Abstract
Background:
There is growing evidence for personalising colorectal cancer (CRC) screening based on risk factors. We compared the cost-effectiveness of personalised CRC screening based on polygenic risk and family history to uniform screening.
Methods:
Using the MISCAN-Colon model, we simulated a cohort of 100 million 40-year-olds, offering them uniform or personalised screening. Individuals were categorised based on polygenic risk and family history of CRC. We varied screening strategies by start age, interval and test and estimated costs and quality-adjusted life years (QALYs). In our analysis we: 1) assessed the cost-effectiveness of uniform screening; 2) developed personalised screening scenarios based on optimal screening strategies by risk group; 3) compared the cost-effectiveness of both.
Results:
At a willingness-to-pay threshold of $50,000/QALY, the optimal uniform screening scenario was annual faecal immunochemical testing (FIT) from 50-74 years, whereas for personalised screening the optimal screening scenario consisted of annual and biennial FIT screening except for those at highest risk who were offered 5-yearly colonoscopy from age 50. Although these scenarios gained the same number of QALYs (17,887), personalised screening was not cost effective, costing an additional $428,953 due to costs associated with determining risk (assumed to be $240 per person). Personalised screening was cost effective when these costs were less than ~$48.
Conclusion:
Uniform CRC screening currently appears more cost effective than personalised screening based on polygenic risk and family history. However, cost-effectiveness is highly dependent on the cost of determining risk.
Impact:
Personalised screening could become increasingly viable as costs for determining risk decrease.
Keywords: colorectal cancer, screening, cost-effectiveness, risk, personalising, polygenic risk, family history
Introduction
Screening has been shown to be a cost-effective method to reduce the incidence and mortality of colorectal cancer (CRC).1–4 In countries with population screening programs, screening for CRC is based on age,5 with separate screening recommendations for those with a positive family history.6 However, genetic susceptibility also plays an important role in CRC risk and it has been suggested that, when combined with family history, this may improve risk prediction and diagnosis.7, 8
Genome-wide association studies have shown that polygenic factors such as common, low risk genetic variants or single-nucleotide polymorphisms (SNPs), play a significant role in defining CRC risk due to their relatively high prevalence in the population.9–12 In isolation, SNPs are only weakly associated with CRC risk, however, cumulatively they explain substantial variation in risk.9, 13 A polygenic test can be used to estimate someone’s polygenic risk score based on the absence or presence of specific risk alleles. Such a risk score can be used to identify individuals at several times lower and greater (0.49-3.40) CRC risk than the average population.14
Compared with age-based screening, personalised screening provides an opportunity to stratify the population allowing screening to be tailored to an individual’s risk.15 This would allow for those at lower risk to start screening later and or have longer screening intervals, while those at higher risk could start screening earlier, undergo more intensive screening or both.9, 15–17 Personalised screening also provides opportunities to detect cancers in younger at-risk individuals, who are currently excluded from age-based screening despite being at increased risk.18–20 In this way, personalised screening has the potential to reduce the harms of screening while maintaining, or even increasing, its benefits in addition to improving its cost-effectiveness.
Previous research has demonstrated the efficacy of stratifying the population for screening based on age and polygenic risk,21, 22 or in combination with other factors including family history.7, 23 However, no studies have evaluated the cost-effectiveness of such risk-stratified screening compared to uniform screening for CRC. To address this gap in knowledge, we investigated the impact of personalising CRC screening, based on polygenic risk and family history and compared its cost-effectiveness to uniform screening.
Methods and Materials
We used the Microsimulation Screening Analysis-Colon (MISCAN-Colon)24 model to estimate the costs, benefits and harms of different uniform screening strategies as well as personalised screening strategies which were based on polygenic risk and family history of CRC.
MISCAN-Colon
MISCAN-Colon is a well-established microsimulation model for CRC developed at the Department of Public Health, Erasmus University Medical Center.24 The structure, underlying assumptions and data sources used to calibrate the model are detailed in Supplementary Methods and Materials. In brief, the model simulates a large population of individuals from birth to death, first without and then with screening for CRC. As each simulated person ages, one or more adenomas may arise and some can progress in size from small (<5 mm) to medium (6- 9 mm) to large (>10 mm). Some adenomas develop into preclinical cancer and subsequently progress through cancer stages I to IV. During each stage symptoms may present and CRC may be diagnosed. The introduction of screening may alter the simulated life histories through detection and removal of adenomas or through detection of CRC at an earlier stage with a more favourable survival. By comparing the life histories of a simulated population being screened to the corresponding life histories in a simulated population not screened, MISCAN-Colon quantifies the effectiveness and the costs of screening.
MISCAN-Colon was calibrated to match the age-specific incidence of CRC in Australia before the introduction of biennial faecal immunochemical test (FIT) screening for those aged 50 to 74 in 2006.25 Stage distribution, localisation of cancers in the colorectum and five-year relative survival after clinical diagnosis of a cancer were based on Australian literature.26, 27 Additional assumptions of the MISCAN-Colon model are presented in Table 1 and Supplementary Methods and Materials.
Table 1:
Model Inputs: Test characteristics, participation assumptions, utility losses and costs associated with colorectal cancer screening and treatment
| TEST CHARACTERISTICS | ||||
|---|---|---|---|---|
| Specificity and sensitivity of FIT a | ||||
| Specificity (per person) | 95.0% | |||
| Sensitivity adenoma 1-5 mm | 0.0% | |||
| Sensitivity adenoma 6-9 mm | 9.0% | |||
| Sensitivity adenoma 10+ mm | 32.0% | |||
| Sensitivity cancer long before clinical diagnosis b | 36.5% | |||
| Sensitivity cancer shortly before clinical diagnosis b | 72.8% | |||
| Specificity and sensitivity of colonoscopy c, d | ||||
| Specificity | 86% | |||
| Sensitivity adenoma 1-5 mm | 75% | |||
| Sensitivity adenoma 6-9 mm | 85% | |||
| Sensitivity adenoma 10+ mm | 95% | |||
| Sensitivity preclinical cancer | 95% | |||
| Complication of colonoscopy e | ||||
| Fatal complication f | 0.040% | |||
| General complication g | ||||
| 50–54 | 0.096% | |||
| 55–59 | 0.080% | |||
| 60–64 | 0.054% | |||
| 65–69 | 0.127% | |||
| 70–74 | 0.073% | |||
| PARTICIPATION | ||||
| Uptake of initial screening offer h | ||||
| 50–54 | 28.5% | |||
| 55–59 | 36.8% | |||
| 60–64 | 43.2% | |||
| 65–69 | 43.5% | |||
| 70–74 | 52.5% | |||
| Uptake of rescreening h | ||||
| Previously attended | 76.0% | |||
| Previously not attended | 19.7% | |||
| Attendance at General Practitioner i | 90.0% | |||
| Uptake of diagnostic test h | ||||
| 50–54 | 72.3% | |||
| 55–59 | 71.6% | |||
| 60–64 | 71.4% | |||
| 65–69 | 70.6% | |||
| 70–74 | 68.2% | |||
| Adherence to surveillance j | 80.0% | |||
| UTILITY LOSS (QALYs) k | ||||
| Per FIT | 0 | |||
| Per colonoscopy l | 0.00274 | |||
| Per complication of colonoscopy m | 0.01918 | |||
| Per LY with CRC Care n, o | Initial Care | Continuing Care | Terminal care (Death CRC) | Terminal care (Death OC) |
| Stage I | 0.12 | 0.05 | 0.70 | 0.05 |
| Stage II | 0.18 | 0.05 | 0.70 | 0.05 |
| Stage III | 0.24 | 0.24 | 0.70 | 0.24 |
| Stage IV | 0.70 | 0.70 | 0.70 | 0.70 |
| COSTS (2016 $AUD) p | ||||
| Per FIT invitation q | 17.35 | |||
| Per returned FIT r | 22.60 | |||
| Per GP visit s | 37.05 | |||
| Per colonoscopy (same day) t | 1,627 | |||
| Polygenic test u | 200 | |||
| Per complication of colonoscopy v | 9,027 | |||
| Treatment by stage and location w, x, y | ||||
| Stage I CC (without bevacizumab) | 31,107 | |||
| Stage I RC (without bevacizumab) | 41,619 | |||
| Stage II CC (without bevacizumab) | 43,776 | |||
| Stage III CC (without bevacizumab) | 79,375 | |||
| Stage II/III RC (without bevacizumab) | 86,317 | |||
| Stage IV CRC without bevacizumab | 71,156 | |||
| Stage IV CRC with bevacizumab | 81,403 | |||
Abbreviations: CC = Colon Cancer; CRC = Colorectal Cancer; Abbreviations: FIT = faecal immunochemical test; GP = General Practitioner; OC = Other Cause; QALY = Quality-Adjusted Life Year; RC = Rectal Cancer; LY = Life Year
Specificity and sensitivity of FIT derived from results of Queensland Health report31
We assume that FIT screening is more sensitive in cancers as they progress towards becoming symptomatic (visible bleeding) and clinically detectable. For preclinical cancers which will become symptomatic within the same stage, assumed test sensitivity is higher
The lack of specificity with endoscopy reflects the detection of non-adenomatous lesions, where the non-adenomatous lesions are removed and therefore induce polypectomy and biopsy or lead to (unnecessary) referral with sigmoidoscopy. The evidence synthesis reported no specificity for endoscopy for any adenoma. Specificity for colonoscopy is therefore based on Schroy et al, 201362
