Supplemental Digital Content is available in the text.
Abstract
Background:
The nasojugal groove or tear trough (TT) area deformity produces visible, pigmented, difficult-to-treat hollows. Hyaluronic acid (HA) filler–based correction yields variable results and complications. We developed an alternative, minimally invasive treatment for this area.
Methods:
Patients with significant, visible TT area pigmentation, and/or those requiring corrections for TT filler removal, were given lignocaine-diluted calcium hydroxyapatite (CaHA) fillers. CaHA boluses were placed deep on the bone, under the origin of the orbicularis retaining ligament, and under the sub–orbicularis oculi fat. Diluted CaHA was used as a subcutaneous biostimulatory wash. Efficacy and complications were assessed using the Global Aesthetic Improvement Scale and a modified Tear Trough Rating Scale, at 4 and 18 months.
Results:
Twelve patients, between 25 and 52 years of age, were treated and showed immediate improvements in hyperpigmentation due to light reflection and some visibility of the filler through skin. Lower eyelid swelling and redness occurred a few days postinjection but resolved spontaneously. Over 4 to 6 months, hyperpigmentation and skin tone, thickness, and color improved noticeably. Global Aesthetic Improvement Scale and modified Tear Trough Rating Scale scores indicated that all patients experienced satisfactory improvements. Three prior HA patients required a second CaHA treatment at 4–6 months for a satisfactory correction, one of whom required a third CaHA “wash” at 10 months. Some mild redness was observed for up to 12 weeks in a few patients; however, no differences in the degree of redness were observed between those treated for dark circles or post-HA correction. One patient experienced a persistent, dull erythema for 8 months; another had overt erythema and swelling following a chest infection which resolved with antibiotics and hydrocortisone cream. No nodules developed in any patient.
Conclusions:
We developed an alternative TT deformity treatment that leverages CaHA unique rheology and neocollagenesis-stimulating ability, which lifted and supported the prolapsing orbicularis retaining ligament, improved skin quality, and rejuvenated the periocular area without direct injections into the TT.
INTRODUCTION
The tear trough deformity (TTD) is the medial periorbital hollow extending from the medial canthus to the midpupillary line.1,2 Dark undereye circles are caused by shadowing from contour deformities which include maxillary hypoplasia with age-related or congenital recessed inferomedial orbital rims, herniation of orbital fat, and malar fat pad deflation and descent.3–5 Changes in skin quality are also implicated,5–7 due to hyperpigmentation, including melasma, postinflammatory hyperpigmentation, and thin translucent lower eyelid skin which increases orbicularis oculi (OO) muscle and underlying venous circulation visibility. Lax, thin skin can also cause significant lower eyelid rhytids.
Treating the TTD is challenging as its vascular supply and lymphatic drainage can produce significant bruising and edema.8 The tear trough (TT) ligament lies between the palpebral and orbital portions of the OO, where it originates from the maxilla and attaches to the skin. It passes from a medial canthal tendon insertion point to near the medial pupil, where it becomes the bilaminar orbicularis retaining ligament (ORL). This ligament lengthens between the bone and its dermal attachment as it progresses centrally, to up to 16 mm when it becomes the ORL.9
Newer, minimally invasive TTD treatments, including direct hyaluronic acid (HA) filler injections, are popular alternatives to traditional surgery. Although minimally invasive techniques are easier to perform, safer, and less painful, results can vary and more complications may occur,10 including nodules, visible lumps, swelling, skin pigmentation, or the “Tyndall effect” optical discoloration.11,12 Patients with deep TTD pigmentation are often disappointed with HA treatments because the discoloration persists and may become more noticeable after contour correction. We developed an alternative approach using a carboxymethylcellulose gel–based filler consisting 25–45 μM calcium hydroxylapatite particles (calcium hydroxyapatite [CaHA], Radiesse; Merz Aesthetics GmbH, Frankfurt, Germany; 30% wt/vol) that stimulates neocollagenesis and is approved for moderate-to-deep facial lines and folds.6 With an understanding of the filler’s high lifting capacity and the anatomic etiologies of the periorbital area and TTD, we exploited CaHA rheology for periorbital rejuvenation. CaHA facilitates the lifting and support of a prolapsing ORL,13 biostimulates to improve skin quality, and rejuvenates the periocular area without direct injections into the TTD.
