Supplemental Digital Content is available in the text.
Abstract
Background:
Adipose stromal vascular fraction (SVF) isolation with enzymatic digestion is the gold standard, but is expensive, having practical and legal concerns. The alternative mechanical SVF isolation methods provide lower cell yields as they employ either centrifugation, emulsification, or digestion steps alone. We combined mechanical processing with buffer incubation and centrifugation steps into an isolation method called “mechanical digestion” and compared the cell yields with that of enzymatic digestion.
Methods:
A total of 40-mL lipoaspirate was harvested from 35 women undergoing liposuction and was submitted to conventional enzymatic digestion for SVF isolation or mechanical digestion using a closed unit harnessing 3 ports with blades, followed by buffer incubation and centrifugation. Culture of the SVFs and flow cytometry were performed.
Results:
The SVF cell yield obtained by enzymatic digestion was significantly higher 3.38 × 106/mL (±3.63; n = 35) than that obtained by mechanical digestion 1.34 × 106/mL (±1.69; n = 35), P = 0.015. The average cell viability was 82.86% ± 10.68 after enzymatic digestion versus 85.86% ± 5.74 after mechanical digestion, which was not significant. Mechanical digested SVF expressed 2-fold higher stem cell surface markers compared with enzymatically digested SVF. Mechanical digestion was less time consuming, cost effective, and did not require a specific laboratory environment.
Conclusions:
Mechanically digested SVF was comparable to enzymatically digested SVF in terms of stromal cell composition and viability. With mechanical digestion, we can isolate 30%–50% SVF cells of that isolated with enzymatic digestion. Further studies are warranted to determine the clinical outcomes.
INTRODUCTION
Autologous adipose-derived stromal vascular fraction (SVF) is a reliable source for regenerative surgery,1 with diverse applications such as bone regeneration, dentistry, hair growth, chronic wounds, and chronic bowel disease among others.2–4 Enzymatic digestion using collagenase is the conventional processing method to isolate adipose SVF. Approximately 100,000–1,300,000 nucleated cells per gram of lipoaspirate can be obtained with >80% viability.5,6 However, this method is expensive, time consuming (90–120 minutes), and raises legal and administrative concerns.7 Therefore, many methods of mechanical isolation of SVF have surfaced. However, the SVF cell yields obtained mechanically based on shaking, vibration, centrifugation, or washing only are significantly lower8 because they lack the combination of digestion and concentration by centrifugation to match the enzymatic isolation techniques.8 We compared the adipose-derived SVF cell yields, obtained through the conventional enzymatic digestion method and a combined mechanical digestion approach consisting in 3 consecutive steps: gradual mechanical dissociation, buffer incubation, and centrifugation.
The aim of our study was to describe an office-based combined processing method for adipose SVF isolation, which gives a cell yield close and comparable to the one obtained with enzymatic digestion.
METHODS