Sensitivity of colonoscopy for the detection of adenomas and CRC within the reach of the endoscope was obtained from a systematic review on miss rates observed in tandem colonoscopy studies63
Complications are conditional on polypectomy, and we assume that polypectomy is only performed if colonoscopy is positive
Fatal perforation taken from Viiala et al, 200364 and includes only deaths from colonoscopies performed in outpatients within 30 days of, and attributed to, colonoscopy
Age-specific rate of complication taken from National Bowel Cancer Screening Monitoring report.33 A complication is considered as an unplanned hospital admission within 30-days of a diagnostic colonoscopy
Uptake of screening, rescreening and participation in diagnostic follow up taken from National Bowel Cancer Screening Monitoring report33
Attendance at general practitioner for referral to colonoscopy taken from Tran et al, 201126
Attendance at surveillance colonoscopies assumed to be 80% based on Colquhoun et al, 200334
The loss of quality of life associated with a particular event
Equal to 2 days per colonoscopy at a utility of 0.5
Complications associated with hospitalisation with 30 days of colonoscopy were assumed to be equal to 14 days at a utility of 0.5
Care for CRC was divided in three clinically relevant phases: the initial, continuing, and terminal care phase. The initial care phase was defined as the first 12 months after diagnosis; the terminal care phase was defined as the final 12 months of life; the continuing care phase was defined as all months in between. In the terminal care phase, we distinguished between CRC patients dying from CRC and CRC patients dying from another cause. For patients surviving less than 24 months, the final 12 months were allocated to the terminal care phase and the remaining months were allocated to the initial care phase
Utility losses for LYs with initial care were derived from a study by Ness and colleagues.42 For LYs with continuing care for stage I and II CRC, we assumed a utility loss of 0.05 QALYs; for LYs with continuing care for stage III and IV CRC, we assumed the corresponding utility losses for LYs with initial care. For LYs with terminal care for CRC, we assumed the utility loss for LYs with initial care for stage IV CRC. For LYs with terminal care for another cause, we assumed the corresponding utility losses for LYs with continuing care
Costs are from a health systems perspective and do not include patient time costs. All costs are presented in Australian dollars ($AUD) and are indexed to 2016 prices
FIT price based on the pricing of a commercially available alternative35
The cost to analyse a specimen based in Australian Medicare Benefits Schedule36
Cost to visit GP taken from Australian Medicare Benefits Schedule37
Costs for colonoscopy are calculated based on information available from Independent Hospital Pricing Authority38
Cost of polygenic test based on a commercially available polygenic test for breast cancer41
Costs for complications of colonoscopy are calculated based on information available from Independent Hospital Pricing Authority38
Cost of treatment taken from Ananda et al, 201639
Proportion of rectal cancer assumed to be 30.81%27
Proportion of Stage IV cancers treated with bevacizumab assumed to be 50%39
Simulated population
For this analysis, we simulated a cohort of 100 million 40-year-olds, with life expectancy as observed in Australia in 2013-2015.28 Individuals were followed for a lifetime, until a maximum age of 100 years, at which point they are all assumed to be dead.
Risk stratification
Using previous research, the population was stratified a priori into five risk groups based on their quintile of polygenic risk score (based on 45 SNPs shown to increase CRC risk) and their first-degree family history of CRC (Table 2).14 The expected prevalence of each of the five categories in the general population was based on a random assignment of 1,000 people given a 20% probability of being in any SNP quintile and a 10% probability of having at least one first-degree relative with CRC.29 The relative risk (RR) of developing CRC (compared with the average population risk) for each risk group was based on the combined RR of each quintile of polygenic risk score and family history based on observed virtual independence of the two factors.14 The five risk groups were defined as: “very low” (RR<0.5), “low” (RR between 0.5-0.9), “average” (RR between 0.9-1.2), “high” (RR between 1.2-1.8), and “very high” (RR >1.8). We assumed no other differences in life expectancy, CRC stage distribution, survival, or screening performance characteristics between the risk groups.
Table 2:
Stratification of individuals according to polygenic risk and family history of colorectal cancer a
| Risk group | Risk category | Description | RR | PP(%) |
|---|---|---|---|---|
| Very Low | 1 | Lowest quintile for polygenic risk and no CRC in first degree relatives | 0.47 | 20 |
| Low | 2 | Second lowest quintile for polygenic risk and no CRC in first degree relatives | 0.72 | 23 |
| Average | 3 | Lowest quintile for polygenic risk and at least one CRC in first degree relative OR middle quintile for polygenic risk and no CRC in first degree relatives | 0.93 | 18 |
| 4 | Second highest quintile for polygenic risk and no CRC in first degree relatives | 1.14 | 14 | |
| High | 5 | Second lowest quintile for polygenic risk and at least one CRC in first degree relatives | 1.45 | 3 |
| 6 | Middle quintile for polygenic risk and at least one CRC in first degree relative OR highest quintile for polygenic risk and no CRC in first degree relatives | 1.70 | 18 | |
| Very High | 7 | Second highest quintile for polygenic risk and at least one CRC in first degree relatives | 2.31 | 2 |
| 8 | Highest quintile for polygenic risk and at least one CRC in first degree relatives | 3.40 | 2 | |
Abbreviations: CRC = Colorectal cancer; PP = Population percentage; RR = Relative risk = risk of colorectal cancer in category compared with the average risk of colorectal cancer
Stratification based on Jenkins et al14
Screening and Surveillance
In addition to a scenario without screening, we modelled 25 different screening strategies, varying screening start age (40, 46, 50, 54 or 60 years), test (FIT or colonoscopy), and interval (annual, biennial or triennial screening for FIT, and every 5 or 10 years for colonoscopy). For all FIT analyses, we assumed a positivity of 7.7% based on rates observed in the Queensland Bowel Cancer Screening Program between August 2006 and December 2010.30, 31 Screening was always assumed to stop at age 74 years. Surveillance intervals and stop-age for all scenarios were based on the Australian National Health and Medical Research Council Clinical Practice Guidelines for Surveillance Colonoscopy.32
Participation
Screening programmes can be assessed under the assumption of perfect adherence or observed adherence. In the first analyses, we assumed perfect adherence to all screening, diagnostic and surveillance tests. Subsequently, we estimated the costs and effects of screening at adherence levels currently observed in Australia.
For the latter analysis, we simulated participation rates as reported by the Australian National Bowel Cancer Screening Program (NBCSP), a biennial FIT screening program, in 2017 (Table 1).33 Participation with annual and triennial FIT and with primary colonoscopy screening was set at the same screening participation rates. Age-specific participation rates were provided in five-year age intervals between 50 and 74 years. As data was not available for screening participation for individuals aged 40-49 years, participation was assumed to be equal to those aged 50-54 years. We assumed that 76.0% of individuals who had previously attended screening would attend again in the next screening round while 19.7% of individuals who had not attended in the previous round would now attend based on data from the NBCSP.33
A positive FIT requires a consultation with a primary care provider, such as a general practitioner (GP) to discuss test results and obtain a referral for colonoscopy. For the observed adherence analyses, it was assumed that 90% of FIT positive cases would attend this appointment.26 In addition, attendance at diagnostic colonoscopy was age-specific ranging from 68.2 to 72.3% based on outcomes from the NBCSP.33 The participation rate for colonoscopy surveillance was assumed to be 80%.34
Assumptions for costs and utilities
The cost of screening with FIT was based on commercially available kits.35 This cost includes the test, postage and test processing fees. The cost to analyse a FIT specimen was based on the Australian Medicare Benefits Schedule (MBS).36 Cost of attending a GP to obtain a referral for colonoscopy (standard consult) is set in the MBS.37 The cost for colonoscopy and complications from colonoscopy were obtained from the Independent Hospital Pricing Authority report on costs in Australian public hospitals.38 Costs for cancer care were based on costs of cancer treatment in the Australian setting.39 All costs are presented in Australian dollars ($AUD), standardised to 2016 prices using the consumer price index where necessary.40
To determine risk, we assumed all individuals underwent assessment for family history of CRC and polygenic testing prior to the commencement of CRC screening. We assumed assessment for family history of CRC would be undertaken by a GP and cost the same as a standard consult (Table 1).37 In addition, we assumed polygenic testing would cost $200 based on a commercially available polygenic test for breast cancer.41
The assumed utility loss due to CRC screening was 0.00274 quality-adjusted life years (QALYs) per colonoscopy (1.5 days at 0.5 utility) and 0.001918 QALYs per complication of colonoscopy (14 days at 0.5 utility) (Table 1). We also assumed that life years (LYs) with CRC care are of lower quality than those without CRC care.42 We assumed no disutility from determining or knowing polygenic risk score.
