Supplemental Digital Content is available in the text.
Abstract
Background:
Hyaluronic acid (HA) fillers for volume augmentation in the anteromedial malar region of Asians have been popular for many years. However, studies on their long-term effectiveness are lacking. This study aimed to evaluate the effectiveness and safety of HA fillers injected into the anteromedial malar region for volume augmentation for up to 52 weeks.
Methods:
Each anteromedial malar region of the subjects was treated with YVOIRE Contour (YVOC) in one side and Restylane Sub-Q (RESS) in the other and followed up at weeks 2, 14, 26, and 52. The volume using the mid-face aesthetic scale (MFAS) ranging from 0 (full) to 4 (very severely sunken) and the subject’s satisfaction and adverse events were evaluated.
Results:
Total 83 subjects were randomized and treated with YVOC and RESS. The LS means (standard error) of MFAS score in the YVOC and RESS groups were both 2.56 (0.05) at baseline, 1.32 (0.07) and 1.39 (0.07) at week 26, and 1.84 (0.10) and 1.89 (0.10) at week 52, respectively. The difference in the LS mean of MFAS score between the groups at week 26 was 0.07 (95% confidence interval, 0.01–0.12), showing the non-inferiority of YVOC to RESS. About 70% of subjects were still satisfied with the results at week 52. No specific safety concern was detected.
Conclusions:
The HA fillers injected for the anteromedial malar augmentation maintained the volume well for up to 52 weeks. Additionally, both YVOC and RESS show similar effectiveness and safety profiles.
INTRODUCTION
Decreased skin elasticity and thickness, the loss of soft tissue volume and bony mass, and redistribution of fat tissue all contribute to the formation of wrinkles and folds, which are characterized as the signs of aging.1 These also change the facial contour, especially around the mid-face due to volume loss.
To make patients look younger, several surgical procedures have been performed in the past, and many surgeons are in search for safer and more effective treatments in these fields. Particularly, hyaluronic acid (HA)-based fillers have been widely used recently not only for the correction of facial lines and folds but also for contour and volume augmentation because of their safety and effectiveness.1–3 Moreover, it can be easily removed with hyaluronidase. Thus, it is used most popularly in fillers.3,4
For the mid-facial volume restoration, various HA fillers including YVOIRE Contour (YVOC; LG Chem, Ltd., Seoul, Republic of Korea) and Restylane Sub-Q (RESS; Q-Med AB, Uppsala, Sweden) are used in many countries. Although Juvederm Voluma (Allergan Inc., Irvine, CA, USA) has been approved firstly for the volume augmentation in the mid-face in 2013 by the US Food and Drug Administration, studies on HA fillers administered for the mid-face augmentation are very limited, and most studies were conducted in Caucasians. However, Asians who have wider, shorter, and flatter faces5 with a different concept of standard beauty from Caucasians6,7 prefer round apple cheeks without zygomatic region in the mid-face.5 Therefore, mid-face filler injection in Asians is made mostly for the restoration of the anterior projection, targeting the medial aspect of the zygoma and submalar regions.5 However, studies on the effectiveness of HA fillers on Asian mid-face augmentation are lacking.
Thus, this study aimed to evaluate long-term effectiveness and safety of two HA fillers, YVOC and RESS, both of which are used widely in the Republic of Korea and injected into the anteromedial malar region of Koreans.
METHODS
Study Design
The study was a multicenter, randomized, active-controlled, split-face design, rater-blind, comparative clinical study conducted in 2 teaching hospitals in the Republic of Korea from June 2014 to October 2015. With split-face of each subject, YVOC or RESS was assigned and injected randomly into the right or left anteromedial malar region by the investigators via the random assignment table generated by a statistician. Then, they were followed up at weeks 2, 14, 26, and 52 after HA filler injection.
The study was conducted in compliance with the ethical guidelines of the Declaration of Helsinki and Good Clinical Practices and was approved by the institutional review board of each study site. Written informed consent was obtained from each participant, and this study was registered at ClinicalTrials.gov, NCT02119780.