METHODS
Patient Selection
Selected candidates had significant, visible TT area pigmentation and/or had undergone HA filler removal with hyaluronidase because of complications but still required corrections for persisting TTDs. Patients had no medical contraindications to dermal fillers and consented to needlestick protocols and CaHA injections. TTDs were assessed using the Global Aesthetic Improvement Scale (GAIS), the Tear Trough Rating Scale (TTRS,5 adapted to our needs), and Fitzpatrick skin typing, for which an equivalent proportion of types II to IV were found. All patients provided written informed consent and permission to publish case details. This study protocol conformed to the 1975 Declaration of Helsinki ethical guidelines.
Preinjection Preparation
Faces were cleansed with Cetaphil (Galderma Laboratories) and 0.5% chlorhexidine. Cannula entry point(s) were injected with 1% lignocaine (Pfizer Pty Ltd, Australia), and Emla Cream (AstraZeneca, Australia) was applied. Target areas were marked, and standardized photos were taken pre- and postinjection at baseline and 2-week, 3- to 4-month, 6-month, and 12- or 18-month follow-up. Patients sat upright during all injections. For ORL support, 0.8 mL of CaHA was mixed with 0.1 mL of 1% lignocaine and injected as “micro-bolus” droplets (described in Injection Technique). This mixture (0.1 mL) was diluted further with 0.4 mL of lignocaine for a subcutaneous biostimulatory wash.
Injection Technique
A 25G cannula was navigated from an entry point in the upper nasolabial fold in a superior direction under the orbicularis and gently upward until resistance was encountered from the ORL (Fig. 1). The cannula tip was aimed toward the ligament base at its attachment to the bone, just inferior to the orbital rim. Minimally diluted CaHA (0.3–0.4 mL) was placed, per side, in micro-bolus droplets along the length of and under the ligament base to provide lift and support (Fig. 1). The total volume of minimally diluted CaHA filler injected varied depending on the TTD severity. To create a CaHA wash, 0.1 mL of residual, minimally diluted CaHA was diluted with 0.5 mL of 1% lignocaine. A 27G cannula was passed subcutaneously from a central point inferior to the lower eyelid, and this hyperdiluted wash was flushed across the lower eyelid and lid–cheek junction periorbital area, in the subcutaneous plane (Fig. 1, bottom; and Fig. 2). The cannula was visible throughout the procedure as the skin is very thin in this area. It is important to maintain the cannula’s position superficial to the orbicularis muscle, just under the dermis, as CaHA can clump in the mobile muscle and create nodules. To distribute the CaHA wash evenly, the area was gently massaged with the fingertips or a Q-tip. CaHA was also not injected directly into the TT area as this can cause visible nodules and plaques. To summarize, micro-boluses of concentrated CaHA were placed deep, on the bone, under the ORL and sub-OO fat. A very dilute wash was flushed over the area, just under the skin. Extra caution was used for eye bags, by staying just under the dermis, so as to avoid accidentally passing through the attenuated orbital septum and overlying orbicularis.
Fig. 1.