A total of 35 consecutive women between 26 and 52 years old with a body mass index range of 25–27 kg/m2 undergoing liposuction were selected for this study, which was approved by our institutional review board. From the lipoaspirate harvested from each patient’s lateral thigh using a 2-mm diameter multihole blunt cannula, 40 mL was used for the study. After decantation, the adipose tissue layer was divided into 2 equal parts and submitted to enzymatic or mechanical digestion for SVF isolation. One part was enzymatically digested using GMP graded collagenase NB6 (Serva Electrophoresis, Heidelberg, Germany) at a concentration of 0.1 U/mL and a ratio of 1:1 (v/v), washed and centrifuged twice at 300 g for 5 minutes, the pellet resuspended and drained. For mechanical digestion, ordinary pistons of 20-cc luer-lock syringes were replaced with custom-made disarmable pistons with concave, cell-adhesive gaskets (see figure, Supplemental Digital Content 1, which displays equipment for mechanical digestion of SVF. Custom-made metallic disarmable pistons, concave cell-adhesive gaskets, closed cubic unit harnessing 3 different-sized sets of blade grids on each luer-lock port, rotating canal at the center of the cube to control the flow of the lipoaspirate, http://links.lww.com/PRSGO/B308). The lipoaspirate was transferred into syringes, connected to a closed unit, harnessing 3 different sets of blade grids on 3 luer-lock ports on a rotating canal. The lipoaspirate was placed in the first port, passed back-and-forth 10 times through the first blade grid containing multiple 1000-micron holes. The direction of the rotating canal was changed to the second port, and the lipoaspirate was passed through the second blade grid containing 750-micron holes and through the 500-micron holes blade grid for full dissociation (Fig. 1). A calcium–magnesium (Ca–Mg) balanced buffer solution was added to the lipoaspirate in the syringes at a ratio of 1:3, incubated and shaken for 10 minutes to wash the erythrocytes and cell debris. The pistons were disattached, and the syringes containing the dissociated lipoaspirate were centrifuged at 2000g for 10 minutes9 with the luer-lock tips directed inward so that the SVF could be collected in concave gaskets (Fig. 2). The pistons were reattached, the supernatant discarded, and the pellet resuspended [See Video 1 [online], which shows the lipoaspirate being transferred into syringes, connected to a closed unit, harnessing 3 different sets of blade grids (1000, 750, and 500 μm) on 3 luer-lock ports on a rotating canal]. The total nucleated cell number and viability of the 2 groups of SVF were determined by flow cytometer (Navios Flow Cytometer - Beckman Coulter, 7AAD Viability Dye) after red blood cell lysis. The characterization of Adipose Derived Stem Cell (ADSC) (CD45−, CD90+/CD73+, CD90+), endothelial cells (CD45, CD31+), macrophages, and monocytes (CD45+, CD14+) was performed, stained with 5 µL of monoclonal antibodies (BD Biosciences, Le Pont de Claix, France) (Table 1). The binding efficiency of surface markers CD13, CD73, CD90, CD146, and CD34 was also examined.
Fig. 1.
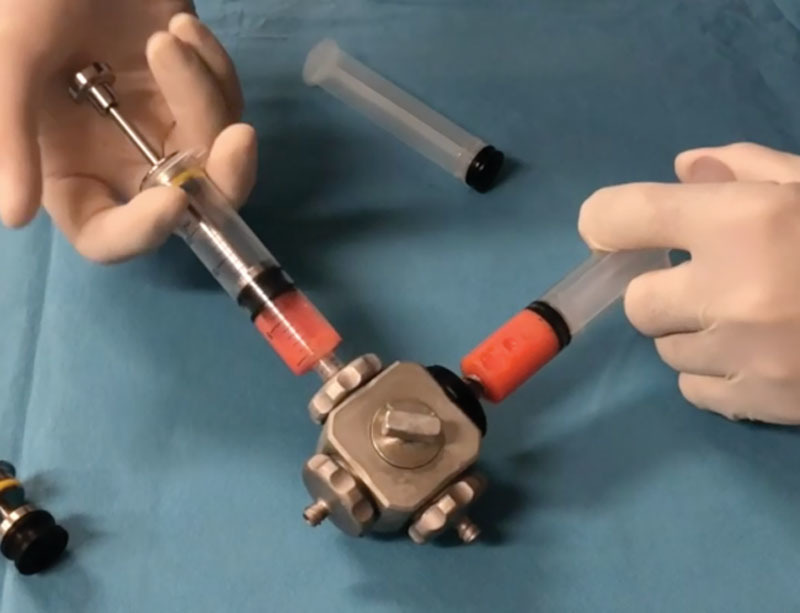
The lipoaspirate connected to the first port is passed back-and-forth 10 times through the first blade grid containing multiple 1000-micron holes. Changing the direction of the rotating canal and the flow in the second port, the lipoaspirate is passed through the second blade grid containing 750-micron holes and through the 500-micron holes blade grid until full dissociation.
Fig. 2.
