rel homology domain (RHD), DNA binding domain (DBD), dimerization domain (DM), nuclear localization signal (NLS), transactivation domains (TD). TA1 and TA2 subdomain of TD presented in RelA and cRel, glycine-rich hinge region (GGG), ankyrin repeats (ANK), double serine phosphorylation sites (SS), leucine zipper (LZ), helix-loop-helix (HLH), NEMO-binding domain (NBD), α-helix (H), coiled coil (CC), and zinc finger (Z).
Figure 3. Different stimuli induce specific formation of known homo and hetero NF-κB dimers.
Different NF-κB dimeric complexes are formed as per cell type and stimulus; some of the physiological important dimers are RelA/p50, cRel/p50 and RelB/p5222. RelA and p50 exists in a wide variety of cell types28; while c-Rel expression is limited to hematopoietic cells and lymphocytes. The RelB expression is highly specific, being found in the thymus, lymph nodes, and Peyer’s patches20. Each NF-κB dimer has the ability to bind with varying affinities to κB sites bearing the consensus sequence GGGRNNYYCC (R, purine: Y, pyrimidine: N, any base) and exhibit their unique functions29. However, NF-κB complexes composed only of the family members lacking TAD, such as the p50 homodimers, are known to impose transcriptional repression30. For all diverse functions of NF-κB in general, the activity is controlled by a family of regulatory proteins, called inhibitors of NF-κB (IκBs; IκB-α, IκB-β, IκB-ε, IκB-z, Bcl-3 etc)14,30 (Figure 2).
Three of “typical” IκBs (IκB-α, IκB-β, and IκB-ε), bind to NF-κB proteins and mask their nuclear translocation and DNA binding activity. IκBs also regulates the export of NF-κB proteins from the nucleus, and are thus known for inhibitory processes in multiple ways31. Recent investigations suggest that p100, when located in a multimeric complex, may also mediate NF-κB inhibition in trans; this activity is termed as IκBδ32,33. The complex of IκBs proteins and NF-κB dimers weas originally thought to be retain in the cytoplasm by the NF-κB super repressor IKK. IKK complex is formed by three different subunits: two catalytic subunits IKKα (IKK1 or CHUK), IKKβ (IKK2) and the regulatory subunit IKKg. IKKg is also known as NF-κB essential modulator (NEMO) protein (Figure 2). Although IKKα and IKKβ cooperate for IκBs phosphorylation, these proteins differ in the signals that they mediate.
3. NF-κB Activation Pathways
There are four models that have been proposed to explain NF-κB activation34. NF-κB is activated by numerous pathological and physiological conditions in a very efficient manner. NF-κB also regulates expression of various genes by modulating promoter activity of targets genes35.
3.1 The IKKβ dependent (classical) pathway
The IKKβ dependent NF-κB activation has been a well studied signaling event. It is also known as the classical or NEMO (IKK-γ)-dependent or canonical pathway (Figure 4). It is induced by several of innate and adaptive immunological agents, and can be turned on within minutes. It principally requires IKKβ components36,37. Phosphorylation of IKKβ at Ser177 and Ser181 may occur after stimulation by TNFR, IL-1R, TLR agonists, radiation exposure, TNF-α (tumour necrosis factor-α), PMA (phorbol 12-myristate 13-acetate), interleukins and other factors, which regulate downstream phosphorylation of IκB-α at Ser32 and Ser36, or IκBβ at Ser19 and Ser23, through the function of ubiquitin-dependent protein kinases. Phosphorylated IκB proteins are then ubiquitinated at nearby lysine residues (lysines 21 and 22 of IκBα and lysine 9 of IκB-β), and thus triggers a rapid degradation of IκB proteins by 26S proteasome38,39. The rapid degradation of IκB-α, IκB-β, and IκB-ε occurs during classical NF-κB signaling pathway. Phosphorylated p65/p50 (phosphorylation of p65 at Ser536) complex quickly translocates into nucleus and binds to 10-bp kB site or interacts with other transcription factors and regulates expression of various target genes. IκBα is a well known regulatory protein (providing a feedback control) for this pathway. The newly synthesized IκBα enters into the nucleus and prevents NF-κB DNA binding activity and transports NF-κB back into the cytoplasm.
Figure 4. There are four proposed NF-κB signaling pathways in response to various stimuli.
(1) The canonical pathway (2), the non-canonical pathway (3), atypical pathway and (4) oxidative stress-induced pathway. Downstream binding of the NF-κB proteins to DNA regulates downstream transcriptional of many potential antioxidant, pro-oxidant, cell cycle regulation and anti-apoptotic targets that have been shown in Supplementary Table (144.4KB, pdf) 1.
3.2 The IKKα dependent (alternative) pathway
Alternative or NEMO-independent or non-canonical pathway is mainly activated during secondary lymphoid organ development, homeostasis and adaptive immunity, and it turns on in few hours40. Senftleben et al. first described IKKα dependent pathway in which processing of p100 and activation of p52/RelB is defined as the alternative pathway (Figure 4)39. In this pathway phosphorylation of IKKα homodimer at Ser176 and Ser180 occurs through the upstream kinase NIK, (NF-κB inducing kinase). This pathway is stimulated by specific TNF receptor family members, such as LTβR, CD40, CD27, CD30, BAFF-R, RANK and others41, that signal through the recruitment of TRAF2 and TRAF3. In the resting cells, continuous degradation of NIK prevents non-canonical NF-κB activation42.
3.3 Atypical pathway
This pathway is essentially independent of IKK and it is mainly triggered in case of UV or chemical-induced DNA damages43,44. Evidence suggests that CK2 (formally known as casein kinase II) is a stress-activated protein kinase involved in the transduction of survival signals (Figure 4)45,46. CK2-mediated IκBα phosphorylation has an important UV-protective function. Jung et al. demonstrated a correlation of ATM with NF-κB in cellular radiosensitivity47 and suggested that the loss of ATM function promotes radiosensitivity by activation of NF-κB47. Recently, Wu et al.48 demonstrated that the cytosolic activation of signaling and sensor complexes (ATM, NEMO, IKK catalytic subunits, and ELKS - an IKK regulatory subunit) are associated with nuclear DNA damage-induced NF-κB activation. This model was proposed on their findings that ATM interacts with NEMO and phosphorylates NEMO at Ser85 after DSBs.
3.4 Oxidative stress-induced pathway
Oxidative stress-induced activation of NF-κB signaling is achieved via IκB-α tyrosine phosphorylation without degradation of IκB-α by Syk protein tyrosine kinase (Figure 4)49,50. H2O2 is one of the central free radical, involved in different cellular processes, including NF-κB activation51.The redox-sensitive pathways triggering this activation may vary with everh cell and cell-type50. NF-κB is also sensitive to oxidative modifications of Cys62 in p50, which are essential for DNA binding52,53. Activation and translocation of NF-κB is stimulated by oxidative circumstances, while its DNA binding affinity is inhibited by the redox sensitive cysteine residue54,55. The tyrosine phosphorylation of IκBα by most agents does not lead to IκBα degradation. However, Pervanadate (it is a protein tyrosine phosphatase inhibitor)-induced activation of NF-κB signaling, tyrosine phosphorylation and degradation of IκB-α has been documented56. Surprisingly, UV-C induced NF-κB activation is mediated through the degradation of IκB-α, that involves neither phosphorylation of serine nor the tyrosine residue of IκB-α57.
4. Post translational modifications of NF-κB proteins
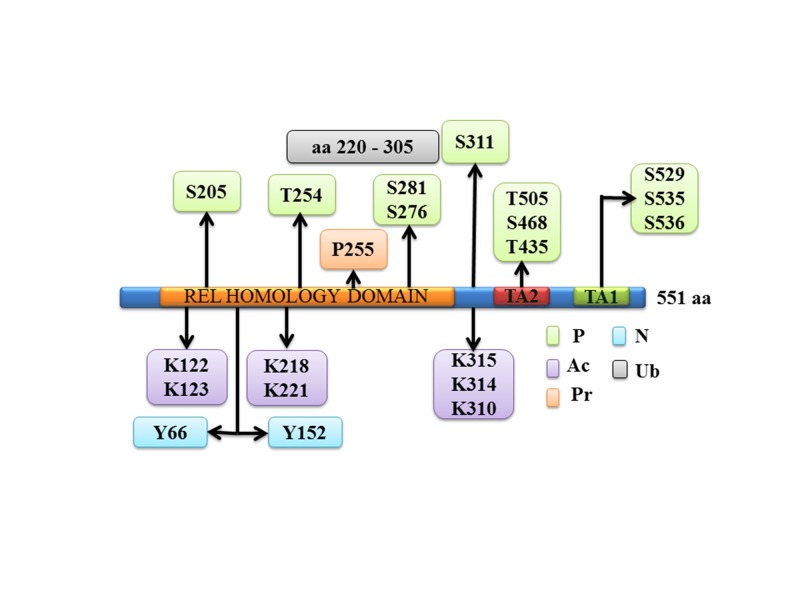
The mammalian transcription factor NF-κB is activated by over 150 diverse stimuli and thousands of potential NF-κB DNA binding sites have been marked across the genome13,58. After degradation of IκBs, activated NF-κB complex moves into nucleus and binds to 10bp defined sequence GGGRNWYYCC (N represents any base, R represents a purine; W represents an adenine or a thymine and Y represents a pyrimidine), which is present in the promoter and enhancer regions of target genes59. Moreover, activity and DNA binding affinity of NF-κB transcription factor are spatially and kinetically controlled, thereby regulating expression of its target genes60. Within the nuclear compartments, various posttranslational modifications (PTMs) of NF-κB occurs, such as: ubiquitination, acetylation and phosphorylation61. Among all NF-κB subunits, most of the post-translational modifications take place in the p65 subunit, which is known to be modified by phosphorylation, acetylation, prolylisomerization, nitrosylation and ubiquitination (Figure 5 and Table 1)12. Phosphorylation of p65 unit takes place either in the cytoplasm or in the nucleus, and is mediated by numerous protein kinases. These sites can be modified in a stimulus- and/or cell type-specific fashion by several kinases (Table 1)62-65.
Figure 5. Phosphorylation and acetylation sites within NF-κB p65.
Eight Serine three Threonine residues phosphorylation and seven acetylation sites have been identified in the NF-κB p65 subunit. Abbreviations: Ac, acetylation; K, lysine; N, tyrosine nitration; P, phosphorylation; Pr, proline isomerization; S, serine; T, threonine; Ub, ubiquitination; Y, tyrosine.
Table 1. The phosphorylation sites of p65, and responsible kinases.
Acetylation sites of p65 and the corresponding enzymes;
* Recently discovered phosphorylation sites
| Site | Location | Kinase | Function | Reference |
|---|---|---|---|---|
| Ser 205* | RHD | unknown | Transcriptional activity | 66 |
| Ser 276 | RHD | PKAc MSK1 | Transcriptional activity Captivator binding Transcriptional activity | 67,68 |
| Ser 281* | RHD | unknown | Transcriptional activity | 66 |
| Ser 311 | RHD | PKCz | Transcriptional activity | 69 |
| Ser 468 | TA2 | GSK3β IKKβ IKKα | Transcriptional activity Transcriptional activity Transcriptional activity | 70-72 |
| Ser 529 | TA1 | CK II | Transcriptional activity | 73 |
| Ser 535 | TA1 | CaMKIV | Transcriptional activity | 74 |
| Ser 536 | TA1 | IKKα IKKβ IKKe TBK1 RSK1 | Transcriptional activity and stabilization Transcriptional activity and nuclear import Transcriptional activity Nuclear localization Affinity to IκBα | 75-81 |
| Ser 547* | unknown | ATM-DSB | Transcriptional inhibition of target genes by HDAC recruitment | 82 |
| Thr 254* | RHD | unknown | Stabilization and Nuclear localization | 83 |
| Thr 435* | TA2 | unknown | Transcriptional activity | 84 |
| Thr 505 | TA2 | ATR ChK1 | Transcriptional activity | 85,86 |
| Tyr 66 Tyr 152 | RHD | NO treatment | p65 dissociation from p50 and association with IκBα | 87 |
| Site | Location | Enzyme | Function | Reference |
| Lys 122 | RHD | P300, PCAF | Inhibition DNA binding | 88 |
| Lys 123 | RHD | P300, PCAF | Inhibition DNA binding | 88 |
| Lys 218 | RHD | CBP/p300 | Unknown | 89 |
| Lys 221 | RHD | CBP/p300 | Promoting DNA binding Inhibition IκBα binding | 89 |
| Lys 310 | RHD | CBP/p300 | Enhancing transactivation | 89 |
| Lys 314 | RHD | P300 | Transcriptional activity | 90 |
| Lys 215 | RHD | P300 | Transcriptional activity | 90 |
PTMs of p65 can regulate the interaction with co-activators91, co-repressors92 promoter-bound degradation93 and interactions with the basal transcriptional machinery94. According to the NF-κB barcode hypothesis the differential modifications of the DNA-binding subunits generate distinct arrays that function through transcription in a highly target gene-specific manner95. Other than p65 post-translation modifications, NIK and IKKα (IKK1)-mediated phosphorylation of p105 NF-κB occurs at multiple sites (Ser921, 923, 927, and 932) on its carboxyl-terminus. SCF/β-TrCP-mediated processing of p105 NF-κB produces the 50 kDa active form product, p5096,97. NF-κB p50 serine 337 is phosphorylated in response to PKA, which regulates the binding affinity of NF-κB p50 and impacts the NF-κB transcriptional activity. In addition to post-translational modifications, recent studies showed the ability of NF-κB to bind the DNA (NF-κB: DNA) is also regulated by other proteins. A recent report suggests that RPS3 (ribosomal protein subunit 3) interacts with RelA via its KH (K Homology) domain and specifically enhances p50: RelA binding affinity with DNA (p50:RelA:DNA)98.
