Abstract
Background:
Distress among newly diagnosed patients with breast cancer is common and may have an impact on their surgical decision-making. The revised Edmonton Symptom Assessment System (ESAS-r) is a validated instrument that provides an estimate of patients’ total distress, and no previous study has related preoperative scores to the choice to have breast reconstruction.
Methods:
Women with breast cancer treated at the Princess Margaret Cancer Centre in 2014 were reviewed, and patient and tumor characteristics were collected from local databases. Breast reconstruction status was obtained from patients’ electronic medical records until April 2017. A multivariable logistic regression model assessed for an independent association between preoperative ESAS-r total distress scores and patients’ decision to have breast reconstruction.
Results:
A total of 312 patients were analyzed. ESAS-r values had an overall median score of 10.0 and ranged from 0 to 69 (interquartile range, 17). Of these patients, 82 chose to undergo breast reconstruction surgery (26.8%). Multivariable logistic regression analysis showed that higher ESAS-r scores were associated with patients forgoing breast reconstruction surgery (lumpectomy-alone group: odds ratio estimate, 1.034 [1.004–1.064], P = 0.025; mastectomy-alone group: odds ratio estimate, 1.031 [1.004–1.059], P = 0.023).
Conclusions:
This study of patients with breast cancer found that higher distress scores as measured by the ESAS-r were associated with reduced breast reconstruction. Distress in patients with breast cancer is important to address, as it is often treatable, and its resolution may unmask a desire for breast reconstruction, which has known benefits psychosocially.
INTRODUCTION
It is estimated that >250,000 women will be diagnosed with breast cancer this year in the United States, making it the most common nonmelanoma malignancy in women.1 Breast cancer imparts both a physical and psychosocial burden in patients.2 Distress is defined as “a multifactorial unpleasant experience of a psychological (ie, cognitive, behavioral, emotional), social, spiritual, and/or physical nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment” by the National Comprehensive Cancer Network. Shortly after routine screening for psychologic disturbances became standard of care, it was proposed that “distress” become the “sixth vital sign” when caring for cancer patients.3 Previous studies have shown that distress can affect patients’ quality of life,4 predict local recurrence, and may persist for years following diagnosis.5,6 Clinically, distress is seen as a continuum, which extends from normal feelings to potentially debilitating levels of anxiety and depression. The prevalence of distress in previous studies ranges from 20% to 30%,7–10 and breast cancer patients score significantly higher on questionnaires quantifying distress than cancer-free controls.2 This is clinically relevant because distress correlates with reduced patient satisfaction in patients’ cancer care,11,12 and some known interventions such as cognitive behavioral therapy and mindfulness can mitigate potential sequelae.7,13 The Canadian Partnership Against Cancer and the National Comprehensive Cancer Network have, therefore, recommended cancer patients be screened during their clinical course for distress, and patients testing positive should be offered targeted interventions.14
One available method for screening is the Edmonton Symptom Assessment System (ESAS), which is an instrument that was developed over 25 years ago as an instrument to assess multiple cancer-related symptoms.15 It has since been translated into >20 languages and validated multiple times.16,17 Studies have also shown that a higher ESAS score was associated with more emergency room visits in the following week and a shorter overall survival in cancer patients.17–19 The current Canadian standard of care as per Accreditation Canada is for cancer patients to complete the ESAS at diagnosis and then at other critical time points during their cancer care.20
Distress after a diagnosis of breast cancer may impact the treatment patients receive after their initial diagnosis. For patients with breast cancer, it is also during this challenging time that they have to consider reconstructive surgery. Patients may be presented with a number of surgical treatments ranging from the least physically invasive lumpectomy option to mastectomy alone, to a more involved procedure such as mastectomy with immediate breast reconstruction. For breast cancer patients with early-stage cancer, there may be no survival difference between the different surgeries; however, there may be differences in long-term quality of life and psychosocial outcomes following the different procedures.21–25
The objective of this study was to determine if a patient’s baseline psychosocial function, such as distress, Eastern Cooperative Oncology Group (ECOG) performance function status, and personal history of anxiety or depression was associated with the type of breast surgery she underwent. We hypothesized that patients with breast cancer who have higher psychosocial burden may be more likely to undergo the less involved procedures such as lumpectomy or mastectomy alone, compared with the more invasive mastectomy with breast reconstruction.
