Abstract
Intercellular junctions of the vascular endothelium are dynamic structures that display a high degree of plasticity, which is required to contribute to their regulation of many physiological and pathological processes including monolayer integrity, barrier function, wound healing and angiogenesis. Vascular endothelial cadherin (VE-cadherin) is connected via catenins to the actin cytoskeleton, both of which are key structures in endothelial junction regulation, and thus are the focus of much investigation. Fluorescence-based live cell imaging is the method of choice to study dynamic remodeling in living cells. Although these methods have been successfully applied to many cell types, investigations of endothelial junction dynamics were for a long time limited as they are largely resistant to transfection using many classical protocols. Application of virus-based gene transduction techniques, together with advanced microscopy, now allows both sufficient expression of fluorescence tagged junction-localized proteins in the endothelium and time-lapse recording over long periods. Using highly spatiotemporally resolved fluorescence microscopy it turned out that endothelial junctions display extensive junction heterogeneity at the subcellular level; a fact that largely limits automated quantification by available software. Recent work describes open software tools to quantitatively analyze large amounts of fluorescence-based image data in either single or confluent epithelial and endothelial cells. Based on quantitative VE-cadherin and actin dynamics novel key players, mechanisms and concepts have been suggested that control endothelial junction dynamics. Here we aim to summarize the recent developments in the field.
Keywords: Quantifying endothelial junction dynamics
SUMMARY
Introduction
Methods to visualize endothelial cell junction dynamics
Endothelial junction dynamics analyzed by fluorescence techniques
Dynamics of VE-cadherin analyzed by spinning disc microscopy
Conclusion
1. Introduction
The vascular endothelium is a highly plastic tissue that covers the inner surface of the entire cardiovascular system. It regulates many physiological functions, such as control of blood pressure and perfusion and balancing coagulation/anticoagulation processes. The endothelium is the critical boundary surface that separates the blood from the interstitial space of tissues, a feature that is generally referred to as endothelial barrier function. Cell integrity and barrier function depend on the formation of cell junctions in an organ and vascular segment specific manner1,2. A systemic loss of cell junction integrity contributes to the pathogenesis of life threatening diseases such as Ebola hemorrhagic fever3 and bacterial sepsis4-7, demonstrating a central role of cell junctions in both physiology and pathology. Endothelial cell junctions, in contrast to epithelial junctions, are complex in nature and display components of tight, adherens and gap junctions8-11. To accomplish the diverse organ and vascular segment specific demands of their multiple roles in physiology, development and diseases such as inflammation, wound healing, angiogenesis and tumor angiogenesis, cell junctions needs to dynamically respond on different time scales; ranging from minutes to many hours.
A main structural and regulatory molecule in endothelial junctions is vascular endothelial (VE)-cadherin that connects endothelial cells to each other in a calcium-dependent manner12,13. VE-cadherin is critical in controlling endothelial junction dynamics as it provides the junction backbone, and is expressed in all types of vascular endothelial cells12,14. The cytosolic domain of VE-cadherin directly interacts with beta-catenin, gamma-catenin and p120. While p120 prevents VE-cadherin endocytosis, β- and γ-catenin bind to α-catenin, which in turn mediates the interaction of the cadherin/catenin complex with the actin cytoskeleton15-17.
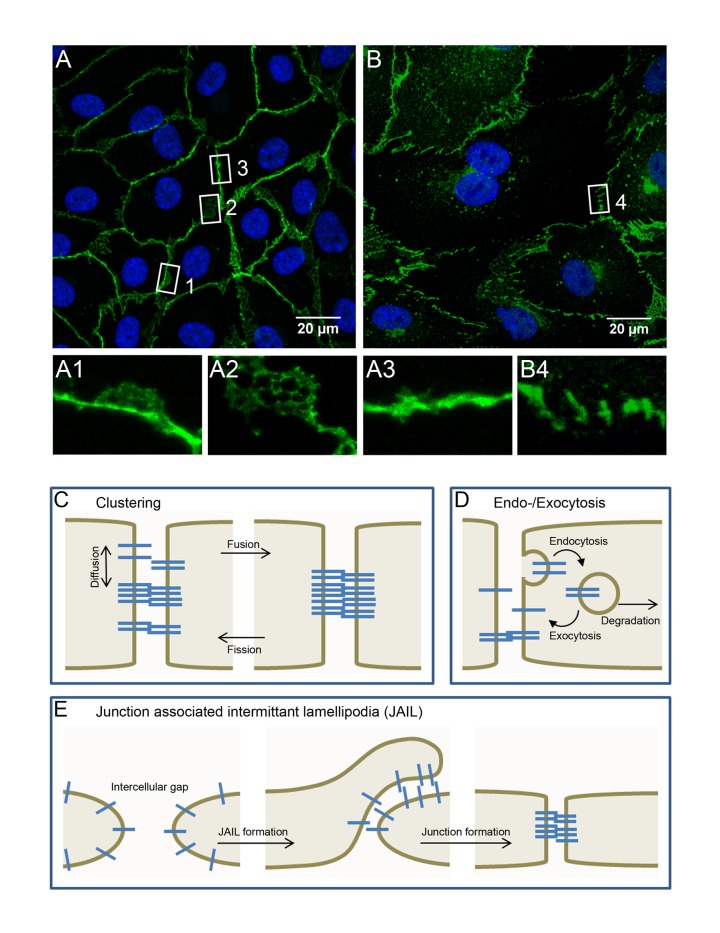
It is generally proposed that control of cell adhesion, barrier function, cell migration and angiogenesis critically depend on the direct and indirect interaction of VE-cadherin with actin filaments, as both structures are remodeled under certain stimulations18-22. When examined by classical laser scanning microscopy (LSM) VE-cadherin displays diverse staining patterns, with linear, reticular, interrupted and plaque morphologies being evident (Figure 1 [A, B]). Accordingly, junction dynamics in the endothelium is obviously not uniform, but rather it display a wide heterogeneity even in one cell within a monolayer23. Super resolution microscopy of epithelial and endothelial adherens junctions indicates the existence of individual cadherin clusters20,24-26 whose size seems to be dynamically regulated by fusion and fission20,25, (Figure 1 [C]).
Figure 1. Heterogeneity of endothelial junctions in HUVEC cultures.
Immune fluorescence microscopy of confluent (A) and subconfluent (B) endothelial cells stained with anti VE-cadherin (green) and DAPI (blue). Cropped areas show the diverse super-structural organization of endothelial junctions. This includes plaque-like structures (A1), reticular networks (A2), linear junctions (A3) and interrupted patterns (B4). These diverse superstructures are constitutively reorganized by dynamic VE-cadherin remodeling via processes such as fusion and fission of VE-cadherin clusters (C), endo- and exocytosis (D) and by locally appearing junction associated intermittent lamellipodia (JAIL), which develop at junction sites with a low VE-cadherin concentration (E).
The molecular mechanistic basis for subcellular junction regulation remains largely unknown, despite the many mechanisms related to cell signaling and junction remodeling, including VE-cadherin and the diverse cytoskeletal filament system, that have been discussed in certain reviews16,17,27-30. While biochemical or biophysical measurements, such as impedance spectroscopy, give mean values of dynamic barrier function regulation, high time resolution live cell imaging additionally uncovers information regarding spatiotemporal regulation at the subcellular level. In addition, the temporal sequence of short duration events are easily perceived in time lapse movies, while those same phenomena are hard to distinguish in snap shots. One such structure are junction associated intermittent lammellipodia (JAIL, see sections below for additional details), which are actin-driven and cause the formation of new VE-cadherin adhesion. In confluent cultures, in which JAIL are small and short lasting, these structures can only be clearly identified in time lapse recordings (available at http://link.springer.com/article/10.1007/s00418-015-1357-8). Therefore, live cell imaging of endothelial cell junction dynamics, followed by quantitative analysis, can significantly upgrade the information output of experiments, but requires methodologically sound techniques and specialized equipment.