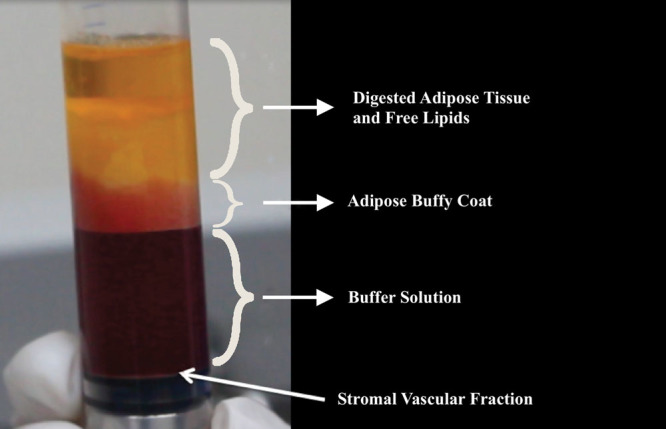
SVF collected over the concave gaskets: from top to bottom, there are 4 layers: buffy coat, adipose tissue, buffer solution, and SVF over the gasket.
Table 1.
Comparison of the Process of Enzymatic Digestion and Mechanical Digestion
| Enzymatic Digestion | Mechanical Digestion | |
|---|---|---|
| Isolation methods | ||
| Addition of the digestive agent | Collagenase | Mincing/filtration with buffer solution |
| Incubation for digestion | 45 min at 37°C | 10 min at room temperature |
| Centrifugation for extraction of the pellet | 300 g for 5 min | 2000 g for 10 min |
| Washing of the reagent | Washing with PBS solution | No washing |
| Cell count and viability | ||
| Cell number | 3.38 × 106/cc | 1.34 × 106/cc |
| Cell viability (%) | 82.86% | 85.82% |
| Flow cytometer results | ||
| CD45(−)/CD73(+) CD90(+)/CD73(+) Adipose-derived stem cell content |
20.22% 31.05% |
42.37% 52.08% |
| CD45(−)/CD31(+) Endothelial cell content |
13.60% | 21.06% |
| CD45(+)/CD14(+) Macrophage/monocyte cell content |
23% | 7.28% |
PBS, phosphate-buffered saline.
Video 1. Mechanical digestion in Three Steps. Video 1 from “A three-step mechanical digestion method to harvest adipose derived stromal vascular fraction: Finding the balance between enzymatic and mechanical isolation techniques”.
Cells were then seeded in T-75 tissue culture plates (Proliferation medium; NutriStem MSC XF Medium/serum free-Biological Industries) at 37°C, at 5% carbon dioxide. After 7 days, cell morphology was observed under light microscopy. The adipogenic differentiation capacity of ADSC in the 2 groups was compared using the StemPro Adipogenesis Differentiation kit and was evaluated by phase contrast microscopy.
Gene expression profile was examined by adipocyte-specific adiponectin and Peroxisome Proliferator-Activated Receptor (PPAR) genes. Primers were designed using Primer-BLAST software from the National Center for Biotechnology (Bethesda, Md.). Total RNA isolation from differentiated cells of the 2 groups was performed using the Total RNA Purification Plus Kit, Norgen, CAN. Student t test was performed to compare cell count and viability parameters with 95% confidence interval and P values <0.05.
RESULTS
The mean number of cells obtained by enzymatic digestion group (EDG) was significantly higher 3.38 × 106/mL (±3.63; n = 35) than that obtained by mechanical digestion group (MDG) 1.34 × 106/mL (±1.69; n = 35), P = 0.015. The average cell viability was 82.86% ± 10.68 after enzymatic digestion versus 85.82% ± 5.74 after mechanical digestion, which was not statistically significant. The CD surface markers of fresh ADSC contents in MDG showed approximately 2-fold increase compared with EDG (52.08% versus 31.05% and 42.37 versus 20.22). The endothelial cell content of MDG was 7.46% higher (21.06% versus 13.60%). The macrophage and monocytes cell content was 3 times higher in EDG (7.28% versus 23%) (Fig. 3).
Fig. 3.