5. NF-κB regulated proteins and their functions in oxidative stress
Exposure of mammalian cells with low doses of ionizing radiation is known to have variable effects and may generate valuable effects within cells99,100. The correct cellular response to ROS following low doses of IR is consequently critical, in order to reduce further oxidative damage, and to maintain cell survival through initiation of cellular signaling, including NFkB pro-survival signaling. Therefore ROS-mediated NF-κB response and thereby regulation of NF-κB target genes may attenuate cell survival. One important way in which NF-κB activity influences ROS levels is by increasing expression of antioxidant and anti-apoptotic proteins. Since NF-κB is known to play a central role in inflammation, some enzymes that promotes the production of ROS are also controlled as well as its targets, particularly in cells of the immune system31. A few known or possible NF-κB target genes that may contribute to the protection of cells from ROS-induced cellular damage are mentioned in Supplementary Table (144.4KB, pdf) 1101-191.
6. Radiation Countermeasures in relation to NF-κB activation
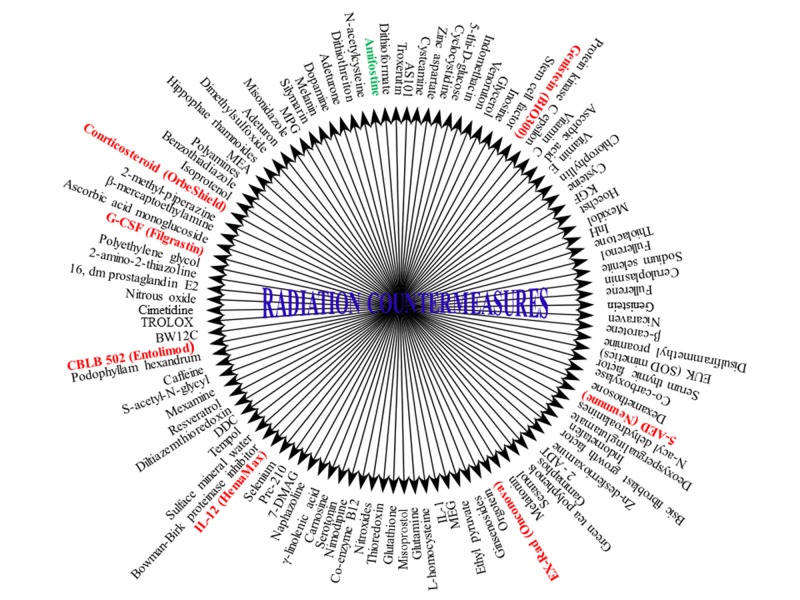
The radioprotective agent can be described as the “molecule(s) or compound(s) that protects against radiation-induced cellular, tissue injury, when applied before, during, or after irradiation in a specified time period”192,193. A number of chemical compounds that are identified and evaluated for radio-protective efficacy may be classified as (Supplementary Table (144.4KB, pdf) 2194-258 and Figure 6):
Figure 6. Schematic representation of chemical compounds that have been evaluated for radio-protective potential until today.
Prophylactic agents,
Mitigators and
Therapeutic agents
To date, there are no safe and effective drugs for the protection against ionizing radiation damage. Therefore, a great need exists to identify and develop non-toxic agents that will be useful as radio-protectors or post-irradiation therapies under a variety of operational scenarios. Suppressing of IR-induced cell death or enhancing survival, proliferation, differentiation of cells are the major ways to obtain protection mechanisms against radiation, addressing the massive cell loss in radiosensitive tissues specifically hematopoietic system (HP) and gastrointestinal tract194-196. Some of radio-protective agents that are currently in clinical trials are listed in Supplementary Table (144.4KB, pdf) 2. NF-κB plays important roles in immunity and cellular survival in response to radiation exposure and oxidative stress. It is known to reduce programmed cell death or apoptosis by promoting the expression of anti-apoptotic proteins and antioxidant molecules associated with enhanced radio-resistance, whereas its deletion in mice results in hypersensitivity to ionizing radiation-induced GI damage21,197.
NF-κB also regulates the production of a wide variety of cytokines in a cell type specific manner. Some of these cytokines induce proliferation and survival of hematopoietic stem cells, thereby promoting bone marrow recovery and tissues regeneration following irradiation. Therefore, pharmacological activation of NF-κB may be considered as a possible approach for radioprotection / mitigation. In this review, we have discussed some radioprotectors/ mitigators, specifically in relation to their efficacy for activation of NF-κB. Great efforts have been directed towards recognizing the role of TLRs (Toll Like Receptor)-mediated responses to microbes (viruses, bacteria, fungi) for the development of novel therapies in autoimmune allergic diseases, malignancy and other infections259. Investigations of TLR agonists are one of the global recent interests, for use in the preparation of immune-modulators. TLR agonists include: small molecules, pathogen derived DNA, RNA, proteins, lipids, which target one or more of the toll-like receptors, including TLR 2-9. Bacterial flagellin, the natural ligand of TLR5, was found to have radioprotective effects in rodents and nonhuman primates260. Recently, Cleveland Bio-Lab has developed the new pharmacological CBLB series, including CBLB502, for radiological emergencies. CBLB502 is a rationally designed derivative of Salmonella flagellin. It is substantially less immunogenic than full length flagellin and possesses its TLR5-dependent NF-κB–inducing activity and radioprotective ability208. Moreover, CBLB502 protected mice from dermatitis and mucositis associated with local fraction irradiation of head and neck area modelling radiation treatment of patients with head and neck cancer and also was shown to be effective as a tissue protectant in mouse models of renal ischemia-reperfusion injury261. A single dose of CBLB502 (0.2mg/kg body weight) 30 min prior to 13 Gy of TBI to NIH-Swiss mouse offered 87% protection. Administration of CBLB502 even up to 1 h post-irradiation results in greater than 90% survival after 9 Gy. CBLB502 also showed radio-protective efficacy in lethally irradiated rhesus monkeys208. Burdelya et al, recently showed that liver was the primary responsive organ for CBLB502 and CBLB502-mediated radioprotection of the HP system. The radioprotection occurred by factors secreted by responsive liver hepatocytes. A strong suppression of growth of tumor cells in the liver, regardless of their TLR5 status, was also observed209.
Recently, a lipopeptide of Mycoplasma arginini has been reported to act as a TLR 2/6 agonist. This novel radiation countermeasure, CBLB 613, has been observed as possible radio-mitigator for humans against radiation induced lethality262. CBLB613 significantly protected mice against a lethal dose of γ-radiation with no observable toxicity at 1.79 mg/kg body weight and 1 mg/kg body weight for single and repeated doses, respectively. In irradiated CD2F1 mice it stimulates bone marrow cellularity, enhances production of cytokines, such as interleukin-1β (IL-1β), IL-6, IL-10, IL-12, keratinocyte-derived chemokine, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumour necrosis factor-1α (TNF- α), and reduces radiation-mediated cytopenia. CBLB613 exhibits substantial dose reduction factor of 1.25.
The baicalein is a bioactive flavonoid, which has been shown to have antioxidant, anti-inflammatory and anti-hepatotoxic properties in both in vitro and in vivo conditions263,264. Treatment with baicalein inhibits the inflammatory signaling pathways involving ERK (extracellular signal-regulated kinase), Akt and nuclear factor-κB (NF-κB) activities in vascular smooth muscle cells265. A recent study showed that γ-irradiation with baicalein reduces lipid and protein oxidation in rat liver. Damaging effects of IR are generally mediated through the production of reactive species (RS), and a substantial increase in RS levels induces cellular damage and decrease in antioxidant enzymes, as well as activates intracellular signaling pathways that activate the expression of many inflammatory genes. The IR induced molecular responses may also be characterized as increased cyclooxygenase-2 (COX-2) level, inducible nitric oxide synthase (iNOS) and vascular adhesion molecule-1 (VCAM-1) expressions that also initiates the activation of the transcription factor NF-κB266. The key role played by NF-κB activation in the process of inflammation has been reported to be closely associated with a redox-sensitive signal cascade that includes MAPKs (ERK, c-Jun N-terminal kinase (JNK] and p38) and Akt266,267. However, activation of the Akt signaling pathway has been known to reduce forkhead box-O (FOXO) transcription activity268, and is involved in cytoprotective effects against oxidative stress269. Irradiation of mice showed an enhancement of NF-κB-mediated inflammatory factors due to the oxidative damage, and the inactivation of FOXO and its target genes, such as catalase and SOD. However, baicalein (5mg/kg bw/day) has the ability to suppress radiation-induced inflammatory consequences, by down regulating NF-κB and up-regulating FOXO activation270. Furthermore, baicalein inhibited radiation induced phosphorylation of MAPKs and Akt, which are upstream kinases of NF-κB and FOXOs. These observations also suggest that baicalein has a radioprotective effect against NF-κB mediated inflammatory response, through MAPKs and the Akt pathway, which is complemented by the protective effects on FOXO and its target genes, such as catalase and SOD.
DNA double-strand breaks (DSBs) are the most deleterious form of DNA damage and numerous in vitro studies have analyzed the DSB repair system that is activated after exposure to ionizing radiation. DSBs rapidly trigger the activation of NF-κB pathway via NEMO48,271. The death-domain protein PIDD was originally identified as an early p53-inducible gene and is implicated in p53-induced apoptosis148. PIDD is a mediator of the DNA-damage-activated stress response and is involved in genotoxic stress-induced NF-κB activation271,272. PIDD expression enhances genotoxic-stress-induced NF-κB activation through augmented sumoylation and ubiquitination of NEMO272. Corilagin (ß-1-O-galloyl-3, 6-(R)-hexahydroxydiphenoyl- D-glucose) is a member of the tannin family and has been isolated from medicinal plants, such as the Phyllanthus sps273. Corilagin has antioxidative, atherogenic, and hypertensive effects in various models273-276. A preliminary in vitro study suggested that corilagin has anti-inflammatory activity277. The activation of microglia and release of pro-inflammatory cytokines post irradiation are regarded as the key effectors of RIBI. Recent, studies demonstrated that corilagin exhibited anti-inflammatory activity in irradiated B7-2 cells by suppressing the release of pro-inflammatory cytokines and mediators. Corilagin suppresses the transcription of pro-inflammatory cytokine genes, through effects on the DSB-triggered NF-κB signaling pathway278.
Ex-RAD employs a novel mode of action, involving the enhancement of internal DNA repair pathways, which significantly reduces the levels of p53, p21, bax, c-abl and p73 proteins-key players in the DNA damage cascade induced upon exposure to 8.0 Gy gamma irradiation207. These mechanisms can cause a halt in cell death pathways and lead to increased recovery and survival of irradiated cells. These novel mechanisms of action attended by minimal side effects suggest that Ex-RAD could be useful both as a prophylactic and mitigative agent. Ex-RAD (4-carboxystyryl-4-chlorobenzylsulfone, sodium salt; or ON 01210.Na] is a synthetic small-molecule radioprotective compound (from Onconova Therapeutics, Inc. (OTI)) that is active in male C3H mice207 when administered 24 h and 15 min (two injections) before total body irradiation (TBI). Although Ex-RAD had been shown to be an inhibitor of apoptosis in vitro, it is not recognized whether a parallel mechanism is occurring in vivo207. In numerous cell-based and complete animal models, Ex-RAD has revealed to have potential for defense from radiation injury when administered either before or after radiation exposure. The drug is currently in Phase I clinical trials in humans. In decision, Ex-RAD usage mitigates potentially life-threatening neutropenia and bone marrow overthrow and, in turn, stimulates bone marrow retrieval, decreases radiation induced phosphorylation of p53 signaling, and enhances survival of acutely irradiated mice279. In addition to mitigating of hematopoietic damage, Ex-RAD also moderates intestinal injury. However, the molecular mechanisms elaborated in Ex-RAD’s promotion of recovery of hematopoietic and GI tissues warrant further study.
