Abstract
Hypothalamic control of fertility is the quintessential homeostatic function. However, fertility is metabolically demanding; so, there must be coordination between energy states and reproductive functions. Because gonadotropin-releasing hormone (GnRH) neurons are devoid of many of the critical metabolic hormone receptors for sensing nutrient levels, it has long been recognized that the sensing of energy stores had to be done by neurons presynaptic to GnRH neurons. Some of the obvious players have been the anorexigenic proopiomelanocortin (POMC) and orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons, both of which are in close apposition to the median eminence, a circumventricular organ. Indeed, POMC and NPY/AgRP neurons are inversely regulated by glucose and metabolic hormones including insulin and leptin. However, their synaptic connections with GnRH neurons are sparse and/or GnRH neurons are lacking the postsynaptic receptors to mediate the appropriate physiological response. Kisspeptin neurons were discovered in the early part of this century and subsequently shown to project to and control GnRH neuronal excitability. In fact, more recently the arcuate kisspeptin neurons have been identified as the command neurons driving pulsatile release of GnRH. Subsequently, it was shown that arcuate kisspeptin neurons express not only steroid hormone receptors but also metabolic hormone receptors such that similar to POMC neurons, they are excited by insulin and leptin. Therefore, based on the premise that arcuate kisspeptin neurons are the key neurons coordinating energy states with reproduction, we will review not only how these vital neurons control pulsatile GnRH release but how they control energy homeostasis through their synaptic connections with POMC and NPY/AgRP neurons and ultimately how E2 can regulate their excitability.
Keywords: kisspeptin neurons, estradiol, glutamate release, POMC neurons, NPY/AgRP neurons, hedonic feeding
Hypothalamic arcuate nucleus kisspeptin (Kiss1ARH) neurons and anteroventral periventricular and periventricular preoptic nucleus Kiss1 (Kiss1(AVPV/PeN)) neurons are regulated in a species-, sex-, and gonadal steroid-specific manner.1-5 Kiss1AVPV/PeN neurons are essential for positive feedback by E2 on gonadotropin-releasing hormone (GnRH) neuronal activity and for generating the luteinizing hormone (LH) surge in rodents.6 These rostral Kiss1 neurons coexpress kisspeptin (Kiss1), vesicular GABA transporter (vGat), glutamic acid decarboxylase 67 (GAD 67), and tyrosine hydroxylase (TH) some of which are upregulated by E27, and Kiss1AVPV/PeN neurons are excited by E2 in part due to upregulation of T-type calcium, h- and persistent sodium (INaP) currents.8,9 In addition, glutamate induces burst firing and pacemaking activity in Kiss1AVPV/PeN neurons.7 However, the effects of E2 on Kiss1ARH neurons are more complex in that mRNA transcripts and corresponding functions are both increased and inhibited by E2.10 Kiss1ARH neurons coexpress the peptide neurotransmitters kisspeptin, neurokinin B (NKB) and dynorphin,5 as well as the vesicular glutamate transporter 2 (vGlut2), and release glutamate.11,12 E2 down-regulates the mRNA expression of the neuropeptides in Kiss1ARH neurons in part via ERα signaling, but also via non–ERE-mediated mechanisms,13 and increases the mRNA expression of Vglut2 and the release of glutamate in females, but not in males,10,12 an important sexual dimorphism.
Because GnRH cell bodies are widely scattered throughout the hypothalamic-septum continuum, the control of synchronous firing of these neurons, the so-called pulse generator activity had been much debated over several decades.14-19 Then in 2003, the puberty peptide kisspeptin was discovered, and its ability to stimulate GnRH release via signaling through GPR54 (a.k.a. Kiss1R) was documented.20-22 Following these and other discoveries, Kiss1ARH neurons were proposed to be the “pulse-generator” neurons that stimulate pulsatile secretion of GnRH,23-25 and it was shown that optogenetic stimulation of Kiss1ARH neurons generated pulsatile release of LH (driven by pulsatile GnRH) in the mouse.26,27 Indeed, the mechanism of synchronization of Kiss1ARH neurons was recently elucidated and involves a combination of coreleased NKB excitation via Tac3 receptors and dynorphin presynaptic inhibition via κ-opioid receptors, which will be further discussed later.28 Besides its role in reproductive functions, Kiss1ARH neurons have been found to be activated by leptin and insulin and communicate directly with neuropeptide Y (NPY)/agouti-related peptide (AgRP) and proopiomelanocortin (POMC) neurons in different species including mouse, guinea pig, and sheep,29-33 which has led to the idea that Kiss1ARH neurons may be integrating metabolic signals and fertility. Optogenetic activation of Kiss1ARH neurons evokes glutamatergic excitatory postsynaptic currents (EPSCs) not only in Kiss1AVPV/PeN neurons28 but also in arcuate POMC and NPY/AgRP neurons,10,12 which further establishes direct functional connections between Kiss1ARH neurons and hypothalamic neurons important for control of reproduction and energy homeostasis, which will be the focus of this review.