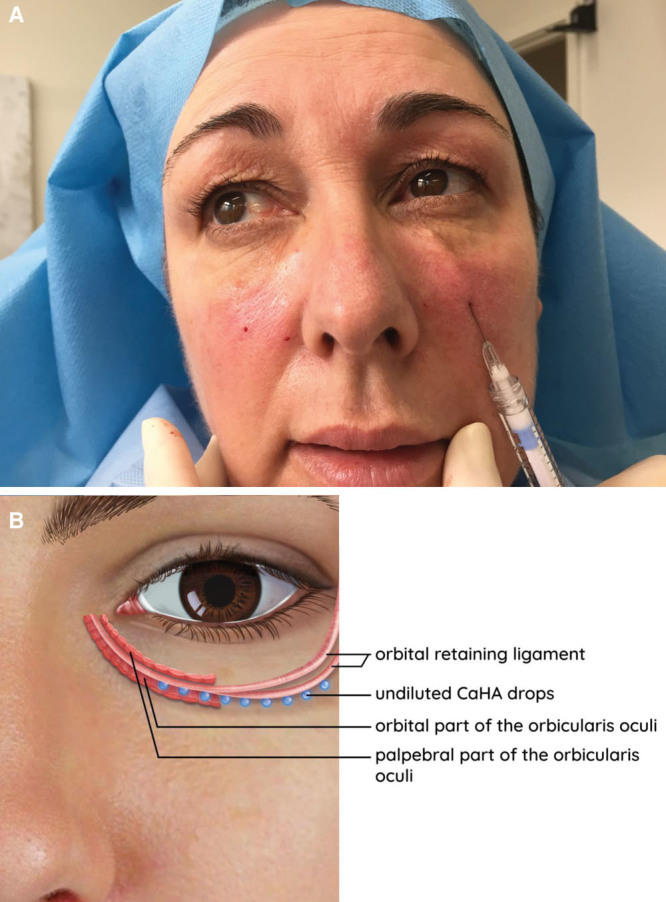
Supporting the ORL. A, A 25G cannula is passed inferiorly from the upper nasolabial fold and beneath the orbicularis before moving upward until resistance was encountered medial to the tear trough ligament and central and lateral to the ORL. B, Schematic of location of CaHA injection in which 0.3–0.4 mL of diluted CaHA were placed, per side, in micro-bolus droplets along the length of and under the ligament base to provide lift and support.
Fig. 2.
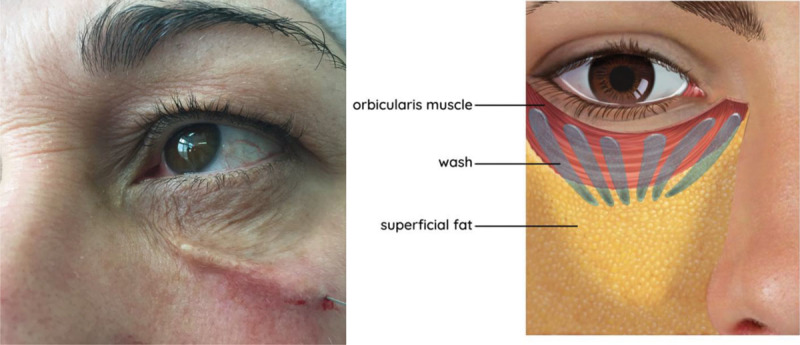
Applying filler wash in subcutaneous plane. A wash consisting of 0.1 mL of CaHA mixed with 0.4 mL of 1% lignocaine was spread over the subcutaneous plane of the entire treatment area using a 27G cannula. Schematic depicts locations and directions of CaHA wash. Undiluted calcium hydroxylapatite is injected in tiny drops (less than 0.03 cc), pushed up against the bony origin of the orbital retaining ligament along its length. A hyperdiluted wash of calcium hydroxylapatite is flushed in the plane between orbicularis and skin.
Postinjection Follow-up and Assessment
Photos were taken at baseline, 2-week, 3- to 4-month, 12-month, and 18-month (17–19 months) follow-up. At 4- and 18-month follow-up, the investigator and a blinded, independent rater assessed the treatment’s clinical effectiveness and adverse events. Various TTD scales and assessment methodologies5,14–17 mostly evaluate hollowness and physical deformities without considering the associated periorbital pigmentation and skin quality issues. For our needs, the TTRS5 was more relevant and practical in a busy clinic. We also modified the Sadick scale5 (SS; Appendix 1) because distance limitations prevent our independent assessor from performing live (nonphotographic) physical assessments or TT depth measurements. Each treatment area was scored at baseline and during follow-up, with aesthetic outcomes compared with baseline. Results were recorded as “worse, −1”; “no change, 0”; “improved, 1”; “much improved, 2”; and “very much improved, 3” on the GAIS.18,19 Using the modified SS, the severities of TT depths, hyperpigmentation or venous pooling, malar fat pad descent, and lower eyelid rhytidosis were scored. Treatment efficacy was evaluated according to investigator and blinded rater assessments of improvements using photographs. Effectiveness end points were improvements in scores at a minimum of 4 months from baseline.
