Abstract
Human induced pluripotent stem cells (hiPSCs) provide a powerful platform for disease modeling and have unlocked new possibilities for understanding the mechanisms governing human biology, physiology, and genetics. However, hiPSC-derivatives have traditionally been utilized in two-dimensional monocultures, in contrast to the multi-systemic interactions that influence cells in the body. We will discuss recent advances in generating more complex hiPSC-based systems including three-dimensional organoids, tissue-engineered constructs, microfluidic organ-chip platforms, and humanized animal systems. While hiPSC differentiation still requires optimization, these next-generation multi-lineage technologies can augment the biomedical researcher’s toolkit and enable more realistic models of human tissue function.
eTOC:
Sharma et al review recent advances in multi-cell lineage, hiPSC-derived platforms for disease modeling and drug screening. These complex model systems, which include organoids, assembloids, organ-chips, and tissue-engineered constructs, are more realistic representations of human tissues containing multiple cell types that interact during development, aging, and disease.
Introduction
The human body is comprised of at least 200 cell types (Regev et al., 2017), and a variety of model systems have been used to study multi-cell type and systemic interactions (Figure 1). However, animal models in particular exhibit shortcomings when extrapolating findings to the human condition due to species-specific differences in physiology, metabolism, and genetics. These differences can lead to inaccurate results in preclinical drug safety or efficacy studies and ultimately lead to drug failure during clinical trials. Indeed, this may give credence to the “valley of death” scenario driving massive attrition during drug development (Butler, 2008). Preclinical human cell-based assays for drug toxicity evaluation traditionally utilize monocultured cells in isolation, such as immortalized cancer cell lines overexpressing a gene or protein of interest. While important for understanding fundamental cellular mechanisms, the relevance of these cells to human disease is questionable. Thus, there is interest in developing a new generation of multi-lineage platforms that can safely, accurately, and simultaneously evaluate interactions between multiple cell types in the context of studying human disease and for drug discovery.
Figure 1: Catalog of cell types and publications associated with hiPSC-derived multi-lineage model systems.
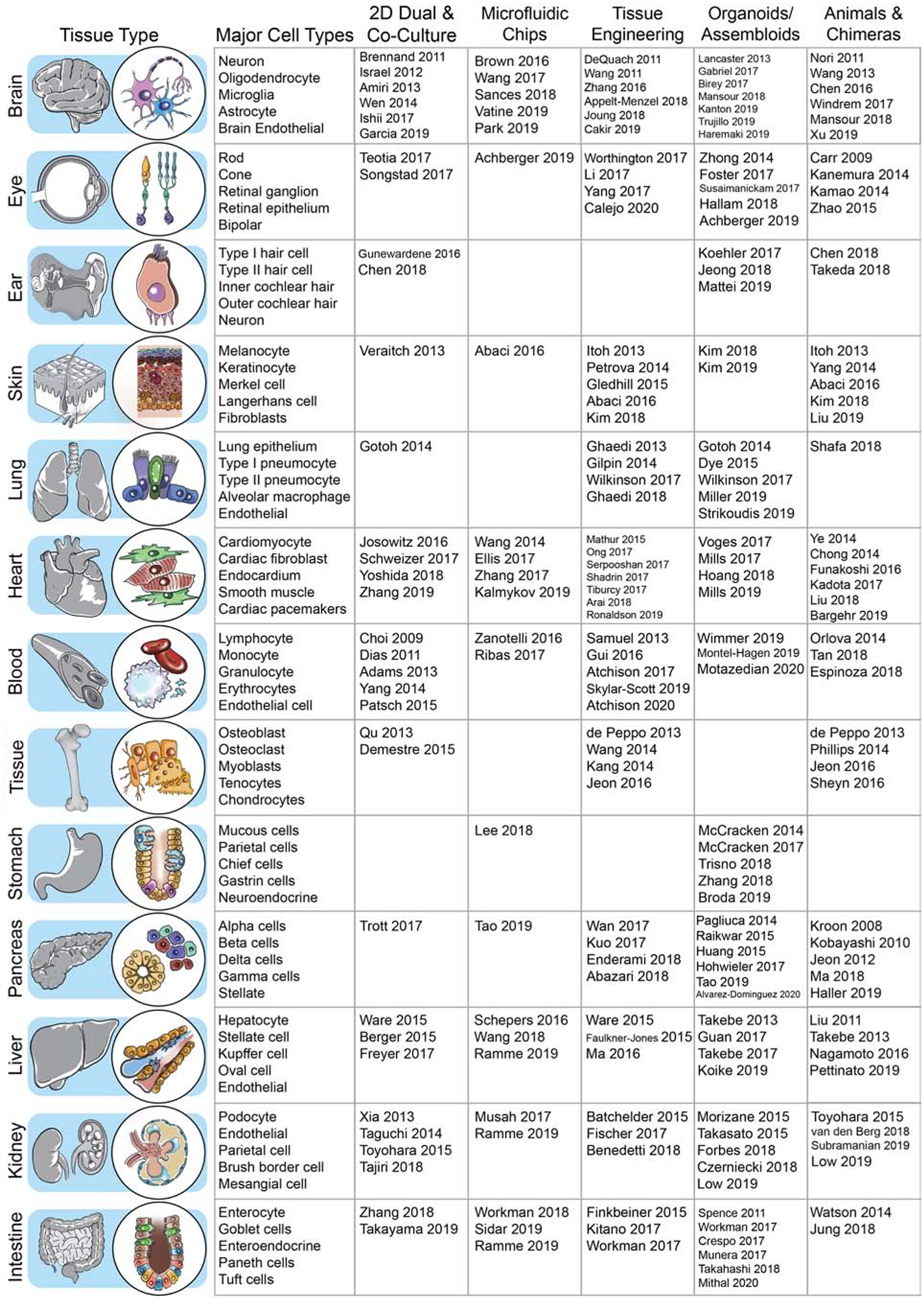
Human iPSCs can model development and disease for a variety of somatic tissues. Differentiation protocols for the cell types comprising these somatic tissues have been developed and refined over the past two decades, initially through two-dimensional culture. In the past 5 years, the hiPSC field has experienced rapid development in organoid, tissue engineering, and microfluidic chip models. Key studies utilizing hiPSC-derived cells in a multi-lineage context are listed according to tissue type and culture platform.
Over the past 15 years, cutting-edge technologies have been developed and refined to model the complex interactions occurring between multiple cell types in the human body. A variety of model systems harboring multiple cell lineages have been employed, ranging from isolated cells in two-dimensional cell co-culture to complex animal models with humanized tissues (Figure 1) (Watson et al., 2017). While several adult human cell types such as neurons and cardiomyocytes are difficult to isolate and culture ex vivo, human induced pluripotent stem cell (hiPSC)-derived cells can now overcome this hurdle (Takahashi et al., 2007; Yu et al., 2007). Treatment with critical pluripotency genes or proteins can transform almost any adult tissue into pluripotent stem cells. These hiPSCs exhibit continued proliferation while maintaining the ability to differentiate into an array of somatic tissues using optimized differentiation protocols. Critically, hiPSCs differentiated into somatic cell types of interest can recapitulate disease phenotypes associated with genetic mutations (Passier et al., 2016).
While analysis of cell subtypes in a cell culture dish provides an excellent resource to study lineage-specific disease mechanisms, in reality cells in the human body do not function in mono-lineage isolation. Thus, understanding disease may require multi-cellular, integrated platforms, which exhibit distinct advantages over mono-lineage analyses (Table 1). Genetic and environmentally-influenced diseases are commonly multi-lineage or multi-systemic in impact, with even a single affected cell type causing a domino-effect that influences downstream pathophysiology in diverse tissues. Similarly, drug toxicity can manifest in multiple organ systems, especially in the case of chemotherapeutic compounds known for their potentially catastrophic off-target toxicities (Burridge et al., 2016). Multi-cell lineage analysis using hiPSCs can also be integrated with personalized medicine, to enable both drug efficacy and toxicity predictions within specific patient populations with unique genetic profiles. A new generation of model systems is being developed to meet rising demand for multi-lineage analysis. These may vary based on their scalability and physiological relevance, but they can be used in combination to elicit a more accurate mechanistic understanding of human biology (Figure 2).
Table 1:
Advantages and challenges of multi-cell lineage model systems using hiPSC-derivative cell types
| Multi-lineage platform | Advantages | Challenges |
|---|---|---|
| 2D co-culture | Highly scalable and amenable to rapid interrogation of cellular, transcriptional, genetic, and signaling mechanisms | Unable to match the 3D physiological complexity associated with other in-vitro systems |
| In-silico | Large computational power enables highly scalable predictive modeling of biological processes and drug responses. | Non-biological platform and dependent on input from accurate biological datasets to establish a useful predictive model |
| Microfluidic organ and body chips | More accurate mimic of in vivo physiological forces than traditional 2D cell culture. Integrates the concept of systemic vasculature through microfluidic channels. Simultaneous interrogation of multiple cell types in a fully-integrated system. | Modest scalability, expensive, and necessary to find appropriate biocompatible materials for cell culture |
| Tissue engineering and 3D printing | Three-dimensionality enables more realistic mimicking of in vivo tissues with potential functional uses for human transplantation | Less scalable than traditional monoculture and cost of bioprinting and bioengineering platforms can be a limiting factor. Biocompatible bioinks must be further refined. |
| Organoids and assembloids | More realistically mimic in vivo developmental processes in an in vitro setting while retaining moderate scalability | Maximum size is limited without adequate vascularization. Potential for tissue necrosis within organoid interior. |
| Humanized animals and chimeras | A physiologically relevant in vivo platform for studying development and potentially serving as a system for mass producing human tissues for transplantation purposes | Expensive and limited throughput. Significant bioethical concerns with growing human reproductive, neural, and other tissues in animals. |
Figure 2: Evolution of multi-lineage hiPSC-derived platforms and comparison of model throughput versus physiological relevance.
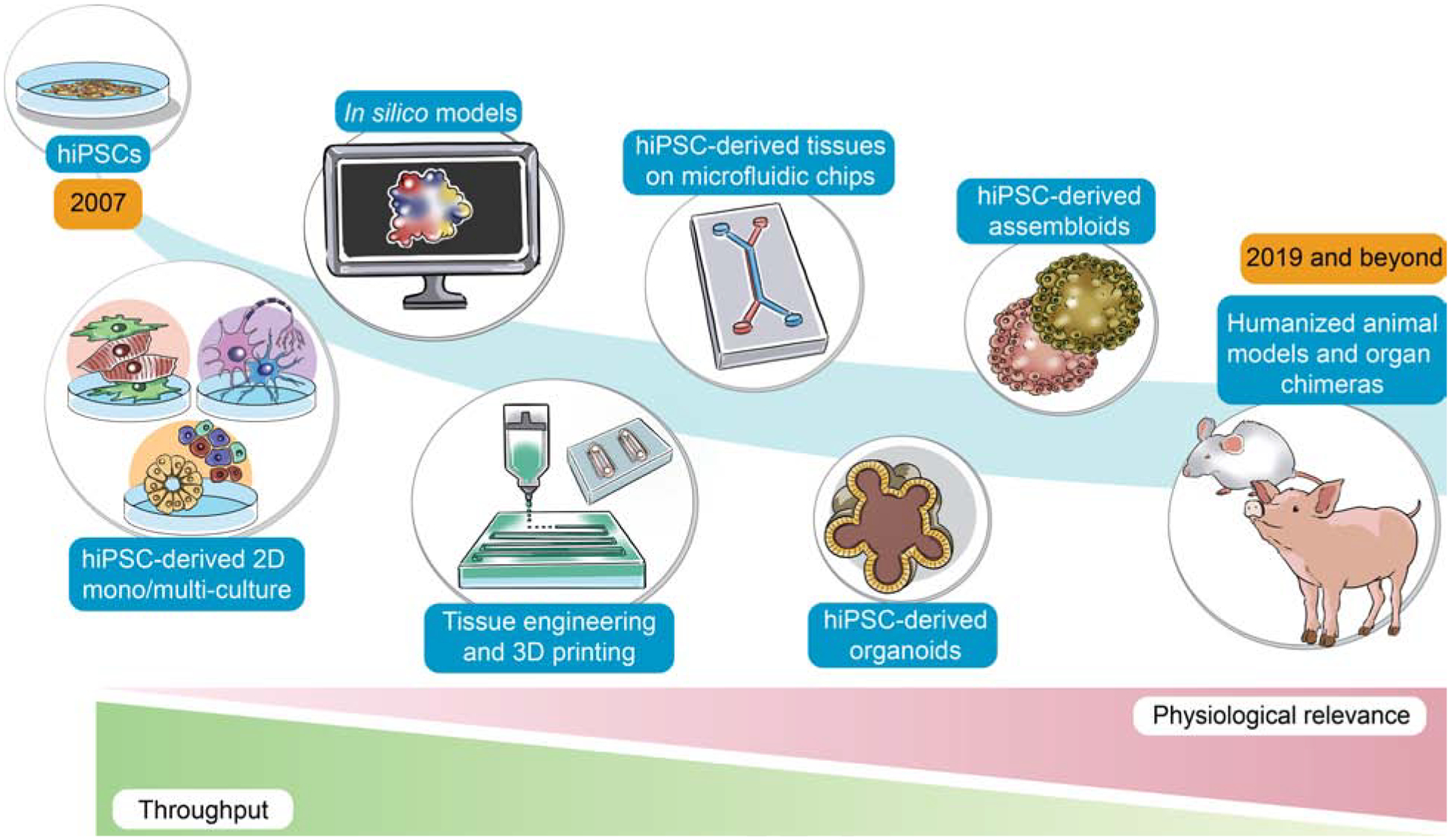
Since the discovery of hiPSCs in 2007, hiPSC-derived multi-lineage platforms have grown in complexity from simple, two-dimensional cultures to engineered platforms and complex organoids. These hiPSC-derived multi-lineage platforms strive to maintain a balance between high throughput and high physiological relevance, with an ideal model exhibiting both characteristics. Future work may incorporate hiPSCs in humanized animal models and organ chimeras to enable human tissue production in large animals.