Kiss1 Regulation of Gonadotropin-Releasing Hormone Neurons
One of the main functions of Kiss1 neurons is to excite GnRH neurons. The somas of GnRH neurons are localized to the medial septum, rostral preoptic area (POA), anterior hypothalamus, and medial basal hypothalamus (MBH) with rodents only expressing a few scattered somas in the MBH.34,35 The majority of these neurons project to the median eminence where they release GnRH peptide into the portal blood in a pulsatile manner to control gonadotropin secretion from the pituitary gland and ultimately fertility.36-38 Kisspeptin is the most potent and efficacious neuropeptide/neurotransmitter to excite GnRH neurons,22,39,40 and Kiss1 neurons may be the presynaptic pacemaker neurons that drive GnRH neurons.8,9,28,41 Although single action potential-generated calcium influx is sufficient to spark the release of classical neurotransmitters, burst firing or tetanic stimulation is required for the release of neuropeptides. Indeed, high-frequency electrical stimulation is required to evoke peptide release as originally demonstrated in the frog ganglion by Jan et al42 (see the study by Arrigoni and Saper43 for review). In this respect, high-frequency electrical stimulation of Kiss1AVPV/PeN neurons increases GnRH cell firing, which is absent in Kiss1R knockout mice and blocked by Kiss1R partial agonist peptide 234.41 High-frequency photostimulation (20 Hz) of Kiss1AVPV/PeN neurons releases kisspeptin, which excites GnRH neurons directly.28 This high-frequency photostimulation of Kiss1AVPV/PeN neurons evokes a slow EPSP in GnRH neurons that is characterized by the tell-tale double rectifying current/voltage (I/V) plot of canonical transient receptor potential 4 (TRPC4) channels and antagonism by the Kiss1R partial agonist peptide 234.22,28,44
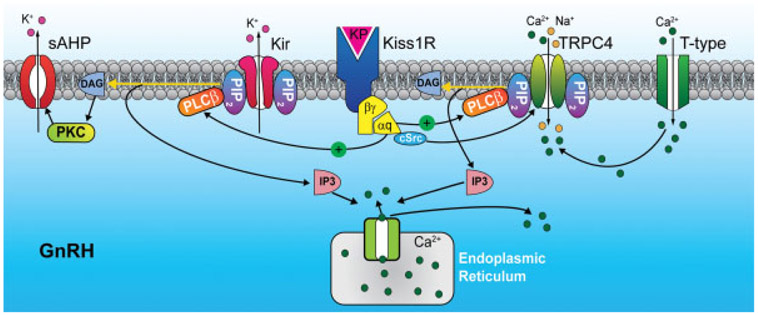
Pharmacologically, kisspeptin-54 and the smaller peptide fragments (e.g., kisspeptin 14, 13, and 10) bind with low nanomolar affinities to rat and human Kiss1R (GPR54) expressed in Chinese hamster ovary cells, and Kiss1R is Gq-coupled to stimulate phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis, Ca2+ mobilization, arachidonic acid release, and phosphorylation of mitogen-activated protein kinase.45 In native GnRH neurons, kisspeptin causes excitation primarily through activation of TRPC4 channels and to a lesser extent inhibition of inwardly rectifying K+ channels.22,39,46 Also, TRPC4 channels in GnRH neurons are receptor operated, and kisspeptin activates TRPC4 channels through PIP2 depletion and cSrc tyrosine kinase activation, which is a potent signaling pathway for sustained excitation of GnRH neurons (Fig. 1).40 Neuronal excitability is also governed by afterhyperpolarization (AHP) that follows an action potential,47,48 and kisspeptin reduces spike frequency adaptation and prolongs firing through the inhibition of a slow AHP (sAHP) current via PKC in GnRH neurons.49 Therefore, kisspeptin signals via multiple pathways for sustained activation of GnRH neurons (Fig. 1).
Fig. 1.
Schematic diagram illustrating the main signaling pathways responsible for kisspeptin-induced depolarization of GnRH neurons. Kisspeptin binds to the Gq-coupled Kiss1 receptor (Kiss1R or GPR 54) to activate PLCβ, which catabolizes PIP2, potentiates canonical transient receptor potential 4 (TRPC 4) channel activity, and inhibits the inwardly rectifying potassium (Kir) channel activity. PKC, activated by the PIP2 hydrolysis product DAG, inhibits the activity of a calcium-activated slow afterhyperpolarization (sAHP) current. Cytoplasmic tyrosine kinase, cSRC, which is activated by kisspeptin/Kiss1R signaling, potentiates the activity of TRPC4 channels. Calcium entering the cell via T-type calcium channels facilitates the activation of TRPC4 channels (modified from Zhang et al40). DAG, diacylglycerol; IP3R, inositol triphosphate receptor; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLCβ, phospholipase Cβ.