RESULTS
Twelve patients, between 25 and 52 years of age, presented with TTDs (Fig. 3 and see figure, Supplemental Digital Content 1, which displays the CaHA filler–mediated correction of the tear trough deformity at 4 and 18 months. One patient developed an infection at 6 weeks that resolved with antibiotics. http://links.lww.com/PRSGO/B298).
Fig. 3.
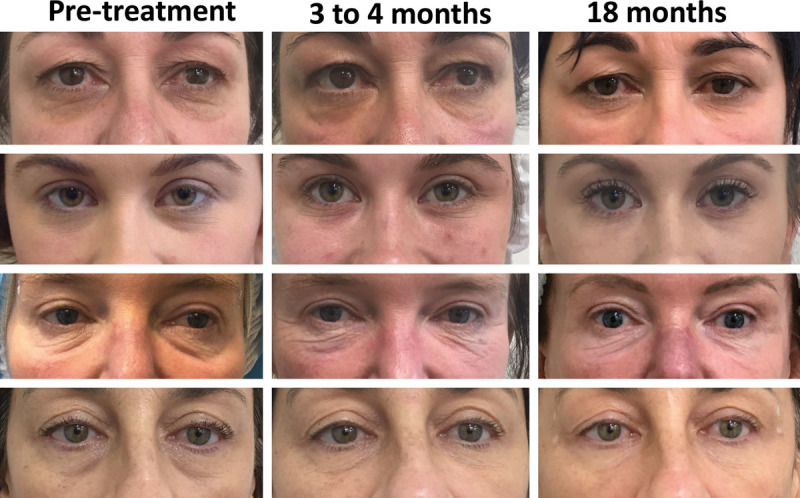
CaHA filler–mediated correction of the TTD at 4 and 18 months. Patients have visible signs of continued improvement in skin quality beyond 4 months. A second treatment was given to patient 1 at 4 months and patient 3 at 6 months.
Four patients previously received HA fillers but developed complications, most commonly edema, swelling, and nodules. Subsequently, 30–50 units of a hyaluronidase mixture (1,500 units in 10 mL of 1% lignocaine) were injected repeatedly in each side until symptoms fully resolved. After 3 months, periorbital areas were retreated with CaHA filler. Six patients had excess periorbital/TT area pigmentation and discoloring that was not improved with HA fillers but was immediately disguised by CaHA, due to CaHA white coloring. Table 1 summarizes our treatments.
Table 1.
Modified Sadick Scale
| Depth of the tear trough at its maximum depth | Mild (1 point), moderate (2 points), severe (3 points) |
| Hyperpigmentation/venous pooling | No hyperpigmentation/venous pooling (1 point) |
| Mild hyperpigmentation/venous pooling (2 points) | |
| Moderate pigmentation/venous pooling (3 points) | |
| Severe hyperpigmentation/venous pooling (4 points) | |
| Descent of malar fat pad | Mild (1 point), moderate (2 points), severe (3 points) |
| Lower eyelid rhytidosis | Mild (1 point), moderate (2 points), advanced (3 points), severe (4 points) |
Summary of patient presentation (by Fitzpatrick Skin Type) and outcomes (by modified TTRS and GAIS). Twelve patients receiving periorbital correction were assessed following treatment with HA injections and 7 deidentified patients are shown here.
A few days postinjection, patients developed swelling and redness over the lower eyelids, which resolved spontaneously. Improvements in hyperpigmentation were immediately observed due to light reflection and some visibility of the white filler through the skin. Patients were followed up for 4–18 months (Table 1 and see table, Supplemental Digital Content 2, which displays a summary of patient presentation (by Fitzpatrick Skin Type) and outcomes (by modified TTRS and GAIS). Twelve patients receiving periorbital correction were assessed following treatment with HA injections, and 5 anonymized patients are shown in http://links.lww.com/PRSGO/B299).