Subject Selection and Treatment
The study included male or female adults aged between 20 and 65 years. Only the subjects who had more than moderate volume loss [ie, score 2–4, rated using the 5-point photograph numeric mid-face aesthetic scale (MFAS); “Upper Cheek Fullness—At Rest”8 (Table 1)] in both anteromedial malar regions symmetrically were included. Major exclusion criteria included subjects with active or infectious skin diseases, uncured wounds or tumors on the mid-face area, subjects with history of radiotherapy in the mid-face area, subjects with history of laser therapy, chemical peeling or dermabrasion in the mid-face area within 3 months before randomization, and subjects who received fillers or botulinum toxin treatment or facial surgery within 12 months before randomization.
Table 1.
MFAS and Upper Cheek Fullness—At Rest
| Score | Degrees of Severity |
|---|---|
| 0 | Full upper cheek |
| 1 | Mildly sunken upper cheek |
| 2 | Moderately sunken upper cheek |
| 3 | Severely sunken upper cheek |
| 4 | Very severely sunken upper cheek |
YVOC is a biphasic HA filler crosslinked with 1,4-Butanediol diglycidyl ether with a HA content of 22 mg/mL. The median particle size (DV50) of YVOC is around 1,200 μm (range 1,000 to 1,500 μm). RESS is also a biphasic HA filler crosslinked with 1,4-Butanediol diglycidyl ether (20 mg/mL of HA) and consists of large particles. The remarkable feature of YVOC unlike RESS is that it consists of large particles mixed with small particles to fill voids between large particles.
According to the random assignment table, YVOC and RESS were injected into the subcutaneous layer of the assigned side of the anteromedial malar region using a 21-gauge cannula by the same treating investigator. If necessary, a local anesthetic was used equally on both anteromedial malar regions at the discretion of the treating investigator. HA fillers were injected to achieve optimal correction for each subject, and one touch-up treatment was allowed after 2 weeks of the initial injection. The maximum acceptable volume was 4.0 mL for one side of the anteromedial malar region for each treatment.
Assessments
The effectiveness of volume restoration was evaluated using MFAS and Global Aesthetic Improvement Scale (GAIS9; Table 2). MFAS, a 5-point photograph numeric validated grading scale8 developed by Merz Pharma GmbH, was used after the written copyright licensing agreement was made between the sponsor (LG Chem, Ltd.) and Merz Pharma GmbH. In this study, “the Upper Cheek Fullness—At Rest” was used (Table 1). Three qualified independent blinded raters who were trained in the MFAS evaluation in advance evaluated the volume of the anteromedial malar region. GAIS, a measure of the relative overall improvement after HA filler injection compared to the pre-injection, was evaluated by the subjects (Table 2).
Table 2.
The Global Aesthetic Improvement Scale
| Score | Rating | Description |
|---|---|---|
| 3 | Very much improved | Optimal cosmetic result for the implant in this patient |
| 2 | Much improved | Marked improvement in appearance from the initial condition, but not completely optimal for this patient. A touch-up would slightly improve the result |
| 1 | Improved | Obvious improvement in appearance from the initial condition, but a touch-up or retreatment is indicated |
| 0 | No change | The appearance is essentially the same as the original condition |
| −1 | Worse | The appearance is worse than the original condition |
The primary parameter was the mean MFAS score at week 26 after HA filler injection. The secondary parameters included the mean MFAS score at weeks 2, 14, and 52 after HA filler injection, changes in mean MFAS score from baseline at each evaluation time point, percentage of the subjects with improvement in MFAS score by at least 1 grade from baseline (MFAS responder rate), mean GAIS score, and percentage of the subjects with improvement in GAIS score by at least 1 grade from baseline (GAIS responder rate).
Adverse events (AEs) were collected at each visit for safety evaluation. Solicited local AEs (pain, tenderness, swelling, redness, bruising, itching, papule, and pigmentation) occurring at the injection site were evaluated for 14 days after HA filler injection through the subject diary.