Kiss1ARH Synchronous Neuronal Firing and LH Release
Kiss1ARH neurons coexpress kisspeptin, the tachykinin NKB, the opioid peptide dynorphin, and the classical neurotransmitter glutamate.5,10-12 The tachykinins comprise a series of structurally related peptides that are derived from alternative processing of three Tac genes and are expressed throughout the nervous and immune systems.50 Tachykinins interact with three neurokinin G-protein-coupled receptors, Tacr1, Tacr2, and Tacr3. Tacr1 and Tacr3, but not Tacr2, mRNAs are expressed in Kiss1ARH neurons based on single-cell RT-PCR analysis,51 and the TacR3 agonist senktide depolarizes Kiss1ARH neurons, which is blocked by TacR3 antagonists.52 Optogenetic high-frequency induced firing of Kiss1ARH neurons results in corelease of NKB and dynorphin.28 NKB binds to TacR3 in neighboring Kiss1ARH neurons to openTRPC5 channels to cause a robust depolarization (slow EPSP); coreleased dynorphin feeds back to bind to presynaptic κ-opioid receptors to limit the release of NKB to discrete bursts of activity and presumably postsynaptic TacR3 desensitization.28,53 Thus, high-frequency autoexcitation of Kiss1ARH neurons ipsilaterally is able to recruit Kiss1ARH neurons bilaterally, which is dependent on NKB activation of TRPC5 channels to induce synchronization of this critical neural network that underlies the pulse generator activity in mammalian females.27,28 The pulsatile LH secretion from NKB activation of Kiss1ARH neurons was first shown in the goat through recording of multiunit activity (MUA) in close proximity of Kiss1 neurons simultaneously with measurements of LH release in the periphery.24 CNS administration of NKB evoked MUA bursting and pulsatile LH release, whereas dynorphin suppressed the activity and LH release. Importantly, the κ-opioid antagonist nor-binaltorphimine increased MUA bursts and LH release. The pulsatile LH secretion from activation of Kiss1ARH neurons was subsequently demonstrated using Cre-dependent ChR2 injection to specifically label Kiss1ARH neurons.27 Male and diestrous female mice were implanted with optic fibers, and were given 5 ms pulses of blue light at a frequency of 10 Hz, which is sufficient to stimulate the release of peptides.28 At the same time, tail blood was collected before and during the optic stimulation. The brief 1 minute ChR2 activation of Kiss1ARH neurons every 30 minutes evoked LH pulses in conscious males and females.27 Therefore, based on experiments in different mammalian animal models, the combination of the two peptide neurotransmitters, NKB and dynorphin, coordinates the synchronous firing of Kiss1ARH neurons causing release of kisspeptin that drives the pulsatile release of GnRH into the median eminence, which is then responsible for pituitary LH pulsatile secretion.24,25,27,28,54,55
Role of Kisspeptin Neurons in Feeding
It has been well documented that energy balance has profound effects on the reproductive system and these effects appear to be mediated, at least in part, by kisspeptin neurons in the hypothalamus.56,57 However, it is less clear which metabolic signals reach Kiss1 neurons, and how Kiss1 neurons communicate with neurons important for controlling energy homeostasis, including POMC and NPY/AgRP neurons.10,32,33,58,59 The adipocyte hormone leptin plays a key role in energy homeostasis and reproduction, and this hormone has an important role in the neuroendocrine adaptation to starvation.60 The sites of the action of leptin in the forebrain include the POA, the ventral premammillary nucleus (PMV), and the arcuate nucleus of the hypothalamus (ARH), and many of the cellular targets for leptin have been identified. Within the ARH, these include NPY/AgRP, POMC, and Kiss1ARH neurons,29,61-63 whereas GnRH neurons and POA Kiss1 neurons do not express leptin receptors.63,64 The PMV, which expresses glutamate and pituitary adenylate cyclase activating polypeptide and responds to leptin treatment with phosphorylation of signal transducer and activator of transcription 3 (STAT3), may communicate nutritional state information to GnRH neurons by modulating the activity of Kiss1 neurons.65 Leptin signals via several pathways in hypothalamic neurons, including phosphorylation of STAT3 and phosphoinositide 3-kinase (PI3K).65-67 Interestingly, mice containing a mutant leptin receptor (LRb) incapable of STAT3 signaling are obese but remain fertile.67 This would indicate that leptin signaling via STAT3 is essential for maintaining normal body weight but not for maintaining normal fertility, which may be dependent on other signaling pathways including PI3K. Studies in the female guinea pig, using whole-cell patch clamp recording and scRT-PCR, established that leptin induces a direct excitatory inward current in Kiss1ARH neurons.31 This current is inhibited by the TRPC channel blocker 2-aminoethoxydiphenyl borate (2-APB), potentiated by the effects of La3+, and LRb mRNA is expressed in individual Kiss1ARH neurons both in guinea pig31 and mouse (Fig. 2). Also, similar to POMC neurons, where LRb couples to the Jak-PI3K signaling pathway,62,66 the Janus kinase (Jak) inhibitor AG490 and the PI3K inhibitor Wortmannin potently block the leptin-induced current in Kiss1ARH neurons.31 Therefore, leptin signals via the Jak-PI3K pathway to activate TRPC5 channels in Kiss1ARH neurons (Fig. 3), which may be important for fertility, given that the TacR3 agonist senktide (and NKB) also excites Kiss1ARH neurons through TRPC5 channels and NKB and TacR3 are critical for fertility.53,68
Fig. 2.
Leptin and insulin receptor expression in mouse Kiss1ARH neurons. Representative gels illustrating (a) insulin receptor (IR) and (b) leptin receptor (LRb) expression in mouse Kiss1ARH neurons. Kiss1ARH neurons (53 neurons from 3 OVX females) were processed for LRb and IR using single-cell RT-PCR. LRb was expressed in 55% and IR was expressed in 47% of Kiss1ARH neurons. MM, molecular marker; −RT Kiss1 cell and BH− (basal hypothalamus) represent negative controls, and BH+ represents positive control processed without (−) and with (+) reverse transcriptase, respectively.