Model outcomes
For all scenarios, the model estimated health effects such as CRC incidence and CRC mortality, and required resources such as the number of screening and surveillance tests performed between ages 40 and 74 years. From these outcomes, we calculated costs, life years, and QALYs lived with each strategy. Costs, life years and QALYs were discounted at an annual rate of 5%, as is recommended in Australia.43 Undiscounted results are presented in Supplementary Results (Table S6–7, Figure S8).
Analyses
Our analysis consisted of four parts. First, we determined costs, benefits and harms of the aforementioned screening strategies applied to the population as a whole (uniform screening). We plotted the uniform screening scenarios in a cost-effectiveness plane and performed an incremental cost-effectiveness analysis to see which scenarios were efficient.
Second, we followed the above steps for each risk group and used these results to determine the efficient screening strategies for each risk group. Then, we combined the efficient screening strategies for all risk groups and ordered them from least expensive to most expensive. Using this list we developed a series of optimal personalised screening scenarios. As each personalised screening scenario can be a combination of different strategies for each risk subgroup, there will be many more personalised screening scenarios.
Third, we compared the outcomes of uniform and personalised screening to establish which method would yield better results. We did this by plotting all uniform and personalised screening scenarios in a single cost-effectiveness plane and by performing an incremental cost-effectiveness analysis to see whether personalised screening or uniform screening was most efficient.
Finally, we applied imperfect participation rates to uniform and personalised screening scenarios to determine their impact in a ‘real-world’ scenario. The benefits and costs of screening were compared to the same population undergoing no screening.
At each step, scenarios with the highest incremental cost-effectiveness ratio (ICER) under a threshold of $50,000 per QALY gained were identified as the optimally cost-effective strategy as this is a commonly used willingness-to-pay (WTP) threshold in Australia.
Sensitivity analyses
In sensitivity analyses, we assessed the impact of weighting QALYs by age44 (we applied age-adjusted health related quality of life so that quality of life deceased with increasing age) and discounting our results at 3% rather than 5% as this is a common international discounting rate.45 In addition, we explored the impact of changes in screening participation for personalised screening, holding the participation of uniform screening at current levels. To do this, we increased and decreased age-specific participation of the initial screening offer by 10 percentage points and adjusted the participation of rescreening.
Due to the uncertainty surrounding costs for determining risk profile, we also included a sensitivity analysis where these costs were excluded. Using this information, we conducted a threshold analysis to estimate the maximum cost for determining risk profile where personalised screening would be considered cost effective compared to uniform screening, at a WTP of $50,000 per QALY gained.
Results
Uniform screening
Compared with no screening, the uniform screening scenarios (Table 3a) reduced CRC incidence by 22-69% (18-58 fewer CRC cases per 1,000 individuals) and mortality by 35-79% (10-23 fewer CRC deaths). These scenarios yielded 0.11-0.32% more QALYs (20-58 additional QALYs) and costs increased by 0.5%-424% ($6,409-$5,277,930) per 1,000 individuals. These screening scenarios increased colonoscopy demand by 383-6,927 colonoscopies per 1,000 individuals (Table 3a). Several uniform screening scenarios were on the efficient frontier (Supplementary Results, Figure S1). Using a WTP threshold of $50,000 per QALY gained, the optimal uniform screening scenario was annual FIT from 50 to 74 years (ICER $43,174). Although close to the efficient frontier, biennial FIT screening from 50 to 74 years, the screening program currently implemented in Australia, was dominated. Colonoscopy screening scenarios were the most effective, however, they also had the highest ICERs.
Table 3:
Costs and effects (discounted at 5%) per 1,000 simulated 40-year-olds of all uniform screening scenarios assuming a) perfect adherence and b) realistic adherence
| a. Effects of uniform screening scenarios assuming perfect adherence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening Strategy |
FITs | Colonoscopies | Complications | CRC Incidence | CRC Mortality | Life Yearsa | Total QALYsa | Total Costsab | ICERab | ||
| Test | Start Age | Interval | |||||||||
| No Screening | 0 | 84 | 0.07 | 84 | 29 | 17,872 | 17,847 | 1,234,089 | |||
| FIT | 60 | 3 | 3,981 | 467 | 0.24 | 66 | 19 | 17,889 | 17,867 | 1,240,498 | 317 |
| FIT | 60 | 2 | 5,935 | 576 | 0.27 | 62 | 16 | 17,892 | 17,871 | 1,256,805 | 4,314 |
| FIT | 54 | 3 | 5,571 | 561 | 0.28 | 64 | 18 | 17,894 | 17,873 | 1,304,332 | Dominated |
| FIT | 60 | 1 | 9,954 | 777 | 0.34 | 56 | 15 | 17,895 | 17,875 | 1,327,965 | Dominated |
| FIT | 54 | 2 | 8,101 | 695 | 0.32 | 59 | 16 | 17,898 | 17,878 | 1,343,651 | 11,768 |
| FIT | 50 | 3 | 6,990 | 625 | 0.30 | 63 | 17 | 17,897 | 17,877 | 1,371,842 | Dominated |
| FIT | 50 | 2 | 9,473 | 759 | 0.34 | 59 | 15 | 17,901 | 17,881 | 1,436,505 | Dominated |
| FIT | 46 | 3 | 7,789 | 660 | 0.29 | 63 | 17 | 17,898 | 17,878 | 1,462,077 | Dominated |
| FIT | 54 | 1 | 13,381 | 953 | 0.40 | 53 | 14 | 17,902 | 17,884 | 1,480,562 | 23,324 |
| FIT | 46 | 2 | 10,767 | 811 | 0.35 | 59 | 16 | 17,902 | 17,883 | 1,556,681 | Dominated |
| FIT | 50 | 1 | 15,397 | 1,042 | 0.43 | 53 | 14 | 17,905 | 17,887 | 1,634,262 | 43,174 |
| FIT | 40 | 3 | 9,187 | 707 | 0.30 | 64 | 18 | 17,899 | 17,879 | 1,635,282 | Dominated |
| FIT | 40 | 2 | 12,532 | 868 | 0.36 | 60 | 16 | 17,903 | 17,884 | 1,789,931 | Dominated |
| COL | 60 | 10 | 0 | 2,198 | 0.60 | 42 | 11 | 17,900 | 17,881 | 1,789,986 | Dominated |
| FIT | 46 | 1 | 17,171 | 1,109 | 0.44 | 54 | 14 | 17,906 | 17,889 | 1,830,442 | 122,612 |
| COL | 60 | 5 | 0 | 3,048 | 0.82 | 37 | 10 | 17,902 | 17,883 | 2,117,448 | Dominated |
| FIT | 40 | 1 | 19,338 | 1,165 | 0.44 | 57 | 16 | 17,906 | 17,888 | 2,201,540 | Dominated |
| COL | 54 | 10 | 0 | 2,928 | 0.86 | 39 | 10 | 17,907 | 17,889 | 2,294,199 | Dominated |
| COL | 50 | 10 | 0 | 3,245 | 0.91 | 37 | 9 | 17,911 | 17,893 | 2,706,770 | Dominated |
| COL | 54 | 5 | 0 | 4,540 | 1.16 | 31 | 7 | 17,911 | 17,894 | 3,016,912 | Dominated |
| COL | 46 | 10 | 0 | 3,341 | 1.01 | 39 | 10 | 17,912 | 17,895 | 3,181,465 | Dominated |