Statistical Analyses
The sample size to demonstrate the non-inferiority of YVOC compared to RESS with regard to the mean MFAS score at week 26 was calculated with a 90% power at the significance level of 2.5% and a one-sided test. The planned enrollment was 83 subjects, assuming a drop-out rate of 20%.
Statistical analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). All effectiveness parameters are based on the descriptive statistics. Wilcoxon’s signed rank test was performed to test for the changes in mean MFAS score in each treatment group from baseline at each evaluation time point including primary time point of week 26. The 2-sided 95% confidence interval (CI) for the difference in mean MFAS score between treatment groups was estimated in the mixed-effects model considering a split-face design, and the non-inferiority of the YVOC in terms of effectiveness at week 26 can be demonstrated if the lower limit of the 95% CI was greater than −0.32. For the responder rates, the 2-sided 95% CI for the difference in rates between treatment groups was summarized. Safety data were presented in descriptive statistics.
RESULTS
Subject Characteristics
A total of 87 subjects were screened, 83 subjects were enrolled, and 68 subjects completed the study up to week 52 (Fig. 1). Fifteen subjects were excluded from the study before week 52 due to follow-up loss (n = 5), withdrawal of consent (n = 9), and deviation from the study protocol (n = 1). The mean age of the subjects who participated in this study was 44 (24–63) years, and 91% of the subjects were female participants. All the randomized subjects were treated with YVOC and RESS in the same way, using the linear threading and fanning technique for both anteromedial malar regions. Table 3 shows a summary of the total HA injection volume.
Fig. 1.
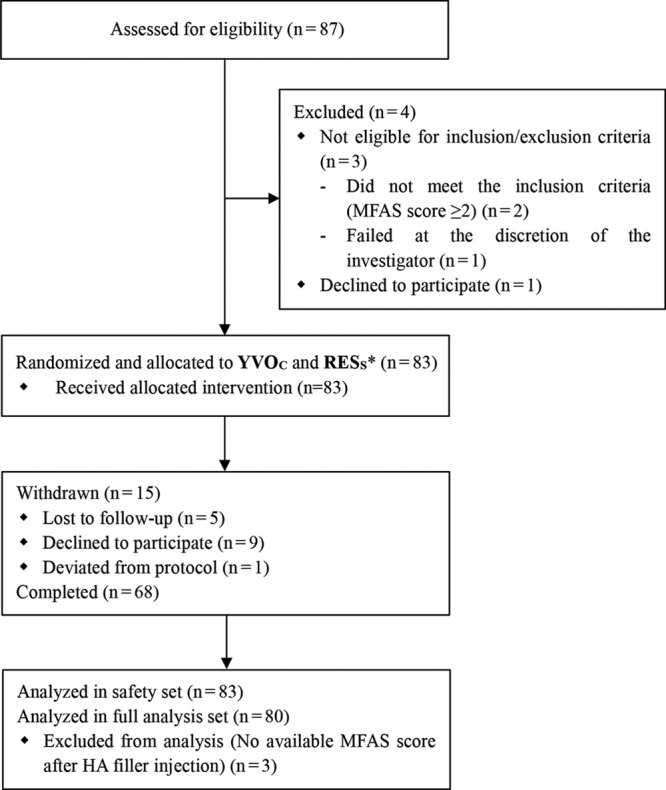
Subject dispositions. *Subjects were randomly assigned to which side of the facial anteromedial malar region they would be injected with either YVOC or RESS.
Table 3.
HA Injection Volume (Safety Set)
| YVOC | RESS | |
|---|---|---|
| Injection volume in subjects who did not receive touch-up treatment (mL) | ||
| N | 75 | 66 |
| Mean (SD) | 1.60 (0.41) | 1.51 (0.36) |
| Min, max | 0.80, 2.80 | 0.80, 2.60 |
| Total injection volume in subjects who received touch-up treatment (mL) | ||
| N | 8 | 17 |
| Mean (SD) | 2.14 (0.22) | 2.17 (0.52) |
| Min, max | 1.80, 2.50 | 1.40, 3.50 |
| Total injection volume in total subjects (mL) | ||
| N | 83 | 83 |
| Mean (SD) | 1.65 (0.42) | 1.65 (0.47) |
| Min, max | 0.80, 2.80 | 0.80, 3.50 |
Max, maximum; Min, minimum.
