Abstract
Background: Gold-induced autologous cytokine (GOLDIC) treatment is usually used in the therapy of the inflammatory musculoskeletal disorders (e.g. osteoarthritis in humans) and is able to modulate the inflammatory reaction. Moreover, governed by chemokines and cytokines, the complex inflammatory response after an acute myocardial infarction (MI), the main cause of death worldwide, plays an important role in the preservation of heart function. Therefore, we hypothesized that GOLDIC could also have an important role in ventricular remodeling after MI. Methods: Myocardial infarction was induced in mice and GOLDIC-enriched serum was directly injected directly in the infarcted tissue. Four weeks later, the function of the heart, as well as the infarction size and the scar composition were analyzed. Statistical analysis was performed with Prism 6.1 software (GraphPad), using 1-way ANOVA, followed by Newman-Keuls post-hoc-test, as indicated. Data are represented as mean ± SEM. Results: Four weeks after MI, GOLDIC-treated mice show significantly decreased heart function and higher infarction size compared to the control group. Immunohistochemistry reveals a significantly increased number of myofibroblasts, correlating with higher collagen content in the infarcted area. Despite impaired heart function, angiogenesis in the GOLDIC-treated group is improved compared with the control, due to the increased vascular endothelial growth factor (VEGF) in the GOLDIC serum. Conclusions: In conclusion, GOLDIC treatment impairs the ventricular remodeling, worsening the heart function. Therefore, these systemic effects should be taken into account when new therapies are designed for the musculoskeletal disorders.
Keywords: GOLDIC, cytokines, myocardial infarction, inflammation
INTRODUCTION
As one of the most frequent causes of morbidity and mortality in western countries, myocardial infarction (MI) triggers a complex inflammatory response, governed by different chemokines and cytokines1. These various factors recruit immune cells, which are responsible for the initiation, the control and the modulation of repair processes, and the remodeling of the injured heart tissue after an infarction2. The mechanism behind this progress defines the scar formation and, consequently, the cardiac function after MI.
Gold has been used for centuries in ancient Egypt and China for the treatment of different mental, spiritual, and physical diseases. Recently, its beneficial effects in patients suffering from rheumatoid arthritis were proved3. Direct administration of gold particles suppressed NF-kappa B pathway activation, reduced production of proinflammatory cytokines, such as TNF-alpha, interleukin-1 and interleukin-6, and enhanced secretion/production of anti-inflammatory factors, such as interleukin-44,5. However, many patients exhibit adverse immune-mediated side effects, such as allergy, renal, skin or oral mucosal complications, necessitating an immediate interruption of the therapy.
Small gold particles incubated with blood lead to an enrichment in different cytokines in the serum. The main effect of this Gold-induced autologous cytokines (GOLDIC) procedure is the significant increase of gelsolin6, a protein responsible for the regulation of actin filaments in particular, and cell structure and metabolism in general7. Thus, it is supposed to decrease the side effects8. GOLDIC are used intra-articular in the treatment of musculoskeletal disorders, such as osteoarthritis, modulating successfully the inflammatory response8.
Therefore, we hypothesized that GOLDIC might be able to influence the inflammatory reaction and to significantly contribute to the ventricular remodeling after MI.
MATERIALS AND METHODS
Production of Serum
The mouse blood was collected in a tube and pretreated with gold particles according to the manufacturer´s protocol (Arthrogen GmbH, Germany)6. Briefly, the blood was incubated at 37°C for 24 hours. After centrifugation at 5000rpm for 10 minutes, the serum containing gold-induced autologous cytokines was extracted. Untreated serum served as control.
Myocardial Infarction Induction and Treatment
Eight to ten weeks old male C57Bl/6N mice were intubated under general anesthesia (100 mg/kg bodyweight ketamine, 10 mg/kg bodyweight xylazine, i.p.) and analgesia (0,1 mg/kg bodyweight buprenorphine, s.c.). They were ventilated using a rodent respirator with positive-pressure and oxygen. Isoflurane was administered at a dose of 0,25-2ml/min during the procedure to assure proper anaesthetization. A left side thoracotomy was performed and MI was induced by permanent ligation of the proximal left anterior descending artery9. After MI induction, visible by a to-grey colour-change of the affected heart tissue, 30µl GOLDIC-enriched or control serum was injected into the border zone using a 33-gauge needle. The ribs, muscle and skin incisions were closed with separate sutures. The analgesia was continued for five days after MI induction by daily injection of 0,1mg/kg bodyweight buprenorphine subcutaneously. All animal experiments and study protocols were approved by local authorities (Landesamt für Natur, Umwelt- und Verbraucherschutz Nordrhein-Westfalen, reference number: 84-02.04.2013.A185), complying with European and German animal protection laws.