METHODS
Population of Interest
Women who were referred to the Princess Margaret Cancer Centre (PMCC), Toronto, Canada, for newly diagnosed nonmetastatic breast cancer during the period from January 1, 2014, to December 31, 2014, and also completed the ESAS questionnaire before their breast cancer surgery were included in this study (Fig. 1). All patients had breast cancer surgery that included lumpectomy, unilateral therapeutic mastectomy, or therapeutic mastectomy with a contralateral prophylactic mastectomy with or without breast reconstruction. Patients were excluded if they opted out of research studies or did not undergo any breast cancer surgery. Patients with breast cancer recurrence were excluded because they have been shown to have significantly different distress levels than patients with a first-time diagnosis and lower levels of functioning.26 All patients with a confirmed genetic predisposition for breast cancer (eg, positive BRCA 1 or BRCA 2 gene status) were excluded because it has been shown that they consistently have higher cancer-related distress than nonhereditary patients with breast cancer.27 In addition, patients undergoing risk reduction surgery would be skewed toward bilateral mastectomy with breast reconstruction.28 Patients who underwent multiple oncologic surgeries (eg, a patient who had a lumpectomy with positive margins necessitating a subsequent mastectomy) were counted only once under the most extensive surgery. Figure 1 depicts the flow of the study diagrammatically. The local institutional ethics review board approved this study (Coordinated Approval Process for Clinical Research identification: 16-5874).
Fig. 1.
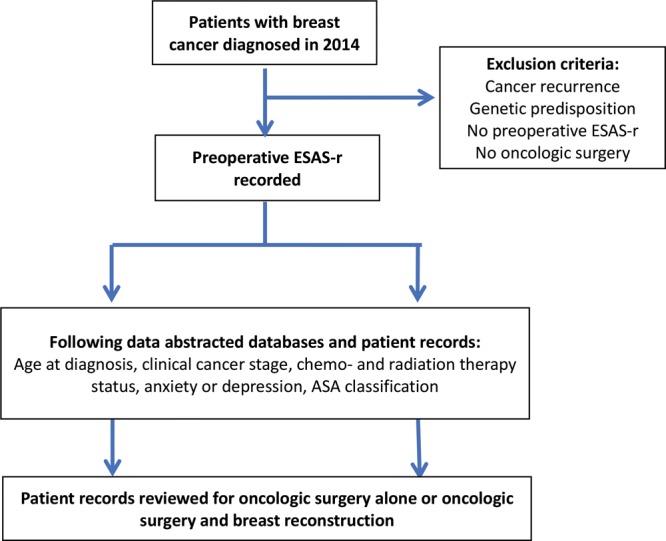
Study design flow chart. ASA indicates American Society of Anesthesiologists.
ESAS Questionnaire
The Distress Assessment Response Tool (DART), which involves a series of questionnaires, was implemented in 2009 at PMCC to identify distress in outpatients with cancer. Currently, the program is implemented in 15 cancer sites and has a 70% screening rate. The ESAS questionnaire assesses symptoms on a numeric rating scale from 0 (no symptoms) to 10 (worst possible) in the following 9 domains: depression, anxiety, pain, tiredness, nausea, drowsiness, appetite, well-being, shortness of breath, and one that is substitutable. All scores are added and the total score represents a patient’s symptom distress score, that similarly ranges from 0 (no distress) to 100 (highest distress).16 Due to the potential for misinterpretation, or confusion when grading appetite and sleep,29,30 a revised ESAS (ESAS-r) was developed, which is more clearly defined. A study comparing the ESAS and ESAS-r showed that the revised version was significantly easier to understand.31 The ESAS-r is used at the PMCC. The questionnaire was originally devised for palliative cancer patients, but Chang et al32 have since validated it in outpatient cancer patients. In a sample of 233 patients they showed that the overall distress score correlated significantly with other summary measures, and that the overall Cronbach α score was 0.79. Additionally, they showed that the test–retest Spearman correlation coefficients for the overall distress score were 0.86 (P < 0.01) at 2 days and 0.45 (P < 0.05) at 1 week. Previous research based on 18 clinical studies has categorized ESAS symptom scores of 0–3, 4–6, and 7–10 as none, moderate, and severe distress, respectively, in clinical practice.33
Outcomes
Patients were considered to have undergone breast reconstruction if they underwent autologous or alloplastic reconstruction following mastectomy in an immediate or delayed fashion. Patients’ breast reconstruction status is up to date as of April 2017.