2. Methods to visualize endothelial cell junction dynamics
Basic problems and requirements for analysis of endothelial junction dynamics
The method of choice to study spatiotemporal dynamics in cells and cell junctions at the subcellular level is live cell imaging of fluorescence tagged proteins. Excellent work conducted during the last years describes the available techniques and methods31,32. This includes the generation and expression of fluorescence tagged proteins33,34, and the development of software tools35-37. In addition, advanced microscopic techniques, such as spinning disc and confocal laser microscopy, Förster or fluorescence resonance energy transfer (FRET), fluorescence recovery after photobleaching (FRAP), single molecule tracking (SMT) and structured illumination microscopy (SIM) have been successfully applied to answer many scientific questions in many different cell types. However, expression of fluorescence tagged proteins in the endothelium is not a simple approach as most endothelial cells, and in particular primary isolates such as HUVEC, resist transfection using classical methods38. Also, electroporation of suspended cells bathed in a plasmid containing medium has been reported to cause cell impairments39 that in turn can perturb cell junction maturation and thus these cells might not exhibit characteristic endothelial cell adhesion and barrier features.
Virus-based gene transfer is sufficient to express fluorescence tagged proteins in the endothelium
A suitable and meanwhile frequently applied alternative for gene expression in endothelial cell lines as well as primary cell cultures is the use of replication-deficient recombinant lenti-, retro- or adeno-viruses40. Since many lentivirus-based vectors include an integrase, the generation of constitutively expressing cultures is also relatively easy to perform. Although the generation and production of replication-deficient recombinant viruses is time and cost intensive it gives excellent transduction efficacy and expression levels can be controlled as well. A few examples in which, viral-based gene transduction was efficiently applied include studies where up to 90% gene transduction was demonstrated for fluorescence tagged caveolin1, proteins of the ARP2/3 complex, LifeAct, GTPases, VE-cadherin, β-actin, β-catenin and γ-catenin41,42. Furthermore, carefully performed virus titration is useful in controlling the protein expression level in the endothelium to achieve either moderate or even strongly overexpressed protein levels. After infection, gene expression from lentiviruses is typically sufficient after three to four days and is best suited to performing spinning disc microscopy over a period of hours23,41,43. Adenoviral mediated gene expression is usually faster, reaching high expression levels after approximately 10 to 16 hours post infection, as demonstrated for the Rho-GTPases and VE-cadherin-EGFP22,23,44,45. Together, virus-based gene transduction is currently the most successful approach available and the method of choice to obtain reliable, reproducible and controlled protein expression levels in the endothelium. An example showing expression of VE-cadherin-mCherry and EGFP-p20 are shown inFigure 2. However, any type of exogenous protein expression in cultured cells has the potential to exhibit toxic effects. Therefore, when using virus mediated gene transfer methods, the respective controls need to be carefully planned. Further, to test if the expression of a particular fluorescence tagged protein disturbs normal cell behavior, functional tests are highly recommended. For example, moderate expression of VE-cadherin-EGFP in the endothelium did not cause cells to behave differently compared to native untreated endothelial cells when tested in a scratch assay. This also holds true for testing the barrier function of VE-cadherin-EGFP or caveolin-1-EGFP expressing cells upon stimulation compared to native non-transduced cells41,46. Those functional tests verify the regular behavior of cells even during fluorescence tagged protein expression. In other cases expression can be used to study the impact of protein concentration on cell functions, as demonstrated by overexpression of VE-cadherin, which leads to clearly reduced cell motility41. Therefore, we suggest choosing functional tests for ensuring regular activity of cells expressing fusion proteins, rather than simple expression of an EGFP or mCherry molecule by itself, because those proteins might also cause artifacts.
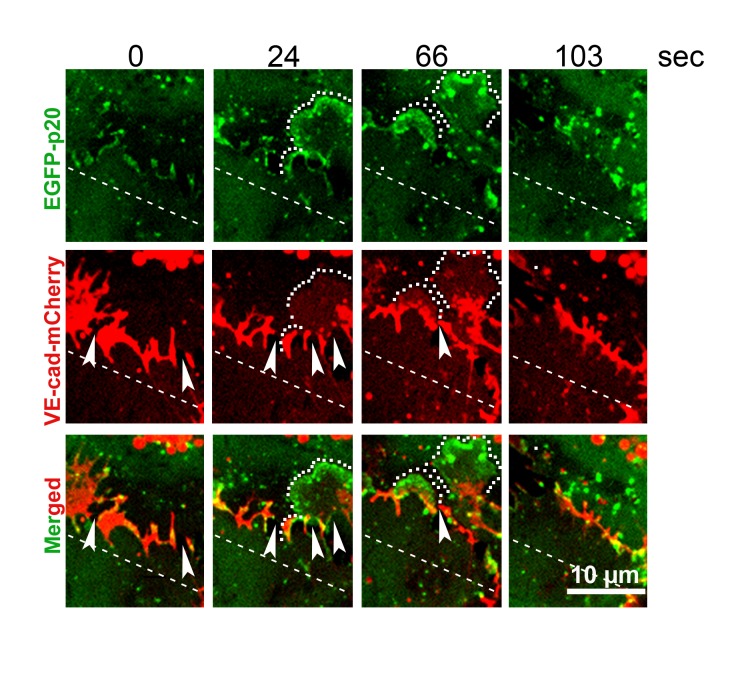
Figure 2. VE-cadherin dynamics is driven by ARP2/3 complex controlled JAIL formation.
Time-lapse recording of sub confluent HUVECs that co-express both VE-cadherin-mCherry and EGFP-p20, a component of the ARP2/3 complex. VE-cadherin remodeling is initiated by ARP2/3-controlled and actin-driven formation of JAIL (dotted line), which then overlap adjacent membranes allowing trans-dimer engagement of free floating VE-cadherin in the membrane (VE-cadherin plaques), followed by JAIL retraction that subsequently clusters and incorporates VE-cadherin into the junctions (reproduced from our previous work41 (Abu Taha A et al. Mol Biol Cell 2014, 25: 245-256) with permission).
Time lapse recordings of fluorescence tagged proteins at endothelial cell junctions
Fluorescence-based live cell imaging is a powerful approach to study protein dynamics and to a certain degree also protein interactions due to its high sensitivity and spatiotemporal resolution. Time-lapse recordings of fluorescence microscopy, however, is limited by photobleaching and phototoxicity due to extensive light excitation. Photobleaching results in reduction or even total loss of the fluorescence signal, while phototoxicity relates to the generation of reactive oxygen species (ROS) that in turn can alter protein- , lipid- and nucleic acid-function47. Investigated cells might, therefore, become impaired and exhibit intercellular disintegration, artificial protein remodeling and/or inactivation of junction molecule dynamics. These problems might then generate misleading findings and lead to incorrect interpretations, and thus have to be taken in consideration. Fortunately, most of these drawbacks can be limited by carefully determining the a) minimal required illumination time to avoid production of high amounts of oxygen radicals, b) minimal image acquisition frequency, which gives the cells the chance to degrade oxygen radicals when combined with anti-oxidant treatment, c) usage of high sensitivity photo-detectors (e.g GaAsP detectors) for confocal laser microscopy, which allows less excitation power to be used, and/or d) use of other advanced microscopy techniques, such as spinning disc microscopy (SPDM)48. Use of these techniques, together with careful adjustment and optimizations of the settings, is in our experience the best approach for performing live cell imaging in the endothelium over many hours without pronounced toxicity or significant loss of signals.
3. Endothelial junction dynamics analyzed by fluorescence techniques
There are a number of excellent techniques that can be used to study cell junction dynamics, including protein turnover, protein displacement or the formation of subcellular structures such as JAIL. Established methods include single molecule tracking, fluorescence correlation spectroscopy (FCS), FRAP, classical confocal laser microscopy, spinning disc microscopy and, with increasing success, high resolution SIM. These techniques have been successfully applied to the study of cytoskeletal dynamics (e.g. actin and vimentin), intercellular adhesion and cell-matrix interaction in various cell types49-53.