Clinical Results
The least squares (LS) mean [standard error (SE)] of MFAS score at baseline was 2.56 (0.05) in both groups. The LS mean (SE) of MFAS score at week 26 was 1.32 (0.07) in the YVOC group and 1.39 (0.07) in the RESS group, and the LS mean change (SE) from baseline was −1.23 (0.06) and −1.16 (0.06) in the YVOC and RESS groups, respectively. The LS mean changes in MFAS score at week 26 were significant in both groups compared with the baseline (P-value <0.0001). In addition, the difference in the LS mean of MFAS score between the groups at week 26 was 0.07 (95% CI, 0.01–0.12), and since the lower limit of the 95% CI of the difference was greater than −0.32, the non-inferiority of YVOC to RESS was demonstrated. The analysis of covariance adjusted for total injection volume was also performed, and the LS means of MFAS score at week 26 were comparable between the groups [see table, Supplemental Digital Content 1, which displays analysis of covariance on the change from baseline at week 26 of MFAS score evaluated by the independent blinded raters (full analysis set), http://links.lww.com/PRSGO/B311].
The LS mean of MFAS score from baseline up to week 52 after HA filler injection is presented in Figure 2 and Table 4. The LS mean (SE) of MFAS score at week 52 was 1.84 (0.10) and 1.89 (0.10), and the LS mean change (SE) from baseline was −0.73 (0.08) and −0.68 (0.08) in the YVOC and RESS groups, respectively (Fig. 3). The LS mean changes in MFAS score at week 52 were significant in both groups compared with the baseline (P-value <0.0001). In addition, the effectiveness of volume restoration was similar between the groups during the 52-week follow-up period.
Fig. 2.
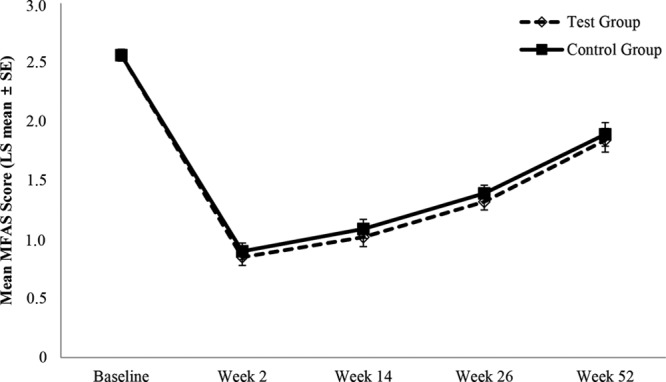
Mean MFAS score over time evaluated by the independent blinded raters (full analysis set).
Table 4.
Mean MFAS Score over Time Evaluated by the Independent Blinded Raters (Full Analysis Set)
| YVOC (N = 80) | RESS (N = 80) | LS mean difference (95% CI) | |
|---|---|---|---|
| Baseline | 2.56 (0.05) | 2.56 (0.05) | 0.00 (−0.04 to 0.04) |
| Week 2 | 0.85 (0.07) | 0.90 (0.07) | 0.05 (0.00 to 0.10) |
| Week 14 | 1.02 (0.08) | 1.09 (0.08) | 0.06 (0.01 to 0.12) |
| Week 26 | 1.32 (0.07) | 1.39 (0.07) | 0.07 (0.01 to 0.12) |
| Week 52 | 1.84 (0.10) | 1.89 (0.10) | 0.05 (−0.02 to 0.13) |
Data are presented as LS mean (SE) unless otherwise indicated; data were analyzed using a mixed effects model including treatment and week as repeated measures effects and the subject as random effect; LS mean difference was calculated as RESs group—YVOC group.
Fig. 3.