Two-dimensional co-culture of hiPSC-derivatives
The traditional two-dimensional co-culture of hiPSCs and cell types derived from them provides the simplest form of a multi-lineage, hiPSC-based, in vitro model. In fact, the first hiPSCs were grown in two-dimensional co-culture with irradiated mouse embryonic fibroblasts, which provided pluripotency-maintaining signaling to hiPSC colonies. In some instances, feeder cells are still used to maintain the multipotency of hiPSC-derived tissue progenitors (Toyohara et al., 2015; Trott et al., 2017). During differentiation, hiPSCs have also been co-cultured with supporting somatic cell types from immortalized cell lines, since morphogens secreted from support cells can enhance differentiation towards a lineage of interest (Choi et al., 2009; Dias et al., 2011; Freyer et al., 2017; Schweizer et al., 2017; Yang et al., 2014a; Zhang et al., 2018a). These support cells can also be derived from primary mouse or human tissues (Qu et al., 2013; Songstad et al., 2017; Taguchi et al., 2014; Tajiri et al., 2018; Teotia et al., 2017; Xia et al., 2013). However, efforts to simplify and standardize hiPSC-derived cell type production have led the stem cell field to shift away from co-culture based methods in favor of chemically or genetically defined, feeder-free approaches. Today, the growth and differentiation of hiPSCs is mainly induced with small molecules, growth factors, or ectopic transcription factor gene expression (Burridge et al., 2014).
HiPSC-derived cell types can be grown together in two dimensions with other cells to mimic a tissue of interest. This has been shown to more accurately recapitulate phenotypes associated with cellular maturity, toxicity, and function (Berger et al., 2015). Astrocytes and neurons have been grown together in two-dimensional co-cultures in an effort to recapitulate the support cell network found in the brain (Brennand et al., 2011; Israel et al., 2012; Wen et al., 2014). Such glial-neuronal co-culture has resulted in improved neural network development as well as enhanced cellular maturity and action potential propagation (Amiri et al., 2013; Ishii et al., 2017). Co-culture with neuronal cell types is used to assess the ability of hiPSC-derivatives to establish synaptic connections (Chen et al., 2018a; Demestre et al., 2015; Gunewardene et al., 2016). For other ectodermal derivatives, hiPSC-derived skin precursors upregulated expression of mature keratinocyte markers when co-cultured with human dermal cells (Veraitch et al., 2013). HiPSC-derived cardiomyocytes (hiPSC-CMs) exhibited improved functionality when co-cultured with mesenchymal stem cells, which secrete soluble growth factors that may enhance cellular maturation through activation of receptor-mediated signaling cascades (Yoshida et al., 2018). Fibroblasts co-cultured with hiPSC-CMs influence cardiomyocyte hypertrophy, function, and gene expression (Josowitz et al., 2016; Zhang et al., 2019). Maturation of hiPSC-CMs in particular has represented an ongoing challenge worthy of being addressed. Since functionally mature hiPSC-CMs would elicit the force output and electrophysiological properties found in adult heart muscle cells, hiPSC-CMs have promising applications for cardiac cell therapy and disease modeling. Although scalability has improved for a majority of hiPSC-derived cell types, immaturity of cells such as hiPSC-CMs can limit their clinical potential (Tu et al., 2018). For the heart, recent work demonstrates that co-cultured hiPSC-CMs and epicardial cells could enhance maturity and potentially lead to more long-term functional integration into heart tissue (Bargehr et al., 2019).
Two-dimensional, multi-lineage co-culture could also represent a more accurate model than monoculture for assessing drug toxicity. Neurotoxicity, hepatotoxicity and cardiovascular toxicity represent major causes of drug withdrawal from the pharmaceutical market, and platforms to accurately assess drug toxicity and efficacy in one or more cell types are in high demand. For example, astrocytes induced a neuroprotective phenotype in neurons subjected to neurotoxic chemicals (Amiri et al., 2013). Co-culture of hiPSC-derived hepatocytes with fibroblasts has shown to more accurately model liver metabolism of toxic drug byproducts (Ware et al., 2015). Indeed, a hepatocyte co-culture may be required for in vitro drug efficacy and toxicity screening assays to mimic liver-mediated metabolism of a prodrug into the active form experienced by the body (Jang et al., 2019; Takayama et al., 2019). Additionally, chemotherapeutic compounds such as tyrosine kinase inhibitors elicit simultaneous cardiac and endothelial toxicity and, therefore, co-culture could be valuable for assessing drug toxicity (Sharma et al., 2017). Endothelial cells represent a major cell type of interest for multi-lineage models, given their integral role in lining the blood vessels that supply nutrients and oxygen to all tissues of the body. However, modeling a barrier-interface system in a two dimensional multi-lineage system is difficult, and may be more amenable to complex three-dimensional and microfluidic model systems. Additionally, immune system-mediated inflammation is associated with numerous cardiovascular, neurological, and infectious disorders (Chen et al., 2018b). Through hiPSC technology, it is now possible to introduce simplified aspects of the immune system into a two-dimensional co-culture system. For example, hiPSC-derived endothelial cells are able to interact with and bind leukocytes both in static conditions and in mimicked blood flow (Adams et al., 2013; Patsch et al., 2015). Overall, two-dimensional co-culture represents an easily accessible platform for studying multi-cell type interactions, though there is clearly a need for more complex, three-dimensional model systems to attain greater physiological relevance.
hiPSC-Derived Organoids
Improvements in the directed differentiation of hiPSC-derived organoids have taken the stem cell research community by storm. Organoids are three-dimensional, miniaturized, self-organizing cellular aggregates containing multiple cell types, and hence represent more structurally accurate analogues of human organs and tissues. Thus, they may provide more faithful models of development and disease, when compared to standard two-dimensional cell culture (Takebe and Wells, 2019). The naturally-occurring counterparts to hiPSC-derived organoids are teratomas, masses containing cells derived from all three germ layers. Teratomas are similar to hiPSC-derived organoids, and indeed can serve as an in vivo assay to validate hiPSC differentiation potential, but arise via spontaneous, undirected differentiation. Under the right growth, survival, and high-density culture environments, three-dimensional hiPSC-organoid differentiation enables the production of an exponentially greater number of cells than in two-dimensional monoculture. Due to their small size, organoids still retain the scalability of traditional two-dimensional monoculture, enabling their use in high-throughput screening (Czerniecki et al., 2018).
Ideally, hiPSC-derived tissue-specific organoids can self-assemble, recapitulate the fundamental developmental mechanisms that ultimately give rise to an organ of interest, and harbor the appropriate cell types found in that tissue. A variety of hiPSC-derived organoids representing different bodily tissues have been recently developed, with the notable exception of cardiac organoids. Spherical cardiac microtissues, although not completely able to self-organize and accurately recapitulate the structural and spatial dynamics of the developing heart, have been developed via bioengineering or forced amalgamation of hiPSC-derived cardiomyocytes and used for drug screening (Hoang et al., 2018; Mills et al., 2019; Mills et al., 2017; Voges et al., 2017). Although these cardiac microtissues do not contain functional vasculature, miniaturized blood vessel organoids containing hiPSC-derived endothelial cells and pericytes have been able to model mechanisms of diabetic vasculopathy (Wimmer et al., 2019). Staying within the realm of blood, T-cells have been generated from pluripotent stem cell-derived organoids in an effort to create off-the-shelf products for chimeric antigen receptor T-cell (CAR-T) therapy (Montel-Hagen et al., 2019; Motazedian et al., 2020). Lung organoids from pluripotent stem cells show rudimentary bronchiole-like structures and express alveolar-cell markers (Dye et al., 2015; Gotoh et al., 2014; Miller et al., 2019; Strikoudis et al., 2019; Wilkinson et al., 2017). Pancreatic organoids are able to secrete insulin in response to glucose and are rapidly advancing towards the clinic as a potential treatment for diabetes (Hohwieler et al., 2017; Pagliuca et al., 2014; Raikwar et al., 2015). Similarly, vascularized liver organoids generate albumin and appropriately metabolize drugs of interest (Guan et al., 2017; Takebe et al., 2013; Takebe et al., 2017). Retinal and corneal organoids are also functionally appropriate and harbor key cell types such as light-responsive photoreceptor cells (Foster et al., 2017; Hallam et al., 2018; Susaimanickam et al., 2017; Zhong et al., 2014). HiPSC-derived kidney organoids have rudimentary nephrons, and 3D culture combined with active fluid flow has led to their enhanced vascularization and maturation (Forbes et al., 2018; Low et al., 2019; Morizane et al., 2015; Takasato et al., 2015; van den Berg et al., 2018). Multiple components of the digestive tract, including the esophagus (Trisno et al., 2018; Zhang et al., 2018b) colon (Crespo et al., 2017; Munera et al., 2017), and stomach (Broda et al., 2019; McCracken et al., 2017; McCracken et al., 2014) have been replicated using hiPSC-derived organoids. Intestinal organoids in particular are noted for their advanced substructure development, and display villus-like and crypt-like structures as well as enterocytes, goblet, enteroendocrine, and Paneth cells (Mithal et al., 2020; Spence et al., 2011; Takahashi et al., 2018; Watson et al., 2014). Directed differentiation of hiPSCs using Wnt and BMP signaling modulation has produced organoids composed of fallopian tube epithelium (Yucer et al., 2017). Even inner ear organoids, containing functional hair cells, have been derived from hiPSCs (Jeong et al., 2018; Koehler et al., 2017; Mattei et al., 2019). These studies demonstrate the explosive growth in the last decade in protocols for differentiating and culturing hiPSC-derived organoids from many lineages.
Although there have been tremendous advancements in hiPSC-derived organoids from a variety of somatic tissue types, the brain organoid field has perhaps experienced the most rapid progress in the past decade. Cerebral organoids contain not only neurons but also the glial support cell types normally found in the brain (Lancaster et al., 2013). For example, oligodendrocytes responsible for myelination have been generated within three-dimensional neural cultures (Madhavan et al., 2018; Marton et al., 2019). Recent studies have been able to recapitulate in a dish, advanced developmental processes such as neurulation using micropatterned organoids stimulated with morphogens under precise temporal control (Haremaki et al., 2019). Organoids can also represent an ideal cellular model to study the effect of developmental teratogens. For instance, Zika virus, known to cause microcephaly, can readily infect hiPSC-derived brain organoids and induce premature differentiation in neural progenitors (Gabriel et al., 2017; Qian et al., 2016; Wells et al., 2016). Similarly, using hiPSC differentiation as an analog for development, organoids can address the divergence in human brain development from other primate species. This is a unique application of organoids towards evolutionary biology, and has revealed that chromatin accessibility differs between human and non-human primate brain organoids in multiple cell types over the course of differentiation (Kanton et al., 2019; Pollen et al., 2019). Thus, brain organoids provide a new opportunity to examine neurodevelopmental processes.
In spite of rapid adoption by the stem cell community, major limitations of organoid cultures include hiPSC differentiation and culture inconsistency and inadequate vascularization. Nutrients are not evenly distributed within an organoid by diffusion alone, thus leading an organoid to often develop a necrotic inner core and limiting the maximum possible size. Cellular, genetic, and bioengineering-based approaches have attempted to address this issue by introducing endothelial cells or printing vascular structures within an organoid (Cakir et al., 2019; Pham et al., 2018). Vascularization can also be induced by transplanting an organoid into an in vivo model system to examine integration of organoids with the host vasculature (Mansour et al., 2018), and these hiPSC-chimera approaches are discussed further later in this review. Vascularization or prolonged culture may improve organoid development and maturation, leading to larger and more functional organoids. Over long-term culture, hiPSC-derived cortical organoids can dynamically change their constituent cell populations and mature electrophysiologically to develop enhanced neural networks (Trujillo et al., 2019). Preliminary comparative studies have shown that these primitive organoid neural networks exhibited characteristics that vaguely resemble those found in electroencephalograms from pre-term human infants. However, using a longer time course to increase organoid and hiPSC-derivative maturation may not always work. If in vitro growth conditions are not comparable to the in vivo situation, cell maturation may create unintended cell types. Further, the maturation of hiPSC-derived multi-lineage organoids, cerebral organoids in particular, raises interesting bioethical questions (Chen et al., 2019). Some in the general public have referred to these immature cerebral organoids as “brains-in-a-dish”, which we believe is a gross oversimplification of the current state of the technology. But should the scientific community more closely consider advanced properties such as higher neural function and consciousness in these brain organoids? And if so, should they be more strictly regulated (Farahany et al., 2018)? Although we must closely evaluate such bioethical concerns, there is no doubting the scientific power of multi-lineage hiPSC-derived organoids in revolutionizing our understanding of human disease and developmental principles.
hiPSC-Derived Forced Aggregates and Assembloids
Taking developmental organoids a step further in complexity, recent studies have focused on artificially assembling multiple organoids or combining organoids together with cells from diverse tissue lineages to create forced-aggregate “assembloids” (Pasca, 2019). These advanced co-cultures can better model the interactions between multiple sub-regions and cell types found in complex organs. For example, hiPSC-derived dorsal and ventral forebrain organoids can be combined in vitro to recapitulate the migration of interneurons in the developing human brain and model neurodevelopmental disorders such as Timothy’s syndrome (Birey et al., 2017; Xiang et al., 2017). Similarly, fusing hPSC-derived thalamic and cortical organoids provides a platform for studying inter-regional circuit organization in the human brain (Xiang et al., 2019). Introducing rudimentary anteroposterior and dorsoventral axes in vitro using so-called “organizers” have attempted to recapitulate the complex three-dimensional neural architecture present in vivo (Cederquist et al., 2019). These organizers could be morphogens or cell types of interest that direct the formation of brain axes or the spinal cord. Creating a signaling gradient of the molecular organizer Sonic Hedgehog in forebrain organoids yielded self-assembly around the aforementioned axes, establishing forebrain subdivisions mimicking in vivo brain organization. Improved polarized assembloids will require a more complete understanding of the developmental signaling mechanisms required to properly specify the spatiotemporal axes in the developing nervous system. Similarly, multi-cell lineage brain assembloids can be made by introducing cell types specified outside the nervous system, such as microglia, endothelial cells comprising the blood vessels in the brain, or other support cells such as pericytes. Such multi-lineage hiPSC-derived assembloids have been used to study neural-glial interplay in Alzheimer’s disease (Lin et al., 2018) and could potentially be used to study the role of the immune system in autoimmune diseases affecting the nervous system, such as multiple sclerosis.