Patient Characteristics
The PMCC Cancer Registry was used to identify patient demographics, breast cancer type, and staging. Other variables of interest not available through the registry were obtained directly from patients’ electronic medical records. The American Society of Anesthesiologists classification was obtained from recorded preoperative assessments. Patients were considered to have a history of anxiety or depression if indicated in the recorded history by a physician. Other known prognostic indicators that were collected were chemotherapy and radiation therapy status. Patient-reported ECOG scale of performance status, which is a standard instrument used to assess function in cancer care,34,35 was obtained through the DART program and also recorded. Clinical cancer stage and American Society of Anesthesiologists classification were both operationalized as low (1 or 2) and high (3 or 4). Similarly, a score of ≥1 on the ECOG performance status, which indicated some level of disability, was considered positive, whereas a score of 0 was negative.
Statistical Analysis
Categorical variables were described as number (percentage) and continuous variables as median (interquartile range) for nonnormally distributed variables or mean (SD) for normally distributed variables. To determine the independent association between symptoms causing severe distress for patients and having breast reconstruction, a multinomial logistic regression model was developed that included the predictors described above and compared our 3 groups: lumpectomy alone, mastectomy alone, and mastectomy with breast reconstruction. Before adding predictor variables to the model, a test for multicollinearity was performed to assess for dependent relationships between the various predictor variables. A tolerance of ≥0.2 was considered acceptable for inclusion in the model. The model was also assessed graphically for influential observations. This model was characterized before data collection (a priori), and, therefore, validation was not conducted. All hypothesis tests were 2-sided, and “P” values <0.05 were considered significant. All analyses were conducted using SAS 9.4 statistical software (SAS Institute Inc, Cary, N.C.).
RESULTS
Patient Characteristics
A total of 478 newly diagnosed patients with breast cancer from January 1, 2014, to December 31, 2014, were identified for potential inclusion in this study (Tables 1 and 2). Of these patients, 166 were excluded; 21 had a genetic predisposition for breast cancer, 8 had recurrent breast cancer, and 137 did not have a completed baseline ESAS-r before surgery. The remaining 312 patients with breast cancer were included in the analysis. Of these patients, 154 underwent lumpectomy (49.4%) and 76 (24.4%) had mastectomy only, and 47 patients (15.1%) had mastectomy with immediate breast reconstruction. Of the mastectomy-only patients, 64 were unilateral mastectomy (84.2%), 9 were unilateral mastectomy with contralateral prophylactic mastectomy (11.8%), and 3 were bilateral mastectomy for bilateral breast cancers (3.95%). Of the immediate breast reconstruction patients, 17 were unilateral (36.2%) and 30 were bilateral (63.8%). In the study period, an additional 35 patients (11.2%) from the mastectomy-alone group went on to undergo delayed breast reconstruction, for a total of 82 patients who underwent breast reconstruction. Of the delayed breast reconstruction patients, 28 were unilateral (80.0%) and 7 were bilateral (20.0%).
Table 1.
Characteristics of the Included Patients Sorted by Group
| Lumpectomy Alone (n = 154) | Mastectomy Alone (n = 76) | Oncological Surgery and Breast Reconstruction (n = 82) | |
|---|---|---|---|
| Age, mean (SD) | 58.2 (11.3) | 58.0 (12.0) | 51.4 (10.2) |
| Anxiety or depression, n (%) | 15 (9.7) | 6 (7.9) | 15 (18.3) |
| ASA classification 3 or 4, n (%) (reference ASA 1 or 2) | 29 (18.8) | 14 (18.4) | 22 (26.8) |
| Cancer stage 3 or 4, n (%) (reference stage 1 or 2) | 1 (0.6) | 15 (19.7) | 45 (54.9) |
| Radiation therapy, n (%) | 136 (88.3) | 49 (64.5) | 34 (41.5) |
| Chemotherapy, n (%) | 52 (33.8) | 50 (65.8) | 50 (61.0) |
| ECOG performance status, median (IQR) | 49 (31.8) | 34 (44.7) | 45 (54.9) |
| Preoperative ESAS distress score, median (IQR) | 10.0 (16.0) | 10.5 (20.5) | 10.5 (16.0) |
ASA, American Society of Anesthesiologists; IQR, interquartile range.