Single molecule (particle) tracking
Single Molecule Tracking (SMT), also referred as Single Particle Tracking (SPT) or Single Dye Tracking (SDT), uses stably tagged molecules (e.g. organic dyes, fluorescent proteins, quantum dots, gold particles) that can be directly followed in real time using high resolution fluorescence microscopy (Nanoscopy). SMT is valuable for analyzing heterogeneities and molecule dynamics, which are usually obscured in averaged data sets54, at the nanoscopic level. For further information we refer the interested reader to several excellent manuscripts that have been recently published54-57.
SMT techniques have been successfully applied to study the mobility of free-floating E-cadherin58,59 and VE cadherin60 in the plasma membrane of living cells. The authors concluded that the lateral motility of cadherins is limited by the membrane cytoskeleton either by binding to the cytosolic domain cadherin (tethering) or by acting as a fence for unbound cadherin (corralling). In addition, it was shown, that E-cadherin forms small oligomers/clusters before the proteins became incorporated into the cell junctions58. So far these techniques have already revealed many details about the complex dynamcis of cadherins outside of the cell-cell contact regions. It is likely that future SMT studies examining cadherins incorporated into the cell junctions will give further valuable information about regulatory mechanisms.
Fluorescence recovery after photobleaching
FRAP is a suitable method for studying dynamic remodeling of fluorescence tagged molecules at the subcellular level, including at cell junctions. A region of interest is bleached using a high laser power without destruction of the molecule itself (bleaching), followed by time-dependent image acquisitions to measure the reappearance of fluorescence in the bleached area (recovery). Based on the biological question FRAP can be successfully used to estimate parameters such as lateral diffusion, protein exchange (e.g. by endo and exocytosis) or cytoskeletal remodeling. For example, actin recruitment to cell junctions due to increases in cAMP resulted in reduced VE-cadherin mobility61, which is accompanied by stabilization of endothelial barrier function62,63. Furthermore, a fluorescence tagged fusion protein consisting of VE-cadherin and α-catenin (VE-cad-a-C-GFP) expressed in endothelial cells displayed a reduced mobility in a latrunculin-dependent manner64. In addition, nectin, another adherens junction protein that forms trans-dimers between adjacent cells, interacts via afadin and drebrin with F-actin. FRAP analyses show a higher mobility of afadin in drebrin depleted cells65. Together, these FRAP data indicate that interaction with the actin cytoskeleton significantly modulates VE-cadherin dynamics.
It is well known that surface expression of junction proteins is dynamic, and like other membrane proteins they undergo both endo- and exocytosis (Figure 1 [D]). Endocytosis of VE-cadherin was described after treatment of cultured cells with bradykinin or even during angiogenesis in vivo in a phosphotyrosine - 658 and - 685 dependent manner66-68. FRAP analyses demonstrated an impaired recovery of VE-cadherin 1) in the presence of endocytosis inhibition, 2) after expression of tyrosine 658 mutated VE-cadherin and 3) after activation of Notch signaling66,67. In contrast, when 3 amino acids that were shown to be necessary for endocytosis are mutated in the juxtamembrane cytoplasmic domain of VE-cadherin, there is no effect on the recovery time following similar treatments69.
FRAP parameters have also been correlated with different VE-cadherin distribution patterns and endothelial barrier function. For example expression of epithelial cadherin (E-cadherin) in endothelial cells shows a heterogeneous distribution with low- and high-density segments. The low density adherens junctions displayed a spotty cadherin density, with higher cadherin mobility and reduced barrier function. In contrast, stimulation of endothelial cells with plasminogen activator inhibitor-1 (PAI-1)70 or thrombin23 reduces the endothelial barrier function, but the mobile fraction remained unchanged (after PAI treatment)70 or were even transiently reduced (after thrombin application)23. In addition, certain changes in VE-cadherin dynamics are necessary for the opening and recovery of endothelial junctions after thrombin treatment23,71. Together the data demonstrate that FRAP is a highly effective method to study local subcellular protein dynamics at cell junctions. Although FRAP gives valuable information about dynamics, it is limited to particular sites of endothelial junctions and estimations of heterogeneity are hard to perform (Figure 1 [E]). This is particularly the case in subconfluent cells in which the dynamics of VE-cadherin and actin are high, due to frequent and large JAIL formation72. In other words, when choosing FRAP analyzes to estimate dynamics of junction proteins in the endothelium, junction heterogeneity must be taken into consideration and the regions of interest must be carefully selected in such a way as to be statistically relevant. Such heterogeneity must also be taken into consideration when analyzing junction dynamics in the endothelium using photo-activatable molecules. For example, in a recent paper rac-1 was suggested to stabilize trans-dimer formation of VE-cadherin in mature endothelial cell junctions by counteracting acto-myosin mediated contraction73. However, in this study cell junction heterogeneity was not taken in consideration, and thus the entire impact of this study is still unclear. In summary, the data demonstrate that a simple correlation between junction protein mobility and endothelial barrier function and VE-cadherin dynamics does not exist.
4. Dynamics of VE-cadherin analyzed by spinning disc microscopy
Dynamic remodeling of VE-cadherin and catenins (i.e. VE-cadherin/catenin complexes) and their interaction with actin filaments are critical in controlling cell junction integrity, permeability and migration activity. Interaction between the VE-cadherin/catenin complex and actin filaments includes many regulatory mechanisms important in physiology and pathology, including activation of kinases, phosphatases, Rho-GTPases and phospho-caveolin-1, to name a few16,22,46,74,75. Published data and the associated mechanism identified to date have mostly been obtained from time-dependent snap shots76. Recent papers, however, have for the first time examined VE-cadherin dynamics directly in the endothelium using live cell imaging of fluorescence tagged VE-cadherin23,41,77. These papers demonstrate a quick and constitutive dynamic remodeling of VE-cadherin that was also strongly related to cell density. In confluent cultures, which comprise small polygonal cells with a small cell junction perimeter and less overall cell motility, VE-cadherin largely appears as a linear densely packed pattern, but also displays reticular, plaques and small interruptions with less dynamic remodeling. This VE-cadherin pattern can be compared to that of venous endothelium in situ. In contrast, VE-cadherin dynamics and cell migration activity is much higher in growing cells and the VE-cadherin pattern quickly switches between linear, interrupted, reticular and plaque like structures (Figure 1 [A, B], movie)23,41. The molecular background for the dramatically different VE-cadherin dynamics in confluent versus growing cultures could at a very fundamental level be directly related to the cell border length. This is due to the equal and cell growth-independent amount of VE-cadherin present in confluent and subconfluent (growing) endothelial cells41,78. Given that a constant amount of VE-cadherin is distributed at the cell junctions, the long cell junction perimeter in growing cells thus contains a reduced relative VE-cadherin concentration at junction sites, while the same amount of VE-cadherin is more concentrated in the junctions of small polygonal confluent cells with a reduced perimeter. Intriguingly, growing cells with high VE-cadherin dynamics display high cell motility, while confluent cells with reduced VE-cadherin dynamics are much less motile. This phenomenon could directly be related to the development of JAIL, which are actin-driven and ARP2/3 complex-dependent actin networks that forming plasma membrane protrusions appearing at subcellular junction sites were VE-cadherin is absent or displays a reduced relative concentration41. JAIL form new VE-cadherin adhesion sites and thus drive VE-cadherin dynamics. The detailed mechanism occurs in a sequential order and was analyzed by co-expression of both VE-cadherin-mCherry and EGFP-p20, a component of the actin related protein complex 2/3 (ARP2/3), which controls the actin network turnover of plasma membrane protrusions, including lamellipodia79 and JAIL41. In particular, at sites with a lower VE-cadherin concentration small locally restricted JAIL are formed that overlap adjacent cells, and in turn induce engagement of free floating VE-cadherin in the membrane, and thus the formation of VE-cadherin adhesion plaques23,41 (Figure 1, [A1, A2]). During JAIL retraction VE-cadherin clusters and becomes incorporated into the plasma membrane41. Thus, this process drives the high levels of VE-cadherin dynamics seen in growing cell cultures, where VE-cadherin interruptions are much more frequent than in confluent cells. The critical impact of VE-cadherin in overall cell junction dynamics was demonstrated by overexpression of VE-cadherin in growing (large perimeter) cells and by application of ARP2/3 inhibitors. Both manipulations blocked JAIL formation and decreased cell motility. It should be noted that JAIL differ significantly from classical lamellipodia, which regulate cell migration on a substrate requiring integrin mediated cell adhesion80,81. In contrast, JAIL form new VE-cadherin adhesion sites and control junction integrity and cell motility within a junction-forming cell layer. These mechanism could be uncovered using double expression of fluorescence tagged molecules and analysis by fluorescence live cell imaging, a procedure that allowed analyses of both spatial and temporal protein dynamics at the same time.