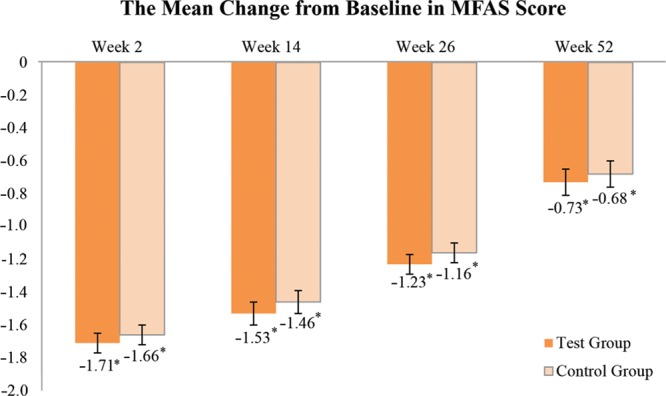
The mean change from baseline in MFAS score evaluated by the independent blinded raters (full analysis set). Data are the LS mean ± SE. *P-value <0.05, P-value was obtained from Wilcoxon’s signed rank test.
In both of the groups, all the subjects showed at least 1 grade improvement in MFAS score up to week 14 after HA filler injection (Fig. 4A). At week 26, the MFAS responder rates were 95% and 93% in the YVOC and RESS groups, respectively. At week 52, the rates were 66% and 61%, respectively.
Fig. 4.
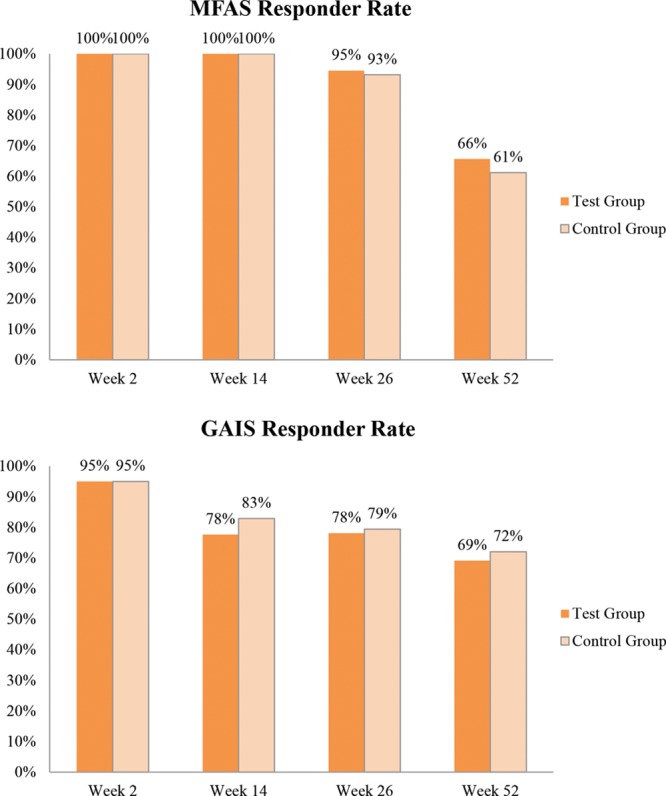
A, MFAS responder rate evaluated by the independent blinded raters (full analysis set); (B) GAIS responder rate evaluated by the subjects (full analysis set).
The GAIS responder rates (improvement by at least 1 grade compared to pre-treatment) were 78% and 79% in the YVOC and RESS groups at week 26 and 69% and 72% at week 52, respectively (Fig. 4B). Moreover, no significant difference was observed between the groups at all time points of measurement. Figure 5 shows a pre- and post-treatment photograph of a representative subject.
Fig. 5.