Table 2.
Analysis of Variance in ESAS among the Three Groups
| Source | Degrees of Freedom | Mean Square | F | P |
|---|---|---|---|---|
| Group | 2 | 257.05 | 1.35 | 0.26 |
| Error | 309 | 190.09 | — | — |
Table 1 presents patient characteristics organized by group, including patients who underwent lumpectomy or mastectomy alone, and patients who had either immediate or delayed breast reconstruction.
Total ESAS-r values for all patients had an overall median score of 10.0 and ranged from 0 to 69 (interquartile range, 17). Preoperative ESAS-r scores did not differ significantly between the 3 groups when testing analysis of variance univariate statistics (P = 0.26) (Table 2).
Multinomial Logistic Regression Model
Multinomial logistic regression analysis showed that patients who had oncologic surgery alone (lumpectomy or mastectomy alone) had reported significantly higher preoperative ESAS-r scores compared with patients having mastectomy with immediate or delayed breast reconstruction, after controlling for other variables (lumpectomy-alone group: odds ratio estimate, 1.034 [1.004–1.064], P = 0.025; mastectomy-alone group: odds ratio estimate, 1.031 [1.004–1.059], P = 0.023) (Table 3). Patients undergoing reconstruction had worse preoperative ECOG performance status (lumpectomy-alone group: odds ratio estimate, 0.344 [0.153–0.775], P = 0.010; mastectomy-alone group: odds ratio estimate, 0.365 [0.160–0.835], P = 0.017) and were more likely to have a history of anxiety or depression (lumpectomy-alone group: odds ratio estimate, 0.223 [0.072–0.687], P = 0.009; mastectomy-alone group: odds ratio estimate, 0.264 [0.085–0.824], P = 0.022). The model also suggested that patients were more likely to undergo breast reconstruction if they were younger or did not require radiation. The Hosmer–Lemeshow test was satisfied. The model convergence criterion was satisfied. Influential observations were checked graphically, and there were no concerning patterns.
Table 3.
Results of Multinomial Regression Analysis
| Predictor | Adjusted Odds Ratio (95% CI) | Test Statistic | P* |
|---|---|---|---|
| Omnibus likelihood ratio (χ2 [df]) | 183.496 (16) | <0.001* | |
| Age at diagnosis | |||
| Lumpectomy alone | 1.057 (1.021–1.094) | 0.055 | 0.002* |
| Mastectomy alone | 1.087 (1.049–1.127) | 0.084 | <0.001* |
| Anxiety or depression | |||
| Lumpectomy alone | 0.223 (0.072–0.687) | −0.751 | 0.009* |
| Mastectomy alone | 0.264 (0.085–0.824) | −0.666 | 0.022* |
| ASA 3 or 4 | |||
| Lumpectomy alone | 0.405 (0.169–0.970) | −0.452 | 0.043* |
| Mastectomy alone | 0.453 (0.189–1.089) | −0.396 | 0.077 |
| Cancer stage 3 or 4 | |||
| Lumpectomy alone | 0.034 (0.004–0.301) | −1.686 | 0.002* |
| Mastectomy alone | 1.272 (0.451–3.589) | 0.120 | 0.650 |
| Radiation therapy | |||
| Lumpectomy alone | 41.165 (16.492–102.753) | 1.859 | <0.001* |
| Mastectomy alone | 4.047 (1.760–9.304) | 0.699 | 0.001* |
| Chemotherapy | |||
| Lumpectomy alone | 0.204 (0.084–0.494) | −0.795 | <0.001* |
| Mastectomy alone | 1.333 (0.556–3.195) | 0.144 | 0.519 |
| ECOG performance status | |||
| Lumpectomy alone | 0.344 (0.153–0.775) | −0.534 | 0.010* |
| Mastectomy alone | 0.365 (0.160–0.835) | −0.503 | 0.017* |
| ESAS distress score | |||
| Lumpectomy alone | 1.034 (1.004–1.064) | 0.033 | 0.025* |
| Mastectomy alone | 1.031 (1.004–1.059) | 0.031 | 0.023* |
*p < 0.05.
