Abstract
Background:
Composite reconstruction with a dermal substitute followed by skin graft is sometimes used for reconstructing high-quality skin while preserving donor sites. This often necessitates 2 separate procedures, additional general anesthetic, and longer hospitalization. Concurrent use of dermal substitutes and skin graft in a single stage has been previously reported in small series. Here, we report our experience with single-stage skin reconstruction with Integra and split-thickness skin graft for coverage of wounds post burn eschar excision and post burn scar contracture release.
Methods:
This is a retrospective review of consecutive operations from 2013 to 2017 in which single-stage bilayer reconstruction (SSBR) was performed. Data were obtained from electronic medical records and perioperative photographs.
Results:
In this 5-year period, 13 surgical sites were identified in which SSBR was used in 8 subjects. Average and median graft take was 86.2% and 95%, respectively. Graft take was over 90% in 10 out of 13 cases. One case required regrafting after initial graft failure.
Conclusions:
In the appropriate setting, SSBR is a practical technique in covering wounds post burn eschar excision and post burn scar contracture release resulting in reasonable graft take. Use of noncontaminated wound beds is crucial. Although there is risk of regrafting, it is not clear whether this risk is any higher than in split-thickness skin grafting alone. This study was unable to evaluate contribution of dermal substitute to contraction, function, and mobility, nor how hypothesized improvement of skin quality compares to the original thick dermal substitute. We recommend further investigation.
INTRODUCTION
Skin defects that result from burn eschar excisions or burn scar contracture releases are typically reconstructed using a skin graft. These wounds associated with burn injuries are a clinically challenging problem that has led to the development of dermal replacement matrices to augment and improve the regeneration of the dermis. Traditionally, these matrices are applied in a 2-stage fashion. This process provides time for the dermal matrix, which is inherently avascular, to incorporate and vascularize before being covered with an overlying split-thickness skin graft (STSG). The 2-stage technique is well described in the literature, and various methods have been used to evaluate outcomes. Landmark studies broadly define success with percentage graft take and recurrence rate. In 2003, Heimbach et al achieved mean 87.7% epidermal autograft take using the traditional 2-stage technique on 589 burn wound sites.1 Frame et al found a 25% recurrence rate—that is, in 35 of 127 sites—after the 2-stage approach in which scar contracture returned to the point of limited functionality after healing.2
Although the 2-stage technique has been largely reliable, it necessitates a 2- to 3-week treatment course and requires a minimum of 2 operations and anesthetic administrations. The 2-stage technique is also associated with a longer hospitalization, and an increased number of outpatient visits.
Consequently, the application of an STSG to a dermal replacement matrix in a single operation is hypothesized to yield substantial reductions in hospital length of stay, operative encounters, outpatient visits, and health-care expenditures. The single-stage technique was initially described over a decade ago and has been repeated with some success in case reports and small cases series among different fields including orthopedic trauma, facial avulsive defects, and others.3–8
In porcine models, we found a single-stage bilayer reconstruction (SSBR) to provide nearly full graft take when the dermal substitute is perforated and is less than 0.4 mm thick. These preliminary results subsequently led us to apply this technique to the patients at our institution with postburn soft-tissue defects. The purpose of this study is to provide an updated account of the single-stage use of Integra and STSG for skin reconstruction.
METHODS
This case series was performed under the auspices of a retrospective study approved by the institutional review board of the US Army Medical Research and Materiel Command. The electronic medical record was reviewed (US Army Institute of Surgical Research and San Antonio Military Medical Center) for procedures in which the SSBR technique was utilized on burn injuries. These wounds ranged from soft-tissue defects following contracture release to excised burn wounds.
Surgical Technique
Each procedure included in this series was performed by the senior author (RKC) using the SSBR technique. For acute wounds, excisional preparation and debridement of any necrotic tissue was completed to prepare each graft site for application of the dermal matrix and STSG. In late reconstructions, wounds were created after a release was performed to traverse a broadband linear contracture. In each case, single-layer Integra wound matrix was perforated using a noncrushing mesher at a 1:1 ratio before application onto the wound bed. The thickness of the Integra matrix used in each case is specified in Table 1. An STSG was then obtained from the patient, at a depth of 10/1,000th of an inch using a pneumatic dermatome (Zimmer Surgical Inc., Dover, OH). The graft was also meshed in a 1:1 ratio and secured onto the Integra matrix overlying the wound bed using dissolvable sutures. Xeroform gauze was then trimmed to cover the graft recipient site. Each of the graft sites was dressed using a negative pressure wound dressing (NPWD), which was placed to continuous suction (–125 mm Hg) for 5 days. When applicable, a prefabricated or custom splint was fashioned to achieve the necessary immobilization during the initial healing stages. After the removal of the NPWD, daily dressing changes were completed using Xeroform under a white gauze dressing secured with medical tape. Immobilization was continued until postoperative days 7–15, at which point passive and active range of motion therapy were begun. Regular follow-ups took place at postoperative day 5 with NPWD removal, at postoperative days 7 and 15, and then at postoperative week 4. Mean follow-up length for the purpose of this study was 4 months.