Assembloid-based studies extend beyond the brain as well. For instance hiPSC-derived intestinal organoids can be combined with neural crest cells to recapitulate intestinal enteric nervous system development and study motility disorders of the gastrointestinal tract (Workman et al., 2017). Similarly, hPSC-derived axial stem cells can simultaneously specify spinal cord neurons and skeletal muscle cells that self-organize to form hybrid neuromuscular organoids (Faustino Martins et al., 2020). Additionally, combining anterior and posterior gut hiPSC-derived organoids enabled the simultaneous, dynamic morphogenesis of hepatic, biliary, and pancreatic structures (Koike et al., 2019). Patient-derived glioblastoma tumor cells have been combined with cerebral organoids to study the mechanisms driving tumor metastasis (Ogawa et al., 2018). Extending this work further, cancer assembloids consisting of tumor cells and hiPSC-derived organoids of various tissue types could be used as a predictive model for metastasis to elucidate tissue types most susceptible to tumor cell migration and invasion, or conversely, chemotherapy. Assembloids could advance targeted, tissue-specific drug treatment in scenarios where drugs differentially affect subsections of an organ or tissue. For example, some drugs more potently affect the atrial versus ventricular chamber cardiomyocytes in the heart, and could cause serious cardiac arrhythmias or atrial fibrillation (Kaakeh et al., 2012). In fact, the heart could be one organ where complex assembloids faithfully recapitulate the multiple chambers that confer its physiological function. As mentioned, existing cardiac microtissues do not accurately recapitulate the three-dimensional, chamber-specific organization of cell types found in the heart, even though atrial and ventricular-like cardiomyocytes can be specified from hiPSCs (Cyganek et al., 2018). Perhaps atrium and ventricle-specific organoids, containing atrial or ventricular hiPSC-derived cardiomyocytes and nodal conduction cells, could be combined to generate a primitive, four-chambered heart consisting of two atrial organoids and two ventricular organoids. While a heart assembloid would not functionally be on par with the in vivo heart, given that hiPSC-derived cardiomyocytes only elicit a fraction of the force output of true cardiomyocytes, it could be a model to study how the heart chambers interact during development. Any organ with multiple chambers, sub-structures, or compartments could be modeled using hiPSC-derived assembloids, so long as the cells in those sub-structures can be produced in culture. The potential of forced-aggregate assembloids as a developmental model is only just beginning to be realized.
Using hiPSCs for Tissue Engineering Applications
At the intersection of bioengineering and stem cell biology is the concept of using hiPSCs for tissue engineering applications. In contrast to tissue-specific organoids that arise by mimicking in vivo developmental processes, tissue-engineered hiPSC-derived platforms are used to arrange cells in specific locations using biocompatible materials that can be natural, synthetic, or a combination of both. While this enables the tissue-engineer to align cells in specific locations, the field must improve how to accurately mimic interactions between different parts of an organ such as the brain. Alternatively, the creation of assembloids relies on nature to establish connections between different parts of an organ. Assembloids are created by the manual forced assembly and fusion of individual organoids or cell types, possibly by using morphogen gradients as substructure “organizers”. In contrast, when combined with hiPSCs or their derivatives, biomaterials can create customizable scaffolds that maintain cell integrity and viability (Wang et al., 2011). Such platforms can harbor multiple cell types and can be used for disease modeling, drug screening, or cell therapy. Bioactive scaffolds can direct stem cell differentiation into a lineage of interest (Willerth and Sakiyama-Elbert, 2008). Scaffolds comprised of cross-linked hydrophilic polymers and matrix proteins are useful for maintaining stem cell pluripotency and can also be injected for in vivo applications (Abazari et al., 2018; Enderami et al., 2018). Hydrogel scaffolds can contain growth factors, nanoparticles, or small molecules that can actively influence hiPSC-derivatives residing within the scaffold (Kuo et al., 2017; Zhang et al., 2016). Other scaffolds can be comprised of protein-based biomaterials such as fibrin or collagen commonly found in natural extracellular matrix (Batchelder et al., 2015; Fischer et al., 2017; Kitano et al., 2017). Fibrin and fibronectin are found naturally during the blood clotting process, making them highly biocompatible materials for stem cell adhesion within scaffolds (Wan et al., 2017). Similarly, collagen-based biomaterial scaffolds are common in stem cell tissue engineering applications, given that collagen is prevalent in the human body and facilitates cell-cell adhesion (Benedetti et al., 2018). Organ decellularization can generate a natural collagenous scaffold upon which hiPSC-derivatives can be introduced, and this process has been particularly useful for generating biomechanically appropriate, but delicate scaffolds for lung tissue engineering (Ghaedi et al., 2013; Ghaedi et al., 2018; Gilpin et al., 2014) Collagen can also be modified to produce gelatin, another material found in biocompatible hydrogels as “GelMA” or gelatin methacrylate (Kang et al., 2014). Improvements in biocompatible materials will facilitate improved cell survival and function in scaffolded and non-scaffolded tissue-engineered constructs.
Three-dimensional bioprinting is an extension of tissue engineering, whereby mechanical devices combine cellular building blocks with biocompatible “bioinks” to deposit scaffolded or scaffold-free constructs (Romanazzo et al., 2019). Using computer-aided design, a mechanical printer can deposit bioink containing hiPSC-derived cells at designated spatial coordinates, velocity, and volume. Bioinks are integral to the printing process as they must maintain cellular viability, and in the context of multi-lineage tissues, must be compatible across multiple cell types. The previously described hydrogels containing biocompatible materials such as collagen can be used as bioinks for printing undifferentiated hiPSCs or hiPSC derivatives.
Over the past 5 years, a number of hiPSC-derived, multi-lineage platforms have been bioprinted. Scaffold-free three-dimensional printing has generated cardiac platforms by printing cardiac spheroids comprised of endothelial cells, fibroblasts, and hiPSC-derived cardiomyocytes. As the native human myocardium is comprised of these cell types, it is hypothesized that a co-cultured, three-dimensional printed system would represent a more accurate model of the human heart. Printed cardiac spheroids were able to function as a rudimentary cardiac pump (Arai et al., 2018). Similarly, multi-lineage cardiac spheroids have been printed into patches and implanted into the native rat myocardium, which elicited some signs of vascularization (Ong et al., 2017). In addition to scaffold-free systems, scaffolded platforms have also gained traction in the cardiac bioprinting field. These include “engineered heart muscle” harboring hiPSC-derived cardiomyocytes and fibroblasts bound by collagen (Tiburcy et al., 2017), cardiac patches co-cultured with hiPSC-derived cardiomyocytes, smooth muscle cells, and fibroblasts, encapsulated in fibrinogen hydrogels (Shadrin et al., 2017), or heteropolar cardiac tissues composed of both atrial and ventricular hiPSC-CMs seeded in hydrogels (Zhao et al., 2019). Scaffolded platforms can also incorporate micropatterning to force cells such as hiPSC-derived cardiomyocytes to adopt more physiologically relevant shapes in hopes of enhancing cell maturity (Ribeiro et al., 2015; Serpooshan et al., 2017). Additionally, endothelial cell incorporation provides not only a potential support structure and enhanced cardiomyocyte survival through cellular crosstalk, but also is the basis for the development of an endogenous network of vasculature in tissue engineered cardiac constructs. As is the case with any large, thick tissue platform, nutrients must be able to diffuse to the tissue interior to prevent necrosis. Alternatively, vasculature itself could be engineered, and cutting-edge work has demonstrated that it is possible to reproducibility create hiPSC-derived vascular channels that could transport blood, air, or other critical nutrients (Grigoryan et al., 2019; Gui et al., 2016; Samuel et al., 2013). Sacrificial bioinks can be degraded to leave behind elaborate vascular channels (Skylar-Scott et al., 2019). The cardiovascular field has seemingly taken the forefront in the realm of multi-lineage tissue engineering and bioprinting, given the interest in remuscularization and revascularization of the heart after injury.
However, other tissue types have also benefitted from recent advances in tissue engineering and 3D bioprinting for multi-lineage construct generation. For example, 3D-cultured hiPSC-derived hepatocytes, when combined with support cells such as endothelial cells, demonstrate improved morphologies, hepatic gene expression, and liver-specific metabolism over two-dimensional cultures (Faulkner-Jones et al., 2015; Ma et al., 2016). Improvements in differentiation and scaffolding of hiPSC-derived osteoclasts and osteoblasts have unlocked new possibilities for generating replacement bone grafts using tissue engineering approaches (de Peppo et al., 2013; Jeon et al., 2016; Wang et al., 2014b). In the neural space, hiPSC-derived spinal neural progenitors and oligodendrocyte progenitors have been co-cultured to develop three-dimensional spinal cord platforms for modeling nervous tissue repair (Joung et al., 2018). HiPSC-derived neural progenitors can be scaffolded in biomaterials of varying stiffness, confirming that matrix stiffness can influence neural differentiation (DeQuach et al., 2011). Clinical trials for macular degeneration and other ocular disorders are being conducted using hiPSC-derived retinal cells as a cell therapy. Tissue engineering approaches enable long-term scaffolding and axonal growth in hiPSC-derived retinal cells, which may be amenable to transplantation (Calejo et al., 2020; Li et al., 2017; Worthington et al., 2017; Yang et al., 2017). Finally, complex skin analogs have been engineered using hiPSC-derived keratinocytes, endothelial cells, and fibroblasts (Abaci et al., 2016; Gledhill et al., 2015; Itoh et al., 2013; Kim and Ju, 2019; Kim et al., 2018; Petrova et al., 2014). The possibilities become endless for creating multi-lineage, hiPSC-derived platforms as bioprinters become more accessible, bioink perfected to be compatible with multiple cell types, and vascularization improved to enhance nutrient diffusion. It remains to be seen whether complex organs can be perfectly replicated using tissue engineering and bioprinting, but for the purpose of disease modeling, these technologies have substantially improved during the past decade. The decision between allowing natural development to do the work of organizing complex tissues or instead artificially engineering the correct orientation of cells remains an exciting and evolving area of scientific discussion. We feel that it is important to continue to advance both areas and evaluate models based on their predictive value to understand and treat human disease.
Organ and Body-on-a-Chip
Advances in biocompatible materials engineering combined with microfabrication technologies have enabled the development of microfluidic organ-chips (Bhatia and Ingber, 2014). Organ-chips are designed for the growth of multiple organ-specific cell types in a fully-integrated system, often with the different cell types being cultured in separate compartments that are interconnected via perforated membranes or juxtaposed microchannels. This approach allows for the co-culture of the multiple cell lineages that underlie an organ’s function and enables fluid flow, and in some cases mimicked stretch, to further simulate the in vivo environment. Since the body is not a static system, but rather perfused by flowing blood vessels, a lymphatic system, mucus flow across the lungs, and fluid flow through the gastric system, organ-chips advance in vitro models to the next level of physiology.
Importantly, these organ-chip systems must be devised from highly biocompatible materials that can sustain long-term culture of multiple cell types. Some studies have used silicon-based chips, comparable to those used in computer processors. Silicon chips have remained useful for high-throughput, microfluidic sorting of blood cells for CAR-T cell therapy (de Wijs et al., 2017). Another commonly used material is polydimethylsiloxane (PDMS), which is a silicon-based organic polymer. PDMS is preferred in organ-chip manufacturing because of its high biocompatibility, optical transparency for facilitating imaging applications, and ease of assembly. Drug permeability and absorbance associated with PDMS may be a concern, although this remains an area of debate since the process is highly drug- and tissue-dependent. Other materials are still frequently used for specialized applications, including polyurethane, glass, and various biopolymers, although the flexibility of PDMS still makes it one of the most attractive candidates in this field.
A major advantage of microfluidic co-culture systems is that they enable evaluation of hydrodynamic forces conferred by flow of the in vivo circulatory systems in a simplified, in vitro format (Table 1). This allows mechanistic interrogation of vessel-organ interfaces, as in the case of the blood-brain barrier (Appelt-Menzel et al., 2018; Vatine et al., 2019; Wang et al., 2017). Cells grown within microfluidic channels can be subjected to controllable shear stresses by altering the flow rate of media into and out of the chip, mimicking the circulatory system. This is particularly valuable for accurately studying the biology and maturation of endothelial and smooth muscle cells, which are constantly subjected to such biomechanical stresses by virtue of residing within blood vessels (Zanotelli et al., 2016). For example, hiPSC-derived smooth muscle cells from patients with progeria exhibited an inflammatory response to strain when cultured on a microfluidic chip (Ribas et al., 2017). Mimicked fluid flow and endothelial cell co-culture has also shown to improve maturation and function of endothelial and neuronal cell types grown within organ-chips (DeStefano et al., 2017; Park et al., 2019; Sances et al., 2018; Vatine et al., 2019). Fluid flow may enhance cellular alignment to mimic physiologically-relevant conditions or induce maturation. Mature cardiomyocytes harbor linearly aligned sarcomeres, a hallmark of adult cardiomyocytes that is typically absent in hiPSC-derived cardiomyocytes grown in two-dimensional culture (Mathur et al., 2015). Recent heart-on-a-chip studies have shown improved alignment of hiPSC-derived cardiovascular cells under flow and have also modeled cardiomyocyte functional abnormalities experienced during cardiomyopathies (Ellis et al., 2017; Wang et al., 2014a). In addition to creating sheer stress similar to the in vivo environment, blood or serum can be flowed through the blood channel of microfluidic channels to mimic a realistic physiological situation (Vatine et al., 2019). This allows the administration of drugs to the blood channel while observing the effects on the tissue channel, further recapitulating the in vivo environment and adding power to the model. Such drug validation have also been demonstrated independently in hiPSC-derived retina-on-a-chip and kidney-on-a-chip systems, which were able to reproduce the retinotoxic and nephrotoxic side-effects of select compounds, respectively (Achberger et al., 2019; Musah et al., 2017). Another enhancement would be the incorporation of pulsatile flow to mimic both the blood flow after a heartbeat and the constant periodic change in sheer force (Atchison et al., 2020; Atchison et al., 2017). Organ-on-a-chip systems can be integrated into standard hiPSC-derived cell analysis pipelines, conferring the possibility of evaluating patient-specific disease mechanisms or drug responses in multiple cell types simultaneously. Primary cells or immortalized cell lines can also be grown in conjunction with hiPSC-derivatives within organ-chips, depending on the research question or application. For example, microfluidic organ-chips harboring hiPSC-derived and primary brain microvascular endothelial cells, human astrocytes, and pericytes have recapitulated the barrier function of the blood-brain barrier (Brown et al., 2016; Park et al., 2019). Similarly, organ-chips containing hiPSC-derived gut organoids were more faithfully able to model human intestinal epithelial signaling responses than organ-chips containing Caco-2 immortalized cells (Workman et al., 2018). As recently demonstrated by intestine-, pancreas-, stomach-, and liver-chips, microfluidic organ-chip and hiPSC-derived organoid technologies can be easily coupled to enable a new generation of more physiologically-relevant cell culture (Lee et al., 2018a; Schepers et al., 2016; Sidar et al., 2019; Tao et al., 2019; Wang et al., 2018). Scalability still remains a concern for organ-chips, as these systems tend to sacrifice throughput for the sake of physiological complexity. However, if concerns related to chip miniaturization and fabrication cost are alleviated, it may be possible to achieve simplified and disposable organ-chips in high-throughput screening pipelines. Microfluidics-enabled, automated systems for media exchange, drug delivery, and collection of the media from the organ-chips will remove manual manipulation as a requirement and, therefore, reduce variability due to human error.