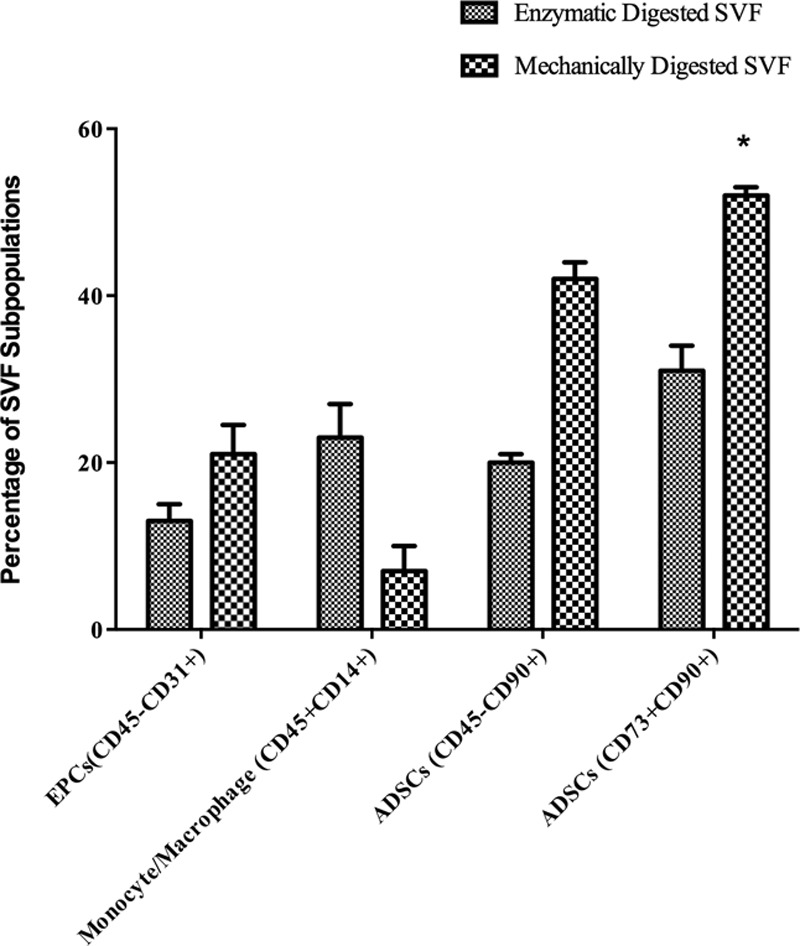
The flow cytometer analysis of SVF subpopulations: endothelial progenitor cells (13.60% vs. 21.06%), monocytes macrophages (23% vs. 7.28%), and ADSCs (31.05% vs. 52.08%–20.22% vs. 42.37%).
MDG demonstrated significantly higher expression of specific phenotypic markers. When ADSC markers of cell activity were compared, we observed a 1.7-fold increase in CD13 (24.75% versus 14.01%), a 1.8-fold increase in CD90 (11.14% versus 6.04%), a 2.3-fold increase in CD146 (17.29% versus 7.4%), and a 2.1-fold increase in CD34 (16.8% versus 7.79%) markers which are commonly used stem cell activity markers [see figure, Supplemental Digital Content 2, which displays the compression of cell phenotypes by flow cytometer. CD34, CD13, CD73, CD90, and CD146 known as universal stem cell markers were 2-fold higher in the mechanically digested SVF group (black line) in comparison with the enzymatically digested SVF group (gray line), http://links.lww.com/PRSGO/B309]. CD13 was used instead of CD105 due to its stability as a known ADSC marker.10
Cell cultures on day 7 showed more cluster formation in the mechanically digested SVF, which may represent better intercellular communication, higher levels of growth factors and neurotransmitters, and consequently better cell growth.11 The messenger RNA (mRNA) expression levels of PPAR2 and adiponectin genes were examined in ADSC after differentiation protocol and were 1.43- and 1.32-fold higher in MDG.
These findings strongly substantiated that mechanical digestion increased the mRNA level of adipocyte complement-related protein, which results in lipid droplets formation, with increase in adipogenic differentiation (see figure, Supplemental Digital Content 3, which displays the differentiation potential: The mechanically digested SVF group had a higher number of lipid droplets examined by phase contrast microscopy. Relative gene expression analysis was performed with adiponectin-related primers such as PPAR and adiponectin. 18S is used for the reference housekeeping gene. mRNA levels showed that the mechanically digested SVF group had approximately 1.3- to 1.4-fold higher PPAR and adiponectin gene expression, http://links.lww.com/PRSGO/B310).