The reference group is oncology surgery with breast reconstruction.
ASA, American Society of Anesthesiologists; df, degrees of freedom.
DISCUSSION
This is the first study to investigate the independent association between preoperative psychosocial well-being and whether patients go on to have breast reconstruction or not. The results show that, after controlling for patient and cancer factors, higher distress in patients with breast cancer measured by the ESAS-r was associated with less frequent utilization of breast reconstruction compared to lumpectomy or mastectomy. In contrast, patients with anxiety or depression and worse functional status, as measured by the ECOG, were more likely to have undergone breast reconstruction. These findings suggest that it is important to consider that patients’ psychosocial status preoperatively may have an impact on subsequent patient care and surgical management in this patient population. This should be considered in the context of previous research that has shown clinicians are poor at recognizing psychosocial disturbances in their patients.36
The period after diagnosis of breast cancer can be extremely difficult. Previous research has shown that distress affects approximately one third of breast cancer patients,37 and it can increase during the first 4 months postdiagnosis.38 Additionally, cancer-related distress seems to persist during the first-year postdiagnosis, regardless of whether patients have a mastectomy with or without reconstruction.39 The sources of distress include high levels of anxiety, loss of identity, and being part of a new system.40 Despite this understanding of distress and how it affects patients after diagnosis, little has been done to investigate whether it affects their medical and surgical care. One previous study by Metcalfe et al41 found that patients who went on to have delayed breast reconstruction actually had higher levels of distress than those who had mastectomy alone. In contrast, our study found patients with higher levels of distress were less likely to have undergone immediate or delayed breast reconstruction in the study period, which may be a result of different instruments for measuring distress, study populations, or variables included in the regression model.
Recognition of cancer-related distress is important because considerable research has shown that effective therapies exist, such as cognitive behavioral therapy42,43 and mindfulness-based recovery.11,44,45 However, it should be noted that the trajectory of distress among breast cancer patients has been shown to decrease over a period of 6 years.46 Some patients seem to benefit from breast reconstruction psychosocially and in terms of quality of life.47
The finding that patients with a history of anxiety or depression were more likely to have had breast reconstruction may be a result of this subset of patients’ tendency to access health care services more often. Previous studies have shown that psychologic distress or illness leads to more frequent access of general practice services.48 A previous study based on a large Taiwanese population of patients with breast cancer showed those with mood disorders were slightly more likely to undergo immediate or delayed breast reconstruction, although that finding was not statistically significant.49
This study showed patients with higher preoperative ECOG scores with more frequently undergoing breast reconstruction. This suggests that patients with a worse performance status are more likely to undergo more invasive surgery after controlling for other factors in the multivariable model. This is an interesting finding, especially when considering previous research that has shown patients with worse performance status are less likely to be offered immediate breast reconstruction, although that may have been a regional finding.50 A previous study by Shi et al51 investigated quality of life over 2 years in patients with breast cancer and compared 3 types of breast surgery. The 3 groups were breast conservation surgery, modified radical mastectomy alone, and modified radical mastectomy with breast reconstruction. They showed that overall change in quality of life was highest in the group undergoing breast reconstruction, but notably, their data also demonstrated that the breast reconstruction group had lower baseline preoperative physical functional status. Interestingly, the reconstructed patients also had the lowest preoperative quality of life globally and scored equally or higher on every other measurement. So, the finding that patients with worse preoperative functional status go on to have breast reconstruction could reflect well-adjusted patients deciding to address a physical well-being deficit. This is supported by a finding from a qualitative study done by Reaby52 that showed the most common reason for women to not have breast reconstruction was if they did not feel it to be essential for their physical well-being. Another consideration is that immediately reconstructed patients have been shown to have worse preoperative functional status when compared with patients undergoing delayed reconstruction,53 and our study included a higher proportion of immediate reconstruction patients than delayed.