Quantitative VE-cadherin and actin dynamics at endothelial cell junctions
The recent advancements in viral-based expression of fluorescence tagged molecules in primary isolates of the endothelium and advanced microscopic techniques such as spinning disc microscopy as well as the development of GaAsP detectors now allow generation of image sequences over relatively long time periods with thousands of images being generated. While time-dependent snap shots can be quantitatively analyzed by manual selection and the use of image analysis software, such as ImageJ, the analysis of long time-lapse recordings is impossible to perform manually. For such experiments quantitative analysis requires software tools that are capable of automatically selecting the regions of interest in image sequences, followed by further mathematical processing to extract the parameters of interest.
Segmentation as a critical step in image analysis
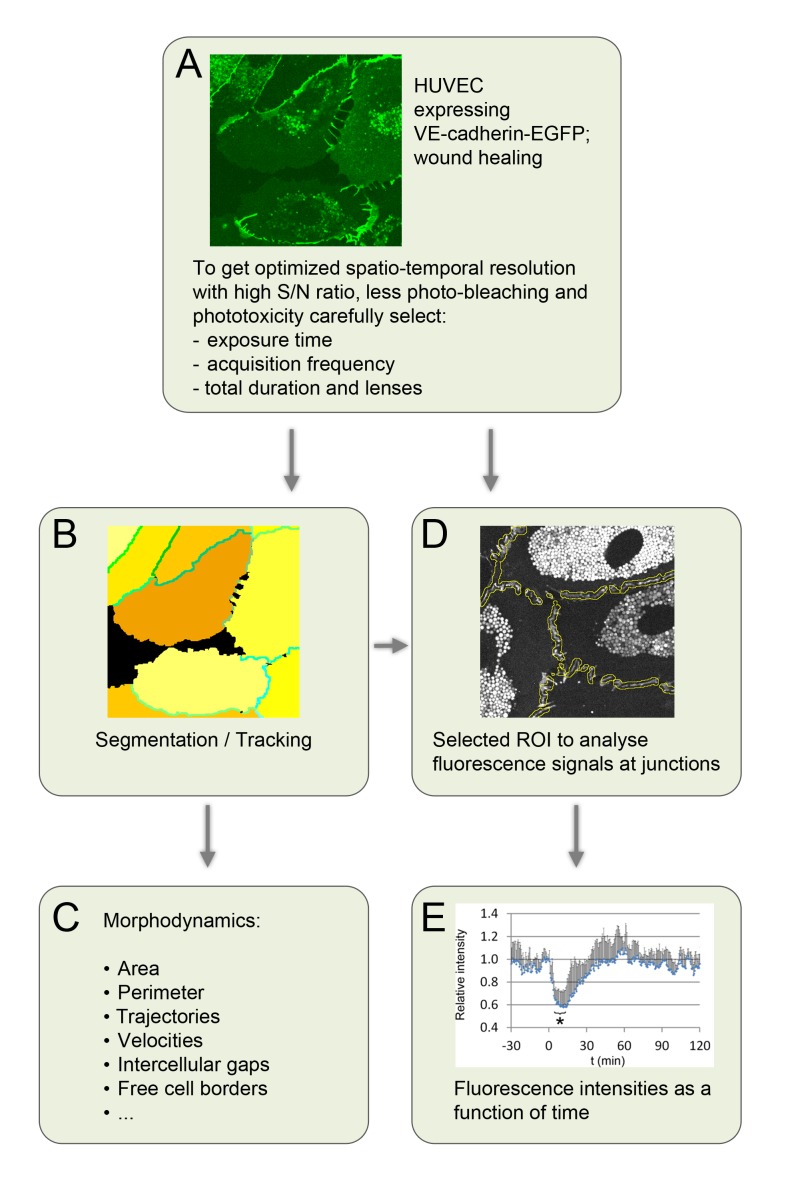
Image segmentation and object tracking is a common approach to the analysis of dynamic processes in time-lapse sequences (Figure 3 [A, B]). This approach has been widely used to analyze the morphological parameters of single cells, such as cell perimeter, cell area, cell motility or plasma protrusions, including lamellipodia and filopodia82-84 (Figure 3 [C]). While the segmentation of fluorescence tagged structures in single cells can often be accomplished using a simple threshold analysis, the segmentation of confluent monolayers is still a challenging task. However, in recent years several excellent segmentation tools have been developed and implemented to analyze time lapse sequences of fluorescence tagged E-cadherin in cultured cell and in drosophila in order to study morphogenesis85-87. Those tools are excellently suited to quantify cell morphology and cell fate, but reveal few or no details regarding subcellular junction organization. An easy to use tool for quantifying subcellular protrusions in single cells, known as Automated Detection and Analysis of Protrusions (ADAPT), was recently developed and is available as open source software as well82.
Figure 3. Scheme illustrating the work-flow for quantitative analyis of endothelial junctions and free cell border dynamics from image segmentations.
Image taken of HUVEC cultures that express VE-cadherin-EGFP after optimization of microscopic settings (A). The cell border extractor was applied and segments both cell junctions and individual cells, and subsequently assigns each cell and each cell junction a unique color-coded identification number (ID), (B). Many parameters can be extracted from the segmentations (C). The identified position of the color-coded intercellular junctions (B, different green colored lines) can be used to generate regions of interest (ROI) (D, yellow outlines) that in turn provide input parameters to quantify various parameters, for example fluorescence intensities as demonstrated for the EGFP-p20 intensity at endothelial junctions upon thrombin applications (E) (reproduced from our previous work23 (Seebach et al, Histochemistry and Cell Biology 2015, 144: 517-532) with permission).
Further, a novel image analyses tool especially developed to study quantitative endothelial cell junction dynamics called CellBorderTracker (CBT) has recently been developed. CBT consists of three basic tools: 1) CellBorderExtractor (CBE), which segments endothelial cells, 2) CellMapper (CM), which identifies and labels individual cells and each cell junction and 3) junction-kymograph (j-kymograph), which allows spatiotemporal analysis of entire cell junctions23 with the possibility to also identify junction heterogeneities23. The basic data obtained are well suited for the extraction of many secondary parameters, such as quantitative protein distribution, the generation of regions of interest, junction displacements, cell shape changes, migration velocities and the visualization of junction dynamics at the subcellular level, to name a few. In particular CBT is able to estimate subcellular activities, such as lamellipodia or JAIL formation, and determine the presence of intercellular gaps. The segmentation data provide a source for defining junctional regions of interest (jROI) (Figure 3 [D]), which together with local fluorescence intensity can be used to determine the amount and dynamics of fluorescence tagged proteins even at the subcellular level23,82. Alternatively, dynamic remodeling can also be quantified by analyzing the differences in gray values for each pixel in a defined jROI23,82.
An example of the application of CBT to the quantitative analyses of spatiotemporal dynamics (i.e. time lapse sequences) of fluorescence tagged proteins at endothelial junctions involves remodeling of the VE-cadherin/catenin complex and actin in response to stimuli such as TNF-a, thrombin and histamine18,19,21,88. Although, the VE-cadherin remodeling is a dynamic process it has until recently been investigated mostly using snap shots in a time-dependent manner. Recent work by independent groups has demonstrated the dynamic remodeling of VE-cadherin and actin using live cell imaging to generate high resolution time lapse recordings23,77. These experiments demonstrated that thrombin application blocks JAIL formation by rebalancing rac-1/rho activity, which in turn leads to VE-cadherin remodeling, stress fiber and gap-formation with a resulting breakdown in barrier function. Resealing of the monolayer was induced by the reappearance of JAIL formations that in turn form new VE-cadherin adhesion sites and closed the thrombin-induced gaps.