Overall, the mechanical process was cost-effective because it did not require any laboratory environment and was less time consuming, as with mechanical mincing, there is no enzyme to wash (Table 1).
We have used mechanically isolated SVF cell-enriched fat grafting in 46 patients, 21–76 years old for various esthetic and reconstructive applications. No complications have been reported over a 24-month period. One patient requested a secondary fat grafting procedure. The results on a 4-point patient satisfaction scale ranged from good to excellent (Fig. 4).
Fig. 4.
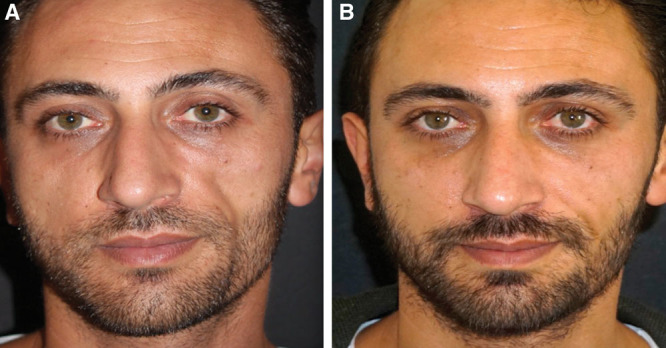
Clinical case of patient affected by Parry-Romberg syndrome. A, Patient with Parry–Romberg syndrome who had previous unsuccessful fat grafting to his left hemiface (preoperative). B, At 12 months postoperative of transfer of mechanically digested SVF cell-enriched fat (80 mL of lipoaspirate was mechanically digested to isolate SVF which was mixed with 46 cc of fat).
DISCUSSION
Many methods of mechanical SVF isolation have surfaced from shaking, vibration, centrifugation, and washing of the lipoaspirate manually and in automated closed devices.12,13 The drawbacks of mechanical SVF isolation methods are the low cell yield (as they consist mostly in centrifuging or vortexing the lipoaspirate, without emulsification or digestion), the high number of peripheral blood cells, and the low number of progenitor cells.9 On the other hand, emulsification of fat alone does not incorporate a concentration step to specifically isolate SVF.14 Because of the high cell yield obtained with enzymatic digestion, it seems crucial to combine mechanical digestion, buffer incubation, and concentration with centrifugation steps to obtain similar cell yields. The cell yield of mechanically digested SVF represented 30%–50% that of enzymatically isolated SVF, probably due to the relative inability with the latter to fully release the stromal cells from the multifaceted connections with the adipose matrix. There was no significant difference in cell viability between the 2 groups.
Cell cultures on day 7 showed more cluster formation and bigger lipid droplets in the mechanically digested SVF, which may represent better intercellular communication, higher levels of growth factors and neurotransmitters, consequently better cell growth.11 Mechanically digested SVF cells had higher differentiation potential in vitro in comparison with enzymatically digested SVF cells. Also, adipocyte content–related gene expression levels of mechanically digested SVF demonstrated higher adipogenesis potential. Some studies have shown that mechanical manipulation and related sheer forces may affect cell functionality and efficacy. The combination of mechanical activation of cells (mincing and emulsification) and our technique of SVF concentration (centrifugation) may explain our sustained preliminary clinical results using mechanically isolated SVF cell-enriched fat grafting.10
Furthermore, this method does not require a specialized environment or personnel, which further contributes to its low cost. The replacement of the pistons of ordinary syringes with disarmable pistons and the closed system harnessing the blades is a practical advantage, which could help reduce contamination. Also, the absence of biologically active substances in this method might ease regulatory constraints.15,16
To our knowledge, the cell yields obtained with this approach is one of the highest reported in the literature, making this approach a viable alternative to enzymatic digestion, which is becoming virtually impossible to use in many countries.16
CONCLUSIONS
Unlike previously described mechanical digestion methods, the combination of sequential mechanical dissociation, buffer incubation, and centrifugation gives comparable results to enzymatic digestion for SVF isolation in terms of stromal vascular cell composition and viability. Even though more lipoaspirate should be processed to harvest the same number of cells, the fact that we can mechanically isolate 30%–50% of stromal vascular cells of that isolated with enzymatic digestion makes this method a viable alternative. Thus, it can be considered as an affordable, safe, and less time-consuming method to isolate SVF. Further studies are being done to optimize the cell counts and determine long-term clinical outcomes.