Perhaps the most exciting culmination of this technology is the body-on-a-chip concept, whereby chips derived from different bodily tissues are connected by a mimicked vascular system. Multiple organ-chips can be interconnected by microfluidics or supernatant media transfer to achieve an analogous body-chip platform (Esch et al., 2011; Ramme et al., 2019; Wikswo et al., 2013). Interconnected chips can enable transfer of metabolites, signaling intermediates, and growth factors from compartments harboring distinct cell types, establishing a more accurate model of how different bodily tissues influence each other in health and disease. Recently, up to 10 independent microphysiological systems mimicking different tissue types have connected in this manner to develop a “physiome-on-a-chip” (Edington et al., 2018; Novak et al., 2020). Body-chip systems also are particularly relevant to study drug toxicity, whereby a drug can be metabolized by hepatocytes in the liver-mimicking compartment of the body-chip (Esch et al., 2011). The metabolized drug can then be introduced to other organ-mimicking compartments on the same chip, establishing a more realistic model of tissue-specific drug toxicity and efficacy. Indeed, fully-integrated body-chips may prove useful for accurate preclinical identification of types of drug toxicity, including cardiotoxicity, hepatotoxicity, and neurotoxicity (Ronaldson-Bouchard and Vunjak-Novakovic, 2018).
The future remains bright for organ-chip technology. Ultimately, long-term success will depend on their integration into preclinical drug discovery and toxicity analysis pipelines for the pharmaceutical industry, as well as using these systems to obtain drug approval from the United States Food and Drug Administration (FDA). Validation of the methodology will require that predictions made in the organ-chip model have direct clinical relevance in human subjects. If successful, hiPSC-based organ-chips have the potential to significantly reduce the need for animals in medical research and increase the efficacy and safety of new medicines. Eventually this may even lead to patient-specific treatments where individual subjects could have their own hiPSC-based organ-chip system for testing drugs prior to use. While this is currently far from reality, the technology is moving at a very fast pace. Both the United States FDA and National Institutes of Health have major initiatives in place to accelerate the integration of organ-chips into drug testing pipelines (Tagle, 2019).
hiPSC Animal Models and Chimeras
In vivo multi-cell type model systems include animal models hybridized with hiPSC-derived tissues. Animal models have the advantage of being living biological systems in which biology and pathophysiology can be interrogated. Mammalian models such as rodents and non-human primates have long served in parallel with human in vitro systems to validate human disease-relevant mechanistic studies. Some of these models have been developed in direct response to regulatory requirements for cellular therapies. Preclinical animal studies are required by the FDA to examine the safety and efficacy of hiPSC-derived cell therapy products prior to use in patients. Indeed, hiPSC-derivatives have routinely been introduced into non-human models, including rodents, pigs, and primates, to investigate mechanisms and treatments for diseases of the heart (Chong et al., 2014; Kadota et al., 2017; Ye et al., 2014), nervous system (Chen et al., 2016; Xu et al., 2019), eye (Carr et al., 2009; Kamao et al., 2014; Kanemura et al., 2014; Zhao et al., 2015) and ear (Takeda et al., 2018). In particular, mice with inhibited immune systems are commonly used as recipients of human donor tissues to test the engraftment of hiPSC-derived cell types including muscle, neural tissues, and pancreatic cells (Funakoshi et al., 2016; Jeon et al., 2012; Kroon et al., 2008; Nori et al., 2011). Animal models can determine whether hiPSC-derivatives have appropriate in vivo functionality, such as the ability of beta cells to secrete insulin under glucose stimulation (Haller et al., 2019; Ma et al., 2018), hematopoietic stem cells to differentiate into blood lineages (Espinoza et al., 2018; Tan et al., 2018) and hepatocytes to metabolize drugs and generate albumin (Liu et al., 2011; Nagamoto et al., 2016; Pettinato et al., 2019). Human iPSC-derived neural tissues have been shown to integrate into rodent central nervous system tissues (Sareen et al., 2014) and in some cases have positive effects in models of neurodegenerative and eye diseases (Nori et al., 2011; Wang et al., 2013). Vascularized hiPSC-derived human brain organoids can merge with mouse host vasculature (Cakir et al., 2019). In a similar fashion, hiPSC-derived tissues have been introduced into non-human primates to study neurodegenerative and musculodegenerative disorders (Sundberg et al., 2013). Pig models have more commonly been used in cardiac cell transplantation studies, due to physiological similarities between human and porcine hearts (Templin et al., 2012). However, long-term functional integration of hiPSC-derived cardiomyocytes and other tissues have been challenging due to the established immaturity of hiPSC-derived cell types. In the case of the heart, human stem cell-derived cardiomyocytes have been able to remuscularize the myocardium after an ischemic event, but in some circumstances, have caused arrhythmias (Liu et al., 2018). Recent work has shown, however, that simultaneously providing epicardial support tissues may enhance remuscularization and integration of hiPSC-derived cardiomyocytes into the heart (Bargehr et al., 2019). Given the propensity of hiPSCs to differentiate into nearly all somatic cell types, other unique therapeutic applications have also been validated in animal models, such as using hiPSC-derivatives for hair growth (Liu et al., 2019; Yang et al., 2014b) and bone regeneration (Phillips et al., 2014; Sheyn et al., 2016). Humanized animal models have been staples of biomedical research for decades and their utility will likely continue to complement in vitro systems.
Large animal models remain critical to evaluating the safety and efficacy of hiPSC-derived cell types used for cell therapy, but use of animal models in this context also requires unique bioethical considerations, in particular for neural and reproductive applications. Human-animal neural chimeras remain an intriguing model system for studying neurological disease and development, but have been heavily regulated and banned in some instances (Sharma et al., 2015). For decades, scientists have considered whether transplanted human brain tissue would confer enhanced cognitive function to other species (Han et al., 2013), although existing data to evaluate this possibility is limited (Mariani et al., 2019; Windrem et al., 2017). Still, animal models engrafted with primary or hiPSC-derived human brain tissue have readily served as in vivo models for studying neurodevelopment or neurodegeneration. Human brain extracts have been injected into the mouse brain for studying tauopathies (Clavaguera et al., 2013) and hiPSC-derived oligodendrocytes have been able to restore myelination in mice with injured spinal cords or congenital hypomyelination (Wang et al., 2013). Similarly, hiPSC-derived glial cells have been injected into the cortex of mice to study the mechanisms of schizophrenia (Windrem et al., 2017). Developmental hiPSC-animal chimeras remain much rarer, although some countries such as Japan have recently eased restrictions on this type of work. An ultimate goal for human-animal developmental chimeras could be the creation of large animals, such as pigs, harboring human organs that could be used for transplantation (Figure 3). The global shortage for human organs is a well-recognized crisis, which could be alleviated by the generation of chimeric pigs containing select human organs such as heart and lungs. To accomplish this, hiPSCs could be introduced at an early developmental timepoint in an animal embryo knocked out for a critical developmental regulator of an organ of interest. This way, the hiPSCs would be able to complement the animal embryo, restoring the developmental potential only in a single organ of interest, and ideally without contributing to neural or reproductive tissues. In reality, hiPSC-animal blastocyst complementation remains extremely technically challenging, although some preliminary studies have been successful. For example, hiPSCs can contribute to early stage pig embryos, suggesting that the human-porcine chimera for human organ generation may be in the realm of possibility (Wu et al., 2017). In addition, mouse blastocysts complemented with hiPSCs are able to generate chimeric human-mouse early stage embryos (Mascetti and Pedersen, 2016). Similarly, mice harboring a rat pancreas have been generated by introducing rat PSCs into a mouse blastocyst knocked out for a key pancreatic developmental regulator, PDX1 (Kobayashi et al., 2010). Importantly, no human chimeric animals have been carried to term due to ethical and governmental restrictions, although this may change as the technology becomes refined. These studies demonstrate the power of hiPSCs to contribute to chimeric development, both for modeling human disease and potentially for translational purposes in organ generation. At the same time, they highlight the challenges of performing ethically acceptable experiments and carefully weighing the risks and benefits of animal chimera studies to advance human therapies.
Figure 3: Assays for interrogating cells within hiPSC-derived multi-lineage model systems and potential downstream applications.
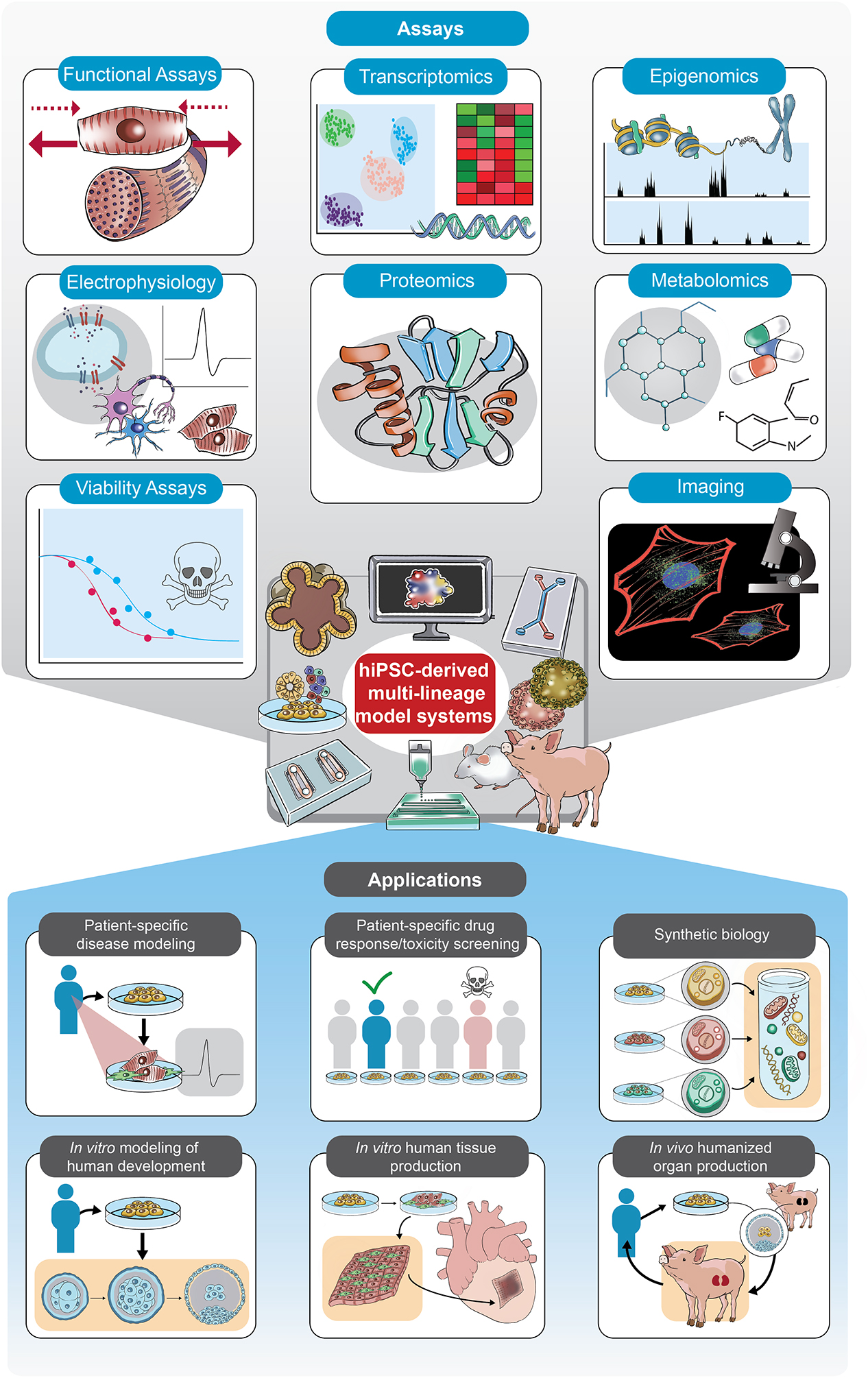
A variety of technical readouts can be considered when evaluating the properties of multiple cell types within an hiPSC-derived multi-lineage model system. Analyses are widely applicable across multi-lineage model systems, in particular those analyses that examine functional, visual, electrophysiological, and –omics level properties of cells. Ideal assays are able to interrogate multiple cell types simultaneously, such as in the case of single-cell transcriptomic, epigenomic, proteomic, and metabolomic analyses. Downstream applications for multi-lineage hiPSC-derived systems include patient-specific disease modeling, personalized drug response screening, and human tissue production.
Readouts to enhance the fidelity of hiPSC models
Assays for evaluating cellular function in multi-lineage, hiPSC-based model systems exhibit significant overlap with those used for evaluating cells in traditional monoculture (Figure 3). Cell viability assays, which could have bioluminescence, fluorescence, or metabolic readouts, can be used to examine cell survival across multiple cell types (Figure 3). However, some multi-lineage platforms may be more amenable to visual evaluation of cellular viability than others. In rodent models containing human cells, modified bioluminescence or fluorescence imaging can be used examine cell engraftment and viability (Jung et al., 2018; Shafa et al., 2018), although zebrafish, given their transparent nature, are more naturally suited for in vivo imaging (Orlova et al., 2014). Organoids are known to vary in cell viability, due to potential necrosis in the center of a spherical organoid depending on nutrient diffusability. This necrotic core may confound results if viability assays are conducted on intact organoids. Indeed, tissue necrosis due to incomplete nutrient diffusability into organoid interiors is one reason why organoids are unable to achieve a diameter above a few millimeters, although bioengineering of vasculature and introduction of artificial microfluidic systems can enhance tissue viability in the organoid interior (Cakir et al., 2019). Similarly, real-time imaging of organoid interiors may be difficult due to tissue thickness, although fluorescence or bioluminescent reporters improve imaging possibilities. Depending on the biomaterial used, cells grown on organ-chips may or may not be amenable to imaging at high resolution. Thus, assays that quantify cell proliferation, for example using imaging-based approaches, may be influenced by limited imaging resolution. To facilitate imaging, custom fluorescent reporter hiPSC lines can be generated using CRISPR/Cas9 genome editing, and can be tailored to cell type-specific expression of fluorescent markers (Sharma et al., 2018). In this manner, transgenic, stably-integrated reporters can be developed to provide cell fluorescence at different wavelengths depending on the differentiated cell types (Kim et al., 2019). This can be useful for live imaging of cell processes in hiPSC-derivatives, as opposed to immunofluorescence as a terminal assay. Functional readouts such as cardiomyocyte contractility or neuronal excitation can also be used in conjunction with fluorescent reporter hiPSC lines (Figure 3) (Toepfer et al., 2019).