7. Conclusion
Radiation-induced injuries and lethality are well described at clinical level and understanding of mechanisms of tissue responses in the event of radiation exposure has gained much attention in recent years. The quest to search a potent radiation countermeasure which can ameliorate radiation syndrome and at the time exhibits no toxicity for human consumption is prevalent since past decades. However, even after the existence of a lot of literature available on radiation counter-measures only handful of identified drugs seem promising for human use. Based on prudent dissection of complicated series of signaling changes within multiple pathways, it might be possible to rationally combine inhibitors of these cascades, to repair damaged bio-molecules, activation of intracellular pathways, stress receptor activation, to achieve radiation protection. As a stress sensor, NF-κB is a crucial component of the cell’s protective response to radiation and therefore an attractive target in the new therapeutic lines to fight cancer or radiological emergencies. NF-κB is now documented as an important player in several critical steps for development of radiation countermeasures.
Recently, focus of radiation protection has shifted to test the radioprotective potential of plant products and herbs in the hope that one day it will be possible to find a suitable pharmacological agent that could protect humans against the deleterious effects of ionizing radiation in clinical and other conditions as well as during nuclear terror attack. Majority of plants and herbs described in this review have medicinal properties and are being used in traditional Ayurvedic or Chinese systems of medicine to treat various ailments in humans. Our review provides a broad idea on the physicochemical role of ionizing radiation on cellular systems and highlights the importance of developing new natural radioprotectants. Medicinal plants like Aconitum heterophyllum, Bergenia stracheyi, Bunium persicum, Dactylorhiza hatgirea, Ephedra gerardiana, Pichorrhiza kurroa, etc., are some of the plants that need elaborate investigations. Furthermore, some radioprotectants may boost their own efficacy in combination therapies Fractionation guided evaluation may result in the development of ideal radioprotectors in the near future.
Supplementary Material
Acknowledgments
The authors are grateful to University Grant Commission for the grant award (to DG). We are also grateful to the Director of INMAS for providing the opportunity and support to carry out our research work and prepare this manuscript.
Footnotes
Conflict of interests: The authors declare no conflict of interest.
Mitogen-activated protein kinase (MAPK); Phosphoinositide 3-kinases (PI3K); Ataxia telangiectasia mutated (ATM); Activator protein 1 (AP1); Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB); Growth arrest and DNA damage-inducible gene 153 (GADD153); Rel Homology Domain (RHD); Dimerization (DM); Nuclear Localization Sequence (NLS); DNA-Binding Domain (DBD); Terminal Transactivation Domain (TAD); B-cell lymphoma 3 (BCL-3); Transcription activation domain 1 (TA1); Transcription activation domain 2 (TA2); Glycine rich hinge region (GGG); Radiation Countermeasures (RC); Ionizing Radiation (IR); Inhibitor kB Kinase (IKK); Reactive Oxygen Species (ROS); Interleukin (IL); Tumour Necrosis Factor a (TNFa); Tumour Growth Factor b (TGFb); Nuclear Export Signals (NES); NF-kB Inducing Kinase (NIK); Receptor activator of NF-kB (RANK); Clusters of Differentiation (CD); Lipopolysaccharide (LPS); Radiation countermeasure agents (RCA); Single stranded Ribonucleic acid (ssRNA); Double strand break (DSB); DNA-binding domain (DBD); C-terminal transactivation domain (TAD); Protein rich in amino acids E, L, K and S (ELKS); TNF receptor-associated factor (TRAF); Forkhead box transcription factor (FOXO); Interleukin-1 (IL-1); B cell-activating factor (BAFF); B cell lymphoma-2 (Bcl-2); Lymphotoxin beta receptor (LTbR); Antioxidant Response Element (ARE)
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Hall Hall, Giaccia Amato. Radiobiology for the Radiologist. Lippincott Williams & Wilkins, 6th edition; 2006. [Google Scholar]
- 2.Identification of radiation-specific responses from gene expression profile. Park Woong-Yang, Hwang Chang-Il, Im Chang-Nim, Kang Min-Ji, Woo Jang-Hee, Kim Ju-Hoon, Kim Yon Su, Kim Ju-Han, Kim Ho, Kim Kyung-A, Yu Hyung-Jin, Lee Sue-Jae, Lee Yun-Sil, Seo Jeong-Sun. Oncogene. 2002;21(55):8521–8. doi: 10.1038/sj.onc.1205977. [DOI] [PubMed] [Google Scholar]
- 3.Stress and radiation-induced activation of multiple intracellular signaling pathways. Dent Paul, Yacoub Adly, Contessa Joseph, Caron Ruben, Amorino George, Valerie Kristoffer, Hagan Michael P, Grant Steven, Schmidt-Ullrich Rupert. Radiation research. 2003;159(3):283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Analysis of gene expression in normal and cancer cells exposed to gamma-radiation. Chaudhry M Ahmad. Journal of biomedicine & biotechnology. 2008;2008:541678. doi: 10.1155/2008/541678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biomarkers for human radiation exposure. Chaudhry M Ahmad. Journal of biomedical science. 2008;15(5):557–63. doi: 10.1007/s11373-008-9253-z. [DOI] [PubMed] [Google Scholar]
- 6.Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Sen R, Baltimore D. Cell. 1986;47(6):921–8. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 7.Function and activation of NF-kappa B in the immune system. Baeuerle P A, Henkel T. Annual review of immunology. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 8.Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. Barnes P J, Karin M. The New England journal of medicine. 1997;336(15):1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 9.NF-kappaB in cancer: from innocent bystander to major culprit. Karin Michael, Cao Yixue, Greten Florian R, Li Zhi-Wei. Nature reviews. Cancer. 2002;2(4):301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 10.NF-kappaB activation in response to toxical and therapeutical agents: role in inflammation and cancer treatment. Bours V, Bonizzi G, Bentires-Alj M, Bureau F, Piette J, Lekeux P, Merville M. Toxicology. 2000;153(1-3):27–38. doi: 10.1016/s0300-483x(00)00302-4. [DOI] [PubMed] [Google Scholar]
- 11.A role for the NF-κB pathway in cell protection from complement-dependent cytotoxicity. Gancz Dana, Lusthaus Michal, Fishelson Zvi. Journal of immunology (Baltimore, Md. : 1950) 2012;189(2):860–6. doi: 10.4049/jimmunol.1103451. [DOI] [PubMed] [Google Scholar]
- 12.Good cop, bad cop: the different faces of NF-kappaB. Perkins N D, Gilmore T D. Cell death and differentiation. 2006;13(5):759–72. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 13.Activators and target genes of Rel/NF-kappaB transcription factors. Pahl H L. Oncogene. 1999;18(49):6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 14.NF-kappaB signaling pathways in mammalian and insect innate immunity. Silverman N, Maniatis T. Genes & development. 2001;15(18):2321–42. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 15.The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Byrd-Leifer C A, Block E F, Takeda K, Akira S, Ding A. European journal of immunology. 2001;31(8):2448–57. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Sequential DNA damage-independent and -dependent activation of NF-kappaB by UV. Bender K, Göttlicher M, Whiteside S, Rahmsdorf H J, Herrlich P. The EMBO journal. 1998;17(17):5170–81. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Huang Tony T, Wuerzberger-Davis Shelly M, Wu Zhao-Hui, Miyamoto Shigeki. Cell. 2003;115(5):565–76. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 18.Series introduction: the transcription factor NF-kappaB and human disease. Baldwin A S. The Journal of clinical investigation. 2001;107(1):3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuclear factor-κB: a friend or a foe in cancer? Shishodia Shishir, Aggarwal Bharat B. Biochemical Pharmacology. 2004;68(6):1071-1080. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 20.NF-kappaB regulation in the immune system. Li Qiutang, Verma Inder M. Nature reviews. Immunology. 2002;2(10):725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 21.NF-kappa B-mediated adaptive resistance to ionizing radiation. Ahmed Kazi Mokim, Li Jian Jian. Free radical biology & medicine. 2008;44(1):1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A single NFκB system for both canonical and non-canonical signaling. Shih Vincent Feng-Sheng, Tsui Rachel, Caldwell Andrew, Hoffmann Alexander. Cell research. 2011;21(1):86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. Schmitz M L, Baeuerle P A. The EMBO journal. 1991;10(12):3805–17. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Structure of the NF-kappa B transcription factor: a holistic interaction with DNA. Kuriyan J, Thanos D. Structure (London, England : 1993) 1995;3(2):135–41. doi: 10.1016/s0969-2126(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 25.Structure of NF-kappa B p50 homodimer bound to a kappa B site. Ghosh G, van Duyne G, Ghosh S, Sigler P B. Nature. 1995;373(6512):303–10. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 26.Transcriptional activation by tetracyclines in mammalian cells. Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Science (New York, N.Y.) 1995;268(5218):1766–9. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 27.Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes & development. 1995;9(22):2723–35. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 28.Proposed NF-kappa B/I kappa B family nomenclature. Nabel G J, Verma I M. Genes & development. 1993;7(11):2063. doi: 10.1101/gad.7.11.2063. [DOI] [PubMed] [Google Scholar]
- 29.Signaling to NF- B. Hayden M. S. Genes & Development. 2004;18(18):2195-2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 30.Rel/NF-kappa B and I kappa B proteins: an overview. May M J, Ghosh S. Seminars in cancer biology. 1997;8(2):63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 31.Crosstalk of reactive oxygen species and NF-κB signaling. Morgan Michael J, Liu Zheng-gang. Cell research. 2011;21(1):103–15. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A fourth IkappaB protein within the NF-kappaB signaling module. Basak Soumen, Kim Hana, Kearns Jeffrey D, Tergaonkar Vinay, O'Dea Ellen, Werner Shannon L, Benedict Chris A, Ware Carl F, Ghosh Gourisankar, Verma Inder M, Hoffmann Alexander. Cell. 2007;128(2):369–81. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Nfkb1 and Nfkb2 proteins p105 and p100 function as the core of high-molecular-weight heterogeneous complexes. Savinova Olga V, Hoffmann Alexander, Ghosh Gourisankar. Molecular cell. 2009;34(5):591–602. doi: 10.1016/j.molcel.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Dejardin Emmanuel. Biochemical pharmacology. 2006;72(9):1161–79. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Regulation of immune responses by NF-kappa B/Rel transcription factor. Sha W C. The Journal of experimental medicine. 1998;187(2):143–6. doi: 10.1084/jem.187.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Delhase M, Hayakawa M, Chen Y, Karin M. Science (New York, N.Y.) 1999;284(5412):309–13. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 37.The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. Li Z W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The Journal of experimental medicine. 1999;189(11):1839–45. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. Chen J Y, Penco S, Ostrowski J, Balaguer P, Pons M, Starrett J E, Reczek P, Chambon P, Gronemeyer H. The EMBO journal. 1995;14(6):1187–97. doi: 10.1002/j.1460-2075.1995.tb07102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reactive oxygen intermediates as second messengers of a general pathogen response. Baeuerle P A, Rupec R A, Pahl H L. Pathologie-biologie. 1996;44(1):29–35. [PubMed] [Google Scholar]