Over 4–6 months, hyperpigmentation and skin tone, thickness, and color improved noticeably in treated areas (see figure, Supplemental Digital Content 3, which displays the CaHA filler–mediated correction of the tear trough deformity at short term. Patient (identity obscured) is shown during pretreatment consultation (left), and at follow-up times (right) after injection with CaHA filler. Improvements in hyperpigmentation and skin tone, thickness, and color were visible in the treated areas at 14–16 weeks. http://links.lww.com/PRSGO/B300). GAIS and modified TTRS scores showed that all patients experienced satisfactory posttreatment improvements.
Three patients requiring dissolution of previous HA fillers needed a second CaHA treatment at 4–6 months for a satisfactory TTD correction, one of whom also had a third wash at 10 months. A dull redness in the area persisted for up to 4 months in these patients. Eight patients (excluding patients 3 and 6) developed a mild, dull redness for 8 to 12 weeks; however, no differences in the severity of redness were observed between patients treated after HA correction or for dark circles. Two complications occurred, one persistent, dull erythema lasting for 8 months and one overt erythema and swelling following a chest infection which resolved within 4 weeks of treatment with antibiotics and hydrocortisone cream. Most patients experienced a dull erythema in the injection area which resolved over 8 to 12 weeks. No nodules were observed. All patients had a mild degree of dull redness for 8 to 12 weeks, except patients 3 and 6 (who had none).
DISCUSSION
Understanding periorbital area aesthetic defects and TTD etiology is essential for correcting its visible signs of aging. The visible TTD groove is observed between the OO cephalad palpebral and caudal orbital portions originating from the maxilla.9 The ORL is longest along the central inferior orbital rim and shortens as it joins the lateral orbital thickening in the lateral canthal area.20 In aging, periorbital volume loss increases TTD visibility, causing the ORL to develop laxity and the orbital fat pads to displace inferiorly.14 Within the medial orbital rim region, the TTD appears as a depression that deepens during aging, with a layer of overlying skin. This skin can be extremely thin and deeply pigmented. Comprehensive information on TTD and undereye shadows are beyond our study scope but widely published elsewhere.1,8,21,22
Our rationale was not to fully correct the TTD, but to improve the periorbital area without recurrence of periorbital swelling in previous HA recipients who seek to avoid further HA treatment, and to prevent further visible hyperpigmentation. HA filler injection into the TTD is a well-described and popular technique. However, this is associated with complications including filler visibility (Tyndall’s sign), edema, and swelling.23–26 Another complication could arise if skin in the depression of the tear trough is deeply pigmented and lifts during filling, resulting in prominent infraorbital discoloration across the lower eyelid, which is difficult to correct. Injecting CaHA directly into this area risks the development of visible nodules if filler is inadvertently placed among the more mobile orbicularis fibers. In thin-skinned patients, such injections also risk filler visibility.
Using our understanding of periorbital anatomy and its treatment challenges, our technique addresses this deformity in patients for whom HA treatment failed and required hyaluronidase or was likely to give a poor outcome due to deep pigmentation. We avoid injecting the TT as our goal was not a full TTD correction, but skin biostimulation and thickening, and support of the ORL from underneath for contour improvements, especially at the lower TTD. It was hoped that improved quality and thickness of the overlying skin here would also avoid the need for volumization or traditional direct injections just cranial to the ORL on the orbital rim. Our technique places CaHA deep in the periorbital area to support the ORL, followed by a very dilute (1:5) wash immediately under the dermis to counteract pigmentation, facilitate biostimulation, and improve skin thickness and pliability. CaHA was chosen due to its high viscosity and elasticity compared with HA, thus requiring only tiny drops to support the prolapsing ORL without risking its spread.27 CaHA was also ideal for our technique as it mediates biostimulation through angiogenesis, elastosis, and type I collagenesis.28 Specifically, volume loss restoration through physiological remodeling and augmentation of extracellular matrix by CaHA has been previously demonstrated through the progressive replacement of type III with type I collagen, alongside increased elastin production. These reported histological changes were observed at dilutions of up to 1:6 and correlated with clinically measured improvements in skin elasticity and pliability,29 while increased skin thickness was demonstrated by microfocused ultrasound,30 which allows tissue depths (or thickness) to be visualized during skin rejuvenation treatments. The biostimulatory effects are still evident in weak dilutions and are the basis for periorbital subcutaneous washes that improve skin quality over the long term. The white CaHA crystals in this dilute wash give an immediate skin lightening effect.