Subcellular heterogeneities of endothelial cell junctions
Analysis of global parameters do not account for the local subcellular heterogeneities of cell junctions in endothelium. We strongly believe, and there is increasing evidence to support, that spatiotemporal diversity of cell junction regulations plays a more prominent role than generally expected. This includes roles in planar polarization, inter-individual cell communication within the cell monolayer and transmigration of tumor cells or leukocytes trough the endothelial cell layer. Further, subcellular heterogeneities at endothelial cell junctions exert a critical influence on characteristic changes associated with stimulation or cell growth23,41. This includes the formation of linear junctions, interrupted junctions, reticular networks and very transient VE-cadherin plaques23. These diverse structures then display diverse dynamics and differentially respond to stimulations, a fact that makes quantitative analysis of cell junction dynamics a challenging task.
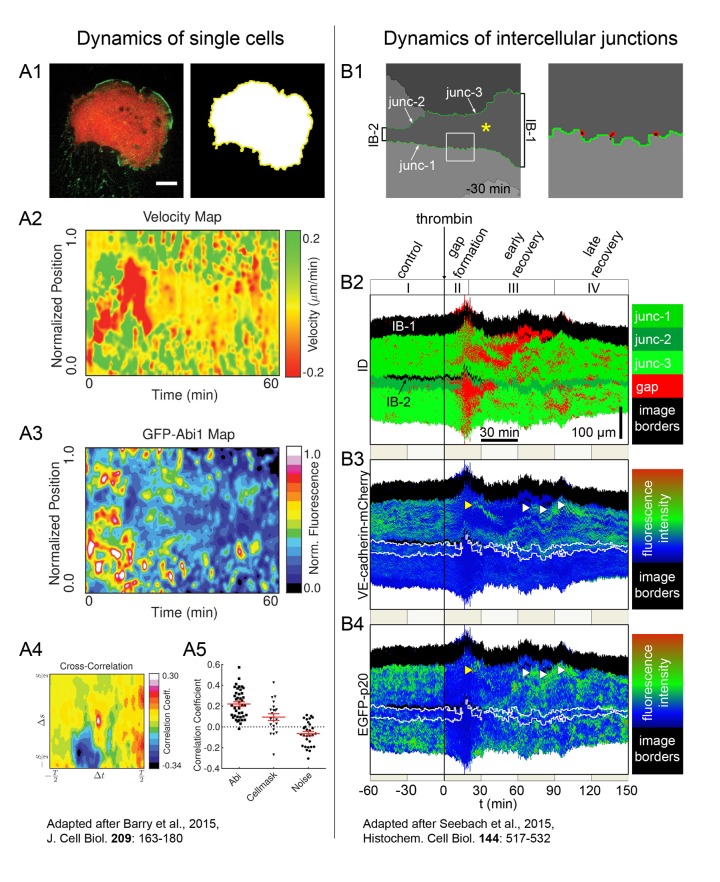
To document some of the spatial aspects of junction heterogeneity, signal intensities along boundaries of the segments can be plotted as a function of time using j-kymograph, in a manner comparable to a conventional kymograph23,82. In single migrating cells those maps have been useful to document subcellular protrusion activities at the cell borders of single cells (Figure 4 [A])82. An example demonstrating a velocity map and a GFP-Abi1 appearance map is shown (Figure 4 [A2, A3]). Furthermore, using a cross-correlation function that includes the velocity of membrane protrusions and the intensity of GFP-Abi1 gives clear information about local heterogeneities (Figure 4 [A4, A5])82. In junction-forming confluent endothelial cell cultures we used comparable approaches (jKymographs) to visualize the spatiotemporal correlation between the presence of the EGFP-p20 (a component of the ARP2/3 complex) and VE-cadherin-mCherry dynamics upon stimulation with the serine protease thrombin, an agent that cause a transient decrease in endothelial barrier function. It was demonstrated that thrombin transiently inhibits ARP2/3 complex mediated JAIL formation23,77, followed by VE-cadherin-mCherry remodeling. The spatiotemporal dynamics can be visualized by jKymographs with separate spatiotemporal appearance of intercellular gaps (Figure 4 [B1, B2]), VE-cadherin-mCherry gaps (Figure 4[B1, B2]), VE-cadherin-mCherry (Figure 4[B3]) and EGFP-p20 (Figure 4[B4])23. Together, the studies clearly demonstrate that subcellular heterogeneities in plasma membrane protrusions, such as filopodia, lamellipodia and JAIL, can be quantitatively estimated. Application of these tools will hopefully help us to better understand membrane dynamics and its regulation in more detail in the future.
Figure 4. Spatiotemporal quantitative analysis of cell protrusions in single HT1080 cells and junction dynamics in confluent endothelial monolayers.
Subcellular protrusion heterogeneities at the plasma membranes of individual GFP-Abi1 expressing HT1080 cells (A) and at cell junctions of HUVEC expressing EGFP-p20 and VE-cadherin-mCherry (B) quantified by generation of kymographs that automatically follow the dynamic displacements of the cell borders or cell junctions, respectively. Fluorescence of GFP-Abi1 expressing HT1080 cells and their segmentation (A1). The depicted example is reproduced from82 (Barry DJ et al., J Cell Biol 2015; 209: 163-180) with permission, and shows kymographs of the membrane protrusion velocity (A2) and the GFP-Abi1 intensity (A3) in single HT1080 cells. A cross correlation of these maps (A4) allows testing of the statistical significance (A5) between these two parameters. The next example is reproduced from our previous work23 (Seebach et al, Histochemistry and Cell Biology 2015, 144: 517-532) with permission, and displays the quantitative dynamics of three endothelial cell junctions of one cell within a monolayer. Cell junctions were segmented using the CBE (B1) and intercellular gaps are identified as red spots, as seen in the cropped area (B1, right panel). j-kymographs display the distribution over time of intercellular gaps (B2, red), VE-cadherin-mCherry intensity (B3) and EGFP-p20 (B4) intensity at the indicated cell junctions upon thrombin application. The spatiotemporal opening of cell junctions and the subsequent recovery is clearly seen.
5. Conclusion
In the last decade scientists have developed a number of ideas and models to understand endothelial cell junction dynamics, and particularly the dynamics of VE-cadherin, and their role in controlling cell integrity under physiological and pathological conditions. However, the regulation of many dynamic processes that control endothelial integrity, barrier function and shape changes are still unexplained. Viral expression systems now allow sufficient expression of fluorescence tagged proteins in the endothelium, and subsequently the performance of fluorescence live cell imaging to investigate and understand junction dynamics. The diverse fluorescence-based methods like FRAP, FRET, SMT, TIRF, SPDM and others that have been established now provide sufficient tools to study the molecular details of junction dynamics in the endothelium. However, understanding spatiotemporal diversity remains a challenging task as locally applied techniques, such as FRAP and FRET, deliver only locally restricted information, and cannot be simply transposed to the other parts of the cells. This becomes even more complicated as those local phenomena most probably depend upon local signaling and regulation. One possibility to better understand both local regulation and local structural remodeling involves tracking of entire cell junctions followed by subcellular analyses. Two nice open source software packages, ADAPT for quantitative analysis of subcellular heterogeneities in single cells82 and CBT for analyses of cell junctions in confluent endothelial cells, are now available23. Application of both tools will help to understand junction dynamics in more detail in the future. Furthermore, combined investigations using quantitative spatiotemporal dissolved high resolution fluorescence microscopy (e.g. two color approaches) together with biophysical methods, such as impedance spectroscopy, will allow us to not only directly observe the sequential steps of junction protein dynamics but also allow causal mechanistic conclusions to be drawn.