- 40.Nrf2 and NF-κB and Their Concerted Modulation in Cancer Pathogenesis and Progression. Bellezza Ilaria, Mierla Anna Lisa, Minelli Alba. Cancers. 2010;2(2):483–97. doi: 10.3390/cancers2020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Xiao G, Harhaj E W, Sun S C. Molecular cell. 2001;7(2):401–9. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 42.Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. Liao Gongxian, Zhang Minying, Harhaj Edward W, Sun Shao-Cong. The Journal of biological chemistry. 2004;279(25):26243–50. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 43.CK2 Is a C-Terminal IkappaB Kinase Responsible for NF-kappaB Activation during the UV Response. Kato Tomohisa, Delhase Mireille, Hoffmann Alexander, Karin Michael. Molecular cell. 2003;12(4):829–39. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 44.IkappaB kinase-independent IkappaBalpha degradation pathway: functional NF-kappaB activity and implications for cancer therapy. Tergaonkar Vinay, Bottero Virginie, Ikawa Masahito, Li Qiutang, Verma Inder M. Molecular and cellular biology. 2003;23(22):8070–83. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joining the cell survival squad: an emerging role for protein kinase CK2. Ahmed Khalil, Gerber Delphine A, Cochet Claude. Trends in cell biology. 2002;12(5):226–30. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 46.Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Litchfield David W. The Biochemical journal. 2003;369(Pt 1):1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correction of radiation sensitivity in ataxia telangiectasia cells by a truncated I kappa B-alpha. Jung M, Zhang Y, Lee S, Dritschilo A. Science (New York, N.Y.) 1995;268(5217):1619–21. doi: 10.1126/science.7777860. [DOI] [PubMed] [Google Scholar]
- 48.Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Wu Zhao-Hui, Shi Yuling, Tibbetts Randal S, Miyamoto Shigeki. Science (New York, N.Y.) 2006;311(5764):1141–6. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 49.Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. Takada Yasunari, Mukhopadhyay Asok, Kundu Gopal C, Mahabeleshwar Ganapati H, Singh Sujay, Aggarwal Bharat B. The Journal of biological chemistry. 2003;278(26):24233–41. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 50.NF-kappaB activation by reactive oxygen species: fifteen years later. Gloire Geoffrey, Legrand-Poels Sylvie, Piette Jacques. Biochemical pharmacology. 2006;72(11):1493–505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 51.The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Schmidt K N, Amstad P, Cerutti P, Baeuerle P A. Chemistry & biology. 1995;2(1):13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 52.Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Matthews J R, Wakasugi N, Virelizier J L, Yodoi J, Hay R T. Nucleic acids research. 1992;20(15):3821–30. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Role of cysteine62 in DNA recognition by the P50 subunit of NF-kappa B. Matthews J R, Kaszubska W, Turcatti G, Wells T N, Hay R T. Nucleic acids research. 1993;21(8):1727–34. doi: 10.1093/nar/21.8.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Functions of glutathione and glutathione disulfide in immunology and immunopathology. Dröge W, Schulze-Osthoff K, Mihm S, Galter D, Schenk H, Eck H P, Roth S, Gmünder H. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1994;8(14):1131–8. [PubMed] [Google Scholar]
- 55.Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Bowie A, O'Neill L A. Biochemical pharmacology. 2000;59(1):13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 56.Pervanadate-induced nuclear factor-kappaB activation requires tyrosine phosphorylation and degradation of IkappaBalpha. Comparison with tumor necrosis factor-alpha. Mukhopadhyay A, Manna S K, Aggarwal B B. The Journal of biological chemistry. 2000;275(12):8549–55. doi: 10.1074/jbc.275.12.8549. [DOI] [PubMed] [Google Scholar]
- 57.Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Li N, Karin M. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(22):13012–7. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Interactions of NF-kappaB with chromatin: the art of being at the right place at the right time. Natoli Gioacchino, Saccani Simona, Bosisio Daniela, Marazzi Ivan. Nature immunology. 2005;6(5):439–45. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- 59.Transcriptional regulation via the NF-kappaB signaling module. Hoffmann A, Natoli G, Ghosh G. Oncogene. 2006;25(51):6706–16. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 60.Shared principles in NF-kappaB signaling. Hayden Matthew S, Ghosh Sankar. Cell. 2008;132(3):344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 61.Diversity and regulation in the NF-kappaB system. Wietek Claudia, O'Neill Luke A J. Trends in biochemical sciences. 2007;32(7):311–9. doi: 10.1016/j.tibs.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Beyond IkappaBs: alternative regulation of NF-kappaB activity. Neumann Manfred, Naumann Michael. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(11):2642–54. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 63.Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Perkins N D. Oncogene. 2006;25(51):6717–30. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 64.Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Viatour Patrick, Merville Marie-Paule, Bours Vincent, Chariot Alain. Trends in biochemical sciences. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Shaping the nuclear action of NF-kappaB. Chen Lin-Feng, Greene Warner C. Nature reviews. Molecular cell biology. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 66.cis-acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-kappa B. Anrather Josef, Racchumi Gianfranco, Iadecola Costantino. The Journal of biological chemistry. 2005;280(1):244–52. doi: 10.1074/jbc.M409344200. [DOI] [PubMed] [Google Scholar]
- 67.Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Zhong H, Voll R E, Ghosh S. Molecular cell. 1998;1(5):661–71. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 68.Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). Vermeulen Linda, De Wilde Gert, Van Damme Petra, Vanden Berghe Wim, Haegeman Guy. The EMBO journal. 2003;22(6):1313–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. Duran Angeles, Diaz-Meco María T, Moscat Jorge. The EMBO journal. 2003;22(15):3910–8. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. Buss Holger, Dörrie Anneke, Schmitz M Lienhard, Frank Ronald, Livingstone Mark, Resch Klaus, Kracht Michael. The Journal of biological chemistry. 2004;279(48):49571–4. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- 71.IKKbeta phosphorylates p65 at S468 in transactivaton domain 2. Schwabe Robert F, Sakurai Hiroaki. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(12):1758–60. doi: 10.1096/fj.05-3736fje. [DOI] [PubMed] [Google Scholar]
- 72.Inducible phosphorylation of NF-kappa B p65 at serine 468 by T cell costimulation is mediated by IKK epsilon. Mattioli Ivan, Geng Hui, Sebald Andrea, Hodel Michael, Bucher Cyril, Kracht Michael, Schmitz M Lienhard. The Journal of biological chemistry. 2006;281(10):6175–83. doi: 10.1074/jbc.M508045200. [DOI] [PubMed] [Google Scholar]
- 73.Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. Wang D, Westerheide S D, Hanson J L, Baldwin A S. The Journal of biological chemistry. 2000;275(42):32592–7. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 74.Phosphorylation of NF-kappa B by calmodulin-dependent kinase IV activates anti-apoptotic gene expression. Bae Jeum Soon, Jang Moon Kyoo, Hong SunHwa, An Won Gun, Choi Yung Hyun, Kim Han Do, Cheong JaeHun. Biochemical and biophysical research communications. 2003;305(4):1094–8. doi: 10.1016/s0006-291x(03)00869-6. [DOI] [PubMed] [Google Scholar]
- 75.The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. Jiang Xu, Takahashi Naoko, Matsui Nobuo, Tetsuka Toshifumi, Okamoto Takashi. The Journal of biological chemistry. 2003;278(2):919–26. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- 76.IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Lawrence Toby, Bebien Magali, Liu George Y, Nizet Victor, Karin Michael. Nature. 2005;434(7037):1138–43. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 77.IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. The Journal of biological chemistry. 1999;274(43):30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 78.Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. Mattioli Ivan, Sebald Andrea, Bucher Cyril, Charles Roch-Philippe, Nakano Hiroyasu, Doi Takahiro, Kracht Michael, Schmitz M Lienhard. Journal of immunology (Baltimore, Md. : 1950) 2004;172(10):6336–44. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 79.Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. Buss Holger, Dörrie Anneke, Schmitz M Lienhard, Hoffmann Elke, Resch Klaus, Kracht Michael. The Journal of biological chemistry. 2004;279(53):55633–43. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 80.Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling. Fujita Fumitaka, Taniguchi Yuko, Kato Takashi, Narita Yasuko, Furuya Akiko, Ogawa Tatsuhiro, Sakurai Hiroaki, Joh Takashi, Itoh Makoto, Delhase Mireille, Karin Michael, Nakanishi Makoto. Molecular and cellular biology. 2003;23(21):7780–93. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. Bohuslav Jan, Chen Lin-Feng, Kwon Hakju, Mu Yajun, Greene Warner C. The Journal of biological chemistry. 2004;279(25):26115–25. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- 82.Phosphorylation of p65(RelA) on Ser(547) by ATM represses NF-κB-dependent transcription of specific genes after genotoxic stress. Sabatel Hélène, Di Valentin Emmanuel, Gloire Geoffrey, Dequiedt Franck, Piette Jacques, Habraken Yvette. PloS one. 2012;7(6):e38246. doi: 10.1371/journal.pone.0038246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Ryo Akihide, Suizu Futoshi, Yoshida Yasuhiro, Perrem Kilian, Liou Yih-Cherng, Wulf Gerburg, Rottapel Robert, Yamaoka Shoji, Lu Kun Ping. Molecular cell. 2003;12(6):1413–26. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 84.Suppression of MEK/ERK signaling pathway enhances cisplatin-induced NF-kappaB activation by protein phosphatase 4-mediated NF-kappaB p65 Thr dephosphorylation. Yeh Pei Yen, Yeh Kun-Huei, Chuang Shuang-En, Song Ying Chyi, Cheng Ann-Lii. The Journal of biological chemistry. 2004;279(25):26143–8. doi: 10.1074/jbc.M402362200. [DOI] [PubMed] [Google Scholar]
- 85.Cisplatin mimics ARF tumor suppressor regulation of RelA (p65) nuclear factor-kappaB transactivation. Campbell Kirsteen J, Witty James M, Rocha Sonia, Perkins Neil D. Cancer research. 2006;66(2):929–35. doi: 10.1158/0008-5472.CAN-05-2234. [DOI] [PubMed] [Google Scholar]
- 86.Regulation of NF-κB function. Campbell Kirsteen J., Perkins Neil D. Biochemical Society Symposium. 2006;73:165-180. doi: 10.1042/bss0730165. [DOI] [PubMed] [Google Scholar]
- 87.Tyrosine Nitration on p65. Park Sung Wook, Huq M. D.Mostaqul, Hu Xinli, Wei Li-Na. Molecular & Cellular Proteomics. 2005;4(3):300-309. doi: 10.1074/mcp.M400195-MCP200. [DOI] [PubMed] [Google Scholar]
- 88.Post-activation Turn-off of NF-κB-dependent Transcription Is Regulated by Acetylation of p65. Kiernan Rosemary, Brès Vanessa, Ng Raymond W. M., Coudart Marie-Pierre, El Messaoudi Selma, Sardet Claude, Jin Dong-Yan, Emiliani Stephane, Benkirane Monsef. Journal of Biological Chemistry. 2002;278(4):2758-2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 89.Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. Chen L.-f. The EMBO Journal. 2002;21(23):6539-6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Functional relevance of novel p300-mediated lysine 314 and 315 acetylation of RelA/p65. Buerki Christine, Rothgiesser Karin M., Valovka Taras, Owen Heather R., Rehrauer Hubert, Fey Monika, Lane William S., Hottiger Michael O. Nucleic Acids Research. 2008;36(5):1665-1680. doi: 10.1093/nar/gkn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.I B Kinase -Mediated Derepression of SMRT Potentiates Acetylation of RelA/p65 by p300. Hoberg J. E., Popko A. E., Ramsey C. S., Mayo M. W. Molecular and Cellular Biology. 2005;26(2):457-471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Repression of gene expression by unphosphorylated NF- B p65 through epigenetic mechanisms. Dong J., Jimi E., Zhong H., Hayden M. S., Ghosh S. Genes & Development. 2008;22(9):1159-1173. doi: 10.1101/gad.1657408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phosphorylation of NF-κB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. Geng Hui, Wittwer Tobias, Dittrich-Breiholz Oliver, Kracht Michael, Schmitz Michael Lienhard. EMBO reports. 2009;10(4):381-386. doi: 10.1038/embor.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.RelA Ser276 Phosphorylation Is Required for Activation of a Subset of NF- B-Dependent Genes by Recruiting Cyclin-Dependent Kinase 9/Cyclin T1 Complexes. Nowak D. E., Tian B., Jamaluddin M., Boldogh I., Vergara L. A., Choudhary S., Brasier A. R. Molecular and Cellular Biology. 2008;28(11):3623-3638. doi: 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Specification of the NF-κB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKKε. Moreno Rita, Sobotzik Jürgen-Markus, Schultz Christian, Schmitz M. Lienhard. Nucleic Acids Research. 2010;38(18):6029-6044. doi: 10.1093/nar/gkq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shared Pathways of I B Kinase-Induced SCF TrCP-Mediated Ubiquitination and Degradation for the NF- B Precursor p105 and I B . Heissmeyer V., Krappmann D., Hatada E. N., Scheidereit C. Molecular and Cellular Biology. 2001;21(4):1024-1035. doi: 10.1128/MCB.21.4.1024-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.SCFbeta-TrCP ubiquitin ligase-mediated processing of NF-kappaB p105 requires phosphorylation of its C-terminus by IkappaB kinase. Orian A. The EMBO Journal. 2000;19(11):2580-2591. doi: 10.1093/emboj/19.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribosomal Protein S3: A KH Domain Subunit in NF-κB Complexes that Mediates Selective Gene Regulation. Wan Fengyi, Anderson D. Eric, Barnitz Robert A., Snow Andrew, Bidere Nicolas, Zheng Lixin, Hegde Vijay, Lam Lloyd T., Staudt Louis M., Levens David, Deutsch Walter A., Lenardo Michael J. Cell. 2007;131(5):927-939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 99.Human lymphocytes exposed to low doses of X-rays are less susceptible to radiation-induced mutagenesis. Kelsey K.T., Memisoglu A., Frenkel D., Liber H.L. Mutation Research Letters. 1991;263(4):197-201. doi: 10.1016/0165-7992(91)90001-k. [DOI] [PubMed] [Google Scholar]