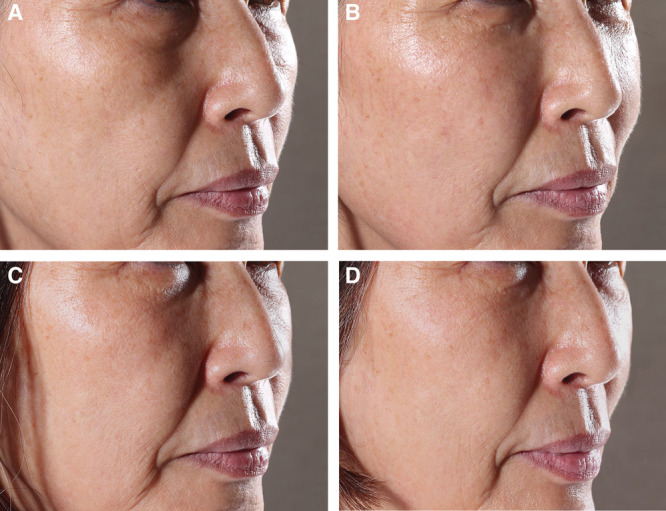
Photographs of a representative subject (A) before treatment, (B) 2 weeks after (optimal state), (C) 26 weeks after, and (D) 52 weeks after HA filler injection into the anteromedial malar region.
The incidence rates of solicited local AEs were in a range of 36%–93%, showing a similar level between the groups (Table 5). All the solicited local AEs were recovered within 14 days after HA filler injection. In addition, the subgroup analysis, divided by the median of the total injection volume in each treatment group, showed that the incidence and duration of solicited local AEs were similar in the low and large volume subgroups [see table, Supplemental Digital Content 2, which displays solicited local AEs by total injection volume after initial injections (safety set), http://links.lww.com/PSYMED/A601).
Table 5.
Solicited Local AEs after Initial Injections (Safety Set)
| YVOC (N = 83) | RESS (N = 83) | |
|---|---|---|
| Any solicited AEs, n (%) | 82 (98.80) | 83 (100.00) |
| Pain, n (%) | 77 (92.77) | 74 (89.16) |
| Tenderness, n (%) | 77 (92.77) | 81 (97.59) |
| Swelling, n (%) | 65 (78.31) | 68 (81.93) |
| Redness, n (%) | 49 (59.04) | 52 (62.65) |
| Bruising, n (%) | 30 (36.14) | 28 (33.73) |
| Itching, n (%) | 27 (32.53) | 31 (37.35) |
| Papule, n (%) | 31 (37.35) | 31 (37.35) |
| Pigmentation, n (%) | 30 (36.14) | 30 (36.14) |
| Duration, days | ||
| Pain, mean (SD) | 3.70 (4.29) | 3.84 (4.13) |
| Tenderness, mean (SD) | 8.57 (9.83) | 6.85 (5.71) |
| Swelling, mean (SD) | 12.71 (16.10) | 10.54 (14.20) |
| Redness, mean (SD) | 4.06 (4.03) | 4.13 (3.78) |
| Bruising, mean (SD) | 9.03 (10.62) | 5.25 (3.95) |
| Itching, mean (SD) | 4.89 (5.50) | 4.87 (5.40) |
| Papule, mean (SD) | 10.48 (15.74) | 9.61 (11.68) |
| Pigmentation, mean (SD) | 6.67 (9.75) | 6.17 (6.43) |
The most frequently reported AEs were gastroenteritis (3.6%) and upper respiratory tract infection (3.6%), which were not related to HA filler injection. Interestingly, delayed-onset swelling of the face was observed in one case. This subject experienced the immediate solicited local AEs including pain, tenderness, swelling, redness, and pigmentation, which recovered completely within 4 days after HA filler injection. However, mild swelling was observed in the RESS injected side at day 31 and also in the YVOC injected side at day 62. This fully recovered within 5 days with administration of steroids and antibiotics.
DISCUSSION
To the best of our knowledge, this was the first clinical study of follow-up for a long-term of 1 year after HA filler injection for the anteromedial malar volume restoration in the mid-face in Korean subjects. In this study, YVOC and RESS were injected into the anteromedial malar region in each side of the mid-face to evaluate the effectiveness and safety of HA fillers injected for volume augmentation. Since both products are large particle-stabilized HA-based fillers, which are most similar in particle form as composed through a biphasic cross-linking procedure of HA and also similar in overall composition and physicochemical properties, they were suitable for assessing the long-term effectiveness and safety of HA fillers and also comparison of the differences in HA fillers.