Fig. 3.
A cellular model of insulin and leptin signaling in Kiss1ARH neurons. Insulin signals via IRS-PI3K to activate canonical transient receptor potential 5 (TRPC5) channels in Kiss1ARH neurons, which generates a robust inward cationic current to depolarize Kiss1ARH neurons and increase their excitability. Similarly, leptin binding to its receptor (LRb) triggers the recruitment of the tyrosine kinase Janus Kinase (Jak) 2, leading to the activation of the Jak/signal transducer and activator of transcription (STAT) 3 signaling pathway and simultaneous activation of PI3K, which also opens TRPC5 channels.31,32 PI3K(p85/p110) will also accelerate the insertion of TRPC5 channels into the plasma membrane. DAG, diacylglycerol; IP3R, inositol triphosphate receptor; IRS, insulin receptor substrate; Jak2, Janus kinase 2; pAKT, phospho-Akt; PDK1, 3-phosphoinositide-dependent kinase 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; PLCγ1, phospholipase Cγ1.
Insulin, like leptin, affects neuropeptide expression in the hypothalamus and food intake.69 Interestingly, insulin, similar to leptin, depolarizes and increases action potential firing in both POMC and Kiss1ARH neurons.32 Moreover, in the presence of the sodium channel blocker tetrodotoxin (TTX) to block presynaptic inputs and under whole-cell voltage clamp conditions, insulin induces an inward current in Kiss1ARH neurons, with a reversal near −10mV, indicative of activation of a nonselective cationic conductance. Also, based on the pharmacological analysis, insulin, like leptin, signals via PI3K to activate TRPC5 channels in both Kiss1ARH and POMC neurons (Fig. 3).32 In addition, insulin receptor mRNA is also expressed in Kiss1ARH neurons as revealed with scRT-PCR (Fig. 2). Therefore, there is some redundancy in the feedback of metabolic hormones onto hypothalamic neurons, including Kiss1ARH neurons, to ensure survival and preserve fertility.70
The stomach-derived hormone ghrelin increases in the circulation with fasting and also increases food intake after hypothalamic administration.71 Ghrelin is the endogenous ligand of the growth hormone secretagogue receptor (GHSR), which is located in different brain regions including the POA and basal hypothalamus.72 Ghsr mRNA is expressed in the majority of NPY/AgRP neurons, but in less than 10% of POMC neurons. Ghrelin induces feeding by robustly activating NPY/AgRP neurons, which then inhibits POMC neurons and POMC-induced αMSH effects in the PVH.73 Moreover, OVX wild-type mice on a regular chow diet gain weight, but global knockout of Ghsr prevents the weight gain.71 Therefore, ghrelin is considered an orexigenic hormone. Recently, it has been shown that Ghsr is also expressed in Kiss1ARH neurons, where its expression is increased by E2.59,72 Given that E2 is anorexigenic and essential for fertility, the E2-induced increased expression of Ghsr in Kiss1ARH neurons could mean that ghrelin helps link metabolism and reproduction; however, the underlying mechanism is currently unknown. Clearly, further studies are needed to elucidate the role of Ghrelin within the kisspeptin circuitries.
Role of Kiss1R (GPR54) and Neuropeptide FF Receptor 1 in Energy Homeostasis
Kiss1 and Kiss1R are essential for pubertal development and reproduction.20,21,74 The Kiss1R is expressed in GnRH neurons, and kisspeptin acting at the Kiss1R potently activates GnRH neurons primarily through action on TRPC4 channels.22,40 The Kiss1R is also expressed in POMC neurons, and kisspeptin directly excites POMC neurons via activation of TRPC5 channels.10,75 High-frequency optogenetic activation of POMC neurons excites Kiss1ARH neurons, an indication that POMC neurons may signal to Kiss1ARH neurons the metabolic status of the organism.76 Chronically, OVX Kiss1R KO females develop obesity, hyperleptinemia, reduced metabolism, and glucose intolerance in comparison to OVX females.58 Therefore, the Kiss1R (GPR54) may play a role to link metabolic changes to reproduction in female rodents.
In contrast, kisspeptin directly inhibits firing and hyperpolarizes NPY/AgRP neurons via activation of a G protein-coupled inwardly rectifying K+ (GIRK) current. The Kiss1 receptor is not expressed in NPY/AgRP neurons; however, NPFFR1 is expressed, and its agonist, RFRP-3, inhibits firing and hyperpolarize NPY/AgRP neurons via activation of a GIRK current.10 Based on evidence that both RFRP-3 and kisspeptin bind to and activate NPFFR1,77 it is likely that the two different neuropeptides inhibit NPY/AgRP neurons by action on the same receptor. Collectively, these data indicate that Kiss1ARH neurons, depending on the hormone status, may utilize kisspeptin to excite POMC neurons but inhibit NPY/AgRP neurons.