- 100.Radiation effects induced by low doses in complex tissue and their relation to cellular adaptive responses. Feinendegen Ludwig E., Bond Victor P., Sondhaus Charles A., Muehlensiepen H. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1996;358(2):199-205. doi: 10.1016/s0027-5107(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 101.Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Jones P L, Ping D, Boss J M. Molecular and Cellular Biology. 1997;17(12):6970-6981. doi: 10.1128/mcb.17.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Activation of NF-kappa B and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Das K. C., Lewis-Molock Y., White C. W. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1995;269(5):L588-L602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- 103.Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Li Yibing, Huang Ting-Ting, Carlson Elaine J., Melov Simon, Ursell Philip C., Olson Jean L., Noble Linda J., Yoshimura Midori P., Berger Christoph, Chan Pak H., Wallace Douglas C., Epstein Charles J. Nature Genetics. 1995;11(4):376-381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 104.Invited review: manganese superoxide dismutase in disease. Macmillan-Crow L A, Cruthirds D L. Free radical research. 2001;34(4):325–36. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 105.CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Elchuri Sailaja, Oberley Terry D, Qi Wenbo, Eisenstein Richard S, Jackson Roberts L, Van Remmen Holly, Epstein Charles J, Huang Ting-Ting. Oncogene. 2005;24(3):367–80. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 106.Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Pham Can G, Bubici Concetta, Zazzeroni Francesca, Papa Salvatore, Jones Joy, Alvarez Kellean, Jayawardena Shanthi, De Smaele Enrico, Cong Rong, Beaumont Carole, Torti Frank M, Torti Suzy V, Franzoso Guido. Cell. 2004;119(4):529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 107.Regulation of ferritin genes and protein. Torti Frank M, Torti Suzy V. Blood. 2002;99(10):3505–16. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 108.Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Schreiber Joerg, Jenner Richard G, Murray Heather L, Gerber Georg K, Gifford David K, Young Richard A. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(15):5899–904. doi: 10.1073/pnas.0510996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diversity of structures and properties among catalases. Chelikani P, Fita I, Loewen P C. Cellular and molecular life sciences : CMLS. 2004;61(2):192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.NF-kappaB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFalpha-treated Ewing sarcoma cells. Djavaheri-Mergny Mojgan, Javelaud Delphine, Wietzerbin Juana, Besançon Françoise. FEBS letters. 2004;578(1-2):111–5. doi: 10.1016/j.febslet.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 111.Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo M M. Developmental biology. 1996;178(1):179–85. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 112.Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. Tanaka Toru, Hosoi Fumihito, Yamaguchi-Iwai Yuko, Nakamura Hajime, Masutani Hiroshi, Ueda Shugo, Nishiyama Akira, Takeda Shunichi, Wada Hiromi, Spyrou Giannis, Yodoi Junji. The EMBO journal. 2002;21(7):1695–703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Nonn Larisa, Williams Ryan R, Erickson Robert P, Powis Garth. Molecular and cellular biology. 2003;23(3):916–22. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.The organization of the human GSTP1-1 gene promoter and its response to retinoic acid and cellular redox status. Xia C, Hu J, Ketterer B, Taylor J B. The Biochemical journal. 1996;313 ( Pt 1):155–61. doi: 10.1042/bj3130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glutathione S-transferase pi1 promotes tumorigenicity in HCT116 human colon cancer cells. Dang Duyen T, Chen Fang, Kohli Manu, Rago Carlo, Cummins Jordan M, Dang Long H. Cancer research. 2005;65(20):9485–94. doi: 10.1158/0008-5472.CAN-05-1930. [DOI] [PubMed] [Google Scholar]
- 116.Mammalian cytosolic glutathione transferases. Dourado Daniel F A R, Fernandes Pedro Alexandrino, Ramos Maria João. Current protein & peptide science. 2008;9(4):325–37. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- 117.Glutathione S-transferases--a review. Salinas A E, Wong M G. Current medicinal chemistry. 1999;6(4):279–309. [PubMed] [Google Scholar]
- 118.Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Hinata Kaede, Gervin Adam M, Jennifer Zhang Y, Khavari Paul A. Oncogene. 2003;22(13):1955–64. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
- 119.Free radical scavenging actions of metallothionein isoforms I and II. Kumari M V, Hiramatsu M, Ebadi M. Free radical research. 1998;29(2):93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- 120.Neuronal growth-inhibitory factor (metallothionein-3): evaluation of the biological function of growth-inhibitory factor in the injured and neurodegenerative brain. Howells Claire, West Adrian K, Chung Roger S. The FEBS journal. 2010;277(14):2931–9. doi: 10.1111/j.1742-4658.2010.07718.x. [DOI] [PubMed] [Google Scholar]
- 121.Involvement of activator protein-1 and nuclear factor-kappaB transcription factors in the control of the DT-diaphorase expression induced by mitomycin C treatment. Yao K S, Hageboutros A, Ford P, O'Dwyer P J. Molecular pharmacology. 1997;51(3):422–30. [PubMed] [Google Scholar]
- 122.Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. Ahn Kwang Seok, Sethi Gautam, Jain Abhinav K, Jaiswal Anil K, Aggarwal Bharat B. The Journal of biological chemistry. 2006;281(29):19798–808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 123.NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Dinkova-Kostova Albena T, Talalay Paul. Archives of biochemistry and biophysics. 2010;501(1):116–23. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Phosphorylation and hypoxia-induced heme oxygenase-1 gene expression in cardiomyocytes. Wu Guimei, Marín-García José, Rogers Terry B, Lakatta Edward G, Long Xilin. Journal of cardiac failure. 2004;10(6):519–26. doi: 10.1016/j.cardfail.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 125.Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Prawan Auemduan, Kundu Joydeb K, Surh Young-Joon. Antioxidants & redox signaling. 2005;7(11-12):1688–703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- 126.Metabolic regulation and function of glutathione peroxidase-1. Lei Xin Gen, Cheng Wen-Hsing, McClung James P. Annual review of nutrition. 2007;27:41–61. doi: 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- 127.Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. Sies H, Sharov V S, Klotz L O, Briviba K. The Journal of biological chemistry. 1997;272(44):27812–7. doi: 10.1074/jbc.272.44.27812. [DOI] [PubMed] [Google Scholar]
- 128.Promoter analysis of a human dihydrodiol dehydrogenase. Ciaccio P J, Walsh E S, Tew K D. Biochemical and biophysical research communications. 1996;228(2):524–9. doi: 10.1006/bbrc.1996.1693. [DOI] [PubMed] [Google Scholar]
- 129.Dihydrodiol dehydrogenases regulate the generation of reactive oxygen species and the development of cisplatin resistance in human ovarian carcinoma cells. Chen Jianli, Adikari Mahesha, Pallai Rajash, Parekh Hemant K, Simpkins Henry. Cancer chemotherapy and pharmacology. 2008;61(6):979–87. doi: 10.1007/s00280-007-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. Anrather Josef, Racchumi Gianfranco, Iadecola Costantino. The Journal of biological chemistry. 2006;281(9):5657–67. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 131.NOX enzymes and the biology of reactive oxygen. Lambeth J David. Nature reviews. Immunology. 2004;4(3):181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 132.Nox proteins in signal transduction. Brown David I, Griendling Kathy K. Free radical biology & medicine. 2009;47(9):1239–53. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH). Xu P, Huecksteadt T P, Hoidal J R. Genomics. 1996;34(2):173–80. doi: 10.1006/geno.1996.0262. [DOI] [PubMed] [Google Scholar]
- 134.NADH oxidase activity of rat liver xanthine dehydrogenase and xanthine oxidase-contribution for damage mechanisms. Maia Luisa, Vala Andrea, Mira Lurdes. Free radical research. 2005;39(9):979–86. doi: 10.1080/10715760500210962. [DOI] [PubMed] [Google Scholar]
- 135.Transcriptional regulation of the human iNOS gene in vascular-smooth-muscle cells and macrophages: evidence for tissue specificity. Kolyada A Y, Savikovsky N, Madias N E. Biochemical and biophysical research communications. 1996;220(3):600–5. doi: 10.1006/bbrc.1996.0449. [DOI] [PubMed] [Google Scholar]
- 136.Statin treatment upregulates vascular neuronal nitric oxide synthase through Akt/NF-kappaB pathway. Nakata Sei, Tsutsui Masato, Shimokawa Hiroaki, Yamashita Takahiro, Tanimoto Akihide, Tasaki Hiromi, Ozumi Kiyoshi, Sabanai Ken, Morishita Tsuyoshi, Suda Osamu, Hirano Hideyasu, Sasaguri Yasuyuki, Nakashima Yasuhide, Yanagihara Nobuyuki. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(1):92–8. doi: 10.1161/01.ATV.0000251615.61858.33. [DOI] [PubMed] [Google Scholar]
- 137.Regulation of neuronal nitric oxide synthase exon 1f gene expression by nuclear factor-kappaB acetylation in human neuroblastoma cells. Li Yinghui, Zhao Yanyan, Li Guangyu, Wang Jun, Li Tingting, Li Wei, Lu Jingyu. Journal of neurochemistry. 2007;101(5):1194–204. doi: 10.1111/j.1471-4159.2006.04407.x. [DOI] [PubMed] [Google Scholar]
- 138.Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Morris Kristin R, Lutz Ryan D, Choi Hyung-Seok, Kamitani Tetsu, Chmura Kathryn, Chan Edward D. Infection and immunity. 2003;71(3):1442–52. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Ahmad Rizwan, Rasheed Zafar, Ahsan Haseeb. Immunopharmacology and immunotoxicology. 2009;31(3):388–96. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- 140.Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. Marnett L J, Rowlinson S W, Goodwin D C, Kalgutkar A S, Lanzo C A. The Journal of biological chemistry. 1999;274(33):22903–6. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 141.The transcriptional regulation of human arachidonate 12-lipoxygenase gene by NF kappa B/Rel. Arakawa T, Nakamura M, Yoshimoto T, Yamamoto S. FEBS letters. 1995;363(1-2):105–10. doi: 10.1016/0014-5793(95)00293-i. [DOI] [PubMed] [Google Scholar]
- 142.Cloning of the guinea pig 5-lipoxygenase gene and nucleotide sequence of its promoter. Chopra A, Ferreira-Alves D L, Sirois P, Thirion J P. Biochemical and biophysical research communications. 1992;185(2):489–95. doi: 10.1016/0006-291x(92)91651-6. [DOI] [PubMed] [Google Scholar]
- 143.Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. Kim Cheolmin, Kim Joo-Young, Kim Jae-Hong. BMB reports. 2008;41(8):555–9. doi: 10.5483/bmbrep.2008.41.8.555. [DOI] [PubMed] [Google Scholar]
- 144.Hydrogen peroxide formation and stoichiometry of hydroxylation reactions catalyzed by highly purified liver microsomal cytochrome P-450. Nordblom G D, Coon M J. Archives of biochemistry and biophysics. 1977;180(2):343–7. doi: 10.1016/0003-9861(77)90047-9. [DOI] [PubMed] [Google Scholar]
- 145.Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Imaoka Susumu, Osada Mayuko, Minamiyama Yukiko, Yukimura Tokihito, Toyokuni Shinya, Takemura Shigekazu, Hiroi Toyoko, Funae Yoshihiko. Cancer letters. 2004;203(2):117–25. doi: 10.1016/j.canlet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 146.Determination of interleukin-4-responsive region in the human cytochrome P450 2E1 gene promoter. Abdel-Razzak Ziad, Garlatti Michèle, Aggerbeck Martine, Barouki Robert. Biochemical pharmacology. 2004;68(7):1371–81. doi: 10.1016/j.bcp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 147.Mechanisms of cytochrome P450 regulation by inflammatory mediators. Morgan Edward T, Li-Masters Tong, Cheng Po-Yung. Toxicology. 2002;181-182:207–10. doi: 10.1016/s0300-483x(02)00283-4. [DOI] [PubMed] [Google Scholar]