Supplementary Material
Footnotes
Published online 11 February 2020.
Presented at the 14th Annual International Federation of Adipose Therapeutics and Science (IFATS) meeting, 2016, San Diego, CA; International Master Course on Aging Science Paris (IMCAS Paris), 2018 and 2019, Las Vegas, NV; and Multi-specialty Meeting, 2018, Las Vegas, NV.
Disclosure: Dr. Tiryaki is an investigator for Mentor, has a royalty agreement for his published book, is on the advisory board of Lipocube Ltd., and holds equity in Mage Group and Lipocube Ltd. Dr. Cohen has stock options and royalties with Millennium Medical Technologies, Carlsbad, CA, and royalties with Tulip Medical. He is a shareholder in the Mage Group and receives royalties. He has been an investigator for Allergan and Ampersand, Inc. and an investigator with Thermigen and Alastin. Ms. Canikyan is a genetic engineer at Lipocube Ltd. Dr. Kocak is a Phd student in Yeditepe University. Neither Dr. Kocak nor Dr. Conde'-Green has any financial disclosures.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Tiryaki T, Findikli N, Tiryaki D. Staged stem cell-enriched tissue (SET) injections for soft tissue augmentation in hostile recipient areas: a preliminary report. Aesthetic Surg J. 2008;28:412–416. [DOI] [PubMed] [Google Scholar]
- 2.Savi M, Bocchi L, Fiumana E, et al. Enhanced engraftment and repairing ability of human adipose-derived stem cells, conveyed by pharmacologically active microcarriers continuously releasing HGF and IGF-1, in healing myocardial infarction in rats. J Biomed Mater Res A. 2015;103:3012–3025. [DOI] [PubMed] [Google Scholar]
- 3.Asatrian G, Pham D, Hardy WR, et al. Stem cell technology for bone regeneration: current status and potential applications. Stem Cells Cloning. 2015;8:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaishi K, Arimura Y, Fujimiya M. Stem cell therapy for inflammatory bowel disease. J Gastroenterol. 2015;50:280–286. [DOI] [PubMed] [Google Scholar]
- 5.Aronowitz JA, Ellenhorn JD. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013;132:932e–939e. [DOI] [PubMed] [Google Scholar]
- 6.Condé-Green A, Baptista LS, de Amorin NF, et al. Effects of centrifugation on cell composition and viability of aspirated adipose tissue processed for transplantation. Aesthet Surg J. 2010;30:249–255. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho PP, Gimble JM, Dias IR, et al. Xenofree enzymatic products for the isolation of human adipose-derived stromal/stem cells. Tissue Eng Part C Methods. 2013;19:473–478. [DOI] [PubMed] [Google Scholar]
- 8.Condé-Green A, Kotamarti VS, Sherman LS, et al. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open. 2016;4:e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conde-Green A, Rodriguez RL, Slezak S, et al. Comparison between stromal vascular cells isolation with enzymatic digestion and mechanical processing of aspirated adipose tissue. Plast Reconstr Surg 2014;134:54. [Google Scholar]
- 10.Banyard DA, Sarantopoulos CN, Borovikova AA, et al. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast Reconstr Surg. 2016;138:237e–247e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cebecauer M, Spitaler M, Sergé A, et al. Signalling complexes and clusters: functional advantages and methodological hurdles. J Cell Sci. 2010;123pt 3309–320. [DOI] [PubMed] [Google Scholar]
- 12.Pallua N, Grasys J, Kim BS. Enhancement of progenitor cells by two step centrifugation of emulsified lipoaspirates. Plast Reconstruct Surg. 2018;142:99–109. [DOI] [PubMed] [Google Scholar]
- 13.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–1026. [DOI] [PubMed] [Google Scholar]
- 15.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiryaki T. A novel three-step mechanical digestion method for ADSC. Paper Presented Oral Presentation at the 14th Annual International Federation of Adipose Therapeutics and Science IFATS Meeting; 2016; San Diego, California. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