Echocardiography
Before and four weeks after MI induction, left ventricular heart function was assessed by echocardiography performed on a small-animal ultrasound imager (Vevo 770, FUJIFILM Visualsonics, Toronto, Canada). Measurements were recorded in B-Mode (2D-realtime) and M-Mode. During the procedure, the mice were anaesthetized by inhalation narcosis, with 2ml/min isoflurane. The ejection fraction (EF), fractional shortening (FS), cardiac output (CO), heart rate (HR) and left ventricular mass were recorded and analyzed using the VisualSonics Software.
Histology and immunohistochemistry
Four weeks after MI induction, infarction size was evaluated. Mice were anaesthetized (100 mg/kg bodyweight ketamine, 10 mg/kg bodyweight xylazine, i.p.). Hearts were excised, fixed in formalin and embedded in paraffin. Serial sections (10-12 sections per heart, 400 µm apart, up to the mitral valve) were stained with Gomori’s 1-step trichrome stain. The infarcted area was determined for all sections using Diskus software (Hilgers, Königswinter, Germany) and expressed as percentage of total left ventricular volume. Blue-stained collagen content was analyzed with Cell P Software (Olympus, Hamburg, Germany) and expressed as a percentage of the infarcted area. Additional sections (3 sections per heart, 400 µm apart) were stained to analyze within the infarcted area the content of myofibroblasts (smooth muscle actin, DAKO, Germany) and CD31-positive capillaries (CD31, Santa Cruz, Santa Cruz, CA). Positive-stained cells or vessels were counted in six different fields per section and expressed as cells or vessels per mm2.
ELISA
Enzyme Linked Immunosorbent Assay (ELISA) was performed from serum samples using the DuoSet ELISA Development System kit for mouse Keratinocyte-derived chemokine (KC) (R&D Systems), vascular endothelial growth factor (VEGF), stromal derived factor (SDF)-1 and monocytes chemotactic protein (MCP)-1.
Statistical analysis
Data are represented as mean ± SEM. Statistical analysis was performed with Prism 6.1 software (GraphPad). Means of two groups were compared with unpaired Student-t test, while for more than two groups, 1-way ANOVA followed by Newman-Keuls post-hoc-test were used, after passing D'Agostino and Pearson omnibus normality test, as indicated. P-values of <0.05 were considered significant.
RESULTS
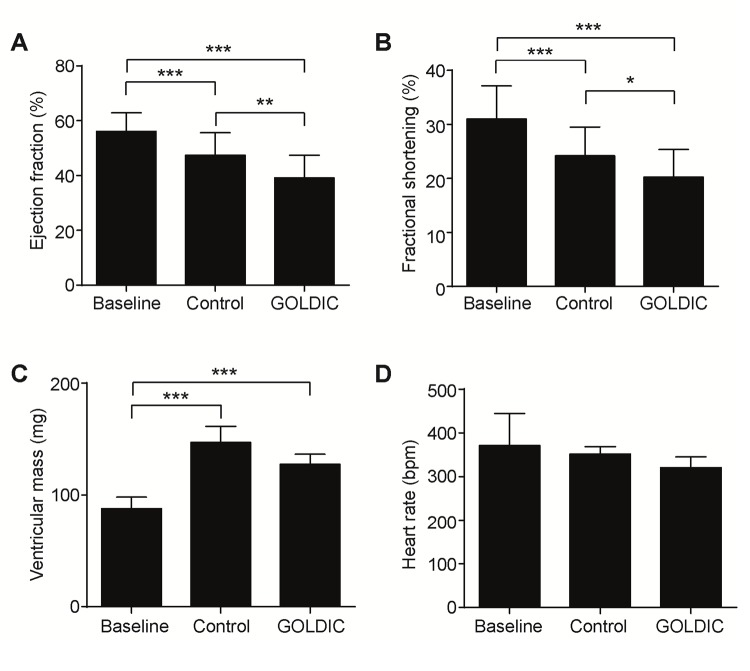
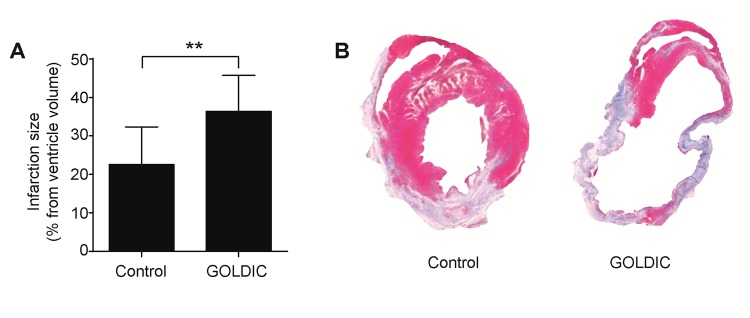