Our strategy targets biostimulation and neocollagenesis to the skin overlying the TTD, especially where there is hyperpigmentation or visibility of underlying muscle or venous circulation. Drops of undiluted CaHA, which has high viscosity and elasticity, placed at the base of the prolapsing ORL, also supports it. In our patients, HA only addressed the contour defect, not the associated pigmentation issues. Our treatments improved skin quality and reduced visible dark circles. Patients 4, 5, 7, and 10 only required 1 treatment for skin quality improvements over 4 to 18 months, suggesting sustained neocollagenesis and biostimulation. Patients 1, 2, and 4 received a second CaHA wash at 4 months but also demonstrated skin quality improvements between 4 and 18 months. One dark eye circle patient (patient 12) had a residual contour deformity and developed a significant infection at week 6, whose impact on treatment outcomes is unknown. Another (patient 11) had no significant improvements but missed her 4-month follow-up due to immunosuppressive treatments for multiple sclerosis, which may have dampened neocollagenesis and dermal biostimulation. Two patients displayed skin improvements at 4 months but missed their 18-month follow-up, whereas 3 patients (including patients with immunosuppression or infections) showed no continuous improvements between 4 and 18 months. Patients 1, 8, and 9 all had post-HA complications but have room for TTD contour improvements. However, all HA-treated patients were secondary referrals, and we lack access to their pre-HA treatment photographs. These patients may suit a combination approach, with minimal quantities of HA fillers injected directly into the TTD22,31,32 and hyperdiluted CaHA to biostimulate overlying skin, or undiluted CaHA for ORL support with minimal volumization where necessary (direct injections of CaHA into the TTD are not recommended). Although our sample size was insufficient to infer clinical trends, all patients with Fitzpatrick type IV skin experienced significant improvements in dark circles.
To avoid lymphatic collecting duct damage or obstruction, filler was not placed within 1 cm of the lateral canthus, where lymphatic collectors are in close proximity33 and at risk of obstruction, potential local edema and swelling, common sequelae of TTD HA injections. Superficial lymphatic drainage of the lower eyelid is directed toward medial and lateral collecting lymphatics that are superficial to the preseptal orbicularis and continue in the superficial nasolabial and lateral orbital fat compartments. However, the deep lymphatic system drains laterally, deep to the preseptal orbicularis to collecting lymphatics that form at the junction of the lateral orbicular thickening and ORL before passing through the ORL, to run in the sub-OO fat in the prezygomatic space. These vessels can be obstructed by filler injections,34 and injectors may benefit from soft tissue anatomical imaging of these areas before minimally invasive procedures. CaHA injections in this area should only be conducted by and under the supervision of experienced clinicians familiar with periorbital anatomy and filler injections.35,36
Familiarity with TTD anatomy and the relationships of the ORL are mandatory, and injectors should review these during cadaver workshops to ensure product placement in correct locations. The blunt cannula tip should be guided underneath the ORL where it originates from the bone. A large-bore, stiff 25G or 22G cannula must be passed gently to the precise target area, without penetrating structures such as infraorbital vessel branches or the ORL itself. A fine 27G cannula can be passed (and is visible) just under the very thin skin of this area to apply subcutaneous washes.
The TTD can be effectively corrected with the direct placement of any suitable HA product; however, CaHA is preferred for patients with thin skin and deep pigmentation, though we emphasize that our goal was not to compare between CaHA and HA. We used CaHA to treat the periorbital area, and consequently the TTD, in 12 consecutive patients, who were counseled to expect a prolonged, dull erythema in the area for up to 12 weeks. In this consecutive series and in other patients, the longest incidence of erythema was 8 months, which was easily and comfortably concealed with cosmetics until resolution. The final outcome of improved skin quality and corrected pigmentation led to high satisfaction in patients who would otherwise achieve poor outcomes when first injected with HA.