Table 1.
Thirteen Wounds in 8 Subjects Were Included in This Case Series
| Patient Age | Sex | Anatomic Site of Wound | Nature of Wound | Bilayer Components | Graft Take (%) |
|---|---|---|---|---|---|
| 21 | M | Posterior knee | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.012 in | 50 |
| 64 | F | Dorsal fourth digit of hand | Acute | Integra Wound Matrix (Thin); STSG 0.01 in | 100 |
| Palm and forearm | Acute | Integra Wound Matrix (Thin); STSG 0.01 in | 100 | ||
| Breast | Acute | Integra Wound Matrix (Thin); STSG 0.01 in | 100 | ||
| 30 | M | Posterior knee | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.012 in | 75 |
| 19 | M | Elbow | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.01 in | 95 |
| Trunk | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.012 in | 100 | ||
| Axilla | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.012 in | 100 | ||
| 23 | M | Elbow | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.01 in | 90 |
| 38 | M | Radial forearm free flap donor defect | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.01 in | 95 |
| 30 | M | Radial forearm free flap donor defect | Postcontracture release | Integra Wound Matrix (Thin); STSG 0.01 in | 100 |
| 47 | M | Posterior knee | Postcontracture release | Integra Wound Matrix; STSG 0.012 in | <25 |
| Posterior knee (regrafting) | Postcontracture release | Integra Wound Matrix; STSG 0.012 in | 90 |
Respective graft take for each site is listed. One patient underwent regrafting for poor initial graft take take; this revision is included as the 13th wound in the series.
RESULTS
Thirteen graft sites in 8 patients were identified within a 5-year period between 2013 and 2017. The spectrum of cases treated in this series was composed largely of postburn scar contracture release in function-limiting areas. Two cases were secondary to wounds that resulted after the acute excision of burn eschar. Two cases were included that demonstrated successful coverage of a free flap donor site with tendon exposure (paratenon preserved).
As previously described, each case was treated using a single-stage STSG and dermal substitute composite. Healing was mostly by primary intention, and any small open areas healed within 2 weeks of the operation.
Outcomes were retrospectively evaluated in terms of percentage of epidermal graft take. The final assessment in each case was made by the senior author (RKC). Mean graft take was 86.2%, with a median of 95%. Ten of the 13 graft sites demonstrated greater than 90% take (Table 1). One site required a revision graft after failure of the initial graft, with less than 25% take by the second postoperative visit. The site was regrafted using the STSG without a dermal matrix and yielded 90% overall take.
Range of motion was not consistently documented or quantitatively assessed in all subjects, and therefore was not included as an outcome measure. However, none of the wounds in the series recontracted to their original state and indeed demonstrated a functionally acceptable outcome without the need for further releases.
Several representative cases were included. Figure 1 depicts the results of the SSBR technique as applied to a full-thickness burn to the hand and distal forearm of a 64-year-old woman. Amputation of the distal aspects of digits 3, 4, and 5 had occurred earlier in her treatment course. She underwent SSBR of the burn wounds. Six months following the procedure, the patients wound had fully healed with 100% graft take. The same patient also underwent SSBR for a burn on her breast, the results of which were equally successful (Fig 2).
Fig. 1.
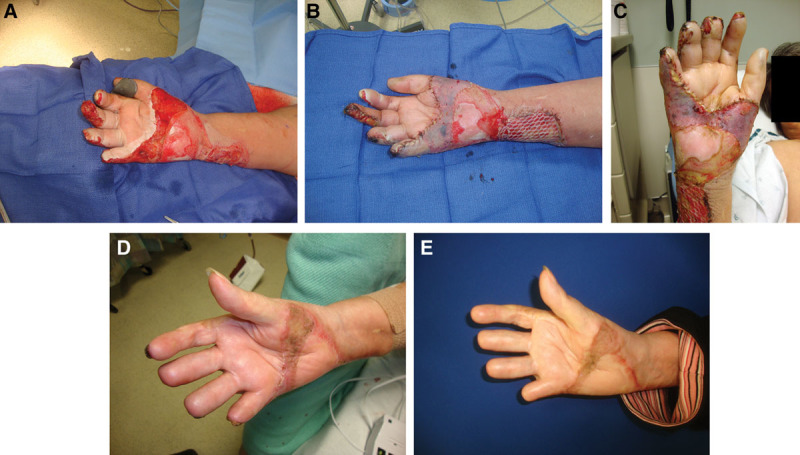
A 64-year-old woman with full-thickness burns to the forearm and hand (A). Patient underwent SSBR and was seen in clinic on postoperative day 4 (B). Follow-up at postoperative week 2 showed good graft take (C). At months 2 and 6 (D and E, respectively), the patient had developed a well-healed scar with no remaining wounds.