This study was conducted as a split-face design in which YVOC and RESS were injected into the anteromedial malar region in each side of a subject, and thus, each subject functioned as the test group as well as the control group, minimizing the difference in characteristics of subjects between the groups. In addition, to eliminate the bias by the treating investigator in the effectiveness evaluation, the MFAS scores evaluated by 3 independent blinded raters were used, and the subjects evaluating the GAIS were also kept blinded.
In the evaluation of MFAS scores, the volume augmentation was in optimal state at week 2 after HA filler injection. Then, the LS means of MFAS score increased gradually up to week 52. At week 52, the LS mean of MFAS score was still lower than those at baseline in both groups, showing the sustained effectiveness of volume augmentation in the anteromedial malar region up to week 52 after HA filler injection. Moreover, at week 52, MFAS responder rates were 66% and 61% in the YVOC and RESS groups, respectively.
Not only the results of the primary effectiveness endpoint, but the results of the other endpoints, including the mean MFAS score and the mean GAIS score at each evaluation time point, show similar trends between the YVOC and RESS groups. As RESS has already been established for its effectiveness through many studies,10–15 the effectiveness and safety of YVOC in anteromedial malar volume restoration of Asian could be confirmed through this study.
Compared with Caucasians’ data16 regarding the improvement rate of 83.2% at month 6 after HA filler injection, the responder rates of this study were higher up to 95% and 93%. Considering the differences in facial structure and optimal correction for each subject between Caucasians and Asians, a little more injected HA volume in this study, and the difference in volume evaluation scales might have resulted in the difference in the responder rates.
As mentioned before, a delayed-onset swelling was reported as shown in the other reports.17–22 The exact causes of this phenomenon have not been identified; it is proposed that it may be due to infection during injection, larger boluses or higher volumes, injection technique and placement, immune system activation of the subject, residual bacterial and/or avian proteins from the HA production process, impurities, or remnants of the chemical agents used in the cross-linking (stabilizing) process.17–22 In this case of the present study, we did not conduct pathologic examination or MRI imaging, because the symptom was mild and the subject did not want to undergo any other procedures. Although the cause of this reaction was not found, it fully recovered within 5 days after administration of steroids and antibiotics, and the causal relationship with HA filler injection was evaluated as unlikely.
In the present study, the subjects were followed up for 1 year after HA filler injection, but the number of subjects who could be evaluated at week 52 was reduced to 68. This may be considered a limitation. Most subjects were withdrawn due to withdrawal of consent or follow-up loss during the follow-up period, suggesting that the subjects dropped out of the study because of the prohibition of additional aesthetic treatments such as other fillers or botulinum toxin treatment on the face during the follow-up period. However, this was inevitable for evaluating the long-term effectiveness and safety of injected HA fillers. In addition, the drop-out rate was assumed to be 20%, but the actual drop-out rate was lower than this, and there was no effect on the effectiveness evaluation.
As an extension of this study, long-term follow-up for up to 2 years after HA filler injection was conducted, and the results will be addressed later.
CONCLUSIONS
It was confirmed that the HA fillers injected in the anteromedial malar region for volume augmentation of Asians are quite effective at week 26 and the volume was well maintained up to week 52. It was also proved that YVOC shows similar effectiveness and safety profiles as RESS.
ACKNOWLEDGMENTS
We thank Won-Serk Kim, MD, PhD (Department of Dermatology, Kangbuk Samsung Hospital, Seoul, Republic of Korea); Sung-Eun Chang, MD, PhD (Department of Dermatology, Asan Medical Center, Seoul, Republic of Korea); Jong Hee Lee, MD, PhD (Department of Dermatology, Samsung Medical Center, Seoul, Republic of Korea); and Je-Ho Mun, MD, PhD (Department of Dermatology, Seoul National University Hospital, Seoul, Republic of Korea) for their contribution as independent blinded raters in this study.
The study was conducted in compliance with the ethical guidelines of the Declaration of Helsinki and Good Clinical Practices and was approved by the institutional review board of each study site.
Supplementary Material
Footnotes
Published online 26 February 2020.