Innovative approaches to other functional readouts have also been developed with an emphasis on real-time monitoring, which offers significant advantages due to minimal invasiveness. Real-time monitoring enables long-term analyses of multi-lineage models over the course of extended differentiation or drug treatment. Organoids harboring integrated multi-electrode arrays enable a real-time readout of electrophysiological function during organoid differentiation (Figure 3) (Kalmykov et al., 2019; Zhang et al., 2017). Similarly, organ-chips with integrated electrophysiological assessment assays enable real-time evaluation of action potentials, particularly important for functionally active cell types such as cardiomyocytes and neurons (Maoz et al., 2017). Recently, metabolomics of secreted extracellular molecules has become a staple assay in multi-lineage systems whereby multiple cell types are connected by an integrated nutrient-supplying system (Figure 3) (McAleer et al., 2019). In organ-chips, which typically harbor only a small amount of cellular material, evaluation of secreted growth factors, bioactivated small molecule drugs, and other metabolites in media can be extremely useful as an alternative approach. For example, organ-chips can model the barrier permeability of metabolites analogous to the blood-brain barrier, and have been able to identify previously unknown metabolic coupling between the blood-brain barrier and neurons (Maoz et al., 2018). Thus, multi-lineage platforms vary in their amenability to downstream cellular readouts, but innovative assays and approaches are constantly being developed and optimized for use with each system.
Computational Analyses for Interrogating Multi-Lineage Models
Over the past two decades, the increased accessibility of next-generation sequencing technologies, and their reduced cost, has been paralleled by dramatic advancement in computational power. Improvements in sequencing and computational power have provided the foundation for the field of bioinformatics, which intersects the two technologies. But perhaps the key advance related to simultaneously evaluating different cell types within hiPSC-derived multi-lineage systems has been the emergence of single-cell transcriptomic analysis. This revolutionary technology has spurred the creation of global consortia to discover and characterize all of the cell types found within individual tissues, organs, and eventually, the entire human body (Regev et al., 2017; Thomsen et al., 2016). “Cell Atlas” projects using single-cell analysis are also useful in the study of developmental processes, such as the differentiation of pluripotent stem cells into multipotent progenitors and terminally-differentiated cells. For example, single-cell analysis is an excellent tool for evaluating the specification of the various lineages found in human blood (Villani et al., 2017). Single-cell transcriptomics can be coupled with any multi-lineage, hiPSC-derived platform, and is particularly useful in analyses of heterogeneous cell populations in organoids mimicking developmental processes (Kanton et al., 2019; Subramanian et al., 2019). Single-cell transcriptomic and epigenomic analyses allow for finer dissection of both the distribution of multiple cell types in a bulk population of hiPSC-derived cells and the defining characteristics of cells in that population. Even within a population of undifferentiated hiPSCs, single-cell transcriptional profiling has revealed heterogeneity in gene expression, something that would not have been detectable by bulk RNA-sequencing (Narsinh et al., 2011). In a multi-lineage co-culture system of hiPSC-derived neurons and astrocytes, single-cell RNA-seq has distinguished cell type-specific responses after treatment with anti-convulsant drugs (Ishii et al., 2017). Single-cell RNA-seq has also identified unique subpopulations in differentiating hiPSC-derived endothelial cells, lending further evidence to the heterogeneity of the hiPSC differentiation process (Paik et al., 2018). Using single-cell expression data from hiPSC differentiation in combination with bioinformatics analysis tools, cell lineages can be artificially recreated, providing a “roadmap” for cellular maturity or a key with which unknown cells can be identified (Gong et al., 2018).
Although transcriptomics is currently the most readily-used single-cell –omics level analysis, recent technical advances have enabled reliable single-cell epigenomic (Bravo Gonzalez-Blas et al., 2019) and proteomic analysis (Bravo Gonzalez-Blas et al., 2019). Data from –omics level, single-cell analyses of multiple cell lineages could be used to establish purely computational blueprints for development, differentiation, or drug response. One could envision a scenario whereby a perturbation of interest, such as a drug or genetic mutation, could be fed into an –omics level in silico dataset compiled from analyses of hiPSC-derivatives. Incorporating machine learning, an algorithm could be able to predict the biological downstream effect, such as disease, developmental abnormality, or drug response, of a perturbation of interest completely in silico. As tantalizing as such a prospect may be, a substantial number of standardized multi-omics datasets from multiple cell lineages, in combination with cellular functional data, will be needed to accurately inform such a predictive algorithm. Nevertheless, recent studies have shown that in silico analyses built upon hiPSC-cardiomyocyte electrophysiological datasets can evaluate and predict drug effects (Paci et al., 2018). Such in silico analyses will likely become more common as disease modeling and drug screening datasets from hiPSC-derived cell types continue to grow in number. Systems-level approaches with integrated analyses of multiple functional readouts will provide the best use of multi-lineage models.
Conclusions and Future Directions
An abundance of recent literature on multi-lineage hiPSC-based models such as organoids, assembloids, microfluidic chips and even animal chimeras demonstrates the excitement around these fields. It is also clear that the biotechnology industry is now utilizing hiPSC-based systems for effective drug production, based on a wealth of unpublished data at leading pharmaceutical companies worldwide. There is hope that these new technologies will more closely resemble tissues of the human body, thus becoming more accurate in both modeling human disease and testing drugs for efficacy and toxicity. During reprogramming, hiPSCs wipe the epigenetic slate clean and allow the researcher to repeatedly replay tissue development. If patient-specific disease phenotypes are found in hiPSC-derived cells, they are likely the result of genetic perturbations within the patient’s DNA (Laperle et al., 2020). Conversely, if the hiPSC model based on patient-specific cells has no observed phenotype, it suggests that there was likely no genetic contribution to the disease, but rather, environmental factors were involved. For most diseases, there will be a complex interaction between the genetics and the environment, potentially with disease initiation from the genetics and exacerbation from the environment. To address this question in models lacking a phenotype, the next step would be to introduce environmental factors to “stress” the system and examine if phenotypes emerge only within the patient cells.
The turning point for multi-lineage systems will be validation in human patients. Did the pathology in the model reflect the pathology in the human disease? Were the -omics changes similar to those seen in patients? Did the hiPSC model accurately predict a drug effect in the same patient? This last point will be invaluable for the future of precision medicine, potentially with a patient’s hiPSCs providing a personalized avatar for personalized disease modeling and drug screening. These validation studies must be performed in advanced models to establish whether they are simply interesting in vitro observations, or an important next step in understanding and treating human disease. This will require a precision health-oriented approach and the further development of “clinical trials in a dish” to predict outcomes in the same patient group. As a related first step, multiple high-profile studies have shown that patient-derived tumor organoids using primary cells can serve as a predictive platform to develop personalized therapies for pancreatic (Huang et al., 2015), colorectal (Vlachogiannis et al., 2018; Yao et al., 2019), prostate (Gao et al., 2014), and bladder cancers (Lee et al., 2018b). Likewise, hiPSC-derived cell types are perfectly poised to help develop personalized drug treatments, given their inherent patient-specificity. Combinations of multi-lineage modeling in vitro, in vivo, and even in silico will likely yield the most accurate, reproducible, and translatable results. Incorporation of other cutting-edge technologies such as genome editing, multi-omics and single-cell gene expression analysis, will enable greater flexibility in both establishing customized models and eliciting more accurate downstream analysis of target cell types. Genome editing in particular unlocks an array of possibilities for generating customized, multi-lineage hiPSC-derived cells for use in personalized medicine or even for synthetic biology approaches, whereby these cells can be engineered to produce useful materials or behave in unique ways.
Against this backdrop of enthusiasm for multi-lineage hiPSC-derived systems, significant questions clearly remain (Table 1). There are skeptics who argue that using these hiPSC-based systems will never replace traditional animal models. We believe that each disease has its own challenges, and all hiPSC-derived models require validation as described. Perhaps first and foremost among the prominent issues (Table 1) is cellular immaturity of hiPSC-derived cell types. Human iPSC-derived tissues are typically fetal-like in nature based on transcriptional and functional analyses, and further maturation often requires complex additions to the culture media or over-expression of aging genes (Ho et al., 2016; Miller et al., 2013). Recent studies have also utilized biophysical stimulation via electromechanical inputs to further mature and age functionally-active hiPSC-derived cell types such as cardiomyocytes (Ronaldson-Bouchard et al., 2019; Ruan et al., 2016). Gradually increasing electromechanical stimulation in hiPSC-derived cardiomyocytes dramatically improves their structural and functional properties. Another approach to maturation could be to transplant hiPSC-derived cell types in vivo so that these tissues could benefit from the systemic milieu and physiologically relevant setting. For example, human intestinal organoids transplanted into mice showed more adult-like phenotypes including increased expression of adult digestive enzymes (Finkbeiner et al., 2015; Watson et al., 2014; Workman et al., 2017). Other unique methods to induce hiPSC-derivative maturation have recently been illustrated, such as training human PSC-derived pancreatic islets according to a circadian rhythm (Alvarez-Dominguez et al., 2020). These studies suggest that it is possible to create more mature hiPSC-derivatives, although the field still has not seen a study demonstrating that an hiPSC-derived tissue is functionally and transcriptionally identical to its adult counterpart. If true adult maturity of hiPSC-derivatives is unattainable, and if a disease is only evident in aging patients, one could argue current hiPSC models will never have any value. However, if the late-onset disease has a genetic component, it could very well affect cells during development and affect other support cells during the lifetime of the patient, both leading to overt clinical phenotypes later in life, as we have recently shown for Huntington’s disease (Garcia et al., 2019; Mathkar et al., 2019). We believe that hiPSC models combined with the multi-lineage technologies described here may reveal very early changes and molecular targets in cells prior to their ultimate dysfunction and death. Indeed, we have discovered a very early molecular phenotype in hiPSC-derived dopaminergic neurons from patients with early onset Parkinson’s disease that can be reversed with phorbal ester compounds (Laperle et al., 2020). These types of studies lead to the future hope of prophylactic drug treatments given early in life to at-risk patients that can mechanistically correct the biological dysfunction and thus resolve the disease before onset. An analogy would be the use of statins that are given years before heart disease in order to lower cholesterol and avoid atherosclerosis. Thus, patient hiPSC-derived models, even with their current immaturity, may provide important clinical information.
For certain cells, such as primary neural and cardiac tissues, it remains exceptionally difficult to obtain and scale in a manner that would be useful for large-scale disease modeling and drug discovery projects. Scalability is not a concern for hiPSC-derived cell types, as hiPSCs can be mass produced in two-dimensional and bioreactor-based platforms and differentiated efficiently. However, the efficiency of directed hiPSC differentiation is still variable between donor lines and even individual experiments and typically leads to varying populations of multiple cell types that arise spontaneously, even in the most refined differentiation protocols (Sances et al., 2016). This remains a serious challenge, which may be helped by better sourcing of small molecules and proteins added to serum free cultures. One step to enhance hiPSC-derived systems is to eliminate extraneous lineages from mixed cultures either manually, metabolically, chemically, or genetically in favor of a single lineage. While simplifying the model and reducing variability, the drawback of this approach is the loss of multiple cell type interactions that may be required for normal tissue function, as emphasized in this review. Multi-lineage in vitro or in silico models, if validated in humans, may serve to reduce a reliance on animals for research purposes, an ethically and societally advantageous situation. But incorporation of these model systems into industrial drug discovery pipelines will certainly depend on the financial feasibility of such platforms. Will organoids, organ-chips, or other systems provide enough scientific utility and value to drug development to justify potentially significant reagent and biomanufacturing costs? These questions remain to be fully addressed, but major advances are on the horizon.
Ultimately, no single model can perfectly replicate the complexity of the human body. However, when used thoughtfully and with well-structured validation studies, multi-lineage model systems offer new tools that can be used in the battle against human disease. In summary, we believe that the multi-lineage, hiPSC-based model systems presented here represent the future of precision medicine.
Acknowledgements
We sincerely appreciate Ryoko Hamaguchi for expertly illustrating the figures for this review. We thank Dr. Soshana Svendsen for editing the manuscript. We acknowledge that this review represents an area of study that is dramatically growing in relevance, and thus we apologize to any authors whose work we were not able to include here. Research from the Svendsen laboratory has been supported by the National Institutes of Health (1UG3TR003148, 5UG3NS105703, T32 HL116273), the Allen Distinguished Investigator Award #12879, the Cedars-Sinai Board of Governor’s Regenerative Medicine Institute, and The ALS Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Cedars-Sinai owns a minority stock interest in Emulate, Inc., a company that produces microfluidic organ-chips. Emulate provided no financial support or guidance for this review.