Fig. 2.
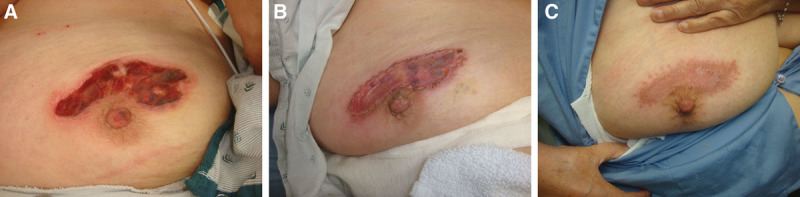
Full-thickness burn to breast adjacent to the nipple-areolar complex (A). Postoperative day 10 showed good take of the overlying STSG (B). The area was completely healed at postoperative month 2 (C).
Figure 3 outlines the course of a 19-year-old man with an axillary burn scar contracture from a 35% total body surface area burn 7 years before presentation to our center. The contracture was released with a combination of a large z-plasty in the axilla and SSBR of the remaining release wound of the lateral trunk. Graft take was 100%, and 2 months postoperatively, the patient had well-healed scars.
Fig. 3.
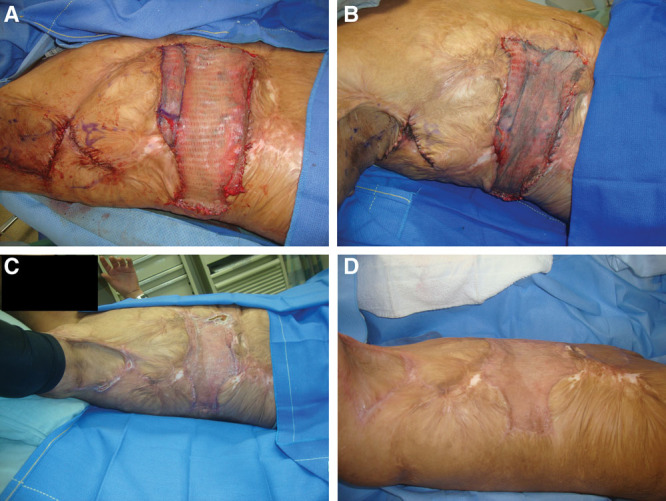
A 19-year-old man with burn scars on the torso. In each image, patient is in left lateral decubitus position with right lateral torso exposed and right axilla located at the top portion of the photograph. Patient underwent axillary release with z-plasty and SSBR of residual burn scar contracture (A). Evaluation on postoperative day 5 showed intact STSG with some underlying hematoma (B). At postoperative month 1, the site was healing well with full graft take (C). There was a well-healed scar at postoperative month 2 (D).
DISCUSSION
Soft-tissue injuries requiring some level of reconstruction result from various mechanisms including burn, ballistic, blast, crush, avulsion, infection, malignancy, and surgically induced defects. Deep wounds often require complex management and a lengthy recovery time. The use of a dermal regeneration template in these cases has been widely described in the literature for well over the past decade.9 Ongoing clinical innovation within this arena has led to ideas to streamline further the use and increase the potential of this surgical tool. One of these improvements has been that of the single-stage approach, as described earlier. The SSBR technique for soft-tissue reconstruction shows promise in reducing the number of operations, episodes of general anesthetic, and length of treatment time.
The goal of SSBR is to provide the patient with the benefit of high-quality skin in which the wound does not contract as much as compared to STSG alone. We define “high-quality skin” as that which has less contraction while possessing increased elasticity, stretch, and improved texture. The advantage of using a dermal matrix is the provision of more dermis to the wound, which theoretically results in better skin. Given the avascular nature of the dermal matrix, it is not advisable to use this material in acutely infected or contaminated wounds. In fact, most acute burn wounds are at the very least contaminated and often times with necrotic tissue still present despite excisional preparation. It is unclear whether there would be any late benefits of using a dermal regeneration template in this setting. Our results have shown that it is best to cover scar contracture release wounds and wounds that are at least clean, if not sterile.