- 148.Oxidative stress, toxicology, and pharmacology of CYP2E1. Caro Andres A, Cederbaum Arthur I. Annual review of pharmacology and toxicology. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 149.CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Cederbaum A I, Wu D, Mari M, Bai J. Free radical biology & medicine. 2001;31(12):1539–43. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 150.IAP family proteins---suppressors of apoptosis. Deveraux Q. L., Reed J. C. Genes & Development. 1999;13(3):239-252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 151.IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. Deveraux Q. L. The EMBO Journal. 1998;17(8):2215-2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Livin, a Novel Inhibitor of Apoptosis Protein Family Member. Kasof Gary M., Gomes Bruce C. Journal of Biological Chemistry. 2000;276(5):3238-3246. doi: 10.1074/jbc.M003670200. [DOI] [PubMed] [Google Scholar]
- 153.X-linked IAP is a direct inhibitor of cell-death proteases. Deveraux Quinn L., Takahashi Ryosuke, Salvesen Guy S., Reed John C. Nature. 1997;388(6639):300-304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 154.Inhibition of JNK activation through NF-κB target genes. Tang Guilin, Minemoto Yuzuru, Dibling Benjamin, Purcell Nicole H., Li Zhiwei, Karin Michael, Lin Anning. Nature. 2001;414(6861):313-317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 155.NF-κB-dependent expression of the antiapoptotic factor c-FLIP is regulated by calpain 3, the protein involved in limb-girdle muscular dystrophy type 2A. Benayoun Béatrice, Baghdiguian Stephen, Lajmanovich Alicia, Bartoli Marc, Daniele Nathalie, Gicquel Evelyne, Bourg Nathalie, Raynaud Fabrice, Pasquier Marie-Anne, Suel Laurence, Lochmuller Hanns, Lefranc Gérard, Richard Isabelle. The FASEB Journal. 2008;22(5):1521-1529. doi: 10.1096/fj.07-8701com. [DOI] [PubMed] [Google Scholar]
- 156.The c-FLIP–NH2terminus (p22-FLIP) induces NF-κB activation. Golks Alexander, Brenner Dirk, Krammer Peter H., Lavrik Inna N. The Journal of Experimental Medicine. 2006;203(5):1295-1305. doi: 10.1084/jem.20051556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Antioxidant c-FLIP Inhibits Fas Ligand-Induced NF- B Activation in a Phosphatidylinositol 3-Kinase/Akt-Dependent Manner. Iyer A. K. V., Azad N., Talbot S., Stehlik C., Lu B., Wang L., Rojanasakul Y. The Journal of Immunology. 2011;187(6):3256-3266. doi: 10.4049/jimmunol.1002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Catz Sergio D, Johnson Jennifer L. Oncogene. 2001;20(50):7342-7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 159.The nuclear transcription factor kappaB/bcl-2 pathway correlates with pathologic complete response to doxorubicin-based neoadjuvant chemotherapy in human breast cancer. Buchholz Thomas A, Garg Amit K, Chakravarti Nitin, Aggarwal Bharat B, Esteva Francisco J, Kuerer Henry M, Singletary S Eva, Hortobagyi Gabriel N, Pusztai Lajos, Cristofanilli Massimo, Sahin Aysegul A. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(23):8398–402. doi: 10.1158/1078-0432.CCR-05-0885. [DOI] [PubMed] [Google Scholar]
- 160.Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Murphy K M, Ranganathan V, Farnsworth M L, Kavallaris M, Lock R B. Cell death and differentiation. 2000;7(1):102–11. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- 161.Modulation of mitochondrial Ca(2+) homeostasis by Bcl-2. Zhu L, Ling S, Yu X D, Venkatesh L K, Subramanian T, Chinnadurai G, Kuo T H. The Journal of biological chemistry. 1999;274(47):33267–73. doi: 10.1074/jbc.274.47.33267. [DOI] [PubMed] [Google Scholar]
- 162.Novel role for JNK as a stress-activated Bcl2 kinase. Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May W S. The Journal of biological chemistry. 2001;276(26):23681–8. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- 163.The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Chen C, Edelstein L C, Gélinas C. Molecular and cellular biology. 2000;20(8):2687–95. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.The Bcl-2 protein family: arbiters of cell survival. Adams J M, Cory S. Science (New York, N.Y.) 1998;281(5381):1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 165.Bcl-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. Minn A J, Kettlun C S, Liang H, Kelekar A, Vander Heiden M G, Chang B S, Fesik S W, Fill M, Thompson C B. The EMBO journal. 1999;18(3):632–43. doi: 10.1093/emboj/18.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. Vander Heiden M G, Li X X, Gottleib E, Hill R B, Thompson C B, Colombini M. The Journal of biological chemistry. 2001;276(22):19414–9. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 167.Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde W A, Chambers T C. The Journal of biological chemistry. 2000;275(39):29980–5. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 168.Bcl-xL is phosphorylated in malignant cells following microtubule disruption. Poruchynsky M S, Wang E E, Rudin C M, Blagosklonny M V, Fojo T. Cancer research. 1998;58(15):3331–8. [PubMed] [Google Scholar]
- 169.NF-kappa B signaling pathway as a target for human tumor radiosensitization. Jung M, Dritschilo A. Seminars in radiation oncology. 2001;11(4):346–51. doi: 10.1053/srao.2001.26034. [DOI] [PubMed] [Google Scholar]
- 170.A high dose of ionizing radiation induces tissue-specific activation of nuclear factor-kappaB in vivo. Zhou D, Brown S A, Yu T, Chen G, Barve S, Kang B C, Thompson J S. Radiation research. 1999;151(6):703–9. [PubMed] [Google Scholar]
- 171.NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S. Molecular and cellular biology. 1999;19(8):5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Hirai H, Roussel M F, Kato J Y, Ashmun R A, Sherr C J. Molecular and cellular biology. 1995;15(5):2672–81. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cancer cell cycles. Sherr C J. Science (New York, N.Y.) 1996;274(5293):1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 174.Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Lukas J, Bartkova J, Bartek J. Molecular and cellular biology. 1996;16(12):6917–25. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Diehl J A, Zindy F, Sherr C J. Genes & development. 1997;11(8):957–72. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 176.Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Fan Ming, Ahmed Kazi Mokim, Coleman Mitchell C, Spitz Douglas R, Li Jian Jian. Cancer research. 2007;67(7):3220–8. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 177.Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. Lorca T, Labbé J C, Devault A, Fesquet D, Capony J P, Cavadore J C, Le Bouffant F, Dorée M. The EMBO journal. 1992;11(7):2381–90. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. Norbury C, Blow J, Nurse P. The EMBO journal. 1991;10(11):3321–9. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Nature. 2001;410(6825):215–20. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- 180.Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Papa Salvatore, Zazzeroni Francesca, Bubici Concetta, Jayawardena Shanthi, Alvarez Kellean, Matsuda Shuji, Nguyen Dung U, Pham Can G, Nelsbach Andreas H, Melis Tiziana, De Smaele Enrico, Tang Wei-Jen, D'Adamio Luciano, Franzoso Guido. Nature cell biology. 2004;6(2):146–53. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 181.Co-activation of ERK, NF-kappaB, and GADD45beta in response to ionizing radiation. Wang Tieli, Hu Yu-Chang, Dong Shaozhong, Fan Ming, Tamae Daniel, Ozeki Munetaka, Gao Qian, Gius David, Li Jian Jian. The Journal of biological chemistry. 2005;280(13):12593–601. doi: 10.1074/jbc.M410982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.GADD45 induction of a G2/M cell cycle checkpoint. Wang X W, Zhan Q, Coursen J D, Khan M A, Kontny H U, Yu L, Hollander M C, O'Connor P M, Fornace A J, Harris C C. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3706–11. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Identification of a functional domain in a GADD45-mediated G2/M checkpoint. Yang Q, Manicone A, Coursen J D, Linke S P, Nagashima M, Forgues M, Wang X W. The Journal of biological chemistry. 2000;275(47):36892–8. doi: 10.1074/jbc.M005319200. [DOI] [PubMed] [Google Scholar]
- 184.The central region of Gadd45 is required for its interaction with p21/WAF1. Zhao H, Jin S, Antinore M J, Lung F D, Fan F, Blanck P, Roller P, Fornace A J, Zhan Q. Experimental cell research. 2000;258(1):92–100. doi: 10.1006/excr.2000.4906. [DOI] [PubMed] [Google Scholar]
- 185.Gadd45 in stress signaling. Liebermann Dan A, Hoffman Barbara. Journal of molecular signaling. 2008;3:15. doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Expression of Ku70 and Ku80 mediated by NF-kappa B and cyclooxygenase-2 is related to proliferation of human gastric cancer cells. Lim Joo Weon, Kim Hyeyoung, Kim Kyung Hwan. The Journal of biological chemistry. 2002;277(48):46093–100. doi: 10.1074/jbc.M206603200. [DOI] [PubMed] [Google Scholar]
- 187.Ku autoantigen: a multifunctional DNA-binding protein. Tuteja R, Tuteja N. Critical reviews in biochemistry and molecular biology. 2000;35(1):1–33. doi: 10.1080/10409230091169177. [DOI] [PubMed] [Google Scholar]
- 188.DNA-dependent ATPase from HeLa cells is related to human Ku autoantigen. Cao Q P, Pitt S, Leszyk J, Baril E F. Biochemistry. 1994;33(28):8548–57. doi: 10.1021/bi00194a021. [DOI] [PubMed] [Google Scholar]
- 189.The life and death of DNA-PK. Collis Spencer J, DeWeese Theodore L, Jeggo Penelope A, Parker Antony R. Oncogene. 2005;24(6):949–61. doi: 10.1038/sj.onc.1208332. [DOI] [PubMed] [Google Scholar]
- 190.NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Cao Ning, Li Shiyong, Wang Zhaoqing, Ahmed Kazi Mokim, Degnan Michael E, Fan Ming, Dynlacht Joseph R, Li Jian Jian. Radiation research. 2009;171(1):9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Muthuswamy S K, Gilman M, Brugge J S. Molecular and cellular biology. 1999;19(10):6845–57. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.New approaches to radiation protection. Rosen Eliot M, Day Regina, Singh Vijay K. Frontiers in oncology. 2014;4:381. doi: 10.3389/fonc.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Radioprotectors and mitigators of radiation-induced normal tissue injury. Citrin Deborah, Cotrim Ana P, Hyodo Fuminori, Baum Bruce J, Krishna Murali C, Mitchell James B. The oncologist. 2010;15(4):360–71. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.The role of p53 in determining sensitivity to radiotherapy. Gudkov Andrei V, Komarova Elena A. Nature reviews. Cancer. 2003;3(2):117–29. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 195.The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Potten C S. Cancer metastasis reviews. 1992;11(2):179–95. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- 196.Radiation and ceramide-induced apoptosis. Kolesnick Richard, Fuks Zvi. Oncogene. 2003;22(37):5897–906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 197.Activation of nuclear factor kappaB In vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Wang Yong, Meng Aimin, Lang Hainan, Brown Stephen A, Konopa Jennifer L, Kindy Mark S, Schmiedt Richard A, Thompson John S, Zhou Daohong. Cancer research. 2004;64(17):6240–6. doi: 10.1158/0008-5472.CAN-04-0591. [DOI] [PubMed] [Google Scholar]
- 198.Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Stone Helen B, Moulder John E, Coleman C Norman, Ang K Kian, Anscher Mitchell S, Barcellos-Hoff Mary Helen, Dynan William S, Fike John R, Grdina David J, Greenberger Joel S, Hauer-Jensen Martin, Hill Richard P, Kolesnick Richard N, Macvittie Thomas J, Marks Cheryl, McBride William H, Metting Noelle, Pellmar Terry, Purucker Mary, Robbins Mike E, Schiestl Robert H, Seed Thomas M, Tomaszewski Joseph E, Travis Elizabeth L, Wallner Paul E, Wolpert Mary, Zaharevitz Daniel. Radiation research. 2004;162(6):711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 199.Priority list of research areas for radiological nuclear threat countermeasures. Pellmar Terry C, Rockwell Sara. Radiation research. 2005;163(1):115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 200.Amifostine inhibits hematopoietic progenitor cell apoptosis by activating NF-kappaB/Rel transcription factors. Romano M F, Lamberti A, Bisogni R, Garbi C, Pagnano A M, Auletta P, Tassone P, Turco M C, Venuta S. Blood. 1999;94(12):4060–6. [PubMed] [Google Scholar]
- 201.Oxidative Stress and Redox Regulation. 2013 [Google Scholar]
- 202.Copp R., Fahl W., Peebles D. Amino thiol compounds and compositions for use in conjunction with cancer therapy. Google Patents. 2005.