Disclosure: Yunae Eom and So Dam Yang are employees of LG Chem, Ltd. The other authors have no conflicts to declare. This study was sponsored by LG Chem, Ltd., Seoul, Republic of Korea.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
The study was registered at ClinicalTrials.gov (identifier: NCT02119780) on April 22, 2014.
REFERENCES
- 1.Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JL, Dayan SH, Brandt FS, et al. Systematic review of clinical trials of small- and large-gel-particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg. 2013;39:205–231. [DOI] [PubMed] [Google Scholar]
- 3.De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39:1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Kim SH, Park ES. The efficacy and safety of HA IDF plus (with lidocaine) versus HA IDF (without lidocaine) in nasolabial folds injection: a randomized, multicenter, double-blind, split-face study. Aesthetic Plast Surg. 2017;41:422–428. [DOI] [PubMed] [Google Scholar]
- 5.Rho NK, Chang YY, Chao YY, et al. Consensus recommendations for optimal augmentation of the Asian face with hyaluronic acid and calcium hydroxylapatite fillers. Plast Reconstr Surg. 2015;136:940–956. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SC. Differences between Caucasian and Asian attractive faces. Skin Res Technol. 2018;24:73–79. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Niddam J, Noel W, et al. Comparison of aesthetic facial criteria between Caucasian and East Asian female populations: an esthetic surgeon’s perspective. Asian J Surg. 2018;41:4–11. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers J, Flynn TC, Geister TL, et al. Validated assessment scales for the mid face. Dermatol Surg. 2012;382 Spec No320–332. [DOI] [PubMed] [Google Scholar]
- 9.Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. [DOI] [PubMed] [Google Scholar]
- 10.DeLorenzi C, Weinberg M, Solish N, et al. The long-term efficacy and safety of a subcutaneously injected large-particle stabilized hyaluronic acid-based gel of nonanimal origin in esthetic facial contouring. Dermatol Surg. 2009;35suppl 1313–321. [DOI] [PubMed] [Google Scholar]
- 11.Verpaele A, Strand A. Restylane subq, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006;261SS10–S17. [DOI] [PubMed] [Google Scholar]
- 12.Belmontesi M, Grover R, Verpaele A. Transdermal injection of restylane subq for aesthetic contouring of the cheeks, chin, and mandible. Aesthet Surg J. 2006;261SS28–S34. [DOI] [PubMed] [Google Scholar]
- 13.Sito G. Transoral injection of restylane subq for aesthetic contouring of the cheeks. Aesthet Surg J. 2006;261SS22–S27. [DOI] [PubMed] [Google Scholar]
- 14.Bugge H, Negaard A, Skeie L, et al. Hyaluronic acid treatment of facial fat atrophy in HIV-positive patients. HIV Med. 2007;8:475–482. [DOI] [PubMed] [Google Scholar]
- 15.Skeie L, Bugge H, Negaard A, et al. Large particle hyaluronic acid for the treatment of facial lipoatrophy in HIV-positive patients: 3-year follow-up study. HIV Med. 2010;11:170–177. [DOI] [PubMed] [Google Scholar]
- 16.Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602–1612. [DOI] [PubMed] [Google Scholar]
- 17.Beleznay K, Carruthers JD, Carruthers A, et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/ml hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41:929–939. [DOI] [PubMed] [Google Scholar]
- 18.O’Reilly P, Malhotra R. Delayed hypersensitivity reaction to restylane ® subq. Orbit. 2011;30:54–57. [DOI] [PubMed] [Google Scholar]
- 19.Baeva LF, Lyle DB, Rios M, et al. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1β production. J Biomed Mater Res A. 2014;102:305–314. [DOI] [PubMed] [Google Scholar]
- 20.Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;3111 pt 21616–1625. [PubMed] [Google Scholar]
- 21.Edwards PC, Fantasia JE. Review of long-term adverse effects associated with the use of chemically-modified animal and nonanimal source hyaluronic acid dermal fillers. Clin Interv Aging. 2007;2:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellman B. Immediate and delayed hypersensitivity reactions to restylane. Aesthet Surg J. 2005;25:489–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