Whenever the use of a dermal substitute is considered, wound bed condition is one of the most important determinants of success, and proper patient selection is crucial. Compared to native skin grafts, dermal matrices do not have integral vascular channels which allow bacteria-fighting dermal blood flow relatively early via inosculation. Until vascular channels can grow into the dermal matrix, it is inert collagen in the plasma environment of the wound, and less likely to resist the development of infection.10,11 Situations in which a traditional 2-stage technique may be appropriate over a single-stage approach include when additional time is needed for a thicker dermal layer to vascularize sufficiently to support an overlying skin graft. Acute wounds in which necrotic tissue may not yet be fully declared would also not be an ideal choice for SSBR, and wounds that are colonized or actively infected, as noted earlier. The 2-stage technique may also be better advised for wounds that involve deeper structures, including tendons or denuded bone, and those wounds overlying or approaching open joints, as the dermal matrix vascularization is slower. Finally, SSBR may not be the best choice for treatment of superficial wounds or patients with ample donor skin for which a traditional STSG would be adequate.
We suggest that the SSBR approach be applied in cases in which the wound indicates it will successfully receive an autograft. Appropriate cases include wounds requiring added tissue thickness or regeneration of the dermis. The benefit of the SSBR approach is that vascularization of the neodermis and epithelialization of the skin graft may occur at the same time on a fresh wound bed. Furthermore, use of an NPWD as the initial dressing has been shown to offset potential complications such as infection, seromas, and hematomas.12 With thin dermal matrix, NPWD most likely allows adequate transfer of plasma through the perforated matrix to keep the overlying STSG nourished early in the healing process.
The gold standard to which all skin substitutes are compared is the autologous full-thickness skin graft (FTSG). The downside of using an FTSG is that, to obtain it, one must cause an iatrogenic full-thickness wound that potentially has the morbidity of the original injury. The advantage of a skin substitute is reducing the morbidity associated with FTSG. The dermal layer of skin plays a key role in wound healing and improved outcomes, and achieving a better scar and comfort is often the most important factor for a patient. Some dermal matrices have been noted to decrease undesirable outcomes, such as hypertrophic scarring and pruritus. As previously cited, when paired with a thin STSG, Integra may give the same result as a wound treated with a thicker STSG alone.1 Unfortunately, despite the advances made over the years in the development of skin substitutes, there is yet to be a product that can be deemed equivalent to autologous skin.13 That being said, even with the introduction of new surgical techniques, there is no conclusive evidence that skin substitutes in any format—that is, whether applied in a single- or 2-stage procedure—are superior to the autologous skin graft.
Our results are consistent with those previously reported in the literature. In 2012, Gabriel et al presented a case series of 20 patients who had undergone single-stage reconstruction on small wounds with an average graft take of 98.3%.14 Our study differs in that our patient base is composed of prior burn injuries, largely complicated by contracture where maintenance of the release is paramount. The main purpose in our surgical approach was to improve patients’ functionality. We therefore placed emphasis on achieving a high-quality reconstruction versus simply wound closure.
Although the majority of patients in this series had a good outcome, there was 1 case that resulted in the failure of graft take. The patient is a 47-year-old man who presented with a posterior knee burn scar contracture with a central chronic open wound. This wound was the site of a Vibrio infection which had occurred 6 months prior and was subsequently debrided. We hypothesized that the wound failed to heal because of tension that has developed on the posterior thigh and leg. We decided that contracture release to relief tension will improve the healing of the chronic wound. The patient underwent excision of the nonhealing wound, cleaning of the wound bed, and then contracture release of the posterior knee with single-stage Integra and STSG. In retrospect, any chronic open wound is colonized by a massive number of bacteria, and even in the absence of clinical infection and despite great care to isolate the contracture release from the chronic wound, it is almost inevitable that cross-contamination occurred and could explain the graft loss. Additionally, a notable difference between this wound and the others is that this was the only case in which the thicker Integra (0.8 versus 0.4 mm) was used. It could be that the thickness of the dermal substitute played a role in this graft’s failure. Either way, this case demonstrated the risk of regrafting following SSBR; however, it is not clear whether this risk is any higher than that of split-thickness skin grafting alone. Interestingly, all grafts in this series achieving <90% take were noted to be in the posterior knee region. The notable degree of tension in this particular area could have contributed to the decreased degree of success with these grafts.