Inhibitory NPY/AgRP Neuronal Input to Kiss1ARH Neurons
It is well known that starvation and excessive physical activity that reduces body fat are associated with infertility, an indication that starvation-activated NPY/AgRP neurons may be involved to communicate metabolic deficits to GnRH neurons.78,79 Also, in lactating rats, NPY has direct inhibitory effects on GnRH neurons by acting through postsynaptic Y5 receptors as demonstrated using whole-cell current clamp recordings and selective Y5 R agonists.80 However, NPY neurons that project to the POA are located in different brain regions including the dorsomedial hypothalamus (DMH) and brainstem in addition to the ARH. To further assess whether NPY/AgRPARH neurons interact directly with GnRH neurons, we used photoactivation of NPY/AGRP neurons that expressed channel-rhodopsin (ChR2) to explore a potential direct input to GnRH neurons in vitro.33 These optogenetic experiments, using both low- and high-frequency stimulation to induce release of GABA or neuropeptides, respectively, reveal that NPY/AgRP neurons do not directly alter the activity of GnRH neurons. Instead, NPY/AgRP neurons evoke inhibitory postsynaptic currents (IPSCs) in Kiss1ARH neurons,33 in support of previous findings of extensive immunoreactive AgRP fiber projections throughout the arcuate nucleus.81 Similarly, optogenetic activation of NPY/AgRP neurons also directly inhibits Kiss1AVPV neurons.33 Therefore, it appears that NPY/AgRP neurons by way of kisspeptin neurons may link nutritional states and fertility.
Kiss1ARH Communication with POMC and NPY/AgRP Neurons
The ability of kisspeptin to activate POMC neurons was initially demonstrated in hypothalamic slices using patch-clamp recording.75 The excitation was inhibited by the partial kisspeptin agonist peptide 234.75 Low-frequency optogenetic stimulation of Kiss1ARH neurons in vitro further documented direct excitatory ionotropic glutamatergic projections to both POMC and NPY/AgRP neurons. The excitatory glutamatergic input to NPY/AgRP neurons is thought to play a key role in the response to fasting, although the origin of the glutamatergic input was not known at the time.82 The low-frequency excitation is blocked by TTX, but reinstated by application of the potassium channel blocker 4-aminopyridine, which is a biophysical evidence of direct synaptic input from Kiss1ARH neurons to POMC and NPY/AGRP neurons. Interestingly, high-frequency stimulation of Kiss1ARH neurons activates POMC and inhibits NPY/AgRP neurons in both males12 and females.10 High-frequency optogenetic stimulation (20 Hz) of Kiss1ARH neurons, which mimics a firing rate that has been observed in these ARH neurons in vivo,83 excites POMC neurons via activation of group I metabotropic glutamate receptors 1 and 5, both of which are expressed in POMC neurons.10 In contrast, high-frequency stimulation of Kiss1ARH neurons inhibits NPY/AgRP neurons, which is mediated primarily by mGluR7 (Gi/o coupled), a glutamatergic type III metabotropic receptor, together with the type II mGluR2 (Gi/o coupled) activation of GIRK current. The metabotropic receptors are thought to be “extrasynaptic” and activated by glutamate spillover with high-frequency stimulation.84 The response in POMC neurons to group I metabotropic agonist 3,5-dihydroxyphenylglycine (DHPG), which activates a TRPC current,85 is not different among OVX, oil-, and E2-treated females. In contrast, the metabotropic receptor-activated GIRK current in NPY/AgRP neurons is augmented by E2-treatment in agreement with earlier findings that E2 inhibits NPY/AgRP neurons through multiple mechanisms.10,86,87 Similar to glutamate, kisspeptin is also released during high-frequency stimulation of Kiss1ARH neurons and excites POMC neurons but inhibits NPY/AgRP neurons. It is important to note that during high physiological E2 conditions, Kiss1 mRNA expression and kisspeptin release in the arcuate nucleus would be quite low in contrast to glutamate, an indication that the effects of glutamate are dominant during this physiological state.10
Vglut2 Deletion in Kiss1 Neurons
The evidence that Kiss1ARH neurons are involved in the regulation of GnRH and LH pulsatility via their peptide neurotransmitters is well documented.23-25,27,28 Although it is known that Slc17a6 (vGlut2) is expressed in Kiss1ARH neurons,11,12 the functional role of glutamate neurotransmission from these neurons has not been extensively investigated. As described earlier, Kiss1ARH neurons send kisspeptin and glutamatergic projections to the orexigenic NPY/AgRP neurons and the anorexigenic POMC neurons, an indication that glutamatergic and/or kisspeptin inputs from Kiss1ARH neurons are involved in regulation of energy homeostasis.