Before and 4 weeks after MI induction and GOLDIC injection, the cardiac function was measured by echocardiography. The ejection fraction (Figure 1 [A]) was significantly decreased in the GOLDIC-treated group (39.2 ± 1.7%, n=8) compared to the control group (47.5 ± 1.6%, n=7, p<0.05), as well as the fractional shortening (Figure 1 [B], 20.2 ± 1.2% vs. control 24.2+-1.1%, p<0.05) in the GOLDIC-treated group in comparison to the control group. The V ventricular mass (Figure 1 [C]), heart rate (Figure 1 [D]) and cardiac output (11.8 ± 1.6 ml/min vs. control 15.5 ± 0.9 ml/min) did not differ between the groups after MI induction. All these data pointed out an unexpected reduction of left ventricular heart function after GOLDIC-treatment. The echocardiographic results were confirmed by histological analysis of the left ventricular infarction size, which was significantly higher in GOLDIC-treated mice than in the control group (36.4 ± 2.9%, n=8, vs. control 22.6 ± 2.8%, n=7, p<0.05, Figure 2).
Figure 1. Echocardiographic measurements.
(A) Ejection fraction measured before (baseline) as well as four weeks after MI showed significant reduction in GOLDIC-treated mice compared to control group. (B) Fractional shortening confirms the reduced pump function of the GOLDIC-treated group. (C) Ventricular mass was reduced after MI, but did not differ between the experimental groups. (D) Heart rate was constant in all mice during the experiment.
Figure 2. Histological assessment of infarction size.
(A) Infarction size was significantly increased in the GOLDIC-treated mice compared to the control group. (B) Representative images of Gomori’ staining from control and GOLDIC-treated mice (red - viable myocardium, blue - infarction scar).
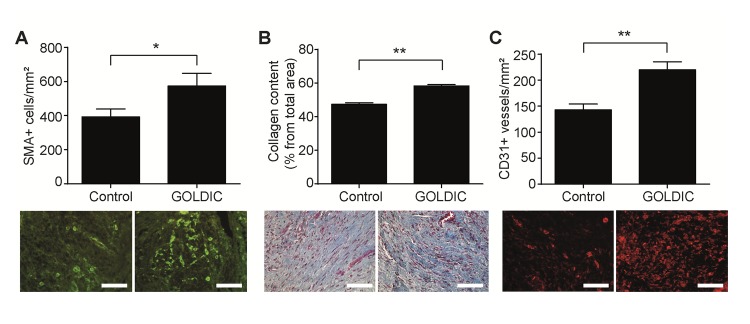
Immunofluorescent staining of smooth muscle actin positive cells showed that the number of myofibroblasts was significantly increased in the GOLDIC-treated group, associated with a higher content of collagen in the scar (Figure 3 [A]). Surprisingly, we found an increase in angiogenesis (CD31 positive newly-formed vessels) in the GOLDIC-treated group (6.0 ± 0.5 vessels/field, p<0.05) compared to the control mice (9.2 ± 0.7 vessels/field, p<0.05, Figure 3 [B]). This was associated with high levels of Vascular Endothelial Growth Factor (VEGF, 555±50 pg/ml) and Stromal-cell Derived Factor (SDF-1, 93±2 pg/ml), in the GOLDIC-serum, measured by ELISA.
Figure 3. Immunohistological analysis of the infarcted area.
(A) Number of myofibroblasts increased in the GOLDIC-treated group compared to the control group, as shown by the smooth muscle actin positive staining (green, scale bar 50 µm). (B) This was associated with increased collagen content (blue, scale bar 50 µm). (C) Angiogenesis determined by counting CD31 positive newly-formed vessels is increased in GOLDIC-treated mice compared to the control group (red, scale bar 50 µm).
CONCLUSION
In this study, mice were injected with GOLDIC-serum into their injured myocardium after MI induction. Four weeks after the procedure, we found an impaired ventricular remodeling and worsening of the heart function after GOLDIC-treatment, despite increased angiogenesis.