| COL | 50 | 5 | 0 | 5,012 | 1.24 | 29 | 7 | 17,916 | 17,899 | 3,664,480 | 184,883 |
| COL | 40 | 10 | 0 | 4,269 | 1.09 | 36 | 9 | 17,916 | 17,898 | 4,319,443 | Dominated |
| COL | 46 | 5 | 0 | 5,700 | 1.35 | 30 | 7 | 17,919 | 17,902 | 4,593,188 | 349,139 |
| COL | 40 | 5 | 0 | 7,011 | 1.57 | 26 | 6 | 17,924 | 17,904 | 6,462,019 | 648,900 |
| b. Effects of uniform screening scenarios assuming realistic adherence | |||||||||||
| Screening Strategy |
FITs | Colonoscopies | Complications | CRC Incidence | CRC Mortality | Life Yearsa | Total QALYsa | Total Costsab | ICERab | ||
| Test | Start Age | Interval | |||||||||
| No Screening | 0 | 84 | 0.07 | 84 | 29 | 17,872 | 17,847 | 1,234,089 | Dominated | ||
| FIT | 60 | 3 | 1,952 | 228 | 0.14 | 77 | 24 | 17,879 | 17,855 | 1,249,846 | 1,936 |
| FIT | 60 | 2 | 3,022 | 285 | 0.16 | 74 | 23 | 17,881 | 17,857 | 1,259,357 | 3,446 |
| FIT | 54 | 3 | 2,488 | 255 | 0.15 | 76 | 24 | 17,880 | 17,857 | 1,278,301 | Dominated |
| FIT | 60 | 1 | 5,427 | 398 | 0.21 | 69 | 20 | 17,885 | 17,863 | 1,292,600 | 6,544 |
| FIT | 54 | 2 | 3,931 | 328 | 0.18 | 73 | 22 | 17,884 | 17,860 | 1,300,838 | Dominated |
| FIT | 50 | 3 | 3,253 | 288 | 0.17 | 75 | 23 | 17,882 | 17,859 | 1,310,216 | Dominated |
| FIT | 50 | 2 | 4,721 | 361 | 0.19 | 72 | 22 | 17,885 | 17,863 | 1,346,432 | Dominated |
| FIT | 46 | 3 | 3,691 | 305 | 0.16 | 75 | 23 | 17,883 | 17,860 | 1,351,925 | Dominated |
| FIT | 54 | 1 | 7,355 | 478 | 0.24 | 67 | 19 | 17,890 | 17,868 | 1,373,779 | 15,702 |
| FIT | 46 | 2 | 5,504 | 390 | 0.20 | 72 | 22 | 17,887 | 17,864 | 1,407,164 | Dominated |
| FIT | 40 | 3 | 4,500 | 332 | 0.17 | 75 | 23 | 17,884 | 17,861 | 1,435,530 | Dominated |
| FIT | 50 | 1 | 8,779 | 529 | 0.26 | 66 | 19 | 17,892 | 17,871 | 1,459,998 | 29,326 |
| COL | 60 | 10 | 0 | 987 | 0.30 | 65 | 21 | 17,884 | 17,862 | 1,460,994 | Dominated |
| FIT | 40 | 2 | 6,651 | 425 | 0.21 | 72 | 22 | 17,888 | 17,866 | 1,528,067 | Dominated |
| COL | 54 | 10 | 0 | 1,048 | 0.35 | 67 | 21 | 17,884 | 17,862 | 1,550,987 | Dominated |
| FIT | 46 | 1 | 10,156 | 573 | 0.27 | 66 | 19 | 17,894 | 17,873 | 1,572,125 | 56,064 |
| COL | 60 | 5 | 0 | 1,413 | 0.45 | 60 | 18 | 17,887 | 17,865 | 1,592,938 | Dominated |
| COL | 50 | 10 | 0 | 1,176 | 0.37 | 65 | 21 | 17,886 | 17,864 | 1,679,843 | Dominated |
| FIT | 40 | 1 | 12,108 | 626 | 0.28 | 67 | 19 | 17,895 | 17,874 | 1,792,630 | 151,031 |
| COL | 54 | 5 | 0 | 1,825 | 0.55 | 57 | 17 | 17,890 | 17,869 | 1,821,556 | Dominated |
| COL | 46 | 10 | 0 | 1,215 | 0.43 | 66 | 21 | 17,887 | 17,865 | 1,831,735 | Dominated |
| COL | 50 | 5 | 0 | 2,040 | 0.60 | 55 | 17 | 17,893 | 17,873 | 2,050,622 | Dominated |
| COL | 40 | 10 | 0 | 1,622 | 0.47 | 62 | 19 | 17,890 | 17,868 | 2,218,841 | Dominated |
| COL | 46 | 5 | 0 | 2,377 | 0.67 | 53 | 16 | 17,896 | 17,876 | 2,395,405 | Dominated |
| COL | 40 | 5 | 0 | 2,986 | 0.79 | 50 | 15 | 17,901 | 17,880 | 3,102,085 | 221,941 |
Abbreviations: COL = colonoscopy; CRC = colorectal cancer, FIT = faecal immunological test; ICER = incremental cost-effectiveness ratio; QALYs = quality-adjusted life years
Grey shading highlights uniform screening scenarios on the efficient frontier prior to considering personalised screening.
Results are discounted at an annual rate of 5%
Costs are presented in Australian Dollars ($AUD)
Optimal screening strategies per risk group
The efficient frontier included many of the same strategies for each risk group, however, the ICERs differed substantially (Supplementary Results Table S1, Figures S2a–e). For example, annual screening with FIT from 54 to 74 years was on the efficient frontier for all risk groups, however, the ICERs ranged from $86,929 for those at very low risk to $3,687 for those at very high risk. Considering a WTP threshold of $50,000 per QALY gained, the optimal screening strategy for those at very low risk was biennial FIT from 54 to 74 years (ICER $33,639), while for those at highest risk, the optimal strategy was 5-yearly colonoscopy from 50 to 74 years (ICER $39,568). Biennial FIT screening was only on the efficient frontier for the very low risk group (ICER $63,911).
Personalised screening
Using these efficient strategies, 39 personalised screening scenarios were created (Table 4). These scenarios (Table 5a) reduced CRC incidence by 4-68% (3-57 fewer CRC cases per 1,000 individuals) and mortality by 5-79% (2-23 fewer deaths). In addition, they yielded 0.02-0.32% more QALYs (3-58 additional QALYs) and increased costs by 19-432% ($233,599-$5,330,249). The personalised screening scenarios increased colonoscopy demand by 45-6,698 colonoscopies per 1,000 individuals (Table 5a).
Table 4:
Specifics of the personalised screening scenarios, when costs and QALYs are discounted at 5%a
| Screening Strategy | Risk Groups |
||||
|---|---|---|---|---|---|
| Very Low | Low | Average | High | Very High | |
| PS1 | NoScr | NoScr | NoScr | NoScr | FIT_60_1 |
| PS2 | NoScr | NoScr | NoScr | NoScr | FIT_54_1 |
| PS3 | NoScr | NoScr | NoScr | FIT_60_2 | FIT_54_1 |
| PS4 | NoScr | NoScr | NoScr | FIT_60_1 | FIT_54_1 |
| PS5 | NoScr | NoScr | NoScr | FIT_54_2 | FIT_54_1 |
| PS6 | NoScr | NoScr | NoScr | FIT_54_1 | FIT_54_1 |
| PS7 | NoScr | NoScr | FIT_60_2 | FIT_54_1 | FIT_54_1 |
| PS8 | NoScr | NoScr | FIT_54_2 | FIT_54_1 | FIT_54_1 |
| PS9 | NoScr | NoScr | FIT_54_2 | FIT_54_1 | FIT_50_1 |
| PS10 | NoScr | FIT_60_2 | FIT_54_2 | FIT_54_1 | FIT_50_1 |
| PS11 | NoScr | FIT_54_2 | FIT_54_2 | FIT_54_1 | FIT_50_1 |
| PS12 | NoScr | FIT_54_2 | FIT_54_1 | FIT_54_1 | FIT_50_1 |
| PS13 | NoScr | FIT_54_2 | FIT_54_1 | FIT_50_1 | FIT_50_1 |
| PS14 | FIT_54_3 | FIT_54_2 | FIT_54_1 | FIT_50_1 | FIT_50_1 |
| PS15 | FIT_54_2 | FIT_54_2 | FIT_54_1 | FIT_50_1 | FIT_50_1 |
| PS16 | FIT_54_2 | FIT_54_2 | FIT_54_1 | FIT_50_1 | COL_54_5 |
| PS17 | FIT_54_2 | FIT_54_2 | FIT_54_1 | FIT_50_1 | COL_50_5 |
| PS18 | FIT_54_2 | FIT_54_1 | FIT_54_1 | FIT_50_1 | COL_50_5 |
| PS19 | FIT_54_2 | FIT_54_1 | FIT_50_1 | FIT_50_1 | COL_50_5 |
| PS20 | FIT_54_2 | FIT_50_1 | FIT_50_1 | FIT_50_1 | COL_50_5 |
| PS21 | FIT_50_2 | FIT_50_1 | FIT_50_1 | FIT_50_1 | COL_50_5 |
| PS22 | FIT_50_2 | FIT_50_1 | FIT_50_1 | COL_50_5 | COL_50_5 |
| PS23 | FIT_50_2 | FIT_50_1 | FIT_50_1 | COL_50_5 | COL_46_5 |
| PS24 | FIT_54_1 | FIT_50_1 | FIT_50_1 | COL_50_5 | COL_46_5 |
| PS25 | FIT_50_1 | FIT_50_1 | FIT_50_1 | COL_50_5 | COL_46_5 |
| PS26 | FIT_50_1 | FIT_50_1 | FIT_46_1 | COL_50_5 | COL_46_5 |
| PS27 | FIT_50_1 | FIT_50_1 | FIT_46_1 | COL_50_5 | COL_40_5 |
| PS28 | FIT_50_1 | FIT_50_1 | FIT_46_1 | COL_46_5 | COL_40_5 |
| PS29 | FIT_50_1 | FIT_50_1 | COL_50_5 | COL_46_5 | COL_40_5 |
| PS30 | FIT_50_1 | FIT_46_1 | COL_50_5 | COL_46_5 | COL_40_5 |
| PS31 | FIT_46_1 | FIT_46_1 | COL_50_5 | COL_46_5 | COL_40_5 |