Bullet Points
◊ Fluorescence-based live cell imaging is the method of choice to study dynamic remodeling in living cells
◊ Live cell imaging uncovers the mechanisms of VE-cadherin dynamics not visible within snapshots
◊ Endothelial live cell imaging can be performed by expression of fluorescent tagged proteins, by using lenti-viral and/or adenoviral gene transfer
◊ Advanced microscopic techniques open new experimentations
◊ Novel software tools are available to quantify endothelial cell junction dynamics and detailed dynamcis of single cells
Acknowledgments
We gratefully acknowledge Allison Groseth for critical reading of the manuscript and editing. This work was supported by grants from the German Research Council to HS (DFG INST 2105/24–1 and SCHN 430/6–2), the Excellence Cluster Cells In Motion (CIM), and WWU-Münster flexible fund to J.S (FF-2016-15) and to HS (FF-2014-15). We apologize that not all published papers related to the current topic could be cited in this short overview.
Footnotes
Conflict of interests: The authors declare no conflicts of interest.
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. Simionescu M, Simionescu N, Palade G E. The Journal of cell biology. 1975;67(3):863–85. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. Simionescu M, Simionescu N, Palade G E. The Journal of cell biology. 1976;68(3):705–23. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebola virus: from discovery to vaccine. Feldmann Heinz, Jones Steven, Klenk Hans-Dieter, Schnittler Hans-Joachim. Nature reviews. Immunology. 2003;3(8):677–85. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- 4.The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Aird William C. Blood. 2003;101(10):3765–77. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 5.Breaking the wall: targeting of the endothelium by pathogenic bacteria. Lemichez Emmanuel, Lecuit Marc, Nassif Xavier, Bourdoulous Sandrine. Nature reviews. Microbiology. 2010;8(2):93–104. doi: 10.1038/nrmicro2269. [DOI] [PubMed] [Google Scholar]
- 6.Inflammation, endothelium, and coagulation in sepsis. Schouten Marcel, Wiersinga Willem Joost, Levi Marcel, van der Poll Tom. Journal of leukocyte biology. 2008;83(3):536–45. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 7.Intravascular immunity: the host-pathogen encounter in blood vessels. Hickey Michael J, Kubes Paul. Nature reviews. Immunology. 2009;9(5):364–75. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 8.The role of VE-cadherin in vascular morphogenesis and permeability control. Dejana Elisabetta, Vestweber Dietmar. Progress in molecular biology and translational science. 2013;116:119–44. doi: 10.1016/B978-0-12-394311-8.00006-6. [DOI] [PubMed] [Google Scholar]
- 9.Evolution: structural and functional diversity of cadherin at the adherens junction. Oda Hiroki, Takeichi Masatoshi. The Journal of cell biology. 2011;193(7):1137–46. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tight junction dynamics: the role of junctional adhesion molecules (JAMs). Garrido-Urbani S, Bradfield P F, Imhof B A. Cell and tissue research. 2014;355(3):701–15. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- 11.VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Harris Elizabeth S, Nelson W James. Current opinion in cell biology. 2010;22(5):651–8. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A novel endothelial-specific membrane protein is a marker of cell-cell contacts. Lampugnani M G, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco L P, Dejana E. The Journal of cell biology. 1992;118(6):1511–22. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Suzuki S, Sano K, Tanihara H. Cell regulation. 1991;2(4):261–70. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Breier G, Breviario F, Caveda L, Berthier R, Schnürch H, Gotsch U, Vestweber D, Risau W, Dejana E. Blood. 1996;87(2):630–41. [PubMed] [Google Scholar]
- 15.Control of endothelial barrier function by regulating vascular endothelial-cadherin. Vestweber Dietmar, Broermann Andre, Schulte Dörte. Current opinion in hematology. 2010;17(3):230–6. doi: 10.1097/MOH.0b013e328338664b. [DOI] [PubMed] [Google Scholar]
- 16.Endothelial cell-cell junctions: happy together. Dejana Elisabetta. Nature reviews. Molecular cell biology. 2004;5(4):261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 17.Actin filament dynamics and endothelial cell junctions: the Ying and Yang between stabilization and motion. Schnittler Hans, Taha Muna, Schnittler Maria Odenthal, Taha Abdallah Abu, Lindemann Nico, Seebach Jochen. Cell and tissue research. 2014;355(3):529–43. doi: 10.1007/s00441-014-1856-2. [DOI] [PubMed] [Google Scholar]
- 18.Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Beckers Cora M L, van Hinsbergh Victor W M, van Nieuw Amerongen Geerten P. Thrombosis and haemostasis. 2010;103(1):40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 19.Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Birukova Anna A, Tian Xinyong, Tian Yufeng, Higginbotham Katherine, Birukov Konstantin G. Molecular biology of the cell. 2013;24(17):2678–88. doi: 10.1091/mbc.E13-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Seebach Jochen, Donnert Gerald, Kronstein Romy, Werth Sebastian, Wojciak-Stothard Beata, Falzarano Darryl, Mrowietz Christof, Hell Stefan W, Schnittler Hans-J. Cardiovascular research. 2007;75(3):596–607. doi: 10.1016/j.cardiores.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. Wójciak-Stothard B, Entwistle A, Garg R, Ridley A J. Journal of cellular physiology. 1998;176(1):150–65. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Rho and Rac but not Cdc42 regulate endothelial cell permeability. Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley A J. Journal of cell science. 2001;114(Pt 7):1343–55. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 23.The CellBorderTracker, a novel tool to quantitatively analyze spatiotemporal endothelial junction dynamics at the subcellular level. Seebach Jochen, Taha Abdallah Abu, Lenk Janine, Lindemann Nico, Jiang Xiaoyi, Brinkmann Klaus, Bogdan Sven, Schnittler Hans-Joachim. Histochemistry and cell biology. 2015;144(6):517–32. doi: 10.1007/s00418-015-1357-8. [DOI] [PubMed] [Google Scholar]
- 24.The adherens junction: a mosaic of cadherin and nectin clusters bundled by actin filaments. Indra Indrajyoti, Hong Soonjin, Troyanovsky Regina, Kormos Bernadett, Troyanovsky Sergey. The Journal of investigative dermatology. 2013;133(11):2546–2554. doi: 10.1038/jid.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Principles of E-cadherin supramolecular organization in vivo. Truong Quang Binh-An, Mani Madhav, Markova Olga, Lecuit Thomas, Lenne Pierre-François. Current biology : CB. 2013;23(22):2197–207. doi: 10.1016/j.cub.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions. Wu Yao, Kanchanawong Pakorn, Zaidel-Bar Ronen. Developmental cell. 2015;32(2):139–54. doi: 10.1016/j.devcel.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Vestweber Dietmar, Winderlich Mark, Cagna Giuseppe, Nottebaum Astrid F. Trends in cell biology. 2009;19(1):8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 28.The Role of VE-Cadherin in Vascular Morphogenesis and Permeability Control. Dejana Elisabetta, Vestweber Dietmar. Progress in Molecular Biology and Translational Science. 2013:119-144. doi: 10.1016/B978-0-12-394311-8.00006-6. [DOI] [PubMed] [Google Scholar]
- 29.Structural and functional aspects of intercellular junctions in vascular endothelium. Schnittler H.-J. Basic Research in Cardiology. 1998;93:s030-s039. doi: 10.1007/s003950050205. [DOI] [PubMed] [Google Scholar]