References
- Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK, and Christiano AM (2016). Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells. Advanced healthcare materials 5, 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abazari MF, Soleimanifar F, Nouri Aleagha M, Torabinejad S, Nasiri N, Khamisipour G, Amini Mahabadi J, Mahboudi H, Enderami SE, Saburi E, et al. (2018). PCL/PVA nanofibrous scaffold improve insulin-producing cells generation from human induced pluripotent stem cells. Gene 671, 50–57. [DOI] [PubMed] [Google Scholar]
- Achberger K, Probst C, Haderspeck J, Bolz S, Rogal J, Chuchuy J, Nikolova M, Cora V, Antkowiak L, Haq W, et al. (2019). Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WJ, Zhang Y, Cloutier J, Kuchimanchi P, Newton G, Sehrawat S, Aird WC, Mayadas TN, Luscinskas FW, and Garcia-Cardena G (2013). Functional vascular endothelium derived from human induced pluripotent stem cells. Stem cell reports 1, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez JR, Donaghey J, Rasouli N, Kenty JHR, Helman A, Charlton J, Straubhaar JR, Meissner A, and Melton DA (2020). Circadian Entrainment Triggers Maturation of Human In Vitro Islets. Cell stem cell 26, 108–122 e110. [DOI] [PubMed] [Google Scholar]
- Amiri M, Hosseinmardi N, Bahrami F, and Janahmadi M (2013). Astrocyte-neuron interaction as a mechanism responsible for generation of neural synchrony: a study based on modeling and experiments. Journal of computational neuroscience 34, 489–504. [DOI] [PubMed] [Google Scholar]
- Appelt-Menzel A, Cubukova A, and Metzger M (2018). Establishment of a Human Blood-Brain Barrier Co-Culture Model Mimicking the Neurovascular Unit Using Induced Pluripotent Stem Cells. Current protocols in stem cell biology 47, e62. [DOI] [PubMed] [Google Scholar]
- Arai K, Murata D, Verissimo AR, Mukae Y, Itoh M, Nakamura A, Morita S, and Nakayama K (2018). Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PloS one 13, e0209162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison L, Abutaleb NO, Snyder-Mounts E, Gete Y, Ladha A, Ribar T, Cao K, and Truskey GA (2020). iPSC-Derived Endothelial Cells Affect Vascular Function in a Tissue-Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome. Stem cell reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison L, Zhang H, Cao K, and Truskey GA (2017). A Tissue Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome Using Human iPSC-derived Smooth Muscle Cells. Scientific reports 7, 8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargehr J, Ong LP, Colzani M, Davaapil H, Hofsteen P, Bhandari S, Gambardella L, Le Novere N, Iyer D, Sampaziotis F, et al. (2019). Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nature biotechnology 37, 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder CA, Martinez ML, and Tarantal AF (2015). Natural Scaffolds for Renal Differentiation of Human Embryonic Stem Cells for Kidney Tissue Engineering. PloS one 10, e0143849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti V, Brizi V, Guida P, Tomasoni S, Ciampi O, Angeli E, Valbusa U, Benigni A, Remuzzi G, and Xinaris C (2018). Engineered Kidney Tubules for Modeling Patient-Specific Diseases and Drug Discovery. EBioMedicine 33, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger DR, Ware BR, Davidson MD, Allsup SR, and Khetani SR (2015). Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology 61, 1370–1381. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, and Ingber DE (2014). Microfluidic organs-on-chips. Nature biotechnology 32, 760–772. [DOI] [PubMed] [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Gonzalez-Blas C, Minnoye L, Papasokrati D, Aibar S, Hulselmans G, Christiaens V, Davie K, Wouters J, and Aerts S (2019). cisTopic: cis-regulatory topic modeling on single-cell ATAC-seq data. Nature methods 16, 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. (2011). Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda TR, McCracken KW, and Wells JM (2019). Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nature protocols 14, 28–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Codreanu SG, Shi M, Sherrod SD, Markov DA, Neely MD, Britt CM, Hoilett OS, Reiserer RS, Samson PC, et al. (2016). Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. Journal of neuroinflammation 13, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmstrom A, Chang AC, Coronado MJ, Ebert AD, et al. (2016). Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nature medicine 22, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, et al. (2014). Chemically defined generation of human cardiomyocytes. Nature methods 11, 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D (2008). Translational research: crossing the valley of death. Nature 453, 840–842. [DOI] [PubMed] [Google Scholar]
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, et al. (2019). Engineering of human brain organoids with a functional vascular-like system. Nature methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calejo MT, Saari J, Vuorenpaa H, Vuorimaa-Laukkanen E, Kallio P, Aalto-Setala K, Miettinen S, Skottman H, Kellomaki M, and Juuti-Uusitalo K (2020). Co-culture of human induced pluripotent stem cell-derived retinal pigment epithelial cells and endothelial cells on double collagen-coated honeycomb films. Acta biomaterialia 101, 327–343. [DOI] [PubMed] [Google Scholar]
- Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, et al. (2009). Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PloS one 4, e8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederquist GY, Asciolla JJ, Tchieu J, Walsh RM, Cornacchia D, Resh MD, and Studer L (2019). Specification of positional identity in forebrain organoids. Nature biotechnology 37, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Kim WY, and Jiang P (2016). Humanized neuronal chimeric mouse brain generated by neonatally engrafted human iPSC-derived primitive neural progenitor cells. JCI insight 1, e88632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Wolf JA, Blue R, Song MM, Moreno JD, Ming GL, and Song H (2019). Transplantation of Human Brain Organoids: Revisiting the Science and Ethics of Brain Chimeras. Cell stem cell 25, 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hong F, Zhang C, Li L, Wang C, Shi H, Fu Y, and Wang J (2018a). Differentiation and transplantation of human induced pluripotent stem cell-derived otic epithelial progenitors in mouse cochlea. Stem cell research & therapy 9, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, and Zhao L (2018b). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, and Slukvin I (2009). Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem cells 27, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, et al. (2013). Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proceedings of the National Academy of Sciences of the United States of America 110, 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, Zhang T, Pipalia NH, Chen HJ, Witherspoon M, et al. (2017). Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nature medicine 23, 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Stauske M, Salinas G, Zimmermann WH, et al. (2018). Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, et al. (2018). High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell stem cell 22, 929–940 e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, and Marolt D (2013). Engineering bone tissue substitutes from human induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America 110, 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wijs K, Liu C, Dusa A, Vercruysse D, Majeed B, Tezcan DS, Blaszkiewicz K, Loo J, and Lagae L (2017). Micro vapor bubble jet flow for safe and high-rate fluorescence-activated cell sorting. Lab on a chip 17, 1287–1296. [DOI] [PubMed] [Google Scholar]
- Demestre M, Orth M, Fohr KJ, Achberger K, Ludolph AC, Liebau S, and Boeckers TM (2015). Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem cell research 15, 328–336. [DOI] [PubMed] [Google Scholar]
- DeQuach JA, Yuan SH, Goldstein LS, and Christman KL (2011). Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue engineering Part A 17, 2583–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano JG, Xu ZS, Williams AJ, Yimam N, and Searson PC (2017). Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids and barriers of the CNS 14, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J, Gumenyuk M, Kang H, Vodyanik M, Yu J, Thomson JA, and Slukvin II (2011). Generation of red blood cells from human induced pluripotent stem cells. Stem cells and development 20, 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington CD, Chen WLK, Geishecker E, Kassis T, Soenksen LR, Bhushan BM, Freake D, Kirschner J, Maass C, Tsamandouras N, et al. (2018). Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Scientific reports 8, 4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BW, Acun A, Can UI, and Zorlutuna P (2017). Human iPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine. Biomicrofluidics 11, 024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderami SE, Kehtari M, Abazari MF, Ghoraeian P, Nouri Aleagha M, Soleimanifar F, Soleimani M, Mortazavi Y, Nadri S, Mostafavi H, et al. (2018). Generation of insulin-producing cells from human induced pluripotent stem cells on PLLA/PVA nanofiber scaffold. Artificial cells, nanomedicine, and biotechnology 46, 1062–1069. [DOI] [PubMed] [Google Scholar]
- Esch MB, King TL, and Shuler ML (2011). The role of body-on-a-chip devices in drug and toxicity studies. Annual review of biomedical engineering 13, 55–72. [DOI] [PubMed] [Google Scholar]
- Espinoza JL, Elbadry MI, Chonabayashi K, Yoshida Y, Katagiri T, Harada K, Nakagawa N, Zaimoku Y, Imi T, Takamatsu H, et al. (2018). Hematopoiesis by iPSC-derived hematopoietic stem cells of aplastic anemia that escape cytotoxic T-cell attack. Blood advances 2, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahany NA, Greely HT, Hyman S, Koch C, Grady C, Pasca SP, Sestan N, Arlotta P, Bernat JL, Ting J, et al. (2018). The ethics of experimenting with human brain tissue. Nature 556, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner-Jones A, Fyfe C, Cornelissen DJ, Gardner J, King J, Courtney A, and Shu W (2015). Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 7, 044102. [DOI] [PubMed] [Google Scholar]
- Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault PL, Kabuss L, Hube I, Gazzerro E, Birchmeier C, et al. (2020). Self-Organizing 3D Human Trunk Neuromuscular Organoids. Cell stem cell 26, 172–186 e176. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SR, Hill DR, Altheim CH, Dedhia PH, Taylor MJ, Tsai YH, Chin AM, Mahe MM, Watson CL, Freeman JJ, et al. (2015). Transcriptome-wide Analysis Reveals Hallmarks of Human Intestine Development and Maturation In Vitro and In Vivo. Stem cell reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I, Westphal M, Rossbach B, Bethke N, Hariharan K, Ullah I, Reinke P, Kurtz A, and Stachelscheid H (2017). Comparative characterization of decellularized renal scaffolds for tissue engineering. Biomedical materials 12, 045005. [DOI] [PubMed] [Google Scholar]
- Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, et al. (2018). Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms. American journal of human genetics 102, 816–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Wahlin K, Adams SM, Birk DE, Zack DJ, and Chakravarti S (2017). Cornea organoids from human induced pluripotent stem cells. Scientific reports 7, 41286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer N, Greuel S, Knospel F, Strahl N, Amini L, Jacobs F, Monshouwer M, and Zeilinger K (2017). Effects of Co-Culture Media on Hepatic Differentiation of hiPSC with or without HUVEC Co-Culture. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi S, Miki K, Takaki T, Okubo C, Hatani T, Chonabayashi K, Nishikawa M, Takei I, Oishi A, Narita M, et al. (2016). Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Scientific reports 6, 19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic M, et al. (2017). Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell stem cell 20, 397–406 e395. [DOI] [PubMed] [Google Scholar]
- Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. (2014). Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia VJ, Rushton DJ, Tom CM, Allen ND, Kemp PJ, Svendsen CN, and Mattis VB (2019). Huntington’s Disease Patient-Derived Astrocytes Display Electrophysiological Impairments and Reduced Neuronal Support. Frontiers in neuroscience 13, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, and Niklason LE (2013). Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. The Journal of clinical investigation 123, 4950–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, Le AV, Hatachi G, Beloiartsev A, Rocco K, Sivarapatna A, Mendez JJ, Baevova P, Dyal RN, Leiby KL, et al. (2018). Bioengineered lungs generated from human iPSCs-derived epithelial cells on native extracellular matrix. Journal of tissue engineering and regenerative medicine 12, e1623–e1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin SE, Ren X, Okamoto T, Guyette JP, Mou H, Rajagopal J, Mathisen DJ, Vacanti JP, and Ott HC (2014). Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. The Annals of thoracic surgery 98, 1721–1729; discussion 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill K, Guo Z, Umegaki-Arao N, Higgins CA, Itoh M, and Christiano AM (2015). Melanin Transfer in Human 3D Skin Equivalents Generated Exclusively from Induced Pluripotent Stem Cells. PloS one 10, e0136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Kwak IY, Koyano-Nakagawa N, Pan W, and Garry DJ (2018). TCM visualizes trajectories and cell populations from single cell data. Nature communications 9, 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh S, Ito I, Nagasaki T, Yamamoto Y, Konishi S, Korogi Y, Matsumoto H, Muro S, Hirai T, Funato M, et al. (2014). Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem cell reports 3, 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, et al. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Xu D, Garfin PM, Ehmer U, Hurwitz M, Enns G, Michie S, Wu M, Zheng M, Nishimura T, et al. (2017). Human hepatic organoids for the analysis of human genetic diseases. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Dash BC, Luo J, Qin L, Zhao L, Yamamoto K, Hashimoto T, Wu H, Dardik A, Tellides G, et al. (2016). Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 102, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunewardene N, Crombie D, Dottori M, and Nayagam BA (2016). Innervation of Cochlear Hair Cells by Human Induced Pluripotent Stem Cell-Derived Neurons In Vitro. Stem cells international 2016, 1781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam D, Hilgen G, Dorgau B, Zhu L, Yu M, Bojic S, Hewitt P, Schmitt M, Uteng M, Kustermann S, et al. (2018). Human-Induced Pluripotent Stem Cells Generate Light Responsive Retinal Organoids with Variable and Nutrient-Dependent Efficiency. Stem cells 36, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller C, Piccand J, De Franceschi F, Ohi Y, Bhoumik A, Boss C, De Marchi U, Jacot G, Metairon S, Descombes P, et al. (2019). Macroencapsulated Human iPSC-Derived Pancreatic Progenitors Protect against STZ-Induced Hyperglycemia in Mice. Stem cell reports 12, 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. (2013). Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell stem cell 12, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haremaki T, Metzger JJ, Rito T, Ozair MZ, Etoc F, and Brivanlou AH (2019). Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nature biotechnology 37, 1198–1208. [DOI] [PubMed] [Google Scholar]
- Ho R, Sances S, Gowing G, Amoroso MW, O’Rourke JG, Sahabian A, Wichterle H, Baloh RH, Sareen D, and Svendsen CN (2016). ALS disrupts spinal motor neuron maturation and aging pathways within gene co-expression networks. Nature neuroscience 19, 1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang P, Wang J, Conklin BR, Healy KE, and Ma Z (2018). Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nature protocols 13, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, Eiseler T, Antony JS, Muller M, Renz S, et al. (2017). Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et al. (2015). Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nature medicine 21, 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii MN, Yamamoto K, Shoji M, Asami A, and Kawamata Y (2017). Human induced pluripotent stem cell (hiPSC)-derived neurons respond to convulsant drugs when co-cultured with hiPSC-derived astrocytes. Toxicology 389, 130–138. [DOI] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. (2012). Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, and Christiano AM (2013). Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PloS one 8, e77673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KJ, Otieno MA, Ronxhi J, Lim HK, Ewart L, Kodella KR, Petropolis DB, Kulkarni G, Rubins JE, Conegliano D, et al. (2019). Reproducing human and cross-species drug toxicities using a Liver-Chip. Science translational medicine 11. [DOI] [PubMed] [Google Scholar]