| PS32 | FIT_46_1 | FIT_46_1 | COL_50_5 | COL_40_5 | COL_40_5 |
| PS33 | FIT_46_1 | FIT_46_1 | COL_46_5 | COL_40_5 | COL_40_5 |
| PS34 | FIT_46_1 | COL_50_5 | COL_46_5 | COL_40_5 | COL_40_5 |
| PS35 | FIT_46_1 | COL_46_5 | COL_46_5 | COL_40_5 | COL_40_5 |
| PS36 | FIT_46_1 | COL_46_5 | COL_40_5 | COL_40_5 | COL_40_5 |
| PS37 | COL_50_5 | COL_46_5 | COL_40_5 | COL_40_5 | COL_40_5 |
| PS38 | COL_50_5 | COL_40_5 | COL_40_5 | COL_40_5 | COL_40_5 |
| PS39 | COL_46_5 | COL_40_5 | COL_40_5 | COL_40_5 | COL_40_5 |
Abbreviations: COL = colonoscopy; FIT = faecal immunochemical test, NoScr = no screening
Screening strategies: Screening test, screening start age, screening interval
All screening ends at or before the age of 74 years
Table 5:
Costs and effects (discounted at 5%) of per 1,000 simulated 40-year-olds of all personalised screening scenarios assuming a) perfect adherence and b) realistic adherence
| a. Effects of personalised
screening scenarios assuming perfect adherence | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening Strategy | FITs | Colonoscopies | Complications | CRC Incidence | CRC Mortality | Life Yearsa | Total QALYsa | Total Costsab | ICERab |
| No Screening | 0 | 84 | 0.07 | 84 | 29 | 17,872 | 17,847 | 1,234,089 | |
| PS01 | 360 | 130 | 0.09 | 81 | 27 | 17,874 | 17,850 | 1,467,668 | Dominated |
| PS02 | 515 | 141 | 0.10 | 81 | 27 | 17,875 | 17,851 | 1,471,312 | Dominated |
| PS03 | 1,722 | 276 | 0.16 | 73 | 23 | 17,882 | 17,859 | 1,505,808 | Dominated |
| PS04 | 2,554 | 324 | 0.18 | 71 | 22 | 17,883 | 17,860 | 1,513,829 | Dominated |
| PS05 | 2,190 | 305 | 0.18 | 72 | 23 | 17,884 | 17,861 | 1,520,843 | Dominated |
| PS06 | 3,322 | 368 | 0.20 | 70 | 22 | 17,885 | 17,863 | 1,542,584 | Dominated |
| PS07 | 5,226 | 525 | 0.27 | 63 | 18 | 17,891 | 17,871 | 1,628,434 | Dominated |
| PS08 | 5,920 | 562 | 0.28 | 63 | 18 | 17,893 | 17,873 | 1,656,571 | Dominated |
| PS09 | 6,017 | 568 | 0.28 | 63 | 18 | 17,894 | 17,873 | 1,662,780 | 15,998 |
| PS10 | 7,406 | 663 | 0.32 | 59 | 16 | 17,897 | 17,877 | 1,734,789 | 19,167 |
| PS11 | 7,898 | 687 | 0.33 | 59 | 16 | 17,898 | 17,878 | 1,756,804 | 19,251 |
| PS12 | 9,596 | 770 | 0.35 | 57 | 15 | 17,899 | 17,880 | 1,801,421 | 24,261 |
| PS13 | 10,057 | 792 | 0.36 | 57 | 15 | 17,900 | 17,881 | 1,834,425 | 28,076 |
| PS14 | 11,189 | 859 | 0.38 | 55 | 14 | 17,902 | 17,884 | 1,911,354 | 32,041 |
| PS15 | 11,700 | 881 | 0.39 | 55 | 14 | 17,902 | 17,884 | 1,924,610 | 33,605 |
| PS16 | 11,089 | 991 | 0.43 | 52 | 14 | 17,903 | 17,885 | 1,952,870 | 38,059 |
| PS17 | 11,089 | 1,023 | 0.44 | 52 | 13 | 17,903 | 17,885 | 1,978,086 | 39,563 |
| PS18 | 12,293 | 1,078 | 0.45 | 51 | 13 | 17,904 | 17,886 | 2,013,777 | 39,692 |
| PS19 | 12,939 | 1,106 | 0.46 | 51 | 13 | 17,905 | 17,887 | 2,063,215 | 45,682 |
| PS20 | 13,382 | 1,125 | 0.47 | 51 | 13 | 17,905 | 17,888 | 2,098,112 | 63,213 |
| PS21 | 13,646 | 1,136 | 0.47 | 51 | 13 | 17,906 | 17,888 | 2,116,904 | 64,062 |
| PS22 | 10,378 | 1,951 | 0.68 | 43 | 11 | 17,909 | 17,893 | 2,487,033 | 81,839 |
| PS23 | 10,378 | 1,969 | 0.69 | 43 | 11 | 17,910 | 17,893 | 2,519,388 | 82,386 |
| PS24 | 11,148 | 2,001 | 0.69 | 43 | 11 | 17,910 | 17,893 | 2,534,168 | 86,970 |
| PS25 | 11,518 | 2,017 | 0.70 | 43 | 11 | 17,910 | 17,894 | 2,564,321 | 95,845 |
| PS26 | 12,085 | 2,037 | 0.70 | 43 | 11 | 17,911 | 17,894 | 2,627,025 | 127,618 |
| PS27 | 12,085 | 2,100 | 0.71 | 43 | 11 | 17,911 | 17,895 | 2,699,821 | 135,361 |
| PS28 | 12,085 | 2,209 | 0.74 | 43 | 11 | 17,912 | 17,896 | 2,880,990 | 172,640 |
| PS29 | 6,575 | 3,465 | 0.99 | 35 | 9 | 17,915 | 17,899 | 3,469,210 | 180,219 |
| PS30 | 6,964 | 3,480 | 0.99 | 35 | 9 | 17,915 | 17,899 | 3,512,866 | 181,642 |
| PS31 | 7,289 | 3,492 | 1.00 | 35 | 9 | 17,916 | 17,899 | 3,549,627 | 226,549 |
| PS32 | 7,289 | 3,804 | 1.05 | 34 | 8 | 17,917 | 17,901 | 3,943,469 | 282,951 |
| PS33 | 7,289 | 4,017 | 1.09 | 34 | 8 | 17,918 | 17,902 | 4,238,838 | 349,821 |
| PS34 | 3,372 | 4,929 | 1.25 | 30 | 7 | 17,920 | 17,903 | 4,694,318 | 370,115 |
| PS35 | 3,372 | 5,105 | 1.28 | 30 | 7 | 17,920 | 17,903 | 4,915,213 | 696,391 |
| PS36 | 3,372 | 5,531 | 1.35 | 29 | 7 | 17,922 | 17,904 | 5,515,822 | 707,094 |
| PS37 | 0 | 6,328 | 1.47 | 27 | 6 | 17,922 | 17,904 | 5,935,615 | 1,808,159 |
| PS38 | 0 | 6,612 | 1.51 | 27 | 6 | 17,923 | 17,904 | 6,365,416 | 2,057,076 |
| PS39 | 0 | 6,782 | 1.54 | 27 | 6 | 17,923 | 17,905 | 6,564,338 | 3,860,049 |
| b. Effects of personalised
screening scenarios assuming realistic adherence | |||||||||
| Screening Strategy | FITs | Colonoscopies | Complications | CRC Incidence | CRC Mortality | Life Yearsa | Total QALYsa | Total Costsab | ICERab |
| No Screening | 0 | 84 | 0.07 | 84 | 29 | 17,872 | 17,847 | 1,234,089 | |
| PS01 | 203 | 107 | 0.08 | 82 | 28 | 17,873 | 17,848 | 1,473,736 | Dominated |
| PS02 | 283 | 112 | 0.08 | 82 | 28 | 17,874 | 17,849 | 1,475,367 | Dominated |
| PS03 | 908 | 168 | 0.11 | 79 | 26 | 17,877 | 17,853 | 1,521,883 | Dominated |
| PS04 | 1,402 | 198 | 0.13 | 77 | 25 | 17,878 | 17,854 | 1,523,703 | Dominated |
| PS05 | 1,101 | 180 | 0.12 | 78 | 25 | 17,878 | 17,854 | 1,529,310 | Dominated |
| PS06 | 1,814 | 218 | 0.14 | 76 | 25 | 17,880 | 17,856 | 1,538,207 | Dominated |
| PS07 | 2,783 | 282 | 0.17 | 73 | 23 | 17,882 | 17,859 | 1,623,617 | Dominated |
| PS08 | 3,074 | 296 | 0.17 | 73 | 22 | 17,883 | 17,860 | 1,637,150 | Dominated |
| PS09 | 3,134 | 299 | 0.18 | 73 | 22 | 17,883 | 17,861 | 1,640,166 | Dominated |
| PS10 | 3,834 | 337 | 0.19 | 71 | 21 | 17,885 | 17,862 | 1,705,874 | Dominated |
| PS11 | 4,042 | 345 | 0.19 | 71 | 21 | 17,885 | 17,863 | 1,716,293 | Dominated |
| PS12 | 5,140 | 393 | 0.21 | 69 | 20 | 17,887 | 17,865 | 1,740,403 | Dominated |
| PS13 | 5,447 | 405 | 0.22 | 69 | 20 | 17,888 | 17,866 | 1,757,876 | 26,955 |
| PS14 | 5,946 | 428 | 0.23 | 68 | 20 | 17,889 | 17,867 | 1,820,048 | Dominated |
| PS15 | 6,239 | 439 | 0.23 | 68 | 20 | 17,889 | 17,867 | 1,827,488 | Dominated |
| PS16 | 5,897 | 479 | 0.25 | 67 | 20 | 17,889 | 17,867 | 1,832,472 | Dominated |
| PS17 | 5,897 | 494 | 0.25 | 67 | 20 | 17,889 | 17,867 | 1,840,654 | Dominated |
| PS18 | 6,688 | 525 | 0.26 | 66 | 19 | 17,890 | 17,869 | 1,861,200 | Dominated |
| PS19 | 7,144 | 541 | 0.27 | 66 | 19 | 17,891 | 17,869 | 1,888,754 | 37,121 |
| PS20 | 7,468 | 552 | 0.27 | 66 | 19 | 17,891 | 17,870 | 1,909,081 | 43,619 |
| PS21 | 7,625 | 557 | 0.27 | 66 | 19 | 17,891 | 17,870 | 1,918,670 | 50,380 |
| PS22 | 5,787 | 873 | 0.37 | 62 | 18 | 17,892 | 17,871 | 2,021,485 | Dominated |
| PS23 | 5,787 | 885 | 0.37 | 62 | 18 | 17,892 | 17,872 | 2,032,352 | Dominated |
| PS24 | 6,320 | 903 | 0.37 | 61 | 18 | 17,893 | 17,872 | 2,043,097 | Dominated |
| PS25 | 6,598 | 911 | 0.38 | 61 | 18 | 17,893 | 17,872 | 2,060,946 | Dominated |