These results sustain the thesis that anti-inflammatory therapies are not always beneficial for the healing of the heart after MI. It was always believed that reducing the initial inflammation, mostly induced by neutrophils, should improve the ventricular scar remodeling and preserve the heart function1. However, more and more new data demonstrate that impairing the neutrophil recruitment might have double-edge effects, since it interferes with the healing response, polarizing macrophages towards a reparative phenotype10-12. Neutrophil depletion in mice induces an increased myofibroblasts proliferation and collagen content, associated with impaired heart function11, which we also observed in the present study. Moreover, in clinical studies the use of anti-inflammatory therapies caused impaired wound healing and increased cardiac rupture, despite a decrease in tissue damage13,14- a situation which forces physicians to be more careful when anti-inflammatory strategies are designed for patients suffering an acute myocardial infarction. In the present study, we demonstrate that the direct transplantation of GOLDIC-enriched serum into the heart exacerbates the worsening effects. However, we pointed out possible and important side effects of the anti-inflammatory therapy, which should be critically scrutinized during treatment to avoid severe complications, especially in patients with cardiac disease.
Interestingly, we found an increased angiogenesis in the infarcted area after GOLDIC treatment. In general, it was believed that increased angiogenesis would automatically preserve or improve heart function after MI. Therefore, many therapies were developed to increase the vessel formation in the infarcted area15-18. However, some studies demonstrate that there is no direct correlation between the neo-angiogenesis and the improved heart function12,19. Thus, we conclude for this study, that the pro-angiogenic factors induced by the GOLDIC-procedure in serum have no beneficial effects on the healing after MI. Furthermore, due to technical reasons, main components of GOLDIC-treated serum identified in human (Gelsolin, G-CSF, S-CSF) could not be determined in mouse, therefore, the applicability of the results is probably critical to the human situation.
In conclusion, since GOLDIC seems to be beneficial in the treatment of many inflammatory diseases, the negative effects on the heart function should be taken into account, when systemic therapies with GOLDIC-serum are performed.
Acknowledgments
This study was supported by the Interdisciplinary Centre for Clinical Research IZKF Aachen (junior research group to E.A.L.). GOLDIC Kits were provided by Arthrogen GmbH, Ringsee, Germany. All animal experiments and study protocols were approved by local authorities (Landesamt für Natur, Umwelt- und Verbraucherschutz Nordrhein-Westfalen, reference number: 84-02.04.2013.A185), complying with European and German animal protection laws. All data generated or analyzed during this study are included in this published article; however, these data can also be provided separately by the corresponding author on reasonable request.
Footnotes
Conflict of interests: There are no conflicts of interest. Ulrich Schneider is an employee of Arthrogen GmbH.
Myocardial infarction (MI); Gold-induced autologous cytokines (GOLDIC); ejection fraction (EF); fractional shortening (FS), left anterior descending artery (LAD); Enzyme Linked Immunosorbent Assay (ELISA); Keratinocyte-derived chemokine (KC); vascular endothelial growth factor (VEGF); stromal derived factor (SDF); monocytes chemotactic protein (MCP).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Repair after myocardial infarction, between fantasy and reality: the role of chemokines. Liehn Elisa A, Postea Otilia, Curaj Adelina, Marx Nikolaus. Journal of the American College of Cardiology. 2011;58(23):2357–62. doi: 10.1016/j.jacc.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Inflammation between defense and disease: impact on tissue repair and chronic sickness. Liehn Elisa A., Hector A. Cabrera-Fuentes. Discoveries. 2015;3(1):e42. doi: 10.15190/d.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.[Molecular mechanisms of action of gold in treatment of rheumatoid arthritis--an update]. Burmester G R. Zeitschrift fur Rheumatologie. 2001;60(3):167–73. doi: 10.1007/s003930170065. [DOI] [PubMed] [Google Scholar]
- 4.In vivo study of spherical gold nanoparticles: inflammatory effects and distribution in mice. Chen Hui, Dorrigan Alisha, Saad Sonia, Hare Dominic J, Cortie Michael B, Valenzuela Stella M. PloS one. 2013;8(2):e58208. doi: 10.1371/journal.pone.0058208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold- and silver-induced murine autoimmunity--requirement for cytokines and CD28 in murine heavy metal-induced autoimmunity. Havarinasab S, Pollard K M, Hultman P. Clinical and experimental immunology. 2009;155(3):567–76. doi: 10.1111/j.1365-2249.2008.03831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider U. International Patent Application 2011; No. PCT/DE2011/001322. USPTO. 2011.