CONCLUSIONS
We describe a novel treatment for periorbital pigmentation and/or discoloration associated with the TTD. HA can give poor results as it can lift and emphasize excess pigmentation. CaHA's unique rheological and biostimulatory properties, and its placement in small quantities beneath a prolapsed tear trough ORL, effectively lifts and supports it without adding excess volume. A CaHA wash thickened and improved the appearance and discoloration of overlying skin.
ACKNOWLEDGMENTS
The author expresses her appreciation to Merz Asia Pacific Pte Ltd for funding the preparation of this article and Shawna Tan for manuscript writing and editorial assistance. The author contributed to the collection and interpretation of data and critical review of the manuscript.
PATIENT CONSENT
Patients provided written consent for the use of their images.
Supplementary Material
Footnotes
Published online 6 February 2020.
The preparation of this article and was sponsored by Merz Asia Pacific.
Disclosure: Niamh Corduff is a consultant for Merz Asia Pacific and Australia.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Stutman RL, Codner MA. Tear trough deformity: review of anatomy and treatment options. Aesthet Surg J. 2012;32:426–440. [DOI] [PubMed] [Google Scholar]
- 2.Huber-Vorländer J, Kürten M. Correction of tear trough deformity with a cohesive polydensified matrix hyaluronic acid: a case series. Clin Cosmet Investig Dermatol. 2015;8:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang YL, Chang SL, Ma L, et al. Clinical analysis and classification of dark eye circle. Int J Dermatol. 2014;53:164–170. [DOI] [PubMed] [Google Scholar]
- 4.Friedmann DP, Goldman MP. Dark circles: etiology and management options. Clin Plast Surg. 2015;42:33–50. [DOI] [PubMed] [Google Scholar]
- 5.Vrcek I, Ozgur O, Nakra T. Infraorbital dark circles: a review of the pathogenesis, evaluation and treatment. J Cutan Aesthet Surg. 2016;9:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadick NS, Bosniak SL, Cantisano-Zilkha M, et al. Definition of the tear trough and the tear trough rating scale. J Cosmet Dermatol. 2007;6:218–222. [DOI] [PubMed] [Google Scholar]
- 7.Haddock NT, Saadeh PB, Boutros S, et al. The tear trough and lid/cheek junction: anatomy and implications for surgical correction. Plast Reconstr Surg. 2009;123:1332–1340; discussion 1341-2. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Wang X, Chen R, et al. Tear trough deformity: different types of anatomy and treatment options. Postepy Dermatol Alergol. 2016;33:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CH, Hsieh MK, Mendelson B. The tear trough ligament: anatomical basis for the tear trough deformity. Plast Reconstr Surg. 2012;129:1392–1402. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31:110–121. [DOI] [PubMed] [Google Scholar]
- 11.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy LL, Emer JJ. Complications of minimally invasive cosmetic procedures: prevention and management. J Cutan Aesthet Surg. 2012;5:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam M, Gladstone H, Kramer EM, et al. ASDS guidelines of care: injectable fillers. Dermatol Surg. 2008;34:115–148. [DOI] [PubMed] [Google Scholar]
- 14.Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699–708. [DOI] [PubMed] [Google Scholar]
- 15.Barton FE, Jr, Ha R, Awada M. Fat extrusion and septal reset in patients with the tear trough triad: a critical appraisal. Plast Reconstr Surg. 2004;113:2115–21; discussion 2122-3. [DOI] [PubMed] [Google Scholar]
- 16.Turkmani MG. New classification system for tear trough deformity. Dermatol Surg. 2017;43:836–840. [DOI] [PubMed] [Google Scholar]