Limitations of this study include small sample size, lack of a control group, and retrospective design. The subjective nature of graft take assessment should be made more objective to improve validity. A randomized controlled trial would be beneficial to further investigate the positive implications of SSBR, particularly in postcontracture release burns. It would also be interesting to evaluate the effect of dermal substitutes on contraction, function, and mobility in these patients. Furthermore, a single brand of dermal matrix (Integra) was used in each case in this series. Consideration was given to the use of other dermal substitutes; however, Integra was selected given our previous experience with this product at our institution. Future studies could compare various dermal substitutes, as we did in our aforementioned porcine models. Given the potential variation in graft take among these products, we decided to use a single brand of matrix in this series for a less confounded assessment.
CONCLUSIONS
Single-stage composite skin reconstruction using a dermal regeneration template is useful to obtain soft-tissue coverage in well-selected adult patients. This single-stage technique decreases treatment time by reducing the number of operations needed to reconstruct a soft-tissue defect. SSBR shows promise in reducing health-care utilization, improving patient comfort, and limiting health-care expenditures while still providing the benefits of augmented dermal regeneration. Further studies are warranted to determine the independent effect of dermal substitutes on contraction, function, mobility, and risk of regrafting as compared to split-thickness skin grafting alone.
ACKNOWLEDGEMENT
The authors would like to thank Dr. Robert Spence for his critical review of the manuscript.
Footnotes
Published online 24 February 2020.
Disclosure: The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, Department of Defense, or the US Government. The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24:42–48. [DOI] [PubMed] [Google Scholar]
- 2.Frame JD, Still J, Lakhel-LeCoadou A, et al. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg. 2004;113:1330–1338. [DOI] [PubMed] [Google Scholar]
- 3.Burd A, Wong PS. One-stage Integra reconstruction in head and neck defects. J Plast Reconstr Aesthet Surg. 2010;63:404–409. [DOI] [PubMed] [Google Scholar]
- 4.Demiri E, Papaconstantinou A, Dionyssiou D, et al. Reconstruction of skin avulsion injuries of the upper extremity with Integra dermal regeneration template and skin grafts in a single-stage procedure. Arch Orthop Trauma Surg. 2013;133:1521–1526. [DOI] [PubMed] [Google Scholar]
- 5.Haifei S, Xingang W, Shoucheng W, et al. The effect of collagen-chitosan porous scaffold thickness on dermal regeneration in a one-stage grafting procedure. J Mech Behav Biomed Mater. 2014;29:114–125. [DOI] [PubMed] [Google Scholar]
- 6.Koenen W, Felcht M, Vockenroth K, et al. One-stage reconstruction of deep facial defects with a single layer dermal regeneration template. J Eur Acad Dermatol Venereol. 2011;25:788–793. [DOI] [PubMed] [Google Scholar]
- 7.Kosutic D, Beasung E, Dempsey M, et al. Single-layer Integra for one-stage reconstruction of scalp defects with exposed bone following full-thickness burn injury: a novel technique. Burns. 2012;38:143–145. [DOI] [PubMed] [Google Scholar]
- 8.Papa G, Pangos M, Renzi N, et al. Five years of experience using a dermal substitute: indications, histologic studies, and first results using a new single-layer tool. Dermatol Surg. 2011;37:1631–1637. [DOI] [PubMed] [Google Scholar]
- 9.Yannas IV, Orgill DP, Burke JF. Template for skin regeneration. Plast Reconstr Surg. 2011;127suppl 160S–70S. [DOI] [PubMed] [Google Scholar]
- 10.Hendrickx B, Vranckx JJ, Luttun A. Cell-based vascularization strategies for skin tissue engineering. Tissue Eng Part B Rev. 2011;17:13–24. [DOI] [PubMed] [Google Scholar]
- 11.Frueh FS, Sanchez-Macedo N, Calcagni M, et al. The crucial role of vascularization and lymphangiogenesis in skin reconstruction. Eur Surg Res. 2018;59:242–254. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin C, Potter M, Clayton E, et al. Topical negative pressure stimulates endothelial migration and proliferation: a suggested mechanism for improved integration of Integra. Ann Plast Surg. 2009;62:92–96. [DOI] [PubMed] [Google Scholar]
- 13.Haddad AG, Giatsidis G, Orgill DP, et al. Skin substitutes and bioscaffolds: temporary and permanent coverage. Clin Plast Surg. 2017;44:627–634. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel A, Wong W, Gupta S. Single-stage reconstruction for soft tissue defects: a case series. Ostomy Wound Manage. 2012;58:30–32. [PubMed] [Google Scholar]