- 203.Selenium pretreatment enhances the radioprotective effect and reduces the lethal toxicity of WR-2721. Weiss J F, Hoover R L, Kumar K S. Free radical research communications. 1987;3(1-5):33–8. doi: 10.3109/10715768709069767. [DOI] [PubMed] [Google Scholar]
- 204.Selenium: a key element that controls NF-kappa B activation and I kappa B alpha half life. Kretz-Remy C, Arrigo A P. BioFactors (Oxford, England) 2001;14(1-4):117–25. doi: 10.1002/biof.5520140116. [DOI] [PubMed] [Google Scholar]
- 205.Bell S., Maniar M. Formulation of Radioprotective α, β Unsaturated Aryl Sulfones. Google Patents. 2007.
- 206.Maniar M., Bell S. Formulations of Radioprotective α, β Unsaturated Aryl Sulfones. Google Patents. 2008.
- 207.Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms. Ghosh Sanchita P, Perkins Michael W, Hieber Kevin, Kulkarni Shilpa, Kao Tzu-Cheg, Reddy E Premkumar, Reddy M V Ramana, Maniar Manoj, Seed Thomas, Kumar K Sree. Radiation research. 2009;171(2):173–9. doi: 10.1667/RR1367.1. [DOI] [PubMed] [Google Scholar]
- 208.An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Burdelya Lyudmila G, Krivokrysenko Vadim I, Tallant Thomas C, Strom Evguenia, Gleiberman Anatoly S, Gupta Damodar, Kurnasov Oleg V, Fort Farrel L, Osterman Andrei L, Didonato Joseph A, Feinstein Elena, Gudkov Andrei V. Science (New York, N.Y.) 2008;320(5873):226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Burdelya Lyudmila G, Brackett Craig M, Kojouharov Bojidar, Gitlin Ilya I, Leonova Katerina I, Gleiberman Anatoli S, Aygun-Sunar Semra, Veith Jean, Johnson Christopher, Haderski Gary J, Stanhope-Baker Patricia, Allamaneni Shyam, Skitzki Joseph, Zeng Ming, Martsen Elena, Medvedev Alexander, Scheblyakov Dmitry, Artemicheva Nataliya M, Logunov Denis Y, Gintsburg Alexander L, Naroditsky Boris S, Makarov Sergei S, Gudkov Andrei V. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(20):E1857–66. doi: 10.1073/pnas.1222805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shakhov A., Gudkov A. Methods of protecting against apoptosis using lipopeptides. Google Patents. 2007.
- 211.Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). Shakhov Alexander N, Singh Vijay K, Bone Frederick, Cheney Alec, Kononov Yevgeniy, Krasnov Peter, Bratanova-Toshkova Troitza K, Shakhova Vera V, Young Jason, Weil Michael M, Panoskaltsis-Mortari Angela, Orschell Christie M, Baker Patricia S, Gudkov Andrei, Feinstein Elena. PloS one. 2012;7(3):e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini , as a novel radiation countermeasure. Singh Vijay K, Ducey Elizabeth J, Fatanmi Oluseyi O, Singh Pankaj K, Brown Darren S, Purmal Andrei, Shakhova Vera V, Gudkov Andrei V, Feinstein Elena, Shakhov Alexander. Radiation research. 2012;177(5):628–42. doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 213.Molecular specificity of 5-androstenediol as a systemic radioprotectant in mice. Whitnall Mark H, Villa Vilmar, Seed Thomas M, Benjack James, Miner Venita, Lewbart Marvin L, Dowding Charles A, Jackson William E. Immunopharmacology and immunotoxicology. 2005;27(1):15–32. doi: 10.1081/iph-51289. [DOI] [PubMed] [Google Scholar]
- 214.5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Stickney Dwight R, Dowding Charles, Authier Simon, Garsd Armando, Onizuka-Handa Nanette, Reading Christopher, Frincke James M. International immunopharmacology. 2007;7(4):500–5. doi: 10.1016/j.intimp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 215.RADIOPROTECTIVE EFFICACY AND ACUTE TOXICITY OF 5-ANDROSTENEDIOL AFTER SUBCUTANEOUS OR ORAL ADMINISTRATION IN MICE. Whitnall Mark H., Wilhelmsen Catherine L., McKinney LuAnn, Miner Venita, Seed Thomas M., Jackson William E. Immunopharmacology and Immunotoxicology. 2002;24(4):595-626. doi: 10.1081/iph-120016038. [DOI] [PubMed] [Google Scholar]
- 216.Geldanamycin Analog 17-DMAG Inhibits iNOS and Caspases in Gamma-Irradiated Human T Cells. Kiang Juliann G., Smith Joan T., Agravante Neil G. Radiation Research. 2009;172(3):321-330. doi: 10.1667/RR1585.1. [DOI] [PubMed] [Google Scholar]
- 217.Up-regulation of autophagy in small intestine Paneth cells in response to total-body γ-irradiation. Gorbunov Nikolai V., Kiang Juliann G. The Journal of Pathology. 2009;219(2):242-252. doi: 10.1002/path.2591. [DOI] [PubMed] [Google Scholar]
- 218.Major Radiation Exposure — What to Expect and How to Respond. Mettler Fred A., Voelz George L. New England Journal of Medicine. 2002;346(20):1554-1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 219.Radiation protection by diethyldithiocarbamate: protection of membrane and DNA in vitro and in vivo against gamma-radiation. Gandhi Nitin Motilal, Nair Cherupally Krishnan Krishnan. Journal of radiation research. 2004;45(2):175–80. doi: 10.1269/jrr.45.175. [DOI] [PubMed] [Google Scholar]
- 220.Role of cytokines in radioprotection. Neta Ruth. Pharmacology & Therapeutics. 1988;39(1-3):261-266. doi: 10.1016/0163-7258(88)90070-8. [DOI] [PubMed] [Google Scholar]
- 221.Utility of interleukin-1 in therapy of radiation injury as studied in small and large animal models. Neta R., Monroy R., MacVittie T. J. Biotherapy. 1989;1(4):301-311. doi: 10.1007/BF02171006. [DOI] [PubMed] [Google Scholar]
- 222.Differential radioprotection of three mouse strains by basic or acidic fibroblast growth factor. Okunieff P, Wu T, Huang K, Ding I. The British journal of cancer. Supplement. 1996;27:S105–8. [PMC free article] [PubMed] [Google Scholar]
- 223.Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Zhang Ming, Qian Jun, Xing Xianying, Kong Feng-Ming, Zhao Lujun, Chen Ming, Lawrence Theodore S. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(6):1868–76. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 224.Mickelson A., Horoho P. Medical Consequences of Radiological and Nuclear Weapons. U.S. Government Printing Office. 2013.
- 225.Novel strategies for protecting mitochondria (the cellular powerhouse) against low-LET radiation: a review. Gupta D., Rajesh A, Mohan R. R., Almasan A., Gudkov A. V., Macklis R. M. Herbal radiomodulators: applications in medicine, homeland defence and space :215-226. [Google Scholar]
- 226.Radiation protection of HepG2 cells by Podophyllum hexandrum Royale. Gupta Damodar, Arora Rajesh, Garg Amar Prakash, Goel Harish Chandra. Molecular and cellular biochemistry. 2003;250(1-2):27–40. doi: 10.1023/a:1024925612233. [DOI] [PubMed] [Google Scholar]
- 227.Antioxidant activity of fractionated extracts of rhizomes of high-altitude Podophyllum hexandrum: role in radiation protection. Chawla Raman, Arora Rajesh, Kumar Raj, Sharma Ashok, Prasad Jagdish, Singh Surendar, Sagar Ravinder, Chaudhary Pankaj, Shukla Sandeep, Kaur Gurpreet, Sharma Rakesh Kumar, Puri Satish Chander, Dhar Kanaya Lal, Handa Geeta, Gupta Vinay Kumar, Qazi Ghulam Nabi. Molecular and cellular biochemistry. 2005;273(1-2):193–208. doi: 10.1007/s11010-005-0821-5. [DOI] [PubMed] [Google Scholar]
- 228.Influence of Podophyllum hexandrum on endogenous antioxidant defence system in mice: possible role in radioprotection. Mittal A, Pathania V, Agrawala P K, Prasad J, Singh S, Goel H C. Journal of ethnopharmacology. 2001;76(3):253–62. doi: 10.1016/s0378-8741(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 229.Podophyllum hexandrum prevents radiation-induced neuronal damage in postnatal rats exposed in utero. Sajikumar S, Goel H C. Phytotherapy research : PTR. 2003;17(7):761–6. doi: 10.1002/ptr.1204. [DOI] [PubMed] [Google Scholar]
- 230.Modification of radiation damage to mitochondrial system in vivo by Podophyllum hexandrum: mechanistic aspects. Gupta Damodar, Arora Rajesh, Garg Amar Prakash, Bala Madhu, Goel Harish Chandra. Molecular and cellular biochemistry. 2004;266(1-2):65–77. doi: 10.1023/b:mcbi.0000049139.05337.40. [DOI] [PubMed] [Google Scholar]
- 231.Protection of mitochondrial system by Hippophae rhamnoides L. against radiation-induced oxidative damage in mice. Goel Harish Chandra, Gupta Damodar, Gupta Shobha, Garg A P, Bala Madhu. The Journal of pharmacy and pharmacology. 2005;57(1):135–43. doi: 10.1211/0022357055218. [DOI] [PubMed] [Google Scholar]
- 232.Radioprotection by a herbal preparation of Hippophae rhamnoides, RH-3, against whole body lethal irradiation in mice. Goel H C, Prasad J, Singh S, Sagar R K, Kumar I Prem, Sinha A K. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2002;9(1):15–25. doi: 10.1078/0944-7113-00077. [DOI] [PubMed] [Google Scholar]
- 233.Protection of jejunal crypts by RH-3 (a preparation of Hippophae rhamnoides) against lethal whole body gamma irradiation. Goel H C, Salin C A, Prakash H. Phytotherapy research : PTR. 2003;17(3):222–6. doi: 10.1002/ptr.1109. [DOI] [PubMed] [Google Scholar]
- 234.Radioprotective effect of sesamol on gamma-radiation induced DNA damage, lipid peroxidation and antioxidants levels in cultured human lymphocytes. Prasad N Rajendra, Menon Venugopal P, Vasudev V, Pugalendi K V. Toxicology. 2005;209(3):225–35. doi: 10.1016/j.tox.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 235.[Prospective double-blind study of prophylaxis of radioxerostomia with Coumarin/Troxerutine in patients with head and neck cancer]. Grötz K A, Henneicke-von Zepelin H H, Kohnen R, al-Nawas B, Bockisch A, Kutzner J, Wüstenberg P, Naser-Hijazi B, Belz G G, Wagner W. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al] 1999;175(8):397–403; discussion 404. doi: 10.1007/s000660050028. [DOI] [PubMed] [Google Scholar]
- 236.Radioprotection of normal tissues in tumor-bearing mice by troxerutin. Maurya Dharmendra Kumar, Salvi Veena Prakash, Krishnan Nair Cherupally Krishnan. Journal of radiation research. 2004;45(2):221–8. doi: 10.1269/jrr.45.221. [DOI] [PubMed] [Google Scholar]
- 237.Ethyl pyruvate, a potentially effective mitigator of damage after total-body irradiation. Epperly Michael, Jin Shunqian, Nie Suhua, Cao Shaonan, Zhang Xichen, Franicola Darcy, Wang Hong, Fink Mitchell P, Greenberger Joel S. Radiation research. 2007;168(5):552–9. doi: 10.1667/RR1009.1. [DOI] [PubMed] [Google Scholar]
- 238.Epperly M., Greenberger J., Mitchell P. Radioprotective agents. Google Patents. 2007.