- 7.Multifunctional roles of gelsolin in health and diseases. Li Guo Hua, Arora Pamela D., Chen Yu, McCulloch Christopher A., Liu Peter. Medicinal Research Reviews. 2010;32(5):999-1025. doi: 10.1002/med.20231. [DOI] [PubMed] [Google Scholar]
- 8.First Results on the Outcome of Gold-induced, Autologous-conditioned Serum (GOLDIC) in the Treatment of Different Lameness-associated Equine Diseases. Ulrich Schneider Georg Veith. Journal of Cell Science & Therapy. 2014;05(01) [Google Scholar]
- 9.Minimal Invasive Surgical Procedure of Inducing Myocardial Infarction in Mice. Curaj Adelina, Simsekyilmaz Sakine, Staudt Mareike, Liehn Elisa. Journal of Visualized Experiments. 2015;(99) doi: 10.3791/52197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blockade of CCR3 retains the neutrophils, preserving their survival during healing after myocardial infarction. Curaj Adelina, Staudt Mareike, Fatu Roxana, Kraaijeveld Andreas O., Jankowski Joachim, Biessen Erik A.L., Liehn Elisa A. Discoveries. 2015;3(2):e45. doi: 10.15190/d.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Horckmans Michael, Ring Larisa, Duchene Johan, Santovito Donato, Schloss Maximilian J., Drechsler Maik, Weber Christian, Soehnlein Oliver, Steffens Sabine. European Heart Journal. 2016:ehw002. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 12.CXC chemokine KC fails to induce neutrophil infiltration and neoangiogenesis in a mouse model of myocardial infarction. Oral Hasan, Kanzler Isabella, Tuchscheerer Nancy, Curaj Adelina, Simsekyilmaz Sakine, Sönmez Tolga Taha, Radu Eugen, Postea Otilia, Weber Christian, Schuh Alexander, Liehn Elisa A. Journal of Molecular and Cellular Cardiology. 2013;60:1-7. doi: 10.1016/j.yjmcc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Meta-analysis of corticosteroid treatment in acute myocardial infarction. Giugliano Gregory R, Giugliano Robert P, Gibson C Michael, Kuntz Richard E. The American journal of cardiology. 2003;91(9):1055–9. doi: 10.1016/s0002-9149(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 14.Relation between use of anti-inflammatory agents and left ventricular free wall rupture during acute myocardial infarction. Silverman H S, Pfeifer M P. The American journal of cardiology. 1987;59(4):363–4. doi: 10.1016/0002-9149(87)90817-4. [DOI] [PubMed] [Google Scholar]
- 15.Controlled intramyocardial release of engineered chemokines by biodegradable hydrogels as a treatment approach of myocardial infarction. Projahn Delia, Simsekyilmaz Sakine, Singh Smriti, Kanzler Isabella, Kramp Birgit K, Langer Marcella, Burlacu Alexandrina, Bernhagen Jürgen, Klee Doris, Zernecke Alma, Hackeng Tilman M, Groll Jürgen, Weber Christian, Liehn Elisa A, Koenen Rory R. Journal of cellular and molecular medicine. 2014;18(5):790–800. doi: 10.1111/jcmm.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effect of SDF-1 α on endogenous mobilized and transplanted stem cells in regeneration after myocardial infarction. Schuh Alexander, Konschalla Simone, Kroh Andreas, Schober Andreas, Marx Nikolaus, Sonmez Tolga Taha, Zenke Martin, Sasse Alexander, Liehn Elisa A. Current pharmaceutical design. 2014;20(12):1964–70. doi: 10.2174/13816128113199990443. [DOI] [PubMed] [Google Scholar]
- 17.Myocardial regeneration by transplantation of modified endothelial progenitor cells expressing SDF-1 in a rat model. Schuh Alexander, Kroh Andreas, Konschalla Simone, Liehn Elisa A, Sobota Radoslav M, Biessen Erik Al, Bot Ilze, Sönmez Tolga Taha, Tolga Taha Sönmez, Schober Andreas, Marx Nikolaus, Weber Christian, Sasse Alexander. Journal of cellular and molecular medicine. 2012;16(10):2311–20. doi: 10.1111/j.1582-4934.2012.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Schuh Alexander, Liehn Elisa A, Sasse Alexander, Hristov Mihail, Sobota Radoslaw, Kelm Malte, Merx Marc W, Weber Christian. Basic research in cardiology. 2008;103(1):69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 19.Novel insights into the mechanism of cell-based therapy after chronic myocardial infarction. Schuh Alexander. Discoveries. 2014;2(1) doi: 10.15190/d.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]