| PS26 | 7,039 | 925 | 0.38 | 61 | 18 | 17,893 | 17,873 | 2,096,862 | Dominated |
| PS27 | 7,039 | 955 | 0.39 | 61 | 18 | 17,894 | 17,873 | 2,123,266 | Dominated |
| PS28 | 7,039 | 1,016 | 0.41 | 60 | 17 | 17,895 | 17,875 | 2,188,845 | 61,316 |
| PS29 | 3,785 | 1,489 | 0.51 | 56 | 17 | 17,895 | 17,875 | 2,342,739 | Dominated |
| PS30 | 4,097 | 1,499 | 0.52 | 56 | 17 | 17,895 | 17,875 | 2,368,540 | Dominated |
| PS31 | 4,366 | 1,507 | 0.52 | 56 | 17 | 17,895 | 17,875 | 2,390,967 | Dominated |
| PS32 | 4,366 | 1,654 | 0.55 | 55 | 16 | 17,897 | 17,877 | 2,539,024 | Dominated |
| PS33 | 4,366 | 1,759 | 0.58 | 55 | 16 | 17,898 | 17,878 | 2,648,884 | 148,949 |
| PS34 | 2,029 | 2,100 | 0.64 | 53 | 16 | 17,898 | 17,878 | 2,770,951 | Dominated |
| PS35 | 2,029 | 2,182 | 0.66 | 53 | 16 | 17,898 | 17,878 | 2,854,071 | Dominated |
| PS36 | 2,029 | 2,380 | 0.69 | 52 | 15 | 17,899 | 17,879 | 3,081,587 | 268,852 |
| PS37 | 0 | 2,675 | 0.74 | 51 | 15 | 17,899 | 17,879 | 3,196,125 | Dominated |
| PS38 | 0 | 2,805 | 0.76 | 50 | 15 | 17,900 | 17,880 | 3,359,342 | Dominated |
| PS39 | 0 | 2,883 | 0.78 | 50 | 15 | 17,900 | 17,880 | 3,434,699 | 677,027 |
Abbreviations: CRC = colorectal cancer; FIT = faecal immunochemical test; PS = personalised screening; QALYs = quality-adjusted life years
Grey shading highlights screening scenarios on the efficient frontier prior to considering uniform screening.
Results are discounted at an annual rate of 5%.
Costs are presented in Australian Dollars ($AUD)
The personalised screening scenarios are described in Table 4
At a WTP threshold of $50,000 per QALY gained, the optimal personalised screening scenario consisted of the following: those at very low risk or low risk screening should start at age 54 with biennial and annual FIT respectively, those at average and high risk screening should start at age 50 with annual FIT and those at very high risk screening should start at age 50 with 5-yearly colonoscopy (ICER $45,682).
Uniform screening versus personalised screening
When compared, personalised and uniform screening scenarios similarly reduced CRC incidence and mortality and yielded similar QALYs. Personalised screening more efficiently allocated colonoscopy demand, however it cost more than uniform screening, due to the cost of determining risk. Although several scenarios from each type of screening were on the efficient frontier (Figure 1a), all of the personalised screening scenarios had an ICER above $100,000 and would therefore not be considered cost effective. At a WTP threshold of $50,000 per QALY gained, the optimal screening scenario was annual FIT screening from 50 to 74 years.
Figure 1:
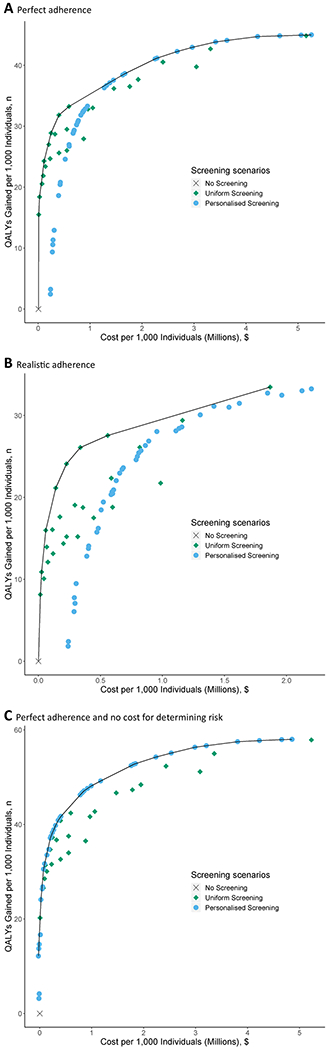
Costs and quality-adjusted life years (discounted at 5%) per 1,000 40-year-olds for all uniform and personalised colorectal cancer screening scenarios and a scenario without screening, with the efficient frontier connecting the economically efficient strategiesa assuming: a) perfect adherence; b) realistic adherence and c) perfect adherence and no costs associated with determining risk.
Abbreviations: QALYs = quality-adjusted life years
Note: A description of the personalised screening scenarios can be found in Table 4.
a. Discounted costs and life years gained reflect total costs and life years gained of a screening program, accounting for time preference for present over future outcomes. Quality-adjusted life years gained are plotted on the y-axis, and total costs are plotted on the x-axis. Each possible screening strategy is represented by a point. Strategies that form the solid line connecting the points lying left and upward are the economically rational subset of choices. This line is called the efficient frontier. The inverse slope of the line represents the incremental cost-effectiveness ratio of the connected strategies. Points lying to the right and beneath the line represent the dominated strategies.
Realistic adherence
As might be expected, the application of realistic participation rates decreased the health benefits as well as the costs of all screening scenarios. At this level of participation, none of the personalised screening scenarios were cost effective compared to uniform screening (Figure 1b).
Sensitivity analyses
Our results were not sensitive to changes in discounting, weighting of QALYs or adjustments to rates of participation (Supplementary Results Tables S2–5, Figures S3–6). However, excluding the costs of determining polygenic risk had a significant impact on our results with personalised screening dominating uniform screening scenarios at both perfect (Figure 1c) and realistic adherence (Supplementary Results Figure S7). The threshold analysis indicated that for personalised screening to be cost effective compared to uniform screening at the WTP of $50,000 per QALY gained, the cost for determining risk should not exceed $47.52 (Supplementary Results Table S8).
Discussion
We investigated the impact of personalising CRC screening based on polygenic risk and family history. We found that uniform screening was equally effective (cancers and deaths prevented) but more cost effective than personalised screening. Although personalised and uniform screening showed similar reductions in CRC incidence and mortality and similar gains in QALYs, personalised screening incurred additional costs resulting from the whole population undergoing testing to determine their CRC risk.
The concept of personalised screening is promising and has previously been shown to be more effective than a strategy based on age alone.7, 21–23 Our results add support to these findings. However, our results do not align with recent findings that risk-stratified screening based on polygenic risk profile for breast cancer is cost effective compared to the standard age-based screening program.46 This discrepancy may be due to differences in the discriminatory performance of risk stratification algorithms or differences in the cost for determining risk, which was substantially lower in this analysis (£50 or ~$90) than in ours (~$240). However, it is difficult to accurately determine how much of the cost for establishing risk should be allocated to a screening program. The cost of polygenic testing varies widely47 and there is potential to combine testing for other cancers. Given this difficulty and because cost-effectiveness of personalised screening is highly dependent on these additional costs, we assessed the impact of excluding them. We found that when these costs were excluded, personalised screening was cost effective. The threshold analysis suggested that at a WTP of $50,000 per QALY gained, the cost to determine risk should not exceed ~$48, which is significantly lower than the cost assumed in this analysis.