- 239.Ethyl pyruvate administration inhibits hepatic tumor growth. Liang Xiaoyan, Chavez Antonio Romo de Vivar, Schapiro Nicole E, Loughran Patricia, Thorne Stephen H, Amoscato Andrew A, Zeh Herbert J, Beer-Stolz Donna, Lotze Michael T, de Vera Michael E. Journal of leukocyte biology. 2009;86(3):599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 240.Cytokine-based treatment of accidentally irradiated victims and new approaches. Hérodin Francis, Drouet Michel. Experimental hematology. 2005;33(10):1071–80. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 241.Radiation countermeasure agents: an update (2011-2014). Singh Vijay K, Newman Victoria L, Romaine Patricia L P, Wise Stephen Y, Seed Thomas M. Expert opinion on therapeutic patents. 2014;24(11):1229–55. doi: 10.1517/13543776.2014.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Integration of filgrastim into chemoradiation for limited small cell lung cancer: a Phase I study. Glisson B, Komaki R, Lee J S, Shin D M, Fossella F, Murphy W K, Kurie J, Perez-Soler R, Schea R, Vadhan-Raj S. International journal of radiation oncology, biology, physics. 1998;40(2):331–6. doi: 10.1016/s0360-3016(97)00709-8. [DOI] [PubMed] [Google Scholar]
- 243.[Use of filgrastim, granulocyte colony stimulating factor (G-CSF), in radiotherapy to reduce drop-outs because of radiogenic leukopenia]. Gava A, Bertossi L, Ferrarese F, Coghetto F, Marazzato G, Andrulli A D, Zorat P L. La Radiologia medica. 1998;95(3):232–6. [PubMed] [Google Scholar]
- 244.Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Davis Thomas A, Clarke Tara K, Mog Steven R, Landauer Michael R. International journal of radiation biology. 2007;83(3):141–51. doi: 10.1080/09553000601132642. [DOI] [PubMed] [Google Scholar]
- 245.Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. Day Regina M, Barshishat-Kupper Michal, Mog Steven R, McCart Elizabeth A, Prasanna P G S, Davis Thomas A, Landauer Michael R. Journal of radiation research. 2008;49(4):361–72. doi: 10.1269/jrr.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Effects of genistein administration on cytokine induction in whole-body gamma irradiated mice. Singh Vijay K, Grace Marcy B, Parekh Vaishali I, Whitnall Mark H, Landauer Michael R. International immunopharmacology. 2009;9(12):1401–10. doi: 10.1016/j.intimp.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 247.Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. Raffoul Julian J, Wang Yu, Kucuk Omer, Forman Jeffrey D, Sarkar Fazlul H, Hillman Gilda G. BMC cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.El-Missiry A., Othman A., Alabdan A. Melatonin for Protection Against Ionizing Radiation. INTECH Open Access Publisher. 2012.
- 249.Can melatonin help us in radiation oncology treatments? Mihandoost Ehsan, Shirazi Alireza, Mahdavi Seied Rabie, Aliasgharzadeh Akbar. BioMed research international. 2014;2014:578137. doi: 10.1155/2014/578137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Carsten Ronald E, Bachand Annette M, Bailey Susan M, Ullrich Robert L. Radiation research. 2008;169(6):633–8. doi: 10.1667/RR1190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Fu Yue, Wang Yan, Du Liqing, Xu Chang, Cao Jia, Fan Tiqiang, Liu Jianxiang, Su Xu, Fan Saijun, Liu Qiang, Fan Feiyue. International journal of molecular sciences. 2013;14(7):14105–18. doi: 10.3390/ijms140714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Vorotnikova Ekaterina, Rosenthal Rosalind A, Tries Mark, Doctrow Susan R, Braunhut Susan J. Radiation research. 2010;173(6):748–59. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. Doctrow Susan R, Lopez Argelia, Schock Ashley M, Duncan Nathan E, Jourdan Megan M, Olasz Edit B, Moulder John E, Fish Brian L, Mäder Marylou, Lazar Jozef, Lazarova Zelmira. The Journal of investigative dermatology. 2013;133(4):1088–96. doi: 10.1038/jid.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Radiation Injury Prevention and Mitigation in Humans. Prasad Kedar. 2012 [Google Scholar]
- 255.Carnosine may reduce lung injury caused by radiation therapy. Guney Yildiz, Ozel Turkcu Ummuhani, Hicsonmez Ayse, Nalca Andrieu Meltem, Guney H. Zafer, Bilgihan Ayse, Kurtman Cengiz. Medical Hypotheses. 2006;66(5):957-959. doi: 10.1016/j.mehy.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 256.Evaluation of radioprotective effect of carnosine (beta- alanyl-1- histidine) on the wound healing in rats. Tanaka Rosana Aramaki, Ramos Flávia Maria de Moraes, Almeida Solange Maria de, Vizioli Mário Roberto, Bóscolo Frab Norberto. Journal of Applied Oral Science. 2005;13(3):253-258. doi: 10.1590/s1678-77572005000300010. [DOI] [PubMed] [Google Scholar]
- 257.IL-12 Facilitates Both the Recovery of Endogenous Hematopoiesis and the Engraftment of Stem Cells after Ionizing Radiation. Chen Tingchao, Burke Kathleen A., Zhan Yuxia, Wang Xingchao, Shibata Darryl, Zhao Yi. Experimental Hematology. 2007;35(2):203-213. doi: 10.1016/j.exphem.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 258.Basile L., Gallaher T., Ellefson D. Il-12 for radiation protection and radiation-induced toxicity mitigation. Google Patents. 2013.
- 259.Update on toll-like receptor-directed therapies for human disease. Tse K., Horner A. A. Annals of the Rheumatic Diseases. 2007;66(Supplement 3 ):iii77-iii80. doi: 10.1136/ard.2007.078998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. Tallant Thomas, Deb Amitabha, Kar Niladri, Lupica Joseph, de Veer Michael J, DiDonato Joseph A. BMC microbiology. 2004;4:33. doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: implications for head-and-neck cancer radiotherapy. Burdelya Lyudmila G, Gleiberman Anatoli S, Toshkov Ilia, Aygun-Sunar Semra, Bapardekar Meghana, Manderscheid-Kern Patricia, Bellnier David, Krivokrysenko Vadim I, Feinstein Elena, Gudkov Andrei V. International journal of radiation oncology, biology, physics. 2012;83(1):228–34. doi: 10.1016/j.ijrobp.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.CBLB613: A TLR 2/6 Agonist, Natural Lipopeptide of Mycoplasma arginini , as a Novel Radiation Countermeasure. Singh V., Ducey E., Fatanmi O., Singh P., Brown D., Purmal A. Radiat Res. 2011 doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 263.Synthesis of baicalein derivatives as potential anti-aggregatory and anti-inflammatory agents. Huang Wen-Hsin, Lee An-Rong, Chien Pei-Yu, Chou Tz-Chong. The Journal of pharmacy and pharmacology. 2005;57(2):219–25. doi: 10.1211/0022357055371. [DOI] [PubMed] [Google Scholar]
- 264.Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. Hwang Jin-Ming, Tseng Tsui-Hwa, Tsai Yu-Ying, Lee Huei-Jane, Chou Feu-Pi, Wang Chau-Jong, Chu Chia-Yih. Journal of biomedical science. 2005;12(2):389–97. doi: 10.1007/s11373-005-1572-8. [DOI] [PubMed] [Google Scholar]
- 265.Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-kappaB activity in vascular smooth-muscle cells. Peng Chieh-Yu, Pan Shiow-Lin, Huang Ying-Wen, Guh Jih-Hwa, Chang Ya-Ling, Teng Che-Ming. Naunyn-Schmiedeberg's archives of pharmacology. 2008;378(6):579–88. doi: 10.1007/s00210-008-0328-1. [DOI] [PubMed] [Google Scholar]
- 266.Molecular activation of NF-kappaB, pro-inflammatory mediators, and signal pathways in gamma-irradiated mice. Ha Young Mi, Chung Sang Woon, Kim Ji Min, Kim Dae Hyun, Kim Ji Young, Lee Eun Kyeong, Lee Jaewon, Kim Young Jin, Yoo Mi Ae, Jeong Kyu Shik, Chung Hae Young. Biotechnology letters. 2010;32(3):373–8. doi: 10.1007/s10529-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 267.The double-stranded RNA-activated protein kinase mediates radiation resistance in mouse embryo fibroblasts through nuclear factor kappaB and Akt activation. von Holzen Urs, Pataer Abujiang, Raju Uma, Bocangel Dora, Vorburger Stephan A, Liu Yanna, Lu Xiaolin, Roth Jack A, Aggarwal Bharat B, Barber Glen N, Keyomarsi Khandan, Hunt Kelly K, Swisher Stephen G. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(20):6032–9. doi: 10.1158/1078-0432.CCR-06-2932. [DOI] [PubMed] [Google Scholar]
- 268.PLK1 is a binding partner and a negative regulator of FOXO3 tumor suppressor. Bucur Octavian, Stancu Andreea Lucia, Muraru Maria Sinziana, Melet Armelle, Petrescu Stefana Maria, Khosravi-Far Roya. Discoveries. 2014;2(2):e16. doi: 10.15190/d.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hydrogen peroxide stress stimulates phosphorylation of FoxO1 in rat aortic endothelial cells. Wang Ye-yu, Chen Si-min, Li Hao. Acta Pharmacologica Sinica. 2010;31(2):160-164. doi: 10.1038/aps.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Modulation of NF-κB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Lee Eun Kyeong, Kim Ji Min, Choi Jehun, Jung Kyung Jin, Kim Dae Hyun, Chung Sang Woon, Ha Young Mi, Yu Byung Pal, Chung Hae Young. Free radical research. 2011;45(5):507–17. doi: 10.3109/10715762.2011.555479. [DOI] [PubMed] [Google Scholar]
- 271.NF-kappaB activation by double-strand breaks. Habraken Yvette, Piette Jacques. Biochemical pharmacology. 2006;72(9):1132–41. doi: 10.1016/j.bcp.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 272.Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Lin Y, Ma W, Benchimol S. Nature genetics. 2000;26(1):122–7. doi: 10.1038/79102. [DOI] [PubMed] [Google Scholar]
- 273.Modulation of PAI-1 and tPA activity and thrombolytic effects of corilagin. Shen Zhi-Qiang, Dong Ze-Jun, Peng Hua, Liu Ji-Kai. Planta medica. 2003;69(12):1109–12. doi: 10.1055/s-2003-45191. [DOI] [PubMed] [Google Scholar]
- 274.Antiatherogenic effects of phyllanthus emblica associated with corilagin and its analogue. Duan Weigang, Yu Yun, Zhang Luyong. Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan. 2005;125(7):587–91. doi: 10.1248/yakushi.125.587. [DOI] [PubMed] [Google Scholar]
- 275.Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2007;14(11):755–62. doi: 10.1016/j.phymed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 276.Antihypertensive effect of corilagin in the rat. Cheng J T, Lin T C, Hsu F L. Canadian journal of physiology and pharmacology. 1995;73(10):1425–9. doi: 10.1139/y95-198. [DOI] [PubMed] [Google Scholar]
- 277.Preliminary exploration on anti-inflammatory mechanism of Corilagin (beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-D-glucose) in vitro. Zhao Lei, Zhang Shu-Ling, Tao Jun-Yan, Pang Ran, Jin Feng, Guo Yuan-Jin, Dong Ji-Hua, Ye Pian, Zhao Hong-Yang, Zheng Guo-Hua. International immunopharmacology. 2008;8(7):1059–64. doi: 10.1016/j.intimp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 278.Corilagin inhibits the double strand break-triggered NF-kappaB pathway in irradiated microglial cells. Dong Xiao-Rong, Luo Ming, Fan Li, Zhang Tao, Liu Li, Dong Ji-Hua, Wu Gang. International journal of molecular medicine. 2010;25(4):531–6. [PubMed] [Google Scholar]
- 279.Amelioration of radiation-induced hematopoietic and gastrointestinal damage by Ex-RAD(R) in mice. Ghosh Sanchita P, Kulkarni Shilpa, Perkins Michael W, Hieber Kevin, Pessu Roli L, Gambles Kristen, Maniar Manoj, Kao Tzu-Cheg, Seed Thomas M, Kumar K Sree. Journal of radiation research. 2012;53(4):526–36. doi: 10.1093/jrr/rrs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.