In this respect, complete silencing of Kiss1ARH neurons, using tetanus toxin treatment to prevent the release of neurotransmitters in adult animals, results in obesity due in part to inactivity and altered day/night eating pattern.88 In contrast, conditional deletion of vGlut2 (Slc17a6, KO) in Kiss1 neurons, which abrogates glutamate release from the ARH population of Kiss1 neurons throughout development, does not alter body weight or normal chow intake. However, lack of glutamatergic neurotransmission by Kiss1 neurons does alter the preference for highly palatable foods in adult estradiol-treated KO females as revealed by the conditioned place preference test.10 Given that Kiss1AVPV/PeN neurons do not express Slc17a6,28 the KO should be specific for Kiss1ARH neurons. Although reward-seeking behaviors have not been specifically associated with Kiss1ARH neurons, the “down-stream” NPY/AgRP and POMC neurons are involved in hedonic feeding in addition to controlling homeostatic food intake (Fig. 4).89-95
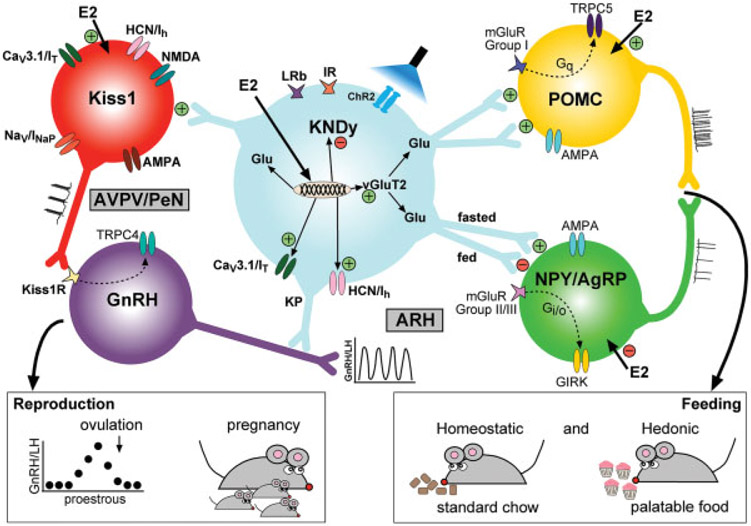
Fig. 4.
Working cellular model of Kiss1ARH role in reproduction and feeding: KNDy (kisspeptin, NKB, dynorphin) and glutamate (Glu) neurons in the ARH express CaV3.1 (IT) and HCN1,2 (Ih) channels and vesicular glutamate transporter 2 (vGlut2) that are upregulated by E2 and contribute to increased excitability of Kiss1ARH neurons and increased glutamate release. The expression of the neuropeptides is decreased by E2. E2 also directly excites Kiss1AVPV/PeN neurons and POMC neurons, but inhibits NPY/AgRP neurons. High-frequency stimulation (photostimulation of channel rhodopsin, ChR2) in Kiss1ARH neurons releases glutamate to excite POMC neurons via group I mGluRs 1,5 and inhibit NPY/AgRP neurons via group II/III mGluRs 2,7; deleting Slc17a6 (vGluT2) in Kiss1ARH neurons abrogates glutamate release from these neurons. The lack of glutamate release from Kiss1ARH neurons promotes preference for highly palatable foods like sucrose in vivo even in high physiological E2 states (right inset). Photostimulation of ChR2 in Kiss1ARH neurons also releases glutamate to excite Kiss1AVPV/PeN neurons via ionotropic glutamate receptors. The excitatory glutamate input induces burst firing activity of Kiss1AVPV/PeN neurons and kisspeptin release, causing excitation of GnRH neurons, preovulatory GnRH/LH release, ovulation, and fertility (left inset).
Interestingly, females with ablation of Slc17a6 (vGluT2) specifically in Kiss1 neurons appeared to exhibit a normal ovulatory cycle, an indication that glutamatergic neurotransmission from Kiss1ARH neurons may not be necessary to support reproductive function.10 In contrast, complete silencing of Kiss1ARH neurons in adults88 produces females with irregular estrus cycles, and the majority of females exhibit persistent diestrus with lower plasma LH levels during diestrus as compared with wild-type controls.88 Since glutamate release from Kiss1ARH onto Kiss1AVPV/PeN neurons in females is increased during high E2 states when kisspeptin and other peptides are decreased, one would predict that glutamate and not the neuropeptides released by Kiss1ARH neurons would be involved in regulating the LH surge and the ovulatory cycle.10,28,96 Indeed, Wang et al96 showed that glutamatergic input (spontaneous EPSCs) to Kiss1AVPV/PeN neurons is significantly increased on proestrus (vs. diestrus and estrus), and this increased excitation is lost with selective deletion of ERα in Kiss1 neurons. Clearly, further experiments, including deletion of Slc17a6 specifically in adult females, which would prevent potential developmental compensation in the conditional knockout models, are necessary to determine whether glutamate neurotransmission from Kiss1ARH neurons to Kiss1AVPV/PeN neurons are involved in reproductive functions and fertility (see Fig. 4).