- Jeon K, Lim H, Kim JH, Thuan NV, Park SH, Lim YM, Choi HY, Lee ER, Kim JH, Lee MS, et al. (2012). Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem cells and development 21, 2642–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon OH, Panicker LM, Lu Q, Chae JJ, Feldman RA, and Elisseeff JH (2016). Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Scientific reports 6, 26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, O’Reilly M, Kirkwood NK, Al-Aama J, Lako M, Kros CJ, and Armstrong L (2018). Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell death & disease 9, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josowitz R, Mulero-Navarro S, Rodriguez NA, Falce C, Cohen N, Ullian EM, Weiss LA, Rauen KA, Sobie EA, and Gelb BD (2016). Autonomous and Non-autonomous Defects Underlie Hypertrophic Cardiomyopathy in BRAF-Mutant hiPSC-Derived Cardiomyocytes. Stem cell reports 7, 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung D, Truong V, Neitzke CC, Guo SZ, Walsh PJ, Monat JR, Meng F, Park SH, Dutton JR, and Parr AM (2018). 3D Printed Stem Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Advanced Functional Materials 28, 1801850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KB, Lee H, Son YS, Lee JH, Cho HS, Lee MO, Oh JH, Lee J, Kim S, Jung CR, et al. (2018). In vitro and in vivo imaging and tracking of intestinal organoids from human induced pluripotent stem cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32, 111–122. [DOI] [PubMed] [Google Scholar]
- Kaakeh Y, Overholser BR, Lopshire JC, and Tisdale JE (2012). Drug-induced atrial fibrillation. Drugs 72, 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota S, Pabon L, Reinecke H, and Murry CE (2017). In Vivo Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Neonatal and Adult Rat Hearts. Stem cell reports 8, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmykov A, Huang C, Bliley J, Shiwarski D, Tashman J, Abdullah A, Rastogi SK, Shukla S, Mataev E, Feinberg AW, et al. (2019). Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Science advances 5, eaax0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, and Takahashi M (2014). Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem cell reports 2, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, Mandai M, Morinaga C, Takahashi M, and Kawamata S (2014). Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PloS one 9, e85336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Shih YV, Hwang Y, Wen C, Rao V, Seo T, and Varghese S (2014). Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta biomaterialia 10, 4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanton S, Boyle MJ, He Z, Santel M, Weigert A, Sanchis-Calleja F, Guijarro P, Sidow L, Fleck JS, Han D, et al. (2019). Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574, 418–422. [DOI] [PubMed] [Google Scholar]
- Kim GB, Rincon Fernandez Pacheco D, Saxon D, Yang A, Sabet S, Dutra-Clarke M, Levy R, Watkins A, Park H, Abbasi Akhtar A, et al. (2019). Rapid Generation of Somatic Mouse Mosaics with Locus-Specific, Stably Integrated Transgenic Elements. Cell 179, 251–267 e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, and Ju JH (2019). Generation of 3D Skin Organoid from Cord Blood-derived Induced Pluripotent Stem Cells. Journal of visualized experiments : JoVE. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park N, Rim YA, Nam Y, Jung H, Lee K, and Ju JH (2018). Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem cell research & therapy 9, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K, Schwartz DM, Zhou H, Gilpin SE, Wojtkiewicz GR, Ren X, Sommer CA, Capilla AV, Mathisen DJ, Goldstein AM, et al. (2017). Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nature communications 8, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS, et al. (2010). Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799. [DOI] [PubMed] [Google Scholar]
- Koehler KR, Nie J, Longworth-Mills E, Liu XP, Lee J, Holt JR, and Hashino E (2017). Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nature biotechnology 35, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iwasawa K, Ouchi R, Maezawa M, Giesbrecht K, Saiki N, Ferguson A, Kimura M, Thompson WL, Wells JM, et al. (2019). Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 574, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature biotechnology 26, 443–452. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Liu YC, and Rajesh R (2017). Pancreatic differentiation of induced pluripotent stem cells in activin A-grafted gelatin-poly(lactide-co-glycolide) nanoparticle scaffolds with induction of LY294002 and retinoic acid. Materials science & engineering C, Materials for biological applications 77, 384–393. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, and Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperle AH, Sances S, Yucer N, Dardov VJ, Garcia VJ, Ho R, Fulton AN, Jones MR, Roxas KM, Avalos P, et al. (2020). iPSC modeling of young-onset Parkinson’s disease reveals a molecular signature of disease and novel therapeutic candidates. Nature medicine. [DOI] [PubMed] [Google Scholar]
- Lee KK, McCauley HA, Broda TR, Kofron MJ, Wells JM, and Hong CI (2018a). Human stomach-on-a-chip with luminal flow and peristaltic-like motility. Lab on a chip 18, 3079–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, et al. (2018b). Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 173, 515–528 e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Zhong X, Yang S, Luo Z, Li K, Liu Y, Cai S, Gu H, Lu S, Zhang H, et al. (2017). HiPSC-derived retinal ganglion cells grow dendritic arbors and functional axons on a tissue-engineered scaffold. Acta biomaterialia 54, 117–127. [DOI] [PubMed] [Google Scholar]
- Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, et al. (2018). APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 98, 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kim Y, Sharkis S, Marchionni L, and Jang YY (2011). In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Science translational medicine 3, 82ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LP, Li YM, Guo NN, Li S, Ma X, Zhang YX, Gao Y, Huang JL, Zheng DX, Wang LY, et al. (2019). Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell reports 27, 455–466 e455. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, et al. (2018). Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nature biotechnology 36, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, et al. (2019). Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network. Cell stem cell 25, 373–387 e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wert KJ, Shvartsman D, Melton DA, and Jaenisch R (2018). Establishment of human pluripotent stem cell-derived pancreatic beta-like cells in the mouse pancreas. Proceedings of the National Academy of Sciences of the United States of America 115, 3924–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Qu X, Zhu W, Li YS, Yuan S, Zhang H, Liu J, Wang P, Lai CS, Zanella F, et al. (2016). Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proceedings of the National Academy of Sciences of the United States of America 113, 2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, et al. (2018). Induction of myelinating oligodendrocytes in human cortical spheroids. Nature methods 15, 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, and Gage FH (2018). An in vivo model of functional and vascularized human brain organoids. Nature biotechnology 36, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, et al. (2018). A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nature biotechnology 36, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoz BM, Herland A, Henry OYF, Leineweber WD, Yadid M, Doyle J, Mannix R, Kujala VJ, FitzGerald EA, Parker KK, et al. (2017). Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab on a chip 17, 2294–2302. [DOI] [PubMed] [Google Scholar]
- Mariani JN, Zou L, and Goldman SA (2019). Human Glial Chimeric Mice to Define the Role of Glial Pathology in Human Disease. Methods in molecular biology 1936, 311–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, and Pasca SP (2019). Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nature neuroscience 22, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascetti VL, and Pedersen RA (2016). Human-Mouse Chimerism Validates Human Stem Cell Pluripotency. Cell stem cell 18, 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathkar PP, Suresh D, Dunn J, Tom CM, and Mattis VB (2019). Characterization of Neurodevelopmental Abnormalities in iPSC-Derived Striatal Cultures from Patients with Huntington’s Disease. Journal of Huntington’s disease 8, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, et al. (2015). Human iPSC-based cardiac microphysiological system for drug screening applications. Scientific reports 5, 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei C, Lim R, Drury H, Nasr B, Li Z, Tadros MA, D’Abaco GM, Stok KS, Nayagam BA, and Dottori M (2019). Generation of Vestibular Tissue-Like Organoids From Human Pluripotent Stem Cells Using the Rotary Cell Culture System. Frontiers in cell and developmental biology 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer CW, Long CJ, Elbrecht D, Sasserath T, Bridges LR, Rumsey JW, Martin C, Schnepper M, Wang Y, Schuler F, et al. (2019). Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Science translational medicine 11. [DOI] [PubMed] [Google Scholar]
- McCracken KW, Aihara E, Martin B, Crawford CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH, and Wells JM (2017). Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature 541, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, and Spence JR (2019). Generation of lung organoids from human pluripotent stem cells in vitro. Nature protocols 14, 518–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, et al. (2013). Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell stem cell 13, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RJ, Parker BL, Quaife-Ryan GA, Voges HK, Needham EJ, Bornot A, Ding M, Andersson H, Polla M, Elliott DA, et al. (2019). Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell stem cell 24, 895–907 e896. [DOI] [PubMed] [Google Scholar]
- Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, et al. (2017). Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proceedings of the National Academy of Sciences of the United States of America 114, E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithal A, Capilla A, Heinze D, Berical A, Villacorta-Martin C, Vedaie M, Jacob A, Abo K, Szymaniak A, Peasley M, et al. (2020). Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nature communications 11, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montel-Hagen A, Seet CS, Li S, Chick B, Zhu Y, Chang P, Tsai S, Sun V, Lopez S, Chen HC, et al. (2019). Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell stem cell 24, 376–389 e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, and Bonventre JV (2015). Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature biotechnology 33, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motazedian A, Bruveris FF, Kumar SV, Schiesser JV, Chen T, Ng ES, Chidgey AP, Wells CA, Elefanty AG, and Stanley EG (2020). Multipotent RAG1+ progenitors emerge directly from haemogenic endothelium in human pluripotent stem cell-derived haematopoietic organoids. Nature cell biology 22, 60–73. [DOI] [PubMed] [Google Scholar]
- Munera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, et al. (2017). Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell stem cell 21, 51–64 e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, et al. (2017). Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nature biomedical engineering 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, Kawabata K, and Mizuguchi H (2016). Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. Journal of hepatology 64, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, Jan T, Wilson KD, Leong D, Rosenberg J, et al. (2011). Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. The Journal of clinical investigation 121, 1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, et al. (2011). Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proceedings of the National Academy of Sciences of the United States of America 108, 16825–16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak R, Ingram M, Marquez S, Das D, Delahanty A, Herland A, Maoz BM, Jeanty SSF, Somayaji MR, Burt M, et al. (2020). Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nature biomedical engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J, Pao GM, Shokhirev MN, and Verma IM (2018). Glioblastoma Model Using Human Cerebral Organoids. Cell reports 23, 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, DiSilvestre D, Vricella L, Conte J, Tung L, et al. (2017). Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Scientific reports 7, 4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova VV, van den Hil FE, Petrus-Reurer S, Drabsch Y, Ten Dijke P, and Mummery CL (2014). Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nature protocols 9, 1514–1531. [DOI] [PubMed] [Google Scholar]
- Paci M, Polonen RP, Cori D, Penttinen K, Aalto-Setala K, Severi S, and Hyttinen J (2018). Automatic Optimization of an in Silico Model of Human iPSC Derived Cardiomyocytes Recapitulating Calcium Handling Abnormalities. Frontiers in physiology 9, 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, and Melton DA (2014). Generation of functional human pancreatic beta cells in vitro. Cell 159, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik DT, Tian L, Lee J, Sayed N, Chen IY, Rhee S, Rhee JW, Kim Y, Wirka RC, Buikema JW, et al. (2018). Large-Scale Single-Cell RNA-Seq Reveals Molecular Signatures of Heterogeneous Populations of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells. Circulation research 123, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TE, Mustafaoglu N, Herland A, Hasselkus R, Mannix R, FitzGerald EA, Prantil-Baun R, Watters A, Henry O, Benz M, et al. (2019). Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nature communications 10, 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP (2019). Assembling human brain organoids. Science 363, 126–127. [DOI] [PubMed] [Google Scholar]
- Passier R, Orlova V, and Mummery C (2016). Complex Tissue and Disease Modeling using hiPSCs. Cell stem cell 18, 309–321. [DOI] [PubMed] [Google Scholar]
- Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, et al. (2015). Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nature cell biology 17, 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova A, Celli A, Jacquet L, Dafou D, Crumrine D, Hupe M, Arno M, Hobbs C, Cvoro A, Karagiannis P, et al. (2014). 3D In vitro model of a functional epidermal permeability barrier from human embryonic stem cells and induced pluripotent stem cells. Stem cell reports 2, 675–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinato G, Lehoux S, Ramanathan R, Salem MM, He LX, Muse O, Flaumenhaft R, Thompson MT, Rouse EA, Cummings RD, et al. (2019). Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Scientific reports 9, 8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, and Waldau B (2018). Generation of human vascularized brain organoids. Neuroreport 29, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MD, Kuznetsov SA, Cherman N, Park K, Chen KG, McClendon BN, Hamilton RS, McKay RD, Chenoweth JG, Mallon BS, et al. (2014). Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays. Stem cells translational medicine 3, 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Bhaduri A, Andrews MG, Nowakowski TJ, Meyerson OS, Mostajo-Radji MA, Di Lullo E, Alvarado B, Bedolli M, Dougherty ML, et al. (2019). Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 176, 743–756 e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Puttonen KA, Lindeberg H, Ruponen M, Hovatta O, Koistinaho J, and Lammi MJ (2013). Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. The international journal of biochemistry & cell biology 45, 1802–1812. [DOI] [PubMed] [Google Scholar]
- Raikwar SP, Kim EM, Sivitz WI, Allamargot C, Thedens DR, and Zavazava N (2015). Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice. PloS one 10, e0116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramme AP, Koenig L, Hasenberg T, Schwenk C, Magauer C, Faust D, Lorenz AK, Krebs AC, Drewell C, Schirrmann K, et al. (2019). Autologous induced pluripotent stem cell-derived four-organ-chip. Future science OA 5, FSO413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, et al. (2017). The Human Cell Atlas. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J, Zhang YS, Pitrez PR, Leijten J, Miscuglio M, Rouwkema J, Dokmeci MR, Nissan X, Ferreira L, and Khademhosseini A (2017). Biomechanical Strain Exacerbates Inflammation on a Progeria-on-a-Chip Model. Small 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, and Pruitt BL (2015). Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America 112, 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanazzo S, Nemec S, and Roohani I (2019). iPSC Bioprinting: Where are We at? Materials 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, and Vunjak-Novakovic G (2018). Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell stem cell 22, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Yeager K, Teles D, Chen T, Ma S, Song L, Morikawa K, Wobma HM, Vasciaveo A, Ruiz EC, et al. (2019). Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nature protocols 14, 2781–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, and Murry CE (2016). Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 134, 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, Buecker C, Schafer R, Han X, Au P, et al. (2013). Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America 110, 12774–12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sances S, Bruijn LI, Chandran S, Eggan K, Ho R, Klim JR, Livesey MR, Lowry E, Macklis JD, Rushton D, et al. (2016). Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nature neuroscience 19, 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sances S, Ho R, Vatine G, West D, Laperle A, Meyer A, Godoy M, Kay PS, Mandefro B, Hatata S, et al. (2018). Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Stem cell reports 10, 1222–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, Gowing G, Sahabian A, Staggenborg K, Paradis R, Avalos P, Latter J, Ornelas L, Garcia L, and Svendsen CN (2014). Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. The Journal of comparative neurology 522, 2707–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers A, Li C, Chhabra A, Seney BT, and Bhatia S (2016). Engineering a perfusable 3D human liver platform from iPS cells. Lab on a chip 16, 2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer PA, Darche FF, Ullrich ND, Geschwill P, Greber B, Rivinius R, Seyler C, Muller-Decker K, Draguhn A, Utikal J, et al. (2017). Subtype-specific differentiation of cardiac pacemaker cell clusters from human induced pluripotent stem cells. Stem cell research & therapy 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpooshan V, Chen P, Wu H, Lee S, Sharma A, Hu DA, Venkatraman S, Ganesan AV, Usta OB, Yarmush M, et al. (2017). Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue. Biomaterials 131, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, and Bursac N (2017). Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nature communications 8, 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafa M, Ionescu LI, Vadivel A, Collins JJP, Xu L, Zhong S, Kang M, de Caen G, Daneshmand M, Shi J, et al. (2018). Human induced pluripotent stem cell-derived lung progenitor and alveolar epithelial cells attenuate hyperoxia-induced lung injury. Cytotherapy 20, 108–125. [DOI] [PubMed] [Google Scholar]
- Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmstrom A, et al. (2017). High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Science translational medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sebastiano V, Scott CT, Magnus D, Koyano-Nakagawa N, Garry DJ, Witte ON, Nakauchi H, Wu JC, Weissman IL, et al. (2015). Lift NIH restrictions on chimera research. Science 350, 640. [DOI] [PubMed] [Google Scholar]
- Sharma A, Toepfer CN, Ward T, Wasson L, Agarwal R, Conner DA, Hu JH, and Seidman CE (2018). CRISPR/Cas9-Mediated Fluorescent Tagging of Endogenous Proteins in Human Pluripotent Stem Cells. Current protocols in human genetics 96, 21 11 21–21 11 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheyn D, Ben-David S, Shapiro G, De Mel S, Bez M, Ornelas L, Sahabian A, Sareen D, Da X, Pelled G, et al. (2016). Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem cells translational medicine 5, 1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidar B, Jenkins BR, Huang S, Spence JR, Walk ST, and Wilking JN (2019). Long-term flow through human intestinal organoids with the gut organoid flow chip (GOFlowChip). Lab on a chip 19, 3552–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, and Lewis JA (2019). Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Science advances 5, eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songstad AE, Worthington KS, Chirco KR, Giacalone JC, Whitmore SS, Anfinson KR, Ochoa D, Cranston CM, Riker MJ, Neiman M, et al. (2017). Connective Tissue Growth Factor Promotes Efficient Generation of Human Induced Pluripotent Stem Cell-Derived Choroidal Endothelium. Stem cells translational medicine 6, 1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikoudis A, Cieslak A, Loffredo L, Chen YW, Patel N, Saqi A, Lederer DJ, and Snoeck HW (2019). Modeling of Fibrotic Lung Disease Using 3D Organoids Derived from Human Pluripotent Stem Cells. Cell reports 27, 3709–3723 e3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Sidhom EH, Emani M, Vernon K, Sahakian N, Zhou Y, Kost-Alimova M, Slyper M, Waldman J, Dionne D, et al. (2019). Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation. Nature communications 10, 5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R, et al. (2013). Improved cell therapy protocols for Parkinson’s disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem cells 31, 1548–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaimanickam PJ, Maddileti S, Pulimamidi VK, Boyinpally SR, Naik RR, Naik MN, Reddy GB, Sangwan VS, and Mariappan I (2017). Generating minicorneal organoids from human induced pluripotent stem cells. Development 144, 2338–2351. [DOI] [PubMed] [Google Scholar]
- Tagle DA (2019). The NIH microphysiological systems program: developing in vitro tools for safety and efficacy in drug development. Current opinion in pharmacology 48, 146–154. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, and Nishinakamura R (2014). Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell stem cell 14, 53–67. [DOI] [PubMed] [Google Scholar]
- Tajiri S, Yamanaka S, Fujimoto T, Matsumoto K, Taguchi A, Nishinakamura R, Okano HJ, and Yokoo T (2018). Regenerative potential of induced pluripotent stem cells derived from patients undergoing haemodialysis in kidney regeneration. Scientific reports 8, 14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Sato S, Kurashima Y, Yamamoto T, Kurokawa S, Yuki Y, Takemura N, Uematsu S, Lai CY, Otsu M, et al. (2018). A Refined Culture System for Human Induced Pluripotent Stem Cell-Derived Intestinal Epithelial Organoids. Stem cell reports 10, 314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, et al. (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568. [DOI] [PubMed] [Google Scholar]
- Takayama K, Negoro R, Yamashita T, Kawai K, Ichikawa M, Mori T, Nakatsu N, Harada K, Ito S, Yamada H, et al. (2019). Generation of Human iPSC-Derived Intestinal Epithelial Cell Monolayers by CDX2 Transduction. Cellular and molecular gastroenterology and hepatology 8, 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, et al. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484. [DOI] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, et al. (2017). Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell reports 21, 2661–2670. [DOI] [PubMed] [Google Scholar]
- Takebe T, and Wells JM (2019). Organoids by design. Science 364, 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Hosoya M, Fujioka M, Saegusa C, Saeki T, Miwa T, Okano H, and Minoda R (2018). Engraftment of Human Pluripotent Stem Cell-derived Progenitors in the Inner Ear of Prenatal Mice. Scientific reports 8, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YT, Ye L, Xie F, Beyer AI, Muench MO, Wang J, Chen Z, Liu H, Chen SJ, and Kan YW (2018). Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proceedings of the National Academy of Sciences of the United States of America 115, 2180–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Wang Y, Chen W, Li Z, Su W, Guo Y, Deng P, and Qin J (2019). Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab on a chip 19, 948–958. [DOI] [PubMed] [Google Scholar]
- Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Muller E, Kuest SM, Cohrs S, Schibli R, et al. (2012). Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation 126, 430–439. [DOI] [PubMed] [Google Scholar]
- Teotia P, Van Hook MJ, and Ahmad I (2017). A Co-culture Model for Determining the Target Specificity of the de novo Generated Retinal Ganglion Cells. Bio-protocol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ER, Mich JK, Yao Z, Hodge RD, Doyle AM, Jang S, Shehata SI, Nelson AM, Shapovalova NV, Levi BP, et al. (2016). Fixed single-cell transcriptomic characterization of human radial glial diversity. Nature methods 13, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, Levent E, Raad F, Zeidler S, Wingender E, et al. (2017). Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 135, 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepfer CN, Sharma A, Cicconet M, Garfinkel AC, Mucke M, Neyazi M, Willcox JAL, Agarwal R, Schmid M, Rao J, et al. (2019). SarcTrack. Circulation research 124, 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyohara T, Mae S, Sueta S, Inoue T, Yamagishi Y, Kawamoto T, Kasahara T, Hoshina A, Toyoda T, Tanaka H, et al. (2015). Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem cells translational medicine 4, 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisno SL, Philo KED, McCracken KW, Cata EM, Ruiz-Torres S, Rankin SA, Han L, Nasr T, Chaturvedi P, Rothenberg ME, et al. (2018). Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell stem cell 23, 501–515 e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott J, Tan EK, Ong S, Titmarsh DM, Denil S, Giam M, Wong CK, Wang J, Shboul M, Eio M, et al. (2017). Long-Term Culture of Self-renewing Pancreatic Progenitors Derived from Human Pluripotent Stem Cells. Stem cell reports 8, 1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, et al. (2019). Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell stem cell 25, 558–569 e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C, Chao BS, and Wu JC (2018). Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation research 123, 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, et al. (2018). Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem cell reports 10, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, et al. (2019). Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell stem cell 24, 995–1005 e1006. [DOI] [PubMed] [Google Scholar]
- Veraitch O, Kobayashi T, Imaizumi Y, Akamatsu W, Sasaki T, Yamanaka S, Amagai M, Okano H, and Ohyama M (2013). Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. The Journal of investigative dermatology 133, 1479–1488. [DOI] [PubMed] [Google Scholar]
- Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. (2017). Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, et al. (2018). Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges HK, Mills RJ, Elliott DA, Parton RG, Porrello ER, and Hudson JE (2017). Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 144, 1118–1127. [DOI] [PubMed] [Google Scholar]
- Wan J, Huang Y, Zhou P, Guo Y, Wu C, Zhu S, Wang Y, Wang L, Lu Y, and Wang Z (2017). Culture of iPSCs Derived Pancreatic beta-Like Cells In Vitro Using Decellularized Pancreatic Scaffolds: A Preliminary Trial. BioMed research international 2017, 4276928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ, and Li S (2011). Induced pluripotent stem cells for neural tissue engineering. Biomaterials 32, 5023–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, et al. (2014a). Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature medicine 20, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhao L, Liu J, Weir MD, Zhou X, and Xu HH (2014b). Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone research 2, 14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. (2013). Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell stem cell 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang H, Deng P, Chen W, Guo Y, Tao T, and Qin J (2018). In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab on a chip 18, 3606–3616. [DOI] [PubMed] [Google Scholar]
- Wang YI, Abaci HE, and Shuler ML (2017). Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnology and bioengineering 114, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware BR, Berger DR, and Khetani SR (2015). Prediction of Drug-Induced Liver Injury in Micropatterned Co-cultures Containing iPSC-Derived Human Hepatocytes. Toxicological sciences : an official journal of the Society of Toxicology 145, 252–262. [DOI] [PubMed] [Google Scholar]
- Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, et al. (2014). An in vivo model of human small intestine using pluripotent stem cells. Nature medicine 20, 1310–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DE, Hunziker R, and Wikswo JP (2017). Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology. Experimental biology and medicine 242, 1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, Kommineni S, Bilican B, Klim JR, Hill EJ, et al. (2016). Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell stem cell 19, 703–708. [DOI] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al. (2014). Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515, 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo JP, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, and Matloff WJ (2013). Scaling and systems biology for integrating multiple organs-on-a-chip. Lab on a chip 13, 3496–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DC, Alva-Ornelas JA, Sucre JM, Vijayaraj P, Durra A, Richardson W, Jonas SJ, Paul MK, Karumbayaram S, Dunn B, et al. (2017). Development of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling. Stem cells translational medicine 6, 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, and Sakiyama-Elbert SE (2008). Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery In StemBook (Cambridge (MA)). [PubMed] [Google Scholar]
- Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hammerle M, Esk C, Bagley JA, et al. (2019). Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, et al. (2017). Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell stem cell 21, 195–208 e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman MJ, Gleeson JP, Troisi EJ, Estrada HQ, Kerns SJ, Hinojosa CD, Hamilton GA, Targan SR, Svendsen CN, and Barrett RJ (2018). Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips. Cellular and molecular gastroenterology and hepatology 5, 669–677 e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, et al. (2017). Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nature medicine 23, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington KS, Wiley LA, Kaalberg EE, Collins MM, Mullins RF, Stone EM, and Tucker BA (2017). Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta biomaterialia 55, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, Suzuki K, Bogliotti YS, Cuello C, Morales Valencia M, et al. (2017). Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 168, 473–486 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, et al. (2013). Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nature cell biology 15, 1507–1515. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, et al. (2019). hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell stem cell 24, 487–497 e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, et al. (2017). Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell stem cell 21, 383–398 e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Brawner AT, Li S, Liu JJ, Kim H, Xue H, Pang ZP, Kim WY, Hart RP, Liu Y, et al. (2019). OLIG2 Drives Abnormal Neurodevelopmental Phenotypes in Human iPSC-Based Organoid and Chimeric Mouse Models of Down Syndrome. Cell stem cell 24, 908–926 e908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CT, French A, Goh PA, Pagnamenta A, Mettananda S, Taylor J, Knight S, Nathwani A, Roberts DJ, Watt SM, et al. (2014a). Human induced pluripotent stem cell derived erythroblasts can undergo definitive erythropoiesis and co-express gamma and beta globins. British journal of haematology 166, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Zheng Y, Burrows M, Liu S, Wei Z, Nace A, Guo W, Kumar S, Cotsarelis G, and Xu X (2014b). Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nature communications 5, 3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC, Chuang JH, Buddhakosai W, Wu WJ, Lee CJ, Chen WS, Yang YP, Li MC, Peng CH, and Chen SJ (2017). Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, Xia F, Fu G, Deng Y, Pan M, et al. (2019). Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell stem cell. [DOI] [PubMed] [Google Scholar]
- Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, et al. (2014). Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell stem cell 15, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, Toyofuku T, Toda K, and Sawa Y (2018). Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Molecular therapy : the journal of the American Society of Gene Therapy 26, 2681–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- Yucer N, Holzapfel M, Jenkins Vogel T, Lenaeus L, Ornelas L, Laury A, Sareen D, Barrett R, Karlan BY, and Svendsen CN (2017). Directed Differentiation of Human Induced Pluripotent Stem Cells into Fallopian Tube Epithelium. Scientific reports 7, 10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotelli MR, Ardalani H, Zhang J, Hou Z, Nguyen EH, Swanson S, Nguyen BK, Bolin J, Elwell A, Bischel LL, et al. (2016). Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta biomaterialia 35, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, Schmuck EG, Raval AN, da Rocha AM, Herron TJ, et al. (2019). Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nature communications 10, 2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RR, Koido M, Tadokoro T, Ouchi R, Matsuno T, Ueno Y, Sekine K, Takebe T, and Taniguchi H (2018a). Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem cell reports 10, 780–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Jiang M, Huang SX, Zhang W, Al Alam D, Danopoulos S, Mori M, Chen YW, Balasubramanian R, et al. (2018b). 3D Modeling of Esophageal Development using Human PSC-Derived Basal Progenitors Reveals a Critical Role for Notch Signaling. Cell stem cell 23, 516–529 e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, Riahi R, Chae S, Hu N, et al. (2017). Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proceedings of the National Academy of Sciences of the United States of America 114, E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZN, Freitas BC, Qian H, Lux J, Acab A, Trujillo CA, Herai RH, Nguyen Huu VA, Wen JH, Joshi-Barr S, et al. (2016). Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proceedings of the National Academy of Sciences of the United States of America 113, 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, Lin T, Rong Z, Kim J, He J, Wang M, et al. (2015). Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell stem cell 17, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, et al. (2019). A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176, 913–927 e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, et al. (2014). Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature communications 5, 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]