This study was subject to a number of limitations. All retrospective reviews are constrained to the variables that were collected, and in this case, there may be other variables related to patients’ psychosocial well-being that were not captured. However, all efforts were made to include variables in the multivariable logistic model that have previously been identified as important confounders of distress in patients with breast cancer. There may have been missing data from this single institutional study, such as patients who underwent breast reconstruction at a different hospital site. The study did benefit though from institutional databases and a review of each patient’s individual electronic personal record, and treatments from surrounding hospitals are often outlined in follow-up clinical notes. There may have been a systematic reason why patients chose not to complete the ESAS-r or participate in research, which might have introduced selection bias into the study. The DART program captures patients approximately 70% across all types of cancer, and patients with breast cancer tend to be motivated to participate in such activities and likely responded higher than this rate.54 Furthermore, there may have been clustering related to patient oncologic management among surgeons, which was not captured, and therefore not possible to adjust for statistically.
CONCLUSIONS
This study of 312 patients with nonmetastatic breast cancer found that preoperative psychosocial measures were significantly associated with patients’ breast reconstruction status. The results reaffirm that psychologic symptoms in patients with breast cancer are important to address, as it is often treatable, and its resolution may change patients’ course of cancer care. It is unclear whether this finding is patient or provider driven. This study underscores the importance of including psychosocial variables in future well-designed prospective studies.
Footnotes
Published online 27 February 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.National Cancer Institute. Breast cancer statistics. https://www.cancer.gov/types/breast. Updated 2014. Accessed June 14, 2017
- 2.Amir M, Ramati A. Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: a preliminary research. J Anxiety Disord. 2002;16:195–206. [DOI] [PubMed] [Google Scholar]
- 3.Howell D, Olsen K. Distress-the 6th vital sign. Curr Oncol. 2011;18:208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold M, Dunn LB, Phoenix B, et al. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur J Oncol Nurs. 2016;20:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton LM, Andersen BL, Carson WE., III Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunol Immunother. 2008;57:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebel S, Rosberger Z, Edgar L, et al. Predicting stress-related problems in long-term breast cancer survivors. J Psychosom Res. 2008;65:513–523. [DOI] [PubMed] [Google Scholar]
- 7.Jassim GA, Whitford DL, Hickey A, Carter B. Psychological interventions for women with non-metastatic breast cancer. Cochrane Database Syst Rev. 2015CD008729. [DOI] [PubMed] [Google Scholar]
- 8.Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. [DOI] [PubMed] [Google Scholar]
- 9.Pickard AS, Jiang R, Lin HW, et al. Using patient-reported outcomes to compare relative burden of cancer: EQ-5D and functional assessment of cancer therapy-general in eleven types of cancer. Clin Ther. 2016;38:769–777. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso G, Graca J, Klut C, et al. Depression and anxiety symptoms following cancer diagnosis: a cross-sectional study. Psychol Health Med. 2016;21:562–570. [DOI] [PubMed] [Google Scholar]
- 11.Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30:1160–1177. [DOI] [PubMed] [Google Scholar]
- 12.Von Essen L, Larsson G, Oberg K, et al. ‘Satisfaction with care’: associations with health-related quality of life and psychosocial function among swedish patients with endocrine gastrointestinal tumours. Eur J Cancer Care (Engl). 2002;11:91–99. [DOI] [PubMed] [Google Scholar]
- 13.Marcus AC, Garrett KM, Cella D, et al. Can telephone counseling post-treatment improve psychosocial outcomes among early stage breast cancer survivors? Psychooncology. 2010;19:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Runowicz CD, Leach CR, Henry NL, et al. American cancer society/american society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–635. [DOI] [PubMed] [Google Scholar]