Conclusions
Although Kiss1ARH neurons have recently been identified as the “pulse generator” neurons that drive the episodic release of GnRH through direct synaptic input, recent findings from several laboratories have identified that these neurons also coordinate energy metabolism with reproductive functions. Indeed, cellular electrophysiological/molecular studies have identified that Kiss1ARH neurons express steroid hormone receptors (ERα) and also metabolic hormone receptors (LRb and insulin receptor), and similar to POMC neurons, they are excited by insulin and leptin. Moreover, they project to and robustly excite POMC neurons via mGluR1 but inhibit NPY/AgRP neurons via mGluR7 metabotropic receptors. Also, the inhibition of NPY/AgRP neurons is ensured by the estrogenic-dependent upregulation of inhibitory mGluR7 receptors. Furthermore, conditional knockout of Slc17a6 (vGlut2) in Kiss1ARH neurons abrogates their glutamatergic input to these vital anorexigenic and orexigenic neurons and thereby increases the drive for highly palatable foods; and complete silencing of Kiss1ARH neurons via tetanus toxin renders females obese after 4 weeks on a normal diet. Clearly, Kiss1ARH neurons are part of an anorexigenic circuit, which is congruent with their vital physiological role in reproduction. In contrast, with insufficient energy stores, the chances of maintaining a successful pregnancy through to parturition are low, and, therefore, the drive for nutrients becomes a higher priority, whereas reproductive activities are attenuated. Furthermore, although the peptides are downregulated in Kiss1ARH neurons during high estrogenic states, endogenous excitatory ion channels (e.g., T-type calcium, HCN channels) and transporters (vGlut2) expression and hence neuronal excitability are upregulated, through multiple estrogenic receptor signaling pathways. All of these channels and transporters contribute to the increased excitability of Kiss1ARH neurons, which would allow them to coordinate reproduction, via ionotropic glutamatergic excitatory input to Kiss1AVPV/PeN neurons, with energy homeostasis via metabotropic excitatory input to POMC neurons and metabotropic inhibitory input to NPY/AgRP neurons. Therefore, although Kiss1ARH neurons were originally thought to be inactive during high estrogenic states, they are clearly the quintessential command neurons to coordinate energy states with reproduction.
Acknowledgments
The authors thank current and previous members of their laboratories who contributed to the work described in the present review. We also thank Ms. Martha Bosch and Dr. Todd Stincic for helping in the preparation of the figures. The research from the authors’ laboratories was supported by the U.S. National Institute of Health (NIH) grants: NS043330, NS038809, and DK068098.
Footnotes
Disclosure statement
The authors have nothing to disclose.
Conflict of Interest
None declared.
References
- 1.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 2006;26(25): 6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol 2006;18(10):806–809 [DOI] [PubMed] [Google Scholar]
- 3.Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Brain Res Rev 2008;57(02): 288–298 [DOI] [PubMed] [Google Scholar]
- 4.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008;149(09):4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol 2013;784:27–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008;28(35):8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Tonsfeldt KJ, Qiu J, et al. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab 2013;305(11):E1384–E1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Bosch MA, Qiu J, Rønnekleiv OK, Kelly MJ. 17β-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice.Mol Endocrinol 2015;29(04):518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, DeFazio RA, Moenter SM. Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro 2016;3(03):. Doi: 10.1523/ENEURO.0094-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu J, Rivera HM, Bosch MA, et al. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 2018;7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011;173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestor CC, Qiu J, Padilla SL, et al. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol 2016;30(06):630–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 2009;29(29): 9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 1978;202(4368):631–633 [DOI] [PubMed] [Google Scholar]
- 15.Knobil E The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 1980;36:53–88 [DOI] [PubMed] [Google Scholar]
- 16.Wilson RC, Kesner JS, Kaufman J-M, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology 1984; 39(03):256–260 [DOI] [PubMed] [Google Scholar]
- 17.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 1997;56(02):293–302 [DOI] [PubMed] [Google Scholar]
- 18.Clarke IJ. Two decades of measuring GnRH secretion. Reprod Suppl 2002;59:1–13 [PubMed] [Google Scholar]
- 19.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol 2003;24(02):79–93 [DOI] [PubMed] [Google Scholar]
- 20.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003;349(17):1614–1627 [DOI] [PubMed] [Google Scholar]
- 21.de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A 2003;100(19):10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 2008;28(17):4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009;29(38):11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010;30(08):3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013;154(11): 4259–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A 2015; 112(42):13109–13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A 2017;114 (47):E10216–E10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu J, Nestor CC, Zhang C, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 2006;18(04):298–303 [DOI] [PubMed] [Google Scholar]
- 30.Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010;151(05):2233–2243 [DOI] [PubMed] [Google Scholar]
- 31.Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 2011;152(04):1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu J, Zhang C, Borgquist A, et al. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab 2014;19(04):682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padilla SL, Qiu J, Nestor CC, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A 2017; 114(09):2413–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman AJ, Krey LC, Zimmerman EA. A comparative study of the luteinizing hormone releasing hormone (LHRH) neuronal networks in mammals. Biol Reprod 1979;20(01):98–110 [DOI] [PubMed] [Google Scholar]
- 35.Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci 1987;7(08):2312–2319 [PMC free article] [PubMed] [Google Scholar]
- 36.Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 1982;111 (05):1439–1448 [DOI] [PubMed] [Google Scholar]
- 37.Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology 1985;117(02):711–721 [DOI] [PubMed] [Google Scholar]
- 38.Clarke IJ, Cummins JT. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology 1985;116(06):2376–2383 [DOI] [PubMed] [Google Scholar]