The effectiveness of personalised screening will be impacted by the precision with which the population is stratified.15 This will be affected by both the accuracy of the metric used to stratify the population and the proportion of the population willing to undertake polygenic testing. Although our results appear unfavourable, the advantage of screening based on polygenic risk and family history remains limited largely because the current contribution of known SNPs to CRC risk is modest.9, 10, 48 As new SNPs are identified, the discriminatory utility of polygenic testing will increase and the performance of risk assessment based on this metric could improve.15 The inclusion of other factors in risk assessment, such as obesity and smoking status, may also enhance the discriminatory performance of personalised screening.13, 49, 50 It may also be pertinent to consider results from an individual’s screening history. As these factors will vary over an individual’s lifespan, assessment of risk may need to become more dynamic in nature and although such inclusions will present challenges, they will likely improve the harm–benefit ratio of CRC screening.
In addition, although genetic testing for CRC has been shown to be acceptable to the community,51 individuals may not always be willing to undergo testing, for a variety of reasons, including concerns over privacy, possible misuse of data, and potential negative psychological impacts of findings.52–54 For this analysis, we assumed all individuals would undergo testing to determine their risk profile however, due consideration of how to manage this issue is required.
The benefits of population screening are largely dependent on participation. With many countries already experiencing suboptimal levels of participation in routine age-based screening for CRC,33, 55 personalised screening presents an interesting proposition. On one hand, increasing the complexity of screening may reduce participation in screening, thereby diminishing the modest benefits. However, individuals at increased risk of CRC have been shown to be more compliant to screening guidelines than those at average risk,56 suggesting that the provision of risk information may assist in screening uptake.16, 57 Coupled with evidence that involvement of GPs improves participation in CRC screening,58 a simple risk assessment has the potential to positively impact screening participation.59 When we applied realistic rates of participation we found that personalised screening remained suboptimal compared to uniform screening, even when participation in personalised screening was improved (Supplementary Results, Figure S6). This suggests that at present, increasing participation in uniform screening will likely yield better results.
Screening effectiveness will also be impacted by the choice of screening test and screening frequency. This will largely be determined by a health systems capacity to provide a given intervention to its population. Our analysis indicates that screening scenarios utilising colonoscopy are the most effective scenarios. However, as would be expected, these scenarios significantly increase colonoscopy utilisation. Although personalised screening more efficiently allocated colonoscopy utilisation compared to uniform screening, such increases in demand will likely be infeasible, especially in countries with limited colonoscopy capacity.
Moving from an age-based screening program will result in a redistribution of the harms and benefits. This raises ethical issues as although personalised screening may be optimal at a population level, individuals may experience increased harms or reduced benefits as a result of their screening protocol. As would be expected, our analysis indicates that when individuals undergo less frequent screening (either by starting screening later or by having a longer screening interval) they experience higher CRC incidence and mortality. However, this will be partly offset by a reduction in other harms such as invasive tests, false-positive test results, adverse events, anxiety and inconvenience. These concerns hold for the inclusion of younger individuals although recent evidence suggests that their inclusion is favourable.60
Limitations exist with our research. First, we only considered a limited number of risk categories. Effectiveness and cost-effectiveness could be further improved as the discriminatory performance of risk stratification improves. Second, we only included a limited number of low intensity screening strategies. It is possible that other low intensity screening strategies, such as one-off colonoscopy or less frequent FIT screening would be more efficient. Third, we did not compare the (cost-)effectiveness of stratifying the population based on family history alone. However, as the aim of this research was to explore the possible implications of combining SNPs and family history in a risk assessment, and, as determining polygenic risk is assumed to be quite expensive, such a comparison would potentially make this analysis look even less cost effective. Finally, there is some uncertainty regarding the assumptions for participation in screening. We assumed that participation in screening of any form would be equal to participation in uniform biennial FIT screening. However this is unlikely as participation in screening varies widely.5 Unfortunately, to date, there is little data examining multiple screening modalities within one population to adequately address this concern.
In summary, this research presents an exploration of the possible impact of personalised screening for CRC based on polygenic risk and family history. Our results suggest that although personalising screening based on CRC risk is slightly more effective than screening based on age alone, it is currently not necessarily cost effective. Cost-effectiveness of personalised screening will depend on the costs of determining risk and the magnitude of the benefits of personalisation. Our analysis suggests that the currently assumed cost of determining risk is too high compared to the gains and costs must be substantially lower for personalised screening to become cost effective. The balance of cost and benefits will be contingent on the discriminatory performance of risk-stratification algorithms on polygenic risk and family history, which remains sub-optimal.
However, we cannot ignore the changing landscape that advances in technology provide and, as improvements in risk stratification occur and costs for polygenic testing decrease, personalising screening will become an increasingly cost-effective and attractive option. This consortium of researchers, and others, have previously called for the concept of personalised screening to be brought to the attention of key stakeholders.15, 61 Our research seeks to highlight the possible implications of personalised screening based on risk assessment, which we believe can and will play a significant role in improving our screening programs. As such, we reiterate our call that key stakeholders carefully consider the evidence for personalised screening in order to effectively plan for the future.
Supplementary Material
Acknowldgements
DR Cenin, DB Preen, HC Ee, P O’Leary and I Lansdorp-Vogelaar received funding from the Cancer Council Western Australia Capacity and Collaboration Building Grant. This research also benefitted from our participation in the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) (grant number: U01-CA199335). MA Jenkins is a Senior Research Fellow for the Australian National Health and Medical Research Council.
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. DR Cenin and I Lansdorp-Vogelaar had full access to all the data and DR Cenin had final responsibility for the decision to submit for publication. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding bodies.
Footnotes
MA Jenkins reports receiving other commercial research support and has ownership interest (including patents) in Genetype Pty Ltd. No potential conflicts of interest were disclosed by the other authors.
All authors have completed the ICMJE Conflict of Interest form (available on request from the corresponding author) and declare no direct conflict of interest.
References
- 1.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–71. [DOI] [PubMed] [Google Scholar]
- 2.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. The Lancet. 1996;348(9040):1472–7. [DOI] [PubMed] [Google Scholar]
- 3.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. The Lancet. 1996;348(9040):1467–71. [DOI] [PubMed] [Google Scholar]
- 4.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–49. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Council Australia Colorectal Cancer Guidelines Working Party. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer [Internet]. Sydney: Cancer Council Australia; 2018. [updated Version URL: https://wiki.cancer.org.au/australiawiki/index.php?oldid=191477. Available from: https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer. [Google Scholar]