- 15.Bruera E, Kuehn N, Miller MJ, et al. The edmonton symptom assessment system (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 16.Hui D, Bruera E. The edmonton symptom assessment system 25 years later: past, present and future developments. J Pain Symptom Manage. 2016;53:630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercadante S, Valle A, Porzio G, et al. ; Home Care–Italy Group Prognostic factors of survival in patients with advanced cancer admitted to home care. J Pain Symptom Manage. 2013;45:56–62. [DOI] [PubMed] [Google Scholar]
- 18.Barbera L, Atzema C, Sutradhar R, et al. Do patient-reported symptoms predict emergency department visits in cancer patients? A population-based analysis. Ann Emerg Med. 2013;61:427.e5–437.e5. [DOI] [PubMed] [Google Scholar]
- 19.Zeng L, Zhang L, Culleton S, et al. Edmonton symptom assessment scale as a prognosticative indicator in patients with advanced cancer. J Palliat Med. 2011;14:337–342. [DOI] [PubMed] [Google Scholar]
- 20.Bultz BD, Groff SL, Fitch M, et al. Implementing screening for distress, the 6th vital sign: a canadian strategy for changing practice. Psychooncology. 2011;20:463–469. [DOI] [PubMed] [Google Scholar]
- 21.Pirro O, Mestak O, Vindigni V, et al. Comparison of patient-reported outcomes after implant versus autologous tissue breast reconstruction using the BREAST-Q. Plast Reconstr Surg Glob Open. 2017;5:e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pusic AL, Matros E, Fine N, et al. Patient-reported outcomes 1 year after immediate breast reconstruction: results of the mastectomy reconstruction outcomes consortium study. J Clin Oncol. 2017;35:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chand ND, Browne V, Paramanathan N, et al. Patient-reported outcomes are better after oncoplastic breast conservation than after mastectomy and autologous reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey CR, Ogbuagu O, Baltodano PA, et al. Quality-of-life outcomes improve with nipple-sparing mastectomy and breast reconstruction. Plast Reconstr Surg. 2017;140:219–226. [DOI] [PubMed] [Google Scholar]
- 25.Eltahir Y, Werners LL, Dreise MM, et al. Which breast is the best? Successful autologous or alloplastic breast reconstruction: patient-reported quality-of-life outcomes. Plast Reconstr Surg. 2015;135:43–50. [DOI] [PubMed] [Google Scholar]
- 26.Yang HC, Thornton LM, Shapiro CL, et al. Surviving recurrence: psychological and quality-of-life recovery. Cancer. 2008;112:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringwald J, Wochnowski C, Bosse K, et al. Psychological distress, anxiety, and depression of cancer-affected BRCA1/2 mutation carriers: a systematic review. J Genet Couns. 2016;25:880–891. [DOI] [PubMed] [Google Scholar]
- 28.Semple J, Metcalfe KA, Lynch HT, et al. ; Hereditary Breast Cancer Clinical Study Group International rates of breast reconstruction after prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers. Ann Surg Oncol. 2013;20:3817–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garyali A, Palmer JL, Yennurajalingam S, et al. Errors in symptom intensity self-assessment by patients receiving outpatient palliative care. J Palliat Med. 2006;9:1059–1065. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe S, Nekolaichuk C, Beaumont C, et al. The edmonton symptom assessment system–what do patients think? Support Care Cancer. 2009;17:675–683. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe SM, Nekolaichuk C, Beaumont C, et al. A multicenter study comparing two numerical versions of the edmonton symptom assessment system in palliative care patients. J Pain Symptom Manage. 2011;41:456–468. [DOI] [PubMed] [Google Scholar]
- 32.Chang VT, Hwang SS, Feuerman M. Validation of the edmonton symptom assessment scale. Cancer. 2000;88:2164–2171. [DOI] [PubMed] [Google Scholar]
- 33.Oldenmenger WH, de Raaf PJ, de Klerk C, et al. Cut points on 0-10 numeric rating scales for symptoms included in the edmonton symptom assessment scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45:1083–1093. [DOI] [PubMed] [Google Scholar]