- 39.Liu X,Lee K,Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 2008; 149 (09):4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology 2013;154(08):2772–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Porteous R, d’Anglemont de Tassigny X, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci 2011;31(07):2421–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jan YN, Jan LY, Kuffler SW. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A 1979;76 (03):1501–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arrigoni E, Saper CB. What optogenetic stimulation is telling us (and failing to tell us) about fast neurotransmitters and neuromodulators in brain circuits for wake-sleep regulation. Curr Opin Neurobiol 2014;29:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roseweir AK, Kauffman AS, Smith JT, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 2009;29(12):3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001;276(37):34631–34636 [DOI] [PubMed] [Google Scholar]
- 46.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 2008; 149(04):1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology 2008;149 (07):3598–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci 2010;30 (18):6214–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Rønnekleiv OK, Kelly MJ. Kisspeptin inhibits a slow afterhyperpolarization current via protein kinase C and reduces spike frequency adaptation in GnRH neurons. Am J Physiol Endocrinol Metab 2013;304(11):E1237–E1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiologyical control and the mechanisms of disease. Physiol Rev 2014;94 (01):265–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navarro VM, Bosch MA, León S, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology 2015;156(02):627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011;152(11):4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly MJ, Qiu J, Rønnekleiv OK. TRPCing around the hypothalamus. Front Neuroendocrinol 2018;51:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 2006;147(02):1007–1013 [DOI] [PubMed] [Google Scholar]
- 55.Campos P, Herbison AE. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A 2014;111(51):18387–18392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Bond JA, Smith JT. Kisspeptin and energy balance in reproduction. Reproduction 2014;147(03):R53–R63 [DOI] [PubMed] [Google Scholar]
- 57.Manfredi-Lozano M, Roa J, Tena-Sempere M. Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front Neuroendocrinol 2018;48:37–49 [DOI] [PubMed] [Google Scholar]
- 58.Tolson KP, Garcia C, Yen S, et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest 2014;124(07):3075–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JA, Yasrebi A, Snyder M, Roepke TA. The interaction of fasting, caloric restriction, and diet-induced obesity with 17β-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Mol Cell Endocrinol 2016;437:35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005; 366(9479):74–85 [DOI] [PubMed] [Google Scholar]
- 61.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411(6836):480–484 [DOI] [PubMed] [Google Scholar]
- 62.Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 2010;30(04):1560–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG Jr. Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology 2011;152(06):2302–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 2009;150(06):2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross RA, Leon S, Madara JC, et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 2018;7:e35960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill JW, Williams KW, Ye C, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 2008;118(05):1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piper ML, Unger EK, Myers MG Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons.Mol Endocrinol 2008;22(03):751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 2009;41(03):354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest 2006;116(07):1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujikawa T, Berglund ED, Patel VR, et al. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab 2013;18(03):431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clegg DJ, Brown LM, Zigman JM, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 2007;56(04):1051–1058 [DOI] [PubMed] [Google Scholar]
- 72.Frazao R, Dungan Lemko HM, da Silva RP, et al. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab 2014;306(06):E606–E614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides 2011;32(11):2248–2255 [DOI] [PubMed] [Google Scholar]
- 74.d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A 2007;104(25):10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 2010;30 (30):10205–10219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stincic TL, Grachev P, Bosch MB, Rønnekleiv OK, Kelly MJ. Estradiol drives the anorexigenic activity of proopiomelanocortin neurons in female mice. eNeuro 2018;5(04): Doi: 10.1523/ENEURO.0103-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonini JA, Jones KA, Adham N, et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem 2000;275(50):39324–39331 [DOI] [PubMed] [Google Scholar]
- 78.Castellano JM, Navarro VM, Fernández-Fernández R, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005;146(09):3917–3925 [DOI] [PubMed] [Google Scholar]
- 79.Fernández-Fernández R, Martini AC, Navarro VM, et al. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol 2006;254–255:127–132 [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide Y. Endocrinology 2009;150(01):333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A 1998;95(25):15043–15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu T, Kong D, Shah BP, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 2012;73(03):511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moss RL, Kelly M, Riskind P. Tuberoinfundibular neurons: dopaminergic and norepinephrinergic sensitivity. Brain Res 1975;89 (02):265–277 [DOI] [PubMed] [Google Scholar]
- 84.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 2010;11(05):301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tozzi A, Bengtson CP, Longone P, et al. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci 2003;18(08): 2133–2145 [DOI] [PubMed] [Google Scholar]
- 86.Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci 2011;31(33):11825–11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab 2013;305(05):E632–E640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padilla SL, Perez JG, Ben-Hamo M, et al. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr Biol 2019;29(04):592–604.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayward MD, Schaich-Borg A, Pintar JE, Low MJ. Differential involvement of endogenous opioids in sucrose consumption and food reinforcement. Pharmacol Biochem Behav 2006;85 (03):601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lippert RN, Ellacott KL, Cone RD. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology 2014;155(05):1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 2013;155(06):1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pandit R, Luijendijk MC, Vanderschuren LJ, la Fleur SE, Adan RA. Limbic substrates of the effects of neuropeptide Yon intake of and motivation for palatable food. Obesity (Silver Spring) 2014;22 (05):1216–1219 [DOI] [PubMed] [Google Scholar]
- 93.Pandit R, van der Zwaal EM, Luijendijk MC, et al. Central melanocortins regulate the motivation for sucrose reward. PLoS One 2015;10(03):e0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 2016;19(02):198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubinstein M, Low MJ. Molecular and functional genetics of the proopiomelanocortin gene, food intake regulation and obesity. FEBS Lett 2017;591(17):2593–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr, Moenter SM. Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor α in adult female mice. J Neurosci 2018;38(05): 1061–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]