- 17.Huang SH, Lin YN, Lee SS, et al. Three simple steps for refining transcutaneous lower blepharoplasty for aging eyelids: the indispensability of micro-autologous fat transplantation. Aesthet Surg J. 2019;39:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stella E, Di Petrillo A. Goisis M. Standard evaluation of the patient: the Merz scale. In: Injections in Aesthetic Medicine: Atlas of Full-face and Full-body Treatment. 2014:Milano: Springer Milan; 33–50. [Google Scholar]
- 19.Carruthers A, Carruthers J. A validated facial grading scale: the future of facial ageing measurement tools? J Cosmet Laser Ther. 2010;12:235–241. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RA, McCann JD, Fiaschetti D, et al. What causes eyelid bags? Analysis of 114 consecutive patients. Plast Reconstr Surg. 2005;115:1395–1402; discussion 1403. [DOI] [PubMed] [Google Scholar]
- 21.Lipp M, Weiss E. Nonsurgical treatments for infraorbital rejuvenation: a review. Dermatol Surg. 2019;45:700–710. [DOI] [PubMed] [Google Scholar]
- 22.Pascali M, Quarato D, Pagnoni M, et al. Tear trough deformity: study of filling procedures for its correction. J Craniofac Surg. 2017;28:2012–2015. [DOI] [PubMed] [Google Scholar]
- 23.Funt DK. Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol. 2011;4:32–36. [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg RA, Fiaschetti D. Filling the periorbital hollows with hyaluronic acid gel: initial experience with 244 injections. Ophthal Plast Reconstr Surg. 2006;22:335–341; discussion 341–3. [DOI] [PubMed] [Google Scholar]
- 25.Morley AM, Malhotra R. Use of hyaluronic acid filler for tear trough rejuvenation as an alternative to lower eyelid surgery. Ophthal Plast Reconstr Surg. 2011;27:69–73. [DOI] [PubMed] [Google Scholar]
- 26.Nestor MS, Ablon GR, Stillman MA. The use of a contact cooling device to reduce pain and ecchymosis associated with dermal filler injections. J Clin Aesthet Dermatol. 2010;3:29–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36:1859–1865. [DOI] [PubMed] [Google Scholar]
- 28.Yutskovskaya Y, Kogan E, Leshunov E. A Randomized, Split-Face, Histomorphologic Study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13:1047–1052. [PubMed] [Google Scholar]
- 29.Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: a Pilot Study. J Drugs Dermatol. 2017;16:68–74. [PubMed] [Google Scholar]
- 30.Kerscher M, Nurrisyanti AT, Eiben-Nielson C, et al. Clinical and biophysical outcomes of combining microfocused ultrasound with visualization and calcium hydroxylapatite filler for facial treatment. Dermatol Ther (Heidelb). 2019;9:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagci B. A new technique for the correction of tear trough deformity via filler injections. Plast Reconstr Surg Glob Open. 2018;6:e1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustak H, Fiaschetti D, Goldberg RA. Filling the periorbital hollows with hyaluronic acid gel: long-term review of outcomes and complications. J Cosmet Dermatol. 2018;17:611–616. [DOI] [PubMed] [Google Scholar]
- 33.Shoukath S, Taylor GI, Mendelson BC, et al. The lymphatic anatomy of the lower eyelid and conjunctiva and correlation with postoperative chemosis and edema. Plast Reconstr Surg. 2017;139:628e–637e. [DOI] [PubMed] [Google Scholar]
- 34.Wong CH, Mendelson B. Newer understanding of specific anatomic targets in the aging face as applied to injectables: aging changes in the craniofacial skeleton and facial ligaments. Plast Reconstr Surg. 2015;1365 Suppl44S–48S. [DOI] [PubMed] [Google Scholar]
- 35.Biesman B. Re: “Calcium hydroxyl-apatite (Radiesse) for the correction of periorbital hollows, dark circles, and lower eyelid bags”. Ophthalmic Plast Reconstr Surg. 2014;30:529. [DOI] [PubMed] [Google Scholar]
- 36.Bernardini FP, Devoto MH, Cetinkaya A, et al. Reply re: “Calcium hydroxyl-apatite (Radiesse) for the correction of periorbital hollows, dark circles, and lower eyelid bags”. Ophthalmic Plast Reconstr Surg. 2014;30:529–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