- 34.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 35.Kelly CM, Shahrokni A. Moving beyond karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vin-Raviv N, Akinyemiju TF, Galea S, et al. Depression and anxiety disorders among hospitalized women with breast cancer. PLoS One. 2015;10:e0129169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schubart JR, Emerich M, Farnan M, et al. Screening for psychological distress in surgical breast cancer patients. Ann Surg Oncol. 2014;21:3348–3353. [DOI] [PubMed] [Google Scholar]
- 38.Liao MN, Chen SC, Chen SC, et al. Change and predictors of symptom distress in breast cancer patients following the first 4 months after diagnosis. J Formos Med Assoc. 2015;114:246–253. [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe KA, Semple J, Quan ML, et al. Changes in psychosocial functioning 1 year after mastectomy alone, delayed breast reconstruction, or immediate breast reconstruction. Ann Surg Oncol. 2012;19:233–241. [DOI] [PubMed] [Google Scholar]
- 40.Jørgensen L, Garne JP, Søgaard M, et al. The experience of distress in relation to surgical treatment and care for breast cancer: an interview study. Eur J Oncol Nurs. 2015;19:612–618. [DOI] [PubMed] [Google Scholar]
- 41.Metcalfe KA, Semple J, Quan ML, et al. Why some mastectomy patients opt to undergo delayed breast reconstruction: results of a long-term prospective study. Plast Reconstr Surg. 2017;139:267–275. [DOI] [PubMed] [Google Scholar]
- 42.Matthews H, Grunfeld EA, Turner A. The efficacy of interventions to improve psychosocial outcomes following surgical treatment for breast cancer: a systematic review and meta-analysis. Psychooncology. 2017;26:593–607. [DOI] [PubMed] [Google Scholar]
- 43.Tsimopoulou I, Pasquali S, Howard R, et al. Psychological prehabilitation before cancer surgery: a systematic review. Ann Surg Oncol. 2015;22:4117–4123. [DOI] [PubMed] [Google Scholar]
- 44.Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31:3119–3126. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Xu R, Wang B, et al. Effects of mindfulness-based therapy for patients with breast cancer: a systematic review and meta-analysis. Complement Ther Med. 2016;26:1–10. [DOI] [PubMed] [Google Scholar]
- 46.Metcalfe KA, Zhong T, Narod SA, et al. A prospective study of mastectomy patients with and without delayed breast reconstruction: long-term psychosocial functioning in the breast cancer survivorship period. J Surg Oncol. 2015;111:258–264. [DOI] [PubMed] [Google Scholar]
- 47.Howard-McNatt MM. Patients opting for breast reconstruction following mastectomy: an analysis of uptake rates and benefit. Breast Cancer (Dove Med Press). 2013;5:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vedsted P, Christensen MB. Frequent attenders in general practice care: a literature review with special reference to methodological considerations. Public Health. 2005;119:118–137. [DOI] [PubMed] [Google Scholar]
- 49.Pan HH, Chu CH, Wu LF, et al. Predictors for reconstruction and mood disorder associated with reconstruction in patients with breast cancer and mastectomy: a retrospective cohort study. Medicine (Baltimore). 2016;95:e2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeevan R, Browne JP, Gulliver-Clarke C, et al. Association between age and access to immediate breast reconstruction in women undergoing mastectomy for breast cancer. Br J Surg. 2017;104:555–561. [DOI] [PubMed] [Google Scholar]
- 51.Shi HY, Uen YH, Yen LC, et al. Two-year quality of life after breast cancer surgery: a comparison of three surgical procedures. Eur J Surg Oncol. 2011;37:695–702. [DOI] [PubMed] [Google Scholar]
- 52.Reaby LL. Reasons why women who have mastectomy decide to have or not to have breast reconstruction. Plast Reconstr Surg. 1998;101:1810–1818. [DOI] [PubMed] [Google Scholar]
- 53.Roth RS, Lowery JC, Davis J, et al. Quality of life and affective distress in women seeking immediate versus delayed breast reconstruction after mastectomy for breast cancer. Plast Reconstr Surg. 2005;116:993–1002; discussion 1003. [DOI] [PubMed] [Google Scholar]
- 54.Mc Grath-Lone L, Day S, Schoenborn C, et al. Exploring research participation among cancer patients: analysis of a national survey and an in-depth interview study. BMC Cancer. 2015;15:618. [DOI] [PMC free article] [PubMed] [Google Scholar]