- 7.So HC, Kwan JS, Cherny SS, Sham PC. Risk prediction of complex diseases from family history and known susceptibility loci, with applications for cancer screening. Am J Hum Genet. 2011;88(5):548–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do CB, Hinds DA, Francke U, Eriksson N. Comparison of family history and SNPs for predicting risk of complex disease. PLoS Genet. 2012;8(10):e1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop MG, Tenesa A, Farrington SM, Ballereau S, Brewster DH, Koessler T, et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42,103 individuals. Gut. 2013;62(6):871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Tassan NA, Whiffin N, Hosking FJ, Palles C, Farrington SM, Dobbins SE, et al. A new GWAS and meta-analysis with 1000Genomes imputation identifies novel risk variants for colorectal cancer. Sci Rep. 2015;5:10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters U, Bien S, Zubair N. Genetic architecture of colorectal cancer. Gut. 2015;64(10):1623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huyghe JR, Bien SA, Harrison TA, Kang HM, Chen S, Schmit SL, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019;51(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee N, Shi J, Garcia-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17(7):392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins MA, Makalic E, Dowty JG, Schmidt DF, Dite GS, MacInnis RJ, et al. Quantifying the utility of single nucleotide polymorphisms to guide colorectal cancer screening. Future Oncol. 2016;12(4):503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury S, Dent T, Pashayan N, Hall A, Lyratzopoulos G, Hallowell N, et al. Incorporating genomics into breast and prostate cancer screening: assessing the implications. Genet Med. 2013;15(6):423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawken SJ, Greenwood CM, Hudson TJ, Kustra R, McLaughlin J, Yang Q, et al. The utility and predictive value of combinations of low penetrance genes for screening and risk prediction of colorectal cancer. Hum Genet. 2010;128(1):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury MJ, Janssens AC, Ransohoff DF. How can polygenic inheritance be used in population screening for common diseases? Genet Med. 2013;15(6):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surveillance Epidemiology and End Results Program. Cancer stat facts: colon and rectum cancer: National Cancer Institute; 2017. [Available from: http://seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 20.Troeung L, Sodhi-Berry N, Martini A, Malacova E, Ee H, O’Leary P, et al. Increasing Incidence of Colorectal Cancer in Adolescents and Young Adults Aged 15-39 Years in Western Australia 1982-2007: Examination of Colonoscopy History. Front Public Health. 2017;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pashayan N, Duffy SW, Chowdhury S, Dent T, Burton H, Neal DE, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104(10):1656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frampton MJ, Law P, Litchfield K, Morris EJ, Kerr D, Turnbull C, et al. Implications of polygenic risk for personalised colorectal cancer screening. Ann Oncol. 2016;27(3):429–34. [DOI] [PubMed] [Google Scholar]
- 23.Hsu L, Jeon J, Brenner H, Gruber SB, Schoen RE, Berndt SI, et al. A model to determine colorectal cancer risk using common genetic susceptibility loci. Gastroenterology. 2015;148(7):1330–9 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32(1):13–33. [DOI] [PubMed] [Google Scholar]
- 25.Australian Institute of Health and Welfare. National Bowel Cancer Screening Program monitoring report: phase 2, July 2008- June 2011 Canberra: Australian Institute of Health and Welfare; 2012. [Available from: https://www.aihw.gov.au/reports/cancer-screening/bowel-cancer-screening-2008-2011/contents/table-of-contents. [Google Scholar]
- 26.Tran B, Keating CL, Ananda SS, Kosmider S, Jones I, Croxford M, et al. Preliminary analysis of the cost-effectiveness of the National Bowel Cancer Screening Program: demonstrating the potential value of comprehensive real world data. Intern Med J. 2011;42(7):794–800. [DOI] [PubMed] [Google Scholar]
- 27.Ananda SS, McLaughlin SJ, Chen F, Hayes IP, Hunter AA, Skinner IJ, et al. Initial impact of Australia’s National Bowel Cancer Screening Program. Med J Aust. 2009;191(7):378–81. [DOI] [PubMed] [Google Scholar]
- 28.Australian Bureau of Statistics; 3302.0.55.001 - Life Tables, States, Territories and Australia, 2013-2015 [Internet]. Canberra: Australian Bureau of Statistics; 2017. [updated 2016 Oct 27 Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/97E435FA3B82A89DCA2570A6000573D3?opendocument. [Google Scholar]
- 29.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331(25):1669–74. [DOI] [PubMed] [Google Scholar]
- 30.Appleyard M, Grimpen F, Spucches C, Si D, A T. Participation in the national bowel cancer screening program and screening outcomes in Queensland. J Gastroenterol Hepatol. 2011;26(Suppl 4):29. [Google Scholar]
- 31.Queensland Health. Queensland Bowel Cancer Screening Program: Statistical Report August 2006 – December 2010. Internet. Brisbane: Queensland Health, 2011. [Google Scholar]
- 32.Cancer Council Australia Colonoscopy Surveillance Working Party. Clinical Practice Guidelines for Surveillance Colonoscopy – in adenoma follow-up; following curative resection of colorectal cancer; and for cancer surveillance in inflammatory bowel disease Sydney: Cancer Council Australia; 2011. [ [Google Scholar]
- 33.Australian Institute of Health and Welfare. National Bowel Cancer Screening Program: monitoring report 2017. Cancer series no. 104. Cat. no. CAN 103 Canberra: AIHW; 2017. [Available from: https://www.aihw.gov.au/reports/cancer-screening/bowel-cancer-screening-program-monitoring-2017/contents/table-of-contents. [Google Scholar]
- 34.Colquhoun P, Chen HC, Kim JI, Efron J, Weiss EG, Nogueras JJ, et al. High compliance rates observed for follow up colonoscopy post polypectomy are achievable outside of clinical trials: efficacy of polypectomy is not reduced by low compliance for follow up. Colorectal Dis. 2004;6(3):158–61. [DOI] [PubMed] [Google Scholar]
- 35.Clinical Genomics. ColoVantage Home Test Kit: Clinical Genomics; 2016. [Available from: http://www.colovantage.com.au/Store/ProdID/1/ColoVantage_Home.
- 36.MBS Online. Medicare Benefits Schedule - Item 73934: Australian Government Department of Health; 2016. [Available from: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=73934&qt=item&criteria=73934%20. [Google Scholar]
- 37.MBS Online. Medicare Benefits Schedule - Item 23: Australian Government Department of Health; 2016. [updated 5 November 2013 Available from: http://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=23&qt=item&criteria=23. [Google Scholar]
- 38.Independent Hospital Pricing Authority. Australian Public Hospitals Cost Report 2013-2014 Round 18 [Internet]. Sydney: Independent Hospital Pricing Authority; 2016. [updated 11 February 2016 Available from: https://www.ihpa.gov.au/publications/australian-public-hospitals-cost-report-2013-2014-round-18. [Google Scholar]
- 39.Ananda S, Kosmider S, Tran B, Field K, Jones I, Skinner I, et al. The rapidly escalating cost of treating colorectal cancer in Australia. Asia Pac J Clin Oncol. 2016;12(1):33–40. [DOI] [PubMed] [Google Scholar]
- 40.Australian Bureau of Statistics. Consumer Price Index Inflation Calculator [Internet]. Canberra: Australian Bureau of Statistics; 2016. [updated 26 July 2016 Available from: http://www.abs.gov.au/websitedbs/d3310114.nsf/home/Consumer+Price+Index+Inflation+Calculator. [Google Scholar]
- 41.BREVAGenplus. Pay for your test: Genetic Technologies Limited; 2018. [updated 2018 Available from: http://www.brevagenplus.com/payment/. [Google Scholar]
- 42.Ness R, Holmes A, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94(6):1650–7. [DOI] [PubMed] [Google Scholar]
- 43.Australian Government Department of Health. The Pharmaceutical Benefits Advisory Committee (PBAC) Guidelines: Australian Government Department of Health; 2016. [updated 2016 Sep. Available from: https://pbac.pbs.gov.au/section-d/section-d-cea/d4-variables-in-the-economic-evaluation.html. [Google Scholar]
- 44.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. [DOI] [PubMed] [Google Scholar]
- 45.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 46.Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and Benefit-to-Harm Ratio of Risk-Stratified Screening for Breast Cancer: A Life-Table Model. JAMA Oncol. 2018;4(11):1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.NIH: US National Library of Medicine. What is the cost of genetic testing, and how long does it take to get the results? 2017. [updated 26 September 2017 Available from: https://ghr.nlm.nih.gov/primer/testing/costresults.
- 48.Jiao S, Peters U, Berndt S, Brenner H, Butterbach K, Caan BJ, et al. Estimating the heritability of colorectal cancer. Hum Mol Genet. 2014;23(14):3898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Win AK, Macinnis RJ, Hopper JL, Jenkins MA. Risk prediction models for colorectal cancer: a review. Cancer Epidemiol Biomarkers Prev. 2012;21(3):398–410. [DOI] [PubMed] [Google Scholar]
- 50.Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology. 2018;154(8):2152–64 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholls SG, Etchegary H, Carroll JC, Castle D, Lemyre L, Potter BK, et al. Attitudes to incorporating genomic risk assessments into population screening programs: the importance of purpose, context and deliberation. BMC Med Genomics. 2016;9(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholls SG, Wilson BJ, Craigie SM, Etchegary H, Castle D, Carroll JC, et al. Public attitudes towards genomic risk profiling as a component of routine population screening. Genome. 2013;56(10):626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor S A population-based survey in Australia of men’s and women’s perceptions of genetic risk and predictive genetic testing and implications for primary care. Public Health Genomics. 2011;14(6):325–36. [DOI] [PubMed] [Google Scholar]
- 54.Hall AE, Chowdhury S, Hallowell N, Pashayan N, Dent T, Pharoah P, et al. Implementing risk-stratified screening for common cancers: a review of potential ethical, legal and social issues. J Public Health (Oxf). 2014;36(2):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altobelli E, Lattanzi A, Paduano R, Varassi G, di Orio F. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med. 2014;62:132–41. [DOI] [PubMed] [Google Scholar]
- 56.Rees G, Martin PR, Macrae FA. Screening participation in individuals with a family history of colorectal cancer: a review. Eur J Cancer Care (Engl). 2008;17(3):221–32. [DOI] [PubMed] [Google Scholar]
- 57.Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013(2):CD001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hewitson P, Ward AM, Heneghan C, Halloran SP, Mant D. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br J Cancer. 2011;105(4):475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Church T Colorectal cancer screening: will non-invasive procedures triumph? Genome Med. 2014;6(6):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cenin D, O’Leary P, Lansdorp-Vogelaar I, Preen D, Jenkins M, Moses E. Integrating personalised genomics into risk stratification models of population screening for colorectal cancer. Aust N Z J Public Health. 2017;41(1):3–4. [DOI] [PubMed] [Google Scholar]
- 62.Schroy PC 3rd, Coe A, Chen CA, O’Brien MJ, Heeren TC. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(2):343–50. [DOI] [PubMed] [Google Scholar]
- 64.Viiala CH, Zimmerman M, Cullen DJ, Hoffman NE. Complication rates of colonoscopy in an Australian teaching hospital environment. Intern Med J. 2003;33(8):355–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