- 30.VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Harris E., Nelson W. Current opinion in cell biology. 2010;22(5):651–658. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Probing cellular processes by long-term live imaging--historic problems and current solutions. Coutu Daniel L, Schroeder Timm. Journal of cell science. 2013;126(Pt 17):3805–15. doi: 10.1242/jcs.118349. [DOI] [PubMed] [Google Scholar]
- 32.Live-cell microscopy - tips and tools. Frigault Melanie M, Lacoste Judith, Swift Jody L, Brown Claire M. Journal of cell science. 2009;122(Pt 6):753–67. doi: 10.1242/jcs.033837. [DOI] [PubMed] [Google Scholar]
- 33.Fluorescent protein tools for studying protein dynamics in living cells: a review. Day Richard N, Schaufele Fred. Journal of biomedical optics. 2008;13(3):031202. doi: 10.1117/1.2939093. [DOI] [PubMed] [Google Scholar]
- 34.Genetically encodable fluorescent biosensors for tracking signaling dynamics in living cells. Newman Robert H, Fosbrink Matthew D, Zhang Jin. Chemical reviews. 2011;111(5):3614–66. doi: 10.1021/cr100002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biological imaging software tools. Eliceiri Kevin W, Berthold Michael R, Goldberg Ilya G, Ibáñez Luis, Manjunath B S, Martone Maryann E, Murphy Robert F, Peng Hanchuan, Plant Anne L, Roysam Badrinath, Stuurman Nico, Stuurmann Nico, Swedlow Jason R, Tomancak Pavel, Carpenter Anne E. Nature methods. 2012;9(7):697–710. doi: 10.1038/nmeth.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caught in the act: quantifying protein behaviour in living cells. Lidke Diane S, Wilson Bridget S. Trends in cell biology. 2009;19(11):566–74. doi: 10.1016/j.tcb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imaging methodologies for systems biology. Smith Sarah E, Slaughter Brian D, Unruh Jay R. Cell adhesion & migration. 2014;8(5):468–77. doi: 10.4161/cam.29152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genetic manipulation of endothelial cells by viral vectors. Lindemann Dirk, Schnittler Hans. Thrombosis and haemostasis. 2009;102(6):1135–43. doi: 10.1160/TH09-10-0724. [DOI] [PubMed] [Google Scholar]
- 39.Bystander Effect Induced by Electroporation is Possibly Mediated by Microvesicles and Dependent on Pulse Amplitude, Repetition Frequency and Cell Type. Prevc Ajda, Bedina Zavec Apolonija, Cemazar Maja, Kloboves-Prevodnik Veronika, Stimac Monika, Todorovic Vesna, Strojan Primoz, Sersa Gregor. The Journal of membrane biology. 2016;249(5):703–711. doi: 10.1007/s00232-016-9915-0. [DOI] [PubMed] [Google Scholar]
- 40.Genetic manipulation of endothelial cells by viral vectors. Lindemann Dirk, Schnittler Hans. Thrombosis and Haemostasis. 2009 doi: 10.1160/TH09-10-0724. [DOI] [PubMed] [Google Scholar]
- 41.ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Taha A. A., Taha M., Seebach J., Schnittler H.-J. Molecular Biology of the Cell. 2013;25(2):245-256. doi: 10.1091/mbc.E13-07-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebola Virus Enters Host Cells by Macropinocytosis and Clathrin-Mediated Endocytosis. Aleksandrowicz Paulina, Marzi Andrea, Biedenkopf Nadine, Beimforde Nadine, Becker Stephan, Hoenen Thomas, Feldmann Heinz, Schnittler Hans-Joachim. The Journal of Infectious Diseases. 2011;204(suppl_3):S957-S967. doi: 10.1093/infdis/jir326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. Huveneers Stephan, Oldenburg Joppe, Spanjaard Emma, van der Krogt Gerard, Grigoriev Ilya, Akhmanova Anna, Rehmann Holger, de Rooij Johan. The Journal of cell biology. 2012;196(5):641–52. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basal-to-apical cadherin flow at cell junctions. Kametani Yoshiko, Takeichi Masatoshi. Nature cell biology. 2007;9(1):92–8. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- 45.Tyrosine phosphorylation and the small GTPase rac cross-talk in regulation of endothelial barrier function. Seebach Jochen, Mädler Hans-Jürgen, Wojciak-Stothard Beata, Schnittler Hans-Joachim. Thrombosis and haemostasis. 2005;94(3):620–9. doi: 10.1160/TH05-01-0015. [DOI] [PubMed] [Google Scholar]
- 46.Caveolin-1 opens endothelial cell junctions by targeting catenins. Kronstein Romy, Seebach Jochen, Grossklaus Sylvia, Minten Carsten, Engelhardt Britta, Drab Marek, Liebner Stefan, Arsenijevic Yvan, Taha Abdallah Abu, Afanasieva Tatiana, Schnittler Hans-Joachim. Cardiovascular research. 2012;93(1):130–40. doi: 10.1093/cvr/cvr256. [DOI] [PubMed] [Google Scholar]
- 47.Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Hoebe R A, Van Oven C H, Gadella T W J, Dhonukshe P B, Van Noorden C J F, Manders E M M. Nature biotechnology. 2007;25(2):249–53. doi: 10.1038/nbt1278. [DOI] [PubMed] [Google Scholar]
- 48.Spinning-disk confocal microscopy -- a cutting-edge tool for imaging of membrane traffic. Nakano Akihiko. Cell structure and function. 2002;27(5):349–55. doi: 10.1247/csf.27.349. [DOI] [PubMed] [Google Scholar]
- 49.Molecular flow quantified beyond the diffraction limit by spatiotemporal image correlation of structured illumination microscopy data. Ashdown George W, Cope Andrew, Wiseman Paul W, Owen Dylan M. Biophysical journal. 2014;107(9):L21–3. doi: 10.1016/j.bpj.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spatiotemporal image correlation spectroscopy (STICS) theory, verification, and application to protein velocity mapping in living CHO cells. Hebert Benedict, Costantino Santiago, Wiseman Paul W. Biophysical journal. 2005;88(5):3601–14. doi: 10.1529/biophysj.104.054874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The Molecular Architecture of Cell Adhesion: Dynamic Remodeling Revealed by Videonanoscopy. Sergé Arnauld. Frontiers in cell and developmental biology. 2016;4:36. doi: 10.3389/fcell.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inside view of cell locomotion through single-molecule: fast F-/G-actin cycle and G-actin regulation of polymer restoration. Watanabe Naoki. Proceedings of the Japan Academy. Series B, Physical and biological sciences. 2010;86(1):62–83. doi: 10.2183/pjab.86.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Helmke B P, Goldman R D, Davies P F. Circulation research. 2000;86(7):745–52. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- 54.Tracking single molecules at work in living cells. Kusumi Akihiro, Tsunoyama Taka A, Hirosawa Kohichiro M, Kasai Rinshi S, Fujiwara Takahiro K. Nature chemical biology. 2014;10(7):524–32. doi: 10.1038/nchembio.1558. [DOI] [PubMed] [Google Scholar]
- 55.A starting point for fluorescence-based single-molecule measurements in biomolecular research. Gust Alexander, Zander Adrian, Gietl Andreas, Holzmeister Phil, Schulz Sarah, Lalkens Birka, Tinnefeld Philip, Grohmann Dina. Molecules (Basel, Switzerland) 2014;19(10):15824–65. doi: 10.3390/molecules191015824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Exploring dynamics in living cells by tracking single particles. Levi Valeria, Gratton Enrico. Cell biochemistry and biophysics. 2007;48(1):1–15. doi: 10.1007/s12013-007-0010-0. [DOI] [PubMed] [Google Scholar]
- 57.A review of progress in single particle tracking: from methods to biophysical insights. Manzo Carlo, Garcia-Parajo Maria F. Reports on progress in physics. Physical Society (Great Britain) 2015;78(12):124601. doi: 10.1088/0034-4885/78/12/124601. [DOI] [PubMed] [Google Scholar]
- 58.Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Iino R, Koyama I, Kusumi A. Biophysical journal. 2001;80(6):2667–77. doi: 10.1016/S0006-3495(01)76236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corralling and tethering by the membrane skeleton. Sako Y, Nagafuchi A, Tsukita S, Takeichi M, Kusumi A. The Journal of cell biology. 1998;140(5):1227–40. doi: 10.1083/jcb.140.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. Baumgartner Werner, Schütz Gerhard J, Wiegand Johannes, Golenhofen Nikola, Drenckhahn Detler. Journal of cell science. 2003;116(Pt 6):1001–11. doi: 10.1242/jcs.00322. [DOI] [PubMed] [Google Scholar]
- 61.Vascular endothelial-cadherin stabilizes at cell-cell junctions by anchoring to circumferential actin bundles through alpha- and beta-catenins in cyclic AMP-Epac-Rap1 signal-activated endothelial cells. Noda Kazuomi, Zhang Jianghui, Fukuhara Shigetomo, Kunimoto Satoshi, Yoshimura Michihiro, Mochizuki Naoki. Molecular biology of the cell. 2010;21(4):584–96. doi: 10.1091/mbc.E09-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Use of electrochemical impedance measurements to monitor beta-adrenergic stimulation of bovine aortic endothelial cells. Wegener J, Zink S, Rösen P, Galla H. Pflugers Archiv : European journal of physiology. 1999;437(6):925–34. doi: 10.1007/s004240050864. [DOI] [PubMed] [Google Scholar]
- 63.cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Schlegel Nicolas, Waschke Jens. Cell and tissue research. 2014;355(3):587–96. doi: 10.1007/s00441-013-1755-y. [DOI] [PubMed] [Google Scholar]
- 64.Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. Schulte Dörte, Küppers Verena, Dartsch Nina, Broermann Andre, Li Hang, Zarbock Alexander, Kamenyeva Olena, Kiefer Friedemann, Khandoga Alexander, Massberg Steffen, Vestweber Dietmar. The EMBO journal. 2011;30(20):4157–70. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drebrin preserves endothelial integrity by stabilizing nectin at adherens junctions. Rehm Kerstin, Panzer Linda, van Vliet Vanessa, Genot Elisabeth, Linder Stefan. Journal of cell science. 2013;126(Pt 16):3756–69. doi: 10.1242/jcs.129437. [DOI] [PubMed] [Google Scholar]
- 66.Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Orsenigo Fabrizio, Giampietro Costanza, Ferrari Aldo, Corada Monica, Galaup Ariane, Sigismund Sara, Ristagno Giuseppe, Maddaluno Luigi, Koh Gou Young, Franco Davide, Kurtcuoglu Vartan, Poulikakos Dimos, Baluk Peter, McDonald Donald, Grazia Lampugnani Maria, Dejana Elisabetta. Nature communications. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Bentley Katie, Franco Claudio Areias, Philippides Andrew, Blanco Raquel, Dierkes Martina, Gebala Véronique, Stanchi Fabio, Jones Martin, Aspalter Irene M, Cagna Guiseppe, Weström Simone, Claesson-Welsh Lena, Vestweber Dietmar, Gerhardt Holger. Nature cell biology. 2014;16(4):309–21. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 68.VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Gavard Julie, Gutkind J Silvio. Nature cell biology. 2006;8(11):1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 69.p120-catenin binding masks an endocytic signal conserved in classical cadherins. Nanes Benjamin A, Chiasson-MacKenzie Christine, Lowery Anthony M, Ishiyama Noboru, Faundez Victor, Ikura Mitsuhiko, Vincent Peter A, Kowalczyk Andrew P. The Journal of cell biology. 2012;199(2):365–80. doi: 10.1083/jcb.201205029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plasminogen Activator Inhibitor-1 Controls Vascular Integrity by Regulating VE-Cadherin Trafficking. Daniel Anna E, Timmerman Ilse, Kovacevic Igor, Hordijk Peter L, Adriaanse Luc, Paatero Ilkka, Belting Heinz-Georg, van Buul Jaap D. PloS one. 2015;10(12):e0145684. doi: 10.1371/journal.pone.0145684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Timmerman Ilse, Hoogenboezem Mark, Bennett Anton M, Geerts Dirk, Hordijk Peter L, van Buul Jaap D. Molecular biology of the cell. 2012;23(21):4212–25. doi: 10.1091/mbc.E12-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Abu Taha Abdallah, Taha Muna, Seebach Jochen, Schnittler Hans-J. Molecular biology of the cell. 2014;25(2):245–56. doi: 10.1091/mbc.E13-07-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. Daneshjou Nazila, Sieracki Nathan, van Nieuw Amerongen Geerten P, Conway Daniel E, Schwartz Martin A, Komarova Yulia A, Malik Asrar B. The Journal of cell biology. 2015;209(1):181. doi: 10.1083/jcb.20140910803202015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.VE-PTP controls blood vessel development by balancing Tie-2 activity. Winderlich Mark, Keller Linda, Cagna Giuseppe, Broermann Andre, Kamenyeva Olena, Kiefer Friedemann, Deutsch Urban, Nottebaum Astrid F, Vestweber Dietmar. The Journal of cell biology. 2009;185(4):657–71. doi: 10.1083/jcb.200811159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. Esser S, Lampugnani M G, Corada M, Dejana E, Risau W. Journal of cell science. 1998;111 ( Pt 13):1853–65. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 76.Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends in cell biology 2009, 19(1): 8-15. Vestweber D., Winderlich M., Cagna G., Nottebaum A. Trends in Cell Biology. 2009;19(1):8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Involvement of Local Lamellipodia in Endothelial Barrier Function. Breslin Jerome W., Zhang Xun E., Worthylake Rebecca A., Souza-Smith Flavia M. PLOS ONE. 2015;10(2):e0117970. doi: 10.1371/journal.pone.0117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). Lampugnani M G, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The Journal of cell biology. 1995;129(1):203–17. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arp2/3 complex interactions and actin network turnover in lamellipodia. Lai Frank P L, Szczodrak Malgorzata, Block Jennifer, Faix Jan, Breitsprecher Dennis, Mannherz Hans G, Stradal Theresia E B, Dunn Graham A, Small J Victor, Rottner Klemens. The EMBO journal. 2008;27(7):982–92. doi: 10.1038/emboj.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.CELL BIOLOGY:Cell Migration--Movin' On. Horwitz A. R. Science. 1999;286(5442):1102-1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 81.Kinetic Model for Integrin-mediated Adhesion Release During Cell Migration. Palecek Sean P., Horwitz Alan F., Lauffenburger Douglas A. Annals of Biomedical Engineering. 1999;27(2):219-235. doi: 10.1114/1.176. [DOI] [PubMed] [Google Scholar]
- 82.Open source software for quantification of cell migration, protrusions, and fluorescence intensities. Barry David J, Durkin Charlotte H, Abella Jasmine V, Way Michael. The Journal of cell biology. 2015;209(1):163–80. doi: 10.1083/jcb.201501081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Analysis of cell movement by simultaneous quantification of local membrane displacement and fluorescent intensities using Quimp2. Bosgraaf Leonard, van Haastert Peter J M, Bretschneider Till. Cell motility and the cytoskeleton. 2009;66(3):156–65. doi: 10.1002/cm.20338. [DOI] [PubMed] [Google Scholar]
- 84.CellGeo: a computational platform for the analysis of shape changes in cells with complex geometries. Tsygankov Denis, Bilancia Colleen G, Vitriol Eric A, Hahn Klaus M, Peifer Mark, Elston Timothy C. The Journal of cell biology. 2014;204(3):443–60. doi: 10.1083/jcb.201306067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segmentation and tracking of adherens junctions in 3D for the analysis of epithelial tissue morphogenesis. Cilla Rodrigo, Mechery Vinodh, Hernandez de Madrid Beatriz, Del Signore Steven, Dotu Ivan, Hatini Victor. PLoS computational biology. 2015;11(4):e1004124. doi: 10.1371/journal.pcbi.1004124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.EpiTools: An Open-Source Image Analysis Toolkit for Quantifying Epithelial Growth Dynamics. Heller Davide, Hoppe Andreas, Restrepo Simon, Gatti Lorenzo, Tournier Alexander L, Tapon Nicolas, Basler Konrad, Mao Yanlan. Developmental cell. 2016;36(1):103–116. doi: 10.1016/j.devcel.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Enabling user-guided segmentation and tracking of surface-labeled cells in time-lapse image sets of living tissues. Mashburn David N, Lynch Holley E, Ma Xiaoyan, Hutson M Shane. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2012;81(5):409–18. doi: 10.1002/cyto.a.22034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rho GTPases and the regulation of endothelial permeability. Wojciak-Stothard Beata, Ridley Anne J. Vascular pharmacology. 2002;39(4-5):187–99. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]